Abstract
Background:
SRY-related HMG box-12, which is associated with the prognosis of cancer, has been frequently described. However, both SRY-related HMG box-12 expression and its relationship with clinicopathological variables and patient survival have not been defined in gastric cancer. The aim of our study was to examine the prognostic value of SRY-related HMG box-12 expression in patients with gastric cancer.
Methods:
In this study, we determined SRY-related HMG box-12 expression in 79 primary gastric cancer tissues and 79 matched adjacent nontumor tissues by immunohistochemistry and then calculated the survival rate using the Kaplan-Meier method. Cox proportional hazard regression model was used to analyze predictors of gastric cancer. Western blot and quantitative real-time polymerase chain reaction were used to investigate the difference in SRY-related HMG box-12 expression between normal gastric epithelial cells and gastric cancer cells at the protein level and RNA level, respectively.
Results:
SRY-related HMG box-12 was downregulated in gastric cancer tissues. Low SRY-related HMG box-12 expression was significantly associated not only with lymph node metastasis (P = .027) and TNM stage (P = .021) but also with disease-specific survival in patients with gastric cancer. Multivariate analysis demonstrated TNM stage was an independent factor predicting poor survival (P = .034).
Conclusions:
Low SRY-related HMG box-12 expression is associated with poor clinical outcomes in gastric cancer.
Keywords: SOX12, gastric cancer, prognosis, survival
Introduction
Gastric cancer (GC) is the most common malignancies in the world.1 Although there are surgical treatment and chemotherapy, the prognosis of GC remains poor, especially for metastasis GC.2 Therefore, it is important to identify new molecules that predict tumor progression and patient survival.
SRY-related HMG box-12 (SOX12) is a member of the SOX gene family with highly conserved high mobility group sequences.3 The SOX proteins are important transcription factors that can maintain the self-renewal and proliferation of embryonic stem cells and various tissue stem cells.4,5 Recent studies indicated that SOX proteins are the leading cause and direct stimulus of invasion, proliferation, and metastasis of tumors, contributing to the high mortality caused by advanced cancers.6,7 For instance, the association between SOX gene expression and the prognosis of various patients with cancer has been reported, including SOX2,8,9 SOX4,10 SOX13,11 SOX 6,12 and SOX 7.13 Likewise, the relationship between SOX12 expression and the prognosis of patients with cancer has also been reported in breast cancer,14 hepatocellular carcinoma,15 lung cancer,16 and colorectal cancer.17 However, it is worth noting that the prognostic value of SOX12 expression in different cancers is highly inconsistent: In colorectal cancer, low SOX12 expression has been shown to be associated with poor prognosis, but in breast cancer, hepatocellular carcinoma, and lung cancer, a positive correlation between high SOX12 expression and poor survival has been reported. These results support the idea that the prognostic value of SOX12 expression in cancers may be organ specific. Yet the role of SOX12 expression in the prognosis of patients with GC is still uncertain. Therefore, we conducted the study to explore the prognostic value of SOX12 expression in patients with GC.
Materials and Methods
Ethics Statement
Ethical approval for the present study was obtained from the Research Ethics Committee of China Medical University, China. All patients providing tumor tissue as well as adjacent nontumor tissues had signed a consent form before surgery to allow for this research to be undertaken.
Tissue Samples and Follow-Up
A total of 79 cases of matched GC and normal tissue samples (3-7 cm away from the tumor margin) were obtained from the First Affiliated Hospital of China Medical University ranging from January 2009 to December 2012. No patients had received any preoperative treatment .The group was composed of 58 men and 21 women with a mean age of 60 ± 15 (range, 22-95) years. All patients-derived specimens were collected and archived under protocols approved by the Institutional Review Boards of the First affiliated Hospital China Medical University. The diagnosis was confirmed by at least 2 pathologists, and the stage was based on pathological outcomes according to the 7th American Joint Committee on Cancer guidelines. Patients with advanced GC received adjuvant chemotherapy after surgery, but no patients received postoperative radiotherapy. Follow-up of the 79 patients was conducted until death or the cutoff date (June 29, 2015). The median of follow-up was 46 months. Disease-specific survival of patients with GC was defined as the time from the initial surgery to the death for cancer-related causes.
