Abstract
Objective:
Gastric cancer is one of the most common malignant tumors worldwide, and for resectable tumors, the most effective treatment is surgery with chemotherapy in neoadjuvant or adjuvant setting. However, the majority of patients fail to achieve the ideal initial response and/or develop resistance to chemotherapy. It was reported that long noncoding RNA regulator of reprogramming (ROR) is highly associated with the progression of gastric cancer. However, the role ROR in multidrug resistance (MDR) remains unclear.
Methods:
The messenger RNA levels of 63 specimens of patients with gastric cancer were determined by real-time polymerase chain reaction analysis and were correlated with drug resistance and survival of patients. To determine the cellular functions of ROR, we generated gastric cancer MDR cells. The effect of ROR depletion on multidrug resistance-associated protein 1 (MRP1) expression and cell apoptosis were examined by immunoblotting analyses, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and flow cytometry.
Results:
We found that ROR expression levels are positively associated with increased MDR and poor prognosis of patients with gastric cancer. Regulator of reprogramming expression is increased in gastric cancer cells resistant to adriamycin (ADR) and vincristine (VCR). Depletion of ROR reduced MRP1 expression and increased apoptosis of drug-resistant gastric cancer cells in response to ADR and VCR treatment.
Conclusions:
We demonstrated that ROR expression promotes MRP1 expression and MDR of gastric cancer cells and is correlated with increased MDR and poor prognosis of patients with gastric cancer. Our finding highlighted the potential of targeting ROR to improve the efficacy of chemotherapy.
Keywords: lncRNA, ROR, MDR, MRP1, gastric cancer
Introduction
Gastric cancer is one of the most common malignant tumors worldwide, particularly in China and other Asian countries.1 For resectable tumors, the most effective treatment is surgery with chemotherapy in neoadjuvant or adjuvant setting, and several chemotherapeutic drugs, such as adriamycin (ADR), vincristine (VCR), and cisplatin (CDDP), were proven to be effective in gastric cancer therapy of some patients.2-4 However, the majority of patients fail to achieve the ideal initial response and/or develop resistance to chemotherapy.5 Multidrug resistance (MDR) significantly restricts the clinical efficacy of gastric cancer chemotherapy, and it is critical to understand the mechanism underlying MDR.6-9
Long noncoding RNAs (lncRNAs) are RNA polymerase II transcripts that are longer than 200 nucleotides and lack an open reading frame. There are more than 10 000 different lncRNAs that are thought to play crucial roles in the development of human diseases, including cancer.10,11 Various lncRNAs, such as MACC1-AS1,12 SFTA1P,13 UCA1,14 CASC15,15 HOTTIP,16 and DANCR,17 were reported to be dysregulated in gastric cancer. The lncRNA regulator of reprogramming (ROR) was first identified in induced pluripotent stem cells (iPSCs).18 Regulator of reprogramming is highly expressed in self-renewing human embryonic stem cells, iPSCs, and many types of cancer cells, including gastric cancer cells,19 breast cancer cells,20 hepatocellular carcinoma cells,21 and pancreatic cancer cells.22 In our previous study,19 we used fluorescence-activated cell sorting to isolate gastric cancer stem cells from MKN-45 gastric cancer cells and demonstrated that ROR was highly expressed in CD133+ gastric cancer stem cells. Regulator of reprogramming increased the expression of several key stemness-promoting transcriptional factors, such as OCT4, SOX2, and NANOG, as well as expression of CD133 in gastric cancer stem cells. In addition, overexpression of ROR significantly increased the proliferation and invasion of gastric cancer stem cells, whereas knockdown of ROR inhibited the proliferation and invasion of these cells. These results revealed a critical role of ROR in the pluripotent state of gastric cancer stem cells. However, the role of ROR in gastric cancer cells MDR remains elusive.
