Abstract
Blood flow restriction training (BFRT) is an increasingly widespread method of exercise that involves imposed restriction of blood flow to the exercising muscle. Blood flow restriction is achieved by inflating a pneumatic pressure cuff (or a tourniquet) positioned proximal to the exercising muscle before, and during, the bout of exercise (i.e., ischemic exercise). Low-intensity BFRT with resistance training promotes comparable increases in muscle mass and strength observed during high-intensity exercise without blood flow restriction. BFRT has expanded into the clinical research setting as a potential therapeutic approach to treat functionally impaired individuals, such as the elderly, and patients with orthopedic and cardiovascular disease/conditions. However, questions regarding the safety of BFRT must be fully examined and addressed before the implementation of this exercise methodology in the clinical setting. In this respect, there is a general concern that BFRT may generate abnormal reflex-mediated cardiovascular responses. Indeed, the muscle metaboreflex is an ischemia-induced, sympathoexcitatory pressor reflex originating in skeletal muscle, and the present review synthesizes evidence that BFRT may elicit abnormal cardiovascular responses resulting from increased metaboreflex activation. Importantly, abnormal cardiovascular responses are more clearly evidenced in populations with increased cardiovascular risk (e.g., elderly and individuals with cardiovascular disease). The evidence provided in the present review draws into question the cardiovascular safety of BFRT, which clearly needs to be further investigated in future studies. This information will be paramount for the consideration of BFRT exercise implementation in clinical populations.
Keywords: autonomic nervous system, blood flow restriction training, exercise pressor reflex, cardiac rehabilitation, Kaatsu training, sympathetic nervous system
INTRODUCTION
Blood flow restriction training (BFRT) is a method of exercise training that involves execution of low-intensity resistance exercise combined with blood flow restriction (LI-BFR) provided by an inflatable cuff or a tourniquet placed proximal to the exercising muscle. In general, the external cuff pressure applied is set relative to the arterial occlusion pressure (AOP; i.e., the pressure required to cease blood flow to a limb), which is sufficient to produce partial restriction of arterial inflow and full occlusion of venous outflow (81, 116). This hypoxic and metabolic-demanding environment augments muscle motor unit recruitment and activates signaling pathways driving protein synthesis and stem cell activation, leading to muscle hypertrophy (for more information see Ref. 119).
BFRT has become increasingly popular in recent years due to its positive effects on muscle size and strength (80). Moreover, the capacity to generate neuromuscular improvements (e.g., muscle hypertrophy and physical function) comparable to more intense resistance training protocols, while employing low-intensity workloads, has generated considerable interest in utilizing BFRT as a potential therapeutic option for functionally impaired populations, which may be incapable of engaging in high-intensity resistance exercise regimens (e.g., older individuals, patients with knee osteoarthritis) (19, 35). Accordingly, several authors have suggested that BFRT could be used as an important therapeutic tool in clinical practice (82, 98, 118), constituting a promising and possibly suitable alternative to high-intensity resistance training for conditions such as postoperative rehabilitation, cardiac rehabilitation, and inflammatory diseases (118). However, for the appropriate implementation of this mode of training in the daily routine of clinical populations, the safety of routine BFRT use in clinical populations has yet to be fully determined.
Previous reviews (53, 82, 118) reported potential negative side effects associated with BFRT, such as increased incidence of blood clots, vein congestion/distension, ischemia-reperfusion injury, muscle damage, and exertional rhabdomyolysis, but concluded that for most of these events BFRT is unlikely to present additional risks in comparison with traditional exercise. A large survey in Japan with BFRT instructors from 232 facilities did not verify any major event, such as cerebral hemorrhage, cerebral infarct, or thrombosis in >120,000 subjects with a large variability of demographical and clinical characteristics (e.g., older adults and people with obesity, diabetes, cerebrovascular and cardiovascular diseases). Reported side effects were generally minor, including transient numbness or dizziness, subcutaneous hemorrhage, and itchiness (98). However, care should be taken with general assumptions about BFRT safety given the paucity of long-term prospective trials with clinical populations, and the possibility of some remaining adverse effects that are still largely overlooked in the literature.
One area requiring further investigation regarding BFRT safety is its potential to promote exacerbated reflex-mediated cardiovascular responses via engagement of the muscle metaboreflex arm of the exercise pressor reflex (154). The metaboreflex is a sympathoexcitatory blood flow- and blood pressure (BP)-raising reflex originating in the contracting skeletal muscle which responds to the increase in muscle metabolites typically occurring during exercise. Upon activation, these afferents relay neural information to the central nervous system (63) which, in turn, produces increases in cardiac output (CO) and BP. While this reflex plays a pivotal role in controlling muscle blood flow during exercise, rapidly correcting any mismatch between oxygen demand and supply (39), an exacerbated activation of the metaboreflex has been shown to produce abnormal cardiovascular responses (20, 28, 73, 140, 152), which may be a matter of concern in specific groups of individuals.
Insufficient O2 delivery to active muscle, and the resulting accumulation of metabolites within muscles fibers during the execution of BFRT, might promote augmented metaboreflex activation, producing increased BP and other abnormal cardiovascular responses, which may increase the risk of adverse cardiovascular events in individuals with cardiovascular risk factors or overt cardiovascular disease. This hypothesis is supported by several experimental and clinical studies demonstrating the role of metaboreflex activation on neural and hemodynamic responses to exercise in healthy and clinical populations (2, 20, 28, 38, 49, 52, 54, 87, 120, 143, 150, 156). Recent studies have assessed cardiovascular responses during typical BFRT sessions (6, 14, 16, 29, 36, 71, 76, 85, 88, 114, 124, 125, 127, 128, 142, 146, 150, 155, 156, 167). The equivocal results of these limited studies suggest that further investigation of the cardiovascular responses to BFRT are necessary and required to properly assess the cardiovascular safety of this method of exercise training. Importantly, some training variables of BFRT exercise might either increase or attenuate potential risks, which also deserve appropriate discussion. Therefore, the aim of this review is to provide experimental and clinical information about the role of the muscle metaboreflex on cardiovascular adjustments to exercise, to present the current evidences on the acute cardiovascular responses to BFRT, and to provide practical consideration regarding implementation of BFRT in clinical populations.
CARDIOVASCULAR ADJUSTMENTS TO EXERCISE: ROLE OF THE AUTONOMIC NERVOUS SYSTEM
During exercise, CO increases mainly due to elevations in heart rate (HR) and relatively smaller increases in stroke volume (SV), while systemic vascular resistance (SVR) may either fall (dynamic aerobic exercise) or increase (static/dynamic resistance exercise). This difference reflects whether skeletal muscle vasodilates substantially (as during dynamic aerobic exercise) or whether there is physical compression of blood vessels in the active muscle (as during strong static/dynamic resistance exercises). During aerobic exercise, the rise in CO is greater than the fall in resistance and, as a result, mean arterial pressure is increased, but the response is much larger during intense resistance exercise (39). Indeed, in experienced bodybuilders, intra-arterial measurements have shown increases in systolic blood pressure in excess of 300 mmHg during high-intensity dynamic contractions (84). Studies with clinical populations and with submaximal resistance exercise intensities have shown more modest increases in BP (50). In parallel to the rise in CO there is a shift in blood flow from inactive vascular beds (e.g., renal and splanchnic) to active (e.g., exercising muscle, heart, and skin). This coordinated hemodynamic response ensures adequate blood flow to all organs and tissues without compromising blood flow and oxygen supply to exercising muscle.
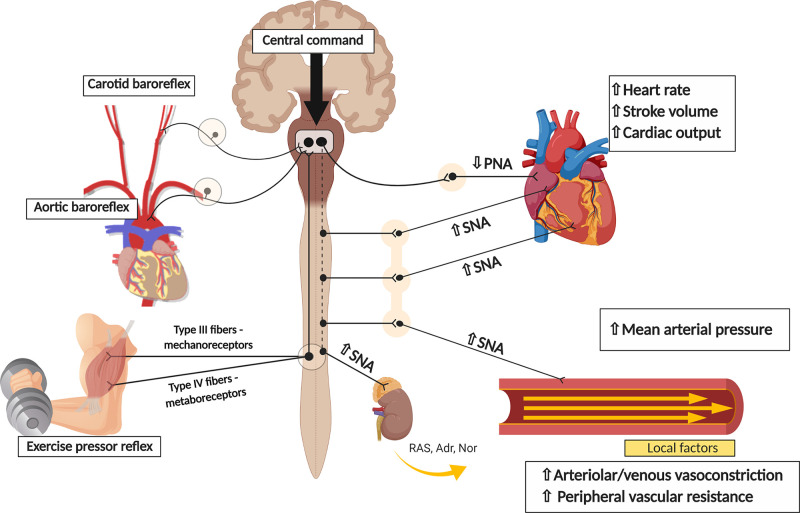
Exercise-induced hemodynamic responses are determined by alterations in the activity of the autonomic nervous system, with increases in sympathetic nerve activity to the heart and vasculature and decreases in cardiac parasympathetic nerve activity. These autonomic adjustments are tightly regulated by the synchronous action of multiple neural mechanisms, including central command (i.e., a feed-forward mechanism originating in higher brain areas involved in volition and effort sensation) (46), the arterial baroreflex (i.e., a negative feedback reflex stimulated by stretch receptors within the carotid sinuses and aortic arch, sensitive to changes in pulsatile blood pressure) (33), and the exercise pressor reflex (63). The exercise pressor reflex (composed of the muscle metaboreflex and muscle mechanoreflex) originates in the contracting skeletal muscle and is triggered by the activation of thinly myelinated (group III) and unmyelinated (group IV) afferent nerve fibers. Group III afferents are mainly stimulated by mechanical stimuli (i.e., mechanoreceptors), whereas group IV afferents are mainly sensitive to changes in the chemical milieu in the interstitial space of skeletal muscle (i.e., metaboreceptors), secondary to the production of exercise metabolites (64, 65). During exercise, descending signals from higher brain areas and afferent signals from baroreceptors and the exercise pressor reflex are integrated within specific areas of the brain stem and, as output, efferent signals carried by parasympathetic and sympathetic nerve fibers produce the abovementioned cardiovascular adjustments to exercise (39, 63, 129) (Fig. 1).
Fig. 1.
Cardiovascular adjustments to exercise: the role of the autonomic nervous system. During exercise, feedforward signals from higher brain areas (i.e., central command) and feedback information arising from different somatic and visceral afferents (i.e., such as the arterial baroreflex and the exercise pressor reflex) convey sensory information to the central nervous system, which then coordinates the efferent response to the cardiovascular system. SNA, sympathetic nerve activity; PNA, parasympathetic nerve activity; RAS, renin–angiotensin system; Adr, adrenaline; Nor, noradrenaline.
MUSCLE METABOREFLEX
As a component of the exercise pressor reflex, the muscle metaboreflex exerts an important role in regulating the cardiovascular response to exercise. Ischemia-induced metabolites produced during moderate- to high-intensity exercise (e.g., proton, lactate, ATP) stimulate metabolically sensitive afferent nerve receptors in the muscle interstitium [e.g., acid-sensing ion channels (ASIC), purinergic receptors (P2X), and transient receptor potential cation channels of the vanilloid type 1 (TRPV1)] (77), which relay information to the central nervous system to produce increases in blood flow to the working muscle (15). However, while optimal activation of the metaboreflex is paramount for regulating muscle blood flow during exercise, an augmented and sustained activation of this reflex might evoke abnormal cardiovascular responses, which could be a matter of concern in populations with increased cardiovascular risks.
Animal Studies
Healthy animals.
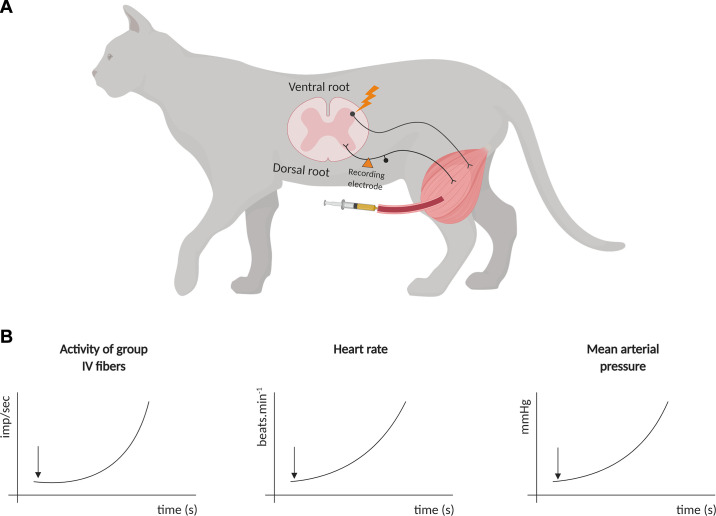
Studies in healthy animals have identified the role and discharge characteristics of the metaboreceptors involved in cardiovascular regulation. A seminal study with anesthetized cats showed that electrically induced contraction (i.e., 30–45 s) of triceps surae increased the activity of unmyelinated group IV muscle afferents, which was accompanied by increases in HR and arterial pressure (64). Of note, these afferents showed a progressive firing increase alongside the stimulation, which is consistent with these types of receptors being sensitive to the accumulation of metabolites in the muscle interstitium (64). In addition, injection of capsaicin (an exogenous agonist TRPv1 receptor primarily localized in type IV fibers) into the abdominal aorta of the cats also increased the impulse activity of type IV fibers. The ischemic dependence of type IV fibers was further evidenced in a subsequent study from the same group that showed that circulatory occlusion to the working muscle increased contraction-induced cardiovascular and nerve firing responses (65) (Fig. 2).
Fig. 2.
Seminal experiments (Refs. 64 and 65) with anesthetized cats showed that electrical stimulation of the ventral root of the spinal cord, or capsaicin injection into the hindlimbs (A), produced an increase in activity of group IV fibers, which was accompanied by increases in heart rate (HR) and mean arterial pressure (B). The black arrow in B illustrates the beginning of stimulation (either by capsaicin or an electrical stimulus).
