Abstract
Recruitment of leukocytes from blood vessels to inflamed zones is guided by biochemical and mechanical stimuli, with the mechanisms only partially deciphered. Here, we studied the guidance by the flow of primary human effector T lymphocytes crawling on substrates coated with ligands of integrins lymphocyte function-associated antigen 1 (LFA-1) (αLβ2) and very late antigen 4 (VLA-4) (α4β1). We reveal that cells segregate in two populations of opposite orientation for combined adhesion and show that decisions of orientation rely on a bistable mechanism between LFA-1-mediated upstream and VLA-4-mediated downstream phenotypes. At the molecular level, bistability results from a differential front-rear polarization of both integrin affinities, combined with an inhibiting cross talk of LFA-1 toward VLA-4. At the cellular level, direction is determined by the passive, flow-mediated orientation of the nonadherent cell parts, the rear uropod for upstream migration, and the front lamellipod for downstream migration. This chain of logical events provides a comprehensive mechanism of guiding, from stimuli to cell orientation.
Significance
Cell guidance is crucial to many biological functions, but the precise mechanisms remain unclear. We have analyzed here an original phenotype of flow-guided cells mimicking leukocytes crawling onto blood vessels and show that the controlling parameter of the cell’s decision to migrate upstream or downstream is the relative number of two specific adhesion molecules, the integrins lymphocyte function-associated antigen 1 and very late antigen 4. The spatial polarization of these integrin affinities plus a feedback loop between them creates a bistable system, in which cells adhere either by their front or their tail to orient upstream or downstream, respectively. This mechanism proposes a complete chain of events from stimuli to cell orientation, which differs strongly from the chemotaxis paradigm because the external stimuli triggers no signaling.
Introduction
Cell guiding is involved in numerous essential functions of living organisms; however, its mechanisms remain partially understood. Chemical guiding, or chemotaxis, has long been studied, and many mechanistic elements have been identified (1,2). In contrast, mechanical guiding, or mechanotaxis, has been acknowledged more recently, and although physiological roles have been identified in cell/organism swimming (3,4), differentiation (5), morphogenesis (6,7), or leukocyte activation (8), its basic functioning remains largely open. The regulation of immune cells trafficking between lymphoid organs, blood system, and inflamed or infected zones involves robust guiding mechanisms by chemical signals (9, 10, 11) and also mechanical signals like hydrodynamic shear stress (12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22). The detection of external force by leukocytes can rely on integrin adhesion proteins (23), which undergo conformational changes by inside-out (24,25) and outside-in (26,27) signaling, and can transduce intracellular signaling when submitted to force. Integrins, which are known to be key players in the recruitment of leukocytes from blood flow (20,28, 29, 30), are also good candidates to play a role in leukocyte mechanotaxis under flow.
Both in vivo and in vitro experiments have reported guiding by the flow of leukocytes crawling on the internal walls of blood vessels. In vivo, on a rat model suffering from autoimmune encephalomyelitis, effector T cells crawl preferentially upstream on the luminal surface of leptomeningeal vessels presenting ICAM-1 and VCAM-1 (12), ligands of lymphocyte function-associated antigen 1 (LFA-1) and very late antigen 4 (VLA-4) integrins, respectively. In vitro on surfaces coated with monolayers of endothelial cells, mouse T cells (13) and human hematopoietic stem and progenitor cells (31) were also crawling upstream in an ICAM-1-required manner. On substrates coated with cell adhesion molecules (CAMs), upstream crawling was reported for human effector T lymphocytes (14, 15, 16,32), human hematopoietic stem and progenitor cells (31) on ICAM-1, and for mouse T cells on ICAM-1 and ICAM-2 (21), whereas downstream crawling was reported for neutrophils and metastatic lymphocytes on ICAM-1 (13,14,21) and for effector T lymphocytes on VCAM-1 (16,31). This variety of mechanotaxis responses versus the types of leukocytes and of integrins suggests the existence of sophisticated mechanosensing mechanisms controlled by integrins.
Two types of orientation mechanisms by flow have been proposed for leukocyte adhering with integrins. The first consists of an “active” mechanism inspired from the chemotaxis machinery. Cue detection (shear stress direction) can involve outside-in signaling at anchoring sites mediated by integrins functioning as molecular force transducers (33, 34, 35, 36). Evidences of integrin-based signaling during migration under flow have been reported for endothelial cells (36,37) and neutrophils (23). Such mechanisms are “active” in the sense that cells develop a specific intracellular signaling activity in response to flow and rely therefore on mechanotransduction. Alternatively, a “passive” model was established for upstream crawling T lymphocytes (15). In this model, flow direction is detected by the passive orientation of cell tail (or uropod), which is not adherent and rotates in the flow like a wind vane in a breeze. Reorientation of the whole cell against the flow follows tail orientation via the realignment of the cell’s front by the ongoing mechanism of front-rear polarization. This mechanism is “passive” in the sense that it requires no signaling triggered by the external cue and therefore no mechanotransduction. Whether the active and passive mechanisms are specific to certain cell types or whether they are alternatively triggered by different microenvironments remains unclear.
A fundamental question in leukocyte mechanotaxis concerns therefore the mechanistic role of integrins in terms of adhesion mediators and/or mechanotransducers. The fact that the mechanotaxis phenotype of T cells changes from upstream to downstream when the substrates are coated by the ligands of integrins LFA-1 or VLA-4 (16,21,31,32) raises fundamental questions such as what decides the upstream versus downstream guiding of leukocytes or how different integrins control different orientations. In this article, we analyzed T lymphocyte migration on substrates with quantified mixtures of ICAM-1 and VCAM-1 molecules. We show that a bistable mechanism triggers upstream or downstream mechanotaxis phenotypes and that bistability relies on a combination of molecular and cellular mechanisms. At the molecular level, a cross talk between integrins LFA-1 and VLA-4 and the contrary polarization of LFA-1 and VLA-4 affinity sustain a differential adhesion of cells either by their leading or trailing edge. At the cellular level, this polarized adhesion passively triggers the upstream phenotype by a mechanism of wind vane uropod or the downstream phenotype by a mechanism of lamellipod flow focusing. This bistable model presents a complete functioning of mechanotaxis controlled by integrins, in which the mechanistic elements are identified from molecular to cellular level and the logical chain of event is continuous from stimulus to cell orientation outcome.
Materials and Methods
Cell culture
Whole blood from healthy adult donors was obtained from the Établissement Français du Sang (Paris, France). Peripheral blood mononuclear cells were recovered from the interface of a Ficoll gradient (Eurobio, Evry, France). T cells were isolated from peripheral blood mononuclear cells with Pan T Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and then were stimulated for 48 h with anti-CD3/anti-CD28 Dynabeads (Gibco by Thermo Fischer Scientific, Waltham, MA) according to the manufacturer’s instructions. T lymphocytes were subsequently cultivated in Roswell Park Memorial Institute Medium (RPMI) 1640 supplemented with 25 mM GlutaMax (Gibco by Thermo Fisher Scientific, Waltham, MA), 10% fetal calf serum (Gibco by Thermo Fisher Scientific) at 37°C, and 5% CO2 in the presence of IL-2 (50 ng/mL−1; Miltenyi Biotec) and used 6–10 days after stimulation. At the time of use, the cells were >99% positive for pan T lymphocyte marker CD3 and assessed for activation and proliferation with CD25, CD45RO, CD45RA, and CD69 markers as judged by flow cytometry.
Microscopy
Bright-field, reflection interference contrast microscopy (RICM), and fluorescent images were performed on a Zeiss Observer Z1 microscope (Carl Zeiss, Oberkachen, Germany) piloted with μManager (38). The microscope was equipped with a CoolSnap HQ CCD camera (Photometrics, Tucson, AZ) and different Zeiss objectives (Plan-Apochromat × 10/0.3, × 20/0.8, and × 63/1.4 and NeoFluar 63/1.25 antiflex. The source was a CoolLED pE-300 (CoolLED, Andover, UK). A narrow band-pass filter (λ = 546 ± 12 nm) was used for RICM.
Total internal reflection fluorescence (TIRF) microscopy images were recorded on Nikon Eclipse Ti microscope (Nikon Instruments, Tokyo, Japan), equipped with an ILas2 system (Roper Scientific, Vianen, the Netherlands) and controlled by MetaMorph software (Molecular Devices, San Jose, CA). Images were taken with an Apo TIRF 60×/1.49 oil objective (Nikon Instruments), a Prime 95TM Scientific CMOS Camera (Photometrics, Tucson, AZ), and an Obis Coherent/ILAS LASER.
Flow devices and surface treatment
Devices consisted in ibidi μ-Slide IV0.4 uncoated (ibidi, Martinsreid, Germany) and in homemade flow devices, fabricated using standard soft lithography routines (39). Briefly, a positive mold was created with SU-8 2100-negative resins (MicroChem Laboratory, Westborough, MA) on silicon wafers (Sil’tronix Silicon Technologies, Archamps, France), and then replicas were molded in polydimethylsiloxane elastomer (Sylgard 184; Dow Corning, Midland, MI) and sealed on glass coverslides via plasma activation (Harrick Plasma, Ithaca, NY). Ports to plug inlet and outlet reservoirs were punched to a 1-mm outer diameter.
