Abstract
Following partial hepatectomy (PH), the complex process of liver regeneration is initiated, which encompasses the synchronized induction of hepatocyte proliferation. Hepatocyte proliferation can be regulated by multiple stimuli, including long non-coding RNAs (lncRNAs) and Wnt/β-catenin signaling, although the underlying mechanism of lncRNA/Wnt in liver regeneration remains unclear. In the present study, a liver regeneration-associated functional lncRNA was identified, and its function was delineated in vitro and in vivo; lncRNA small nucleolar RNA host gene 12 (SNHG12) was revealed to be upregulated at various time-points after 2/3 PH. The expression of SNHG12 was also increased in normal liver cell lines treated with different concentrations of hepatocyte growth factor (HGF). Functionally, SNHG12 enhanced hepatocyte proliferation in vitro and in vivo, and the liver/body weight ratio of SNHG12-overexpressing mice was significantly higher than that of the control mice. Overexpression of SNHG12 promoted the activation of Wnt/β-catenin signaling in hepatocytes. Furthermore, specific inhibition of Wnt/β-catenin signaling significantly attenuated SNHG12-induced hepatocyte proliferation and the affected liver/body weight ratio. Collectively, the results of the present study indicated that SNHG12 contributes to liver regeneration by activating Wnt/β-catenin signaling. Therefore, drugs that regulate the SNHG12/Wnt axis may be beneficial for liver regeneration following PH.
Keywords: long non-coding RNA, small nucleolar RNA host gene 12, Wnt/β-catenin, liver regeneration, partial hepatectomy
Introduction
Liver regeneration occurs after events that could lead to the loss of liver mass, including surgery, trauma, infection and liver transplantation (1). During liver regeneration, and following hepatectomy, liver cell proliferation and apoptosis occur, and liver size is largely controlled by liver cell proliferation (2,3). Subsequent hepatocyte stimulation is triggered by multiple feedback signals, allowing hepatocytes to enter a rapid growing phase. The clinical implications of liver regeneration are extremely valuable, as it enables recovery from the symptoms of surgical damage, such as reduced liver size and dysfunction; conversely, poor liver regeneration is likely to result in patient fatality (4). Thus, the explicit functional mechanism of liver regeneration is required in order to amplify its full clinical potential following surgery. Previous studies have highlighted a variety of regulatory factors for the hepatocyte cell cycle and the appropriate progression of liver regeneration, including the release of inflammatory cytokines (5), hepatocyte growth factor (HGF) (6) and metabolic regulatory factors, and cell cycle regulation. It has also been demonstrated that certain types of non-coding RNAs such as long non-coding RNAs (lncRNAs) and micro (mi)RNAs, play important roles in liver regeneration (7,8), though the precise molecular mechanisms of the associated pathways remain unclear.
lncRNAs are non-coding transcripts (>200 nucleotides) which have been widely demonstrated as vital regulators of numerous cellular responses, developmental processes and disease (9). Emerging evidence has shown that lncRNAs may be functionally significant in the progression of liver regeneration (10,11). For instance, lncRNA Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) was recently reported to promote hepatocyte proliferation by stimulating the Wnt/β-catenin signaling pathway (1).
Small nucleolar RNA host gene 12 (SNHG12) is a newly identified lncRNA that is associated with various malignancies, and is considered to be a useful tumor biomarker (12). It was recently demonstrated that SNHG12 promoted cellular proliferation and metastasis, which regulated the Wnt/β-catenin signaling pathway in papillary thyroid carcinoma cells; the same interplay was detected in an in vivo disease model (13). In addition, other studies have revealed that SNHG12 plays a regulatory role in glioma (14), osteosarcoma (15), and gastric carcinoma (16), by acting as a sponge for associated micro (mi)RNAs. However, the precise mechanism by which SNHG12 regulates hepatocyte proliferation in liver regeneration is yet to be elucidated. The aim of the present study was to investigate the underlying mechanism of SNHG12-mediated hepatocyte proliferation during liver regeneration.
Materials and methods
Animals and 2/3 partial hepatectomy (PH) model
Prior to animal experimentation, the present study was approved by the Institutional Animal Ethics Committee of the Second Military Medical University. Male BALB/c mice (6–8 weeks of age) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. The mice were maintained in sterile conditions with free access to food and water, under a 12-h light/dark cycle. 2/3 PH was performed according to previously described methods (17), where the left and median liver lobes were removed under isoflurane anesthesia (2% v/v). Euthanasia was carried out by CO2 inhalation for 5 min. Humane endpoints included weight loss of >20%, dehydration, severe lameness, ruffled fur, and hunched appearance. None of mice exhibited the humane endpoint criteria. The liver tissues were collected and the liver/body weight ratio was calculated on days 0, 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 after 2/3 PH.
