Abstract
Although the generation of ETV2-induced endothelial cells (iECs) from human fibroblasts serves as a novel therapeutic strategy in regenerative medicine, the process is inefficient, resulting in incomplete iEC angiogenesis. Therefore, we employed chromatin immunoprecipitation (ChIP) sequencing and identified molecular mechanisms underlying ETV2-mediated endothelial transdifferentiation to efficiently produce iECs retaining appropriate functionality in long-term culture. We revealed that the majority of ETV2 targets in human fibroblasts are related to vasculature development and signaling transduction pathways, including Rap1 signaling. From a screening of signaling pathway modulators, we confirmed that forskolin facilitated efficient and rapid iEC reprogramming via activation of the cyclic AMP (cAMP)/exchange proteins directly activated by cAMP (EPAC)/RAP1 axis. The iECs obtained via cAMP signaling activation showed superior angiogenesis in vivo as well as in vitro. Moreover, these cells could form aligned endothelium along the vascular lumen ex vivo when seeded into decellularized liver scaffold. Overall, our study provided evidence that the cAMP/EPAC/RAP1 axis is required for the efficient generation of iECs with angiogenesis potential.
Keywords: direct reprogramming, transdifferentiation, ETV2, endothelial cells, angiogenesis, cAMP/EPAC/RAP1 pathway
Graphical Abstract

Kim et al. identify that ETV2 directly binds to RAPGEF3, encoding EPAC1, during endothelial transdifferentiation by using genome-wide ChIP-seq. Given these results, they employ forskolin, a cAMP activator, to increase the efficiency of endothelial reprogramming through activation of the cAMP/EPAC1/RAP1 pathway. They also report that forskolin-treated iECs acquire functional features of vascular ECs following long-term culture.
Introduction
The therapeutic use of endothelial cells (ECs) is an attractive strategy to treat patients with traumatic and ischemic vascular diseases. In spite of the variability of clinical outcomes after autologous cell transplantation, some studies have demonstrated the clinical efficacy and safety of autologous CD34-positive cells in improving the symptoms of patients with cardiovascular diseases, such as limb ischemia,1 angina,2 and dilated cardiomyopathy.3 Thus, many attempts have been made to attain patient-specific ECs from induced pluripotent stem cells (iPSCs). However, the directed endothelial differentiation of iPSCs has been challenging due to its inefficiency and poor stability of differentiated ECs in long-term culture.4,5
Direct reprogramming technology, which transduces lineage transcription factors into terminally differentiated cells, has emerged as an alternative strategy for the production of patient-specific ECs.6, 7, 8 It has been reported that introducing a combination of ETS transcription factors, i.e., ETV2, FLI1, and ERG, directly transdifferentiates human amniotic cells into functional induced ECs (iECs).8 Subsequent work by the same group revealed that the reprogramming efficiency and functionality of mouse amniotic cell-derived iECs could be promoted through the addition of protein kinase Akt1 or the transcription factor Sox17.9 However, other researchers recently reported that ETV2 alone could directly convert human fibroblasts into functional iECs, albeit slowly and inefficiently.6,7 To address such limitations, ETV2-mediated endothelial conversion may require enhancements in efficiency and further elucidation of underlying molecular mechanisms.
ETV2, one of the ETS family transcription factors, is a critical regulator of hematopoietic and EC development.10,11 ETV2 deficiency even leads to embryonic lethality of animals due to the defects in blood vessel development.10 Moreover, ETV2 could directly activate other ETS family genes, consequently establishing the ETS hierarchy.11 Distinct from other ETS factors, ETV2 is only transiently expressed in the early phase of development.10
Cyclic AMP (cAMP), a second messenger generated from ATP by adenylyl cyclases, regulates a plethora of biological processes.12 The accumulation of cAMP leads to CREB phosphorylation by cAMP-dependent protein kinase A (PKA), resulting in the activation of cAMP-responsive genes. The cAMP/PKA pathway is known to play a key role in the endothelial differentiation of mouse embryonic stem cells (mESCs) through increased expression of VEGFR2 and NRP113 or the direct upregulation of Etv2.14 Furthermore, another cAMP effector protein, exchange proteins directly activated by cAMP (EPAC), functions as a guanine-nucleotide-exchange factor (GEF) for small G protein Rap and acts in a PKA-independent manner.15 However, the potential influence of cAMP signaling and its downstream effectors on iEC reprogramming from human fibroblasts has yet to be explored.
In the present study, to elucidate the mechanisms by which ETV2 drives endothelial reprogramming, we employed genome-wide analyses to define downstream targets of ETV2 in human dermal fibroblasts (hDFs). We also reported the first small molecule screen aimed at dissecting the intracellular signaling pathways that play roles in ETV2-mediated direct reprogramming. We demonstrated that forskolin, a cAMP signaling activator, enables highly efficient and rapid iEC reprogramming. Moreover, we utilized in vitro and in vivo endothelial assays to show how cAMP signaling activation can be applied to obtain stable and functional iECs.
Results
Ectopic Expression of ETV2 Induces Endothelial Development Program from hDFs
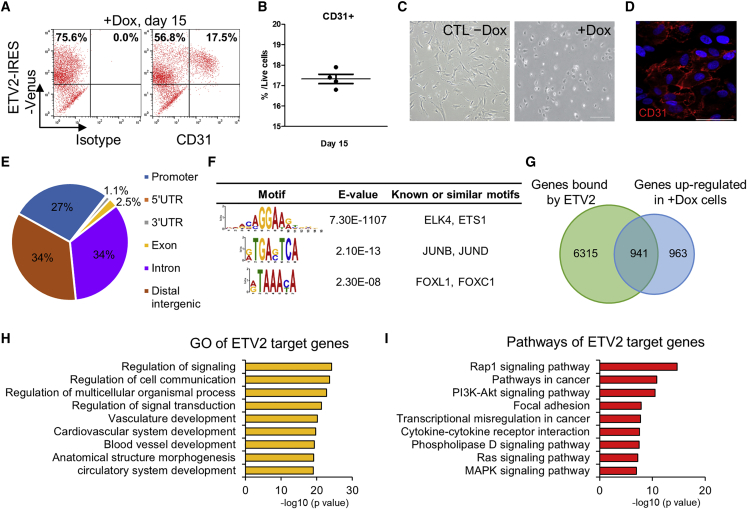
The transduction of ETV2 into hDFs enables direct conversion to iECs.6,7 To optimize this process, we infected hDFs with doxycycline (dox)-inducible lentivirus containing hemagglutinin (HA)-ETV2-internal ribosome entry site (IRES)-Venus (ETV2-hDFs).7 We checked that ETV2-hDFs expressed ETV2 only when treated with dox (Figure S1A). Then, we observed that the population of CD31-positive cells was about 17.5% (range, 16.8%–17.9%) among the live cells on day 15 of continuous dox administration (Figures 1A and 1B). ETV2-hDFs did not express EC-specific surface markers in the absence of dox (Figure S1B). Subsequently, we obtained a pure population of CD31-positive cells by magnetic-activated cell sorting (MACS) and characterized these cells as iECs by evaluating cobblestone morphology (Figure 1C) and anti-CD31 immunostaining (Figure 1D).
Figure 1.
Characterization of ETV2’s Role during Reprogramming from hDFs to iECs
(A and B) FACS analysis results (A) showing ETV2-IRES-Venus+/CD31+-induced ECs at day 15 with dox treatment and quantification (B) of the reprogramming efficiency. (C) Phase-contrast images of ETV2-hDFs without dox (left) and sorted CD31+ iECs (right) on day 15. CTL −Dox, control cells without dox. Scale bars, 200 μm. (D) Immunocytochemical image of CD31+ iECs sorted from ETV2-hDFs on day 15. Cells were immunostained for CD31 (red) and DAPI (blue). Scale bar, 50 μm. (E) ETV2 peaks genomic classification from ChIP-seq in +Dox cells. (F) Motif analysis of the ETV2 peaks. The peaks are ordered by significance. (G) Venn diagram representing the overlap of genes bound in their regulatory region (−5 kb to +1 kb plus extension up to 1 Mb distal analyzed by GREAT) and upregulated (≥2-fold) by ETV2 overexpression. (H) Gene ontology terms enriched in ETV2 target genes during reprogramming. (I) KEGG pathway analysis of ETV2 target genes.