Immunohistochemical Methodology
Tissue blocks were fixed in 10% buffered formalin, embedded in paraffin, and serially sectioned at 5-µm thickness. Endogenous peroxidase activity was suppressed by 10-minute incubation with 3% hydrogen peroxide. The slides were then blocked with 5% bobovine serum albumin (BSA) (Boster Bioengineering, Wuhan, China). The tissue sections were treated with primary antibodies against SOX12 (Sigma, Shanghai, China) in a dilution of 1:200 and then incubated overnight in a humidified chamber at 4°C. The sections were visualized with 3,3′-diaminobenzidine and counterstained with hematoxylin for microscopic examination.18
Evaluation of Immunohistochemical Staining
Immunoreactivity was evaluated independently by 2 researchers who were blinded to patient outcomes. The evaluation was based on the staining intensity and extent of staining. Staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The scoring system was as follows: 0, no positive cells; 1-3, positive cells with yellowish, light-brown, and dark-brown staining, respectively. The percentage of positive cells was scored as follows: 0, no positive cells; 1, ≤10% positive cells; 2, 11% to 50% positive cells; 3, >50% positive cells. Expression of SOX12 was scored by multiplying the percentage of positive tumor cells and the staining intensity and ranged from 0 to 9.19
Cell Culture
Normal gastric epithelial cell line (GES-1) and 3 human gastric cancer cell lines (SGC-7901 and AGS) were obtained from the Institute of Biochemistry and Cell Biology at the Chinese Academy of Sciences (Shanghai, China). Gastric epithelial cell line was maintained in Dulbecco modified Eagle medium with 10% fetal bovine serum. Both SGC-7901 and AGS were maintained in RPMI 1640 medium with 10% fetal bovine serum. Cell lines were cultured at 37°C in a humidified atmosphere of 5% CO2.
Western Blot
Whole-cell extracts were prepared by lysing cells with Radio Immunoprecipitation Assay lysis buffer with a proteinase inhibitor cocktail (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Protein concentrations were quantified using a Pierce Protein Assay kit (Thermo Fisher Scientific, Inc, Waltham, Massachusetts). Protein lysates (20 mg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and target proteins were detected by Western blot analysis with antibodies against SOX12 (1:500 dilution; Sigma) and β-actin (1:5000) as a control.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from the cells. Reverse transcription of 1 μg RNA was performed using the RT reagent Kit (Takara, Dalian, China). Quantitative real-time polymerase chain reaction (PCR) was performed using SYBR Green Premix Ex Taq (Takara). The sequence of the primer pairs are as follows: SOX12 forward, 5′-CCGAGCGGCCACATCAAGAG-3′; SOX12 reverse, 5′-GCCACTGGTCCATGATCTTCCG-3′. Glyceraldehyde-3-phosphate dehydrogenase was used as a reference gene. The experiment was repeated 3 times.
Statistical Analysis
Statistical analysis was performed using SPSS version 22 software. The χ2 test or Fisher exact test was used to analyze the difference in SOX12 expression between adjacent nontumor tissues and GC tissues and the relationship between SOX12 expression in GC and clinicopathological variables. According to the immunohistochemical score of cancer and para-cancer tissue, the receiver operating characteristic (ROC) curve was made, and the cutoff value was found. The survival rates were calculated by the Kaplan-Meier method, and the differences between the survival curves were examined by the log-rank test. Prognostic factors were assessed in univariate and multivariate analyses using Cox proportional hazards regression models. Only the significant prognostic factors in the univariate analyses were included to the multivariate analysis. All the other data were analyzed using Student t test. All P values were based on the 2-sided statistical analysis, and P < .05 was considered to be statistically significant in difference.
