Abstract
Impairment in glucose regulation is an indicatory effect capable of mediating multiple dysfunction such as cerebrovascular disorder with ischemia and brain damage inclusive. This study aims at investigating the glucose-lowering and neuroprotective capability of Diosgenin (DG) towards hyperglycemia-induced cerebral injury in a developed type 2 diabetes mellitus (T2DM) Zebrafish (ZF) model. T2DM was developed in ZF with 20 mg/kg body weight (b.w) multiple-low dose (MLD) Streptozotocin (STZ) for 28 days. Different doses of 20 mg/kg b.w (DG1) and 40 mg/kg b.w (DG2) DG was intraperitoneally administered twice in 7 days for a period of 28 days after T2DM was completely developed. Weight and behavioral changes were monitored and the catalytic activity including the plasma glucose level of diseased and treated ZF was spectrometrically estimated. Histopathological studies were employed to image the brain pathological condition during disease and treatment. SPSS was used as the statistical tool for result analysis and comparison of data obtained. STZ significantly (###p < 0.001) induced hyperglycemia when compared to control as plasma glucose increases from 101.56 ± 4.52 mgdL−1 to 175.87 ± 6.00 mg/dL. Our results have indicated a marked reduction in glucose concentration from a mean average of 175.87 ± 6.00 mgdL−1 to 105.68 ± 4.48 mgdL−1 and 82.06 ± 7.27 mgdL−1 in DG 1 and DG 2 respectively. Catalytic activity significantly decreases (p < 0.05) from 206.42 ± 30.77 unit/mL to 123.85 ± 29.99 unit/mL at a minimum and maximum value of 103.21 and 275.23 in diseased ZF respectively. On DG treatment, catalytic activity significantly (p < 0.01) rise from 101.58 ± 11.29 and 130.73 ± 27.52 to 130.98 ± 17.13 and 255.96 ± 30.34 with DG1 and DG2 treatment respectively. Studies on the behavioral pattern of STZ-induced anxiolytic effect on ZF confirmed changes in the number of transitions and time spent in both Novel tank test (NTT) and Dark/light test (LDT). Histopathological analysis confirmed the cerebral cortex with inflammatory brain cells in the diseased condition and an attenuation of damage posed revealed in diseased state was largely reversed with DG. As compared to the normal control, a significant (#p < 0.05 and ###p < 0.001) changes in weight of fishes were recorded and DG1 and DG2 significantly promotes (***p < 0.001) body weight and improves the irregularities in weight of ZF during disease progression. Our study confirms that the potential of DG towards the management of hyperglycemia and hyperglycemia–mediated cerebral ischemic injury is through its blood glucose-lowering properties, anti-inflammatory activity, antioxidant effect, and anxiolytic capabilities.
Keywords: Diosgenin, Type 2 diabetes mellitus, Hyperglycaemia, Zebrafish, Streptozotocin, Neuroprotection, Ischemia, Brain injury, Novel tank test, Dark/light test, Chemistry, Drug development, Neuroscience, Behavioral neuroscience, Neurotoxicology, Pharmaceutical science, Pharmacology, Drug delivery
Diosgenin; Type 2 diabetes mellitus; Hyperglycaemia; Zebrafish; Streptozotocin; Neuroprotection; Ischemia; Brain injury; Novel tank test; Dark/light test; Chemistry; Drug development; Neuroscience; Behavioral neuroscience; Neurotoxicology; Pharmaceutical science; Pharmacology; Drug delivery
1. Introduction
Type 2 diabetes mellitus (T2DM) can be characterized as a hyperglycaemic, non-infective metabolic disorder that is associated with a dysfunctional β-cell, deficit in insulin secretion as well as a decreased cell response and insulin sensitivity (Costes, 2018). The recent edition on diabetes prevalence as reported by the International Diabetes Federation (IDF) estimated the possibility of an increase in the number of diabetes mellitus patients from 424.9 million in 2017 to 628.6 million by 2045 with more than 90 % occupying Type 2 Diabetes Mellitus (IDF, 2017). This morbid condition increases the risk of triggering many biochemical pathways that aids both micro and macrovascular complications, with cerebrovascular accidents and insults such as ischemic stroke and brain injury inclusive. Propagation of this disorder can be associated with the delicate vascular, metabolic and endocrine disorientation mediated by diabetes since impaired glucose regulation related to type 2 diabetes mediates various complications, though, their role in cerebral insults and complications seems unclear (Geijselaers et al., 2015). For example, cerebral arteries contain insulin receptors with its vasodilating effect being dependent on the concentration of insulin (Hardigan et al., 2016). Insulin resistance found in type 2 diabetes mellitus decreases the concentration of nitric oxide (NO) responsible for vasorelaxation, thereby, deregulating the constant cerebral perfusion required by the brain. Also, episodes of hypoglycemia and hyperglycaemic occurrence in diabetes condition has a deletory effect on the cerebral function (Biessels et al., 1994).
Increased oxidative stress accompanies cell damage, cell dysfunction, as well as cell death since their generation during hyperglycaemic state, is triggered, thereby exceeding cellular antioxidant defense mechanism. However, treatment of this troubling condition requires not only an agent that proves safety, bioavailability and therapeutic capability but that which will enhance insulin sensitivity and abruptly discontinue pancreatic β-cell failure and possibly address micro and macro-vascular associated complexities. Available therapeutic strategies include the sulfonylureas, Biguanides/metformin, alpha-glucosidase inhibitors, thiazolidinediones, and the meglitinides but studies and data collected from diabetic patients have revealed the potential side effects posed by these common diabetes drugs. This ranges from severe blood glucose reduction (hypoglycemia), risk of liver disease, gastrointestinal diseases, kidney complications, weight gain and many more.
Plant-derived phytomolecules represents the new area of focus in the health industry owing to their unique phytochemistry. Various research have proven their ability to moderate different series of chemical reaction required in the treatment of numerous metabolic conditions such as cancer, diabetes, Kidney diseases, Inflammatory diseases, etc (Sajid et al., 2019). Diosgenin (DG) is a phytosteroidal sapogenin that is majorly found in edible pulses and root including roots of wild yam also known as the Dioscoroceae. Efficacy of diosgenin has been explored in various diseases such as Metabolic dysfunction (Wang et al., 2015) where diosgenin displayed critical activity in adipocyte differentiation. In Liver injury, diosgenin has been reported to have undergone a protective role against ethanol-induced liver injury (Xu et al., 2014). Also, the Renoprotective effect of diosgenin (Kanchan et al., 2016) and protective role against Colorectal cancer (Ahmad Hidayat et al., 2018) was also reported. The neuroprotective effect of diosgenin glycosides was recently studied and its selective suppression of inflammatory factors such as IL-6 and NO shows its potential effectiveness in slowing down the development of certain neurodegenerative disorders (Wang et al., 2017).
Various animal models have been used to study disease development and therapeutic potential these include, mice, rats, and rodents but their labor-intensive property has represented its limitation (Zang et al., 2017). Zebrafish (Danio rerio) is undergoing wide exploration as an animal model in disease discovery, disease development and drug discovery due to their fertility and unique genetic and physiological similarity as that of mammals (Elo et al., 2007). Various models have been established such as visceral adiposity (Oka et al., 2010), impaired glucose metabolism (Capiotti et al., 2014), non-alcoholic steatohepatitis (Asaoka et al., 2013) including diabetes (Kinkel and Prince, 2009), using zebrafishes. This study aims at studying the glucose-regulating effect of Diosgenin in an STZ-induced type 2 diabetes Zebrafish model including the possibility of hyperglycemic-mediated cerebral ischemic damage while presenting a mild exploration of DG towards its neuroprotective role against resulting traces of brain injury.
