Abstract
The aim of the present study was to investigate the effect of urantide on collagen metabolism in the hearts of rats with atherosclerosis (AS) by evaluating the expression of Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) pathway constituents. Urantide was delivered to rats with AS via tail vein injection for 3, 7 and 14 days. Serological indicators were identified by an automated biochemical analyzer. Histomorphological changes in the cardiac tissue of rats were observed by pathological staining techniques. The expression of genes and proteins was assessed using reverse transcription-quantitative PCR and western blot analysis, respectively. Localization of proteins was detected by immunofluorescence. Overexpression of urotensin II (UII) and its receptor, G protein-coupled receptor 14 (GPR14), was observed in the hearts of rats with AS and the expression of both proteins significantly declined after urantide administration. Triglyceride, total cholesterol, low-density lipoprotein, high-density lipoprotein and calcium levels were improved in rats with AS following treatment with urantide. Notably, urantide was able to antagonize the UII/GPR14 system. Urantide treatment resulted in markedly decreased expression levels of matrix metalloproteinase 2 (MMP-2), collagen type I/III, and genes and proteins in the JAK2/STAT3 pathway. By contrast, TIMP metallopeptidase inhibitor 2 (TIMP-2) levels were increased. In addition, the MMP-2/TIMP-2 protein ratio was significantly decreased in rats treated with urantide compared with AS rats with no urantide treatment. Constituents of the JAK2/STAT3 pathway and collagen type I/III were found to be localized in the diseased tissue and blood vessels of the hearts of rats with AS. In conclusion, urantide was able to effectively block the UII/GPR14 system by regulating the JAK2/STAT3 pathway and collagen metabolism. Inhibition of the UII/GPR14 system may prevent and potentially treat atherosclerotic myocardial fibrosis. Based on the current results, it was hypothesized that collagen metabolism may be associated with the JAK2/STAT3 pathway.
Keywords: urantide, atherosclerosis, urotensin II, collagen metabolism, myocardial fibrosis, Janus kinase 2/signal transducer and activator of transcription 3 pathway
Introduction
Atherosclerosis (AS) is a serious disease that causes hypoxic ischemia of the heart and leads to disordered collagen metabolism during its progression (1,2). Multiple studies have revealed that the urotensin II/urotensin report (UII/UT) system, which is composed of UII and its receptor, G protein-coupled receptor 14 (GPR14), serves an important role in various diseases, including AS and heart disease (3,4). Therefore, the prevention and treatment of AS via regulation of the UII/UT system may prove to be effective for the improvement of myocardial collagen metabolism disorders.
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway is a key cytokine signaling pathway that regulates various pathophysiological processes, including inflammation and myocardial fibrosis (5–7). In addition, the proteolytic enzymes matrix metalloproteinase (MMP) and tissue inhibitor of matrix metalloproteinase can regulate the expression of collagen, which serves an important role in myocardial fibrosis and cardiac dysfunction (8). Study has reported that the JAK2/STAT3 pathway, MMP-2 and MMP-9 are strongly associated with the prevention of myocardial injury by reducing inflammation and fibrosis (9). In the present study, the state of collagen metabolism disorders was monitored in rats with AS by observing changes in the JAK2/STAT3 pathway and the MMP-2/TIMP metallopeptidase inhibitor 2 (TIMP-2) protein ratio.
Urantide, a UII receptor antagonist derived from human UII (hUII), competitively antagonizes the contraction of the rat thoracic aorta and the mitogenic effect of UII on vascular smooth muscle cells (10,11). The main reason for conducting the present study was the fact that little research regarding the association between the effects of urantide on the prevention and treatment of AS and AS-related heart disease has been performed. Based on the successful replication of an AS rat model, it was demonstrated that urantide was able to significantly reduce myocardial fibrosis in rats with AS. This effect may have been associated with the JAK2/STAT3 pathway and the MMP-2/TIMP-2 protein ratio.
Materials and methods
Animals and treatment groups
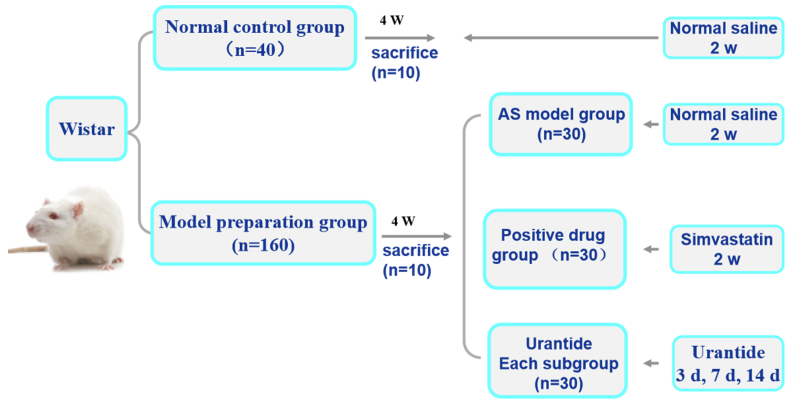
A total of 200 Wistar male rats (age, 4 weeks) weighing 180–200 g were obtained from Charles River Laboratories, Inc. and housed at a constant temperature of 22±2°C with a humidity of 40–60% and under a 12-h light/dark cycle. The rats were randomly divided into two groups: A normal control group consisting of 40 rats and an AS model preparation group consisting of 160 rats. Rats in the normal control group were supplied with normal feed daily and rats in the AS model preparation group followed a high-fat diet according to an AS rat preparation method, as previously described (12,13). The high-fat diet was purchased from Beijing Keao Xieli Feed Co., Ltd. The experimental period lasted 4 weeks.
