Abstract
Ischemic post-conditioning (IPO) and diazoxide post-conditioning (DPO) has been proven to reduce myocardial ischemia reperfusion injury (MIRI); however, the mechanisms of IPO/DPO are still not clear. The present study aimed to investigate whether mitochondrial ATP-sensitive potassium channels (mitoKATP) channels are activated by IPO/DPO, which may further activate the hypoxia inducible factor 1/hypoxic response element (HIF-1/HRE) pathway to mitigate MIRI. Using a Langendorff perfusion device, healthy male (250–300 g) Sprague Dawley rat hearts were randomly divided into the following groups. Group N was aerobically perfused with K-H solution for 120 min. Group ischaemia/reperfusion (I/R) was aerobically perfused for 20 min, then subjected to 40 min hypoxia plus 60 min reperfusion. Group IPO was treated like the I/R group, but with 10 sec of hypoxia plus 10 sec of reperfusion for six rounds before reperfusion. Group DPO was exposed to 50 µM diazoxide for 5 min before reperfusion and otherwise treated the same as group I/R. In groups IPO+5-hydroxydecanoic acid (5HD), DPO+5HD and I/R+5HD, exposure to 100 µM 5HD (a mitoKATP channel specific blocker) for 5 min before reperfusion as described for groups IPO, DPO and I/R, respectively. In groups IPO+2-methoxyestradiol (2ME2), DPO+2ME2 and I/R+2ME2, exposure to 2 µM 2ME2 (a HIF-1α specific blocker) for 10 min before reperfusion as described for groups IPO, DPO and I/R respectively. Cardiac hemodynamics, myocardial injury and the expression of HIF-1/HRE pathway [HIF-1α, heme oxygenase (HO-1), inducible nitric oxide synthase (iNOS) and vascular endothelial growth factor (VEGF)] were detected in each group. The infarct size and mitochondrial Flameng scores of groups IPO/DPO were significantly decreased compared with the I/R group (P<0.05), but the myocardial protective effects of IPO/DPO could be eliminated by 5HD or 2ME2 (P<0.05). In addition, IPO/DPO could increase the mRNA expression of HIF-1α and the downstream factors of the HIF-1/HRE pathway (the mRNA and protein expression of HO-1, iNOS and VEGF; P<0.05). However, the myocardial protective effects and the activation the HIF-1/HRE pathway mediated by IPO/DPO could be eliminated by 5HD or 2ME2 (P<0.05). Therefore, the activation of the HIF-1/HRE pathway by opening mitoKATP channels may work with the mechanism of IPO/DPO in reducing MIRI.
Keywords: diazoxide post-conditioning, hypoxia inducible factor-1, ischemic post-conditioning, mitoKATP channels, myocardial ischemia
Introduction
Myocardial ischemia is a serious threat to patients with cardiovascular disease. Even the process of restoring the blood supply to the myocardium after the treatment of cardiovascular disease tends to have a serious effect on the recovery of the heart, as a result of the sudden recovery of coronary blood supply. This is known as myocardial ischemia reperfusion injury (MIRI) (1). This is especially true for patients after heart surgery. MIRI is a cause of the increasing rate of myocardial infarction, heart failure and mortality (2). Therefore, the reduction of MIRI in patients is key to alleviating myocardial infarction rates, heart failure and mortality, and to improving quality of life.
Research into alleviating MIRI has demonstrated that, numerous methods, such as ischemic pre-conditioning, ischemic post-conditioning, drug pre-conditioning and drug post-conditioning can play a role in myocardial protection (3–7). At the same time, a variety of myocardial protection mechanisms are involved. This includes the hypoxia inducible factor 1/hypoxia response element pathway (HIF-1/HRE) and the mitochondrial ATP-sensitive potassium channels (mitoKATP channels) (8,9).
The study of Zhao et al (10) in vivo showed that ischemic post-conditioning (IPO) could increase the expression of inducible nitric oxide synthase (iNOS) by activating the HIF-1α pathway and reduce the infarct size of the myocardium. In myocardial cells and isolated heart perfusion experiments, drug post-conditioning can increase HIF-1α, and then activate iNOS to reduce MIRI. This process can be reversed by HIF-1α small interfering (si)RNA or 2-methoxyestradiol (2ME2; a HIF-1α subunit blocker) (11). All these studies suggest that both IPO and drug post-conditioning can alleviate MIRI by activating the HIF-1/HRE pathway.
A study by Jin et al (12) has also shown that IPO can open mitoKATP channels to play a role in myocardial protection. Diazoxide (a specific mitoKATP channel opener) post-conditioning (DPO) can also alleviate MIRI (13). MitoKATP channels are potassium channels in the mitochondrial membrane, which are composed of Kir and SUR subunits. The regulation of mitoKATP channels is primarily related to the regulatory subunit SUR, which is mainly controlled by ATP. Other metabolites, such as protein kinase A, protein kinase C and PIP2, can also regulate mitoKATP channels (14). As signaling molecules, the reactive oxygen species (ROS) produced by mitoKATP channels can activate downstream signaling pathways and ultimately reduce MIRI by reducing calcium overload. Thus, whether the downstream signaling pathways activated by mitoKATP channels include the HIF-1/HRE pathway in IPO and whether DPO can also activate the HIF-1/HRE pathway through mitoKATP channel opening merits investigation.
In order to study the above problems, a rat heart perfusion model was established using a Langendorff experiment device (15). Using cardioplegia, cardiac arrest was simulated in clinical cardiopulmonary bypass and the myocardial protective effect of IPO/DPO was observed. The present study aimed to assess whether the HIF-1/HRE pathway participates in the myocardial protection mechanism of DPO. Also, 5-hydroxydecanoic acid (5HD; a specific mitoKATP channels blocker) and 2ME2 (a HIF-1α subunit blocker) were used to observe the expression changes in the HIF-1/HRE pathway, and whether or not IPO/DPO could open mitoKATP channels and then activate the HIF-1/HRE pathway. The present study will provide a theoretical basis for the clinical application of diazoxide to the treatment of MIRI.
Materials and methods
Materials
The experimental animals used were 80 healthy male Sprague Dawley (SD) rats (weight, 250–300 g; 16–20 weeks old), which were provided by the laboratory animal center of DaPing Hospital, Chongqing. Before the experiment, the rats were housed in groups of three or four for at least 1 week (12-h light/dark cycle, free access to food and water, temperature 20–25°C, humidity 50–65%).
