Abstract
Dexmedetomidine (DEX) was widely used in clinical work. However, the effectiveness of DEX on postoperative cognitive dysfunction (POCD) was still need to be confirmed. The aim of this meta-analysis was to explore whether DEX can reduce the incidence of POCD on the first day and seventh postoperative day. The results showed that lower incidence of POCD associated with DEX treatment on the first (odds ratio [OR]: 0.41; 95% confidence interval [CI]: 0.31-0.54) or seventh postoperative day (OR: 0.53; 95% CI: 0.36-0.77). Mini-Mental State Examination scores on the first (mean difference [MD]: 4.67; 95% CI: 1.72-7.63) and seventh postoperative days (MD: 3.71; 95% CI: 2.51-4.90) were higher in DEX use group than that in physiological saline group. Meanwhile, neuron-specific enolase (NSE; MD: −3.99; 95% CI: −6.20 to −1.78) and interleukin 6 (IL-6) levels (MD: −17.53; 95% CI: −21.51 to −13.54) on the first postoperative day in DEX group were lower than that in the physiological saline group. This meta-analysis suggested that DEX use could reduce the risk of POCD and the reduction in levels of NSE and IL-6 can improve long-term cognitive dysfunction and anti-inflammation.
Keywords: cognition disorders, dexmedetomidine, inflammation, postoperative
Introduction
Postoperative cognitive dysfunction (POCD) is a serious complication of anesthesia and surgery. The symptoms associated with POCD include confusion, anxiety, personality changes, and impaired memory.1 Postoperative cognitive dysfunction is associated with increased mortality, prolonged hospital stays, increased postoperative complications, and worse short-term quality of life.2,3 Although most frequent after cardiac surgery, studies indicate a POCD incidence of 7% to 40% and 7% to 15% 1 and 3 months postoperatively, respectively, for noncardiac procedures.4 Existing studies confirmed that the incidence of POCD is not significantly different between general and regional anesthesia5 and different types of surgery.6 Although the exact etiopathogenesis of POCD remains unknown, the majority of researchers consider that neuroinflammation plays an important role in the development of POCD. Previous studies have shown that inhalation anesthesia7 and surgery7,8 induce neuroinflammation through disruption of the blood–brain barrier (BBB) and migration of macrophages into the brain.
Dexmedetomidine (DEX), a highly selective α2-receptor agonist, is a useful adjuvant during general anesthesia that promotes hemodynamic stability and decreases the required doses of anesthetics and analgesics.9 Recent data have shown that DEX protects against tissue damage through anti-inflammatory pathways10 because DEX reduces the expression of various inflammatory molecules, including interleukin 6 (IL-6) and tumor necrosis factor-α.11 Although DEX may relieve central nervous system (CNS) damage through antioxidant12 and anti-inflammatory pathways, how DEX reduces the POCD rate remains uncertain. We conducted a meta-analysis to determine the association between DEX and physiological saline with the incidence of POCD.
Materials and Methods
This meta-analysis was conducted and reported according to the 2016 Preferred Reporting Items for Systematic Review and Meta-Analysis statement.13 Ethical approval for this study was not necessary because the study was a review of existing literature and did not involve the processing of any individual patient data.
Search Methods
Two review authors (D.L. and Y.S.) independently searched the PubMed, EMBASE, and Web of Science databases using MeSH terms combined with text words and full texts of suitable studies that were retrieved without language restriction. The online literature was searched from database inception until April 30, 2019. The online literature was searched using the following combination of medical subject heading terms: “POCD” or “postoperative cognitive dysfunction” or “cognitive” or “cognitive dissonance” and “surgery” or “postoperative” and “dexmedetomidine.” Discrepancies between 2 authors were resolved through discussion until reaching a general consensus.
