Abstract
Adenosine is an endogenous neuroprotectant that modulates vasodilation in the central nervous system. Oxygen changes occur when there is an increase in local cerebral blood flow and thus are a measure of vasodilation. Transient oxygen events following rapid adenosine events have been recently discovered, but the relationship between adenosine and blood flow change during ischemia/reperfusion (I/R) has not been characterized. Caffeine is a nonselective adenosine receptor antagonist that can modulate the effects of adenosine in the brain, but how it affects adenosine and oxygen levels during I/R is also unknown. In this study, extracellular changes in adenosine and oxygen were simultaneously monitored using fast-scan cyclic voltammetry during bilateral common carotid artery occlusion (BCCAO) and the effects of a specific A2A antagonist, SCH 442416, or general antagonist, caffeine, were studied. Measurements were made in the caudate-putamen for one hour of normoxia, followed by 30 min of BCCAO and 30 min of reperfusion. The frequency and number of both adenosine and oxygen transient events significantly increased during I/R. The specific A2A antagonist, SCH 442416 (3 mg/kg, i.p.), eliminated the increase in adenosine and oxygen events caused by I/R. The general adenosine receptor antagonist, caffeine (100 mg/kg, i.p.), decreased the frequency of adenosine and oxygen transient events during I/R. These results demonstrate that during BCCAO, there are more rapid release events of the neuromodulator adenosine and correlated local oxygen changes, and these rapid, local effects are dampened by caffeine and other A2A antagonists.
Keywords: Adenosine, Oxygen, ischemia/reperfusion injury, in vivo, blood flow, BCCAO, Fast-scan cyclic voltammetry, FSCV, Caffeine, A2A receptor
Introduction
Ischemic brain injury is one of the leading causes of death and disability.1 It occurs when cerebral blood flow is reduced, either transiently or permanently, which causes decreased delivery nutrients to the brain. In particular, oxygen levels change with blood flow and thus, ischemic tissue experiences severe hypoxia. Reestablishment of blood flow will deliver nutrients, but this reperfusion process also generates oxygen-derived free radicals that can actually increase ischemic tissue damage.2 The brain is particularly sensitive to ischemia as complete blockade of blood flow to the brain for only 5 minutes can cause the death of vulnerable neurons.3
Adenosine is a major neuromodulator with neuroprotective properties in the central nervous system that plays an important role during ischemic injury. Adenosine has a cerebral vasodilatory effect,4–6 causing increased blood flow and oxygen supply. Adenosine levels in plasma acutely rise during transient ischemic attack and stroke in human.7 In the rat brain, intracerebral infusion of adenosine into the ischemic striatum using microdialysis significantly improves the neurological outcome and reduces the infarct volume after transient focal cerebral ischemia.8 The protective effects are attributed to the activation of A2A receptors, and peripheral administration of adenosine A2A agonist, CGS 21680, protects against neuronal loss during ischemia.9 Our lab has recently identified transient increases in adenosine,10 which increases in frequency during ischemia, and are dependent on A2a receptors11. These adenosine events are correlated with transient oxygen changes,12 a measure of local blood flow, and the time-course of both adenosine and oxygen transient events are fast, lasting only about 3 seconds. However, the extent to which adenosine can cause oxygen changes during ischemia, when blood flow is occluded, is unknown.
Caffeine is a nonselective adenosine receptor antagonist that can act at A2A receptors to affect cerebral blood flow. For example, a high dose of caffeine (50 μM) significantly attenuates the dose-dependent vasodilation caused by extraluminal application of adenosine.13 Magnetic resonance imaging (MRI) study shows that caffeine consumption (184 mg daily) globally reduces cerebral blood flow in humans.14 Field et al. suggest a linear correlation between daily caffeine intake and cerebral blood flow response.15 Caffeine also reduces the intensity and duration of the cerebral hyperemia during anoxia.16 In addition, caffeine regulates adenosine levels as it increases plasma adenosine in a dose dependent manner.17 However, the effect of caffeine on transient adenosine release has not been studied and there are still many open questions about how caffeine regulates adenosine and blood flow changes. These questions are important to address so that we understand how caffeine might modulate potential neuroprotective effects of adenosine during I/R.
Here, we investigated the extent to which adenosine events are correlated with oxygen transient events during cerebral ischemia/reperfusion (I/R) in vivo and the effect of an A2A antagonist or caffeine on transient adenosine and oxygen changes. Fast-scan cyclic voltammetry at carbon-fiber microelectrodes was used to simultaneously detect extracellular changes in adenosine and oxygen during normoxia or ischemia with bilateral common carotid artery occlusion (BCCAO). The frequency of spontaneous adenosine events increased during I/R and that increase was accompanied by an increase in the frequency of oxygen events. A large dose of the adenosine A2A antagonist, SCH 442416, eliminated the increase in adenosine and oxygen events during I/R while a large dose of caffeine significantly decreased the frequency of adenosine and oxygen events during I/R. This study shows that even when major arteries are occluded, adenosine can act to transiently increase local blood flow during ischemia and that large doses of caffeine can dampen that effect. Thus, caffeine may hinder the neuroprotective effects of adenosine during I/R, particularly by limiting transient adenosine release which signals for increase in local blood flow.
Results
Adenosine and oxygen detection with fast-scan cyclic voltammetry in vivo
Fast-scan cyclic voltammetry at a carbon-fiber microelectrode was used to monitor rapid fluctuations of adenosine and oxygen in the caudate-putamen of anesthetized rats in vivo.12 The carbon-fiber microelectrode was ramped from 0 V to 1.45 V, then to −1.4 V and back to 0 V every 100 ms at a scan rate of 450 V/s. The double layer charging at the electrode produces a large and stable background current that can be subtracted to reveal smaller faradaic currents due to reactions of electroactive analytes. Fig. 1A shows a background-subtracted cyclic voltammogram (CV) with peaks for adenosine and oxygen (Figure 1A). Adenosine has two oxidation peaks; the primary oxidation occurs on the downward scan slightly after the switching potential and secondary oxidation occurs at 1.2 V on the upward scan. The oxygen reduction peak occurs at −1.3 V on the cathodic scan. Concentration vs. time traces were extracted from the primary oxidation potential for adenosine and reduction potential for oxygen (Figure 1B) in the color plot (Figure 1C). The green/purple ovals in the color plot are adenosine oxidation currents; the top oval is the primary oxidation and the bottom oval directly below is the secondary oxidation of adenosine. The dark blue area around −1.3 V is the reduction of oxygen. Three spontaneous adenosine events and one oxygen event are identified and marked by a star. Only some adenosine events are correlated with an oxygen event and both adenosine and oxygen events are fast, lasting about 3 seconds in the extracellular space. Figure 1D shows the placement of electrodes in the caudate-putamen.
Figure 1. Spontaneous adenosine and oxygen changes in the caudate-putamen.
