Abstract
Dysbiosis of the enteric microbiota causes gastrointestinal diseases, including colitis. The present study investigated the beneficial effect of Lactobacillus plantarum 06CC2 in experimental colitis in mice. An experimental colitis model in C57BL6 mice was induced using dextran sulfate sodium. Mice were orally administered 06CC2 (06CC2 group) or PBS only (control group) by gavage. The disease activity index (DAI), histological grading, and colon tissue and colonic lamina propria mononuclear cells (LPMCs) were examined macroscopically and histopathologically, and the expression levels of inflammation-associated cytokines (IL-6, IL-12, TNF-α and IL-10) in these samples were determined. Compared with the control group, the 06CC2 group exhibited a significantly lower DAI (1.5±0.8 vs. 0.2±0.3, respectively; P<0.05) and pathology score (6.3±1.5 vs. 3.8±1.3, respectively; P<0.05). IL-10 expression in colonic LPMCs was higher in the 06CC2 group than in the control group, although there was no significant difference in IFN-γ, IL-6 or IL-12 expression in colonic LPMCs between the two groups. In addition, 06CC2 stimulated the production of IL-10 from CD11b-positive cells and CD11c-positive cells in the colon. The 06CC2 strain induced IL-10 production in the colon and attenuated colon inflammation.
Keywords: colitis, inflammatory bowel disease, Lactobacillus, microbiota, interleukin-10, Lactobacillus plantarum 06CC2
Introduction
Inflammatory bowel diseases (IBDs) such as ulcerative colitis and Crohn's disease are refractory disorders characterized by chronic relapsing inflammation of the gastrointestinal tract. The mucosal lesions in IBD are generated by an excessive or dysregulated immune response against commensal microbes in the gut.
Genome-wide association studies have reported that IBD-associated single nucleotide polymorphisms (SNPs) in genes such as NOD2, CARD9, and ATG16L1 interact with the microbiota and are involved in the pathogenesis of IBD (1). Several studies have revealed that microbial imbalance causes pro-inflammatory immunological responses and induces gut inflammation (2,3). Further, it has been reported that specific commensal bacteria affect the differentiation of mucosal immunocompetent cells. It is possible that some commensal bacterial strains can also induce anti-inflammatory immunological responses. To maintain intestinal homeostasis it is important that the commensal bacterial flora remains consistent (4–7) Microbial dysbiosis is essential for driving inflammation in IBD (8), and can be induced by several factors that affect the complexity and stability of the microbiome, including genetics, birth route, stress, nutrition, and drugs (9). The gut commensal bacteria regulate mucosal inflammation by several mechanisms, including inducing regulatory T cells, down-regulating inflammatory cytokines, and regulating nuclear factor (NF)-κB activation (4,10,11). Lactobacillus bacteria, which constitute a significant component of the gut microbiota, downregulate inflammatory signaling in the mucosa of IBD patients and also ameliorate dextran sulfate sodium (DSS)-induced colitis in mice by reducing the production of pro-inflammatory cytokines (12).
The Lactobacillus plantarum (LP) 06CC2 strain, isolated from traditional Mongolian dairy products, has shown probiotic potential in mice and has immune modulating effects. Oral administration of the 06CC2 strain exerted a significant immunomodulatory effect in influenza virus-infected mice (13,14). However, the involvement of the 06CC2 strain in intestinal inflammation remains unknown. Therefore, we assessed the impact of the 06CC2 strain in experimental colitis and its impact on intestine-specific, antigen-presenting cell phenotypes such as macrophages.
Materials and methods
Preparation of the LP 06CC2 strain
The 06CC2 strain, a lactic acid bacterium with probiotic potential, was isolated from Mongolian dairy cheese (Aaruul) as described previously (13). The 06CC2 cells were cultured at 37°C for 24 h in Man, Rogosa, and Sharpe (MRS) broth (Merck KGaA), cells were harvested by centrifugation at 10,000 × g for 5 min, washed twice with PBS, and then boiled for 1 h (14). The boiled 06CC2 cells were washed again with PBS and lyophilized. The lyophilized 06CC2 cells were suspended in Roswell Park Memorial Institute (RPMI)-1640 medium or Dulbecco's modified Eagle's medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS) for co-culture. For oral administration to mice, heat-killed 06CC2 cells were lyophilized and powdered, and the 06CC2 powder was suspended in distilled water before use.
Animals
Specific-pathogen-free C57BL/6 mice, 8 to 10 weeks of age and weighing between 22 to 26 g, were sourced from Kyudo (Saga). Mice were maintained under standard conditions (22°C, 50–60% humidity, and 12-h light/dark cycle) with free access to standard mouse/rat chow diet (LabDiet Autoclavable Rodent Diet 5010; PMI Nutrition International,). We monitored the health of mice daily, and all efforts were made to minimize animal suffering. We monitored body and stool conditions and measured the body weight of the mice each day. Mice were euthanized by cervical dislocation following anesthesia intraperitoneally with pentobarbital (30–50 mg/kg), and the colonic tissue was collected following euthanasia. Animal experimental procedures were approved by the institutional animal care and use committees of Kagoshima University (permit no. MD14106), and were performed in accordance with these committees' guidelines for animal experiments.
Induction and assessment of colitis
DSS-induced colitis is an established experimental model that allows the investigation of the signs and symptoms of UC, including diarrhea, weight loss, bloody stools, mucosal ulceration, and shortening of the large intestine (15). The Acute experimental colitis was induced using 1.0% DSS. DSS powder was manufactured by Wako Pure Chemical Industries, Ltd., and the average molecular weight was 5,000 Da. Control mice were given distilled water. A 06CC2 suspension (20 mg per mouse) or PBS was inoculated twice a day into experimental mice 3 days before oral DSS administration. 06CC2-treated mice were administered 1.0% DSS for 15 days and were euthanized on day 20. Whole colon tissue was collected and lamina propria mononuclear cells (LPMCs) were extracted.