Results
Expression of SOX12
SOX12 was expressed in both primary GC tissues and matched adjacent nontumor tissues (Figure 1). In 79 GC tissues, the immunohistochemical score of SOX12 was 3.55 ± 1.863. In 79 matched adjacent nontumor tissues, the score was 6.70 ± 1.449. According to the immunohistochemical score of cancer and matched adjacent nontumor tissues, the ROC curve was made, and the cutoff value was found (cutoff value = 5). So, we classified a pattern of SOX12 expression ranging from high expression (scored as ≥5) to low expression (scored as ≥5; Figure 2). Then, the differential expression of SOX12 between GC tissues and normal tissues was statistically analyzed. As shown in Figure 1, SOX12 was lowly expressed in GC tissues when compared to the paired nontumor tissues. We found that in 79 pairs of GC tissues and adjacent nontumor tissues, the high expression rate of SOX12 in cancer tissues was 77.22% (61/79), which was significantly lower than 93.67% (74 /79) in adjacent tissues. The difference in SOX12 staining between adjacent nontumor tissues and GC tissues was statistically significant (P = .006).
Figure 1.
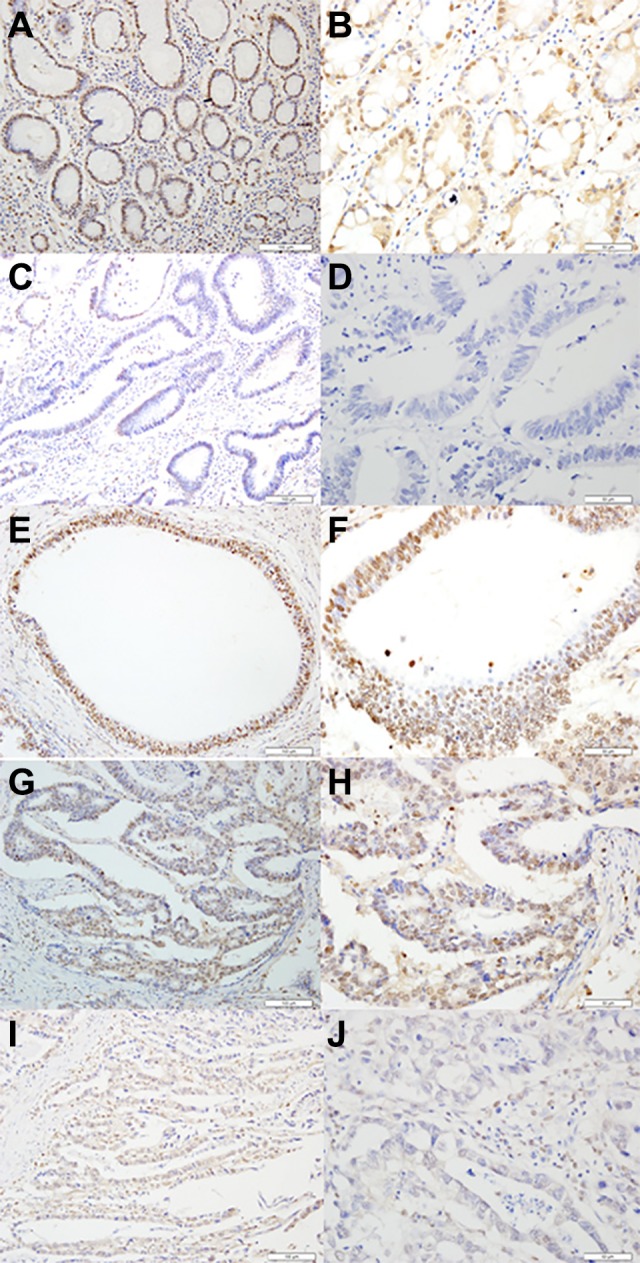
Representative of SRY-related HMG box-12 (SOX12) expression in adjacent nontumor tissues and primary gastric cancer tissues detected by immunostaining with anti-SOX12 antibody (×200 or ×400) .The evaluation was based on the staining intensity and extent of staining. Staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). A and B, Staining status of adjacent nontumor tissues (strong staining). C and D, Staining of SOX12 in gastric cancer tissues (negative). E and F, Staining of SOX12 in gastric cancer tissues (strong staining). G and H, Staining of SOX12 in gastric cancer tissues (medium staining). I and J, Staining of SOX12 in gastric cancer tissues (weak staining).
Figure 2.