2. Materials and methods
2.1. Collection, experimental design, and maintenance of zebra fishes
The animal protocol was designed to minimize pain or discomfort to zebrafishes. 3–5 cm long adult healthy wild type zebra fishes (Danio rerio), Tubingen background of both sexes were procured from a certified supplier in India. The experimental protocol for the development of type 2 diabetes mellitus was approved by the “Institutional Animal Ethics Committee”, Amity Institute of Pharmacy, Amity University, Noida, Uttar Pradesh as stated in the guidelines of the “Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA/AIP/2013/004) under chapter 4 section 15, subsection 1 of the prevention of Cruelty to Animals Act 1960 (CPCSEA, Ministry of Environment, Forest and Climatic Change, Government of India, Animal Welfare Division, 5th floor, Vayu Wing, Indira Paryavaran Bhawan Jor Bagh Road, New Delhi-110003. The fishes were housed in our zebra-fish experimental room and acclimatized for 15 days. Fishes were allowed free movement in a 5L tank with a maximum of 15 fishes in each tank at a temperature of 28 ± 2 °C at a pH of 7.0–8.0. Fishes were kept under aeration and a 12h/12h dark and light photocycle was maintained. Fishes were fed twice daily with normal fish meal that comprises soya bean meal, wheat flour and rice flour supplemented with fish and fish derivatives (Toya fish food, India). The water in the tanks was changed every 3days throughout the experiment and the weight and behavioral analysis was also investigated and recorded twice every week. Fishes were randomly selected in this study.
2.2. Drugs and reagents
Diosgenin was procured from Chromadex, Irvine, CA 92618 USA and stored at room temperature for further use. Previously extracted BG oil was used as the drug solubilizing agent in this study. Streptozotocin (STZ) was procured from Sisco Research Laboratories Pvt Ltd, Maharashtra, India and stored at 4 °C for further use. Glucose test kit was procured from Transasia Biochemical limited, Solan and was also stored between 2-8 °C. All other chemicals and reagents used in this study were of analytical grade.
2.3. Induction of diabetes
A stock of 1 mg/mL STZ solution was prepared in 0.85 % normal saline. Fishes were grouped into Control (n = 10) and Disease (n = 36). The diseased group were distributed into different 5L capacity tanks with 12 fishes distributed in each. STZ was administered through the tail vein of the zebrafishes. A multi-low dose of 20 mg/kg b.w of streptozotocin was intravenously administered to ZF with a 1mL syringe having a 1-inch needle at a 7-day interval for a period of 28 days. At every 7th day, fishes were collected and sacrificed by placing on ice to initiate hypothermia. The liver and brain tissues were collected and stored appropriately for biochemical and histopathological analysis. Signs of stress and anxiety were observed by carrying out the behavioral changes in fishes. Weight of fishes with disease progression was also studied continuously throughout the study.
2.4. Experimental treatment procedure
Diseased fishes were further divided into four groups: Positive standard control (n = 5), DG 1 treated group (n = 5), DG2 treated group (n = 6) and BG (n = 6). The positive standard control received 500mg metformin while the treated group received 20 and 40mg/BW DG respectively, while 5μL oil was administered to the BG group. Treatment with DG and Metformin commences after 28 days of induction of diabetes. 1mg DG was weighed and allowed to dissolve in BG oil. Dose of each respective drug was administered at every 3rd day for a period of 28 days. After each 7th day, fishes were collected and sacrificed for biochemical and histopathological analysis.
The study group is as indicated below.
-
•
Group 1 - Normal Control (Naked)
-
•
Group 2 - Negative Control (n = 6) STZ
-
•
Group 3 - Vehicle (n = 6) BG Vehicle (5 μL)
-
•
Group 4 - Positive Control (n = 6) 500 mg/kg Metformin
-
•
Group 4–20 mg/kg DG (ip)
-
•
Group 5–40 mg/kg DG (ip)
At the end of the study, blood glucose level, weight and behavioral pattern, including the catalytic activity of the fishes were studied.
2.5. Determination of blood glucose
The blood glucose of STZ and DG treated fishes was determined by using the GOD-POD endpoint method (Trinder, 1969). On the night of each 7th day, fishes were left unfed over the night against sacrifice on the following day. Fishes were sacrificed by placing on ice until they become completely still. Tissue samples were removed and stored at -20 °C in phosphate-buffered saline (PBS) of pH 7.4. Tissues stored were collected from storage and homogenized with a manual crusher. The resulting homogenized tissue solution was centrifuged at 14,000 rpm for 15 min. Post mitochondrial Supernatant (PMS) was collected and used for the analysis. The concentration of glucose was determined spectrometrically using the Malvern UV-Visible Spectrophotometer and absorbance was recorded at 505 nm. The concentration of glucose in mg/dL of control and diseased samples was calculated as shown.
Concentration above 100 mg/dL and 125 mg/dl is taken as the onset and diabetes establishment respectively as STZ dosing progresses till the 28th day.
2.6. Catalytic activity
The catalase activity was measured according to the method described by Claiborne (1985) (Claiborne, 1985). Briefly, 1.99 mL of 0.05M phosphate buffer (pH 7) was introduced into each well-labeled control and test tubes in duplicates. Subsequently, 10μL PMS was added and 1mL of freshly prepared 20mM H2O2 was added. Absorbance was recorded at 340 nm kinetically for 120 s. The activity of oxidant defense and enzymes in each sample was calculated as shown
Where OD = Optical Density; TRV = Total reaction volume; TSV = Total Sample Volume.
2.7. Histopathological analysis
Microscopic analysis of the tissue was done in order to ascertain the manifestation of the disease. Brain and liver tissue samples were collected from sacrificed zebra fishes and fixed in 10 % neutral buffered formalin solution. Fixed specimen were further dehydrated in different alcohol series, acetone and xylene consecutively before embedding in paraffin. Afterward, paraffin blocks were trimmed and sectioned at 4 μm. Deparaffinization followed by hematoxylin and eosin staining was performed, processed into a thin microscopic section and slides were examined under light microscopy at 400X magnification. Images were further taken for biopsy and pathological examination. Only the histopathological report of the brain sample was given in this study. Image J software was further used for the counting of cells.
2.8. Statistical analysis
From data obtained, values are represented as mean ± standard error of mean (SEM). The statistical analysis involving two groups was evaluated using SPSS by means of one-way analysis of variance (ANOVA). Tukey's multiple comparison test was used for statistical comparison between Normal control, Disease control, and various treated groups. The homogeneity of the various groups were further analyzed by Tukey's post hoc test. Statistical significance was accepted at the ρ < 0.05 values. Statistical significance was represented as *, **, and *** when ρ < 0.01, ρ < 0.01 and ρ < 0.001when compared to diabetic group and ### when compared to control.
3. Results
3.1. Antidiabetic activity
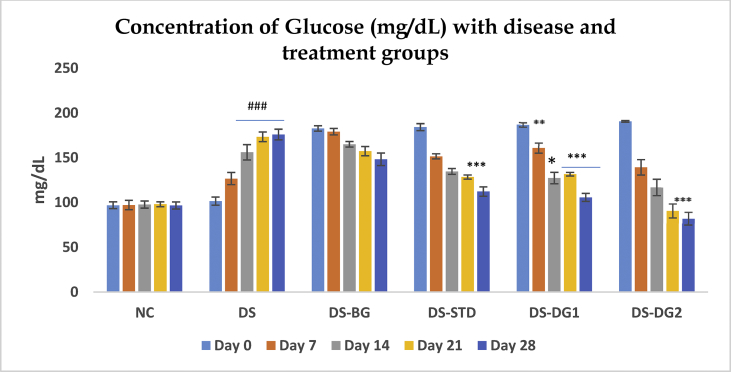
20 mg/kg b.w multi-low dose STZ significantly (###ρ < 0.001) induced hyperglycemia when compared to normal control as represented in Table 1 and Figure 1. The plasma glucose increases from 101.56 ± 4.523 mg/dL at day 0–175.87 ± 5.998 mg/dL after the completion of the quarterly administration of STZ on the 28th day with hyperglycemia persisting after second dose of STZ (Day 14). At a Diosgenin concentration of 20mg (DG 1), glucose concentration significantly (***ρ < 0.001) reduced from 186.82 ± 2.451 at a minimum value of 178 and a maximum of 192 to 105.682 ± 4.485 at a minimum and maximum value of 99.76 and 123.45 respectively by the 28th day of treatment. 40 mg/kg Diogenin (DG2) also showed significant (ρ < 0.01) decrease in the level of plasma glucose at day 14 and an increased significance (***ρ < 0.001) on the 21st and 28th day (190.60 ± 0.892 mg/dL to 116.806 ± 9.183 mg/dL; 90.85 ± 7.796 mg/dL and 82.06 ± 7.270 mg/dL at day 14, Day 21 and day 28 respectively). At 40 mg/kg Diosgenin (DG 2), a minimum and maximum value of 188.23 and 193.27 at day 0 and 59.27 and 101.30 at day 28 were obtained respectively.