In another experiment, a 06CC2 suspension or PBS was inoculated into mice for 30 days. Beginning 3 days after inoculation, 1.0% DSS was administered for 27 days, and neutralizing anti-IL-10 monoclonal antibodies (clone JES5-16E3; BioLegend) or control rat IgG2a (BioLegend) (0.4 mg/200 ml) was injected intraperitoneally for the last 8 days. The DAI was assessed blindly in both mouse groups. The DAI is based on clinical scores for weight loss, stool consistency, and bleeding, as previously described (16). Each clinical parameter was scored on a scale from 0 to 4, and the parameter values were summed.
Isolation of murine colonic LPMCs
Colonic LPMCs were isolated according to a previous report (17). Briefly, colon samples were cut into small pieces in Ca2+/Mg2+-free 1.5% Hank's balanced salt solution (1.5% HBSS). The colon samples were then placed in 1.5% HBSS and washed three times at 37°C, then incubated with HBSS containing 1 mM dithiothreitol (#15-508-031; Invitrogen; Thermo Fisher Scientific, Inc.) and 5 mM EDTA (#15-575-020; Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min at 37°C to remove the epithelial layer. The samples were then placed in digestion solution containing 1.5% HBSS, 1.0–3.0 mg/ml collagenase A (#11-088-793-001; Roche Diagnostics GmbH) and 0.1 mg/ml DNase I (#11-284-932-001; Roche Diagnostics GmbH) for 1–2 h at 37°C. The samples were passed through a 100-µm strainer and transferred to a 50-ml Falcon tube and centrifuged for 5 min at 4°C. The supernatant was washed, and the pellets were resuspended in 40% Percoll and overlaid on a 75% Percoll fraction. Mononuclear cells were collected at the interphase, washed, and resuspended in RPMI-1640 medium (#11875-093; Life Science Products, Inc.) containing 10% FBS and 1% penicillin/streptomycin.
Histological assessment and evaluation
Colon tissue was fixed in 10% phosphate-buffered formalin, and was embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Colitis stages were scored via blinded histopathological analysis according to previously described morphological criteria: Epithelial properties, depth of inflammation, and degree of ulceration (18). Epithelial properties were scored as follows: 0, intact crypt; 1, loss of the basal one-third of the crypt; 2, loss of the basal two-thirds of the crypt; 3, loss of the entire crypt but with intact surface epithelium; and 4, loss of the entire crypt and surface epithelium (erosion). Depth of inflammation was scored as follows: 0, no infiltration; 1, crypt base; 2, mucosa; 3, submucosa; and 4, submucosa (extensive). The degree of ulceration was scored as follows: 0, none; 2, positive; and 4, positive (extensive). Each specimen was assigned a histological score ranging from 0 to 12.
Cell separation of LPMCs by magnetic-activated cell sorting (MACS)
Colonic LPMCs were separated by MACS. LPMCs with specific CD antibodies were magnetically labeled with their respective magnetic beads (CD11c, #130-108-338; CD11b, #130-049-601; CD4, #130-049-201; Miltenyi Biotec). The cell suspension was loaded onto a MACS® LS Column (#130-042-401; Miltenyi Biotec), which was placed in the magnetic field of a MACS Separator (#130-042-301; Miltenyi Biotec). The MACS Separation Buffer contained a final BSA (Miltenyi Biotec) concentration of 0.5%. After cells were separated, they were analyzed for gene expression.
RNA isolation and gene expression assay
Total RNA was extracted from colon tissues or LPMCs using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (0.5 µg) was reverse transcribed using a PrimeScript RT Reagent kit (Takara Bio Inc.) at 37°C for 15 min and 85°C for 5 sec. Each reaction mixture for RT (total volume 20 µl) consisted of total RNA (adjusted to 500 ng, 2.5 µl), oligo dT PCR primer (50 µM, 1 µl), 5X PrimeScript buffer (4 µl), PrimeScript RT enzyme mix (1 µl), random 6 mers (100 µM, 1 µl), and RNase free distilled H2O (10.5 µl). The synthesized cDNA was amplified by RT-qPCR using SYBR (Applied Biosystems; Thermo Fisher Scientific, Inc.). Each reaction mixture for PCR (total volume 20 µl) consisted of cDNA (adjusted to 500 ng, 2 µl), forward and reverse PCR primers (10 µM, 0.8 µl each), SYBR (10X, 10 µl), ROX reference dye (50X, 0.4 µl), and distilled H2O (6 µl). The cycling conditions were as follows: One cycle at 95°C for 30 sec followed by 35 cycles each at 95°C for 5 sec and 60°C for 34 sec. The relative expression levels of target genes were normalized to rRNA (Applied Biosystems; Thermo Fisher Scientific, Inc.) and were calculated using the 2−∆∆Cq method (19). Primers for the following genes were used: GAPDH, IL-6, IL-10, IL-12α, IL-23α, TGF-β, TNF-α, and Toll-like receptor (TLR)2, TLR3, and TLR4 (Table I).
Table I.
List of primers.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| IL-6 | CCACTTCACAAGTCGGAGGCTTA | CCAGTTTGGTAGCATCCATCATTTC |
| IL-10 | GACCAGCTGGACAACATACTGCTAA | GATAAGGCTTGGCAACCCAAGTAA |
| IL-12α | AATGTTCCA GTGCCTCAACC | CTAGAGTTTGT CTGGCCTTCTG |
| TNF-α | TATGGCCCAGACCCTCACA | GGAGTAGACAAGGTACAACCCATC |
| TLR2 | CTCCCAGCAGGAACATCTGCTA | CCAGGAATGAAGTCCCGCTTA |
| TLR3 | TGTCTGGAAGAAAGGGACTTTGA | GTTGAACTGCATGATGTACCTTGA |
| TLR4 | CTGGGTGTGTTTCCATGTCTCA | TGCGGACACACACACTTTCAAATA |
| Foxp3 | AGTGCCTGTGTCCTCAATGGTC | AGGGCCAGCATAGGTGCAAG |
| TGF-β | GCTGAGCCAGCCAGATATAACAAGA | ACAGGCAAGTAGCTGATCCCAAAC |
| GAPDH | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
IL, interleukin; TGF-β, transforming growth factor β; TLR, toll-like receptor; TNF-α, tumor necrosis factor α.
