Introduction
Excessive bleeding is a major problem in pediatric open heart surgery with cardiopulmonary bypass (CPB), especially in neonates,1–6 consequences of which include higher mortality rates and significant morbidity.7–9 Effective prevention and treatment of excessive bleeding is therefore needed to improve outcomes in the pediatric cardiac surgery population.
The causes of excessive bleeding following pediatric cardiac surgery are multifactorial. Many are attributable to the effects of CPB on the hemostatic system. During complex cardiovascular procedures, CPB is used to maintain blood flow and oxygen delivery to vital organs while diverting blood flow away from the surgical field. Consequences of priming the CPB pump and of extracorporeal circulation of blood through the bypass circuit include hemodilution, as well as activation and consumption of circulating cells and coagulation factors, which collectively impair hemostasis (reviewed in10–13). Defects in platelet aggregation, which are partly attributable to thrombocytopenia, are among the most reproducible hemostatic changes induced by CPB in children and have been associated with excessive post-operative bleeding.5, 14–19 Transfusion with appropriate blood products has been shown to correct CPB-induced defects in platelet count, coagulation factor levels and in vitro measures of hemostasis, but was only partially effective at decreasing perioperative bleeding and reducing the requirement for additional transfusions in the post-operative period for pediatric cardiac surgery patients.14, 15, 20, 21 The factors responsible for excessive post-CPB bleeding in children despite apparent normalization of hemostatic parameters with blood product transfusion are not well characterized.
The goal of this study was to explore the contribution of platelet count and function defects to excessive bleeding following CPB surgery in neonates treated with protocolized platelet and cryoprecipitate transfusions to correct platelet and fibrinogen deficiencies.20, 21 We examined the responsiveness of platelets to agonist stimulation in blood samples obtained at various times before, during, and after CPB surgery. We describe the mechanisms that underlie changes in agonist-induced platelet responsiveness at different times during surgery and clarify the extent to which decreases in platelet count and changes in platelet function correlate with excessive post-operative bleeding in this high-risk population.
Materials and Methods
Study Protocol and Sample Acquisition
Data were obtained from patients enrolled in a prospective observational study of neonates (≤30 days of age) undergoing surgery with CPB. Children’s Hospital of Wisconsin Institutional Review Board (IRB) approved the study and informed consent was obtained from legal guardians of all enrolled subjects. The study protocol, patient demographics, and clinical characteristics of patients with normal vs. excessive levels of post-operative bleeding have been described previously.20,21 Briefly, surgery was performed with CPB on heparinized patients under hypothermic conditions. All patients were transfused with ¼ unit of apheresis-derived single donor platelets (SDP) and 1 unit of cryoprecipitate after aortic cross clamp (AoX) removal and rewarming to 32oC but prior to termination of CPB. Following separation from CPB, protamine was administered to completely reverse the effects of heparin and an additional ¼ unit SDP from the same donor was transfused. Additional transfusion and treatment decisions were based on clinical laboratory test results and evidence of ongoing surgical bleeding. Platelet counts and research laboratory tests of platelet phenotype and function were performed in whole blood (WB) samples, anticoagulated with acid-citrate-dextrose, obtained at four time points, including (1) prior to surgical incision (Baseline), (2) following AoX removal and rewarming to 32°C but prior to SDP and cryoprecipitate transfusion and while still on CPB (On CPB), (3) following separation from CPB, administration of protamine, and transfusion of SDP and cryoprecipitate (Post-CPB), and (4) upon admission to the cardiac intensive care unit (CICU). Patients were separated into two groups on the basis of whether they did or did not bleed excessively following surgery according to the multi-part definition described by Bercovitz, et al.22 Patients who bled excessively met one or more of the following criteria: 1) chest tube output ≥ 7 mL/kg/hour for ≥2 consecutive hours in the first 12 post-operative hours (1 patient), 2) chest tube output ≥84 mL/kg total in the first 24 post-operative hours (2 patients), 3) surgical re-exploration for bleeding or tamponade physiology in the first 24 post-operative hours (3 patients), 4) a combination of chest tube output ≥ 7 mL/kg/hour for ≥2 consecutive hours in the first 12 post-operative hours and surgical re-exploration for bleeding or tamponade in the first 24 post-operative hours (2 patients), 5) a combination of chest tube output ≥84 mL/kg total in the first 24 post-operative hours and surgical re-exploration for bleeding or tamponade in the first 24 post-operative hours (3 patients), or 6) a combination of chest tube output ≥ 7 mL/kg/hour for ≥2 consecutive hours in the first 12 post-operative hours, chest tube output ≥84 mL/kg total for the first 24 post-operative hours, and surgical re-exploration for bleeding or tamponade in the first 24 post-operative hours (6 patients).
Hemostatic Management
All patients were treated according to an institutional transfusion strategy for neonatal congenital cardiac surgery requiring CPB, which has been described previously.20 To reiterate here, all neonates received a CPB prime consisting of either non-fresh whole blood (2–5 days of storage) or reconstituted whole blood composed of a 1:1 ratio of packed red blood cells and fresh frozen plasma. All patients received tranexamic acid as antifibrinolytic therapy (30 mg/kg loading dose, 10 mg/kg/h infusion and 0.1 mg/mL in CPB prime). The appropriate heparin dose for bypass was determined after establishing a heparin dose-response curve using the patient’s own blood and calculating the heparin dose that would achieve an activated clotting time of at least 480 seconds, and the blood heparin concentration was measured after heparinization prior to initiation of CPB. After aortic cross-clamp removal and rewarming immediately prior to termination of CPB, all neonates were transfused with ¼ unit of single-donor apheresis platelets (SDPs) and 1 donor unit of cryoprecipitate. Following separation from CPB, protamine was administered, and both activated clotting time and heparin concentration measurements were performed to confirm complete heparin reversal. An additional ¼ SDP from the same donor was transfused immediately after protamine reversal. Transfusion of packed red blood cells was performed to achieve a hematocrit ≥ 45% in neonates with cyanotic lesions and ≥ 35% in neonates with acyanotic lesions. Additional transfusion decisions were based on clinical laboratory test results and evidence of ongoing bleeding. Neonates with active bleeding were transfused to maintain a platelet count >100 × 103/μl, fibrinogen concentration >200 mg/dl, and prothrombin time <1.5 times normal. When residual heparin effect was confirmed by elevated activated clotting time, heparin concentration or activated partial thromboplastin time, additional protamine (1 mg/kg) was given. If blood product transfusion and surgical intervention failed to control bleeding, the anesthesiologist and surgeon jointly made decisions regarding recombinant activated factor VII administration.
Rotational Thromboelastometry (ROTEM) Analyses
The ROTEM extrinsically activated (ExTEM) and fibrinogen polymerization (FibTEM) maximal clot firmness (MCF) assays were performed as instructed by the manufacturer (ROTEM delta; Tem Systems, Inc., Munich, Germany). A platelet-specific ROTEM (PlTEM) value was calculated by subtracting the FibTEM from the ExTEM value as previously described.23
Antibodies and Agonists
Monoclonal antibodies (mAb) specific for human αIIbβ3 (clone 312.2) or glycoprotein VI (GPVI; clone 11a12) were purchased from the Versiti – Blood Research Institute Hybridoma Core (Milwaukee, WI). MAb 312.2 was conjugated to AlexaFluor-488 or AlexaFluor-660, and mAb 11a12 was conjugated to AlexaFluor 647, using an antibody labeling kit (Thermo Fisher, Waltham, MA). The phycoerythrin (PE)-conjugated mAb specific for human P-selectin (anti-human CD62P) was purchased from BioLegend (San Diego, CA). Mouse IgG1/kappa isotype control antibodies conjugated to PE, AlexaFluor-647, or AlexaFluor-488 were purchased from BioLegend and used as negative controls. The AlexaFluor-660-conjugated mouse IgG1/kappa isotype control antibody was purchased from Thermo Fisher. Thrombin receptor activating peptide (TRAP) and collagen-related peptide (CRP) were purchased from the Versiti – Blood Research Institute Protein Core. The thromboxane A2 mimetic U46619 was purchased from Millipore Sigma (St Louis, MO). Adenosine diphosphate (ADP) was purchased from Chrono-Log Corp (Havertown, PA).
