Abstract
Biomolecular simulations are intrinsically high dimensional and generate noisy data sets of ever-increasing size. Extracting important features from the data is crucial for understanding the biophysical properties of molecular processes, but remains a big challenge. Machine learning (ML) provides powerful dimensionality reduction tools. However, such methods are often criticized as resembling black boxes with limited human-interpretable insight. We use methods from supervised and unsupervised ML to efficiently create interpretable maps of important features from molecular simulations. We benchmark the performance of several methods, including neural networks, random forests, and principal component analysis, using a toy model with properties reminiscent of macromolecular behavior. We then analyze three diverse biological processes: conformational changes within the soluble protein calmodulin, ligand binding to a G protein-coupled receptor, and activation of an ion channel voltage-sensor domain, unraveling features critical for signal transduction, ligand binding, and voltage sensing. This work demonstrates the usefulness of ML in understanding biomolecular states and demystifying complex simulations.
Significance
Understanding how biomolecules function requires resolving the ensemble of structures they visit. Molecular dynamics simulations compute these ensembles and generate large amounts of data that can be noisy and need to be condensed for human interpretation. Machine learning methods are designed to process large amounts of data but are often criticized for their black-box nature and have historically been modestly used in the analysis of biomolecular systems. We demonstrate how machine learning tools can provide an interpretable overview of important features in a simulation data set. We develop a protocol to quickly perform data-driven analysis of molecular simulations. This protocol is applied to identify the molecular basis of ligand binding to a receptor and of voltage sensitivity of an ion channel.
Introduction
Molecular dynamics (MD) simulations of biological systems provide a unique atomistic insight into many important biological processes, such as a protein’s conformational change between functional states, the folding of a soluble protein, or the effect of ligand binding to a receptor. These systems can be extremely high dimensional, with pairwise interactions between tens to hundreds of thousands of atoms at every snapshot in time. As system sizes, as well as the reachable timescales of simulations, have increased significantly over the last decade, a conventional simulation can now generate terabytes of raw data that need to be condensed for human interpretation. Data-driven methods reduce the risk for researchers to overlook important properties in a simulation or even misinterpret the computational experiment and introduce human bias in the analysis.
A system of N particles has 3N spatial degrees of freedom. Fortunately, restraints in the system because of, for example, the force field and steric hindrance restrict biomolecules to only adopt a subset of all possible atomic rearrangements. Thus, the system typically moves on a manifold of much lower dimensionality than its actual number of degrees of freedom. Finding this manifold is not easy, and even when it is found, it can still be difficult to interpret it. Researchers often seek reaction coordinates or collective variables (CVs) to describe important features of this manifold. An optimal set of CVs should help answering a specific question. For example, if the aim is to enhance sampling of transitions from one functional state to another, we typically seek the CVs that best describe the slowest motion of the system. In other situations, we might seek answers to biophysically relevant questions by chasing subtle differences on the molecular level between slightly perturbed states. Examples include establishing the molecular signatures of a protein when bound to different ligands, comparing structural changes associated with different protonation states or evaluating the effect of a point mutation. Regardless of the biological problem at hand, analysis typically involves processing a large set of high-dimensional data in search of important features. This type of dimensionality reduction problem may be addressed with machine learning (ML) methods.
The increasing amounts of data, as well as limited time and resources for processing and analysis, are not only a challenge in the field of biomolecular simulations. ML methods have gained enormous interest in recent years and are now applied in a wide range of research areas within biology, medicine, and health care (1, 2, 3) such as genomics (4), network biology (5), drug discovery (6), and medical imaging (7,8). In molecular simulations, such methods have, for example, eminently been used to enhance sampling by identifying CVs or the intrinsic dimensionality of biomolecular system in a data-driven manner (9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30), as an interpolation or exploratory tool for generating new protein conformations (23,31,32), as well as providing a framework for learning biomolecular states and kinetics (11,33,34). However, many tools borrowed from ML, most notably nonlinear models such as neural networks (NNs), are criticized for their resemblance to a black box, which obstructs human-interpretable insights (1, 2, 3,5). Therefore, making this opaque black box transparent is an active area of research (35,36).
In this study, we have demonstrated how to learn ensemble properties from molecular simulations and provide easily interpretable metrics of important features with prominent ML methods of varying complexity, including principal component analysis (PCA), random forests (RFs), and three types of neural networks (NNs): autoencoders (AEs), restricted Boltzmann machines (RBMs), and multilayer perceptrons (MLPs). For different types of data sets, we considered supervised methods, in which each simulation frame belongs to a known class, as well as unsupervised methods, in which information is extracted from unlabeled data. To show how the methods perform under various circumstances, we first evaluated them on a toy model designed to mimic real macromolecular behavior, in which functional states were defined by state-dependent random displacement of atoms. This approach enabled us to understand and illustrate the different methods’ properties and shortcomings in a quantitative manner. In particular, we find that all methods perform equally well on the toy model’s simple setup, in which optimal inverse interatomic distances are used as an input. However, although supervised methods are still able to identify important features for a more complex input such as Cartesian coordinates, unsupervised methods fail to do so.
We further applied our protocol for identifying key features of three biologically interesting processes that involve systems sampled using extensive conventional MD or enhanced sampling techniques: the conformational rearrangements occurring within the C-terminal domain of the Ca2+-bound soluble protein calmodulin, the effect of ligand binding to a G protein-coupled receptor (GPCR), and the response of an ion channel voltage-sensor domain (VSD) to a change in the transmembrane potential. In all three cases, the ML methods pinpointed molecular features that are known to be important for the function of these proteins. In general, our results demonstrate the ability of ML methods to reveal valuable insights into biomolecular systems. We anticipate that this straightforward, yet powerful, approach can become useful for many researchers when demystifying complex simulations.
Methods
PCA
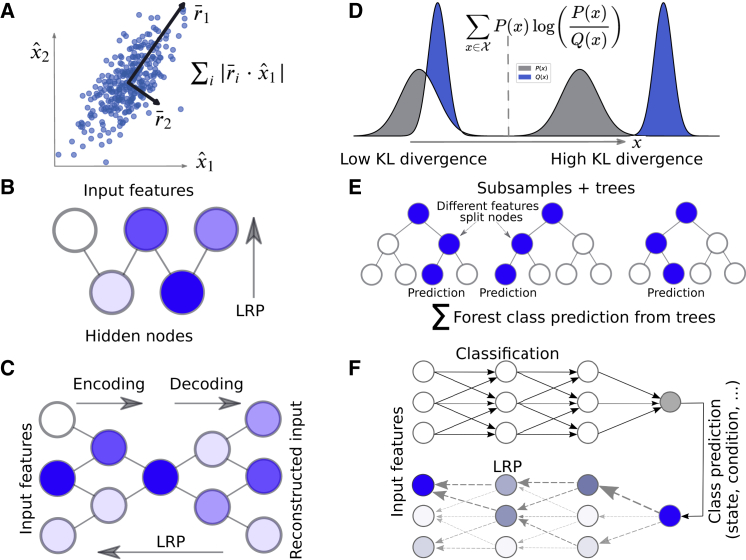
PCA converts a set of input features, X, to a set of orthogonal linearly uncorrelated variables (Fig. 1 A), T, corresponding to the eigenvectors of XTX with normalized eigenvalues λ (37). The eigenvector with the highest eigenvalue covers the largest variance possible in the data set and is called the first principal component (PC), and the second PC covers the largest variance in the orthogonal space of the first component etc. The feature importance, R, was taken to be the projection of the first PCs and eigenvalues onto the original input features up to a certain threshold in accumulated variance (Fig. 1 A): , where i is the index of the input features, and j is the index of the PCs. jmax was determined by three different approaches: 1) to a fixed number of PCs, 2) so that the sum was below a certain threshold, or 3) set to the first λj that fulfilled the condition λj − 1/λj ≥ 10. Finally, the importance was normalized between 0 and 1.
Figure 1.
Machine learning (ML) models used in this study. (A) Principal component analysis (PCA) converts the input features to an orthogonal set of linearly uncorrelated variables called principal components (PCs) through an orthogonal transformation. The first component covers the largest variance possible. The feature importance is taken to be the components’ coefficients of a feature times the variance covered by (i.e., the eigenvalues) the components. (B) Restricted Boltzmann machine (RBM) is a generative stochastic NN trained to maximize the likelihood of the data using a graphical model with a layer of hidden nodes connected to the input nodes. Important features can be derived using layer-wise relevance propagation (LRP), an algorithm originally developed for image classification problems (39). (C) Autoencoder (AE) is a NN trained to reconstruct the input features through a set of hidden layers of lower dimensionality. Important features can be derived using LRP. (D) Kullback-Leibler (KL) divergence (also called relative entropy): this metric computes the difference between two distributions P(x) and Q(x) along every individual feature. We compute the KL divergence between one class and all other classes as an indication of the importance of a specific feature. (E) Random forest (RF) classifier: a prediction is taken as an ensemble average over many decision trees. Average relevance per feature is computed as the mean decrease impurity (44). (F) A Multilayer perceptron (MLP) is a feedforward artificial NN with fully connected layers. Important features can be derived using LRP. To see this figure in color, go online.
RBM
An RBM (38) is a generative stochastic NN trained to maximize the likelihood of the data using a graphical model with a layer of hidden nodes connected to the input nodes (Fig. 1 B). We used an implementation from scikit-learn of a Bernoulli RBM. Training was performed with stochastic maximum likelihood. To find important features, layer-wise relevance propagation (LRP; explained in the following section) was performed from the output of the hidden layer to the input layer.