Diazoxide and 5HD were purchased from Sigma-Aldrich; Merck KGaA. 2ME2 was purchased from Selleck Chemicals. The VEGF antibody (cat. no. NB100-664) and HIF-1α antibody (cat. no. NB100-105) were purchased from Novus Biologicals, LLC. The HO-1 antibody (cat. no. ab13248) and iNOS antibody (cat. no. ab49999) were purchased from Abcam. β-actin antibody (cat. no. 66009-1-Ig) was purchased from Proteintech Group, Inc. IRDye 800CW secondary antibodies (cat. no. 926-32210) was purchased from LI-COR Biosciences. The Sensiscript RT kit and Real-Time amplification kit were purchased from Takara Bio, Inc. The primers were purchased from Shanghai Generay Biotech Co., Ltd.
Methods
Establishment of rat heart perfusion model in vitro
SD rats were anesthetized by intraperitoneal injection of 1% pentobarbital sodium (60 mg/kg) and heparin (500 U/kg), and then a thoracotomy was performed. The aorta was removed from the heart quickly and completely. After that, the heart was fixed on the Langendorff system via the aorta and low flow retrograde perfusion of K-H solution (oxygenated with 95% O2 and 5% CO2; 37°C) was performed in the aortic root. A small incision was made on the left atrial appendage and the piezometric tube was inserted into the left ventricle through the small opening. The left ventricular end diastolic pressure was adjusted to 2–5 mmHg using the PowerLab physiological experiment system. The K-H solution and the ambient temperature of the heart are controlled at 37°C. Thereafter, the flow rate of aortic perfusion was regulated and the perfusion pressure increased slowly and stabilized at ~70 mmHg. After the heart was perfused for 20 min, the left ventricular developed pressure (LVDP), heart rate (HR) and arrhythmia were observed. At LVDP >80 mmHg, HR >250 times/min and arrhythmia <2/min, follow-up experiments were carried out.
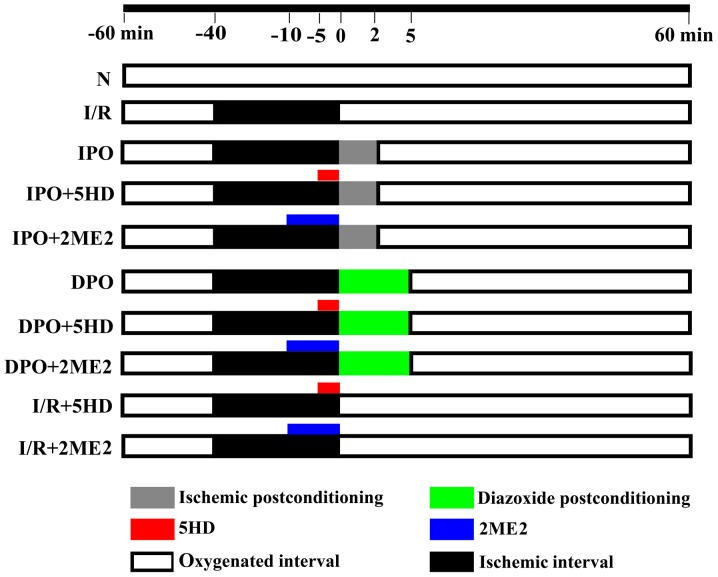
A total of 80 hearts were randomly divided into the following 10 groups (n=8, each group): Group N, group I/R, group IPO, group IPO+2ME2, group IPO+5HD, group DPO, group DPO+2ME2, group DPO+5HD, group I/R+2ME2 and group I/R+5HD (Fig. 1). Group N was aerobically perfused with K-H solution for 120 min. Group I/R, after being aerobically perfused for 20 min, was subjected to 40 min hypoxia and 60 min reperfusion. Group IPO was treated the same as group I/R, but 10 sec hypoxia plus 10 sec reperfusion was performed six times prior to reperfusion. Group IPO+5HD, was treated the same as group IPO, but perfused with 100 µM 5HD for 5 min before ischemic post-conditioning (13). Group IPO+2ME2 was treated the same as group IPO, but perfused with 2 µM 2-methoxyestradiol for 10 min before ischemic post-conditioning (11). Group DPO was treated the same as group I/R, but perfused with 50 µM diazoxide for 5 min before reperfusion (13). Group DPO+5HD was treated the same as group DPO, but perfused with 100 µM 5HD for 5 min before diazoxide post-conditioning. Group DPO+2ME2 was treated the same as group DPO, but perfused with 2 µM 2ME2 for 10 min before diazoxide post-conditioning. Group I/R+5HD was treated the same as group I/R, but the heart was perfused with 100 µM 5HD for 5 min at 35 min after stopping the perfusion. Group I/R+2ME2 was treated the same as group I/R, but the heart was perfused with 2 µM 2ME2 for 10 min at 30 min after stopping the perfusion.
Figure 1.
Perfusion of the isolated heart. Using a Langendorff perfusion device and K-H solution, the rat hearts were randomly divided into the following 10 groups (n=8/group): Group N, group I/R, group IPO, group IPO+2ME2, group IPO+5HD, group DPO, group DPO+2ME2, group DPO+5HD, group I/R+2ME2 and group I/R +5HD. The K-H solution contained (in g/l) 6.8959 NaCl, 2.1799 D-glucose, 2.1003 NaHCO3, 0.2952 MgSO4 7H2O, 0.3504 KCl, 0.1633 KH2PO4 and 0.22198 CaCl2. The St. Thomas cardioplegic solution contained (in g/l) 6.4284 NaCl, 1.6264 MgCl2 6H2O, 1.1928 KCl, 0.8401 NaHCO3 and 0.1332 CaCl2. IPO, ischemic post-conditioning; DPO, diazoxide post-conditioning; I/R, ischemia/reperfusion; 5HD, 5-hydroxydecanoic acid; 2ME2, 2-methoxyestradiol.
Except for in group N, perfusion of the K-H solution in all other groups was stopped after 20 min and cardiac arrest was attained by immediately perfusing the St. Thomas cardioplegic solution (4°C). After that, the hearts were maintained in a 32°C environment for 40 min.
Cardiac function monitoring
HR, LVDP, left ventricular end diastolic pressure (LVEDP) and maximal left ventricular pressure (+dp/dtmax) were measured using the PowerLab system at 20 min and 2 h.
Detection of infarct size in the heart
First, 1% TTC dye solution was prepared for incubation in the dark at 37°C for ~25 min.
The heart was quickly removed and placed into a −80°C refrigerator for 7 min. Along the transverse section of the heart, it was cut into five thick circular slices (0.1–0.3 cm). After that, the cardiac slices were immersed in the above 1% TTC dye solution and incubated at 37°C in the dark for 25 min. After 25 min, the cardiac slices were placed in 10% formaldehyde for 7 days at 20–25°C. The cardiac slices were arranged from big to small in order and images were captured using a Sony camera. Finally, the infarct size was calculated using ImageJ software (version 1.46R; National Institutes of Health).
