Abstract
Long non-coding RNAs (lncRNAs) are a group of non-coding transcripts of >200 nucleotides. They can act as competing endogenous RNAs (ceRNAs) and suppress microRNA (miRNA) function by preventing them from binding to and interacting with target mRNAs. However, the specific role of the lncRNA-associated ceRNA network in the pathogenesis of glaucoma has not yet been elucidated. To study this, data were downloaded from the Gene Expression Omnibus database (GSE126170), which contained three human trabecular meshwork cell (HTMC) samples treated with 300 µm hydrogen peroxide and three control samples treated with vehicle. Differentially expressed lncRNAs and mRNAs of HTMCs were obtained using the R package limma. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway analyses of differentially expressed mRNAs were performed using the R package clusterProfiler. Finally, the ceRNA network was constructed using the mircode, miRDB, miRTarBase and TargetScan databases, and visualized using Cytoscape v3.6.1. The results showed that 70 lncRNAs and 558 mRNAs were identified to be significantly dysregulated (|log2FoldChange| >1 and adjusted P<0.05) in HTMCs under oxidative stress compared to those in HTMCs under control conditions. Moreover, 24 lncRNAs, 24 miRNAs and 40 mRNAs were closely connected, and were part of the ceRNA network. Among these, the expression levels of 19 lncRNAs were upregulated, and those of 5 lncRNAs were downregulated. To conclude, using bioinformatics analysis, the differential expression profiles of lncRNAs were reported and a lncRNA-associated ceRNA network in HTMCs under oxidative stress was constructed. These results may bring to light a new pathological mechanism or a potential therapeutic target for glaucoma.
Keywords: glaucoma, lncRNA, ceRNA, HTMC, oxidative stress
Introduction
Glaucoma is the leading cause of irreversible blindness and is characterized by the progressive degeneration of the optic nerve and loss of the visual field (1). A total of 76 million and 111.8 million individuals worldwide are estimated to have glaucoma by 2020 and 2040, respectively (2,3). Increased intraocular pressure (IOP) has been identified as the main risk factor for glaucoma (4). IOP is maintained by the aqueous humor, which is produced by the ciliary epithelium; the aqueous humor enters into the posterior chamber around the lens and iris, and then goes through the trabecular meshwork (TM) to the Schlemm's canal, eventually entering the episcleral venous circulation via the conventional route (5). The TM presents as a porous, sponge-like structure, and is composed of strands or beams of connective tissues that contain a core of collagenous and elastic fibers, and is covered by flat cells (6). Since the TM is an important structure in the aqueous humor outflow tract, it plays a major role in maintaining IOP balance. Variations in IOP are usually a result of changes in the physiological status of the TM (6).
Reactive oxygen species (ROS) is a collective term used to define oxygen radicals such as superoxide and non-radicals such as hydrogen peroxide (H2O2) (7). Excessive ROS can not only cause direct damage to the DNA, protein and lipids, but it can also lead to oxidative stress via the activation of various signaling pathways, such as the mitogen-activated protein kinase (MAPK), PI3K/AKT, NF-κB and p53 signaling pathways (8). These pathways play important roles in determining cell death and survival (8). Although there are multiple hypotheses, oxidative stress has been widely accepted as the pathological mechanism of glaucoma (9). Exposure of eyes to sunlight can result in the acceleration of the generation of ROS due to ultraviolet radiation (7), while also resulting in oxidative stress to the ocular structures (10). Previous research has also demonstrated that oxidative stress can result in pathological alteration of the anterior chamber and TM (10). The TM has shown greater sensitivity to oxidative damage compared to other ocular tissues, possibly due to the lack of an effective antioxidant mechanism in the TM (9). Furthermore, oxidative DNA damage has been shown to be significantly higher in the TM of glaucoma patients (11). It has been hypothesized that repetitive oxidative stress due to in vivo H2O2 can impair TM cell adhesion and cause cell loss, resulting in elevated resistance to aqueous humor outflow (12). Thus, oxidative stress is an important mechanism that regulates TM pathology.
Long non-coding RNAs (lncRNAs) are a group of non-coding transcripts of >200 nucleotides in length (13). lncRNAs form the largest percentage of mammalian non-coding transcriptomes (13). lncRNAs play a significant role in regulating gene expression, protein modification, cell differentiation, immune response and other critical biochemical pathways (14,15). Studies have identified roles for lncRNAs in several neurodegenerative diseases, such as Alzheimer's, Parkinson's and Huntington's disease (16–19). In 2011, a hypothesis was proposed, which suggested that lncRNAs were microRNA (miRNA) sponges that suppressed miRNA interactions with mRNAs and affected translation of protein-coding genes (20). The study referred to the miRNA sponges as competing endogenous RNAs (ceRNAs). Given this lncRNA-miRNA-mRNA regulation network, in the present study a ceRNA network that further describes the interactions of these RNAs was elucidated.
Over the years, there have been extensive studies into the effects of lncRNAs on various types of diseases (19,21). Nevertheless, the effects of the lncRNA-associated ceRNA network on human trabecular meshwork cells (HTMCs) under oxidative stress have not yet been completely described. In this study, the aim was to analyze the effects of a lncRNA-associated ceRNA network on HTMCs under oxidative stress.