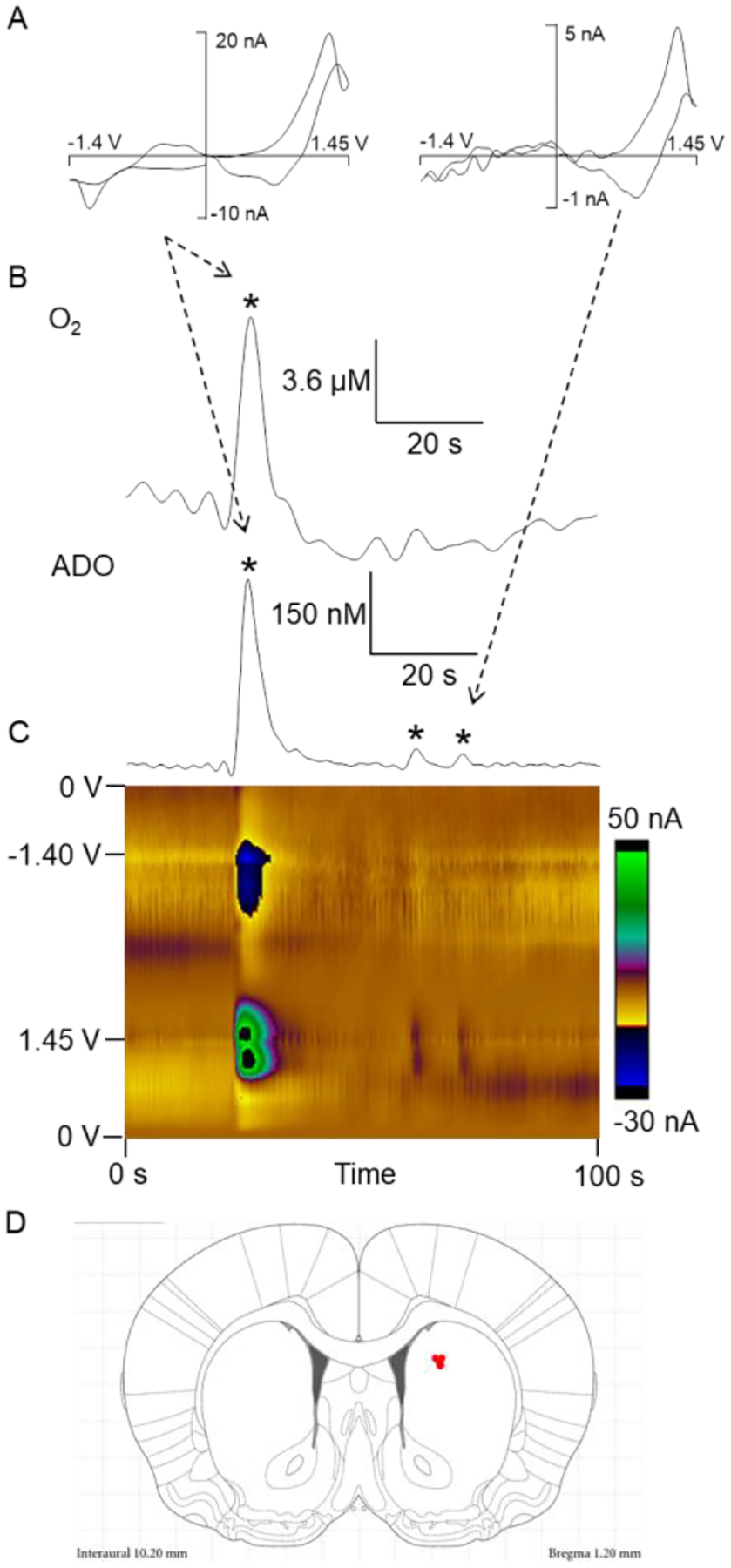
(A) CV for adenosine and oxygen events collected in vivo. The primary oxidation of adenosine occurs at 1.27 V on the cathodic scan and the secondary oxidation occurs at 1.26 V on the anodic scan. The reduction of oxygen occurs at −1.3 V on the cathodic scan. (B) Concentration vs time traces of adenosine (bottom) and oxygen (top) events. (C) Color plot of adenosine and oxygen events in vivo. One oxygen and three adenosine events are starred, where one adenosine is correlated with an oxygen event. Adenosine oxidations are the green/purple in the middle of the plot while reduction of oxygen is dark blue at the top. (D) Microelectrode recording locations (red dots) in the caudate putamen. Drawing from the stereotaxic atlas of Paxinos and Watson.58
Adenosine and oxygen changes are correlated during ischemia/reperfusion
To investigate simultaneous adenosine and oxygen changes during ischemia and reperfusion, bilateral common carotid artery occlusion (BCCAO) was performed to induce cerebral ischemia/reperfusion (I/R) in anesthetized rats. To confirm that BCCAO and reperfusion were successful in the caudate-putamen, blood flow was measured by a laser Doppler probe during normoxia and I/R. BCCAO resulted in immediate cerebral blood flow reduction of about 50% (Figure 2A). Releasing the occluders to allow reperfusion led to an increase in blood flow, but the blood flow levels did not return to normal levels (Figure 2A). The blood flow for normoxia and BCCAO were significantly different (Figure 2E, paired t-test, n = 3 animals, p = 0.027).
Figure 2. Cerebral blood flow changes in rat brain.
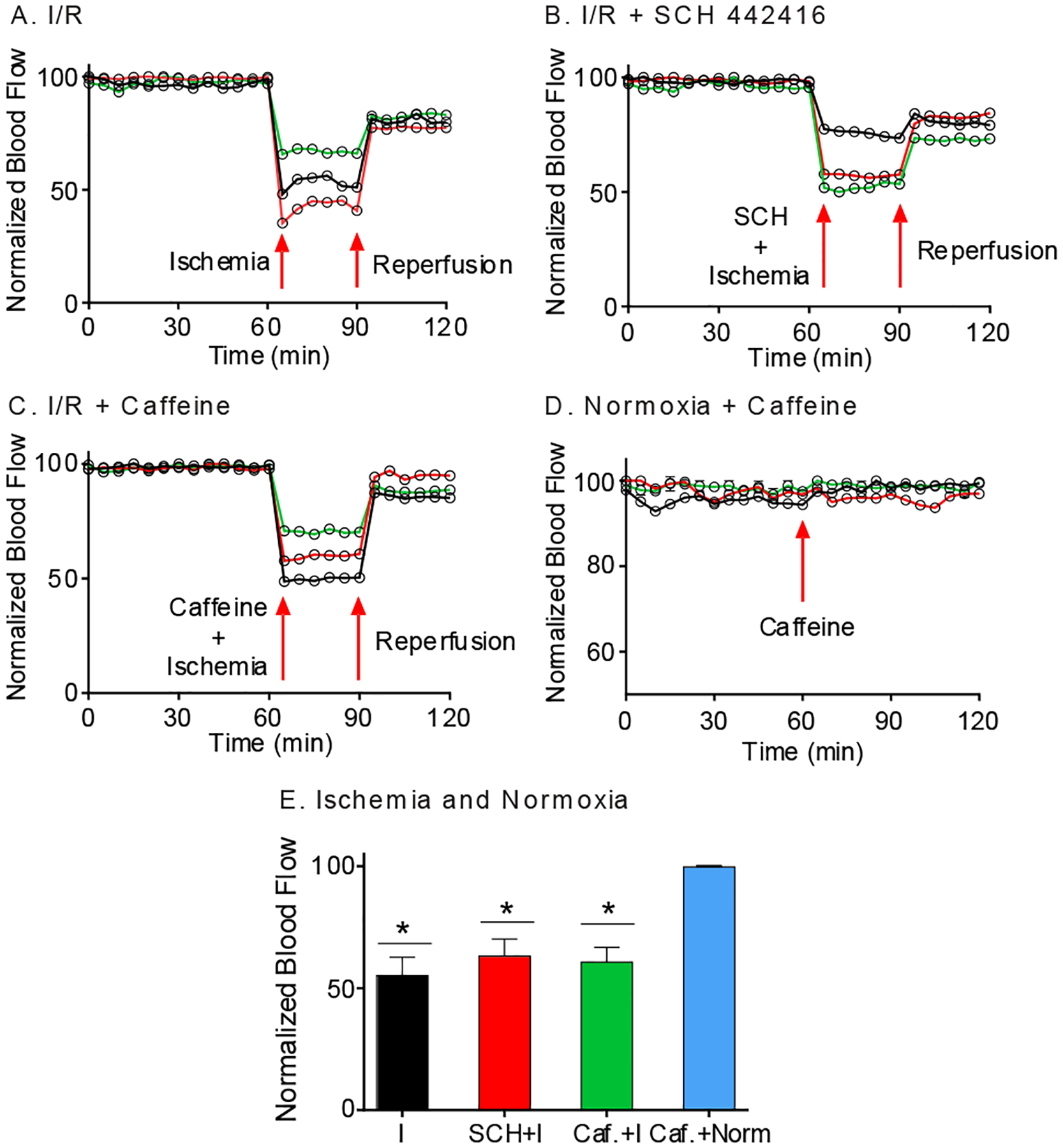
(A) Blood flow change during normoxia and I/R (n = 3 animals). Blood flow decreased during BCCAO and is restored, but not to baseline, during reperfusion. (B) Blood flow change during I/R with SCH 442416 (3 mg/kg) administration is similar to plot without drug (n = 3 animals). (C) Blood flow change during I/R with caffeine (100 mg/kg) administration is similar to that without caffeine. (D) Caffeine control (n = 3 animals). Blood flow was measured before and after caffeine (100 mg/kg) during normoxia. Blood flow did not change. (E) Average changes in blood flow. Blood flow significantly decreased during 30 min of ischemia (I) (*p = 0.027), SCH+ ischemia (*p = 0.034) and caffeine + ischemia (*p = 0.023) groups compared to 30 min of normoxia (Norm) Caffeine administration itself did not affect blood flow under normoxia (p = 0.94). Statistical analyses are paired t-test, where each treatment is compared with its own control.
Spontaneous adenosine and oxygen events were continuously monitored for 1 hr normoxia followed by 30 minutes of BCCAO and 30 minutes of reperfusion. Example data show the difference between normoxia (Figure 3A) and ischemia (Figure 3B). During normoxia, three adenosine events were observed during a 100 s window, one of which is followed by an oxygen event, indicating local blood flow was transiently increased. However, during ischemia, five adenosine events were observed in the same time frame, two of which have corresponding oxygen events. The number of events was measured continuously and averaged in 6 min bins for each animal to understand the effects over time (Figure 3C and 3D). A two-way ANOVA (time × I/R) revealed no main effect of time on the number of adenosine and oxygen transient events [adenosine: F(9, 54) = 0.96, p = 0.48 and oxygen: F(9, 54) = 1.19, p = 0.32], but a main effect of I/R [adenosine: F(1, 6) = 17.24, p = 0.01 and oxygen: F(1, 6) = 11.54, p = 0.01]. There was no interaction between time and I/R [adenosine: F(9, 54) = 0.82, p = 0.60 and oxygen: F(9, 54) = 0.76, p = 0.65]. The average number of events per hour significantly increased for both adenosine and oxygen during I/R compared to normoxia (Fig. 3C, D inset, paired t-test, n = 7 animals, adenosine: p = 0.0066 and oxygen: p = 0.022). During normoxia, 43% of adenosine event are correlated with an oxygen event and during I/R, 49% of adenosine events were followed by oxygen events, but this increase is not significant (Figure 4, paired t-test, n = 7 animals, p = 0.34). To examine the frequency, the time interval between consecutive events, termed the inter-event time, was plotted as a histogram. I/R decreased the mean inter-event time for adenosine transients from 77 to 48 s and for oxygen from 177 to 96 s. The distributions of inter-event times were significantly different for normoxia and I/R for both adenosine and oxygen events (Figure 3E and 3F, Kolmogorov-Smirnov (KS) test, n = 7 animals, adenosine: p < 0.0001 and oxygen: p < 0.0001).
