Abstract
Treating gastrointestinal (GI) fistulas endoscopically is challenging owing to an established epithelial tract. The variety of endoscopic approaches is transforming endoscopy into a first-line therapy. However, many sessions are often required, with variable success rates. Owing to these limitations, the off-label use of cardiac septal occluders (CSOs) has been reported.
We searched for articles related to CSOs in the MEDLINE, EMBASE, Cochrane Library, and LILACS databases and gray literature. The primary outcomes included technical success, clinical success, and safety of CSOs in GI fistula management.
A total of 25,574 records were identified, and 19 studies ultimately satisfied the inclusion criteria. Technical success was achieved in all cases. Of the 22 fistulas, 77.27% had successful closure, with a mean follow-up period of 32.02 weeks. The adverse event rate was 22.72%, with no associated mortality. Univariable and multivariable regression analyses showed no significant difference in the success of closure and adverse events in relation to several variables among the subgroups.
The use of CSOs appeared to be technically feasible, effective, and safe in the treatment of GI fistulas. The satisfactory results derived from this sparse literature suggest that it can be an option in the management of GI fistulas.
Keywords: Cardiac septal occluder, Endoscopy, Fistula, Gastrointestinal fistula
INTRODUCTION
Gastrointestinal (GI) fistulas can occur after surgery and can also be caused by chronic inflammation in the tissues (e.g., inflammatory bowel diseases), untreated long-term leak, malignancy complications, radiation therapy, and chemotherapy medications. Fistulas can be divided into either internal or external. An internal fistula occurs between an abdominal organ and another organ; conversely, an external fistula occurs from an abdominal organ to the skin surface. The established epithelial tract near unhealthy tissues makes GI fistulas one of the most challenging complications to treat endoscopically [1-3].
The variety of endoscopic approaches and devices, including closure, covering, and drainage methods, is transforming endoscopy as the first-line approach for the treatment of these conditions. Closure and covering endoscopic therapies include the use of clips, cap mounted clips, self-expandable metal stents (SEMSs), tissue sealants, and endoscopic sutures. Draining therapies and techniques include internal endoscopic drainage using double pigtail stents and endoscopic vacuum therapy. However, the literature shows that many sessions are often required, with variable success rates. Owing to the limitations of the current therapeutic approaches, the off-label use of cardiac septal occluders (CSOs) has been reported [4-15].
Dr. Kurt Amplatz, an interventional radiologist, invented the AMPLATZERTM cardiac septal defect occluder for closing atrial septal defects (ASDs). In 1958, he performed one of the first percutaneous catheterizations of the heart. The AMPLATZERTM cardiac septal defect occluder (St. Jude Medical, Plymouth, MN, USA) is a shape-memory, self-expanding double-disc device composed of nitinol and interwoven polyester, which promotes occlusion and tissue in-growth. The thick waist portion serves to self-center the device during deployment to close the defect. The disc diameter varies from 9 mm to 54 mm, and the waist size varies from 4 mm to 38 mm. Although CSOs are intended for percutaneous closure of ASDs or ventricular septal defects (VSDs), they have been used repeatedly in other situations, mainly concerning other cardiovascular defects, such as arteriovenous fistulas, aberrant or dilated vessel occlusion, and aortic pseudoaneurysms [16-18]. Further, there have been reports of CSOs being used to close extravascular defects, including bronchopleural and GI fistulas [7,8,19].
In this review, we describe the role of the off-label use of CSOs in the treatment of GI fistulas.
MATERIAL AND METHODS
This systematic review of the literature was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [20]. The study was registered in the International Prospective Register of Systematic Reviews (www.crd.york.ac.uk/prospero/).
Data sources and searches
We thoroughly searched the MEDLINE, EMBASE, Scopus, Web of Science, Cochrane Library, OVID, CINAHL/EBSCO, and LILACS/Bireme databases and gray literature (references from the selected articles) from inception to August 20, 2018. The search strategy for the MEDLINE database included the use of the following keywords: (fistula OR leak OR defect) AND (cardiac septal defect closure device OR AMPLATZER OR occluder OR closure device OR endoscopic). A similar search strategy was used for the other databases. Literature screening and data extraction were independently performed by three authors (DM, AB and EM). Disagreement regarding final study inclusion was resolved via discussion. If a consensus could not be reached, the senior author (CT) served as the final arbiter.
Study selection
Studies were selected following the PICO method:
- Participants: Randomized clinical trials, observational cohort studies, case series, and case reports studying the use of CSOs in GI fistulas were considered eligible. Conference abstracts were also included if they met the eligibility criteria.
- Intervention: Use of CSOs in patients with GI fistulas, including those who underwent malignant tumor resection. Studies that did not use the AMPLATZERTM cardiac septal defect occluder in the management of GI fistulas, those with non-human subjects, and those where the fistula site involved malignant tissues were excluded.
- Comparison: There was no comparative group in any of the studies included in this systematic review.
- Outcomes: Efficacy and safety of CSOs in the treatment of GI fistulas.
CSOs
CSOs are a shape-memory, self-expanding double-disc closure device. They have a thick waist to accommodate tissues and are composed of nitinol and interwoven polyester, which promotes occlusion and tissue in-growth (Fig. 1). These devices can be easily recaptured and redeployed for optimal placement.
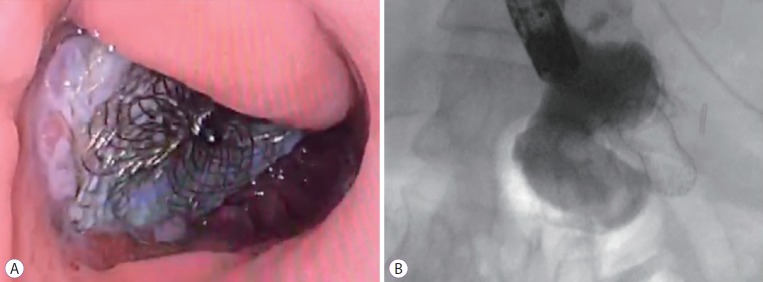
Fig. 1.
(A) Endoscopic image of a cardiac septal occluder (CSO) with tissue in-growth. (B) Fluoroscopic image of a CSO with contrast injection showing occlusion of the fistula.
There are two types of CSOs: ASD closure device and VSD closure device. Both are used for treating GI fistulas, depending on the size. To select the correct device, it is important to understand the characteristics of each, as well as the delivery systems, as summarized in Fig. 2 and Tables 1 and 2.