Study Selection and Eligibility Criteria
Two reviewers (D.L. and Y.S.) independently screened the search results and identified studies that were potentially relevant based on the title and abstract. Relevant studies were read in full text and selected according to eligibility criteria. The following elements were used to define the eligibility criteria: (1) study contains 2 groups: control and case group, (2) participants (adult patients undergoing surgery), (3) comparisons (intraoperative DEX vs saline control), (4) primary outcome (incidence of POCD), and secondary outcomes (Mini-Mental State Examination [MMSE] score and neuron-specific enolase [NSE] and IL-6 levels). The exclusion criteria were as follows: (1) only have case group but no control group; (2) reviews, letters, abstracts, or editorials; and (3) studies that reported insufficient data. Patients using DEX compared with other sedative agents (benzodiazepines, midazolam, and propofol) were excluded.
Data Synthesis and Data Analysis
The following data were extracted from the included studies on an Excel spreadsheet (Microsoft Corporation, Washington, USA): authors’ names, year of publication, American Standards Association (ASA) classification, mean age, sample size, and type of surgery. A random effects model was used to define all pooled outcome measures, as described by DerSimonian and Laird.14 The effect size of an intervention, risk ratios with 95% confidence intervals (CIs) were calculated for dichotomous data. The mean difference (MD) and the associated 95% CI were calculated for continuous variables using the same methodology. Heterogeneity was measured and expressed as I2, with an I2 > 50% indicating significant heterogeneity.15 The percentage of total variation across studies reflected heterogeneity rather than chance. The absence of statistical heterogeneity was indicated by a value of 0%, whereas larger values indicated increasing heterogeneity. A fixed-effect model was used for statistical analysis when there was no significant statistical heterogeneity (P > .1 and I2 < 50%). Analyses were conducted using Review Manager (version 5.3; The Cochrane Collaboration, Copenhagen, Denmark). The quality of the included studies was evaluated by the Newcastle-Ottawa Scale.
Results
Retrieved Studies
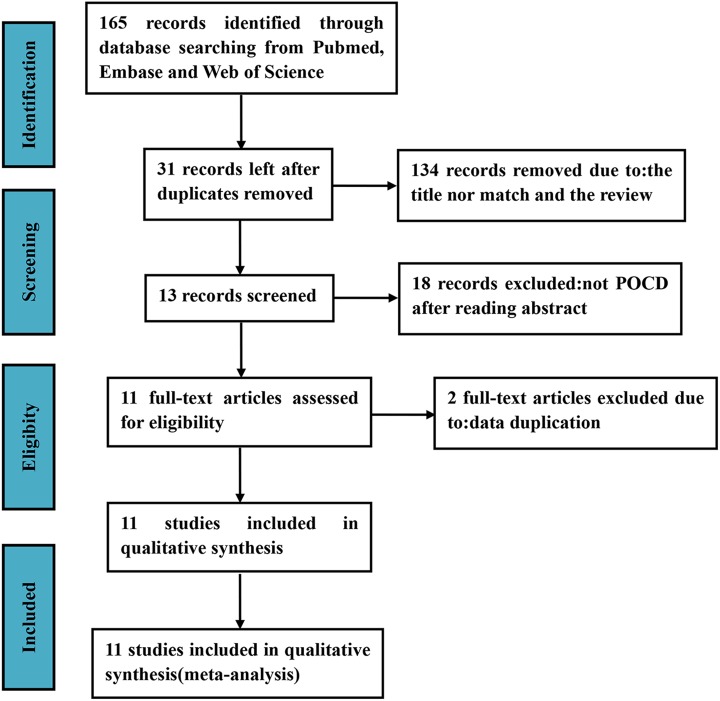
Based on our search strategy, 165 potentially eligible records were identified. The titles and abstracts of these records were screened for inclusion. Exclusion at this stage resulted from articles that clearly did not meet the inclusion criteria and therefore being not deemed potentially relevant. Finally, 13 full-text articles were assessed for eligibility. We found that 2 articles reported on identical patient samples; therefore, we selected 1 article for each patient sample for inclusion. In total, 11 studies were included in our analysis16-26 (Figure 1).
Figure 1.
Summary of the process for identifying candidate studies. Inclusion and exclusion criteria were described in the study selection and eligibility criteria. POCD indicates postoperative cognitive dysfunction.