Figure 3. Spontaneous adenosine and oxygen changes in the caudate-putamen during I/R injury.
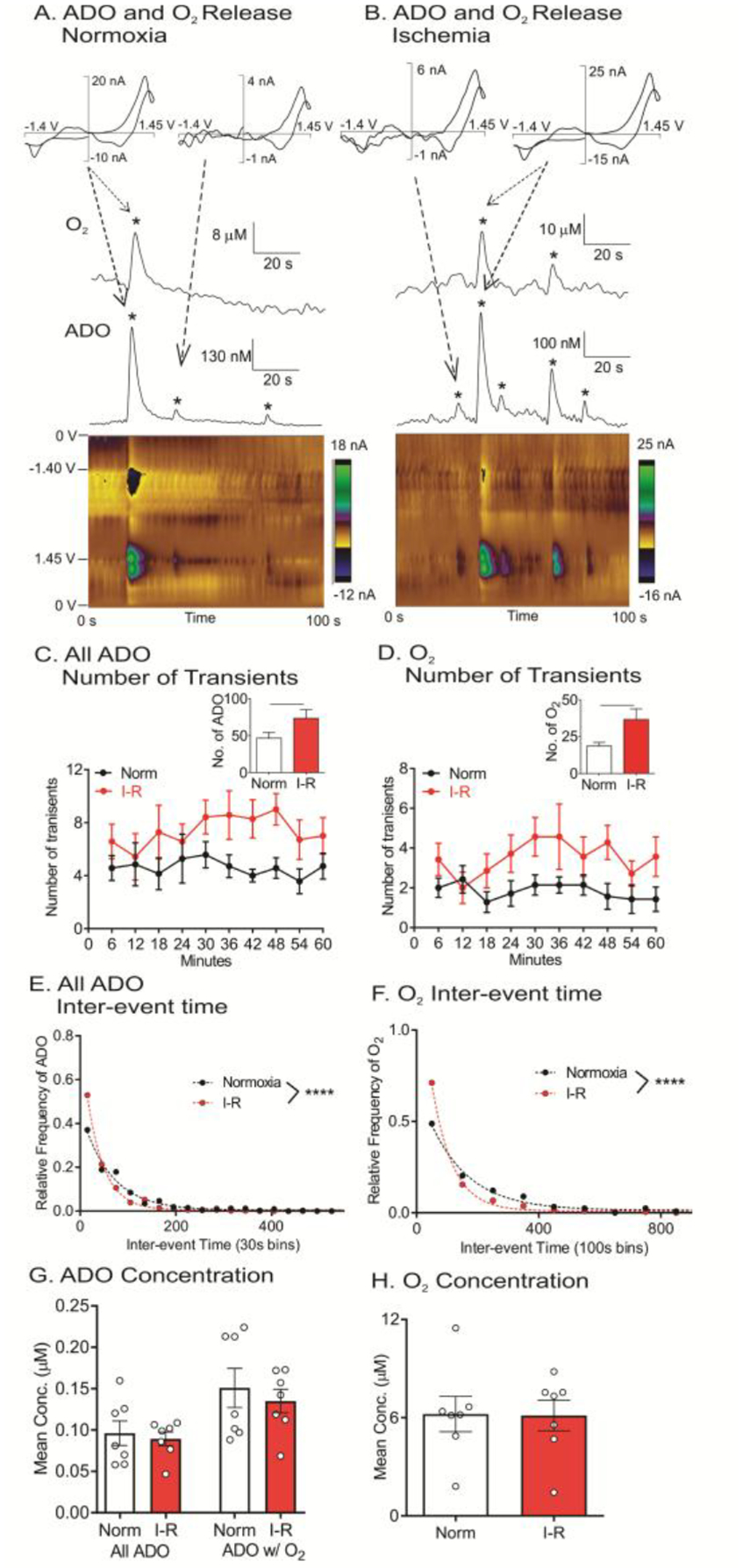
(A) Example adenosine and oxygen events during normoxia. Three adenosine and one oxygen events (starred) were observed. (B) Example adenosine and oxygen changes during ischemia, where five adenosine and two oxygen events were observed. (C) Number of adenosine events, in 6 min bins, during normoxia and I/R injury. Inset: Average number of adenosine events increases during I/R (paired t-test, n = 7 animals, **p = 0.0066 <0.01). (D) Number of oxygen events in 6 min bins. Inset: Oxygen events increased during I/R injury (paired t-test, n = 7 animals, *p = 0.022 < 0.01). (E) Inter-event time histogram for adenosine. Underlying distributions were significantly different between normoxia and I/R injury (KS test, n = 7 animals, ****p < 0.0001). (F) Oxygen inter-event time distributions were significantly different for I/R injury (KS test, n = 7 animals, ****p < 0.0001). (G) Mean event concentration of adenosine events was not different during I/R injury for all adenosine events or adenosine events with oxygen event (ADO w/O2)(paired t-test, n = 7 animals, p = 0.62 and p = 0.42, respectively). (H) The mean concentration of each oxygen event was not significantly different during I/R injury (paired t-test, n = 7 animals, p = 0.92).
Figure 4. Percent of transient adenosine events that correlate with an oxygen event.
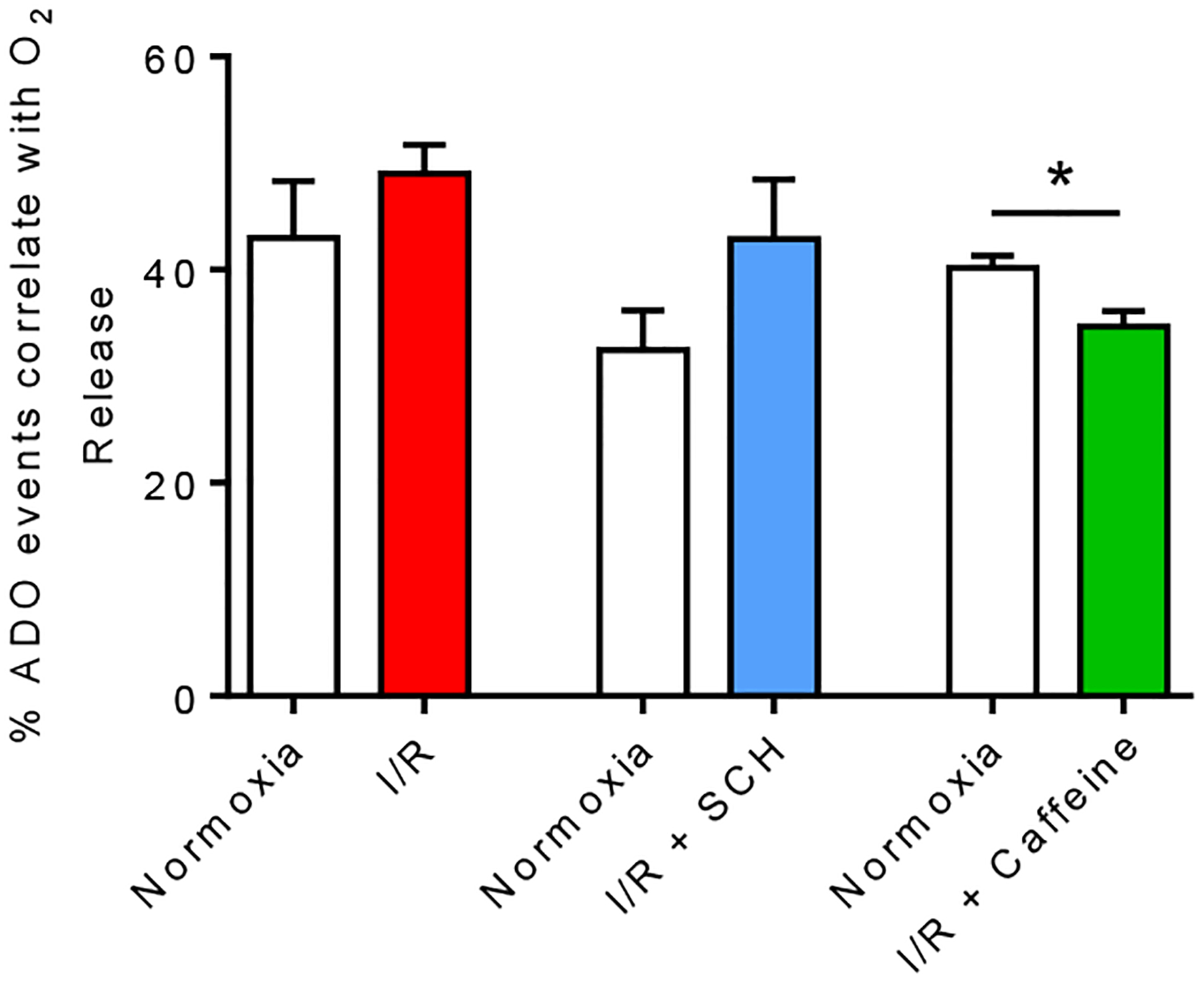
Each animal was used as its own control to compare percentage of correlated adenosine and oxygen events during normoxia and I/R with/without drug treatment. The percent of transient adenosine events that correlates with an oxygen event did not significantly change under I/R injury (Paired t-test, n = 7 animals, p = 0.34) and I/R injury with SCH 442416 administration (Paired t-test, n = 6 animals, p = 0.059). However, the percent of adenosine events that correlates with an oxygen event significantly decreased under I/R injury with caffeine administration (Paired t-test, n = 6 animals, *p = 0.043).