Mouse primary hepatocytes isolation
Mouse primary hepatocytes were isolated from the livers of 2/3 PH model mice and control mice. Liver tissues were fragmented using ophthalmic scissors, and the resulting tissue pieces were washed with PBS containing with 100 U/ml of penicillin and 100 mg/ml of streptomycin. The liver tissues were then digested (0.25% trypsin; 0.04% EDTA), filtrated through a 4 µm cell strainer and centrifuged for 5 min at 350 × g at 4°C. Then, mouse primary hepatocytes (5–6×105/ml) were suspended and cultured in high glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin at 37°C in a humidified atmosphere of 5% CO2, with medium replaced every 2 days. Cells were subcultured for 3–4 days until confluence reached to 70–80%. Mouse primary hepatocytes were treated with different concentrations of HGF (5, 10, 20, 30, and 40 ng/µl).
Cell culture and transfection
The NCTC 1469 and BNL CL.2 mouse hepatocyte cell lines were purchased from the American Type Culture Collection, and cultured in DMEM containing 10% fetal bovine serum at 37°C with (5% CO2). NCTC 1469 cells were treated with HGF (20 ng/µl) or the Wnt inhibitor IWR-1 (60 µM) at 37°C for 24 h. Recombinant lentivirus (Lv)-SNHG12 and the scrambled control (Lv-NC) were constructed by Shanghai GeneChem Co., Ltd. The cells were transfected using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) by exposure to diluted viral supernatant containing polybrene for 48 h, as previously described (18). SNHG12 small interfering (si)RNA was purchased from ZHBY Biotech Co. Ltd. and scramble siRNA was used as the control.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA from tissues and cells was extracted using a TRIzol® kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Reverse transcription (RT) was carried out using a PrimeScript RT reagent Kit (Takara Bio, Inc., Tokyo, Japan). The RT system of 10 µl was carried out according to the manufacturer's instructions. The RT conditions were 37°C for 15 min and 85°C for 5 sec. mRNA and lncRNA expression levels were determined using the SYBR Green Supermix (Invitrogen; Thermo Fisher Scientific, Inc.) on an Applied Biosystems 7300 real-time PCR system. Thermocycling conditions were 95°C for 10 min, following by 35 cycles at 95°C for 10 sec, 58°C for 15 sec and 72°C for 20 sec, and a final 72°C for 20 min. β-actin was used as the internal control. All qPCR experiments were performed at ≥3 times, and the primer sequences were as follows: SNHG12 forward, 5′-CATCAAGACTGAGAAAAAGCACACC-3′ and reverse, 5′-TACCTTAAAGCACAGCTCCAGAAAC-3′; Axin2 forward, 5′-GATGTTGGAGAGTGAGCGGCAG-3′ and reverse, 5′-TGTTGGGTGGGGTAAGGGGAG-3′; β-actin forward, 5′-CAACTGGGACGACATGGAG-3′ and reverse, 5′-TAGCACAGCCTGGATAGCAAC-3′.
Western blot analysis
The total protein from liver tissues and hepatocytes was extracted and homogenized in RIPA buffer (Beijing Solarbio Science & Technology Co., Ltd.). The concentrations of the extracted nuclear and cytoplasmic fractions were quantified using the bicinchoninic acid assay method. A total of 50 µg protein per sample was separated by SDS-PAGE (10%) and then transferred to a PVDF membrane, prior to blocking with 5% non-fat milk in 1X TBST, overnight at 4°C. The membrane was then incubated with anti-β-catenin (1:1,000; product no. ab32572), anti-β-actin (1:5,000; product no. ab8227), anti-histone 3 (1:2,000; product no. ab62642; all from Abcam) primary antibodies for 2 h at room temperature. After washing three times in 1X TBST, the membranes were incubated with the corresponding HRP-conjugated secondary antibody (1:5,000; cat. no. A6154-1 ml; Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. The membranes were washed once more, exposed to ECL (Sangon Biotech Co., Ltd.), and then photographed by ChemiDoc™ XRS+ Imaging system (Bio-Rad Laboratories, Inc.). The protein expression levels were quantified using ImageJ software version 1.46 (National Institutes of Health). Histone 3 and β-actin were used as internal controls.
Cell proliferation assay
Cell Counting Kit (CCK)-8 reagent (Biotech Well) was used to determine the level of cellular proliferation, per the manufacturer's protocol. Briefly, NCTC 1469 or BNL CL.2 cells were seeded into 96-well plates at a density of 3×105/ml, and transfected with the aforementioned constructs for 48 h. CCK-8 reagent was then added to each well and the cells were incubated for ~30 min at 37°C. The absorbance was measured by a microplate reader (Bio-Rad Laboratories, Inc.) at a wavelength of 450 nm. The number of cells was calculated in reference to a standard curve obtained under the same conditions.
NCTC 1469 or BNL CL.2 cells were seeded at 3×103 cells/well in 96-well plates. After 2 days of culture, proliferation was assessed using Cell Proliferation ELISA, BrdU (colorimetric) kit (product no. 11647229001; Roche), per the manufacturer's protocol. Briefly, BrdU-labeling solution (100 µM) was added into each well and incubated for 4 h at 37°C. The absorbance was measured at 450 nm by a microplate reader (Infinite 200; Tecan Group).