In this report, we demonstrate that ROR expression is increased in gastric cancer cells resistant to the treatment with ADR and VCR. Depletion of ROR reduced multidrug resistance-associated protein 1 (MRP1) expression and increased apoptosis of drug-resistant gastric cancer in response to ADR and VCR treatment. In addition, we demonstrated that ROR expression levels are positively associated with increased MDR and poor prognosis of patients with gastric cancer.
Materials and Methods
Human Gastric Cancer Tissues Collection
Tumors from 63 patients with gastric cancer with pathological information were obtained after surgery during 2014 to 2016 at the Affiliated Hospital of Qingdao University. All patients had locally advanced gastric cancer. XELOX (capecitabine plus oxaliplatin) was used as neoadjuvant chemotherapy for 2 to 4 cycles. Thirty-six patients’ diseases progressed, whereas the remaining 27 patients did not exhibit disease progression after the neoadjuvant chemotherapy. The clinicopathological features are listed in Table 1. All patients received open D2 radical gastrectomy 15 to 20 days after the completion of the neoadjuvant chemotherapy and their tumor specimens were examined by real-time polymerase chain reaction (RT-PCR) analyses. The resected gastric cancer tissues were fixed in formalin and embedded in paraffin for pathological diagnosis or snap-frozen immediately in liquid nitrogen for RNA extraction. The protocol was approved by the Affiliated Hospital of Qingdao University.
Table 1.
The Clinical Parameters of Patients With Gastric Cancer.
| Variable | Nonrefractory (n = 27) | Refractory (n = 36) | P |
|---|---|---|---|
| Age | 58.63 ± 4.98 | 55.95 ± 6.84 | .896 |
| ≥60 years | 17 | 21 | .798 |
| <60 years | 10 | 15 | |
| Sex | .450 | ||
| Male | 15 | 16 | |
| Female | 12 | 20 | |
| Tumor size | .801 | ||
| ≥5cm | 13 | 19 | |
| <5cm | 14 | 17 | |
| Location | .798 | ||
| Upper one-third | 7 | 11 | |
| Middle one-third | 8 | 12 | |
| Lower one-third | 12 | 13 | |
| TNM stage | 1.000 | ||
| I + II | 5 | 10 | |
| III + IV | 12 | 26 |
Cell Lines and Culture
SGC-7901 human gastric cancer cells were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). SGC-7901 cells were cultured in RPMI-1640 medium supplemented with 10% of fetal bovine serum (Gibco, Invitrogen, Paisley, UK), 100 U/mL of penicillin, and 100 mg/mL of streptomycin and were grown at 37°C in a humidified incubator with 5% CO2. Both ADR and VCR were obtained from Sigma-Aldrich (St Louis, Missouri). The drug-resistant SGC-7901/ADR and SGC-7901/VCR gastric cancer cells were obtained by increasing drug dosages gradually according to previous studies.23-25 To maintain the drug-resistant cell phenotype, ADR and VCR were added to a final concentration of 0.5 and 1 mg/mL, respectively.
Real-Time Polymerase Chain Reaction Analysis
Cells were harvested in TRIzol reagent (Life Technologies, Invitrogen, Carlsbad, California), and the RNA was extracted. The RT-PCR analyses were conducted by following the manufacturer’s instructions, as described in a previous study.19 The primers used for the PCR were listed as follows26: ROR, forward, 5′-CTTGATGGCATTGTCGCTAA-3′; reverse, 5′-TCCAGTGGCTGTGCTAGATG-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward, 5′-TGTTCGTCATGGGTGTGAAC-3′; reverse 5′-ATGGCATGGACTGTGGTCAT-3′.