Studies using conscious, chronically instrumented dogs support the role of the metaboreflex in the control of circulation during exercise. Wyss et al. (169) originally placed a vascular occluder around the terminal aorta of these animals to limit blood supply to active muscles during exercise. These authors showed that a protocol of mild to vigorous dynamic exercise with restriction of blood flow to the hindlimbs evoked a powerful BP increase, which was mainly due to increases in HR and CO. Later studies supported these findings and showed that an increase in cardiac sympathetic activity is the main mechanism behind these metaboreflex-mediated hemodynamic responses (105). Importantly, CO increases as a result of enhanced ventricular performance and central blood volume mobilization, which preserves SV despite the reduced filling time promoted by tachycardia (107, 147). This cardiac response is also accompanied by sympathetically mediated vasoconstriction of renal (8, 9) and nonischemic vascular beds (8), which helps redirect blood flow to the exercising muscle. On the other hand, the vascular response to the ischemic muscle is under opposing vasoconstrictor and vasodilatory influences, and the resulting response is a function of the interaction between contrasting neural, hormonal and local inputs (66, 67, 69, 108). Nevertheless, metaboreflex activation increases perfusion pressure to the exercising muscle, partially restoring blood flow and O2 delivery to the active skeletal muscle (106, 111).
Animal models of cardiovascular disease.
While metaboreflex-induced increases in CO increase blood supply to the ischemic working muscle, the accompanying increase in BP, and resulting neurovascular adjustments, might be particularly unfavorable in groups at risk for cardiovascular events. For instance, activation of the metaboreflex, by either ischemic muscle contraction or by graded administration of capsaicin into the arterial supply of the hindlimb, elicits increased BP and renal sympathetic nerve activity responses in hypertensive rats (73, 93). In addition, expression of TRPV1 receptors within dorsal root ganglia was increased in hypertensive rats, and administration of a TRPV1 antagonist attenuated these increased metaboreflex-induced cardiovascular responses (93). These findings demonstrate increased metaboreflex sensitivity in hypertensive rats and advocate for an important role of TRPV1 receptors in the increased BP responses during exercise in hypertension (HTN).
Studies in conscious hypertensive animals also show an altered metaboreflex response to exercise. Sala-Mercado et al. (140) and Spranger et al. (153) reported attenuated metaboreflex-induced increase in CO increase and a shift toward enhanced peripheral vasoconstriction in dogs with renovascular HTN. In this model, the rise in BP is reduced, and this attenuated pressor response is due to a smaller increase in CO, which is associated with decreased ventricular performance. Interestingly, exaggerated α-adrenergic-mediated sympathetic coronary vasoconstriction might underline this reduced ventricular performance in the hypertensive animals (152), which raises additional questions about the safety of excessive metaboreflex activation during exercise in specific populations. Exaggerated coronary vasoconstriction coupled with increased myocardial oxygen demand might favor ischemic episodes and lead to arrhythmias and sudden cardiac death.
A hallmark of diseases such as heart failure (HF) and peripheral artery disease (PAD) is underperfusion of skeletal muscle. As the metaboreflex is tonically active is these diseases, further stimulation of this reflex might generate abnormal cardiovascular responses to exercise. For instance, in HF animals, metaboreflex activation during exercise impairs increases in cardiac performance and CO (5, 7, 48, 109, 139). As with HTN, this response seems to be mediated either by increased coronary vasoconstriction (5) (which may also lead to electrical abnormalities in the failing heart, thereby increasing cardiovascular risks) or by impaired systolic and/or diastolic function (109, 139). Despite diminished CO responses during exercise, animals with HF do exhibit metaboreflex-induced increases in BP, and this response seems to be primarily mediated by a marked increase in peripheral vasoconstriction (7). Importantly, in HF this metaboreflex-induced vasoconstriction also occurs in the ischemic active skeletal muscle, via increased α-adrenergic sympathetic vasoconstriction, coupled with potential impaired functional sympatholysis (68). This response might further limit blood flow to working muscle, promoting additional engagement of the metaboreflex, thereby leading to a vicious cycle of progressive impairment in skeletal muscle blood flow.
PAD is characterized by transient ischemia in skeletal muscle during physical exertion, which may evoke pain while walking (i.e., intermittent claudication) and tonic activation of the metaboreflex. In support of the latter, femoral artery ligation (i.e., model of simulated PAD) has shown to produce increased BP and sympathetic responses to exercise (163, 164), which is mediated by an increase in protein expression of TRPV1, P2X3, and ASIC3 receptors in sensory afferent neurons (78, 79, 170, 171). Furthermore, stimulation of these receptors, either by the injection of specific metabolites (e.g., ATP, lactic acid, capsaicin) into the arterial blood supply of hindlimbs or by electrically induced muscle contraction, also leads to increased sympathetic and cardiovascular responses in PAD models (78, 79, 157, 170). Collectively, these findings suggest that an increase in either the expression and/or the sensitivity of metaboreceptors takes place in PAD models, leading to augmented reflex-mediated cardiovascular responses during physical effort. Further stimulation of the metaboreflex might generate abnormal cardiovascular responses to exercise.
Studies in Humans
Healthy humans.
Studies in healthy humans support the important role of the metaboreflex in the control of hemodynamic responses during exercise. The classic method to test the role of the metaboreflex in cardiovascular responses in humans is the employment of suprasystolic circulatory occlusion immediately after static or dynamic exercise (2, 38, 40, 54, 55, 87, 89, 120, 133). This protocol traps contraction-induced metabolites within previously working muscle, allowing the assessment of cardiovascular responses elicited by the metaboreflex in isolation, without concurrent influence of other mechanisms engaged during exercise (e.g., central command and the muscle mechanoreflex). Other studies have performed partial or total circulatory occlusion during exercise to engage the metaboreflex in a typical exercise condition (2, 37, 49, 89).
The seminal study of Alam and Smirk (2) showed that circulatory arrest during and following dynamic calf and forearm exercises elicits an increase in systolic and diastolic BP compared with exercise under free-flow conditions. According to these authors, this reflex-mediated BP response ensures an increase in blood supply to ischemic working muscles, thereby postponing fatigue. Subsequent studies reinforced this marked pressor response associated with the engagement of the metaboreflex, and further studies investigated the neural and hemodynamic mechanisms responsible for this response (87, 130, 134, 149). Mark et al. (87) employed microneurography to directly record sympathetic activity during handgrip exercise followed by postexercise muscle ischemia (PEMI). This study showed that the powerful pressor response associated with metaboreflex activation is paralleled by marked sympathetic activation to the peripheral vasculature. In another landmark study, Joyner et al. (60) found a marked rise in muscle sympathetic activity during heavy handgrip and PEMI in healthy humans, with this response being significantly attenuated by the application of suction around the forearm to artificially increase blood flow to active muscle. These findings indicate that metaboreflex activation drives increases in muscle sympathetic activity to the peripheral vasculature during exercise, even in unrestricted blood flow conditions.
As previously mentioned, the neural responses associated with the engagement of the metaboreflex are important mechanisms in the regulation of blood flow to exercising muscle. Accordingly, studies in humans have shown that the increased sympathetic activation to the peripheral vasculature produces vasoconstriction in renal and splanchnic vascular beds (91, 94, 149) as well as nonactive muscle vasculature (122, 134), directing blood flow to the exercising muscles (106). In addition, metaboreflex-induced sympathetic venoconstriction increases preload and central blood volume mobilization, which favors increases in cardiac performance, leading to increases in SV and CO (15, 149).
The role of metaboreflex on HR in humans at one time was debatable (37, 38, 40, 54–56, 101, 120, 133). Previous studies showed a complete return of HR to baseline levels during PEMI after moderate intensity static or rhythmic handgrip in healthy humans (40, 55, 56, 133), which initially led to the suggestion that, despite producing a robust peripheral sympathetic response, activation of the metaboreflex did not seem to increase cardiac sympathetic activity during exercise (137). On the other hand, more recent studies using either more intense exercise (38), or exercise recruiting larger muscle masses (e.g., leg exercise) (37, 49, 120), demonstrate moderate elevations in HR during PEMI, which is consistent with the notion that activation of the metaboreflex is able to produce at least modest cardiac sympathetic responses in humans.
The prevailing explanation for the return of HR toward baseline levels in the face of persistent metaboreflex activation is that the increase in cardiac sympathetic activity during PEMI may be overpowered by a concurrent parasympathetic reactivation which occurs upon the cessation of exercise (56, 101, 105). Parasympathetic reactivation could result from 1) deactivation of central command and the mechanoreflex (18, 121) and/or 2) stimulation of the arterial baroreflex due to increased BP during ischemia (56). Indeed, parasympathetic blockade increases HR during PEMI after moderate-intensity handgrip exercise (38), while sympathetic blockade abolishes this response. These results indicate that cardiac sympathetic tone is augmented as a result of metaboreflex activation, but a concurrent parasympathetic activation might diminish the sympathetic effects on HR after exercise.
Elevations in HR in response to metaboreflex activation have also been reported when the metaboreflex is engaged via partial blood flow restriction during (not after) exercise (32, 49). During exercise, the activation of central command and the mechanoreflex, and the resetting of the arterial baroreflex to higher BP values, lead to a reduction in cardiac parasympathetic activity (102, 104a, 104b). In this scenario, further parasympathetic blockade does not seem to accentuate the metaboreflex-mediated increases in HR. On the other hand, sympathetic blockade significantly attenuates the HR responses to partial blood flow restriction during exercise (37), indicating an important cardiac sympathetic activation in response to the engagement of the muscle metaboreflex during exercise.
Humans with cardiovascular disease.
The studies in humans reviewed herein are in line with the studies in animals showing significant sympathetic and pressor responses associated with the engagement of the metaboreflex in healthy populations. As previously reported, these neural and hemodynamic responses are paramount for providing adequate blood flow to exercising muscle. However, supraphysiological activation of the metaboreflex might produce abnormal cardiovascular responses, and this could be potentially deleterious in some populations. Indeed, recent studies in humans confirm previous findings in animals showing that metaboreflex-induced cardiovascular responses could be more accentuated in subjects with cardiovascular and metabolic diseases, such as HTN, diabetes, HF, and PAD, as reviewed below.
Hypertensive patients present with increased BP responses to exercise (11, 20), and this is partly due to increased metaboreflex-mediated cardiovascular responses (20). Studies using isometric or rhythmic handgrip exercise followed by PEMI have shown increased BP and muscle sympathetic responses in young (143) and elderly (28) hypertensive subjects. Interestingly, the increased BP response to dynamic exercise is normalized after the partial blockade of muscle afferents using lumbar intrathecal injection of fentanyl (a selective μ-opioid receptor agonist) (11), which further supports the role of reflex-based signals arising from skeletal muscle in producing increased BP responses during exercise in hypertension.
It is important to point out that the increased BP response to exercise in hypertensive individuals occurs even in those with well-controlled resting BP levels, and a recent study demonstrated that this response is mediated by an increased metaboreflex sensitivity (20). Interestingly, in this study the systolic BP responses during isolated metaboreflex activation (via PEMI after static handgrip) in the treated control patients were similar to the treated uncontrolled or untreated patients. It is hypothesized that part of this abnormal response is due to the fact that most, if not all, antihypertensive drugs do not directly influence the metaboreflex-related pathways (ASIC, TRPV1, P2X, as previously mentioned). Therefore, the excessive engagement of the metaboreflex might produce exaggerated BP responses even in those individuals with properly controlled resting BP.
Hypertensive individuals have also shown to present with an attenuated reduction in HR during PEMI compared with their normotensive counterparts (120). Slower HR recovery during metaboreflex activation in hypertensive individuals suggests sustained sympathetic activation and reduced parasympathetic reactivation (121), which may increase cardiovascular risks in certain conditions where the metaboreflex is overactivated (23). Indeed, a reduced HR recovery after exercise has been shown to be a predictor of ventricular fibrillation after myocardial infarct (151) and to be independently associated with cardiovascular disease (58, 121) and mortality (23, 121).
Dysfunction in cardiovascular autonomic regulation is intrinsically involved in the pathophysiology of HF, being associated with clinical symptoms (34, 123) and implicated in the disease progression and mortality (13, 24). HF patients present with increased sympathetic and decreased parasympathetic activity under baseline conditions (13, 74), and also increased sympathetic responses to exercise (104). Interestingly, this sympathetic overactivation may be a compensatory response to preserve CO in the face of reduced ventricular function (41). However, as the disease progresses, this persistent sympathetic activation exacerbates ventricular dysfunction, further increasing cardiovascular (24) and overall mortality risks (13, 70).
The muscle hypothesis contends that part of the functional incapacity and exercise intolerance in HF patients comes from insufficient blood flow/oxygen supply to active and respiratory muscles, which may be related to exaggerated sympathetic-mediated vasoconstriction in these areas (22, 123). Importantly, vasoconstriction in active skeletal muscle is driven by, and drives, a persistent engagement of the metaboreflex, further compromising muscle perfusion and thereby exacerbating the symptoms of fatigue and exertional intolerance (123). Clinical studies partially support the role of exaggerated metaboreflex-induced sympathetic activity (122, 123, 145). Piepoli et al. (122) reported increased BP responses during PEMI following dynamic handgrip exercise in stable HF patients, and this response was supported by an increase in a sympathetic-related index of HR variability. Similar results were found by Notarius et al. (103), who reported increased sympathetic nerve traffic and HR during PEMI in HF patients following either static or dynamic handgrip exercise. On the other hand, Sterns et al. (156) observed blunted muscle sympathetic nerve activity responses during PEMI following static handgrip in NYHA class II-IV HF patients. Differences in exercise protocol (dynamic vs. static handgrip, muscle group involved in the exercise), and in characteristics of the population (e.g., sex, disease etiology, severity of disease) might underline these reported differences in sympathetic activity (150). The association between metaboreflex-induced cardiovascular responses and the severity of HF was further explored by Negrão et al. (99), who reported a preserved muscle sympathetic response to PEMI after moderate static handgrip exercise in mild HF patients and diminished responses in severe HF patients.