Flow Cytometric Assessment of Receptor Expression Levels and Platelet Activation
To measure levels of expression of αIIbβ3 and GPVI, WB samples were diluted 1:10 with HEPES/Tyrode’s buffer (20mM HEPES, 137mM NaCl, 2.7mM KCl, 1mM MgCl2, 5.6mM Glucose, 1 mg/mL bovine serum albumin, pH 7.4). The diluted WB samples were incubated in the dark for 20 minutes at room temperature with a combination of the αIIbβ3-specific mAb 312.2–488 and the GPVI-specific mAb 11a12–647 to allow the level of expression of GPVI to be normalized to that of αIIbβ3. Levels of expression of GPVI were normalized to those of αIIbβ3 to control for the tendency of platelets to expose a large amount of membrane from the open canalicular system upon activation;24 however, levels of expression of αIIbβ3 did not change over the course of neonatal cardiac surgery (Supplementary Figure 1).
To measure platelet activation in response to agonist stimulation, WB samples were incubated with the αIIbβ3-specific mAb 312.2–660, which was used to identify platelets, and the P-selectin-specific mAb CD62P-PE, which was used to assess the level of platelet activation, in the dark for 20 minutes at room temperature in the presence of 10 μM TRAP, 2.5 μg/mL CRP, 1 μM U46619, or 20 μM ADP to stimulate platelets or in the absence of agonist as an unstimulated negative control. When added to amplify platelet activation by TRAP or U46619, epinephrine was used at a final concentration of 2 μM. Samples were then diluted with a 10X excess volume of HEPES/Tyrode’s buffer to quench the reactions and analyzed immediately on an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA). Median fluorescence intensities were determined using FlowJo Ver10.0 software (FlowJo LLC, Ashland, OR).
Preparation of WB Samples with Defined Platelet Counts
WB samples with defined platelet counts were prepared to determine how thrombocytopenia affected agonist-induced platelet P-selectin exposure. Freshly drawn, citrated WB samples (5 mL) were obtained from healthy adult volunteers. The Versiti – Blood Research Institute IRB approved protocols involving the use of human subjects, and informed consent was obtained from healthy adult volunteers. Thromboctyopenic WB samples were prepared as previously described.25 Briefly, a small volume of WB was reserved, and the remaining blood was centrifuged and fractionated to obtain platelet poor plasma (PPP) and a red blood cell (RBC) concentrate. Reserved WB, RBC concentrate, and PPP were mixed to achieve WB samples with a hematocrit of 30% and the desired platelet count. In some cases, platelets from unexpired, de-identified apheresis-derived SDP units, obtained from Versiti – Blood Center of Wisconsin, were added to thrombocytopenic WB to achieve the desired platelet count. As shown in Supplementary Figure 2, platelet counts were significantly lower, whereas levels of P-selectin exposure on unstimulated platelets were similarly low, in WB samples with low vs. normal platelet counts.
Statistics
For the categorical variables in Table 1, the Chi-square test was used to compare the percentages of patients of each gender and of those that were exposed to different types of CPB prime (WB or PBRC + FFP), deep hypothermic circulatory arrest (DHCA) or selective cerebral perfusion (SCP); the Fisher’s exact test was used to compare the percentages of patients who experienced Aox or different types of procedures (Norwood, ASO ± VSD, or other. For continuous variables, the distribution of the data was examined using histograms and the Kolmogorov-Smirnov test with Lilliefors significance correction. Most of the continuous variables shown in Table 1 were not normally distributed; therefore, for consistency, the Mann-Whitney test was used to compare the two groups for all continuous variables in Table 1. For other data that were not normally distributed, Generalized Estimating Equation (GEE) with maximum likelihood estimation method was used to model the variables over time accounting for bleeding status and the appropriateness of the model was assessed using the residual plots. The remaining data were normally distributed; therefore, the paired t-test was used to compare thrombocytopenic whole blood samples to those with normal platelet counts for these variables. Depending on the distributions of the variables that were tested, normal distribution with identity link function, lognormal distribution with identity link function, or gamma distribution with log link function was used for comparisons as detailed in the footnotes for the tables and in the figure legends. The AR(1) covariance structure was specified to account for repeated measurements within each subject. Post-hoc analyses were adjusted by the Tukey-Kramer method and p<0.05 was considered statistically significant. Instances in which data were missing for one or more patients are indicated in the footnotes of the tables and the figure legends. Because the cause of the missing data was different in each instance, we assumed that the data were missing completely at random. All statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC).
Table 1:
Baseline demographics and surgical characteristics
| Demographics | Total | Bleeding Type | p value | |
|---|---|---|---|---|
| Normal (n=27) | Excessive (n=17) | |||
| Age (days), median (range) | 7 (1 – 24) | 7 (1 – 24) | 7 (1 – 23) | 0.47 |
| Weight (kg), median (range) | 3.27 (2.57 – 4.35) | 3.34 (2.57 – 4.35) | 3.01 (2.58 – 3.94) | 0.12 |
| Height (cm), median (range) | 50.5 (39 – 55.5) | 51.0 (39.0 – 55.5) | 49.0 (46.5 – 49.0) | 0.039 |
| Gender, n (%) | 0.43 | |||
| Female | 20 (45.5) | 11 (40.7) | 9 (52.9) | |
| Male | 24 (54.5) | 16 (59.3) | 8 (47.1) | |
| Perioperative Data | ||||
| CPB Prime, n (%) | 0.98 | |||
| WB | 18 (40.9) | 11 (40.7) | 7 (41.2) | |
| PBRC + FFP | 26 (59.1) | 16 (59.3) | 10 (58.8) | |
| CPB Time (min), median (range) | 195 (87 – 696) | 190 (87 – 373) | 202 (88 – 696) | 0.55 |
| Aox, n (%) | 42 (95.5) | 26 (96.3) | 16 (94.1) | >0.99 |
| Aox Time (min), median (range) | 100 (38 – 449) | 103.5 (38 – 194) | 97.5 (68 – 449) | 0.83 |
| DHCA, n (%) | 27 (61.3) | 13 (48.2) | 14 (82.4) | 0.023 |
| DHCA Time (min), median (range) | 16 (7 – 111) | 16 (7 – 111) | 17.5 (10 – 81) | 0.51 |
| SCP, n (%) | 22 (50.0) | 11 (40.7) | 11 (64.7) | 0.12 |
| SCP Time (min), median (range) | 59.5 (2 – 106) | 60 (3 – 88) | 58 (2 – 106) | >0.99 |
| Lowest Temp (°C), median (range) | 17.5 (14.1 – 34.2) | 24.7 (14.1 – 34.2) | 17.3 (15.1 – 33.6) | 0.071 |
| RACHS-1, median (range) | 4 (0 – 6)1 | 4 (0 – 6) | 5.5 (0 – 6)1 | 0.24 |
| Procedure, n (%) | 0.042* | |||
| Norwood | 21 (47.7) | 11 (40.7) | 10 (58.8) | |
| ASO +/− VSD* | 8 (18.2) | 8 (29.6) | 0 (0) | |
| Other2 | 15 (34.1) | 8 (29.6) | 7 (41.2) | |
Abbreviations: CPB – cardiopulmonary bypass; WB – whole blood; PRBC – packed red blood cells; FFP – fresh frozen plasma; Aox – aortic cross clamp; DHCA – deep hypothermic circulatory arrest; SCP – selective cerebral perfusion; RACHS – risk adjustment for congenital heart surgery; ASO – arterial switch operation; VSD – ventricular septal defect
The Mann-Whitney test was used for continuous variables and the Chi-square or Fisher’s exact test was used for categorical variables as described in Methods.