Flow devices (ibidi μ-slide and homemade) were precoated for 1 h at 37°C with 50 μg/mL−1 of Protein A (Sigma-Aldrich, St. Louis, MO). Surfaces were then blocked with 2.5% bovine serum album (BSA) (Axday, Dardilly, France) in phosphate-buffered saline (PBS) (Gibco by Thermo Fisher Scientific) for 30 min at room temperature. Channels were subsequently functionalized by an overnight incubation at 4°C with 10 μg/mL−1 of either human ICAM-1-Fc or VCAM-1-Fc (R&D Systems, Minneapolis, MN) in PBS or mixture of the CAMs. Channels were rinsed with PBS. Cells were then added in complete RPMI medium, allowed to equilibrate for 10 min, and then rinsed with medium.
Cells in the flow chambers were observed at 37°C with the Zeiss Z1 automated microscope. Flow of prewarmed and CO2 equilibrated culture media through the flow chamber was controlled using an ibidi pump system (ibidi). Bright-field images (EC Plan-NEoFluar 10×/0.3 Ph1 objective) were collected every 10 s over the time frame indicated. The field of view represents 1740 × 1300 μm2.
Fluorescence quantification of adhesion molecules
Anti-human CD106-PE and anti-human CD54-PE (eBioscience by Thermo Fischer Scientific, Waltham, MA) antibody were used for the quantification of substrates coatings with mixed ICAM-1 and VCAM-1. First, we set up bulk calibration data by measuring the fluorescence intensity of 41-μm-thick channels pretreated with 1% Pluronic F127 (Sigma-Aldrich) and filled with antibody solutions at concentrations of 1.5, 3, 5, and 7 μg/mL−1. Samples with mixed ICAM-1/VCAM-1 were then stained either with CD106-PE or with CD54-PE at 10 μg/mL−1 overnight at 4°C, and fluorescent images were then taken the next day and analyzed with Fiji software (40). The average intensity at five different positions was converted into surface density using the bulk calibration data.
Cell tracking and data analysis
A homemade program was developed with MATLAB software (The MathWorks, Natick, MA) to track migrating cells and analyze their pathway properties. In all flow experiments, the flow is directed from the top to the bottom of the images presented here. To get an indication of the amount of motion in a particular direction, a migration index (MI) is calculated by dividing the distance the cells travel in the flow direction by the total distance traveled by the cells. The average speed of a cell, V, is calculated as the ratio between the total trajectory length and the corresponding time of migration. All calculations were performed at least in triplicate for each substrate. Image Analysis was performed with ImageJ (National Institutes of Health, Bethesda, MD).
Fixation under flow and immunofluorescence staining
Cells migrating under flow were instantly fixed by quick injection of 4% paraformaldehyde (Affymetrix, Cleveland, Ohio) into the device. After a 10-min incubation, the device was rinsed with PBS-Tween 0.1% and either stained or kept at 4°C until used. For the staining, cells were initially permeabilized with Triton 0.5% (Sigma-Aldrich) in PBS for 5 min and rinsed three times with PBS-Tween 0.1%. Free Fc-binding sites of the Protein A layer were blocked with human serum IgG 100 μg/mL−1 solution for 20 min; samples were then further blocked with BSA 2% for 20 min. After three washes with PBS-Tween, samples were incubated with mAb B44 (antibody at a concentration of 5 μg/mL−1) for 20 min, washed three times, and then incubated with a secondary anti-mouse antibody (20 μg/mL−1) for another 20 min before imaging mAb B44 alone as a control. For the double staining of mAb B44 and mAb24, free binding sites of the secondary antibody were blocked by incubating the samples for at least 1 h with mouse IgG1 isotype control (10 μg/mL−1). Primarily labeled mAb24 antibody was finally added (4 μg/mL−1) and kept in solution during the imaging process.
The antibodies used were mouse anti-integrin β1, clone name B44 (MilliporeSigma, Temecula, CA); goat anti-mouse IgG, CF647 conjugated (Sigma-Aldrich); mouse IgG1 isotype control (BioLegend, San Diego, CA); and mouse anti-human CD11a/CD18, Alexa Fluor 488 conjugated, clone name mAb24 (BioLegend). All antibodies were diluted in PBS-Tween 0.1%.
For the histograms of Fig. 5 B, raw values were normalized by applying the following formula:
| (1) |
where Imin is the minimal value recorded on each image and Imax is the maximal value recorded for each fluorophore in either condition.
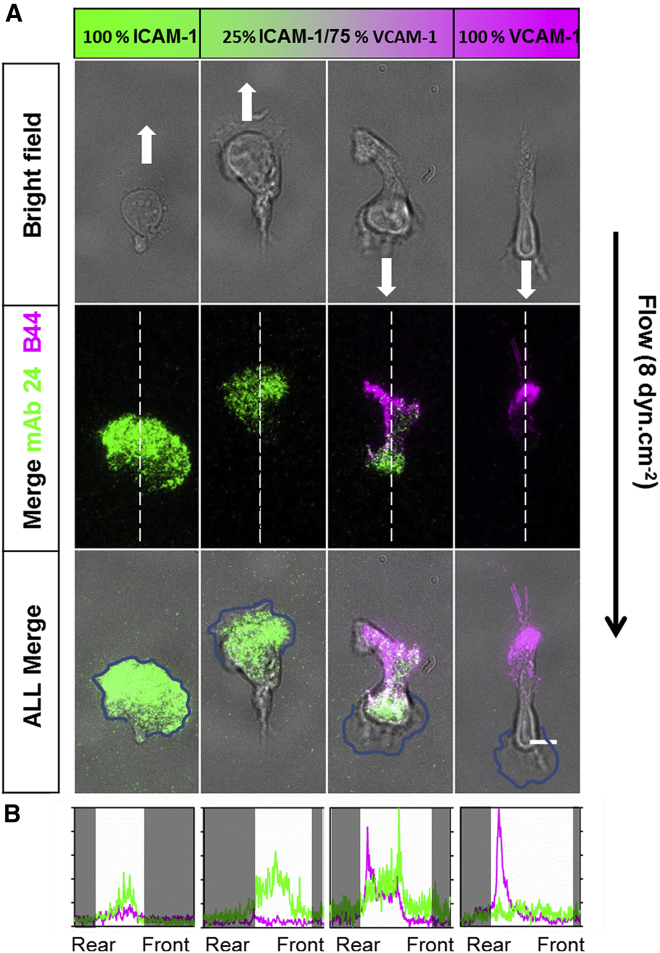
Figure 5.
Imaging of high affinity LFA-1 and VLA-4 in cell contact zone by TIRF reveals complex regulation mechanism of their affinity. (A) Shown are representative microscopic images in bright field (top) and TIRF (center) of crawling T cells fixed under a shear stress of 8 dyn/cm−2 and stained for high affinity LFA-1 with mAb24 (green) and high affinity VLA-4 with mAb B44 (magenta). Scale bars, 10 μm. (B) Intensity profiles performed for each fluorescent channel highlight integrin distribution along the cell axis. Values were normalized to the highest value recorded on either condition. White regions indicate the cell body area. (Additional representative profiles are reported in Fig. S5). To see this figure in color, go online.
Fluorescent detection of calcium flux
For calcium imaging experiments, cells were first seeded in channels with RPMI medium and were incubated for 10 min at 37°C to allow adhesion, and then they were rinsed with Hank’s Balanced Salt Solution (HBSS) plus 1% BSA and incubated with Oregon Green 488™ BAPTA-1, AM (Thermo Fisher Scientific) diluted in HBSS + 1% BSA (5 μM) for 15 min at 37°C in the dark. After rinsing with HBSS + 1% BSA, the medium was replaced by HBSS + 10% fetal bovine serum (FBS). Control experiment was achieved by an injection of ionomycin (Thermo Fisher Scientific) at a concentration of 1 μg/mL−1.
Flow cytometry
100,000 cells were taken from the cultured population and pelleted by centrifugation for 5 min at 1000 rpm. The cells were resuspended in 100 μL PBS+ 2% FBS, containing the premixed antibodies (CD11a-FITC, clone Hi111 (eBioscience by Thermo Fischer Scientific) and CD29-PE, clone TS2/16 (BioLegend)) to the desired concentration and incubated for 30 min at 4°C in the dark. The cells were washed with PBS + 2% FBS and then resuspended in 0.5 mL of PBS + 2% FBS. For dose responses with blocking antibodies against integrins, we used TS1/22 (Thermo Fisher Scientific) and a recombinant monoclonal antibody to integrin α 4 (CD49) clone natalizumab (Absolute Antibody, Boston, MA) to block, respectively, LFA-1 and VLA-4. Blocked LFA-1 were measured with secondary antibody against TS1/22. Available functional VLA-4 was measured with antibody against CD49 (clone HP2/1). All flow cytometry was performed on a BD LSR II flow cytometer (BD Biosciences, San Jose, CA).
