Abstract
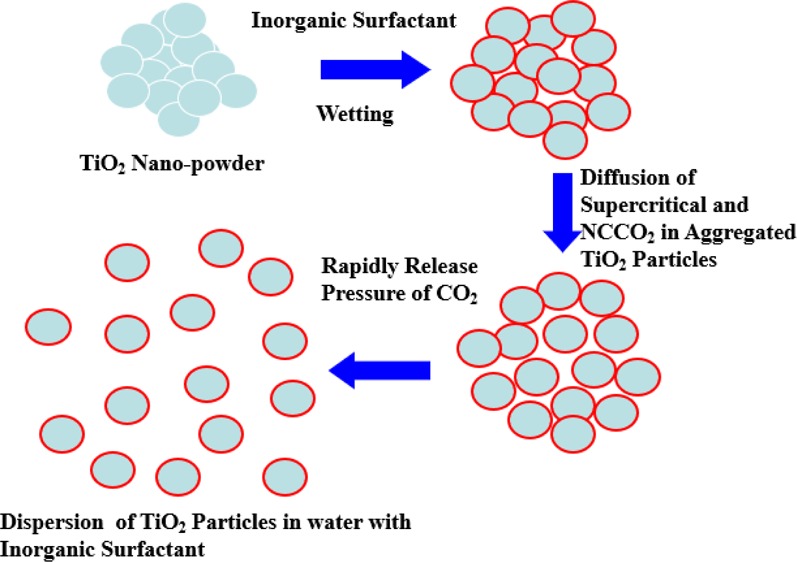
In this study, we investigated the effects of near supercritical carbon dioxide (SCCO2) parameters, including pressure, temperature, and saturation time on titanium dioxide (TiO2) nanopowder dispersion in water-containing sodium hexametaphosphate (SHMP). The stability and morphology of TiO2 particles dispersed in an aqueous solution were examined using a zeta potential instrument, dynamic light scattering, and transmission electron microscopy. As shown in the results, of particular interest, it was found that near SCCO2’s pressure and saturation time had the strongest impact on TiO2 dispersion in water-containing SHMP. This finding indicated that TiO2’s secondary average particle size was significantly reduced with an increase in near SCCO2’s pressure and saturation time. Additionally, in the presence of SHMP, the zeta potential of the as-prepared dispersion solution reached −53.7 mV because of production of the larger negative static charge repulsion force (resulting from SHMP dissociation) on the TiO2 particle surface. The secondary average size was 127 ± 68 nm, indicating good stability of TiO2 dispersed in water containing an inorganic dispersant.
1. Introduction
Titanium dioxide (TiO2) has been widely used for photocatalytic coating,1,2 cosmetic,3 antibacterial,4 self-cleaning,5 and biological6 purposes. Furthermore, TiO2’s smaller particle size has a specific surface area effect,7 resulting in outstanding light absorption, catalytic, and magnetic qualities. However, the small particle size is easily agglomerated because of van der Waals forces,8 decreasing the stability of the TiO2 dispersion solution and making it difficult to detect the advantages of the small particle size.9 Therefore, the process to reduce the secondary average particle size and enhance the stability of TiO2 dispersion solution is a very important topic in application sectors.
Generally, the dispersion processes involve three steps: wetting, mechanical dispersion, and stability. In order to understand TiO2 dispersion in solution, it is essential to understand the formation and properties of the solid–liquid interface. Dispersion may be defined as a two-phase system, in which one is the dispersion of the small particle phase and the other is the continued fluid phase. Following particle wetting, a mechanical process, such as ultrasonic dispersion,10 is usually required to complete their separation. Typically, a mechanical method, such as a continuous stirring recirculating media mill,11 is applied to disperse the wetted particles into a separating unit. Ultrasonic waves have also been proven as useful tools for dispersing nanoparticles and eliminating agglomeration in aqueous suspensions.12 Ultrasonic irradiation generates shock waves by causing cavitation collapse, leading to interparticle collisions. The agglomerated particles thus become eroded and split by the collisions. Many studies have applied ultrasonic wave to disperse TiO2 particles in various solutions for application in different fields.13−18
Supercritical fluids (SCFs) are involved in numerous industrial processes and have a potentially wide field of new applications.19 The SCF has both liquid- and gas-like properties, so that it concurrently exhibits good diffusion and wettability. Additionally, SCFs have lower surface tensions, resulting in faster permeations than those seen with a liquid.20 As a result, a SCF dispersion process was developed in order to separate the aggregated particles. The supercritical carbon dioxide (SCCO2) dispersing method was first studied by Kamiwano et al.20 for dispersing carbon black particles in water without dispersants. The results showed that the samples of 2 wt % carbon black were uniformly dispersed throughout the solutions after standing for 100 h and had a secondary average particle size of <5 μm. Cheng et al.21 used SCCO2 to disperse aminoanthraquinone red, green 36, and blue 15:6 organic pigments in propylene glycol monomethyl ether acetate (PGMEA) in which the organic pigment concentration was 0.001 wt % in order to obtain secondary average particle sizes of 178.5, 93.5, and 188.7, respectively, in the dispersion solution. Wu et al.22 used SCCO2 to disperse 1 wt % violet 23 organic pigment in PGMEA. They found that under the favorable conditions of 328.2 K and 20 MPa, the secondary average particle size of pigment dispersion containing dispersants in PGMEA was as small as 175 nm. Cheng and Wu23 studied a cyclohexanone and PGMEA mixture as a binary solvent for assisting SCCO2 dispersion and stabilizing organic nanoparticles in the presence of a polyester/polyamine copolymer as a capping agent. As shown in the results, a secondary average particle sizes of 59 and 64 nm were obtained for diketopyrrolopyrrole (red 254) and copper phthalocyanine (green 36) organic pigments, respectively.