Note that in all computations, the input features were scaled between values of 0 and 1. The RBM used in this study assumes a probabilistic interpretation of the input features with values between 0 and 1, in which the upper limit indicates that a specific input node is activated. When feeding inverse distances to the RBM, and when larger input values correspond to residues in contact with each other, this model makes physical sense as long as the values are scaled properly. Cartesian coordinates, even after scaling, do not fulfill the assumptions made about the input features and have been taken into consideration only for the sake of consistency.
LRP or deep Taylor decomposition
LRP, or deep Taylor decomposition when applied to networks with rectifier activation (ReLU) (39), is a method originally developed for image classification problems with the aim to produce a decomposition (an explanation) of a prediction that can be visualized in the same way as the input data. Given a trained NN, it is possible to perform a backward pass through the network (Fig. 1 F), keeping track of which nodes contributed to a certain classification decision. More specifically, for a prediction with input features X, true labels T, and a network output T′, the relevance vector, R, is initialized as Ri = TiT′i. The relevance is iteratively propagated from nodes in one layer (index k) to the nodes in a previous layer (index j) with the following iterative rule: . Here, aj is the activation of the current node, ε is a small increment set to 10−9, wjk are the weights connecting the two layers, and ρ is a function that transforms the weights. For layers of bounded values, such as the input layer if X is scaled between fixed values, ρ = ajwjk − ljmax(0, wjk) − hjmin(0, wjk) where lj and hj are the layer’s lower and upper bounds. For unbounded values, ρ = ajmax(0, wjk). The importance of a feature was derived by computing the average relevance over all input samples in the training set and normalizing the importance for all features and frames to have an upper bound of 1. Unlike other methods in this study, it is possible to compute the importance of a feature for a specific simulation frame in this way.
AE
An AE is a generative NN trained to reconstruct the input through a set of hidden layers of lower dimensionality (Fig. 1 C; (40)). In short, the model can be broken down into two parts: encoding and decoding. In the encoding part, the layers decrease in size, causing the network to ignore noise during training. In the decoding part, the layers increase in size up to the output layer, which consists of reconstructed input features.
The AE was implemented by training a scikit-learn MLP regressor (41) with the same data as input and output. The encoding layers and the decoding layers were always set to be of the same shape. To find important features, LRP was performed from the reconstructed output to the actual input.
Kullback-Leibler divergence
Kullback-Leibler (KL) divergence (Fig. 1 D; (42)), also called relative entropy, is a measure of the difference between two distributions P(x) and Q(x) along a feature X: . The importance of a feature was set to be a symmetric version of the KL divergence, , between one state and all other states along that feature. All importance scores were normalized between 0 and 1.
RF classifier
An RF classifier (Fig. 1 E; (43)) is an ML model in which a prediction is taken as an ensemble average over many decision trees, each tree fitted to subsamples of the data set. Important features were identified by computing the forest’s normalized mean decrease impurity (44), using scikit-learn’s (41) implementation. Mean decrease impurity is essentially a measure of how often a feature is used to split nodes in the underlying decision trees. It is computed by identifying all nodes split by that feature and averaging the metric ρΔi over all trees in the forest, where ρ is the fraction of samples reaching the node and Δi is the decrease in impurity by the split (45). In this study, we used the Gini impurity, computed for a data set with N classes as , where pj is the probability of a sample in class j reaching that node. To compute the feature importance for a specific state, the RF was alternatively used as a one-versus-the-rest binary classifier trained to distinguish one state from all the others.
MLP classifier
An MLP classifier (Fig. 1 F) is a feedforward artificial NN with fully connected layers (42). An implementation from scikit-learn (41) of an MLP classifier was used throughout this study, trained with the Adam solver (46). To find important features, LRP was performed from the output to the input layer.
Toy model
To construct model systems, atoms were randomly placed in a box to set up an initial configuration. To define states, a set of atoms unique to every state were labeled as important and displaced relative to the initial configuration. The toy model equivalents of simulation frames in a trajectory were generated by displacing every labeled atom’s position with uniform noise and randomly rotating the entire system around the origin. In silico systems are typically rotation invariant, but aligning different configurations is often not trivial. In addition to the number of atoms and frames in the generated trajectory, the number of states, the number of important atoms per state, and the strength of the noise were all configurable parameters of the toy model.
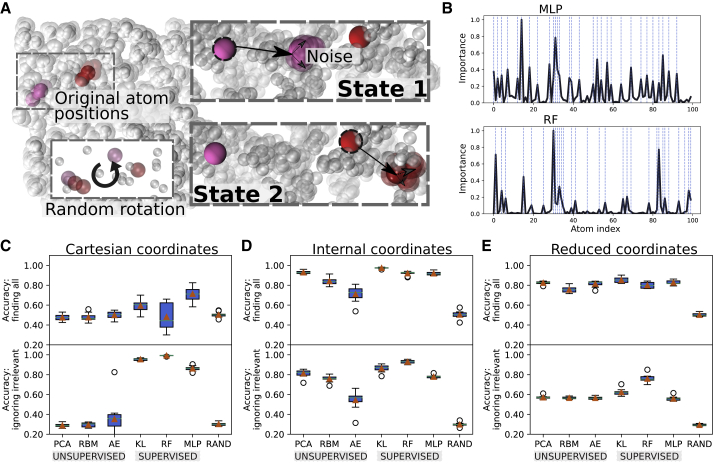
Moreover, we constructed two kinds of displacements: a linear displacement of atoms from their original position (Fig. 2 A) as well as a nonlinear displacement method (Fig. S1 and Supporting Materials and Methods). In the linear method, the magnitude and direction of the displacement were the same for all atoms. In the nonlinear method, one atom per state was displaced from its equilibrium position, with the size and the direction of the displacement depending on the state index. Subsequent atoms were rotated around the position of the previous atom in the same state (see Supporting Materials and Methods). From a biomolecular perspective, this can be seen as a model of how inter-residue contacts are broken in a linear (for example, with the displacement of one subdomain away from another) or nonlinear manner (for example, with the twist of a helix).
Figure 2.
(A) Toy model used to benchmark different ML approaches to extract important features from simulation data. Atomic coordinates are randomly generated, and a subset of the atoms, unique to every state, are displaced linearly from their initial positions. Artificial simulation frames are generated by adding noise to all atoms’ positions. Because only the relative and not the absolute positions of atoms are of significance in real biological systems, the system is rotated randomly around the origin. (B) Importance per atom for single instances of the toy model is shown. The index of the displaced atoms is highlighted as dashed vertical lines, coinciding with all peaks in importance in the case of an MLP and some of them for an RF. (C–E) Box plots of the performance of the different methods using either Cartesian coordinates (C), the full set of inverse interatomic distances (D), or a reduced set of inverse interatomic distances (E) are given as input features sampled over different instances of the toy model with linear displacement and 10% noise level. A high accuracy at finding all atoms signifies that every displaced atom has been identified as important and that other atoms have low importance. A high accuracy at ignoring irrelevant atoms signifies that only displaced atoms, although not necessarily all of them, have been marked important (Methods and Fig. S10). The best performing set of hyperparameters found after benchmarking every method (Figs. S4–S9) have been used. RAND stands for random guessing. The boxplots show the median (orange horizontal line), the interquartile range (box), the upper and lower whiskers (vertical lines), as well as the outliers (circles). To see this figure in color, go online.
The magnitude of the displacement was 0.1, and the strength of the noise was 0.01, except for Fig. S2, where it was increased to 0.05. 1200 frames were generated for each state. To obtain statistics, we generated 10 toy models with 100 atoms in a box with a side length stretching from −1 to 1, with the exception of Fig. S3, for which we evaluated systems of varying size. In total, there were three states with 10 displaced atoms per state. For every ML method, we computed the performance for different combinations of hyperparameters (Figs. S4–S9). For every instance of the toy model, the feature importance was computed 10 times. The parameters that gave the best performance were included in Figs. 2, S1, and S2.
When using inverse distances as features, the relevance per atom was computed by summing the average importance of all distances involving an atom and normalizing the values between 0 and 1. To take into account the smallest number of input features possible without losing information regarding the positions of the atoms, we also constructed a reduced set of internal coordinates. We did so by including the distances from atom i to atoms i + 1, i + 2, i + 3, and i + 4 only; this is akin to triangulation and suffices to reconstruct the coordinates of the entire system. Cartesian coordinates generated 3N input features. To compute the importance per atom, the importance of every triplet of xyz coordinates was summed and normalized.
Accuracy quantification
We used two scores to estimate a method’s performance on the toy model. Given a measured distribution of importance and a true distribution , we computed the accuracy at finding all important residues as 1 − . This mean squared error-based metric gave high scores to methods that identified all displaced atoms in the toy model and could tolerate some noise. The ignoring irrelevant accuracy score, designed to give high scores to methods that only identified truly important atoms without necessarily identifying all of them, was defined as . To illustrate these scoring methods, the accuracy scores of various distributions are highlighted in Fig. S10.
Calmodulin analysis
The data set consisted of six calmodulin (CaM) C-terminal states extracted with spectral clustering from a mix of regular MD (3600 ns) and temperature-enhanced simulations (570 ns replica-exchange MD and 460 ns replica-exchange solute tempering) (47). We used inverse Cɑ distances between C-terminal domain residues and a total of 7600 frames, yielding a data set size of 7600 × 2145. The data were first shuffled in blocks of 100 frames and filtered before feature extraction. Only residue pairs with Cɑ distance less than 1.0 nm in at least one frame and more than 1.0 nm in another were kept. This cutoff filtering decreased the number of features to 688. All features were normalized with scikit-learn’s (41) min-max scaler. To pick the number of components in PCA, a variance cutoff of 75% was used. The AE and MLP both had hidden layers of 120 and 100 nodes, respectively. Both were trained with an Adam optimizer and used ReLU activation functions. The RF classifier used 500 estimators and was trained with a one-versus-the-rest approach to obtain important features for every individual state. We used threefold cross validation and averaged the residue profile over five independent iterations.