Perturbation experiments
Cells were incubated with an anti-CD11a monoclonal antibody clone TS1/22 or a recombinant monoclonal antibody to integrin α 4 (CD49) clone natalizumab for 10 min at 37°C. Cells were first seeded in channels with RPMI medium and were incubated for 10 min at 37°C to allow adhesion before starting the experiment.
Ethics statement
Human subjects: Blood from healthy volunteers was obtained through a formalized agreement with French Blood Agency (Établissement Français du Sang, agreement 2017-7222). Blood was obtained by the agency after informed consent of the donors, in accordance with the Declaration of Helsinki. All experiments were approved by the INSERM Institutional Review Board and Ethics Committee.
Results
Flow fosters a variety of migration phenotypes on mixtures of ICAM-1 and VCAM-1
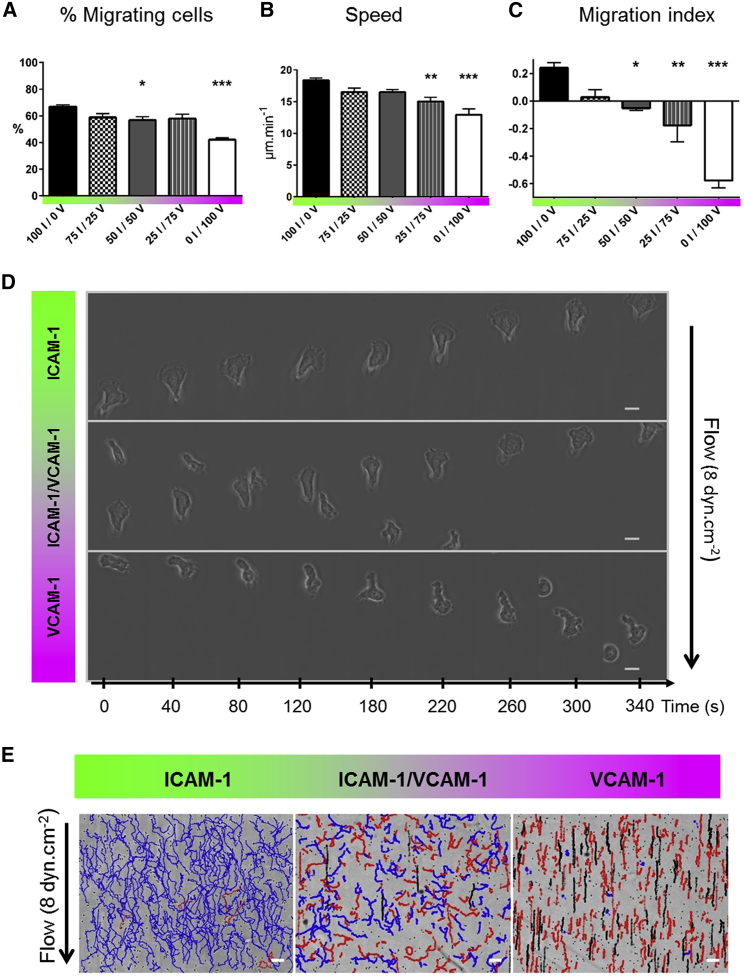
To examine the respective role of LFA-1 and VLA-4 on the orientation of T lymphocytes crawling under flow, the concentrations of ICAM-1 and VCAM-1 on substrates were tightly controlled and quantified. Coatings were prepared by adsorption of Fc-ICAM-1 and Fc-VCAM-1 molecules in a channel precoated with protein A. The common Fc fragment (ligand of protein A) promoted a similar binding affinity of ICAM-1 and VCAM-1 with the substrate, and the ratio of ICAM-1/VCAM-1 on the substrates was tuned by adjusting the concentrations in the adsorbing solution. The absolute amount of each ligand on surfaces was further quantified by fluorescence (Fig. S1). On these substrates and in the absence of flow, the percentage of migrating cells and their speed decreased only moderately with a decrease in the ICAM-1 fraction (Fig. 1, A and B). These observations suggest that the crawling machinery does not significantly depend on the type of integrin engaged with the substrate. In contrast, when flow was applied, cells exhibited sharply different responses depending on the surface composition. They migrated mainly upstream on ICAM-1-treated substrates and mainly downstream on VCAM-1-treated substrates (Video S1), as previously reported in the literature (14,16,21,31,32). To quantify motion directionality, we calculated a MI as the ratio between the end-to-end and curvilinear displacement of cells. Fig. 1 C shows a smooth transition from upstream (positive MI) to downstream (negative MI) phenotype versus an increasing fraction in VCAM-1. Flow therefore revealed a critical interplay between the integrin-mediated adhesion and the crawling machinery. However, this analysis on population-average data is missing important features of the system. On mixed ICAM-1/VCAM-1 substrates and for a given set of flow rates, cells displayed phenotypes of upstream crawling, downstream crawling, or downstream rolling (Fig. 1 D; Video S2). The system was therefore not homogeneous but multiphasic, with individual cells choosing opposite phenotypes. To refine average data analysis, we therefore sorted cells according to their adhesion/migration phenotype under flow. Cells were defined as “migrating cells” if they moved more than 30 μm during the whole acquisition (100 frames, 17 min). Under flow condition, we distinguished rolling from crawling according to the following criteria: 1) direction remained within 10° of flow direction in a 17-min path and 2) SD of direction upon 20-s steps remained within 25°. Only the cells that rolled at velocities lower than 90 μm/min−1 could be tracked because of the frame acquisition rate (Fig. 1 E, black trajectories). Finally, all remaining cells were considered as “crawling” (at this point of investigation) and sorted into upstream (Fig. 1 E, blue trajectories) or downstream crawling (Fig. 1 E, red trajectories). This description shows that the transition on mixed substrates from upstream to downstream crawling phenotypes is biphasic.
Figure 1.
Flow reveals different migration modes on mixed ICAM-1/VCAM-1 substrates. (A) Shown is the percentage of migrating cells and (B) speed versus substrate composition in shear-free conditions. XI/YV stands for X% ICAM-1/Y% VCAM-1. (C) Shown is the direction of T lymphocytes under shear flow expressed as the MI (positive values indicating motion upstream and negative values indicating motion downstream) versus substrates composition at shear stresses of 4 dyn/cm−2. The MI was calculated for all cells as the ratio between the end-to-end displacement and the cumulative curvilinear traveled distance. All data are mean ± SE; n = 6 independent experiments with at least 500 cells in each experiment, ∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.001 with respect to substrate composition, 100% ICAM-1/0% VCAM-1, one-way ANOVA with post hoc Dunnett’s test. (D) Shown are bright-field images sequence of cells crawling on pure ICAM-1, pure VCAM-1, and mixed ICAM-1/VCAM-1 substrates. Scale bars, 10 μm, with time laps of 40 s. (E) Shown are trajectories of mobile cells on pure ICAM-1, pure VCAM-1, and mixed ICAM-1/VCAM-1 substrates, with a color code for cells crawling upstream (blue), crawling downstream (red), and rolling (black). Time span is 17 min. Scale bars, 100 μm. To see this figure in color, go online.
Bright-field images with magnification x10.
Transmission images with magnification x10.
ICAM-1 imposes strong adhesion and crawling, whereas VCAM-1 allows transient adhesion and rolling
To better understand the coupling between integrins and flow mechanotaxis, we further characterized adhesion properties. Focusing on the case of mobile and polarized cells, we saw stronger adhesion on ICAM-1-rich than on VCAM-1-rich substrates (Fig. 2 A). This difference was consistent with a larger expression of LFA-1 than VLA-4 revealed by quantitative flow cytometry (Fig. S2). However, other parameters are also determinant for adhesion control, such as affinity, avidity, or clustering properties of integrins. Interestingly, mobile cells only crawled and never rolled on ICAM-1 (Fig. 2 B), in line with a robust adhesion mediated by ICAM-1/LFA-1. In contrast, the fraction of rolling cells increased with the fraction of VCAM-1, up to a maximum of 25% on pure VCAM-1 substrates, revealing weaker and potentially transient adhesion. Altogether, these results on adhesion strength of immobile and motile cells support that ICAM-1 promotes strong adhesion and a high propensity for crawling, whereas VCAM-1 promotes transient adhesion and a coexistence of rolling and crawling cells.