An AS rat model was established by intraperitoneal (i.p.) injection of 150 U/kg vitamin D3 (Harbin Huasheng Science and Technology Animal Medicine Factory) for 3 days, and rats were continuous feeding a high-fat diet to damage the arterial intima (14). In the fourth week, 10 rats from each of the two experimental groups were randomly sacrificed by i.p. injection of sodium phenobarbital (150 mg/kg) in order to detect whether the AS rat model had been successfully replicated. Subsequently, the rats with AS were randomly divided into the following groups: i) An AS model group (30 rats); ii) a simvastatin group (30 rats); and iii) three urantide groups (30 rats in each of the three subgroups). Saline (30 µg/kg) was injected into the tail veins of rats in the normal control group and the AS model group for 14 consecutive days. Rats in the simvastatin group were intraperitoneally injected with 5 µg/kg simvastatin (Lunan Beite Pharmaceutical Co., Ltd.) for 14 days. Urantide (30 µg/kg; Shanghai Qiangyao Biological Technology Co., Ltd.) was injected into the tail veins of rats with AS in the three urantide groups daily for 3, 7 and 14 days, respectively. In the present study, the dosages of simvastatin and urantide were based on two preliminary studies by Zhao et al (10,11). The rats were given ad libitum access to food and water. The experimental design is presented in Fig. 1.
Figure 1.
Experimental design. The setting of the experimental group, the interventions of each group and the intervention time. AS, atherosclerosis; w, weeks; d, days; n, number.
Sample collection and testing
All rats were sacrificed using 150 mg/kg sodium pentobarbital (Tianjin Fuchen Chemical Co., Ltd.). The rat abdominal aortas were punctured to obtain blood, and following centrifugation at 1,500 × g for 10 min at 4°C, the supernatant was stored at −20°C. In the present study, automated biochemical analysis (BS-480; Shenzhen Mindray Bio-Medical Electronics Co., Ltd.) at Hebei Province 266 Hospital was performed to determine triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL) and calcium (Ca2+) levels.
Morphological evaluation
The thoracic aortas and hearts were dissected from the rats and divided into two parts; one part was fixed with 4% paraformaldehyde at 4°C for 24 h for morphological examination, while the other part was stored directly in liquid nitrogen for molecular biological detection.
Paraffinized, 4.5-µm sections of rat thoracic aortas and cardiac tissue were prepared and hematoxylin and eosin (H&E) staining (Nanchang Yulu Experimental Equipment Co., Ltd.) was performed according to the manufacturer's instructions. A total of 10 heart sections were randomly selected from each group. Each section was examined for evidence of mononuclear and polymorphic cell infiltration, necrosis and mineralization and given a histological score of 0 to 4 as follows: i) 0, no change; ii) 1, changes found in 0–25% of cells; iii) 2, changes found in 26–50% of cells; iv) 3, changes found in 51–75% of cells; and v) 4, changes found in 76–100% of cells (15).
Masson trichrome staining and immunolocalization
Collagen content in the cardiac tissue was determined using Masson trichrome staining (Fuzhou Maixin Biotech Co., Ltd.). The ratio of collagen positive area to total area was calculated using a computer imaging system. The distribution of phosphorylated (p)-JAK2 (cat. no. ab32101; Abcam), p-STAT3 (phosphorylated-STAT3; cat. no. 9145; Cell Signaling Technology, Inc.) and collagen type I/III (cat. no. bs-0578R/0948R; Bioss) proteins was determined using immunofluorescence. The isolated paraffinized heart sections were dewaxed by xylene, and following their dehydration, stained for collagen at 22±2°C for 5 min and incubated with antibodies against p-JAK2 (cat. no. ab32101; Abcam) and p-STAT3 (cat. no. 9145; Cell Signaling Technology, Inc.) and collagen type I/III (cat. no. bs-0578R/0548R; BIOSS) at 1:300 at 4°C for 12 h according to a standard protocol (16). Next, the sections which were immunoblotted for p-JAK2/p-STAT3 and collagen type I/III were incubated with FITC-labeled goat anti-rabbit IgG antibody (cat. no. A0562; Beyotime Institute of Biotechnology) at 1:1,000 at 37°C for 60 min. Then, the myocardial collagen volume (%) was calculated using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.). Images were captured using a fluorescence microscope (Olympus Corporation).
Quantification of mRNA expression levels by reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the cardiac tissues using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and quantified by UV spectrophotometry. Subsequently, total RNA was reverse transcribed into cDNA using a FastQuant RT kit (Tiangen Biotech Co., Ltd.) at 42°C for 15 min and 95°C for 3 min. qPCR was subsequently performed using the SuperReal PreMix Plus (SYBR Green) fluorescence quantitative premixing kit (Tiangen Biotech Co., Ltd.). The related gene primers were synthesized by Takara Biotechnology Co., Ltd. and their respective sequences are presented in Table I. According to the manufacturer's protocol, the following thermocycling conditions were used for qPCR: 95°C for 15 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 32 sec. mRNA expression levels were quantified using the 2−ΔΔCq method and normalized to the internal reference gene β-actin (17,18).
Table I.
Primer sequences used for the reverse transcription-quantitative PCR analysis of relative mRNA expression levels.
| Gene | Species | Primer sequence (5′→3′) |
|---|---|---|
| UII | Rat | F: GGAGGAGCTGGAGAGGACTG |
| R: GAGTCTCGGCACTGGGATCT | ||
| GPR14 | Rat | F: AATGGCTCTAGGGTCCTCCT |
| R: AACAGCCTCTGTGATGGACA | ||
| JAK2 | Rat | F: TTTGGATCCCTGGATACATACCTGA |
| R: TGGCACACACATTCCCATGA | ||
| STAT3 | Rat | F: TTTGAGACAGAGGTGTACCACCAAG |
| R: ACCACAGGATTGATGCCCAAG | ||
| MMP-2 | Rat | F: GGACAGTGACACCACGTGACAA |
| R: TTTCCAAAGTGCTGGCAGAATAGAC | ||
| TIMP-2 | Rat | F: GACACGCTTAGCATCACCCAGA |
| R: CTGTGACCCAGTCCATCCAGAG | ||
| Collagen I | Rat | F: CCCTGCTGGAGAAGAAGGAA |
| R: AGGAGAACCTTTGGGACCAG | ||
| Collagen III | Rat | F: ACTGGTGAACGTGGCTCTAA |
| R: GGACCTGGATGTCCACTTGA | ||
| β-actin | Rat | F: CAGGCATTGCTGACAGGATG |
| R: TGCTGATCCACATCTGCTGG |
UII, urotensin II; GPR14, G protein-coupled receptor 14; JAK2, Janus kinase 2; STAT3, signal transducer and activator of transcription 3; MMP-2, matrix metalloproteinase 2; TIMP-2, TIMP metallopeptidase inhibitor 2; F, forward; R, reverse.