GPCR analysis
The data set contained the output coordinates from every 10th short 10 ps trajectory initiated from points along the most probable transition path between β2 adrenergic receptor (β2AR)’s active and inactive state. Trajectories from every 10th of the 200–300 iterations of a string-of-swarms simulation (10) were used. Inverse Cɑ distances between all 284 protein residues were normalized with scikit-learn’s min-max scaler and were chosen as input features.
All PCs until the corresponding eigenvalue differed by at least an order of magnitude compared to the next eigenvalue were used to perform PCA. An RF classifier with 1000 estimators was trained to discriminate between ligand-bound and unbound frames. In total, 2674 frames were used in training with fourfold cross validation. The final importance profiles were averaged over 30 independent iterations.
VSD analysis
Snapshots corresponding to configurations making up five metastable states were extracted from a metadynamics trajectory taken from our previous study (48,49). The five states were previously identified using the network of salt bridges between the S4 positive residues and their negative counterparts as a collective variable (50). The overall length of the trajectory was 12,175 frames with 1087, 1900, 2281, 4359, and 2548 frames corresponding to E, Δ, Γ, B, and A states, respectively. Inverse heavy-atoms distances between all VSD residues were used as input features (13,041 in total). Distances larger than 0.7 nm or smaller than 0.5 nm throughout the entire trajectory were filtered out, yielding a final number of 3077 input features. To identify important residues, an RF classifier was trained with 100 estimators and threefold cross validation. The final residue importance profile was averaged over five independent iterations.
Software availability
The software used in this study is available for download in Delemottelab’s GitHub repository (https://github.com/delemottelab/demystifying; Table 1).
Table 1.
Resources—Data and Software
| Source | Identifier | |
|---|---|---|
| Deposited Data | ||
| Calmodulin trajectory | (47) | https://doi.org/10.1371/journal.pcbi.1006072 |
| β2 trajectory | (10) | https://doi.org/10.1101/627026 |
| VSD trajectory | (49) | https://doi.org/10.1073/pnas.1416959112 |
| Extracted data for this work | this work | https://drive.google.com/open?id=19V1mXz7Yu0V_2JZQ8wtgt7aZusAKs2Bb |
| Software | ||
| Demystifying | this work | https://github.com/delemottelab/demystifying |
| VMD | (84) | http://www.ks.uiuc.edu/Research/vmd/ |
| Python 2.7 | open source software | https://www.python.org/ |
| scikit-learn | (41) | https://scikit-learn.org/ |
| MDTraj | (85) | http://mdtraj.org/ |
| Numpy | open source software | http://www.numpy.org/ |
| BioPandas | (86) | http://rasbt.github.io/biopandas/ |
Results and Discussion
Benchmarking with a toy model provides quantitative measures of ML methods’ performance and guidance for analysis of real systems
Biomolecular systems consist of a large number of atoms or molecules. At each functional state, these fluctuate around their equilibrium positions because of thermal motion. As time progresses, a typical biomolecule transitions between a number of metastable states, either spontaneously or because of changes in its environment. For real systems, identifying important features of such metastable states is desirable, and ML offers data-driven methods for this purpose.
To evaluate the performance of a certain method, one needs to truly know how the states are defined. We have designed and used a toy model that mimics a biomolecular system and for which we are able to control the parameters describing states (Fig. 2 A). In short, the atoms were first randomly placed within a box at their equilibrium positions. To define new states, certain atoms were displaced from their original positions (Fig. 2 A) in either a linear (Fig. 2, C and D) or nonlinear manner (Fig. S1, Methods, and Supporting Materials and Methods, Section S1). Artificial simulation frames were then generated by adding noise to the atomic coordinates. The accuracy of a method at finding all displaced (relevant) atoms was evaluated using a metric based on the mean squared error between the estimated and the true feature importance profiles. In addition to this, we measured the ability of a method to ignore irrelevant atoms (i.e., minimizing false positives; Methods). The way these scoring methods describe known distributions is shown in Fig. S10. In this way, the toy model allowed us to test and quantify how successfully a method identified true positives. We completed this analysis by evaluating the performance of the different methods when considering Cartesian or internal coordinates as input features in a full or reduced set (Fig. 2, C–E) and at different noise levels (Fig. S2).
Given a trajectory consisting of many atoms and frames, with no or very little prior knowledge of the system, a widespread first approach in the biomolecular simulation community would be to apply PCA (Fig. 1 A) to all atomic coordinates and evaluate the ability of the first PCs to identify the features that contributed most to the variance in the data. Figs. 2 C and S1 illustrate some major drawbacks of this unsupervised approach. Because PCA intrinsically performs a linear mapping of input features to a low-dimensional representation (37), the performance of the method is highly dependent on the choice of input coordinates (13). For example, PCA failed to detect the important features of the toy system and even performed worse than random guessing when using raw, unaligned Cartesian coordinates as input (Figs. 2 C and Document S1. Supporting Materials and Methods, Figs. S1–S14, and Tables S1–S6, Video S1. Per-frame Feature Importance Projected onto the Molecular Ensemble of Structures in the Δ State of the Kv1.2 VSD). To remedy this, we performed PCA on internal coordinates (Fig. 2 D), namely inverse interatomic distances, using either the full set of all pairwise distances or a reduced set (Fig. 2 E) only connecting an atom to the four atoms before and after it in the sequence (Methods). The interatomic displacement that was performed to construct the states is easily described by such input coordinates, and PCA was able to successfully identify all the correct atoms for this simpler setup of the toy model (Fig. 2 D).
In addition to PCA, two alternative unsupervised learning methods were evaluated: a Bernoulli RBM (Fig. 1 B; (38)) and an AE (Fig. 1 C; (40)). For every set of input features and labels, important features for a single classification decision were derived using LRP (Fig. 1 F and Methods; (39)). RBM and AE were both poor at distinguishing important features from irrelevant ones using Cartesian coordinates (Fig. 2 C). They were able, however, to perform with higher accuracy for linear as well as nonlinear toy model displacements using internal coordinates (Figs. 2 D and S1 B). Although AE is suggested to be a promising unsupervised method for data sets that require nonlinear transformation, our results showed that it had a performance similar to or, at best, marginally better than PCA for Cartesian coordinates and a reduced set of internal coordinates, as well as for systems of smaller size (Figs. 2 and S3). For the full set of internal coordinates from larger systems, PCA performs better (Figs. 2 and S3). This outcome indicates that AE is difficult to train on data sets of high dimensionality such as biomolecular simulation trajectories and that to use this method successfully, one should reduce the number of input features to a minimum.
The main limitation of PCA and other unsupervised learning techniques is that when the conformational states are known and each frame is labeled according to the state it belongs to, they fall short in utilizing this information. Supervised learning techniques, on the other hand, are designed for this purpose. In principle, supervised ML should be able to identify even the most subtle differences between states. We have applied three methods of different character and complexity (Figs. 2, C–E, S1, and S2) that take known class labels into account. As a simple first step, we computed the KL (Fig. 1 D) divergence (42) between one state and all other states for every individual input feature. A high KL divergence indicates that the feature is good at differentiating between states and hence important. Next, we trained an RF (Fig. 1 E) classifier (43) to discriminate between states and computed feature importance using mean decrease impurity (44). The third method we evaluated was an MLP classifier (Fig. 1 F), a prototypical NN (42), in combination with LRP (39).
Computing the KL divergence between atomic coordinates does not transform the features in any way and therefore failed to identify important atoms from the raw Cartesian coordinates (Fig. 2 C), especially when the noise in the toy model was strong (Fig. S2 A). On the other hand, it did not inaccurately score irrelevant atoms. RF also performed well for only one of the two accuracy metrics, specifically to ignore irrelevant atoms and map out some important ones, albeit not all of them (Figs. 2, B and C and S1 A). This means that the classifier successfully learned to predict states without taking all relevant features into account. In many situations, this is an appealing characteristic; RF and KL provide quick overviews with a close-to-minimal set of features to describe the system and give highly interpretable importance profiles. This, however, comes at the risk of ignoring other significant features in the data. MLP, which has the ability to approximate nonlinear classification functions because of its multilayer architecture and use of activation functions, successfully identified the majority of the important features from unaligned Cartesian coordinates (Figs. 2, B and C and S1 A). Its performance, compared to the other methods, was found to be superior when states were defined by nonlinear instead of linear displacement between atoms (Fig. S1 A) or after increasing the strength of the noise in the toy model (Fig. S2). On the other hand, it was not as good at ignoring irrelevant features because it inaccurately scores some nondisplaced atoms as relatively important by mapping out overfitted nonlinearities.
All three supervised methods were excellent at identifying important features using the optimal distance-based features (Figs. 2, D and E and S1 B). RF and KL often outperformed MLP, although all three supervised methods were close to perfect at identifying the important features for this setup. Note that to find good discriminators between states, one should use a classifier such as MLP or RF instead of KL divergence. However, seeing that a high KL divergence correlates with a proper choice of input coordinates suggests that it can be used as a separation score to describe how good a few selected features are at separating states.
