Abstract
BACKGROUND
Esophageo-gastro-duodenoscopy (EGD) is an important procedure used for detection and diagnosis of esophago-gastric lesions. There exists no consensus on the technique of examination.
AIM
To identify recent advances in diagnostic EGDs to improve diagnostic yield.
METHODS
We queried the PubMed database for relevant articles published between January 2001 and August 2019 as well as hand searched references from recently published endoscopy guidelines. Keywords used included free text and MeSH terms addressing quality indicators and technological innovations in EGDs. Factors affecting diagnostic yield and EGD quality were identified and divided into the follow segments: Pre endoscopy preparation, sedation, examination schema, examination time, routine biopsy, image enhanced endoscopy and future developments.
RESULTS
We identified 120 relevant abstracts of which we utilized 67 of these studies in our review. Adequate pre-endoscopy preparation with simethicone and pronase increases gastric visibility. Proper sedation, especially with propofol, increases patient satisfaction after procedure and may improve detection of superficial gastrointestinal lesions. There is a movement towards mandatory picture documentation during EGD as well as dedicating sufficient time for examination improves diagnostic yield. The use of image enhanced endoscopy and magnifying endoscopy improves detection of squamous cell carcinoma and gastric neoplasm. The magnifying endoscopy simple diagnostic algorithm is useful for diagnosis of early gastric cancer.
CONCLUSION
There is a steady momentum in the past decade towards improving diagnostic yield, quality and reporting in EGDs. Other interesting innovations, such as Raman spectroscopy, endocytoscopy and artificial intelligence may have widespread endoscopic applications in the near future.
Keywords: Upper endoscopy, Gastroscopy, Quality indicators, Gastric cancer
Core tip: In this article, we aim to provide a comprehensive review to identify factors affecting diagnostic yield and esophageo-gastro-duodenoscopy quality. These are divided into pre endoscopy preparation, sedation, examination schema, examination time, routine biopsy, image enhanced endoscopy and future developments. There is a steady momentum in the past decade towards improving diagnostic yield, quality and reporting in esophageo-gastro-duodenoscopys. Other interesting innovations, such as Raman spectroscopy, endocytoscopy and artificial intelligence will also be discussed.
INTRODUCTION
Gastric cancer is the fifth most common malignancy in the world and the third leading cause of cancer death worldwide[1]. Despite recent advances in the treatment of gastric cancer, the 5 years survival of gastric cancer patients with locoregional or distant disease remains dismal[2]. Early detection is the key strategy to improve patient survival[3].
Although population based screening for gastric cancer has not been found to be cost-effective outside high risk populations[4,5], an esophagogastroduodenoscopy (EGD) examination is an ubiquitous first line tool to investigate upper gastrointestinal symptoms in most countries worldwide. There exists no worldwide consensus on the technique of examination to optimize its diagnostic yield[6,7]. Guidelines that suggest standardized endoscopic examination and documentation exist[8,9] but are cumbersome and often not followed. In absence of a widely recognized and accepted protocol for a systemic examination and objective measurement of quality in upper gastrointestinal endoscopy, false-negative rates of EGD examinations are estimated to vary between 10% and 20%[10-12].
Following Barclay et al[13]’s study demonstrating that endoscopists with a mean withdrawal time of six min or more consistently detected more adenomas and colon cancers, our group demonstrated that endoscopists with a mean examination time of 7 min or more were more likely to uncover premalignant and neoplastic lesions during diagnostic EGDs[14]. In addition to examination time, several other factors may also affect the diagnostic yield of EGDs. Through this review, we aim to provide a succinct summary of the recent literature on the advances in diagnostic EGDs.
MATERIALS AND METHODS
The PubMed database was queried for relevant articles published between January 2001 and August 2019. A deliberate longer period was chosen so that the literature review would also include dated guidelines in order to reflect how practices of endoscopy have changed over the past 2 decades. The keywords are listed as follows: Type of intervention: Gastroscopy, esophagogastroduodenoscopy, upper endoscopy, narrow band imaging, image enhanced endoscopy, chromoendoscopy, artificial intelligence, biopsy; outcomes: Quality indicators, standardization, quality, quality indicators, gastric cancer, gastric neoplasia. The references of recently published endoscopy guidelines were hand-searched to include studies that were missed by the above search strategy. All guidelines, retrospective, case-control studies, randomized controlled studies as well as systemic review/meta-analysis captured by the search strategy were assessed for suitability. The literature review was performed by two reviewers and novel articles were included into this systemic review.
RESULTS
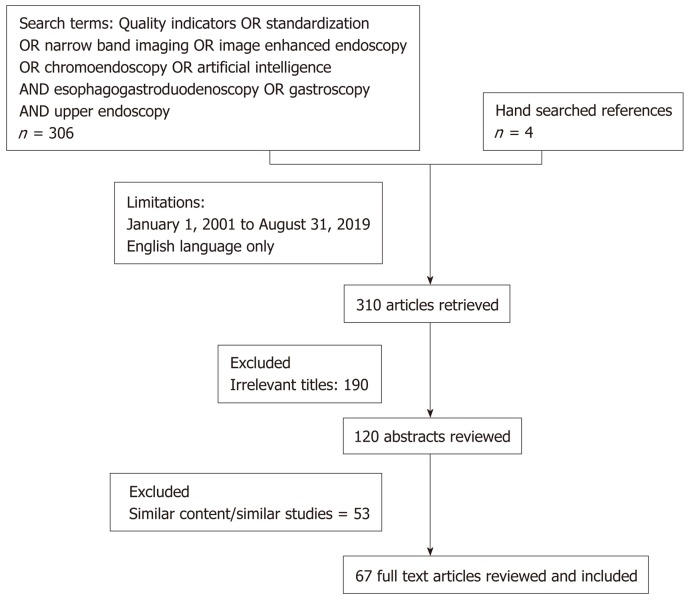
Using the above search terms, we identified 306 articles from PubMed based on the above search terms. Four additional articles were retrieved by hand searching recent published endoscopy guidelines, 190 articles were excluded after screening for irrelevant titles, 120 abstracts were reviewed and subsequently 67 articles were included in this review of recent advances in diagnostic endoscopy. Articles were excluded if both authors agreed that those study findings were not novel and/or findings did not present additional knowledge to already published guidelines or literature. A PRISMA diagram is presented in Figure 1. The major subheadings were divided as follows: Pre endoscopic preparation, endoscopy sedation, systemic examination, duration of examination, routine endoscopic biopsy, image enhanced endoscopy as well as future developments. The key recommendations and findings are summarized in Table 1.
Figure 1.
PRISMA diagram of literature review.
Table 1.