Irrespective of augmented or blunted sympathetic responses to metaboreflex activation, most studies show a preserved BP increase to metaboreflex activation in HF (12, 27, 122, 132). However, as previously reported in animal studies, HF individuals have an impaired capacity to increase SV and CO during metaboreflex activation; thus the metaboreflex-mediated BP increase is largely dependent upon an increase in SVR (12, 27, 122, 132, 165). Accentuated peripheral vasoconstriction occurs in nonactive vascular beds (122) and in active muscle (3), with the latter reducing skeletal muscle perfusion during exercise, thereby increasing fatigue-related symptoms and stimulating further metaboreflex responses. Indeed, Piepoli et al. (122) reported an increase in non-exercising leg vascular resistance during PEMI following dynamic handgrip in HF patients. The muscle reflex dependence of this response was reinforced by Amann et al. (3), who observed powerful increases in exercising leg blood flow and conductance during exercise after blockade of group III and IV muscle afferent central projections with intrathecal fentanyl. While not yet investigated in humans, animal studies support the contention that metaboreflex-mediated vasoconstriction might also occur in the coronary vasculature (5, 110), potentially leading to coronary vasospasms, ventricular fibrillation, and myocardial infarct.
HF patients also suffer with exertional dyspnea, which might be related to changes in pulmonary vascular dynamics (83, 165), which is an important mediator of exercise intolerance in this population (31). Importantly, activation of the metaboreflex may promote pulmonary vasoconstriction (83, 165) and abnormal ventilatory responses during exercise in HF (122, 123, 126, 144, 145). Piepoli et al. (122) reported increased ventilatory responses during PEMI after leg cycling exercise in patients with HF. This increased ventilatory metaboreflex sensitivity is associated with disease severity as assessed by NYHA functional class, and with exercise intolerance measured by peak V̇o2 (126, 144). Therefore, an augmented activation of the metaboreflex in HF patients might produce dyspnea responses in this population.
Studies in other clinical populations have also showed abnormal cardiovascular effects produced by altered metaboreflex responses. Coronary artery disease patients without overt clinical signs and symptoms of HF also present with the inability to increase CO during metaboreflex activation (86). This may be related, in part, to paradoxical sympathetic coronary vasoconstriction in response to the engagement of the metaboreflex (110, 152). Indeed, increased epicardial vasoconstriction during exercise has been reported to produce impairment of left-ventricular performance and an increased risk of cardiovascular events in these patients (17). In humans with PAD, chronic limb ischemia sensitizes muscle afferents, and this has been shown to potentiate BP and sympathetic responses to exercise (96). This response seems to be partially mediated by increased SVR, as demonstrated by augmented renal, coronary, and muscle vasoconstriction during exercise in these patients (30, 72, 135). Importantly, this increased sympathetic activity during exercise in PAD promotes a further reduction in blood flow to exercising muscle, likely increasing exercise intolerance in this population (75). Interestingly, a recent study showed that the increased pressor response to exercise is attenuated after leg revascularization (92), which further supports the role of limb ischemia in promoting abnormal cardiovascular responses during exercise. Finally, type 2 diabetes mellitus patients also present with increased BP and sympathetic responses to exercise, and this has been attributed to increased muscle metaboreflex sensitivity (52). On the other hand, subjects with type 1 diabetes mellitus (131), and with Parkinson’s disease (138), have shown blunted hemodynamic and sympathetic responses to metaboreflex activation, which may be related to central and/or peripheral neural denervation/loss of sensitivity in these groups (43, 90, 160). It should be acknowledged that a blunted cardiovascular response to the engagement of the metaboreflex and other reflexes might help explain the autonomic dysreflexia and the high prevalence of postural hypotension and hemodynamic abnormalities in this group of patients (90, 161).
CARDIOVASCULAR RESPONSES TO BFR EXERCISE: CURRENT EVIDENCE
Methodological Considerations
Collectively, experimental data in animals and humans demonstrate negative cardiovascular outcomes due to excessive metaboreflex activation as the result of limiting blood flow during exercise. These negative cardiovascular outcomes are particularly noteworthy in subjects with chronic diseases such as HF, HTN, and PAD. It must be highlighted though, that these data were collected during in vivo experimental or clinical laboratory studies assessing the integrated role of the metaboreflex on cardiovascular regulation. Therefore, discretion should be taken when extrapolating these findings to the real-world BFRT setting. Recently, studies have begun to assess the hemodynamic responses during typical BFRT sessions in healthy and clinical populations. These studies have compared BP and other hemodynamic responses to LI-blood flow restriction(LI-BFR; 3–4 sets, 10–15 repetitions, 20–40% 1 RM), low-intensity free flow resistance training (LI; 3–4 sets, 10–15 repetitions, 20–40% 1 RM), and high-intensity free flow resistance training (HI; 3–4 sets, 8–10 repetitions, 60–80% 1 RM) (Table 1).
Table 1.
Review of the studies assessing cardiovascular responses to blood flow-restricted resistance exercise
| N, Sex | Exercise Protocol | BFR Protocol | CV Outcome (Method) | Main Results | |
|---|---|---|---|---|---|
| Healthy young subjects | |||||
| Poton and Polito (127) | 12 men | Unilateral knee extension; HI, 3 × 8 reps (80% 1RM) LI, 3 × 15 reps (20% 1RM) LI-BFRC, 3 × 15 reps (20% 1RM) |
OP: 100% AOP Cuff width: 18 cm |
SBP, DBP, CO, SV, HR, TPR (finger photoplethysmography) |
SBP: HI > LI-BFRC > LI DBP: HI > (LI-BFRC = LI) CO: HI > LI; HI = LI-BFRC; LI = LI-BFRC HR: HI > LI-BFRC> LI SV: HI = LI-BFRC = LI TPR: HI > LI; HI = LI-BFRC; LI = LI-BFRC |
| May et al. (88) | 14 men | 45° leg press; HI, 4 × 8 reps (80% 1RM) LI, 1 × 30+3 × 15 reps (20% 1RM) LI-BFRC, 1 × 30+3 × 15 reps (20% 1RM) |
OP: 80% AOP Cuff width: 10.5 cm |
SBP and DBP (oscillometric) CO (gas rebreathing technique) HR (HR monitor) SV (CO/HR) |
SBP: HI = LI-BFRC; LI = LI-BFR; HI > LI DBP: LI-BFRC > (HI = LI) CO: HI > (LI-BFRC = LI) HR: HI > LI-BFRC > LI SV: HI < LI; HI = LI-BFRC; LI = LI-BFR |
| Poton and Polito (128) | 17; 11 men 6 women |
45° leg press; HI, 3 × 8 reps (80% 1RM) LI, 3 × 15 reps (20% 1RM) LI-BFRC, 3 × 15 reps (20% 1RM) |
OP: 100% AOP Cuff width: 18 cm |
SBP, DBP, CO, SV, HR, TPR (finger photoplethysmography) |
During sets: SBP: HI > LI-BFRC > LI DBP: HI > LI-BFRC > LI CO: HI > (LI-BFRC = LI) HR: (HI = LI-BFRC) > LI SV: (HI = LI) > LI-BFRC TPR: HI > LI-BFRC > LI Interval between sets: SBP: LI-BFRC = LI = HI DBP: LI-BFRC = LI = HI CO: LI-BFRC = LI = HI SV: LI-BFRC = LI = HI HR: LI-BFRC = LI = HI TPR: LI-BFRC = LI = HI |
| Libardi et al. (76) | 12 men | 45° leg press; HI, 4 × max reps (80% 1RM) LI, 4 × max reps (30% 1RM) LI-BFRC, 4 × 15 reps (30% 1RM) |
OP: 50% AOP Cuff width: 17.5 cm |
SBP, DBP, CO, SV, TPR (finger photoplethysmography) HR (ECG) |
SBP: HI > LI > LI-BFRC DBP: HI > LI > LI-BFRC CO: LI > (HI = LI-BFRC) HR: LI > HI > LI-BFR SV: LI > (HI = LI-BFRC) TPR: LI > LI-BFR; LI = HI; HI = LI-BFRC |
| Brandner et al. (16) | 12 men | Unilateral elbow flexion; HI 4 × 6–8 reps (80% 1RM) LI 1 × 30+3 × 15 reps (20% 1RM) LI-BFRC 1 × 30+3 × 15 reps (20% 1RM) LI-BFRI 1 × 30+3 × 15 reps (20% 1RM) |
OP: LI-BFRC = 80% AOP OP: LI-BFRI = 130% AOP Cuff width: 10.5 cm |
SBP, DBP (auscultatory) CO (gas-rebreathing technique) HR (heart rate monitor) SV (CO/SV) TPR (MAP/CO) |
SBP: (HI = LI-BFRI) > LI-BFRC > LI DBP: (HI = LI-BFRI) > LI; LI-BFRC = all CO: HI > (LI-BFRI = LI-BFRC = LI) HR: (HI = LI-BFRI) > (LI-BFRC = LI) SV: HI = LI-BFRI = LI-BFRC = LITPR: LI-BFRI > (LI-BFRC = LI) > HI |
| Kilgas et al. (71) | 10 men | Rhythmic handgrip; LI, 1 × 30 reps (30% MVC) LI-BFRC, 1 × 30 reps (30% MVC) |
OP: 60, 80, 100, and 120% AOP Cuff width: 10 cm |
MAP, CO (finger photoplethysmography) HR (ECG) |
MAP: LI = LI-BFRC CO: LI = LI-BFRC HR: LI = LI-BFRC |
| Bazgir et al. (14) | 16 men | Unilateral eccentric leg extension; LI, 4 × 15 reps (30% MVC) LI-BFRC, 4 × 15 reps (30% MVC) |
OP: 90–100 mmHg Cuff width: 13 cm |
SBP, DBP (auscultatory) HR (heart rate monitor) |
SBP: LI-BFRC > LI DBP: LI-BFRC > LI HR: LI-BFRC = LI |
| Downs et al. (29) | 13; 5 men 8 women |
Leg press and heel raise; HI, 3 × max (80% 1RM) LI, 3 × max (20% 1RM) LI-BFRC1, 3 × max (20% 1RM) LI-BFRC2, 3 × max (20% 1RM) |
OP: C1 = 1.3 × resting DBP C2 = 1.3 × resting SBP Cuff width: 6 cm |
SBP, DBP (finger photoplethysmography) SV (echocardiography) HR (ECG) CO (HR × SV) |
SBP: LI-BFRC2 > (LI-BFRC1 = HI) > LI DBP: LI-BFRC2 > LI-BFRC1 > (HI = LI) CO: (HI = LI) > LI-BFRC1 > LI-BFRC2 HR: (HI = LI) > (LI-BFRC1 = LI-BFRC2) SV: (HI = LI = LI-BFRC1) > LI-BFRC2 |
| Ozaki et al. (114) | 14 men (7 per group) | Bench press; HI, 3 × 10 reps (75% 1RM) LI-BFRC, 3 × 15 reps (30% 1RM) |
OP: 160 mmHg Cuff width: 3 cm |
SBP, HR (oscillometric) | SBP: HI > LI-BFRC HR: HI > LI-BFRC |
| Takano et al. (159) | 11 men | Leg extension; LI 1 × 30+3 × max (20% 1RM) LI-BFRC 1 × 30+3 × max (20% 1RM) |
OP: 1.3 times resting SBP Cuff width: 3.3 cm |
SBP, DBP (finger photoplethysmography) SV (impedance cardiography) HR (ECG) CO = SV x HR TPR = MAP/CO |
SBP: LI-BFRC > LI DBP: LI-BFRC > LI CO: LI-BFRC = LI HR: LI-BFRC > LI SV: LI-BFRC < LI TPR: LI-BFRC = LI |
| Vieira et al. (167) | 15 men | Unilateral biceps curl; LI, 1 × 3 min (30% 1RM) LI-BFRC, 1 × 3 min (30% 1RM) |
OP: 120 mmHg Cuff width: NR |
SBP, DBP (oscillometric) HR (heart rate monitor) CBF, CVR (venous occlusion plethysmography) |
SBP: LI-BFRC > LI DBP: LI-BFRC > LI HR: LI-BFRC > LI CBF: LI-BFRC < LI CVR: LI-BFRC > LI |
| Figueroa and Vicil (36) | 23; 11 men 12 women |
Bilateral leg extension and flexion; LI, 3 × max (40% 1RM) LI-BFRI, 3 × max (40% 1RM) |
OP: 100 mmHg Cuff width: NR |
SBP, DBP (oscillometric) HR (pulse interval from a tonometer) |
SBP: LI-BFR = LI DBP: LI-BFR = LI HR: LI-BFR = LI |
| Staunton et al. (155) | 11 men | 45° leg press; LI, 1 × 30+3 × 15 reps (20% 1RM) LI-BFRC, 1 × 30+3 × 15 reps (20% 1RM) |
OP: 60% AOP Cuff width: 10.5 cm |
SBP, DBP (auscultatory) CO (gas rebreathing technique) HR (heart rate monitor) SV = CO/HR TPR = MAP/CO |
SBP: LI-BFRC > LI DBP: LI-BFRC > LI CO: LI-BFRC = LI HR: LI-BFRC > LI SV: LI-BFRC = LI TPR: LI-BFRC = LI |
| Kacin and Strazar (61) | 10 men | Unilateral knee extension LI, 1 × max (15% 1RM) LI-BFRTC, 1 × max (15% 1RM) |
OP: 230 mmHg Cuff width: 13 cm |
HR (ECG) SBP, DBP (finger photoplethysmography) |
SBP: LI-BFRC = LI DBP: LI-BFRC = LI HR: LI-BFRC = LI |
| Middle-aged/elderly subjects | |||||
| Sardeli et al. (142) | 21 NR | 45° Leg press; HI, 4 × max (80% 1RM) LI, 4 × max (30% 1RM) LI-BFRC, 1 × 30+3 × 15 reps (30% 1RM) |
OP: 50% AOP Cuff width: 17.5 cm |
SBP, DBP, HR (finger photoplethysmography) |
SBP: LI-BFRC > HI; LI-BFRC = LI; LI = HI DBP: LI-BFRC > HI; LI-BFRC = LI; LI = HI HR: LI-BFRC = LI = HI |
| Scott et al. (146) | 15 women | Leg press and leg extension; HI, 3 × 10 reps (70% 1RM) LI, 1 × 20+2 × 15 reps (20% 1RM) LI-BFRC, 1 × 20+2 × 15 reps (20% 1RM) |
OP: 50% AOP Cuff width: 10 cm |
SBP, DBP (auscultatory) CO, HR and SV (impedance cardiography) TPR = MAP/TPR |
In both exercises: SBP: LI-BFRC > (LI = HI) DBP: LI-BFRC > (LI = HI) CO: LI-BFRC = LI = HI HR: (LI-BFRC = HI) > LI SV: LI-BFRC = LI = HI TPR: LI-BFRC = LI = HI |
| Vieira et al. (167) | 12 men | Unilateral biceps curl; LI, 1 × 3 min (30% 1RM) LI-BFRC, 1 × 3 min (30% 1RM) |
OP: 120 mmHg Cuff width: NR |