Lentivirus Infection
The lentivirus expressing ROR shRNA (shRNA-ROR) and control shRNA (shRNA-CON) were synthesized from Ribo Company (Guangzhou, China). The primer sequences of shRNA-ROR and shRNA-CON were similar to the previous study.27 Cells were infected with lentivirus in the enhanced infection solution supplemented with polybrene, according to the manufacturer’s instructions. These cells were then selected with puromycin (Sigma-Aldrich) for 3 weeks to obtain stable cell lines.28
Western Blot Analysis
Proteins were extracted from cultured cells using RIPA buffer (Beyotime, Haimen, China) supplemented with protease inhibitor cocktail (Roche, Basel, Switzerland), and Western blot analysis was performed as described previously.29 Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control, and MRP1 (ab230948; Abcam, Cambridge, United Kingdom) and GAPDH (ab181602; Abcam) were used as primary antibodies. Moreover, goat antirabbit Alexa Fluor® 405 (IgG H&L) (horseradish peroxidase, ab6721; Abcam) was used as the secondary antibody.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)Analysis
Cells were seeded at a density of 5 × 103cells/well into 96-well culture plate and maintained overnight. Then cells were treated by different concentrations of ADR or VCR for 48 hours. Following treatment, 20 μL of MTT solution (5 mg/mL in phosphate-buffered saline [PBS]) was added to each well and the plates were incubated for 4 hours at 37°C. At the end of incubation, the supernatants were aspirated and 150 μL of dimethyl sulfoxide was added into each well for dissolving the formazan crystals. Absorbance at 570 nm was measured using a Bio-Rad microplate reader (Hercules, California). Each assay was performed in triplicate with 3 independent replicates.
Flow Cytometry Analysis
Apoptosis was determined using an Annexin V apoptosis kit (Biosea Biotech, Beijing, China), according to the manufacturer’s instructions. In brief, cells (1 × 105) treated by 1 mg/mL ADR or 2 mg/mL VCR for 48 hours were washed twice with ice-cold PBS and resuspended in binding buffer. Cell pellets were collected and incubated with 5 μL Annexin V-fluorescein isothiocyanate (FITC) and 1 μL of propidium iodide (PI) in dark. The cells were analyzed by an FACS Caliber instrument (BD Biosciences, San Jose, CA, United States). The data of flow cytometry were analyzed with FlowJ software. Three independent experiments were conducted simultaneously.
Statistical Analysis and Reproducibility
The mean values obtained for the control and experimental groups were analyzed for significant differences. Pair-wise comparisons were performed using a 2-tailed Student t test. P values less than .05 were considered significant.
Results
Expression Levels of ROR Are Positively Associated With Increased MDR and Poor Prognosis of Patients With Gastric Cancer
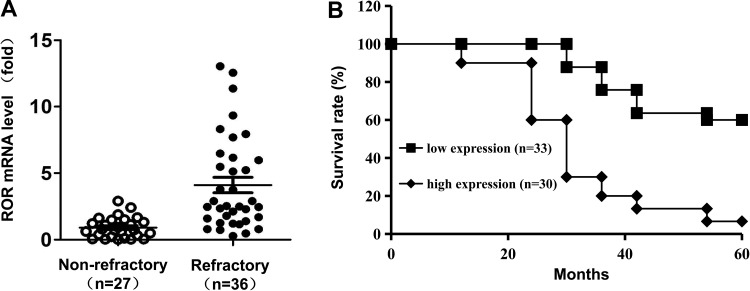
A total of 63 patients with locally advanced gastric cancer were examined. XELOX (capecitabine plus oxaliplatin) was used as neoadjuvant chemotherapy for 2 to 4 cycles. Thirty-six patients’ diseases progressed, whereas the remaining 27 patients did not exhibit disease progression after the neoadjuvant chemotherapy. All patients received open D2 radical gastrectomy 15 to 20 days after the completion of the neoadjuvant chemotherapy and their tumor specimens were examined by RT-PCR analyses. We found that refractory patient tumor specimens after chemotherapy exhibited a significant increase in messenger RNA (mRNA) levels of ROR (Figure 1A). Patients with gastric cancer were classified as low expression group when ROR expression was lower than mean ROR level in all patients. Kaplan-Meier survival curve analyses showed that the overall survival period of the patients with gastric cancer with high ROR expression (n = 33) were substantially decreased compared with that of the patients with gastric cancer with low ROR expression (n = 30; Figure 1B). These results suggested that ROR expression levels are positively associated with increased MDR and poor prognosis of patients with gastric cancer.
