Abstract
Viral infections are responsible for many illnesses, and recent outbreaks have raised public health concerns. Despite the availability of many antiviral drugs, they are often unsuccessful due to the generation of viral mutants and less effective against their target virus. Identifying novel antiviral drugs is therefore of critical importance and natural products are an excellent source for such discoveries. Coumarin is one such natural compound that is a potential drug candidate owing to its properties of stability, solubility, and low toxicity. There are numerous evidences showing its inhibitory role against infection of various viruses such as HIV, Influenza, Enterovirus 71 (EV71) and coxsackievirus A16 (CVA16). The mechanisms involve either inhibition of proteins essential for viral entry, replication and infection or regulation of cellular pathways such as Akt-Mtor (mammalian target of rapamycin), NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), and anti-oxidative pathway including NrF-2 (The nuclear factor erythroid 2 (NFE2)-related factor 2). This review summarizes the present state of understanding with a focus on coumarin's antiviral effect and their possible molecular mechanisms against Influenza virus, HIV, Hepatitis virus, Dengue virus and Chikungunya virus.
Keywords: Microbiology, Pharmaceutical science, Health sciences, Public health, Pharmacology, Coumarin, Antiviral, Viral replication, Natural product, Inhibitor, Mechanism
Microbiology; Pharmaceutical science; Health sciences; Public health; Pharmacology; Coumarin; Antiviral; Viral replication; Natural product; Inhibitor; Mechanism.
1. Introduction
Viruses pose a global threat and add serious medical and social problems to the mankind. They are the main contributors in many minor and major outbreaks, epidemics and pandemics worldwide (Table 1), like Swine Influenza, Avian Influenza, Dengue fever. Even though various types of treatment methods are available to cure viral diseases, like chemotherapy [1, 2, 3, 4] but owing to their potential for mutation and emergence of new strains and developing resistance towards drugs [5, 6] viruses are evolving fast [7]. This necessitates the search for new antiviral compounds that are more potent and effective against viruses with no or less adverse side effects [8, 9, 10].
Table 1.
Overview of outbreaks of some viral diseases across the world.
| Viral diseases | Period of occurrence | Estimated number of deaths | References |
|---|---|---|---|
| Influenza | H1N1 Spanish Influenza (1918) | 50-100 million | [79] |
| H1N1 Swine Influenza (2009) | 284,500 | [80] | |
| H5N1 Avian (bird) Influenza- (2003–2019) | 455 | [81] | |
| Seasonal Influenza (Annually) | 0.29–0.65 million | [82] | |
| Viral Hepatitis B &C | Annually | 1.4 million | [83] |
| HIV | 1981–2018 | 32 million | [84] |
| Dengue | Annually | 25,000 | [85] |
Various natural compounds including herbal products and plant extracts have been investigated for their antiviral activities against several viruses [11, 12, 13, 14]. One natural compound that has been studied is coumarin, and the factors which makes it a good candidate for an anti-viral drug, are its role in targeting various cellular pathways, inhibiting the growth and replication of viruses. Antiviral activity of coumarin and its derivatives has been observed against a wide range of viruses such as influenza viruses, HIV, Enterovirus 71 (EV71), coxsackievirus A16 (CVA16), dengue virus and chikungunya virus [15, 16, 17, 18].
This review will elucidate the antiviral aspects of coumarins and possible targets and mechanisms underlying its inhibitory effects and will also illustrate its potential relevance in viral diseases and antiviral therapies (See Figure 1, Figure 2).
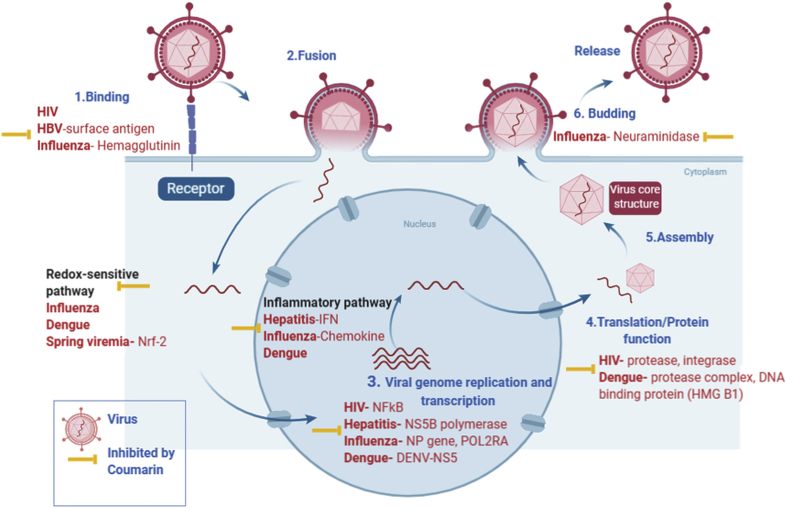
Figure 1.
Antiviral Mechanism of coumarin in Infected Host cells: Viral infection involves various stages (1) binding of virion with receptor present at the cell surface, followed by (2) fusion and entry, (3) viral genome replication and transcription, (4) translation and (5) virion assembly, and ultimately budding and release. Chemotherapeutics target these critical stages of viral life cycle. Likewise, coumarin inhibits many of the proteins involved in the transcription/translation machineries required in virus life cycle. Additionally, coumarin modulates the host cell signaling pathways, NF-κB, and inflammatory redox-sensitive pathways, which block the virus replication. Many facets of anti-viral effect of coumarin has been demonstrated in numerous viruses as indicated. (Figure was made using Biorender Online tool and Microsoft powerpoint).
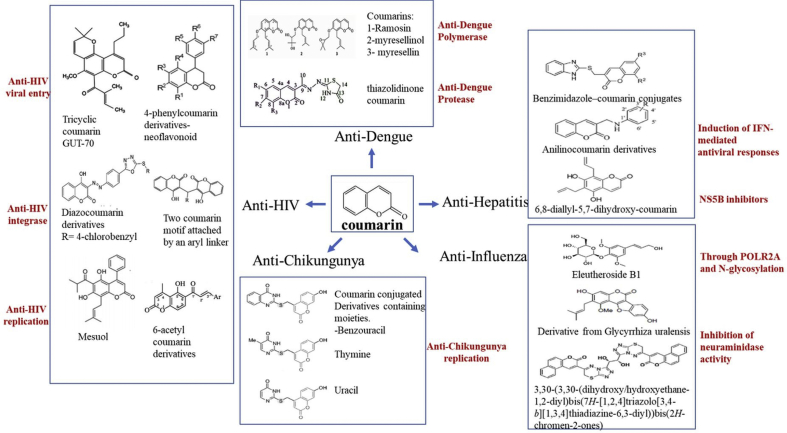
Figure 2.