To investigate the role of ETV2 in reprogramming, we sought to identify the downstream targets of ETV2 by chromatin immunoprecipitation (ChIP) followed by DNA sequencing (ChIP-seq). HA-tagged ETV2 was immunoprecipitated from ETV2-hDFs after 7 days of dox administration. We identified 8,565 ETV2-occupied regions that corresponded mostly to the intronic (34%) and intergenic (34%) regions followed by the promoter (27%) region (Figure 1E). De novo motif discovery identified a highly enriched binding motif (GGAA/T) similar to a previously proposed motif in mESCs11 (Figure 1F). Additionally, we found significant enrichment of the AP-1 motif (E = 2.1 × 10−13) and FOX motif (E = 2.3 × 10−8) in ETV2-bound regions. Next, we identified potential target genes of ETV2 by associating the ChIP peaks with nearby genes using GREAT16 (Figure 1G). By comparing the gene-expression profiles of dox-treated cells with ChIP-seq data, 941 genes (49.4%) were found to be associated with ETV2 binding sites among the 1,904 differentially upregulated genes. Then, we functionally annotated ETV2-activated target genes and found that many of the enriched gene ontology (GO) terms were associated with vasculature development and signaling transduction (Figure 1H). KEGG pathway analyses demonstrated that ETV2 target genes were associated with Rap1, phosphatidylinositol 3-kinase (PI3K)-Akt, Ras, and mitogen-activated protein kinase (MAPK) signaling pathways, which are known to be required for normal vasculature development and angiogenesis17,18 (Figure 1I; Table S1).
As Etv2 and its downstream targets regulate hemato-endothelial commitment of mESCs,11 we next investigated whether key genes involved in endothelial differentiation were also bound and activated by ETV2 in hDFs. Consistent with our ChIP-seq data, we observed that ETV2 bound to regulatory elements of transcription factor-encoding genes (SOX7, SOX18, ERG, FLI1, LMO2, and TAL1) and vascular endothelial growth factor (VEGF) signaling-associated genes (VEGFR2 and FLT1) (Figure S1C). We further examined whether ETV2 could modulate the epigenetic state of these genes by analyzing the level of histone H3 lysine 27 acetylation (H3K27ac), which marks active promoters and enhancers.19,20 By ChIP-qPCR analysis, we found that ETV2 binding was accompanied with the acquisition of the active H3K27ac marks (Figure S1D). These results suggest that ETV2 converts hDFs to endothelial fate by targeting genes and signaling pathways linked to vascular development.
cAMP Signaling Pathway Activation Promotes the Reprogramming of iECs from Human Fibroblasts
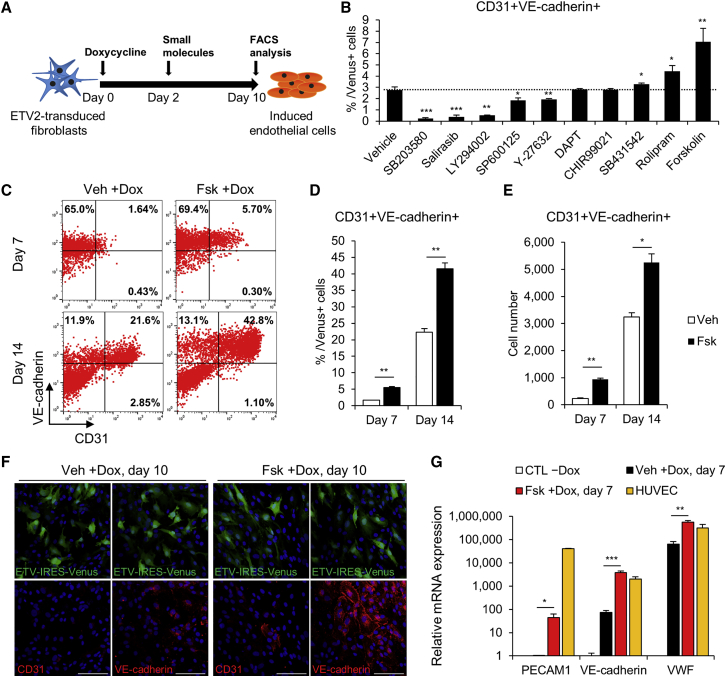
Next, we attempted to overcome the slow and inefficient process of iEC generation (Figures 1A and 1B) by modulating the signaling pathways underlying ETV2-mediated reprogramming. We screened 10 small molecules based on signaling pathways linked to ETV2 targets and endothelial differentiation for their involvement in endothelial reprogramming (Figure 1I; Table S1). We quantified reprogramming efficiency by evaluating the number of CD31 and vascular endothelial (VE)-cadherin double-positive cells within the Venus (ETV2)-positive population at day 10 (Figure 2A; Table S2). Notably, SB203580, salirasib, and LY294002 inhibited the generation of CD31+/VE-cadherin+ iECs compared with vehicle control (DMSO), indicating that target signaling pathways of ETV2, such as MAPK, Ras, and Akt signaling, are required for hDF-to-iEC reprogramming. Interestingly, we observed that the phosphodiesterase-4 inhibitor rolipram and the cAMP signaling activator forskolin were most efficient at increasing the iEC population (Figure 2B). Combining forskolin with other small molecules did not affect the efficiency of iEC reprogramming (Figure S2A). We then varied the concentration of forskolin in the medium and determined that continuous treatment with 5 μM forskolin was optimal (Figure S2B).
Figure 2.
Small Molecule Screening Identifies the cAMP Signaling Pathway as a Facilitator of iEC Reprogramming
(A) Schematic diagram of the strategy for the screening of small molecules during iEC reprogramming. (B) Quantification of the averaged percent iECs from flow analysis at day 10 in each treatment. Gated on Venus+ cells. n = 3 independent experiments; mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle. (C–E) Representative FACS results (C), quantification (D), and cell numbers (E) of CD31+/VE-cadherin+ iECs at the indicated time points. Veh +Dox, vehicle-treated with dox; Fsk +Dox, forskolin-treated with dox. n = 3 independent experiments; mean ± SD. *p < 0.05, **p < 0.01. (F) Representative immunochemistry images for CD31 (red) and VE-cadherin (red) expression in Veh +Dox and Fsk +Dox 10 days after dox treatment. Note that the images show a heterogeneous cell population that includes either ETV2-infected (green) or uninfected cells. Scale bars, 100 μm. (G) qRT-PCR of EC-specific genes in CTL −Dox, Veh +Dox, Fsk +Dox, and HUVECs on day 7 of induction. For each group, n = 3; mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 versus Veh +Dox.
To further monitor the effects of forskolin on ETV2-mediated endothelial reprogramming, we analyzed the iEC population by flow cytometry in a time-dependent manner. Forskolin treatment increased both the proportion (Figures 2C and 2D) and absolute number (Figure 2E) of CD31+/VE-cadherin+ iECs approximately 3.2-fold on day 7 and 1.9-fold on day 14. Moreover, forskolin did not preferentially increase the population of ETV2-infected (Venus-positive) cells (Figures S2C–S2E). This indicated that forskolin enhanced reprogramming efficiency via mechanisms other than increasing the proliferation rate of ETV2-infected cells. Immunostaining analysis on day 10 showed that forskolin-treated cultures yielded greater numbers of CD31 and VE-cadherin-positive cells (Figure 2F). Subsequently, we examined the mRNA expression levels of EC marker genes by quantitative RT-PCR (qRT-PCR) and observed increased expression levels of PECAM1, VE-cadherin, and VWF in forskolin-treated cells (Figure 2G). We also confirmed that ETV2-expressing cells expressed other EC surface markers, such as VEGFR2, CD34, TIE2, CLDN5, and VWF by fluorescence-activated cell sorting (FACS) analysis (Figure S2F). In addition, to explore whether forskolin could affect the reprogramming of other cell sources, we transduced retroviral ETV2 in umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs). After 7 days of culture, we observed that forskolin-treated UCB-MSCs expressed higher levels of endothelial markers than did vehicle-treated UCB-MSCs (Figures S2G–S2J). Taken together, we demonstrated that forskolin, a cAMP signaling activator, enhances the endothelial reprogramming.