ELISA for IL-10
LPMCs were co-cultured with 06CC2 at concentrations of 0, 1, 10 and 100 µg/ml, and the supernatant was collected. IL-10 concentrations were measured with an ELISA kit (#M1000B; R&D Systems) according to the manufacturer's instructions, and analyzed in duplicate using a microplate reader (Bio-Rad Laboratories, Inc.) at 450 nm. The concentration of IL-10 in the supernatant was calculated according to a standard curve.
DNA extraction from fecal samples and intestinal bacteria assay
For quantification by quantitative PCR, DNA was extracted from fecal samples obtained from 06CC2-treated and control mice on day 20 using an ISOFECAL for Beads Beating kit (Nippon Gene) according to the manufacturer's instructions as described in a previous report (20). DNA was also extracted from standard strains for each group, including Bifidobacterium longum JCM 1217 for the Bifidobacterium subspecies, the 06TCa19 strain for the Lactobacillus group, Clostridium coccoides JCM 1395 for the Clostridium group, and Bacteroides fragilis JCM 11019 for the Bacteroides-Prevotella group. Except for strain 06TCa19, these strains were cultured at 37°C for 24–48 h in GAM broth and on GAM agar (Nissui) containing 0.5% glucose in jars with anaerobic packs for enumeration of colony-forming units (CFUs). Strain 06TCa19 was cultured at 37°C for 24 h in MRS broth and on MRS agar. Bifidobacterium subspecies, C. coccoides, Bacteroides-Prevotella, and Lactobacillus groups were detected by SYBR-Green quantitative PCR. Specific primers are shown in Table II (20). Bacterial CFUs were calculated using a standard regression curve of the threshold cycle values generated from 10-fold serial dilutions of DNA samples from the standard strains with known numbers of CFUs. Each fecal sample was diluted 103-fold and stained with 4′,6-diamidino-2-phenylindole (DAPI) solution (Dojindo Laboratories), and the bacteria were counted with a hemocytometer for bacteria (ASONE) using a fluorescence microscope (Olympus).
Table II.
Primer list for target bacteria.
| Target bacteria | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| Bifidobacterium subspecies | TCGCGTCYGGTGTGAAAG | CCACATCCAGCRTCCAC |
| Lactobacillus group | AGCAGTAGGGAATCTTCCA | CACCGCTACACATGGAG |
| Bcteroides-Prevotella group | GAAGGTCCCCCACATTG | CAATCGGAGTTCTTCGTG |
| Clostridium coccoides group | AAATGACGGTACCTGACTAA | TTTGAGTTTCATTCTTGCGAA |
Statistical analysis
All of the data are expressed as the mean and standard error of the mean. Differences between two or three groups were appropriately analyzed using one-way ANOVA followed by the Tukey HSD, Dunnet T3, Mann-Whitney U test, and Steel-Dwass tests (IBM SPSS version 23.0). P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of genes for inflammation-related cytokines and concentrations of LPMCs co-cultured with LP 06CC2
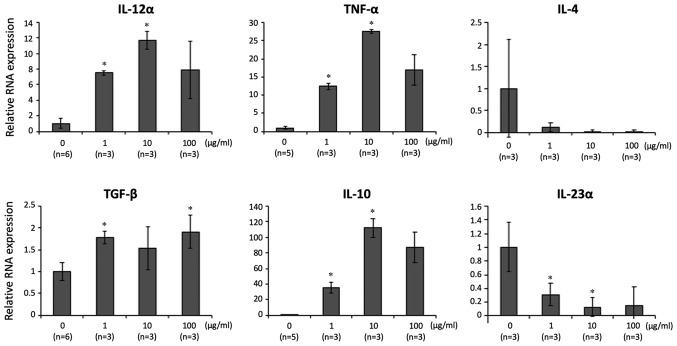
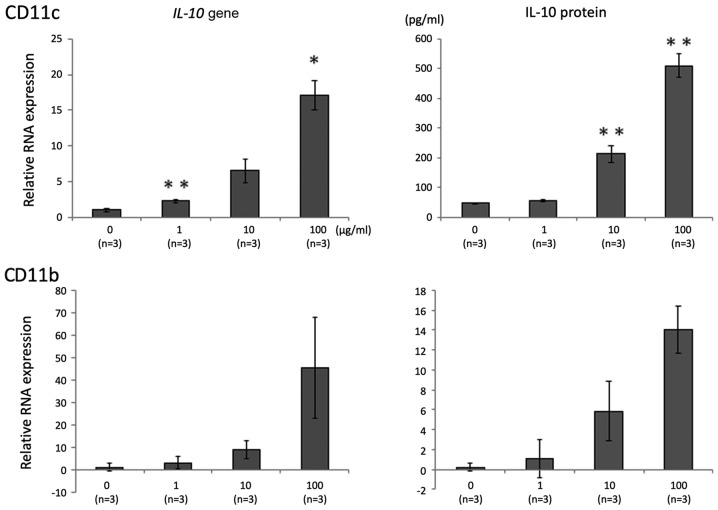
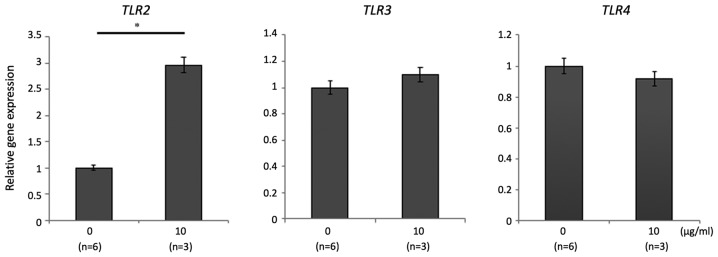
The effect of 06CC2 on LPMCs isolated from whole colon samples of mice without DSS-induced colitis was investigated. The expression of genes for the following pro- and anti-inflammatory cytokines was assessed: IL-12α, TNF-α, IL-4, IL-23α, TGF-β, and IL-10. Gene expressions of IL-12α, TNF-α, TGF-β, and IL-10 were significantly and dose-dependently higher in LPMCs exposed to 06CC2. Since the gene expression of IL-10 was induced at particularly high levels, we focused on results related to IL-10 (Fig. 1). LPMCs were separated by MACS as follows: CD4-positive cells, CD11b-positive cells, and CD11c-positive cells. The gene expression and protein concentration of IL-10 were increased by 06CC2 stimulation in a dose-dependent manner in CD11b-positive and CD11c-positive cells (Fig. 2), but not in CD4-positive cells (data not shown). The production of IL-10 was especially high in CD11c-positive cells. These results show that 06CC2 induced expression of a regulatory cytokine in LPMCs. TLR gene expression was significantly increased by 06CC2 administration only in the case of TLR2 in CD11c-positive cells; gene expressions of TLR3 and TLR4 were unchanged (Fig. 3).