After dispersing the wetted particles into a separated unit, sufficient stability should be maintained for a long time for application purposes. Many studies have focused on the TiO2 particle dispersion stability in water. Mou et al.24 studied the TiO2 dispersion solution’s solubility by adding ethanol, tetrahydrofuran, polyvinylpyrrolidone (PVP), and sodium hexametaphosphate (SHMP) as dispersants. The results showed that SHMP and tetrahydrofuran as dispersants were the most stable ones. Almusallam et al.25 explored the stability of TiO2 particles suspended in various aqueous solutions with and without salicylic acid as an organic contaminant. They found that stable TiO2 dispersion solution could be obtained under basic solution conditions. However, when changing the pH values of the solution from basic to acidic, it was observed that TiO2 particles aggregated at pH values below the isoelectric point.26 It was also shown that TiO2 aggregation was accelerated by an increase in the concentration of particles in solution. Mahlalela et al.27 examined the stability of the dispersion solution by using different conducting liquids (deionized water, NaCl, CaCl2, and MgCl2) in the various solutions with different pH values. Agglomeration and zeta potentials were influenced by ionic strength, electrolyte type, and the presence of dyestuff. Tsai et al.28 investigated SHMP and polyacrylic acid (PAA) dispersant effects on TiO2 dispersion stability. The results illustrated that the SHMP was more stable than PAA for facilitating TiO2 dispersion in water. In addition, ammonium polyacrylate (PAA-NH4) has been investigated and compared with respect to the dispersion stabilities of TiO2 powders with different particle sizes and surface chemistries in aqueous suspensions containing a common water-based dispersant.29
According to the studies described in the literature, the present study examined the use of near SCCO2 dispersing TiO2 nanopowder in water-containing SHMP as a dispersant, which could be called as a green process because the used materials are nontoxic chemicals. The effects of near SCCO2 conditions, including pressure, temperature, and saturated time, on the stability and morphology of TiO2 particles dispersed in aqueous solution was examined by zeta potential instruments, a pH meter, dynamic light scattering (DLS), transmission electron microscopy (TEM), and ultraviolet–visible absorption spectroscopy (UV–vis) associated with high-speed centrifugation and natural sedimentation in this study.
2. Results and Discussion
2.1. Ratio of the Dispersant to TiO2 and Effect of the pH Value on TiO2 Dispersion Solution
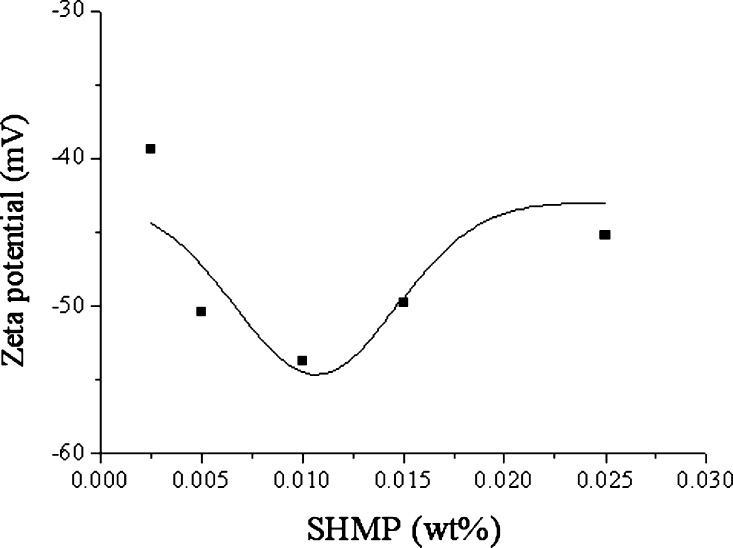
Figure 1 shows that the zeta potential of dispersed TiO2 in water varied with different dispersant concentrations under specified conditions of near SCCO2. As observed in the figure, when the ratio of the dispersant is two times that of TiO2 in weight percentage, the most stable dispersion solution exists because SHMP in water has been dissociated into negatively charged phosphate ions and adsorbed on the surface of TiO2 particles to form a perfect electrical double layer. A small amount of inorganic dispersants cannot form an effective electrical double layer on the surface of TiO2 particles, indicating that the electrostatic repulsion force is small, which results in unstable TiO2 dispersion in the solution; however, an excess amount of inorganic dispersants would compress the electrical double layer on the surface of TiO2 particles leading to a low negative zeta potential. Therefore, the weight ratio of the dispersant to TiO2 was determined as 2/1 in this study.
Figure 1.
Zeta potential varying with concentration of the SHMP dispersant in 0.005 wt % TiO2 solution after being dispersed via SCCO2 under conditions of 35 °C, 1200 psi, and a saturation time of 30 min, in which the residual CO2 in the TiO2 dispersion solution was removed with a ultrasonic bath for 10 min after depressurizing.
In addition, the effect of pH values on zeta potential and the secondary average particle size estimated by DLS, for the dispersion of 0.05 wt % TiO2 in solution with 0.1 wt % SHMP through near SCCO2 under conditions of 35 °C, 1200 psi, and a saturation time of 30 min, is shown in Figure 2. As seen in the figure, it was found that the maximum negative zeta potential reached −53.7 mV at a pH of 6.25, corresponding to a minimum secondary average particle size of about 200 nm. This is because the acidic conditions cause TiOH2+ formation on the TiO2 particles’ surfaces; in the meantime, adsorption of phosphate ions occurs, resulting in a decrease in the negative charge on particles’ surface. In other words, with an increase in the pH value, the TiO– formation on the surface of TiO2 particles would couple with the adsorbed phosphate ion to enhance negative charges on TiO2 particles’ surfaces, thus inducing dispersed solution stabilization. The Na+ ion in the solution would also increase and absorb the negative charge on the TiO2 surface, causing a decrease in the electrical double layer thickness and resulting in an unstable TiO2 dispersion solution.