Figure 1.
Expression levels of ROR are positively associated with increased MDR and poor prognosis of patients with gastric cancer. A, The expression of ROR was compared by real-time PCR analyses of the tumor specimens from patients with refractory gastric cancer after chemotherapy (n = 36) and patients with nonrefractory gastric cancer (n = 27), P = .0069. B, Kaplan-Meier survival curves stratified a total of 63 gastric cancer patients according to ROR expression. P = .026 between low ROR (n = 33) and high ROR (n = 30) groups. MDR indicates multidrug resistance; PCR, polymerase chain reaction; ROR, regulator of reprogramming.
Depletion of ROR Reduces the MRP1 Expression in Gastric Cancer Cells
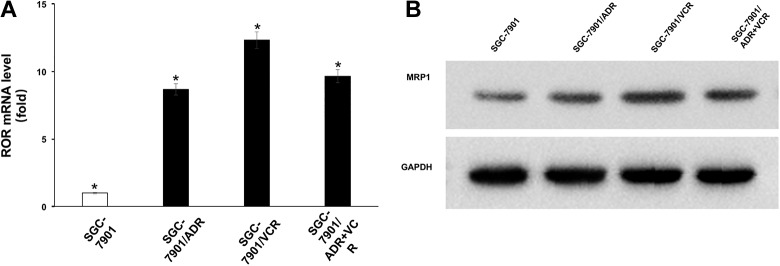
Multidrug resistance-associated protein 1 is critical for MDR of cancer cells.30 To determine the role of ROR in MRP1 expression, we treated SGC-7901 human gastric cancer cells with ADR and/or VCR and generated drug-resistant cells: SGC-7901/ADR, SGC-7901/VCR, and SGC-7SGC901/ADR + VCR, which were resistant to ADR, VCR, and combined ADR and VCR treatment, respectively. We found that the mRNA (Figure 2A) and protein (Figure 2B) levels of MRP1 were significantly increased in these drug-resistant gastric cancer cells compared with their parental cells.
Figure 2.
The expression of ROR was upregulated in gastric cancer MDR cells. A, The ROR mRNA expression in SGC7901, SGC7901/ADR, SGC7901/VCR, and SGC7901/ADR + VCR cells was determined by real-time PCR. *P < .05. B, Protein expression of MRP1 in SGC7901, SGC7901/ADR, SGC7901/VCR, and SGC7901/ADR + VCR cells was determined by immunoblotting analyses with the indicated antibodies. ADR indicates adriamycin; MDR, multidrug resistance; mRNA, messenger RNA; MRP1, multidrug resistance-associated protein 1; PCR, polymerase chain reaction; ROR, regulator of reprogramming; VCR, vincristine.
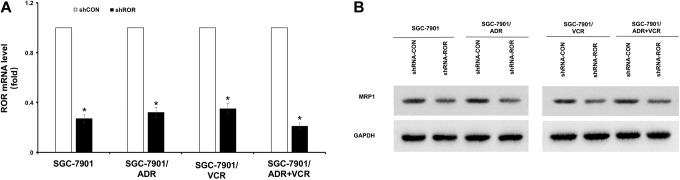
We next depleted ROR expression by expressing its shRNA. Real-time PCR analyses revealed that the shRNA expression reduced mRNA levels of ROR in SGC-7901, SGC-7901/ADR, SGC-7901/VCR, and SGC-7SGC901/ADR + VCR cells (Figure 3A). Regulator of reprogramming depletion reduced the expression of MRP1 in these cells, especially in drug-resistant cells (Figure 3B). These results indicated that ROR regulates MRP1 expression in gastric cancer cells.
Figure 3.