Basic Features and Quality Assessment of Included Studies
Overall, data from 1400 patients were analyzed in this systematic review and meta-analysis; 751 patients received DEX and 689 patients received saline. Eight studies16-19,22,24-26 included elderly people (≥65 years of age), 2 studies20,23 involved middle-aged people, and 1 study21 primarily involved children or students from the general population (Table 1). The ASA classification of all patients was lower than IV. Studies involved in this analysis included different surgical procedures (abdominal, lower extremities, cardiovascular, eyes, and other minor surgeries) but did not report the outcomes separately for each procedure (Table 1).
Table 1.
Characteristics of the Included Studies.
| Author (Publication Year) | Dexmedetomidine (Experimental) | Physiological Saline Group (Control) | Surgical Setting | Newcastle- Ottawa Scale | ||||
|---|---|---|---|---|---|---|---|---|
| Headcount | Age (Years) | ASA Classification (I/II/III, n) | Headcount | Age (Years) | ASA Classification (I/II/III, n) | |||
| Li et al24 | 20 | 65.8 ± 4.28 | 22/18/0 | 20 | 66.1 ± 3.96 | 9/11/0 | Femoral head replacement | 8 |
| Xu et al20 | 40 | 51.03 ± 6.39 | 16/15/9 | 40 | 48.83 ± 6.52 | 19/13/8 | Orthotopic liver transplantation | 6 |
| Ding et al19 | 50 | 69.7 ± 4.4 | 28/19/3 | 50 | 73.9 ± 6.3 | 24/24/2 | Robot-assisted laparoscopicradical prostatectomy | 6 |
| Cheng et al25 | 269 | 71 ± 5.6 | 196/69/4 | 266 | 70 ± 4.2 | 195/68/3 | Gastrointestinal laparotomy | 8 |
| Zhang et al17 | 20 | 72.3 ± 5.6 | 5/13/2 | 20 | 71.5 ± 4.4 | 4/14/2 | Laparoscopic surgery | 7 |
| Chen et al16 | 59 | 66.2 ± 7.5 | 28/31/0 | 63 | 67.9 ± 6.6 | 29/34/0 | Laparoscopic cholecystectomy | 7 |
| Gong et al23 | 40 | 42.3 ± 1.6 | 18/14/8 | 40 | 42.4 ± 1.5 | 20/18/2 | Coronary artery bypass grafting | 8 |
| Chen et al18 | 87 | 70.6 ± 4.2 | 31/47/9 | 61 | 71.4 ± 4.9 | 22/32/7 | Fracture surgery; prostate removal; gallbladder surgery; radical resection of rectal carcinoma | 6 |
| Xu et al22 | 48 | 71.89 ± 31.36 | 30/18/0 | 48 | 72.06 ± 32.17 | 29/19/0 | Laparoscopic ovarian cystectomy | 7 |
| Jia et al21 | 31 | 13.3 ± 5.5 | 16/15/0 | 31 | 12.4 ± 5.3 | 19/12/0 | General anesthesia | 7 |
| Mansouri et al26 | 50 | 66.5 ± 1.6 | 40/10/0 | 50 | 64.02 ± 7.25 | 43/7/0 | Cataract surgery | 7 |
Abbreviation: ASA, American Standards Association.
Primary Outcome
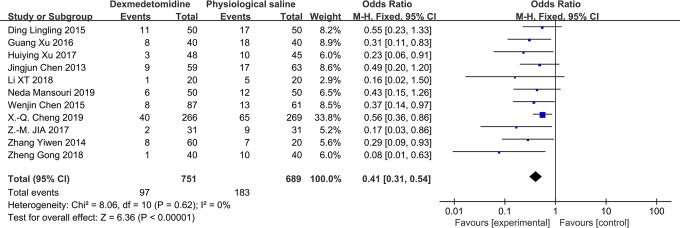
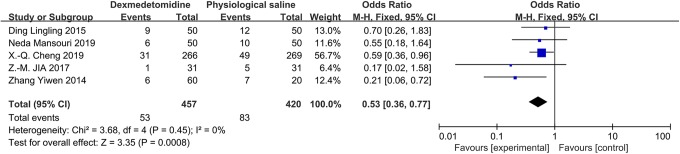
Pooling data from 11 studies demonstrated a significant reduction in the risk of POCD associated with the administration of DEX on the first (odds ratio [OR]: 0.41; 95% CI: 0.31-0.54; P < .0001; I 2 = 0%; Figure 2) and seventh postoperative days (OR: 0.53; 95% CI: 0.36-0.77; P = .008; I 2 = 0%; Figure 3).
