Abstract
Acute respiratory distress syndrome (ARDS) is characterized by lung inflammation and pulmonary edema, leading to arterial hypoxemia and death if the hypoxemia is severe. Strategies to correct hypoxemia have the potential to improve clinical outcomes in ARDS. The goal of this study was to evaluate the potential of hemoglobin modification as a novel therapy for ARDS-induced hypoxemia. The therapeutic effect of two different doses of GBT1118, a compound that increases the oxygen affinity of hemoglobin, was evaluated in a murine model of acute lung injury induced by intratracheal LPS instillation 24 h before exposure to 5% or 10% hypoxia (n = 8–15 per group). As expected, administration of GBT1118 to mice significantly increased the oxygen affinity of hemoglobin. Compared with mice receiving vehicle control, mice treated with GBT1118 had significantly lower mortality after LPS + 5% hypoxia (47% with vehicle vs. 22% with low-dose GBT1118, 13% with high-dose GBT1118, P = 0.032 by log rank) and had reduced severity of illness. Mice treated with GBT1118 showed a sustained significant increase in SpO2 over 4 h of hypoxia exposure. Treatment with GBT1118 did not alter alveolar-capillary permeability, bronchoalveolar lavage (BAL) inflammatory cell counts, or BAL concentrations of IL-1β, TNF-α, or macrophage inflammatory protein-1α. High-dose GBT1118 did not affect histological lung injury but did decrease tissue hypoxia as measured intensity of pimonidazole (Hypoxyprobe) staining in liver (P = 0.043) and kidney (P = 0.043). We concluded that increasing the oxygen affinity of hemoglobin using GBT1118 may be a novel therapy for treating hypoxemia associated with acute lung injury.
NEW & NOTEWORTHY In this study, we show that GBT1118, a compound that increases hemoglobin affinity for oxygen, improves survival and oxygen saturation in a two-hit lung injury model of intratracheal LPS without causing tissue hypoxia. Modulation of hemoglobin oxygen affinity represents a novel therapeutic approach to treatment of acute lung injury and acute respiratory distress syndrome, conditions characterized by hypoxemia.
Keywords: acute respiratory distress syndrome, acute lung injury, GBT1118, hemoglobin, hypoxia, oxygen affinity
INTRODUCTION
Hypoxemia attributable to impaired gas exchange is a key pathogenic feature of acute respiratory distress syndrome (ARDS) (29). Present treatments for severe ARDS-associated hypoxemia are limited to mechanical ventilation with manipulation of the fraction of inspired oxygen, positive end-expiratory pressure (5, 10, 14), and mean airway pressure, prone positioning (12), therapies to reduce ventilation perfusion mismatch such as inhaled nitric oxide (1) and neuromuscular blockade (20), or extracorporeal membrane oxygenation (21). Other therapeutic approaches for ARDS include low tidal volume mechanical ventilation (2) and conservative fluid management strategies (3), approaches that do not directly address hypoxemia. Given the continued high morbidity and mortality of patients with ARDS, new therapies for ARDS are still needed to further improve patient outcomes.
One novel strategy to improve oxygenation during critical illness is to increase the capacity for hemoglobin (Hb) to bind and transport oxygen. This can be accomplished in several different ways. For example, in sickle cell disease, induction of fetal Hb, which has a higher oxygen-binding affinity than native Hb, increased the proportion of oxygenated Hb and reduced Hb polymerization without hindering oxygen delivery (16, 17, 26, 28). More recently, a class of novel small molecules that reversibly bind to the NH2-terminal α-chain of Hb and increase Hb-O2 affinity (8) has been developed and may provide a promising new treatment for disorders associated with hypoxemia. One such compound, GBT440, increased Hb-O2 affinity in a murine model of sickle cell disease, resulting in the prolongation of red blood cell half-life and a decrease in sickling ex vivo (15, 18). Although these compounds are effective in models of sickle cell disease, their effects in lung disease are not well studied. Recently, Geng et al. (11) showed that GBT1118, an analog of GBT440 that also increases Hb-O2 affinity, improved oxygenation and decreased lung fibrosis in a murine model of bleomycin-induced pulmonary fibrosis. On the basis of these studies, we hypothesized that enteral administration of GBT1118 would improve arterial oxygen saturation and clinical outcomes in a mouse model of acute lung injury caused by intratracheal instillation of LPS with concurrent hypoxia exposure.
METHODS
Drug information.
GBT1118 (provided by Global Blood Therapeutics, San Francisco, CA) is a small molecule that reversibly binds to the NH2-terminal chain of Hb and increases Hb-O2 affinity. GBT1118 was formulated in dimethylacetamide:polyethylene glycol 400 (PEG400): 40% cavitron at a 1:5:4 ratio (11). For all experiments, the control group received vehicle solution of PEG400:40% cavitron.
Lung injury model.