Western blot analysis
Western blotting was conducted to evaluate cardiac protein expression. Total protein was extracted from the heart tissue using RIPA protein lysate (Beijing Solarbio Science & Technology Co., Ltd.). Protein content was quantified using the bicinchoninic acid Protein Concentration assay kit (Beijing Solarbio Science & Technology Co., Ltd.). A total of 45 µg protein/lane was separated via 8% SDS-PAGE and transferred to an immobilon-P PVDF membrane, which was blocked with 5% skimmed milk powder at 22±2°C for 1.5 h. Next, the membrane was incubated with primary antibodies against UII (cat. no. orb352970; Biorbyt Ltd.), GPR14 (cat. no. sc-514460; Santa Cruz Biotechnology, Inc.) and p-STAT3 (cat. no. 9145; Cell Signaling Technology, Inc.) at a 1:800 dilution; JAK2 and STAT3 (cat. nos. 380903 and 310003; http://www.zen-bio.cn/; Zen Bioscience) at a 1:500 dilution; p-JAK2 (cat. no. ab32101; Abcam) and MMP-2/TIMP-2 (cat. no. BS1236/1366; https://www.bioworlde.com/Search.aspx?keyword=1366&Category=233&x=7&y=10; Bioworld Technology, Inc.) at a 1:1,000 dilution; collagen type I/III (cat. no. bs-0578R/0548R; Bioss) at a 1:500 dilution; or GAPDH (cat. no. bs-10900R; Bioss) at a 1:1,000 dilution at 4°C for 14–16 h. The membranes were washed with TBST (0.1% Tween-20; Beijing Solarbio Science & Technology Co., Ltd.) three times and then incubated with horseradish peroxidase-labeled goat anti-rabbit or anti-mouse secondary antibodies (cat. no. 074-1056/074-1086; KPL, Inc.) at a 1:5,000 dilution at room temperature for 1 h. Protein bands were visualized using a hypersensitive ECL chemiluminescence kit (Beyotime Institute of Biotechnology). The intensities of each protein band were analyzed using Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.) with GAPDH as the loading control.
Statistical analysis
Data are expressed as the mean ± SEM of the mean of ≥3 independent experiments and were analyzed using SPSS software (version 20; IBM Corp.). The serum index contents, and gene and protein expression levels in each group of rats were analyzed by one-way analysis of variance followed by Tukey's post hoc test. Cardiac injury scores were analysed by Friedman followed by Nemenyi post hoc test for multiple comparisons. GraphPad Prism (version 7; GraphPad Software, Inc.) software was used to generate the charts. P<0.05 was considered to indicate a statistically significant difference.
Results
Assessing the rat model of AS
After 4 weeks, the thoracic aortas dissected from rats in the AS model preparation group were found to contain diverse characteristics typical of AS lesions, in contrast to the thoracic aortas of rats in the normal control group. These characteristics included: i) Intimal inflammatory cell infiltration; ii) obvious calcification; iii) vascular smooth muscle cell proliferation and disorder; iv) foam-like cell formation; v) elastic fiber degeneration; and vi) rupture and disintegration. The specific results from H&E staining are presented in Fig. 2.
Figure 2.
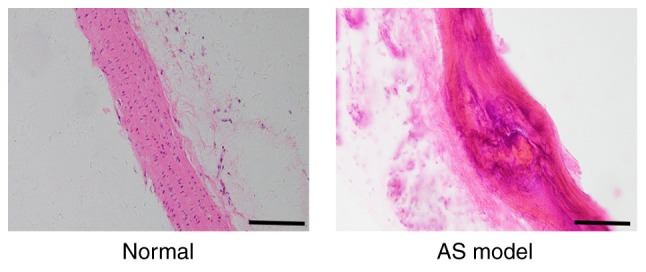
Histological changes in the thoracic aortas of atherosclerotic rats induced by a high-fat diet identified by hematoxylin and eosin staining. In the normal group, the arterial intima was intact and smooth, and the cells were orderly arranged. In the AS model group, atherosclerotic plaque of the intima was present and the arrangement of the cells was disordered. Scale bars, 100 µm. AS, atherosclerosis.
Blood lipid and Ca2+levels
Compared with the levels in the normal group, the serum TG, TC, LDL and Ca2+ levels in the AS model preparation group were significantly increased, whereas HDL levels were decreased. Furthermore, the serum TG, TC, LDL and Ca2+ levels in the simvastatin and urantide groups were obviously decreased, in contrast to the HDL content, which was significantly increased. It was revealed that urantide and simvastatin significantly improved blood lipid and Ca2+ levels in rats with AS. The specific results are displayed in Table II.
Table II.
Comparison of the levels of blood lipid components and Ca2+ in the sera of atherosclerotic and non-atherosclerotic rats.
| Group | TC (mmol/l) | TG (mmol/l) | HDL (mmol/l) | LDL (mmol/l) | Ca2+ (mmol/l) |
|---|---|---|---|---|---|
| Normal | 2.07±0.61 | 0.28±0.08 | 2.95±0.93 | 0.27±0.05 | 2.60±0.56 |
| AS model | 11.18±3.12a | 0.77±0.12a | 0.93±0.23a | 1.70±0.31a | 3.95±0.42a |
| Simvastatin | 4.06±1.51a,b | 0.41±0.12b,c | 2.16±0.35b,c | 0.77±0.19a,b | 3.63±0.13a,d |
| Urantide treatment | |||||
| 3 days | 7.52±1.59a,b,e | 0.55±0.20a,d,f | 1.48±0.37a,b,e | 1.05±0.31a,b,e | 3.46±0.32a,d |
| 7 days | 5.28±0.59a,b,f | 0.37±0.15b | 1.91±0.66a,d | 0.84±0.27a,b | 3.22±0.29a,b,f |
| 14 days | 8.33±1.43a,b,e | 0.59±0.21a,f | 1.02±0.44a,e | 1.27±0.27a,b,e | 3.68±0.30a |
TC, total cholesterol; TG, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Ca2+, calcium. Differences between groups were detected by an automatic biochemical analyzer (mmol/l).
