Abstract
Older adults after hip fracture surgery experience progressive muscle atrophy and weakness, limiting full recovery. Further understanding of the molecular mechanisms in muscle with adaptation to exercise training in this vulnerable population is necessary. Therefore, we conducted a pilot study to investigate the skeletal muscle inflammatory and ceramide biosynthesis gene expression levels associated with the toll-like receptor (TLR) pathway before (Pre) and following a 3-mo multicomponent exercise training program in older adults (3M, 4F; 78.4 ± 13.3 yr; 25.5 ± 2.3 kg/m2) ~4 mo after repair from hip fracture (HipFx). Vastus lateralis biopsies from the surgical limb were obtained before (Pre) and after training. Molecular end points and muscle function data were also compared with matched nonexercise healthy controls (CON). As a follow-up analysis, we evaluated specific sphingolipid pools in HipFx and CON muscle. Following training, quadriceps cross-sectional area, strength, and 6-min walk (6MW) increased in the surgical limb (P < 0.05). Additionally, MYD88, TAK1, NFKB1, IL6, SPT2, and CERS1 gene expression decreased after training (P ≤ 0.05), but some remained elevated above CON levels. Interestingly, MYD88 mRNA was inversely correlated to quadriceps CSA, strength, and 6MW. Finally, muscle dihydroceramides and phosphoceramides in HipFx were lower than CON at Pre (P ≤ 0.05), but after training differences from CON were removed. Together, our pilot data support that exercise training alters skeletal muscle inflammation and ceramide metabolism associated with TLR signaling in older adults recovering from hip fracture surgery and may be related to improvements in muscle function recovery.
NEW & NOTEWORTHY These pilot data demonstrate that 3 mo of exercise training in older adults recovering from hip fracture surgery was able to mitigate skeletal muscle gene expression related to inflammation and ceramide metabolism while also improving surgical limb lean tissue, strength, and physical function.
Keywords: atrophy, resistance exercise, TLR4, sphingolipids, inflammation
hip fracture is a common and costly injury for hundreds of thousands of U.S. older adults every year (7), resulting in devastating health consequences (12, 35) for many. Loss of skeletal muscle mass that persists at least 2–6 mo after surgery (24, 47, 62) is common. This is alarming considering that there is little muscle reserve to spare in this population (34). Consequently, many are not likely to return to prefracture functional level one year after fracture (63, 64). Rehabilitation following hip fracture has remained largely unchanged over the past several decades (41), despite evidence (41) suggesting that older adults recovering from hip fracture could benefit from enhanced rehabilitation practices at higher exercise intensities (3, 40, 56) with emphasis on restoring unilateral limb deficits in muscle size, strength, and function (32). There is also a paucity of information on the specific cellular and molecular events in skeletal muscle of older adults recovering from hip fracture in response to exercise training. This information may help identify key factors important in the adaptation to exercise that may lead to improved rehabilitation strategies to maximize muscle and function recovery in older adults following hip fracture surgery.
Inflammation has received considerable attention as an underlying mechanism contributing to a decline in skeletal muscle health of older adults with physical function deficits (15, 51, 57). Skeletal muscle inflammation is heightened in older adults (8, 48) and further in older adults who are inactive (1, 6, 19). Therefore, it is not surprising that the severe physical trauma and associated physical inactivity that accompanies recovery from hip fracture surgery is characterized by a robust muscle inflammatory response (2). Although inflammation is clearly important for proper muscle regeneration (59), prolonged inflammation can lead to atrophy (25, 29, 45, 61) possibly interfering with muscle and functional recovery in older adults after hip fracture. However, it is unclear if exercise training could lessen the muscle inflammatory response in this unique and vulnerable population.
The source of skeletal muscle inflammation is complex but may partly originate from toll-like receptor (TLR) signaling. The toll-like receptors, such as TLR4, are pattern recognition innate immune receptors expressed on a variety of membrane cell surfaces including skeletal muscle (39, 49). In response to ligands released from damaged tissue, TLR4 activates a proinflammatory response most commonly through the transcription factor, nuclear factor kappa beta (NFκB). In parallel to a release of proinflammatory cytokines, TLR4/NFκB signaling also upregulates mRNAs of key enzymes associated with ceramide biosynthesis (31). Ceramides are a subset of bioactive sphingolipids that have traditionally been linked to metabolic disease through their influence on impairing insulin signaling (10). However, ceramides also play a part in cellular function such as inhibiting proliferation and increasing apoptosis (65).
TLR4 is found to be upregulated in muscle fibers under chronic inflammatory conditions such as in older (27) and diabetic adults (49) and those with inflammatory diseases (46, 66). Additionally, we have shown that disuse models in rodents and older adults display elevated skeletal muscle levels of TLR4, NFκB, IL6, and ceramide biosynthesis signaling (18, 37). Importantly, exercise training has been shown to reduce TLR signaling in muscle (38) and in blood cells in older adults (22, 23, 50, 54, 55). However, it is unknown if TLR signaling is dysregulated in skeletal muscle of older adults recovering from hip fracture surgery and if these molecular responses can be manipulated in response to exercise training.
Surprisingly, beyond the point of surgery (2, 42), very little information is available at the molecular level in muscle in older adults recovering from hip fracture. We sought to expand on data within this unique older adult population by conducting a pilot study to examine if a 3-mo high-intensity, multimodal exercise program ~4 mo following hip fracture could reduce skeletal muscle inflammatory and ceramide biosynthesis gene expression related to the TLR signaling pathway. Second, we investigated whether exercise training could alter these molecular profiles in comparison to muscle from matched healthy older adults. We hypothesized that a 3-mo exercise training program would reduce skeletal muscle gene expression levels associated with the TLR signaling pathway in older adults recovering from hip fracture and that these responses would be more comparable to healthy older adults.
MATERIALS AND METHODS
Participants.
A sample of 7 community-dwelling older adults (Table 1) recovering from hip fracture (HipFx), and recently discharged from ~8–12 wk of usual-care physical therapy, participated in the study. Each of the participants had incurred a hip fracture in the past 2-6 mo and was discharged from physical therapy in the preceding 1–12 wk (~2.4 wk). Medical history revealed participants current medication including treatment for blood pressure, high cholesterol, osteoporosis, depression, and pain. Muscle tissue from a subset of age-, sex-, and body mass index (BMI)-matched controls (CON; n = 8) from a prior study (1) was used as a comparator to HipFx for the tissue and muscle function analysis. As expected, there were no demographic differences between HipFx and CON groups (P > 0.05). Before any data collection, all participants were provided with written and oral details about the experimental procedures and associated risks. Study procedures were approved by the University of Utah Institutional Review Board, and all participants provided informed consent.