Summary of of key recommendations for improving quality in diagnostic endoscopy
| Pre endoscopic preparation |
| Premedication with simethicone or simethicone and N-acetylcysteine improves visualisation in the stomach and oesophagus |
| Pronase, a proteolytic agent, increases gastric visibility scores |
| Allowing clear liquids up to 2 h of endoscopy improves patient anxiety and patient comfort |
| Use of antispasmodic agents to enhance detection of high risk superficial neoplasms is recommended |
| Sedation |
| Patients should be counselled adequately regarding sedation options. Reported satisfaction is higher after endoscopy with sedation |
| Propofol sedation decreases sedation time and improves the detection of early stage pharyngeal and upper gastrointestinal cancers |
| Propofol use is associated with better inspection during oesophageogastroduodenoscopy (OGD) and hence offers better quality examination compared to midazolam |
| In patients undergoing sedation with midazolam, routine fentanyl use reduces additional midazolam doses and shortens procedural times and reduces patient retching |
| In low risk patients and procedures, the use of a target controlled infusion of propofol and alfentanil administered by a nurse anesthetist has been shown to be safe and improves anesthesia quality |
| In patients who prefer not to undergo sedation, small caliber OGD performed via transnasal or transoral route may offer better patient tolerability with similar level of diagnostic accuracy |
| Systemic examination |
| A mandatory set of systemic images in endoscopy reports may increase quality of reports and reduce variability in interpretation |
| There is currently no consensus how many pictures should be recorded for an adequate OGD |
| The use of systemic alphanumeric coded endoscopy approach during endoscopy increases yield of high risk lesions |
| Endoscopists with high rates of ampulla photo documentation were more likely to detect upper gastrointestinal neoplasms and dysplasia and ampulla photo documentation may be used a quality indicator for thorough gastroscopy |
| Duration of examination |
| Endoscopists with average Barrett’s inspection time (BIT) exceeding 1 min per centimeter detected more endoscopically suspicious lesions; A longer BIT correlated with high grade dysplasia and adenocarcinoma detection |
| Endoscopists with a mean examination time exceeding 7 min for a normal examination were twice as likely to detect high risk lesions and neoplastic lesions compared to their faster counterparts |
| The effect of longer examination time may be diminished in very experienced endoscopists who are able to readily recognise neoplastic lesions |
| Various societies and consensus guidelines now recommend at least 7–8 min for an adequate upper endoscopic examination |
| Routine endoscopy biopsy |
| No studies have demonstrated that routine biopsy improves detection of high risk lesions during endoscopy |
| Endoscopists with high biopsy rates were less likely to miss a cancer in patients who undergo interval endoscopy |
| Image enhanced endoscopy |
| Detection of oesophageal lesions |
| Absence of iodine staining on chromoendoscopy, even when negative for dysplasia on initial histology, identifies esophageal lesions with high sensitivity for dysplasia or cancer in later follow ups |
| Non-magnifying narrow band imaging (NBI) was found to have similar sensitivity with superior accuracy and specificity compared to iodine staining for early squamous cell carcinoma |
| Endoscopists should be trained in the NBI use. NBI Sensitivity was higher in the hands of more experienced endoscopists |
| Blue laser imaging (BLI) is comparable to magnifying NBI as well as Lugol iodine chromoendoscopy for detection of early esophageal cancer |
| Detection of gastric lesions |
| Newer generation NBI improves pick up rate of focal gastric lesions and intestinal metaplasia compared to high definition white light endoscopy |
| The magnifying endoscopy simple diagnostic algorithm guideline should be followed to identify early cancers |
| In the presence of a demarcation line as well as irregular micro surface and/or irregular microvascular pattern, a diagnosis of early gastric cancer can be confidently made |
| High specificity in excluding gastric neoplasms may reduce the need for unnecessary biopsies if magnifying endoscopy (ME) and NBI is employed |
| ME-NBI improves visualization of the horizontal margin of early gastric cancer compared to low magnification NBI and chromoendoscopy |
| BLI- Bright was demonstrated to be superior to white light endoscopy (WLE) in the real-time detection of early gastric cancers |
| Linked color imaging (LCI) identifies confidently Helicobacter pylori infection, gastric intestinal metaplasia and early gastric cancer |
| The diagnostic accuracy of magnifying LCI with indigo carmine for small depressed gastric lesions has been shown to be better than both conventional WLE and magnifying BLI |
| Future developments |
| Raman spectroscopy differentiates normal gastric tissue from premalignant and malignant tissue and allows real time diagnosis and reduces need for biopsy |
| Endocytoscopy allows real time diagnosis of Helicobacter pylori positivity, intestinal metaplasia, atrophic gastritis and early gastric cancer. There is good interobserver agreement between endoscopists and pathologists |
| Neural network based artificial intelligence can now be trained to identify oesophageal squamous cell carcinoma and gastric cancer with high sensitivity and specificity |
DISCUSSION
Pre endoscopy preparation
Premedication with simethicone or simethicone and N-acetylcysteine improved visualization in the esophagus and stomach significantly compared to pre-medication with water alone in the esophagus and stomach[15]. Addition of pronase, a proteolytic agent, to simethicone improves gastric visibility scores (73% vs 49%) with the need for lesser water flushes with no increase in endoscopic examination time[16]. The use of pronase in endoscopic flushing during biopsy decreases the thickness of mucus, depth of biopsy and improved diagnostic assessment[17] when performing endoscopic biopsies. In performing gastroscopy, we conventionally advise patients to remain fasted for 4-6 h to prevent aspiration; however newer data suggest that allowing clear liquids up to 2 h of endoscopy improves anxiety (8% vs 25%, P = 0.029), patient comfort (18% vs 42%, P = 0.01), reduces hunger (44% vs 67%, P = 0.024) without increasing regurgitation of gastric contents[18]. In a randomized trial, the use of lidocaine spray alone compared to lidocaine spray plus a lidocaine viscous solution resulted in a statistically significant but clinically insignificant reduction in the number of pharyngeal sites observed; there were no difference in the number of gag reflexes between the 2 groups when sedation was administered[19].
The Asian consensus on standards of diagnostic upper endoscopy for neoplasia recommends the use of antispasmodic agents to enhance detection rates of superficial neoplasms during oesophageogastroduodenoscopy (OGD) and image enhanced endoscopy (IEE)[20] but acknowledges the lack of evidence behind the re-commendation.
Sedation
There is conflicting data on which is the recommended sedation regime for patients undergoing upper endoscopy. A closed ended questionnaire found that patients undergoing OGD did not have sufficient information prior to endoscopic examination to make an informed choice regarding sedation option and that patient satisfaction was higher in patients who received sedation[21]. The use of propofol sedation decreased the sedation time and improved the detection of earlier pharyngeal and upper gastrointestinal (GI) superficial squamous cell carcinoma compared to when no sedation was used in upper GI endoscopies utilizing narrow band imaging (NBI)[22]. In patients who prefer not to undergo sedation, small caliber OGD performed via the transnasal or transoral route may offer better patient tolerability with similar level of diagnostic accuracy[23-25].
The use of propofol compared to midazolam in a randomized controlled trial has demonstrated that sedation with propofol is associated with better inspection of multiple areas during OGD and hence results in a better quality examination[26]. Patients who undergo endoscopy with propofol sedation have a significantly shortened time between injection and intubation of the esophagus, and a lengthened time between intubation of the esophagus and procedure completion, suggesting that the use of propofol allows the endoscopist to perform a more detailed examination[26]. In a recently published randomized controlled trial comparing routine use of 100 mcg of fentanyl compared to placebo on top of a regular dose of IV 2 mg midazolam, routine administration of 100 mcg of fentanyl resulted in less midazolam use, shorter procedural times (8.5 min vs 11.1 min, P < 0.001) and significantly lesser retching observed in patients[27]. The use of propofol improves quality image acquisition compared to the use of midazolam/fentanyl in a randomized trial of patients undergoing upper GI confocal laser endomicroscopy[28]. An improvement in the diagnostic accuracy of mucosal lesions was observed but not found to be statistically significant[28]. The use of propofol shortened examination times and led to better patient satisfaction[28]. In another randomized trial comparing midazolam and propofol versus propofol in low risk patients, the use of midazolam and propofol reduced the dose of propofol used and allowed the patients to enter the desired state of sedation more quickly but did not improve patient satisfaction or quality of endoscopic evaluation[29]. The use of the propofol after midazolam induction was found to be safe, even in the absence of an anesthesiologist[30]; however, it was associated with 10% risk of arterial hypotension, 2% risk of bradycardia and hypoxaemia[30]. One patient required tracheal intubation as a result[30]. A meta-analysis did not find any differences in terms of anaesthesia duration, recovery time and mean arterial pressure at intubation and patient satisfaction, but the use of etomidate reduced hypoxaemia and injection pain compared to propofol[31]. The use of a target controlled infusion (TCI) of propofol and alfentanil administered by a nurse anaesthetist has been shown to improve anaesthesia quality as measured by response to stimulation during maintenance, hemodynamic stability, EtC02 levels and recovery time compared to manual administration[32,33].The European Society Gastrointestinal Endoscopy advocates that non anaesthesiologist administered propofol (NAAP) is possible with NAAP trained endoscopists and nurses but caution against NAAP in patients with American society of anesthesiologists category ≥ 3, a Mallampati’s class of 3, risk factors for airway obstruction or anticipated prolonged procedures[34,35].
Systemic examination during gastroscopy
Asfeldt et al[36] reported in 2008 that there was great variability in the inter-observer and intra-observer interpretation of pathology in endoscopic images, which appears to be independent of endoscopist seniority. A mandatory set of systemic images into endoscopy reports may increase quality of reports and reduce variability in interpretation[36].
The recent Asian consensus advocates that systemic examination of the stomach and the esophagus may improve detection rates of upper GI superficial neoplasms[20] to avoid missing neoplastic lesions especially at high risk areas of the esophagus[37] or stomach[38]. Several societies and research groups have published recommendations, but a large disparity exists amongst these guidelines[39]. The European Society of Gastrointestinal Endoscopy, in 2001, advocates 8 pictures to be taken during any gastroscopy examination, with 2 pictures in the esophagus, 4 in the stomach and 2 in the duodenum[8]. Yao[40], on the other hand, suggests a protocol where 22 photos are taken but no well-designed study has been performed to ascertain the efficacy of this protocol and it may be too complicated to follow in clinical practice[40]. Using a systemic alphanumeric coded endoscopy approach where 5 regions in the stomach and 21 areas are imaged, overall detection rate of gastric neoplasia was found to be 2.8%, with 31% of patients having a premalignant finding and 57% with Helicobacter pylori infection[41].
Interestingly, a retrospective review also identified that endoscopists with high rates of ampulla photo documentation were more likely to detect upper GI neoplasms (0.26% vs 0.20%, P = 0.03), more dysplasia (0.17% vs 0.11%, P = 0.004) and smaller neoplasms (0.14% vs 0.09%, P = 0.01) compared to endoscopists with low rates of ampulla photo documentation[42]. Duration of examination and seniority of endoscopists did not correlate with ampulla photo-documentation rates[42]. Ampulla photo documentation may be used as a quality indicator for a thorough gastroscopy examination.