Detection of myocardium under transmission electron microscope
At the end of perfusion, several 0.1 cm3 pieces of myocardial tissue were cut rapidly from the left ventricle of the heart. The myocardial tissues were fixed with 4°C precooled 2.5% glutaraldehyde fixative (for no more than 2 weeks). They were rinsed in phosphate buffer (washed once at intervals of 2 h, three times) and then fixed with 4°C precooled osmic acid (1%) for 2 h. After successive gradient acetone dehydration, epoxy resin embedding polymerization (45°C for 12 h and 60°C for 48 h), UltracutE ultrathin sectioning (50–70 nm each slice), double staining with acetic acid uranium dioxide (20–25°C for 20 min) and lead citrate (20–25°C for 10 min), the ultrastructure of the cardiomyocytes was observed under a Hitachi H7500 transmission electron microscope.
The mitochondrial Flameng score criteria under transmission electron microscope is as follows (16). The greater the myocardial mitochondria damage, the higher the score and injury is scored as 0–4 points: 0, the structure of mitochondria is normal and they are full of particles; 1, the structure of mitochondria is essentially normal, but the matrix particles are lost; 2, mitochondrial swelling and matrix transparency are apparent; 3, rupture of mitochondrial cristae with matrix transparency and concentration; 4, the mitochondrial cristae are split, the integrity of the mitochondria inside and outside the membrane has been lost, and they appear vacuolated.
In each electron microscopy experiment, 100 mitochondria were observed and 20 mitochondria were randomly selected from each field. A total of five visual fields were selected randomly. The mitochondrial Flameng score was calculated as the mean of the 100 mitochondrial total scores.
Reverse transcription-quantitative PCR (RT-qPCR)
At the end of the experiment, the left ventricular myocardium was placed in an enzyme-free cryopreservation tube (2 ml) and frozen in liquid nitrogen (−196°C). The frozen myocardium was transferred to a −80°C refrigerator.
RNA isoPlus solution, chloroform, isopropanol and ethanol were used to extract the RNA from the myocardium. The concentration and purity of the RNA were determined using a Varioskan Flash (Thermo Fisher Scientific, Inc.). After that, cDNA was synthesized using a reverse transcription kit (Takara Bio, Inc.) and 2400 PCR instrument (Bio-Rad Laboratories, Inc.). The reverse transcriptase temperature profile was 37°C for 15 min, 85°C for 5 min and maintenance at 4°C. To evaluate the target genes, the cDNA was amplified using a Takara Bio, Inc., qPCR kit and CFX Connect instrument (Bio-Rad, Laboratories, Inc.). The amplification reaction temperature profile was 95°C for 3 min, followed by 95°C for 10 min and 61.5°C for 30 min for a total of 40 cycles. The Cq values of samples were taken to analyze and calculate the relative expression of the target genes (17).
The primer sequence for each gene were as follows: HIF-1α (forward, 5′-CCCATTCCTCATCCATCAAACATT-3′ and reverse, 5′-CTTCTGGCTCATAACCCATCAACTC-3′); HO-1 (forward, 5′-ATGAGGAACTTTCAGAAGGGTC-3′ and reverse, 5′-GGAAGTAGAGTGGGGCATAGAC-3′); VEGF (forward, 5′-CCTCTCCCTACCCCACTTCCT-3′ and reverse, 5′-CACTTTCTCTTTTCTCTGCCTCCAT-3′); iNOS (forward, 5′-TCCTCAGGCTTGGGTCTTGTTAG-3′ and reverse, 5′-GGGTTTTCTCCACGTTGTTGTT-3′); β-actin (forward, 5′-CTGAACCCTAAGGCCAACCG-3′ and reverse, 5′-GACCAGAGGCATACAGGGACAA-3′).
Western blotting
Myocardial proteins were extracted using RIPA lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.). After the protein concentration was determined by bicinchoninic acid, various target proteins were assessed by western blot analysis. Equivalent amounts of protein (40 µg/5 µl) from the experimental groups were analyzed by SDS-PAGE (the gel percentages were 10% for separating gel and 4% for stacking gel) and the proteins were transferred to PVDF membranes.. Then, membranes were blocked using western blocking buffer (Beijing Solarbio Science & Technology Co., Ltd.) at room temperature for 2 h. After incubation with the primary (4°C, 12 h) and secondary (20–25°C, 2 h) antibodies in succession, the PVDF membranes were scanned and detected by the Odyssey Infrared Imaging System (LI-COR Biosciences). The dilution ratio of each antibody was 1:500 (HIF-1 α), 1:1,000 (VEGF), 1:250 (HO-1), 1:1,000 (iNOS), 1:5,000 (β-actin) and 1:10,000 (IRDye 800cw secondary antibody).
Statistical methods
All the data are presented as the mean ± standard error of the mean and were analyzed using SPSS 17.0 statistical software (SPSS, Inc.) One-way ANOVA followed by Dunnetts's T3 test were used for intergroup comparison. P<0.05 was considered to indicate a statistically significant difference.
Results
Cardiac function
After the heart was aerobically perfused for 20 min, there were no significant differences in HR, LVDP, LVEDP and +dp/dtmax among the groups (P>0.05; Table I). At the end of the experiment, there were no significant differences in HR, LVDP, LVEDP and +dp/dtmax among the groups (P>0.05; Table II).
Table I.
Cardiac function indexes after isolated heart aerobically perfused for 20 min.
| Groups | LVDP (mmHg) | HR (bpm) | LVEDP (mmHg) | +dp/dtmax (mmHg/s) |
|---|---|---|---|---|
| N | 96.9±7.4 | 306±33 | 2.63±0.92 | 3,810±300 |
| I/R | 93.0±6.6 | 312±45 | 2.43±1.27 | 3,590±146 |
| IPO | 96.4±7.1 | 301±19 | 2.37±0.92 | 3,530±364 |
| IPO+5HD | 96.8±7.0 | 287±33 | 2.43±1.27 | 3,700±141 |
| IPO+2ME2 | 94.6±5.2 | 303±34 | 2.89±0.60 | 3,550±360 |
| DPO | 96.8±8.8 | 306±26 | 2.38±0.74 | 3,550±267 |
| DPO+5HD | 93.3±8.5 | 309±28 | 2.38±0.92 | 3,810±294 |
| DPO+2ME2 | 93.9±5.2 | 308±41 | 2.22±0.67 | 3,810±457 |
| I/R+5HD | 90.8±6.2 | 298±22 | 2.33±0.52 | 3,590±370 |
| I/R+2ME2 | 96.7±5.7 | 284±17 | 2.14±1.07 | 3,510±408 |
The results are expressed as the mean ± standard error of the mean, n=8. IPO, ischemic post-conditioning; DPO, diazoxide post-conditioning; I/R, ischemia/reperfusion; 5HD, 5-hydroxydecanoic acid; 2ME2, 2-methoxyestradiol; HIF, hypoxia inducible factor; HO-1, heme-oxygenase-1; iNOS, inducible nitric oxide; VEGF, vascular endothelial growth factor; HR, heart rate; LVEDP, left ventricular end diastolic pressure; LVDP, left ventricular developed pressure.