Table 1.
Subject characteristics
| HipFx | CON | |
|---|---|---|
| Sex | 3M, 4F | 4M, 4F |
| Age, yr | 78.4 ± 13.3 | 76.4 ± 4.8 |
| BMI, kg/m2 | 25.5 ± 2.3 | 25.2 ± 2.3 |
| Time since fracture, mo | 3.5 ± 1.4 |
Data are means ± SD. HipFx participants were taking medications for hypertension (n = 4), cholesterol (n = 3), depression (n = 1), osteoporosis (n = 1), and pain (n = 2).
Exercise training.
A 3-mo high-intensity, task-oriented, resistance training program with an emphasis on improving surgical limb lean mass, muscle, and physical function and whole body balance was incorporated in this study. Hip fracture participants attended three 60–80 min supervised exercise sessions per week for 12 wk. The sessions included a 5-min warm-up on a recumbent ergometer (Nustep, Ann Arbor, MI) or gait training on a treadmill, six lower extremity strength exercises (straight leg raise, prone knee flexion, standing hip abduction, standing hip extension, seated knee extension, and seated leg press) performed 3 sets × 8 repetitions at 85% of the surgical limb 1-repetition maximum (1-RM), balance/mobility exercises (group Tai-Chi, sit-to-stand repetitions, task-oriented balance, and gait training) with emphasis on restoring confidence and movement pattern symmetry, and 1–2×/weekly 5–10 min lower extremity eccentric ergometer resistance training with emphasis placed on surgical limb (Eccentron, BTE Tech, Hanover, MD). Following each exercise session, participants were provided a 17-g protein-rich drink (~5 g Leu; Pepform BCAA Peptide; Glanbia Nutritionals, Twin Falls, ID) to maximize muscle mass and strength gains by enhancing the adaptive physiological response to resistance training (9, 60). 1-RM values were measured and recorded after the initial 3 wk of training, then retested every 3 wk to maximize resistance training stimuli on the surgical limb. Depending on the specific exercise, individuals improved an average of 40–65% in 1-RM in the surgical limb over the 3 mo course of training, similar to lower extremity gains documented after other post-hip fracture resistance training trials (3). Adherence to the 3-mo exercise protocol remained high for HipFx participants (92 ± 5%; range 71–100%).
Quadriceps cross sectional area, muscle strength, and physical performance.
Magnetic resonance imaging (MRI; Siemens Trio, Siemens Medical, Erlangen, Germany) was performed for determination of quadriceps cross-sectional area (CSA) as previously described (1). An imaging transfer error limited complete MRI data sets (Pre and Post) in two hip fracture participants, and one CON participant did not undergo MRI testing. Therefore quadriceps CSA data were only available for n = 5 HipFx and n = 7 CON participants. An isokinetic dynamometer (KinCom, Chattanooga) was used to determine the maximum unilateral voluntary isometric contraction (MVIC) force of the knee extensors. The average MVIC of three trials (with 30-s rest between trials) was used for analysis. Lower extremity power was measured using the Nottingham Leg Extension Power Rig (Queen’s Medical Centre, Nottingham, UK). Following 4–5 practice trials, the participant performed 5 leg extension maximal efforts and the average maximal effort leg extension power was used for analysis. The 6-min walk (6MW) test was used to assess overall locomotor ability/endurance (distance covered in 6 min) and is a reliable performance-based measure of older adults’ physical function.
Skeletal muscle biopsies.
Muscle biopsies (~40 mg) were obtained from the vastus lateralis (VL) of the surgical limb using aseptic technique, local anesthesia (1% lidocaine), and a 5 mm Bergström biopsy needle with manual suction. Immediately, the muscle sample was washed with cold saline and dissected of visible connective and fat tissue. The tissue sample was then frozen in liquid nitrogen and stored at −80°C until analysis. The biopsy procedure was conducted 2–3 days before (Pre) any exercise intervention and again 2–3 days following the 36th exercise session (Post). The Post biopsy was taken immediately proximal to the Pre biopsy. Additionally, participants refrained from food ~10 h before the muscle biopsy procedure. Due to difficulties in extracting an optimal amount of muscle tissue in this population of older adults, we limited our tissue analysis to skeletal muscle gene expression, and the remaining tissue was used as a follow-up analysis for sphingolipids.
RNA Isolation and qPCR.
RNA was isolated from skeletal muscle samples (~10 mg) as demonstrated previously (17). Muscle tissue was homogenized in TriReagent following specific manufacturer instructions for aqueous phase separation and precipitation and washing of the RNA pellet (RT 111; Molecular Research Center, Cincinnati, OH). RNA concentration was quantified and assessed for RNA purity using the 260/280 nm ratio (1.88 ± 0.04). Approximately 1 μg of total RNA was synthesized using a commercially available kit (iScript, Bio-Rad, Hercules, CA). Real-time PCR was carried out with a CFX Connect real-time PCR cycler (Bio-Rad) under similar protocol conditions as reported previously (17) using either TaqMan fluorescence predesigned or SYBR green custom designed primers. Each reaction tube consisted of a SYBR Green or TaqMan master mix along with ultrafiltered water and specific forward and reverse primers for a total reaction volume of either 25 or 20 μl, respectively. For SYBR green, we used the following PCR temperature conditions: 1 cycle for 3 min at 95°C followed by 40 cycles at 95°C for 20 s and at 55°C (or 60°C) for 30 s. TaqMan PCR assays (Thermo Fisher Scientific, Waltham, MA) were performed under the recommended PCR temperature guidelines: 1 cycle for 10 min at 95°C followed by 40 cycles at 95°C for 15 s and at 60°C for 60 s. The following TaqMan primers were used: TLR2 (Hs01872448_s1), RAGE (Hs00179504_m1), TAK1 (Hs00177373_m1), IL6 (Hs00985639_m1), and HMGB1 (Hs1923466_g1). MYD88, MCP1, NFKB1 and β2M primers have been designed and published previously (18, 37). The remaining SYBR green primers were designed for this study using standard optimization guidelines (58): TLR4 (NM_138554) (Fwd: 5′-TTCTTCTAACTTCCTCTCCTGTGA-3′, Rev: 5′-AGCGGCAACCTTAGCATTC-3′), TNF associated factor 6, E3 ubiquitin protein ligase (TRAF6; NM_145803) (Fwd: 5′-TTTGCTCTTATGGATTGTCCCC-3′ Rev: 5′-CATTGATGCAGCACAGTTGTC-3′), serine palmitoyltransferase long chain base subunit 1 (SPT1; NM_006415) (Fwd: 5′-TTCCAGTCTTCGTTGTGT-3′, Rev: 5′-GGCTAAGGATGCTCAGAT-3′), serine palmitoyltransferase long chain base subunit 1 (SPT2; NM_004863) (Fwd: 5′-AAGAATCCAGCCATCGTA-3′, Rev: 5′-ATGACTACAGAAGCAAGAATC-3′), ceramide synthase 1 (CERS1; NM_021267) (Fwd: 5′-CCATCTCCGTGCTCTTCTT-3′, Rev: 5′-CACTCGTCCACCACCATG-3′), ceramide synthase 2 (CERS2; NM_181746) (Fwd: 5′-TGGCAATAAGTGTCAGAC-3′, Rev: 5′-GCAAGGAAGGCATAAGAA-3′), and sphingosine kinase 1 (SPHK1; NM_021972) (Fwd: 5′-TATGAATGCCCCTACTTG-3′, Rev: 5′-TCGCTAACCATCAATTCC-3′). All designed primers were carefully optimized for efficiency (between 90 and 110%) and verified by melt analysis and product size using a DNA agarose gel. Cycle threshold values were normalized to beta 2-microglobulin (β2M) for comparisons of Pre vs. Post exercise training in HipFx. However, due to difficulties identifying a stable housekeeping gene (β2M, GAPDH, and HMBS) or calculating geometric means between HipFx and CON and in the interests of preserving limited cDNA, we used chemokine receptor type 2 (CCR2; Hs00704702_s1) as a normalizing gene because it remained stable across the exercise intervention and between groups. Fold change values were calculated using the 2−ΔΔCt method. In the case of comparing Pre vs. Post HipFx gene expression, Pre values were used as the comparator; thus Pre HipFx values were approximately set to 1. In the case of comparing HipFx samples against CON, the CON values were used as the comparator; thus CON values were approximately set to 1.