cAMP/EPAC/RAP1 Signaling Regulates Endothelial Reprogramming
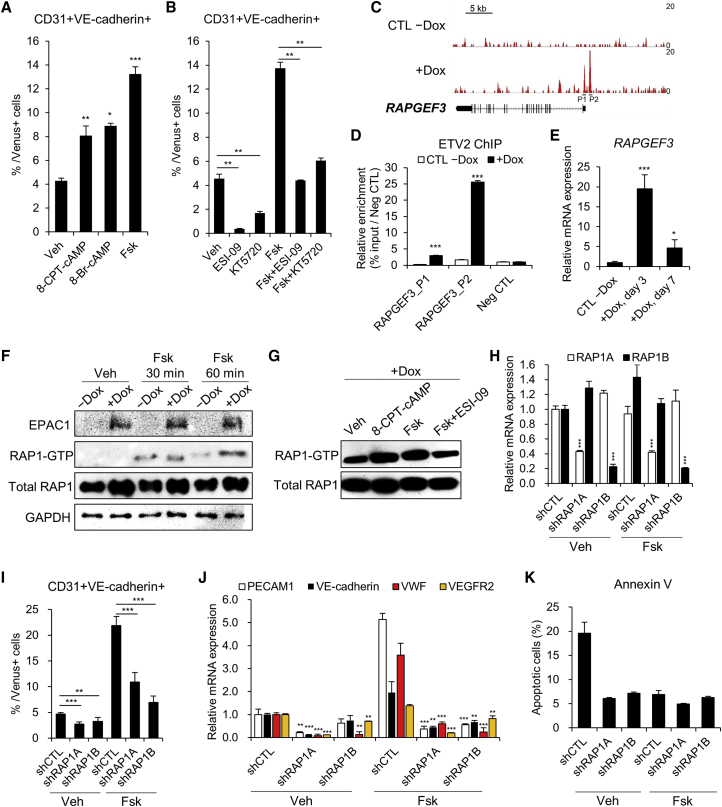
To confirm whether the effect of forskolin was through major downstream targets of cAMP, we applied two different cAMP analogs that activated EPAC or PKA.21 Both 8-(4-chlorophenylthio)-2′-O-methyladenosine (8-CPT-2Me)-cAMP, which selectively activates EPAC, and 8-bromoadenosine (8-Bromo)-cAMP, which activates both PKA and EPAC, increased iEC populations after 10 days of exposure (Figure 3A). We also analyzed endothelial reprogramming efficiency in the presence of two inhibitors of cAMP signaling: ESI-09, which specifically inhibits EPAC, and KT5720, which is a specific inhibitor of PKA. Both specific inhibitors reduced the iEC populations and also diminished the effects of forskolin on reprogramming (Figure 3B).
Figure 3.
The cAMP/EPAC/RAP1 Axis Modulates ETV2-Mediated Reprogramming
(A) Quantification of flow cytometry analysis in iECs treated with vehicle, 100 μM 8-CPT-cAMP, 25 μM 8-Br-cAMP, or 5 μM forskolin for 10 days. After 2 days of dox treatment, the small molecules were supplemented in the medium until day 10. n = 3 independent experiments; mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle. (B) Quantification of flow cytometry analysis in iECs treated with vehicle, 2.5 μM ESI-09, 3 μM KT5720, 5 μM forskolin, 5 μM forskolin + 2.5 μM ESI-09, or 5 μM forskolin + 3 μM KT5720 for 10 days. n = 3 independent experiments; mean ± SD. **p < 0.01. (C) ETV2 occupancy profile at the RAPGEF3 locus. P1 and P2 indicate primers designed for ChIP-qPCR analysis. (D) ChIP-qPCR results showing enrichment of ETV2 at promoters of RAPGEF3. The regulatory region of the POU5F1 gene was used as a negative control. For each group, n = 3; mean ± SD. ***p < 0.001 versus CTL −Dox. (E) qRT-PCR analysis of RAPGEF3 in ETV2-hDFs without or with dox treatment on day 3 and 7 of induction. For each group, n = 3; mean ± SD. *p < 0.05, ***p < 0.001 versus CTL −Dox. (F) Western blot analysis of EPAC1, RAP1-GTP, and total RAP1 levels after the indicated treatments in −Dox and +Dox cells starved overnight. (G) Western blot analysis of RAP1-GTP and total RAP1 levels in +Dox cells treated with vehicle, 100 μM 8-CPT-cAMP, 10 μM forskolin, or 10 μM forskolin + 2.5 μM ESI-09 for 1 h. (H) qRT-PCR validation for silencing of RAP1A and RAP1B in iECs transduced with each shRNA. For each group, n = 3; mean ± SD. ***p < 0.001 versus shCTL. (I) FACS analysis of CD31+/VE-cadherin+ population in iECs transduced with shCTL, shRAP1A, and shRAP1B on day 10 of induction. For each group, n = 3; mean ± SD. **p < 0.01, ***p < 0.001. (J) qRT-PCR analysis of PECAM1, VE-cadherin, VWF, and VEGFR2 in iECs transduced with shCTL, shRAP1A, and shRAP1B on day 7 of induction. For each group, n = 3; mean ± SD. **p < 0.01, ***p < 0.001 versus shCTL. (K) Apoptosis analysis of iECs transduced with shCTL, shRAP1A, and shRAP1B by annexin V staining on day 10 of induction. For each group, n = 3; mean ± SD.
cAMP-regulated EPAC1, which is encoded by RAPGEF3, is known to activate small GTPase RAP1 by inducing guanosine diphosphate (GDP)/guanosine triphosphate (GTP) exchange.15 Notably, we identified that RAPGEF3 is directly bound and activated by ETV2, implying the involvement of cAMP/EPAC1 signaling in ETV2-mediated reprogramming (Figures 1I and 3C–3E). To investigate whether the activity of RAP1, a downstream effector of EPAC1, is regulated by ETV2, we performed pull-down assays to quantify the expression of active GTP-bound RAP1 (RAP1-GTP) protein. Interestingly, after stimulation with forskolin, the expression of RAP1-GTP was higher in ETV2-expressing cells compared with control cells (Figure 3F). Furthermore, 8-CPT-2Me-cAMP and ESI-09 regulated the amount of RAP1-GTP in ETV2-expressing cells (Figure 3G). We thus explored whether RAP1 is a key mediator of endothelial reprogramming by knocking down two isoforms of RAP1 protein, RAP1A and RAP1B (Figure 3H). After 10 days of induction, knockdown of these two genes led to a significant decrease in the generation of CD31+/VE-cadherin+ iECs (Figure 3I). Similar to the FACS analysis, knockdown of RAP1 caused a decrease in the mRNA levels of endothelial genes, such as PECAM1, VE-cadherin, VWF, and VEGFR2 at day 7 of induction (Figure 3J). Given the possibility that cells were in apoptosis during knockdown of RAP1 and thereby expressing lower endothelial markers, we confirmed that knockdown of RAP1A or RAP1B did not affect the viability of the cells by annexin V staining (Figure 3K). With these data, we found that the cAMP/EPAC/RAP1 signaling axis is an important regulatory pathway for endothelial reprogramming.
CREB Target Genes Partly Mediate iEC Reprogramming
To determine other possible mechanisms by which forskolin regulates ETV2-mediated reprogramming, we first assessed the known role of forskolin in modulating reactive oxygen species (ROS).22,23 We observed that the exogenous expression of ETV2 during reprogramming upregulated ROS levels, whereas forskolin treatment was unable to reduce these levels (Figures S3A and S3B). We next elucidated the potential effect of forskolin on the Akt signaling pathway during endothelial reprogramming.24 Western blot analyses of starved cells showed that forskolin, in contrast to VEGFA, could not increase phosphorylated (phospho)-AKT expression (Figure S3C).