Duration of examination
Since the landmark paper describing withdrawal times on adenoma detection rates in colonoscopy[13], there has been considerable interest on the effect of examination time on the detection rates of neoplastic lesions in upper endoscopy. In Barrett’s esophagus, endoscopists with average Barrett’s inspection time (BIT) exceeding 1 min per centimeter during examination detected more endoscopically suspicious lesions (54.2% vs 13.3%, P = 0.04) and a direct correlation between mean BIT per centimeter of BE and detection of high grade dysplasia and early adenocarcinoma (ρ = 0.63, P = 0.03) was found. These studies prompted our group to perform a study on the effect of examination time on endoscopy yield during upper endoscopy. In our study, we divided endoscopists as “fast endoscopists” or “slow endoscopists”, depending on whether their mean examination times for a normal examination exceeded 7 min. We found that slow endoscopists were twice as likely to detect high risk lesions as fast endoscopists (OR = 2.50, 95%CI: 1.52–4.12) and thrice as likely to detect neoplastic lesions (OR = 3.42, 95%CI: 1.25–10.38). Our findings were affirmed by 2 large database reviews from Japan and Korea respectively (Table 2). The Japanese study comprised of 55786 examinations[43] which demonstrated that moderate endoscopists (mean examination time 5-7 min) and slow endoscopists (mean examination time > 7 min) were twice as likely to detect neoplastic lesions compared to fast endoscopists ( mean examination time < 5 min). The second study involved 111962 subjects who underwent EGD as part of a healthy screening exercise between January 2009 to December 2016 in Korea[44]. Using a cut off of 3 min, Park et al[44] determined that slow endoscopists were more likely to detect gastric adenomas or carcinomas compared to fast endoscopists; slow endoscopists also had higher neoplasm detection rates compared to faster endoscopists (0.28% vs 0.20%, P = 0.0054)[44]. In 2018, Yoshimizu et al[45] found that inspection time did not affect neoplasm detection rates, but were higher in endoscopists with more than 1 year of intensive training (2.2% vs 3.7%, OR = 1.65, 95%CI: 1.02–2.68). All endoscopists included in this study, regardless of their length of intensive training, were seasoned endoscopists who had performed at least 1000 EGDs. The findings of these studies have been summarized in Table 2. There is currently no consensus what the minimum time for a quality endoscopic examination should be, although 3 studies suggest that a longer examination results in higher endoscopic yield. The Asian consensus recommends a minimum examination time of 8 min[20] whereas the British Society of Gastroenterology guidelines recommends a minimum examination time of at least 7 min[46]. The effect of longer examination time, may be diminished in very experienced endoscopists who possess skills to readily recognize a neoplastic lesion[45].
Table 2.
Summary of studies reporting effect of endoscopy examination time on detection rates during upper endoscopy
| Ref. | Country of origin | Study design | Sample size | Classification timings | Findings |
| Teh et al[14], 2015 | Singapore | Retrospective database | 837 | Fast < 7 min | Slow endoscopists twice likely yo detect high risk lesions than fast endoscopist (OR = 2.5, 95%CI: 1.52-4.12) |
| Slow > 7 min | |||||
| Kawamura et al[43], 2017 | Japan | Retrospective database | 15763 | Fast < 5 min | OR for neoplastic lesion detection for moderate and slow group was 1.9 (95%CI: 1.06–3.4 ) and 1.89 (95%Cl: 0.98–3.64) respectively |
| Moderate 5-7 min | |||||
| Slow > 7 min | |||||
| Park et al[44], 2017 | South Korea | Retrospective database | 111962 | Fast < 3 min | Slow endoscopists more likely to detect gastric adenomas/carcinomas than fast endoscopist (OR = 1.52, 95%CI: 1.17–1.97) |
| Slow > 3 min | |||||
| Yoshimizu et al[45], 2018 | Japan | Retrospective database | 3925 | Fast < 7 min | No difference in neoplasm pick up rates amongst the 3 groups |
| Moderate 7-10 min | |||||
| Endoscopists > 1 yr of intensive training picked up more lesions | |||||
| Slow > 10 min |
Routine endoscopy biopsy
There are no studies on the efficacy of routine biopsy during upper endoscopy. Januszewicz et al[47] recently published data demonstrating that endoscopists with high endoscopist biopsy rate (EBR > 43.8%) were lesser likely to miss a cancer which was detected up 3 years after the index gastroscopy; this association was also validated in a second endoscopy unit[47].
Image enhanced endoscopy
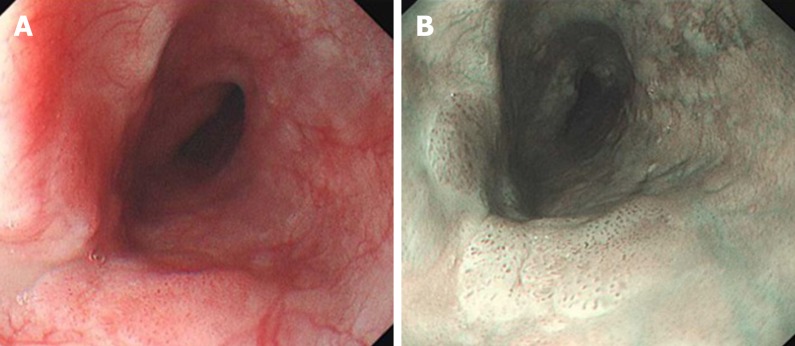
There are 2 forms of image enhanced endoscopy, namely dyed based technique and equipment image-based techniques. Iodine has been traditionally used as chro-moendoscopy to detect superficial squamous lesions not easily detected by white light endoscopy. Lugol’s iodine stains glycogen in normal esophageal epithelium and absence of staining occurs in glycogen depleted dysplasic epithelium, resulting in the “pink-color sign”[48]. Non magnifying narrow band imaging identifies suspicious areas by identification of areas with brownish discoloration; and magnifying narrow band imaging is utilized in these suspicious areas to further characterize the malignant risk of these lesions by observing the intrapapillary capillary loops according to the Japanese Esophageal Society classification[49] and avascular areas[50]. Figure 2 demonstrates a superficial squamous cell carcinoma depicted on white light endoscopy and narrow band imaging. Absence of iodine staining on chromoendoscopy, even when negative for dysplasia on initial histology, identifies esophageal lesions with high sensitivity for dysplasia, carcinoma in situ or esophageal squamous cell carcinoma (ESCC) in later follow ups[51]. In a prospective, propensity matched study, non-magnifying NBI was found to have similar sensitivity with superior accuracy and specificity compared to chromo-endoscopy with iodine staining for early squamous cell carcinoma[52]. A recent meta-analysis identified that NBI was comparable to Lugol chromoendoscopy to identify high -grade dysplasia and squamous cell carcinoma, with high summary receiver operating characteristics curves of 0.9587 and 0.9685 respectively. Training in the use of NBI is important, as it has been demonstrated that sensitivity of NBI was higher in the hands of more experienced endoscopists (100% vs 53%, P < 0.001) and sensitivity of NBI in less experienced endoscopists improved from 43% to 60% with training[52]. Blue laser imaging, a recent innovation by Fujifilm, which combines laser light wavelengths of 410 nm and 450 nm and fluorescent light, has been shown to be comparable to magnifying NBI as well as Lugol iodine chromoendoscopy for detection of early esophageal cancer[53].
Figure 2.
White light endoscopy and narrow band imaging of superficial esophageal squamous cell carcinoma. A: White light endoscopy of superficial esophageal squamous cell carcinoma; B: Narrow band imaging of superficial esophageal squamous cell carcinoma.
In performing OGD to detect gastric lesions, the use of NBI is especially useful in magnifying endoscopy. NBI utilizes 2 narrow band illumination (415 nm and 540 nm) without conventional white light[54]. The resultant image is hence too dark to be used for gastroscopy[54] in the stomach which has a large lumen when sufficiency insufflated. Even so, NBI was demonstrated to be effective in the detection of atrophic gastritis and intestinal metaplasia when compared to white light in a randomized cross over study[55]. Tri-modal imaging endoscopy with white light endoscopy, autofluorescence imaging and narrow band imaging had previously demonstrated its efficacy in improving detection of premalignant gastric lesions compared to white light endoscopy alone[56,57]. Comparing the use newer generation NBI which is twice as bright as the previous version to high definition white light endoscopy, the use of NBI resulted in higher pick up rate of focal gastric lesions (40.6% vs 29%, P = 0.003) and intestinal metaplasia (17.7% vs 7.7%, P < 0.001) with no difference in the detection of gastric cancers (1% vs 2.4%, P = 0.189)[58].