SBP, DBP (oscillometric) HR (heart rate monitor) CBF, CVR (venous occlusion plethysmography) |
SBP: LI-BFRC > LI DBP: LI-BFRC > LI HR: LI-BFRC > LI CBF: LI-BFRC < LI CVR: LI-BFRC > LI |
| Staunton et al. (155) | 13 men | 45° leg press; LI, 1 × 30+3 × 15 reps (20% 1RM) LI-BFRC, 1 × 30+3 × 15 reps (20% 1RM) |
OP: 60% AOP Cuff width: 10.5 cm |
SBP, DBP (auscultatory) CO (gas rebreathing technique) HR (heart rate monitor) SV = CO/HR TPR = MAP/CO |
SBP: LI-BFRC > LI DBP: LI-BFRC > LI CO: LI-BFRC = LI HR: LI-BFRC > LI SV: LI-BFRC = LI TPR: LI-BFRC = LI |
| Subjects with chronic diseases | |||||
| Araújo et al. (6) | 14 women (7 per group); (HT) | Leg extension; HI, 3 × 15 (50% 1RM) LI-BFRC, 3 × 15 (30% 1RM) |
OP: 80% AOP Cuff width: 18 cm |
SBP, DBP (oscillometric) HR (heart rate monitor) |
SBP: LI-BFRC > HI DBP: LI-BFRC > HI HR: LI-BFRC = HI |
| Pinto et al. (124) | 18 women; (HT) |
Knee extension; HI, 3 × 10 reps (65% 1RM) LI-BFRC, 3 × 10 reps (20% 1RM) |
OP: 80% AOP Cuff width: 18 cm |
SBP, DBP, CO, SV, HR, TPR (finger photoplethysmography) |
During sets: SBP: LI-BFRC = HI DBP: LI-BFRC = HI CO: LI-BFRC = HI SV: LI-BFRC = HI HR: LI-BFRC = HI TPR: LI-BFRC = HI Interval between sets: SBP: LI-BFRC > HI DBP: LI-BFRC > HI CO: LI-BFRC < HI SV: LI-BFRC < HI HR: LI-BFRC = HI TPR: LI-BFRC > HI |
| Pinto and Polito (125) | 12 women; (HT) |
45° leg press; HI, 3 × 8 reps (65% 1RM) LI, 3 × 15 reps (20% 1RM) LI-BFRC, 3 × 15 reps (20% 1RM) |
100% AOP Cuff width: 18 cm |
SBP, DBP, CO, SV, HR, TPR (finger photoplethysmography) |
During sets: SBP: LI-BFRC > HI > LI DBP: LI-BFRC > (HI = LI) CO: (HI = LI) > LI-BFRC HR: LI-BFRC > (HI = LI) SV: LI-BFRC = HI = LI TPR: LI-BFRC > (HI = LI) Interval between sets: SBP: LI-BFRC > (HI = LI) DBP: LI-BFRC > (HI = LI) CO: (HI = LI) > LI-BFRC HR: LI-BFRC> HI; LI-BFR = LI; HI = LI SV: LI-BFRC = HI = LI TPR: LI-BFRC > (HI = LI) |
| Madarame et al. (85) | 9; 7 men 2 women (stable patients with CAD) |
Knee extension; LI, 1 × 30+3 × 15 reps (20% 1RM) LI-BFRC, 1 × 30+3 × 15 reps (20% 1RM) |
OP: 200 mmHg Cuff width = 5 cm |
HR (oxymeter) | HR: LI-BFRC > LI |
LI, low-intensity resistance exercise; HI, high-intensity resistance exercise; LI-BFRC, low-intensity resistance exercise with blood flow restriction continuously applied throughout exercise sets and interset pauses; LI-BFRI, low-intensity resistance exercise with blood flow restriction interrupted during interset pauses; HT, hypertensives; CAD, coronary artery disease; BFR, blood flow restriction; CV, cardiovascular; LOP, limb occlusion pressure; CON, control session; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; CO, cardiac output; SV, stroke volume; TPR, total peripheral resistance; OP, occlusion pressure; AOP, arterial occlusion pressure; CBF, calf blood flow; CVR, calf vascular resistance; NR, not reported; max, maximal repetitions. MAP was only presented when no information about SBP or DBP was provided.
As depicted in Table 1, most of these studies were performed with young, healthy subjects and used lower limb exercises (i.e., leg press or leg extension); however, there are also studies with middle-aged/elderly subjects with or without chronic diseases, and studies that used upper limb exercises (i.e., bench press or biceps curl). Aspects related to the blood flow restriction protocol, such as the cuff pressure and width, also present large variability between studies. Some studies employed absolute occlusion pressures (i.e., 100–200 mmHg), while others used a percentage of the AOP (i.e., 50–130% of AOP). Similarly, cuff widths ranged from narrow (3–5 cm) to wider cuffs (10–18 cm). Finally, different methods have been used to assess hemodynamic responses during exercise, which may also affect interpretation of the results, as discussed below.
Studies Comparing LI-BFR with LI Free Flow Exercise
The majority of the trials (14 of 19, 74%) comparing LI-BFR with LI (i.e., comparison matched by load) showed increased BP responses in the LI-BFR group (14, 16, 29, 85, 88, 125, 127, 128, 146, 155, 159, 167). These results were reproduced in young or middle-aged/elderly men (14, 16, 88, 127, 155, 159, 167) and women (125, 146), and also in populations with chronic diseases (85, 125). Moreover, these trials employed a wide variety of exercise (e.g., lower or upper limbs) and blood flow restriction protocols (e.g., wide or narrow cuffs; low or high occlusion pressure levels). On the other hand, there was no difference in BP responses between LI-BFR and LI in four trials (36, 45, 71, 142), and only one trial showed higher responses in LI (76). These studies employed either exercise protocols of repetitions until fatigue (36, 45, 76, 142), performed exercise with a small muscle mass (i.e., handgrip) (71), or an intermittent blood flow restriction protocol (i.e., blood flow restriction interrupted during the interval between sets) (36). When repetitions until fatigue are used, the number of repetitions and, therefore, the volume are expected to be higher in LI due to less fatigue in this type of exercise, which might help to promote a larger mechanical and/or metabolic stimulus in LI. In addition, the use of a small muscle mass and intermittent BFR might limit the metabolic impact of BFR, which may generate attenuated hemodynamic responses.
Studies Comparing LI-BFR with HI Free Flow Exercise
The data are more equivocal when LI-BFR and HI are compared, with 6 of 12 (50%) trials showing greater BP responses in LI-BFR (6, 29, 88, 125, 142, 146), 5 (42%) showing greater BP responses in HI (16, 76, 114, 127, 128), and 1 (8%) showing no differences (124). Differences in exercise and/or blood flow restriction protocols might help explain such differences. For instance, greater responses in LI-BFR have been found when an elevated number of repetitions were employed (from 15 to fatigue) (29, 88, 142), and when the occlusion pressures were higher than resting systolic pressure (29). Regarding this latter aspect, previous studies showed increased BP responses when higher AOPs (e.g., 80 vs. 40%) are employed (59). These conditions might increase the production and/or reduce the rate of removal of muscle metabolites, which might help to explain increased hemodynamic responses in LI-BFR.
An important BFRT protocol-related parameter that might affect the comparison between LI-BFR and HI is the cuff width. For absolute occlusion pressures (e.g., 100 mmHg), wider cuffs tend to promote greater arterial occlusion compared with thinner ones. For instance, Rossow et al. (136) found greater BP responses using 13.5-cm compared with 5.0-cm-wide cuffs. In the present review, the only study comparing LI-BFR with HI that used absolute occlusion pressures observed reduced BP responses to LI-BFR compared with HI using a 3-cm cuff, which support the notion that thinner cuffs might attenuate BP responses during LI-BFR. On the other hand, when the occlusion pressure is made relative to AOP, the cuff width may not affect significantly the BP responses during blood flow restriction (95).
The comparison between LI-BRF and HI is also influenced by the population studied. Among healthy young subjects, most of the studies (5 of 7, 71%) either showed decreased BP responses to LI-BFR or did not show differences between trials (16, 76, 114, 127, 128). On the other hand, four of five (i.e., 80%) of the studies with hypertensive subjects or middle-aged/elderly subjects showed greater BP responses in LI-BFR compared with HI (6, 125, 142, 146). These findings corroborate the concern raised by the current review, that patients with chronic diseases (e.g., HTN) or with increased cardiovascular risks (e.g., middle-aged/elderly) might present with exaggerated BP responses to blood flow restriction protocols, which might be ascribed to an increased metaboreflex sensitivity in these groups.
Additional Aspects To Be Considered
There is only one pilot study that has assessed cardiovascular-related responses to BFRT in patients with heart disease. Madarame et al. (85) compared the responses of HR, plasma noradrenaline, and markers of hemostasis and inflammation between LI-BFR and LI in coronary artery disease patients. Interestingly, although there were no differences in inflammatory and hemostatic responses between groups, HR and noradrenaline concentration were greater in LI-BFR compared with LI. These results support the animal and human studies showing greater sympathetic responses to metaboreflex activation in patients with heart disease (68, 103). Moreover, this study provides preliminary data that the increased HR response to the BFRT in populations with cardiovascular disease might involve increased overall adrenergic activation.
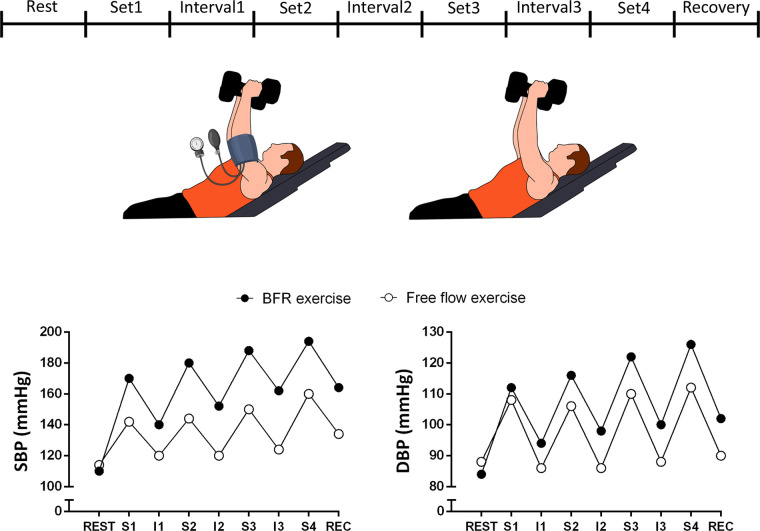
The information reviewed thus far is restricted to the responses occurring during exercise. However, three studies also compared the hemodynamic responses to the above-mentioned exercise protocols during intervals between sets, and in two of them (124, 125) BP was increased in LI-BFR compared with LI, while in the other (128) there was no difference between groups. The absence of a proper reduction in BP during recovery might help to explain increased BP responses in the subsequent sets in BFRT. It is worth mentioning that in these three studies, blood flow restriction was maintained during intervals, which might help to explain the increased BP responses during intervals and subsequent sets. Sustained increases in BP throughout the BFRT exercise protocol result in increased cardiovascular overload, which is a matter of concern in cardiovascular disease populations.
Several studies have investigated the hemodynamic determinants of the BP responses to BFRT comparatively to free flow exercise, but the overall data are inconclusive. Most of the studies showed increased CO during HI compared with LI and LI-BFR (16, 29, 88, 125, 128). Decreased CO responses in LI-BFR compared with HI might be related to a reduction in the SV observed with LI-BFR (29, 128), while the reduced response in LI seems to be related to a decreased HR response (88, 128, 146). Paradoxically, the analysis of the reviewed studies does not support the contention that the reduced CO in LI-BFR is compensated by greater increases in peripheral vascular resistance, providing similar or higher BP responses in this mode of training. However, it is worth considering that the aforementioned studies used different methods to estimate/calculate CO and peripheral vascular resistance, some less reliable than the others, which might have affected the consistency of the results. Among the trials using more robust methods to assess vascular resistance during exercise, two trials using venous plethysmography and one trial with a gas-rebreathing technique have, respectively, showed increased forearm/calf vascular resistance and SVR in LI-BFR compared with LI and HI (16, 167). These results suggest that an excessive engagement of the metaboreflex might produce increases in α-adrenergic-mediated peripheral vasoconstriction. However, given the apparent inconsistency of this finding (155), more studies with robust measurements of local vascular resistance and SVR are necessary to confirm this hypothesis.
Summary of the Evidence
The results of the studies assessing cardiovascular responses to BFRT support the concerns raised by the review of animal and human studies assessing the neural and hemodynamic responses to metaboreflex activation. Almost unanimously, LI-BFR seems to evoke greater BP responses comparatively to LI when the regimes are performed at the same intensity and matched by the total work, which is likely caused by greater metaboreflex activation during LI-BFR. Moreover, when HI is used as a reference, the LI-BFR group seems to produce greater BP/HR responses specifically in individuals with increased cardiovascular risks (i.e., middle-aged and elderly subjects, hypertensive and coronary artery disease patients), which is likely related to a greater metaboreflex sensitivity in these groups.