Figure 1.
LPMCs isolated from entire mouse colons were co-cultured with 06CC2 (0, 1, 10 and 100 µg/ml). The gene expression levels of IL-12α, TNF-α, TGF-β and IL-10 were significantly and dose-dependently higher in LPMCs exposed to 06CC2; IL-10 gene expression was particularly high compared with the other genes. IL-12α, IL-4 and IL-23α were analyzed using Tukey's test. TNF-α, TGF-β and IL-10 were analyzed using Dunnet T3 test. *P<0.05 vs. 0 µg/ml. IL, interleukin; LPMCs, lamina propria mononuclear cells; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α.
Figure 2.
IL-10 expression in CD11c-positive and CD11b-positive cells in colonic lamina propria mononuclear cells under co-culture with 06CC2. Top panel, CD11c; lower panel, CD11b. Data were analyzed using Tukey's test. *P<0.05 and **P<0.01 vs. 0 µg/ml. IL-10, interleukin 10.
Figure 3.
TLR2, TLR3 and TLR4 expression in CD11c-positive cells in colonic lamina propria mononuclear cells under co-culture with 06CC2. Data were analyzed using Mann-Whitney's U test. *P<0.05. TLR, toll-like receptor.
Effects of oral administration of LP 06CC2 on the immune system of the murine large intestine
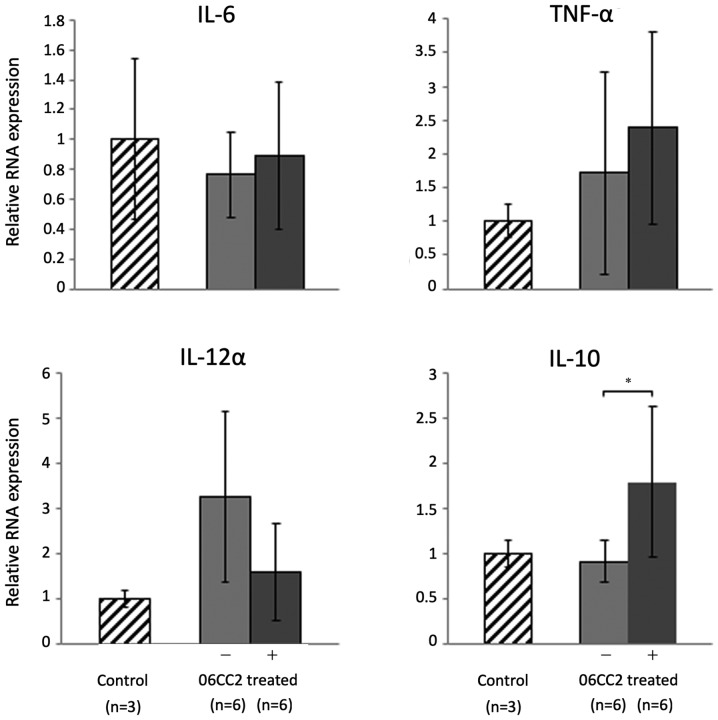
06CC2 was orally administered to mice twice a day for 4 days; the mice were subsequently euthanized, and colon tissue was collected for analysis. Pro-inflammatory and regulatory cytokines in the large intestine were investigated by quantitative PCR. The expression of IL-10 was significantly increased in mice treated with 06CC2 compared to control mice (P=0.048). However, administration of 06CC2 did not affect the expression of three pro-inflammatory genes, namely IL-6, TNF-α, and IL-12α (Fig. 4).
Figure 4.
Inflammatory and anti-inflammatory cytokine gene expression in the large intestine. The expression of IL-10 was significantly increased in 06CC2-treated mice compared with control. IL-6 and IL-10 were analyzed by Tukey's test. TNF-α and IL-12α were analyzed by Dunnet T3 test. *P<0.05. IL, interleukin; TNF-α, tumor necrosis factor α.