Table II.
Cardiac function indexes at the end of the experiment.
| Groups | LVDP (mmHg) | HR (bpm) | LVEDP (mmHg) | +dp/dtmax (mmHg/s) |
|---|---|---|---|---|
| N | 82.1±8.3 | 295±36 | 3.50±0.76 | 3,350±279 |
| I/R | 73.4±5.2 | 322±41 | 3.29±0.48 | 3,220±539 |
| IPO | 84.6±7.5 | 297±19 | 3.38±0.52 | 3,410±387 |
| IPO+5HD | 76.3±4.8 | 297±16 | 3.14±0.90 | 3,110±153 |
| IPO+2ME2 | 76.0±8.2 | 320±26 | 3.67±0.71 | 3,380±431 |
| DPO | 80.6±5.3 | 317±30 | 3.13±0.99 | 3,140±405 |
| DPO+5HD | 77.6±8.1 | 321±20 | 3.25±1.16 | 3,400±313 |
| DPO+2ME2 | 74.0±6.3 | 322±18 | 3.11±0.78 | 3,200±435 |
| I/R+5HD | 73.2±6.4 | 322±51 | 3.00±0.63 | 3,150±625 |
| I/R+2ME2 | 77.9±6.8 | 304±20 | 3.14±1.07 | 3,330±539 |
The results are expressed as the mean ± standard error of the mean, n=8. IPO, ischemic post-conditioning; DPO, diazoxide post-conditioning; I/R, ischemia/reperfusion; 5HD, 5-hydroxydecanoic acid; 2ME2, 2-methoxyestradiol; HIF, hypoxia inducible factor; HO-1, heme-oxygenase-1; iNOS, inducible nitric oxide; VEGF, vascular endothelial growth factor; HR, heart rate; LVEDP, left ventricular end diastolic pressure; LVDP, left ventricular developed pressure.
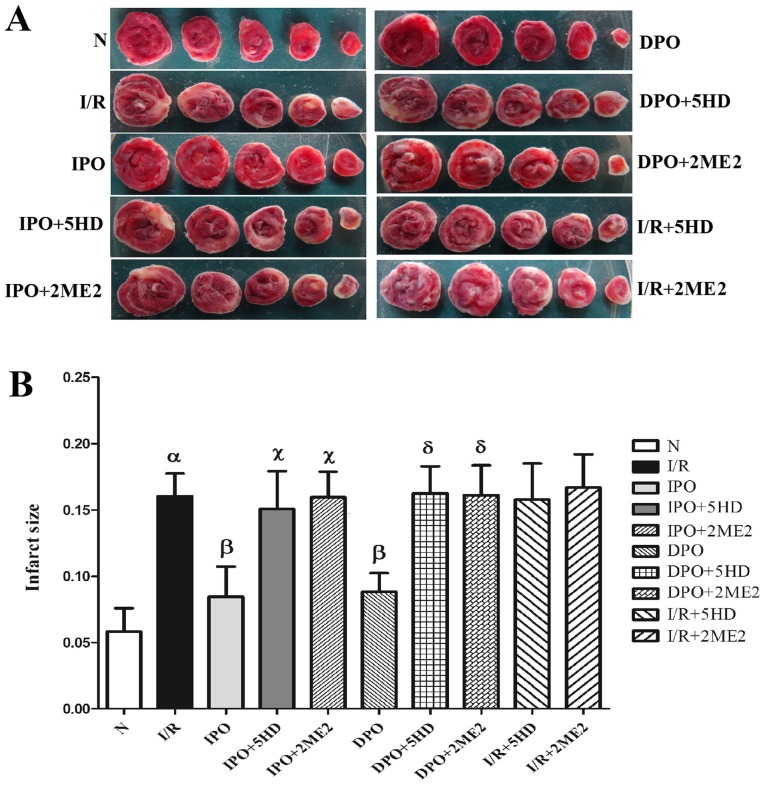
Infarct area of the myocardium
The infarct area of group N was 5.83±1.77%. In comparison, the infarct size of group I/R increased significantly (P<0.05; Fig. 2). The infarct areas in groups IPO and DPO were significantly decreased compared with group I/R (P<0.05), and the infarct areas in groups IPO+5HD and IPO+2ME2 were significantly increased compared with group IPO (P<0.05; Fig. 2). The infarct sizes in groups DPO+5HD and DPO+2ME2 were significantly increased compared with group DPO (P<0.05; Fig. 2). Compared with the I/R group, there were no significant differences in infarct size between groups I/R+5HD and I/R+2ME2 (P>0.05; Fig. 2B).
Figure 2.
Infarct size in each group after cardiac TTC staining. (A) White area represents the heart infarct area; the red area is the normal heart tissue. (B) DPO/IPO significantly reduced the infarct size caused by I/R, while 5HD/2ME2 reversed the cardioprotective effect of DPO/IPO. The results are expressed as the mean ± standard error of the mean, n=6. αP<0.05 vs. group N; βP<0.05 vs. group I/R; χP<0.05 vs. group IPO; δP<0.05 vs. group DPO. IPO, ischemic post-conditioning; DPO, diazoxide post-conditioning; I/R, ischemia/reperfusion; 5HD, 5-hydroxydecanoic acid; 2ME2, 2-methoxyestradiol.