Figure 2.
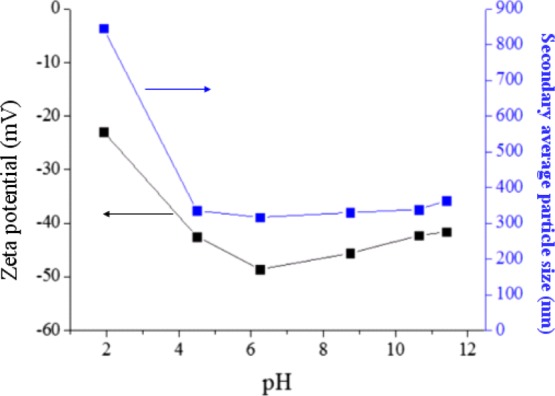
Zeta potential (black) and the secondary average particle size (blue) estimated by DLS of dispersed 0.005 wt % TiO2 in water varying with the pH value of dispersion solution containing 0.01 wt % SHMP through SCCO2 under the conditions of 35 °C, 1200 psi, and a saturation time of 30 min.
2.2. Effects of Near SCCO2 Conditions on Dispersion of TiO2 in Water
2.2.1. Temperature
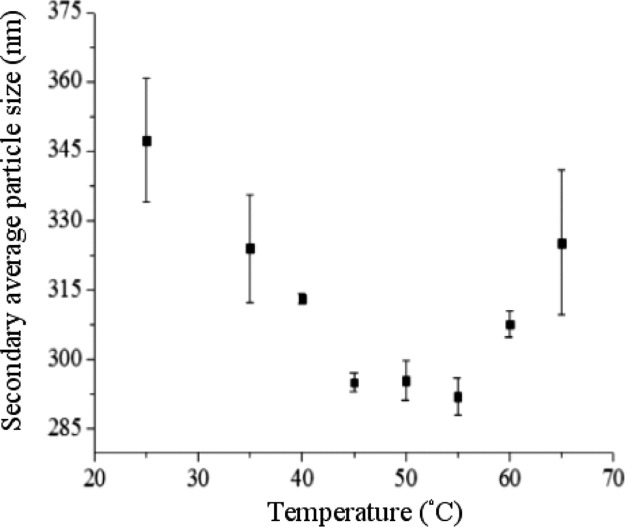
In this study, we changed the SCCO2 temperature in the dispersion process at a pressure of 1200 psi and the saturation time of 30 min followed by CO2 removal in the dispersion solution using an ultrasonic bath for 10 min after depressurization. As seen in Figure 3, the temperature had a significant effect on the secondary average particle size of the dispersed TiO2 in solution. As the temperature increased, the secondary average particle size of TiO2 was decreased to 292 ± 4 nm at 55 °C followed by an increase in particle size to 325 ± 16 nm at 65 °C, indicating that the temperature should be optimized for TiO2 dispersion in water through near SCCO2. This process occurs because the elevated temperature can input energy and disrupt van der Waals forces between the particles followed by enhancement of particle mobility in the solution leading to formation of smaller and more uniform secondary average particle sizes; in contrast, an increase in temperature would reduce carbon dioxide solubility in water,30 which would yield in weakening of aggregated TiO2 particle dispersion by near SCCO2.
Figure 3.
Secondary average particle size estimated from DLS of 0.005 wt % TiO2 dispersion solution involving 0.01 wt % SHMP as a function of temperature of SCCO2 under a pressure of 1200 psi and a saturation time of 30 min.
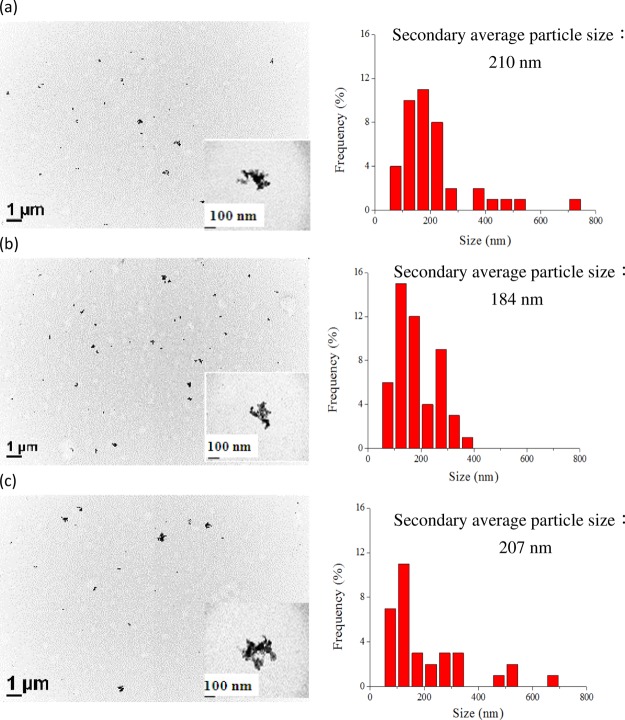
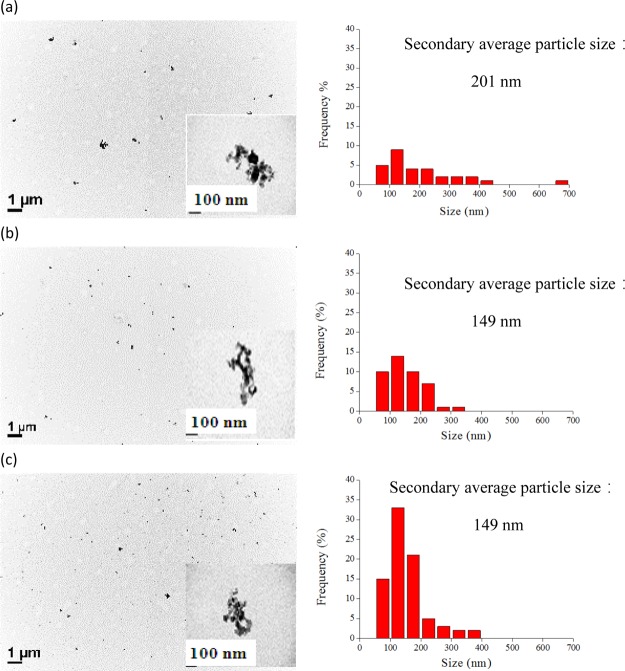
In order to further understand the temperature effects of near SCCO2, we increased the TiO2 concentrations in solution from 0.005 to 0.1 wt % in addition to selecting three different temperatures: (1) 25; (2) 55; and (3) 65 °C. As estimated from the TEM images shown in Figure 4, the secondary average particle sizes of dispersed TiO2 in water by near SCCO2 were 210 ± 135, 184 ± 81, and 207 ± 153 nm at the temperatures of 25, 55, and 65 °C, respectively. This suggests that applying energy to enhance separation of aggregated particles and solubility of near SCCO2 in water would be traded for a smaller secondary average particle size of dispersed TiO2 in solution.