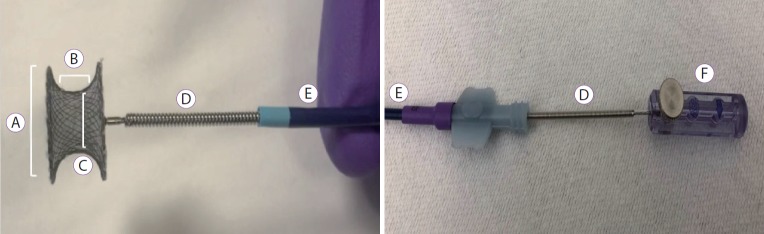
Fig. 2.
Description of the cardiac septal defect occluder and the delivery system. (A) Disc diameter. (B) Waist length. (C) Device size (Waist diameter). (D) Delivery cable. (E) Sheath. (F) Plastic vise.
Table 1.
Description of the Cardiac Septal Defect Occluders
| Characteristics | Atrial septal defet | Ventricular septal defect |
|---|---|---|
| Disc diameter (mm) | Right atrial disc: 12–48 | 9–26 |
| Left atrial disc: 16–54 | ||
| Waist length (mm) | 3–4 | 7 |
| Device size/Waist diameter (mm) | 4–38 | 4–18 |
| Delivery system (Fr) | 6–12 | 5–9 |
Table 2.
Description of the Delivery System of the Cardiac Septal Defect Occluders
| Sheath size | 5–12 Fr |
| Tip angle | 45° and 180° |
| Usable length | 60 cm and 80 cm |
Notably, the CSO delivery system has a maximal length of 80 cm. Therefore, the delivery system cannot be used through the channels of most available endoscopes. The CSO is usually delivered over a guidewire under direct endoscopic visualization with or without fluoroscopic examination. Another technique can also be used. The CSO is separated from the delivery system, allowing the stent to be back-loaded into an adapted endoscopic biliary catheter (7 Fr to 10 Fr) to provide enough length to be deployed through a therapeutic endoscope. This can be performed by placing a pediatric biopsy forceps down a biliary catheter and then grabbing the stent to be deployed and recaptured as needed through the working channel of a therapeutic endoscope.
Outcomes
The main outcomes in this study were the technical success, clinical success, and safety profile of CSOs in GI fistula management.
Data synthesis and analysis
For the qualitative analysis, technical success, clinical success, and adverse events were assessed. The averages and standard deviations were calculated using Microsoft Excel (https://products.office.com/pt-br/excel). To calculate the fistula duration and follow-up period, we transformed years and months into weeks (1 year=12 months; 1 month=4.3 weeks). In this analysis, a fistula duration of <4 weeks was considered acute, and that of >4 weeks was considered chronic.
For the quantitative analysis, the Student’s t-test (for continuous variables) and chi-squared test (for categorical variables) were used to find any association between successful fistula closure and several factors, including age, fistula size, fistula duration, prior treatment, and adjunctive therapy. Additionally, the association between adverse events and these factors was determined. Univariable and multivariable logistic regression analyses were then performed to assess the predictors of successful fistula closure and adverse events. In the univariable analyses, patient age, fistula size, fistula duration, prior treatment, and adjunctive therapy were used as the predictors of successful closure and adverse events. Given the number of cases, two predictors (i.e., prior treatment and fistula duration) were allowed in each multivariable model and were selected as a priori. P-values of <0.05 were deemed statistically significant. Statistical analysis was performed using SAS 9.4 (Cary, NC, USA).
RESULTS
A total of 25,574 records were identified in the initial search. After title/abstract assessment, 29 articles were selected for complete evaluation. After individual review, 19 studies satisfied the inclusion criteria and were finally included in the analysis. To summarize the study selection process, an adapted PRISMA flow diagram was used (Fig. 3) [20].
Fig. 3.
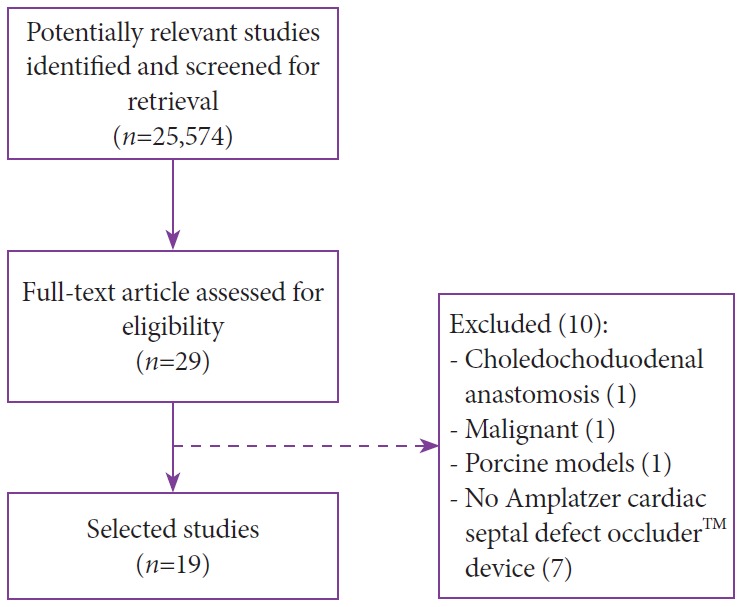
Search strategy. Adapted from Moher et al. [20].
Results of the individual studies
Rabenstein et al. (2006) [21]
A 70-year-old woman with an esophagorespiratory (right pulmonary segment 6) fistula that occurred after swallowing a fishbone (diagnosed 20 years previously) was investigated. Bronchial application of methylene blue into the right segment allowed esophagoscopic identification of the GI portion of the fistula. Cytology brush and fibrin glue injection were performed; however, the fistula recurred after 5 months. At this point, a VSD device was selected. An AMPLATZERTM super-stiff guidewire was placed from the esophagus to the bronchus and was captured via forceps biopsy. Thereafter, the VSD device was deployed over the guidewire and the delivery sheath from the esophagus to the bronchus. After 4 weeks, the symptoms recurred. After 9 weeks, the patient experienced minor hemoptysis. Chest radiography showed that the VSD device has dislocated toward the bronchial system, and the esophageal end was incompletely expanded. Bronchoscopy revealed the site of bleeding to be segment 6; however, the VSD device could not be reached for extraction. The patient recovered under antibiotic therapy. After 6 months, the patient had no more symptoms, and contrast studies revealed complete closure of the fistula. At the 1-year follow-up, the patient reported minor hemoptysis and expectorations, which were treated successfully with antibiotics. New endoscopic and radiologic evaluations showed that the fistula was closed by the completely expanded CSO, although the esophageal umbrella was lying in the mediastinum below the esophageal wall.