Increased BP responses to BFRT occur both during exercise sets and also in interset intervals (Fig. 3). Collectively, these findings do not support the general assumption that BFRT confers similar cardiovascular risks compared with unrestricted resistance exercise, which draws into question whether BFRT is a feasible alternative to high-load resistance training exercise in clinical populations.
Fig. 3.
Hypothetical time course of blood pressure responses occurring during resistance exercise with and without blood flow restriction (BFR). Absence of interruption of BFR during interset intervals may promote progressive increase in systolic (SBP) and diastolic (DBP) blood pressure throughout exercise in BFR exercise. S1–S4, sets 1 to 4; I1–I4, interset intervals 1 to 4.
POTENTIAL CLINICAL RESPONSES THAT MAY ARISE FROM EXCESSIVE METABOREFLEX ENGAGEMENT DURING BFRT
Considering the reviewed neural and cardiovascular responses to metaboreflex activation, and the current data on the cardiovascular responses to BFRT exercise, measured caution is warranted when employing BFRT. There are potential cardiovascular risks that must be considered before employing a BFRT protocol, particularly in a clinical setting with certain disease populations. As previously reported, hypertensive individuals seem to present with exacerbated BP responses during BFRT sessions, which is partly related to increased metaboreflex sensitivity (20). It is important to underscore that increased metaboreflex sensitivity also occurs in hypertensive individuals with good control of resting BP values (20); thus even uncomplicated hypertensive individuals might exhibit increased BP responses during BFRT. This increased BP response to BFRT might increase the risks of vascular events (e.g., hemorrhagic stroke, artery dissection, or myocardial ischemia) during exercise. Indeed, previous reports have identified the occurrence of dissection of aortic and craniocervical arteries and subarachnoid hemorrhage in response to strenuous activities, which is probably triggered by a marked rise in BP occurring during such tasks (1, 42, 51). Microvascular events might also be triggered by acute elevations in BP (21), and there is a report of retinal hemorrhage in hypertensive and diabetic middle-aged men after a single session of BFRT (115). Finally, the ACC/AHA 2002 Guideline Update for Exercise Testing warns that “severe systemic hypertension may cause exercise-induced ST depression in the absence of atherosclerosis” (44). Although these events are rare in nature and may also occur in nonrestricted exercise, the evidence presented in this review supports the contention that this frequency might increase during BFRT in subjects with increased risks for vascular events.
For patients with increased risk for ischemic events, the potential coronary vasoconstriction that could occur in response to metaboreflex engagement (5, 152) might create a mismatch between myocardial blood demand and supply, favoring episodes of ischemia and arrhythmia and increasing the risks of ischemic events and sudden death (97). Although coronary blood flow responses have not been directly tested by studies employing BFRT, the available evidence gives support to the proposed hypothesis. For instance, PAD patients present with reduced coronary hyperemia during exercise (135); however, this response is partially reverted 1 mo after leg revascularization (92), suggesting that episodes of leg ischemia leading to increased metaboreflex activation during exercise is the primary mechanism behind the marked coronary vasoconstriction during exercise in this disease. Studies showing increased forearm vascular resistance during BFRT (167) reinforce this hypothesis, as previous studies have shown associations between peripheral and coronary vasoreactivity (4). The sympathetic origin of such a response is supported by experimental studies showing increased α-adrenergic coronary vasoconstriction during exercise with blood flow restriction (152), and also by evidence in humans showing increased plasma noradrenaline levels during BFRT (57, 85).
The increase in limb vascular resistance during BFRT might be a matter of concern in patients with exercise intolerance due to the impairment in peripheral blood flow. Indeed, patients with HF and PAD present with reduced leg vascular conductance, which is partly mediated by enhanced metaboreflex-mediated adrenergic activation (3, 75, 122). Additional engagement of the metaboreflex during BFRT might exacerbate this sympathetic response, further reducing limb blood flow and increasing exercise intolerance. Although it could be argued that this response will terminate upon cessation of BFRT, experimental data show that femoral artery ligation (a model of chronic limb ischemia) increases the expression of chemically sensitive receptors (i.e., TRPV1, P2X3, and ASIC3) in afferent neurons (78, 79, 170, 171), which might evoke a sustained increase in metaboreflex responsivity. On the other hand, a recent study has shown that BFRT in healthy and young male humans did not exacerbate metaboreflex responsitivity (26), which discourages such a hypothesis. Future studies should investigate the effects of repeated sessions of BFRT in metaboreflex responses and exercise tolerance in chronic disease populations.
CONCLUSION AND FUTURE STEPS
BFRT is a mode of resistance exercise training that has recently gained popularity due to its proved effectiveness in promoting increases in muscle strength, mass, and functionality. The capacity to generate clinically relevant neuromuscular gains with relatively reduced workloads has made BFRT a highly attractive mode of exercise to populations with functional incapacity and/or with certain chronic diseases. Indeed, recent guidelines have suggested that this mode of training might be a viable alternative to HI in patients with chronic diseases and/or during postsurgery rehabilitation. Despite the desirable effects promoted by this type of training on skeletal muscle function, the present review synthesizes evidence supporting the hypothesis that BFRT may evoke increased BP and other abnormal cardiovascular responses secondary to the augmented and sustained activation of the muscle metaboreflex. Based on the reviewed studies that provided peak BP values during the exercise protocols (14, 29, 36, 61, 88, 124, 155, 159), it is estimated that BFRT exercise might add 5–10 mmHg to the usual BP response during resistance exercise. These increased BP responses might trigger major cardiovascular events in populations with increased cardiovascular risks. These potential adverse outcomes do not support the general claims about safety of BFRT for populations with chronic diseases or under cardiac rehabilitation.
It is important to underscore that the present review does not negate the hypothesis that in parallel to potentially increasing acute cardiovascular risks, BFRT might also promote subacute (i.e., reduction of BP hours after exercise) and chronic (i.e., reduction of BP weeks/months after exercise) benefits to the cardiovascular system (6, 27, 100, 158). This acute-chronic paradox has been extensively discussed with other types of exercise (e.g., HI interval training (47)), and this discussion has only been recently initiated for the BFRT (154). More studies assessing the cardiovascular responses during BFRT in populations with increased cardiovascular risks are necessary to better elucidate the impact of this method of exercise training on the cardiovascular system. For instance, all BFRT studies with subjects with chronic diseases were conducted in women, whereas the studies in young subjects mainly involved men. Sex-based differences in cardiovascular responses have already been reported in metaboreflex studies (141), and this should be further investigated in BFRT studies. In addition, the effects of BFRT on intra- and extracranial circulation, coronary blood flow, and muscle sympathetic nerve activity remain to be characterized.
It is also likely that some aspects of BFRT prescription (e.g., using continuous vs. intermittent blood flow restriction protocols, types of exercise) might improve the benefits and reduce the risks of this mode of exercise. For instance, reduced BP responses during BFRT have been reported when lower AOPs (e.g., 40 vs. 80%) are employed (168). It is worth considering that, besides promoting lower circulatory arrest, the use of lower AOPs might also generate lower pain/discomfort levels (25), which per se might alleviate BP responses during BFRT (162). The metaboreflex literature also indicates lower cardiovascular responses when exercising with small vs. larger muscle masses (37, 39), and this may also occur in BFRT. Further investigation on the effects of different combinations of BFRT variables (e.g., cuff pressure and width, occlusion protocol, exercise intensity, and number of repetitions) on hemodynamic responses are necessary to better characterize the isolated effects of each of these variables on cardiovascular function during BFRT.
Even with further clarification, it should be emphasized that some training recommendations may not be practical in the real world. For instance, a recent review has recommended that cuff pressure should be established according to cuff width and based on previous measurements of AOP (i.e., from 40 to 80% of AOP) (118). However, despite our agreement with this recommendation, the use of tourniquets instead of BP cuffs, the occasional unavailability of Doppler ultrasound to personalize AOP, and absence of trained professionals may hamper this standardization. For instance, Patterson et al. (117) conducted a survey among BFRT practitioners and verified that only 11.5% of them based the cuff pressure according to the AOP, with the vast majority using previously defined cuff pressures. It should also be highlighted the challenges associated with accurately assessing BP responses during resistance exercise as the auscultatory method underestimate the peak BP during resistance exercise by 30–35% (168). More accurate methods require the use of expensive noninvasive devices (45) or arterial catheterization (50, 84, 168), making it impractical in the real world.
Finally, although some recent clinical trials with patients with heart (62) and kidney disease (10) or elderly subjects (25, 148, 162, 166, 172, 173) have not shown adverse cardiovascular effects during BFRT, long-term and powered prospective clinical trials in cardiovascular disease patients, directly targeting cardiovascular outcomes and including a detailed report of adverse effects, must also be conducted to identify potentially overlooked cardiovascular side effects associated with this type of exercise training. The safety profile of BFRT in a cardiovascular-related clinical setting can only come from a comprehensive investigation of the cardiovascular responses to BFRT in different disease populations (e.g., patients in cardiac rehabilitation, coronary artery disease patients, HF, HTN, PAD, inflammatory diseases, etc.). Inasmuch as these data are virtually nonexistent, it may be considered clinical malpractice to employ BFRT in a cardiovascular-related clinical setting without performing the appropriate risk assessment.
GRANTS
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo Grant FAPESP 2016/23319-0, Conselho Nacional de Desenvolvimento Científico e Tecnológico Grant CNPq 406196/2018-4, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Grant CAPES-PROEX, Finance Code 001.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.C.-O. and T.P. conceived and designed research; M.C.-O., K.M., and T.P. analyzed data; T.P. prepared figures; M.C.-O., K.M., M.D.S., D.S.O., H.R., and T.P. drafted manuscript; M.C.-O., K.M., M.D.S., D.S.O., H.R., and T.P. edited and revised manuscript; M.C.-O., K.M., M.D.S., D.S.O., H.R., and T.P. approved final version of manuscript.