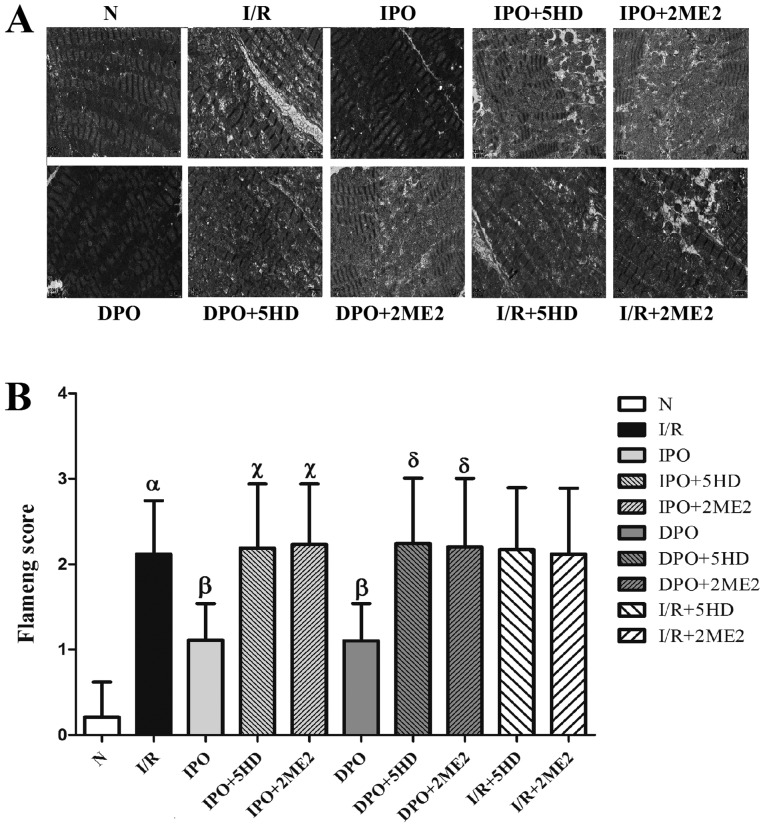
Myocardial morphology
Using the transmission electron microscope, the myocardial morphology in each group was assessed. The mitochondrial structure of group N was essentially normal and the mitochondrial score was 0.21±0.409. The mitochondrial score in group I/R was significantly increased compared with group N (P<0.05; Fig. 3). The mitochondrial scores of groups IPO and DPO were significantly decreased compared with the I/R group (P<0.05), and the mitochondrial score of groups IPO+5HD and IPO+2ME2 was significantly increased compared with group IPO (P<0.05). The mitochondrial scores in groups DPO+5HD and DPO+2ME2 were significantly increased compared with group DPO (P<0.05). There were no significant differences in the mitochondrial scores among groups I/R+5HD, I/R+2ME2 and I/R (P>0.05; Fig. 3).
Figure 3.
Myocardial morphology. (A) Myocardial ultrastructures of the 10 groups were analyzed by transmission electron microscopy (magnification, ×20,000). (B) Flameng scores for myocardial mitochondria in each group at the end of perfusion. DPO/IPO significantly reduced the myocardial damage caused by I/R, while 5HD/2ME2 reversed the cardioprotective effect of DPO/IPO. The results are expressed as the mean ± standard error of the mean, n=100. αP<0.05 vs. group N; βP<0.05 vs. group I/R; χP<0.05 vs. group IPO; δP<0.05 vs. group DPO. IPO, ischemic post-conditioning; DPO, diazoxide post-conditioning; I/R, ischemia/reperfusion; 5HD, 5-hydroxydecanoic acid; 2ME2, 2-methoxyestradiol.
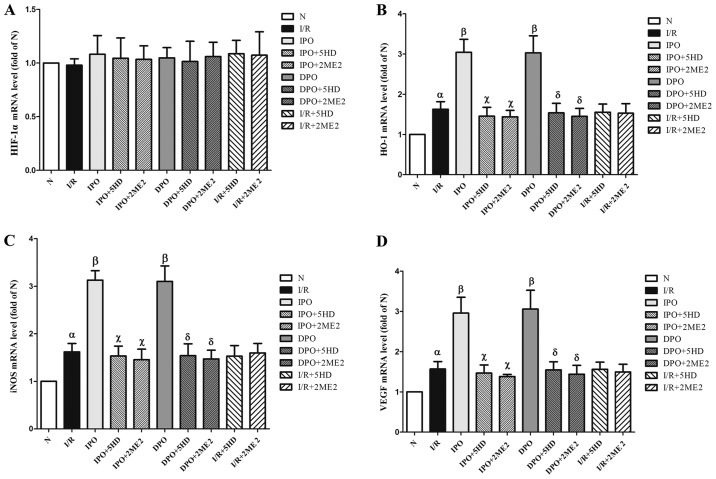
Expression of HIF-1/HRE related genes
There was no significant difference in HIF-1α mRNA expression among the groups (P>0.05; Fig. 4A). The expression levels of HO-1, iNOS and VEGF mRNA in group I/R were significantly increased compared with group N (P<0.05). In groups IPO and DPO, the expression levels (HO-1, iNOS and VEGF mRNA) were significantly increased compared with group I/R (P<0.05). In groups IPO+5HD and IPO+2ME2, the expression levels (HO-1, iNOS and VEGF mRNA) were significantly decreased compared with group IPO (P<0.05). In groups DPO+5HD and DPO+2ME2, the expression levels (HO-1, iNOS and VEGF mRNA) were significantly decreased compared with group DPO (P<0.05; Fig. 4).
Figure 4.
Expression of HIF-1/hypoxic response element-related genes in rats following myocardial ischemia/diazoxide post-conditioning. (A) Expression level of HIF-1α mRNA had no significant effect among the groups. The expression levels of (B) HO-1, (C) iNOS and (D) VEGF mRNA respectively. Compared with group I/R, group DPO/IPO significantly increased the expression levels of HO-1, iNOS and VEGF mRNA; while the blockers 5HD/2ME2 could reverse the above effects of DPO/IPO. The results are expressed as the mean ± standard error of the mean, n=6. αP<0.05 vs. group N; βP<0.05 vs. group I/R; χP<0.05 vs. group IPO; δP<0.05 vs. group DPO. IPO, ischemic post-conditioning; DPO, diazoxide post-conditioning; I/R, ischemia/reperfusion; 5HD, 5-hydroxydecanoic acid; 2ME2, 2-methoxyestradiol; HIF, hypoxia inducible factor; HO-1, heme-oxygenase-1; iNOS, inducible nitric oxide; VEGF, vascular endothelial growth factor.
In groups I/R+5HD and I/R+2ME2, compared with group I/R, there were no significant differences in the mRNA expression of HO-1, iNOS and VEGF (P>0.05; Fig. 4B-D).