Figure 2.
The forest plot: the incidence of POCD of dexmedetomidine versus physiological saline group on the first postoperative day. POCD indicates postoperative cognitive dysfunction; total, all surgical patients.
Figure 3.
The forest plot: the incidence of POCD of dexmedetomidine versus physiological saline group on the seventh postoperative day. POCD indicates postoperative cognitive dysfunction; total, all surgical patients.
Secondary Outcomes
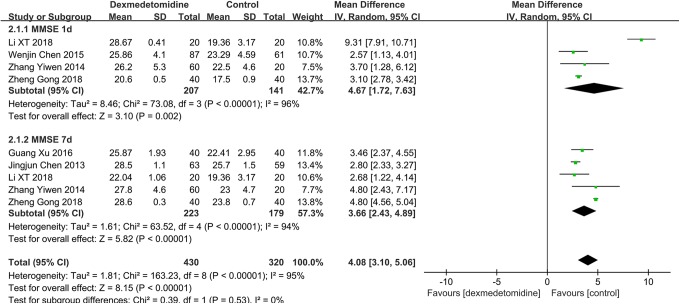
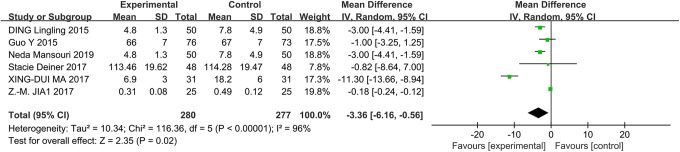
A total of 550 participants (DEX group [n = 310] and physiological saline group [n = 240]) from 6 studies were eligible. A random effects model was used with an I 2 of 95%. This finding suggested that DEX patients had an impaired MMSE score compared with physiological saline patients on the first (MD: 4.67; 95% CI: 1.72-7.63) and seventh postoperative days (MD: 3.66; 95% CI: 0.36-0.77; Figure 4). Heterogeneity was high in the subgroup with MMSE scores on the first (χ2 = 73.08; P = .002; I 2 = 96%) and seventh postoperative days (χ2 = 63.52; P < .00001; I 2 = 94%).
Figure 4.
The forest plot: MMSE score of dexmedetomidine versus physiological saline on the first and seventh postoperative day. MMSE indicates Mini-Mental State Examination.
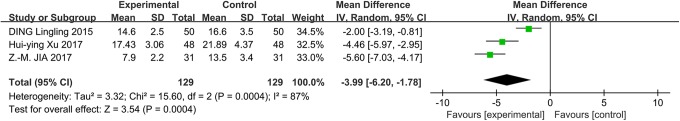
In addition to the incidence of POCD, the NSE levels were also measured in the included studies. Three studies reported NSE levels on the first postoperative day. A random effects model was used with an I 2 of 87%, and the results suggested that the NSE levels were significantly lower on the first postoperative day in the DEX group than the physiological saline group (MD: −3.99; 95% CI: −6.20 to −1.78; Figure 5). Heterogeneity was high in the NSE levels (χ2 = 15.60; P = .0004; I 2 = 87%). As a result of the small number of trials, further subgroup analysis was not conducted.
Figure 5.
The forest plot: NSE level of dexmedetomidine versus physiological saline group on the first postoperative day. NSE indicates neuron-specific enolase.
The IL-6 levels were reported in 6 trials. A random effects model was used with an I 2 of 77%, and the results were significantly lower in the DEX group than the physiological saline group (control group) on the first postoperative day (MD: −17.53; 95% CI: −21.51 to −13.54), as shown in Figure 6. Heterogeneity was high in the IL-6 levels (χ2 = 21.82; P = .0006; I 2 = 77%). As a result of the small number of trials, further subgroup analysis was not conducted.
Figure 6.
The forest plot: IL-6 level of dexmedetomidine versus physiological saline group on the first postoperative day. IL-6 indicates interleukin 6.