Green et al. (2008) [22]
A 69-year-old man who underwent esophagectomy after primary radiation and chemotherapy for adenocarcinoma of the esophagus 14 months earlier was investigated. Computerized tomography (CT) showed a fistula between the right mainstem bronchus and the neo-esophagus. Esophagoscopy could not be performed owing to a stricture at the cervical anastomosis. Bronchoscopy revealed a 6-mm fistula tract with no evidence of recurrent malignancy. The fistula was transverse, confirming a direct communication with the neo-esophagus. Initial treatment with an uncovered SEMS failed. Thereafter, the uncovered SEMS was removed, and a CSO was deployed. After 5 weeks, a repeated bronchoscopy revealed a good position of the CSO and no evidence of any leak at the fistula site.
Boulougouri et al. (2009) [16]
A 57-year-old man underwent right hemicolectomy for small bowel obstruction due to sclerosing mesenteritis. During surgery, an injury to the third duodenal portion occurred, which was repaired using two absorbable sutures. Thereafter, the patient had an upper GI hemorrhage, and two endoscopic clips were placed. After 27 days, a fistulogram revealed findings of a duodenocutaneous fistula. Percutaneous access was required as the endoscope could not reach the third portion of the duodenum, and a CSO was placed. There was significant output through the drain for 8 days, with gradual reduction until complete closure at 30 days. In the 5-month follow-up, no leak was observed.
Melmed et al. (2009) [6]
An 82-year-old woman underwent percutaneous endoscopic gastrostomy (PEG) tube placement. After 1 year, she presented with diarrhea and feculent vomiting. Upper GI series (swallow) revealed a 1.5-cm gastrocolic fistula. Endoscopic repair was attempted for four times, including cauterization of the fistula tract, hemoclip use, new G-tube placement, endoloop + hemoclip technique, and biodegradable plug use. However, none of these techniques successfully closed the fistula. A final attempt using a CSO was performed via passage of a guidewire under endoscopic and fluoroscopic views, followed by CSO placement. At this point, contrast study showed a leak through the proximal disk into the colon, and 3 mL of cyanoacrylate glue was then injected to the proximal disk to provide a watertight seal. A contrast enema confirmed fistula closure. After 4 months, her symptoms recurred, and contrast radiography confirmed that the fistula returned and showed that the device collapsed into the colon. The device was retrieved via colonoscopy, and a similar device, i.e., CardioSEAL septal repair implant (NMT Medical, Boston, MA, USA), was implanted together with hemoclips and fibrin glue to avoid migration. In the 18-month follow-up, the patient had no signs of recurrence and died of cardiopulmonary and renal diseases.
Coppola et al. (2010) [8]
An 83-year-old patient with a benign tracheoesophageal fistula induced by accidental ingestion of dental amalgam was investigated. The patient had unsuccessful endoscopic treatments, including the use of covered self-expandable plastic stent and clips and injection of fibrin glue. Thereafter, a CSO was selected. During endoscopy, a guidewire was placed into the fistula and then recaptured in the hypopharynx; thereafter, a catheter was introduced over the guidewire from the esophagus to the trachea, and the CSO was inserted through the catheter. The distal umbrella was released on the tracheal site and proximally on the esophagus site. After 2 months, the fistula orifice became larger, and the device migrated into the bronchial three; it was then removed from the middle bronchus. After 2 months and two partial covered self-expandable metal stent (CSEMS) placements, the patient became asymptomatic for 10 months.
Kouklakis et al. (2010) [23]
A 58-year-old man underwent distal gastric resection with Billroth II reconstruction for perforated duodenal ulcer 21 years previously. For the past year, the patient complained of fecal-smelling eructation, diarrhea, and weight loss. Barium swallow revealed a fistulous tract between the transverse colon, upper jejunum, and gastric remnant. After malignancy was excluded, the patient refused surgery, and ASD device placement was attempted to occlude both fistulas. The device was introduced via a modified technique using an endoscope to load, guide, and deploy the device. After 1 week, the patient’s condition improved, with cessation of symptoms; further, endoscopy showed that the device was on an adequate site without fecal material in the gastric remnant lumen. However, a small leak was noted on contrast imaging.
Baron (2010) [24]
A 38-year-old woman who underwent Roux-en-Y gastric bypass (RYGB) 8 years previously complained of severe diarrhea and intermittent vomiting since the surgery. Barium swallow revealed a gastrocolonic fistula, with the origin at the gastric pouch. Treatment with endoclips failed. A guidewire and a dedicated sheath were passed across the fistula with endoscopic and fluoroscopic guidance. Thereafter, a VSD device was deployed. At the 6-week follow-up, the patient became asymptomatic.
Repici et al. (2010) [25]
A 58-year-old man underwent distal esophageal resection with gastric pull-up and intrathoracic anastomosis from an adenocarcinoma in the distal esophagus. After 1 month, a tracheoesophageal fistula developed. The three prior endoscopic therapies, including the use of hemoclips and CSEMS and injection of fibrin glue, failed. Surgical repair also failed. After these treatment failures, a CSO was placed. A guidewire was first placed through the esophagus to the trachea and recaptured in the mouth. Thereafter, the CSO was placed under endoscopic and fluoroscopic control. At 8 months, endoscopic and fluoroscopic tests confirmed complete fistula closure, and the CSO was partially covered by re-epithelized mucosa.
Lee et al. (2011) [7]
A 68-year-old man underwent esophagectomy and gastric pull-up surgery. After 18 months, CT revealed a fistula between the left main bronchus and the neo-esophagus. Endoscopic clip placement, fibrin glue injection, and clip with detachable snare placement were attempted; however, the fistula did not close. Finally, an ASD device was placed over a guidewire through the esophagus into the fistula tract. Barium study on day 5 showed complete closure of the fistula. At the 1-month follow-up, endoscopic evaluation confirmed successful closure.
Cardoso et al. (2012) [26]
A 60-year-old man underwent distal esophageal resection with gastric pull-up and intrathoracic anastomosis for esophageal cancer. On the 8th postoperative day, he was noted to develop an intrathoracic collection and a 50% anastomotic dehiscence resulting in a 35-mm diameter defect. A reoperation presented a very high risk, and endoscopic treatment was selected. After suture removal, 50 mL of fibrin glue was injected. Thereafter, a guidewire was placed into the cavity under fluoroscopic and endoscopic visualization, and a CSO was deployed. An fully covered self-expandable metal stent (FCSEMS) was then placed. One day after the procedure, an oral contrast study showed a small leak; thus, a jejunostomy tube was used to feed the patient. After 6 weeks, CT with oral contrast showed no evidence of any leak.