Chemical structure of coumarin and its derivatives: Anti-viral activity of coumarin derivatives against several viruses. (Figure was made using Microsoft Powerpoint).
2. Coumarin
Coumarin is a therapeutic agent, found as a naturally occurring secondary metabolite in plants, bacteria, fungi, essential oils and can also be chemically synthesized [19]. Coumarins have been isolated from different families of plant kingdom like Clusiaceae, Umbelliferae and Rutaceae [20]. It was isolated from Tonka beans in 1820 independently by A. Vogel of Munich, Germany and by Nicholas Guibourt of France [21]. William Henry Perkin, an English chemist first synthesized coumarin in 1868 [22]. Coumarin is basically made up of a benzene moiety fused with an alpha-pyrene ring named as benzopyrene [19]. Coumarin derivatives are synthesized using various synthetic pathways such as Perkin condensation, Knoevenagel condensation, Pechmann reaction and metal-catalyzed Cyclization [23]. They are stable, soluble, low molecular weight compounds without any adverse side effects and toxicity. These and several other properties of coumarins make them a potential drug candidate against many viral and bacterial diseases. Many of natural, synthetic, conjugated, hybrid potential candidate lead compounds possessing coumarin scaffold have been studied and are in different stages of drug development [18]. Their biological activity can be changed depending upon the combination of various substituents and conjugates. On top of this, Coumarin motifs can be foresighted as a privileged scaffold and model framework for the design and synthesis of several pharmacological compounds having significant binding affinity with the different biological targets. They can be easily modified to satisfy “the rule of 5 “of Lipinski to make them a drug-like molecule by applying a privileged structure approach of drug discovery using combinatorial chemistry [24]. Coumarin as an antiviral agent, widely studied in anti-HIV therapy [13, 25, 26, 27, 28], attracts attention from scientists to study its significance in the prevention of other viral diseases.
3. Coumarin as anti-viral agent
3.1. Anti-hepatitis virus
The available therapeutic treatment for infection caused by the hepatitis C virus have many adverse effects (Headache, fatigue, nausea, diarrhea, depression, hemolytic anemia) [29, 30, 31] and the cost of twelve-week treatment amounts to approximately $84,000 [32]. Researchers are focusing on synthesizing new compounds using coumarin and its derivatives to overcome the shortcomings associated with anti-HCV drugs [33].
Hepatocarcinoma (HCC) is associated with chronic hepatitis C virus (HCV) infection which involves increase in plasma alanine transferase (ALT) levels [34, 35]. In 2001 Okamoto and co-workers found coumarin, a probable model chemical to bring shift in the hypercarcinogenic state of the liver to a hypocarcinogenic state which was detected by lower levels of plasma ALT by using mouse liver injury models [36]. Benzimidazole-coumarin conjugates were synthesized by connecting benzimidazole and coumarin derivatives with methylenethio linker. Their role as anti-hepatitis C virus agents was evaluated by studying its effect on HCV replication and proliferation in Huh 5-2 cells. Two of these conjugates, 2-[(6_-bromocoumarin-3_-yl) methylenethio]-5-fluorobenzimidazole and its derivative 1-[(2__,3__,4__,6__-tetra-O-acetyl) glucopyranos-1__-yl]-2-[(6_-bromocoumarin-3_-yl) methylenethio] benzimidazole inhibited HCV RNA replication up to 90% exhibiting a potential anti-HCV activity [37]. In another case new conjugates were synthesized by attaching coumarin with naphthalene moiety and by adding benzimidazole with 5,6-benzocoumarin, these compounds displayed a remarkable anti-viral activity, mechanism of action of which still needs further studies [38]. Likewise, various heterobicycle-coumarin conjugates were synthesized using -SCH2- linker and their structure-activity relationship was studied for measuring their anti-hepatitis potential [39]. In similar manner, conjugates of coumarin attached with purine ribofuranosides found to inhibit the replication of HCV subgenomic replicon in the Huh 5-2 cells [40]. These studies displayed the essentiality of the scaffold in the form of heterobicycles and other aromatic rings on coumarin in their inhibitory activity against HCV [39].
Recently, imidazole-coumarin conjugates appeared as novel anti-HCV compounds showing increased potency and selectivity. The anti-viral activity was enhanced when substituents like F, Cl, Br, Me, and OMe was attached to the coumarin molecule. The mechanism behind this enhanced anti-HCV activity needs further studies (20).
Resembling many of the currently used anti-viral drugs which act on polymerase components of virus to inhibit its replication, NS5B, a hepatitis C virus polymerase is a novel target for newly synthesized coumarin derivative 6,8-diallyl-5,7-dihydroxycoumarin. This compound was found to act as anti-viral by binding in thumb pocket-1 (TP-1) of NS5B and inhibit the viral polymerase activity [41]. The first study, in which coumarins act against HCV replication by enhancing IFN-mediated antiviral responses, was reported in 2013. In this report, a synthesized derivative of anilinocoumarins having OMe as a strong electron-donating substituent, showed enhanced anti-HCV activity by targeting host factors of the IFN pathway [42]. Coumarin's anti-viral activity doesn't restrict to HCV, many studies have shown its activity against Hepatitis B virus (HBV) also. Natural pyranocoumarins nordentatin and clausarin were isolated from the medicinal plant Clausena excavate [43]. These compounds were found to inhibit hepatitis B virus surface antigen (HBsAg) in HepA2 cells. Their analogues were synthesized using hydrogenation, methylation and epoxidation reactions by Chung-Ren Su et.al in 2008 and their potency as anti-HBV was studied. They have observed that analogues of pyranocoumarin consisting of dimethylallyl or dimethylpropyl side chain along with functional groups attached to pyran ring, were showing the highest anti-HBV activity becoming a potential future candidate to be anti-HBV drug [44].