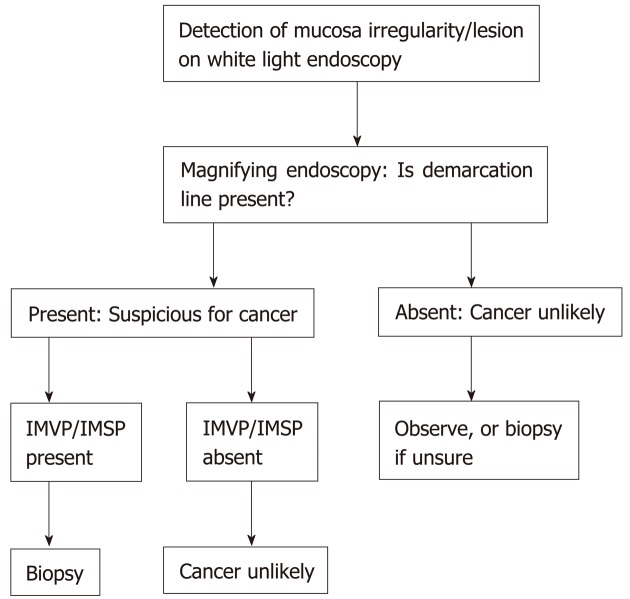
On magnifying endoscopy, NBI allows the inspection of a demarcation line, the micro-vessel and micro-surface patterns. We follow the magnifying endoscopy simple diagnostic algorithm (Figure 3) proposed by Muto et al[59]. First, irregularity (depression or elevation) as well as colour changes (pale areas or erythema) is closely examined on the mucosa surface. Once this is identified, we attempt to identify a demarcation line between the lesion and background mucosa using magnifying endoscopy and narrow band imaging (ME-NBI). The lesion is most likely benign if no demarcation line is present. In the presence of a demarcation line as well as irregular micro surface and/or irregular microvascular pattern, a diagnosis of early gastric cancer can be confidently made[59]. In a pooled analysis of 14 studies, the sensitivity and specificity of ME-NBI was 0.86 and 0.96 respectively with high specificity of 0.96 and 0.98 for depressed gastric lesions and lesions less than 10 mm[60]. High specificity in excluding gastric neoplasms may reduce the need for unnecessary biopsies if ME-NBI is employed[60]. In a meta-analysis of 10 studies comparing white light endoscopy (WLE) and magnifying endoscopy-narrow band imaging (ME-NBI) for the detection of early gastric lesions, the pooled sensitivity, specificity and Area under Curve (AUC) using ME-NBI was superior at 0.83, 0.96 and 0.96 compared to 0.48, 0.67 and 0.62 for WLE[61]. A recent randomized controlled study showed that ME-NBI had similar delineation rates of tumor margin compared to indigo carmine chromoendoscopy (88.0% vs 85.7%, P = 0.63). The use of ME-NBI may however improve visualization of the horizontal margin of early gastric cancer[62] compared to low magnification NBI or chromoendoscopy by better visualization of the number and depth of subepithelial capillaries[63]. A prospective study previously demonstrated that magnifying-blue laser imaging (BLI) had improved sensitivity, specificity and accuracy compared to white light imaging[64] for detection of early gastric cancers. In a randomized controlled study, BLI-bright was demonstrated to be superior to WLE in the real- time detection of early gastric cancers (93.1% vs 50.0%, P = 0.01)[65]. BLI may be a promising addition to IEE to improve the detection of gastric neoplasms.
Figure 3.
Magnifying endoscopy simple diagnostic algorithm for diagnosis of early gastric cancer. Adapted from Muto et al[59]’s magnifying endoscopy simple diagnostic algorithm of early gastric cancer. IMVP: Irregular microvascular pattern; IMSP: Irregular microsurface pattern.
Another innovation recently by Fujifilm corp is linked color imaging (LCI) which is derived from a combination of white light and narrow band short wavelength light[66]. The reflectance of this combination of light on abnormal tissue and surrounding mucosa is then acquired and LCI reallocates the acquired color to a color similar to the background mucosa but of greater contrast so that minute differences can be detected and closer examination of lesion architecture and vascularity is possible[66]. In a retrospective review of 60 patients, the LCI finding of red appearance of fundic gland mucosa was 85.8% sensitive and 93.3% specific for Helicobacter pylori infection, higher than WLE[67]. The classical patchy lavender color on LCI is classically described in patients with gastric intestinal metaplasia[68-70]. For early gastric cancer, the use of LCI was superior for lesion recognition and the color difference between cancer and non-cancer areas was demonstrated to be greater[71] with higher blood vessel density appreciated using LCI (5.96% vs 4.15%, P = 0.004)[72]. The diagnostic accuracy of magnifying LCI with indigo carmine for small depressed gastric lesions has been shown to be better than both conventional WLE and magnifying BLI[73], especially for non-expert endoscopists[74]. The evidence of LCI use for esophageal squamous cell carcinoma is currently limited but it may be useful for assessment of invasion depth for superficial ESCC.
LCI, especially magnifying LCI, may become a mainstream modality for diagnosis of early gastric cancers in the near future.
Future developments
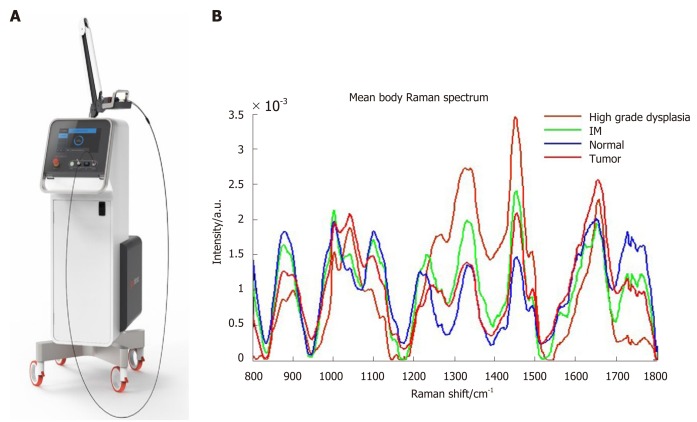
There are several new technological advancements to aid endoscopists identify high risk gastric lesions as well as reduce the need for routine biopsies. Raman spectroscopy utilizes optical vibrational technique where there is inelastic scattering of the incident laser light after the incident laser light polarizes the target tissue molecules. The Raman probe is depicted in Figure 4A. The characteristic biochemical tissue and molecular make up of premalignant tissue (intestinal metaplasia, high grade dysplasia and tumour) allows it to be differentiated from normal tissue after Raman spectroscopy is applied (Figure 4B). A fibre-optic Raman endoscopic technique allows real time acquisition of the Raman spectra within 0.5 s[75], with a high sensitivity (89.3%), specificity (92.2%) for gastric intestinal metaplasia[76], reducing the need for biopsies.
Figure 4.
Raman spectroscopy probe and different Raman spectrum according to normal tissue, intestinal metaplasia, high grade dysplasia and tumor tissue. A: Raman spectroscopy probe; B: Different Raman spectrum according to normal tissue, intestinal metaplasia, high grade dysplasia and tumor tissue.
Olympus has recently introduced the endocytoscopy (Olympus GIF-H290EC) with up to 520 × magnification to allow cellular assessment. Prior to examination, the lesion is washed with saline and spraying with crystal violet and methylene blue. Endocytoscopy findings in the antrum correlates with Helicobacter pylori positivity[77] with high sensitivity and specificity for intestinal metaplasia[78] and atrophic gastritis[77] as well as gastric cancer diagnosis[79]. Good interobserver agreement with a pathologist[80] and inter-endoscopist agreement of endocytoscopy findings for early gastric cancer[81] has since been demonstrated. Recently, the “enlarged nuclear sign” seen on endocytoscopy for early gastric cancer diagnosis was also published[81].
Recently, endocytoscopy coupled with artificial Intelligence and machine learning has several promising applications for diagnostic endoscopy. Using a database of 4715 esophageal images, a neural network-based artificial intelligence was constructed and achieved a sensitivity of 92.6% and sensitivity of 89.3% for esophageal squamous cell carcinoma. Similarly, in gastric cancer, the neural network trained to detect gastric cancer in endoscopic images was able to identify gastric cancer lesions with a sensitivity of 92.2%, with 98.6% sensitivity for lesions measuring ≥ 6 mm[82]; missed lesions were all early depressed lesions which might present a challenge for the expert endoscopist[82]. In another deep learning system developed by the Zhongshan group from China, the network was able to identify tumors of more than SM1 infiltration with sensitivity of 76% and specificity of 96%, higher than human endoscopists[83], allowing better selection of patients who may benefit from endoscopic resection and reducing the need for endoscopic ultrasonic examination and invasive gastrectomy.