Materials and methods
Tissue samples from Gene Expression Omnibus (GEO) database and bioinformatics analysis
The RNA expression data were downloaded from the NCBI GEO database (GSE126170). The series contained three HTMC samples that were treated with 300 µm H2O2 in serum-free medium for 2 h and three control samples that were treated with vehicle. Agilent-078298 human ceRNA array V1.0 4X180K (Agilent Technologies, Inc.) was utilized to perform all the treatments. Approval from an ethics committee was not required as the data were downloaded from the GEO database.
Analysis of differentially expressed genes
The limma package in the R software (version 3.5.2; www.R-project.org) (22) was used to identify differentially expressed lncRNAs and mRNAs with |log2 Fold Change (FC)| thresholds >1.0 and adjusted P<0.05 in the treatment and control groups. The volcano map was obtained using the pheatmap package in the R software (version 3.5.2; www.R-project.org). The Encyclopedia of DNA Elements (version 38; http://www.encodeproject.org/) and Ensembl (version 96; htps://www.ensembl.org/) were used in order to define and annotate the differentially expressed RNAs (mRNAs and lncRNAs).
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analysis
In order to explore the underlying biological functions and processes of these differentially expressed genes, the GO database (http://www.geneontology.org), KEGG (http://www.kegg.jp/), and the clusterProfilerGOpackage and clusterProfilerKEGG packages in R software (version 3.5.2; www.R-project.org) (23) were used to perform functional enrichment analysis. Records with P<0.05 and enrichment >2.0 were defined as ‘conserved’.
Construction of the lncRNA-miRNA-mRNA ceRNA networks
The miRNA targets of lncRNAs were predicted using the mircode database (version 11; http://www.mircode.org/) (24) and mRNA targets of miRNAs using miRTarBase (version 7.0; http://mirtarbase.mbc.nctu.edu.tw/) (25), miRDB (version 5.0, http://mirdb.org/) (26) and TargetScan (version 7.2; http://www.targetscan.org/) (27). Targeted mRNAs were cross-matched with differentially expressed mRNAs. The lncRNAs, miRNAs and mRNAs with |log2FC| >1.0 and P<0.05 were recorded. Finally, a lncRNA-associated ceRNA network was constructed and visualized using Cytoscape v3.6.1 (28).
Results
Differential expression of lncRNAs induced by oxidative stress in HTMCs
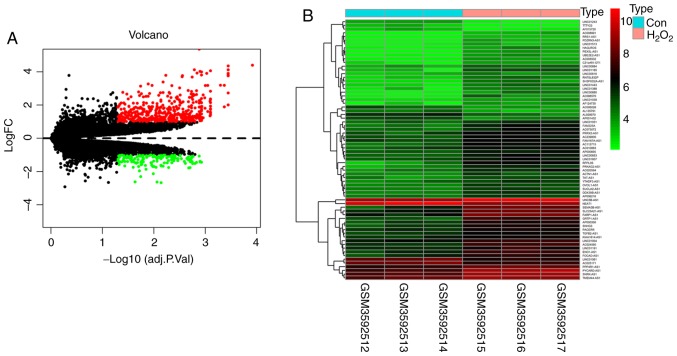
A total of 70 differentially expressed lncRNAs were discovered from HTMCs treated with 300 µm H2O2 in serum-free medium for 2 h and HTMCs treated with vehicle and having |log2FC| >1 and adjusted P<0.05 (Table SI). Of these differentially expressed lncRNAs, 24 were part of the ceRNA network that was constructed using the following steps (Table I). The volcano map of differentially expressed lncRNAs and mRNAs was obtained from the limma package and pheatmap package in the R software (Fig. 1A). The heatmap of differentially expressed lncRNAs showed clear differences in expression between the two groups (Fig. 1B).
Table I.
Differentially expressed lncRNAs in the ceRNA network.
| lncRNA | Gene ID | Regulation | LogFC (T/N) | adj.P.Val |
|---|---|---|---|---|
| ACTN1-AS1 | 161159 | Up | 1.129092 | 0.016997 |
| AC005532 | – | Up | 1.406952 | 0.002765 |
| AC020594 | – | Up | 1.518925 | 0.026473 |
| AC024560 | – | Up | 1.922623 | 0.001163 |
| AC073072 | – | Up | 1.532428 | 0.013798 |
| AC096570 | – | Up | 1.747278 | 0.025237 |
| AF124730 | – | Up | 1.346528 | 0.042546 |
| AP000356 | – | Up | 1.720098 | 0.005243 |
| AP000695 | – | Up | 1.10531 | 0.004826 |
| AP006216 | – | Up | 1.004154 | 0.009124 |
| C21orf91-OT1 | 246312 | Up | 1.157345 | 0.013636 |
| DDX39B-AS1 | 106478957 | Up | 1.315506 | 0.001423 |
| ENO1-AS1 | 100505975 | Up | 1.829472 | 0.006297 |
| FARP1-AS1 | 100874080 | Up | 2.605723 | 0.003072 |
| GRTP1-AS1 | 100874068 | Up | 2.368667 | 0.003501 |
| PDZRN3-AS1 | 101927249 | Up | 1.152825 | 0.010978 |
| PEX5L-AS1 | 100874040 | Up | 1.606603 | 0.001311 |
| SNHG3 | 8420 | Up | 1.669148 | 0.00915 |
| SNRK-AS1 | 100873954 | Up | 1.375921 | 0.002028 |
| AC006026 | – | Down | −1.1049 | 0.031015 |
| AC025171 | – | Down | −1.13395 | 0.009238 |
| AF015720 | – | Down | −1.01988 | 0.041587 |
| NEAT1 | 283131 | Down | −1.47837 | 0.006227 |
| TTTY23 | 252955 | Down | −1.10446 | 0.021033 |
-, no corresponding gene ID in PubMed; T, human trabecular meshwork cells treated with 300 µM H2O2 for 2 h; N, human trabecular meshwork cells treated with vehicle; lncRNA, long non-coding RNA; AS, antisense; ceRNA, competing endogenous RNA; adj.P.Val, adjusted P-value; FC, fold change.