Figure 4.
TEM images (1) and particle size distribution (2) calculated from TEM of dispersed 0.1 wt % TiO2 in water containing 0.2 wt % SHMP through SCCO2 under temperatures of (a) 25, (b) 55, and (c) 65 °C, respectively, as well as at a pressure of 1200 psi and a saturation time of 30 min. The inset is the morphology of aggregated TiO2 particles in solution.
2.2.2. Pressure
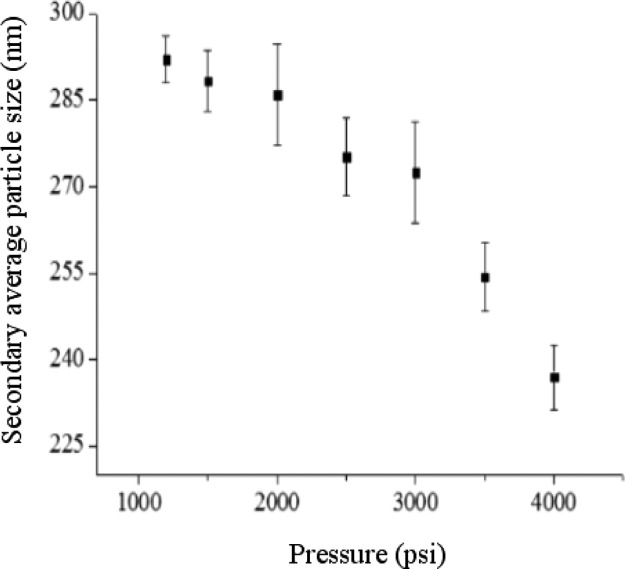
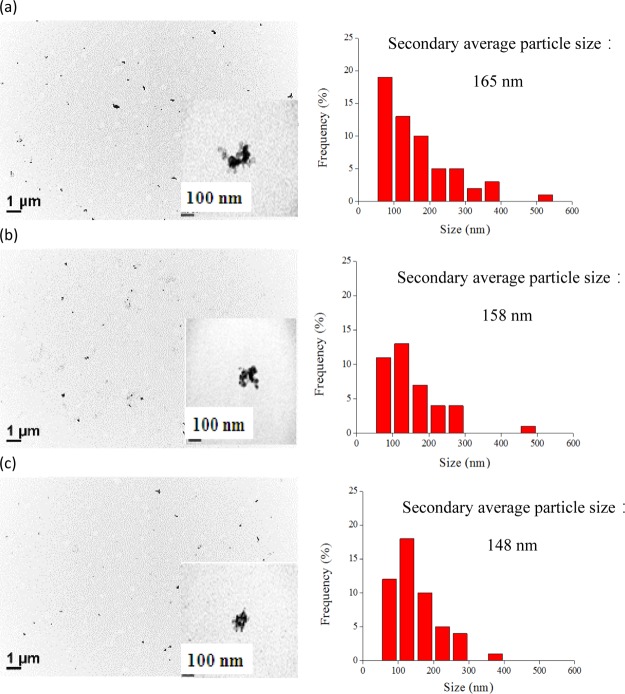
At a temperature of 55 °C and a saturation time of 30 min, we changed the pressure of near SCCO2 in the dispersion process followed by CO2 removal from the dispersion solution using an ultrasonic bath for 10 min. As shown in Figure 5, with an increase in pressure from 1200 to 4000 psi, the secondary average particle size was reduced from 292 ± 4 to 237 ± 6 nm as measured by DLS. It was indicated that higher near SCCO2 pressure was favorable for the formation of smaller TiO2 particles in solution. This process probably occurs because an elevation in pressure leads to an increase in CO2 solubility in water, resulting in facilitating near SCCO2 penetration into the pores of aggregated particles in solution. Additionally, Figure 6 shows the TEM of dispersed TiO2 particles in solution under different near SCCO2 pressures. As exhibited in the images, the secondary average particle size of thedispersed TiO2 particle decreases with increasing near SCCO2 pressure. When the pressure was increased from 2000 to 4000 psi, the secondary average particle size of dispersed TiO2 particle was reduced from 165 ± 98 to 148 ± 68 nm. The standard deviation was decreased, indicating that the narrow particle size distribution of dispersed TiO2 in solution could be obtained by increasing the pressure of near SCCO2.
Figure 5.
Secondary average particle size estimated from DLS of 0.005 wt % TiO2 dispersion solution involving 0.01 wt % SHMP varying with pressure of SCCO2 under a temperature of 55°C and a saturation time of 30 min.