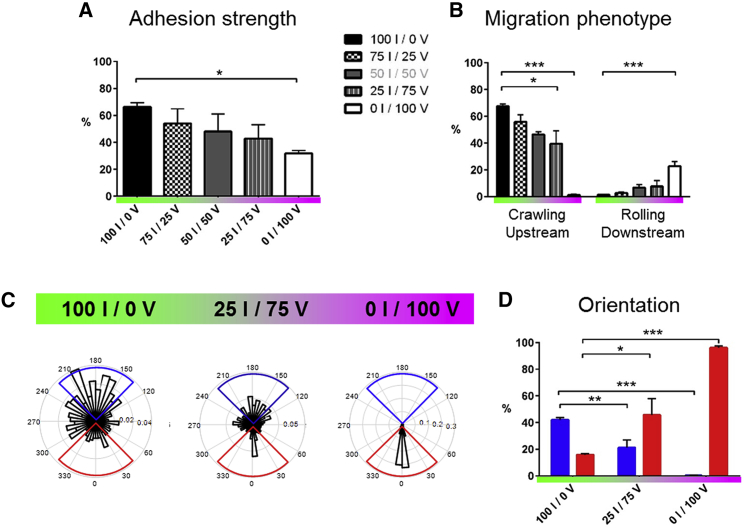
Figure 2.
ICAM-1 imposes strong adhesion and upstream crawling, whereas VCAM-1 allows transient adhesion and downstream crawling/rolling. (A) Shown is the adhesion strength of mobile cells, measured as the percentage of cells resistant to a shear stress of 4 dyn/cm−2 with respect to the initial number of mobile cells on the substrate. XI/YV stands for X% ICAM-1/Y% VCAM-1. (B) Shown are percentages of cells crawling upstream and rolling downstream with respect to the total number of cells migrating on the surface, under a shear stress of 4 dyn/cm−2 and for a different substrate composition. (C) Shown are rose plots of cell directions at different substrate compositions. (D) Shown is the percentage of upstream (blue) and downstream (red) crawling cells, determined here by cumulating data in the blue and red quadrant of the rose plots of cell migration for, respectively, upstream and downstream crawling cells. All data are mean ± SE; n = 6 independent experiments with at least 500 cells in each experiment. ∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.001 with respect to substrate composition, 100% ICAM-1/0% VCAM-1, one-way ANOVA with post hoc Dunnett’s test. To see this figure in color, go online.
ICAM-1 promotes upstream crawling, whereas VCAM-1 allows downstream crawling
To further characterize the guiding of crawling cells versus the integrin-ligand pair involved, we then quantified the population of cells with downstream or upstream phenotype by taking into account trajectories making an angle ±45°, respectively, with and against the flow direction (Fig. 2, C and D). Upstream phenotype was maximum on pure ICAM-1 substrates (50% of crawling cells at 4 dyn/cm−2, 1 dyn/cm−2 = 0.1 Pa in SI units) and absent on VCAM-1 substrates, which suggests that upstream phenotype is associated to ICAM-1-mediated cell adhesion. In contrast, the fraction of cells with downstream phenotype increased with the fraction of VCAM-1 on the substrates up to 100% on pure VCAM-1 substrates, which suggests that VCAM-1 mediates downstream phenotype or inhibits upstream phenotype. Although these general trends are robust, they do not explain the biphasic behavior on mixed substrates. A finer characterization of the properties of upstream and downstream phenotypes at the single cell level was therefore necessary to shed light on opposite behaviors in a given population.
Speed remains constant for upstream crawling cells
Fig. 3 A shows that the speed of rolling cells increased with shear stress on mixed ICAM-1/VCAM-1 substrates as well as on VCAM-1 substrates, which is consistent with rolling being passively powered by the action of flow on transiently adherent cells. In contrast, upstream crawling T cells on ICAM-1 substrates had a constant speed versus shear stress. The apparent slight decrease of speed on ICAM-1 substrates was previously identified as a population selection effect, and the velocity of single cells was shown to be constant up to shear stress of 60 dynes/cm−2 (14). Cells crawling upstream on mixed ICAM-1/VCAM-1 substrates also had a constant velocity versus flow, exactly like on pure ICAM-1 substrate. The hydrodynamic force on cells (<0.1 nN) is indeed negligible as compared to the force developed by the cells crawling machinery (several nN) (14). Hence, upstream crawling is generally characterized by high adhesion strength and strong migration power on both ICAM-1 and mixed ICAM-1/VCAM-1 substrates.
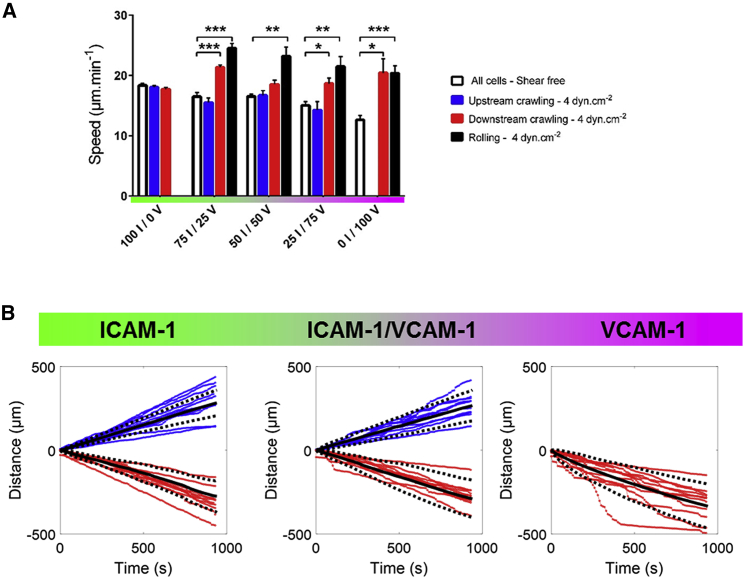
Figure 3.
Speed increases with flow for rolling cells but remains constant for upstream crawling cells. (A) Shown is speed versus substrate composition of all cells in shear-free condition and of upstream crawling cells and downstream crawling cells and rolling cells under a shear stress of 4 dyn/cm−2. X I/Y V stands for X% ICAM-1/Y% VCAM-1. All data are mean ± SE; n = 6 independent experiments with at least 500 cells in each experiment. ∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.001, one-way ANOVA with post hoc Dunnett’s test. (B) Shown are cumulative distance traveled by individual crawling cells on ICAM-1 (left), mixed ICAM-1/VCAM-1 (center), and VCAM-1 (right) substrates. The color of each curve indicates the migration mode of the corresponding cell tracked—blue for upstream and red for downstream crawling cells. Black solid lines represent the mean, and black dotted lines represent the SD. To see this figure in color, go online.
Speed increases with flow for downstream crawling cells
For cells crawling downstream, the population-averaged data (Fig. 3 A) showed a significant increase of speed when flow increases. Because flow actuation is orders of magnitude weaker than the power of the crawling machinery, as previously argued, this effect is not a straightforward action of flow push. A closer look at tracks of individual cells (Fig. 3 B) revealed that curvilinear displacements versus time had a constant slope for cells crawling upstream (on ICAM-1 and mixed ICAM-1/VCAM-1) but were composed of segments with different slopes for cells crawling downstream (on VCAM-1 and mixed ICAM-1/VCAM-1). For upstream crawling cells, the speed was different from one cell to another but constant versus time for each given cell, which is in line with a flow-independent speed set by the stable motility machinery of each individual cell. In contrast, for downstream crawling, low speed sequences corresponded to crawling, whereas high speed sequences corresponded to mixed crawling and rolling with detachment of the cell rear (Video S3). The distribution of crawling steps was widened toward large steps corresponding to mixed crawling-rolling steps (Fig. S3). Taking into account the fact that VLA-4 mediates transient and less robust adhesion than LFA-1, these observations suggest that upstream and downstream crawling cells adhere mostly via LFA-1 and VLA-4, respectively.
Transmission (left) and RICM (right) images with magnification x63.
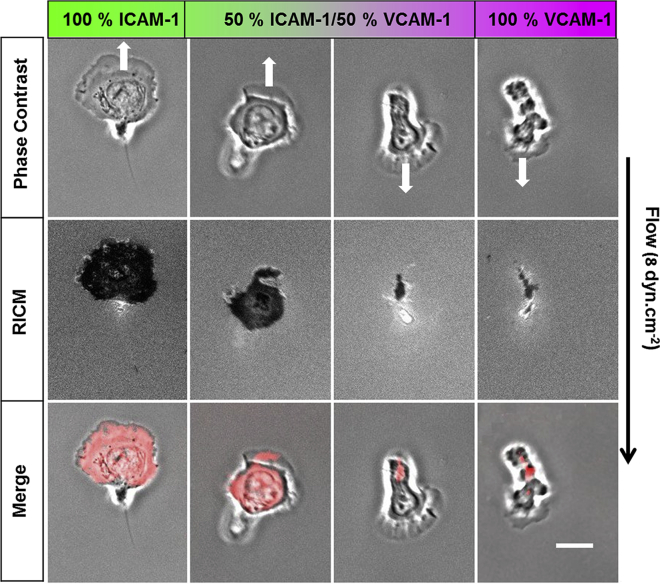
Uropod is detached for upstream bound cells and attached for downstream-bound cells
To shed further light on the mechanism underlying orientation under flow, we used RICM to image the adhesion footprint of cells during migration. On all types of substrate coatings, uropods were found to be nonadherent for upstream crawling cells and markedly adherent for downstream crawling cells (Fig. 4; Video S5). These observations support that the uropod-wind vane mechanism functions independently of the substrate composition. Cells migrate upstream whenever the uropod-wind vane mechanism is ON (detached uropod). Conversely, they have no reason to go upstream when the uropod-wind vane mechanism is OFF (attached uropod). An alternative mechanism must, however, be at work to foster the orientation of downstream crawling cells. Fig. 4 shows that lamellipods, which were strongly adherent for cells crawling upstream, were markedly nonadherent for cells crawling downstream. As previously suggested for neutrophils and keratocytes (13,41), a lamellipod loosely connected to a substrate can be passively funneled by flow, yielding a preferential downstream orientation of cells. Experiments with devices allowing instant changes of flow direction supported further that the cell front rotates around its rearward attachment zone on VCAM-1 (Video S4), opposite to the previously reported phenotype of cell uropods rotating around the adherent cell front on ICAM-1 (15). Altogether, the characteristics of cell adhesion patterns on ICAM-1 and VCAM-1 support that two mechanisms are at work to guide cells versus flow; the uropod-wind vane mechanism guides cells upstream whenever the uropod is detached, and a lamellipod-focusing mechanism guides cells downstream whenever the uropod is attached. These two mechanisms are in fact closely related and based on the cell adhesion footprint. They provide a self-consistent mechanistic picture for upstream and downstream mechanotaxis phenotypes. Noticeably, they are both passive in the sense that they do not require mechanotransduction but mere mechanical orientation with the flow of cells parts that are loosely attached.