Adult, 8- to 10-wk-old male C57Bl/6J mice (n = 8 to 15 per group) (Jackson Laboratory, Bar Harbor, ME) were anesthetized with isoflurane and treated with 100 µg LPS (Sigma, St. Louis, MO) in 100 µL PBS by direct intratracheal injection (4, 23–25). After 24 h, mice were given GBT1118 (70 or 140 mg/kg) or vehicle (5 µl/g) by oral gavage 2 h before placement in a hypoxia chamber to induce a clinically relevant degree of hypoxemia. These doses of GBT1118 were selected based on prior studies of this drug in mice (11). Mice were exposed to either 5% (lethal) or 10% (nonlethal) O2 for 4 h (Fig. 1). Before and after hypoxia exposure, clinical markers were used to determine the composite severity of illness score. This score was adapted from a score that has been validated for evaluation of mice with experimental sepsis (27). The composite score is a sum of four parameters of illness as follows: 1) response to finger poke [4 = normal response, 3 = decreased response, 2 = severely decreased response, 1 = minimal response, 0 = no response (deceased)], 2) signs of encephalopathy [4 = normal, 3 = tremors, staggering, 2 = twisting, 1 = turning and flipping, 0 = no response (deceased)], and 3) appearance (4 = normal, with 1 point subtracted for each of piloerection, periorbital exudates, respiratory distress, diarrhea) (27). The composite severity of illness score ranged from 12 (healthy) to 0 (deceased). During hypoxic exposure, oxygen saturation was measured with a pulse oximeter (STARR Life Sciences, Oakmont, PA) at baseline and hourly during hypoxic exposure. Time to death was assessed by time to moribund status or death. All animal experiments were approved by the Vanderbilt University Institute for Animal Care and Use Committee.
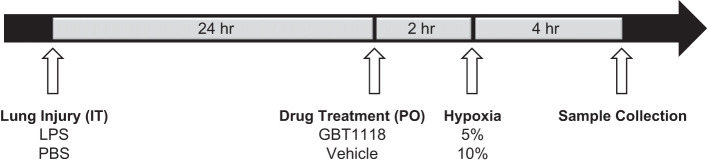
Fig. 1.
Experimental timeline. Mice received an intratracheal (IT) injection of 100 μg of LPS. After 24 h mice received GBT1118 (70 mg/kg or 140 mg/kg or vehicle control) via oral gavage (PO). 2 h later, mice were exposed to hypoxia (5% or 10%) for 4 h followed by sample collection.
Sample collection.
For sample collection, mice were euthanized with pentobarbital overdose. Blood was collected by retroorbital puncture into 4% sodium citrate. A bronchoalveolar lavage (BAL) was performed with 900 μl of normal saline. Lungs were flash frozen in liquid nitrogen. Plasma and BAL fluid were frozen after centrifugation at 2,000 g for 10 min. All samples were stored at −80°C until further study.
Hb occupancy of GBT1118 in whole blood.
Blood samples were analyzed for GBT1118 concentration using liquid chromatography tandem mass spectrometry (LC-MS). Standards and quality control samples were preincubated at 37°C. These samples were then diluted 1:2 with water to match experimental sample conditions. In a 96-well plate, 10 µl of diluted sample was added to 240 µl of sodium citrate (pH 3) and vortexed for 10 min. A sample (500 µl) of an internal standard, GBT1592 (GBT440-D7), in acetonitrile was added to each well at a concentration of 200 ng/ml followed by gentle agitation of the plate for 20 min. The sample plate was then centrifuged at 4,000 revolutions/min for 10 min. Ten microliters of supernatant was transferred to an injection plate and diluted with 190 µl of 50% acetonitrile (acetonitrile:water = 1:1) before injection in to the LC-MS. Separation of GBT1118 and GBT1592 was achieved using a Thermo Aquasil C18 column (2.1 × 20, 5 μm). The mobile phase was started with a mixture of 85% mobile phase A (0.1% formic acid in water) and 15% mobile phase B (0.1% formic acid in 100% acetonitrile) from minute 0.0–0.5. Next a gradient mixture of 95% mobile phase B was programmed from minute 0.5–1.5 and held until minute 1.8. At minute 1.9, the mobile phase went back to 15% mobile phase B and held until the process was complete. The peak area of m/z 341 → 203 product ion (GBT1118) was measured against that of the m/z 345 → 159 product ion (GBT1592) in positive ion mode. The analytical range was 50 to 100,000 ng/ml.
Whole blood hemoximetry.
Oxygen equilibrium curves for mouse blood were obtained by deoxygenation of O2-equilibrated samples in a HEMOX buffer at 37°C, using a HEMOX Analyzer (TCS Scientific, New Hope, PA). Blood samples placed in the hemoximeter were saturated with compressed air, followed by deoxygenation using pure nitrogen. The absorbance at wavelengths that correspond to the isosbestic point (570 nm) and deoxy Hb (560 nm) was recorded as a function of the sample O2 tension (Po2). During deoxygenation, the Po2 and percent O2 saturation values were collected to obtain the oxygen equilibrium curves and the p50 values (partial pressure of O2 at which Hb is 50% saturated with O2) (8, 11, 18).