Immunohistochemistry (IHC)
The liver specimens were fixed using formalin (10%) and embedded in paraffin. Serial sections of 5-µm thickness were sliced from the paraffin embedded blocks, and used for subsequent experiments. The sections were incubated overnight at 4°C with an anti-proliferating cell nuclear antigen (PCNA) primary antibody (1:500; product no. ab29; Abcam), then washed thrice prior to subsequent incubation with an HRP-conjugated goat anti-rabbit antibody (1:200; product no. 5571; Cell Signaling Technology, Inc.). The sections were then stained with DAB (Sangon Biotech Co., Ltd.) for 5 min at 37°C to observe the expression of PCNA.
Luciferase reporter assay
NCTC 1469 or BNL CL.2 cells (5×104/well) were seeded into 24-well plates. TOP-FLASH or FOP-FLASH constructs and Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) were used to transfect the cells and luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega Corporation) as previously described (19). Luciferase activity was normalized to that in cells transfected with TOPFlash/FOPFlash.
Statistical analysis
Statistical analysis was conducted using SPSS software version 16 (SPSS, Inc.). All data are presented as the mean ± SD (standard deviation) from three or more separate experiments. Statistical significances between two groups were analyzed using one-way ANOVA followed by the Scheffé test (Figs. 2C-F, 3 and 5), or two-tailed Student's t-test (Figs. 1, 2A and B, 4 and Fig. S1). P<0.05 was considered to indicate a statistically significant difference.
Figure 2.
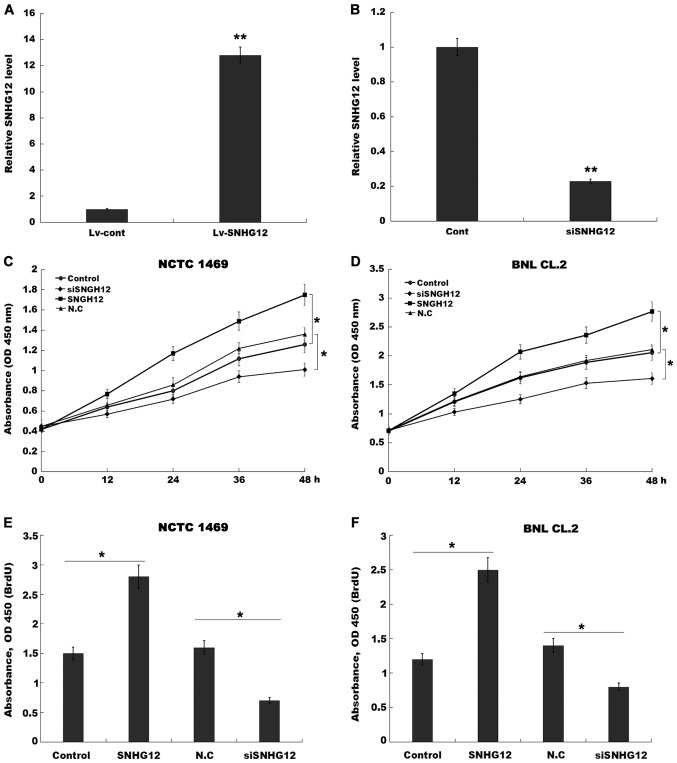
SNHG12 promotes hepatocyte proliferation in vitro. (A and B) RT-qPCR analysis of SNHG12 expression after SNHG12 overexpression and knockdown in NCTC 1469 cells. CCK-8 analysis was carried out to assess (C) NCTC 1469 and (D) BNL CL.2 cell proliferation after SNHG12 overexpression and knockdown. BrdU ELISA was conducted to assess (E) NCTC 1469 and (F) BNL CL.2 cell proliferation after SNHG12 overexpression and knockdown. *P<0.05 and **P<0.01. SNHG12, small nucleolar RNA host gene 12; RT-qPCR, reverse transcription-quantitative PCR; CCK-8, Cell Counting Kit-8.
Figure 3.
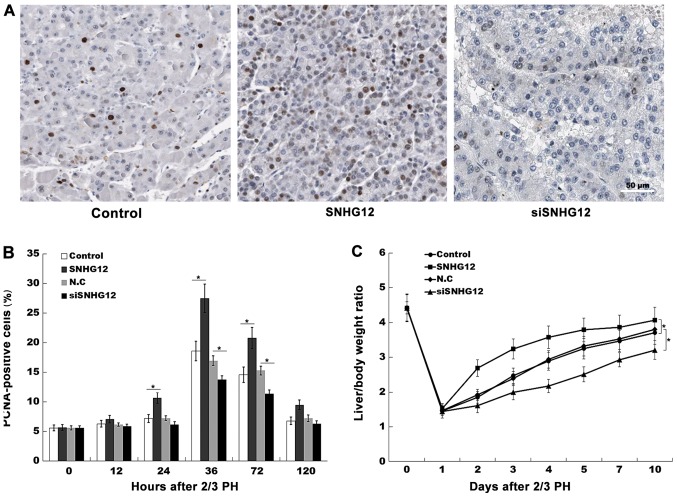
SNHG12 promotes liver regeneration in vivo. (A) Immunohistochemical analysis of liver cell proliferation after SNHG12 overexpression and knockdown in vivo, using a PCNA-specific antibody. Scale bar, 50 µm. (B) Quantification of PCNA-positive cells at different time-points after 2/3 PH. (C) The liver/body weight ratio in SNHG12 or siSNHG12-treated mice following 2/3 PH (n=5). *P<0.05. SNHG12, small nucleolar RNA host gene 12; PH, partial hepatectomy; PCNA, proliferating cell nuclear antigen.