In conclusion, there is a steady move in the past decade towards improving diagnostic yield, quality and reporting in EGDs over the past decade. Diagnostic yield from EGDs can be improved via improvements in several domains, including proper pre-endoscopy preparation, adequate sedation, conforming to a systemic photo-documentation schema, dedicating sufficient time to endoscopic examination as well as the use of magnifying endoscopy and image enhanced endoscopy. While our review does not aim to replace the comprehensive guidelines published by gastrointestinal societies, we hope to provide endoscopists with a succinct summary of up to date advances in diagnostic upper endoscopy and identify recent innovation in endoscopy. Amongst these, artificial intelligence and convoluted neural networks may have widespread endoscopic applications in the near future.
ARTICLE HIGHLIGHTS
Research background
Currently, there is no consensus on upper endoscopic examination technique to improve diagnostic yield. In recent years, quality of endoscopy is a hotly discussed topic and several papers and guidelines including the Asian consensus on standards of diagnostic upper endoscopy and the British Society of Gastroenterology guidelines have been published.
Research motivation
Despite recent advances in the surgical and oncological treatment of gastric cancer, it remains one of the leading causes of cancer death. It is imperative to improve detection of early gastric cancer in order to improve patient survival. An esophagogastroduodenoscopy (EGD) examination is an ubiquitous first line tool to investigate upper gastrointestinal symptoms in most countries worldwide and allows detection of early gastric cancer by direct inspection of the mucosa. Several factors may affect the quality of endoscopic examination itself; this includes but is not limited to pre-procedural preparation for endoscopy, appropriate sedation and use of image enhanced endoscopy. Educating endoscopists on examination methods to improve diagnostic yield during upper endoscopy is therefore the most effective intervention to improve detection of early gastric cancer in order to improve patient outcomes and survival. We are also at the dawn of the artificial intelligence age, and application of AIs into EGDs will greater enhance the ability of endoscopists to identify and diagnose early gastric and esophageal lesions.
Research objectives
Through this review, we aim to provide a succinct yet comprehensive summary of the recent literature on the advances in diagnostic EGDs. The authors hope that, through the article, endoscopists can identify potential areas of improvement to better their quality of upper endoscopy.
Research methods
The PubMed database was queried for relevant articles published between January 2001 and August 2019 using several keywords that were relevant to upper endoscopy. References of selected articles were hand searched to include any studies that may have been omitted by the PubMed search. Studies which presented relevant or novel data were included into this review.
Research results
Pre-endoscopic preparation, endoscopy sedation, systemic examination, duration of examination, routine endoscopic biopsy and image enhanced endoscopy are factors which may improve quality of EGD examination. Premedication with simethicone or simethicone and N-acetylcysteine, use of Pronase and antispasmodics improves visualization in the stomach and esophagus. There is currently no evidence that taking more photos improves diagnostic yield, but a mandatory set of systemic images such as the systemic alphanumeric coded endoscopy approach may increase yield of high-risk lesions and may also reduce variability in inter-endoscopist interpretation of endoscopic reports. Several studies have shown that endoscopists with longer inspection times during EGD consistently detect more high risk and neoplastic lesions compared to counterparts with shorter examination times; however, the beneficial of longer examination time may be diminished in very experienced endoscopists. Novel image enhanced endoscopy techniques such as Blue laser imaging (BLI) and linked colour imaging (LCI) enhances detection of early esophageal cancer and gastric cancers. When approaching a suspicious gastric lesion, the magnifying endoscopy simple diagnostic algorithm helps the endoscopist further characterize the lesion. The presence of a demarcation time, irregular micro-surface and micro-vascular pattern is highly suspicious for an early gastric cancer.
Research conclusions
Our review provides a succinct summary of the advances in diagnostic endoscopy in the past 2 decades. Several advances have been made recently in the field of image enhanced endoscopy with introduction of magnifying NBI, BLI and LCI. Being well acquainted with these techniques will allow the endoscopist to detect early gastric lesions more confidently. There is, however, still an urgent need to identify and standardize quality indicators and reporting in EGDs in order to better audit endoscopic quality and reduce variability in inter-endoscopist interpretation of endoscopic pictures.
Research perspectives
More studies are required in order to demonstrate whether a systemic method of photo-documentation during EGD, routine endoscopic biopsy and use of image enhanced techniques will indeed improve diagnostic yield during endoscopy. Having a standardized set of quality indicators for every endoscopic examination will reassure patients and physicians that a quality endoscopy and inspection had been performed so that the risk of a missed lesion is minimized. Artificial Intelligence is extremely promising to aid endoscopists detect suspicious lesions, may reduce need for biopsy and assist physicians plan further treatment for suspicious lesions. Further innovation and research will improve the sensitivity and specificity of these AIs systems as well as the best way to incorporate the use of these systems in the current endoscopic workflow.
ACKNOWLEDGEMENTS
We would like to thank Professor Lawrence HKY, National University Health System, Singapore for contributing images of the Raman probe, Raman Spectrum and endoscopic images.
Footnotes
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: All the authors declare that they have no competing interests.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Peer-review started: October 13, 2019
First decision: December 5, 2019
Article in press: January 14, 2020
P-Reviewer: Tsoulfas G S-Editor: Gong ZM L-Editor: A E-Editor: Zhang YL
Contributor Information
Jun-Liang Teh, Department of Surgery, National University Hospital System, Singapore 119228, Singapore; Department of Surgery, Jurong Health Campus, National University Health System, Singapore 609606, Singapore.
Asim Shabbir, Department of Surgery, National University Hospital System, Singapore 119228, Singapore.
Soon Yuen, Department of Surgery, National University Hospital System, Singapore 119228, Singapore; Department of Surgery, Jurong Health Campus, National University Health System, Singapore 609606, Singapore.
Jimmy Bok-Yan So, Department of Surgery, National University Hospital System, Singapore 119228, Singapore; Department of Surgery, National University of Singapore, Singapore 119074, Singapore. jimmy_so@nuhs.edu.sg.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Asplund J, Kauppila JH, Mattsson F, Lagergren J. Survival Trends in Gastric Adenocarcinoma: A Population-Based Study in Sweden. Ann Surg Oncol. 2018;25:2693–2702. doi: 10.1245/s10434-018-6627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, Gotoda T, Lin JT, You WC, Ng EK, Sung JJ Asia Pacific Working Group on Gastric Cancer. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 4.Tashiro A, Sano M, Kinameri K, Fujita K, Takeuchi Y. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol. 2006;12:4873–4874. doi: 10.3748/wjg.v12.i30.4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dan YY, So JB, Yeoh KG. Endoscopic screening for gastric cancer. Clin Gastroenterol Hepatol. 2006;4:709–716. doi: 10.1016/j.cgh.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Sasako M, Inoue M, Lin JT, Khor C, Yang HK, Ohtsu A. Gastric Cancer Working Group report. Jpn J Clin Oncol. 2010;40 Suppl 1:i28–i37. doi: 10.1093/jjco/hyq124. [DOI] [PubMed] [Google Scholar]