BAL studies.
BAL inflammatory cell counts and differentials were determined manually after staining cytospins with DiffQuik (4, 23, 25). BAL total protein was measured in duplicate by BCA assay (Thermo Scientific, Waltham, MA). Levels of TNF-α, IL-1β, and macrophage inflammatory protein-1α (MIP-1α) were measured in BAL in duplicate by ELISA (R&D Systems, Minneapolis, MN).
Histological assessment of lung injury.
Ten nonoverlapping fields of hematoxylin and eosin-stained lung tissue were blindly assessed using a four-component lung injury score as previously described (24, 25). Each field was scored for inflammation, septal thickening, edema, and hemorrhage, and a composite total lung injury score was calculated.
Hypoxyprobe staining and quantification.
Pimonidazole (Hypoxyprobe) staining was perfomed on fixed tissue as previously described (6). Briefly, mice were given a single intraperitoneal injection of pimonidazole HCl (Hypoxyprobe, Burlington, MA) at 60 mg/kg inside the hypoxia chamber, 1.5 h before sample collection. Tissues were perfused and fixed in 10% formalin and embedded in paraffin. Slides were stained using an FITC-conjugated mouse IgG1 monoclonal antibody (FITC-Mab1, 1:50 dilution) followed by a rabbit anti-FITC secondary antibody conjugated with horseradish peroxidase (anti-FITC-Mab, 1:50 dilution). The slides were developed using the VECTOR NovaRED peroxidase substrate kit (Vector Laboratories, Burlingame, CA) and were counterstained with hematoxylin. The slides were blinded, and ten images were taken of each slide at ×40 magnification using an Olympus BX43 microscope with an Olympus DP70 camera. The intensity of Hypoxyprobe staining per high-powered field was determined using a previously described method using ImageJ (NIH, Bethesda, MD) (9).
Statistical analysis.
Continuous variables were compared by one-way ANOVA with post hoc Tukey’s testing. Mortality was compared using log-rank analysis of Kaplan-Meier survival curves. Statistical significance was determined using a two-sided P value of 0.05. p50 values were calculated using nonlinear regression analysis. Statistical analysis was done with SPSS version 23 (IBM, Armonk, North Castle, NY).
RESULTS
Pharmacology of GBT1118 treatment in our lung injury model.
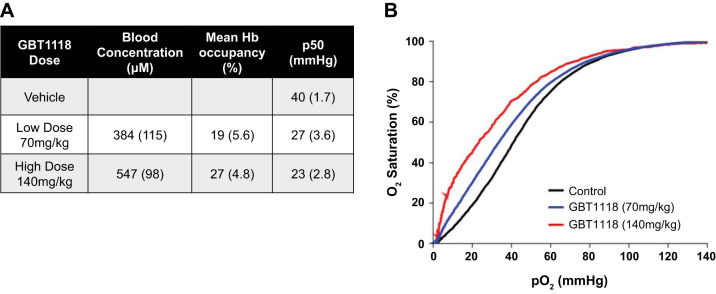
To confirm that LPS and hypoxia did not alter the pharmacology of GBT1118, we measured Hb occupancy by GBT1118 and the effects of GBT1118 on Hb-O2 affinity. Hb occupancy of GBT1118 in whole blood of GBT1118-treated mice was 19% with low-dose GBT1118 (70 mg/kg) and 27% with high-dose GBT1118 (140 mg/kg) as measured by pharmacokinetic analysis after 4 h hypoxia exposure (Fig. 2A). The p50, which is the Po2 at which blood is 50% saturated with O2, decreased from 40 mmHg with vehicle control to 27 mmHg and 23 mmHg with low- and high-dose GBT1118, respectively (Fig. 2A). Representative Hb-O2 dissociation curves were determined using whole blood from mice treated with intratracheal LPS and GBT1118 or vehicle after 4 h of 5% hypoxia exposure. As expected, GBT1118 shifted the Hb dissociation curve to the left in a dose-dependent manner (Fig. 2B).
Fig. 2.
Pharmacology of GBT1118 treatment. A: blood concentration and hemoglobin (Hb) occupancy of GBT1118. B: representative Hb-oxygen dissociation curves in the presence and absence of GBT1118. Administration of GBT1118 shifted the Hb dissociation curve to the left and decreased the p50 (Po2 at which blood is 50% saturated with O2) by 13 and 17 mmHg, respectively.
GBT1118 improves clinical outcomes of mice exposed to LPS with hypoxia.