From the aforementioned studies, we can deduce that in hepatitis virus infection, coumarin has shown to target a wide range of proteins, like binding antigens present at the surface of the cell, proteins that are related to polymerase responsible for viral replication & factors involved in interferon signaling pathways.
3.2. Anti-HIV
Currently, anti-HIV approaches are to target several steps in virus life cycle including virus-host cell attachment, cell membrane fusion, integration, assembly besides the conventional target like inhibition of the reverse transcriptase, protease, integrase [45]. Chemical compound coumarins have been shown from many research studies to have anti-HIV effects. Coumarin derivatives, 4-Hydroxycoumarins (warfarin, 4-HC tetramer), Pyranocoumarins (Khellactone, Calanolide), Furanocoumarin, 3-phenylcoumarins, 4-Phenylcoumarins, Hybrid coumarin analogue, Toddacoumaquinone have shown pharmacological effect against HIV infection. They inhibit HIV protease, integrase, reverse transcriptase, viral DNA replication, vpr, sp1-related genes (cell cycle arrest), Tat, Rev, glycosylation [18].
Anti-viral action and mechanism of 6-acetyl-coumarin analogues have been studied on human C8166 cells. It has been found that electronegative substituent at the phenyl ring connected with coumarin central motif plays a very important role in the anti-HIV activity of coumarin [46]. Further, QSAR and Docking approaches indicated that Coumarin negatively regulates 3’ processing and integrase enzyme of HIV. Two coumarin motif attached by an aryl linker were found to be very crucial for the anti-HIV-integrase activity. In this coumarin derivative, lipophilicity of aromatic ring of the linker was responsible for the inhibitory activity [47]. Another research group studied the therapeutic potential of synthesized coumarin carbohydrazide derivatives. Cytotoxicity of coumarin hydrazide derivatives was found to be significantly improved in human cells when salicyl moiety was replaced with a coumaryl moiety. Anti-HIV-1 Integrase activity has been shown by hydrogen/chlorine on salicyl moiety and halogen/alkoxy added on coumarin. Molecular docking reveals fitting of the aromatic ring in the hydrophobic pocket and acyl hydrazide at the metal centers [48]. Further, Alimi Livani et al., designed synthetic new coumarin derivative that mimic the integrase strand transfer inhibitors by adding 1,3,4-oxidiazole ring as a chelating motif on its halobenzyl moiety. Coumarin derivatives having 4-chlorobenzyl ring showed very significant anti-HIV activity exhibiting inhibition rate almost near to AZT (Azidothymidine), an anti-HIV drug. Many physicochemical properties were found to match with that of FDA approved Integrase strand transfer inhibitors (INSTIs) except aqueous solubility that was low, this needs further structural modifications [49]. The study of Chiang et al., suggested that spatial distance between coumarin unit and linker moiety was crucial for anti-HIV integrase activity [50].
Coumarin can act as an inhibitor for HIV proteases [51]. Temitope et al., synthesized a series of novel N-benzylated coumarin-AZT conjugates that have inhibitory activity against both HIV-protease and Reverse transcriptase [52]. Membrane fusion by binding of viral envelop protein and CD4 of T cells/Macrophages is the crucial step in HIV infection [53]. Tricyclic coumarin GUT70, a natural compound isolated from Callophyllum brasiliense demonstrated strong inhibitory activity on HIV-1 replication by the suppression of NF-kB activation in both acutely and chronically virus-infected cells [28]. GUT70 has the ability to reduce the membrane fluidity that affects viral entry. Matsuda et al., found that GUT-70 reduced membrane fluidity, inhibited membrane fusion and thus inhibited HIV infection [53]. Further, expression of the HIV-1 receptor (CD4) and co-receptors (CXCR4 and CCR5) was downregulated which may also contribute to blocking the HIV entry [53, 55]. 4-phenylcoumarin derivatives called neoflavanoids were synthesized and their anti-HIV effects were assessed on MT-2 infected cells in vitro [46]. It suggested that the acyl group was essential for the inhibition of both the Tat and NF-kB pathway [56]. The HIV-replication inhibitory activity of a 4-phenyl coumarins mesouol (extracted from Marila pluricostata) has been studied in Jurkat T cells. Mesuol attenuated TNF- α induced HIV-1-LTR (Long terminal repeat) activity via phosphorylation of p65 (NF-kB). Mesuol suppressed NF-kB p65 subunit phosphorylation and its transcriptional activity in TNF-α induced T cells but displayed neutral response on integration and reverse transcription [57].
One of the major problems associated with antiretroviral therapy is that it cannot be fully effective until the problem of latent viral reservoir is resolved. To solve this problem Coumarin derivatives, hymecromone and scoparone which have the ability to reactivate HIV latent reservoir can be used in HIV-1 reservoir elimination approaches [58].
3.3. Anti-influenza virus
Anti-viral therapy which are currently used for the treatment of influenza viruses, have the limitations on vaccine design, efficacy and delay in vaccine production because of the evolution of drug-resistant viral mutants attributed to the mutation or reassortment potential of this virus [59, 60]. Studies are taking place to isolate new antiviral agents from natural compounds.