We then proceeded to test the role of forskolin in regulating the PKA/CREB pathway during iEC reprogramming. Upon Ser133 phosphorylation by PKA, CREB translocates to the nucleus, initiating transcription of its target genes.25 By performing co-immunoprecipitation assays, we found that phospho-CREB acts on its targets independently of a direct interaction with ETV2 (Figure S3D). Then, we tested whether forskolin increases the endogenous expression levels of ETV2 because phospho-CREB directly binds to the ETV2 promoter during hemato-endothelial differentiation from mESCs.14 RT-PCR analysis demonstrated that endogenous ETV2 was not induced by forskolin treatment, indicating that phospho-CREB does not regulate ETV2 transcription during iEC reprogramming (Figure S3E). To further investigate the role of CREB, we selected two transcription factors (NR4A1 and JUNB), which have promoters that harbor the CRE motif, as candidate regulators of iEC reprogramming.26,27 Forskolin treatment increased the expression of these genes by regulating the phopho-CREB binding to their promoter regions (Figures S3F–S3H). Subsequently, we transduced cells with short hairpin RNAs (shRNAs) targeting each gene to evaluate whether these genes are required in mediating iEC commitment from fibroblasts (Figure S3I). Knockdown of these two genes reduced the population of CD31+/VE-cadherin+ iECs and decreased the expression of endothelial-specific genes compared with control shRNA (shCTL)-infected cells (Figures S3J and S3K). Taken together, these results suggest that target genes of transcription factor CREB, in part, mediate iEC reprogramming.
cAMP Signaling Activation Induces a Vascular Genetic Program and High Angiogenesis Potential during Endothelial Reprogramming
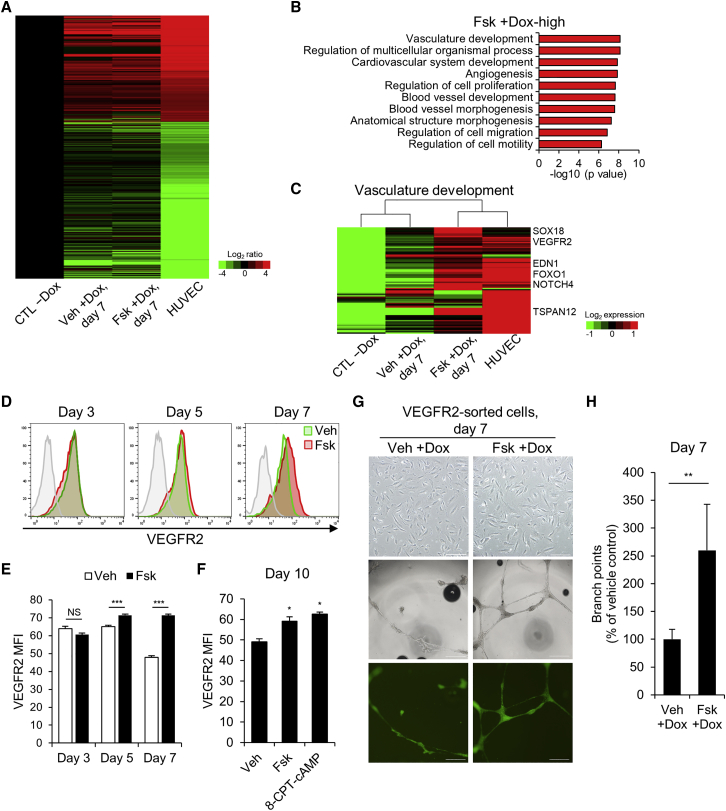
To gain insight into the molecular-level effects of cAMP signaling activation during endothelial reprogramming, we performed RNA sequencing. In addition to human umbilical vein endothelial cells (HUVECs) and control cells not treated with dox (CTL −Dox), we compared the gene expression profiles of vehicle- and forskolin-treated ETV2-hDFs after 7 days of dox induction (Veh +Dox and Fsk +Dox). The heatmap image revealed a shift in the global gene expression patterns of ETV2-hDFs from a hDF state toward an endothelial state in 7 days of dox treatment (Figure 4A). Subsequently, we applied GO analysis to 2-fold upregulated 483 genes in forskolin-treated cells compared with vehicle-treated cells (Figure 4B). This analysis revealed that the upregulated genes in forskolin-treated cells were primarily enriched for vasculature development and angiogenesis. Furthermore, gene set enrichment analysis (GSEA) revealed a significant enrichment of vasculature development gene set in forskolin-treated cells (Figure S4A). By analyzing leading-edge genes that drive enrichment for a particular gene set, we identified forskolin-regulated leaders and associated pathways, such as angiogenesis and Notch signaling pathways (Figure S4B; Table S3). Notably, forskolin-treated cells and HUVECs showed distinct gene expression patterns compared to vehicle-treated cells in the vasculature development gene set, suggesting that forskolin enhanced endothelial reprogramming (Figure 4C; Figure S4C). To identify cell fates adopted during forskolin-mediated endothelial reprogramming, we examined the mRNA expression levels of arterial marker and venous marker genes by qRT-PCR. Interestingly, forskolin-treated cells displayed increased expression levels of arterial marker genes (EFNB2, HEY1, and NOTCH4) compared with vehicle-treated cells (Figures S4D and S4E). This finding suggests that forskolin-treated cells were preferentially specified to arterial fates through the activation of the Notch signaling pathway.28
Figure 4.
cAMP Signaling Activation Induces Endothelial Gene Expression and Enhances Angiogenesis Potential
(A) Heatmap representing the log-fold changes of group Veh +Dox, Fsk +Dox, and HUVECs compared to CTL −Dox. ETV2-hDFs were treated without or with dox for 7 days. Genes that were differentially expressed by more than 4-fold between CTL −Dox and HUVECs are represented. CTL −Dox, control cells without dox. (B) GO term enrichment analysis of the genes that were differentially expressed by ≥2-fold in Fsk +Dox compared with Veh +Dox. The top 10 GO terms for biological processes are shown. (C) Heatmap showing the expression of representative genes involved in vasculature development. (D and E) Representative FACS profiles (D) and quantification (E) of mean fluorescence intensity (MFI) of VEGFR2 in Veh +Dox (green) and Fsk +Dox (red) at day 3, 5, and 7 post-induction. For each group, n = 3; mean ± SD. ***p < 0.001. (F) MFI of VEGFR2 in iECs treated with vehicle, 100 μM 8-CPT-cAMP, or 5 μM forskolin for 10 days. For each group, n = 3; mean ± SD *p < 0.05. (G) Phase-contrast images (top) of VEGFR2-sorted cells from ETV2-hDFs on day 7 of induction. Scale bars, 200 μm. Representative phase-contrast images (middle) and ETV2-Venus fluorescence images (bottom) of tubular structures 24 h after plating on Matrigel. Scale bars, 500 μm. (H) Quantification of branching points formed during in vitro tubulogenesis. n = 4 independent experiments; mean ± SD. **p < 0.01.
Given that forskolin increased the expression of several genes associated with angiogenesis (Figures 4B and 4C; Table S3), we next determined whether forskolin enhances the function of iECs by using a Matrigel tubule formation assay. Because the heterogeneous nature of the reprogramming hindered the direct application of the cells in the assay, we initially sorted the cells with CD31 microbeads at day 14 of induction and observed that forskolin treatment enabled the formation of tubule structures in Matrigel (Figure S4F). However, because CD31 was barely expressed during reprogramming, we attempted to sort the reprogrammed cells with other surface markers to obtain a large number of cells. VEGFR2-positive cells have been characterized as endothelial progenitors during mesoderm specification of PSCs and have been shown to have the potential to differentiate into multiple lineages, including ECs, smooth muscle cells, and cardiomyocytes.29 During endothelial reprogramming, we observed that the size of the VEGFR2-positive population was comparable in vehicle- and forskolin-treated ETV2-hDFs. However, the expression level of VEGR2 was higher in forskolin-treated cells from day 5 of induction (Figures 4D and 4E). Of interest, 8-CPT-2Me-cAMP promoted the expression of VEGFR2, suggesting a molecular role of the EPAC/RAP1 axis in ETV2-mediated reprogramming (Figure 4F). Thus, we sorted VEGFR2-positive cells on day 6 to remove unreprogrammed cells, and the overall purity of sorted cells was about 91% (Figures S4G and S4H). Then, the sorted cells were embedded in Matrigel to investigate angiogenesis capabilities. Even though the amount of tubules formed in Matrigel by HUVECs was considerable, forskolin-treated VEGFR2-sorted cells showed more enhanced neo-angiogenesis than did vehicle-treated VEGFR2-sorted cells (Figures 4G and 4H; Figure S4I). We additionally confirmed that forskolin-treated fibroblasts and VEGFR2-negative cells could not form the tubule structure in Matrigel, implying that the tubular networks were distinctly formed by iECs (Figures S4J and S4K). Collectively, these data demonstrated that this cAMP signaling activator plays a role in improving the reprogramming efficiency and in vitro angiogenesis capacity of iECs.