- 7.Bisschops R, Areia M, Coron E, Dobru D, Kaskas B, Kuvaev R, Pech O, Ragunath K, Weusten B, Familiari P, Domagk D, Valori R, Kaminski MF, Spada C, Bretthauer M, Bennett C, Senore C, Dinis-Ribeiro M, Rutter MD. Performance measures for upper gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2016;48:843–864. doi: 10.1055/s-0042-113128. [DOI] [PubMed] [Google Scholar]
- 8.Rey JF, Lambert R ESGE Quality Assurance Committee. ESGE recommendations for quality control in gastrointestinal endoscopy: guidelines for image documentation in upper and lower GI endoscopy. Endoscopy. 2001;33:901–903. doi: 10.1055/s-2001-42537. [DOI] [PubMed] [Google Scholar]
- 9.Emura F MJ, Mejía M, Osorio C, Hernández C, González I, et al. Utilidad de la cromoendoscopia sistemática en el diagnóstico del cáncer temprano y lesiones gástricas premalignas: Resultado de dos campañas masivas consecutivas de tamización en Colombia (2006-2007) Revista Colombiana de Gastroenterologia. 2010;25:19–30. [Google Scholar]
- 10.Hosokawa O, Tsuda S, Kidani E, Watanabe K, Tanigawa Y, Shirasaki S, Hayashi H, Hinoshita T. Diagnosis of gastric cancer up to three years after negative upper gastrointestinal endoscopy. Endoscopy. 1998;30:669–674. doi: 10.1055/s-2007-1001386. [DOI] [PubMed] [Google Scholar]
- 11.Amin A, Gilmour H, Graham L, Paterson-Brown S, Terrace J, Crofts TJ. Gastric adenocarcinoma missed at endoscopy. J R Coll Surg Edinb. 2002;47:681–684. [PubMed] [Google Scholar]
- 12.Yalamarthi S, Witherspoon P, McCole D, Auld CD. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy. 2004;36:874–879. doi: 10.1055/s-2004-825853. [DOI] [PubMed] [Google Scholar]
- 13.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 14.Teh JL, Tan JR, Lau LJ, Saxena N, Salim A, Tay A, Shabbir A, Chung S, Hartman M, So JB. Longer examination time improves detection of gastric cancer during diagnostic upper gastrointestinal endoscopy. Clin Gastroenterol Hepatol. 2015;13:480–487. e2. doi: 10.1016/j.cgh.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 15.Elvas L, Areia M, Brito D, Alves S, Saraiva S, Cadime AT. Premedication with simethicone and N-acetylcysteine in improving visibility during upper endoscopy: a double-blind randomized trial. Endoscopy. 2017;49:139–145. doi: 10.1055/s-0042-119034. [DOI] [PubMed] [Google Scholar]
- 16.Kim GH, Cho YK, Cha JM, Lee SY, Chung IK. Effect of pronase as mucolytic agent on imaging quality of magnifying endoscopy. World J Gastroenterol. 2015;21:2483–2489. doi: 10.3748/wjg.v21.i8.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SY, Han HS, Cha JM, Cho YK, Kim GH, Chung IK. Endoscopic flushing with pronase improves the quantity and quality of gastric biopsy: a prospective study. Endoscopy. 2014;46:747–753. doi: 10.1055/s-0034-1365811. [DOI] [PubMed] [Google Scholar]
- 18.Koeppe AT, Lubini M, Bonadeo NM, Moraes I, Jr, Fornari F. Comfort, safety and quality of upper gastrointestinal endoscopy after 2 hours fasting: a randomized controlled trial. BMC Gastroenterol. 2013;13:158. doi: 10.1186/1471-230X-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi T, Asahina Y, Waseda Y, Kitamura K, Kagaya T, Seike T, Okada K, Inada Y, Takabatake H, Orita N, Yanase Y, Yamashita T, Ninomiya I, Yoshimura K, Kaneko S. Lidocaine spray alone is similar to spray plus viscous solution for pharyngeal observation during transoral endoscopy: a clinical randomized trial. Endosc Int Open. 2017;5:E47–E53. doi: 10.1055/s-0042-120414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu PWY, Uedo N, Singh R, Gotoda T, Ng EKW, Yao K, Ang TL, Ho SH, Kikuchi D, Yao F, Pittayanon R, Goda K, Lau JYW, Tajiri H, Inoue H. An Asian consensus on standards of diagnostic upper endoscopy for neoplasia. Gut. 2019;68:186–197. doi: 10.1136/gutjnl-2018-317111. [DOI] [PubMed] [Google Scholar]
- 21.Quinn L, Kelly ME, Khan A, Irwin R, Khan W, Barry K, Waldron R, Khan IZ. Sedation for gastroscopy: Is it an adequately understood and informed choice? Ir J Med Sci. 2016;185:785–789. doi: 10.1007/s11845-015-1354-x. [DOI] [PubMed] [Google Scholar]
- 22.He Y, Zhao Y, Fu K, Du Y, Yu J, Wang J, Jin P, Zhao X, Li N, Guo H, Li J, Zhao F, Sheng J. Propofol sedation versus no sedation in detection of pharyngeal and upper gastrointestinal superficial squamous cell carcinoma using endoscopic narrow band imaging: a multicenter prospective trial. Int J Clin Exp Med. 2015;8:18647–18655. [PMC free article] [PubMed] [Google Scholar]
- 23.Thota PN, Zuccaro G Jr, Vargo JJ 2nd, Conwell DL, Dumot JA, Xu M. A randomized prospective trial comparing unsedated esophagoscopy via transnasal and transoral routes using a 4-mm video endoscope with conventional endoscopy with sedation. Endoscopy. 2005;37:559–565. doi: 10.1055/s-2005-861476. [DOI] [PubMed] [Google Scholar]
- 24.Preiss C, Charton JP, Schumacher B, Neuhaus H. A randomized trial of unsedated transnasal small-caliber esophagogastroduodenoscopy (EGD) versus peroral small-caliber EGD versus conventional EGD. Endoscopy. 2003;35:641–646. doi: 10.1055/s-2003-41513. [DOI] [PubMed] [Google Scholar]
- 25.Stroppa I, Grasso E, Paoluzi OA, Razzini C, Tosti C, Andrei F, Biancone L, Palmieri G, Romeo F, Pallone F. Unsedated transnasal versus transoral sedated upper gastrointestinal endoscopy: a one-series prospective study on safety and patient acceptability. Dig Liver Dis. 2008;40:767–775. doi: 10.1016/j.dld.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 26.Meining A, Semmler V, Kassem AM, Sander R, Frankenberger U, Burzin M, Reichenberger J, Bajbouj M, Prinz C, Schmid RM. The effect of sedation on the quality of upper gastrointestinal endoscopy: an investigator-blinded, randomized study comparing propofol with midazolam. Endoscopy. 2007;39:345–349. doi: 10.1055/s-2006-945195. [DOI] [PubMed] [Google Scholar]
- 27.Khan KJ, Fergani H, Ganguli SC, Jalali S, Spaziani R, Tsoi K, Morgan DG. The Benefit of Fentanyl in Effective Sedation and Quality of Upper Endoscopy: A Double-Blinded Randomized Trial of Fentanyl Added to Midazolam Versus Midazolam Alone for Sedation. J Can Assoc Gastroenterol. 2019;2:86–90. doi: 10.1093/jcag/gwy041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo XL, Li Z, Liu XP, Li CQ, Ji R, Wang P, Zhou CJ, Liu H, Li YQ. Propofol vs midazolam plus fentanyl for upper gastrointestinal endomicroscopy: a randomized trial. World J Gastroenterol. 2012;18:1814–1821. doi: 10.3748/wjg.v18.i15.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julián Gómez L, Fuentes Coronel A, López Ramos C, Ochoa Sangrador C, Fradejas Salazar P, Martín Garrido E, Conde Gacho P, Bailador Andrés C, García-Alvarado M, Rascarachi G, Castillo Trujillo R, Rodríguez Gómez SJ. A clinical trial comparing propofol versus propofol plus midazolam in diagnostic endoscopy of patients with a low anesthetic risk. Rev Esp Enferm Dig. 2018;110:691–698. doi: 10.17235/reed.2018.5289/2017. [DOI] [PubMed] [Google Scholar]
- 30.Akyuz U, Pata C, Senkal V, Erzin Y. Is propofol sedation with midazolam induction safe during endoscopic procedures without anesthesiologist? Hepatogastroenterology. 2010;57:685–687. [PubMed] [Google Scholar]
- 31.Ye L, Xiao X, Zhu L. The Comparison of Etomidate and Propofol Anesthesia in Patients Undergoing Gastrointestinal Endoscopy: A Systematic Review and Meta-Analysis. Surg Laparosc Endosc Percutan Tech. 2017;27:1–7. doi: 10.1097/SLE.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 32.Chang YT, Tsai TC, Hsu H, Chen YM, Chi KP, Peng SY. Sedation for gastrointestinal endoscopy with the application of target-controlled infusion. Turk J Gastroenterol. 2015;26:417–422. doi: 10.5152/tjg.2015.0206. [DOI] [PubMed] [Google Scholar]