The average event concentration, which was calculated by averaging the mean concentration from each animal, is plotted in Figures 3G and 3H for all adenosine events (All ADO), adenosine events correlated with an oxygen event (ADO w/O2), and oxygen concentrations. I/R did not significantly change the concentration of events (paired t-test, n = 7 animals, All ADO: p =0.62, ADO w/O2: p = 0.42 and oxygen: p = 0.92). Thus, I/R did not affect the concentration of adenosine and oxygen events but local adenosine and oxygen changes occur more frequently during I/R, even when blood flow was decreased during ischemia.
A2A receptor modulation of adenosine and oxygen change during ischemia/reperfusion
To evaluate the effects of A2A receptors on rapid adenosine and oxygen dynamics during I/R, we tested a specific A2A receptor antagonist, SCH 442416 (SCH) (3 mg/kg, i.p.). The drug was administered right before I/R, after 1 hour of normoxia. Blood flow was measured to investigate if SCH 442416 affects cerebral blood flow under I/R (Figure 2B), but the pattern of blood flow with SCH 442416 was similar to that of I/R without drug administration. The blood flow during normoxia and after BCCAO + SCH 442416 administration were significantly different (Figure 2E, paired t-test, n = 3 animals, p = 0.034), just like I/R. Thus, administration of the A2A antagonist, SCH 442416, did not affect global cerebral blood flow during I/R.
Figures 5A and 5B show the average number of adenosine and oxygen events in 6 minute bins. A two-way ANOVA (time × I/R with SCH treatment) revealed no main effect of time on number of adenosine events [F (9, 45) = 0.73, p = 0.68] nor effect of SCH + I/R [F (1, 5) =0.22, p =0.66]. However, there was a main effect of SCH + I/R on number of oxygen events [F(1, 5) =1.87, p = 0.023] but no main effect of time [F(9, 45) = 0.67, p = 0.73]. There was no interaction between time and SCH + I/R for either number of adenosine or oxygen events [adenosine: F(9, 45) = 0.79, p = 0.63 and oxygen: F(9, 45) = 0.98, p = 0.47]. The average number of adenosine events did not significantly change after SCH + I/R (Figure 5A inset, paired t-test, n = 6 animals, p = 0.64) and the number of oxygen events also did not significantly increase (Figure 5B inset, paired t-test, n = 6 animals, p = 0.24). In addition, there was no significant difference in the percentage of adenosine events with a subsequent oxygen event between normoxia and I/R + SCH (Figure 4, paired t-test, n = 6 animals, p = 0.059). The mean inter-event time decreased slightly from 48 to 44 s for adenosine and 135 to 103 s for oxygen, but there was no significant change in frequency distributions for either adenosine or oxygen events with SCH under I/R (Figure 5C and 5D, KS test, n = 6 animals, adenosine: p = 0.63 and oxygen: p = 0.46). The concentration of all ADO, ADO w/O2 and oxygen events did not differ (Figure 5E and 5F, paired t-test, n = 6 animals, All ADO: p = 0.55, ADO w/O2: p = 0.63 and oxygen: p = 0.96). Therefore, with the A2A antagonist SCH 442416, the number of adenosine and oxygen events no longer increase during I/R.
Figure 5. Effect of the A2A antagonist, SCH 442416 (3 mg/kg, i.p.), on adenosine and oxygen changes during I/R injury.
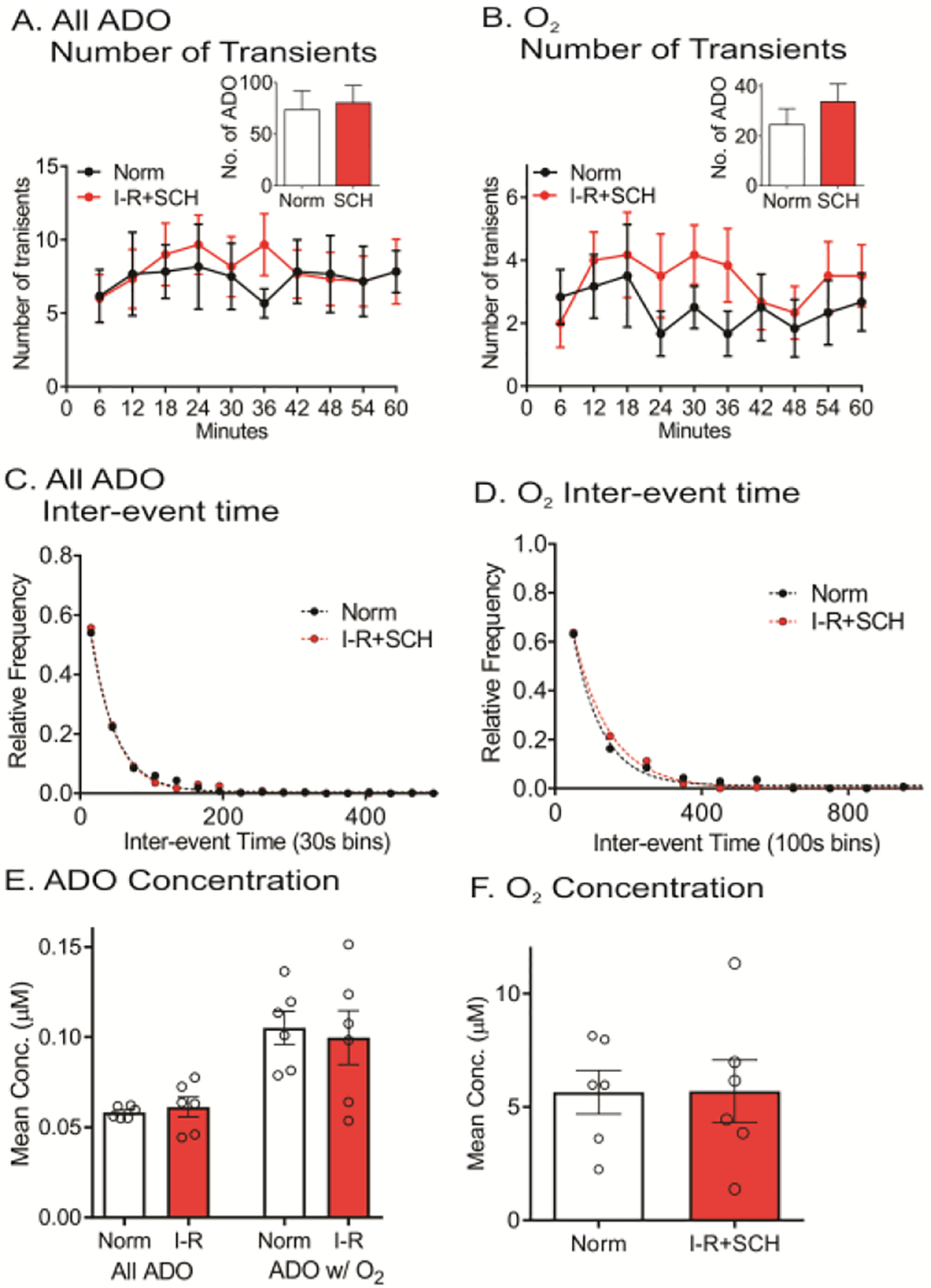
(A) Number of adenosine event release traces during every 6 min during normoxia and I/R injury. Inset: No change in number of adenosine events between normoxia and I/R injury (paired t-test, n = 6 animals, p = 0.64). (B) Number of oxygen events in 6 min. bins. Inset: Number of oxygen events did not change during I/R (paired t-test, n = 6 animals, p = 0.24). (C) For adenosine, there are no differences in inter-event times between normoxia and I/R injury (KS test, n = 6 animals, p = 0.63). (D) For oxygen, there were no changes in inter-event times during I/R (KS test, n = 6 animals, p = 0.46). (E) Mean event concentration of all adenosine (ADO) and ADO w/O2 were not significantly different during I/R injury (paired t-test, n = 6 animals, p = 0.55 and p = 0.63, respectively). (F) The mean event concentration of oxygen was not significantly different during I/R injury (paired t-test, n = 6 animals, p = 0.96).