cAMP Signaling Activated iECs Increase Vascularity in the Ischemic Hindlimb
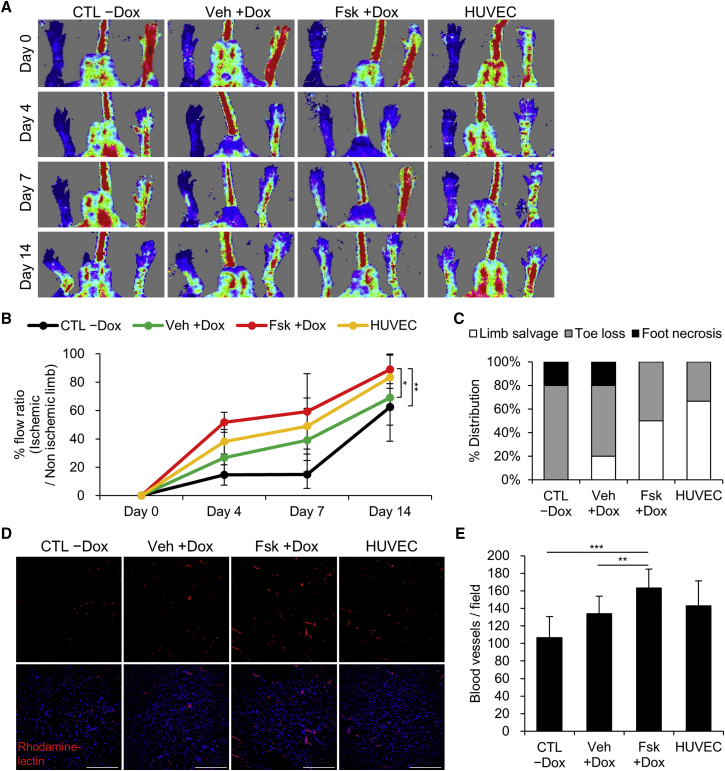
On the basis of in vitro results, we further evaluated whether cAMP signaling activation enhances angiogenic activity of iECs in vivo. We surgically ligated and transected the femoral artery in nude mice to establish a hindlimb ischemia model for efficient blood vessel regeneration.30,31 Four types of cells were intramuscularly transplanted into the ischemic hindlimbs: control cells without dox (CTL −Dox), vehicle- and forskolin-treated VEGFR2-sorted cells (Veh +Dox and Fsk +Dox), and HUVECs. For 14 days post-surgery, mice implanted with forskolin-treated cells and HUVECs showed improved recovery of blood flow compared to control cell-implanted mice (Figure 5A). Notably, transplantation of forskolin-treated cells significantly enhanced blood perfusion and limb salvage in the ischemic hindlimb compared with vehicle-treated cells (Figures 5B and 5C). After isolectin B4 was intravenously administered, the gastrocnemius muscles of each group were dissected and analyzed via blood vessel quantification in the ischemic regions. The number of vessels (rhodamine-positive) was quantified from randomly selected images. The results showed greater levels of angiogenesis in the forskolin-treated cell group than those observed in the vehicle-treated cell group (Figures 5D and 5E). These findings indicate that cAMP signaling activated iECs retain superior angiogenic capacity, augmenting neovascularization in vivo.
Figure 5.
cAMP Signaling-Activated iECs Improve Angiogenesis in Hindlimb Ischemia
(A and B) After transplantation of CTL −Dox, Veh +Dox and Fsk +Dox (VEGFR2-sorted after 5 days of dox induction), and HUVECs, blood reperfusion of ischemic limbs was analyzed via laser Doppler imaging (LDI) by 14 days post-surgery. Representative images of LDI analysis (A) and the ratio of blood perfusion (left injured limb/right normal limb) (B) are shown. For each group, n = 5–6; mean ± SD. *p < 0.05, **p < 0.01 by two-way ANOVA. (C) At 18 days post-surgery, ischemic limbs were examined visually to determine severity of toe and foot necrosis in each group. (D and E) Representative immunofluorescence images (D) and quantification (E) of rhodamine-lectin-stained blood vessels (red) in the gastrocnemius muscle sections on day 18 post-surgery. Nuclei were counterstained with DAPI (blue). Scale bars, 100 μm. For each group, n = 12; mean ± SD. **p < 0.05, ***p < 0.001.
Long-Term Cultured iECs Exhibit Morphological and Functional Characteristics of ECs
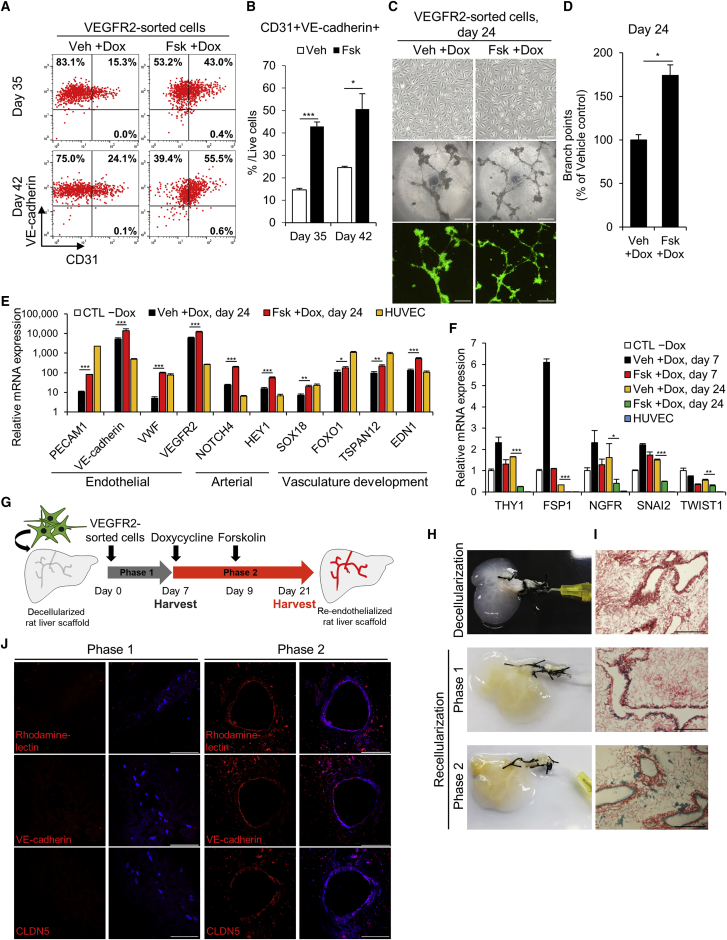
We next determined whether iECs show additional functional characteristics other than angiogenesis potential. VEGFR2-sorted iECs showed reduced proliferation, thereby limiting its long-term passaging and culture. Considering the ChIP-seq data, we reasoned that ETV2 may directly repress cell cycle-related genes, such as KIF14 and CKS2, in hDFs (Figure S5). Thus, following a protocol used to generate late iECs,6 we cultured and passaged VEGFR2-sorted iECs without dox and then retreated them with dox from day 14. We observed that forskolin-treated cultures yielded a larger population of CD31+/VE-cadherin+ cells during a 42-day period compared with vehicle-treated cultures (Figures 6A and 6B). We also checked that VEGFR2-sorted iECs expressed other endothelial markers, such as TIE2 and CD34 (Figure S6A). Interestingly, VEGFR2-sorted iECs on day 24 displayed a more homogeneous cobblestone morphology than did cells on day 7 (Figures 4G and 6C). Additionally, forskolin treatment maintained the expression of VEGFR2 (Figure S6B) and increased the angiogenesis phenotype, as shown by the Matrigel tubule formation assay (Figures 6C and 6D; Figure S6C). To characterize these cells further, we performed qRT-PCR analysis and observed that forskolin upregulated the expression of endothelial marker genes (PECAM1, VE-cadherin, VWF, and VEGFR2), arterial marker genes (NOTCH4 and HEY1), and vasculature development-associated genes (SOX18, FOXO1, TSPAN12, and EDN1) in VEGFR2-sorted iECs (Figure 6E). Of interest, forskolin treatment reduced the expression of mesenchymal marker genes, such as THY1, FSP1, NGFR, SNAI2, and TWIST1, in VEGFR2-sorted iECs, implying the silence of fibroblasts gene signature (Figure 6F). Additionally, to investigate the mesenchymal identity of VEGFR2-sorted iECs, we checked the expressions of THY1 and FSP1 proteins after 35 days of culture. Although THY1-positive cells remained among the CD31-positive population, forskolin significantly reduced the number of THY1-positive cells compared to vehicle treatment (Figures S7A–S7C). We also observed that FSP1 was barely expressed in forskolin-treated cells, suggesting the suppression of mesenchymal identity by forskolin (Figure S7D). Based on these results, we cultivated the forskolin-treated VEGFR2-sorted iECs (Fsk-iECs) without dox treatment in large quantities and assessed other vascular functions of the cells.