Figure 1.
Differentially expressed genes and lncRNAs in human trabecular meshwork cells between the oxidative stress and control group. (A) Volcano map of differentially expressed genes; black dots represent non-differentially expressed genes. (B) Heatmap of differentially expressed lncRNAs; each column represents a sample and each row represents a specific lncRNA. |log2FC| >1 and adjusted P<0.05. Red indicates upregulation and green indicates downregulation of expression. Con, control; H2O2, hydrogen peroxide; lncRNA, long non-coding RNA; FC, fold change.
GO enrichment and KEGG pathway analysis of differentially expressed mRNAs
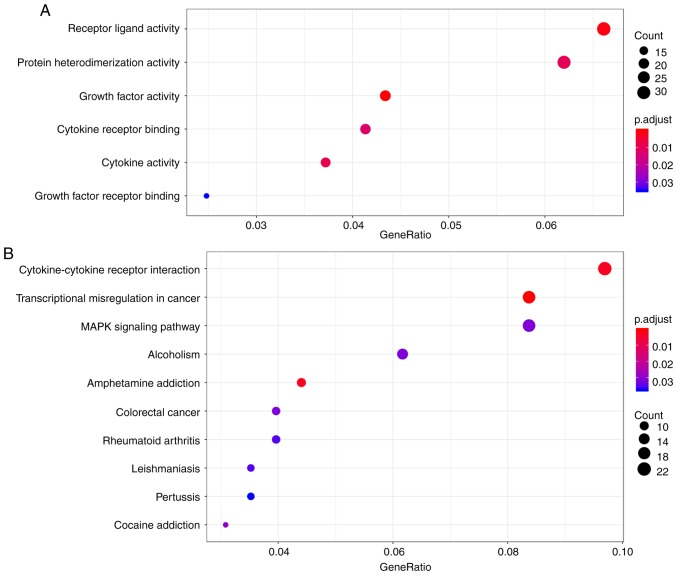
As shown in the heatmap in Fig. 2, a total of 518 differentially expressed mRNAs were identified. In order to explore the functions and signaling pathways of these genes GO and KEGG analyses were carried out. A total of 6 GO enrichment terms were listed (Fig. 3A) and the functions that were the most enriched were receptor ligand activity (GO: 0048018), protein heterodimerization activity (GO: 0046982) and growth factor activity (GO: 0008083) in 32, 21 and 20 genes, respectively. As shown in the bubble chart in Fig. 3B, 10 KEGG pathways were also identified. Among the KEGG pathways, cytokine-cytokine receptor interaction, transcriptional misregulation in cancer and the MAPK signaling pathway ranked top 3, and were implicated in 22, 19 and 19 genes, respectively.
Figure 2.
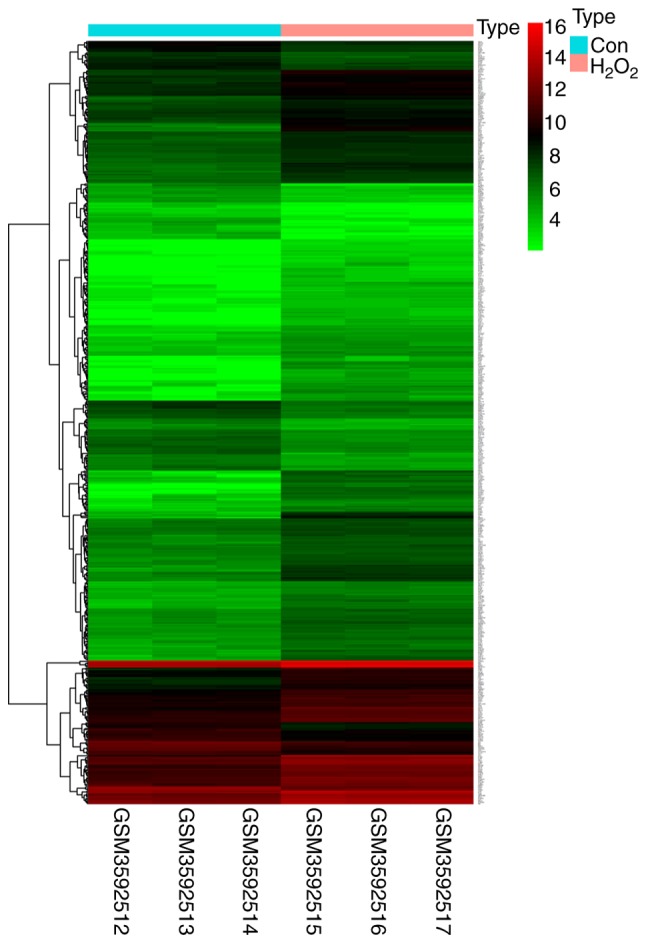
Heatmap of differentially expressed mRNAs. Red blocks indicate upregulation and green blocks indicate downregulation of expression levels. Con, control; H2O2, hydrogen peroxide.