Liquid chromatography mass spectrometric (LC-MS) sphingolipid quantification.
A follow-up analysis was performed with the limited skeletal muscle tissue (Pre vs. Post, n = 5; Healthy Con n = 8). Approximately 30 mg of tissue was isolated for lipids following procedures reported previously (21, 36). A known quantity of C17 Ceramide (d18:1/17:0) was spiked in during lipid extraction and used as an internal standard (Avanti Polar Lipids, Alabaster AL). Following extraction, the lipid pellets were solubilized in 200 µl of methanol, transferred to LC-MS vials, and analyzed by UPLC-ESI-MS. An Agilent 1290 UPLC system fit with an Acquity UPLC CSH C18 1.7 µm, 2.1 × 50 mm column (Waters, Beverly MA) was employed to fractionate the lipid mixture. An Agilent 6490 triple quadrupole mass spectrometer operated in the positive mode with the transitions listed below was used for detection. Mobile phase A consisted of acetonitrile in H2O (60% vol/vol) and mobile phase B consists of isopropanol in H2O (90% vol/vol), both containing 10 mM ammonium formate and 0.1% formic acid. The gradient initiated at 60% B, held for 1 min, then ramped to 99% B over 9 min and maintained for 10 min. Flow rate was 0.2 ml/min and the injection volume was 3 µl. The source gas temperature was set to 150°C, with a drying gas flow of 14 l/min and a nebulizer pressure of 20 psi. Sheath gas temp was 350°C, sheath gas flow was 11 l/min, capillary voltage of 3,000 V, high-pressure radiofrequency 150 V, and low-pressure radiofrequency 60 V. All collision energies were 29 and cell accelerator voltages were 4. Data analysis was performed using Mass Hunter Quant B.07.00 (Agilent Technologies, Santa Clara CA).
Statistical analysis.
Dependent variable data are plotted as individual points in figures. Data in text and tables are expressed as means ± SD. Dependent t-tests were used for comparisons of Pre vs. Post exercise in HipFx. Independent t-tests were used to determine differences at baseline between HipFx vs. healthy age-, sex-, BMI-matched controls (HipFx Pre vs CON) and separately to determine if HipFx dependent variables after exercise training (Post) were modulated in comparison to CON (exercise training vs. CON). Finally, nonparametric Spearman correlations were conducted on molecule indexes and muscle function data. Statistical significance was set at P ≤ 0.05.
RESULTS
Subject characteristics and muscle, strength, and physical performance before and after exercise training.
As expected, there were no differences in age or BMI between HipFx and CON groups (Table 1). Leg power and 6MW in HipFx were lower than CON at Pre. However, in response to 3 mo of exercise training in HipFx, surgical limb quadriceps lean tissue CSA increased ~8%, surgical limb strength increased 20%, and the distance covered during the 6MW increased by 23%. There were no changes in surgical limb power or muscle quality after the exercise intervention. After exercise training in HipFx participants, only 6MW remained different between HipFx and CON (Table 2).
Table 2.
Muscle, strength, and physical performance in HipFx before and after 3 mo of a multicomponent exercise training program and in comparison to matched, healthy older controls (CON)
| Pre | Post | |
|---|---|---|
| HipFx | ||
| Quad CSA, cm2 | 35.5 ± 13.7 | 39.3 ± 13.4* |
| Muscle quality, N/cm2 | 8.6 ± 1.9 | 9.1 ± 1.3 |
| Force, N | 315.7 ± 168.5 | 358.2 ± 125.6* |
| Power, W | 108.7 ± 73.8# | 118.8 ± 55.8 |
| 6MW, m | 370.0 ± 159.0# | 454.4 ± 149.0*# |
| CON | ||
| Quad CSA, cm2 | 45.5 ± 15.4 | |
| Muscle quality, N/cm2 | 8.7 ± 2.3 | |
| Force, N | 404.2 ± 196.3 | |
| Power, W | 164.7 ± 104.2 | |
| 6MW, m | 539.8 ± 69.2 |
Data are means ± SD. Quadriceps (Quad) cross-sectional area (CSA), force and power in HipFx (n = 7) are data reported in the surgical leg, whereas in the healthy CON (n = 7) the data are an average of left and right legs. Quad CSA data were only acquired in 5 HipFx and 7 CON participants (see materials and methods). 6MW, 6-min walk.
Different from Pre.
Different from CON.
Skeletal muscle gene expression in HipFx in response to 3 mo of exercise training.