Figure 4.
Cell rear is detached for upstream-bound cells and cell front for downstream-bound cells. Shown is the representative image sequence of crawling cells under a flow of 8 dyn/cm−2 in phase contrast (top) and reflection interference contrast microscopy (RICM) (center). On the merged images, the RICM image is contrast inverted and colored in red. The black arrow indicates flow direction, and the white arrow indicates the direction of cell migration. The adhesion zone (dark in RICM, red in merge) is positioned in cell front for upstream crawling cells and in cell rear for downstream crawling cells. Scale bars, 10 μm. To see this figure in color, go online.
VCAM-1 panel: The right cell is representative of cells displaying mostly crawling and the left cell of cells displaying a combination of crawling and rolling. ICAM-1 panel: Cells displaying representative crawling phenotype on ICAM-1. The yellow arrows indicate the direction of flow (8 dyn.cm-2), which is from top to bottom in the beginning of the sequence and from right to left in the end. n > 20 cells/experiments. nexp = 3.
Top: Transmission images with magnification x63. Bottom: RICM images with magnification x63.
Flow triggers no calcium signaling
Although passive mechanisms explain cell orientation under flow, one cannot discard a role of active mechanisms based on the signaling triggered by integrins or other mechanotransduction events. We therefore monitored the intracellular calcium activity during flow stimulation (23). Fig. S4 (and Video S6) shows that calcium activity upon flow onset remained below the detection level on all substrates tested, whereas control with ionomycin (42) showed a strong signal. Because calcium signaling is shared by many intracellular signaling pathways (43), these data support that mechanotransduction may not be involved in T cell guidance by flow.
Last part of the movie is the control experiment with ionomycin which increases calcium release by increasing the cell membrane permeability.
The affinities of LFA-1 and VLA-4 are polarized in opposite directions
RICM revealed a different positioning of the cell adhesion footprint depending on the substrate type, but it provided no information on the type and amount of integrins involved in the adhesion zones. To perform functional imaging of adhesion zones under flow, we used TIRF microscopy and the antibodies mAb 24 and mAb B44 against the high affinity states of integrins LFA-1 and VLA-4, respectively (Fig. 5 A). TIRF experiments were performed on fixed cells because these antibodies instantly alter the regulation of integrins affinity and cell motility in live conditions. We observed that LFA-1 and VLA-4 had their affinities strongly polarized from front to rear, with opposite directions. Adhesion of the frontward zone on pure ICAM-1 contained mostly high affinity LFA-1, whereas adhesion of the rearward zone on pure VCAM-1 contained mostly high affinity VLA-4. The absence of high affinity LFA-1 in VCAM-1-mediated contact zones or high affinity VLA-4 in ICAM-1-mediated contact zones suggests that activation of integrins in contact zones requires local engagement with their respective ligand. However, ligand-induced activation does not promote adhesion of the cell rear on ICAM-1 substrates nor of the cell front on VCAM-1 substrates. Therefore, ligand-induced activation is not the sole mechanism involved. Our observations suggest also an upregulation of the affinity of LFA-1 in cell front and of VLA-4 in cell rear and, conversely, downregulation of the affinity of LFA-1 in cell rear and of VLA-4 in cell front.
Bistability between upstream crawling with LFA-1 and downstream crawling with VLA-4
On mixed ICAM-1/VCAM-1 substrates, both integrins are stimulated by their ligands in the contact zones so that cells with high affinity LFA-1 in their front and high affinity VLA-4 in their rear could a priori adhere by their two poles. Considering upstream crawling cells, RICM imaging showed that cells adhered only by their front, and TIRF revealed that such frontal adhesion zones involved almost exclusively LFA-1 (Fig. 5 B). This observation cannot be explained straightforwardly. Downregulation of VLA-4 affinity by cell polarization signaling, previously evidenced, explains the absence of activated VLA-4 in the cell front but not the absence of the adhesion of cell rear. An additional mechanism is therefore at play to hamper the adhesion of the cell rear by VLA-4. An inhibiting cross talk of activated LFA-1 toward VLA-4 could play this role, and its existence has indeed been reported in the literature (24). Considering now the downstream crawling phenotype, RICM showed that cells were strongly attached by their rear, and TIRF further revealed that high affinity VLA-4 was dominant in this posterior adhesion zone. High affinity LFA-1 was mostly present in the cell central zone and partially also in the cell rear (Fig. 5 B). These observations go against an inhibiting cross talk of activated VLA-4 toward LFA-1 and rather in favor of an activating cross talk because LFA-1 affinity is usually downregulated in the cell rear on the ICAM-1 substrate. Altogether, these data show that polarization of integrins and ligand activation alone cannot explain the differential orientation under flow, and other mechanisms involving cross talk between integrins must be involved.
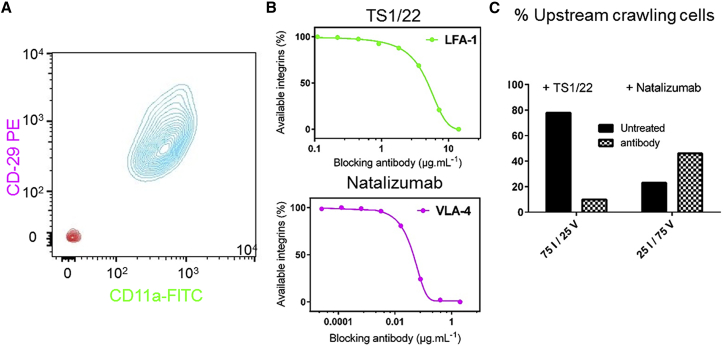
The level of high affinity integrins dictates orientation decision
Integrins LFA-1 and VLA-4 are crucial for cell orientation under flow, but the decision process for upstream or downstream orientation remains unclear. To challenge the existence of populations with different LFA-1 and VLA-4 expression levels, we performed flow cytometry experiments with double staining of αL (for LFA-1) and β2 (for VLA-4) but found a single population (Fig. 6 A). Therefore, opposite orientations under flow cannot arise from two populations with sharply different levels of integrins LFA-1 and VLA-4 expression. However, a bistable system can trigger sharply distinct responses within a single population. To challenge this mechanism, we performed perturbation experiments to address the correlation between effective integrin levels and migration phenotype under flow. The effective number of available LFA-1 and VLA-4 integrins at cell surfaces was tuned by the addition of blocking antibodies against high affinity LFA-1 or VLA-4, and cytometry dose-response analysis was performed to quantitatively determine the percentage of integrin blockage (Fig. 6 B). On mixed ICAM-1/VCAM-1 substrates, the blocking of 50% of LFA-1 integrins changed the phenotype distribution from upstream to downstream migration. Conversely, blocking of 50% of VLA-4 integrins changed the phenotype distribution from downstream to upstream migration (Fig. 6 C; Video S7). These data support the hypothesis that the bistable choice for a given cell to go up- or downstream depends on its relative amounts of VLA-4 and LFA-1. A cell rich in LFA-1 will adopt the upstream migration phenotype like T cells on ICAM-1 substrates, whereas a cell rich in VLA-4 will adopt the general downstream migration phenotype like T cells on VCAM-1 substrates.
Figure 6.