Kadlec et al. (2013) [27]
A 63-year-old woman underwent left pneumonectomy for bronchopulmonary carcinoid. After 3 weeks, CT revealed a 10-mm fistula in the distal esophagus communicating with the left pneumonectomy space. She underwent surgical drainage but refused surgical options to treat the fistula. A nasojejunal feeding tube was inserted to optimize caloric intake. After 3 months, CT confirmed that the fistula size was unchanged. Thereafter, a 12-mm ASD device using the dual approach via the thoracic window was used, and endoscopy was performed. The guidewire was introduced by the flexible endoscope in the thoracic window, followed by the device. The first flange was deployed in the chest and the second flange in the esophagus. After 12 days, the ASD device was found to be dislodged, and the fistula had increased in size. The ASD device was then removed; however, owing to the initiated granulation growth, the defect size decreased to 2 mm. Thereafter, the defect regained its original site with epithelized edges, and the patient had successful surgical repair after 6 months. At the 9-month follow-up, the findings were unremarkable.
Kumbhari et al. (2014) [28]
A 50-year-old woman with a history of sleeve gastrectomy 4 weeks prior to presentation was admitted with a 6×4-mm leak immediately distal to the gastroesophageal junction along the staple line. An over-the-scope clip, FCSEMS, and endoscopic suturing all failed to achieve closure. Therefore, a CSO was selected. A 12-Fr delivery catheter (80 cm in length) was placed over a guidewire into the fistula, and a preloaded CSO was deployed under endoscopic and fluoroscopic visualization. After 1 week, the external drain was removed. At 8 weeks, follow-up endoscopy with contrast injection demonstrated no leak.
Kumbhari et al. (2014) [29]
A 72-year-old woman presented with a chronic 10-mm iatrogenic tracheoesophageal fistula as a complication of tracheal stenting for stenosis due to prolonged intubation. A CSO was deployed across the fistula using a combined bronchoscopic, endoscopic, and fluoroscopic approach. At the 6-week follow-up, a contrast swallow test revealed no fistula.
Wiest et al. (2014) [30]
A 40-year-old man underwent sleeve gastrectomy. On postoperative day 7, a leak was found. He was returned to the operating room where an endoluminal stent was placed, and a re-laparoscopic switch to RYGB was performed. However, the fistula persisted. After 7 months, a 12-mm (7 mm in length) VSD device, which was ~3-mm larger than the leak size, was placed under endoscopic and fluoroscopic guidance. The delivery system was inserted over a guidewire, followed by VSD device deployment. At the 1-year follow-up, the fistula was completely closed, and the device was fully integrated into the tissue.
Odemis et al. (2015) [31]
A 35-year-old man underwent laparoscopic sleeve gastrectomy. On postoperative day 4, the patient was found to have a 15×15-mm defect at the proximal edge of the staple line. After 4 weeks of conservative treatment with a nasogastric tube, the fistula still persisted, and endoscopic treatment was considered. Over a period of 5 days, two attempts at closure with an over-the-scope clip failed, and a VSD device was selected. Under endoscopic and fluoroscopic views, a guidewire was placed through the fistula, and an 18-mm VSD device was delivered. At the 6-month follow-up, the patient remained asymptomatic.
Cohen-Atsmoni et al. (2015) [32]
Two mechanically ventilated patients with tracheoesophageal fistulas due to prolonged intubation were investigated. The first patient had a 4.5-mm fistula. An ASD device number 6 was inserted through the delivery system from the esophagus to the trachea under endoscopic and fluoroscopic visualization. Four years after the procedure, the patient remained stable without any signs of leak. The second patient had an ASD device number 12 introduced through the delivery system transnasally under bronchoscopic and fluoroscopic visualization. Two weeks after the procedure, the symptoms recurred, and bronchoscopy demonstrated dislocation of the ASD device. The patient became critically ill and died a few weeks later due to fungal sepsis.
Subtil et al. (2016) [33]
A 63-year-old man underwent neoadjuvant chemotherapy and esophagectomy for adenocarcinoma of the esophagogastric junction. Postoperatively, he developed two esophagotracheal fistulas, 8 mm in diameter and 1 cm apart. Attempted treatment with two SEMSs, feeding jejunostomy tube, and tracheal stent failed. Thus, fistula closure with an ASD device was pursued. The endoscope was first inserted to the trachea, and access was gained to the esophageal lumen with a guidewire. Thereafter, with the endoscope in the esophagus, the first ASD device was deployed. Three weeks after endoscopy, the ASD device placement procedure was repeated to treat the second fistula. Endoscopic follow-up after 4 months confirmed fistula closure.
Fernandez-Urien et al. (2016) [34]
A 51-year-old man underwent chemotherapy and esophagectomy complicated by a 5-mm esophagobronchial fistula at the esophagogastric anastomosis. Surgery was deemed to present a very high risk, and conventional endoscopic approaches failed. Thus, a CSO was indicated. A guidewire was deployed from the esophagus through the fistula into the airway and was captured. A 5-Fr catheter was introduced via the oral route to release the CSO; the first umbrella was placed in the airway and the second in the esophagus under endoscopic visualization. Four weeks later, the patient developed a second fistula at a separate site, and a second CSO was placed. At the 9-month follow-up, the patient had no complications from CSO placement, but ultimately died from brain metastases.
Mejia Perez et al. (2016) [35]
A 55-year-old man presented with an esophagopleural fistula due to an adverse event of endoscopic balloon dilation for a radiation-induced esophageal stricture. After 9 months of CSEMS treatment, the fistula persisted. The patient underwent an Eloesser flap thoracostomy, and 2 months after surgery, an ASD device was deployed. Under endoscopic visualization, an ASD device attached to the delivery system was passed percutaneously through the fistula into the esophagus. The first umbrella was deployed into the esophagus, while the second umbrella was deployed externally and was sutured to the chest wall to prevent migration. One week after deployment, an esophagram showed a persistent leak; however, 4 weeks after tissue in-growth, closure was confirmed.
Qualitative analysis
All of the 19 studies included in this analysis were case reports, including 22 fistulas in 20 patients; 11 were men (55%), and six were women (30%); the sex of the three remaining patients was not specified. The patients had an average age of 59.3±13.0 years. Thirteen fistulas were esophagus-respiratory fistulas (seven esophagotracheal, three esophagopleural, and three esophagobronchial fistulas); three fistulas occurred after bariatric surgery (two after sleeve gastrectomy and one gastrocolonic fistula after RYGB). There were also two cutaneous (one duodenocutaneous and one gastrocutaneous fistulas), one gastrocolonic, one gastrojejunocolonic, and one gastrotracheal fistulas; one fistula was also found at the site of a gastroesophageal anastomosis. The mean size of the fistulas was 11.42 (SD, 7.98) mm, and the mean fistula duration was 64.54 (SD, 132.43) weeks. Among the 22 fistulas, 16 (72.72%) had failed closure attempted with other endoscopic techniques (Table 3).