Patient 34 excluded because of missing data
Procedures included and the number of patients who underwent each procedure were as follos: Interupted Aortic Arch (3), Tetralogy of Fallot (3), Central Shunt (2), Total Anomalous Pulmonary Venous Return (2), Ventricular Septal Defect (2), Aortopulmonary Window (1), Double Outlet Right Ventricle (1), Truncus Arteriosus (1), Atrial Septal Defect (1)
Results
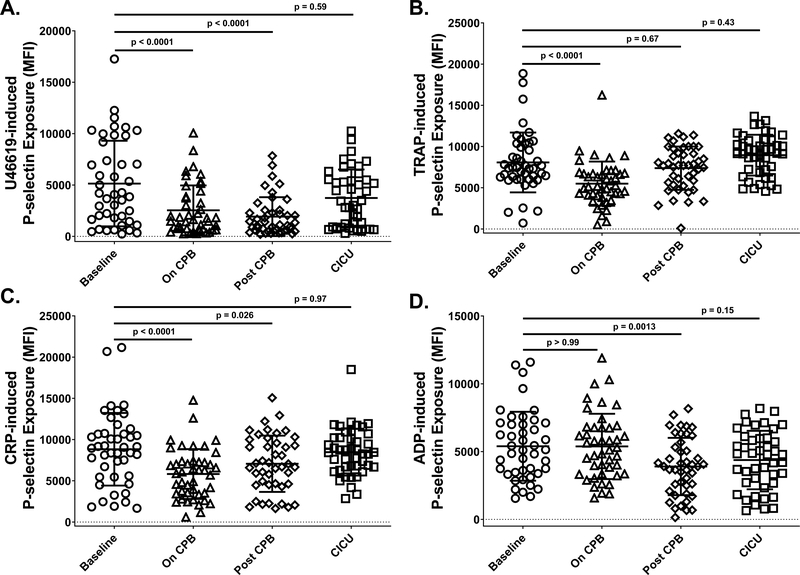
To determine how platelet function changes over the course of CPB surgery in neonates, we assessed the activation state of platelets, as measured by exposure of P-selectin, following stimulation with several agonists at four time points during surgery, including prior to surgery (Baseline), while patients were on CPB (On CPB), after patients were removed from CPB and administered platelet transfusions (Post-CPB), and after patients had been admitted to the CICU (CICU). We found that the thromboxane A2 analogue U46619, the thrombin receptor agonist peptide TRAP, and the collagen receptor agonist CRP all induced levels of P-selectin exposure that were significantly reduced On CPB relative to Baseline, and that platelet responsiveness to these agonists recovered to Baseline levels either Post-CPB or by the time of admission to the CICU (Figure 1A–C). In contrast, levels of P-selectin exposure induced by ADP were significantly lower than Baseline only at the Post-CPB time point (Figure 1D).
Figure 1. Exposure to cardiopulmonary bypass (CPB) affected responsiveness of neonatal platelets to in vitro stimulation in an agonist-dependent manner.
Whole blood (WB) was recovered from neonates at four times during CPB surgery, including prior to initiation of surgery (Baseline; circles), after completion of surgery but before separation from CPB (On CPB; triangles), after separation from CPB and prophylactic transfusion of platelets (Post-CPB; diamonds), and immediately after admission to the cardiac intensive care unit (CICU; squares). Platelets in WB samples were stimulated with (A) thromboxane A2 analog U46619 (1 μM), (B) thrombin receptor activating peptide (TRAP; 10 μM), (C) collagen-related peptide (CRP; 2.5 μg/mL), or (D) adenosine diphosphate (ADP; 20 μM). Activated platelets were identified by flow cytometry following staining with a phycoerythrin (PE)-tagged antibody specific for the platelet α-granule constituent, P-selectin. Results are reported as PE median fluorescence intensity (MFI). Each symbol represents the result for a single patient, bars denote means ± standard deviations, and dotted lines represent MFI = 0 (n=44 for TRAP, CRP and ADP; n=43 for U46619 - patient 26 excluded for technical reasons). Generalized Estimating Equation (GEE) with maximum likelihood estimation method was used to model the variables over time accounting for bleeding status. Normal distribution with identity link function (A), lognormal distribution with identity link function (B and C), and gamma distribution with log link function (D) were used for comparisons. Statistically significant differences between groups are indicated by p values. Note that platelet responses to stimulation with U46619, TRAP and CRP all dropped significantly On CPB relative to Baseline and returned to Baseline values after platelet transfusion and by the time of admission to the CICU. In contrast, platelet responses to stimulation with ADP were significantly lower than Baseline values only at the Post-CPB time point.
The mechanisms underlying decreased platelet responsiveness to agonist stimulation at different time points during cardiac surgery are poorly characterized and likely multifactorial. One possibility is that the platelets that remain in circulation during CPB surgery have already been activated by exposure to the bypass circuit, rendering them relatively insensitive to agonist stimulation.26–29 Consistent with this possibility, we found that, in the absence of agonist stimulation, the percentage of platelets that were P-selectin positive (Figure 2A) and the magnitude of P-selectin exposure (Figure 2B) were significantly higher On CPB and Post-CPB relative to Baseline. Nevertheless, the magnitude of P-selectin exposure observed in the absence of agonist stimulation was much less than that observed upon stimulation with agonists (compare the y-axis values in Figure 2B with those in Figure 1A–D). These findings indicate that the mild activation experienced by platelets following exposure to the bypass circuit does not render them insensitive to additional agonist stimulation and may not be the only factor contributing to decreased platelet responsiveness to agonist stimulation at different time points during cardiac surgery.
Figure 2. Exposure to CPB resulted in mild platelet activation.
WB was recovered from neonates at four times during CPB surgery, including prior to initiation of surgery (Baseline; circles), after completion of surgery but before separation from CPB (On CPB; triangles), after separation from CPB and prophylactic transfusion of platelets (Post CPB; diamonds), and immediately after admission to the cardiac intensive care unit (CICU; squares). Activated platelets were identified by flow cytometry following staining with a phycoerythrin (PE)-tagged antibody specific for the platelet α-granule constituent, P-selectin. Results are reported as (A) the percentage of platelets that were PE positive and (B) PE median fluorescence intensity (MFI). Each symbol represents the result for a single patient, bars denote means ± standard deviations, and dotted lines represent 0 percent PE-positivity (A) or PE MFI = 0 (B). Generalized Estimating Equation (GEE) with maximum likelihood estimation method was used to model the variables over time accounting for bleeding status. Lognormal distribution with identity link function was used for comparisons. Statistically significant differences between groups are indicated by p values. Note that, while the percentage of platelets that were P-selectin positive was significantly greater On CPB relative to Baseline, the MFI of P-selectin-positive platelets was, albeit significantly, only slightly greater than Baseline at the On CPB and Post CPB time points. (n=42 - patient 26 excluded for technical reasons and patient 38 excluded because of high Baseline PE MFI)
Pediatric cardiac surgery patients are known to experience a significant drop in platelet count on CPB.4, 14–19 Similarly, we have previously demonstrated that platelet counts are significantly lower at the On CPB, Post-CBP, and CICU time points in the patient population included in this study, with the lowest counts observed On CPB.20 We therefore sought to determine whether low platelet counts similar to those observed in neonates On CPB might contribute to decreased platelet responsiveness to U46619, TRAP and CRP. To this end, we measured agonist-induced platelet P-selectin exposure in normal vs. thrombocytopenic WB samples, the latter of which possessed platelet counts similar to those observed in neonates On CPB. We found that levels of P-selectin exposure induced by U46619 (Figure 3A), and to a lesser extent TRAP (Figure 3B), were lower in thrombocytopenic relative to unmanipulated WB samples, and that addition of exogenous epinephrine restored these responses to normal (Supplementary Figure 3). In contrast, levels of P-selectin exposure induced by CRP were not reduced in thrombocytopenic relative to unmanipulated WB samples (Figure 3C).