Table 1.
Effect of DG on glucose level.
| Effect of multi-low dose STZ and DG on glucose concentration of Zebra fishes (n = 5) | ||||||
|---|---|---|---|---|---|---|
| Glucose Concentration (mg/dL) | ||||||
| S/N | Group | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
| I | NC | 96.95 ± 3.74 | 97.19 ± 5.25 | 97.70 ± 3.99 | 98.13 ± 2.72 | 96.62 ± 3.96 |
| II | DS | 101.56 ± 4.52 | 106.68 ± 6.76# | 156.03 ± 8.62### | 173.34 ± 5.32### | 175.87 ± 6.00### |
| III | DS-BG | 182.71 ± 3.02 | 179.08 ± 3.63*** | 165.05 ± 3.12 | 157.32 ± 5.23 | 148.20 ± 6.95* |
| IV | DS-STD | 184.17 ± 3.88 | 151.23 ± 2.82 | 134.67 ± 3.28 | 128.41 ± 2.33*** | 112.25 ± 5.24*** |
| V | DS-DG1 | 186.82 ± 2.45 | 160.90 ± 5.79** | 127.32 ± 6.45* | 131.65 ± 2.02*** | 105.68 ± 4.49*** |
| VI | DS-DG2 | 190.60 ± 0.89 | 139.29 ± 8.59 | 116.81 ± 9.18** | 90.85 ± 7.80*** | 82.06 ± 7.27*** |
Data is presented as Mean ± SEM for Normal control (NC), Disease (DS), DS with BG vehicle (DS-BG), DS with positive metformin control (DS-STD), DS with 20 mg/kg diosgenin (DS-DG1) and 40 mg/kg diosgenin (DS-DG2). The significance of each is indicated as *ρ < 0.05, **ρ < 0.01 and ***ρ < 0.001 when Disease control (Group II) is compared with Group III, IV, V and VI and #ρ < 0.05, ##ρ < 0.01 and ###ρ < 0.001 when normal control (Group 1) is compared with Disease (Group 2).
Figure 1.
Effect of STZ and DG on blood glucose level. Data is presented as Mean ± SEM for Normal control (NC), Disease (DS), DS with BG vehicle (DS-BG), DS with positive metformin control (DS-STD), DS with 20 mg/kg diosgenin (DS-DG1) and 40 mg/kg diosgenin (DS-DG2). The significance of each is indicated as *ρ < 0.05, **ρ < 0.01 and ***ρ < 0.001 when Disease control (Group II) is compared with Group III, IV, V and VI and #ρ < 0.05, ##ρ < 0.01 and ###ρ < 0.001 when normal control (Group 1) is compared with Disease (Group 2).
3.2. Effect on body weight
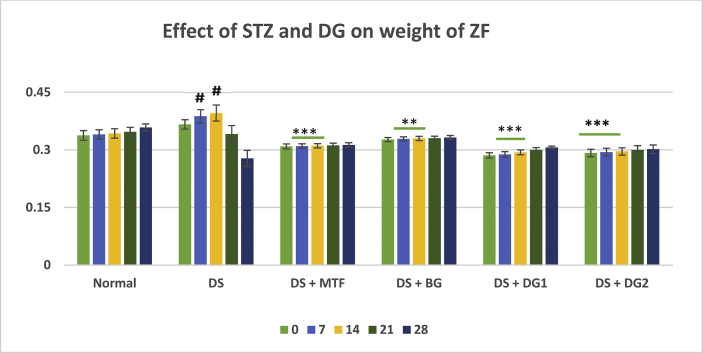
The changes in body weight of Zebrafishes during disease and different treatment conditions were analyzed for this study. As presented in Table 2, a continuous increase in weight between day 0 till day 14, followed by a marked reduction in weight of diabetic fishes was observed (i.e Day 0 = 0.336 ± 0.0123 g; Day 7 = 0.387 ± 0.0178 g; Day 14 = 0.396 ± 0.0213 g; Day 21 = 0.341 ± 0.022 g and Day 28 = 0.278 ± 0.0210 g. As compared to the normal control, a significant (#p < 0.05 and ###p < 0.001) changes in weight of fishes was recorded at day 21 and 28. Diosgenin, (20 and 40 mg/kg) gave a significant (***p < 0.01) increase in body weight of diseased fishes till day 28 from an average weight of 0.286 ± 0.00663 and 0.292 ± 0.00967 at day 0–0.306 ± 0.00368 and 0.3018 ± 0.0110 at day 28 in DG1 and DG2 respectively. BG vehicle shows a slight (**p < 0.01) increase in weight of zebra fishes at days 7 and 14 with no significant difference in subsequent treatment days (Figure 2).
Table 2.
Weight changes in Zebrafish with disease and treatment progression.
| Effect of multi-low dose STZ (DS) and DG on the weight of Zebra fishes | ||||||
|---|---|---|---|---|---|---|
| Weight in g | ||||||
| S/N | Group | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
| I | NC | 0.338 ± 0.01 | 0.340 ± 0.01 | 0.343 ± 0.01 | 0.347 ± 0.01 | 0.358 ± 0.01 |
| II | DS | 0.366 ± 0.01 | 0.387 ± 0.02# | 0.396 ± 0.02# | 0.341 ± 0.02 | 0.278 ± 0.02### |
| III | DS-BG | 0.327 ± 0.01 | 0.329 ± 0.02*** | 0.330 ± 0.01*** | 0.330 ± 0.01 | 0.332 ± 0.01* |
| IV | DS-STD | 0.309 ± 0.01** | 0.310 ± 0.01** | 0.311 ± 0.01** | 0.312 ± 0.01 | 0.313 ± 0.01 |
| V | DS-DG1 | 0.286 ± 0.01** | 0.288 ± 0.01*** | 0.294 ± 0.01*** | 0.300 ± 0.01 | 0.306 ± 0.00 |
| VI | DS-DG2 | 0.292 ± 0.01** | 0.294 ± 0.01*** | 0.296 ± 0.01*** | 0.300 ± 0.01 | 0.302 ± 0.01 |
Data is presented as Mean ± Standard Error of Mean for Normal control (NC), Disease (DS), DS with BG vehicle (DS-BG), DS with positive metformin control (DS-STD), DS with 20 mg/kg diosgenin (DS-DG1) and 40 mg/kg diosgenin (DS-DG2). The significance of each is indicated as *ρ < 0.05, **ρ < 0.01 and ***ρ < 0.001 when Disease control (Group II) is compared with Group III, IV, V, VI and #ρ < 0.05, ##ρ < 0.01 and ###ρ < 0.001 when normal control (Group 1) is compared with Disease (Group 2).
Figure 2.
Changes in weight of ZF with multi-low dose STZ administration and treatment groups. Data is presented as Mean ± SEM for Normal control (NC), Disease (DS), DS with BG vehicle (DS-BG), DS with positive metformin control (DS-STD), DS with 20 mg/kg diosgenin (DS-DG1) and 40 mg/kg diosgenin (DS-DG2). The significance of each is indicated as *ρ < 0.05, **ρ < 0.01 and ***ρ < 0.001 when Disease control (Group II) is compared with Group III, IV, V and #ρ < 0.05, ##ρ < 0.01 and ###ρ < 0.001 when normal control (Group 1) is compared with Disease (Group 2) using ANOVA followed by Tukey's test.