Table 3.
Description of the included Studies
| Study | n | Age (yr) | Fistula site | Fistula size/Period | CSO (size) | Access | Failed previous treatment | Adjunctive therapy | Successful closure | Adverse events | Follow-up period |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rabenstein et al. (2006) [21] | 1 | 70 | Esophagopleural | N/A | VSD (6 mm) | Oral/Tracheal | Fibrin glue | No | Yes | Yes; Migration | 1 yr |
| Chronic | |||||||||||
| Green et al. (2008) [22] | 1 | 69 | Bronchoesophageal | 6 mm | N/A | Bronchial | Uncovered SEMS | No | Yes | No | 5 wk |
| 14 mo | |||||||||||
| Boulougouri et al. (2009) [16] | 1 | 57 | Duodenocutaneous (third portion) | Wide | N/A (10 mm; waist) | Percutaneous | Two endoclips | No | Yes | No | 5 mo |
| 27 days | |||||||||||
| Melmed et al. (2009) [6] | 1 | 82 | Gastrocolonic | 15 mm | N/A | Oral | Cauterization, endoclips, endoloop, and biodegradable plug | Cyano-acrylate glue after both procedures | No | Yes; Migration | 18 mo |
| 1 yr after PEG tube placement | |||||||||||
| Coppola et al. (2010) [8] | 1 | 83 | Tracheoesophageal | N/A | N/A | Oral | FCSEMS, endoclips, and fibrin glue | No | No | Yes; Fistula enlargement and migration | 10 mo |
| Chronic | |||||||||||
| Kouklakis et al. (2010) [23] | 1 | 58 | Gastrojejunocolonic | N/A | ASD (9 mm) | Oral | No | No | No | No | 1 wk |
| Baron (2010) [24] | 1 | 38 | Gastrocolonic after RYGB | N/A | VSD | Oral | Endoclips | No | Yes | No | 6 wk |
| 8 yr | |||||||||||
| Repici et al. (2010) [25] | 1 | 58 | Esophagotracheal | N/A | N/A | Oral | Endoclips, FCSEMS, fibrin glue, and surgery | No | Yes | No | 8 mo |
| Chronic | |||||||||||
| Lee et al. (2011) [7] | 1 | 68 | Gastrotracheal | N/A | ASD | Oral | Endoclips and fibrin glue | No | Yes | No | 1 mo |
| Chronic | |||||||||||
| Cardoso et al. (2012) [26] | 1 | 60 | Gastroesophageal anastomosis | 35 mm | N/A | Oral | No | fibrin glue + PCSEMS | Yes | No | 6 wk |
| 8 days | |||||||||||
| Kadlec et al. (2013) [27] | 1 | 63 | Esophagopleural | 10 mm | ASD (12 mm) | Thoracic/Oral | Nasoenteral tube | No | No | Yes; Fistula enlargement | 9 mo |
| 3 mo | |||||||||||
| Kumbhari et al. (2014) [28] | 1 | 50 | Gastric (after sleeve gastrectomy) | 6 mm | N/A | Oral | OTSC, FCSEMS, and | No | Yes | No | 8 wk |
| 4 weeks after sleeve gastrectomy | |||||||||||
| Kumbhari et al. (2014) [29] | 1 | 72 | Tracheoesophageal | 10 mm | N/A | Oral | No | No | Yes | No | 6 wk |
| Chronic | |||||||||||
| Wiest et al. (2014) [30] | 1 | 40 | Gastric (after sleeve gastrectomy) | 9 mm | VSD (12 mm) | Oral | FCSEMS, surgery to switch to gastric bypass | No | Yes | No | 1 yr |
| 7 mo | |||||||||||
| Odemis et al. (2015) [31] | 1 | 35 | Gastrocutaneous | 15 mm | VSD (18 mm) | Oral | Nasogastric tube and OTSC | No | Yes | No | 6 mo |
| 4 weeks | |||||||||||
| Cohen-Atsmoni et al. (2015) [32] | 2 | N/A | Tracheoesophageal | 1) 4.5 mm N/A | 1) ASD (6 mm waist) | 1) Oral | 1) No | 1) No | 1) Yes | 1) No | 1) 4 yr |
| 2) N/A N/A | 2) ASD (12 mm waist) | 2) Bronchial | 2) No | 2) No | 2) No | 2) Yes; Migration | 2) More than 2 wk | ||||
| Subtil et al. (2016) [33] | 2 | 63 | Tracheoesophageal | 1) 8 mm Chronic | 1) ASD (8 mm) | Oral/Tracheal | Two FCSEMSs, nasogastric tube, and tracheal prosthesis | No | 1) Yes | 1) No | 4 mo |
| 2) 1 cm Chronic | 2) ASD (13 mm) | 2) Yes | 2) No | ||||||||
| Fernandez-Urien et al. (2016) [34] | 2 | 51 | Esophagobronchial | 1) 5 mm Chronic | 1) N/A | Tracheal/Oral | 1) Traditional endoscopic approaches | 1) No | 1) Yes | 1) No | 9 mo |
| 2) N/A Chronic | 2) N/A | 2) No | 2) No | 2) Yes | 2) No | ||||||
| Mejia Perez el al. (2016) [35] | 1 | 55 | Esophagopleural | 5 mm Chronic | ASD | Percutaneous (chest wall) | FCSEMS | Fixation with suture to the chest | Yes | No | 4 wk |
ASD, atrial septal defect; CSO, cardiac septal occluder; FCSEMS, fully covered self-expandable metal stent; N/A, not available; OTSC, over-the-scope clip; PCSEM, partially covered self-expandable metal stent; PEG, percutaneous endoscopic gastrostomy; RYGB, Roux-en-Y gastric bypass; SEMS, self-explandable metal stent; VSD, ventricular septal defect.
Technical success was achieved in all cases (100%). Of the 22 fistulas, 17 had successful closure (77.27%). In four cases (18.18%), adjunctive therapy was used with a CSO. Three of these cases (75%) achieved success closure, and one had failed closure. Adverse events occurred in five cases (22.72%), including three migration (13.63%), one fistula enlargement (4.54%), and one migration owing to fistula enlargement (4.54%). No death was related to the use of CSOs. The mean follow-up period was 33.02 (SD, 44.09) weeks.
Regression analysis
There were no correlations of patient age, fistula size, fistula duration, prior treatment, and adjunctive therapy with successful fistula closure and adverse events in the univariable regression analysis (Tables 4, 5).
Table 4.
Possible Predictors of Successful Fistula Closure
| Variables | p-value (p<0.05) |
|---|---|
| Age | 0.71 |
| Fistula size | 0.93 |
| Fistula duration (acute/chronic) | 0.36 |
| Fistula duration (continuous) | 0.35 |
| Prior treatment | 0.47 |
| Adjunctive therapy | 0.90 |
Table 5.