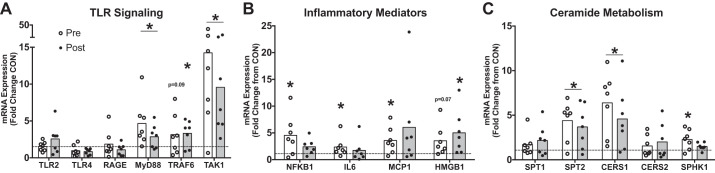
Characterization of the muscle inflammatory signature associated with the TLR signaling cascade in the muscle of HipFx in response to a 3-mo exercise training program revealed that MYD88 (0.7 ± 0.4-fold change vs. Pre) and TAK1 (0.8 ± 0.6-fold change vs. Pre) mRNA decreased after 3 mo of exercise training in skeletal muscle of HipFx (Fig. 1A). These downstream toll-like receptor gene expression responses were also accompanied by a marginal decrease (P = 0.06) in RAGE gene expression, a parallel inflammatory pathway that shares many of the same downstream intermediates as toll receptor signaling (33). Additionally, NFKB1 (0.7 ± 0.5-fold relative to Pre) and IL6 (0.5 ± 0.3-fold change vs. Pre) mRNA were reduced in comparison to Pre after exercise training (Fig. 1B). No changes were noted for TLR2, TLR4, TRAF6, MCP1, CCR2, or HMGB1 mRNA in response to 3 mo of exercise training. Finally, exercise training decreased gene expression of SPT2 (0.7 ± 0.2-fold change vs. Pre) and CERS1 (0.6 ± 0.3-fold change vs. Pre), mRNAs coding for rate-limiting enzymes responsible for ceramide biosynthesis. No changes were noted for SPT1, CERS2, or SPHK1 gene expression after exercise training (Fig. 1C).
Fig. 1.
Genes related to TLR signaling, inflammation, and ceramide metabolism in HipFx in response to exercise training. Individual data values for genes related to toll-like receptor signaling (A), inflammatory mediators (B), and ceramide metabolism (C) in HipFx (n = 7) skeletal muscle after 3 mo of exercise training (relative to Pre). Data were normalized to beta2-microglobulin (B2M) using the 2–ΔΔCt method; therefore values for Pre were ~1 (dotted line). Open bar represents the mean. *Different from Pre (P ≤ 0.05). TLR2, toll-like receptor 2; TLR4, toll-like receptor 4; RAGE, receptor for age-related glycation; MYD88, myeloid differentiation primary response gene 88; TRAF6, TNF receptor-associated factor 6; TAK1, transforming growth factor beta-activated kinase 1; NFKB1, nuclear factor kappa beta 1; IL6, interleukin 6; MCP1, monocyte chemoattractant protein-1; HMGB1, high-mobility group box protein 1; SPT1, serine palmitoyltransferase 1 (SPT1); SPT2, serine palmitoyltransferase 2; CERS1, ceramide synthase 1; CERS2, ceramide synthase 2; SPHK1, sphingosine kinase 1.
Skeletal muscle gene expression levels in HipFx compared with matched, healthy older adult controls (CON).
MYD88, TRAF6, TAK1, and HMGB1 mRNA expression levels in HipFx remained elevated above CON levels both before and after exercise training (Fig. 2, A and B). Skeletal muscle IL6, MCP1, and NFKB1 mRNA were also elevated in HipFx at Pre in comparison to CON; however, 3 mo of exercise training reduced the gene expression of these inflammatory readouts to CON levels (Fig. 2B). Finally, SPT2, CERS1, and SPHK1 mRNA (Fig. 2C) were elevated at Pre compared with CON, but only SPHK1 mRNA was reduced to CON levels after exercise training.
Fig. 2.
Genes related to TLR signaling, inflammation, and ceramide metabolism in HipFx in comparison to matched, healthy older adult control. Individual data values for genes related to toll-like receptor signaling (A), inflammatory mediators (B), and ceramide metabolism (C) in HipFx (n = 7) before (Pre; open circles) and after (Post; closed circles) 3 mo of exercise training relative to healthy older adult control levels (CON; n = 8). Data were normalized to chemokine receptor type 2 (CCR2) using the 2 –ΔΔCt method; therefore values for CON were ~1 (dotted line). Open bar represents the mean for Pre. Grey bar represents the mean for Post. *Different from CON (P ≤ 0.05).
Muscle sphingolipids in HipFx before and after exercise training and compared with CON.
As a follow-up analysis to the genes related to ceramide metabolism, we measured specific pools and individual species of sphingolipids in skeletal muscle samples (ceramides, dihydroceramides, phosphoceramides, sphingomyelin) in HipFx before and after 3 mo of exercise training and in healthy older adult controls. We found that dihydroceramides and phosphoceramides were lower at Pre in HipFx (vs. CON) (Fig. 3B). These data were further supported by lower dihydroceramide individual species in HipFx muscle samples at Pre (vs. CON) (DiHydro Cer16, 0.49 ± 0.34-fold; DiHydro Cer18, 0.51 ± 0.37-fold; DiHydro Cer24, 0.51 ± 0.42-fold) (P < 0.001). Similarly, phosphoceramide 24:1 was lower in HipFx samples at Pre (vs. CON) (0.24 ± 0.28-fold) (P < 0.001). After exercise training total dihydroceramides and individual species (DiHydro Cer16, 1.92 ± 0.84-fold, DiHydro Cer18, 1.94 ± 0.84-fold) tended to increase in HipFx muscle after exercise training compared with Pre (P = 0.07) (Fig. 3A). Finally, when comparing post-exercise training HipFx samples to CON, total and individual dihydroceramides and phosphoceramides were no longer different (Fig. 3B). There were no differences in ceramides or sphingomyelin as a result of exercise training or HipFx Pre and Post differences compared with CON.
Fig. 3.

Skeletal muscle sphingolipids. Individual data points representing total abundance of specific skeletal muscle sphingolipids in HipFx participants (n = 5) after 3 mo exercise training (fold change relative to Pre) (A) and Pre (open circles) and Post (closed circles) abundance levels compared with matched, healthy older adult controls (n = 8; fold change relative to CON) (B). Cer, ceramide; DiHydro, dihydroceramide; CerP, phosphoceramide; SM, sphingomyelin. In Fig. 3B, open bar represents the mean for Pre and grey bar represents the mean for Post. $P = 0.07 (vs. Pre). *Different from CON (P ≤ 0.05).
Skeletal muscle gene expression and muscle function correlations.
First, we attempted to determine if any relationships exist between mRNAs associated with the TLR signaling cascade (TLR2, TLR4, MYD88, TRAF6, TAK1, SPT1, SPT2, CERS1, CERS2) with those notably associated with inflammation (NFKB1, IL6). As a result, several gene expression markers associated to TLR signaling were strongly related to inflammatory mediators (data not reported). For example, MYD88 mRNA was positively correlated to NFKB1 (R = 0.94, P = <0.01) and IL6 (R = 0.74, P = <0.01) mRNA. Next, we attempted to establish potential relationships between quadriceps CSA, force and 6MW data with key mRNAs associated with TLR signaling, inflammation, and ceramide metabolism. Interestingly, we found an inverse relationship between quadriceps CSA (R = −0.60, P = 0.01), force (R = −0.42, P = 0.05) and 6MW (R = −0.48; P = 0.02) and MYD88 mRNA.