3.3. Catalase (CAT)
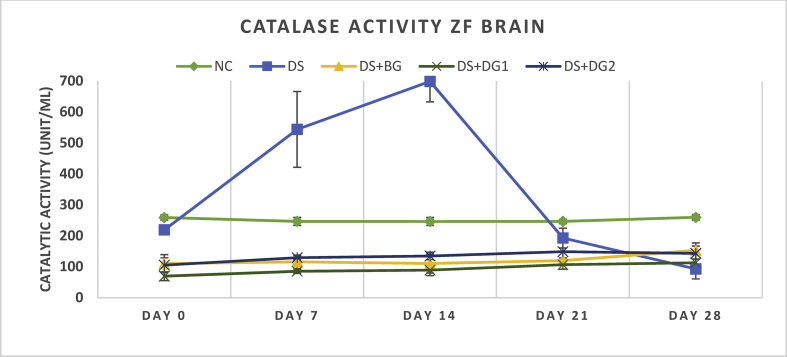
The catalase activity as a measure of enzymatic catalase antioxidant activity was kinetically determined in response to its ability to consume hydrogen peroxide in the liver (Table 3) and brain tissues (Table 4, Figure 3). The activity of catalase in the liver expressed as unit catalase activity in 1 mL protein (1unit/mL) was shown to significantly (p < 0.05) reduce from 206.42 ± 30.771unit/mL at day 0–123.85 ± 29.991unit/mL at day 28 at a minimum and maximum value of 103.21 and 275.23 respectively. A significant difference of 13.76 ± 34.872; -82.99 ± 23.884 ρ < 0.05; -97.68 ± 21.47 ρ < 0.01; -97.69 ± 29.64 ρ < 0.05; -110.09 ± 33.93 ρ < 0.05 was obtained at day 0, 7, 14, 21 and 28 when disease group (DS) was compared with Normal control (NC). Further activity with 20 and 40 mg/kg DG showed significant improvement in catalytic activity but and a more effective activity was observed in DG2 (p < 0.01). A pronounced significant increase (p < 0.01) in catalytic activity from a mean activity of 130.73 ± 27.52 unit/mL at a minimum and maximum value 206.42 and 330.28 at day 0 to a mean activity of 255.96 ± 30.34 at a minimum and maximum value of 206.42 and 330.28 at day 28 was shown in DG2. In ZF brain catalytic activity in DS was found to significantly reduce (ρ < 0.001) from an average of 219.10 ± 7.81 unit/mL at day 0–92.89 ± 32.46 after day 28 of disease induction. Treatment with DG 1 and DG 2 showed a significantly improved elevation from 69.49 ± 14.20 unit/mL (34.40 min/120.40 max value) and 105.28 ± 23.27 (34.40 minimum/151.38 maximum) at day 0 to an average catalytic activity of 112.85 ± 11.01 unit/mL (82.57 minimum/151.38 maximum) and 143.12 ± 33.60 unit/mL (96.33 minimum and 275.23 maximum) at Day 28 (Figure 3, Table 4).
Table 3.
Catalytic activity in ZF Liver.
| Effect of multi-low dose STZ (DS) and DG on the antioxidant activity of Zebra fishes | ||||||
|---|---|---|---|---|---|---|
| Catalytic Liver activity in Unit/mL | ||||||
| S/N | Group | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
| I | NC | 192.66 ± 31.90 | 220.18 ± 17.54* | 221.19 ± 19.98** | 214.75 ± 22.76* | 233.94 ± 20.06* |
| II | DS | 206.42 ± 30.77 | 137.19 ± 10.89* | 123.51 ± 8.29** | 117.06 ± 16.73* | 123.85 ± 29.99* |
| III | DS + BG | 106.70 ± 13.80 | 134.86 ± 6.74 | 116.42 ± 7.58 | 235.79 ± 20.30** | 295.23 ± 19.02** |
| IV | DS + DG1 | 101.58 ± 11.29 | 79.81 ± 12.61 | 95.31 ± 3.22 | 100.79 ± 8.13 | 130.98 ± 17.13 |
| V | DS + DG2 | 130.73 ± 27.52 | 123.85 ± 28.20 | 212.92 ± 24.83** | 214.68 ± 30.34* | 255.96 ± 30.34** |
Catalytic activity of ZF with multi-low dose STZ administration and treatment groups represented herein. Data is presented as Mean ± SEM for Normal control (NC), Disease (DS), DS with BG vehicle (DS-BG), DS with 20 mg/kg diosgenin (DS-DG1) and 40 mg/kg diosgenin (DS-DG2). The significance of each is indicated as *ρ < 0.05, **ρ < 0.01 and ***ρ < 0.001 when Disease control (Group II) is compared with Group III, IV, V and #ρ < 0.05, ##ρ < 0.01 and ###ρ < 0.001 when normal control (Group 1) is compared with Disease (Group 2) using ANOVA followed by Tukey's test.
Table 4.
Catalytic activity in ZF Brain.
| Effect of multi-low dose STZ (DS) and DG on the antioxidant activity of Zebra fishes | ||||||
|---|---|---|---|---|---|---|
| Catalytic Brain activity in Unit/mL | ||||||
| S/N | Group | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
| I | NC | 259.80 ± 8.89 | 246.46 ± 12.97 | 246.22 ± 12.84 | 259.79 ± 7.03 | 259.79 ± 8.89 |
| II | DS | 219.10 ± 7.81 | 543.58 ± 122.31# | 698.65 ± 65.99### | 192.66 ± 31.90 | 92.89 ± 32.46### |
| III | DS + BG | 110.09 ± 29.09** | 115.83 ± 22.00*** | 110.32 ± 30.77*** | 119.72 ± 8.80 | 152.75 ± 13.83 |
| IV | DS + DG1 | 69.49 ± 14.20** | 85.32 ± 7.02*** | 89.05 ± 17.87*** | 106.65 ± 14.51 | 112.85 ± 11.01 |
| V | DS + DG2 | 105.28 ± 23.27** | 129.36 ± 7.02*** | 134.86 ± 11.84*** | 148.62 ± 33.00 | 143.12 ± 33.60 |
Catalytic activity of ZF Brain with multi-low dose STZ administration and treatment groups represented herein. Data is presented as Mean ± SEM for Normal control (NC), Disease (DS), DS with BG vehicle (DS-BG), DS with 20 mg/kg diosgenin (DS-DG1) and 40 mg/kg diosgenin (DS-DG2). The significance of each is indicated as *ρ < 0.05, **ρ < 0.01 and ***ρ < 0.001 when Disease control (Group II) is compared with Group III, IV, V and #ρ < 0.05, ##ρ < 0.01 and ###ρ < 0.001 when normal control (Group 1) is compared with Disease (Group 2) using ANOVA followed by Tukey's test.
Figure 3.
Catalytic activity of ZF brain with multi-low dose STZ administration and treatment groups is represented herein. Data is presented as Mean ± SEM. The significance of each is indicated as *p < 0.05, **p < 0.01 and ***p < 0.001 when Disease control (Group II) is compared with Group III, IV, V and #p < 0.05, ##p < 0.01 and ###p < 0.001 when normal control (Group 1) is compared with Disease (Group 2) using ANOVA followed by Tukey's test.