Possible Predictors of Adverse Events Following Cardiac Septal Occluder Placement
| Variables | p-value (p<0.05) |
|---|---|
| Age | 0.12 |
| Fistula size | 0.88 |
| Fistula duration (acute/chronic) | 0.36 |
| Fistula duration (continuous) | 0.35 |
| Prior treatment | 0.75 |
| Adjunctive therapy | 0.75 |
Multivariable regression analyses were also performed, and no significant predictors of fistula closure or adverse events were found.
DISCUSSION
A GI fistula is defined as an abnormal communication between two epithelized surfaces. The most common causes include chronic inflammation, malignancy, and untreated long-term leaks [1-3].
Endoscopic closure of a GI fistula represents a major advancement in the treatment of patients. An appropriate endoscopic approach to fistula closure includes several basic principles. Undrained cavities and collections of fluid must be initially drained radiologically, surgically, or endoscopically. In many cases, endoscopic therapy can be used to interrupt or drain the flow of luminal contents through a GI defect. Several features must be considered to optimize outcomes, including size of the defect, shape of the margin, viability of the surrounding tissue, and location of the wall defect. After this, the best endoscopic therapy for the patient can be selected, which involves either closure, covering, or draining techniques [36-38]. However, the literature shows variable success rates of these techniques including several failures, showing that other devices are necessary.
The off-label use of CSOs has been reported, mainly after conventional endoscopic techniques fail, with satisfactory efficacy and safety. The properties of CSOs and early results suggest they could be useful in treating fistulas that are otherwise difficult to manage using available endoscopic techniques. The nitinol structure with interwoven polyester liner is available in multiple waist and disk sizes and is thought to promote tissue in-growth while sealing the fistula tract. These features may allow the device to manage fistulas with irregular margins and epithelized tracts and those in edematous or scarred tissues, which are less amenable to clipping, suturing, or stenting.
In this review, technical success was achieved in all cases (100%), proving that it is a feasible procedure. Of the 22 fistulas, 17 had successful closure (77.27%), with a mean follow-up period of 32.02 weeks (approximately 8 months). When used in conjunction with an adjunctive therapy, the CSO success rate was 75%, showing no additional advantage. The mean fistula duration prior to attempted closure with CSOs was 64.54 weeks; 16 of the 22 fistulas (72.72%) had failed closure attempted with other endoscopic techniques, showing that CSOs are mostly used in chronic, challenging cases.
Univariable and multivariable regression analyses for assessing the success of fistula closure and adverse events in relation to several variables, including patient age, size, fistula duration prior to CSO placement, prior therapies, and adjunctive therapy, were performed. These analyses did not reveal any significant factor related to successful fistula closure or adverse events, which may be attributed to the scarcity of data in the literature.
We considered five cases to have failed closures (22.72%) [6,8,23,27,32]; however, in two of these cases, the authors considered the results to be successful [6,23]. Melmed et al. [6] used the AMPLATZERTM CSO in a gastrocolonic fistula 1 year after PEG tube placement and found immediate success; however, after 4 months, the device migrated, and the fistula recurred. After migration, a similar device (CardioSEAL septal repair implant [NMT Medical]) was used, and successful closure was achieved. Kouklakis et al. [23] described the use of a CSO (ASD device) in a complex gastrojejunocolonic fistula with only 1 week of follow-up and reported clinical success; however, imaging showed that the fistula did not close completely. The three other failures were reported in esophagus-respiratory fistulas and were related to fistula enlargement and CSO migration [8,27,32]. Of these cases, one was treated with surgical repair27 and one with partially covered SEMSs [8], and the third patient died of fungal sepsis [32].
After selecting 28 studies for complete evaluation, we included 19 reports and excluded 10 reports because they did not meet the inclusion criteria. Ell et al. [39] reported the successful use of an ASD device in choledochoduodenostomy for sump syndrome 35 years ago. Perretta et al. [40] demonstrated the efficacy of CSOs in the closure of gastrostomy defects in porcine models, showing a 100% success closure rate, without any adverse events. Malespin et al. [41] used an ASD device to close a malignant gastrocolonic fistula with adjunctive therapy and duodenal stenting and reported successful closure after 6 weeks, suggesting that success was achieved because of the ASD device. The other seven studies were excluded because they did not use ASD or VSD AMPLATZERTM occluder devices [42-48]. Li et al. [42] conducted a case series on six patients with esophageal-respiratory fistulas (four benign and two malignant fistulas), used similar CSOs and a vessel plug (Lifetech Scientific Co., China), and presented a 100% immediate successful closure rate; however, four fistulas recanalized (two due to malignance). Two other studies [44,48] also used a similar ASD device (Gore, Flagstaff, AZ, USA) in patients with tracheoesophageal fistulas with successful closure. The AMPLATZERTM vascular plug is indicated to embolize the vessels; however, similar with other septal occluders, it has been used for closure of GI fistula defects [43,45-47]. Three case reports [43,45,46], including three patients, reported the efficacy of the AMPLATZERTM vascular plug for closure of trachea-respiratory fistulas with a 100% successful closure rate. However, another case report demonstrated its failure in rectovaginal fistulas [47]. More studies should be performed using these devices to assess their efficacy and safety better.
The major limitation of our study is that the evidence is limited, and the literature consists of only case reports. Thus, publication bias is a concern, given the fact that most authors publish favorable case reports and not individual cases where treatment failed. As such, the quality of the literature did not allow us to perform a meta-analysis. Therefore, a systematic review with a pooled analysis was conducted to assess the combined outcomes of these case reports. Additionally, despite combining these case reports, this analysis was still likely underpowered owing to the small number of cases, which may explain the statistically insignificant findings in the subgroup and regression analyses.
In summary, CSOs are thought to promote fistula closure by occluding the fistula tract and stimulating tissue in-growth. This analysis found CSOs to have a 100% technical success rate, 77.27% clinical success rate, and 22.72% adverse event rate, with no death related to their off-label use. The satisfactory efficacy and safety results derived from this sparse literature suggest that CSOs can be an option in the management of GI fistulas. However, prospective studies are necessary to clarify their indications better before they can be considered a first-line therapy.
Footnotes
Conflicts of Interest: Christopher Thompson received grants and personal fees from Apollo Endosurgery, USGI Medical, and Boston Scientific and grants, personal fees, and non-financial support from Olympus. The other authors have no financial conflicts of interest.