Figure 6.
TEM images (1) and particle size distribution (2) calculated from TEM of dispersed 0.1 wt % TiO2 in water containing 0.2 wt % SHMP through SCCO2 under pressures of (a) 2000, (b) 3000, and (c) 4000 psi, respectively, as well as at a temperature of 55 °C and a saturation time of 30. The inset is the morphology of aggregated TiO2 particles in solution.
2.2.3. Saturation Time
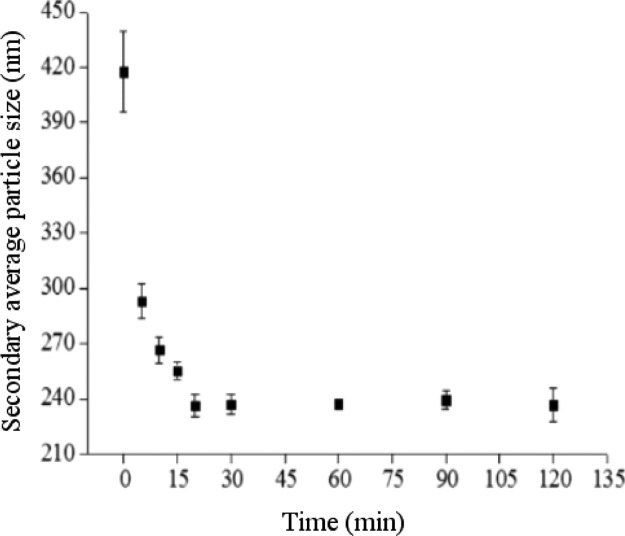
Figure 7 illustrates the effect of near SCCO2 saturation time on the secondary average particle size of dispersed TiO2 particles in water, in which CO2 pressure and temperature in addition to the time of CO2 removal from the dispersion solution after ultrasonication were 4000 psi, 55 °C, and 10 min, respectively. As determined from the figure, an increase in saturation time from 0 to 120 min led to a reduction in the secondary average particle sizes of dispersed TiO2 in solution from 418 ± 22 to 237 ± 9 nm as determined by DLS. This finding indicated that more of the near SCCO2 permeated the pores in-between TiO2 particles as the saturation time increased, resulting in full wetting of aggregated particles in solution. Meanwhile, we also found that the secondary average particle size of TiO2 in solution would present a constant trend when the saturation time of near SCCO2 reached 30 min, indicating that the agglomerated particle pores were completely filled with near SCCO2. Figure 8 shows the TEM of dispersed TiO2 in solution with near SCCO2 at different saturation times. As observed from the figure, the secondary average particle size of dispersed TiO2 in solution containing near SCCO2 decreased with an increase in saturation time. When the saturation time was increased from 5 to 120 min, the average particle size of dispersed TiO2 particles in solution through near SCCO2 was reduced from 201 ± 135 to 149 ± 65 nm as estimated by TEM.
Figure 7.
Secondary average particle size estimated from DLS of 0.005 wt % TiO2 dispersion solution involving 0.01 wt % SHMP varying with saturation time of SCCO2 under a temperature of 55°C and a pressure of 4000 psi.
Figure 8.
TEM images (1) and particle size distribution (2) calculated from TEM of dispersed 0.1 wt % TiO2 in water containing 0.2 wt % SHMP through SCCO2 under the saturation times of (a) 5, (b) 20, and (c) 120 min, respectively, as well as at a temperature of 55 °C and a pressure of 4000 psi. The inset is the morphology of aggregated TiO2 particles in solution.
2.3. Stability of TiO2 Dispersion Solution
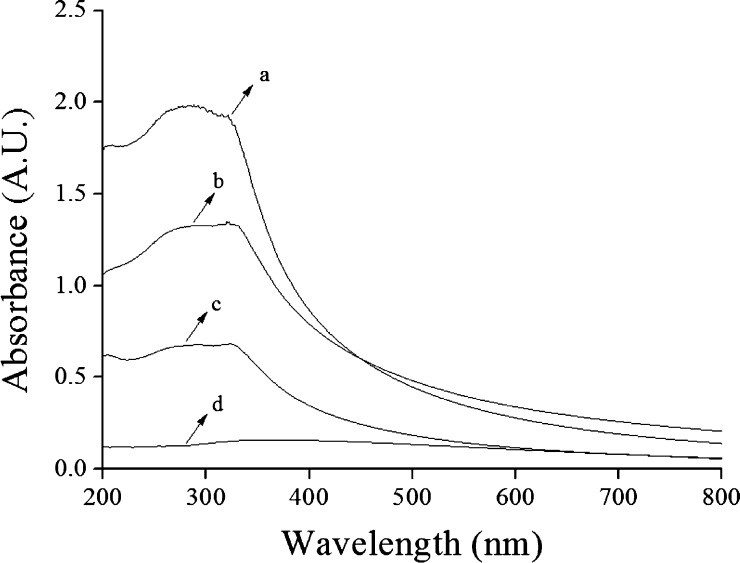
The UV–vis light absorption associated with centrifugation was used to examine stability of dispersed TiO2 in solution without and with inorganic dispersants via near SCCO2 in this study. Figure 9 presents the UV–vis light absorption spectra of four samples before and after centrifugation at a rotational speed of 3000 rpm for 30 min. As observed from Figure 9d, the UV–vis light absorption value of dispersed TiO2 in water without dispersants by only magnetic stirring for one day was the lowest because of aggregation seriously leading to particle precipitation. The UV–vis light absorption spectra of the sample dispersed by near SCCO2 in the absence of dispersants is shown in Figure 9c, in which the absorption value was obviously higher than the sample without dispersion by SCCO2, resulting from TiO2 particles suspended in water stably.