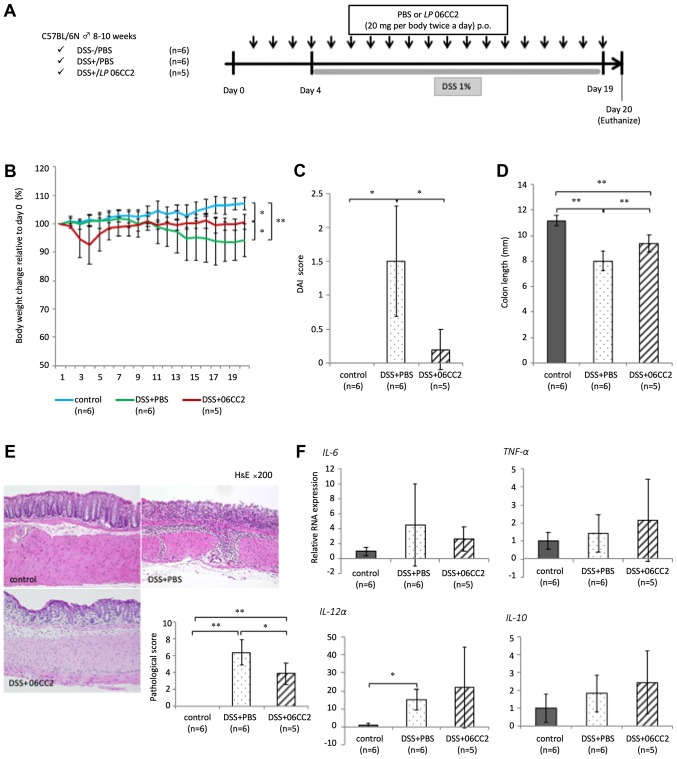
Effects of oral administration of LP 06CC2 in mice with DSS-induced colitis
The ability of 06CC2 to prevent colitis was investigated. After the administration of 06CC2 or phosphate-buffered saline (PBS) as vehicle by oral gavage for 20 days. Approximately 20 mm of the distal colon was collected and fixed by 10% phosphate-buffered formalin for histological analysis. LPMCs were extracted from the remaining colon. Mice with DSS-induced colitis that received vehicle demonstrated significantly decreased body weight, while those administered 06CC2 showed marked attenuation of average body weight loss (a 6.5% decrease in body weight relative to day 0 body weight) and reduced colon shortening. Thus, we confirmed a transient decrease in the body weights of DSS+06CC2 treated mice during our preliminary tests. We performed these experiments several times, and this phenomenon was easily reproducible, although we note that this weight loss could also be induced by the physical irritation associated with oral 06CC2 administration. The disease activity index (DAI) and pathological score in the 06CC2 group were significantly lower than in the vehicle group. The expression of inflammatory cytokine genes such as IL-6 and TNF-α in colonic LPMCs was elevated in colitis mouse models. The expression of IL-10 was also slightly higher in the 06CC2 group, but these differences were not significant (Fig. 5). The symptoms of clinical and pathological colitis were lessened in the 06CC2 group, despite the fact that the expression of proinflammatory cytokines such as IL-6 and TNF-α were increased. Therefore, IL-10 expression may act to mitigate the symptoms of colitis.
Figure 5.
Effects of oral administration of LP 06CC2 in mice with DSS-induced colitis. (A) In a mouse experimental colitis model, 06CC2 was administered by oral gavage prior to the induction of DSS-colitis. (B) Final body weight relative to Day 0 (%). (C) DAI scores. (D) Colon lengths (mm). (E) Histopathology of the distal large intestine and the pathological score. Magnification, ×200. (F) Cytokine gene expression in colonic lamina propria mononuclear cells. Data were analyzed by (B and D) Tukey's test, (C) Dunnett T3 test and (E and F) Games-Howell test. *P<0.05 and **P<0.01, as indicated. DAI, disease activity index; DSS, dextran sulfate sodium; IL, interleukin; TNF-α, tumor necrosis factor α.
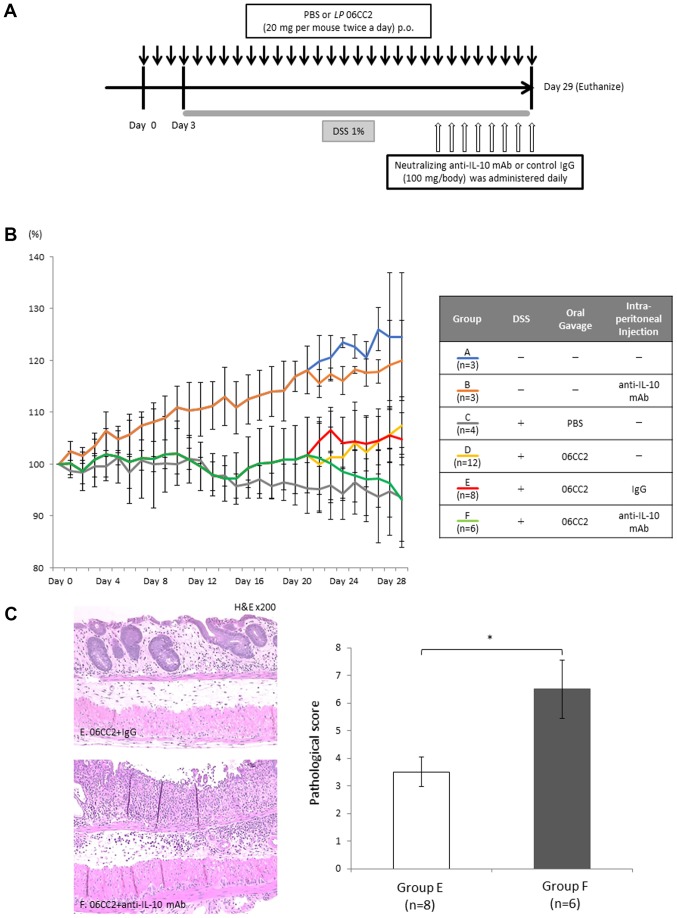
LP 06CC2-treated colitis model mice: Effect of neutralizing anti-IL-10 antibody
To confirm whether IL-10 was required for the protective effect of 06CC2 against DSS-induced colitis, mice were orally administered PBS or 06CC2 twice a day before drinking DSS, and neutralizing anti-IL-10 monoclonal antibody (100 mg/mouse, once a day) or control antibody were intraperitoneally administered after the second administration of PBS or 06CCs, as previously described (21).
Group A constituted a control group that did not receive DSS, 06CC2, or intraperitoneal antibody administration; Group B received only anti-IL-10 neutralizing antibody without DSS or 06CC2; Group C received DSS and PBS gavage; Group D received DSS and 06CC2 gavage but no intraperitoneal injection; Group E received DSS, 06CC2 gavage, and control IgG intraperitoneal injection; and Group F received DSS, 06CC2 gavage, and intraperitoneal injection of anti-IL-10 neutralizing antibody. Group F showed attenuated average body weight loss (93.1% of day 0 body weight), while Group E mice exhibited no significant changes in body weight. Pathological examination showed that mucosal layer crypts still existed in Group E, whereas in Group F these crypts had disappeared and inflammatory cells had invaded the submucosal layer. The pathology scores of Group F were worse than those of Group E. These findings may indicate that the protective probiotic effect of 06CC2 against colitis depends on IL-10 (Fig. 6).