The ROR depletion inhibits the MRP expression in gastric cancer MDR cells. A, The ROR mRNA expression in SGC7901, SGC7901/ADR, SGC7901/VCR, and SGC7901/ADR + VCR cells with or without ROR shRNA expression was determined by real-time PCR. *P < .05. shCON, a control shRNA. shROR, ROR shRNA. B, The MRP1 expression in SGC7901, SGC7901/ADR, SGC7901/VCR, and SGC7901/ADR + VCR cells with or without shROR expression was determined by immunoblotting analyses with the indicated antibodies. ADR indicates adriamycin; MDR, multidrug resistance; mRNA, messenger RNA; MRP1, multidrug resistance-associated protein 1; PCR, polymerase chain reaction; ROR, regulator of reprogramming; shRNA, short hairpin RNA; VCR, vincristine.
Depletion of ROR Reduces MDR in Gastric Cancer Cells
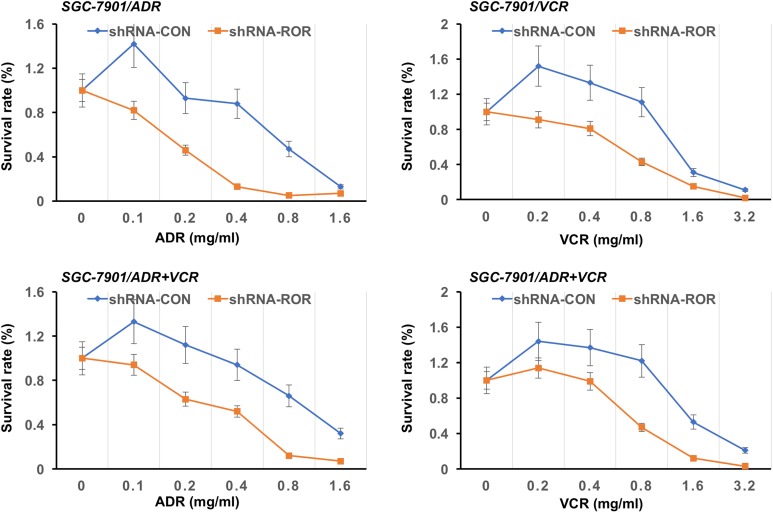
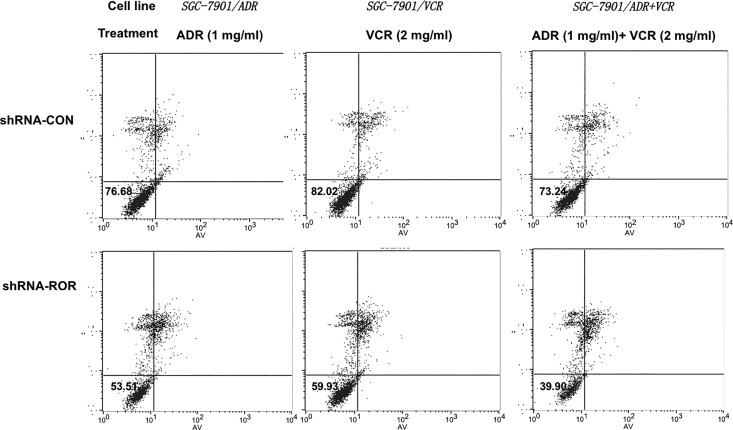
To determine the role of ROR in MDR in gastric cancer cells, we treated SGC-7901/ADR, SGC-7901/VCR, and SGC-7SGC901/ADR + VCR cells, which expressed a control shRNA or ROR shRNA, with different dosages of ADR or VCR for 24 hours. Compared to the cells expressing control shRNA, ROR shRNA expression greatly sensitized SGC-7901/ADR and SGC-7SGC901/ADR + VCR cells to ADR treatment and SGC-7901/VCR and SGC-7SGC901/ADR + VCR cells to VCT treatment and reduced cell survival rates of these cells, as determined by MTT assay (Figure 4). Similar results were also obtained by an apoptosis assay using Annexin V-FITC/PI staining followed by flow cytometry analyses (Figure 5). These results indicated that ROR expression promotes the resistance of gastric cancer cells to chemotherapeutic agents.