P<0.01 vs. the normal group
P<0.01 vs. the AS model group
P<0.05 vs. the normal group
P<0.05 vs. the AS model group
P<0.01
P<0.05 vs. the simvastatin group. Data are presented as the mean ± SEM (n=6–8 rats in each group).
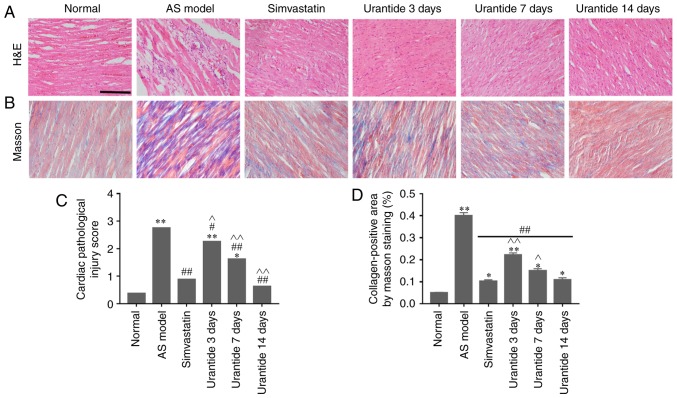
Cardiac pathological changes in rats with AS
Morphological methods were employed to assess AS-related cardiac damage and collagen metabolism. In the normal group, neatly arranged myocardial fibers, oval-shaped and centered nuclei, no atrophy or hypertrophy of the myocardial cells, and the microscopic expression of collagen fibers were observed. By contrast, the AS model preparation group exhibited cardiomyocyte hypertrophy, interstitial fibrosis, increased neutrophil infiltration between cells, scattered foam-like cell formation, hyperemia and abundant fibers (Fig. 3A and B). Furthermore, both cardiac damage scores and myocardial collagen volumes were significantly higher in the AS model group than in the control group (Fig. 3C and D). Compared with the AS model group, the simvastatin and urantide groups were shown to have significantly reduced cardiomyocyte fibrosis and neutrophil infiltration, and lower cardiac injury scores and myocardial collagen volumes. In addition, these improvements were positively associated with treatment time and the effect of 14-day urantide treatment was similar to that observed in the positive control group.
Figure 3.
Alleviation of heart disease in rats with AS following urantide treatment. The following cardiac parameters improved with treatment time: (A) Pathological symptoms, (B) collagen expression, (C) cardiac pathological injury score, and (D) myocardial collagen volume. Scale bar, 50 µm. *P<0.05, **P<0.01 vs. the normal group; #P<0.05, ##P<0.01 vs. the AS model group; ^P<0.05, ^^P<0.01 vs. the simvastatin group. Data are presented as the mean ± SEM (n=6–8 rats in each group). H&E, hematoxylin & eosin; AS, atherosclerosis.
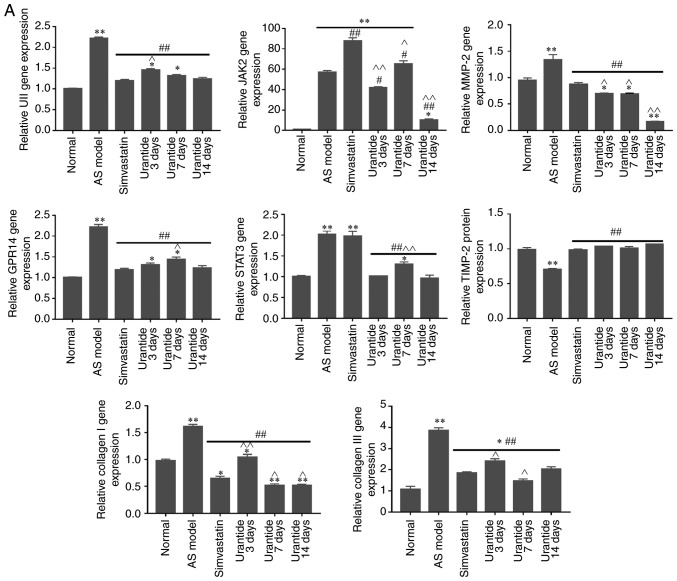
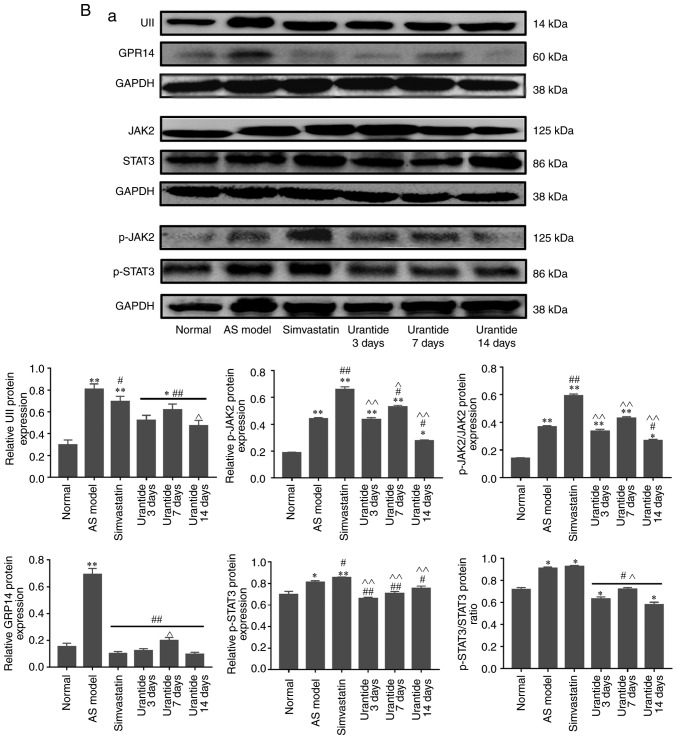
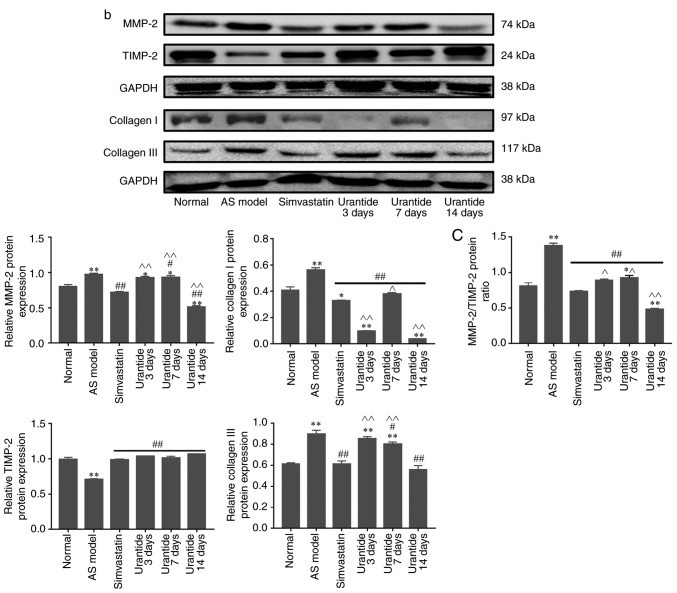
Alterations in the expression levels of genes and proteins in cardiac tissue
Molecular biology techniques were used to detect gene and protein expression in cardiac tissue. Compared with that in the normal group, the expression of UII, GPR14, JAK2, STAT3, MMP-2 and collagen type I/III was higher in the cardiac tissue of the AS model group, whereas TIMP-2 expression was lower. Furthermore, the expression levels of UII, GPR14, JAK2, STAT3, MMP-2 and collagen type I/III were downregulated in the cardiac tissue of rats in the urantide groups compared with the AS model group; however, TIMP-2 