Caffeine modulation of adenosine and oxygen changes during ischemia/reperfusion
Caffeine has been proposed as a treatment to reduce the risk of stroke and is a nonselective antagonist of adenosine receptors.18,19 Previous studies suggested that low doses of caffeine are stimulatory but high doses are inhibitory on neuronal activity.20 We employed a large dose of caffeine (100 mg/kg, i.p.) to understand how it affects transient adenosine change during I/R. Blood flow during normoxia was compared to I/R with caffeine administration (Figure 2C, n = 3 animals). Blood flow change during I/R with caffeine treatment was similar to those during I/R without drug administration (Figure 2E, paired t-test, n = 3 animals, p = 0.023). We also tested the same dose of caffeine (100 mg/kg i.p.) during normoxia and caffeine did not affect global blood flow (Figure 2D, paired t-test, n = 3 animals, p = 0.94). Thus, administration of caffeine did not affect global blood flow during normoxia or I/R.
Figure 6A shows example data for normoxia and after caffeine during ischemia, where the number of adenosine events decreases. There are 4 adenosine events during normoxia, two of which are correlated with oxygen changes (Figure 6A), while during ischemia with caffeine, there are only two adenosine and one oxygen event (Figure 6B). The number of events for both adenosine and oxygen decreased during I/R following caffeine injection (Figure 6C and 6D). A two-way ANOVA (time × I/R with caffeine treatment) revealed a significant effect of time for number of adenosine and oxygen events [adenosine: F(9, 45) = 3.93, p = 0.001 and oxygen: F(9, 45) = 2.61, p =0.02] and a main effect on caffeine + I/R [adenosine: F(1, 5) =12.04, p = 0.02 and oxygen: F(1, 5) = 20.24, p = 0.01]. There was no interaction between time and caffeine + I/R [adenosine: F(9, 45) = 1.52, p = 0.17 and oxygen: F(9, 45) = 1.34, p = 0.24]. The number of both adenosine and oxygen events significantly decreased compared to normoxia (Figure 6C and 6D insets, paired t-test, n = 6 animals, adenosine: p = 0.022 and oxygen: p = 0.0069). Caffeine + I/R did not significantly change the percentage of adenosine events with a subsequent oxygen event (Figure 4, paired t-test, n = 6 animals, p = 0.043). In addition, caffeine increased the mean inter-event time for adenosine events from 34 to 63 s and for oxygen from 83 to 177 s during I/R and the underlying inter-event time distributions were significantly different than normoxia (Figure 6E and 6F, KS test, n = 6 animals, adenosine: p < 0.0001 and oxygen: p < 0.0001). However, caffeine + I/R did not affect the concentration of either adenosine or oxygen (Figure 6G and 6H, paired t-test, n = 6 animals, All ADO: p = 0.75, ADO w/O2: p = 0.56 and oxygen: p = 0.96). Therefore, while caffeine treatment did not affect global blood flow, it markedly reduced the frequency of transient adenosine and oxygen changes during I/R.
Figure 6. Effect of caffeine (100 mg/kg, i.p.) on adenosine and oxygen changes during I/R injury.
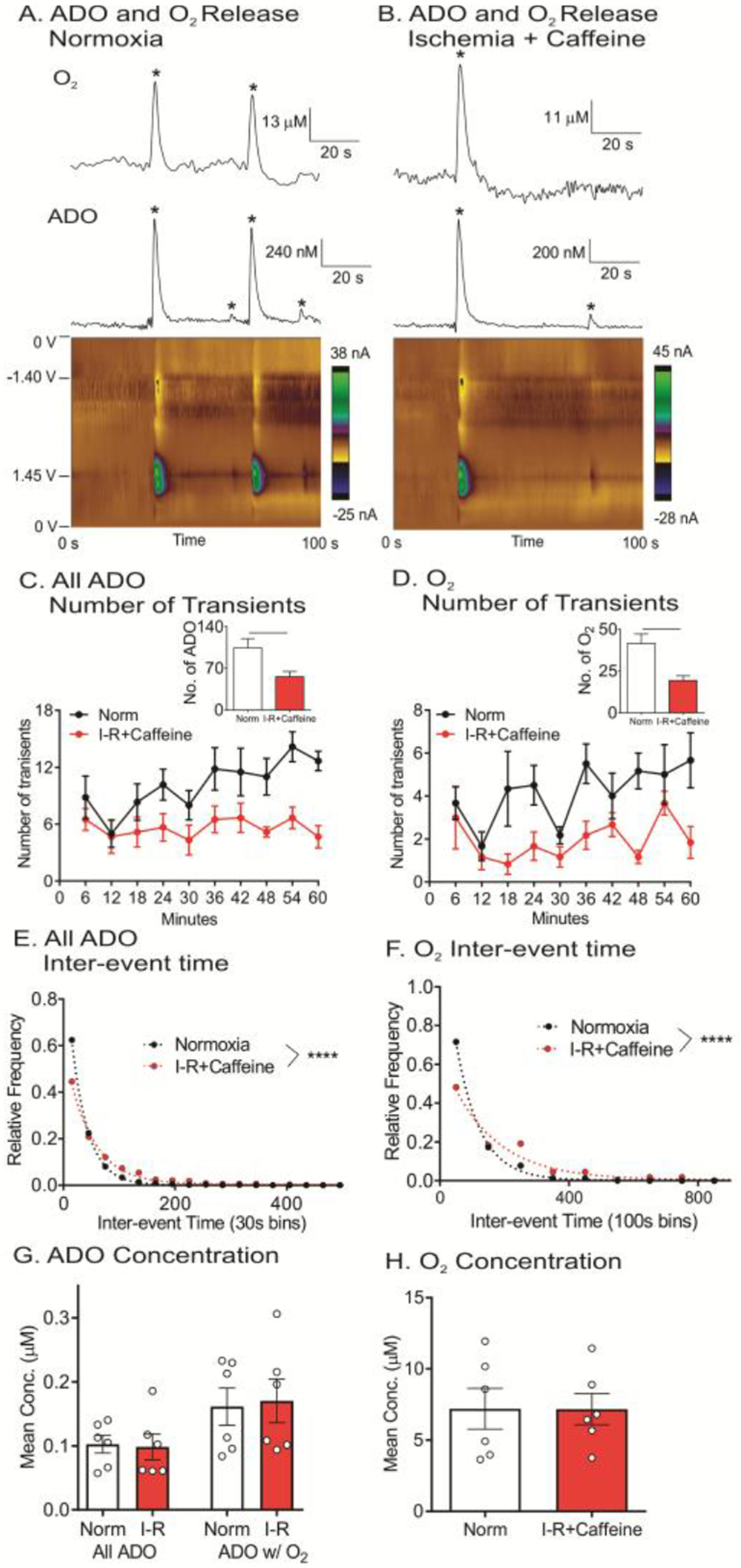
(A) Example adenosine and oxygen changes during normoxia, where four adenosine and two oxygen events (starred) were observed. (B) Example adenosine and oxygen changes during I/R injury with caffeine treatment, where two adenosine and one oxygen events were observed. (C) Number of adenosine event release traces during every 6 min during normoxia and I/R injury. Inset: Average number of adenosine release events decreased during caffeine+I/R (paired t-test, n = 6 animals, *p = 0.022). (D) Number of oxygen events in 6 min bins. Inset: Average number of oxygen events decreased during caffeine+ I/R injury (paired t-test, n = 6 animals, *p = <0.01). (E) For adenosine, underlying distributions of inter-event times were significantly different between normoxia and caffeine + I/R injury (KS test, n = 6 animals, ****p < 0.0001). (F) For oxygen, the distributions of inter-event times were significantly different for caffeine+I/R (KS test, n = 6 animals, ****p < 0.0001). (G) Average event concentration of adenosine events was not significantly different (paired t-test, n = 6 animals, all ADO: p = 0.75 and ADO w/O2: p =0.56). (H) Average oxygen events concentration was not significantly (paired t-test, n = 6 animals, p = 0.96).
Discussion
In this study, we demonstrate that transient adenosine changes are correlated with transient oxygen changes, and that there are more transient adenosine and oxygen events during BCAAO and reperfusion, periods of reduced global blood flow. Thus, adenosine could act as a signal to transiently increase local blood flow even when some major arteries are blocked during BCCAO. The increases in oxygen and adenosine events during I/R are mediated by A2A receptors. The specific A2A receptor antagonist, SCH 442416, blocks the increase in the number of adenosine and oxygen events detected with I/R, while a high dose of caffeine actually decreases the number of adenosine and oxygen events during I/R. Thus, adenosine is a rapid signal that transiently increases local blood flow, especially during I/R, and caffeine dampens that rapid neuromodulatory response of adenosine.