Figure 4.
The ROR depletion reduces MDR of gastric cancer cells. Cell survival rates of SGC7901, SGC7901/ADR, SGC7901/VCR, and SGC7901/ADR + VCR cells with or without shROR expression in the presence of the indicated dosages of ADR or VCR were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. ADR indicates adriamycin; MDR, multidrug resistance; ROR, regulator of reprogramming; shROR, ROR short hairpin RNA; VCR, vincristine.
Figure 5.
The ROR depletion induces apoptosis of gastric cancer MDR cells. Cell apoptosis rates of SGC7901, SGC7901/ADR, SGC7901/VCR, and SGC7901/ADR + VCR cells with or without shROR expression in the presence of the ADR (1 mg/mL) or VCR (2 mg/mL) for 48 hours were determined. ADR indicates adriamycin; MDR, multidrug resistance; ROR, regulator of reprogramming; shROR, ROR short hairpin RNA; VCR, vincristine.
Discussion
Gastric cancer is one of the most common malignant tumors.31 Chemotherapy is still a major treatment option for advanced gastric cancer. However, chemotherapy often inevitably induced MDR of gastric cancer.32 Although intensive studies on the progression of MDR in gastric cancer have been conducted,33 the exact mechanism underlying MDR occurrence remains unclear. Previous reports showed that lncRNAs play important roles in tumor development,34 and we previously found that lncRNA ROR was highly expressed in gastric cancer stem cells and demonstrated that abnormally high expression of ROR promoted proliferation and invasion of gastric cancer stem cells.19 In the breast cancer cells, Chen and colleagues have found that overexpression of ROR presented decreased sensibility of 5-fluorouracil (5-FU) and paclitaxel with decreased E-cadherin expression and increased vimentin, N-cadherin expression, and invasion ability, which suggested that ROR is an important marker for MDR of breast cancer, and its upregulation is important for chemotherapy tolerance and invasion of breast cancer.35 Shi et al also indicated that silencing ROR could improve the sensitivity of non-small cell lung cancer to CDDP resistance by inhibiting PI3K/Akt/mTOR signaling pathway.36 In addition, ROR was proved to be closely related to diabetes drug resistance in various kinds of cancers, such as pancreatic cancer,37 colorectal cancer,38 and osteosarcoma.26 However, whether ROR was closely related to drug resistance in gastric cancer remained unknown.
In the present study, we revealed a novel function of ROR in gastric cancer MDR. We demonstrated that high level of ROR is a poor prognostic factor for patients with gastric cancer. In addition, ROR expression regulates MRP1 expression in gastric cancer cells. Regulator of reprogramming depletion inhibited the cell proliferation and reduced the resistance of gastric cancer cells to ADR and VCR treatment. Thus, our finding elucidated an important mechanism underlying MDR in gastric cancer and highlighted the potential of targeting ROR to improve the efficacy of chemotherapeutic treatment.
Footnotes
Authors’ Note: Our study was approved by the ethical committee of The Affiliated Hospital of Qingdao University (approval No. QDHB-2017ABS6TD). All patients provided written informed consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by China Postdoctoral Science Project (2019M652331, 2018M642619), Qingdao Postdoctoral Application Project (2018121236, 2018121238), Department of Health of Shandong Province (2018WS068), Weifang City Science and Technology Bureau (2017YX053), Department of Health of Weifang (2017wsjs122).