expression was upregulated (Fig. 4A and B). Most importantly, when the rats with AS received treatment, the protein ratio of p-JAK2/JAK2, p-STAT3/STAT3 and MMP-2/TIMP-2 was effectively improved as the corresponding protein ratio decreased after urantide treatment (Fig. 4Ba and C). Moreover, improved myocardial collagen metabolism was positively associated with the urantide treatment time and the effects of urantide were similar to those of the positive control drug. The specific results are shown in Fig. 4. However, the protein ratio of p-JAK2/JAK2 fluctuated at different times of urantide treatment, and the trend was consistent with serological indicators. In addition, simvastatin had little effect on the protein ratio of p-JAK2/JAK2 and p-STAT3/STAT3, as opposed to urantide treatment. Therefore, the specific mechanism underlying these effects require further study.
Figure 4.
Effect of urantide on UII/GPR14, the JAK2/STAT3 pathway and collagen metabolism in the cardiac tissue of rats with AS. Changes in the expression of the following genes and proteins with increasing drug administration time: (A) relative expression level of genes. Changes in the expression of the following genes and proteins with increasing drug administration time: (B-a) relative expression level of signaling pathway proteins, (B-b) relative expression level of collagen metabolism related proteins and (C) MMP-2/TIMP-2 protein ratio. *P<0.05, **P<0.01 vs. the normal group; #P<0.05, ##P<0.01 vs. the AS model group; ^P<0.05, ^^P<0.01 vs. the simvastatin group. Data are presented as the mean ± SEM (n=6–8 rats in each group). AS, atherosclerosis; UII, urotensin II; G protein-coupled receptor 14; JAK2, Janus kinase 2; p, phosphorylated; STAT3, signal transducer and activator of transcription 3; MMP-2, matrix metalloproteinase 2; TIMP-2, TIMP metallopeptidase inhibitor 2.
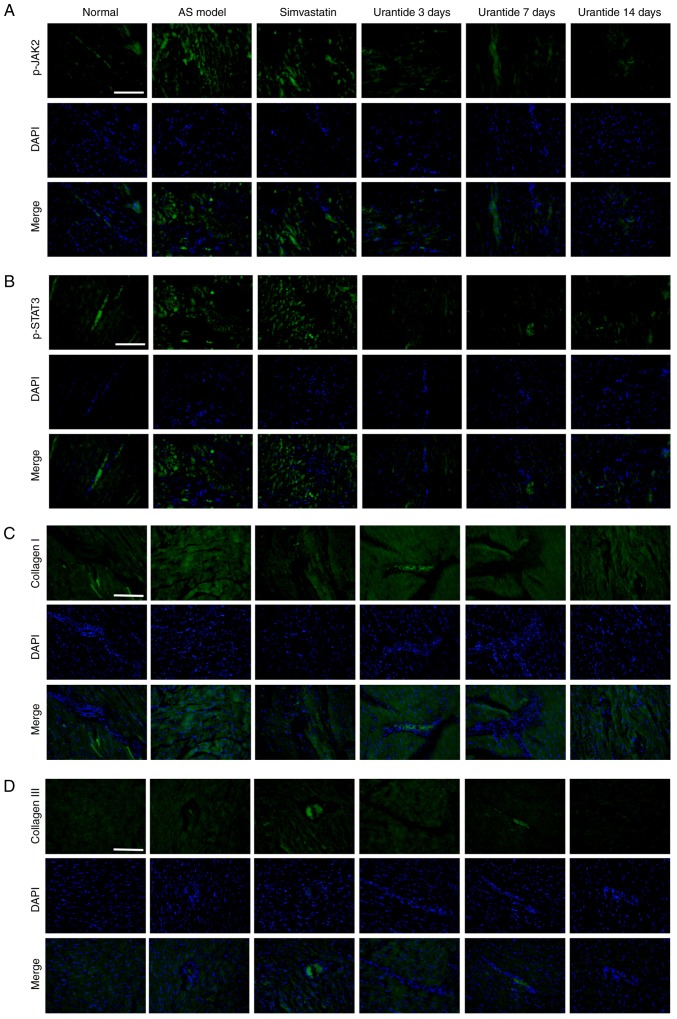
Localization of p-JAK2/p-STAT3 and collagen type I/III expression in cardiac tissue
Localization of the p-JAK2/p-STAT3 and collagen type I/III proteins was determined by immunofluorescence. The expression of p-JAK2, p-STAT3, and collagen types I and III in the cardiac tissue of rats in the AS model group was increased compared with the normal group. In addition, vascular and myocardial degeneration, and necrosis were identified in the AS model group (Fig. 5). Compare with the AS model group, the expression levels of p-JAK2, p-STAT3, collagen types I and III were gradually restored in AS rats treated with urantide, which mainly exhibited perivascular and myocardial degeneration and necrosis. Moreover, this effect was positively associated with treatment time.