Figure 3. Low platelet counts equivalent to those experienced by neonates on CPB reduced platelet responsiveness to stimulation via thromboxane A2 and thrombin, but not collagen, receptors.
The platelet count in WB samples obtained from healthy adult subjects was left unmodified (closed circles) or reduced to thrombocytopenic levels observed in neonates on CPB (open circles) prior to stimulation with (A) thromboxane A2 analog U46619 (1 μM), (B) thrombin receptor activating peptide (TRAP; 10 μM), or (C) collagen-related peptide (CRP; 2.5 μg/mL). Activated platelets were identified by flow cytometry following staining with a phycoerythrin (PE)-tagged antibody specific for the platelet α-granule constituent, P-selectin. Results are reported as PE median fluorescence intensity (MFI). Lines represent paired results for unmodified and thrombocytopenic WB samples from the same subject (n=10). Because the data were normally distributed, the paired t-test was used to compare thrombocytopenic whole blood samples to those with normal platelet counts. Statistically significant differences between groups are indicated by p values. Note that platelet responses to stimulation with U46619 were dramatically reduced, responses to TRAP were slightly but significantly reduced, and responses to CRP were not reduced in thrombocytopenic relative to non-thrombocytopenic WB samples.
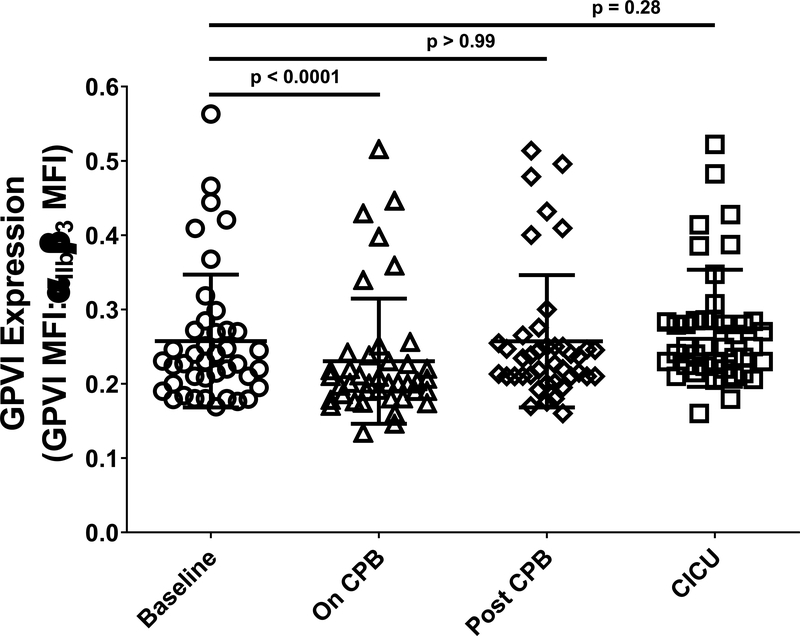
We next sought to determine a potential cause of the decreased platelet responsiveness to CRP that was observed in neonates on CPB. The GPVI collagen receptor is responsible for platelet activation by collagen and CRP,30 and the level of GPVI expression on the platelet surface, which varies as a consequence of genetic polymorphism31–33 and antibody- or activation-induced cleavage events,34 affects the magnitude of platelet responses to GPVI agonists. We therefore asked whether decreased levels of GPVI on the platelet surface coincided with decreased responsiveness to CRP of neonatal platelets On CPB. We found that platelet GPVI levels were decreased in all but 6 of 41 patients On CPB relative to Baseline, which resulted in a slight but significant reduction in the average level of GPVI expressed by platelets obtained from neonates at the On CPB time point relative to Baseline (Figure 4).
Figure 4. Reduced levels of expression of the GPVI collagen receptor accompanied reduced platelet responsiveness to stimulation with collagen related peptide (CRP) in neonates on CPB.
WB samples drawn from patients prior to initiation of CPB surgery (Baseline, circles), after completion of surgery but before separation from CPB (On CPB, triangles), after separation from CPB and transfusion of platelets (Post-CPB, diamonds), and immediately after admission to the cardiac intensive care unit (CICU, squares) were used for assessment of levels of platelet GPVI expression. Flow cytometry was performed following staining of platelets with an Alexafluor 488-tagged antibody specific for αIIbβ3 and an Alexafluor 647-tagged antibody specific for human GPVI. Results are reported as the ratio of Alexafluor 647/Alexaflour 488 median fluorescence intensity (MFI). Each symbol represents the result for a single patient and bars denote means ± standard deviations (n = 41; patients 26 and 33 were excluded because of missing data for all time points; patient 38 was excluded because of abnormally high levels of expression of GPVI relative to αIIbβ3). Generalized Estimating Equation (GEE) with maximum likelihood estimation method was used to model the variables over time accounting for bleeding status. Lognormal distribution with identity link function was used for comparisons. Statistically significant differences between groups are indicated by p values. Note that platelet GPVI expression levels were significantly lower in neonates On CPB relative to Baseline, and returned to baseline values following platelet transfusion.
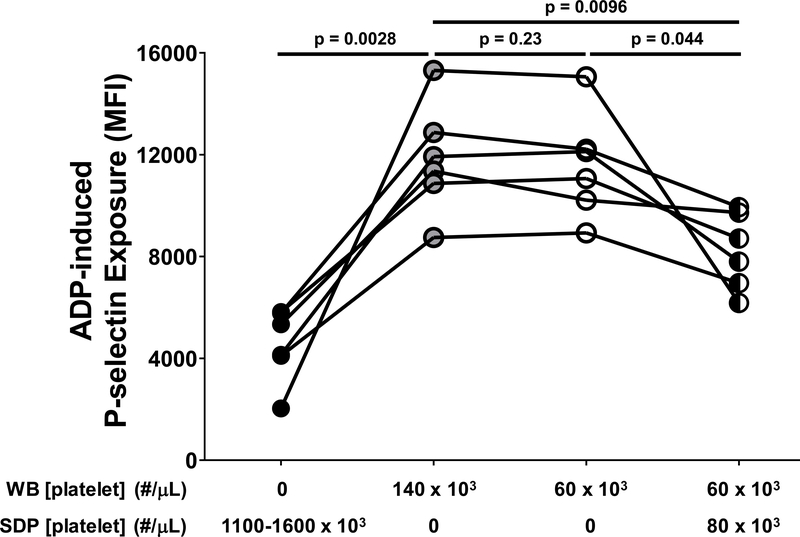
Defective platelet responsiveness to ADP occurred at the Post CPB time point (Figure 1D), which coincided with platelet transfusion in our study. We therefore sought to determine whether transfused platelets might contribute to decreased platelet responsiveness to ADP. Consistent with previous reports that the responsiveness to ADP of platelets in platelet concentrates is reduced relative to that of platelets in freshly prepared WB or platelet-rich plasma,35–39 we found that responsiveness to ADP of platelets in SDP units was significantly lower than that of platelets in freshly isolated WB, the latter of which was not affected by thrombocytopenia (Figure 5). Interestingly, addition of SDP to thrombocytopenic WB to approximate ratios of SDP and WB platelets that were present in neonates at the Post CPB time point (80:60 × 103 platelets/μL) resulted in significantly lower levels of ADP-induced P-selectin exposure than were observed in WB samples in which the same platelet count was achieved with only WB platelets (Figure 5).
Figure 5. Single donor platelet (SDP) units reduced the responsiveness of platelets in fresh whole blood to activation by adenosine diphosphate (ADP).