- 33.Chiang MH, Wu SC, You CH, Wu KL, Chiu YC, Ma CW, Kao CW, Lin KC, Chen KH, Wang PC, Chou AK. Target-controlled infusion vs. manually controlled infusion of propofol with alfentanil for bidirectional endoscopy: a randomized controlled trial. Endoscopy. 2013;45:907–914. doi: 10.1055/s-0033-1344645. [DOI] [PubMed] [Google Scholar]
- 34.Dumonceau JM, Riphaus A, Schreiber F, Vilmann P, Beilenhoff U, Aparicio JR, Vargo JJ, Manolaraki M, Wientjes C, Rácz I, Hassan C, Paspatis G. Non-anesthesiologist administration of propofol for gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates Guideline--Updated June 2015. Endoscopy. 2015;47:1175–1189. doi: 10.1055/s-0034-1393414. [DOI] [PubMed] [Google Scholar]
- 35.Dumonceau JM, Riphaus A, Aparicio JR, Beilenhoff U, Knape JT, Ortmann M, Paspatis G, Ponsioen CY, Racz I, Schreiber F, Vilmann P, Wehrmann T, Wientjes C, Walder B NAAP Task Force Members. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: Non-anesthesiologist administration of propofol for GI endoscopy. Endoscopy. 2010;42:960–974. doi: 10.1055/s-0030-1255728. [DOI] [PubMed] [Google Scholar]
- 36.Asfeldt AM, Straume B, Paulssen EJ. Impact of observer variability on the usefulness of endoscopic images for the documentation of upper gastrointestinal endoscopy. Scand J Gastroenterol. 2007;42:1106–1112. doi: 10.1080/00365520701259240. [DOI] [PubMed] [Google Scholar]
- 37.Enestvedt BK, Lugo R, Guarner-Argente C, Shah P, Falk GW, Furth E, Ginsberg GG. Location, location, location: does early cancer in Barrett's esophagus have a preference? Gastrointest Endosc. 2013;78:462–467. doi: 10.1016/j.gie.2013.03.167. [DOI] [PubMed] [Google Scholar]
- 38.Pimenta-Melo AR, Monteiro-Soares M, Libânio D, Dinis-Ribeiro M. Missing rate for gastric cancer during upper gastrointestinal endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:1041–1049. doi: 10.1097/MEG.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 39.Marques S, Bispo M, Pimentel-Nunes P, Chagas C, Dinis-Ribeiro M. Image Documentation in Gastrointestinal Endoscopy: Review of Recommendations. GE Port J Gastroenterol. 2017;24:269–274. doi: 10.1159/000477739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol. 2013;26:11–22. [PMC free article] [PubMed] [Google Scholar]
- 41.Machaca Quea NR, Emura F, Barreda Bolaños F, Salvador Arias Y, Arévalo Suárez FA, Piscoya Rivera A. Effectiveness of systematic alphanumeric coded endoscopy for diagnosis of gastric intraepithelial neoplasia in a low socioeconomic population. Endosc Int Open. 2016;4:E1083–E1089. doi: 10.1055/s-0042-115408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JM, Lim CH, Cho YK, Lee BI, Cho YS, Song HJ, Choi MG. The effect of photo-documentation of the ampulla on neoplasm detection rate during esophagogastroduodenoscopy. Endoscopy. 2019;51:115–124. doi: 10.1055/a-0662-5523. [DOI] [PubMed] [Google Scholar]
- 43.Kawamura T, Wada H, Sakiyama N, Ueda Y, Shirakawa A, Okada Y, Sanada K, Nakase K, Mandai K, Suzuki A, Kamaguchi M, Morita A, Nishioji K, Tanaka K, Mochizuki N, Uno K, Yokota I, Kobayashi M, Yasuda K. Examination time as a quality indicator of screening upper gastrointestinal endoscopy for asymptomatic examinees. Dig Endosc. 2017;29:569–575. doi: 10.1111/den.12804. [DOI] [PubMed] [Google Scholar]
- 44.Park JM, Huo SM, Lee HH, Lee BI, Song HJ, Choi MG. Longer Observation Time Increases Proportion of Neoplasms Detected by Esophagogastroduodenoscopy. Gastroenterology. 2017;153:460–469. e1. doi: 10.1053/j.gastro.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Yoshimizu S, Hirasawa T, Horiuchi Y, Omae M, Ishiyama A, Yoshio T, Tsuchida T, Fujisaki J. Differences in upper gastrointestinal neoplasm detection rates based on inspection time and esophagogastroduodenoscopy training. Endosc Int Open. 2018;6:E1190–E1197. doi: 10.1055/a-0655-7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, Rodriguez-Justo M, Novelli MR, Ragunath K, Shepherd N, Dinis-Ribeiro M. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545–1575. doi: 10.1136/gutjnl-2018-318126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Januszewicz W, Wieszczy P, Bialek A, Karpinska K, Szlak J, Szymonik J, Rupinski M, Mroz A, Regula J, Kaminski MF. Endoscopist biopsy rate as a quality indicator for outpatient gastroscopy: a multicenter cohort study with validation. Gastrointest Endosc. 2019;89:1141–1149. doi: 10.1016/j.gie.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Ishihara R, Kanzaki H, Iishi H, Nagai K, Matsui F, Yamashina T, Matsuura N, Ito T, Fujii M, Yamamoto S, Hanaoka N, Takeuchi Y, Higashino K, Uedo N, Tatsuta M, Tomita Y, Ishiguro S. Pink-color sign in esophageal squamous neoplasia, and speculation regarding the underlying mechanism. World J Gastroenterol. 2013;19:4300–4308. doi: 10.3748/wjg.v19.i27.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yip HC, Chiu PW. Endoscopic diagnosis and management of early squamous cell carcinoma of esophagus. J Thorac Dis. 2017;9:S689–S696. doi: 10.21037/jtd.2017.06.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oyama T, Inoue H, Arima M, Momma K, Omori T, Ishihara R, Hirasawa D, Takeuchi M, Tomori A, Goda K. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017;14:105–112. doi: 10.1007/s10388-016-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu M, Liu Z, Liu F, Guo C, Xu R, Li F, Liu A, Yang H, Zhang S, Shen L, Duan L, Wu Q, Cao C, Pan Y, Liu Y, Li J, Cai H, He Z, Ke Y. Absence of Iodine Staining Associates With Progression of Esophageal Lesions in a Prospective Endoscopic Surveillance Study in China. Clin Gastroenterol Hepatol. 2019 doi: 10.1016/j.cgh.2019.08.058. [DOI] [PubMed] [Google Scholar]
- 52.Ishihara R, Takeuchi Y, Chatani R, Kidu T, Inoue T, Hanaoka N, Yamamoto S, Higashino K, Uedo N, Iishi H, Tatsuta M, Tomita Y, Ishiguro S. Prospective evaluation of narrow-band imaging endoscopy for screening of esophageal squamous mucosal high-grade neoplasia in experienced and less experienced endoscopists. Dis Esophagus. 2010;23:480–486. doi: 10.1111/j.1442-2050.2009.01039.x. [DOI] [PubMed] [Google Scholar]
- 53.Diao W, Huang X, Shen L, Zeng Z. Diagnostic ability of blue laser imaging combined with magnifying endoscopy for early esophageal cancer. Dig Liver Dis. 2018;50:1035–1040. doi: 10.1016/j.dld.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 54.Kim JW. Usefulness of Narrow-Band Imaging in Endoscopic Submucosal Dissection of the Stomach. Clin Endosc. 2018;51:527–533. doi: 10.5946/ce.2018.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dutta AK, Sajith KG, Pulimood AB, Chacko A. Narrow band imaging versus white light gastroscopy in detecting potentially premalignant gastric lesions: a randomized prospective crossover study. Indian J Gastroenterol. 2013;32:37–42. doi: 10.1007/s12664-012-0246-5. [DOI] [PubMed] [Google Scholar]
- 56.Kato M, Kaise M, Yonezawa J, Goda K, Toyoizumi H, Yoshimura N, Yoshida Y, Kawamura M, Tajiri H. Trimodal imaging endoscopy may improve diagnostic accuracy of early gastric neoplasia: a feasibility study. Gastrointest Endosc. 2009;70:899–906. doi: 10.1016/j.gie.2009.03.1171. [DOI] [PubMed] [Google Scholar]
- 57.So J, Rajnakova A, Chan YH, Tay A, Shah N, Salto-Tellez M, Teh M, Uedo N. Endoscopic tri-modal imaging improves detection of gastric intestinal metaplasia among a high-risk patient population in Singapore. Dig Dis Sci. 2013;58:3566–3575. doi: 10.1007/s10620-013-2843-2. [DOI] [PubMed] [Google Scholar]
- 58.Ang TL, Pittayanon R, Lau JY, Rerknimitr R, Ho SH, Singh R, Kwek AB, Ang DS, Chiu PW, Luk S, Goh KL, Ong JP, Tan JY, Teo EK, Fock KM. A multicenter randomized comparison between high-definition white light endoscopy and narrow band imaging for detection of gastric lesions. Eur J Gastroenterol Hepatol. 2015;27:1473–1478. doi: 10.1097/MEG.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 59.Muto M, Yao K, Kaise M, Kato M, Uedo N, Yagi K, Tajiri H. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G) Dig Endosc. 2016;28:379–393. doi: 10.1111/den.12638. [DOI] [PubMed] [Google Scholar]