Figure 9.
UV–vis light absorption spectra of 0.005 wt % TiO2 in water (a) with both 0.01 wt % SHMP and dispersion by SCCO2, (b) with 0.01 wt % SHMP but without dispersion by SCCO2, (c) without SHMP but with dispersion by SCCO2, and (d) without both SHMP and dispersion by SCCO2. The conditions of SCCO2 are a temperature of 55 °C, a pressure of 4000 psi, and a saturation time of 30 min.
We further investigated the UV–vis light absorption of TiO2 with and without dispersion by near SCCO2 in the presence of SHMP. As shown in Figure 9a,b, the absorption peak was blue-shifted31 for the sample dispersion by near SCCO2, indicating that the secondary average particle size of dispersed TiO2 in water was reduced in order to induce an absorption peak shift to a shorter wavelength. This could be used to demonstrate that the near SCCO2 is an effective method for TiO2 dispersion in water with inorganic dispersants.
Figure 10 shows photographs of the natural sedimentation experiment in which sample (a) was made from 0.1 wt % TiO2 in water; sample (b) was prepared from blending 0.1 wt % TiO2 and 0.2 wt % SHMP in water; sample (c) was fabricated by mixing 0.1 wt %TiO2 and 0.2 wt % SHMP and dispersed by near SCCO2; and sample (d) was water for comparison, as well as the SCCO2 dispersion conditions were 55 °C, 4000 psi, and 30 min saturation time. As observed from the photographs, without inorganic dispersants in water, the TiO2 particles had completely precipitated in the bottom of the scintillation flask after dispersion for about one day; on the other hand, adding SHMP could significantly enhance dispersed TiO2 stability in solution, which clearly indicated that the TiO2 dispersion solution with dispersants was more stable than that without dispersants. This difference resulted from smaller secondary average particle size of dispersed TiO2 in solution through near SCCO2 in the presence of dispersants. The lifetime of dispersed TiO2 in water-containing SHMP was over two weeks in this study.
Figure 10.

Photographs of the natural sedimentation experiment for the samples: (a) TiO2 in water; (b) TiO2 and SHMP in water; (c) TiO2 and SHMP in water after being dispersed by SCCO2; and (d) water for comparison.
3. Conclusions
In this study, near SCCO2 was successfully employed to disperse TiO2 particles in water involving inorganic dispersants. First, the amount of the SHMP dispersant was determined by zeta potential analysis. The effects of different temperatures, pressures, and saturation times on the dispersed TiO2 in solution through near SCCO2 were then examined by DLS and TEM. Finally, the stability of dispersed TiO2 in solution was characterized via UV–vis light absorption associated with centrifugation and natural sedimentation method. As shown in the results, we summarize the study by highlighting several significant findings:
-
(1)
As the addition of SHMP was twice the concentration of TiO2 in weight, the maximum zeta potential of the dispersion solution was −53.7 mV as measured by a zeta potential instrument, suggesting that the SHMP as a dispersant was well dissociated from the phosphate ions and adsorbed on the surface of the TiO2 particles. This finding implied that TiO2 dispersion in solution had a large negative charge from the electrostatic repulsive force and had good stability at a pH of 6.25 in order to prevent TiO2 particle precipitation under operating conditions of near SCCO2.
-
(2)
When the concentration of TiO2 in solution with SHMP was 0.1 wt %, the secondary average particle size was 366 ± 295 and 148 ± 68 nm as measured by TEM before and after dispersion by near SCCO2, respectively, indicating that near SCCO2 was validated as having penetrated the pores of aggregated particles, and then rapidly depressurized to effectively separate aggregated particles in solution.
-
(3)
It was found that applying energy to promote separation of aggregated TiO2 particles and solubility of CO2 in water should be compromised, namely, the temperature of near SCCO2 must be optimized in this work.
-
(4)
Raising the pressure could increase the density of near SCCO2 in order to elevate wettability of the near SCF on the surface of particles and enhance the separation of aggregated TiO2 particles during the rapid depressurization process.
-
(5)
As analyzed from DLS, when the concentration of TiO2 ranged from 0.005 to 0.1 wt %, a 30 min SCCO2 saturation time was enough to completely wet the surface of the aggregated particles.
-
(6)
As measured by natural sedimentation, the storage time of the as-prepared 0.1 wt % TiO2 dispersion solution could be over two weeks.
Hopefully, the above results promise to facilitate fabrication of colloid inorganic oxide suspension through a green process.
4. Materials and Method
4.1. Chemicals
TiO2 powder (P25) with a primary average particle size of 21 nm and a spherical shape was purchased from Degussa. SHMP (99%) was used as an inorganic surfactant (SHOWA) in order to enhance dispersion stability. Deionized water (18.1 Ω), which was mechanically filtered and processed to remove impurities, was used as a continuous phase. Hydrochloric acid (32.4%) and sodium hydroxide (96%) were obtained from Choneye Pure Chemicals and SHOWA, respectively. Both were diluted to 0.1 wt % for adjusting the pH values of the dispersion solutions.