Expression of HIF-1/HRE related proteins
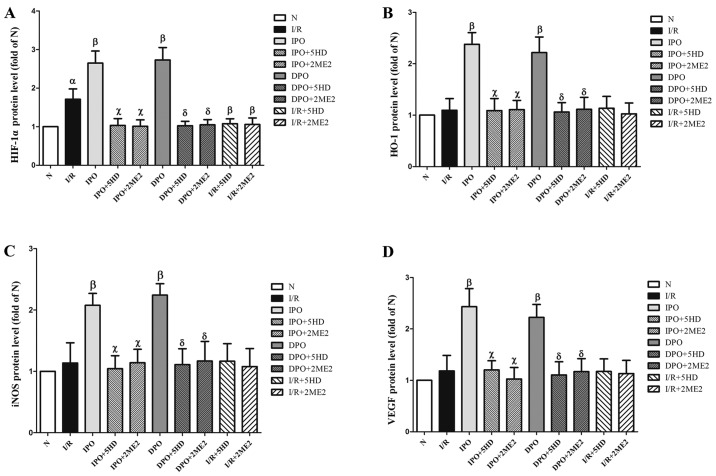
The expression of HIF-1α protein in group I/R was significantly increased compared with group N (P<0.05; Figs. 5 and 6A). The expression levels of HIF-1, HO-1, iNOS and VEGF in group IPO and DPO were significantly increased compared with those in group I/R (P<0.05; Figs. 5 and 6B-D). In groups IPO+5HD and IPO+2ME2, the expression levels (HIF-1, HO-1, iNOS and VEGF) were significantly decreased compared with those in group IPO (P<0.05; Figs. 5 and 6). In groups DPO+5HD and DPO+2ME2, the expression levels (HIF-1, HO-1, iNOS and VEGF) were significantly decreased compared with those in group DPO (P<0.05; Figs. 5 and 6).
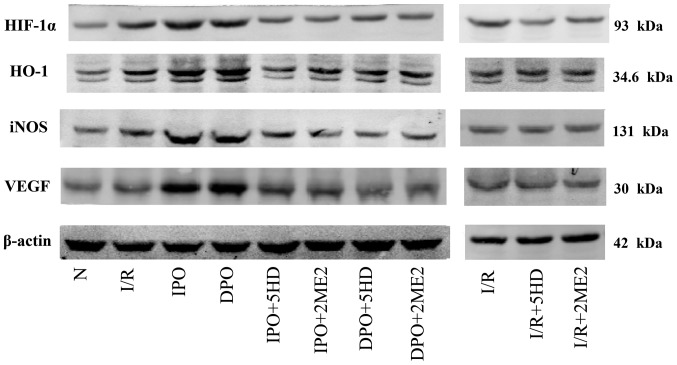
Figure 5.
Western blotting to evaluate the levels of hypoxia inducible factor/hypoxic response element pathway proteins. IPO, ischemic post-conditioning; DPO, diazoxide post-conditioning; I/R, ischemia/reperfusion; 5HD, 5-hydroxydecanoic acid; 2ME2, 2-methoxyestradiol; HO-1, heme-oxygenase-1; iNOS, inducible nitric oxide; VEGF, vascular endothelial growth factor.
Figure 6.
Expression of HIF-1/hypoxic response element-related proteins in rats following myocardial ischemia/diazoxide post-conditioning. The expression of (A) HIF-1α, (B) HO-1, (C) iNOS and (D) VEGF protein respectively. Compared with group I/R, group DPO/IPO significantly increased the expression levels of each protein; while the blockers 5HD/2ME2 could reverse the above effects of DPO/IPO. The results are expressed as the mean ± standard error of the mean, n=6. αP<0.05 vs. group N; βP<0.05 vs. group I/R; χP<0.05 vs. group IPO; δP<0.05 vs. group DPO. IPO, ischemic post-conditioning; DPO, diazoxide post-conditioning; I/R, ischemia/reperfusion; 5HD, 5-hydroxydecanoic acid; 2ME2, 2-methoxyestradiol; HIF, hypoxia inducible factor; HO-1, heme-oxygenase-1; iNOS, inducible nitric oxide; VEGF, vascular endothelial growth factor.
In groups I/R+5HD and I/R+2ME2, the protein expression of HO-1, iNOS and VEGF was not significantly different from that in group I/R (P>0.05; Figs. 5 and 6B-D), however, the expression of HIF-1α protein in groups I/R+5HD and I/R+2ME2 was decreased compared with group I/R (P<0.05; Figs. 5 and 6A).
Discussion
In the present study, the rat heart perfusion model was established with the aid of the Langendorff experimental device. The mechanism of action of IPO/DPO on MIRI was investigated using cardioplegia to simulate cardioversion after cardiopulmonary bypass.
In the present experiment, it was observed that compared with group N, the infarct size of group I/R and the mitochondrial Flameng score increased. Compared with group I/R, both the infarct size and mitochondrial Flameng score decreased in groups IPO and DPO. In addition, IPO/DPO increased the expression (genes and proteins) of the HIF-1/HRE pathway (HO-1, iNOS and VEGF) and HIF-1α protein. The myocardial protective effects of IPO/DPO and their activation of the HIF-1/HRE pathway-related products could be eliminated after the use of 5HD or 2ME2.
In addition, when groups I/R+5HD and I/R+2ME2 were compared to group I/R, it was found that 5HD and 2ME2 had no effect on MIRI. This was manifested by the size of the myocardial infarct and the mitochondrial Flameng scores of myocardial cells. Although HR, LVDP, LVEDP and +dp/dtmax directly reflect the state of cardiac function, changes in cardiac function may take a certain period of reperfusion to show. Therefore, there was no significant change in cardiac functional indexes at the end of perfusion in this experiment.
As an oxygen sensitive transcription factor, HIF can make aerobic organisms adapt to anoxia. HIF-1 is one of the most important factors in the HIF family and it is also an important target for the study of myocardial protection (18–20). HIF-1 is composed of α and β subunits. HIF-1α is HIF-1's regulatory protein, which is very sensitive to changes in the oxygen concentration and plays a key role in the regulation of HIF-1 function. HIF-1β is the basic expression protein and is not regulated by oxygen (21). Under normal oxygen supply, the proline and asparagine residues on HIF-1α are hydroxylated. This promotes binding to the ligase complex pVHL- ubiquitin E3 and rapid degradation. Under hypoxic conditions, the hydroxylase activity is inhibited, thereby preventing the degradation of HIF-1α. As a result, HIF-1α in the cytoplasm increased and then transferred into the nucleus, combining with HIF-1β. HIF in the nucleus can be combined with target gene promoters related to the hypoxia response element (22). HIF-1 can regulate numerous genes, including VEGF, HO-1 and iNOS. These factors are involved in a number of physiological responses, such as anaerobic metabolism, angiogenesis, erythrocyte production, cell proliferation and apoptosis (23,24).