Figure 3.
Functional enrichment of differentially expressed mRNAs. (A) Enrichment of Gene Ontology analysis for differentially expressed mRNAs. (B) Enrichment of Kyoto Encyclopedia of Genes and Genomes pathway analysis for differentially expressed mRNAs. The size of the bubbles indicates the number of mRNAs; the larger the bubble, the greater the number of mRNAs. The color represents the adjusted P-value; the redder the bubble, the smaller the adjusted P-value.
Construction of ceRNA network
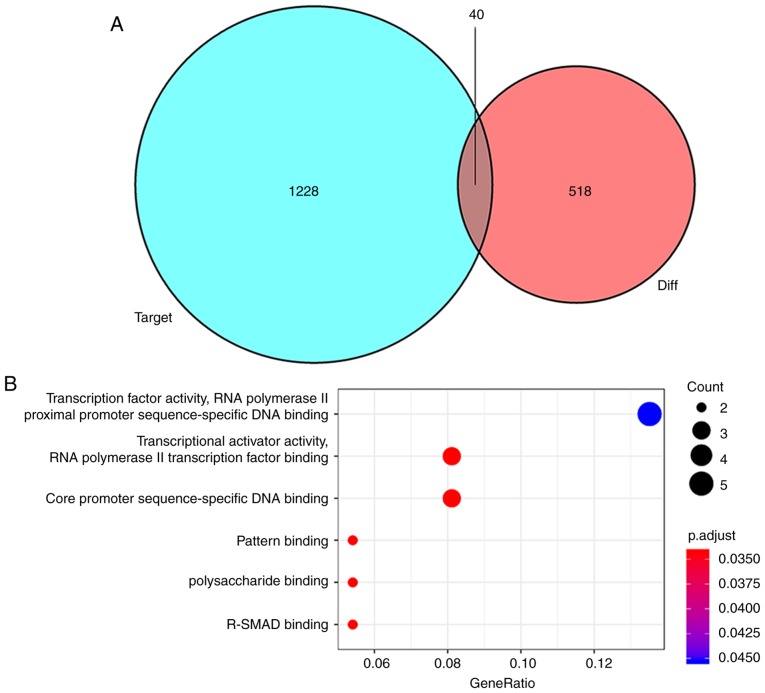
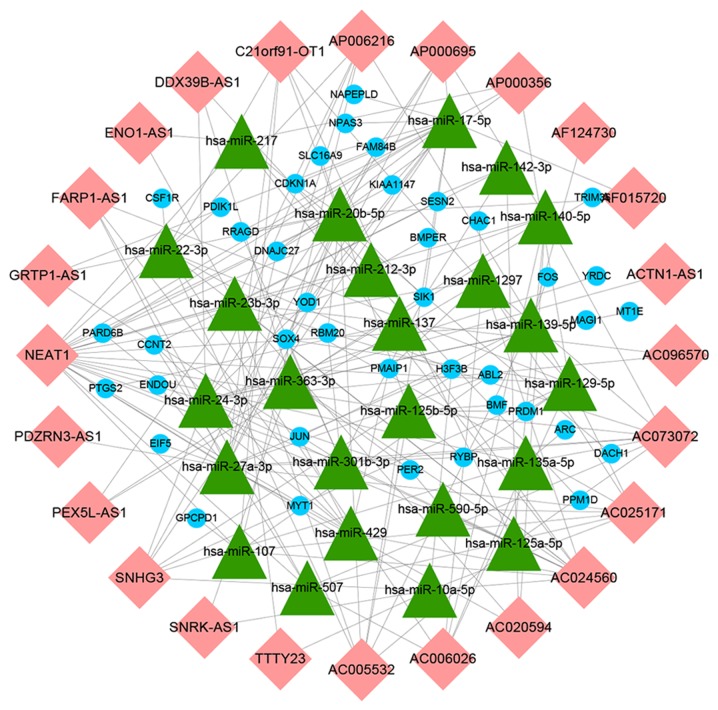
To further understand how lncRNAs and miRNAs regulate mRNA in HTMCs under oxidative stress, an lncRNA-miRNA-mRNA network was designed. Using mircode, 24 miRNAs that interacted with 24 lncRNAs were predicted (Table II). It was found that the lncRNA, nuclear enriched abundant transcript 1 (NEAT1), was downregulated the most and was predicted to interact with 21 miRNAs. A total of 1,228 mRNA targets of these 24 miRNAs were predicted using miRDB, miRTarBase and TargetScan databases. miRNA targets of mRNAs that were not included in the 518 differentially expressed mRNAs were discarded and 40 mutual mRNAs were preserved (Fig. 4A; Table III). Among the mRNAs that were preserved, the expressions of 27 mRNAs, including ABL2, activity regulated cytoskeleton associated protein, fos proto-oncogene, AP-1 transcription factor subunit and phorbol-12-myristate-13-acetate-induced protein 1 were upregulated, and that of 13 mRNAs, including dachshund family transcription factor 1, KIAA1147, membrane associated guanylate kinase, WW and PDZ domain containing 1, and SOX4, were downregulated (Table SII). Moreover, the GO enrichment analysis of mRNAs that were a part of the ceRNA network demonstrated the role of transcription factor activity, RNA polymerase II transcription factor binding, core promoter sequence-specific DNA binding and pattern binding (Fig. 4B). Finally, based on these lncRNA-miRNA and miRNA-mRNA interactions, a ceRNA network containing 24 lncRNAs, 24 miRNAs and 40 mRNAs was constructed and visualized using Cytoscape v3.7.0 (Fig. 5).