For every method, we chose a set of hyperparameters to tune using cross validation (Figs. S4–S9; Tables S1–S6) and included the results for the parameter set with best average performance in Figs. 2, S1, and S2. For PCA, we varied the number of components to consider in the aggregated importance per feature (Fig. S4; Table S1) and found that it is better to include all the PCs with corresponding eigenvalues (i.e., variance covered) within the same order of magnitude than to only include the first PC. For all three neural-network-based methods (RBM, AE, and MLP), we evaluated different shapes of the hidden layers (Figs. S5–S7; Tables S2–S4) and found that increasing the number of hidden nodes or layers does not necessarily increase performance in general. For the MLP and AE, we considered the regularization parameter, and for the RBM, we evaluated the performance for different values of the learning rate. For the AE, we also varied the number of epochs used in training, the learning rate and the batch size. Neither had a critical impact on the overall performance in this case, with the exception of having a too-high learning rate equal to one for the RBM. We also computed the KL divergence with different discretization parameters (Fig. S8; Table S5) and trained RF classifiers as either a set of binary or multiclass classifiers with varying the number of estimators, the maximal tree depth, and the minimum number of samples to be a leaf (Fig. S9; Table S6). A proper discretization size for KL seemed to be ∼1% of the range of values covered in the data. Tuning the number of minimum samples to be a leaf and the number of estimators in RF classifiers tended to increase the accuracy to some extent. Otherwise, both of these methods were moderately sensitive to the choice of hyperparameters, although a set of binary RF classifiers were, unsurprisingly, considerably better at identifying important atoms of a specific state compared to a multiclass classifier, which was only able to select the important atoms for the entire ensemble. In general, we found that a small change in a method’s hyperparameter value for this application typically resulted in a small change in the importance profile and that even a significantly different set of hyperparameter values performed with comparable accuracy.
We note that it is unlikely that the best-performing set of parameters in this study is optimal for all biological problems. In fact, it is unlikely that the optimal set of hyperparameters has been found even for the toy model. Moreover, although important atoms in the toy model were successfully identified with distance-based features, it is not sufficient to assume inter-residue distances to form an adequate set of input features for all biological problems. For example, it is possible that backbone dihedral angles are better indicators of certain types of conformational changes or that local root mean square deviation to experimentally resolved structures better describes how computationally sampled states agree with the experiments. To have a toolbox of methods that are not as sensitive to the choice of input coordinates in combination with metrics such as a separation score makes it straightforward to compare different sets of features and methods hyperparameters and avoid making poor choices. Based on the learnings from the toy model benchmarks, we outlined our protocol in the form of a checklist (Box 1) that we followed as we transitioned into biological applications.
Box 1. Checklist for interpreting molecular simulations with ML.
-
1)
Identify the problem to investigate.
-
2)Decide whether you should use supervised or unsupervised ML (or both).
-
a)The best choice depends on what data are available and the problem at hand.
-
b)If you chose unsupervised learning, consider also clustering the simulation frames to label them and perform supervised learning.
-
a)
-
3)Select a set of features and scale them.
-
a)For many processes, protein internal coordinates are adequate. To reduce the number of features, consider filtering distances with a cutoff.
-
b)Consider other features that can be expressed as a function of internal coordinates that you suspect to be important for the process of interest (dihedral angles, cavity or pore hydration, ion or ligand binding, etc.).
-
a)
-
4)Chose a set of ML methods to derive feature importance.
-
a)To quickly get a clear importance profile with little noise, consider RF or KL for supervised learning. RF may perform better for noisy data.
-
b)For unsupervised learning, consider PCA, which is fast and relatively robust when conducted on internal coordinates.
-
c)To find all important features, including those requiring nonlinear transformations of input features, also use neural-network-based approaches such as MLP. This may come at the cost of more peaks in the importance distribution.
-
d)Decide whether you seek the average importance across the entire data set (all methods), the importance per state (KL, a set of binary RF classifiers, or MLP), or the importance per single configuration (MLP, RBM, AE).
-
e)Chose a set of hyperparameters that give a reasonable tradeoff between performance and model prediction accuracy.
-
a)
-
5)
Ensure that the selected methods and hyperparameter choice perform well under cross validation.
-
6)
Average the importance per feature over many iterations.
-
7)
Check that the distribution of importance has distinguishable peaks.
-
8)
To select low-dimensional, interpretable CVs for plotting and enhanced sampling, inspect the top-ranked features.
-
9)
For a holistic view, average the importance per residue or atom and visualize the projection on the three-dimensional system.
-
10)
If necessary, iterate over steps 3–9 with different features, ML methods, and hyperparameters.
Supervised and unsupervised learning pinpoint conserved residues and frequent target-protein binders of calmodulin
Calmodulin is a ubiquitous messenger protein activated by calcium ions (51). The Ca2+-bound state typically resembles a dumbbell with the N-terminal and C-terminal domains that are separated by a fully helical linker (Fig. 3 A). When activated, clefts of hydrophobic residues are exposed in the two domains. The exposed hydrophobic surfaces enable binding to a large number of target proteins. Through regulation of these target proteins, CaM plays an important part in many physiologically vital pathways (52).
Figure 3.
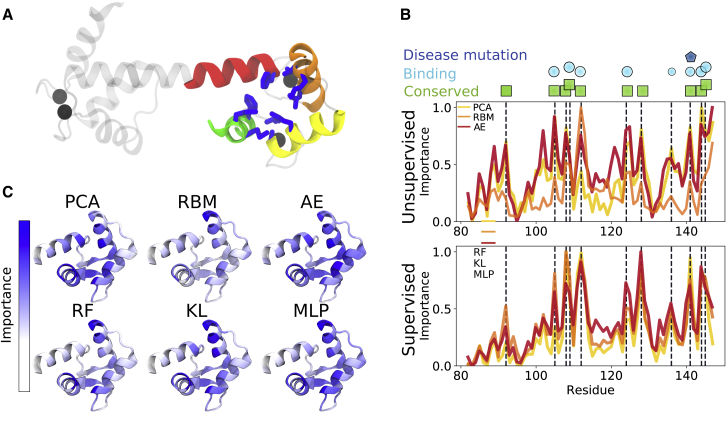
Important residues in the calmodulin C-terminal domain according to the feature extractors. (A) A molecular structure of Ca2+-bound CaM. The helices of the C-terminal domain are represented by colored ribbons, and Ca2+ ions are shown as black spheres. The residues identified as important in the analysis are shown with blue sticks. (B) The importance plotted along the residue sequence of unsupervised (upper) and supervised (lower) methods is shown. The peaks identified through visual inspection of the supervised method results are marked by vertical dashed lines, showing that most extractors give consistent profiles. The peak residues are marked as being conserved (green box), involved in binding (cyan circle), or known to be mutated in a disease (blue pentagon). (C) Molecular structures of the CaM C-terminal domain with important residues identified by the six extractors highlighted in blue are shown. To see this figure in color, go online.
We made a qualitative comparison between different methods (extractors) applied to a data set obtained with spectral clustering enhanced sampling trajectories of Ca2+-bound (holo) CaM. The data set contains the six states of the C-terminal domain of CaM (47). We intended to use unsupervised and supervised methods to identify the biological descriptors underpinning the different states that were identified in a data-driven manner.
Fig. 3, B and C show the average estimated feature importance obtained from the CaM data set with six states, using internal coordinates as input. The peaks obtained from the different extractors are shown as sticks on the molecular structure (Fig. 3 A) and highlighted with vertical dashed lines in the importance profiles (Fig. 3 B). Notably, the supervised methods agree well, whereas the unsupervised methods provide noisy profiles. Moreover, the residues that were found important are either highly conserved across phylogenetic groups (53), involved in binding target proteins (54), or known to cause severe cardiac diseases upon mutation (55). M109, M124, and M144-145, for example, are highly conserved (53) and recognized to play important roles for target-protein binding through various packings (56). Another example is F141, a well-studied mutation linked to long QT syndrome and decrease in Ca2+ affinity (55,57). We note that two peculiar peaks appeared in the AE profile of the CaM data set: A102 and V121. The implications of these peaks and their dependence on AE architecture are discussed in Supporting Materials and Methods, Section S2.
To monitor the dependency of the average importance profiles on the chosen number of states, a qualitative comparison between the RF importance profiles using five to eight states was carried out (Fig. S11). Evidently, the profiles obtained from six or more states overlap. In the five-state data set, however, an extra peak appears at residue V121 (Fig. S11 B). This residue was specifically identified as important for states 2 and 4 in the five-state data set, whereas it was only important for state 6 in the six-state data set (Fig. S12, A–C). Hence, it is assigned a large peak in the averaged profile of the five-cluster data set and a smaller peak in the six-state data set. We further note that both states 2 and 4 in the five-cluster data set are poorly populated and merged into state 6 in the six-state data set (Fig. S12, D–F). Therefore, the profiles with a smaller peak at V121, which was obtained with the six-or-more-state data sets, appears more reasonable than that of the five-state data set.
Comparing the unsupervised and supervised profiles reveals two residues specifically identified by the supervised methods: V108 and A128. These two residues are not typical target-protein binders. However, both residues are well conserved (53) and thus likely play stabilizing roles for exposing binding surfaces. In fact, the packing and repacking of V108 between F89 and F92 is linked to the transition between apo and holo-like C-terminal states (56). A third surprising peak is that of V136, which is not highly conserved (53) and only participates in binding to some extent (54). This residue is, however, part of the C-terminal domain β-sheet that was shown to be disrupted in an extra-open state of the analyzed data set (47). Thus, by contrasting the output of the different methods, it is evident that the supervised methods show more distinguishable peaks, and the ensemble of methods successfully uncovers important residues of the CaM C-terminal domain.