Figure 5.
Effect of urantide on the distribution and localization of p-JAK2/p-STAT3 and collagen type I/III. Immunofluorescence staining of: (A) p-JAK2, (B) p-STAT3, (C) collagen I and (D) collagen III indicating the location and intensity of the positive expression of these proteins in cardiac tissue derived from each group. Scale bars, 50 µm. Blue, nucleus; green, positive expression. AS, atherosclerosis; p-JAK2, phosphorylated-Janus kinase 2; p-STAT3, phosphorylated-signal transducer and activator of transcription 3.
Discussion
In the early stages of AS, type III collagen has been reported to have important compensatory effects on cardiac rearrangement and muscle bundle formation, which maintain the normal function of he tissue in the early stage of the disease (19,20). However, in the advanced stages of AS, the accumulation of type I collagen in the myocardial interstitium occludes vasodilation and blood flow (19,21). It was hypothesized that the effective regulation of disordered collagen metabolism may have important clinical significance in the treatment of AS.
The results demonstrated significant hyperlipidemia and myocardial hypertrophy, neutrophil infiltration, hyperemia and hemorrhage in the cardiac tissue of rats with AS, accompanied by abundant collagen. In addition, the UII/UT system in the cardiac tissue of rats with AS was activated. Luo et al (22) found that UII induces the synthesis of type I collagen fibers and promotes the occurrence of myocardial fibrosis in rats. Previous studies performed by the same research group have reported that urantide had a protective effect on the thoracic aortas and livers of rats with AS following inhibition of the UII/UT system (6,23). The possibility of urantide treatment having a reparative effect on disordered collagen metabolism caused by AS was further investigated in the present study.
AS was shown to induce JAK2/STAT3 pathway activation and MMP-2/TIMP-2 protein ratio imbalance, consequently causing disordered collagen metabolism mainly due to increased collagen type I/III expression. This finding is consistent with results from studies on cardiac pathology and similar to the results of current studies on myocardial fibrosis (24,25). Ahn et al (26) reported that the JAK2/STAT3 pathway may regulate MMP-2 expression, and promote endometrial cell proliferation and migration. Furthermore, it has been demonstrated that abnormal proliferation of cardiac fibroblasts causes an imbalance in the MMP-2/TIMP-2 protein ratio, leading to disordered collagen metabolism (27,28). To investigate whether inhibition of the JAK2/STAT3 pathway would ameliorate the symptoms of myocardial fibrosis, rats with AS were treated with the peptide urantide.
Recent studies identified that contrast-enhanced cardiac magnetic resonance imaging of patients with AS indicated that individuals who had a history of cardiovascular events were more likely to present with myocardial fibrosis than those who did not (29,30). In the present study, disordered collagen metabolism was significantly improved in rats with AS following treatment. This effect exhibited significant time dependence. To verify the mechanism of this effect, the UII/UT system and the JAK2/STAT3 pathway were examined, and it was determined that they had undergone significant changes. The results demonstrated that urantide effectively inhibited the expression of the UII/UT system and the JAK2/STAT3 pathway. Inhibition of the JAK2/STAT3 pathway may be associated with alleviated symptoms of myocardial fibrosis, as after urantide treatment the pathological symptoms of the heart were alleviated, which is consistent with reports from relevant studies (7,22). It has been reported that simvastatin is capable of reducing myocardial fibrosis by downregulating related proteins, including transforming growth factor, MMP-2, MMP-9, TIMP-1 and TIMP-2 (31). Nevertheless, studies on the relationship of simvastatin with the JAK2/STAT3 pathway are limited. Importantly, simvastatin was not found to inhibit the activation of the JAK2/STAT3 signaling pathway in the cardiac tissue of rats with AS. The results from the experiment in the present study are inconsistent with those observed following administration of simvastatin alone for the treatment of cardiac hypertrophy (32). To this end, future studies should establish a rat model of myocardial fibrosis to further explore the association of simvastatin with the JAK2/STAT3 signaling pathway.
A recent study by Cho et al (33) indicated that the JAK2/STAT3 pathway regulates MMP-2 expression, thus follow-up research was conducted on collagen mechanisms, such as changes in the expression levels of related MMPs and TIMPs after myocardial fibrosis. Another study by Wang et al (25) demonstrated that an imbalance in the MMP-2/TIMP-2 protein ratio affected the synthesis and degradation of myocardial collagen. In the present study, an increase in MMP-2 expression was observed in rats with AS, whereas TIMP-2 expression was decreased. Treatment with urantide for a prolonged administration time resulted in a significant improvement in the MMP-2/TIMP-2 protein ratio. By contrast, the expression levels of collagen type I/III tended to decrease. The aforementioned findings indicated that the JAK2/STAT3 pathway affected the expression of collagen type I/III by regulating the balance of MMP-2/TIMP-2, thereby alleviating the symptoms of disordered collagen metabolism.
In addition, p-JAK2/p-STAT3 and collagen type I/III were abundantly expressed in AS lesions. Urantide treatment led to a decrease in the expression and range of these proteins. Moreover, their localization indicated that the JAK2/STAT3 pathway may be involved in the process of collagen metabolism.