DISCUSSION
This is the first study to show that 3 mo of supervised exercise training in older adults recovering from hip fracture surgery (HipFx) reduced the expression of several skeletal muscle inflammatory and ceramide metabolism genes related to the TLR signaling cascade. Although skeletal muscle expression of inflammation and ceramide metabolism genes were reduced after exercise training in the surgical limb of HipFx participants, several gene markers remained above healthy older adult control levels after training. Interestingly, MYD88 inversely corresponded to quadriceps CSA, strength, and 6MW. Finally, select sphingolipid pools were lower in HipFx (compared with CON), but after exercise training these differences compared with CON were negligible. Together, our data support that 3 mo of multimodal exercise training is capable of dampening skeletal muscle gene expression levels related to TLR signaling and may be related to muscle function of older adults who are recovering from a hip fracture.
Our findings are in line with Bamman et al. (2) supporting that at the time of surgery following a traumatic hip fracture, middle-aged adults (~44 yr) have a heightened skeletal muscle inflammatory response as noted by increased muscle expression of IL6 and NFκB. We add to these data and show in a much older (~77 yr) population while also several months after hip fracture surgery that NFκB and IL6 are elevated and are in part associated to increased TLR signaling. This was noted by an upregulation of surgical limb skeletal muscle mRNAs associated to the TLR signaling pathway: MyD88, TAK1, and ceramide biosynthesis signaling (SPT2 and CERS1). Strong positive associations between mRNAs related to key TLR signaling molecules, and NFKB1 and IL6 mRNA expression further support the role of TLR and inflammation in the muscle of these patients. Interestingly, the muscle inflammatory signals were elevated above matched, healthy older adult levels after ~4 mo of recovery and even after formally completing standard rehabilitation. These data suggest that a heightened local inflammatory environment in the involved leg continues to exist several months after surgery, possibly limiting ample recovery of skeletal muscle tissue and physical function in older adults following hip fracture surgery.
A novel component of this study was that we gathered skeletal muscle biopsies before and after exercise training in this unique older adult population; a population that is generally classified as sarcopenic (34). We hypothesized that exercise training would attenuate muscle inflammation (28) particularly associated with the TLR pathway. Indeed, we show that 3 mo of a multicomponent exercise training program consisting of resistance and balance exercises reduced the expression of several skeletal muscle genes related to inflammation and ceramide metabolism. These findings are in agreement with Rodriguez-Miguelez et al. (50) who found that 12 wk of resistance exercise training downregulated protein expression of TLR-related molecules in peripheral blood mononuclear cells of healthy male and female older adults. Similarly, Lambert et al. (38) conducted a 12-wk exercise training program consisting of resistance, aerobic, and flexibility exercises in obese older adults. These authors found that skeletal muscle TLR4, IL-6, and tumor necrosis factor alpha mRNA expression robustly decreased after exercise training (but not with 12 wk of weight loss) and these responses were independent of any inflammatory cytokine changes in the serum (38), indicating that exercise training had local impacts on inflammation. In contrast to our findings, Ghosh and colleagues (27) conducted a 16-wk aerobic exercise trial in sedentary older adults and did not observe any changes in skeletal muscle TLR signaling after training, including TLR4 and NFκB protein expression. The discrepant findings in Ghosh et al. vs. those of our current study and others (23, 38, 50) suggest that TLR signaling may be modulated by a specific exercise stimulus that increases lean mass such as high-intensity resistance exercise rather than aerobic exercise. Even though exercise training in HipFx reduced muscle expression of genes related to inflammation and ceramide metabolism compared with Pre levels, these levels were still largely elevated above healthy older adult control levels. Intriguingly, we demonstrate an inverse relationship between MYD88 mRNA (a critical TLR signaling adaptor molecule) and quadriceps CSA, strength, and 6MW, suggesting that TLR signaling may regulate muscle function. Future studies are needed to determine if muscle inflammatory status and muscle function in HipFx could be further improved with additional exercise training.
As a follow-up analysis to the ceramide biosynthesis gene expression data, we evaluated sphingolipid pools in the remaining skeletal muscle biopsy samples to determine if specific sphingolipids, such as ceramides, were likewise altered with exercise training and different from healthy older adult controls. In contrast to our data in which we observed a downregulation of genes related to ceramide biosynthesis (SPT2, CERS1), we did not detect any changes in the total abundance (or individual species) of muscle ceramides in HipFx following exercise training or in comparison to healthy older adult controls. The ability of exercise training to modulate muscle ceramide pools in humans has been somewhat controversial with some exercise training studies showing a decrease (11, 20) whereas others show no change (16, 30). A possible explanation for the discrepant ceramide findings (gene expression vs. ceramide pools) in our study may be that alternate pathways that regulate ceramide turnover may have compensated for lower expression of enzymes involved with de novo ceramide synthesis. For example, SPHK1 has been reported to reduce ceramide accumulation in skeletal muscle (4, 5, 44) by participating in the enzymatic degradation of ceramide into sphingosine-1-phosphate. In our study, muscle SPHK1 mRNA expression in HipFx samples was lower compared with healthy older adult controls after exercise training, thus possibly indicating a maintenance of ceramide pools (by a decrease in ceramide degradation) in skeletal muscle tissue following an exercise-trained state.
Despite unchanged skeletal muscle ceramide levels in HipFx after training, we surprisingly observed a lower total (and individual species) abundance of muscle dihydroceramides and phosphoceramides in HipFx (vs. healthy older adult controls). Interestingly, after training, the muscle sphingolipid differences compared with healthy controls were nonexistent. The role of phosphoceramides in cellular function in response to exercise training is unclear but may be related to enhanced muscle regeneration as noted by a study showing that ceramide-1-phosphate was capable of increasing proliferation of C2C12 myoblasts (26). Dihydroceramides, in addition to being a precursor for ceramide synthesis, are recognized to have various roles in cellular function, including the activation of autophagy (52, 53), a cellular process to remove damaged protein aggregates and organelles, suggesting that autophagy may be impaired in this population. In support, Marzetti et al. (42) recently demonstrated that postsurgical skeletal muscle samples from sarcopenic older adults who had a hip fracture were characterized with lower gene expression levels of markers associated with autophagy and mitophagy. Together, 3 mo of exercise training was able to increase skeletal muscle dihydroceramide and phosphoceramide abundance in HipFx to levels comparable to healthy older adults, leading one to speculate that an increase in these sphingolipid pools might be related to a restoration of muscle function during recovery from surgery.