Figure 6.
Effect of anti-IL-10 neutralizing antibody on LP 06CC2-treated colitis model mice. Mice were allocated into six groups: Group A is a control; Group B received only anti-IL-10 neutralizing antibody without DSS or 06CC2; Group C received DSS and PBS gavage; Group D received DSS and 06CC2 gavage but no intraperitoneal injection; Group E received DSS, 06CC2 gavage, and control IgG intraperitoneal injection; and Group F received DSS, 06CC2 gavage, and intraperitoneal injection of anti-IL-10 neutralizing antibody. (A) Experimental design. (B) Alterations in body weight. (C) Histopathology of the distal large intestine and the pathological score. Magnification, ×200. *P<0.01. DSS, dextran sulfate sodium; IL-10, interleukin 10.
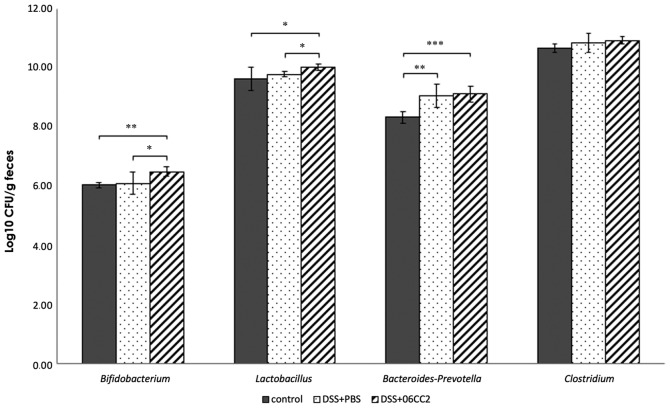
LP 06CC2-treated colitis model mice: Alteration of the microbiome
The total bacterial counts measured by DAPI staining did not differ between the control group, the colitis and PBS group, and the colitis and 06CC2 group. The Lactobacillus and the Bifidobacterium counts in feces from 06CC2-treated colitis model mice were significantly higher than those in control and PBS-treated colitis model mice. Bacteroides-Prevotella and Clostridium counts were similar regardless of the administration of 06CC2. Lactobacillus and Bifidobacterium counts increased significantly in the 06CC2 group during the intake period (Fig. 7).
Figure 7.
Bacterial counts of Lactobacillus, Bifidobacterium, Bacteroides-Prevotella and Clostridium in feces of 06CC2-treated colitis model mice. Bifidobacterium, Bacteroides-Prevotella and Clostridium data were analyzed by Tukey's test. Lactobacillus data were analyzed by Dunnet T3 test. *P<0.05, **P<0.01 and ***P<0.001, as indicated. DSS, dextran sulfate sodium.
Discussion
We showed that the 06CC2 strain attenuated DSS-induced colitis and stimulated the production of IL-10 from CD11b-positive and CD11c-positive cells in the colon. The 06CC2 strain, which was isolated and identified from traditional Mongolian dairy products, has beneficial probiotic properties (13,14). 06CC2 is a useful probiotic lactic acid bacteria strain for activating immunocompetent cells in the large intestine. Our data regarding the beneficial effects of 06CC2 are the first to suggest that this strain may prevent colitis.
Several reports demonstrated that intestinal bacteria in the Firmicutes phylum, which is divided primarily into the Clostridia and Bacilli classes, also attenuated DSS-induced colitis (22,23). In particular, Faecalibacterium prausnitzii, a species in the Firmicutes phylum, was shown to ameliorate DSS-induced colitis through the TLR2-dependent modulation of IL-12 and IL-10 production (22). Clostridium cluster IV and XIVa strains promoted the accumulation of IL-10-producing Treg cells in the colon and induced resistance to acute DSS-induced colitis (23). Clostridium butyricum exerted a protective effect on the epithelial barrier by maintaining the expression of tight junction proteins; it also increased the expression of the anti-inflammatory cytokine IL-10, and decreased levels of pro-inflammatory cytokines such as IL-1β, TNF, and IL-13 (17,24). In terms of Lactobacillus members of the Bacilli class, orally administered LP-K68 ameliorated DSS-induced colitis in BALB/c mice by reducing the production of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 (12). Lactobacillus brevis K65 reduced the levels of the pro-inflammatory cytokines TNF-α, IL-6, and IL-1β (25). Lactobacillus reuteri F-9-35 prevented DSS-induced colitis by inhibiting the expression of several pro-inflammatory genes, namely those for TNF-α, cyclooxygenase-2, and IL-6 (26). Therefore, we focused on 06CC2 under the hypothesis that its probiotic effect would augment intestinal immunity in DSS-induced colitis model mice.
We analyzed the levels of anti-inflammatory and pro-inflammatory cytokines, namely IL-6, TNF-α, IL-12α, and IL-10, in mice without DSS-induced colitis that were orally administered 06CC2. Regulation of the production of pro-inflammatory mediators is critical for normal physiological immune function and for controlling inflammation. The expression levels of IL-6, TNF-α, IL-12α, and IL-10 showed no significant difference between DSS-induced colitis mice treated with and without 06CC2 (Fig. 5). Although the expressions of IL-6, TNF-α, and IL-12 genes were not changed by 06CC2 stimulation, the expression of IL-10 was significantly increased in 06CC2-treated mice compared to controls (Fig. 4). Furthermore, the expression of the inflammatory cytokine genes IL-12α and TNF-α were significantly associated with 06CC2 concentration (expression was 10 to 30 times higher relative to the 0 ng/ml). However, the expression of IL-10 following 06CC2 culture was much higher relative to the control (80 to 120 times higher), as shown in Fig. 1. Therefore, we considered that IL-10 played a key role in these experiments, and focused on how its expression was altered by 06CC2 stimulation.