Figure 6.
Vasculogenic Ability of Long-Term Cultured Fsk-iECs
(A and B) Representative FACS results (A) and quantification (B) of CD31+/VE-cadherin+ iECs that are derived from VEGFR2-sorted cells at the indicated time points. Note that dox was readministered with vehicle or forskolin from day 14. For each group, n = 3; mean ± SD. *p < 0.05, ***p < 0.001. (C) Phase-contrast images of VEGFR2-sorted cells at day 24 post-induction (top). Scale bars, 200 μm. Representative phase-contrast images (middle) and ETV2-Venus fluorescence images (bottom) of tubular structures 24 h after plating on Matrigel are shown. Scale bars, 500 μm. (D) Quantification of branch points formed during in vitro tubulogenesis. For each group, n = 4; mean ± SD. *p < 0.05. (E and F) qRT-PCR of endothelial, arterial and vasculature development-related genes (E) and mesenchymal marker genes (F) in CTL −Dox, HUVECs, and VEGFR2-sorted cells on day 24 of induction. For each group, n = 3; mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. (G) Schematic diagram of the strategy used for recellularization of VEGFR2-sorted cells into rat acellular liver scaffolds. (H and I) Gross images (H) and representative picrosirius red-stained sections (I) of rat liver extracellular matrix. Decellularized liver (top); recellularized liver with VEGFR2-sorted cells after phase 1 (middle); recellularized liver harvested at the end of phase 2 (bottom). For each group, n = 2. Scale bars, 200 μm. (J) Representative confocal microscopic images of recellularized vessel structures on day 7 and day 21. The sections of each group were stained with rhodamine-lectin (top), VE-cadherin (middle), and CLDN5 (bottom). Nuclei were stained with DAPI (blue). Scale bars, 100 μm.
First, we measured barrier tightness using fluorescein isothiocyanate (FITC)-dextran in Fsk-iECs and HUVECs after treatment with various inflammatory cytokines and observed increased permeability across the monolayer (Figure S6D). We confirmed the uptake of fluorescently labeled acetylated low-density lipoprotein (LDL) in Fsk-iECs and HUVECs, which is characteristic of normal ECs (Figure S6E). Moreover, to demonstrate the integration of Fsk-iECs into vascular networks in vivo, carboxyfluorescein diacetate succinimidyl ester (CFDA)-labeled Fsk-iECs were embedded in Matrigel and subcutaneously injected into the flanks of BALB/c nude mice. We observed that Fsk-iECs (CFDA and rhodamine double-positive) could engraft into existing host vasculature (Figure S6F). Given these results, Fsk-iECs appear to acquire functional features of vascular ECs via direct reprogramming.
Fsk-iECs Can Re-endothelialize the Acellular Rat Liver Scaffold
In our previous study, we successfully reconstructed vasculature trees within decellularized liver scaffolds using EA.hy926 ECs derived from immortalized HUVECs.32 In an effort to prove the vasculogenesis of long-term cultured Fsk-iECs, we developed an ex vivo two-phase culture strategy, which facilitates the vascular modeling of decellularized rat liver scaffold. In this strategy, VEGFR2-sorted iECs, expanded without dox treatment, were seeded and cultured in the scaffold using perfusion bioreactor systems to maintain their viability. In phase 1, the cells were injected to engraft into the scaffold and expanded for 7 days without dox. In phase 2, resupplementation of dox and forskolin in the perfusion medium promoted functional maturation of the cells to form confluent endothelium along the vascular channel in another 14 days (Figure 6G).
To measure the extent of endothelialization, we harvested the cell-seeded scaffolds after phase 1 and phase 2 culture (Figure 6H). Picrosirius red staining was carried out to evaluate cellular distribution within the scaffold for each group. After phase 1 culture, the cells engrafted into the scaffold but did not form elaborate vascular networks. After phase 2 culture, Fsk-iECs developed complete endothelialization, surrounding the vessel lumen of the scaffold (Figure 6I). To determine the proportion of reprogrammed cells contributing to endothelialization, we performed immunostaining of the tissue sections. Consistent with histological examinations, vessel-like structures comprising isolectin B4, VE-cadherin, and CLDN5-positive cells were detected after phase 2 culture (Figure 6J). Taken together, these results support that Fsk-iECs are capable of maintaining their characters and forming aligned vascular networks in tissue-engineered constructs.
Fsk-iECs Show Protective Effects on Ischemia-Induced Severe Injury
To further assess whether long-term cultured Fsk-iECs are applicable for therapeutic uses, we intramusculary transplanted the cells after induction of severe hindlimb ischemia by simultaneous excision of the femoral artery, vein, and its branches. Mice implanted with Fsk-iECs and HUVECs exhibited limb tissues partially protected from necrosis, whereas mice implanted with CTL −Dox experienced severe ischemia resulting in massive muscle degeneration (Figures S8A–S8D). Quantification of blood vessels in gastrocnemius muscle showed greater levels of angiogenesis in the Fsk-iECs and HUVECs groups than those observed in the CTL −Dox group (Figures S8E and S8F). Moreover, we confirmed that Fsk-iECs and HUVECs could be engrafted into the newly generated vessels in ischemic himdlimbs (Figures S8G and S8H). Collectively, we corroborated that Fsk-iECs displayed angiogenic potential in vivo and could be incorporated to host vasculature, both of which are certainly necessary for therapeutic applications.
Discussion
ETV2 is a known master regulator of hemato-endothelial development;33 however, little is known about its molecular role in hDF-to-iEC reprogramming. In this study, by integrating genome-wide ChIP-seq and RNA sequencing (RNA-seq) analyses, we revealed target genes of ETV2, yielding insights into the mechanisms by which ETV2 drives endothelial reprogramming and the molecular links between ETV2 and signaling pathways. The indispensable roles of ETV2-regulated Notch and VEGF signaling during development have been well described.34 However, the finding that ETV2 directly regulates EPAC1, an important regulator of Rap1 signaling, allowed us to infer its novel role in ETV2-mediated endothelial reprogramming. The cAMP/EPAC pathway elevates endothelial barrier function and regulates remodeling of ECs.35 In the context of development, EPAC promotes hematopoietic differentiation from human PSCs by reducing ROS levels.23 Moreover, although EPAC-deficient mice do not exhibit lethal phenotypes,15 the knockout of Rap1 in mice leads to cardiovascular defects and impaired angiogenesis,36 suggesting compensatory roles of other RapGEFs in maintaining Rap1 functions in EPAC-deficient mice. Therefore, considering that selective activation and blockade of EPAC altered the population of iECs, we surmise that the cAMP/EPAC/Rap1 pathway may be involved in ETV2-related pathological angiogenesis, such as tumor angiogenesis37 and ischemic injury-mediated neo-angiogenesis.38
To date, considerable progress has been made to generate ECs by directed differentiation from PSCs.39, 40, 41 Researchers have investigated various signaling pathways involved in this process and have attempted to enhance the efficiency and quality of ECs.4,29,39 Thus, in an effort to improve the reprogramming process, we screened small molecules based on signaling pathways linked to endothelial differentiation and found that the cAMP signaling activator forskolin enabled a highly rapid and efficient generation of iECs from hDFs. However, we observed that some signaling modulators showed distinct effects on directed differentiation and ETV2-mediated reprogramming. This implies that these two processes may generate ECs via different molecular and signaling cascades. Furthermore, one report described that inhibition of transforming growth factor β (TGF-β) signaling enabled iEC reprogramming from amniotic cells by regulating VEGFR2 phosphorylation.8 However, we observed that treatment of cells with SB431542 alone or with forskolin had minimal effects on the efficiency of generating CD31+/VE-cadherin+ populations. This may have resulted from different cell sources and combination of transcription factors used in each study; specifically, our study used ETV2 alone-infected human fibroblasts.