Supervised learning of the β2AR identifies ligand interaction sites and reveals allosteric coupling, whereas unsupervised methods learn the main characteristics of GPCR activation
GPCRs form a large family of receptors in the human genome and act as an important class of modern drug targets (58). The β2AR (Fig. 4 A) is a class A GPCR often used as a prototype in studies of GPCR activation. In its native state without any binding partner, the intracellular part of the β2AR undergoes interconversion between an inactive and an active state, allowing a G-protein to bind to the intracellular region, a process called basal activity. Agonist ligands interact with residues in the orthosteric site and shift the equilibrium between states to make the active state more energetically favorable.
Figure 4.
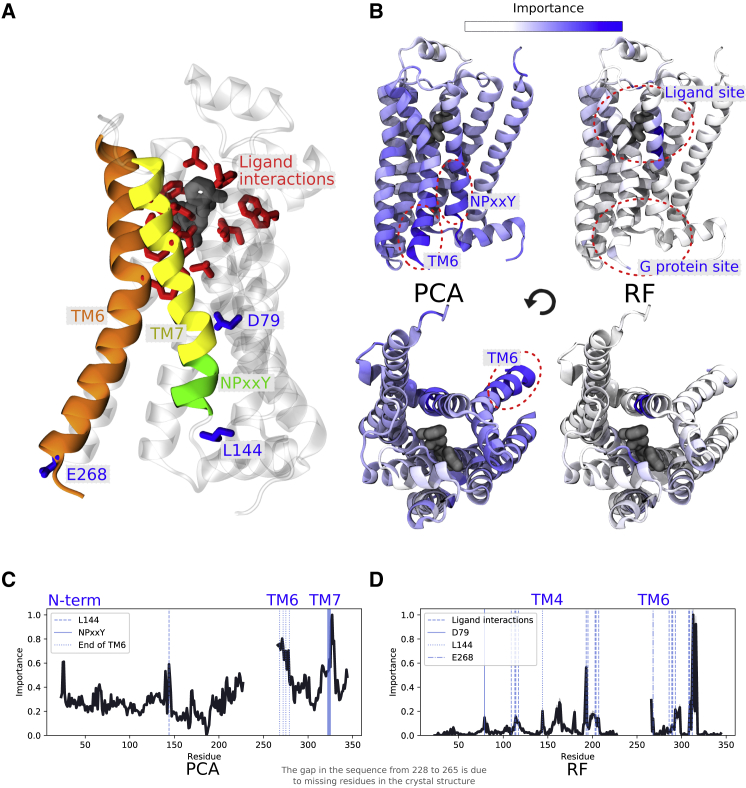
Important residues of the β2 adrenergic receptor using frames from a string-of-swarms simulation of the receptor’s activation (10). (A) A molecular structure of the β2AR bound to an agonist ligand is shown. Transmembrane helices TM6 and TM7, as well the NPxxY motif, are represented as colored ribbons. Important residues known to interact with ligands are shown with red sticks, and other key residues identified in the analysis are colored in blue. (B) Active state structures with important residues highlighted in blue are shown. (C) Unsupervised learning with PCA is shown. The G-protein binding site undergoes the most significant conformational change upon activation, especially TM6 and the NPxxY motif at the bottom of TM7. (D) Supervised learning with an RF classifier trained to identify protein conformations as either being ligand bound or ligand unbound is shown. Residues known to interact with the ligand binding site (65) coincide with the residues identified as important. Important residues to discriminate between the two conditions are also located in the G-protein binding site, an indication of allosteric coupling between the intracellular part of the receptor and the orthosteric site. To see this figure in color, go online.
We analyzed the conformational ensemble of short trajectories from a string-of-swarms simulation of β2AR activation in the presence and absence of an agonist ligand (10). Unsupervised learning with PCA on the entire internal coordinate data set revealed known hallmarks of activation (Fig. 4, B and C), including significant rearrangements of helices around the G-protein binding site, especially the twist of the conserved NPxxY motif at the bottom of TM7 (59,60). Another protruding hallmark of GPCR activation identified by unsupervised learning is the displacement of the intracellular part of TM6, including E268, which is the residue that undergoes the largest conformational change upon activation (61), as well as residues L272, L275, and M279 on TM6, whose side chains are rotated upon activation (62). The third most important region consisted of residues just below the extracellular N-terminus, a disordered region consistently changing its orientation throughout the simulation. Its large variance in interactions with other residues makes it prone to be identified as important by these methods, even though its movement is not considered a typical characteristic of GPCR activation. Finally, L144 at intracellular loop 2 was shown as important, and it is known to be implicated in β2AR/Gs coupling (63,64).
Following the guidelines in our checklist (Box 1), we trained an RF classifier to discriminate between the ligand-bound and ligand-unbound simulation frames within the same data set. After training, the most important residues were identified close to the ligand binding site (Fig. 4, B and D), most of which are well known to interact with agonist ligands (65). Other parts of the receptor interacting directly with these residues were also identified as important, especially residues along transmembrane helix 4 (TM4). T164, for example, interacts with ligand-binding-site residues S203, S207, and V114. In general, residues further away from the orthosteric site were considered less important (Fig. 4 D). One exception is the local peak at the D79 residue on TM2, the most conserved residue in all class A GPCRs. This residue is also thought to change protonation state during activation (Fig. 4 D; (10)). Remarkably, important residues were further identified around the G-protein binding site: L144 and E268 at the bottom of TM6, which sample both larger as well as smaller values of displacement in the apo trajectories (10). This result is indicative of allosteric coupling between the two sites and shows that the classifier is able to detect subtle local changes in the G-protein binding site that are induced by agonist binding. It suggests that ligand binding not only shifts the energy of the active state but forces the G-protein binding site to adopt alternative conformations. The intracellular end of TM6 and the NPxxY motif do not appear as important, on the other hand, presumably because they are observed in similar conformations both in the ligand-bound and the ligand-unbound ensemble.
In Fig. 4, B–D, it can clearly be seen that RF gives a more easily interpretable profile compared to PCA, in which the peaks are distinct, and the average importance is low. This is not merely a result of the chosen methods; it is also due to the kind of insights they provide. GPCR activation involves conformational change throughout the protein, whereas ligand binding and allosteric signaling are more local effects. To conclude, unsupervised ML was useful on this data set to study the general large-scale activation mechanism, whereas supervised ML successfully showed the effect of agonist binding throughout the conformational ensemble.
Supervised learning reveals state-dependent residues allosterically coupled to the gating charge
The VSD is a common module of voltage-gated ion channels responsible for reacting to changes in the membrane potential (66). It is usually composed of four transmembrane segments named S1–S4 and a short surface C-terminal helix, the S4–S5 linker (Fig. 5 A; (67, 68, 69)). A few positively charged residues on the S4 segment confer voltage sensitivity to the VSD function (Fig. 5 A). Because they are located in a low dielectric medium, these residues are specifically sensitive to an externally applied electric field and thus adapt their positions according to the field direction and strength (50,70,71). For instance, when the membrane is depolarized, they move toward the extracellular solution. This motion engages the entire S4 segment, which promotes the voltage-sensor activation: the S4 transition from the resting (inward-facing) to the activated (outward-facing) state (72, 73, 74, 75, 76). To ensure the stability of the positive charges inside the low dielectric medium, they are paired with negative charges of the three remaining segments. During activation, the positive charges switch negatively charged counterparts so that a network of salt bridges between S4 and S1–S3 is maintained in every conformational state of the VSD (72).
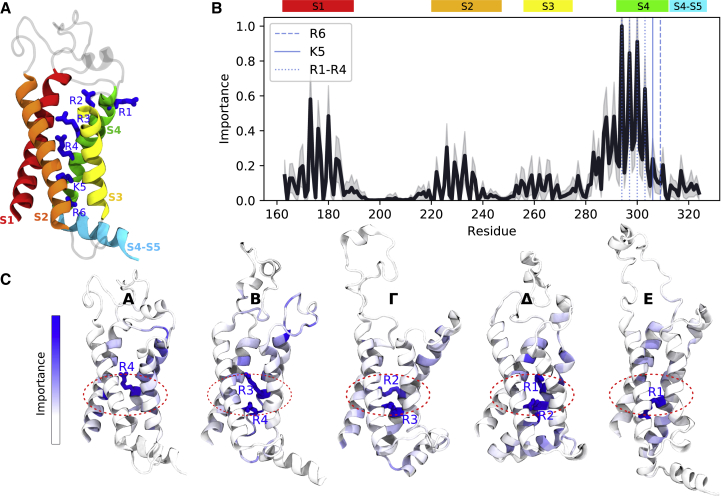
Figure 5.
Identifying key residues for Kv1.2 voltage-sensor function. (A) A cartoon representation of the Kv1.2 voltage sensor is given. The four membrane segments S1–S4 and the surface S4–S5 helix are shown. S4 positive charges are represented as blue sticks. (B) Per-residue importance obtained using RF classifiers and averaged over the five states of the Kv1.2 VSD is shown. The colored rectangles denote the helical segments. S4 charges (R1, R2, R3, R4, K5 and R6) are marked with the blue dashed lines. The average importance and its standard deviation are shown as the black line and the shadow around it, respectively. (C) Per-state per-residue importance is shown. The top-ranked residues are shown as sticks. The area of the hydrophobic plug is marked as a red dashed zone. To see this figure in color, go online.