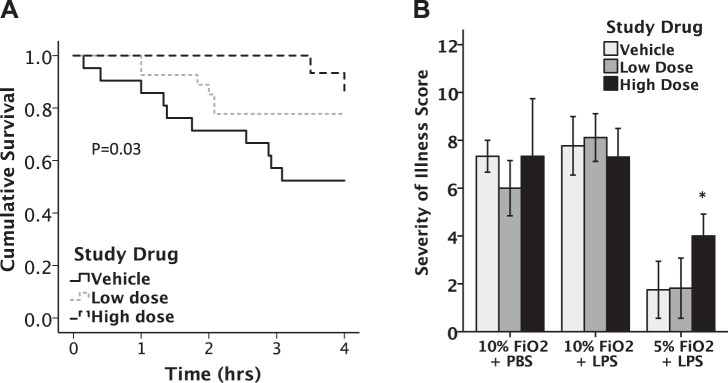
Mice treated with intratracheal LPS were treated with GBT1118 or vehicle before exposure to lethal (5%) or nonlethal (10%) hypoxia and monitored for mortality and severity of illness. Vehicle-treated mice given LPS and exposed to 5% O2 for 4 h had 55% mortality. In 5% O2, mortality was reduced to 40% in mice treated with low-dose GBT1118 and to 14% with high-dose GBT1118 (140 mg/kg, P = 0.032 by log rank) (Fig. 3A). Neither the 70 mg/kg nor 140 mg/kg dose of GBT1118 improved the severity of illness scores of mice after exposure to 10% hypoxia. In contrast, mice treated with 140 mg/kg GBT1118, but not 70 mg/kg, had significant improvement of severity of illness scores compared with vehicle-treated mice (mean score 4.00 vs. 1.75) after 5% hypoxia (P = 0.014) (Fig. 3B).
Fig. 3.
Treatment with GBT1118 improved outcomes of mice exposed to LPS + 5% hypoxia. GBT1118 significantly improved survival with LPS + hypoxia compared with vehicle-treated mice (A), P = 0.032 by log rank. Treatment with 140 mg/kg GBT1118 improved severity of illness scores (B) compared with vehicle at 5% hypoxia but had no significant effects on severity of illness in 10% hypoxia, *P = 0.014, n = 15–18 in each group.
Administration of GBT1118 increased SpO2 during hypoxia exposure.
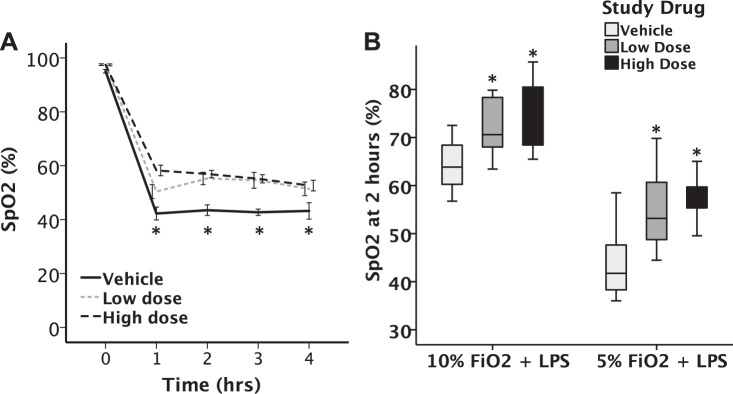
Oxygen saturation was measured before and hourly during hypoxic exposure using a pulse oximeter collar. Although all mice had a drop in SpO2, mice that received either 70 mg/kg or 140 mg/kg GBT1118 had significantly higher SpO2 over the first 4 h of 10% hypoxia compared with control mice (P < 0.02) (Fig. 4A). After 2 h in 5% hypoxia, mice that received either high- or low-dose GBT1118 had significantly higher SpO2 compared with control mice (P < 0.001) (Fig. 4B).
Fig. 4.
Administration of GBT1118 increased SpO2 during hypoxia exposure. A: GBT1118 is effective within 1 h, as shown by a time course of SpO2 measurements over 4 h at 5% hypoxia. *P < 0.05. B: treatment with GBT1118 significantly increased average SpO2 at 2 h during both 5% and 10% hypoxia exposure, *P < 0.05 vs. control, n = 11–18 in each group. Horizontal line of each boxplot indicates the median value. Top and bottom on the boxplot indicate the 75th and 25th percentile values, respectively.
GBT1118 did not attenuate lung inflammation or permeability.