It has generally been reported that IL-10 is produced by CD11b-, CD11c-, and CD4-positive cells, including Treg cells. We investigated CD11b-, CD11c-, and CD4-positive colonic LPMCs co-cultured with 06CC2. CD11b- and CD11c-positive cells produced IL-10 following stimulation by 06CC2. IL-10 production was much higher in CD11c-positive cells relative to CD11b-positive cells (Fig. 2), whereas IL-10 production was unaffected in CD4-positive cells (data not shown). In addition, the transcription factor Foxp3 is specifically expressed by naturally occurring Treg cells and plays a key role in their development and function (27–29). As we observed no changes in the expression of Foxp3 following the co-culturing of colonic LPMCs with 06CC2 in this study (data not shown), we suggest that the number of Treg cells in LPMCs was unaffected by co-culturing, and that other cells were responsible for IL-10 production. We believe that the main locus of IL-10 production may be CD11c-positive cells.
The function of IL-10 is important for regulating gut homeostasis during host defense, and IL-10 suppresses the function of T-lymphocytes and mononuclear cells (30,31). A genome-wide association study in humans showed that a single nucleotide polymorphism in the IL-10 gene was closely associated with IBD (32,33). Thus, the association between IL-10 and IBD has been demonstrated by several findings in both humans and animal models (34). In fact, IL-10 knockout mice spontaneously developed colitis in the presence of commensal bacteria (35). There are reports that various immune cell types, including regulatory T cells (Treg cells), macrophages, and dendritic cells, are a major source of IL-10 in the gut (36–38).
This study used a colitis mouse model to investigate the effects of prolonged DSS treatment. We used prolonged DSS treatment because, in terms of the duration of DSS treatment, the short-term administration of DSS has been used as an acute colitis model in previous studies, while low-dose and long-term administration for periods of around 10 days have often been used as model for chronic colitis (39,40). We found no differences between the PBS group and the 06CC2 group after DSS administration for 10 days in our preliminary experiments. We decided that a 10-day DSS treatment period would be sufficient to identify any changes in mouse weight.
In this study we co-cultured LPMCs with 06CC2 and investigated which cells produced IL-10. LPMCs were separated by MACS as follows: CD11b-positive cells, CD11c-positive cells, and CD4-positive cells. It has been reported that IL-10 is produced by CD11b-positive cells, CD11c-positive cells, and Treg cells. We confirmed that 06CC2 exposure dose-dependently increased the gene expression of IL-10 in CD11c-positive cells, and to a lesser extent, CD11b-positive cells (Fig. 2), but not CD4-positive cells (data not shown). Since CD11b and CD11c are mainly expressed by macrophages and dendritic cells, respectively, we believe that 06CC2 mainly affected dendritic cells.
The 06CC2 administered to mice with DSS-induced colitis resulted in increased numbers of Lactobacillus and Bifidobacterium bacteria compared with mice not treated with 06CC2. This result occurred despite the fact that heat-killed 06CC2 was administered. Lactobacillus and Bifidobacterium, both of which have probiotic characteristics, play a pivotal role in the intestinal environment by preventing pathogen colonization and maintaining normal mucosal immunity. Increased numbers of Lactobacillus and Bifidobacterium lead to downregulation of the gene expression of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β (25–41). Thus, as a result of changing the composition of the intestinal microflora, heat-killed 06CC2 may indirectly prevent colitis.
There were some limitations to this study. First, we demonstrated that 06CC2 suppressed colon inflammation by altering IL-10 production, but this should be confirmed using genetically engineered animals such as IL-10 knockout mice. However, administration of a neutralizing IL-10 antibody to DSS-induced colitis model mice demonstrated that the protective effects of 06CC2 were mediated by IL-10. Further study should aim to determine whether the number of IL-10-producing LPMCs is increased in mice following 06CC2 treatment. Unfortunately, we did not count the total number of LPMCs because we focused primarily on LPMC gene expression. However, we believe that our results are valid because of the number of experimental replicates we performed. MACS could not be used to strictly classify LPMCs, though CD11b-positive cells and CD11c-positive cells were considered to be macrophages and dendritic cells, respectively. It is thus not exactly clear how 06CC2 directly or indirectly affects immune cells, although TLR2 gene expression in CD11c-positive cells was increased by 06CC2 stimulation. We showed that 06CC2 may act on macrophages or dendritic cells to induce IL-10 via TLR2 in vitro, but we have not demonstrated how the mechanism of 06CC2 differs between in vivo and in vitro experiments. Data concerning 06CC2 monotherapy in mice is almost non-existent. However, we have performed a similar study in humans. Participants ingested strain 06CC2 daily for 28 days, resulting in the elevation of fecal Bifidobacterium abundance (data not shown). Although our study was limited to only humans, 06CC2 may facilitate Bifidobacterium abundance in other organisms.
In conclusion, oral administration of the 06CC2 strain suppressed colon inflammation by altering IL-10 production in colonic dendritic cells and macrophages. We expect that 06CC2 will be available clinically as a therapeutic agent in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
AT and SK were responsible for the experimental design. AT, SK, YM and KK performed the animal experiments. AT and YM performed the colonic histochemical examinations, reverse transcription-quantitative PCR assays, and colonic ELISAs and were involved in the analysis and interpretation of data. AT, YM, MT and ST extracted DNA from fecal samples, performed the intestinal bacteria assays, and were involved in the analysis and interpretation of data. SA, FS, YN, ST and SH were responsible for statistical analyses. AI made substantial contributions to the conception and design of the study and the drafting of the manuscript. AT and SK wrote the manuscript. All authors were involved in manuscript revisions.