In a previous study, we reported five conformational states of the Kv1.2 VSD called E (resting), A (activated), and Δ, Γ, and B (intermediate states) (49,50). Here, we followed the provided checklist (Box 1) and trained an RF classifier to compare these states and elucidate their defining structural elements using more than 12,000 frames from an enhanced sampling trajectory (see Methods). The simulation frames were extracted from basins in the free-energy landscape constructed by metadynamics. Thus, even though the trajectory was not sampled from a Boltzmann distribution, the derived simulations snapshots represent conformations one would expect to see in an unbiased MD trajectory. Fig. 5 B shows the per-residue importance obtained using RF classifiers averaged over all five states. The first top-ranked residues (R1–R4) are four out of six positive charges of the S4 segment (R1, R2, R3, R4, K5, and R6). Thus, in agreement with the classical view, the positively charged residues are suggested to be crucial in delineating the five conformational states of the Kv1.2 VSD. Analysis of the per-residue importance of each state (Fig. 5 C) reveals that although the top-ranked charges are indeed state specific (R4 in A, R3 and R4 in B, R2 and R3 in Γ, R1 and R2 in Δ, and R1 in E), their localization is state independent: all of them are placed near the so-called hydrophobic plug, the central part of the VSD where the response to the external electric field is the largest (50,77,78). Other highly ranked residues are located along transmembrane segments and facing the protein lumen. LRP performed on the MLP classifier further offers the possibility to attribute feature importance to each individual trajectory frame. Video S1 specifically shows per-frame feature importance profiles and provide comparable insight regarding the localization of important residues around the hydrophobic plug. Taken together, our data suggest that the interaction with the hydrophobic plug is a key aspect to distinguish between the metastable activation states in the Kv1.2 VSD.
Supervised and unsupervised learning play different roles in identifying important features
Supervised and unsupervised methods are designed for different purposes, and the optimal choice of analysis method is case dependent. Sometimes the labels are inherent to the computational experiment, such as in the example of comparing ligand-bound and ligand-unbound simulations of β2AR or when comparing the conformational ensemble of a wild-type protein with a specific mutant. For such cases, supervised learning techniques may reveal subtle differences invisible to unsupervised techniques. The performance evaluated with the toy model also shows that supervised learning generally outperforms unsupervised learning on labeled data sets.
Standalone unsupervised learning becomes more attractive when simulation data cannot be labeled directly. To use supervised learning for such cases, one would need to preprocess the data—for example, by manual labeling or with a clustering method—to assign each frame to a state. Given adequate states, supervised learning will provide useful insights about the data, as was done for calmodulin and the Kv1.2 VSD in this study. However, to arrive at biologically relevant insights requires the states themselves to contain useful information, and it is important to bear this limitation in mind for practical applications. Unsupervised learning techniques can also be used as prior feature extractors to supervised classifiers or provide a projection onto a low-dimensional space where it is straightforward to cluster structures into states. A strength of having different tools for computing the importance per feature within the same framework is that it becomes straightforward to combine supervised and unsupervised learning to give a coherent view of what features are important in a system. It is simple to extend this framework to include more methods as long as there is an algorithm to derive the importance per input feature.
For practical applications, the best choice of method depends on the type of data available. Regardless, the results should be averaged over many runs and cross validated to reduce the variance induced by stochastic solvers, as well as the risk of introducing inaccuracies due to overfitting. However, unless a good set of input features is known or can be validated, nonlinear ML methods such as NNs will be more reliable than linear methods in general.
We also note that the protocol presented in this study is in itself not restricted to study data sampled from a Boltzmann distribution, as illustrated by the VSD application case. The biophysical conclusions one can draw from the analysis, on the other hand, will depend on the nature of the data set. If one were to look for important features for an entire ensemble in a data set containing highly biased samples, then taking the Boltzmann weight into account might aid interpretation. In general, this can be achieved by balancing the data set according to the weight of the samples, and some models such as the RF classifier used in this study even support weighting samples during training out of the box.
Feature selection and model parameter choice can enhance performance but are not obstacles for interpretability
We used inverse interatomic distances as features for the biological systems, which puts more emphasis on local changes than mere distances, and normalized the values to enable the different ML methods to perform well (Methods). The choice of internal coordinates may seem straightforward, following the results obtained from the toy model. For large systems, however, it may be computationally intractable to consider all internal coordinates as features and, in the case of limited amount of simulation frames, can also increase the chance of overfitting the feature extractors. In addition to performing cross validation to overcome this problem, distances that did not reach below a certain cutoff in the trajectory were prefiltered in the case of CaM and the VSD (Methods). This also has a physical significance because atoms close to each other have a stronger interaction energy. Long-range allosteric effects may still be discovered and propagated through nonlinear methods.
In principle, any intuitively interesting reaction coordinate can be included in the set of input features. In protein folding, dihedral angles are often reported as important and should thus be included. Other CVs such as hydration, ion binding, etc. may be considered as part of the input feature data set. We note here too that we have only taken structural (and not dynamic) information into account, following the nature of the data set we had at hand. To account for dynamics, for example, a time-lagged AE (11) could be used on continuous MD simulation trajectories such that a NN is trained to reconstruct features using features from previous times as input and derive the resulting importance profiles from the trained model. In general, given a Markov state model with a number of connected Markov states (32,33,79,80), our approach provides the means to make a data-driven interpretation on a molecular basis of the dynamics behind these states.
For supervised learning, one should be aware of the difference between choosing a multiclass RF or MLP in contrast to using KL or RF with a one-versus-the-rest splitting of the data. One-versus-the-rest splitting in learning enables identifying important features for specific states as opposed to only reporting the important features across the entire ensemble (Figs. S7–S9). Furthermore, the difference between multiclass and one versus the rest may have a major effect when dealing with unbalanced data sets. In this regard, algorithms such as LRP are powerful because they allow us to compute the feature importance for every individual frame (Video S1) and thereby enhance interpretability at a very fine-grained level.
Figs. S4–S9 demonstrate that the performance of the methods are affected by choosing different hyperparameters, such as the regularization constraints for a NN, the size of the discretization parameters to compute the KL divergence, or the minimum number of samples to be a leaf in a RF classifier. To include too many parameters in a model by adding an unnecessarily large number of hidden nodes in an MLP or to neglect parts of the data set by considering too few PCs are both examples of what may reduce performance. We found it particularly challenging to obtain high accuracy with the AE, and it is possible that additional effort in parameter tuning may improve its performance. Seeing that they are both unsupervised neural-network-based methods, it seems reasonable for the AE to obtain a performance comparable to the RBM, even though the AE used in this study—unlike the RBM or, for example, a variational AE (81)—does not make any strong assumptions about the distribution of input or hidden-layer latent variables. Incidentally, an optimally trained AE with sigmoid activation and a single hidden layer is strongly related to PCA (82,83). For smaller sets of input features, we do indeed observe a similar performance between PCA and the AE (Fig. S3). Thus, from a practical point of view, it is probably a more rewarding effort to reduce the number of input features (for example, by using a distance-based cutoff) than to expand the hyperparameter search space. In general, NN models have many tunable hyperparameters as well as a large number of weights that need to be fitted, whereas simpler methods such as PCA and KL have fewer parameters. This drawback of the complicated models needs to be taken into account for practical applications.
Putting some effort into crude parameter tuning should be a rewarding effort, whereas excessive fine tuning may not reveal any new insights. Moreover, even though it is most likely possible to further improve the performance of these methods with extended efforts of parameter tuning, the optimal set of hyperparameters for a certain classifier likely depends on the choice of input features and the biological system. From a practical point of view, it seems instead to be more useful to follow the guidelines presented here using an adequate configuration of every ML method, ensure that it is robust to cross validation, and apply it to investigate the performance of different input features. Our checklist (Box 1), which covers the most important aspects of interpreting molecular simulations with ML (Methods), makes this process easier.
The fact that many models will yield the same conclusion irrespective of hyperparameter setup increases the credibility of these methods for practical purposes. For neural-network-based models, changing the network architecture leads to trained models with completely different underlying weight matrices. As shown in this study, networks of different shape and regularization constraints nevertheless identify the same important features in a simulation system. Although similar arguments hold for other methods, this is a particularly important result because of the enormous interest and skepticism toward deep learning. This contradicts any criticism of the black-box nature of such methods: the biological properties propagated from the input to the output are clearly consistent and traceable, as well as interpretable.
Conclusions
In this study, we have applied various ML methods to identify important features from molecular simulations. We constructed a toy model that mimics real macromolecular behavior to perform a quantitative comparison between the methods and derive insights regarding their applicability for practical purposes.
When optimizing the performance of different ML methods, we found that the choice of proper input features had larger effect than scrupulous hyperparameter tuning. Specifically, a great performance increase was observed when switching from raw Cartesian to internal distance-based features (potentially with a cutoff). Supervised learning methods, in particular, outperform unsupervised methods on a suboptimal Cartesian data set. Furthermore, an MLP classifier was shown to be significantly better at identifying all important features of a system compared to an RF or computing the KL divergence. This indicates that a NN is able to learn the transformation of input data to an optimal representation and that more complex deep learning approaches are robust analysis tools when little, or even no, prior knowledge is available to guide the choice of input features. However, MLP’s output was arguably more difficult to interpret than that of the other supervised methods. For unsupervised learning on an optimal set of internal coordinates, all methods (PCA, RBM, and AE) identified important residues with similar accuracies on smaller data sets. On larger data sets, however, PCA outperformed both RBMs and AEs with regards to accuracy as well as computational efficiency.
Finally, we successfully applied this protocol to derive key insights into three distinct types of biomolecular processes: the conformational rearrangements of the soluble protein calmodulin, the effect of ligand binding to a GPCR, and the allosteric coupling of an ion channel VSD to a transmembrane potential. Rather than trying to identify one optimal method for all practical applications, we condensed our learnings into a protocol in the form of a checklist. In short, the ML methods were designed for different purposes, and the best choice of analysis methods depends on the question at hand. Supervised learning should be used when applicable, for example, for studying the small allosteric switches induced by ligand binding to a receptor or comparing distinct metastable states, whereas unsupervised methods can derive ensemble features of unlabeled data, thus capturing the major conformational change of a protein.