HIF-1 is an important factor in the hypoxia response and the HIF-1/HRE signaling pathway also plays an important role in myocardial protection (25,26). Zhao et al (27) found that IPO could alleviate MIRI by upregulating HIF-1α in normal/hyperlipidemic rats. In this experiment, it was also demonstrated that IPO could reduce MIRI by activating the HIF-1/HRE signal pathway. IPO increased the expression (genes and proteins) of the HIF-1/HRE signaling pathway (HO-1, iNOS and VEGF), and it also increased the protein level of HIF-1α. After the use of 2ME2, the myocardial protection of IPO disappeared and the infarct area in group IPO+2ME2 was increased compared with in group IPO. The myocardial mitochondrial Flameng score in group IPO+2ME2 was increased compared with in group IPO. The myocardial structure of group IPO+2ME2 was largely the same as that in the I/R group and the structure of the myocardium was damaged, in that vacuoles had formed and mitochondria were swollen and ruptured. In group IPO+2ME2, the downstream expression related proteins and genes (HO-1, iNOS and VEGF) of the HIF-1/HRE pathway and HIF-1α were lower than in group IPO. This experiment also showed that DPO was similar to IPO.
Therefore, the present study showed that both IPO and DPO can reduce the area of myocardial infarction caused by MIRI, and reduce the damage to the myocardial cell structure by activating the HIF-1/HRE pathway.
IPO has been the basis of the theoretical system of ‘trigger-regulating medium-terminal effectors’. IPO induces the release of trigger factors, mediates the signaling pathway, acts on a variety of effectors and exerts protective effects on cardiac myocytes (28,29). A previous study showed that IPO could activate the protein kinase C and reperfusion injury salvage kinase pathways via the intracellular adenosine and NO concentration, finally acting on mitochondrial permeability transition pores and mitoKATP channels to play a role in myocardial protection (30). DPO can also open mitoKATP channels to reduce MIRI and the blocking of the opening of mitoKATP channels can eliminate myocardial protection (31). Therefore, as a result, mitoKATP channels may play a key role in mitigating MIRI.
As an eight-polymer channel located in the mitochondrial inner membrane of the cell, the mitoKATP channel allows inward access to potassium ions. The opening of the mitoKATP channels leads to the influx of potassium and the efflux of hydrogen, further alkalifying the mitochondrial matrix. Mitochondrial matrix alkalinization can cause the respiratory chain to produce ROS (32). ROS can regulate a variety of signaling factors, which includes nuclear transcription factor HIF-1 (33). In the present study, it was also shown that both IPO and DPO could alleviate MIRI by activating the HIF-1/HRE pathway. Therefore, it could be speculated that the activation of the HIF-1/HRE pathway may be related to the opening of mitoKATP channels in IPO/DPO.
Furthermore, the effects of 5HD (mitoKATP channel specific inhibitor) on MIRI and the expression of HIF-1/HRE pathway components were observed in the IPO/DPO groups. IPO/DPO reduced the myocardial infarct area and mitigated myocardial mitochondrial damage, increasing the expression of HIF-1/HRE pathway components (HO-1, iNOS and VEGF) and HIF-1α protein. After the use of 5HD, the above myocardial protective effect of IPO/DPO disappeared and the expression (genes and proteins) of the HIF-1/HRE pathway and HIF-1α protein decreased. It is suggested that IPO/DPO can activate the HIF-1/HRE pathway to relieve MIRI by opening the mitoKATP channels.
Based on the results of the present study, it is possible to speculate that both IPO and DPO may open mitoKATP channels and in turn activate the HIF-1/HRE pathway to alleviate MIRI.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science Technology Fund Projects of Guizhou Province (grant nos. qian ke he SY zi 2014 and 2188).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JL, WJZ, WC, YZ, HYW and TY participated in the study design. JL, WC and WJZ performed the experiments. JL, WJZ, WC, YZ, HYW and TY performed the data analysis. JL, WJZ and WC wrote the manuscript. JL, WJZ, WC, YZ, HYW and TY read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee on Animal Laboratory of Zunyi Medical College. The use and processing of animals was in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institute of Health (NIH Publication 88.23, revised 1996).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960;70:68–78. [PubMed] [Google Scholar]
- 2.Hausenloy DJ, Boston-Griffiths E, Yellon DM. Cardioprotection during cardiac surgery. Cardiovasc Res. 2012;94:253–265. doi: 10.1093/cvr/cvs131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frankenreiter S, Groneberg D, Kuret A, Krieg T, Ruth P, Friebe A, Lukowski R. Cardioprotection by ischemic postconditioning and cyclic guanosine monophosphate-elevating agents involves cardiomyocyte nitric oxide-sensitive guanylyl cyclase. Cardiovasc Res. 2018;114:822–829. doi: 10.1093/cvr/cvy039. [DOI] [PubMed] [Google Scholar]
- 4.Pachauri P, Garabadu D, Goyal A, Upadhyay PK. Angiotensin (1–7) facilitates cardioprotection of ischemic preconditioning on ischemia-reperfusion-challenged rat heart. Mol Cell Biochem. 2017;430:99–113. doi: 10.1007/s11010-017-2958-4. [DOI] [PubMed] [Google Scholar]
- 5.Hao YL, Fang HC, Zhao HL, Li XL, Luo Y, Wu BQ, Fu MJ, Liu W, Liang JJ, Chen XH. The role of microRNA-1 targeting of MAPK3 in myocardial ischemia-reperfusion injury in rats undergoing sevoflurane preconditioning via the PI3K/Akt pathway. Am J Physiol Cell Physiol. 2018;315:C380–C388. doi: 10.1152/ajpcell.00310.2017. [DOI] [PubMed] [Google Scholar]