Apheresis-derived SDP units were obtained from Versiti – Blood Center of Wisconsin and left unmodified (black circles). Whole blood (WB) samples were obtained from healthy adult volunteers and manipulated to achieve a normal platelet count (140 × 103/μL; gray circles) or were made thrombocytopenic to approximate platelet counts observed in neonates on cardiopulmonary bypass (60 × 103/μL; open circles). SDP units were mixed with thrombocytopenic WB samples to achieve a final platelet count of 140 × 103/μL, with 60 × 103/μL platelets derived from WB and 80 × 103/μL platelets derived from SDP units (black and white circles). Each of these platelet preparations was stimulated with ADP (20 μM). Activated platelets were identified by flow cytometry following staining with a phycoerythrin (PE)-tagged antibody specific for the platelet α-granule constituent, P-selectin. Results are reported as PE median fluorescence intensity (MFI). Each symbol represents the result for a single experiment and lines represent relationships between SDP and WB samples studied in a given experiment (n=6). Because the data were normally distributed, the paired t-test was used to compare thrombocytopenic whole blood samples to those with normal platelet counts. Statistically significant differences between groups are indicated by p values. Note that, compared to WB with a platelet count of 140 × 103/μL, the response to stimulation with ADP was lower in SDP units, the same in WB samples with a thrombocytopenic platelet count of 60 × 103/μL, and reduced in thrombocytopenic WB samples to which platelets from SDP units were added to achieve a platelet count of 140 × 103/μL.
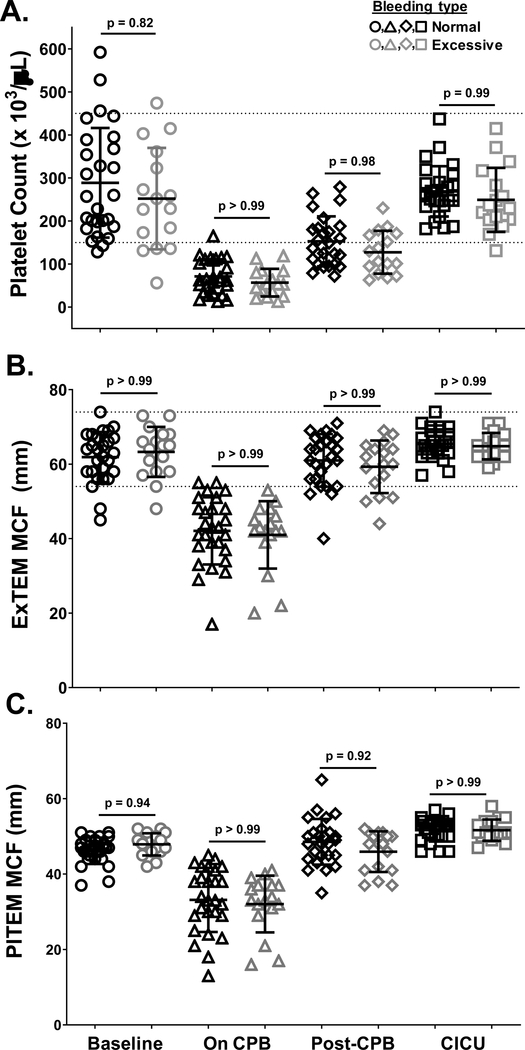
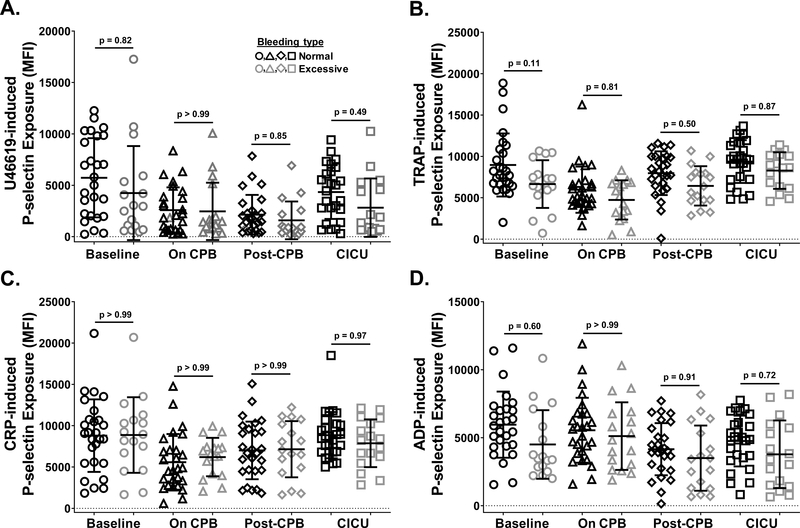
Finally, we sought to determine whether changes in platelet count and function correlate with the severity of post-surgical bleeding experienced by patients. To address this question, patients were separated into two groups according to severity of postoperative bleeding, which was based on the recently proposed standardized definition of postoperative bleeding described by Bercovitz, et al.22 Patients who bled excessively and those who did not were not significantly different with respect to age, gender, type of product used for priming the bypass pump, incidence or duration of aortic cross clamp (Aox), duration of deep hypothermic circulatory arrest (DHCA) or selective cerebral perfusion (SCP), or risk adjustment for congenital heart surgery (RACHS-1) score (Table 1). In contrast, patients who bled excessively were significantly shorter, more likely to have undergone DHCA, and less likely to have undergone arterial switch operations (ASO). These differences in patient demographics and surgical experience did not, however, translate to differences in hemostatic values such as hematocrit, fibrinogen level, prothrombin time or partial thromboplastin time at any time during surgery (Table 2). Similarly, neither platelet count (Figure 6A), ROTEM values reflective of platelet contribution to clot formation (Figure 6B–C), nor platelet responsiveness to stimulation with U46619 (Figure 7A), TRAP (Figure 7B), CRP (Figure 7C) or ADP (Figure 7D) differed significantly at any time point during surgery between patients who experienced normal vs. excessive levels of bleeding.
Table 2:
Hemostatic Variables
| Total | Bleeding Type | p value | ||
|---|---|---|---|---|
| Normal (n=27) | Excessive (n=17) | |||
| Hematocrit (%), median (range) | ||||
| Baseline | 42.75 (32.1 – 60.5) | 42.8 (33.4 – 60.5) | 41 (32.1 – 58.4) | >0.99 |
| CICU | 40.55 (22.9 – 56.4) | 38 (25.2 – 56.4) | 42.3 (22.9 – 51.3) | 0.98 |
| Fibrinogen (mg/dL), median (range) | ||||
| Baseline | 223.5 (141 – 466) | 211 (152 – 389) | 242 (141 – 466) | 0.83 |
| On CPB | 188 (108 – 340)1 | 181 (110 – 312)2 | 210 (108 – 340)3 | 0.74 |
| Post-CPB | 292 (152 – 513)4 | 282 (152 – 489) | 293.5 (175 – 513)4 | >0.99 |
| CICU | 267.5 (161 – 445) | 266 (190 – 445) | 273 (161 – 379) | >0.99 |
| Prothrombin Time (sec), median (range) | ||||
| Baseline | 15.8 (13.3 – 18.9) | 16.2 (13.3 –18.9) | 15.6 (13.5 – 17.2) | 0.73 |
| Post-CPB | 17.3 (13.3 – 23.1)4 | 17.3 (13.3 – 23.1) | 17.2 (15.2 – 19.7)4 | >0.99 |
| CICU | 15.4 (10 – 18.7) | 15.6 (10 – 18.7) | 15.3 (11.1 – 17.1) | 0.95 |
| Partial Thromboplastin Time (sec), median (range) | ||||
| Baseline | 50.75 (29.8 – 200) | 52.5 (29.8 – 200) | 46.2 (34.2 – 79.6) | 0.72 |
| Post-CPB | 41.9 (29.8 – 200)5 | 41.2 (29.8 – 49.9)6 | 45.5 (34 – 200)4 | 0.56 |
| CICU | 43.15 (30.6 – 177.9) | 44.2 (30.6 – 86.9) | 41.3 (34.3 – 177.9) | 0.99 |
Generalized Estimating Equation (GEE) with maximum likelihood estimation method was used to model the variables over time accounting for bleeding status. Normal distribution with identity link function (hematocrit), lognormal distribution with identity link function (fibrinogen and partial thromboplastin time), and gamma distribution with log link function (prothrombin time) were used for comparisons.