Publication Bias
A funnel plot of the 7 studies reporting an incidence of POCD did not suggest publication bias (Figure 7), with all the studies within the 95% CI of the funnel.
Figure 7.
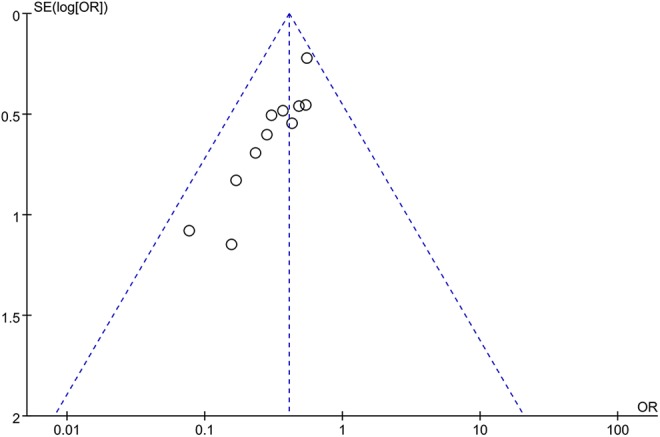
Funnel plot of all the studies reporting incidence of POCD. POCD indicates postoperative cognitive dysfunction.
Discussion
Based on this meta-analysis, evidence from studies showed that DEX is a potential effective treatment for reducing the incidence of POCD and increasing the MMSE score. Comparisons of DEX with physiological saline alternatives suggested that reducing the IL-6 and NSE levels may be beneficial for anti-inflammation and neuroprotection.
Neuron-specific enolase, which is secreted by the neurons or neuroendocrine cells, is an enzyme that catalyzes the conversion of 2-phospho-d-glycerate to phosphoenolpyruvate in the glycolytic pathway. Neuron-specific enolase has potential use as an indicator of cognitive decline and is beneficial for perioperative care and identification of individuals at risk of long-term cognitive dysfunction.27 Our included studies observed the incidence of POCD and NSE level in DEX group was significantly lower than the control group within the first day postoperatively, which suggested that DEX may attenuate POCD by inhibiting the generation of NSE.
Neuroinflammation has a substantial role in the pathogenesis of POCD.28 Pro-inflammatory cytokines can upregulate COX-2 in neurovascular endothelial cells and disrupt BBB permeability,29 allowing pro-inflammatory cytokines to enter the CNS. Interleukin 6 has been found in hippocampal tissue and cerebrospinal fluid following surgical trauma, suggesting a breakdown in the BBB. Available evidence has shown that peripheral surgery can induce the release of pro-inflammatory cytokines, such as IL-6, which could enter the brain to cause further neuroinflammatory responses and brain injury.30 In addition, serum IL-6 levels among individuals are a significant correlate of memory performance.31 Furthermore, IL-6 is strongly expressed in the hippocampus, which is well placed to modulate memory after manipulations in the periphery.32 The relationship between DEX reduction in the incidence of POCD and the decrease in the IL-6 level requires more research to further reveal the function of IL-6 in memory.
As a potent α2-adrenergic agonist, DEX acts on the locus caeruleus of the brain and stimulates the presynaptic α2-adrenoceptors, thus inhibiting the release of noradrenaline at the sympathetic synapses.33 The reduction in norepinephrine induces the release of neurotransmitters, including γ-aminobutyric acid and galanin by the ventrolateral preoptic nucleus. These neurotransmitters further inhibit norepinephrine release by the locus ceruleus and suppress histamine secretion by the tuberomamillary nucleus.34 Reducing the release of histamine is beneficial to inflammation caused by tissue damage. In addition, DEX suppresses microglia-mediated release of nitric oxide, interleukin 1β, monocyte chemoattractant protein-1, prostaglandin E2, and other factors integral to the pro-inflammatory cascade, which play an integral role in the pro- and anti-inflammatory cascades.35 Dexmedetomidine possesses an anti-inflammatory effect reducing the central sympathetic outflow and surgical stress response. All these findings indicate that the intraoperative use of DEX can reduce the incidence of POCD after surgery.