Eleutheroside B1, a coumarin compound has been earlier demonstrated to inhibit expression of many chemokine genes and gene for Nucleoprotein, an influenza structural protein that is a constituent of vRNPs viral ribonucleoprotein complex important for viral replication [61]. RNA sequencing technology has confirmed that the eleutheroside B1 acts on chemokine signaling pathways and cytokine-cytokine receptor interactions. Recently, the mechanism of action against influenza viral infection of eleutheroside B1 has been assessed showing that the target for this compound is POLR2A gene which is responsible for the expression of viral polymerase [62]. Thiazolyl-coumarin hybrids synthesized by Osman et al., were found to show promising antiviral against the H3N2 and H1N1 strains of influenza in MDCK cell-based assays. Molecular docking studies has shown this anti-influenza activity is probably because of inhibition of viral envelope protein neuraminidase [63]. Similarly, derivatives of coumarin, bis(triazolothiadiazinyl coumarin) was synthesized and evaluated for their anti-influenza activity. Site of target of this synthesized coumarin was neuraminidase protein, this was observed by measuring binding affinity between the coumarin derivative and target protein of influenza virus using molecular docking [64]. Another research study has shown the neuraminidase inhibitory action of coumarins isolated from methanol extracts from the roots of a plant, Glycyrrhiza uralensis. Glycyrol, a ring-closed coumarin was shown to have the strongest inhibitory activity, the presence of the five-membered furan rings was essential for the potency of polyphenols against neuraminidase [65]. Other probable targets of a compound containing both coumarin and monoterpenoid derivative in influenza was viral hemagglutinin HA which is involved in viral entry in host cell by membrane fusion. The activities of these compounds against virus is thought to be due to presence of bulky polycyclic moiety which is responsible to block the changes in conformation of HA molecule which is essential for the entry of viruses into host cell. Thus, these derivatives inhibit the activity of the virus by blocking the early stages of viral reproduction [60]. Due to the high mutation tendency of influenza viral proteins [66], redox-sensitive pathways which are useful for viral replication and are independent of variability of the strains, are gaining importance as the new target to inhibit viral infection. Coumarins oxidized with 2-iodobenzoic acid (IBX) with different catechol and pyrogallol derivatives have shown antiviral activity against influenza A H1N1 by modulating proteins involved in redox pathways [67].
Thus, these observed effects of synthesized coumarin derivatives on viruses indicate that anti-viral therapy can be developed in the future, targeting those pathways which are essential for the establishment and multiplication of viruses in the host cell.
3.4. Anti-dengue virus
Coumarin has the potential to significantly suppress the Dengue and chikungunya viral infections in vitro. However, the need is to investigate the mechanism and other effects on in vivo model [68]. Flaviviruses like Dengue virus need their polyprotein processing by viral NS3 protease called NS3pro for its replication activities. Activation of NS3pro requires a 47-residue catalytic part of NS2B cofactor. NS2B stabilizes and completes substrates-binding pocket of NS3pro [69]. Therefore, targeting binding site of NS2B/NS3 complex could be a better approach in the development of a drug molecule. With this idea, molecular docking approaches using synthetic coumarin derivatives as ligands have been applied to check their anti-dengue protease activity that is known in the case of HIV [51]. Molecular Docking study indicates 4-thiazolidinone-coumarin derivatives have significant binding affinity with the binding site of viral protease complex suggesting its inhibitory role [70].
In a study, from the plant Myrtopsis corymbosa major compounds of coumarins (ramosin, myrsellinol, myresellin) and alkaloids (skimmianine, gama-fagarin and haplopin) were extracted and their inhibitory activities were checked against Dengue infection. They exhibited weak anti-dengue activity when used separately, but showed very significant activity when their crude product was applied that contain all the components. Specifically, they showed very low activity against dengue virus RNA dependent RNA polymerase (DENV-NS5) when applied separately to cells. It indicated the possibility that anti-dengue effect was due to synergetic effect of many compound or because of some other molecules present in small quantities [71]. Subsequently, potential and mechanism of Resvertrol (RESV), a phytoalexin (hydroxylated derivative of coumarin) against dengue viral infection were investigated. In case of Dengue infection, a DNA binding protein namely, high mobility group box 1 (HMGB1) translocates from nucleus to cytoplasm that triggers many inflammatory responses. RESV attenuated migration of HMGB1 out of nucleus (HMGB1 retention in nucleus mediated by Sirt1 deacetylase) this leads to suppression of dengue virus replication by increasing the production of interferon-stimulated genes. HGB1 that is known as inducer of inflammatory responses, its inhibition by RESV indicated the possibility that RESV may act as a potential anti-inflammatory agent. However, this needs further confirmation [72].
3.5. Anti-chikungunya virus
Synthetic coumarin conjugates have been used to see their inhibitory role against chikungunya viruses. Jih Ru Hwu et al., used the triply conjugated uracil-coumarin-arenes compound to see structure-activity relationship against chikungunya virus. In this research, coumarin central moiety was found to be essential for the anti-viral activity. There was a positive correlation between size of conjugated moiety (benzouracil > thymine > uracil) and chikungunya inhibitory action [3]. Same research group have synthesized thioguanosine-coumarin conjugates and explored their anti-chikungunya action in Vero cells. In this case also, they found that central moiety of guanosine-coumarin conjugates was an essential component for anti-viral activity. In addition, conjugates having –OMe moiety were found to be more potent than conjugates having other substitutes [73].
3.6. Against other viruses
Filoviruses family include Ebola virus, Marburg virus, Ceuvavirus which are responsible for many diseases in human beings like severe hemorrhagic fever. After screening of anti-histamine library in an attempt to search for the compound that has capacity to inhibit flaviviral entry, two potential Histamine receptor antagonist compounds were found. Interestingly both the compounds contain coumarin fused ring system that was crucial for their inhibitory activity. This study indicated the possibility that active compound containing hydrophobic moiety at position 3, 4 may act as an interacting part with the target molecule [74]. Similar reports were found in the viremia infection caused by spring viremia of carp virus (SVCV), a rhabdovirus found in freshwater aquatic environment. The potency of imidazole-coumarin derivative 7-(4-(4-methyl-imidazole))-coumarin and another benzimidazole coumarin derivative, 7-(4-benzimidazole-butoxy)-coumarin (BBC) [75], as anti-SVCV was studied in vivo in zebrafish. The anti-viral activity was displayed in the form of reduced horizontal transmission of SVCV, thus decreasing the mortality and viral titer in the fish body [76]. 7-[6-(2-methylimidazole) hexyloxy] coumarin, a newly synthesized coumarin derivative were found to damage the glycoprotein structure thus, inhibiting the entry of virus into host cell. It also suppressed the virus-induced autophagy, by regulating Akt-mTOR pathway and in this way exhibiting anti-viral activity [77]. These results are indicative of the potential therapeutic role of coumarin derivative by acting on anti-oxidative pathways [78].
4. Conclusions and future perspectives
Coumarin is a natural product that are derived from various parts (seeds, leaves and roots) of the plant families such as Apiaceae, Rutaceae and many other sources in the form of fungi, bacteria etc. Due to its wide distribution and property of being stable, soluble, low molecular weight compound without any adverse side effects and toxicity and feasibility of chemical modification to generate new semisynthetic derivatives, coumarin is gaining attention in the field of medicinal chemistry. The purpose of this review was to provide a significant insight into the development of new coumarin-based antiviral compounds and their likely mechanism of action that will refine our knowledge of viral treatment.