Patients 2, 17 and 27 excluded because of missing data
Patient 17 excluded because of missing data
Patients 2 and 27 excluded because of missing data
Patient 4 excluded because of missing data
Patients 4 and 32 excluded because of missing data
Patient 32 excluded because of missing data
Figure 6. Platelet counts and platelet-dependent Rotational Thromboelastometry (ROTEM) values changed similarly over the course of CPB surgery in neonates who bled excessively following surgery relative to those who did not.
WB samples were drawn from patients prior to initiation of CPB surgery (Baseline, circles), after completion of surgery but before separation from CPB (On CPB, triangles), after separation from CPB and prophylactic transfusion of platelets and cryoprecipitate (Post-CPB, diamonds), and immediately after admission to the cardiac intensive care unit (CICU, squares). Platelet counts (A) and ROTEM ExTEM (B) and PlTEM (C) values were determined immediately after blood samples were drawn. Results from patients who experienced normal (black symbols) vs. excessive (gray symbols) levels of bleeding were plotted separately. Each symbol represents the result for a single patient, bars denote means ± standard deviations, and dotted lines represent the normal ranges in healthy infants as described previously20 (n=44 for ROTEM ExTEM and PlTEM values; n = 41 for platelet counts - patients 2, 3, and 4 were excluded because of missing data). Generalized Estimating Equation (GEE) with maximum likelihood estimation method was used to model the variables over time accounting for bleeding status. Normal distribution with identity link function was used for comparisons. Note that platelet counts and ROTEM values did not differ significantly between patients who bled excessively vs. those who did not at any time point.
Figure 7. Platelet responsiveness to agonist stimulation changed similarly in neonates who bled excessively following CPB surgery relative to those who did not.
WB was recovered from neonates at four times during CPB surgery, including prior to initiation of surgery (Baseline; circles), after completion of surgery but before separation from CPB (On CPB; triangles), after separation from CPB and prophylactic transfusion of platelets (Post CPB; diamonds), and immediately after admission to the cardiac intensive care unit (CICU; squares). Platelets in WB samples were stimulated with (A) thromboxane A2 analog U46619 (1 μM), (B) thrombin receptor activating peptide (TRAP; 10 μM), (C) collagen-related peptide (CRP; 2.5 μg/mL), or (D) adenosine diphosphate (ADP; 20 μM). Activated platelets were identified by flow cytometry following staining with a phycoerythrin (PE)-tagged antibody specific for the platelet α-granule constituent, P-selectin. Results are reported as PE median fluorescence intensity (MFI). Results observed in patients who experienced normal (black symbols) vs. excessive (gray symbols) levels of bleeding were plotted separately. Each symbol represents the result for a single patient, bars denote means ± standard deviations, and dotted lines represent MFI = 0 (n=44 for TRAP, CRP and ADP; n=43 for U46619 - patient 26 excluded for technical reasons). Generalized Estimating Equation (GEE) with maximum likelihood estimation method was used to model the variables over time accounting for bleeding status. Normal distribution with identity link function (A), lognormal distribution with identity link function (B and C), and gamma distribution with log link function (D) were used for comparisons. Statistically significant differences between groups are indicated by p values. Note that platelet responses to stimulation with U46619, TRAP, CRP, and ADP did not differ significantly between patients who bled excessively vs. those who did not at any time point.
Discussion
It is well established that CPB induces thrombocytopenia in neonates, the correction of which is a target to prevent post-surgical bleeding.5, 14–19 It has also been proposed that activation of platelets by the bypass circuit renders the platelets that remain in circulation hypo-responsive to agonist stimulation, which may impair their hemostatic effectiveness.26–29 The mechanisms underlying CPB-induced defects in platelet responsiveness and the extent to which thrombocytopenia and platelet dysfunction contribute to excessive post-surgical bleeding in neonatal cardiac surgery patients are, however, not known. In the present study, we expand upon our recent demonstration that CPB is associated with decreased platelet counts and platelet-dependent clot formation in neonates20 by demonstrating that CPB surgery also affects platelet responsiveness to agonist stimulation in these same neonates. Mechanistically, low platelet counts similar to those experienced by patients on CPB decreased platelet responsiveness to U46619 and TRAP, whereas reduced levels of expression of the GPVI collagen receptor were associated with decreased platelet responsiveness to CRP, in patients on CPB. In addition, poor responsiveness to ADP of transfused platelets may have contributed to impaired platelet responsiveness to ADP in neonates following platelet transfusion. We also demonstrate that prophylactic platelet transfusions corrected platelet count and function defects but did not prevent excessive bleeding in all patients. This finding indicates that the excessive bleeding that occurs following CPB surgery in neonates whose platelet count and function defects are corrected by platelet transfusion is attributable either to factors that are unrelated to platelets or to platelet-related factors that were not detectable by the assays used in these studies.
It is important to point out that platelet P-selectin exposure,40 rather than platelet aggregation,41 was used as the measure of platelet activation in the present study. This choice was made in part because in vitro measurement of platelet aggregation in response to multiple agonists requires quantities of whole blood that cannot be safely drawn from neonates. The choice was also made because platelet P-selectin exposure is thought to be relatively insensitive to changes in platelet count,42 which are known to occur over the course of CPB surgery in our patients.21 Platelet aggregation in response to stimulation with U46619 or TRAP is known to require amplification of the initial platelet activating signal by secondary agonists like ADP and epinephrine, which are released from platelet granules, bind to Gαi/z-coupled P2Y12 and α2-adrenergic receptors, respectively, and inhibit adenylate cyclase43, 44, 45. Similarly, U46619-induced release of platelet granule contents, of which P-selectin exposure is a measure, has been shown to require binding of released ADP to the P2Y12 receptor.46 Low platelet counts have been shown to reduce the amounts of secondary agonists that are available to amplify initial platelet activation by at least some agonists.47, 48 Furthermore, we found that addition of exogenous epinephrine restored P-selectin exposure by platelets in thrombocytopenic WB to levels observed in WB samples with normal platelet counts. Collectively, these results suggest that thrombocytopenia might contribute to decreased platelet P-selectin exposure in response to stimulation by U46619 and TRAP by reducing the amounts of secreted secondary agonists that are available for amplification of the initial response to these agonists.
Previous studies performed in pediatric populations that encompassed patients with a wider range of ages demonstrated that demographic and surgical characteristics such as age, weight, height, surgical conditions encountered, and type of surgery performed, were associated with excessive post-surgical bleeding.1, 2, 4, 5, 14, 19, 49–51 In this study, which included only neonates, the demographic and surgical characteristics that were strongly associated with excessive bleeding included shorter height, higher incidence of exposure to DHCA, and decreased likelihood of undergoing ASO procedures. A previous study of these same patients21 did not evaluate differences in the incidences of exposure to DHCA or ASO procedures, and did not find a significant difference in height between patients who bled excessively and those who did not. A possible explanation for the latter disparity is that our study adopted a literal definition of excessive bleeding that resulted in inclusion of one more patient in the excessive bleeding group than was included in the previous study. In addition, our study compared median values rather than means to account for the fact that some of the demographic data were not normally distributed. The results of our study enhance our understanding of the factors that contribute to excessive bleeding following CPB surgery by demonstrating that even amongst neonates, who are relatively uniform in age and size, smaller patients and those who undergo more complex surgeries that require DHCA and/or more hypothermic CPB may be at increased risk for excessive bleeding following CPB surgery.