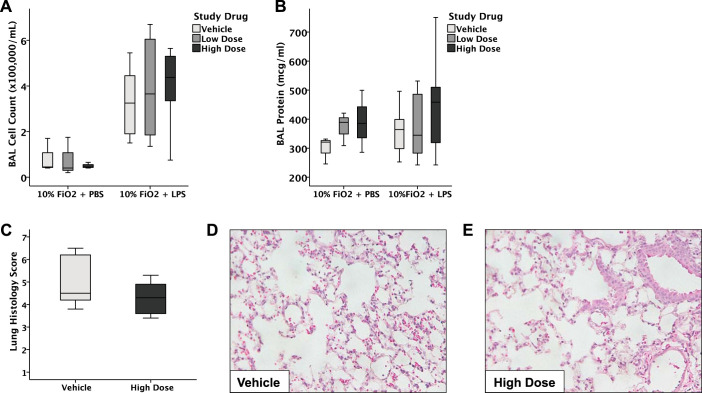
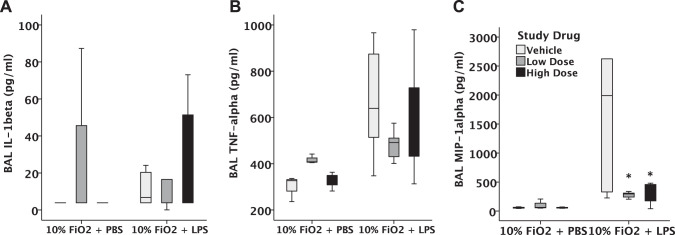
We next tested whether the benefits of GBT1118 on SpO2 and survival were due to effects of GBT1118 on the severity of acute lung injury as measured by lung inflammation and permeability. For comparison, we have previously reported that both BAL protein (400 μg/ml) and inflammatory cell counts (300,000 cells/ml) 24 h after intratracheal LPS alone (24) are similar to those measured in this study. After administration of intratracheal LPS in the setting of 10% hypoxia, mice developed lung inflammation, as measured by increased BAL cell counts (Fig. 5A). Treatment with GBT1118 did not diminish the number of inflammatory cells in the airspace. Furthermore, alveolar-capillary barrier permeability, as measured by BAL total protein levels, was not affected by GBT1118 (Fig. 5B). Furthermore, high-dose GBT1118 did not affect histological lung injury compared with vehicle-treated mice (Fig. 5, C–E). Although GBT1118 was associated with reduced levels of MIP-1α in BAL (Fig. 6C) compared with vehicle control, there were no significant effects of GBT1118 on IL-1β (Fig. 6A) or TNF-α (Fig. 6B) in the BAL.
Fig. 5.
GBT1118 did not affect alveolar-capillary permeability or lung inflammation. Total bronchoalveolar lavage (BAL) cell counts (A) and BAL protein (B) were not affected by the administration of GBT1118 (n = 13–15 in each group). Horizontal line of each boxplot indicates the median value. Top and bottom on the boxplot indicate the 75th and 25th percentile values, respectively. C–E: high-dose GBT1118 had no effect on the composite histological lung injury score compared with vehicle-treated mice.
Fig. 6.
Measurement of bronchoalveolar lavage (BAL) cytokines after 4-h exposure to PBS + hypoxia or LPS + hypoxia. GBT1118 decreased macrophage inflammatory protein-1α (MIP-1α) compared with vehicle (C) but had no effect on BAL levels of IL-1β (A) and TNF-α (B),*P < 0.01 vs. control, n = 13–15 in each group. Horizontal line of each boxplot indicates the median value. Top and bottom on the boxplot indicate the 75th and 25th percentile values, respectively.
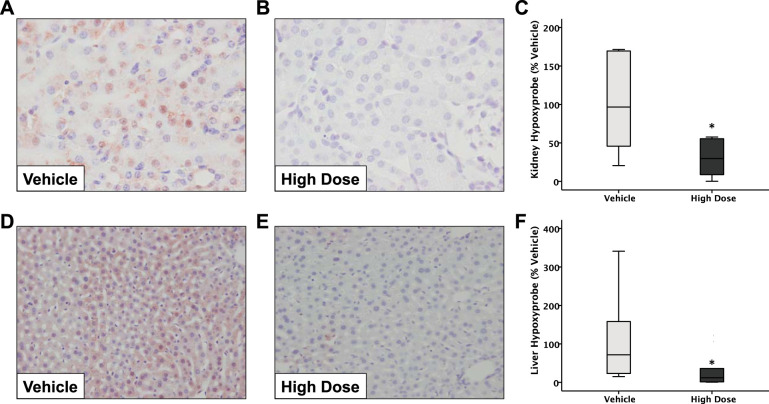
GBT1118 attenuates tissue hypoxia in the liver and kidney.
Because GBT1118 improved survival in this model of acute lung injury but did not have major effects on lung injury or inflammation, we next tested whether GBT1118 altered the degree of hypoxia of the liver and kidney. To assess this, we quantified the intensity of Hypoxyprobe staining, which assesses the degree of tissue hypoxia (6). LPS + hypoxia resulted in Hypoxyprobe staining in both the kidney (Fig. 7A) and liver (Fig. 7D). Administration of high-dose GBT1118 significantly attenuated the intensity of Hypoxyprobe staining in the kidney (Fig. 7, A–C) (P = 0.043) and liver (Fig. 7, D–F) (P = 0.043). These results confirm that, despite an increased oxygen loading of Hb with GBT1118, there is no evidence of tissue hypoxia.
Fig. 7.
Administration of GBT1118 decreases tissue hypoxia. Mice were treated with LPS and exposed to 10% hypoxia. Administration of high-dose GBT1118 significantly decreased the intensity of Hypoxyprobe staining compared with vehicle-treated mice in both the kidney (A–C) and liver (D–F), n = 9–10 in each group. *P = 0.043.