Perturbation experiments support that integrin expression level dictates the decision of orientation versus flow. (A) Shown are two-dimensional cytometry graphs of activated T cells versus expression of heterodimer αL (Ab α -CD11a) for LFA-1 and β2 (Ab α-CD29) for VLA-4 (stained cells, Blue; unstained cells, red). (B) Shown are percentages of available integrins on effector T lymphocytes versus the concentration of blocking antibodies in solution, as determined by cytometry. Blocking antibodies were TS1/22 for LFA-1 and natalizumab for VLA-4. (C) Shown are percentages of upstream crawling cells on mixed ICAM-1/VCAM-1 substrates with and without the addition of blocking antibodies TS1/22, against integrins LFA-1 (left), and natalizumab, against VLA-4 (right). Blocking of LFA-1 displaces phenotype distribution toward the downstream phenotype, and blocking of VLA-4 displaces phenotype distribution toward the upstream phenotype. To see this figure in color, go online.
First part of the movie (left) shows that on a mixed substrate 75% ICAM-1 – 25% VCAM-1, cells are mainly crawling upstream. By adding blocking antibody against LFA-1 (TS1/22) and decreasing the ratio LFA-1/ VLA-4, cells are mainly crawling downstream (right). Second part of the movie (left) shows that on a mixed substrate 25% ICAM-1 – 75% VCAM-1, cells are mainly crawling downstream. By adding blocking antibody against VLA-4 (Natalizumab) and decreasing the ratio LFA-1/ VLA-4, cells are mainly crawling upstream (right).
Discussion
Upon recruitment from the blood stream, lymphocytes crawl on the intraluminal surface of blood vessels presenting ICAM-1 and VCAM-1 adhesion molecules under a shear stress of 5–10 dynes/cm−2 (44). Shear stress has recently been recognized in vivo and in vitro as an efficient stimulus to guide crawling T lymphocytes, albeit the function and mechanism of this guiding phenomenon remains poorly understood. We confirmed here that lymphocytes display upstream crawling on ICAM-1 (12,14, 15, 16,45) and downstream migration by rolling and crawling on VCAM-1 (16,28,32) and that a transition occurs from upstream to downstream migration when the VCAM-1 to ICAM-1 ratio increases (16). Our analysis at the single cell level has, however, shown that individual cells do not adopt intermediate phenotypes between upstream and downstream migration modes. Instead of a smooth phenotype transition by a homogenous population, we observed a biphasic system with two distinct populations of upstream and downstream phenotypes. From a physical point of view, the separation of a system in two distinct phases may rely on a first-order phase transition or on a bistable mechanism. There seems to be no thermodynamic-like phase transition here because individual cells do not exchange between the two different states (phenotypes), at least in the time frame of our experiments. A bistable mechanism is, however, plausible with individual cells remaining in distinct states. We indeed deciphered the chain of mechanistic elements at the molecular and cellular level that allow the emergence of a bistable system.
Integrin regulation plays a central role in cell response to flow, and our results bring new insights in the spatial distribution of high affinity integrins along polarized migrating T cells. Previous investigations on ICAM-1 substrates have reported a spatial segregation of LFA-1 affinity state, with intermediate states exclusively in the lamellipod and high affinity states confined in the central or “focal” zone (46). In this work, we found that high affinity LFA-1 was strongly present in the focal zone but also in the lamellipod of T cells that crawled upstream (on ICAM-1 and ICAM-1/VCAM-1 substrates) and in the central and rear zones of cells that crawled downstream (on ICAM-1/VCAM-1 substrates). This more precise cartography of integrin affinity was achieved partly from the improvement of staining protocols with mAB 24 but also from the distinctive analysis of subpopulations of T cells, depending on their phenotypes of mechanotaxis under flow.
Guiding of migrating cells by an external stimulus is generally assumed to result from a sophisticated mechanism evolved for a specific function. Multiple specific functions are identified for chemotaxis, which undoubtedly relies on specific cue detection, signal transduction, and complex (although still unknown) signal processing to trigger cytoskeleton reorganization and cell orientation (1). In the case of mechanotaxis, various observations support that similar complex mechanisms of signal transduction and processing may be important and linked to integrins. Mechanosignaling by integrins and further downstream signaling events have been identified, like the dephosphorylation of p130Cas (crk assocoated substrate) under tension and the inactivation of Rho-GTPase Rac1 in the side of cell-facing flow (36). Furthermore, Cas domain was directly identified in vitro as a primary molecular force (35). Mechanotransduction by integrin adhesion complexes seems therefore to play a key role in mechanotaxis of cells forming mature focal adhesion complexes, like endothelial cells (37). In the case of amoeboid cells like leukocytes, migration occurs without maturation of integrin focal contacts, and the role of mechanotransduction by integrins remains largely questionable. Dixit et al. (23) hypothesized that shear forces used high affinity LFA-1 transmission to facilitate the cooperation with the calcium release-activated channel Orai1 in directing localized cytoskeletal activation and subsequent directed migration. Besides, Artemenko et al. or Niethammer (43,47) have shown that flow could activate internal signaling networks common with chemotaxis. These works support, therefore, the hypothesis that leukocyte flow guiding may be mediated by active signal transduction and processing, like for chemotaxis, but the mechanistic link between mechanotransduction and mechanotaxis is not fully established.
In contrast, we previously proposed a model without mechanotransduction for upstream migration of T lymphocytes mediated by one integrin, LFA-1 (15). This mechanism was based on two established properties of crawling effector T cells, a detached tail (or uropod) acting as a wind vane and a robustly maintained front-rear polarization. These two elements exist in the absence of flow and the mechanotaxis mechanism requires no signal triggered by flow. This model was generalized here to a more complex system of flow mechanotaxis controlled by two integrins, LFA-1 and VLA-4, and of cells displaying opposite choices between upstream and downstream directions. The wind vane mechanism is preserved for cells with a detached uropod and systematically promotes cell migration against the flow, whereas a flow-focusing mechanism explains downstream migration for cells with loosely adherent lamellipod as already observed with keratocytes (41). Altogether, upstream and downstream mechanotaxis of effector T lymphocytes adhering via LFA-1 or VLA-4 integrins can rely on passive mechanisms without mechanotransduction. Hence, although overwhelming biological processes rely on sophisticated signaling pathways, passive mechanisms are also emerging to support various mechanotaxis phenotypes, such as flowtaxis (15) and barotaxis (48) of leukocytes or rheotaxis of swimming sperm cells (4) and worms (3).
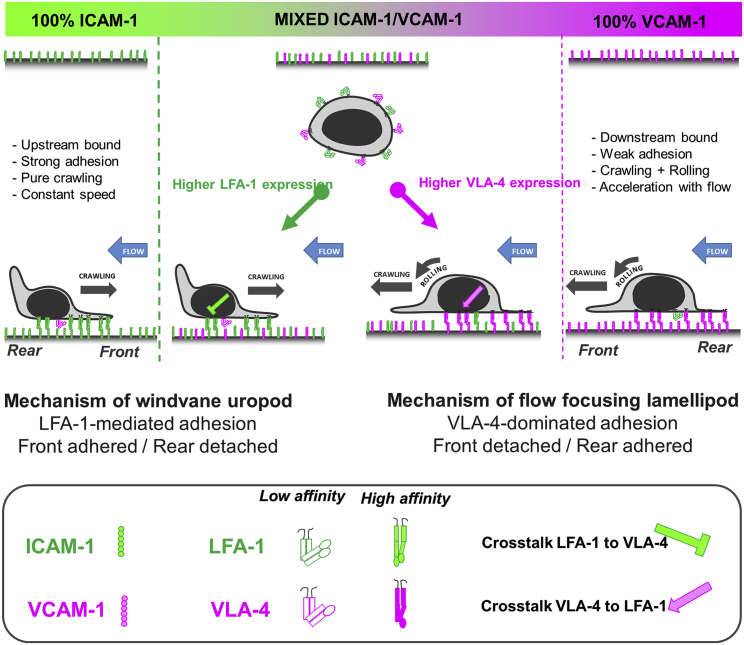
The analysis of the cell adhesion footprint at the cellular level explains upstream and downstream phenotypes with the uropod/wind vane and lamellipod flow-focusing mechanisms; however, the understanding of different cell adhesion properties requires analysis at the molecular level. The bistability of cell adhesion either in the front or in the rear was found to rely in large part on the opposite polarization of LFA-1 and VLA-4 affinities along the cells rear-front axis. Integrins in the high affinity state were found polarized toward the cell front for LFA-1 and toward the cell rear for VLA-4. This is in agreement with literature data reporting affinity upregulation for LFA-1 in the cell front (46,49) and of VLA-4 in the cell rear (50), on the one hand, and affinity downregulation of LFA-1 in the cell rear (46,51,52) and of VLA-4 in the cell front (24), on the other hand. These opposite polarizations of integrins explained directly the selective anterior adhesion of cells on ICAM-1 (with associated upstream phenotype) and the selective posterior adhesion of cells on VCAM-1 (with associated downstream phenotype). On mixed substrates, perturbation experiments with blocking antibodies of LFA-1 and VLA-4 confirmed a link between the cell orientation under flow and the implication of LFA-1 or VLA-4 in cell adhesion. Higher levels of high affinity LFA-1 versus VLA-4 on cells and/or of ICAM-1 versus VCAM-1 on substrates favors the upstream state, whereas lower ratios favors the downstream state (Fig. 7).