This review had several limitations. First, only 11 articles involving 1400 patients were selected; the sample sizes of these studies were relatively small. Second, it was not possible to assess the long-term associations of DEX with POCD because no trial followed patients for >3 months. Third, none of the included studies provided rates of developing DEX use disorder and doses of other α2-receptor drugs used during surgery.
Conclusions
This meta-analysis showed that DEX use was associated with reduction in the incidence of POCD and increased the MMSE score compared to physiological saline group. The reduction in NSE and IL-6 levels suggested that DEX may be beneficial for long-term cognitive dysfunction and anti-inflammation.
Footnotes
Authors’ Note: D.L. contributed to the design of the project. C.H. contributed to the administrative support. D.L., Y.S., S.W., S.X., L.L., and C.H. contributed to the collection and assembly of data. D.L. and C.H. contributed to data analysis. All authors wrote the first draft of the manuscript. All authors taken part in the final approval of the manuscript. D.L. and Y.S. contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors thank Professor Hong Zhou for his generous support. This work was funded by Fund of Six Best Talent of Jiangsu, 2016 (grant number WSW-113), and Fund of Science and Technology of Yixing (grant number 2016-11).
ORCID iD: Daoyun Lei  https://orcid.org/0000-0001-9105-9021
https://orcid.org/0000-0001-9105-9021
References
- 1. Cascella M, Bimonte S. The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction. Neural Regen Res. 2017;12(11):1780–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. [DOI] [PubMed] [Google Scholar]
- 3. Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS, Group I. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110(3):548–555. [DOI] [PubMed] [Google Scholar]
- 4. Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology. 2007;106(3):572–590. [DOI] [PubMed] [Google Scholar]
- 5. Guay J. General anaesthesia does not contribute to long-term post-operative cognitive dysfunction in adults: a meta-analysis. Indian J Anaesth. 2011;55(4):358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112(5):1179–1185. [DOI] [PubMed] [Google Scholar]
- 7. Wu X, Lu Y, Dong Y, et al. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-alpha, IL-6, and IL-1beta. Neurobiol Aging. 2012;33(7):1364–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vacas S, Degos V, Feng X, Maze M. The neuroinflammatory response of postoperative cognitive decline. Br Med Bull. 2013;106:161–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guler G, Akin A, Tosun Z, Eskitascoglu E, Mizrak A, Boyaci A. Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anaesthesiol Scand. 2005;49(8):1088–1091. [DOI] [PubMed] [Google Scholar]
- 10. Zeng X, Wang H, Xing X, Wang Q, Li W. Dexmedetomidine protects against transient global cerebral ischemia/reperfusion induced oxidative stress and inflammation in diabetic rats. PLoS One. 2016;11(3):e0151620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xianbao L, Hong Z, Xu Z, Chunfang Z, Dunjin C. Dexmedetomidine reduced cytokine release during postpartum bleeding-induced multiple organ dysfunction syndrome in rats. Mediators Inflamm. 2013;2013:627831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cho JS, Shim JK, Soh S, Kim MK, Kwak YL. Perioperative dexmedetomidine reduces the incidence and severity of acute kidney injury following valvular heart surgery. Kidney Int. 2016;89(3):693–700. [DOI] [PubMed] [Google Scholar]
- 13. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 14. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(pt A):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen J, Yan J, Han X. Dexmedetomidine may benefit cognitive function after laparoscopic cholecystectomy in elderly patients. Exp Ther Med. 2013;5(2):489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang Y, Xing Z, Xu Y, Xu S. Effects of different doses of dexmedetomidine on cognitive dysfunction in elderly patients early after laparoscopic surgery for colorectal cancer [in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34(5):743–746. [PubMed] [Google Scholar]