As summarized here, coumarin (and its derivatives) can target various enzymes and pathways that are essential for viral entry, survival and infection. These studies have given many fresh biological perspectives based on the analysis of the relationship between structure and its activity, giving a reason to be hopeful that antiviral coumarin derivatives will eventually be created with broad clinical applications.
A major limitation of the current literature on the anti-viral effects of coumarins is that these studies have been mainly performed in silico and in vitro and only in few cases, studies have been done on animal models. These preliminary studies in this area necessitated the need for further exploration of efficacy and potential application of coumarins in vivo followed by clinical trials to develop effective antiviral treatments.
While some coumarin derivatives have been reported to indicate a particular biological activity, the task would be to design and synthesize new coumarin derivatives with significant specific activity for other pharmacological targets and identify their mechanism of action to obtain new medicinal anti-viral drugs.
Declarations
Author contribution statement
Shruti Mishra, Achyut Pandey, Siddharth Manvati: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by fellowships received by the first and second authors by the Council of Scientific and Industrial Research (CSIR), Goverment of India.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Sagar S., Kaur M., Minneman K.P. Antiviral lead compounds from marine sponges. Mar. Drugs. 2010:2619–2638. doi: 10.3390/md8102619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye N., Chen H., Wold E.A., Shi P., Zhou J. Therapeutic potential of spirooxindoles as antiviral agents. ACS Infect. Dis. 2016 doi: 10.1021/acsinfecdis.6b00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ru J., Kapoor M., Tsay S., Lin C., Chu K. Benzouracil – coumarin – arene conjugates as inhibiting agents for chikungunya virus. Antivir. Res. 2015:1–6. doi: 10.1016/j.antiviral.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Bandarage U.K., Clark M.P., Perola E., Gao H., Jacobs M.D., Tsai A. Novel 2-substituted 7-azaindole and 7-azaindazole analogues as potential anti-viral agents for the treatment of influenza. ACS Med. Chem. Lett. 2017;8(2):261–265. doi: 10.1021/acsmedchemlett.6b00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cagno V., Donalisio M., Civra A., Cagliero C., Rubiolo P., Lembo D. In vitro evaluation of the antiviral properties of Shilajit and investigation of its mechanisms of action. J. Ethnopharmacol. 2015;166:129–134. doi: 10.1016/j.jep.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 6.V Vernekar S.K., Tang J., Wu B., Huber A.D., Casey M.C., Myshakina N., Wilson D.J., Kankanala J., Kirby K.A., Parniak M.A., Sara S.G., Wang Z. Double-winged 3 - hydroxypyrimidine-2,4-diones: potent and selective inhibition against HIV - 1 RNase H with signi fi cant antiviral activity. J. Med. Chem. 2017 doi: 10.1021/acs.jmedchem.7b00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy D.A., Read A.F. Why does drug resistance readily evolve but vaccine resistance does not? Proc. R. Soc. B: Biol. Sci. 2017;284:20162562. doi: 10.1098/rspb.2016.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Field H.J., Wainberg M.A. Antiviral drug development. Future Virol. 2011;6(5):545–547. [Google Scholar]
- 9.De Clercq E. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016;29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pour P.M., Fakhri S., Asgary S., Farzaei M.H., Echeverría J. The signaling pathways, and therapeutic targets of antiviral agents: focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front. Pharmacol. 2019;10:1–23. doi: 10.3389/fphar.2019.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mycol V., Kotwal G.J., Longum P. Natural Antivirals against Human Viruses Bitter melon. Virol. Mycol. 2014;3:3–5. [Google Scholar]
- 12.Lin L.T., Hsu W.C., Lin C.C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014;4:24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathew D., Hsu W. Antiviral potential of curcumin. J. Funct. Foods. 2018;40:692–699. [Google Scholar]
- 14.Kannan S., Kolandaivel P. Antiviral potential of natural compounds against influenza virus hemagglutinin. Comput. Biol. Chem. 2017 doi: 10.1016/j.compbiolchem.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang C.Y., Huang S.C., Zhang Y., Lai Z.R., Kung S.H., Chang Y.S., Lin C.W. Antiviral ability of Kalanchoe gracilis leaf extract against Enterovirus 71 and coxsackievirus A16, Evidence-Based Complement. Altern. Med. 2012;2012 doi: 10.1155/2012/503165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riveiro M.E., De Kimpe N., Moglioni A., Vázquez R., Monczor F., Shayo C., Davio C. Coumarins: old compounds with novel promising therapeutic perspectives. Curr. Med. Chem. 2010:1325–1338. doi: 10.2174/092986710790936284. [DOI] [PubMed] [Google Scholar]
- 17.Li G., Gao Q., Yuan S., Wang L., Altmeyer R., Lan K., Yin F., Zou G. Characterization of three small molecule inhibitors of enterovirus 71 identified from screening of a library of natural products. Antivir. Res. 2017;143:85–96. doi: 10.1016/j.antiviral.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Hassan M.Z., Osman H., Ali M.A., Ahsan M.J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 2016 doi: 10.1016/j.ejmech.2016.07.056. AC SC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venugopala K.N., Rashmi V., Odhav B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013 doi: 10.1155/2013/963248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stefanachi A., Leonetti F., Pisani L., Catto M., Carotti A. Coumarin: a natural, privileged and versatile scaffold for bioactive compounds. Molecules. 2018 doi: 10.3390/molecules23020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarker S.D., Nahar L. Progress in the chemistry of naturally occurring coumarins. Prog. Chem. Org. Nat. Prod. 2017 doi: 10.1007/978-3-319-59542-9_3. [DOI] [PubMed] [Google Scholar]