Table II.
lncRNAs and target miRNAs in the competing endogenous RNA network.
| Key lncRNAs | miRNAs |
|---|---|
| AC005532 | miR-125a-5p, miR-125b-5p, miR-139-5p, miR-17-5p, miR-20b-5p, miR-22-3p, miR-23b-3p |
| AC006026 | miR-129-5p, miR-1297, miR-135a-5p, miR-140-5p, miR-22-3p, miR-507 |
| AC020594 | miR-140-5p, miR-142-3p, miR-23b-3p, miR-27a-3p, miR-107 |
| AC024560 | miR-10a-5p, miR-1297, miR-137, miR-217, miR-22-3p, miR-23b-3p, miR-24-3p, miR-301b-3p, miR-363-3p, miR-429, miR-590-5p |
| AC025171 | miR-107, miR-10a-5p, miR-129-5p, miR-1297, miR-22-3p, miR-27a-3p, miR-507, miR-590-5p |
| AC073072 | miR-125a-5p, miR-125b-5p, miR-1297, miR-135a-5p, miR-140-5p, miR-23b-3p, miR-27a-3p, miR-301b-3p, miR-507 |
| AC096570 | miR-23b-3p, miR-24-3p |
| ACTN1-AS1 | miR-24-3p |
| AF015720 | miR-17-5p, miR-20b-5p |
| AF124730 | miR-139-5p |
| AP000356 | miR-129-5p, miR-17-5p, miR-20b-5p, miR-217 |
| AP000695 | miR-140-5p, miR-17-5p, miR-20b-5p, miR-24-3p, miR-27a-3p |
| AP006216 | miR-107, miR-129-5p, miR-22-3p, miR-23b-3p, miR-24-3p, miR-27a-3p |
| C21orf91-OT1 | miR-107, miR-125a-5p, miR-125b-5p, miR-129-5p, miR-22-3p, miR-27a-3p |
| DDX39B-AS1 | miR-212-3p, miR-24-3p, miR-363-3p |
| ENO1-AS1 | miR-217, miR-23b-3p |
| FARP1-AS1 | miR-129-5p, miR-27a-3p, miR-429, miR-507 |
| GRTP1-AS1 | miR-17-5p, miR-20b-5p, miR-301b-3p |
| NEAT1 | miR-107, miR-10a-5p, miR-125a-5p, miR-125b-5p, miR-129-5p, miR-1297, miR-135a-5p, miR-139-5p, miR-140-5p, miR-142-3p, miR-17-5p, miR-20b-5p, miR-212-3p, miR-217, miR-22-3p, miR-23b-3p, miR-24-3p, miR-27a-3p, miR-301b-3p, miR-429, miR-507 |
| PDZRN3-AS1 | miR-27a-3p |
| PEX5L-AS1 | miR-1297, miR-140-5p, miR-23b-3p |
| SNHG3 | miR-10a-5p, miR-129-5p, miR-135a-5p, miR-139-5p, miR-17-5p, miR-20b-5p, miR-24-3p |
| SNRK-AS1 | miR-10a-5p, miR-363-3p |
| TTTY23 | miR-10a-5p, miR-23b-3p |
lncRNA, long non-coding RNA; miR/miRNA, microRNA; AS, antisense.
Figure 4.
Mutual mRNAs between differentially expressed and predicted mRNAs, and their GO analysis. (A) Venn diagram of differentially expressed mRNAs and predicted mRNAs. (B) GO enrichment analysis of the mRNAs involved in the competing endogenous RNA network. GO, Gene Ontology.
Table III.
miRNAs and target mRNAs in the competing endogenous RNA network.
| miRNA | mRNA |
|---|---|
| miR-107 | ABL2, GPCPD1 |
| miR-10a-5p | H3F3B |
| miR-125a-5p | PRDM1, BMF, PRDM1, RYBP |
| miR-129-5p | PRDM1, RYBP, SOX4 |
| miR-1297 | CHAC1, PMAIP1 |
| miR-135a-5p | ARC |
| miR-137 | PTGS2 |
| miR-139-5p | FOS, JUN |
| miR-140-5p | PRDM1, YOD1 |
| miR-142-3p | CCNT2, SIK1 |
| miR-17-5p | CDKN1A, DNAJC27, KIAA1147, NAPEPLD, NPAS3, PARD6B, RRAGD, SIK1, SLC16A9, SOX4, YOD1 |
| miR-20b-5p | CDKN1A, DNAJC27, KIAA1147, PARD6B, RBM20, RRAGD, SIK1, SLC16A9, SOX4, YOD1 |
| miR-212-3p | BMPER |
| miR-217 | DACH1, PPM1D |
| miR-22-3p | CSF1R, H3F3B, PDIK1L |
| miR-23b-3p | SESN2 |
| miR-24-3p | MAGI1, MT1E, PER2, SESN1, YOD1, YRDC |
| miR-27a-3p | ABL2, DNAJC27, EIF5, ENDOU, FAM84B, H3F3B, MYT1, RYBP |
| miR-301b-3p | RBM20, RRAGD, SIK1, SOX4 |
| miR-363-3p | PER2, SOX4, TRIM36 |
| miR-429 | CCNT2, JUN, PARD6B, PMAIP1 |
| miR-507 | CCNT2 |
| miR-590-5p | PER2 |
miR/miRNA, microRNA.