REFERENCES
- 1.Ahmadi H, Shirani S, Yazdanifard P. Aortic dissection type I in a weightlifter with hypertension: a case report. Cases J 1: 99, 2008. doi: 10.1186/1757-1626-1-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MAH, Jonathan Groot H, Walter Wray D, Stehlik J, Richardson RS. Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol 174: 368–375, 2014. doi: 10.1016/j.ijcard.2014.04.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 5.Ansorge EJ, Augustyniak RA, Perinot ML, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, O’Leary DS. Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. Am J Physiol Heart Circ Physiol 288: H1381–H1388, 2005. doi: 10.1152/ajpheart.00985.2004. [DOI] [PubMed] [Google Scholar]
- 6.Araújo JP, Silva ED, Silva JCG, Souza TSP, Lima EO, Guerra I, Sousa MSC. The acute effect of resistance exercise with blood flow restriction with hemodynamic variables on hypertensive subjects. J Hum Kinet 43: 79–85, 2014. doi: 10.2478/hukin-2014-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Augustyniak RA, Ansorge EJ, Kim JK, Sala-Mercado JA, Hammond RL, Rossi NF, O’Leary DS. Cardiovascular responses to exercise and muscle metaboreflex activation during the recovery from pacing-induced heart failure. J Appl Physiol (1985) 101: 14–22, 2006. doi: 10.1152/japplphysiol.00072.2006. [DOI] [PubMed] [Google Scholar]
- 8.Augustyniak RA, Ansorge EJ, O’Leary DS. Muscle metaboreflex control of cardiac output and peripheral vasoconstriction exhibit different latencies. Am J Physiol Heart Circ Physiol 278: H530–H537, 2000. doi: 10.1152/ajpheart.2000.278.2.H530. [DOI] [PubMed] [Google Scholar]
- 9.Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O’Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001. doi: 10.1152/ajpheart.2001.280.4.H1645. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa JB, Maia TO, Alves PS, Bezerra SD, Moura EC, Medeiros AIC, Fuzari HK, Rocha LG, Marinho PE. Does blood flow restriction training increase the diameter of forearm vessels in chronic kidney disease patients? A randomized clinical trial. J Vasc Access 19: 626–633, 2018. doi: 10.1177/1129729818768179. [DOI] [PubMed] [Google Scholar]
- 11.Barbosa TC, Vianna LC, Fernandes IA, Prodel E, Rocha HNM, Garcia VP, Rocha NG, Secher NH, Nobrega AC. Intrathecal fentanyl abolishes the exaggerated blood pressure response to cycling in hypertensive men. J Physiol 594: 715–725, 2016. doi: 10.1113/JP271335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett-O’Keefe Z, Lee JF, Berbert A, Witman MA, Nativi-Nicolau J, Stehlik J, Richardson RS, Wray DW. Metaboreceptor activation in heart failure with reduced ejection fraction: linking cardiac and peripheral vascular haemodynamics. Exp Physiol 103: 807–818, 2018. doi: 10.1113/EP086948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrão CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135: 302–307, 2009. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 14.Bazgir B, Rezazadeh Valojerdi M, Rajabi H, Fathi R, Ojaghi SM, Emami Meybodi MK, Neto GR, Rahimi M, Asgari A. Acute cardiovascular and hemodynamic responses to low intensity eccentric resistance exercise with blood flow restriction. Asian J Sports Med 7: e38458, 2016. doi: 10.5812/asjsm.38458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boushel R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol (Oxf) 199: 367–383, 2010. doi: 10.1111/j.1748-1716.2010.02133.x. [DOI] [PubMed] [Google Scholar]
- 16.Brandner CR, Kidgell DJ, Warmington SA. Unilateral bicep curl hemodynamics: low-pressure continuous vs high-pressure intermittent blood flow restriction. Scand J Med Sci Sports 25: 770–777, 2015. doi: 10.1111/sms.12297. [DOI] [PubMed] [Google Scholar]
- 17.Brown BG, Lee AB, Bolson EL, Dodge HT. Reflex constriction of significant coronary stenosis as a mechanism contributing to ischemic left ventricular dysfunction during isometric exercise. Circulation 70: 18–24, 1984. doi: 10.1161/01.CIR.70.1.18. [DOI] [PubMed] [Google Scholar]
- 18.Carter R 3rd, Watenpaugh DE, Wasmund WL, Wasmund SL, Smith ML. Muscle pump and central command during recovery from exercise in humans. J Appl Physiol (1985) 87: 1463–1469, 1999. doi: 10.1152/jappl.1999.87.4.1463. [DOI] [PubMed] [Google Scholar]
- 19.Centner C, Wiegel P, Gollhofer A, König D. Effects of blood flow restriction training on muscular strength and hypertrophy in older individuals: a systematic review and meta-analysis. Sports Med 49: 95–108, 2019. [Erratum in: Sports Med 49: 109–111, 2019.] doi: 10.1007/s40279-018-0994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chant B, Bakali M, Hinton T, Burchell AE, Nightingale AK, Paton JF, Hart EC. Antihypertensive treatment fails to control blood pressure during exercise. Hypertension 72: 102–109, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11076. [DOI] [PubMed] [Google Scholar]
- 21.Christensen CK. Abnormal albuminuria and blood pressure rise in incipient diabetic nephropathy induced by exercise. Kidney Int 25: 819–823, 1984. doi: 10.1038/ki.1984.95. [DOI] [PubMed] [Google Scholar]
- 22.Coats AJ. The “muscle hypothesis” of chronic heart failure. J Mol Cell Cardiol 28: 2255–2262, 1996. doi: 10.1006/jmcc.1996.0218. [DOI] [PubMed] [Google Scholar]
- 23.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341: 1351–1357, 1999. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 24.Colucci WS. The effects of norepinephrine on myocardial biology: implications for the therapy of heart failure. Clin Cardiol 21, Suppl 1: 20–24, 1998. doi: 10.1002/clc.4960211305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook SB, LaRoche DP, Villa MR, Barile H, Manini TM. Blood flow restricted resistance training in older adults at risk of mobility limitations. Exp Gerontol 99: 138–145, 2017. doi: 10.1016/j.exger.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crisafulli A, de Farias RR, Farinatti P, Lopes KG, Milia R, Sainas G, Pinna V, Palazzolo G, Doneddu A, Magnani S, Mulliri G, Roberto S, Oliveira RB. Blood flow restriction training reduces blood pressure during exercise without affecting metaboreflex activity. Front Physiol 9: 1736, 2018. doi: 10.3389/fphys.2018.01736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- 28.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299: H1318–H1327, 2010. doi: 10.1152/ajpheart.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Downs ME, Hackney KJ, Martin D, Caine TL, Cunningham D, O’Connor DP, Ploutz-Snyder LL. Acute vascular and cardiovascular responses to blood flow-restricted exercise. Med Sci Sports Exerc 46: 1489–1497, 2014. doi: 10.1249/MSS.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 30.Drew RC, Muller MD, Blaha CA, Mast JL, Heffernan MJ, Estep LE, Cui J, Reed AB, Sinoway LI. Renal vasoconstriction is augmented during exercise in patients with peripheral arterial disease. Physiol Rep 1: e00154, 2013. doi: 10.1002/phy2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubé BP, Agostoni P, Laveneziana P. Exertional dyspnoea in chronic heart failure: the role of the lung and respiratory mechanical factors. Eur Respir Rev 25: 317–332, 2016. doi: 10.1183/16000617.0048-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eiken O, Bjurstedt H. Dynamic exercise in man as influenced by experimental restriction of blood flow in the working muscles. Acta Physiol Scand 131: 339–345, 1987. doi: 10.1111/j.1748-1716.1987.tb08248.x. [DOI] [PubMed] [Google Scholar]
- 33.Fadel PJ, Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol 97: 39–50, 2012. doi: 10.1113/expphysiol.2011.057554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson DW, Berg WJ, Sanders JS, Kempf JS. Clinical and hemodynamic correlates of sympathetic nerve activity in normal humans and patients with heart failure: evidence from direct microneurographic recordings. J Am Coll Cardiol 16: 1125–1134, 1990. doi: 10.1016/0735-1097(90)90544-Y. [DOI] [PubMed] [Google Scholar]
- 35.Ferraz RB, Gualano B, Rodrigues R, Kurimori CO, Fuller R, Lima FR, DE Sá-Pinto AL, Roschel H. Benefits of resistance training with blood flow restriction in knee osteoarthritis. Med Sci Sports Exerc 50: 897–905, 2018. doi: 10.1249/MSS.0000000000001530. [DOI] [PubMed] [Google Scholar]
- 36.Figueroa A, Vicil F. Post-exercise aortic hemodynamic responses to low-intensity resistance exercise with and without vascular occlusion. Scand J Med Sci Sports 21: 431–436, 2011. doi: 10.1111/j.1600-0838.2009.01061.x. [DOI] [PubMed] [Google Scholar]
- 37.Fisher JP, Adlan AM, Shantsila A, Secher JF, Sørensen H, Secher NH. Muscle metaboreflex and autonomic regulation of heart rate in humans. J Physiol 591: 3777–3788, 2013. doi: 10.1113/jphysiol.2013.254722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher JP, Seifert T, Hartwich D, Young CN, Secher NH, Fadel PJ. Autonomic control of heart rate by metabolically sensitive skeletal muscle afferents in humans. J Physiol 588: 1117–1127, 2010. doi: 10.1113/jphysiol.2009.185470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Compr Physiol 5: 475–512, 2015. doi: 10.1002/cphy.c140022. [DOI] [PubMed] [Google Scholar]
- 40.Fisher JP, Young CN, Fadel PJ. Effect of muscle metaboreflex activation on carotid-cardiac baroreflex function in humans. Am J Physiol Heart Circ Physiol 294: H2296–H2304, 2008. doi: 10.1152/ajpheart.91497.2007. [DOI] [PubMed] [Google Scholar]
- 41.Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54: 375–385, 2009. doi: 10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 42.Fragoso YD, Adoni T, do Amaral LL, Braga FT, Brooks JB, Campos CS, Comini-Frota ER, Ferreira NP, Giacon LM, Gomes S, Goncalves MV, Magalhaes PS, Matta AP, de Oliveira FT, de Oliveira JF, Pierucettti MA, Pereira SL, Pontes ME, Siquineli F. Cerebrum-cervical arterial dissection in adults during sports and recreation. Arq Neuropsiquiatr 74: 275–279, 2016. doi: 10.1590/0004-282X20150150. [DOI] [PubMed] [Google Scholar]
- 43.Gerson MC, Caldwell JH, Ananthasubramaniam K, Clements IP, Henzlova MJ, Amanullah A, Jacobson AF. Influence of diabetes mellitus on prognostic utility of imaging of myocardial sympathetic innervation in heart failure patients. Circ Cardiovasc Imaging 4: 87–93, 2011. doi: 10.1161/CIRCIMAGING.110.954784. [DOI] [PubMed] [Google Scholar]
- 44.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, Winters WL Jr, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC Jr; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) . ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 106: 1883–1892, 2002. doi: 10.1161/01.CIR.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 45.Gomides RS, Dias RM, Souza DR, Costa LA, Ortega KC, Mion D Jr, Tinucci T, de Moraes Forjaz CL. Finger blood pressure during leg resistance exercise. Int J Sports Med 31: 590–595, 2010. doi: 10.1055/s-0030-1252054. [DOI] [PubMed] [Google Scholar]
- 46.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226: 173–190, 1972. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halle M. Letter by Halle regarding article, “Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients”. Circulation 127: e637, 2013. doi: 10.1161/CIRCULATIONAHA.112.150441. [DOI] [PubMed] [Google Scholar]
- 48.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O’Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000. doi: 10.1152/ajpheart.2000.278.3.H818. [DOI] [PubMed] [Google Scholar]
- 49.Hartwich D, Dear WE, Waterfall JL, Fisher JP. Effect of muscle metaboreflex activation on spontaneous cardiac baroreflex sensitivity during exercise in humans. J Physiol 589: 6157–6171, 2011. doi: 10.1113/jphysiol.2011.219964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haslam DR, McCartney N, McKelvie RS, MacDougall JD. Direct measurements of arterial blood pressure during formal weightlifting in cardiac patients. J Cardiopulm Rehabil Prev 8: 213–225, 1988. doi: 10.1097/00008483-198806000-00002. [DOI] [Google Scholar]
- 51.Haykowsky MJ, Findlay JM, Ignaszewski AP. Aneurysmal subarachnoid hemorrhage associated with weight training: three case reports. Clin J Sport Med 6: 52–55, 1996. doi: 10.1097/00042752-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP, Fadel PJ. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. Am J Physiol Heart Circ Physiol 310: H300–H309, 2016. doi: 10.1152/ajpheart.00636.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hughes L, Paton B, Rosenblatt B, Gissane C, Patterson SD. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med 51: 1003–1011, 2017. doi: 10.1136/bjsports-2016-097071. [DOI] [PubMed] [Google Scholar]
- 54.Ichinose M, Nishiyasu T. Muscle metaboreflex modulates the arterial baroreflex dynamic effects on peripheral vascular conductance in humans. Am J Physiol Heart Circ Physiol 288: H1532–H1538, 2005. doi: 10.1152/ajpheart.00673.2004. [DOI] [PubMed] [Google Scholar]
- 55.Ichinose M, Saito M, Wada H, Kitano A, Kondo N, Nishiyasu T. Modulation of arterial baroreflex dynamic response during muscle metaboreflex activation in humans. J Physiol 544: 939–948, 2002. doi: 10.1113/jphysiol.2002.024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iellamo F, Pizzinelli P, Massaro M, Raimondi G, Peruzzi G, Legramante JM. Muscle metaboreflex contribution to sinus node regulation during static exercise: insights from spectral analysis of heart rate variability. Circulation 100: 27–32, 1999. doi: 10.1161/01.CIR.100.1.27. [DOI] [PubMed] [Google Scholar]
- 57.Iida H, Kurano M, Takano H, Kubota N, Morita T, Meguro K, Sato Y, Abe T, Yamazaki Y, Uno K, Takenaka K, Hirose K, Nakajima T. Hemodynamic and neurohumoral responses to the restriction of femoral blood flow by KAATSU in healthy subjects. Eur J Appl Physiol 100: 275–285, 2007. doi: 10.1007/s00421-007-0430-y. [DOI] [PubMed] [Google Scholar]
- 58.Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M, Kamada T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 24: 1529–1535, 1994. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 59.Jessee MB, Dankel SJ, Buckner SL, Mouser JG, Mattocks KT, Loenneke JP. The cardiovascular and perceptual response to very low load blood flow restricted exercise. Int J Sports Med 38: 597–603, 2017. doi: 10.1055/s-0043-109555. [DOI] [PubMed] [Google Scholar]