Our transcriptome analyses allowed us to elucidate the molecular effects of cAMP signaling activation in endothelial reprogramming. Treatment of forskolin seems to preferentially lead to the formation of endothelial-like cells via upregulation of vascular development-related genes. Above all, it is noteworthy that the activation of EPAC increases the expression of VEGFR2, as phosphorylated VEGFR2 upon VEGF binding triggers several signaling cascades and critically regulates vascular development and angiogenesis.42 Considering the finding of a previous report that a constitutive active form of PKA regulated the expression of VEGFR2 in a directed differentiation of mESCs to ECs,13 our observation supports a possible role of cAMP/EPAC signaling in the regulation of differentiation.
Furthermore, forskolin is known to enhance other types of reprogramming, such as induced neurons22 or iPSCs,43,44 through regulation of anti-oxidative pathways22 or chromatin accessibility.45 More than that, a recent study revealed that the addition of cAMP promoted DNA demethylation by regulating the amount of 5-hydroxymethylcytosine.46 Thus, it will be interesting to assess in future studies whether forskolin affects metabolism or chromatin architecture underlying ETV2-mediated reprogramming.
Our findings demonstrated an advanced method that could enhance the reprogramming efficiency and quality of iECs. We proposed diverse applications of these cells. Specifically, these cells could be used for the treatment of ischemic diseases and for various endothelial platforms. By applying Matrigel plug assays and a hindlimb ischemia model, Fsk-iECs were shown to be capable of integrating into host vasculature in vivo. However, these assays merely recapitulate angiogenesis, which is the growth of new vasculature extended from existing vessels. In this regard, we provided a newly developed platform for validating long-term endothelialization by using a decellularized liver scaffold. By extension, this approach may lay the groundwork for engineering human hepatic tissue with functional vasculature because well-vascularized networks are indispensable for liver regeneration. Thus, these characteristics of Fsk-iECs provide a special advantage for stably producing iECs for applications in regenerative medicine.
Even though there was a limitation that iECs were not readily expandable, we could overcome this issue by adopting the strategy that VEGFR2-sorted cells were maintained with dox-withdrawn medium for proliferation and then re-induced for rapid maturation. With this approach, we could secure sufficient scales without cell senescence and the loss of characteristics. To this end, with the scalability of iECs, future applications of iECs for the GMP system and therapeutics will be considerable.
Materials and Methods
Generation of iECs
To generate iECs, human dermal fibroblasts transduced with ETV2 were seeded on collagen type I-coated (20 μg/mL, Corning) plates containing FGM-2. After expansion of the cells, the medium was changed to EGM-2 supplemented with doxycycline (Sigma) and replenished every other day. For small molecules treatment, reagents were purchased from the following sources: SB203570, Salirasib, LY294002, SP600125, CHIR99021, and SB431542 from Sigma; rolipram, forskolin, DAPT, KT5720, and ESI-09 from Cayman Chemical; and 8-Bromo-cAMP and 8-CPT-2Me-cAMP from Tocris.
Author Contributions
J.-J.K. and D.-H.K. designed and performed the study. J.-J.K., D.-H.K., J.Y.L, B.-C.L., D.K., and I.K. performed the experiments. M.G.K. helped to analyze ROS levels. S.W.C., D.-I.K., and H.-M.W. contributed discussion. K.-S.K. supervised the study and contributed to writing.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We thank Dr. Rimpei Morita at Keio University for the CSII-EF-HA-ETV2-IRES-Venus plasmid. We are grateful to Dr. Young-sup Yoon at Emory University School of Medicine and Dr. Kyung-Rok Yu at Catholic University of Korea for experimental advice. We also thank Dr. Byeong-Cheol Kang, Dr. Won-Woo Lee, and Dr. Jae-Hak Park at Seoul National University for helpful discussions. This work was carried out with the support of the Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01100201), Rural Development Administration, Republic of Korea.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.11.019.
Supplemental Information
References
- 1.Losordo D.W., Kibbe M.R., Mendelsohn F., Marston W., Driver V.R., Sharafuddin M., Teodorescu V., Wiechmann B.N., Thompson C., Kraiss L., Autologous CD34+ Cell Therapy for Critical Limb Ischemia Investigators A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ. Cardiovasc. Interv. 2012;5:821–830. doi: 10.1161/CIRCINTERVENTIONS.112.968321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velagapudi P., Turagam M., Kolte D., Khera S., Hyder O., Gordon P., Aronow H.D., Leopold J., Abbott J.D. Intramyocardial autologous CD34+ cell therapy for refractory angina: a meta-analysis of randomized controlled trials. Cardiovasc. Revasc. Med. 2019;20:215–219. doi: 10.1016/j.carrev.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Vrtovec B., Poglajen G., Lezaic L., Sever M., Domanovic D., Cernelc P., Socan A., Schrepfer S., Torre-Amione G., Haddad F., Wu J.C. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ. Res. 2013;112:165–173. doi: 10.1161/CIRCRESAHA.112.276519. [DOI] [PubMed] [Google Scholar]
- 4.Israely E., Ginsberg M., Nolan D., Ding B.S., James D., Elemento O., Rafii S., Rabbany S.Y. Akt suppression of TGFβ signaling contributes to the maintenance of vascular identity in embryonic stem cell-derived endothelial cells. Stem Cells. 2014;32:177–190. doi: 10.1002/stem.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James D., Nam H.S., Seandel M., Nolan D., Janovitz T., Tomishima M., Studer L., Lee G., Lyden D., Benezra R. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFβ inhibition is Id1 dependent. Nat. Biotechnol. 2010;28:161–166. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S., Park C., Han J.W., Kim J.Y., Cho K., Kim E.J., Kim S., Lee S.J., Oh S.Y., Tanaka Y. Direct reprogramming of human dermal fibroblasts into endothelial cells using ER71/ETV2. Circ. Res. 2017;120:848–861. doi: 10.1161/CIRCRESAHA.116.309833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morita R., Suzuki M., Kasahara H., Shimizu N., Shichita T., Sekiya T., Kimura A., Sasaki K., Yasukawa H., Yoshimura A. ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells. Proc. Natl. Acad. Sci. USA. 2015;112:160–165. doi: 10.1073/pnas.1413234112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsberg M., James D., Ding B.S., Nolan D., Geng F., Butler J.M., Schachterle W., Pulijaal V.R., Mathew S., Chasen S.T. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFβ suppression. Cell. 2012;151:559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schachterle W., Badwe C.R., Palikuqi B., Kunar B., Ginsberg M., Lis R., Yokoyama M., Elemento O., Scandura J.M., Rafii S. Sox17 drives functional engraftment of endothelium converted from non-vascular cells. Nat. Commun. 2017;8:13963. doi: 10.1038/ncomms13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee D., Park C., Lee H., Lugus J.J., Kim S.H., Arentson E., Chung Y.S., Gomez G., Kyba M., Lin S. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F., Li D., Yu Y.Y., Kang I., Cha M.J., Kim J.Y., Park C., Watson D.K., Wang T., Choi K. Induction of hematopoietic and endothelial cell program orchestrated by ETS transcription factor ER71/ETV2. EMBO Rep. 2015;16:654–669. doi: 10.15252/embr.201439939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrigan R.M., Gründling A. Cyclic di-AMP: another second messenger enters the fray. Nat. Rev. Microbiol. 2013;11:513–524. doi: 10.1038/nrmicro3069. [DOI] [PubMed] [Google Scholar]
- 13.Yamamizu K., Kawasaki K., Katayama S., Watabe T., Yamashita J.K. Enhancement of vascular progenitor potential by protein kinase A through dual induction of Flk-1 and Neuropilin-1. Blood. 2009;114:3707–3716. doi: 10.1182/blood-2008-12-195750. [DOI] [PubMed] [Google Scholar]
- 14.Yamamizu K., Matsunaga T., Katayama S., Kataoka H., Takayama N., Eto K., Nishikawa S., Yamashita J.K. PKA/CREB signaling triggers initiation of endothelial and hematopoietic cell differentiation via Etv2 induction. Stem Cells. 2012;30:687–696. doi: 10.1002/stem.1041. [DOI] [PubMed] [Google Scholar]
- 15.Lezoualc’h F., Fazal L., Laudette M., Conte C. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ. Res. 2016;118:881–897. doi: 10.1161/CIRCRESAHA.115.306529. [DOI] [PubMed] [Google Scholar]