- 22.PERKIN W.H. On the artificial production of coumarin and formation of its homologues. J. Chem. Soc. 1868:53–63. A. Production, By. F.R.S. IT. [Google Scholar]
- 23.Jadhav N.H., Sakate S.S., Rasal N.K., Shinde D.R., Pawar R.A. Heterogeneously catalyzed Pechmann condensation employing the tailored Zn0. 925Ti0. 075O NPs: synthesis of coumarin. ACS Omega. 2019;4:8522–8527. doi: 10.1021/acsomega.9b00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desimone R.W., Currie K.S., Mitchell S.A., Darrow J.W., Pippin D.A. Privileged Structures ;: applications in drug discovery. Comb. Chem. High Throughput Screen. 2004:473–493. doi: 10.2174/1386207043328544. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J., Jiang J. Pharmacological and nutritional effects of natural coumarins and their structure-activity relationships. Mol. Nutr. Food Res. 2018:1–74. doi: 10.1002/mnfr.201701073. [DOI] [PubMed] [Google Scholar]
- 26.Laila U., Akram M., Ali M., Mehmmod A., Naheed H., Imtiaz A., Tahir M., Owais A., Naveed G., Muhammad M., Naheed R., Shaheen G., Ullah Q., Zahid R. Role of medicinal plants in HIV/AIDS therapy. Clin. Exp. Pharmacol. Physiol. 2019:1–11. [Google Scholar]
- 27.Srikrishna D. A review on pharmacological properties of coumarins. Mini Rev. Med. Chem. 2017 doi: 10.2174/1389557516666160801094919. Scanned by CamScanner. [DOI] [PubMed] [Google Scholar]
- 28.Kudo E., Taura M., Matsuda K., Shimamoto M., Kariya R., Goto H., Hattori S., Kimura S., Okada S. Inhibition of HIV-1 replication by a tricyclic coumarin GUT-70 in acutely and chronically infected cells. Bioorg. Med. Chem. Lett. 2013;23:606–609. doi: 10.1016/j.bmcl.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 29.Sandmann L., Schulte B., Manns M.P., Maasoumy B. Treatment of chronic hepatitis C: efficacy, side effects and complications. Vis. Med. 2019;35:161–170. doi: 10.1159/000500963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manns M.P., Wedemeyer H., Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ly T., Permanente K., California N., Dermatol A.A., Tinea M.S., Ges D.D., Management G.R., Cosmet C., Dermatol I., Sc H., Tinea B.J., Js M., Infect E., Lm D., Bg R., Kh R., Control I., Epidemiol H., Pc S., Rc S., Journal B. Cutaneous side-effects of antihepatitis C treatment;: the U. K. experience. Br. J. Dermatol. 2014:2014–2015. doi: 10.1111/bjd.13227. [DOI] [PubMed] [Google Scholar]
- 32.Henry B. Drug pricing & challenges to hepatitis C treatment access. J. Heal. Biomed. Law. 2018;14:265–283. http://www.ncbi.nlm.nih.gov/pubmed/30258323%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC6152913 [PMC free article] [PubMed] [Google Scholar]
- 33.Tsay S., Lin S., Huang W., Hsu M., Hwang K.C., Lin C., Horng J., Chen I., Hwu J.R., Shieh F., Leyssen P., Neyts J. Synthesis and Structure-activity relationships of imidazole-coumarin conjugates against hepatitis C virus. Molecules. 2016 doi: 10.3390/molecules21020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rino Y., Ohkawa S., Shimizu A., Tamai S., Miyakawa K., Aoki H., Imada T., Shindo K., Okamoto N., Totsuka S. Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer. 1999:589–595. doi: 10.1002/(sici)1097-0142(19990815)86:4<589::aid-cncr7>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 35.Close association between high serum ALT and more rapid recurrence of hepatocellular carcinoma in hepatectomized patients with HCV-associated liver cirrhosis and hepatocellular carcinoma. Intervirology. 2000:20–26. doi: 10.1159/000025019. O. Paper. 0815. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto T., Kajino K., Hino O. Hepatoprotective drugs for the treatment of virus-induced chronic hepatitis;: from hypercarcinogenic state to hypocarcinogenic state. Jpn. J. Pharmacol. 2001;180:177–180. doi: 10.1254/jjp.87.177. REVIEW — Current Perspective. [DOI] [PubMed] [Google Scholar]
- 37.Ru J., Singha R., Ching S., Hsiung Y., Das A.R., Vliegen I., De Clercq E., Neyts J. Synthesis of new benzimidazole – coumarin conjugates as anti-hepatitis C virus agents. Antivir. Res. 2008;77:157–162. doi: 10.1016/j.antiviral.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Hsu M., Shieh F., Lin C., Hwang K.C. Coumarins hinged directly on benzimidazoles and their ribofuranosides to inhibit hepatitis C virus. Eur. J. Med. Chem. 2013 doi: 10.1016/j.ejmech.2013.02.008. SC. [DOI] [PubMed] [Google Scholar]
- 39.Neyts J., De Clercq E., Singha R., Chang Y.H., Das A.R., Chakraborty S.K. Structure - activity relationship of new anti-hepatitis C virus agents: heterobicycle - coumarin conjugates. J. Med. Chem. 2009:1486–1490. doi: 10.1021/jm801240d. [DOI] [PubMed] [Google Scholar]
- 40.Hwu J.R., Lin S., Tsay S., De Clercq E., Leyssen P., Neyts J. Coumarin - purine ribofuranoside conjugates as new agents against hepatitis C virus. J. Med. Chem. 2011:2114–2126. doi: 10.1021/jm101337v. [DOI] [PubMed] [Google Scholar]
- 41.Nichols D.B., Le R.A.C., Basu A., Chudayeu M., De F P., Moraes D., Talele T.T., Paulo R.R., Kaushik-basu N. Evaluation of coumarin and neoflavone derivatives as HCV NS 5 B polymerase inhibitors. Chem. Biol. Drug Des. 2013;81(5):607–614. doi: 10.1111/cbdd.12105. [DOI] [PubMed] [Google Scholar]