3.4. Histopathological analysis
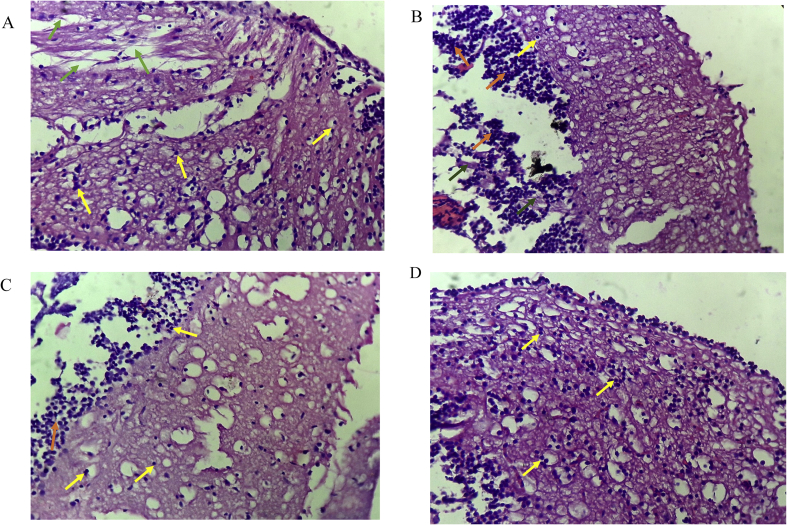
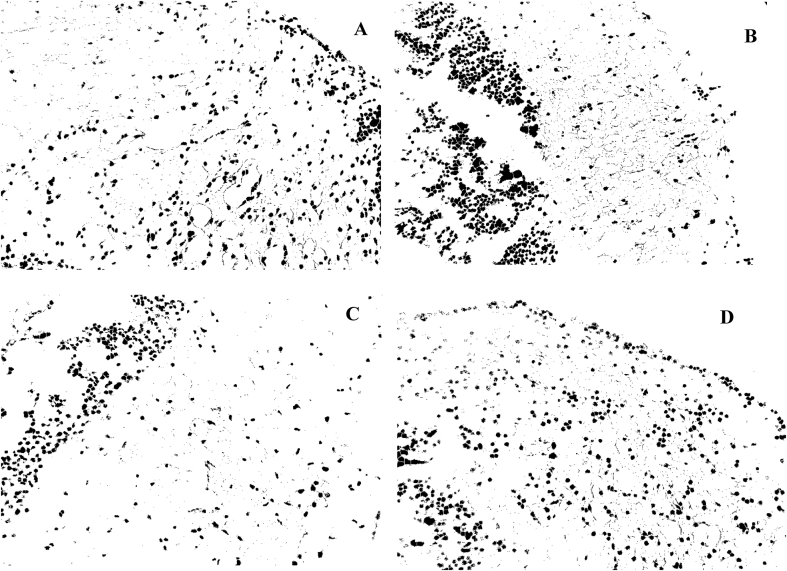
Histology of the brain of zebra fishes is presented in the photomicrographs below (Figure 4). Microscopic investigation to further identify the structural and morphological transformations exerted by DG and STZ Diabetic ZF at day 21 and 28 (Figure 4a and b) showed the occurrence of cerebral damage via the tissue degeneration, scattered inflammatory cells, activation of glial cells with increased cell population. After an array of treatment with DG 1 and DG 2 (Figure 4c and d), the cerebral cortex featured few oligodendrocytes and moderate inflammatory cell infiltrates. Cell quantification and size is also presented in Table 5, Figure 6 in order to feature the cellular environment. Treatment with STZ at day 21 and 28 (Figures 5a and 5b) showed an increased cell volume and cellular aggregation was activated at day 28. DG treated groups (Figures 5c and 5d) of DG1 and DG2 showed a slight difference in the orientation with a decrease in cell volume and size.
Figure 4.
Photomicrographs of histopathological analysis of brain by H&E staining; (A) Diabetic-STZ Dose 3 (B) diabetic-STZ Dose 4, (C) and (D) 20 and 40 mg/kg diosgenin respectively. Arrows: yellow (Astrocytes), green (tissue degeneration of cerebral cortex), orange (glial cells aggregate).
Table 5.
Cell quantification analysis of histopathological photomicrographs.
| Slice | Cell count | Total Area | Average size | % Area |
|---|---|---|---|---|
| Zf-DS 1 | 3044 | 58413 | 19.19 | 7.80 |
| Zf-DS 2 | 3662 | 91704 | 25.04 | 9.24 |
| Zf-DS + DG1 | 2055 | 44563 | 15.685 | 5.37 |
| Zf-Ds + DG2 | 2813 | 49784 | 16.698 | 7.17 |
Table showing the number of cells with their mean size during ZF treatment with STZ and DG (DG1 and DG2). Day 21(A) and Day 28 (B) represents day 21 and 28 of STZ induced T2DM development. The progressive effect of STZ on the number of brain cells and morphology represents Slice Zf-DS1 and ZF-DS2 in the table. ZF-DS1 and ZF-DS2 treatment indicates an increased volume of astrocytes (glial cells GC). ZF-DS + DG1 and ZF-DS + DG2 progressively reduce cell volume and mean size.
Figure 6.
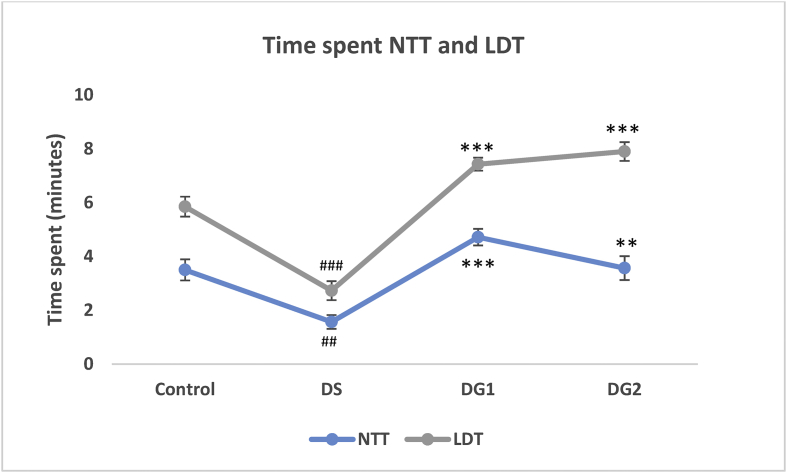
Effect of STZ and DG on the number of time spent in the water surface (NTT) and lit region (LDT) within a maximum of 10 min. The significant difference is represented as ##p < 0.01 and ###p < 0.001when disease control is compared with normal control ***p < 0.001 when disease is compared with DG 1 and DG 2 using ANOVA followed by Tukey's test.
Figure 5.
Image analysis showing the number of cells during STZ and DG (DG1 and DG2) treated ZF. Day 21(A) and Day 28 (B) of STZ treatment indicates scattered and increased volume of astrocytes (glial cells GC) with activation of aggregation of GC at day 28 (B). DG 1 (C) and DG2 (C) progressively reduce cell volume as shown in the image.
3.5. Behavioral analysis
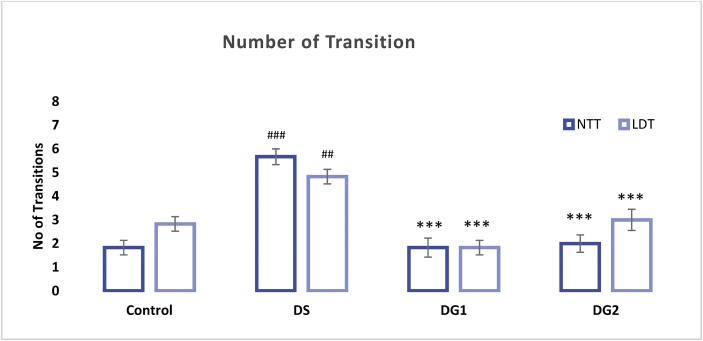
The result of Zebra fishes' behavioral pattern after undergoing a Novel tank diving test (NTT) and Light-dark test (LDT) is presented in Figures 6 and 7 below. Time spent up and in the light region including a number of transitions up and toward the light region in NTT and LDT are represented in Figures 6 and 7 below. Result indicated a significant (##P < 0.01) decrease in time spent in the upper region of the tank (NTT) when Diabetic ZF were compared to control ZF after exposure to a multi low dose of STZ (20 mg/kg b.w) four times for 28 days (1.567 ± 0.258; df = 1.933; p-value = 0.005) at a 0.5156 and 3.3511 lower and upper bound respectively. After diabetic fishes were treated with diosgenin, time spent in the upper region of the tank significantly elevated (***p < 0.001 and **p < 0.01) to 4.717 ± 0.309 and 3.567 ± 0.443 at a mean difference (df) of 3.150 and 2.000 in DG1 and DG2 respectively (see Figure 6). During LDT, Diabetic fishes spent approximately 2.733 ± 0.354 min; (df = 3.117 ± 0.472) in the light region of the tank. In comparison with the control fishes, a significant (###p < 0.001) decrease in time spent in the light region was observed in Diabetic ZF (Figure 6). DG1 and DG2 treated ZF displayed an increased time spent when exposed to LDT test (7.433 ± 0.244 df = 4.70 ± 0.472; 7.900 ± 0.348 df = 5.17 ± 0.472). The number of transitions in diseased ZF is shown in Figure 7. Diseased fishes displayed number of transition up the tank (5.67 ± 0.333, ###p < 0.001) and light region (4.83 ± 0.307, ##p < 0.01) as compared to the control fishes (1.83 ± 0.307) at a mean difference of -3.833 (NTT) and -2.00 (LDT) between the control and diseased groups. DG 1 and DG 2 showed significantly fewer transitions in the two tests (NTT and LDT) when compared to disease group (***p < 0.001) but no significant difference was observed between DG1 and DG2. In NTT, a mean transition of 1.83 ± 0.401 and 2.00 ± 0.365 with a mean difference of 3.83 and 3.67 was recorded for DG1 and DG 2 respectively while LDT gave a mean transition number of 1.83 ± 0.307, df = 3.00 and 3.00 ± 0.447 df = 2.83 (see Figure 7).