- 6.Zhang FW, Tong J, Yan YS, Chen QQ, Zhao XP. ω-3 polyunsaturated fatty acid postconditioning protects the isolated perfused rat heart from ischemia-reperfusion injury. Cardiorenal Med. 2018;8:173–182. doi: 10.1159/000487490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YH, Zhang Y, Chen W, Wang Y, Cao S, Yu T, Wang H. Pinacidil-postconditioning is equivalent to ischemic postconditioning in defeating cardiac ischemia-reperfusion injury in rat. Eur J Pharmacol. 2016;780:26–32. doi: 10.1016/j.ejphar.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Testai L, Marino A, Piano I, Brancaleone V, Tomita K, Di Cesare Mannelli L, Martelli A, Citi V, Breschi MC, Levi R, et al. The novel H2S-donor 4-carboxyphenyl isothiocyanate promotes cardioprotective effects against ischemia/reperfusion injury through activation of mitoKATP channels and reduction of oxidative stress. Pharmacol Res. 2016;113:290–299. doi: 10.1016/j.phrs.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Hnatiuk AP, Ong SG, Olea FD, Locatelli P, Riegler J, Lee WH, Jen CH, De Lorenzi A, Giménez CS, Laguens R, et al. Allogeneic mesenchymal stromal cells overexpressing mutant human hypoxia-inducible factor 1-α (HIF1-α) in an ovine model of acute myocardial infarction. J Am Heart Assoc. 2016;5:e003714. doi: 10.1161/JAHA.116.003714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao HX, Wang XL, Wang YH, Wu Y, Li XY, Lv XP, Zhao ZQ, Zhao RR, Liu HR. Attenuation of myocardial injury by postconditioning: Role of hypoxia inducible factor-1alpha. Basic Res Cardiol. 2010;105:109–118. doi: 10.1007/s00395-009-0044-0. [DOI] [PubMed] [Google Scholar]
- 11.Si J, Wang N, Wang H, Xie J, Yang J, Yi H, Shi Z, Ma J, Wang W, Yang L, et al. HIF-1α signaling activation by post-ischemia treatment with astragaloside IV attenuates myocardial ischemia-reperfusion injury. PLoS One. 2014;9:e107832. doi: 10.1371/journal.pone.0107832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin C, Wu J, Watanabe M, Okada T, Iesaki T. Mitochondrial K+ channels are involved in ischemic postconditioning in rat hearts. J Physiol Sci. 2012;62:325–332. doi: 10.1007/s12576-012-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penna C, Perrelli MG, Tullio F, Angotti C, Camporeale A, Poli V, Pagliaro P. Diazoxide postconditioning induces mitochondrial protein S-nitrosylation and a redox-sensitive mitochondrial phosphorylation/translocation of RISK elements: No role for SAFE. Basic Res Cardiol. 2013;108:371. doi: 10.1007/s00395-013-0371-z. [DOI] [PubMed] [Google Scholar]
- 14.Flagg TP, Enkvetchakul D, Koster JC, Nichols CG. Muscle KATP channels: Recent insights to energy sensing and myoprotection. Physiol Rev. 2010;90:799–829. doi: 10.1152/physrev.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olejnickova V, Novakova M, Provaznik I. Isolated heart models: Cardiovascular system studies and technological advances. Med Biol Eng Comput. 2015;53:669–678. doi: 10.1007/s11517-015-1270-2. [DOI] [PubMed] [Google Scholar]
- 16.Flameng W, Borgers M, Daenen W, Stalpaert G. Ultrastructural and cytochemical correlates of myocardial protection by cardiac hypothermia in man. J Thorac Cardiovasc Surg. 1980;79:413–424. doi: 10.1016/S0022-5223(19)37950-4. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Xie P, Wu J, Yu J, Yu T, Wang H, Wang J, Xia Z, Zheng H. Sevoflurane postconditioning improves myocardial mitochondrial respiratory function and reduces myocardial ischemia-reperfusion injury by up-regulating HIF-1. Am J Transl Res. 2016;8:4415–4424. [PMC free article] [PubMed] [Google Scholar]
- 19.Eckle T, Kohler D, Lehmann R, El KK, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: A new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 20.Ong SG, Hausenloy DJ. Hypoxia-inducible factor as a therapeutic target for cardioprotection. Pharmacol Ther. 2012;136:69–81. doi: 10.1016/j.pharmthera.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Xie J, Liao Y, Yang L, Wu J, Liu C, Xuan W, Li M, Zhang L, Liu Y, Wu P, Bin J. Ultrasound molecular imaging of angiogenesis induced by mutant forms of hypoxia-inducible factor-1α. Cardiovasc Res. 2011;92:256–266. doi: 10.1093/cvr/cvr229. [DOI] [PubMed] [Google Scholar]
- 22.Tian YM, Mole DR, Ratcliffe PJ, Gleadle JM. Characterization of different isoforms of the HIF prolyl hydroxylase PHD1 generated by alternative initiation. Biochem J. 2006;397:179–186. doi: 10.1042/BJ20051996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tekin D, Dursun AD, Xi L. Hypoxia inducible factor 1 (HIF-1) and cardioprotection. Acta Pharmacol Sin. 2010;31:1085–1094. doi: 10.1038/aps.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimna A, Kurpisz M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: Applications and therapies. Biomed Res Int. 2015;2015:549412. doi: 10.1155/2015/549412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poynter JA, Manukyan MC, Wang Y, Brewster BD, Herrmann JL, Weil BR, Abarbanell AM, Meldrum DR. Systemic pretreatment with dimethyloxalylglycine increases myocardial HIF-1α and VEGF production and improves functional recovery after acute ischemia/reperfusion. Surgery. 2011;150:278–283. doi: 10.1016/j.surg.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Zhao H, Wu Y, Zhang S, Zhao X, Zhang Y, Wang J, Wang J, Liu H. Up-regulation of hypoxia-inducible factor-1alpha enhanced the cardioprotective effects of ischemic postconditioning in hyperlipidemic rats. Acta Biochim Biophys Sin (Shanghai) 2014;46:112–118. doi: 10.1093/abbs/gmt132. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Wang Y, Wu Y, Li X, Yang G, Ma X, Zhao R, Liu H. Hyperlipidemia does not prevent the cardioprotection by postconditioning against myocardial ischemia/reperfusion injury and the involvement of hypoxia inducible factor-1alpha upregulation. Acta Biochim Biophys Sin (Shanghai) 2009;41:745–753. doi: 10.1093/abbs/gmp063. [DOI] [PubMed] [Google Scholar]
- 28.Granfeldt A, Jiang R, Wang NP, Mykytenko J, Eldaif S, Deneve J, Zhao ZQ, Guyton RA, Tønnesen E, Vinten-Johansen J. Neutrophil inhibition contributes to cardioprotection by postconditioning. Acta Anaesthesiol Scand. 2012;56:48–56. doi: 10.1111/j.1399-6576.2011.02577.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang ZH, Liu JL, Wu L, Yu Z, Yang HT. Concentration-dependent wrestling between detrimental and protective effects of H2O2 during myocardial ischemia/reperfusion. Cell Death Dis. 2014;5:e1297. doi: 10.1038/cddis.2014.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 31.Ichinomiya T, Cho S, Higashijima U, Matsumoto S, Maekawa T, Sumikawa K. High-dose fasudil preserves postconditioning against myocardial infarction under hyperglycemia in rats: Role of mitochondrial KATP channels. Cardiovasc Diabetol. 2012;11:28. doi: 10.1186/1475-2840-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol. 2006;291:H2067–H2074. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- 33.Koshikawa N, Hayashi J, Nakagawara A, Takenaga K. Reactive oxygen species-generating mitochondrial DNA mutation up-regulates hypoxia-inducible factor-1alpha gene transcription via phosphatidylinositol 3-kinase-Akt/protein kinase C/histone deacetylase pathway. J Biol Chem. 2009;284:33185–33194. doi: 10.1074/jbc.M109.054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.