4.2. Determination of the Ratio of the Dispersant to TiO2 in Dispersion Solution
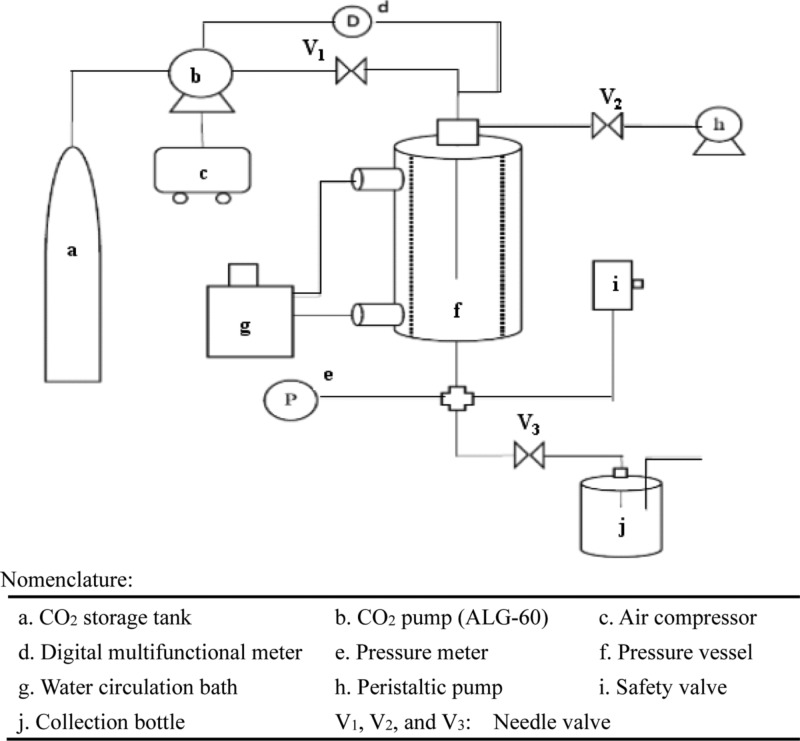
In order to stabilize the dispersed TiO2 and prevent it from precipitating in water under near SCCO2 operating conditions, the experiments were conducted by varying the amounts of SHMP from 0.0025 to 0.025 wt % (0.0025, 0.005, 0.01, 0.015, and 0.025 wt %) in 50 mL deionized water containing 0.005 wt % TiO2 with continuous magnetic stirring (Ciamarec 2 Thermolyne, 950 rpm) for 24 h followed by transfering to a 300 mL supercritical vessel through a peristaltic pump (Cole-Parmer Masterflex L/S) at normal atmospheric pressure and ambient temperature. A schematic diagram of the experimental system used in this study is shown in Figure 11. For each experiment, the supercritical vessel was operated according to the following procedure; after introducing 50 mL of the dispersion solution, the supercritical vessel was closed, and the temperature was controlled at 35 °C via a circulating water bath (Deng Yang Water Bath D-606). The carbon dioxide concentration was then increased up to 1200 psi in the supercritical vessel using an air driven pump (Haskel ALG-60), followed by maintaining it at this pressure for 30 min, and then followed by rapid depressurization of the near SCCO2 to disperse the TiO2 particles in the solution. In order to remove the residual carbon dioxide in the TiO2 dispersion solution after the near SCCO2 dispersion process, an ultrasonic bath was used for 10 min before characterization. The pH of the TiO2 dispersion solution was varied by addition of sodium hydroxide or hydrochloric acid. In addition, a zeta potential instrument (Malvern Zetasizer Nano Series) was used for measuring the zeta potential of the TiO2 particles in the dispersion solution after the pH was measured using a pH meter (SUNTEX SP-701).
Figure 11.
Schematic diagram of experimental apparatus in this study.
4.3. Effects of Near SCF Conditions on TiO2 Dispersed in Water with the SHMP Dispersant
The experimental samples were prepared by using 0.01 wt % SHMP in 50 mL deionized water, followed by adding 0.005 wt % TiO2 with continuous magnetic stirring for 24 h. The subsequent procedure was similar to the dispersion method used in the previous experiment, but the near SCCO2 dispersion temperature was varied from 25 to 65 °C, the pressure was regulated from 1200 to 4000 psi, and the saturation time was limited between 0 and 120 min. We used the same conditions and steps to disperse TiO2 in solution in three separate experiments.
A DLS analyzer (Brookhaven 90Plus Particle Sizer) was used for measuring the secondary average particle size and dispersion solution distribution. For further validation, the dispersed TiO2 particles in solution were characterized by TEM (JEOL JEM-1200CX). The TEM images of dispersed TiO2 particles were analyzed with ImageJ.31
4.4. Stability of As-Dispersed TiO2 in Water through Near SCCO2
The stability of the dispersed TiO2 in solutions with and without dispersants was evaluated by ultraviolet–visible absorption spectroscopy (UV-1800 SHIMADZU) together with high-speed centrifugation (CN-3302 HSIANGTAI) in addition to natural sedimentation.
Acknowledgments
The authors sincerely appreciate the financial support from the Ministry of Science and Technology (MOST: 104-2221-E-005-084-), Taiwan.
The authors declare no competing financial interest.