- 18. Chen W, Liu B, Zhang F, Xue P, Cui R, Lei W. The effects of dexmedetomidine on post-operative cognitive dysfunction and inflammatory factors in senile patients. Int J Clin Exp Med. 2015;8(3):4601–4605. [PMC free article] [PubMed] [Google Scholar]
- 19. Ding L, Zhang H, Mi W, et al. Effects of dexmedetomidine on recovery period of anesthesia and postoperative cognitive function after robot-assisted laparoscopicradical prostatectomy in the elderly people [in Chinese]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;40(2):129–135. [DOI] [PubMed] [Google Scholar]
- 20. Xu G, Li LL, Sun ZT, Zhang W, Han XP. Effects of dexmedetomidine on postoperative cognitive dysfunction and serum levels of b-amyloid and neuronal microtubule-associated protein in orthotopic liver transplantation patients. Ann Transplant. 2016;21:508–515. [DOI] [PubMed] [Google Scholar]
- 21. Jia ZM, Hao HN, Huang ML, Ma DF, Jia XL, Ma B. Influence of dexmedetomidine to cognitive function during recovery period for children with general anesthesia. Eur Rev Med Pharmacol Sci. 2017;21(5):1106–1111. [PubMed] [Google Scholar]
- 22. Xu HY, Fu GH, Wu GS. Effect of dexmedetomidine-induced anesthesia on the postoperative cognitive function of elder patients after laparoscopic ovarian cystectomy. Saudi J Biol Sci. 2017;24(8):1771–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gong Z, Li J, Zhong Y, Guan X, Huang A, Ma L. Effects of dexmedetomidine on postoperative cognitive function in patients undergoing coronary artery bypass grafting. Exp Ther Med. 2018;16(6):4685–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li XT, Jiang XM, Zheng ZY, Huang HS. Effect of dexmedetomidine on inflammatory factors level and cognitive function after femoral head replacement in elderly patients [in Chinese]. Zhongguo Gu Shang. 2018;31(12):1091–1095. [DOI] [PubMed] [Google Scholar]
- 25. Cheng XQ, Mei B, Zuo YM, et al. A multicentre randomised controlled trial of the effect of intra-operative dexmedetomidine on cognitive decline after surgery. Anaesthesia. 2019;74(6):741–750. [DOI] [PubMed] [Google Scholar]
- 26. Mansouri N, Nasrollahi K, Shetabi H. Prevention of cognitive dysfunction after cataract surgery with intravenous administration of midazolam and dexmedetomidine in elderly patients undergoing cataract surgery. Adv Biomed Res. 2019;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones EL, Gauge N, Nilsen OB, et al. Analysis of neuron-specific enolase and S100B as biomarkers of cognitive decline following surgery in older people. Dement Geriatr Cogn Disord. 2012;34(5-6):307–311. [DOI] [PubMed] [Google Scholar]
- 28. Lu SM, Yu CJ, Liu YH, et al. S100A8 contributes to postoperative cognitive dysfunction in mice undergoing tibial fracture surgery by activating the TLR4/MyD88 pathway. Brain Behav Immun. 2015;44:221–234. [DOI] [PubMed] [Google Scholar]
- 29. Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70(6):986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kannan S, Saadani-Makki F, Muzik O, et al. Microglial activation in perinatal rabbit brain induced by intrauterine inflammation: detection with 11C-(R)-PK11195 and small-animal PET. J Nucl Med. 2007;48(6):946–954. [DOI] [PubMed] [Google Scholar]
- 31. Elderkin-Thompson V, Irwin MR, Hellemann G, Kumar A. Interleukin-6 and memory functions of encoding and recall in healthy and depressed elderly adults. Am J Geriatr Psychiatry. 2012;20(9):753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burton MD, Sparkman NL, Johnson RW. Inhibition of interleukin-6 trans-signaling in the brain facilitates recovery from lipopolysaccharide-induced sickness behavior. J Neuroinflammation. 2011;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gu J, Chen J, Xia P, Tao G, Zhao H, Ma D. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta Anaesthesiol Scand. 2011;55(10):1272–1278. [DOI] [PubMed] [Google Scholar]
- 34. Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol. 2008;21(4):457–461. [DOI] [PubMed] [Google Scholar]
- 35. Lannes N, Eppler E, Etemad S, Yotovski P, Filgueira L. Microglia at center stage: a comprehensive review about the versatile and unique residential macrophages of the central nervous system. Oncotarget. 2017;8(69):114393–114413. [DOI] [PMC free article] [PubMed] [Google Scholar]