- 60.Hu YY, Lian QW, Lin ZH, Zhong J, Xue M, Wang LJ. Diagnostic performance of magnifying narrow-band imaging for early gastric cancer: A meta-analysis. World J Gastroenterol. 2015;21:7884–7894. doi: 10.3748/wjg.v21.i25.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Q, Wang F, Chen ZY, Wang Z, Zhi FC, Liu SD, Bai Y. Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: a meta-analysis. Gastric Cancer. 2016;19:543–552. doi: 10.1007/s10120-015-0500-5. [DOI] [PubMed] [Google Scholar]
- 62.Uchita K, Yao K, Uedo N, Shimokawa T, Iwasaki T, Kojima K, Kawada A, Nakayama M, Okazaki M, Iwamura S. Highest power magnification with narrow-band imaging is useful for improving diagnostic performance for endoscopic delineation of early gastric cancers. BMC Gastroenterol. 2015;15:155. doi: 10.1186/s12876-015-0385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Makazu M, Hirasawa K, Sato C, Ikeda R, Fukuchi T, Ishii Y, Kobayashi R, Kaneko H, Taguri M, Tateishi Y, Inayama Y, Maeda S. Histological verification of the usefulness of magnifying endoscopy with narrow-band imaging for horizontal margin diagnosis of differentiated-type early gastric cancers. Gastric Cancer. 2018;21:258–266. doi: 10.1007/s10120-017-0734-5. [DOI] [PubMed] [Google Scholar]
- 64.Dohi O, Yagi N, Majima A, Horii Y, Kitaichi T, Onozawa Y, Suzuki K, Tomie A, Kimura-Tsuchiya R, Tsuji T, Yamada N, Bito N, Okayama T, Yoshida N, Kamada K, Katada K, Uchiyama K, Ishikawa T, Takagi T, Handa O, Konishi H, Naito Y, Yanagisawa A, Itoh Y. Diagnostic ability of magnifying endoscopy with blue laser imaging for early gastric cancer: a prospective study. Gastric Cancer. 2017;20:297–303. doi: 10.1007/s10120-016-0620-6. [DOI] [PubMed] [Google Scholar]
- 65.Dohi O, Yagi N, Naito Y, Fukui A, Gen Y, Iwai N, Ueda T, Yoshida N, Kamada K, Uchiyama K, Takagi T, Konishi H, Yanagisawa A, Itoh Y. Blue laser imaging-bright improves the real-time detection rate of early gastric cancer: a randomized controlled study. Gastrointest Endosc. 2019;89:47–57. doi: 10.1016/j.gie.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 66.Fukuda H, Miura Y, Hayashi Y, Takezawa T, Ino Y, Okada M, Osawa H, Lefor AK, Yamamoto H. Linked color imaging technology facilitates early detection of flat gastric cancers. Clin J Gastroenterol. 2015;8:385–389. doi: 10.1007/s12328-015-0612-9. [DOI] [PubMed] [Google Scholar]
- 67.Dohi O, Yagi N, Onozawa Y, Kimura-Tsuchiya R, Majima A, Kitaichi T, Horii Y, Suzuki K, Tomie A, Okayama T, Yoshida N, Kamada K, Katada K, Uchiyama K, Ishikawa T, Takagi T, Handa O, Konishi H, Naito Y, Itoh Y. Linked color imaging improves endoscopic diagnosis of active Helicobacter pylori infection. Endosc Int Open. 2016;4:E800–E805. doi: 10.1055/s-0042-109049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen H, Wang H, Wu X, Liu Y, Wu Q, Lu Y, Lin X, Fan D, Li C. Predictability of gastric intestinal metaplasia by patchy lavender color seen on linked color imaging endoscopy. Lasers Med Sci. 2019;34:1791–1797. doi: 10.1007/s10103-019-02775-8. [DOI] [PubMed] [Google Scholar]
- 69.Min M, Dong TH, Liu Y, Bi YL, Ma CY. Novel endoscopic findings as visualized by non-magnification endoscopy with linked color imaging are indicative of gastric intestinal metaplasia. Chin Med J (Engl) 2019;132:782–788. doi: 10.1097/CM9.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ono S, Kato M, Tsuda M, Miyamoto S, Abiko S, Shimizu Y, Sakamoto N. Lavender Color in Linked Color Imaging Enables Noninvasive Detection of Gastric Intestinal Metaplasia. Digestion. 2018;98:222–230. doi: 10.1159/000489454. [DOI] [PubMed] [Google Scholar]
- 71.Kanzaki H, Takenaka R, Kawahara Y, Kawai D, Obayashi Y, Baba Y, Sakae H, Gotoda T, Kono Y, Miura K, Iwamuro M, Kawano S, Tanaka T, Okada H. Linked color imaging (LCI), a novel image-enhanced endoscopy technology, emphasizes the color of early gastric cancer. Endosc Int Open. 2017;5:E1005–E1013. doi: 10.1055/s-0043-117881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujiyoshi T, Miyahara R, Funasaka K, Furukawa K, Sawada T, Maeda K, Yamamura T, Ishikawa T, Ohno E, Nakamura M, Kawashima H, Nakaguro M, Nakatochi M, Hirooka Y. Utility of linked color imaging for endoscopic diagnosis of early gastric cancer. World J Gastroenterol. 2019;25:1248–1258. doi: 10.3748/wjg.v25.i10.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshifuku Y, Sanomura Y, Oka S, Kurihara M, Mizumoto T, Miwata T, Urabe Y, Hiyama T, Tanaka S, Chayama K. Evaluation of the visibility of early gastric cancer using linked color imaging and blue laser imaging. BMC Gastroenterol. 2017;17:150. doi: 10.1186/s12876-017-0707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kitagawa Y, Hara T, Ikebe D, Nankinzan R, Takashiro H, Kobayashi R, Nakamura K, Yamaguchi T, Suzuki T. Magnified endoscopic observation of small depressed gastric lesions using linked color imaging with indigo carmine dye. Endoscopy. 2018;50:142–147. doi: 10.1055/s-0043-119212. [DOI] [PubMed] [Google Scholar]
- 75.Huang Z, Teh SK, Zheng W, Lin K, Ho KY, Teh M, Yeoh KG. In vivo detection of epithelial neoplasia in the stomach using image-guided Raman endoscopy. Biosens Bioelectron. 2010;26:383–389. doi: 10.1016/j.bios.2010.07.125. [DOI] [PubMed] [Google Scholar]
- 76.Lin K, Wang J, Zheng W, Ho KY, Teh M, Yeoh KG, Huang Z. Rapid Fiber-optic Raman Spectroscopy for Real-Time In Vivo Detection of Gastric Intestinal Metaplasia during Clinical Gastroscopy. Cancer Prev Res (Phila) 2016;9:476–483. doi: 10.1158/1940-6207.CAPR-15-0213. [DOI] [PubMed] [Google Scholar]
- 77.Sato H, Inoue H, Hayee B, Ikeda H, Sato C, Phalanusitthepha C, Santi EG, Kobayashi Y, Kudo SE. In vivo histopathology using endocytoscopy for non-neoplastic changes in the gastric mucosa: a prospective pilot study (with video) Gastrointest Endosc. 2015;81:875–881. doi: 10.1016/j.gie.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 78.Chiu PW, Ng EK, To KF, Teoh AY, Lam CC, Chan FK, Sung JJ, Lau JY. Recognition of goblet cells upon endocytoscopy indicates the presence of gastric intestinal metaplasia. Dig Endosc. 2014;26:52–56. doi: 10.1111/den.12050. [DOI] [PubMed] [Google Scholar]
- 79.Kaise M, Ohkura Y, Iizuka T, Kimura R, Nomura K, Kuribayashi Y, Yamada A, Yamashita S, Furuhata T, Kikuchi D, Ogawa O, Matsui A, Mitani T, Hoteya S. Endocytoscopy is a promising modality with high diagnostic accuracy for gastric cancer. Endoscopy. 2015;47:19–25. doi: 10.1055/s-0034-1377965. [DOI] [PubMed] [Google Scholar]
- 80.Tsurudome I, Miyahara R, Funasaka K, Furukawa K, Matsushita M, Yamamura T, Ishikawa T, Ohno E, Nakamura M, Kawashima H, Watanabe O, Nakaguro M, Satou A, Hirooka Y, Goto H. In vivo histological diagnosis for gastric cancer using endocytoscopy. World J Gastroenterol. 2017;23:6894–6901. doi: 10.3748/wjg.v23.i37.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abad MRA, Inoue H, Ikeda H, Manolakis A, Rodriguez de Santiago E, Sharma A, Fujiyoshi Y, Fukuda H, Sumi K, Onimaru M, Shimamura Y. Utilizing fourth-generation endocytoscopy and the 'enlarged nuclear sign' for in vivo diagnosis of early gastric cancer. Endosc Int Open. 2019;7:E1002–E1007. doi: 10.1055/a-0957-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hirasawa T, Aoyama K, Tanimoto T, Ishihara S, Shichijo S, Ozawa T, Ohnishi T, Fujishiro M, Matsuo K, Fujisaki J, Tada T. Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer. 2018;21:653–660. doi: 10.1007/s10120-018-0793-2. [DOI] [PubMed] [Google Scholar]
- 83.Zhu Y, Wang QC, Xu MD, Zhang Z, Cheng J, Zhong YS, Zhang YQ, Chen WF, Yao LQ, Zhou PH, Li QL. Application of convolutional neural network in the diagnosis of the invasion depth of gastric cancer based on conventional endoscopy. Gastrointest Endosc. 2019;89:806–815. e1. doi: 10.1016/j.gie.2018.11.011. [DOI] [PubMed] [Google Scholar]