- 16.McLean C.Y., Bristor D., Hiller M., Clarke S.L., Schaar B.T., Lowe C.B., Wenger A.M., Bejerano G. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 2010;28:495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chrzanowska-Wodnicka M., Kraus A.E., Gale D., White G.C., 2nd, Vansluys J. Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficient mice. Blood. 2008;111:2647–2656. doi: 10.1182/blood-2007-08-109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawasaki K., Watabe T., Sase H., Hirashima M., Koide H., Morishita Y., Yuki K., Sasaoka T., Suda T., Katsuki M. Ras signaling directs endothelial specification of VEGFR2+ vascular progenitor cells. J. Cell Biol. 2008;181:131–141. doi: 10.1083/jcb.200709127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pataskar A., Jung J., Smialowski P., Noack F., Calegari F., Straub T., Tiwari V.K. NeuroD1 reprograms chromatin and transcription factor landscapes to induce the neuronal program. EMBO J. 2016;35:24–45. doi: 10.15252/embj.201591206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goode D.K., Obier N., Vijayabaskar M.S., Lie-A-Ling M., Lilly A.J., Hannah R., Lichtinger M., Batta K., Florkowska M., Patel R. Dynamic gene regulatory networks drive hematopoietic specification and differentiation. Dev. Cell. 2016;36:572–587. doi: 10.1016/j.devcel.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pattabiraman D.R., Bierie B., Kober K.I., Thiru P., Krall J.A., Zill C., Reinhardt F., Tam W.L., Weinberg R.A. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science. 2016;351:aad3680. doi: 10.1126/science.aad3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gascón S., Murenu E., Masserdotti G., Ortega F., Russo G.L., Petrik D., Deshpande A., Heinrich C., Karow M., Robertson S.P. Identification and successful negotiation of a metabolic checkpoint in direct neuronal reprogramming. Cell Stem Cell. 2016;18:396–409. doi: 10.1016/j.stem.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Saxena S., Rönn R.E., Guibentif C., Moraghebi R., Woods N.B. Cyclic AMP signaling through Epac axis modulates human hemogenic endothelium and enhances hematopoietic cell generation. Stem Cell Reports. 2016;6:692–703. doi: 10.1016/j.stemcr.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Namkoong S., Kim C.-K., Cho Y.-L., Kim J.-H., Lee H., Ha K.-S., Choe J., Kim P.H., Won M.H., Kwon Y.G. Forskolin increases angiogenesis through the coordinated cross-talk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signaling. Cell. Signal. 2009;21:906–915. doi: 10.1016/j.cellsig.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 25.Taylor S.S., Ilouz R., Zhang P., Kornev A.P. Assembly of allosteric macromolecular switches: lessons from PKA. Nat. Rev. Mol. Cell Biol. 2012;13:646–658. doi: 10.1038/nrm3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng H., Qin L., Zhao D., Tan X., Manseau E.J., Van Hoang M., Senger D.R., Brown L.F., Nagy J.A., Dvorak H.F. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J. Exp. Med. 2006;203:719–729. doi: 10.1084/jem.20051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Licht A.H., Pein O.T., Florin L., Hartenstein B., Reuter H., Arnold B., Lichter P., Angel P., Schorpp-Kistner M. JunB is required for endothelial cell morphogenesis by regulating core-binding factor β. J. Cell Biol. 2006;175:981–991. doi: 10.1083/jcb.200605149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamizu K., Matsunaga T., Uosaki H., Fukushima H., Katayama S., Hiraoka-Kanie M., Mitani K., Yamashita J.K. Convergence of Notch and β-catenin signaling induces arterial fate in vascular progenitors. J. Cell Biol. 2010;189:325–338. doi: 10.1083/jcb.200904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahara M., Hansson E.M., Wernet O., Lui K.O., Später D., Chien K.R. Manipulation of a VEGF-Notch signaling circuit drives formation of functional vascular endothelial progenitors from human pluripotent stem cells. Cell Res. 2014;24:820–841. doi: 10.1038/cr.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welten S.M., Bastiaansen A.J., de Jong R.C., de Vries M.R., Peters E.A., Boonstra M.C., Sheikh S.P., La Monica N., Kandimalla E.R., Quax P.H., Nossent A.Y. Inhibition of 14q32 microRNAs miR-329, miR-487b, miR-494, and miR-495 increases neovascularization and blood flow recovery after ischemia. Circ. Res. 2014;115:696–708. doi: 10.1161/CIRCRESAHA.114.304747. [DOI] [PubMed] [Google Scholar]
- 31.Rao X., Zhong J., Zhang S., Zhang Y., Yu Q., Yang P., Wang M.H., Fulton D.J., Shi H., Dong Z. Loss of methyl-CpG-binding domain protein 2 enhances endothelial angiogenesis and protects mice against hind-limb ischemic injury. Circulation. 2011;123:2964–2974. doi: 10.1161/CIRCULATIONAHA.110.966408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussein K.H., Park K.M., Kang K.S., Woo H.M. Heparin-gelatin mixture improves vascular reconstruction efficiency and hepatic function in bioengineered livers. Acta Biomater. 2016;38:82–93. doi: 10.1016/j.actbio.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 33.Sumanas S., Choi K. ETS transcription factor ETV2/ER71/Etsrp in hematopoietic and vascular development. Curr. Top. Dev. Biol. 2016;118:77–111. doi: 10.1016/bs.ctdb.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Wythe J.D., Dang L.T., Devine W.P., Boudreau E., Artap S.T., He D., Schachterle W., Stainier D.Y., Oettgen P., Black B.L. ETS factors regulate Vegf-dependent arterial specification. Dev. Cell. 2013;26:45–58. doi: 10.1016/j.devcel.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuhara S., Sakurai A., Sano H., Yamagishi A., Somekawa S., Takakura N., Saito Y., Kangawa K., Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol. Cell. Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chrzanowska-Wodnicka M. Rap1 in endothelial biology. Curr. Opin. Hematol. 2017;24:248–255. doi: 10.1097/MOH.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabir A.U., Lee T.J., Pan H., Berry J.C., Krchma K., Wu J., Liu F., Kang H.K., Hinman K., Yang L. Requisite endothelial reactivation and effective siRNA nanoparticle targeting of Etv2/Er71 in tumor angiogenesis. JCI Insight. 2018;3:97349. doi: 10.1172/jci.insight.97349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park C., Lee T.J., Bhang S.H., Liu F., Nakamura R., Oladipupo S.S., Pitha-Rowe I., Capoccia B., Choi H.S., Kim T.M. Injury-mediated vascular regeneration requires endothelial ER71/ETV2. Arterioscler. Thromb. Vasc. Biol. 2016;36:86–96. doi: 10.1161/ATVBAHA.115.306430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harding A., Cortez-Toledo E., Magner N.L., Beegle J.R., Coleal-Bergum D.P., Hao D., Wang A., Nolta J.A., Zhou P. Highly efficient differentiation of endothelial cells from pluripotent stem cells requires the MAPK and the PI3K pathways. Stem Cells. 2017;35:909–919. doi: 10.1002/stem.2577. [DOI] [PubMed] [Google Scholar]
- 40.Patsch C., Challet-Meylan L., Thoma E.C., Urich E., Heckel T., O’Sullivan J.F., Grainger S.J., Kapp F.G., Sun L., Christensen K. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 2015;17:994–1003. doi: 10.1038/ncb3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prasain N., Lee M.R., Vemula S., Meador J.L., Yoshimoto M., Ferkowicz M.J., Fett A., Gupta M., Rapp B.M., Saadatzadeh M.R. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat. Biotechnol. 2014;32:1151–1157. doi: 10.1038/nbt.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simons M., Gordon E., Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016;17:611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 43.Hou P., Li Y., Zhang X., Liu C., Guan J., Li H., Zhao T., Ye J., Yang W., Liu K. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 44.Fritz A.L., Adil M.M., Mao S.R., Schaffer D.V. cAMP and EPAC signaling functionally replace OCT4 during induced pluripotent stem cell reprogramming. Mol. Ther. 2015;23:952–963. doi: 10.1038/mt.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith D.K., Yang J., Liu M.L., Zhang C.L. Small molecules modulate chromatin accessibility to promote NEUROG2-mediated fibroblast-to-neuron reprogramming. Stem Cell Reports. 2016;7:955–969. doi: 10.1016/j.stemcr.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camarena V., Sant D.W., Huff T.C., Mustafi S., Muir R.K., Aron A.T., Chang C.J., Renslo A.R., Monje P.V., Wang G. cAMP signaling regulates DNA hydroxymethylation by augmenting the intracellular labile ferrous iron pool. eLife. 2017;6:e29750. doi: 10.7554/eLife.29750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.