Figure 5.
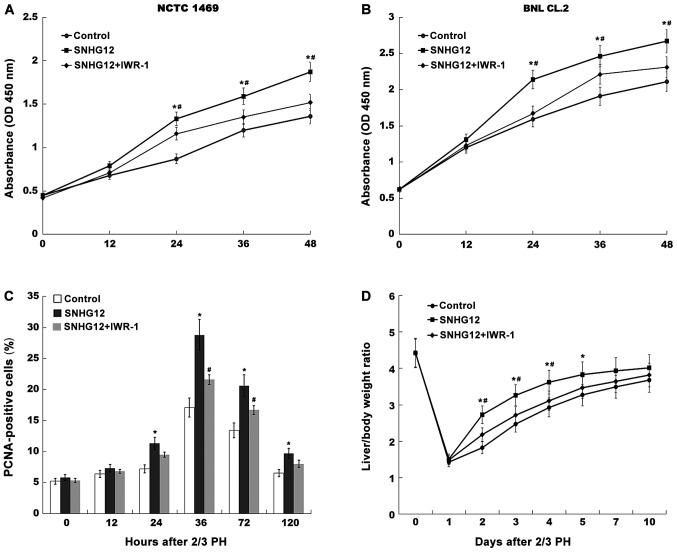
SNHG12 accelerates liver regeneration by activating Wnt signaling. CCK-8 analysis was carried out to assess (A) NCTC 1469 and (B) BNL CL.2 cell proliferation after SNHG12 overexpression in the presence or absence of 20 µM IWR-1 (an inhibitor of Wnt signaling). (C) Quantification of PCNA-positive cells at different time-points after 2/3 PH following SNHG12 overexpression in the presence or absence of 60 µM IWR-1. (D) The liver/body weight ratio of SNHG12-treated mice was assessed after 2/3 PH in the presence or absence of 60 µM IWR-1 (n=5). *P<0.05 vs. control. #P<0.05 vs. SNHG12. SNHG12, small nucleolar RNA host gene 12; PH, partial hepatectomy; CCK-8, Cell Counting Kit-8; PCNA, proliferating cell nuclear antigen.
Figure 1.
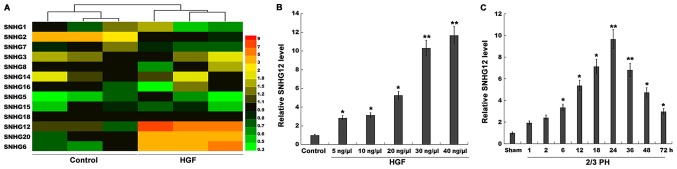
SNHG12 expression is significantly upregulated after 2/3 PH. (A) Heatmap of RT-qPCR data for the expression of 13 liver cancer-related SNHGs (SNHG1, SNHG2, SNHG3, SNHG5, SNHG6, SNHG7, SNHG8, SNHG12, SNHG14, SNHG15, SNHG16, SNHG18, and SNHG20). NCTC 1469 cells were treated with HGF (20 ng/µl), and then total RNA was extracted and used to perform RT-qPCR analysis. (B) RT-qPCR analysis of SNHG12 expression level in primary mouse hepatocytes (2×105 cells/well) after treatment with different concentrations of HGF (5, 10, 20, 30 and 40 ng/µl). (C) RT-qPCR analysis of SNHG12 expression in liver tissues (n=5) at different time-points after 2/3 PH. *P<0.05 and **P<0.01. SNHG12, small nucleolar RNA host gene 12; PH, partial hepatectomy; RT-qPCR, reverse transcription-quantitative PCR; HGF, hepatocyte growth factor.
Figure 4.