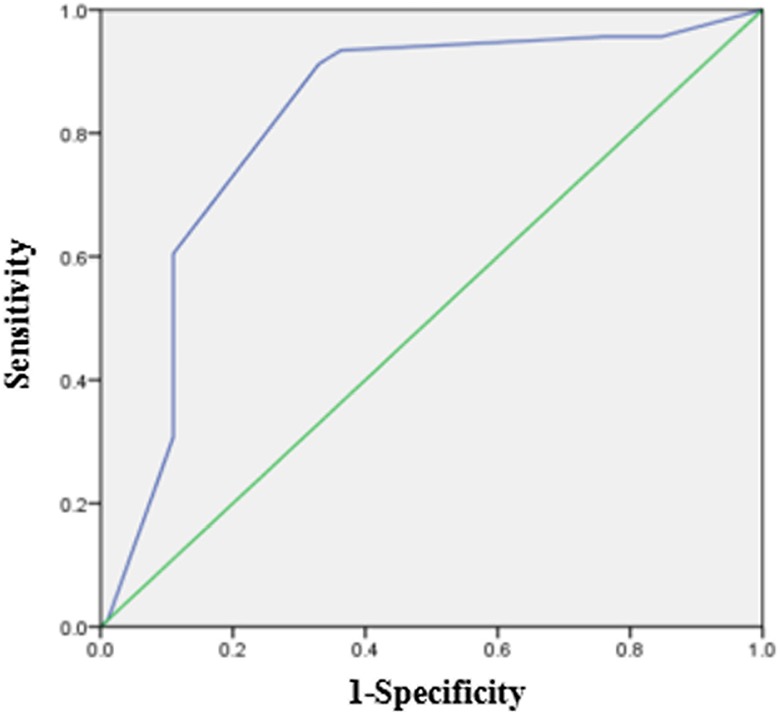
SRY-related HMG box-12 (SOX12) expression level receiver operating characteristic (ROC) curve.
Subsequently, we analyzed the protein and messenger RNA (mRNA) expression of SOX12 between 1 normal GES-1 and 2 GC cell lines (AGS and SGC-7901) by analyzing Western blot and quantitative real-time PCR. In concordance with our immunohistochemistry results, we found that expression levels of SOX12 mRNA and protein in GC cell lines were significant lower than the expression levels in the GES-1 (Figure 3A and B).
Figure 3.
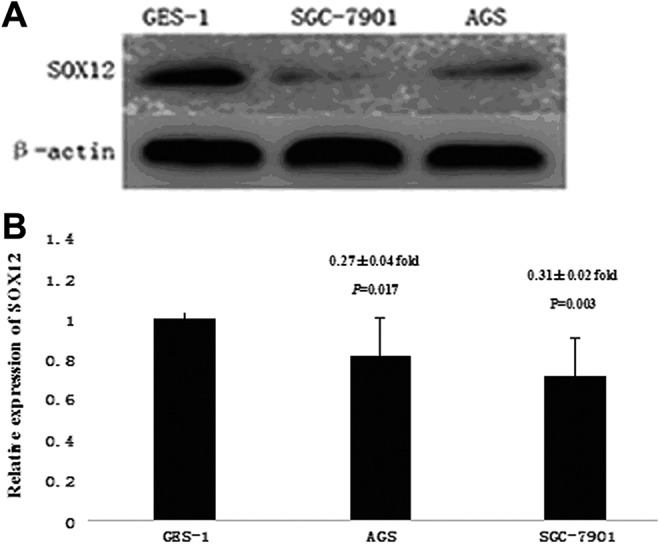
Expression of SRY-related HMG box-12 (SOX12) in gastric cancer cell lines. Western blot analysis (A) and quantitative real-time polymerase chain reaction (PCR) analysis (B) of SOX12 expression in 1 gastric epithelial cell line (GES-1) and 2 gastric cancer cell lines (AGS and SGC-7901). SOX12 was downregulated in AGS and SGC-7901 cell lines compared to GES-1 cell line. Data of quantitative real-time PCR analysis were presented in gastric cancer cell lines relative to GES-1.
Correlation Between SOX12 Expression and Clinicopathological Variables in Patients With GC
To investigate the clinicopathological significance of SOX12 in GC, we analyzed the potential correlations between SOX12 expression and the clinicopathological characteristics of patients with GC. As shown in Table 1, low SOX12 expression was significantly correlated with the lymph node metastasis (P = .027) and TNM stage (P = .021) but not significantly associated with age, invasive depth, vascular invasion, and histological type.