Figure 5.
Competing endogenous RNA network in human trabecular meshwork cells under oxidative stress. Pink rhombuses represent lncRNAs, green triangles represent miRNAs and blue dots are mRNAs. Grey lines indicate lncRNA-miRNA-mRNA interactions. lncRNA, long non-coding RNA; miR/miRNA, microRNA.
Discussion
lncRNAs can act as ceRNA and suppress miRNA function, thus preventing miRNAs from binding to and interacting with target mRNAs (20). In the present analysis, 24 dysregulated lncRNAs that have miRNA targets were identified. Among these, NEAT1 was downregulated the most, and was predicted to interact with 21 miRNAs. NEAT1 plays an important role in the formation of nuclear structure (29). A previous study suggested that the downregulation of NEAT1 lowers cell viability in hepatocellular carcinoma and esophageal squamous cell carcinoma by serving as a ceRNA and influencing the expression of miR-129-5p (30,31). Another study showed that NEAT1 regulates the miR-107-mediated expression of cyclin-dependent kinase 6 in laryngeal squamous cell cancer (32). A number of studies have demonstrated that the expression of NEAT1 can be activated by NF-κB and p53, and activate PI3K/AKT pathways (33–35). However, the function of NEAT1 in the pathogenesis of glaucoma has not yet been investigated. Apart from directly participating in biological processes, lncRNAs play an important role in various signal pathways, such as NF-κB, PI3K/AKT, Notch and Wnt/β-catenin (36–40). Multiple types of NF-κB-associated lncRNAs, including NEAT1, have been reported to interact with different sites in the NF-κB/IκB complex in order to orchestrate opposite effects. For example, the interaction of NF-κB with NF-κB interacting lncRNA (NKILA) can result in the binding and inhibition of the phosphorylation site of IκB, thus suppressing NF-κB activation (37). NKILA can, in turn, be upregulated by NF-κB (37). p50-associated cyclooxygenase-2 extragenic RNA enhances NF-κB signaling by combining with free p50 to decrease the concentration of p50-p50 heterodimers and increase the concentration of p65-p50 heterodimers (40). lncRNA AK023948 was found to stabilize p85 by interacting with p85 and DExH-box helicase 9, as well as elevating AKT activity in the PI3K/AKT pathway (38).
miRNAs, such as miR-107, miR-137, miR-22 and miR-590, have been reported to regulate several biochemical pathways in glaucoma (41). HTMCs injured by oxidative stress can release miR-107, miR-149, miR-21 and miR-450 into the aqueous humor, which then travels via the uveoscleral pathway to the peripapillary retina, thus resulting in the transmission of damaging signals to the optic nerve (42). miR-27a was demonstrated to play a critical role in cell proliferation, differentiation and apoptosis by regulating f-box and WD repeat domain containing 7 (43). The upregulation of miR-24 can be a limitation in the activation of transforming growth factor β (TGFβ)1 under mechanical stress, which can in turn contribute to the pathogenesis of glaucoma (44). Additionally, miR-23-27-24 gene clusters can enhance angiogenesis (45) and inhibit neuronal apoptosis by suppressing the expression of apoptotic peptidase activating factor 1 (46). These miRNAs were detected in the present analysis, suggesting that miRNAs in the ceRNA network play an important role in the pathological changes associated with glaucoma.
The functional enrichment of differentially expressed mRNAs was mainly associated with the activities of cytokines and cytokine receptors. Previously, it was demonstrated that the TGFβ pathway plays an important role in the regulation of aqueous humor by promoting the expression of genes related to cell contractility and senescence of TM (47). These processes are coordinated via numerous signaling molecules and crosstalk communication between various pathways, including MAPK, JNKs and Rho GTPase signaling pathways (47). Other pathways, such as the brain-derived neurotrophic factor, JNK, PI3K/AKT, PTEN, Bcl-2, caspase and calcium-calpain pathways have been shown to participate in the pathological process of glaucoma (48). Consistent with previous studies, the pathway analysis that was performed also demonstrated an enrichment of these cytokine-cytokine receptor activities and the MAPK pathway, thus indicating the effect of these signaling pathways in the progression of glaucoma.
The majority of lncRNAs target more than one miRNA, and miRNAs target a number of mRNAs as well (Tables II and III). For example, lncRNAs such as NEAT1, AC005532, AC006026, AC024560, AC025171, AC073072, AP006216, C21orf91-OT1 and small nucleolar RNA host gene 3 all interact with more than 6 miRNAs (Table II), implying the complex interaction between these genes in biological processes. There is a similar relationship between miRNAs and mRNAs, such as miR-125a-5p, miR-17-5p, miR-20b-5p, miR-27a-3p, miR-301b-3p and miR-429, thus creating a comprehensive network of lncRNA-miRNA-mRNA. By establishing the ceRNA network, associated genes were linked together.