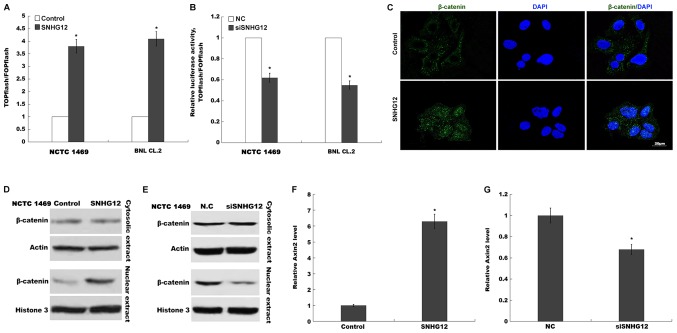
SNHG12 activates Wnt/β-catenin signaling in hepatocytes. Activation of canonical Wnt/β-catenin signaling was assessed in NCTC 1469 and BNL CL.2 cells using TOPflash/FOPflash after (A) SNHG12 overexpression and (B) SNHG12 knockdown. RLUs were calculated using ≥3 results. (C) Nuclear translocation of β-catenin was assessed using an immunofluorescence assay. Scale bar, 20 µm. Nuclear translocation of β-catenin induced by (D) SNHG12 overexpression or (E) SNHG12 knockdown was indicated using western blot analysis. Relative Axin2 mRNA expression was analyzed using RT-qPCR in BNL CL.2 cells after (F) SNHG12 overexpression and (G) SNHG12 knockdown. *P<0.05. SNHG12, small nucleolar RNA host gene 12; RLU, Relative Luciferase Unit; RT-qPCR, reverse transcription-quantitative PCR.
Results
SNHG12 expression is significantly upregulated after 2/3 PH
lncRNAs play crucial roles in the regulation of omnigenous cellular activities, which may provide unique functions and improve the regenerative ability of the liver. Recently, the role of SNHGs in tumor progression was verified (20–22), but their role in liver regeneration remains unexplored. In the present study, 13 liver cancer-related SNHGs (SNHG1, SNHG2, SNHG3, SNHG5, SNHG6, SNHG7, SNHG8, SNHG12, SNHG14, SNHG15, SNHG16, SNHG18 and SNHG20) were assayed using RT-qPCR. Since HGF is a key factor in liver regeneration (23,24), the expression of these SNHGs was assessed in NCTC 1469 cells following treatment with HGF. Among the 13 SNHGs, the expression level of SNHG2 was decreased, and that of SNHG6, 12 and 20 was significantly increased after HGF treatment (Fig. 1A). SNHG12 was selected for further analysis, since its level of upregulation was the most significant.
Mouse primary hepatocytes were isolated and treated with HGF. Fig. 1B revealed that in primary hepatocytes, the expression level of SNHG12 was upregulated in a dose-dependent manner following HGF treatment. Therefore, 2/3 PH was performed and the SNHG12 level was assessed once more. The SNHG12 expression was also significantly increased at various time-points after 2/3 PH (Fig. 1C); 2/3 PH in mice resulted in enhanced SNHG12 levels that were detectable at 6 h, that peaked between 24 h, and that had returned to almost normal levels by 72 h. Thus, the time-dependent alterations in SNHG12 expression indicated that SNHG12 may potentially be involved in liver regeneration.
SNHG12 promotes hepatocyte proliferation in vitro and in vivo
To investigate the role of SNHG12 in hepatocyte proliferation, a CCK-8 assay was performed using normal mouse liver cell lines (NCTC 1469 cells and BNL CL.2 cells) following SNHG12-overexpression or -knockdown. Fig. 2A and B revealed that SNHG12 expression was significantly increased in NCTC 1469 cells after treatment with Lv-SNHG12, and decreased after treatment with SNHG12-specific siRNA (siSNHG12). Furthermore, NCTC 1469 hepatocyte proliferation was significantly increased by the overexpression of SNHG12 (Fig. 2C), which also promoted BNL CL.2 cell proliferation (Fig. 2D), whereas SNHG12-knockdown decreased NCTC 1469 and BNL CL.2 cell proliferation (Fig. 2C and D).
The results of the BrdU ELISA assays also indicated that hepatocyte proliferation was increased after SNHG12-overexpression, and suppressed by SNHG12-knockdown (Fig. 2E and F). Subsequently, to assess hepatocyte proliferation in vivo, the expression level of PCNA was investigated by IHC in mouse-model tissues. At 36 h post-2/3 PH, SNHG12-treated mice exhibited a higher number of PCNA-positive nuclei in the hepatocytes (Fig. 3A and B). Furthermore, the role of SNHG12 in regulating liver regeneration was assayed. As revealed in Fig. 3C, after 2/3 PH the liver/body weight ratio of mice overexpressing SNHG12 was significantly greater than that of the control mice. Conversely, SNHG12-inhibition suppressed hepatocyte proliferation in vivo and reduced the liver/body weight ratio (Fig. 3A-C). These data indicated that SNHG12 upregulation contributed to hepatocyte proliferation in vitro and in vivo.