REFERENCES
- 1.Caulfield H, Hyman NH. Anastomotic leak after low anterior resection: a spectrum of clinical entities. JAMA Surg. 2013;148:177–182. doi: 10.1001/jamasurgery.2013.413. [DOI] [PubMed] [Google Scholar]
- 2.de Moura DTH, Sachdev AH, Thompson CC. Endoscopic full-thickness defects and closure techniques. Curr Treat Options Gastroenterol. 2018;16:386–405. doi: 10.1007/s11938-018-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640–1655. doi: 10.1038/ajg.2017.241. [DOI] [PubMed] [Google Scholar]
- 4.Haito-Chavez Y, Kumbhari V, Ngamruengphong S, et al. Septotomy: an adjunct endoscopic treatment for post-sleeve gastrectomy fistulas. Gastrointest Endosc. 2016;83:456–457. doi: 10.1016/j.gie.2015.08.065. [DOI] [PubMed] [Google Scholar]
- 5.Haito-Chavez Y, Law JK, Kratt T, et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video) Gastrointest Endosc. 2014;80:610–622. doi: 10.1016/j.gie.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 6.Melmed GY, Kar S, Geft I, Lo SK. A new method for endoscopic closure of gastrocolonic fistula: novel application of a cardiac septal defect closure device (with video) Gastrointest Endosc. 2009;70:542–545. doi: 10.1016/j.gie.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Lee HJ, Jung ES, Park MS, et al. Closure of a gastrotracheal fistula using a cardiac septal occluder device. Endoscopy. 2011;43 Suppl 2 UCTN:E53–E54. doi: 10.1055/s-0030-1256058. [DOI] [PubMed] [Google Scholar]
- 8.Coppola F, Boccuzzi G, Rossi G, Gaia S, Cosimato M, Recchia S. Cardiac septal umbrella for closure of a tracheoesophageal fistula. Endoscopy. 2010;42 Suppl 2:E318–E319. doi: 10.1055/s-0030-1255822. [DOI] [PubMed] [Google Scholar]
- 9.Kantsevoy SV, Bitner M, Hajiyeva G, et al. Endoscopic management of colonic perforations: clips versus suturing closure (with videos) Gastrointest Endosc. 2016;84:487–493. doi: 10.1016/j.gie.2015.08.074. [DOI] [PubMed] [Google Scholar]
- 10.Kantsevoy SV, Bitner M, Davis JM, Hajiyeva G, Thuluvath PJ, Gushchin V. Endoscopic suturing closure of large iatrogenic colonic perforation. Gastrointest Endosc. 2015;82:754–755. doi: 10.1016/j.gie.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Lippert E, Klebl FH, Schweller F, et al. Fibrin glue in the endoscopic treatment of fistulae and anastomotic leakages of the gastrointestinal tract. Int J Colorectal Dis. 2011;26:303–311. doi: 10.1007/s00384-010-1104-5. [DOI] [PubMed] [Google Scholar]
- 12.Mukewar S, Kumar N, Catalano M, et al. Safety and efficacy of fistula closure by endoscopic suturing: a multi-center study. Endoscopy. 2016;48:1023–1028. doi: 10.1055/s-0042-114036. [DOI] [PubMed] [Google Scholar]
- 13.de Moura DTH, Brunaldi VO, Minata M, Riccioppo D, Santo MA, de Moura EGH. Endoscopic vacuum therapy for a large esophageal perforation after bariatric stent placement. VideoGIE. 2018;3:346–348. doi: 10.1016/j.vgie.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Moura EG, Silva GL, de Moura ET, et al. Esophageal perforation after epicardial ablation: an endoscopic approach. Endoscopy. 2015;47 Suppl 1 UCTN:E592–E593. doi: 10.1055/s-0034-1393594. [DOI] [PubMed] [Google Scholar]
- 15.Okazaki O, Bernardo WM, Brunaldi VO, et al. Efficacy and safety of stents in the treatment of fistula after bariatric surgery: a systematic review and meta-analysis. Obes Surg. 2018;28:1788–1796. doi: 10.1007/s11695-018-3236-6. [DOI] [PubMed] [Google Scholar]
- 16.Boulougouri K, Theodoropoulos E, Karydas G, Tachtaras E, Hatzinikolaou A, Georgountzos V. Combined endoscopic and percutaneous treatment of a duodenocutaneous fistula using an Amplatzer septal occluder. Cardiovasc Intervent Radiol. 2009;32:356–360. doi: 10.1007/s00270-008-9433-2. [DOI] [PubMed] [Google Scholar]
- 17.Uthaman B, Al-Qbandi M, Abushaban L, Rathinasamy J. Transcatheter closure of large pulmonary arteriovenous fistula including pulmonary artery to left atrial fistula with Amplatzer septal occluder. Catheter Cardiovasc Interv. 2007;70:422–428. doi: 10.1002/ccd.21163. [DOI] [PubMed] [Google Scholar]
- 18.Jolly N, Garg RK, Raman J, Hijazi ZM. Amplatzer septal occluder device for closure of aortic pseudoaneurysms. Catheter Cardiovasc Interv. 2007;70:619–620. doi: 10.1002/ccd.21188. author reply 621. [DOI] [PubMed] [Google Scholar]
- 19.Gómez López A, García Luján R, De Pablo Gafas A, et al. First use of Amplatzer device for bronchopleural fistula after lung transplantation. Thorax. 2017;72:668–670. doi: 10.1136/thoraxjnl-2016-209543. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 21.Rabenstein T, Boosfeld C, Henrich R, Ell C. First use of ventricular septal defect occlusion device for endoscopic closure of an esophagorespiratory fistula using bronchoscopy and esophagoscopy. Chest. 2006;130:906–909. doi: 10.1378/chest.130.3.906. [DOI] [PubMed] [Google Scholar]
- 22.Green DA, Moskowitz WB, Shepherd RW. Closure of a broncho-neo-esophageal fistula using an amplatzer® septal occluder device. Chest. 2008;134(4 Suppl 2):23C. doi: 10.1016/j.athoracsur.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 23.Kouklakis G, Zezos P, Liratzopoulos N, et al. Billroth II gastrectomy complicated by gastrojejunocolonic fistulas, treated endoscopically with a cardiac septal defect closure device. Endoscopy. 2010;42 Suppl 2:E134–E135. doi: 10.1055/s-0029-1244058. [DOI] [PubMed] [Google Scholar]
- 24.Baron TH. Endoscopic closure of a gastrocolonic fistula using a cardiac ventricular septal defect occlusion device. Gastrointest Endosc. 2010;71:AB104. [Google Scholar]