Although our study adds to the body of literature describing the muscle inflammatory status (presumably through the TLR pathway) of hip fracture participants after exercise training, our study is not without limitations. First, we were not able to deduce whether exercise training and/or the course of time contributed to improved muscle and physical function and reduced muscle inflammatory signals since we did not have a nonexercise hip fracture group. However, the functional improvements that were observed in our study with exercise training are consistent with a well-cited extended rehabilitation program in a similar cohort of patients initiated at approximately the same postoperative period using a home-based exercise program as their control (3). A second limitation was that the data gathered from this pilot study were likely underpowered, thus warranting the need to conduct a larger clinical trial to validate these findings. Finally, the low amounts of skeletal muscle tissue sampled from these participants limited the interpretation to primarily gene expression.
In conclusion, this pilot study is the first to demonstrate that exercise training modulates skeletal muscle inflammation and ceramide metabolism (possibly through inhibiting TLR activation) in older adults following hip fracture, while also demonstrating that older adults recovering from hip fracture several months after surgery can further improve muscle and physical function. Continuation of exercise training in patients recovering from hip fracture may be necessary to further reduce skeletal muscle inflammation and improve muscle function.
GRANTS
This study was supported by a grant from the Orthopedic Trauma Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.I.M., R.A.B., K.M.B., D.S.N., O.S.K., R.L.M., and M.J.D. performed experiments; A.I.M., R.A.B., K.M.B., P.N.H., and M.J.D. analyzed data; A.I.M., K.M.B., R.L.M., and M.J.D. interpreted results of experiments; A.I.M. and M.J.D. prepared figures; A.I.M., R.L.M., and M.J.D. drafted manuscript; A.I.M., R.A.B., K.M.B., D.S.N., O.S.K., R.L.M., and M.J.D. edited and revised manuscript; A.I.M., R.A.B., K.M.B., D.S.N., O.S.K., T.F.H., R.L.M., and M.J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants who volunteered for muscle biopsies and diligently devoted their time to the 3-mo exercise intervention. We also acknowledge B. Powell for help with exercise training the participants. We also thank B. Petersen and E. Bastian from Glanbia Nutritionals for generously providing the protein supplements.
REFERENCES
- 1.Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA, Marcus RL. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging 18: 532–538, 2014. doi: 10.1007/s12603-014-0019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamman MM, Ferrando AA, Evans RP, Stec MJ, Kelly NA, Gruenwald JM, Corrick KL, Trump JR, Singh JA. Muscle inflammation susceptibility: a prognostic index of recovery potential after hip arthroplasty? Am J Physiol Endocrinol Metab 308: E670–E679, 2015. doi: 10.1152/ajpendo.00576.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. JAMA 292: 837–846, 2004. doi: 10.1001/jama.292.7.837. [DOI] [PubMed] [Google Scholar]
- 4.Bruce CR, Risis S, Babb JR, Yang C, Kowalski GM, Selathurai A, Lee-Young RS, Weir JM, Yoshioka K, Takuwa Y, Meikle PJ, Pitson SM, Febbraio MA. Overexpression of sphingosine kinase 1 prevents ceramide accumulation and ameliorates muscle insulin resistance in high-fat diet-fed mice. Diabetes 61: 3148–3155, 2012. doi: 10.2337/db12-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce CR, Risis S, Babb JR, Yang C, Lee-Young RS, Henstridge DC, Febbraio MA. The sphingosine-1-phosphate analog FTY720 reduces muscle ceramide content and improves glucose tolerance in high fat-fed male mice. Endocrinology 154: 65–76, 2013. doi: 10.1210/en.2012-1847. [DOI] [PubMed] [Google Scholar]
- 6.Buford TW, Cooke MB, Manini TM, Leeuwenburgh C, Willoughby DS. Effects of age and sedentary lifestyle on skeletal muscle NF-kappaB signaling in men. J Gerontol A Biol Sci Med Sci 65: 532–537, 2010. doi: 10.1093/gerona/glp196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 22: 465–475, 2007. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 8.Caldow MK, Cameron-Smith D, Levinger P, McKenna MJ, Levinger I. Inflammatory markers in skeletal muscle of older adults. Eur J Appl Physiol 113: 509–517, 2013. doi: 10.1007/s00421-012-2458-x. [DOI] [PubMed] [Google Scholar]
- 9.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 96: 1454–1464, 2012. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 10.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab 15: 585–594, 2012. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Coen PM, Menshikova EV, Distefano G, Zheng D, Tanner CJ, Standley RA, Helbling NL, Dubis GS, Ritov VB, Xie H, Desimone ME, Smith SR, Stefanovic-Racic M, Toledo FG, Houmard JA, Goodpaster BH. Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning, and insulin sensitivity after gastric bypass surgery. Diabetes 64: 3737–3750, 2015. doi: 10.2337/db15-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cree M, Soskolne CL, Belseck E, Hornig J, McElhaney JE, Brant R, Suarez-Almazor M. Mortality and institutionalization following hip fracture. J Am Geriatr Soc 48: 283–288, 2000. doi: 10.1111/j.1532-5415.2000.tb02647.x. [DOI] [PubMed] [Google Scholar]
- 13.Cuervo AM, Bergamini E, Brunk UT, Dröge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy 1: 131–140, 2005. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 14.Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem 275: 31505–31513, 2000. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- 15.Degens H. The role of systemic inflammation in age-related muscle weakness and wasting. Scand J Med Sci Sports 20: 28–38, 2010. doi: 10.1111/j.1600-0838.2009.01018.x. [DOI] [PubMed] [Google Scholar]
- 16.Devries MC, Samjoo IA, Hamadeh MJ, McCready C, Raha S, Watt MJ, Steinberg GR, Tarnopolsky MA. Endurance training modulates intramyocellular lipid compartmentalization and morphology in skeletal muscle of lean and obese women. J Clin Endocrinol Metab 98: 4852–4862, 2013. doi: 10.1210/jc.2013-2044. [DOI] [PubMed] [Google Scholar]