The most crucial metabolic process in the body is the utilization of molecular oxygen and the formation of adenosine triphosphate. ROS are generated from the metabolic pathways, such as the tricarboxylic acid cycle and the respiratory chain. Oxidative stress occurs once ROS overloads in the tissue (7). H2O2 was used to mimic the oxidative stress condition in glaucoma (12). The interplay of lncRNAs and signaling pathways has been partly demonstrated in previous studies. When oxidative stress occurs, PI3K/Akt, NF-κB and p53 signaling pathways are directly activated by ROS, thus causing damage to ocular structures (7,9). The ROS regulate lncRNAs, and some lncRNAs influence these pathways (33–40). A total of 70 differentially expressed lncRNAs were obtained, of which 24 participate in the lncRNA-miRNA-mRNA interaction network. As a ‘sponge’ of miRNA, lncRNAs play an important role in this network.
A total of 24 lncRNAs, 24 miRNAs and 40 mRNAs made up the ceRNA network. As stated before, the functions and associations of some remarkable RNAs have been discussed. lncRNA could not only have effects on various signaling pathways, but also combine with miRNAs to regulate the expression of mRNA, which might lead to morphotype or property changes of TM under oxidative stress. By constructing the ceRNA network, it was possible to clearly demonstrate the complex association between these RNAs and different signaling pathways. Thus, this contributed to the understanding of their interactions and the pathological processes of TM under oxidative stress. Once a typical association to explain pathogenesis is discovered, a new treatment theory for glaucoma could be found.
There were also limitations in the present analysis. Due to the value of HTMCs, the sample size was not large. This also limits the ability to carry out cell experiments to confirm lncRNA function and lncRNA-miRNA-mRNA interactions. There are plans to conduct further experiments, providing that HTMCs are available, in order to explore the role of lncRNA-associated ceRNA networks in glaucoma.
To conclude, the differential expression profiles of lncRNAs were demonstrated, and a lncRNA-associated ceRNA network was constructed in HTMCs under oxidative stress. This could contribute to finding a new pathological mechanism or a potential therapeutic target for glaucoma.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- lncRNA
long non-coding RNA
- miRNA
microRNA
- ceRNA
competing endogenous RNA
- HTMC
human trabecular meshwork cell
- IOP
intraocular pressure
- TM
trabecular meshwork
- ROS
reactive oxygen species
- MAPK
mitogen-activated protein kinase
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YY, KY and HZ conceived and designed the study. KY, YY, PJ, CD and FL contributed analysis tools and carried out the data analysis. YY, KY and CD wrote the paper. KY, YY and FL reviewed and edited the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: A review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol. 2015;5:439–473. doi: 10.1002/cphy.c140014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braunger BM, Fuchshofer R, Tamm ER. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur J Pharm Biopharm. 2015;95:173–181. doi: 10.1016/j.ejpb.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Bayir H. Reactive oxygen species. Crit Care Med. 2005;33(12 Suppl):S498–S501. doi: 10.1097/01.CCM.0000186787.64500.12. [DOI] [PubMed] [Google Scholar]
- 8.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: Signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 9.Izzotti A, Sacca SC, Longobardi M, Cartiglia C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Invest Ophthalmol Vis Sci. 2009;50:5251–5258. doi: 10.1167/iovs.09-3871. [DOI] [PubMed] [Google Scholar]
- 10.Sacca SC, Gandolfi S, Bagnis A, Manni G, Damonte G, Traverso CE, Izzotti A. From DNA damage to functional changes of the trabecular meshwork in aging and glaucoma. Ageing Res Rev. 2016;29:26–41. doi: 10.1016/j.arr.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Izzotti A, Sacca SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003;114:638–646. doi: 10.1016/S0002-9343(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Li Y, Yue BY. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: The trabecular meshwork. J Cell Physiol. 1999;180:182–189. doi: 10.1002/(SICI)1097-4652(199908)180:2<182::AID-JCP6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 13.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: Insights into functions. Nature reviews. Genetics. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 14.Caley DP, Pink RC, Trujillano D, Carter DR. Long noncoding RNAs, chromatin, and development. ScientificWorldJournal. 2010;10:90–102. doi: 10.1100/tsw.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akhade VS, Pal D, Kanduri C. Long noncoding RNA: Genome organization and mechanism of action. Adv Exp Med Biol. 2017;1008:47–74. doi: 10.1007/978-981-10-5203-3_2. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Xu J. Identification of Alzheimer's disease-associated long noncoding RNAs. Neurobiol Aging. 2015;36:2925–2931. doi: 10.1016/j.neurobiolaging.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Gstir R, Schafferer S, Scheideler M, Misslinger M, Griehl M, Daschil N, Humpel C, Obermair GJ, Schmuckermair C, Striessnig J, et al. Generation of a neuro-specific microarray reveals novel differentially expressed noncoding RNAs in mouse models for neurodegenerative diseases. RNA. 2014;20:1929–1943. doi: 10.1261/rna.047225.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y, Hayden MR, Xu B. BDNF overexpression in the forebrain rescues Huntington's disease phenotypes in YAC128 mice. J Neurosci. 2010;30:14708–14718. doi: 10.1523/JNEUROSCI.1637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan P, Su W, Zhuo Y. The role of long noncoding RNAs in neurodegenerative diseases. Mol Neurobiol. 2017;54:2012–2021. doi: 10.1007/s12035-016-9793-6. [DOI] [PubMed] [Google Scholar]