Table 1.
Relationship Between SOX12 Expression and Clinicopathological Variables in Patients With Gastric Cancer.
| Characteristics | SOX12 Expression | P Value | |
|---|---|---|---|
| Low (%) | High (%) | ||
| Age, years | .518 | ||
| ≥60 | 11 (22.0) | 39 (78.0) | |
| ≥60 | 7 (24.1) | 22 (75.9) | |
| Sex | .552 | ||
| Male | 12 (20.7) | 46 79.3) | |
| Female | 6 (28.6) | 15 (71.4) | |
| Histological type | .582 | ||
| Differentiated | 9 (23.1) | 30 (76.9) | |
| Undifferentiated | 9 (22.5) | 31 (77.5) | |
| Lymph node metastasis | .027 | ||
| N1 | 0 (0.0) | 12 (100) | |
| N2 | 4 (23.5) | 13 (76.5) | |
| N3 | 9 (42.9) | 13 (57.1) | |
| N0 | 4 (14.3) | 24 (85.7) | |
| Invasive depth | .543 | ||
| T1, T2 | 5 (15.2) | 28 (84.8) | |
| T3, T4 | 13 (28.3) | 33 (71.7) | |
| Vascular invasion | .317 | ||
| Negative | 12 (17.6) | 56 (82.4) | |
| Positive | 8 (42.1) | 11 (57.9) | |
| TNM stage | .021 | ||
| I, II | 4 (11.1) | 32 (88.9) | |
| III, IV | 14 (32.6) | 29 (67.4) | |
Abbreviation: SOX12, SRY related HMG box-12.
Survival Analysis
We then performed a Kaplan-Meier survival analysis to assess whether SOX12 expression in GC tissues could be used as a prognostic factor for patients with GC. As shown in Figure 4, the disease-specific survival in patients with high SOX12 expression was shown to be significantly greater than that of patients with low SOX12 expression (P = .025; Figure 4).
Figure 4.
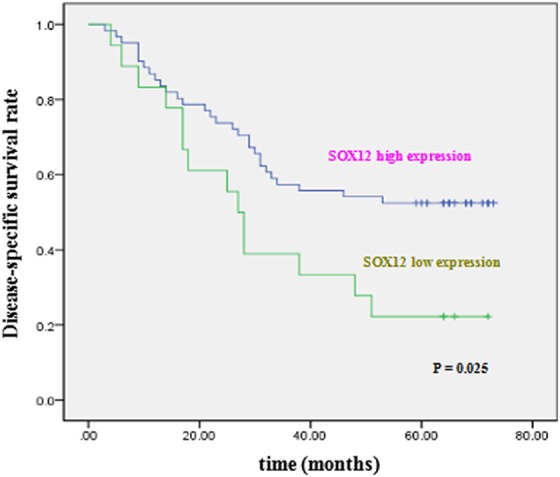
Kaplan-Meier postoperative survival curve for disease-specific survival of patients with gastric cancer.
To further determine the prognostic value of SOX12 expression in GC, univariate and multivariate analyses were performed. On univariate analysis, SOX12 expression (P = .025), lymph node metastasis (P = .001), T stage (P = .003), and TNM stage (P < .001) were all demonstrated to be prognostic factors. Multivariate analysis demonstrated that TNM stage was independent prognostic factor (P = .034; Table 2).
Table 2.
Univariate and Multivariate Analysis of Prognostic Factors for Disease-Specific Survival in Patients With Gastric Cancer.
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Age | 1.032 (1.005-1.061) | .822 | ||
| Histological type | 0.817 (0.449-1.488) | .509 | ||
| T stage | 2.875 (1.442-5.733) | .003 | ||
| N stage | 3.561 (1.645-7.708) | .001 | ||
| TNM stage | 4.358 (2.180-8.711) | <.001 | 3.951 (1.108-14.083) | .034 |
| SOX12 expression | 0.481 (0.253-0.914) | .025 | ||
Abbreviations: SOX12, SRY-related HMG box-12; CI, confidence interval.