SNHG12 activates the Wnt/β-catenin signaling pathway in hepatocytes
Previous studies have demonstrated the Wnt/β-catenin pathway as the key factor in triggering liver regeneration after PH (25). Furthermore, lncRNAs can control liver regeneration by regulating Wnt/β-catenin signalling (10). lncRNA SNHG12 was revealed to promote cellular proliferation and metastasis by activating the Wnt/β-catenin pathway in papillary thyroid cancer (13). Therefore, the present study sought to investigate whether SNHG12 regulated Wnt/β-catenin in hepatocytes following PH. A TOPflash/FOPflash assay was first carried out to verify the role of SNHG12 in Wnt regulation. Fig. 4A and B indicated that the relative luciferase activity of BNL CL.2 and NCTC 1469 cells was significantly increased following SNHG12 overexpression, whereas SNHG12 knockdown suppressed the luciferase activity, compared with the control. When Wnt signaling is activated, β-catenin is dephosphorylated and translocates into the nucleus, where it promotes the expression of downstream target genes (26,27). In the present study, the effects of the SNHG12-induced nuclear translocation of β-catenin were also investigated. SNHG12-induced nuclear translocation was further investigated using immunofluorescence (Fig. 4C). When SNHG12 was overexpressed in NCTC 1469 cells, β-catenin nuclear translocation was markedly increased (Fig. 4D), whereas SNHG12-knockdown inhibited the nuclear translocation of β-catenin (Fig. 4E). Axin-2, a downstream target of Wnt/β-catenin signaling, was also enhanced in BNL CL.2 cells after SNHG12 overexpression, yet suppressed following SNHG12 knockdown, compared with the control (Fig. 4F and G). These results demonstrated that SNHG12 is a regulator of Wnt/β-catenin signaling.
SNHG12 accelerates liver regeneration by influencing Wnt signaling
To investigate the role of the SNHG12/Wnt pathway in the regulation of liver regeneration following 2/3 PH, SNHG12 was overexpressed and Wnt was concurrently inhibited using a specific inhibitor (IWR-1); hepatocyte proliferation and liver regeneration were then assessed in vitro and in vivo. As revealed in Fig. 5A and B, the cell proliferation of mouse hepatocytes was significantly increased after SNHG12 overexpression, whereas IWR-1-induced Wnt inhibition attenuated SNHG12-associated cellular proliferation. As anticipated, SNHG12 overexpression was revealed in vivo, as indicated by increased nuclear translocation of β-catenin (Fig. S1). The effect of SNHG12 overexpression on hepatocyte proliferation and liver regeneration was thus investigated in vivo. After 2/3 PH, the SNHG12-treated mice exhibited a larger number of PCNA-positive hepatocyte nuclei than those in the control group (Fig. 5C). However, Wnt inhibition partially suppressed SNHG12-associated hepatocyte proliferation in vivo. Furthermore, the liver/body weight ratio of SNHG12-treated mice was significantly increased. Conversely, Wnt inhibition attenuated SNHG12-induced liver regeneration after 2/3 PH (Fig. 5D). These results demonstrated that PH-induced upregulation of SNHG12 accelerated hepatocyte proliferation and liver regeneration by activating Wnt/β-catenin signaling.
Discussion
In the present study, the role of SNHG12-associated hepatocyte proliferation and liver regeneration after 2/3 PH was verified. The data demonstrated that: i) SNHG12 expression was significantly increased following 2/3 PH; ii) SNHG12 enhanced hepatocyte proliferation in vitro and in vivo, and promoted liver regeneration in vivo; iii) SNHG12 contributed to the activation of Wnt signaling; and iv) pharmacological inhibition of Wnt partially attenuated SNHG12-induced liver regeneration. These results demonstrated the function of the SNHG12/Wnt axis in promoting hepatocyte proliferation and liver regeneration after 2/3 PH, and indicate a novel therapeutic method for liver failure and transplantation.
It is acknowledged that different cytokines, growth factors and miRNAs regulate the genes responsible for cellular proliferation during liver regeneration (28), whereas lncRNAs have rarely been discussed in this context. In the present study, a comprehensive analysis of the expression of 13 SNHG lncRNAs in HGF-treated cell lines was performed, which indicated an upregulation in SNHG12 expression. The regulatory role of SNHG12 was then investigated in association with hepatocyte proliferation. In vitro results displayed a clear positive relationship between hepatocyte proliferation and SNHG12. This relationship was also demonstrated in vivo, as SNHG12 overexpression increased the liver mass of 2/3 PH mice. A similar finding was previously reported, where lncRNA MALAT1 was indicated as an activator of cellular proliferation and cell cycle progression in hepatocytes (1). Collectively, the significance of lncRNAs in liver regeneration is associated with their ability to promote hepatocyte proliferation.
Previous studies have widely reported that the Wnt/β-catenin pathway plays a primary role in organogenesis, homeostasis and various human diseases (29). In terms of liver regeneration, several studies have revealed the functional role of Wnt/β-catenin signaling; the literature demonstrated that the Wnt/β-catenin pathway is involved in Brahma-related gene 1-mediated liver regeneration (30), and there is direct evidence that Wnt agonists promote liver regeneration after small-for-size liver transplantation in vivo (31). Given that the interplay between the Wnt/β-catenin pathway and lncRNAs has been reported in numerous diseases, including liver cancer, it is likely that SNHG12 accelerates liver regeneration by activating Wnt signaling. In the present study, the activation of Wnt signaling was induced by SNHG12 overexpression, whereas siRNA-SNHG12 suppressed the nuclear translocation of β-catenin, decreasing Wnt/β-catenin activity. Consistent with previous findings of lncRNA-LALR1-associated activation of Wnt/β-catenin signaling in vivo (10), these results indicated a similar regulatory role for SNHG12 in liver cells.