Figure 7.
Effect of STZ and DG on the number of time spent in the water surface (NTT) and lit region (LDT) within a maximum of 10 min. The significant difference is represented as ##p < 0.01 and ###p < 0.001when disease control is compared with normal control and ***p < 0.001 when disease group is compared with DG 1 and DG 2 using ANOVA followed by Tukey's test.
4. Discussion
Insulin resistance and defective insulin secretion can be linked to the persistent hyperglycaemic state in the pathogenesis of type 2 diabetes mellitus (Dedoussis et al., 2007; Reaven, 2011). Elevated blood glucose results from impaired insulin secretion from β-cells and a showcase of inadequate compensation for non-insulin sensitivity in peripheral tissue. Also, This condition influences the overproduction of reactive oxygen species (ROS), leading to depleted antioxidant defense capacity, hence, oxidative stress by activating the stress transduction factor pathways (Weyer et al., 1999). During these multifactorial complications, behavioral patterns tend to be influenced (Sweetnam et al., 2012), including body weight. The increased ROS triggers non-genetic changes and dysfunctional endothelial cells, thereby, stimulating more ROS production that causes cell instability in cell membrane due to lipid peroxidation, finally leading to inflammation and damage to the affected organs and tissue (Brownlee, 2001; Ceriello et al., 2009).
Streptozotocin (STZ) is widely known to depict a hyperglycaemic condition due to its ability to directly cause destruction to the pancreatic β-cell, thereby resulting in an inconsistent and impaired insulin production (Shafrir, 2003; Rerup, 1970). The zebrafish model is developed to elucidate these complications. After the third dose of administration, fishes showed an increased blood glucose concentration with a maximum significance (p < 0.01) after the last STZ dose as shown in Table 1. This confirms the development of hyperglycemia related to type 2 diabetes induction by STZ. Diosgenin at a concentration of 20 and 40 mg/kg b.w in Bottle gourd (BG) seed oil as a vehicle decreases the glucose concentration significantly (p < 0.01) to a mean difference of -27.60 ± 8.174, -70.184 ± 8.174 and -82.490 ± 6.67 for BG, DG1, and DG2 (Group 4, 5 and 6) respectively as compared to the Disease group (Group 2) after 28th day of treatment. This supports the previous report on the blood-glucose-lowering property of Diosgenin due to its alpha-glucosidase inhibitory effect and the presence of B-sitosterol (McAnuff et al., 2005; Prasanna, 2000; Sharma et al., 1990; Cayen and Dvornik, 1979).
From the studies carried out on body weight, the significant decrease (p < 0.001) in body weight of ZF after the last dose of STZ indicates an acute induction of diabetes. Elevated loss in body weight in diabetic condition is an indication of a profound breakdown of muscle tissue, protein oxidation, including gluconeogenesis which explains decreased body weight as a metabolic impairment associated with diabetes (Patel et al., 2014). This can also be associated with pancreatic Beta cell damage as reported by Junod et al. (1969) (Junod et al., 1969). During treatment, diosgenin and BG oil, decreased body weight was altered. This explains the possibility of Diosgenin in BG oil to promote muscular tissue repair, alter gluconeogenesis and enhance insulin production.
The behavioral pattern of zebra fishes was also studied with disease progression since clinical and pre-clinical data have confirmed the association of diabetes in aggravating the incidence of cognitive decline (Dash, 2013), with a possibility of two times likelihood of anxiety and anxiety-like behavior of hyperglycemic history. The NDT and LDT as a robust and relevant measure for anxiety in zebrafish reveals the anxious-like behavior in zebra fishes after the third dose of diosgenin as fishes spent more time at the bottom of the tank with less vertical exploration. Our result confirmed that i.p dose of 20 mg/kg multiple low dose streptozotocin significantly promotes the anxiety-like behavior induced by STZ as reported in a previous study (Gupta et al., 2014; Dos Santos et al., 2018). In contrast, Diosgenin attenuates the effect caused by STZ by increasing the number of vertical transitions and time spent on the water surface and lit compartment as reported in our tank diving results and LDT.
Since persistent and prolonged hyperglycemia through inhibition of insulin signal causes increased production of reactive oxygen species and imbalance with antioxidant defense system, oxidative stress becomes elevated. Catalase is an heme-containing ubiquities enzyme that uses up hydrogen peroxide produced by superoxide dismutase during dismutation reaction in order to prevent the generation of hydroxyl radicals responsible for oxidative damage (Hunt et al., 1990; Ugochukwu and Cobourne, 2003). Our result confirmed the reduced activity of catalase enzyme in diabetic fishes. This can be attributed to the high probability of awakening of numerous oxidative stress pathways such as glucose autooxidation, polyol pathway, AGE formation and PKC β ½ Kinase in a hyperglycaemic state (Brownlee, 2005). After the third dose of 40 mg/kg Diosgenin in BGsO, the effect of reactive oxygen species was attenuated by improving the activity of the antioxidants. The ability displayed by diosgenin can be attributed to the body's response to reactive oxygen species indicating that diosgenin can revive the antioxidant enzymatic activities owning to the presence of a hydroxyl group in its structural composition (Pari et al., 2012; Al-Matubsi et al., 2011).
Histopathological studies were carried out to further compensate our studies. Histology of the brain tissues of zebra fishes was studied since critical pathological activities tend to occur in the brain during hyperglycemia and diabetes because of reduced glucose utilization during this period (McCall, 1992; Nagayach et al., 2014). Cerebrovascular inflammation is also reported as one of the major pathophysiological features in diabetes and its vascular complication (Goldberg, 2009; Drake et al., 2011). In our study, Diabetic rats showed cerebral cortex with astrocytes, few oligodendrocytes, hyperglycemia-induced and scattered inflammatory cells in a fibrillary background with very occasional neuron seen at day 21 and severe neuronal loss after the 28th day of STZ administration. Since glial cells are easily influenced by any change or damage in the brain, its activation in terms of cell number and morphology is a significant sign of severe inflammation (Nagayach et al., 2014). From our histology report of the diabetic brain, aggregation of glial cells was reported as a biomarker indicating severe brain tissue damage. These astrocytes and glial cell activation can be attributed to the hyperglycaemic effect followed by pancreatic β-cell damage, thereby mediating inflammatory pathways and cytotoxic products such as Interleukin-1 (IL-1) and ROS responsible for cerebral damage (Di Napoli et al., 2011; Arend, 1991; Shukla et al., 2017). Li et al. (2011) reported acute cerebral infarction in the presence of diabetes mellitus (Li et al., 2011). After the last treatment dose, the section examined showed cerebral cortex with focal densely scattered inflammatory cells in DG1 and moderate inflammatory cells infiltrate in DG2 and Vehicle but with neuronal loss. This shows the potential of DG in improving the pathological features of the damaged brain by altering the neuronal cell death, improving the antioxidant capacity and cerebral function. This means although, activation of microglia is a response to a brain insult such as inflammation and neurotoxins but its overstimulation can promote the already-existing neuronal damage via increased production of neurotoxins. Meanwhile, the neuroprotective effect of diosgenin is presented through its ability to regulate microglial activation as previously reported (Wang et al., 2017). Further image analysis on cell quantification revealed the presence of numerous neuronal cells in diseased ZF with some large cellular globules compared to DG treated ZF groups. This alteration in cell morphology, size and volume is in agreement with a previous study that when the brain is insulted chemically and mechanically, hypertrophy and proliferation is enhanced due to structural and functional changes (Selim and Selim, 2013). This contributes to neurotoxicity from inflammatory cytokines and free radical with subsequent neuronal damage that severely contributes to the pathogenesis of neurodegenerative disease (Baydas et al., 2006; Bates et al., 2002).