- 60.Joyner MJ, Wieling W. Increased muscle perfusion reduces muscle sympathetic nerve activity during handgripping. J Appl Physiol (1985) 75: 2450–2455, 1993. doi: 10.1152/jappl.1993.75.6.2450. [DOI] [PubMed] [Google Scholar]
- 61.Kacin A, Strazar K. Frequent low-load ischemic resistance exercise to failure enhances muscle oxygen delivery and endurance capacity. Scand J Med Sci Sports 21: e231–e241, 2011. doi: 10.1111/j.1600-0838.2010.01260.x. [DOI] [PubMed] [Google Scholar]
- 62.Kambič T, Novaković M, Tomažin K, Strojnik V, Jug B. Blood flow restriction resistance exercise improves muscle strength and hemodynamics, but not vascular function in coronary artery disease patients: a pilot randomized controlled trial. Front Physiol 10: 656, 2019. doi: 10.3389/fphys.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaufman MP. The exercise pressor reflex in animals. Exp Physiol 97: 51–58, 2012. doi: 10.1113/expphysiol.2011.057539. [DOI] [PubMed] [Google Scholar]
- 64.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 65.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- 66.Kaur J, Alvarez A, Hanna HW, Krishnan AC, Senador D, Machado TM, Altamimi YH, Lovelace AT, Dombrowski MD, Spranger MD, O’Leary DS. Interaction between the muscle metaboreflex and the arterial baroreflex in control of arterial pressure and skeletal muscle blood flow. Am J Physiol Heart Circ Physiol 311: H1268–H1276, 2016. doi: 10.1152/ajpheart.00501.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaur J, Machado TM, Alvarez A, Krishnan AC, Hanna HW, Altamimi YH, Senador D, Spranger MD, O’Leary DS. Muscle metaboreflex activation during dynamic exercise vasoconstricts ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 309: H2145–H2151, 2015. doi: 10.1152/ajpheart.00679.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaur J, Senador D, Krishnan AC, Hanna HW, Alvarez A, Machado TM, O’Leary DS. Muscle metaboreflex-induced vasoconstriction in the ischemic active muscle is exaggerated in heart failure. Am J Physiol Heart Circ Physiol 314: H11–H18, 2018. doi: 10.1152/ajpheart.00375.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaur J, Spranger MD, Hammond RL, Krishnan AC, Alvarez A, Augustyniak RA, O’Leary DS. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in β2-mediated vasodilation. Am J Physiol Heart Circ Physiol 308: H524–H529, 2015. doi: 10.1152/ajpheart.00648.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol 26: 1257–1263, 1995. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 71.Kilgas MA, McDaniel J, Stavres J, Pollock BS, Singer TJ, Elmer SJ. Limb blood flow and tissue perfusion during exercise with blood flow restriction. Eur J Appl Physiol 119: 377–387, 2019. doi: 10.1007/s00421-018-4029-2. [DOI] [PubMed] [Google Scholar]
- 72.Kruse NT, Ueda K, Hughes WE, Casey DP. Eight weeks of nitrate supplementation improves blood flow and reduces the exaggerated pressor response during forearm exercise in peripheral artery disease. Am J Physiol Heart Circ Physiol 315: H101–H108, 2018. doi: 10.1152/ajpheart.00015.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leal AK, Williams MA, Garry MG, Mitchell JH, Smith SA. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am J Physiol Heart Circ Physiol 295: H1429–H1438, 2008. doi: 10.1152/ajpheart.01365.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leimbach WN Jr, Wallin BG, Victor RG, Aylward PE, Sundlöf G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986. doi: 10.1161/01.CIR.73.5.913. [DOI] [PubMed] [Google Scholar]
- 75.Li J, Xing J. Muscle afferent receptors engaged in augmented sympathetic responsiveness in peripheral artery disease. Front Physiol 3: 247, 2012. doi: 10.3389/fphys.2012.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Libardi CA, Catai AM, Miquelini M, Borghi-Silva A, Minatel V, Alvarez IF, Milan-Mattos JC, Roschel H, Tricoli V, Ugrinowitsch C. Hemodynamic responses to blood flow restriction and resistance exercise to muscular failure. Int J Sports Med 38: 134–140, 2017. doi: 10.1055/s-0042-115032. [DOI] [PubMed] [Google Scholar]
- 77.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol 299: H1357–H1364, 2010. doi: 10.1152/ajpheart.00612.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu J, Li JD, Lu J, Xing J, Li J. Contribution of nerve growth factor to upregulation of P2X3 expression in DRG neurons of rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol 301: H1070–H1079, 2011. doi: 10.1152/ajpheart.00188.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lixandrão ME, Ugrinowitsch C, Berton R, Vechin FC, Conceição MS, Damas F, Libardi CA, Roschel H. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med 48: 361–378, 2018. doi: 10.1007/s40279-017-0795-y. [DOI] [PubMed] [Google Scholar]
- 81.Loenneke JP, Fahs CA, Rossow LM, Sherk VD, Thiebaud RS, Abe T, Bemben DA, Bemben MG. Effects of cuff width on arterial occlusion: implications for blood flow restricted exercise. Eur J Appl Physiol 112: 2903–2912, 2012. doi: 10.1007/s00421-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loenneke JP, Wilson JM, Wilson GJ, Pujol TJ, Bemben MG. Potential safety issues with blood flow restriction training. Scand J Med Sci Sports 21: 510–518, 2011. doi: 10.1111/j.1600-0838.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- 83.Lykidis CK, White MJ, Balanos GM. The pulmonary vascular response to the sustained activation of the muscle metaboreflex in man. Exp Physiol 93: 247–253, 2008. doi: 10.1113/expphysiol.2007.039487. [DOI] [PubMed] [Google Scholar]
- 84.MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol (1985) 58: 785–790, 1985. doi: 10.1152/jappl.1985.58.3.785. [DOI] [PubMed] [Google Scholar]
- 85.Madarame H, Kurano M, Fukumura K, Fukuda T, Nakajima T. Haemostatic and inflammatory responses to blood flow-restricted exercise in patients with ischaemic heart disease: a pilot study. Clin Physiol Funct Imaging 33: 11–17, 2013. doi: 10.1111/j.1475-097X.2012.01158.x. [DOI] [PubMed] [Google Scholar]
- 86.Magnani S, Roberto S, Sainas G, Milia R, Palazzolo G, Cugusi L, Pinna V, Doneddu A, Kakhak SA, Tocco F, Mercuro G, Crisafulli A. Metaboreflex-mediated hemodynamic abnormalities in individuals with coronary artery disease without overt signs or symptoms of heart failure. Am J Physiol Heart Circ Physiol 314: H452–H463, 2018. doi: 10.1152/ajpheart.00436.2017. [DOI] [PubMed] [Google Scholar]
- 87.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985. doi: 10.1161/01.RES.57.3.461. [DOI] [PubMed] [Google Scholar]
- 88.May AK, Brandner CR, Warmington SA. Hemodynamic responses are reduced with aerobic compared with resistance blood flow restriction exercise. Physiol Rep 5: e13142, 2017. doi: 10.14814/phy2.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McNulty CL, Moody WE, Wagenmakers AJM, Fisher JP. Effect of muscle metaboreflex activation on central hemodynamics and cardiac function in humans. Appl Physiol Nutr Metab 39: 861–870, 2014. doi: 10.1139/apnm-2013-0414. [DOI] [PubMed] [Google Scholar]
- 90.Micieli G, Tosi P, Marcheselli S, Cavallini A. Autonomic dysfunction in Parkinson’s disease. Neurol Sci 24, Suppl 1: s32–s34, 2003. doi: 10.1007/s100720300035. [DOI] [PubMed] [Google Scholar]
- 91.Middlekauff HR, Nitzsche EU, Nguyen AH, Hoh CK, Gibbs GG. Modulation of renal cortical blood flow during static exercise in humans. Circ Res 80: 62–68, 1997. doi: 10.1161/01.RES.80.1.62. [DOI] [PubMed] [Google Scholar]
- 92.Miller AJ, Luck JC, Kim DJ, Leuenberger UA, Aziz F, Radtka JF 3rd, Sinoway LI, Muller MD. Peripheral revascularization attenuates the exercise pressor reflex and increases coronary exercise hyperemia in peripheral arterial disease. J Appl Physiol (1985) 125: 58–63, 2018. doi: 10.1152/japplphysiol.01046.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol 589: 6191–6204, 2011. doi: 10.1113/jphysiol.2011.214429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Momen A, Leuenberger UA, Ray CA, Cha S, Handly B, Sinoway LI. Renal vascular responses to static handgrip: role of muscle mechanoreflex. Am J Physiol Heart Circ Physiol 285: H1247–H1253, 2003. doi: 10.1152/ajpheart.00214.2003. [DOI] [PubMed] [Google Scholar]
- 95.Mouser JG, Dankel SJ, Jessee MB, Mattocks KT, Buckner SL, Counts BR, Loenneke JP. A tale of three cuffs: the hemodynamics of blood flow restriction. Eur J Appl Physiol 117: 1493–1499, 2017. doi: 10.1007/s00421-017-3644-7. [DOI] [PubMed] [Google Scholar]
- 96.Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590: 6237–6246, 2012. doi: 10.1113/jphysiol.2012.241281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Myerburg RJ, Kessler KM, Mallon SM, Cox MM, deMarchena E, Interian A Jr, Castellanos A. Life-threatening ventricular arrhythmias in patients with silent myocardial ischemia due to coronary-artery spasm. N Engl J Med 326: 1451–1455, 1992. doi: 10.1056/NEJM199205283262202. [DOI] [PubMed] [Google Scholar]
- 98.Nakajima T, Kurano M, Iida H, Takano H, Oonuma H, Morita T, Meguro K, Sato Y, Nagata T; KAATSU Training Group . Use and safety of KAATSU training: results of a national survey. Int J KAATSU Train Res 2: 5–13, 2006. doi: 10.3806/ijktr.2.5. [DOI] [Google Scholar]
- 99.Negrão CE, Rondon MU, Tinucci T, Alves MJ, Roveda F, Braga AM, Reis SF, Nastari L, Barretto AC, Krieger EM, Middlekauff HR. Abnormal neurovascular control during exercise is linked to heart failure severity. Am J Physiol Heart Circ Physiol 280: H1286–H1292, 2001. doi: 10.1152/ajpheart.2001.280.3.H1286. [DOI] [PubMed] [Google Scholar]
- 100.Neto GR, Sousa MS, Costa PB, Salles BF, Novaes GS, Novaes JS. Hypotensive effects of resistance exercises with blood flow restriction. J Strength Cond Res 29: 1064–1070, 2015. doi: 10.1519/JSC.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 101.Nishiyasu T, Tan N, Morimoto K, Nishiyasu M, Yamaguchi Y, Murakami N. Enhancement of parasympathetic cardiac activity during activation of muscle metaboreflex in humans. J Appl Physiol (1985) 77: 2778–2783, 1994. doi: 10.1152/jappl.1994.77.6.2778. [DOI] [PubMed] [Google Scholar]
- 102.Nóbrega AC, Williamson JW, Friedman DB, Araújo CG, Mitchell JH. Cardiovascular responses to active and passive cycling movements. Med Sci Sports Exerc 26: 709–714, 1994. doi: 10.1249/00005768-199406000-00009. [DOI] [PubMed] [Google Scholar]
- 103.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol 280: H969–H976, 2001. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- 104.Notarius CF, Millar PJ, Murai H, Morris BL, Marzolini S, Oh P, Floras JS. Divergent muscle sympathetic responses to dynamic leg exercise in heart failure and age-matched healthy subjects. J Physiol 593: 715–722, 2015. doi: 10.1113/jphysiol.2014.281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104a.Ogoh S, Fisher JP, Dawson EA, White MJ, Secher NH, Raven PB. Autonomic nervous system influence on arterial baroreflex control of heart rate during exercise in humans. J Physiol 566: 599–611, 2005. doi: 10.1113/jphysiol.2005.084541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104b.Ogoh S, Wasmund WL, Keller DM, O-Yurvati A, Gallagher KM, Mitchell JH, Raven PB. Role of central command in carotid baroreflex104. Notarius CF, Millar PJ, Murai H, Morris BL, Marzolini S, Oh P, Floras JS. Divergent muscle sympathetic responses to dynamic leg exercise in heart failure and age-matched healthy subjects. J Physiol 593: 715–722, 2015. doi: 10.1113/jphysiol.2014.281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O’Leary DS. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol (1985) 74: 1748–1754, 1993. doi: 10.1152/jappl.1993.74.4.1748. [DOI] [PubMed] [Google Scholar]
- 106.O’Leary DS. Point: the muscle metaboreflex does restore blood flow to contracting muscles. J Appl Physiol (1985) 100: 357–361, 2006. doi: 10.1152/japplphysiol.01222.2005. [DOI] [PubMed] [Google Scholar]
- 107.O’Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol 275: H220–H224, 1998. doi: 10.1152/ajpheart.1998.275.1.H220. [DOI] [PubMed] [Google Scholar]
- 108.O’Leary DS, Rossi NF, Churchill PC. Muscle metaboreflex control of vasopressin and renin release. Am J Physiol 264: H1422–H1427, 1993. doi: 10.1152/ajpheart.1993.264.5.H1422. [DOI] [PubMed] [Google Scholar]
- 109.O’Leary DS, Sala-Mercado JA, Augustyniak RA, Hammond RL, Rossi NF, Ansorge EJ. Impaired muscle metaboreflex-induced increases in ventricular function in heart failure. Am J Physiol Heart Circ Physiol 287: H2612–H2618, 2004. doi: 10.1152/ajpheart.00604.2004. [DOI] [PubMed] [Google Scholar]
- 110.O’Leary DS, Sala-Mercado JA, Hammond RL, Ansorge EJ, Kim JK, Rodriguez J, Fano D, Ichinose M. Muscle metaboreflex-induced increases in cardiac sympathetic activity vasoconstrict the coronary vasculature. J Appl Physiol (1985) 103: 190–194, 2007. doi: 10.1152/japplphysiol.00139.2007. [DOI] [PubMed] [Google Scholar]
- 111.O’Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol 268: H980–H986, 1995. doi: 10.1152/ajpheart.1995.268.3.H980. [DOI] [PubMed] [Google Scholar]
- 114.Ozaki H, Yasuda T, Ogasawara R, Sakamaki-Sunaga M, Naito H, Abe T. Effects of high-intensity and blood flow-restricted low-intensity resistance training on carotid arterial compliance: role of blood pressure during training sessions. Eur J Appl Physiol 113: 167–174, 2013. doi: 10.1007/s00421-012-2422-9. [DOI] [PubMed] [Google Scholar]
- 115.Ozawa Y, Koto T, Shinoda H, Tsubota K. Vision loss by central retinal vein occlusion after kaatsu training: a case report. Medicine (Baltimore) 94: e1515, 2015. doi: 10.1097/MD.0000000000001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Partsch B, Partsch H. Calf compression pressure required to achieve venous closure from supine to standing positions. J Vasc Surg 42: 734–738, 2005. doi: 10.1016/j.jvs.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 117.Patterson SD, Brandner CR. The role of blood flow restriction training for applied practitioners: a questionnaire-based survey. J Sports Sci 36: 123–130, 2018. doi: 10.1080/02640414.2017.1284341. [DOI] [PubMed] [Google Scholar]