ORCID iD: Dongming Xing  https://orcid.org/0000-0001-7427-0861
https://orcid.org/0000-0001-7427-0861
References
- 1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015.CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 2. Zhang YW, Zhang YL, Pan H, et al. Chemotherapy for patients with gastric cancer after complete resection: a network meta-analysis. World J Gastroenterol. 2014;20(2):584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koizumi W. Chemotherapy for advanced gastric cancer: review of global and Japanese status. Gastrointest Cancer Res. 2007;1(5):197–203. [PMC free article] [PubMed] [Google Scholar]
- 4. Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma Y, Wei X, Wu Z. HNF-4α promotes multidrug resistance of gastric cancer cells through the modulation of cell apoptosis. Oncol Lett. 2017;14(6):6477–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou L, Li X, Zhou F, et al. Downregulation of leucine-rich repeats and immunoglobulin-like domains 1 by microRNA-20a modulates gastric cancer multidrug resistance. Cancer Sci. 2018;109(4):1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ilson DH. Advances in the treatment of gastric cancer: 2019.Curr Opin Gastroenterol. 2019;35(6):551–554. [DOI] [PubMed] [Google Scholar]
- 8. Russi S, Verma HK, Laurino S, et al. Adapting and surviving: intra and extra-cellular remodeling in drug-resistant gastric cancer cells. Int J Mol Sci. 2019;20(15). doi:10.3390/ijms20153736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marin JJ, Al-Abdulla R, Lozano E, et al. Mechanisms of resistance to chemotherapy in gastric cancer. Anticancer Agents Med Chem. 2016;16(3):318–334. [DOI] [PubMed] [Google Scholar]
- 10. Wang DQ, Fu P, Yao C, et al. Long non-coding RNAs, novel culprits, or bodyguards in neurodegenerative diseases. Mol Ther Nucleic Acids. 2018;10:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiao Z, Shen J, Zhang L, Li M, Hu W, Cho C. Therapeutic targeting of noncoding RNAs in hepatocellular carcinoma: recent progress and future prospects. Oncol Lett. 2018;15(3):3395–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao Y, Liu Y, Lin L, et al. The lncRNA MACC1-AS1 promotes gastric cancer cell metabolic plasticity via AMPK/Lin28 mediated mRNA stability of MACC1. Mol Cancer. 2018;17(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma H, Ma T, Chen M, Zou Z, Zhang Z. The pseudogene-derived long non-coding RNA SFTA1P suppresses cell proliferation, migration, and invasion in gastric cancer. Biosci Rep. 2018;38(2). doi:10.1042/BSR20171193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gu L, Lu LS, Zhou DL, Liu ZC. UCA1 promotes cell proliferation and invasion of gastric cancer by targeting CREB1 sponging to miR-590-3p. Cancer Med. 2018;7(4):1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu Q, Xiang S, Ma J, et al. Long non-coding RNA CASC15 regulates gastric cancer cell proliferation, migration and epithelial mesenchymal transition by targeting CDKN1A and ZEB1. Mol Oncol. 2018;12(6):799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao R, Zhang Y, Zhang X, et al. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol Cancer. 2018;17(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pan L, Liang W, Gu J, et al. Long noncoding RNA DANCR is activated by SALL4 and promotes the proliferation and invasion of gastric cancer cells. Oncotarget. 2018;9(2):1915–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loewer S, Cabili MN, Guttman M, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet. 2010;42(12):1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang S, Liu F, Deng J, Cai X, Han J, Liu Q. Long noncoding RNA ROR regulates proliferation, invasion, and stemness of gastric cancer stem cell. Cell Reprogram. 2016;18(5):319–326. [DOI] [PubMed] [Google Scholar]