Adenosine and oxygen changes are correlated during I/R
We performed ischemia with a BCCAO model in this study, which blocks the common carotid arties of the rat bilaterally and causes cerebral hypoperfusion. Blood flow decreased by about 50% during BCCAO in the cortex, which is expected to be similar to the caudate-putamen where measurements were performed. Both the carotid and vertebral arteries of rat provide the blood supply to the brain, so blocking the carotid arties does not completely occlude blood flow to the brain.23–27 Because basal cerebral oxygen levels correlate with blood flow,28 BCCAO is expected to decrease the basal oxygen levels as well and future studies could examine the correlation between basal, slow changes in oxygen during ischemia and the fast changes observed here. BCCAO and subsequent reperfusion cause significant microvascular alterations in rats,21,22 and our previous study showed 30 minutes of BCCAO produced cell nucleus shrinkage and mitochondria swelling, but not cell death11. BCCAO is also used to model ischemic preconditioning, where a small stroke can induce brain tolerance to larger ischemic injury.29 Other models of stroke could be used in the future to provide global ischemia, either 4-vessel occlusion, with occlusion of the vertebral and carotid arteries,30 or BCCAO with hypotension, where the mean arterial blood pressure is reduced below 50 mmHg.31,32 However, BCCAO is a good model of a partial, transient ischemic attack.24
During BCCAO and early reperfusion, adenosine and oxygen events were still correlated and oxygen events occurred more frequently during ischemia and reperfusion compared to normoxia. Thus, even when major arteries were occluded, local oxygen can still increase, which is a sign of greater local blood flow. The duration of adenosine and oxygen events only last about 3 s, so adenosine promotes transient, local vasodilation even when global blood flow is reduced. The likely cause of the transient increases in oxygen is capillaries dilating to provide local increases in blood flow. Capillary dilation produces 84% of the blood flow increase in response to neural activity,33 which is generated by a relaxation of pericytes. Pericytes constrict capillaries and often die during ischemia,34 which may explain why blood flow did not restore to the same level as normoxia condition during reperfusion (Figures 2A–2C).
The percentage of adenosine events followed by an oxygen event are also similar during normoxia and I/R (Figure 4). The similar percentage of adenosine events causing oxygen is likely because the concentration of each adenosine event did not change during I/R, and a high concentration of adenosine is correlated with an oxygen event.12 Therefore, the increase in local blood flow during I/R was primarily caused by an increase in adenosine event frequency, and more adenosine and oxygen events occurred. These results demonstrate that adenosine could be a stress signal that causes local blood flow and oxygen increase to help mitigate against I/R injury. However, causation has not been definitively proven and there are other molecules, such as NO and CO2, that might also be co-released to induce vasodilation. Our previous study showed that NO synthase inhibitor, L-NAME, did not affect adenosine or subsequent oxygen events, suggesting NO is not the cause of oxygen release.12 Future research could investigate whether other vasodilatory molecules are correlated with these oxygen and adenosine changes.
A2A receptors diminish adenosine and oxygen events during I/R
Extracellular adenosine plays an important role during cerebral ischemia, and drugs that target the adenosine system have been proposed as stroke treatments.35 A2A antagonists are neuroprotective during stroke, although their effects can vary by dose, with lower doses sometimes being more effective.36,37 However, the high dose of SCH 442416 administered here is protective against cell swelling and death.11 A2A receptors are highly expressed in the cerebral vasculature38–40 and are located on endothelial as well as smooth muscle cells.41 Adenosine regulates vasodilation through A2A receptors, which are coupled to excitatory G proteins.42,43 Treatment with an A2A antagonist, ZM-241385, reduces the vasodilation caused by neuronal activation44 and A2A receptor knockout mice have a significantly reduced capacity for vasodilation of cerebral blood vessels during short periods of hypotension.44 The objective of this study was to determine whether an A2A antagonist affects adenosine for oxygen increases and our hypothesis was that there would be fewer oxygen events after an A2A antagonist.
While I/R increased the number of adenosine and oxygen events in controls, that increase was not observed when the A2A antagonist SCH 442416 was administered. These results are also similar to our previous finding on non-ischemic rats, where blocking A2A receptors with SCH 442416 decreased the number of both adenosine and oxygen events but did not change the concentration.12 There was no significant change in the percentage of adenosine events with a subsequent oxygen event under I/R + SCH (Figure 4). Thus, the main driver for the lower number of oxygen events with the A2A antagonist is that there are fewer adenosine events to cause them. However, oxygen events were not completely eliminated by the A2A antagonist, and thus there may also be other molecules co-released that stimulate endothelial second messengers systems, such as prostaglandins45 or CO2.46 Future studies could clarify the role of A2A receptors on adenosine-induced transient blood flow change by using A2A knockout mice. Even though blockade of A2A receptors by SCH 442416 did not suppress global cerebral blood flow (i.e Figure 2), it reduced the extent of local, transient vasodilation. Therefore, the A2A antagonist suppressed the release of rapid adenosine and the subsequent oxygen events so there was not as much delivery of local blood oxygen to the brain.
Caffeine diminishes adenosine and oxygen events during I/R
Caffeine is a nonselective adenosine receptor antagonist that can reduce the physical, cellular, and molecular damage caused by a stroke.19 Vasoconstrictive effects of caffeine were investigated,47,48 and dietary caffeine with a mean dose of 250 mg decreased 30% cerebral blood flow in humans.49 Another study demonstrated that high caffeine users (950 mg/day) had less cerebral blood flow than the low (45 mg/day) or moderate users (405 mg/day).50 In rats, caffeine produces a dose-dependent reduction in cerebral blood flow during neuronal activation51 and attenuates exogenous adenosine-induced vasodilation during somatosensory stimulation.13 Previous experiments suggest that various caffeine dosages could either increase or attenuate ischemia induced brain damage depending on the treatment paradigm.52,53 Here, we examined the effects of caffeine on local blood flow changes caused by transient adenosine release during I/R.
A large dose of caffeine 100 mg/kg (i.p.) before I/R significantly reduced the number and frequency of adenosine and oxygen events. In addition, the percentage of adenosine events that were followed by an oxygen event significantly decreased during caffeine + I/R (Figure 4). The average spontaneous adenosine event concentration was a couple hundred nM,54 which was sufficient to activate high affinity A1 (approximately 70 nM) and A2A receptors (150 nM) receptors, but not A2B (5100 nM) and A3 receptors (6500 nM).55,56 The potency of caffeine for adenosine receptors is similar (A1, KD = 20 μM and A2A, KD = 8.1 μM)57 and we used a high dose that would bind to both receptors. However, the data are in line with caffeine acting primarily to block the excitatory A2A receptors, and it had even more of an effect of blocking adenosine events than the A2A antagonist. The blood flow data indicate that global blood flow is still attenuated during I/R with a large dose of caffeine, but the decrease in local adenosine and oxygen events indicate that this caffeine dose is likely not locally neuroprotective and could exacerbate the effects of ischemia. Future work is needed to link the transient changes in adenosine and blood flow to ischemic damage, but a high dose of caffeine depresses the local increase in oxygen normally observed during I/R.
Conclusions
Our results demonstrate that during I/R, the number of spontaneous adenosine events increases and that they continue to be correlated with transient changes in oxygen. Thus, even when a major artery is occluded, local, transient oxygen events continue to occur. The specific A2A antagonist SCH 442416 and non-selective antagonist caffeine decreased the amount of adenosine and oxygen events during I/R. This rapid mode of adenosine release likely functions as a local neuromodulator, which produces a local, rapid increase in blood flow in response to I/R. Caffeine dampens these changes in adenosine and oxygen and thus, may dampen the neuromodulatory action of adenosine during I/R. Future studies of more severe stroke will reveal if oxygen can increase when more arteries are blocked or blood pressure is low, and how local actions of adenosine contribute to its neuroprotective effects.
Materials and Methods
Animals Care
Male Sprague-Dawley rats (250–350 g; Charles River, Wilmington, MA, USA) were pair-housed on a 12:12 light-dark cycle and were given food and water ad libitum. All experiments were performed in accordance with the Institutional Animal Care and Use Committee guidelines of the University of Virginia.
Rats were initially anesthetized with isoflurane (1 mL/100g rat weight) in a desiccator and then injected with urethane (1.0 g/kg, i.p.). Additional doses of urethane (<3/10 of the induction dose) were administered as necessary in order to maintain deep anesthesia. The surgical site was shaved and a ventral midline incision was made to expose the common carotid arteries; the right and left common carotid arteries were isolated. Vascular occluder cuffs (DOCXS, Ukiah, CA, USA) were wrapped around the exposed common carotid arteries and secured in place using suture material passed through the eyelets. Liquid was injected into the actuating tube by a syringe to inflate the diaphragm and compress the vessel into full occlusion to induce ischemia.
For adenosine and oxygen measurement, rats were placed in a stereotaxic frame (David Kopf instruments, Tujunga, CA, USA). Bupivacaine (0.25 mL) (Sensorcaine® MPF; APP Pharmaceuticals, LLC, Schaumburg, IL, USA) was administered under the skin for local anesthesia. The surgical site was shaved and holes were drilled above the caudate-putamen and the electrode was placed (AP +1.2 mm, ML +2.0 mm and DV −4.5 mm) based on the atlas of Paxinos and Watson.58 A Ag/AgCl reference electrode was inserted on the contralateral side of brain. The body temperature of the rat was maintained at 37 °C using an isothermal pad and a rectal probe (FHC, Bowdoin, ME, USA).