- 118.Patterson SD, Hughes L, Warmington S, Burr J, Scott BR, Owens J, Abe T, Nielsen JL, Libardi CA, Laurentino G, Neto GR, Brandner C, Martin-Hernandez J, Loenneke J. Blood flow restriction exercise position stand: considerations of methodology, application, and safety. Front Physiol 10: 533, 2019. [Erratum in: Front Physiol 10: 1332, 2019.] doi: 10.3389/fphys.2019.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pearson SJ, Hussain SR. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med 45: 187–200, 2015. doi: 10.1007/s40279-014-0264-9. [DOI] [PubMed] [Google Scholar]
- 120.Peçanha T, de Brito LC, Fecchio RY, de Sousa PN, da Silva Junior ND, de Abreu AP, da Silva GV, Mion-Junior D, Forjaz CL. Metaboreflex activation delays heart rate recovery after aerobic exercise in never-treated hypertensive men. J Physiol 594: 6211–6223, 2016. doi: 10.1113/JP272851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peçanha T, Silva-Júnior ND, Forjaz CL. Heart rate recovery: autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin Physiol Funct Imaging 34: 327–339, 2014. doi: 10.1111/cpf.12102. [DOI] [PubMed] [Google Scholar]
- 122.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation 93: 940–952, 1996. doi: 10.1161/01.CIR.93.5.940. [DOI] [PubMed] [Google Scholar]
- 123.Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A, Coats AJ. A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. Am Heart J 137: 1050–1056, 1999. doi: 10.1016/S0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- 124.Pinto RR, Karabulut M, Poton R, Polito MD. Acute resistance exercise with blood flow restriction in elderly hypertensive women: haemodynamic, rating of perceived exertion and blood lactate. Clin Physiol Funct Imaging 38: 17–24, 2018. doi: 10.1111/cpf.12376. [DOI] [PubMed] [Google Scholar]
- 125.Pinto RR, Polito MD. Haemodynamic responses during resistance exercise with blood flow restriction in hypertensive subjects. Clin Physiol Funct Imaging 36: 407–413, 2016. doi: 10.1111/cpf.12245. [DOI] [PubMed] [Google Scholar]
- 126.Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ, Piepoli MF. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation 104: 2324–2330, 2001. doi: 10.1161/hc4401.098491. [DOI] [PubMed] [Google Scholar]
- 127.Poton R, Polito MD. Hemodynamic response to resistance exercise with and without blood flow restriction in healthy subjects. Clin Physiol Funct Imaging 36: 231–236, 2016. doi: 10.1111/cpf.12218. [DOI] [PubMed] [Google Scholar]
- 128.Poton R, Polito MD. Hemodynamic responses during lower-limb resistance exercise with blood flow restriction in healthy subjects. J Sports Med Phys Fitness 55: 1571–1577, 2015. [PubMed] [Google Scholar]
- 129.Potts JT. Inhibitory neurotransmission in the nucleus tractus solitarii: implications for baroreflex resetting during exercise. Exp Physiol 91: 59–72, 2006. doi: 10.1113/expphysiol.2005.032227. [DOI] [PubMed] [Google Scholar]
- 130.Ray CA, Secher NH, Mark AL. Modulation of sympathetic nerve activity during posthandgrip muscle ischemia in humans. Am J Physiol 266: H79–H83, 1994. doi: 10.1152/ajpheart.1994.266.1.H79. [DOI] [PubMed] [Google Scholar]
- 131.Roberto S, Marongiu E, Pinna M, Angius L, Olla S, Bassareo P, Tocco F, Concu A, Milia R, Crisafulli A. Altered hemodynamics during muscle metaboreflex in young type 1 diabetes patients. J Appl Physiol (1985) 113: 1323–1331, 2012. doi: 10.1152/japplphysiol.00280.2012. [DOI] [PubMed] [Google Scholar]
- 132.Roberto S, Mulliri G, Milia R, Solinas R, Pinna V, Sainas G, Piepoli MF, Crisafulli A. Hemodynamic response to muscle reflex is abnormal in patients with heart failure with preserved ejection fraction. J Appl Physiol (1985) 122: 376–385, 2017. doi: 10.1152/japplphysiol.00645.2016. [DOI] [PubMed] [Google Scholar]
- 133.Rondon MU, Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrão CE. Abnormal muscle metaboreflex control of sympathetic activity in never-treated hypertensive subjects. Am J Hypertens 19: 951–957, 2006. doi: 10.1016/j.amjhyper.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 134.Roseguini BT, Alves CN, Chiappa GR, Stein R, Ribeiro JP. Muscle metaboreflex contribution to resting limb haemodynamic control is preserved in older subjects. Clin Physiol Funct Imaging 27: 335–339, 2007. doi: 10.1111/j.1475-097X.2007.00756.x. [DOI] [PubMed] [Google Scholar]
- 135.Ross AJ, Gao Z, Luck JC, Blaha CA, Cauffman AE, Aziz F, Radtka JF 3rd, Proctor DN, Leuenberger UA, Sinoway LI, Muller MD. Coronary exercise hyperemia is impaired in patients with peripheral arterial disease. Ann Vasc Surg 38: 260–267, 2017. doi: 10.1016/j.avsg.2016.05.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rossow LM, Fahs CA, Loenneke JP, Thiebaud RS, Sherk VD, Abe T, Bemben MG. Cardiovascular and perceptual responses to blood-flow-restricted resistance exercise with differing restrictive cuffs. Clin Physiol Funct Imaging 32: 331–337, 2012. doi: 10.1111/j.1475-097X.2012.01131.x. [DOI] [PubMed] [Google Scholar]
- 137.Rowell LB, O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol (1985) 69: 407–418, 1990. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- 138.Sabino-Carvalho JL, Teixeira AL, Samora M, Daher M, Vianna LC. Blunted cardiovascular responses to exercise in Parkinson’s disease patients: role of the muscle metaboreflex. J Neurophysiol 120: 1516–1524, 2018. doi: 10.1152/jn.00308.2018. [DOI] [PubMed] [Google Scholar]
- 139.Sala-Mercado JA, Hammond RL, Kim JK, McDonald PJ, Stephenson LW, O’Leary DS. Heart failure attenuates muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 292: H2159–H2166, 2007. doi: 10.1152/ajpheart.01240.2006. [DOI] [PubMed] [Google Scholar]
- 140.Sala-Mercado JA, Spranger MD, Abu-Hamdah R, Kaur J, Coutsos M, Stayer D, Augustyniak RA, O’Leary DS. Attenuated muscle metaboreflex-induced increases in cardiac function in hypertension. Am J Physiol Heart Circ Physiol 305: H1548–H1554, 2013. doi: 10.1152/ajpheart.00478.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Samora M, Incognito AV, Vianna LC. Sex differences in blood pressure regulation during ischemic isometric exercise: the role of the β-adrenergic receptors. J Appl Physiol (1985) 127: 408–414, 2019. doi: 10.1152/japplphysiol.00270.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sardeli AV, do Carmo Santos L, Ferreira MLV, Gáspari AF, Rodrigues B, Cavaglieri CR, Chacon-Mikahil MP. Cardiovascular responses to different resistance exercise protocols in elderly. Int J Sports Med 38: 928–936, 2017. doi: 10.1055/s-0043-115737. [DOI] [PubMed] [Google Scholar]
- 143.Sausen MT, Delaney EP, Stillabower ME, Farquhar WB. Enhanced metaboreflex sensitivity in hypertensive humans. Eur J Appl Physiol 105: 351–356, 2009. doi: 10.1007/s00421-008-0910-8. [DOI] [PubMed] [Google Scholar]
- 144.Scott AC, Davies LC, Coats AJ, Piepoli M. Relationship of skeletal muscle metaboreceptors in the upper and lower limbs with the respiratory control in patients with heart failure. Clin Sci (Lond) 102: 23–30, 2002. doi: 10.1042/cs1020023. [DOI] [PubMed] [Google Scholar]
- 145.Scott AC, Francis DP, Davies LC, Ponikowski P, Coats AJ, Piepoli MF. Contribution of skeletal muscle ‘ergoreceptors’ in the human leg to respiratory control in chronic heart failure. J Physiol 529: 863–870, 2000. doi: 10.1111/j.1469-7793.2000.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scott BR, Peiffer JJ, Thomas HJ, Marston KJ, Hill KD. Hemodynamic responses to low-load blood flow restriction and unrestricted high-load resistance exercise in older women. Front Physiol 9: 1324, 2018. doi: 10.3389/fphys.2018.01324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sheriff DD, Augustyniak RA, O’Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol 275: H767–H775, 1998. doi: 10.1152/ajpheart.1998.275.3.H767. [DOI] [PubMed] [Google Scholar]
- 148.Shimizu R, Hotta K, Yamamoto S, Matsumoto T, Kamiya K, Kato M, Hamazaki N, Kamekawa D, Akiyama A, Kamada Y, Tanaka S, Masuda T. Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur J Appl Physiol 116: 749–757, 2016. doi: 10.1007/s00421-016-3328-8. [DOI] [PubMed] [Google Scholar]
- 149.Shoemaker JK, Mattar L, Kerbeci P, Trotter S, Arbeille P, Hughson RL. WISE 2005: stroke volume changes contribute to the pressor response during ischemic handgrip exercise in women. J Appl Physiol (1985) 103: 228–233, 2007. doi: 10.1152/japplphysiol.01334.2006. [DOI] [PubMed] [Google Scholar]
- 150.Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol (1985) 99: 5–22, 2005. doi: 10.1152/japplphysiol.01405.2004. [DOI] [PubMed] [Google Scholar]
- 151.Smith LL, Kukielka M, Billman GE. Heart rate recovery after exercise: a predictor of ventricular fibrillation susceptibility after myocardial infarction. Am J Physiol Heart Circ Physiol 288: H1763–H1769, 2005. doi: 10.1152/ajpheart.00785.2004. [DOI] [PubMed] [Google Scholar]
- 152.Spranger MD, Kaur J, Sala-Mercado JA, Krishnan AC, Abu-Hamdah R, Alvarez A, Machado TM, Augustyniak RA, O’Leary DS. Exaggerated coronary vasoconstriction limits muscle metaboreflex-induced increases in ventricular performance in hypertension. Am J Physiol Heart Circ Physiol 312: H68–H79, 2017. doi: 10.1152/ajpheart.00417.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Spranger MD, Kaur J, Sala-Mercado JA, Machado TM, Krishnan AC, Alvarez A, O’Leary DS. Attenuated muscle metaboreflex-induced pressor response during postexercise muscle ischemia in renovascular hypertension. Am J Physiol Regul Integr Comp Physiol 308: R650–R658, 2015. doi: 10.1152/ajpregu.00464.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spranger MD, Krishnan AC, Levy PD, O’Leary DS, Smith SA. Blood flow restriction training and the exercise pressor reflex: a call for concern. Am J Physiol Heart Circ Physiol 309: H1440–H1452, 2015. doi: 10.1152/ajpheart.00208.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Staunton CA, May AK, Brandner CR, Warmington SA. Haemodynamics of aerobic and resistance blood flow restriction exercise in young and older adults. Eur J Appl Physiol 115: 2293–2302, 2015. doi: 10.1007/s00421-015-3213-x. [DOI] [PubMed] [Google Scholar]
- 156.Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB, Sinoway LI. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation 84: 2034–2039, 1991. doi: 10.1161/01.CIR.84.5.2034. [DOI] [PubMed] [Google Scholar]
- 157.Stone AJ, Copp SW, McCord JL, Kaufman MP. Femoral artery ligation increases the responses of thin-fiber muscle afferents to contraction. J Neurophysiol 113: 3961–3966, 2015. doi: 10.1152/jn.00288.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sundblad P, Kölegård R, Rullman E, Gustafsson T. Effects of training with flow restriction on the exercise pressor reflex. Eur J Appl Physiol 118: 1903–1909, 2018. doi: 10.1007/s00421-018-3911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Takano H, Morita T, Iida H, Asada K, Kato M, Uno K, Hirose K, Matsumoto A, Takenaka K, Hirata Y, Eto F, Nagai R, Sato Y, Nakajima T. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol 95: 65–73, 2005. doi: 10.1007/s00421-005-1389-1. [DOI] [PubMed] [Google Scholar]
- 160.Takatsu H, Nishida H, Matsuo H, Watanabe S, Nagashima K, Wada H, Noda T, Nishigaki K, Fujiwara H. Cardiac sympathetic denervation from the early stage of Parkinson’s disease: clinical and experimental studies with radiolabeled MIBG. J Nucl Med 41: 71–77, 2000. [PubMed] [Google Scholar]
- 161.Tang M, Donaghue KC, Cho YH, Craig ME. Autonomic neuropathy in young people with type 1 diabetes: a systematic review. Pediatr Diabetes 14: 239–248, 2013. doi: 10.1111/pedi.12039. [DOI] [PubMed] [Google Scholar]
- 162.Thiebaud RS, Loenneke JP, Fahs CA, Rossow LM, Kim D, Abe T, Anderson MA, Young KC, Bemben DA, Bemben MG. The effects of elastic band resistance training combined with blood flow restriction on strength, total bone-free lean body mass and muscle thickness in postmenopausal women. Clin Physiol Funct Imaging 33: 344–352, 2013. doi: 10.1111/cpf.12033. [DOI] [PubMed] [Google Scholar]
- 163.Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010. doi: 10.1152/ajpheart.00141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. J Physiol 589: 6173–6189, 2011. doi: 10.1113/jphysiol.2011.217851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Van Iterson EH, Snyder EM, Johnson BD, Olson TP. Influence of the metaboreflex on pulmonary vascular capacitance in heart failure. Med Sci Sports Exerc 48: 353–362, 2016. doi: 10.1249/MSS.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vechin FC, Libardi CA, Conceição MS, Damas FR, Lixandrão ME, Berton RP, Tricoli VA, Roschel HA, Cavaglieri CR, Chacon-Mikahil MP, Ugrinowitsch C. Comparisons between low-intensity resistance training with blood flow restriction and high-intensity resistance training on quadriceps muscle mass and strength in elderly. J Strength Cond Res 29: 1071–1076, 2015. doi: 10.1519/JSC.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 167.Vieira PJC, Chiappa GR, Umpierre D, Stein R, Ribeiro JP. Hemodynamic responses to resistance exercise with restricted blood flow in young and older men. J Strength Cond Res 27: 2288–2294, 2013. doi: 10.1519/JSC.0b013e318278f21f. [DOI] [PubMed] [Google Scholar]
- 168.Wiecek EM, McCartney N, McKelvie RS. Comparison of direct and indirect measures of systemic arterial pressure during weightlifting in coronary artery disease. Am J Cardiol 66: 1065–1069, 1990. doi: 10.1016/0002-9149(90)90506-V. [DOI] [PubMed] [Google Scholar]
- 169.Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol 245: H481–H486, 1983. doi: 10.1152/ajpheart.1983.245.3.H481. [DOI] [PubMed] [Google Scholar]
- 170.Xing J, Gao Z, Lu J, Sinoway LI, Li J. Femoral artery occlusion augments TRPV1-mediated sympathetic responsiveness. Am J Physiol Heart Circ Physiol 295: H1262–H1269, 2008. doi: 10.1152/ajpheart.00271.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol Heart Circ Physiol 296: H1380–H1387, 2009. doi: 10.1152/ajpheart.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yasuda T, Fukumura K, Tomaru T, Nakajima T. Thigh muscle size and vascular function after blood flow-restricted elastic band training in older women. Oncotarget 7: 33595–33607, 2016. doi: 10.18632/oncotarget.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yasuda T, Ogasawara R, Sakamaki M, Ozaki H, Sato Y, Abe T. Combined effects of low-intensity blood flow restriction training and high-intensity resistance training on muscle strength and size. Eur J Appl Physiol 111: 2525–2533, 2011. doi: 10.1007/s00421-011-1873-8. [DOI] [PubMed] [Google Scholar]