In order to assess the diabetic-related behavioral changes in ZF, the NTT and LDT were considered. NTT and LDT have been widely explored and reported as an analog to study stressors, anxiety-like behaviors and drug responses in zebra fishes since the former targets exploration and locomotive pattern of fishes while the latter is capable of displaying the natural tendency of light avoidance and open anxiety-like phenotypes (Scototaxis) in fishes (Stewart et al., 2010, 2014; Maximino et al., 2010; Hascoet et al., 2001). In our study, time spent up and time spent in the light including the number of transitions up and towards the light region in both NTT and LDT respectively were reported as the endpoint. From our results, diseased ZF showed a much longer avoidance of the water surface as much time was spent at the bottom of the tank. Similarly, diseased fishes showed a high level of light avoidance by spending higher time in the dark region. Further studies towards the number of transition within the scheduled 10 min revealed that diseased fishes transited to the top and to the light region twice as much as the treatment group. This indicated that although diseased fishes spent less time in the light and on the water surface, but they displayed an unrestful state by an increased number of urgent transitions with immediate return. On comparing with the treatment group, DG1 and DG2 significantly increased the time spent in both cases with a decreased number of transitions. The difference in the behavioral pattern of both diseased and treated groups signifies a comparable ground. This unusual behavioral pattern in both groups can be tailored to the influence of diabetic complications in relation to dysfunctional neurobehavior (Biessels et al., 1994).
5. Conclusion
Diosgenin selectively lowers the blood glucose concentration in a hyperglycemia-induced diabetes condition. It improves the weight and growth pattern of diabetic zebrafishes and also displayed enhanced catalytic activity. Its activity towards protecting the brain from the possibility of hyperglycemic-mediated brain damage and apoptotic brain cell death is visible from the suppressed inflammation. This study set forth the possibility and potential of diosgenin as an anti-inflammatory and antidiabetic and neuroprotective agent. Its neuroprotectant potential can be positively explored in future clinical studies.
Declarations
Author contribution statement
O. Oyelaja-Akinsipo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
D. Katare: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
E. Dare: Conceived and designed the experiments.
Funding statement
This work was supported by Department of Biotechnology (DBT) in collaboration with The World Academy of Science (TWAS), Fellowship Award No. 3240300002 and the Amity Institute of Biotechnology, Amity University, Uttar Pradesh, India.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Footnotes
Supported by Department of Biotechnology (DBT) in collaboration with The World Academy of Science (TWAS), No. 3240300002.
References
- Ahmad Hidayat A.F., Chan C.K., Mohamad J., Abdul Kadir H. 'Dioscorea bulbifera induced apoptosis through inhibition of ERK 1/2 and activation of JNK signaling pathways in HCT116 human colorectal carcinoma cells. Biomed. Pharmacother. 2018;104:806–816. doi: 10.1016/j.biopha.2018.05.073. [DOI] [PubMed] [Google Scholar]
- Al-Matubsi H.Y., Nasrat N.A., Oriquat G.A., Abu-Samak M., Al-Mzain K.A., Salim M. 'The hypocholesterolemic and antioxidative effect of dietary diosgenin and chromium chloride supplementation on high-cholesterol fed Japanese quails. Pakistan J. Biol. Sci. 2011;14:425–432. doi: 10.3923/pjbs.2011.425.432. [DOI] [PubMed] [Google Scholar]
- Arend W.P. 'Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J. Clin. Invest. 1991;88:1445–1451. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka Y., Terai S., Sakaida I., Nishina H. 'The expanding role of fish models in understanding non-alcoholic fatty liver disease. Dis. Model Mech. 2013;6:905–914. doi: 10.1242/dmm.011981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates K.A., Fonte J., Robertson T.A., Martins R.N., Harvey A.R. Chronic gliosis triggers Alzheimers disease-like processing of amyloid precursor protein. Neuroscience. 2002;113:785–796. doi: 10.1016/s0306-4522(02)00230-0. [DOI] [PubMed] [Google Scholar]
- Baydas G., Ozer M., Yasar A., Koz S.T., Tuzcu M. 'Melatonin prevents oxidative stress and inhibits reactive gliosis induced by hyperhomocysteinemia in rats. Biochemistry (Mosc.) 2006;71(suppli):S91–S95. doi: 10.1134/s0006297906130153. [DOI] [PubMed] [Google Scholar]
- Biessels G.J., Kappelle A.C., Bravenboer B., Erkelens D.W., Gispen W.H. Cerebral function in diabetes mellitus. Diabetologia. 1994;37:643–650. doi: 10.1007/BF00417687. [DOI] [PubMed] [Google Scholar]
- Brownlee M. 'Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Capiotti K.M., Antonioli R., Jr., Kist L.W., Bogo M.R., Bonan C.D., Da Silva R.S. 'Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014;171:58–65. doi: 10.1016/j.cbpb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Cayen M.N., Dvornik D. 'Effect of diosgenin on lipid metabolism in rats. J. Lipid Res. 1979;20:162–174. [PubMed] [Google Scholar]
- Ceriello A., Ihnat M.A., Thorpe J.E. 'Clinical review 2: the "metabolic memory": is more than just tight glucose control necessary to prevent diabetic complications? J. Clin. Endocrinol. Metab. 2009;94:410–415. doi: 10.1210/jc.2008-1824. [DOI] [PubMed] [Google Scholar]
- Claiborne A. 'Catalase activity. In: A Greenwald R., editor. Handbook of Methods for Oxygen Radical Research. CRC Press; Boca Raton: 1985. [Google Scholar]
- Costes S. 'Targeting protein misfolding to protect pancreatic beta-cells in type 2 diabetes. Curr. Opin. Pharmacol. 2018;43:104–110. doi: 10.1016/j.coph.2018.08.016. [DOI] [PubMed] [Google Scholar]
- Dash S.K. 'Cognitive impairment and diabetes. Recent Pat. Endocr. Metab. Immune Drug Discov. 2013;7:155–165. doi: 10.2174/1872214811307020009. [DOI] [PubMed] [Google Scholar]
- Dedoussis, George V.Z., Kaliora Andriana C., Panagiotakos Demosthenes B. 'Genes, diet, and type 2 diabetes mellitus: a review. Rev. Diabet. Stud.: RDS. 2007;4:13–24. doi: 10.1900/RDS.2007.4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Napoli Mario, Imtiaz M., Shah 'Neuroinflammation and cerebrovascular disease in old age: a translational medicine perspective. J. aging Res. 2011;2011 doi: 10.4061/2011/857484. 857484-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos M.M., de Macedo G.T., Prestes A.S., Loro V.L., Heidrich G.M., Picoloto R.S., Rosemberg D.B., Barbosa N.V. 'Hyperglycemia elicits anxiety-like behaviors in zebrafish: protective role of dietary diphenyl diselenide. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2018;85:128–135. doi: 10.1016/j.pnpbp.2018.04.017. [DOI] [PubMed] [Google Scholar]
- Drake Caroline, Boutin Hervé, Jones Matthew S., Adam Denes, McColl Barry W., Selvarajah Johann R., Hulme Sharon, Georgiou Rachel F., Hinz Rainer, Gerhard Alexander, Vail Andy, Prenant Christian, Peter Julyan, Renaud Maroy, Brown Gavin, Smigova Alison, Herholz Karl, Kassiou Michael, Crossman David, Francis Sheila, Proctor Spencer D., Russell James C., Hopkins Stephen J., Tyrrell Pippa J., Rothwell Nancy J., Allan Stuart M. 'Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav. Immun. 2011;25:1113–1122. doi: 10.1016/j.bbi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo B., Villano C.M., Govorko D., White L.A. 'Larval zebrafish as a model for glucose metabolism: expression of phosphoenolpyruvate carboxykinase as a marker for exposure to anti-diabetic compounds. J. Mol. Endocrinol. 2007;38:433–440. doi: 10.1677/JME-06-0037. [DOI] [PubMed] [Google Scholar]
- Geijselaers Stefan L.C., Sep Simone J.S., Stehouwer Coen D.A., Biessels Geert Jan. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. The Lancet Diabetes Endocrinol. 2015;3:75–89. doi: 10.1016/S2213-8587(14)70148-2. [DOI] [PubMed] [Google Scholar]