In summary, the treatment of rats with AS with urantide was able to relieve cardiac symptoms and also significantly improve collagen metabolism. The underlying mechanism responsible for these effects may be antagonization of the UII/UT system and inhibition of the JAK2/STAT3 pathway by urantide. This combined mechanism is capable of regulating the balance between MMP-2/TIMP-2 and decreasing collagen type I/III expression. The regulation of collagen metabolism was restored and, consequently, the degree of myocardial fibrosis in rats with AS was reduced. In addition, differences were identified in the gene and protein expression levels of collagen I between the 3- and 7-day subgroups receiving urantide treatment. These differences could be considered an asynchronous phenomenon, with differences in expression time, related to the processes of transcription and/or translation. Regarding this matter, in-depth research should be conducted to clarify the relevant mechanisms. However, whether the UII/UT system directly affects the JAK2/STAT3 pathway and how this pathway regulates collagen metabolism remains unclear. The main limitation of the present study is the lack of in vitro cell experiments examining how the UII/UT system affects the JAK2/STAT3 signaling pathway and collagen metabolism. Therefore, the downstream genes regulated by the JAK2/STAT3 pathway that directly or indirectly affect collagen metabolism should be studied to further elucidate the mechanism by which the JAK2/STAT3 pathway affects collagen metabolism.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Hebei Youth Talents Project (grant. no. Hebei[2016]9), the Hebei Provincial Department of Education Outstanding Youth Fund Project (grant. no. YQ2013005) and the Key Discipline Construction Projects in Hebei Province (grant. no. Hebei[2013] 4).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
JZ, TW and XS designed the study. TW and XS performed the experiments. HPC and KL performed the animal model experiment. JZ, TW, XS, HC and KL analyzed the data. TW and XS wrote the manuscript. All authors critically reviewed the manuscript, and read and approved the final version of the manuscript.
Ethics approval and consent to participate
All animal procedures were ethically approved by the Experimental Animal Care and Use Committee of Chengde Medical University and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Istratoaie O, OfiTeru AM, Nicola GC, Radu RI, Florescu C, Mogoant L, Streba CT. Myocardial interstitial fibrosis-histological and immunohistochemical aspects. Rom J Morphol Embryol. 2015;56:1473–1480. [PubMed] [Google Scholar]
- 2.Chen X, Tang Y, Gao M, Qin S, Zhou J, Li X. Prenatal exposure to lipopolysaccharide results in myocardial fibrosis in rat offspring. Int J Mol Sci. 2015;16:10986–10996. doi: 10.3390/ijms160510986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross B, McKendy K, Giaid A. Role of urotensin II in health and disease. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1156–R1172. doi: 10.1152/ajpregu.00706.2009. [DOI] [PubMed] [Google Scholar]
- 4.Leprince J, Chatenet D, Dubessy C, Fournier A, Pfeiffer B, Scalbert E, Renard P, Pacaud P, Oulyadi H, Ségalas-Milazzo I, et al. Structure-activity relationships of urotensin II and URP. Peptides. 2008;29:658–673. doi: 10.1016/j.peptides.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Kiu H, Nicholson SE. Biology and significance of the JAK/STAT signalling pathways. Growth Factors. 2012;30:88–106. doi: 10.3109/08977194.2012.660936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu M, Li Y, Liang B, Li Z, Jiang Z, Chu C, Yang J. Hydrogen sulfide attenuates myocardial fibrosis in diabetic rats through the JAK/STAT signaling pathway. Int J Mol Med. 2018;41:1867–1876. doi: 10.3892/ijmm.2018.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L, Wu D, Sang M, Xu Y, Liu Z, Wu Q. Stachydrine ameliorates isoproterenol-induced cardiac hypertrophy and fibrosis by suppressing inflammation and oxidative stress through inhibiting NF-κB and JAK/STAT signaling pathways in rats. Int Immunopharmacol. 2017;48:102–109. doi: 10.1016/j.intimp.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 8.El Hajj EC, El Hajj MC, Voloshenyuk TG, Mouton AJ, Khoutorova E, Molina PE, Gilpin NW, Gardner JD. Alcohol modulation of cardiac matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs favors collagen accumulation. Alcohol Clin Exp Res. 2014;38:448–456. doi: 10.1111/acer.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai R, Yin X, Feng X, Cao Y, Wu Y, Zhu Z, Li C, Tu P, Chai X. Corydalis hendersonii Hemsl. protects against myocardial injury by attenuating inflammation and fibrosis via NF-κB and JAK2-STAT3 signaling pathways. J Ethnopharmacol. 2017;207:174–183. doi: 10.1016/j.jep.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Zhang SF, Shi Y, Ren LQ. Effects of urotensin II and its specific receptor antagonist urantide on rat vascular smooth muscle cells. Bosn J Basic Med Sci. 2013;13:78–83. doi: 10.17305/bjbms.2013.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J, Xie LD, Song CJ, Mao XX, Yu HR, Yu QX, Ren LQ, Shi Y, Xie YQ, Li Y, et al. Urantide improves atherosclerosis by controlling C-reactive protein, monocyte chemotactic protein-1 and transforming growth factor-β expression in rats. Exp Ther Med. 2014;7:1647–1652. doi: 10.3892/etm.2014.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang ZY, Yang PY, Almofti MR, Yu YL, Rui YC, Yang PY. Comparative analysis of the proteome of left ventricular heart of arteriosclerosis in rat. Life Sci. 2004;75:3103–3115. doi: 10.1016/j.lfs.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 13.Jiao YB, Rui YC, Li TJ, Yang PY, Qiu Y. Expression of pro-inflammatory and anti-inflammatory cytokines in brain of atherosclerotic rats and effects of Ginkgo biloba extract. Acta Pharmacol Sin. 2005;26:835–839. doi: 10.1111/j.1745-7254.2005.00106.x. [DOI] [PubMed] [Google Scholar]