For blood flow measurement, cerebral blood flow was monitored by laser Doppler flow meter with a 1.5 mm diameter probe (Model DRT4, Moor Instruments, Delaware, DE, USA). Rat was placed in a stereotaxic frame, anesthetized, and holes drilled in similar places as for the electrochemical experiments. The Doppler probe was placed over the hole in the skull in the cortex and blood flow measured every 5 minutes under normoxia and I/R, and with pharmacological agents.
Chemicals and Drugs
All components of the phosphate-buffered saline (PBS) solution (in mM: 3.0 KCl, 10.0 NaH2PO4, 2.0 Na2SO4, 1.2 MgCl2, 131. 25 NaCl and 1.2 CaCl2, with pH adjust to 7.4 using concentrated HCl or NaOH) were purchased from Fisher Scientific (Fair Lawn, NJ, USA) and PBS was used for electrode calibration. A 10.0 mM stock solution of adenosine (Fisher Scientific, Fair Lawn, NJ, USA) was prepared in 0.1 M HClO4 once a month and stored at 4°C. 1.0 μM adenosine solution for electrode calibration was prepared daily in PBS buffer. All drugs were administered intraperitoneally (i.p). Caffeine (100 mg/kg, Sigma Aldrich, St. Louis, MO, USA) was dissolved in 1 mL warm saline. SCH 442416 (3 mg/kg; Tocirs, Bristol, UK) was dissolved in 300 μL dimethyl sulfoxide (DMSO; Amresco, Solon, OH, USA). Doses were selected based on previous experiments in the literature.12,59
Voltammetric Adenosine and Oxygen Measurements
Fabrication of carbon fiber microelectrode with T-650 carbon fiber was previously described.12 Cylinder electrodes were used which were 150–200 μm long and 7 μm in diameter. For adenosine and oxygen measurement, electrodes were scanned from 0 V to 1. 45 V, then – 1.4 V and back to 0 V every 100 ms with a scan rate of 450 V/s. Electrodes were implanted and equilibrated for 1 hour with the applied waveform prior to data collection. Data were excluded if fewer than 20 events were observed in the initial 1 hour of equilibration and a new electrode was inserted. After equilibration, one hour of normoxia data were collected, BCCAO was induced for 30 min by inflating the occluders, and then 30 min of reperfusion data were collected after the occulders were deflated. For pharmacology experiments, the drug was injected directly before BCCAO was induced.
Electrodes were calibrated with 1.0 μM adenosine solution. To calibrate oxygen concentration, various mixtures of nitrogen- and air-saturated PBS buffer with volume ratios of 1:0, 2:1, 5:1, 10:1 and 15:1 were prepared and oxygen concentration measured using a D.O. 6+ dissolved oxygen meter (Eutech Instruments Pte Ltd., Singapore). The microelectrode response was calibrated by running the flow cell analysis of different concentrations, with nitrogen saturated PBS buffer as the comparison for background subtraction. This calibration curve was used to calculate oxygen concentration in vivo.
Principal component regression (PCR) was used to identify adenosine and oxygen events. A training set was obtained based on previous procedure except each adenosine event was first identified by color plot and CV.10 Briefly, adenosine event was identified by color plot and CV with clear primary and secondary oxidation peaks. The five largest and most definitive adenosine were picked as a training set from one animal and used it for data analysis for the same animal only, so that each animal had its own training set using PCR. The CV for the training set was taken at the maximum current of the primary oxidation peak. Principal components were calculated based on the training set. This provided an adenosine concentration vs. time trace and was used to determine the peak concentration of primary oxidation of adenosine and time of oxidation peak location. For oxygen, a training set was compiled from the five different concentrations of oxygen that were measured in vitro.12
Statistical Analysis
All statistics were performed in GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA). All data were presented as mean ± SEM. The distributions of inter-event times of adenosine and oxygen events were analyzed using a KS test. For inter-event time graphs, a few data points with long times were not shown in order highlight the changes at lower inter-event times. However, all data for inter-event times were used for statistical analyses. Averages were analyzed with two-way ANOVA, one-way ANOVA, or paired t-tests, depending on the number of variables in the data sets. p values < 0.05 were considered significant.
Acknowledgments
We would like to thank Dr. Zhiyi Zuo and Dr. Jun Li for kind advice for this project and allowing us to use the blood flow meter.
Funding
This research was supported by a grant from NIH (R01NS076875 and R01EB026497) to BJV.
Footnotes
The authors declare no competing financial interests.
References
- 1.Heron M Deaths: Leading Causes for 2011. Natl Vital Stat Rep. 2015;64(7):1–96. [PubMed] [Google Scholar]
- 2.Williams-Karnesky RL, Stenzel-Poore MP. Adenosine and stroke: maximizing the therapeutic potential of adenosine as a prophylactic and acute neuroprotectant. Curr Neuropharmacol. 2009;7(3):217–227. doi: 10.2174/157015909789152209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000;106(6):723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heistad DD, Marcus ML, Gourley JK, Busija DW. Effect of adenosine and dipyridamole on cerebral blood flow. Am J Physiol. 1981;240(5):H775–80. [DOI] [PubMed] [Google Scholar]
- 5.Phillis JW. Adenosine in the control of the cerebral circulation. Cerebrovasc Brain Metab Rev. 1989;1(1):26–54. [PubMed] [Google Scholar]
- 6.Pelligrino DA, Vetri F, Xu H-L. Purinergic mechanisms in gliovascular coupling. Semin Cell Dev Biol. 2011;22(2):229–236. doi: 10.1016/j.semcdb.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laghi Pasini F, Guideri F, Picano E, et al. Increase in plasma adenosine during brain ischemia in man: A study during transient ischemic attacks, and stroke. Brain Res Bull. 2000;51(4):327–330. doi: 10.1016/S0361-9230(99)00240-3. [DOI] [PubMed] [Google Scholar]
- 8.Kitagawa H, Mori A, Shimada J, Mitsumoto Y, Kikuchi T. Intracerebral adenosine infusion improves neurological outcome after transient focal ischemia in rats. Neurol Res. 2002;24(3):317–323. doi: 10.1179/016164102101199819. [DOI] [PubMed] [Google Scholar]
- 9.Sheardown MJ, Knutsen LJS. Unexpected neuroprotection observed with the adenosine A2A receptor agonist CGS 21680. Drug Dev Res. 1996;39(1):108–114. doi:. [DOI] [Google Scholar]
- 10.Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI, Venton BJ. Characterization of spontaneous, transient adenosine release in the caudate-putamen and prefrontal cortex. PLoS One. 2014;9(1):e87165. doi:19326203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganesana M, Venton BJ. Early changes in transient adenosine during cerebral ischemia and reperfusion injury. PLoS One. 2018;13(5):e0196932. doi: 10.1371/journal.pone.0196932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Venton BJ. Correlation of transient adenosine release and oxygen changes in the caudate-putamen. J Neurochem. 2017;140(1):13–23. doi: 10.1111/jnc.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meno JR, Nguyen TK, Jensen EM, et al. Effect of Caffeine on Cerebral Blood Flow Response to Somatosensory Stimulation. J Cereb Blood Flow Metab. 2005;25(6):775–784. doi: 10.1038/sj.jcbfm.9600075. [DOI] [PubMed] [Google Scholar]
- 14.Vidyasagar R, Greyling A, Draijer R, Corfield DR, Parkes LM. The effect of black tea and caffeine on regional cerebral blood flow measured with arterial spin labeling. J Cereb Blood Flow Metab. 2013;33(6):963–968. doi: 10.1038/jcbfm.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field AS, Laurienti PJ, Yen Y-F, Burdette JH, Moody DM. Dietary Caffeine Consumption and Withdrawal: Confounding Variables in Quantitative Cerebral Perfusion Studies? Radiology. 2003;227(1):129–135. doi: 10.1148/radiol.2271012173. [DOI] [PubMed] [Google Scholar]
- 16.Phillis JW, Preston G, DeLong RE. Effects of Anoxia on Cerebral Blood Flow in the Rat Brain: Evidence for a Role of Adenosine in Autoregulation. J Cereb Blood Flow Metab. 1984;4(4):586–592. doi: 10.1038/jcbfm.1984.83. [DOI] [PubMed] [Google Scholar]
- 17.Conlay LA, Conant JA, deBros F, Wurtman R. Caffeine alters plasma adenosine levels. Nature. 1997;389(6647):136–136. doi: 10.1038/38160. [DOI] [PubMed] [Google Scholar]
- 18.Larsson SC, Männistö S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Coffee and Tea Consumption and Risk of Stroke Subtypes in Male Smokers. Stroke. 2008;39(6). [DOI] [PubMed] [Google Scholar]