- 42.Peng H., Chen W., Lee J., Yang S., Tzeng C., Lin Y., Yang S. Novel anilinocoumarin derivatives as agents against hepatitis C virus by the induction of IFN-mediated antiviral responses. Org. Biomol. Chem. 2013:1858–1866. doi: 10.1039/c2ob26860d. [DOI] [PubMed] [Google Scholar]
- 43.Huang S.C., Wu P.L., Wu T.S. Two coumarins from the root bark of Clausena excavata. Phytochemistry. 1997;44(1):179–181. [Google Scholar]
- 44.Su C., Farn S., Miem C., Damu A.G., Kuo T., Chiang P., Bastow K.F., Lee K., Wu T. Bioorganic & Medicinal Chemistry Anti-HBV and cytotoxic activities of pyranocoumarin derivatives. Bioorg. Med. Chem. 2009;17:6137–6143. doi: 10.1016/j.bmc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Moore J.P., Stevenson M. New targets for inhibitors of HIV-1 replication. Nat. Rev. Mol. Cell Biol. 2000;1:40–49. doi: 10.1038/35036060. [DOI] [PubMed] [Google Scholar]
- 46.Srivastav V.K., Tiwari M., Zhang X., Yao X.J. Synthesis and antiretroviral activity of 6-Acetyl-coumarin derivatives against HIV-1 infection. Indian J. Pharm. Sci. 2018;80:108–117. [Google Scholar]
- 47.Srivastav V.K., Tiwari M. QSAR and docking studies of coumarin derivatives as potent HIV-1 integrase inhibitors. Arab. J. Chem. 2017;10:S1081–S1094. [Google Scholar]
- 48.Jesumoroti O.J., Mnkandhla D., Isaacs M., Hoppe H.C., Klein R. Evaluation of novel N′-(3-hydroxybenzoyl)-2-oxo-2H-chromene-3-carbohydrazide derivatives as potential HIV-1 integrase inhibitors. MedChemComm. 2018 doi: 10.1039/c8md00328a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alimi Z., Safakish M., Hajimahdi Z., Soleymani S. Design, synthesis, molecular modeling, in silico ADME studies and anti- HIV-1 assay of new diazocoumarin derivatives. Iran. J. Pharm. Res. (IJPR): 2018;17:65–77. [PMC free article] [PubMed] [Google Scholar]
- 50.Chiang C.-C., Mouscadet J.-F., Tsai H.-J., Liu C.-T., Hsu L.-Y. Synthesis and HIV-1 integrase inhibition of novel bis- or tetra-coumarin analogues. Chem. Pharm. Bull. 2007;55:1740–1743. doi: 10.1248/cpb.55.1740. (Tokyo) [DOI] [PubMed] [Google Scholar]
- 51.Lunney E.A., Hagen S.E., Domagala J.M., Humblet C., Kosinski J., Tait B.D., Warmus J.S., Wilson M., Ferguson D., Hupe D., Tummino P.J., Baldwin E.T., Bhat T.N., Liu B., Ericksod J.U.T. A novel nonpeptide HIV-1 protease inhibitor: elucidation of the binding mode and its application in the design of related analogs. J. Med. Chem. 1994:2664–2677. doi: 10.1021/jm00043a006. [DOI] [PubMed] [Google Scholar]
- 52.Olomola T.O., Klein R., Mautsa N., Sayed Y., Kaye P.T. Bioorganic & Medicinal Chemistry Synthesis and evaluation of coumarin derivatives as potential dual-action HIV-1 protease and reverse transcriptase inhibitors. Bioorg. Med. Chem. 2013:1–8. doi: 10.1016/j.bmc.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 53.Matsuda K., Hattori S., Kariya R., Komizu Y., Kudo E., Goto H., Taura M., Ueoka R., Kimura S., Okada S. Inhibition of HIV-1 entry by the tricyclic coumarin GUT-70 through the modification of membrane fluidity. Biochem. Biophys. Res. Commun. 2015;457:288–294. doi: 10.1016/j.bbrc.2014.12.102. [DOI] [PubMed] [Google Scholar]
- 55.Harada S., Yusa K., Monde K., Akaike T., Maeda Y. Influence of membrane fluidity on human immunodeficiency virus type 1 entry. Biochem. Biophys. Res. Commun. 2005;329:480–486. doi: 10.1016/j.bbrc.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Olmedo D.A., Luis L., Olmo E., Bedoya L.M., Muñoz E., Feliciano A.S., Gupta M.P. Neoflavonoids as inhibitors of HIV-1 replication by targeting the Tat and NF- κ B pathways. Molecules. 2017:2–14. doi: 10.3390/molecules22020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Márquez N., Sancho R., Bedoya L.M., Alcamí J., López-Pérez J.L., San Feliciano A., Fiebich B.L., Muñoz E. Mesuol, a natural occurring 4-phenylcoumarin, inhibits HIV-1 replication by targeting the NF-κB pathway. Antivir. Res. 2005;66:137–145. doi: 10.1016/j.antiviral.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Li X., Zeng H., Wang P., Lin L., Liu L., Zhen P., Fu Y., Lu P., Zhu H. Reactivation of latent HIV-1 in latently infected cells by coumarin compounds: hymecromone and scoparone. Curr. HIV Res. 2016:484–490. doi: 10.2174/1570162x14666161003152458. [DOI] [PubMed] [Google Scholar]
- 59.Spanakis N., Pitiriga V. A review of neuraminidase inhibitor susceptibility in influenza strains. Expert Rev. Anti-infect. Ther. 2014:1325–1336. doi: 10.1586/14787210.2014.966083. [DOI] [PubMed] [Google Scholar]
- 60.Khomenko T.M., V Zarubaev V., Orshanskaya I.R., Kadyrova R.A., Sannikova V.A., V Korchagina D., Volcho K.P., Salakhutdinov N.F. Bioorganic & medicinal chemistry letters anti-influenza activity of monoterpene-containing substituted coumarins. Bioorg. Med. Chem. Lett. 2017;27:2920–2925. doi: 10.1016/j.bmcl.2017.04.091. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y., Yan W., Chen Q., Huang W., Yang Z., Li X., Wang X. Inhibition viral RNP and anti-in fl ammatory activity of coumarins against in fl uenza virus. Biomed. Pharmacother. 2017;87:583–588. doi: 10.1016/j.biopha.2016.12.117. ScienceDirect. [DOI] [PubMed] [Google Scholar]
- 62.Yan W.E.N., Zheng C., He J., Zhang W., Huang X.I.N.A.N. Eleutheroside B1 mediates its anti-influenza activity through POLR2A and N-glycosylation. Int. J. Mol. Med. 2018:2776–2792. doi: 10.3892/ijmm.2018.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osman H., Yusufzai S.K., Khan M.S., Razik B.M.A., Sulaiman O., Mohamad S., Gansau J.A., Ezzat M.O., Parumasivam T., Hassan M.Z. New thiazolyl-coumarin hybrids: design, synthesis, characterization, X-ray crystal structure, antibacterial and antiviral evaluation. J. Mol. Struct. 2018 AC. [Google Scholar]