- 14.Gou SH, Liu BJ, Han XF, Wang L, Zhong C, Liang S, Liu H, Qiang Y, Zhang Y, Ni JM. Anti-atherosclerotic effect of Fermentum Rubrum and Gynostemma pentaphyllum mixture in high-fat emulsion- and vitamin D3-induced atherosclerotic rats. J Chin Med Assoc. 2018;81:398–408. doi: 10.1016/j.jcma.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Rezkalla S, Kloner RA, Khatib FG, Smith FE, Khatib R. Effect of metoprolol in acure coxsackievirus B3 murine myocarditis. Am Coll Cardiol. 1988;12:412–414. doi: 10.1016/0735-1097(88)90414-7. [DOI] [PubMed] [Google Scholar]
- 16.Hayes AJ, Hughes CE, Caterson B. Antibodies and immunohistochemistry in extracellular matrix research. Methods. 2008;45:10–21. doi: 10.1016/j.ymeth.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Li X, Luo R, Wang W, Wang T, Tang H. Detection of KIT Genotype in Pigs by TaqMan MGB Real-time quantitative polymerase chain reaction. DNA Cell Biol. 2018;37:457–464. doi: 10.1089/dna.2017.4070. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Collier P, Watson CJ, van Es MH, Phelan D, McGorrian C, Tolan M, Ledwidge MT, McDonald KM, Baugh JA. Getting to the heart of cardiac remodeling; how collagen subtypes may contribute to phenotype. J Mol Cell Cardiol. 2012;52:148–153. doi: 10.1016/j.yjmcc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Lopez B, Gonzalez A, Hermida N, Valencia F, de Teresa E, Diez J. Role of lysyl oxidase in myocardial fibrosis: From basic science to clinical aspects. Am J Physiol Heart Circ Physiol. 2010;299:H1–H9. doi: 10.1152/ajpheart.00335.2010. [DOI] [PubMed] [Google Scholar]
- 21.Lopez B, Gonzalez A, Varo N, Laviades C, Querejeta R, Diez J. Biochemical assessment of myocardial fibrosis in hypertensive heart disease. Hypertension. 2001;38:1222–1226. doi: 10.1161/hy1101.098549. [DOI] [PubMed] [Google Scholar]
- 22.Luo SY, Chen S, Qin YD, Chen ZW. Urotensin-II Receptor antagonist SB-710411 protects rat heart against ischemia-reperfusion injury via RhoA/ROCK pathway. PLoS One. 2016;11:e0146094. doi: 10.1371/journal.pone.0146094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J, Yu QX, Kong W, Gao HC, Sun B, Xie YQ, Ren LQ. The urotensin II receptor antagonist, urantide, protects against atherosclerosis in rats. Exp Ther Med. 2013;5:1765–1769. doi: 10.3892/etm.2013.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aboulhoda BE. Age-related remodeling of the JAK/STAT/SOCS signaling pathway and associated myocardial changes: From histological to molecular level. Ann Anat. 2017;214:21–30. doi: 10.1016/j.aanat.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Li J, Li D. Losartan reduces myocardial interstitial fibrosis in diabetic cardiomyopathy rats by inhibiting JAK/STAT signaling pathway. Int J Clin Exp Patho. 2015;8:466–473. [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn JH, Choi YS, Choi JH. Leptin promotes human endometriotic cell migration and invasion by up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol Hum Reprod. 2015;21:792–802. doi: 10.1093/molehr/gav039. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Xu YX, Du XJ, Sun QG, Tian YJ. Dynamic expression profiles of MMPs/TIMPs and collagen deposition in mechanically unloaded rat heart: Implications for left ventricular assist device support-induced cardiac alterations. J Physiol Biochem. 2013;69:477–485. doi: 10.1007/s13105-013-0235-x. [DOI] [PubMed] [Google Scholar]
- 28.Guo C, Piacentini L. Type I collagen-induced MMP-2 activation coincides with up-regulation of membrane type 1-matrix metalloproteinase and TIMP-2 in cardiac fibroblasts. J Biol Chem. 2003;278:46699–46708. doi: 10.1074/jbc.M307238200. [DOI] [PubMed] [Google Scholar]
- 29.Ambale-Venkatesh B, Liu CY, Liu YC, Donekal S, Ohyama Y, Sharma RK, Wu CO, Post WS, Hundley GW, Bluemke DA, Lima JAC. Association of myocardial fibrosis and cardiovascular events: The multi-ethnic study of atherosclerosis. Eur Heart J Cardiovasc Imaging. 2019;20:168–176. doi: 10.1093/ehjci/jey140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu CY, Heckbert SR, Lai S, Ambale-Venkatesh B, Ostovaneh MR, McClelland RL, Lima JAC, Bluemke DA. Association of Elevated NT-proBNP with myocardial fibrosis in the Multi-Ethnic study of atherosclerosis (MESA) J Am Coll Cardiol. 2017;70:3102–3109. doi: 10.1016/j.jacc.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing XQ, Xu J, Lu XW, Huang Y, Zhu PL. Effects of losartan and simvastatin on collagen content, myocardial expression of MMP-2 mRNA, MMP-9 mRNA and TIMP-1 mRNA, TIMP-2 mRNA in pressure overload rat hearts. Zhonghua Xin Xue Guan Bing Za Zhi. 2009;37:887–891. (In Chinese) [PubMed] [Google Scholar]
- 32.Al-Rasheed NM, Al-Oteibi MM, Al-Manee RZ, Al-Shareef SA, Al-Rasheed NM, Hasan IH, Mohamad RA, Mahmoud AM. Simvastatin prevents isoproterenol-induced cardiac hypertrophy through modulation of the JAK/STAT pathway. Drug Des Devel Ther. 2015;9:3217–3229. doi: 10.2147/DDDT.S86431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho HJ, Park JH, Nam JH, Chang YC, Park B, Hoe HS. Ascochlorin suppresses MMP-2-mediated migration and invasion by targeting FAK and JAK-STAT signaling cascades. J Cell Biochem. 2018;119:300–313. doi: 10.1002/jcb.26179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.