DISCUSSION
Our study demonstrates for the first time the potential of pharmacological manipulation of the oxygen affinity of Hb as a novel treatment for acute lung injury with hypoxemia. A single oral dose of GBT1118 increased the affinity of Hb for O2, as measured by a decrease in the p50 of Hb. Furthermore, a single dose of GBT1118 significantly improved oxygenation, survival, and severity of illness in the setting of acute lung injury with beneficial effects from both the 70 mg/kg and 140 mg/kg doses of GBT1118. These beneficial effects are associated with reduced hypoxia in the kidney and liver but were independent of airspace inflammation and alveolar-capillary barrier permeability.
The potential use of Hb modification as a treatment in ARDS has not been well studied. However, this treatment strategy has been studied in sickle cell disease and pulmonary fibrosis models (11, 18). In a murine model of sickle cell disease, treatment with an analog of GBT1118 (GBT440) was shown to increase Hb-O2 affinity, which extended the half-life of red blood cells and decreased reticulocyte counts (18). GBT1118 also had a profound therapeutic effect in a model of chronic fibrotic lung injury caused by oropharyngeal bleomycin and hypoxia. Mice that received GBT1118 had arterial oxygen saturation levels restored to near normal levels and attenuation of pulmonary collagen accumulation, weight loss, and leukocyte infiltration (11). The present study adds important new information to this field by demonstrating that GBT1118 can improve survival during acute lung injury with concurrent hypoxia. Together, these studies present strong evidence to support the potential of Hb modification as a novel therapeutic strategy for the treatment of severe hypoxemia.
Because GBT1118 administration daily for 8 days improved lung fibrosis, collagen accumulation, and leukocyte infiltration in a bleomycin fibrosis model (11), we hypothesized that administration of GBT1118 might reduce inflammation and permeability defects caused by LPS-induced inflammation in the acute lung injury model. However, in contrast to the bleomycin studies, GBT1118 had no effect on inflammation or permeability in response to LPS and hypoxia exposure. One explanation is that the severity of the LPS-induced inflammation and permeability defects in this model masked any benefits of GBT1118. A second potential explanation is that the antifibrotic and anti-inflammatory effects of GBT1118 require chronic administration rather than the single dose administered in the present study. Finally, the differences between the acute and chronic lung injury models could be due to timing of observations. Although we did not see any differences in lung inflammation after 4 h of hypoxia, this does not rule out a difference at later time points. Understanding the kinetics of the effects of GBT1118 will be an important focus of future studies. Regardless, the lack of effects of GBT1118 on the severity of lung inflammation despite improved survival suggests that the increased survival is not due to a reduction in the severity of acute lung injury.
One potential concern with pharmacological manipulation of Hb affinity for oxygen is an unintended decrease in O2 delivery in peripheral tissues. However, previous in vitro studies have demonstrated that Hb treated with GBT1118 is capable of effectively offloading O2 to tissues in low pH environments (11, 18) despite the leftward shift of the O2 dissociation curve. Furthermore, the therapeutic strategy of increasing Hb-O2 affinity has been studied in a variety of experimental hypoxia models; tissue extraction of oxygen and consumption of O2 increased, and no tissue hypoxia was observed (30). Our assessment of tissue hypoxia using Hypoxyprobe staining is consistent with these reports and demonstrates that GBT1118 administration reduces tissue hypoxia, confirming that GBT1118 does not affect oxygen offloading in this acute lung injury model. We propose that reduction of peripheral hypoxia may be one mechanism through which GBT1118 improves survival in LPS + hypoxia.
This study has some limitations. First, we used SpO2 to measure the severity of hypoxemia, rather than arterial blood gas analysis, because it could be measured noninvasively and repetitively in the hypoxic environment. We attempted to obtain blood gases by several methods in the hypoxia chamber, but it was not technically feasible. Previous studies in patients with ARDS, and in other critically ill patients, have shown that oxygen saturation measured by pulse oximetry and arterial blood gas analysis provide comparable assessments of severity of hypoxemia (7, 19, 22). Second, the specific physiological mechanisms through which GBT1118 improves survival and tissue hypoxia during acute lung injury remain uncertain and will require further study. This is a limitation of studies of acute illness in mice, wherein it is not possible to identify a specific mechanism of death. The mice in our study are quite ill, which leads us to speculate that they may be dying from shock and/or multisystem organ failure. We do show that GBT1118 attenuates tissue hypoxia, which may be particularly relevant to acute kidney injury because renal tissue is highly susceptible to hypoxic injury (13). Next, because of limitations of drug availability, we were not able to study GBT1118 in both male and female mice. Finally, we were unable to show significant disruption of the alveolar-capillary barrier or inflammation in the airspace. It may be that administration of intratracheal LPS preconditioned the lungs to the subsequent hypoxic exposure, thereby attenuating the inflammation and permeability responses in the lung.
In summary, a single oral dose of GBT1118, a compound that increases Hb-O2 affinity, was sufficient to shift the oxy-Hb dissociation curve and improve the survival of mice in an LPS-induced acute lung injury model with concurrent hypoxia. In combination with other studies showing benefit of altering Hb-O2 affinity in other model systems, these findings suggest that therapeutic approaches to modify the oxygen affinity of Hb may be a promising new approach to treat severe hypoxemia attributable to acute lung injury.