Figure 7.
A bistable mechanism of cell adhesion spatial regulation explains integrin control of T cell flow mechanotaxis. On pure substrates of ICAM-1 or VCAM-1, the T cell population has homogeneous phenotypes with an opposite orientation on ICAM-1 and VCAM-1. On mixed substrates of ICAM-1 or VCAM-1, T cells distribute in two populations with opposite orientations and characteristics similar to phenotypes on pure substrates. Decisions of orientation on mixed substrates are controlled by the expression level of integrins LFA-1 and VLA-4 via a bistable polarization of cell adhesion; a higher LFA-1 expression leads to a LFA-1-dominated adhesion of cell front (very similar to upstream crawling cells on ICAM-1), whereas a higher expression of VLA-4 leads to adhesion of cell rear and center (very similar to downstream crawling cells on VCAM-1). Inhibiting cross talk of LFA-1 toward VLA-4 reinforces adhesion polarization toward cell front, which favors wind vane mechanism and upstream phenotype. Activating cross talk of VLA-4 toward LFA-1 reinforces the adhesion of cell uropod, which hampers the wind vane mechanism and favors the downstream phenotype. To see this figure in color, go online.
If the attachment of a single pole of cells was essential to explain the possibility of different orientations under flow, the polarization of integrin affinity alone could not fully explain bistability. On mixed substrates, the attachment of cells by their front only, as revealed in our experiments for upstream cells, required an inhibitory cross talk of activated LFA-1 toward VLA-4 to detach the uropod. The existence of this cross talk, which plays a crucial role in our mechanistic model of upstream guiding, is also attested to in the literature (24,53). This inhibitory cross talk acts as an amplifier of integrin expression imbalance, allowing cells with the dominant expression of LFA-1 versus VLA-4 to behave like cells bearing only LFA-1 (Fig. 7). For the case of downstream crawling cells, a symmetric inhibitory cross talk effect from VLA-4 toward LFA-1 could promote symmetric orientation versus flow for cells with a dominant VLA-4 versus LFA-1 expression. However, we observed the presence of high affinity LFA-1 and VLA-4 in the cell rear on mixed ICAM-1/VCAM-1. Because polarization signaling inhibits LFA-1 in the cell rear, these results suggest an activation cross talk of VLA-4 toward LFA-1 that counterbalances polarization effect. This conclusion is consistent with literature data showing that VLA-4 promotes the activation of LFA-1 (54,55). Attachment of the cell rear is therefore enhanced by the combined adhesion of VLA-4 and LFA-1, which reinforces the inhibition of the wind vane mechanism and of the upstream phenotype. In the end, provided that the cell rear is attached, the extreme front edge of the lamellipod, before its attachment, can always promote downstream guiding. Fig. 7 summarizes the interplay between integrin affinity regulation and cross talk on cell-directed migration under flow. It illustrates a unique model linking mechanisms from the molecular to the cellular levels. Flow mechanotaxis decisions and bistability of guidance under flow rely on a polarized inside-out regulation of integrins LFA-1 and VLA-4 affinity and on an inhibitory cross talk mechanism between LFA-1 and VLA-4 at the molecular levels relayed by a wind vane uropod mechanism and a lamellipod flow focusing at the cellular level. This model shows that several integrins working synergistically can mediate multiphasic mechanotaxis by acting as switchable immobilizing anchors rather than as force transducers (49).
During recruitment of leukocytes from the blood system to inflamed zones, integrins are known to control several crucial functions. First, for cell arrest in blood vessels, VLA-4 (α4β1) contributes to rolling, and LFA-1 (αLβ2) is essential for crawling. Then, for cell extravasation, endothelial overexpression of integrin ligands is arguably guiding leukocytes into specialized portals of transmigration (56). Finally, for tissue migration, integrins αV condition the proper homing of lymphocytes to inflamed zones. The mechanism of lymphocyte guidance by flow enriches, therefore, the panel of integrin functionalities in the sequence of leukocyte recruitment. Although passive mechanisms suggest the possibility of a fortuitous phenotype, the robustness and sophistication of a mechanism with synergistic regulation and cross talk of multiple integrins supports instead a system evolved for a given function in leukocyte recruitment (32). More generally, a complete knowledge of integrin functions in leukocyte recruitment may also be valuable for therapeutic purposes—for instance, to modulate the immune response by integrins blocking antibodies in the treatment of pathologies such as multiple sclerosis.
Author Contributions
Conceptualization, M.P.V. and O.T.; Flow migration experiments, A.H. and T.S.; TIRF experiments, T.S. and N.G.-S.; Calcium experiments, L.A. and X.L.; Cytometry experiments, M.B.-P.; Data analysis, T.S., N.G.-S., and M.P.V.; and Manuscript writing, O.T. and M.P.V.
Acknowledgments
We are grateful to Laurent Limozin and Alphée Michelot for their support and advice with TIRF-fluorescence photobleaching recovery imaging and to Laurence Borge for assistance with the use of the Cell Culture Platform facility (Luminy TPR2-INSERM).
The project leading to this publication has received funding from the French Agence Nationale de la Recherche (ANR– ‐10– ‐INBS– ‐04– ‐01, (Investments for the future)), LABEX INFORM, the Région PACA, Institute CENTURI, Excellence Initiative of Aix-Marseille University A∗MIDEX, a French “Investissements d’Avenir” program ( A-M-AAP-ID-17-68-170301-11.33-VALIGNAT-HLS-SAT), and Alvéole.
Editor: Alexander Dunn.
Footnotes
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2019.12.013.
Supporting Material
References
- 1.Swaney K.F., Huang C.H., Devreotes P.N. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu. Rev. Biophys. 2010;39:265–289. doi: 10.1146/annurev.biophys.093008.131228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insall R.H. Understanding eukaryotic chemotaxis: a pseudopod-centred view. Nat. Rev. Mol. Cell Biol. 2010;11:453–458. doi: 10.1038/nrm2905. [DOI] [PubMed] [Google Scholar]
- 3.Yuan J., Raizen D.M., Bau H.H. Propensity of undulatory swimmers, such as worms, to go against the flow. Proc. Natl. Acad. Sci. USA. 2015;112:3606–3611. doi: 10.1073/pnas.1424962112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z., Liu J., Sun Y. Human sperm rheotaxis: a passive physical process. Sci. Rep. 2016;6:23553. doi: 10.1038/srep23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engler A.J., Sen S., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Mitrossilis D., Röper J.C., Farge E. Mechanotransductive cascade of Myo-II-dependent mesoderm and endoderm invaginations in embryo gastrulation. Nat. Commun. 2017;8:13883. doi: 10.1038/ncomms13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertet C., Sulak L., Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 8.Feng Y., Brazin K.N., Lang M.J. Mechanosensing drives acuity of αβ T-cell recognition. Proc. Natl. Acad. Sci. USA. 2017;114:E8204–E8213. doi: 10.1073/pnas.1703559114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber M., Hauschild R., Sixt M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- 10.Castellino F., Huang A.Y., Germain R.N. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich L.I., Oh D.Y., Lewis R.S. Differential contribution of chemotaxis and substrate restriction to segregation of immature and mature thymocytes. Immunity. 2009;31:986–998. doi: 10.1016/j.immuni.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartholomäus I., Kawakami N., Flügel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 13.Smith L.A., Aranda-Espinoza H., Hammer D.A. Interplay between shear stress and adhesion on neutrophil locomotion. Biophys. J. 2007;92:632–640. doi: 10.1529/biophysj.105.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valignat M.P., Theodoly O., Lellouch A.C. T lymphocytes orient against the direction of fluid flow during LFA-1-mediated migration. Biophys. J. 2013;104:322–331. doi: 10.1016/j.bpj.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valignat M.P., Nègre P., Theodoly O. Lymphocytes can self-steer passively with wind vane uropods. Nat. Commun. 2014;5:5213. doi: 10.1038/ncomms6213. [DOI] [PubMed] [Google Scholar]
- 16.Dominguez G.A., Anderson N.R., Hammer D.A. The direction of migration of T-lymphocytes under flow depends upon which adhesion receptors are engaged. Integr. Biol. 2015;7:345–355. doi: 10.1039/c4ib00201f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumagin R., Sarelius I.H. Intercellular adhesion molecule-1 enrichment near tricellular endothelial junctions is preferentially associated with leukocyte transmigration and signals for reorganization of these junctions to accommodate leukocyte passage. J. Immunol. 2010;184:5242–5252. doi: 10.4049/jimmunol.0903319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillipson M., Heit B., Kubes P. Vav1 is essential for mechanotactic crawling and migration of neutrophils out of the inflamed microvasculature. J. Immunol. 2009;182:6870–6878. doi: 10.4049/jimmunol.0803414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumagin R., Prizant H., Sarelius I.H. LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J. Immunol. 2010;185:7057–7066. doi: 10.4049/jimmunol.1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rainger G.E., Buckley C.D., Nash G.B. Neutrophils sense flow-generated stress and direct their migration through alphaVbeta3-integrin. Am. J. Physiol. 1999;276:H858–H864. doi: 10.1152/ajpheart.1999.276.3.H858. [DOI] [PubMed] [Google Scholar]