- 17.Drummond MJ, Addison O, Brunker L, Hopkins PN, McClain DA, LaStayo PC, Marcus RL. Downregulation of E3 ubiquitin ligases and mitophagy-related genes in skeletal muscle of physically inactive, frail older women: a cross-sectional comparison. J Gerontol A Biol Sci Med Sci 69: 1040–1048, 2014. doi: 10.1093/gerona/glu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drummond MJ, Timmerman KL, Markofski MM, Walker DK, Dickinson JM, Jamaluddin M, Brasier AR, Rasmussen BB, Volpi E. Short-term bed rest increases TLR4 and IL-6 expression in skeletal muscle of older adults. Am J Physiol Regul Integr Comp Physiol 305: R216–R223, 2013. doi: 10.1152/ajpregu.00072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond MJ, Timmerman KL, Markofski MM, Walker DK, Dickinson JM, Jamaluddin M, Brasier AR, Rasmussen BB, Volpi E. Short-term bed rest increases TLR4 and IL-6 expression in skeletal muscle of older adults. Am J Physiol Regul Integr Comp Physiol 305: R216–R223, 2013. doi: 10.1152/ajpregu.00072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubé JJ, Amati F, Toledo FG, Stefanovic-Racic M, Rossi A, Coen P, Goodpaster BH. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 54: 1147–1156, 2011. doi: 10.1007/s00125-011-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erickson KA, Smith ME, Anthonymuthu TS, Evanson MJ, Brassfield ES, Hodson AE, Bressler MA, Tucker BJ, Thatcher MO, Prince JT, Hancock CR, Bikman BT. AICAR inhibits ceramide biosynthesis in skeletal muscle. Diabetol Metab Syndr 4: 45, 2012. doi: 10.1186/1758-5996-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Gonzalo R, De Paz JA, Rodriguez-Miguelez P, Cuevas MJ, González-Gallego J. Effects of eccentric exercise on toll-like receptor 4 signaling pathway in peripheral blood mononuclear cells. J Appl Physiol (1985) 112: 2011–2018, 2012. doi: 10.1152/japplphysiol.01499.2011. [DOI] [PubMed] [Google Scholar]
- 23.Flynn MG, McFarlin BK, Phillips MD, Stewart LK, Timmerman KL. Toll-like receptor 4 and CD14 mRNA expression are lower in resistive exercise-trained elderly women. J Appl Physiol (1985) 95: 1833–1842, 2003. doi: 10.1152/japplphysiol.00359.2003. [DOI] [PubMed] [Google Scholar]
- 24.Fox KM, Magaziner J, Hawkes WG, Yu-Yahiro J, Hebel JR, Zimmerman SI, Holder L, Michael R. Loss of bone density and lean body mass after hip fracture. Osteoporos Int 11: 31–35, 2000. doi: 10.1007/s001980050003. [DOI] [PubMed] [Google Scholar]
- 25.Frost RA, Lang CH. Regulation of muscle growth by pathogen-associated molecules. J Anim Sci 86, Suppl: E84–E93, 2008. doi: 10.2527/jas.2007-0483. [DOI] [PubMed] [Google Scholar]
- 26.Gangoiti P, Bernacchioni C, Donati C, Cencetti F, Ouro A, Gómez-Muñoz A, Bruni P. Ceramide 1-phosphate stimulates proliferation of C2C12 myoblasts. Biochimie 94: 597–607, 2012. doi: 10.1016/j.biochi.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh S, Lertwattanarak R, Garduño JJ, Galeana JJ, Li J, Zamarripa F, Lancaster JL, Mohan S, Hussey S, Musi N. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J Gerontol A Biol Sci Med Sci 70: 232–246, 2015. doi: 10.1093/gerona/glu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 11: 607–615, 2011. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 29.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985) 98: 911–917, 2005. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 30.Helge JW, Stallknecht B, Drachmann T, Hellgren LI, Jiménez-Jiménez R, Andersen JL, Richelsen B, Bruun JM. Improved glucose tolerance after intensive life style intervention occurs without changes in muscle ceramide or triacylglycerol in morbidly obese subjects. Acta Physiol (Oxf) 201: 357–364, 2011. doi: 10.1111/j.1748-1716.2010.02180.x. [DOI] [PubMed] [Google Scholar]
- 31.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 121: 1858–1870, 2011. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houck J, Kneiss J, Bukata SV, Puzas JE. Analysis of vertical ground reaction force variables during a Sit to Stand task in participants recovering from a hip fracture. Clin Biomech (Bristol, Avon) 26: 470–476, 2011. doi: 10.1016/j.clinbiomech.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim ZA, Armour CL, Phipps S, Sukkar MB. RAGE and TLRs: relatives, friends or neighbours? Mol Immunol 56: 739–744, 2013. doi: 10.1016/j.molimm.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Ji HM, Han J, Jin DS, Suh H, Chung YS, Won YY. Sarcopenia and Sarcopenic Obesity in Patients Undergoing Orthopedic Surgery. Clin Orthop Surg 8: 194–202, 2016. doi: 10.4055/cios.2016.8.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ 307: 1248–1250, 1993. doi: 10.1136/bmj.307.6914.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon OS, Nelson DS, Barrows KM, O’Connell RM, Drummond MJ. Intramyocellular ceramides and skeletal muscle mitochondrial respiration are partially regulated by Toll-like receptor 4 during hindlimb unloading. Am J Physiol Regul Integr Comp Physiol 311: R879–R887, 2016. doi: 10.1152/ajpregu.00253.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon OS, Tanner RE, Barrows KM, Runtsch M, Symons JD, Jalili T, Bikman BT, McClain DA, O’Connell RM, Drummond MJ. MyD88 regulates physical inactivity-induced skeletal muscle inflammation, ceramide biosynthesis signaling, and glucose intolerance. Am J Physiol Endocrinol Metab 309: E11–E21, 2015. doi: 10.1152/ajpendo.00124.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol (1985) 105: 473–478, 2008. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lang CH, Silvis C, Deshpande N, Nystrom G, Frost RA. Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1beta, −6, and high-mobility-group protein-1 in skeletal muscle. Shock 19: 538–546, 2003. doi: 10.1097/01.shk.0000055237.25446.80. [DOI] [PubMed] [Google Scholar]
- 40.Mangione KK, Craik RL, Palombaro KM, Tomlinson SS, Hofmann MT. Home-based leg-strengthening exercise improves function 1 year after hip fracture: a randomized controlled study. J Am Geriatr Soc 58: 1911–1917, 2010. doi: 10.1111/j.1532-5415.2010.03076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangione KK, Lopopolo RB, Neff NP, Craik RL, Palombaro KM. Interventions used by physical therapists in home care for people after hip fracture. Phys Ther 88: 199–210, 2008. doi: 10.2522/ptj.20070023. [DOI] [PubMed] [Google Scholar]