The differences in patient demographics and surgical experience that were observed between neonates who bled excessively and those who did not in this study, did not translate to differences in any of the hemostatic values tested. These included measures of coagulation function such as hematocrit, fibrinogen level, prothrombin time or partial thromboplastin time, as was also reported previously.52 Unique to this study is the report that platelet count and platelet responses to stimulation with agonists that activate platelets through the thromboxane A2, thrombin, collagen, or P2Y1 and P2Y12 receptors also did not differ significantly between patients who bled excessively and those who did not. Platelet responses to epinephrine and ristocetin, which activate platelets through the α2-adrenergic and GPIb/IX/V receptors, respectively, were also tested in this study but found to be too low to allow for meaningful comparisons (data not shown). Other coagulation parameters, such as those related to von Willebrand disease, clot lysis and FXIII levels, were not assessed in the present study. Consequently, it remains to be determined whether defects in platelet responses to agonists other than those studied here, aberrant levels or function of von Willebrand factor, hyperfibrinolysis, or lower levels of coagulation factors not evaluated in this study explain excessive bleeding in neonates following CPB surgery.
There are several limitations to our study. The first is that whereas clinical data were obtained from neonates, whole blood samples obtained from healthy adult volunteers were manipulated to recapitulate, for in vitro analysis, the thrombocytopenic conditions experienced by neonates during CPB surgery. This choice was made because ethical and practical considerations preclude acquisition of whole blood from healthy neonates purely for the purposes of research. The in vitro data reported in this study may therefore not faithfully represent the responses of which neonatal platelets are capable, especially given the well-established differences between neonatal and adult platelet phenotype and function.53, 54 Additional limitations of our study are that bleeding severity was assessed using only one definition of excessive bleeding and that the study included only a small number of patients. Future studies should be done to determine whether the findings reported herein are reproducible with other bleeding definitions (reviewed in Bercovitz, et al.22) and whether they generalize to a larger, but equally well-characterized, group of neonatal cardiac surgery patients.
In conclusion, we found that neonatal patients experienced both thrombocytopenia and defects in platelet responsiveness to U46619, TRAP, CRP, and ADP while undergoing surgery requiring CPB. Evidence is provided that, on CPB, mild platelet activation and thrombocytopenia may contribute to defective platelet responses to U46619 and TRAP, whereas mild platelet activation and reduced expression of GPVI may explain defective platelet responses to CRP. Following platelet transfusion, defective platelet responses to ADP may be explained by poor responsiveness of transfused platelets to this agonist. Neither thrombocytopenia nor platelet function defects correlated with excessive post-operative bleeding. Future studies are needed to identify platelet-independent factors, or platelet-related factors that were not evaluate herein, that correlate with excessive post-surgical bleeding and may therefore serve as targets for therapeutic intervention.
Supplementary Material
Acknowledgments
The authors acknowledge all members of The Herma Heart Institute Pediatric Cardiac Thrombosis and Hemostasis Research Program. We specifically thank James Tweddell, Michael Mitchell, Alan Mast, Susan Maroney, Wes Zwifelhofer, Robert Montgomery, Jeremy Mattson, Rowena Punzalan and Shawn Jobe for helpful discussions, and Michelle Brenner and Brook Fricke for technical help.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to report.
References
- 1.Manno CS, Hedberg KW, Kim HC, et al. Comparison of the hemostatic effects of fresh whole blood, stored whole blood, and components after open heart surgery in children. Blood 1991; 77(5): 930–936. [PubMed] [Google Scholar]
- 2.Petaja J, Lundstrom U, Leijala M, et al. Bleeding and use of blood products after heart operations in infants. J Thorac Cardiovasc Surg 1995; 109(3): 524–529. [DOI] [PubMed] [Google Scholar]
- 3.Chambers LA, Cohen DM, Davis JT. Transfusion patterns in pediatric open heart surgery. Transfusion 1996; 36(2): 150–154. [DOI] [PubMed] [Google Scholar]
- 4.Williams GD, Bratton SL, Riley EC, et al. Association between age and blood loss in children undergoing open heart operations. Ann Thorac Surg 1998; 66(3): 870–875; discussion 875–876. [DOI] [PubMed] [Google Scholar]
- 5.Williams GD, Bratton SL, Ramamoorthy C. Factors associated with blood loss and blood product transfusions: a multivariate analysis in children after open-heart surgery. Anesth Analg 1999; 89(1): 57–64. [DOI] [PubMed] [Google Scholar]
- 6.Moganasundram S, Hunt BJ, Sykes K, et al. The relationship among thromboelastography, hemostatic variables, and bleeding after cardiopulmonary bypass surgery in children. Anesth Analg 2010; 110(4): 995–1002. [DOI] [PubMed] [Google Scholar]
- 7.Unsworth-White MJ, Herriot A, Valencia O, et al. Resternotomy for bleeding after cardiac operation: a marker for increased morbidity and mortality. Ann Thorac Surg 1995; 59(3): 664–667. [DOI] [PubMed] [Google Scholar]
- 8.Guay J, Rivard GE. Mediastinal bleeding after cardiopulmonary bypass in pediatric patients. Ann Thorac Surg 1996; 62(6): 1955–1960. [DOI] [PubMed] [Google Scholar]
- 9.Guzzetta NA, Allen NN, Wilson EC, et al. Excessive postoperative bleeding and outcomes in neonates undergoing cardiopulmonary bypass. Anesth Analg 2015; 120(2): 405–410. [DOI] [PubMed] [Google Scholar]
- 10.McEwan A Aspects of bleeding after cardiac surgery in children. Paediatr Anaesth 2007; 17(12): 1126–1133. [DOI] [PubMed] [Google Scholar]
- 11.Eaton MP, Iannoli EM. Coagulation considerations for infants and children undergoing cardiopulmonary bypass. Paediatr Anaesth 2011; 21(1): 31–42. [DOI] [PubMed] [Google Scholar]
- 12.Arnold PD. Coagulation and the surgical neonate. Paediatr Anaesth 2014; 24(1): 89–97. [DOI] [PubMed] [Google Scholar]
- 13.Whiting D, Yuki K, DiNardo JA. Cardiopulmonary bypass in the pediatric population. Best Pract Res Clin Anaesthesiol 2015; 29(2): 241–256. [DOI] [PubMed] [Google Scholar]
- 14.Miller BE, Mochizuki T, Levy JH, et al. Predicting and treating coagulopathies after cardiopulmonary bypass in children. Anesth Analg 1997; 85(6): 1196–1202. [DOI] [PubMed] [Google Scholar]
- 15.Straub A, Schiebold D, Wendel HP, et al. Using reagent-supported thromboelastometry (ROTEM) to monitor haemostatic changes in congenital heart surgery employing deep hypothermic circulatory arrest. Eur J Cardiothorac Surg 2008; 34(3): 641–647. [DOI] [PubMed] [Google Scholar]
- 16.Velik-Salchner C, Maier S, Innerhofer P, et al. An assessment of cardiopulmonary bypass-induced changes in platelet function using whole blood and classical light transmission aggregometry: the results of a pilot study. Anesth Analg 2009; 108(6): 1747–1754. [DOI] [PubMed] [Google Scholar]
- 17.Ranucci M, Carlucci C, Isgro G, et al. A prospective pilot study of platelet function and its relationship with postoperative bleeding in pediatric cardiac surgery. Minerva Anestesiol 2012; 78(5): 556–563. [PubMed] [Google Scholar]
- 18.Bonding Andreasen J, Hvas AM, Ravn HB. Marked changes in platelet count and function following pediatric congenital heart surgery. Paediatr Anaesth 2014; 24(4): 386–392. [DOI] [PubMed] [Google Scholar]