Discussion
The abnormal expression of SOX12, which has a close association with the development, progression, metastasis, and prognosis of cancer, has been frequently reported.20-22 However, both SOX12 expression and its association with clinicopathological findings and the reported 5-year survival rate have not been defined in GCs. In this study, we found that SOX12 was downregulated in GC tissues compared to adjacent nontumor tissues. Our Western blot and quantitative real-time PCR analyses also confirmed this result in gastric cell lines. In addition, we found that low expression of SOX12 was associated with the presence of lymph node metastasis and advanced TNM stage. Survival analysis showed that low expression of SOX12 was significantly associated with poor disease-specific survival in patients with GC. These results suggest that downregulation of SOX12 may lead to poor prognosis in patients with GC.
In the current study, we found low SOX12 expression was significantly associated with lymph node metastasis and advanced TNM stage. Previous work has suggested that SOX12 may serve as a metastasis suppressor by affecting Wnt signaling in colon cancer.17 Therefore, given the versatility of SOX12 and the critical role of Wnt in tumorigenesis, we speculate that the tumor suppressor role of SOX12 in GC can be ascribed to this mechanism. In the future work, the metastasis suppressor characteristics of Wnt signaling should be examined for GC in the context of low SOX12 expression.
Although there has been an agreement that the functions of SOX12 differ in different cancers, conflict observations were reported. For instance, Huang et al showed that survival rate of patients with hepatocellular carcinoma having higher SOX12 expression was significantly shorter than patients with lower SOX12 expression.15 On the contrary, Duquet et al demonstrated that survival rate of patients with colon cancer having lower SOX12 expression was significantly shorter than patients with higher SOX12 expression.17 The discrepancies may arise from different organs involved in the studies: the observations of Huang et al were mainly made in hepatocellular carcinoma, whereas in the study of Duquet et al, the role of SOX12 was studied in colon cancer. Therefore, to unequivocally illustrate the specific role of SOX12 in GC, we think it more appropriate to directly investigate the prognostic value of SOX12 expression in patients with GC. Consequently, in our study, Cox proportional hazard regression model was used to analyze predictors of GC. Our results clearly show the survival rate of patients with GC having lower SOX12 expression was significantly shorter than patients with higher SOX12 expression.
Interestingly, the results obtained by Du et al 23 are contrary to our results. This difference may be caused by a series of factors, such as experimental reagents, experimental population, and so on. The specific reason remains to be further verified by experiments.
Conclusions
In summary, in this study, we showed that SOX12 was downregulated in GC tissues and correlated with poor prognosis in patients with GC. Our finding implies that SOX12 could be a potential prognostic marker in patients with GC.
Abbreviations
- GC
gastric cancer
- GES-1
gastric epithelial cell line
- mRNA
messenger RNA
- PCR
polymerase chain reaction
- ROC
receiver operating characteristic
- SOX12
SRY-related HMG box-12.
Footnotes
Authors’ Note: Kan-kan Yang and Hui-mian Xu assisted in study concept and design and writing the article; Kan-kan Yang, Jin-yu Huang, and Yu-xuan Guo assisted in analysis and interpretation of data; Kan-kan Yang and Jin-yu Huang assisted in cell experiment; Zhen-ning Wang assisted in data collection; Hui-mian Xu assisted in obtaining funding. The ethical approval of this study came from the ethics committee of China Medical University (No. 2011415052). Consent to participate in the study was obtained from all patients.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was fund by National Science Foundation of China [No.81272550].