- 19.Rivera-Oliver M, Díaz-Ríos M. Using caffeine and other adenosine receptor antagonists and agonists as therapeutic tools against neurodegenerative diseases: a review. Life Sci. 2014;101(1–2):1–9. doi: 10.1016/j.lfs.2014.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svenningsson P, Nomikos GG, Fredholm BB. The stimulatory action and the development of tolerance to caffeine is associated with alterations in gene expression in specific brain regions. J Neurosci. 1999;19(10):4011–4022. doi: 10.1523/JNEUROSCI.19-10-04011.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapi D, Vagnani S, Pignataro G, et al. Protective effects of quercetin on rat pial microvascular changes during transient bilateral common carotid artery occlusion and reperfusion. 2012. doi: 10.3389/fphys.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapi D, Vagnani S, Pignataro G, Esposito E, Paterni M, Colantuoni A. Rat Pial Microvascular Responses to Transient Bilateral Common Carotid Artery Occlusion and Reperfusion: Quercetin’s Mechanism of Action. Front Physiol. 2012;3:99. doi: 10.3389/fphys.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grotta JC, Albers G (Gregory), Broderick JP, et al. Stroke : Pathophysiology, Diagnosis, and Management. Elsevier; 2016. [Google Scholar]
- 24.Vanella A, Sorrenti V, Castorina C, et al. Lipid peroxidation in rat cerebral cortex during post-ischemic reperfusion: Effect of exogenous antioxidants and Ca++-antagonist drugs. Int J Dev Neurosci. 1992;10(1):75–80. doi: 10.1016/0736-5748(92)90008-N. [DOI] [PubMed] [Google Scholar]
- 25.Shaheen AA, Abd-el-Fattah AA, Seif-el-Nasr M. Influence of verapamil on the efficacy of vitamin E in preventing the ischemia-reperfusion-induced biochemical dearrangement in cerebral cortex of rat. Arzneimittelforschung. 1996;46(7):670–673. [PubMed] [Google Scholar]
- 26.Aabdallah DM, Eid NI. Possible neuroprotective effects of lecithin and α-tocopherol alone or in combination against ischemia/reperfusion insult in rat brain. J Biochem Mol Toxicol. 2004;18(5):273–278. doi: 10.1002/jbt.20037. [DOI] [PubMed] [Google Scholar]
- 27.Ghoneim AI, Abdel-Naim AB, Khalifa AE, & El-Denshary ES. Protective effects of curcumin against ischaemia/reperfusion insult in rat forebrain. Pharmacol Res. 2002;46(3):273–279. doi: 10.1016/S1043-6618(02)00123-8. [DOI] [PubMed] [Google Scholar]
- 28.Lowry JP, Fillenz M. Evidence for uncoupling of oxygen and glucose utilization during neuronal activation in rat striatum. J Physiol. 1997;498 (Pt 2)(Pt 2):497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speetzen LJ, Endres M, Kunz A. Bilateral common carotid artery occlusion as an adequate preconditioning stimulus to induce early ischemic tolerance to focal cerebral ischemia. J Vis Exp. 2013;(75):e4387. doi: 10.3791/4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11(5):491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 31.Plaschke K, Bardenheuer HJ, Martin E, Sartor K, Heiland S. Evolution of apparent diffusion coefficient and transverse relaxation time (T2) in the subchronic stage of global cerebral oligemia in different rat models. Exp Brain Res. 2006;169(3):361–368. doi: 10.1007/s00221-005-0146-3. [DOI] [PubMed] [Google Scholar]
- 32.Smith M-L, Bendek G, Dahlgren N, Rosén I, Wieloch T, Siesjö BK. Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Neurol Scand. 1984;69(6):385–401. doi: 10.1111/j.1600-0404.1984.tb07822.x. [DOI] [PubMed] [Google Scholar]
- 33.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Attwell D, Mishra A, Hall CN, O ‘farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2016;36(2):451–455. doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedata F, Corsi C, Melani A, Bordoni F, Latini S. Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann N Y Acad Sci. 2001;939:74–84. doi: 10.1111/j.1749-6632.2001.tb03614.x. [DOI] [PubMed] [Google Scholar]
- 36.Pintor A, Quarta D, Pèzzola A, Reggio R, Popoli P. SCH 58261 (an adenosine A(2A) receptor antagonist) reduces, only at low doses, K(+)-evoked glutamate release in the striatum. Eur J Pharmacol. 2001;421(3):177–180. [DOI] [PubMed] [Google Scholar]
- 37.Popoli P, Pintor A, Domenici MR, et al. Blockade of Striatal Adenosine A 2A Receptor Reduces, through a Presynaptic Mechanism, Quinolinic Acid-Induced Excitotoxicity: Possible Relevance to Neuroprotective Interventions in Neurodegenerative Diseases of the Striatum. J Neurosci. 2002;22(5):1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Regan M Adenosine and the regulation of cerebral blood flow. Neurol Res. 2005;27(2):175–181. doi: 10.1179/016164105X21931. [DOI] [PubMed] [Google Scholar]
- 39.Pelligrino DA, Xu H-L, Vetri F. Caffeine and the control of cerebral hemodynamics. J Alzheimers Dis. 2010;20 Suppl 1:S51–62. doi: 10.3233/JAD-2010-091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mills JH, Alabanza L, Weksler BB, Couraud P-O, Romero IA, Bynoe MS. Human brain endothelial cells are responsive to adenosine receptor activation. Purinergic Signal. 2011;7(2):265–273. doi: 10.1007/s11302-011-9222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coney AM, Marshall JM. Role of adenosine and its receptors in the vasodilatation induced in the cerebral cortex of the rat by systemic hypoxia. J Physiol. 1998;509 (Pt 2:507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillis JW. Adenosine and adenine nucleotides as regulators of cerebral blood flow: roles of acidosis, cell swelling, and KATP channels. Crit Rev Neurobiol. 2004;16(4):237–270. [DOI] [PubMed] [Google Scholar]
- 43.Miekisiak G, Kulik T, Kusano Y, Kung D, Chen J-F, Winn HR. Cerebral Blood Flow Response in Adenosine 2a Receptor Knockout Mice during Transient Hypoxic Hypoxia. J Cereb Blood Flow Metab. 2008;28(10):1656–1664. doi: 10.1038/jcbfm.2008.57. [DOI] [PubMed] [Google Scholar]
- 44.Kusano Y, Echeverry G, Miekisiak G, et al. Role of adenosine A2 receptors in regulation of cerebral blood flow during induced hypotension. J Cereb Blood Flow Metab. 2010;30(4):808–815. doi: 10.1038/jcbfm.2009.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine Contributes to Blood Flow Regulation in the Exercising Human Leg by Increasing Prostaglandin and Nitric Oxide Formation. doi: 10.1161/HYPERTENSIONAHA.109.130880. [DOI] [PubMed] [Google Scholar]
- 46.Yang H-J, Dey D, Sykes J, et al. Arterial CO2 as a Potent Coronary Vasodilator: A Preclinical PET/MR Validation Study with Implications for Cardiac Stress Testing. J Nucl Med. 2017;58(6):953–960. doi: 10.2967/jnumed.116.185991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terai N, Spoerl E, Pillunat LE, Stodtmeister R. The effect of caffeine on retinal vessel diameter in young healthy subjects. Acta Ophthalmol. 2012;90(7):e524–e528. doi: 10.1111/j.1755-3768.2012.02486.x. [DOI] [PubMed] [Google Scholar]
- 48.Addicott MA, Yang LL, Peiffer AM, et al. The effect of daily caffeine use on cerebral blood flow: How much caffeine can we tolerate? Hum Brain Mapp. 2009;30(10):3102–3114. doi: 10.1002/hbm.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cameron OG, Modell JG, Hariharan M. Caffeine and human cerebral blood flow: A positron emission tomography study. Life Sci. 1990;47(13):1141–1146. doi: 10.1016/0024-3205(90)90174-P. [DOI] [PubMed] [Google Scholar]
- 50.Addicott MA, Yang LL, Peiffer AM, et al. The Effect of Daily Caffeine Use on Cerebral Blood Flow: How Much Caffeine Can We Tolerate? Hum Brain Mapp. 2009;10(10):3102–3114. doi: 10.1002/hbm.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gotoh J, Kuang TY, Nakao Y, et al. Regional differences in mechanisms of cerebral circulatory response to neuronal activation. Am J Physiol Heart Circ Physiol. 2001;280(2):H821–9. [DOI] [PubMed] [Google Scholar]
- 52.Jacobson KA, von Lubitz DK, Daly JW, Fredholm BB. Adenosine receptor ligands: differences with acute versus chronic treatment. Trends Pharmacol Sci. 1996;17(3):108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB. Neuroprotective role of adenosine in cerebral ischaemia. Trends PharmacolSci. 1992;13:439–445. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI, Venton BJ. Characterization of spontaneous, transient adenosine release in the caudate-putamen and prefrontal cortex. PLoS One. 2014;9(1):e87165. doi:19326203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Svenningsson P Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59(4):355–396. doi: 10.1016/S0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 56.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 57.Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of Caffeine in the Brain with Special Reference to Factors That Contribute to Its Widespread Use. Pharmacol Rev. 1999;51(1). [PubMed] [Google Scholar]
- 58.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- 59.Sitkovsky Akio Ohta M, Lukashev D, Jackson EK, Ohta A, Fredholm BB, Sitkovsky M. 1,3,7-Trimethylxanthine (Caffeine) May Exacerbate Acute Inflammatory Liver Injury by Weakening the Physiological Immunosuppressive Mechanism. J Immunol. 2007;179(11):7431–7438. doi: 10.4049/jimmunol.179.11.7431. [DOI] [PubMed] [Google Scholar]