GRANTS
This work was supported by a grant from Global Blood Therapeutics.
DISCLOSURES
Although this study was sponsored by Global Blood Therapeutics (GBT), who provided the GBT1118 and vehicle control solutions, all analysis was done by our group without any input from GBT. Measurements of hemoglobin occupancy by GBT1118 and hemoglobin oxygen affinity were performed by GBT on blinded samples, and data were not unblinded until results were sent back to our laboratory.
AUTHOR CONTRIBUTIONS
N.D.P., K.D., C.-M.L., Q.X., A.H., J.L.-G., L.B.W., and J.A.B. conceived and designed research; N.D.P., K.D., C.-M.L., Q.X., A.H., and J.L.-G. performed experiments; N.D.P., C.M.S., S.M.M., and J.A.B. analyzed data; N.D.P., C.M.S., S.M.M., L.B.W., and J.A.B. interpreted results of experiments; N.D.P. and J.A.B. drafted manuscript; N.D.P., C.M.S., K.D., C.-M.L., Q.X., A.H., J.L.-G., L.B.W., and J.A.B. edited and revised manuscript; N.D.P., C.M.S., K.D., C.-M.L., Q.X., A.H., J.L.-G., S.M.M., L.B.W., and J.A.B. approved final version of manuscript; C.M.S. and J.A.B. prepared figures.
REFERENCES
- 1.Angus DC, Clermont G, Linde-Zwirble WT, Musthafa AA, Dremsizov TT, Lidicker J, Lave JR, Investigators NO; NO-06 Investigators . Healthcare costs and long-term outcomes after acute respiratory distress syndrome: a phase III trial of inhaled nitric oxide. Crit Care Med 34: 2883–2890, 2006. doi: 10.1097/01.CCM.0000248727.29055.25. [DOI] [PubMed] [Google Scholar]
- 2.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A; Acute Respiratory Distress Syndrome Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network . Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354: 2564–2575, 2006. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 4.Bastarache JA, Sebag SC, Clune JK, Grove BS, Lawson WE, Janz DR, Roberts LJ II, Dworski R, Mackman N, Ware LB. Low levels of tissue factor lead to alveolar haemorrhage, potentiating murine acute lung injury and oxidative stress. Thorax 67: 1032–1039, 2012. doi: 10.1136/thoraxjnl-2012-201781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network . Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 351: 327–336, 2004. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 6.Bryant AJ, Carrick RP, McConaha ME, Jones BR, Shay SD, Moore CS, Blackwell TR, Gladson S, Penner NL, Burman A, Tanjore H, Hemnes AR, Karwandyar AK, Polosukhin VV, Talati MA, Dong HJ, Gleaves LA, Carrier EJ, Gaskill C, Scott EW, Majka SM, Fessel JP, Haase VH, West JD, Blackwell TS, Lawson WE. Endothelial HIF signaling regulates pulmonary fibrosis-associated pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310: L249–L262, 2016. doi: 10.1152/ajplung.00258.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Janz DR, Shaver CM, Bernard GR, Bastarache JA, Ware LB. Clinical characteristics and outcomes are similar in ARDS diagnosed by oxygen saturation/Fio2 ratio compared with Pao2/Fio2 ratio. Chest 148: 1477–1483, 2015. doi: 10.1378/chest.15-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dufu K, Lehrer-Graiwer J, Ramos E, Oksenberg D. GBT440 inhibits sickling of sickle cell trait blood under in vitro conditions mimicking strenuous exercise. Hematol Rep 8: 6637, 2016. doi: 10.4081/hr.2016.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaskill CF, Carrier EJ, Kropski JA, Bloodworth NC, Menon S, Foronjy RF, Taketo MM, Hong CC, Austin ED, West JD, Means AL, Loyd JE, Merryman WD, Hemnes AR, De Langhe S, Blackwell TS, Klemm DJ, Majka SM. Disruption of lineage specification in adult pulmonary mesenchymal progenitor cells promotes microvascular dysfunction. J Clin Invest 127: 2262–2276, 2017. doi: 10.1172/JCI88629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Pelosi P, Crotti S, Valenza F. Effects of positive end-expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med 151: 1807–1814, 1995. doi: 10.1164/ajrccm.151.6.7767524. [DOI] [PubMed] [Google Scholar]
- 11.Geng X, Dufu K, Hutchaleelaha A, Xu Q, Li Z, Li CM, Patel MP, Vlahakis N, Lehrer-Graiwer J, Oksenberg D. Increased hemoglobin-oxygen affinity ameliorates bleomycin-induced hypoxemia and pulmonary fibrosis. Physiol Rep 4: 4, 2016. doi: 10.14814/phy2.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerin C, Gaillard S, Lemasson S, Ayzac L, Girard R, Beuret P, Palmier B, Le QV, Sirodot M, Rosselli S, Cadiergue V, Sainty JM, Barbe P, Combourieu E, Debatty D, Rouffineau J, Ezingeard E, Millet O, Guelon D, Rodriguez L, Martin O, Renault A, Sibille JP, Kaidomar M. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA 292: 2379–2387, 2004. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 13.Haase VH. Mechanisms of hypoxia responses in renal tissue. J Am Soc Nephrol 24: 537–541, 2013. doi: 10.1681/ASN.2012080855. [DOI] [PubMed] [Google Scholar]