- Goldberg Ronald B. 'Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J. Clin. Endocrinol. Metab. 2009;94:3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- Gupta D., Radhakrishnan M., Kurhe Y. 'Insulin reverses anxiety-like behavior evoked by streptozotocin-induced diabetes in mice. Metab. Brain Dis. 2014;29:737–746. doi: 10.1007/s11011-014-9540-5. [DOI] [PubMed] [Google Scholar]
- Hardigan Trevor, Abdul Yasir, Abdelsaid Mohammed, Couch Maha, El-Shaffey Sally, Li Weiguo, Johnson Maribeth H., Ergul Adviye. 'Linagliptin treatment improves cerebrovascular function and remodeling and restores reduced cerebral perfusion in Type 2 diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;311:R466–R477. doi: 10.1152/ajpregu.00057.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascoet M., Bourin M., Nic Dhonnchadha B.A. 'The mouse light-dark paradigm: a review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2001;25:141–166. doi: 10.1016/s0278-5846(00)00151-2. [DOI] [PubMed] [Google Scholar]
- Hunt James V., Smith Christopher C.T., Wolff Simon P. 'Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990;39:1420–1424. doi: 10.2337/diab.39.11.1420. [DOI] [PubMed] [Google Scholar]
- IDF . International Diabetes Federation. 2017. International diabetes atlas. Brussels, Belgium. [Google Scholar]
- Junod A., Lambert A.E., Stauffacher W., Renold A.E. 'Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J. Clin. Invest. 1969;48:2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchan D.M., Somani G.S., Peshattiwar V.V., Kaikini A.A., Sathaye S. 'Renoprotective effect of diosgenin in streptozotocin-induced diabetic rats. Pharmacol. Rep. 2016;68:370–377. doi: 10.1016/j.pharep.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Kinkel M.D., Prince V.E. On the diabetic menu: zebrafish as a model for pancreas development and function. Bioessays. 2009;31:139–152. doi: 10.1002/bies.200800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Xu X., Wang D., Wang J., Wang Y., Yu J. 'Microglial activation during acute cerebral infarction in the presence of diabetes mellitus. Neurol. Sci. 2011;32:1075–1079. doi: 10.1007/s10072-011-0632-2. [DOI] [PubMed] [Google Scholar]
- Maximino C., Marques de Brito T., Dias C.A., Gouveia A., Jr., Morato S. 'Scototaxis as anxiety-like behavior in fish. Nat. Protoc. 2010;5:209–216. doi: 10.1038/nprot.2009.225. [DOI] [PubMed] [Google Scholar]
- McAnuff M.A., Omoruyi F.O., Morrison E.Y., Asemota H.N. 'Changes in some liver enzymes in streptozotocin-induced diabetic rats fed sapogenin extract from bitter yam (Dioscorea polygonoides) or commercial diosgenin. W. Indian Med. J. 2005;54:97–101. doi: 10.1590/s0043-31442005000200002. [DOI] [PubMed] [Google Scholar]
- McCall Anthony L. The impact of diabetes on the CNS. Diabetes. 1992;41:557–570. doi: 10.2337/diab.41.5.557. [DOI] [PubMed] [Google Scholar]
- Nagayach Aarti, Patro Nisha, Patro Ishan. 'Experimentally induced diabetes causes glial activation, glutamate toxicity and cellular damage leading to changes in motor function. Front. Cell. Neurosci. 2014;8 doi: 10.3389/fncel.2014.00355. 355-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Nishimura Y., Zang L., Hirano M., Shimada Y., Wang Z., Umemoto N., Kuroyanagi J., Nishimura N., Tanaka T. 'Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010;10:21. doi: 10.1186/1472-6793-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pari L., Monisha P., Mohamed Jalaludeen A. 'Beneficial role of diosgenin on oxidative stress in aorta of streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2012;691:143–150. doi: 10.1016/j.ejphar.2012.06.038. [DOI] [PubMed] [Google Scholar]
- Patel A.N., Bandawane D.D., Mhetre N.K. 'Pomegranate (Punica granatum Linn.) leaves attenuate disturbed glucose homeostasis and hyperglycemia mediated hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. Eur. J. Integr. Med. 2014;6:307–321. [Google Scholar]
- Prasanna M. 'Hypolipidemic effect of fenugreek: a clinical study. J. Pharmacol. 2000;32:34–36. [Google Scholar]
- Reaven G.M. 'Insulin resistance: the link between obesity and cardiovascular disease. Med. Clin. North Am. 2011;95:875–892. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Rerup C.C. 'Drugs producing diabetes through damage of the insulin-secreting cells. Pharmacol. Rev. 1970;22:485–518. [PubMed] [Google Scholar]
- Sajid M., Cameotra S.S., Ahmad Khan M.S., Ahmad I. Nanoparticle-based delivery of phytomedicines: challenges and opportunities. In: Sajjad Mohd, Khan Ahmad, Ahmad Iqbal, Chattopadhyay Debprasad., editors. New Look to phytomedicine. Academic Press; 2019. pp. 597–623. [Google Scholar]
- Selim A.S., Selim A.O. 'Effect of streptozotocin-induced diabetes mellitus on the cerebellar cortex of adult male albino rats: histological and immunohistochemical study. Egypt. J. Histol. 2013;36:103–113. [Google Scholar]
- Shafrir E. 'Diabetes in Animals: contribution to the understanding of diabetes by study of its etiopathology in animal models. In: Porte D., Sherwin R.S., Baron A., editors. Diabetes Mellitus. McGraw Hill; New York: 2003. [Google Scholar]
- Sharma R.D., Raghuram T.C., Rao N.S. 'Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur. J. Clin. Nutr. 1990;44:301–306. [PubMed] [Google Scholar]
- Shukla Vibha, Kumar Shakya Akhalesh, Perez-Pinzon Miguel A., Dave Kunjan R. 'Cerebral ischemic damage in diabetes: an inflammatory perspective. J. Neuroinflammation. 2017;14 doi: 10.1186/s12974-016-0774-5. 21-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A.M., Braubach O., Spitsbergen J., Gerlai R., Kalueff A.V. 'Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci. 2014;37:264–278. doi: 10.1016/j.tins.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart Adam, Adam Ferdous Kadri, Ferdous John Dileo, John Chung, Min Kyung, Cachat Jonathan, Jonathan, Goodspeed Jason, Jason Suciu, Shaw Christopher, Roy Sudipta, Gaikwad Siddharth, Siddharth Wong, Allan V. 'The developing utility of zebrafish in modeling neurobehavioral disorders. Int. J. Comp. Psychol. 2010;23:104. [Google Scholar]
- Sweetnam Danielle, Holmes Andrew, Tennant Kelly A., Zamani Akram, Walle Mark, Jones Paul, Wong Charles, Craig E., Brown 'Diabetes impairs cortical plasticity and functional recovery following ischemic stroke. J. Neurosci.: Off. J. Soc. Neurosci. 2012;32:5132–5143. doi: 10.1523/JNEUROSCI.5075-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinder P. 'Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol. 1969;22:158–161. doi: 10.1136/jcp.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugochukwu N.H., Cobourne M.K. 'Modification of renal oxidative stress and lipid peroxidation in streptozotocin-induced diabetic rats treated with extracts from Gongronema latifolium leaves. Clin. Chim. Acta. 2003;336:73–81. doi: 10.1016/s0009-8981(03)00325-5. [DOI] [PubMed] [Google Scholar]
- Wang Shaoxia, Wang Fujiang, Yang Hongyun, Li Ruilin, Guo Hong, Hu Limin. Diosgenin glucoside provides neuroprotection by regulating microglial M1 polarization. Int. Immunopharm. 2017;50:22–29. doi: 10.1016/j.intimp.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Wang X., Liu J., Long Z., Sun Q., Liu Y., Wang L., Zhang X., Hai C. 'Effect of diosgenin on metabolic dysfunction: role of ERbeta in the regulation of PPARgamma. Toxicol. Appl. Pharmacol. 2015;289:286–296. doi: 10.1016/j.taap.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Weyer C., Bogardus C., Mott D.M., Pratley R.E. 'The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Zheng L., Xu L., Yin L., Qi Y., Xu Y., Han X., Peng J. 'Protective effects of dioscin against alcohol-induced liver injury. Arch. Toxicol. 2014;88:739–753. doi: 10.1007/s00204-013-1148-8. [DOI] [PubMed] [Google Scholar]
- Zang L., Shimada Y., Nishimura N. 'Development of a Novel zebrafish model for type 2 diabetes mellitus. Sci. Rep. 2017;7:1461. doi: 10.1038/s41598-017-01432-w. [DOI] [PMC free article] [PubMed] [Google Scholar]