Finally, the use of the Wnt inhibitor IWR-1 revealed that the promotion of hepatocyte proliferation was partially attenuated, further confirming that SNHG12 may exert its regenerative role by activating Wnt signaling. Subsequent in vivo studies confirmed the aforementioned conclusions, where the number of hepatocytes, as well as liver mass, were decreased upon IWR-1 administration.
Collectively, the results of the present study demonstrated the potential regulatory role of SNHG12 in liver regeneration. In vitro and in vivo studies revealed that SNHG12 promoted hepatocyte proliferation by activating the Wnt signaling pathway. This indicates that SNHG12 may be used as a biomarker of prognosis for liver diseases and the success of resection.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 81770613) and the Science and Technology Commission of Shanghai Municipality (grant no. 19411967000).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YaZ and ZQ participated in the design of the main research ideas and manuscript correction. YiZ edited the manuscript and conducted the experiments. BL and XJ performed statistical evaluation of the final data and agreed to publish the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Animal Ethics Committee of the Second Military Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing of interests.
References
- 1.Li C, Chang L, Chen Z, Liu Z, Wang Y, Ye Q. The role of lncRNA MALAT1 in the regulation of hepatocyte proliferation during liver regeneration. Int J Mol Med. 2017;39:347–356. doi: 10.3892/ijmm.2017.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tannuri AC, Tannuri U, Wakamatsu A, Mello ES, Coelho MC, Dos Santos NA. Effect of the immunosuppressants on hepatocyte proliferation and apoptosis in a young animal model of liver regeneration: An immunohistochemical study using tissue microarrays. Pediatr Transplant. 2008;12:40–46. doi: 10.1111/j.1399-3046.2007.00766.x. [DOI] [PubMed] [Google Scholar]
- 3.Mangnall D, Bird NC, Majeed AW. The molecular physiology of liver regeneration following partial hepatectomy. Liver Int. 2003;23:124–138. doi: 10.1034/j.1600-0676.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- 4.Dutkowski P, Linecker M, Deoliveira ML, Mullhaupt B, Clavien P. Challenges to liver transplantation and strategies to improve outcomes. Gastroenterology. 2015;148:307–323. doi: 10.1053/j.gastro.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 5.Selzner N, Selzner M, Tian Y, Kadry Z, Clavien PA. Cold ischemia decreases liver regeneration after partial liver transplantation in the rat: A TNF-alpha/IL-6-dependent mechanism. Hepatology. 2002;36:812–818. doi: 10.1016/S0270-9139(02)00087-3. [DOI] [PubMed] [Google Scholar]
- 6.Pediaditakis P, Lopeztalavera JC, Petersen BE, Monga SPS, Michalopoulos GK. The processing and utilization of hepatocyte growth factor/scatter factor following partial hepatectomy in the rat. Hepatology. 2001;34:688–693. doi: 10.1053/jhep.2001.27811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shu J, Kren BT, Xia Z, Wong PY, Li L, Hanse EA, Min MX, Li B, Albrecht JH, Zeng Y, et al. Genomewide microRNA down-regulation as a negative feedback mechanism in the early phases of liver regeneration. Hepatology. 2011;54:609–619. doi: 10.1002/hep.24421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro RE, Ferreira DMS, Zhang X, Borralho PM, Sarver AL, Zeng Y, Steer CJ, Kren BT, Rodrigues CM. Identification of microRNAs during rat liver regeneration after partial hepatectomy and modulation by ursodeoxycholic acid. Am J Physiol Gastrointest Liver Physiol. 2010;299:G887–G897. doi: 10.1152/ajpgi.00216.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boon RA, Jae N, Holdt LM, Dimmeler S. Long noncoding RNAs: From clinical genetics to therapeutic targets? J Am Coll Cardiol. 2016;67:1214–1226. doi: 10.1016/j.jacc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 10.Xu D, Yang F, Yuan JH, Zhang L, Bi HS, Zhou CC, Liu F, Wang F, Sun SH. Long noncoding RNAs associated with liver regeneration 1 accelerates hepatocyte proliferation during liver regeneration by activating Wnt/β-catenin signaling. Hepatology. 2013;58:739–751. doi: 10.1002/hep.26361. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto Y, Nishikawa Y, Tokairin T, Omori Y, Enomoto K. Increased expression of H19 non-coding mRNA follows hepatocyte proliferation in the rat and mouse. J Hepatol. 2004;40:808–814. doi: 10.1016/j.jhep.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Zhou B, Li L, Li Y, Sun H, Zeng C. Long noncoding RNA SNHG12 mediates doxorubicin resistance of osteosarcoma via miR-320a/MCL1 axis. Biomed Pharmacother. 