This work sheds, to our knowledge, new light on ML as a pillar in interpretation of biomolecular systems. The possible applications of our approach spans from interpreting large amounts of simulation or experimental ensemble data to selecting CVs for enhanced sampling simulations. Compared to the computationally expensive MD simulations, these insights are cheap: all results in this study were computed on commodity hardware using software published in the public repository “demystifying” on GitHub (Table 1).
Humans did not evolve to interpret large sets of high-dimensional data by mere visual inspection. ML methods were, on the contrary, specifically designed to process big data sets. Nevertheless, despite the increasing interest and faith put into ML, current state-of-the-art methods are not at a stage at which computers are able to set up, run, and analyze simulations autonomously. Instead, we have shown how statistical models and algorithms commonly used to solve ML problems provide a powerful toolbox to efficiently make data-driven interpretations of biomolecular systems. As the timescales accessible by simulations increase, and the popularity of ML tools continue to thrive across many scientific areas, we anticipate that our approach can be useful to aid many researchers in demystifying complex simulations.
Author Contributions
O.F., M.A.K., and L.D. conceptualized the project. O.F., M.A.K., and A.M.W. wrote the code and performed different parts of data curation and formal analysis: A.M.W. for CaM, O.F. for β2, and M.A.K. for the VSD. O.F. and M.A.K. designed and analyzed the toy model. L.D. supervised the project. O.F., M.A.K., and A.M.W. wrote the first draft of the manuscript. All authors reviewed and edited the manuscript.
Acknowledgments
This work was supported by grants from the Gustafsson Foundation and Science for Life Laboratory to L.D. The simulations were performed on resources provided by the Swedish National Infrastructure for Computing at the Parallelldatorcentrum (PDC) Centre for High Performance Computing.
Editor: Philip Biggin.
Footnotes
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2019.12.016.
Supporting Material
References
- 1.Ching T., Himmelstein D.S., Greene C.S. Opportunities and obstacles for deep learning in biology and medicine. J. R. Soc. Interface. 2018;15:20170387. doi: 10.1098/rsif.2017.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zitnik M., Nguyen F., Hoffman M.M. Machine learning for integrating data in biology and medicine: principles, practice, and opportunities. Inf. Fusion. 2019;50:71–91. doi: 10.1016/j.inffus.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akay A., Hess H. Deep learning: current and emerging applications in medicine and technology. IEEE J. Biomed. Health Inform. 2019;23:906–920. doi: 10.1109/JBHI.2019.2894713. [DOI] [PubMed] [Google Scholar]
- 4.Zou J., Huss M., Telenti A. A primer on deep learning in genomics. Nat. Genet. 2019;51:12–18. doi: 10.1038/s41588-018-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camacho D.M., Collins K.M., Collins J.J. Next-generation machine learning for biological networks. Cell. 2018;173:1581–1592. doi: 10.1016/j.cell.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Chen H., Engkvist O., Blaschke T. The rise of deep learning in drug discovery. Drug Discov. Today. 2018;23:1241–1250. doi: 10.1016/j.drudis.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Lundervold A.S., Lundervold A. An overview of deep learning in medical imaging focusing on MRI. Z. Med. Phys. 2019;29:102–127. doi: 10.1016/j.zemedi.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Jang H.J., Cho K.O. Applications of deep learning for the analysis of medical data. Arch. Pharm. Res. 2019;42:492–504. doi: 10.1007/s12272-019-01162-9. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro J.M.L., Tiwary P. Achieving reversible ligand-protein unbinding with deep learning and molecular dynamics through RAVE. bioRxiv. 2018 doi: 10.1101/400002. [DOI] [PubMed] [Google Scholar]
- 10.Fleetwood O., Matricon P., Delemotte L. Energy landscapes reveal agonist’s control of GPCR activation via microswitches. bioRxiv. 2019 doi: 10.1101/627026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wehmeyer C., Noé F. Time-lagged autoencoders: deep learning of slow collective variables for molecular kinetics. J. Chem. Phys. 2018;148:241703. doi: 10.1063/1.5011399. [DOI] [PubMed] [Google Scholar]
- 12.Sittel F., Stock G. Perspective: identification of collective variables and metastable states of protein dynamics. J. Chem. Phys. 2018;149:150901. doi: 10.1063/1.5049637. [DOI] [PubMed] [Google Scholar]
- 13.Sittel F., Jain A., Stock G. Principal component analysis of molecular dynamics: on the use of Cartesian vs. internal coordinates. J. Chem. Phys. 2014;141:014111. doi: 10.1063/1.4885338. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad M., Helms V., Lengauer T. Relative principal components analysis: application to analyzing biomolecular conformational changes. J. Chem. Theory Comput. 2019;15:2166–2178. doi: 10.1021/acs.jctc.8b01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung H., Covino R., Hummer G. Artificial intelligence assists discovery of reaction coordinates and mechanisms from molecular dynamics simulations. arXiv. 2019 https://arxiv.org/abs/1901.04595 arXiv:1901.04595. [Google Scholar]
- 16.Sultan M.M., Kiss G., Pande V.S. Automatic selection of order parameters in the analysis of large scale molecular dynamics simulations. J. Chem. Theory Comput. 2014;10:5217–5223. doi: 10.1021/ct500353m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidky H., Whitmer J.K. Learning free energy landscapes using artificial neural networks. J. Chem. Phys. 2018;148:104111. doi: 10.1063/1.5018708. [DOI] [PubMed] [Google Scholar]
- 18.Hashemian B., Millán D., Arroyo M. Modeling and enhanced sampling of molecular systems with smooth and nonlinear data-driven collective variables. J. Chem. Phys. 2013;139:214101. doi: 10.1063/1.4830403. [DOI] [PubMed] [Google Scholar]
- 19.Mittal S., Shukla D. Recruiting machine learning methods for molecular simulations of proteins. Mol. Simul. 2018;44:891–904. [Google Scholar]
- 20.Ribeiro J.M.L., Bravo P., Tiwary P. Reweighted autoencoded variational Bayes for enhanced sampling (RAVE) J. Chem. Phys. 2018;149:072301. doi: 10.1063/1.5025487. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J., Chen M. Unfolding hidden barriers by active enhanced sampling. Phys. Rev. Lett. 2018;121:010601. doi: 10.1103/PhysRevLett.121.010601. [DOI] [PubMed] [Google Scholar]
- 22.Trapl D., Horvacanin I., Spiwok V. Anncolvar: approximation of complex collective variables by artificial neural networks for analysis and biasing of molecular simulations. Front. Mol. Biosci. 2019;6:25. doi: 10.3389/fmolb.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W., Tan A.R., Ferguson A.L. Collective variable discovery and enhanced sampling using autoencoders: innovations in network architecture and error function design. J. Chem. Phys. 2018;149:072312. doi: 10.1063/1.5023804. [DOI] [PubMed] [Google Scholar]
- 24.Chen W., Sidky H., Ferguson A.L. Nonlinear discovery of slow molecular modes using state-free reversible VAMPnets. J. Chem. Phys. 2019;150:214114. doi: 10.1063/1.5092521. [DOI] [PubMed] [Google Scholar]
- 25.Chen W., Ferguson A.L. Molecular enhanced sampling with autoencoders: on-the-fly collective variable discovery and accelerated free energy landscape exploration. J. Comput. Chem. 2018;39:2079–2102. doi: 10.1002/jcc.25520. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez A., d’Errico M., Laio A. Computing the free energy without collective variables. J. Chem. Theory Comput. 2018;14:1206–1215. doi: 10.1021/acs.jctc.7b00916. [DOI] [PubMed] [Google Scholar]
- 27.Facco E., d’Errico M., Laio A. Estimating the intrinsic dimension of datasets by a minimal neighborhood information. Sci. Rep. 2017;7:12140. doi: 10.1038/s41598-017-11873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendels D., Piccini G., Parrinello M. Collective variables from local fluctuations. J. Phys. Chem. Lett. 2018;9:2776–2781. doi: 10.1021/acs.jpclett.8b00733. [DOI] [PubMed] [Google Scholar]
- 29.Pietrucci F., Andreoni W. Graph theory meets ab initio molecular dynamics: atomic structures and transformations at the nanoscale. Phys. Rev. Lett. 2011;107:085504. doi: 10.1103/PhysRevLett.107.085504. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Ribeiro J.M.L., Tiwary P. Past-future information bottleneck for sampling molecular reaction coordinate simultaneously with thermodynamics and kinetics. Nat. Commun. 2019;10:3573. doi: 10.1038/s41467-019-11405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degiacomi M.T. Coupling molecular dynamics and deep learning to mine protein conformational space. Structure. 2019;27:1034–1040.e3. doi: 10.1016/j.str.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Wu H., Mardt A., Noe F. Deep generative Markov state models. arXiv. 2018 https://arxiv.org/abs/1805.07601 arXiv:1805.07601. [Google Scholar]
- 33.Mardt A., Pasquali L., Noé F. VAMPnets for deep learning of molecular kinetics. Nat. Commun. 2018;9:5. doi: 10.1038/s41467-017-02388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez A., Laio A. Machine learning. Clustering by fast search and find of density peaks. Science. 2014;344:1492–1496. doi: 10.1126/science.1242072. [DOI] [PubMed] [Google Scholar]
- 35.Olah C., Satyanarayan A., Mordvintsev A. The building blocks of interpretability. Distill. 2018;3:e10. doi: 10.23915/distill.00010. [DOI] [Google Scholar]