- 19.Zubair MM, Bailly DK, Lantz G, et al. Preoperative platelet dysfunction predicts blood product transfusion in children undergoing cardiac surgery. Interact Cardiovasc Thorac Surg 2015; 20(1): 24–30. [DOI] [PubMed] [Google Scholar]
- 20.Scott JP, Niebler RA, Stuth EAE, et al. Rotational Thromboelastometry Rapidly Predicts Thrombocytopenia and Hypofibrinogenemia During Neonatal Cardiopulmonary Bypass. World J Pediatr Congenit Heart Surg 2018; 9(4): 424–433. [DOI] [PubMed] [Google Scholar]
- 21.Maroney SA, Peterson JA, Zwifelhofer W, et al. Plasma Proteolytic Cascade Activation during Neonatal Cardiopulmonary Bypass Surgery. Thromb Haemost 2018; 118(9): 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bercovitz RS, Shewmake AC, Newman DK, et al. Validation of a definition of excessive postoperative bleeding in infants undergoing cardiac surgery with cardiopulmonary bypass. J Thorac Cardiovasc Surg 2018; 155(5): 2112–2124 e2112. [DOI] [PubMed] [Google Scholar]
- 23.Ji SM, Kim SH, Nam JS, et al. Predictive value of rotational thromboelastometry during cardiopulmonary bypass for thrombocytopenia and hypofibrinogenemia after weaning of cardiopulmonary bypass. Korean J Anesthesiol 2015; 68(3): 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bull BS, Zucker MB. Changes in platelet volume produced by temperature, metabolic inhibitors, and aggregating agents. Proc Soc Exp Biol Med 1965; 120(2): 296–301. [DOI] [PubMed] [Google Scholar]
- 25.Bercovitz RS, Brenner MK, Newman DK. A whole blood model of thrombocytopenia that controls platelet count and hematocrit. Ann Hematol 2016; 95(11): 1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferroni P, Speziale G, Ruvolo G, et al. Platelet activation and cytokine production during hypothermic cardiopulmonary bypass--a possible correlation? Thromb Haemost 1998; 80(1): 58–64. [PubMed] [Google Scholar]
- 27.Ichinose F, Uezono S, Muto R, et al. Platelet hyporeactivity in young infants during cardiopulmonary bypass. Anesth Analg 1999; 88(2): 258–262. [DOI] [PubMed] [Google Scholar]
- 28.Guay J, Ruest P, Lortie L. Cardiopulmonary bypass induces significant platelet activation in children undergoing open-heart surgery. Eur J Anaesthesiol 2004; 21(12): 953–956. [DOI] [PubMed] [Google Scholar]
- 29.Straub A, Smolich J, d’Udekem Y, et al. Activation of platelets in young infants during cardiopulmonary bypass. Thromb Haemost 2010; 103(2): 466–469. [DOI] [PubMed] [Google Scholar]
- 30.Watson SP, Herbert JM, Pollitt AY. GPVI and CLEC-2 in hemostasis and vascular integrity. J Thromb Haemost 2010; 8(7): 1456–1467. [DOI] [PubMed] [Google Scholar]
- 31.Joutsi-Korhonen L, Smethurst PA, Rankin A, et al. The low-frequency allele of the platelet collagen signaling receptor glycoprotein VI is associated with reduced functional responses and expression. Blood 2003; 101(11): 4372–4379. [DOI] [PubMed] [Google Scholar]
- 32.Trifiro E, Williams SA, Cheli Y, et al. The low-frequency isoform of platelet glycoprotein VIb attenuates ligand-mediated signal transduction but not receptor expression or ligand binding. Blood 2009; 114(9): 1893–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neeves KB, Onasoga AA, Hansen RR, et al. Sources of variability in platelet accumulation on type 1 fibrillar collagen in microfluidic flow assays. PLoS One 2013; 8(1): e54680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bender M, Hofmann S, Stegner D, et al. Differentially regulated GPVI ectodomain shedding by multiple platelet-expressed proteinases. Blood 2010; 116(17): 3347–3355. [DOI] [PubMed] [Google Scholar]
- 35.Murphy S, Gardner FH. Platelet storage at 22 degrees C; metabolic, morphologic, and functional studies. The Journal of clinical investigation 1971; 50(2): 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiMinno G, Silver MJ, Murphy S. Stored human platelets retain full aggregation potential in response to pairs of aggregating agents. Blood 1982; 59(3): 563–568. [PubMed] [Google Scholar]
- 37.Shapira S, Friedman Z, Shapiro H, et al. The effect of storage on the expression of platelet membrane phosphatidylserine and the subsequent impacton the coagulant function of stored platelets. Transfusion 2000; 40(10): 1257–1263. [DOI] [PubMed] [Google Scholar]
- 38.Curvers J, van Pampus EC, Feijge MA, et al. Decreased responsiveness and development of activation markers of PLTs stored in plasma. Transfusion 2004; 44(1): 49–58. [DOI] [PubMed] [Google Scholar]
- 39.Koessler J, Weber K, Koessler A, et al. Expression and function of purinergic receptors in platelets from apheresis-derived platelet concentrates. Blood Transfus 2016; 14(6): 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stenberg PE, McEver RP, Shuman MA, et al. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol 1985; 101(3): 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michelson AD, Frelinger AL 3rd, Furman MI. Current options in platelet function testing. Am J Cardiol 2006; 98(10A): 4N–10N. [DOI] [PubMed] [Google Scholar]
- 42.Frelinger AL 3rd, Grace RF, Gerrits AJ, et al. Platelet function tests, independent of platelet count, are associated with bleeding severity in ITP. Blood 2015; 126(7): 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paul BZ, Jin J, Kunapuli SP. Molecular mechanism of thromboxane A(2)-induced platelet aggregation. Essential role for p2t(ac) and alpha(2a) receptors. J Biol Chem 1999; 274(41): 29108–29114. [DOI] [PubMed] [Google Scholar]
- 44.Trumel C, Payrastre B, Plantavid M, et al. A key role of adenosine diphosphate in the irreversible platelet aggregation induced by the PAR1-activating peptide through the late activation of phosphoinositide 3-kinase. Blood 1999; 94(12): 4156–4165. [PubMed] [Google Scholar]
- 45.Adam F, Verbeuren TJ, Fauchere JL, et al. Thrombin-induced platelet PAR4 activation: role of glycoprotein Ib and ADP. J Thromb Haemost 2003; 1(4): 798–804. [DOI] [PubMed] [Google Scholar]
- 46.Li Z, Zhang G, Le Breton GC, et al. Two waves of platelet secretion induced by thromboxane A2 receptor and a critical role for phosphoinositide 3-kinases. J Biol Chem 2003; 278(33): 30725–30731. [DOI] [PubMed] [Google Scholar]
- 47.Knofler R, Weissbach G, Kuhlisch E. Critical evaluation of the quantification of ATP release reaction in whole blood. Thromb Res 1996; 84(3): 157–165. [DOI] [PubMed] [Google Scholar]
- 48.Knofler R, Weissbach G, Kuhlisch E. Release of adenosine triphosphate by adenosine diphosphate in whole blood and in erythrocyte suspensions. Am J Hematol 1997; 56(4): 259–265. [DOI] [PubMed] [Google Scholar]
- 49.Eisses MJ, Chandler WL. Cardiopulmonary bypass parameters and hemostatic response to cardiopulmonary bypass in infants versus children. J Cardiothorac Vasc Anesth 2008; 22(1): 53–59. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi T, Sakurai Y, Fukuda K, et al. Correlations between global clotting function tests, duration of operation, and postoperative chest tube drainage in pediatric cardiac surgery. Paediatr Anaesth 2011; 21(8): 865–871. [DOI] [PubMed] [Google Scholar]
- 51.Krajewski S, Kurz J, Neumann B, et al. Short-acting P2Y12 blockade to reduce platelet dysfunction and coagulopathy during experimental extracorporeal circulation and hypothermia. Br J Anaesth 2012; 108(6): 912–921. [DOI] [PubMed] [Google Scholar]
- 52.Peterson JA, Maroney SA, Zwifelhofer W, et al. Heparin-protamine balance after neonatal cardiopulmonary bypass surgery. J Thromb Haemost 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sola-Visner M Platelets in the neonatal period: developmental differences in platelet production, function, and hemostasis and the potential impact of therapies. Hematology Am Soc Hematol Educ Program 2012; 2012: 506–511. [DOI] [PubMed] [Google Scholar]
- 54.Margraf A, Nussbaum C, Sperandio M. Ontogeny of platelet function. Blood Adv 2019; 3(4): 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.