- 21.Steiner O., Coisne C., Lyck R. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood-brain barrier endothelium. J. Immunol. 2010;185:4846–4855. doi: 10.4049/jimmunol.0903732. [DOI] [PubMed] [Google Scholar]
- 22.Lee H.J., Diaz M.F., Wenzel P.L. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat. Commun. 2017;8:14122. doi: 10.1038/ncomms14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixit N., Yamayoshi I., Simon S.I. Migrational guidance of neutrophils is mechanotransduced via high-affinity LFA-1 and calcium flux. J. Immunol. 2011;187:472–481. doi: 10.4049/jimmunol.1004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grönholm M., Jahan F., Gahmberg C.G. LFA-1 integrin antibodies inhibit leukocyte α4β1-mediated adhesion by intracellular signaling. Blood. 2016;128:1270–1281. doi: 10.1182/blood-2016-03-705160. [DOI] [PubMed] [Google Scholar]
- 25.Ishibashi M., Miyanaga Y., Ueda M. Integrin LFA-1 regulates cell adhesion via transient clutch formation. Biochem. Biophys. Res. Commun. 2015;464:459–466. doi: 10.1016/j.bbrc.2015.06.155. [DOI] [PubMed] [Google Scholar]
- 26.Nordenfelt P., Elliott H.L., Springer T.A. Coordinated integrin activation by actin-dependent force during T-cell migration. Nat. Commun. 2016;7:13119. doi: 10.1038/ncomms13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schürpf T., Springer T.A. Regulation of integrin affinity on cell surfaces. EMBO J. 2011;30:4712–4727. doi: 10.1038/emboj.2011.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alon R., Kassner P.D., Springer T.A. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J. Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huttenlocher A., Horwitz A.R. Integrins in cell migration. Cold Spring Harb. Perspect. Biol. 2011;3:a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verma N.K., Kelleher D. Not just an adhesion molecule: LFA-1 contact tunes the T lymphocyte program. J. Immunol. 2017;199:1213–1221. doi: 10.4049/jimmunol.1700495. [DOI] [PubMed] [Google Scholar]
- 31.Buffone A., Jr., Anderson N.R., Hammer D.A. Migration against the direction of flow is LFA-1-dependent in human hematopoietic stem and progenitor cells. J. Cell Sci. 2018;131:jcs205575. doi: 10.1242/jcs.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson N.R., Buffone A., Jr., Hammer D.A. T lymphocytes migrate upstream after completing the leukocyte adhesion cascade. Cell Adhes. Migr. 2019;13:163–168. doi: 10.1080/19336918.2019.1587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman B.D., Grashoff C., Schwartz M.A. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.del Rio A., Perez-Jimenez R., Sheetz M.P. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawada Y., Tamada M., Sheetz M.P. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaidel-Bar R., Kam Z., Geiger B. Polarized downregulation of the paxillin-p130CAS-Rac1 pathway induced by shear flow. J. Cell Sci. 2005;118:3997–4007. doi: 10.1242/jcs.02523. [DOI] [PubMed] [Google Scholar]
- 37.Urbich C., Dernbach E., Dimmeler S. Shear stress-induced endothelial cell migration involves integrin signaling via the fibronectin receptor subunits alpha(5) and beta(1) Arterioscler. Thromb. Vasc. Biol. 2002;22:69–75. doi: 10.1161/hq0102.101518. [DOI] [PubMed] [Google Scholar]
- 38.Edelstein A., Amodaj N., Stuurman N. Computer control of microscopes using μmanager. Curr. Protoc. Mol. Biol. 2010;Chapter 14:Unit14.20. doi: 10.1002/0471142727.mb1420s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia Y., Whitesides G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998;28:153–184. [Google Scholar]
- 40.Schindelin J., Arganda-Carreras I., Cardona A. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohnet S., Ananthakrishnan R., Verkhovsky A.B. Weak force stalls protrusion at the leading edge of the lamellipodium. Biophys. J. 2006;90:1810–1820. doi: 10.1529/biophysj.105.064600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kao J.P., Li G., Auston D.A. Practical aspects of measuring intracellular calcium signals with fluorescent indicators. Methods Cell Biol. 2010;99:113–152. doi: 10.1016/B978-0-12-374841-6.00005-0. [DOI] [PubMed] [Google Scholar]
- 43.Artemenko Y., Axiotakis L., Jr., Devreotes P.N. Chemical and mechanical stimuli act on common signal transduction and cytoskeletal networks. Proc. Natl. Acad. Sci. USA. 2016;113:E7500–E7509. doi: 10.1073/pnas.1608767113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston B., Issekutz T.B., Kubes P. The alpha 4-integrin supports leukocyte rolling and adhesion in chronically inflamed postcapillary venules in vivo. J. Exp. Med. 1996;183:1995–2006. doi: 10.1084/jem.183.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorina R., Lyck R., Engelhardt B. β2 integrin-mediated crawling on endothelial ICAM-1 and ICAM-2 is a prerequisite for transcellular neutrophil diapedesis across the inflamed blood-brain barrier. J. Immunol. 2014;192:324–337. doi: 10.4049/jimmunol.1300858. [DOI] [PubMed] [Google Scholar]
- 46.Smith A., Stanley P., Hogg N. The role of the integrin LFA-1 in T-lymphocyte migration. Immunol. Rev. 2007;218:135–146. doi: 10.1111/j.1600-065X.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 47.Niethammer P. Neutrophil mechanotransduction: a GEF to sense fluid shear stress. J. Cell Biol. 2016;215:13–14. doi: 10.1083/jcb.201609101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreau H.D., Blanch-Mercader C., Lennon-Duménil A.M. Macropinocytosis overcomes directional bias in dendritic cells due to hydraulic resistance and facilitates space exploration. Dev. Cell. 2019;49:171–188.e5. doi: 10.1016/j.devcel.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 49.Ghandour H., Cullere X., Mayadas T.N. Essential role for Rap1 GTPase and its guanine exchange factor CalDAG-GEFI in LFA-1 but not VLA-4 integrin mediated human T-cell adhesion. Blood. 2007;110:3682–3690. doi: 10.1182/blood-2007-03-077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laudanna C., Campbell J.J., Butcher E.C. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- 51.Morin N.A., Oakes P.W., Kim M. Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J. Exp. Med. 2008;205:195–205. doi: 10.1084/jem.20071543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pouwels J., De Franceschi N., Ivaska J. SHARPIN regulates uropod detachment in migrating lymphocytes. Cell Rep. 2013;5:619–628. doi: 10.1016/j.celrep.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porter J.C., Hogg N. Integrin cross talk: activation of lymphocyte function-associated antigen-1 on human T cells alters α4β1- and α5β1-mediated function. J. Cell Biol. 1997;138:1437–1447. doi: 10.1083/jcb.138.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.May A.E., Neumann F.J., Preissner K.T. VLA-4 (alpha(4)beta(1)) engagement defines a novel activation pathway for beta(2) integrin-dependent leukocyte adhesion involving the urokinase receptor. Blood. 2000;96:506–513. [PubMed] [Google Scholar]
- 55.Chan J.R., Hyduk S.J., Cybulsky M.I. α 4 β 1 integrin/VCAM-1 interaction activates α L β 2 integrin-mediated adhesion to ICAM-1 in human T cells. J. Immunol. 2000;164:746–753. doi: 10.4049/jimmunol.164.2.746. [DOI] [PubMed] [Google Scholar]
- 56.Carman C.V., Springer T.A. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bright-field images with magnification x10.
Transmission images with magnification x10.
Transmission (left) and RICM (right) images with magnification x63.
VCAM-1 panel: The right cell is representative of cells displaying mostly crawling and the left cell of cells displaying a combination of crawling and rolling. ICAM-1 panel: Cells displaying representative crawling phenotype on ICAM-1. The yellow arrows indicate the direction of flow (8 dyn.cm-2), which is from top to bottom in the beginning of the sequence and from right to left in the end. n > 20 cells/experiments. nexp = 3.
Top: Transmission images with magnification x63. Bottom: RICM images with magnification x63.
Last part of the movie is the control experiment with ionomycin which increases calcium release by increasing the cell membrane permeability.
First part of the movie (left) shows that on a mixed substrate 75% ICAM-1 – 25% VCAM-1, cells are mainly crawling upstream. By adding blocking antibody against LFA-1 (TS1/22) and decreasing the ratio LFA-1/ VLA-4, cells are mainly crawling downstream (right). Second part of the movie (left) shows that on a mixed substrate 25% ICAM-1 – 75% VCAM-1, cells are mainly crawling downstream. By adding blocking antibody against VLA-4 (Natalizumab) and decreasing the ratio LFA-1/ VLA-4, cells are mainly crawling upstream (right).