- 42.Marzetti E, Calvani R, Lorenzi M, Tanganelli F, Picca A, Bossola M, Menghi A, Bernabei R, Landi F. Association between myocyte quality control signaling and sarcopenia in old hip-fractured patients: Results from the Sarcopenia in HIp FracTure (SHIFT) exploratory study. Exp Gerontol 80: 1–5, 2016. doi: 10.1016/j.exger.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Marzetti E, Lees HA, Manini TM, Buford TW, Aranda JM Jr, Calvani R, Capuani G, Marsiske M, Lott DJ, Vandenborne K, Bernabei R, Pahor M, Leeuwenburgh C, Wohlgemuth SE. Skeletal muscle apoptotic signaling predicts thigh muscle volume and gait speed in community-dwelling older persons: an exploratory study. PLoS One 7: e32829, 2012. doi: 10.1371/journal.pone.0032829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikłosz A, Łukaszuk B, Baranowski M, Górski J, Chabowski A. Effects of inhibition of serine palmitoyltransferase (SPT) and sphingosine kinase 1 (SphK1) on palmitate induced insulin resistance in L6 myotubes. PLoS One 8: e85547, 2013. doi: 10.1371/journal.pone.0085547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mourkioti F, Kratsios P, Luedde T, Song YH, Delafontaine P, Adami R, Parente V, Bottinelli R, Pasparakis M, Rosenthal N. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J Clin Invest 116: 2945–2954, 2006. doi: 10.1172/JCI28721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muth IE, Zschüntzsch J, Kleinschnitz K, Wrede A, Gerhardt E, Balcarek P, Schreiber-Katz O, Zierz S, Dalakas MC, Voll RE, Schmidt J. HMGB1 and RAGE in skeletal muscle inflammation: Implications for protein accumulation in inclusion body myositis. Exp Neurol 271: 189–197, 2015. doi: 10.1016/j.expneurol.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 47.Neander G, Adolphson P, von Sivers K, Dahlborn M, Dalén N. Bone and muscle mass after femoral neck fracture. A controlled quantitative computed tomography study of osteosynthesis versus primary total hip arthroplasty. Arch Orthop Trauma Surg 116: 470–474, 1997. doi: 10.1007/BF00387579. [DOI] [PubMed] [Google Scholar]
- 48.Peake J, Della Gatta P, Cameron-Smith D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol 298: R1485–R1495, 2010. doi: 10.1152/ajpregu.00467.2009. [DOI] [PubMed] [Google Scholar]
- 49.Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 57: 2595–2602, 2008. doi: 10.2337/db08-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Miguelez P, Fernandez-Gonzalo R, Almar M, Mejías Y, Rivas A, de Paz JA, Cuevas MJ, González-Gallego J. Role of Toll-like receptor 2 and 4 signaling pathways on the inflammatory response to resistance training in elderly subjects. Age (Dordr) 36: 9734, 2014. doi: 10.1007/s11357-014-9734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M; Health ABC Study . Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci 64: 1183–1189, 2009. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siddique MM, Li Y, Chaurasia B, Kaddai VA, Summers SA. Dihydroceramides: From Bit Players to Lead Actors. J Biol Chem 290: 15371–15379, 2015. doi: 10.1074/jbc.R115.653204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siddique MM, Li Y, Wang L, Ching J, Mal M, Ilkayeva O, Wu YJ, Bay BH, Summers SA. Ablation of dihydroceramide desaturase 1, a therapeutic target for the treatment of metabolic diseases, simultaneously stimulates anabolic and catabolic signaling. Mol Cell Biol 33: 2353–2369, 2013. doi: 10.1128/MCB.00226-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, McFarlin BK, Timmerman KL, Coen PM, Felker J, Talbert E. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun 19: 389–397, 2005. doi: 10.1016/j.bbi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Timmerman KL, McFarlin BK, Coen PM, Talbert E. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc 39: 1714–1719, 2007. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- 56.Sylliaas H, Brovold T, Wyller TB, Bergland A. Progressive strength training in older patients after hip fracture: a randomised controlled trial. Age Ageing 40: 221–227, 2011. doi: 10.1093/ageing/afq167. [DOI] [PubMed] [Google Scholar]
- 57.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci 55: M709–M715, 2000. doi: 10.1093/gerona/55.12.M709. [DOI] [PubMed] [Google Scholar]
- 58.Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M. A practical approach to RT-qPCR-Publishing data that conform to the MIQE guidelines. Methods 50: S1–S5, 2010. doi: 10.1016/j.ymeth.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Tidball JG, Dorshkind K, Wehling-Henricks M. Shared signaling systems in myeloid cell-mediated muscle regeneration. Development 141: 1184–1196, 2014. doi: 10.1242/dev.098285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC, van Loon LJ. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 13: 713–719, 2012. doi: 10.1016/j.jamda.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 61.Van Gammeren D, Damrauer JS, Jackman RW, Kandarian SC. The IkappaB kinases IKKalpha and IKKbeta are necessary and sufficient for skeletal muscle atrophy. FASEB J 23: 362–370, 2009. doi: 10.1096/fj.08-114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Visser M, Harris TB, Fox KM, Hawkes W, Hebel JR, Yahiro JY, Michael R, Zimmerman SI, Magaziner J. Change in muscle mass and muscle strength after a hip fracture: relationship to mobility recovery. J Gerontol A Biol Sci Med Sci 55: M434–M440, 2000. doi: 10.1093/gerona/55.8.M434. [DOI] [PubMed] [Google Scholar]
- 63.Wehren LE, Hawkes WG, Hebel JR, Orwig DL, Magaziner J. Bone mineral density, soft tissue body composition, strength, and functioning after hip fracture. J Gerontol A Biol Sci Med Sci 60: 80–84, 2005. doi: 10.1093/gerona/60.1.80. [DOI] [PubMed] [Google Scholar]
- 64.Young Y, Fried LP, Kuo YH. Hip fractures among elderly women: longitudinal comparison of physiological function changes and health care utilization. J Am Med Dir Assoc 11: 100–105, 2010. doi: 10.1016/j.jamda.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou K, Blom T. Trafficking and functions of bioactive sphingolipids: lessons from cells and model membranes. Lipid Insights 8, Suppl 1: 11–20, 2015. doi: 10.4137/LPI.S31615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zong M, Bruton JD, Grundtman C, Yang H, Li JH, Alexanderson H, Palmblad K, Andersson U, Harris HE, Lundberg IE, Westerblad H. TLR4 as receptor for HMGB1 induced muscle dysfunction in myositis. Ann Rheum Dis 72: 1390–1399, 2013. doi: 10.1136/annrheumdis-2012-202207. [DOI] [PubMed] [Google Scholar]