Ethics approval and consent to participate
All experiments were approved by the Animal Care and Use Committee of Kagoshima University (approval no. MD14106).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 5.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 9.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology. 2014;146:1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llopis M, Antolin M, Carol M, Borruel N, Casellas F, Martinez C, Espín-Basany E, Guarner F, Malagelada JR. Lactobacillus casei downregulates commensals' inflammatory signals in Crohn's disease mucosa. Inflammatory Bowel Dis. 2009;15:275–283. doi: 10.1002/ibd.20736. [DOI] [PubMed] [Google Scholar]
- 11.Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, Pettersson S, Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 12.Liu YW, Su YW, Ong WK, Cheng TH, Tsai YC. Oral administration of Lactobacillus plantarum K68 ameliorates DSS-induced ulcerative colitis in BALB/c mice via the anti-inflammatory and immunomodulatory activities. Int Immunopharmacol. 2011;11:2159–2166. doi: 10.1016/j.intimp.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Takeda S, Yamasaki K, Takeshita M, Kikuchi Y, Tsend-Ayush C, Dashnyam B, Ahhmed AM, Kawahara S, Muguruma M. The investigation of probiotic potential of lactic acid bacteria isolated from traditional Mongolian dairy products. Anim Sci J. 2011;82:571–579. doi: 10.1111/j.1740-0929.2011.00874.x. [DOI] [PubMed] [Google Scholar]
- 14.Takeda S, Takeshita M, Kikuchi Y, Dashnyam B, Kawahara S, Yoshida H, Watanabe W, Muguruma M, Kurokawa M. Efficacy of oral administration of heat-killed probiotics from Mongolian dairy products against influenza infection in mice: Alleviation of influenza infection by its immunomodulatory activity through intestinal immunity. Int Immunopharmacol. 2011;11:1976–1983. doi: 10.1016/j.intimp.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Büchler MW. Characterisation of acute murine dextran sodium sulphate colitis: Cytokine profile and dose dependency. Digestion. 2000;62:240–248. doi: 10.1159/000007822. [DOI] [PubMed] [Google Scholar]
- 16.Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–1734. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi A, Sato T, Kamada N, Mikami Y, Matsuoka K, Hisamatsu T, Hibi T, Roers A, Yagita H, Ohteki T, et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13:711–722. doi: 10.1016/j.chom.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Takeda S, Fujimoto R, Takenoyama SI, Takedhita M, Kikuchi Y, Tsend-Ayush C, Dashnyam B, Muguruma M, Kawahara S. Application of probiotics from Mongolian dairy products to fermented dairy products and its effects on human defecation. Food Sci Technol Res. 2013;19:245–253. doi: 10.3136/fstr.19.245. [DOI] [Google Scholar]
- 21.Mabalirajan U, Dinda AK, Kumar S, Roshan R, Gupta P, Sharma SK, Ghosh B. Mitochondrial structural changes and dysfunction are associated with experimental allergicasthma. J Immunol. 2008;181:3540–3548. doi: 10.4049/jimmunol.181.5.3540. [DOI] [PubMed] [Google Scholar]
- 22.Rossi O, Khan MT, Schwarzer M, Hudcovic T, Srutkova D, Duncan SH, Stolte EH, Kozakova H, Flint HJ, Samsom JN, et al. Faecalibacterium prausnitzii strain HTF-F and its extracellular polymeric matrix attenuate clinical parameters in DSS-induced colitis. PLoS One. 2015;10:e0123013. doi: 10.1371/journal.pone.0123013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atarashi K, Honda K. Microbiota in autoimmunity and tolerance. Curr Opin Immunol. 2011;23:761–768. doi: 10.1016/j.coi.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Gong Y, Xie Y, Sun Q, Li Y. Clostridium butyricum protects the epithelial barrier by maintaining tight junction protein expression and regulating microflora in a murine model of dextran sodium sulfate-induced colitis. Scand J Gastroenterol. 2018;53:1031–1042. doi: 10.1080/00365521.2016.1192678. [DOI] [PubMed] [Google Scholar]
- 25.Liu YW, Ong WK, Su YW, Hsu CC, Cheng TH, Tsai YC. Anti-inflammatory effects of Lactobacillus brevis K65 on RAW 264.7 cells and in mice with dextran sulphate sodium-induced ulcerative colitis. Benef Microbes. 2016;7:387–396. doi: 10.3920/BM2015.0109. [DOI] [PubMed] [Google Scholar]
- 26.Sun MC, Zhang FC, Yin X, Cheng BJ, Zhao CH, Wang YL, Zhang ZZ, Hao HW, Zhang TH, Ye HQ. Lactobacillus reuteri F-9-35 prevents DSS-induced colitis by inhibiting proinflammatory gene expression and restoring the gut Microbiota in mice. J Food Sci. 2018;83:2645–2652. doi: 10.1111/1750-3841.14326. [DOI] [PubMed] [Google Scholar]
- 27.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 28.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 29.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 30.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 31.Sydora BC, MacFarlane SM, Lupicki M, Dmytrash AL, Dieleman LA, Fedorak RN. An imbalance in mucosal cytokine profile causes transient intestinal inflammation following an animal's first exposure to faecal bacteria and antigens. Clin Exp Immunol. 2010;161:187–196. doi: 10.1111/j.1365-2249.2010.04140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 33.Coelho T, Andreoletti G, Ashton JJ, Pengelly RJ, Gao Y, RamaKrishnan A, Batra A, Beattie RM, Williams AP, Ennis S. Immuno-genomic profiling of patients with inflammatory bowel disease: A systematic review of genetic and functional in vivo studies of implicated genes. Inflamm Bowel Dis. 2014;20:1813–1819. doi: 10.1097/MIB.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 34.Santana G, Bendicho MT, Santana TC, Reis LB, Lemaire D, Lyra AC. The TNF-α-308 polymorphism may affect the severity of Crohn's disease. Clinics (Sao Paulo) 2011;66:1373–1378. doi: 10.1590/S1807-59322011000800011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hedges SR, Sibley DA, Mayo MS, Hook EW, III, Russell MW. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: Effects of concomitant infections. J Infect Dis. 1998;178:742–751. doi: 10.1086/515372. [DOI] [PubMed] [Google Scholar]
- 36.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 38.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-H. [DOI] [PubMed] [Google Scholar]
- 40.Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflammatory Bowel Dis. 2009;15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh MC, Tsai WH, Jheng YP, Su SL, Wang SY, Lin CC, Chen YH, Chang WW. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: A randomized, double-blinded, placebo-controlled trial. Sci Rep. 2018;8:16791. doi: 10.1038/s41598-018-35014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.