- 25.Repici A, Presbitero P, Carlino A, et al. First human case of esophagus-tracheal fistula closure by using a cardiac septal occluder (with video) Gastrointest Endosc. 2010;71:867–869. doi: 10.1016/j.gie.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 26.Cardoso E, Silva RA, Moreira-Dias L. Use of cardiac septal occluder device on upper GI anastomotic dehiscences: a new endoscopic approach (with video) Gastrointest Endosc. 2012;76:1255–1258. doi: 10.1016/j.gie.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 27.Kadlec J, Turner K, Van Leuven M. Attempted closure of a post-pneumonectomy oesophagopleural fistula with an Amplatzer atrial septal occluder. Interact Cardiovasc Thorac Surg. 2013;16:538–540. doi: 10.1093/icvts/ivs530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumbhari V, Storm AC, Saxena P, Okolo PI., 3rd Closure of a persistent gastric leak using a cardiac septal occluder. Endoscopy. 2014;46 Suppl 1 UCTN:E147–E148. doi: 10.1055/s-0034-1364948. [DOI] [PubMed] [Google Scholar]
- 29.Kumbhari V, Azola A, Okolo PI, 3rd, et al. Closure of a chronic tracheoesophageal fistula by use of a cardiac septal occluder. Gastrointest Endosc. 2014;80:332. doi: 10.1016/j.gie.2014.05.335. [DOI] [PubMed] [Google Scholar]
- 30.Wiest R, Tutuian R, Meier B, Nett P. Use of a cardiac occluder for closure of a complex gastric leak after bariatric surgery. Endoscopy. 2014;46 Suppl 1 UCTN:E487–E488. doi: 10.1055/s-0034-1377591. [DOI] [PubMed] [Google Scholar]
- 31.Odemis B, Beyazit Y, Torun S, Kayacetin E. Endoscopic closure of gastrocutaneous fistula with an AMPLATZERTM septal occluder device. Therap Adv Gastroenterol. 2015;8:239–242. doi: 10.1177/1756283X15578609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen-Atsmoni S, Tamir A, Avni Y, Priel IE, Roth Y. Endoscopic occlusion of tracheoesophageal fistula in ventilated patients using an Amplatzer septal occluder. Indian J Otolaryngol Head Neck Surg. 2015;67:196–199. doi: 10.1007/s12070-015-0842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subtil JC, Valenti V, Cienfuegos JA, Calabuig J, Hernández-Lizoain JL, Muñoz-Navas M. Successful endoscopic closure of multiple tracheoesophageal fistulas following implantation of two atrial septal defect occluders. Endoscopy. 2016;48:E346–E347. doi: 10.1055/s-0042-116432. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Urien I, Lezaun R, Hernández M, Lainez B, Leitão C, Vila J. Esophagobronchial fistula closed by a cardiac septal occluder device. Endoscopy. 2016;48 Suppl 1:E289–E290. doi: 10.1055/s-0042-112974. [DOI] [PubMed] [Google Scholar]
- 35.Mejia Perez LK, Confer B, Veniero J, Raymond D, Bhatt A. Closure of a persistent esophagopleural fistula by use of an atrial septal occluder device. VideoGIE. 2016;1:27–28. doi: 10.1016/j.vgie.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willingham FF, Buscaglia JM. Endoscopic management of gastrointestinal leaks and fistulae. Clin Gastroenterol Hepatol. 2015;13:1714–1721. doi: 10.1016/j.cgh.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Merrifield BF, Lautz D, Thompson CC. Endoscopic repair of gastric leaks after Roux-en-Y gastric bypass: a less invasive approach. Gastrointest Endosc. 2006;63:710–714. doi: 10.1016/j.gie.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Ribeiro IB, Bernardo WM, Martins BDC, et al. Colonic stent versus emergency surgery as treatment of malignant colonic obstruction in the palliative setting: a systematic review and meta-analysis. Endosc Int Open. 2018;6:E558–E567. doi: 10.1055/a-0591-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ell C, Boosfeld C, Henrich R, Rabenstein T. Endoscopic treatment of the “sump syndrome” after choledochoduodenostomy: a new technique using an amplatzer septal occluder. Z Gastroenterol. 2006;44:1231–1235. doi: 10.1055/s-2006-927168. [DOI] [PubMed] [Google Scholar]
- 40.Perretta S, Sereno S, Forgione A, et al. A new method to close the gastrotomy by using a cardiac septal occluder: long-term survival study in a porcine model. Gastrointest Endosc. 2007;66:809–813. doi: 10.1016/j.gie.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 41.Malespin M, Gaspar JP, Boulay B. Palliation of a malignant gastrocolic fistula with the use of an atrial septal defect occlusion device. Endoscopy. 2014;46 Suppl 1 UCTN:E4. doi: 10.1055/s-0033-1358931. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Gao X, Chen J, Lao M, Wang S, Zeng G. Endoscopic closure of acquired oesophagorespiratory fistulas with cardiac septal defect occluders or vascular plugs. Respir Med. 2015;109:1069–1078. doi: 10.1016/j.rmed.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Sun M, Pan R, Kong X, Cao D. Successful closure of postoperative esophagobronchial fistula with amplatzer vascular plug. Ann Thorac Surg. 2015;99:1453. doi: 10.1016/j.athoracsur.2014.11.070. [DOI] [PubMed] [Google Scholar]
- 44.Vivacqua A, Malankar D, Idrees JJ, Rice TW, Raymond DP, Roselli EE. Endoscopic repair of recurrent tracheoesophageal fistula with an atrial septal occluder device. Ann Thorac Surg. 2016;102:e485–e487. doi: 10.1016/j.athoracsur.2016.04.090. [DOI] [PubMed] [Google Scholar]
- 45.Young JA, Shimi SM, Alijani A, Patil PV, Bhat R. Occlusion of a neo-esophageal-bronchial fistula using the Amplatzer vascular plug 2. Diagn Interv Radiol. 2013;19:259–262. doi: 10.5152/dir.2013.026. [DOI] [PubMed] [Google Scholar]
- 46.Koo JH, Park KB, Choo SW, Kim K, Do YS. Embolization of postsurgical esophagopleural fistula with AMPLATZER vascular plug, coils, and Histoacryl glue. J Vasc Interv Radiol. 2010;21:1905–1910. doi: 10.1016/j.jvir.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Kılıçkesmez Ö, Andıç C, Oğuzkurt L. Delayed failure of rectovaginal fistula embolization with Amplatzer vascular plug 2. Diagn Interv Radiol. 2014;20:511–512. doi: 10.5152/dir.2013.14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodrigues AJ, Scordamaglio PR, Tedde ML, Minamoto H, de Moura EG, Pedra CA. Bronchoscopic closure of tracheoesophageal fistulas. Gastrointest Endosc. 2011;74:1173. doi: 10.1016/j.gie.2011.07.001. [DOI] [PubMed] [Google Scholar]