- 64.Pavurala S., Vaarla K., Kesharwani R., Liekens S., Vedula R.R. Bis coumarinyl bis triazolothiadiazinyl ethane derivatives: synthesis, antiviral activity evaluation, and molecular docking studies ARTICLE HISTORY. Synth. Commun. 2018;48:1494–1503. [Google Scholar]
- 65.Bae Y., Hoon J., Park S., Sun J., Rho M., Bae K., Hun K., Song W. Bioorganic & Medicinal Chemistry Letters Inhibition of neuraminidase activity by polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioorg. Med. Chem. Lett. 2010;20:971–974. doi: 10.1016/j.bmcl.2009.12.106. [DOI] [PubMed] [Google Scholar]
- 66.Escuret V., Frobert E., Bouscambert-duchamp M., Sabatier M., Grog I., Valette M., Lina B., Morfin F., Ferraris O. Detection of human influenza A ( H1N1 ) and B strains with reduced sensitivity to neuraminidase inhibitors. J. Clin. Virol. 2008;41:25–28. doi: 10.1016/j.jcv.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 67.Bizzarri B.M., Botta L., Capecchi E., Celestino I., Checconi P., Palamara A.T., Nencioni L. Regioselective IBX-mediated synthesis of coumarin derivatives with antioxidant and anti-in fl uenza activities. J. Nat. Prod. 2017 doi: 10.1021/acs.jnatprod.7b00665. [DOI] [PubMed] [Google Scholar]
- 68.Gómez-calderón C., Mesa-castro C., Robledo S., Gómez S., Bolivar-avila S. Antiviral effect of compounds derived from the seeds of Mammea americana and Tabernaemontana cymosa on Dengue and Chikungunya virus infections. BMC Complement Altern. Med. 2017:1–12. doi: 10.1186/s12906-017-1562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erbel P., Schiering N., Arcy A.D., Renatus M., Kroemer M., Lim S.P., Yin Z., Keller T.H., Vasudevan S.G., Hommel U. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat. Struct. Mol. Biol. 2006;13:2005–2006. doi: 10.1038/nsmb1073. [DOI] [PubMed] [Google Scholar]
- 70.Yusufzai S.K., Osman H., Khan M.S., Razik B.M.A. 4 - thiazolidinone coumarin derivatives as two - component NS2B/NS3 DENV flavivirus serine protease inhibitors: synthesis, molecular docking, biological evaluation and structure – activity relationship studies. Chem. Cent. J. 2018:1–16. doi: 10.1186/s13065-018-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coulerie P., Maciuk A., Lebouvier N., Hnawia E., Guillemot J., Canard B., Figadère B., Nour M. Phytochemical study of Myrtopsis corymbosa, perspectives for Anti-dengue natural compound research. Rec. Nat. Prod. 2013;3:250–253. [Google Scholar]
- 72.Zainal N., Chang C., Cheng Y., Wu Y. Resveratrol treatment reveals a novel role for HMGB1 in regulation of the type 1 interferon response in dengue virus infection. Nat. Publ. Gr. 2017:1–12. doi: 10.1038/srep42998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ru J., Huang W., Lin S., Tan K. European Journal of Medicinal Chemistry Chikungunya virus inhibition by synthetic coumarin e guanosine conjugates. Eur. J. Med. Chem. 2019;166:136–143. doi: 10.1016/j.ejmech.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 74.Cheng H., Schafer A., Soloveva V., Gharaibeh D., Kenny T., Zamani R., Bavari S., Peet N.P., Rong L. Identification of a coumarin-based antihistamine as an anti-filoviral entry inhibitor. Antivir. Res. 2017 doi: 10.1016/j.antiviral.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shen Y., Liu L., Feng C., Hu Y., Chen C., Wang G., Zhu B. Fish and Shell fi sh Immunology Synthesis and antiviral activity of a new coumarin derivative against spring viraemia of carp virus. Fish Shellfish Immunol. 2018;81:57–66. doi: 10.1016/j.fsi.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 76.Liu G., Wang C., Wang H., Zhu L., Zhang H., Wang Y. Antiviral e ffi ciency of a coumarin derivative on spring viremia of carp virus in vivo. Virus Res. 2019;268:11–17. doi: 10.1016/j.virusres.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 77.Chen W., Liu L., Shen Y., Hu Y., Ling F., Wang X., Zhu B. PT key laboratory of applied marine biotechnology of ministry of education, ningbo. Cell. Signal. 2018 [Google Scholar]
- 78.Liu L., Hu Y., Lu J., Wang G. An imidazole coumarin derivative enhances the antiviral response to spring viremia of carp virus infection in zebrafish Authors: SC. Virus Res. 2019 doi: 10.1016/j.virusres.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 79.Morens D.M., Taubenberger J.K., Harvey H.A., Memoli M.J. The 1918 influenza pandemic: lessons for 2009 and the future. Crit. Care Med. 2010;38:e10–e20. doi: 10.1097/CCM.0b013e3181ceb25b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dawood F.S., Iuliano A.D., Reed C., Meltzer M.I., Shay D.K., Cheng P.Y., Bandaranayake D., Breiman R.F., Brooks W.A., Buchy P., Feikin D.R. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect. Dis. 2012;12(9):687–695. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 81.World Health Organization . World Health Organization; Geneva, Switzerland: 2019. Cumulative Number of Confirmed Human Cases for Avian Influenza A (H5N1) Reported to WHO, 2003-2019. [Google Scholar]
- 82.World Health Organization . WHO News release; 2017. Up to 650 000 people die of respiratory diseases linked to seasonal flu each year. [Google Scholar]
- 83.WHO, W . World Health Organization; Geneva: 2017. Global Hepatitis Report 2017. [Google Scholar]
- 84.Global, H. I. V. AIDS Statistics–2018 Fact Sheet. UNAIDS; 2019. [Google Scholar]
- 85.World Health Organization . 2018. Global Strategy for Dengue Prevention and Control: 2012–2020.http://www.who.int/denguecontrol/9789241504034/en/ Avilable from: Accessed, 18. [Google Scholar]