- 36.Montavon G., Samek W., Müller K.-R. Methods for interpreting and understanding deep neural networks. Digit. Signal Process. 2018;73:1–15. [Google Scholar]
- 37.Tipping M.E., Bishop C.M. Probabilistic principal component analysis. J. R. Stat. Soc. B. 1999;61:611–622. doi: 10.1162/089976699300016728. [DOI] [PubMed] [Google Scholar]
- 38.Smolensky P. Information processing in dynamical systems: foundations of Harmony theory. In: Rumelhart D.E., McClelland J.L., editors. Parallel Distributed Processing: Explorations in the Microstructure of Cognition. MIT Press; 1986. pp. 194–281. [Google Scholar]
- 39.Montavon G., Lapuschkin S., Müller K.-R. Explaining nonlinear classification decisions with deep Taylor decomposition. Pattern Recognit. 2017;65:211–222. [Google Scholar]
- 40.Hinton G.E., Salakhutdinov R.R. Reducing the dimensionality of data with neural networks. Science. 2006;313:504–507. doi: 10.1126/science.1127647. [DOI] [PubMed] [Google Scholar]
- 41.Pedregosa F., Varoquaux G., Gramfort A. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 2011;12:2825–2830. [Google Scholar]
- 42.Bishop C.M. Springer Science+ Business Media, New York; 2006. Pattern Recognition and Machine Learning. [Google Scholar]
- 43.Ho T.K. Random decision forests. In: Kavanaugh M., Storms P., editors. Vol. 1. 1995. pp. 278–282. (Proceedings of the 3rd International Conference on Document Analysis and Recognition). [Google Scholar]
- 44.Breiman L., Friedman J.H., Stone C.J. Wadsworth & Brooks/Cole Advanced Books & Software; Monterey, CA: 1984. Classification and Regression Trees. [Google Scholar]
- 45.Louppe G. Understanding random forests: from theory to practice. arXiv. 2014 https://arxiv.org/abs/1407.7502 arXiv:1407.7502. [Google Scholar]
- 46.Kingma D.P., Ba J. Adam: a method for stochastic optimization. arXiv. 2014 https://arxiv.org/abs/1412.6980 arXiv:1412.6980. [Google Scholar]
- 47.Westerlund A.M., Delemotte L. Effect of Ca2+ on the promiscuous target-protein binding of calmodulin. PLoS Comput. Biol. 2018;14:e1006072. doi: 10.1371/journal.pcbi.1006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delemotte L., Kasimova M.A., Carnevale V. Free-energy landscape of ion-channel voltage-sensor-domain activation. Proc. Natl. Acad. Sci. USA. 2015;112:124–129. doi: 10.1073/pnas.1416959112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delemotte L., Kasimova M.A., Tarek M. Exploring the complex dynamics of an ion channel voltage sensor domain via computation. bioRxiv. 2017 doi: 10.1101/108217. [DOI] [Google Scholar]
- 50.Delemotte L., Tarek M., Treptow W. Intermediate states of the Kv1.2 voltage sensor from atomistic molecular dynamics simulations. Proc. Natl. Acad. Sci. USA. 2011;108:6109–6114. doi: 10.1073/pnas.1102724108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuboniwa H., Tjandra N., Bax A. Solution structure of calcium-free calmodulin. Nat. Struct. Biol. 1995;2:768–776. doi: 10.1038/nsb0995-768. [DOI] [PubMed] [Google Scholar]
- 52.Kanehisa M., Furumichi M., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halling D.B., Liebeskind B.J., Aldrich R.W. Conserved properties of individual Ca2+-binding sites in calmodulin. Proc. Natl. Acad. Sci. USA. 2016;113:E1216–E1225. doi: 10.1073/pnas.1600385113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villarroel A., Taglialatela M., Areso P. The ever changing moods of calmodulin: how structural plasticity entails transductional adaptability. J. Mol. Biol. 2014;426:2717–2735. doi: 10.1016/j.jmb.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 55.Limpitikul W.B., Dick I.E., Yue D.T. Calmodulin mutations associated with long QT syndrome prevent inactivation of cardiac L-type Ca(2+) currents and promote proarrhythmic behavior in ventricular myocytes. J. Mol. Cell. Cardiol. 2014;74:115–124. doi: 10.1016/j.yjmcc.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shukla D., Peck A., Pande V.S. Conformational heterogeneity of the calmodulin binding interface. Nat. Commun. 2016;7:10910. doi: 10.1038/ncomms10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crotti L., Johnson C.N., George A.L., Jr. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation. 2013;127:1009–1017. doi: 10.1161/CIRCULATIONAHA.112.001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hauser A.S., Attwood M.M., Gloriam D.E. Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 2017;16:829–842. doi: 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weis W.I., Kobilka B.K. The molecular basis of G protein-coupled receptor activation. Annu. Rev. Biochem. 2018;87:897–919. doi: 10.1146/annurev-biochem-060614-033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Latorraca N.R., Venkatakrishnan A.J., Dror R.O. GPCR dynamics: structures in motion. Chem. Rev. 2017;117:139–155. doi: 10.1021/acs.chemrev.6b00177. [DOI] [PubMed] [Google Scholar]
- 61.Rasmussen S.G., DeVree B.T., Kobilka B.K. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dror R.O., Arlow D.H., Shaw D.E. Activation mechanism of the β2-adrenergic receptor. Proc. Natl. Acad. Sci. USA. 2011;108:18684–18689. doi: 10.1073/pnas.1110499108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moro O., Lameh J., Sadée W. Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J. Biol. Chem. 1993;268:22273–22276. [PubMed] [Google Scholar]
- 64.Moreira I.S. Structural features of the G-protein/GPCR interactions. Biochim. Biophys. Acta. 2014;1840:16–33. doi: 10.1016/j.bbagen.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 65.Chan H.C., Filipek S., Yuan S. The principles of ligand specificity on beta-2-adrenergic receptor. Sci. Rep. 2016;6:34736. doi: 10.1038/srep34736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hille B. Sinauer Associates; Sunderland, MA: 2001. Ion Channels of Excitable Membranes. [Google Scholar]
- 67.Long S.B., Campbell E.B., Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 68.Payandeh J., Scheuer T., Catterall W.A. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swartz K.J. Sensing voltage across lipid membranes. Nature. 2008;456:891–897. doi: 10.1038/nature07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Treptow W., Tarek M. Environment of the gating charges in the Kv1.2 Shaker potassium channel. Biophys. J. 2006;90:L64–L66. doi: 10.1529/biophysj.106.080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Treptow W., Tarek M., Klein M.L. Initial response of the potassium channel voltage sensor to a transmembrane potential. J. Am. Chem. Soc. 2009;131:2107–2109. doi: 10.1021/ja807330g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vargas E., Yarov-Yarovoy V., Roux B. An emerging consensus on voltage-dependent gating from computational modeling and molecular dynamics simulations. J. Gen. Physiol. 2012;140:587–594. doi: 10.1085/jgp.201210873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khalili-Araghi F., Jogini V., Schulten K. Calculation of the gating charge for the Kv1.2 voltage-activated potassium channel. Biophys. J. 2010;98:2189–2198. doi: 10.1016/j.bpj.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwaiger C.S., Bjelkmar P., Lindahl E. 310-helix conformation facilitates the transition of a voltage sensor S4 segment toward the down state. Biophys. J. 2011;100:1446–1454. doi: 10.1016/j.bpj.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yarov-Yarovoy V., DeCaen P.G., Catterall W.A. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc. Natl. Acad. Sci. USA. 2012;109:E93–E102. doi: 10.1073/pnas.1118434109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jensen M.Ø., Jogini V., Shaw D.E. Mechanism of voltage gating in potassium channels. Science. 2012;336:229–233. doi: 10.1126/science.1216533. [DOI] [PubMed] [Google Scholar]
- 77.Tao X., Lee A., MacKinnon R. A gating charge transfer center in voltage sensors. Science. 2010;328:67–73. doi: 10.1126/science.1185954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kasimova M.A., Lindahl E., Delemotte L. Determining the molecular basis of voltage sensitivity in membrane proteins. J. Gen. Physiol. 2018;150:1444–1458. doi: 10.1085/jgp.201812086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chodera J.D., Noé F. Markov state models of biomolecular conformational dynamics. Curr. Opin. Struct. Biol. 2014;25:135–144. doi: 10.1016/j.sbi.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prinz J.H., Wu H., Noé F. Markov models of molecular kinetics: generation and validation. J. Chem. Phys. 2011;134:174105. doi: 10.1063/1.3565032. [DOI] [PubMed] [Google Scholar]
- 81.Kingma D.P., Welling M. Auto-encoding variational Bayes. arXiv. 2013 https://arxiv.org/abs/1312.6114 arXiv:1312.6114. [Google Scholar]
- 82.Bourlard H., Kamp Y. Auto-association by multilayer perceptrons and singular value decomposition. Biol. Cybern. 1988;59:291–294. doi: 10.1007/BF00332918. [DOI] [PubMed] [Google Scholar]
- 83.Chicco D., Sadowski P., Baldi P. Deep autoencoder neural networks for gene ontology annotation predictions. In: Baldi P., Wang W., editors. Proceedings of the 5th ACM Conference on Bioinformatics, Computational Biology, and Health Informatics - BCB ’14. ACM Press; 2014. pp. 533–540. [Google Scholar]
- 84.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38, 27–28. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 85.McGibbon R.T., Beauchamp K.A., Pande V.S. Mdtraj: a modern open library for the analysis of molecular dynamics trajectories. Biophys. J. 2015;109:1528–1532. doi: 10.1016/j.bpj.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raschka S. BioPandas: working with molecular structures in pandas DataFrames. JOSS. 2017;2:279. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.