- 20. Eades G, Wolfson B, Zhang Y, Li Q, Yao Y, Zhou Q. lincRNA-RoR and miR-145 regulate invasion in triple-negative breast cancer via targeting ARF6. Mol Cancer Res. 2015;13(2):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takahashi K, Yan IK, Kogure T, Haga H, Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 2014;4:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhan HX, Wang Y, Li C, et al. LincRNA-ROR promotes invasion, metastasis and tumor growth in pancreatic cancer through activating ZEB1 pathway. Cancer Lett. 2016;374(2):261–271. [DOI] [PubMed] [Google Scholar]
- 23. Traverso N, Ricciarelli R, Nitti M, et al. Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev. 2013. doi:10.1155/2013/972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li DQ, Wang ZB, Bai J, et al. Reversal of multidrug resistance in drug-resistant human gastric cancer cell line SGC7901/VCR by antiprogestin drug mifepristone. World J Gastroenterol. 2004;10(12):1722–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yin F, Shi YQ, Zhao WP, Xiao B, Miao JY, Fan DM. Suppression of P-gp induced multiple drug resistance in a drug resistant gastric cancer cell line by overexpression of Fas. World J Gastroenterol. 2000;6(5):664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng FH, Zhao ZS, Liu WD. Long non-coding RNA ROR regulated ABCB1 to induce cisplatin resistance in osteosarcoma by sponging miR-153-3p. Eur Rev Med Pharmacol Sci. 2019;23(17):7256–7265. [DOI] [PubMed] [Google Scholar]
- 27. Hou P, Zhao Y, Li Z, et al. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 2014;5:e1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Y, Jiang H, Zhou H, et al. Lentivirus-mediated silencing of HOTAIR lncRNA restores gefitinib sensitivity by activating Bax/Caspase-3 and suppressing TGF-α/EGFR signaling in lung adenocarcinoma. Oncol Lett. 2018;15(3):2829–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu Z, Liu D, Hornia A, Devonish W, Pagano M, Foster DA. Activation of protein kinase C triggers its ubiquitination and degradation. Mol Cell Biol. 1998;18(2):839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dhasmana D, Singh A, Shukla R, Tripathi T, Garg N. Targeting nucleotide binding domain of multidrug resistance-associated protein-1 (MRP1) for the reversal of multi drug resistance in cancer. Sci Rep. 2018;8(1):11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39(7). doi:10.1177/1010428317714626. [DOI] [PubMed] [Google Scholar]
- 32. Xu W, Yang Z, Lu N. Molecular targeted therapy for the treatment of gastric cancer. J Exp Clin Cancer Res. 2016;35:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nie H, Mu J, Wang J, Li Y. miR-195-5p regulates multi-drug resistance of gastric cancer cells via targeting ZNF139. Oncol Rep. 2018;40(3):1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu J, Zhao W, Wang Z, Xiang X, Zhang S, Liu L. Long non-coding RNA SNHG20 promotes the tumorigenesis of oral squamous cell carcinoma via targeting miR-197/LIN28 axis. J Cell Mol Med. 2019;23(1):680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen YM, Liu Y, Wei HY, Lv KZ, Fu P. Linc-ROR induces epithelial-mesenchymal transition and contributes to drug resistance and invasion of breast cancer cells. Tumour Biol. 2016;37(8):10861–10870. [DOI] [PubMed] [Google Scholar]
- 36. Shi H, Pu J, Zhou XL, Ning YY, Bai C. Silencing long non-coding RNA ROR improves sensitivity of non-small-cell lung cancer to cisplatin resistance by inhibiting PI3K/Akt/mTOR signaling pathway. Tumour Biol. 2017;39(5). doi:10.1177/1010428317697568 [DOI] [PubMed] [Google Scholar]
- 37. Li C, Zhao Z, Zhou Z, Liu R. Linc-ROR confers gemcitabine resistance to pancreatic cancer cells via inducing autophagy and modulating the miR-124/PTBP1/PKM2 axis. Cancer Chemother Pharmacol. 2016;78(6):1199–1207. [DOI] [PubMed] [Google Scholar]
- 38. Yang P, Yang Y, An W, et al. The long noncoding RNA-ROR promotes the resistance of radiotherapy for human colorectal cancer cells by targeting the p53/miR-145 pathway. J Gastroenterol Hepatol. 2017;32(4):837–845. [DOI] [PubMed] [Google Scholar]