- 14.Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ, Group CTSAS; CT Scan ARDS Study Group . Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1444–1450, 2001. doi: 10.1164/ajrccm.163.6.2005001. [DOI] [PubMed] [Google Scholar]
- 15.Metcalf B, Chuang C, Dufu K, Patel MP, Silva-Garcia A, Johnson C, Lu Q, Partridge JR, Patskovska L, Patskovsky Y, Almo SC, Jacobson MP, Hua L, Xu Q, Gwaltney SL II, Yee C, Harris J, Morgan BP, James J, Xu D, Hutchaleelaha A, Paulvannan K, Oksenberg D, Li Z. Discovery of GBT440, an orally bioavailable R-state stabilizer of sickle cell hemoglobin. ACS Med Chem Lett 8: 321–326, 2017. doi: 10.1021/acsmedchemlett.6b00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozzarelli A, Hofrichter J, Eaton WA. Delay time of hemoglobin S polymerization prevents most cells from sickling in vivo. Science 237: 500–506, 1987. doi: 10.1126/science.3603036. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi CT, Rodgers GP, Serjeant G, Schechter AN. Levels of fetal hemoglobin necessary for treatment of sickle cell disease. N Engl J Med 318: 96–99, 1988. doi: 10.1056/NEJM198801143180207. [DOI] [PubMed] [Google Scholar]
- 18.Oksenberg D, Dufu K, Patel MP, Chuang C, Li Z, Xu Q, Silva-Garcia A, Zhou C, Hutchaleelaha A, Patskovska L, Patskovsky Y, Almo SC, Sinha U, Metcalf BW, Archer DR. GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease. Br J Haematol 175: 141–153, 2016. doi: 10.1111/bjh.14214. [DOI] [PubMed] [Google Scholar]
- 19.Pandharipande PP, Shintani AK, Hagerman HE, St Jacques PJ, Rice TW, Sanders NW, Ware LB, Bernard GR, Ely EW. Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Crit Care Med 37: 1317–1321, 2009. doi: 10.1097/CCM.0b013e31819cefa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guérin C, Prat G, Morange S, Roch A, Investigators AS; ACURASYS Study Investigators . Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 363: 1107–1116, 2010. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 21.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D; CESAR Trial Collaboration . Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374: 1351–1363, 2009. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 22.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB; National Institutes of Health, National Heart, Lung, and Blood Institute ARDS Network . Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 132: 410–417, 2007. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 23.Shaver CM, Grove BS, Clune JK, Mackman N, Ware LB, Bastarache JA. Myeloid tissue factor does not modulate lung inflammation or permeability during experimental acute lung injury. Sci Rep 6: 22249, 2016. doi: 10.1038/srep22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaver CM, Grove BS, Putz ND, Clune JK, Lawson WE, Carnahan RH, Mackman N, Ware LB, Bastarache JA. Regulation of alveolar procoagulant activity and permeability in direct acute lung injury by lung epithelial tissue factor. Am J Respir Cell Mol Biol 53: 719–727, 2015. doi: 10.1165/rcmb.2014-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaver CM, Upchurch CP, Janz DR, Grove BS, Putz ND, Wickersham NE, Dikalov SI, Ware LB, Bastarache JA. Cell-free hemoglobin: a novel mediator of acute lung injury. Am J Physiol Lung Cell Mol Physiol 310: L532–L541, 2016. doi: 10.1152/ajplung.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinberg MH. Modulation of fetal hemoglobin in sickle cell anemia. Hemoglobin 25: 195–211, 2001. doi: 10.1081/HEM-100104028. [DOI] [PubMed] [Google Scholar]
- 27.Su G, Atakilit A, Li JT, Wu N, Luong J, Chen R, Bhattacharya M, Sheppard D. Effective treatment of mouse sepsis with an inhibitory antibody targeting integrin αvβ5. Crit Care Med 41: 546–553, 2013. doi: 10.1097/CCM.0b013e3182711b1e. [DOI] [PubMed] [Google Scholar]
- 28.Sunshine HR, Hofrichter J, Eaton WA. Requirement for therapeutic inhibition of sickle haemoglobin gelation. Nature 275: 238–240, 1978. doi: 10.1038/275238a0. [DOI] [PubMed] [Google Scholar]
- 29.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 30.Yalcin O, Cabrales P. Increased hemoglobin O2 affinity protects during acute hypoxia. Am J Physiol Heart Circ Physiol 303: H271–H281, 2012. doi: 10.1152/ajpheart.00078.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]