- 20.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 22.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu G, Wang LG, Han Y, He QY. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeggari A, Marks DS, Larsson E. miRcode: A map of putative microRNA target sites in the long non-coding transcriptome. Bioinformatics. 2012;28:2062–2063. doi: 10.1093/bioinformatics/bts344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou CH, Shrestha S, Yang CD, Chang NW, Lin YL, Liao KW, Huang WC, Sun TH, Tu SJ, Lee WH, et al. miRTarBase update 2018: A resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46:D296–D302. doi: 10.1093/nar/gkx1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong N, Wang X. miRDB: An online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015;43((Database Issue)):D146–D152. doi: 10.1093/nar/gku1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sunwoo H, Dinger ME, Wilusz JE, Amaral PP, Mattick JS, Spector DL. MEN epsilon/beta nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res. 2009;19:347–359. doi: 10.1101/gr.087775.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang L, Sun J, Pan Z, Song Y, Zhong L, Zhang Y, Liu Y, Zheng X, Huang P. Long non-coding RNA NEAT1 promotes hepatocellular carcinoma cell proliferation through the regulation of miR-129-5p-VCP-IκB. Am J Physiol Gastrointest Liver Physiol. 2017;313:G150–G156. doi: 10.1152/ajpgi.00426.2016. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Chen D, Gao X, Li X, Shi G. LncRNA NEAT1 regulates cell viability and invasion in esophageal squamous cell carcinoma through the miR-129/CTBP2 axis. Dis Markers. 2017;2017:5314649. doi: 10.1155/2017/5314649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang P, Wu T, Zhou H, Jin Q, He G, Yu H, Xuan L, Wang X, Tian L, Sun Y, et al. Long noncoding RNA NEAT1 promotes laryngeal squamous cell cancer through regulating miR-107/CDK6 pathway. J Exp Clin Cancer Res. 2016;35:22. doi: 10.1186/s13046-016-0297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng N, Guo Y. Long noncoding RNA NEAT1 promotes nasopharyngeal carcinoma progression through regulation of miR-124/NF-κB pathway. Onco Targets Ther. 2017;10:5843–5853. doi: 10.2147/OTT.S151800. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Fuschi P, Carrara M, Voellenkle C, Garcia-Manteiga JM, Righini P, Maimone B, Sangalli E, Villa F, Specchia C, Picozza M, et al. Central role of the p53 pathway in the noncoding-RNA response to oxidative stress. Aging (Albany NY) 2017;9:2559–2586. doi: 10.18632/aging.101341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H, Li J, Zhou ZG. NEAT1 promotes cell proliferation in multiple myeloma by activating PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2018;22:6403–6411. doi: 10.26355/eurrev_201810_16053. [DOI] [PubMed] [Google Scholar]
- 36.Zhang A, Xu M, Mo YY. Role of the lncRNA-p53 regulatory network in cancer. J Mol Cell Biol. 2014;6:181–191. doi: 10.1093/jmcb/mju013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X, Lin L, Yao H, Su F, Li D, et al. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27:370–381. doi: 10.1016/j.ccell.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Koirala P, Huang J, Ho TT, Wu F, Ding X, Mo YY. LncRNA AK023948 is a positive regulator of AKT. Nat Commun. 2017;8:14422. doi: 10.1038/ncomms14422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trimarchi T, Bilal E, Ntziachristos P, Fabbri G, Dalla-Favera R, Tsirigos A, Aifantis I. Genome-wide mapping and characterization of Notch-regulated long noncoding RNAs in acute leukemia. Cell. 2014;158:593–606. doi: 10.1016/j.cell.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romano GL, Platania CB, Forte S, Salomone S, Drago F, Bucolo C. MicroRNA target prediction in glaucoma. Prog Brain Res. 2015;220:217–240. doi: 10.1016/bs.pbr.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Izzotti A, Ceccaroli C, Longobardi MG, Micale RT, Pulliero A, La Maestra S, Saccà SC. Molecular damage in glaucoma: From anterior to posterior eye segment. The MicroRNA role. Microrna. 2015;4:3–17. doi: 10.2174/2211536604666150707124640. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Ye X, Liu Y, Wei W, Wang Z. Aberrant regulation of FBW7 in cancer. Oncotarget. 2014;5:2000–2015. doi: 10.18632/oncotarget.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. MicroRNA-24 regulates the processing of latent TGFβ1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J Cell Physiol. 2011;226:1407–1414. doi: 10.1002/jcp.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Q, Gallagher R, Ufret-Vincenty R, Li X, Olson EN, Wang S. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters. Proc Natl Acad Sci USA. 2011;108:8287–8292. doi: 10.1073/pnas.1105254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Q, Xu J, Li L, Li H, Mao S, Zhang F, Zen K, Zhang CY, Zhang Q. MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis. 2014;5:e1132. doi: 10.1038/cddis.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pervan CL. Smad-independent TGF-β2 signaling pathways in human trabecular meshwork cells. Exp Eye Res. 2017;158:137–145. doi: 10.1016/j.exer.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Gauthier AC, Liu J. Epigenetics and signaling pathways in glaucoma. Biomed Res Int. 2017;2017:5712341. doi: 10.1155/2017/5712341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.