ORCID iD: Hui-mian Xu  https://orcid.org/0000-0002-2184-6510
https://orcid.org/0000-0002-2184-6510
References
- 1. Miller KD, Goding Sauer A, Ortiz AP, et al. Cancer statistics for Hispanics/Latinos, 2018. CA Cancer J Clin. 2018;68(6):425–445. [DOI] [PubMed] [Google Scholar]
- 2. Li W, Ng JM, Wong CC, Ng EKW, Yu J. Molecular alterations of cancer cell and tumour microenvironment in metastatic gastric cancer. Oncogene. 2018;37(36):4903–4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12(1):15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhattaram P, Penzo-Mendez A, Sock E, et al. Organogenesis relies on SoxC transcription factors for the survival of neural and mesenchymal progenitors. Nat Commun. 2010;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lang D, Powell SK, Plummer RS, Young KP, Ruggeri BA. PAX genes: roles in development, pathophysiology, and cancer. Biochem Pharmacol. 2007;73(1):1–14. [DOI] [PubMed] [Google Scholar]
- 6. Kamachi Y, Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Development. 2013;140(20):4129–4144. [DOI] [PubMed] [Google Scholar]
- 7. Castillo SD, Sanchez-Cespedes M. The SOX family of genes in cancer development: biological relevance and opportunities for therapy. Expert Opin Ther Targets. 2012;16(9):903–919. [DOI] [PubMed] [Google Scholar]
- 8. Camilo V, Barros R, Celestino R, et al. Immunohistochemical molecular phenotypes of gastric cancer based on SOX2 and CDX2 predict patient outcome. BMC Cancer. 2014;14:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Otsubo T, Akiyama Y, Yanagihara K, Yuasa Y. SOX2 is frequently downregulated in gastric cancers and inhibits cell growth through cell-cycle arrest and apoptosis. Br J Cancer. 2008;98(4):824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reichling T, Goss KH, Carson DJ, et al. Transcriptional profiles of intestinal tumors in Apc(Min) mice are unique from those of embryonic intestine and identify novel gene targets dysregulated in human colorectal tumors. Cancer Res. 2005;65(1):166–176. [PubMed] [Google Scholar]
- 11. Schlierf B, Friedrich RP, Roerig P, Felsberg J, Reifenberger G, Wegner M. Expression of SoxE and SoxD genes in human gliomas. Neuropathol App Neurobiol. 2007;33(6):621–630. [DOI] [PubMed] [Google Scholar]
- 12. Qin YR, Tang H, Xie F, et al. Characterization of tumor-suppressive function of SOX6 in human esophageal squamous cell carcinoma. Clin Cancer Res. 2011;17(1):46–55. [DOI] [PubMed] [Google Scholar]
- 13. Stovall DB, Cao P, Sui G. SOX7: from a developmental regulator to an emerging tumor suppressor. Histol Histopathol. 2014;29(4):439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ding H, Quan H, Yan W, Han J. Silencing of SOX12 by shRNA suppresses migration, invasion and proliferation of breast cancer cells. Biosci Rep. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Huang W, Chen Z, Shang X, et al. Sox12, a direct target of FoxQ1, promotes hepatocellular carcinoma metastasis through up-regulating Twist1 and FGFBP1. Hepatology. 2015;61(6):1920–1933. [DOI] [PubMed] [Google Scholar]
- 16. Wang L, Hu F, Shen S, et al. Knockdown of SOX12 expression inhibits the proliferation and metastasis of lung cancer cells. Am J Transl Res. 2017;9(9):4003–4014. [PMC free article] [PubMed] [Google Scholar]
- 17. Duquet A, Melotti A, Mishra S, et al. A novel genome-wide in vivo screen for metastatic suppressors in human colon cancer identifies the positive WNT-TCF pathway modulators TMED3 and SOX12. EMBO Mol Med. 2014;6(7):882–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tie J, Pan Y, Zhao L, et al. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet. 2010;6(3):e1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tokunaga Y, Shirahase H, Hoppou T, Kitaoka A, Tokuka A, Ohsumi K. Density of Helicobacter pylori infection evaluated semiquantitatively in gastric cancer. J Clin Gastroenterol. 2000;31(3):217–221. [DOI] [PubMed] [Google Scholar]
- 20. Dy P, Penzo-Mendez A, Wang H, Pedraza CE, Macklin WB, Lefebvre V. The three SoxC proteins—Sox4, Sox11 and Sox12—exhibit overlapping expression patterns and molecular properties. Nucleic Acids Res. 2008;36(9):3101–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinner D, Kordich JJ, Spence JR, et al. Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol. 2007;27(22):7802–7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wan H, Cai J, Chen F, Zhu J, Zhong J, Zhong H. SOX12: a novel potential target for acute myeloid leukaemia. Br J Haematol. 2017;176(3):421–430. [DOI] [PubMed] [Google Scholar]
- 23. Du F, Weibo F, Chen S, et al. Sex determining region Y-box 12 (SOX12) promotes gastric cancer metastasis by upregulating MMP7 and IGF1. Cancer Lett. 2019;452:103–118. [DOI] [PubMed] [Google Scholar]