2018;106:850–857. doi: 10.1016/j.biopha.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Ding S, Qu W, Jiao Y, Zhang J, Zhang C, Dang S. LncRNA SNHG12 promotes the proliferation and metastasis of papillary thyroid carcinoma cells through regulating wnt/β-catenin signaling pathway. Cancer Biomark. 2018;22:217–226. doi: 10.3233/CBM-170777. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Zheng J, Xue Y, Qu C, Chen J, Wang Z, Li Z, Zhang L, Liu Y. Inhibition of TDP43-mediated SNHG12-miR-195-SOX5 feedback loop impeded malignant biological behaviors of glioma cells. Mol Ther Nucleic Acids. 2018;10:142–158. doi: 10.1016/j.omtn.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou S, Yu L, Xiong M, Dai G. LncRNA SNHG12 promotes tumorigenesis and metastasis in osteosarcoma by upregulating Notch2 by sponging miR-195-5p. Biochem Biophys Res Commun. 2018;495:1822–1832. doi: 10.1016/j.bbrc.2017.12.047. [DOI] [PubMed] [Google Scholar]
- 16.Yang BF, Cai W, Chen B. LncRNA SNHG12 regulated the proliferation of gastric carcinoma cell BGC-823 by targeting microRNA-199a/b-5p. Eur Rev Med Pharmacol Sci. 2018;22:1297–1306. doi: 10.26355/eurrev_201803_14471. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167–1170. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Jin C, Zheng Y, Li X, Zhang S, Zhang Y, Jia L, Li W. Knockdown of lncRNA MIR31HG inhibits adipocyte differentiation of human adipose-derived stem cells via histone modification of FABP4. Sci Rep. 2017;7:8080. doi: 10.1038/s41598-017-08131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou B, Guo H, Tang J. Long Non-Coding RNA TFAP2A-AS1 inhibits cell proliferation and invasion in breast cancer via miR-933/SMAD2. Med Sci Monit. 2019;25:1242–1253. doi: 10.12659/MSM.912421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan T, Ma W, Hong Z, Wu L, Chen X, Yuan Y. Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12) promotes tumorigenesis and metastasis by targeting miR-199a/b-5p in hepatocellular carcinoma. J Exp Clin Cancer Res. 2017;36:11. doi: 10.1186/s13046-016-0486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo T, Wang H, Liu P, Xiao Y, Wu P, Wang Y, Chen B, Zhao Q, Liu Z, Liu Q. SNHG6 Acts as a genome-wide hypomethylation trigger via coupling of miR-1297-Mediated S-adenosylmethionine-dependent positive feedback loops. Cancer Res. 2018;78:3849–3864. doi: 10.1158/0008-5472.CAN-17-3833. [DOI] [PubMed] [Google Scholar]
- 22.Shan Y, Ma J, Pan Y, Hu J, Liu B, Jia L. LncRNA SNHG7 sponges miR-216b to promote proliferation and liver metastasis of colorectal cancer through upregulating GALNT1. Cell Death Dis. 2018;9:722. doi: 10.1038/s41419-018-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 24.Oe H, Kaido T, Mori A, Onodera H, Imamura M. Hepatocyte growth factor as well as vascular endothelial growth factor gene induction effectively promotes liver regeneration after hepatectomy in Solt-Farber rats. Hepatogastroenterology. 2005;52:1393–1397. [PubMed] [Google Scholar]
- 25.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 26.Qu Y, Olsen JR, Yuan X, Cheng PF, Levesque MP, Brokstad KA, Hoffman PS, Oyan AM, Zhang W, Kalland KH, Ke X. Small molecule promotes β-catenin citrullination and inhibits Wnt signaling in cancer. Nat Chem Biol. 2018;14:94–101. doi: 10.1038/nchembio.2510. [DOI] [PubMed] [Google Scholar]
- 27.Zheng CH, Wang JB, Lin MQ, Zhang PY, Liu LC, Lin JX, Lu J, Chen QY, Cao LL, Lin M, et al. CDK5RAP3 suppresses Wnt/β-catenin signaling by inhibiting AKT phosphorylation in gastric cancer. J Exp Clin Cancer Res. 2018;37:59. doi: 10.1186/s13046-018-0716-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng R, Song G, Roll GR, Frandsen NM, Willenbring H. A microRNA-21 surge facilitates rapid cyclin D1 translation and cell cycle progression in mouse liver regeneration. J Clin Invest. 2012;122:1097–1108. doi: 10.1172/JCI46039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol. 2012;74:299–323. doi: 10.1146/annurev-physiol-020911-153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li N, Kong M, Zeng S, Hao C, Li M, Li L, Xu Z, Zhu M, Xu Y. Brahma related gene 1 (Brg1) contributes to liver regeneration by epigenetically activating the Wnt/β-catenin pathway in mice. FASEB J. 2018;33:327–338. doi: 10.1096/fj.201800197R. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y, Lv X, He J, Liu T, Wen S, Wang L. Wnt agonist stimulates liver regeneration after small-for-size liver transplantation in rats. Hepatol Res. 2016;46:E154–E64. doi: 10.1111/hepr.12553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.