References
- Othman S. H.; Abdul Rashid S.; Mohd Ghazi T. I.; Abdullah N. Dispersion and Stabilization of Photocatalytic TiO2 Nanoparticles in Aqueous Suspension for Coatings Applications. J. Nanomater. 2012, 2012, 718214. 10.1155/2012/718214. [DOI] [Google Scholar]
- Khalilzadeh A.; Fatemi S. Spouted Bed Reactor for VOC Removal by Modified Nano-TiO2 Photocatalytic Particles. Chem. Eng. Res. Des. 2016, 115, 241. 10.1016/j.cherd.2016.10.004. [DOI] [Google Scholar]
- Lu P.-J.; Huang S.-C.; Chen Y.-P.; Chiueh L.-C.; Shih D. Y.-C. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug. 2015, 23, 587. 10.1016/j.jfda.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besinis A.; De Peralta T.; Handy R. D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine onStreptococcus mutansusing a suite of bioassays. Nanotoxicology 2014, 8, 1. 10.3109/17435390.2012.742935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal N.; Rastogi D.; Jassal M.; Agrawal A. K. Dispersion Stabilization of Titania Nanoparticles for Textile: Aggregation Behavior and Self-Cleaning Activity. J. Disper. Sci. Tecnol. 2013, 34, 611. 10.1080/01932691.2012.685842. [DOI] [Google Scholar]
- Nia M. H.; Rezaei-Tavirani M.; Nikoofar A. R.; Masoumi H.; Nasr R.; Hasanzadeh H.; Jadidi M.; Shadnush M. Stabilizing and Dispersing Methods of TiO2 Nanoparticles in Biological Studies. J. Paramed. Sci. 2015, 6, 96. [Google Scholar]
- Song X.; Zhang Y.; Chang C. Novel Method for Preparing Activated Carbons with High Specific Surface Area from Rice Husk. Ind. Eng. Chem. Res. 2012, 51, 15075. 10.1021/ie3012853. [DOI] [Google Scholar]
- Hamaker H. C. The London-van der Waals attraction between spherical particles. Physica 1937, 4, 1058. 10.1016/s0031-8914(37)80203-7. [DOI] [Google Scholar]
- Ji X.; Jin X.; George S.; Xia T.; Meng H.; Wang X.; Suarez E.; Zhang H.; Hoek E. M. V.; Godwin H.; Nel A. E.; Zink J. I. Dispersion and Stability Optimization of TiO2 Nanoparticles in Cell Culture Media. Environ. Sci. Technol. 2010, 44, 7309. 10.1021/es100417s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H. Y.; Hemingway M.; Xia F.; Li S. N.; Zhao Y. P. Production of β-Carotene Nanoparticles by the Solution Enhanced Dispersion with Enhanced Mass Transfer by Ultrasound in Supercritical CO2. Ind. Eng. Chem. Res. 2011, 50, 13475. 10.1021/ie2011565. [DOI] [Google Scholar]
- Simpson A. B. G.; Byrne J. A.; McLaughlin J. A. D.; Strawhorne M.; Strawhorne M. Effect of Solids Concentration on Particle Size Distribution of Deagglomerated Barium Titanate in Stirred Media Mills. Chem. Eng. Res. Des. 2015, 93, 287. 10.1016/j.cherd.2014.04.006. [DOI] [Google Scholar]
- Sato K.; Li J.-G.; Kamiya H.; Ishigaki T. Ultrasonic Dispersion of TiO2 Nanoparticles in Aqueous Suspension. J. Am. Ceram. Soc. 2008, 91, 2481. 10.1111/j.1551-2916.2008.02493.x. [DOI] [Google Scholar]
- Liu Y.; Yu Z.; Zhou S.; Wu L. Deagglomeration and Dispersion of NanoTiO2 in an Agitator Bead Mill. J. Disper. Sci. Technol. 2006, 27, 983. 10.1080/01932690600766975. [DOI] [Google Scholar]
- Ghadimi A.; Metselaar I. H. The influence of surfactant and ultrasonic processing on improvement of stability, thermal conductivity and viscosity of titania nanofluid. Exp. Therm. Fluid Sci. 2013, 51, 1. 10.1016/j.expthermflusci.2013.06.001. [DOI] [Google Scholar]
- Qi X.; Jia Z.; Yang Y. Influence of the dispersion of nano titanium dioxide on the tribological performance of fabric self-lubricating liner. J. Appl. Polym. Sci. 2013, 130, 2100. 10.1002/app.39343. [DOI] [Google Scholar]
- Wu Y.; Zhao J.; Li Y.; Lu K. Preparation and Freezing Behavior of TiO2 Nanoparticle Suspensions. Ceram. Int. 2016, 42, 15597. 10.1016/j.ceramint.2016.07.012. [DOI] [Google Scholar]
- He H.; Cheng Y.; Yang C.; Zeng G.; Zhu C.; Yan Z. Influences of Anion Concentration and Valence on Dispersion and Aggregation of Titanium Dioxide Nanoparticles in Aqueous Solutions. J. Environ. Sci. 2017, 54, 135. 10.1016/j.jes.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Bałdyga J.; Kubicki D.; Shekunov B. Y.; Smith K. B. Mixing Effects on Particle Formation in Supercritical Fluids. Chem. Eng. Res. Des. 2010, 88, 1131. 10.1016/j.cherd.2010.02.016. [DOI] [Google Scholar]
- Beckman E. J. Supercritical and Near-critical CO2 in Green Chemical Synthesis and Processing. J. Supercrit. Fluids 2004, 28, 121. 10.1016/s0896-8446(03)00029-9. [DOI] [Google Scholar]
- Kamiwano M.; Nishi K.; Inoue Y.. Dispersion Method and Dispersing Apparatus using Supercritical State. EP0850682A1, 1999.
- Cheng W. T.; Hsu C. W.; Chih Y. W. Dispersion of Organic Pigments using Supercritical Carbon Dioxide. J. Colloid Interface Sci. 2004, 270, 106. 10.1016/j.jcis.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Wu H.-T.; Huang K.-Y.; Lee K.-T. Supercritical fluid-assisted dispersion of C.I. pigment violet 23 in an organic medium. Powder Technol. 2011, 206, 322. 10.1016/j.powtec.2010.09.037. [DOI] [Google Scholar]
- Cheng W. T.; Wu J. C. Organic Nano-particle Capping with Polymeric Surfactant Dispersed by Supercritical Carbon Dioxide in the Binary Solvent. Chem. Eng. Process. 2013, 66, 44. 10.1016/j.cep.2013.01.002. [DOI] [Google Scholar]
- Mou Y.; Lü K. M.; Gao D. M. Influence of Dispersants on Dispersion Stability of TiO2 Suspensions. Adv. Mater. Res. 2011, 356, 476. 10.4028/www.scientific.net/amr.356-360.476. [DOI] [Google Scholar]
- Almusallam A. S.; Abdulraheem Y. M.; Shahat M.; Korah P. Aggregation Behavior of Titanium Dioxide Nanoparticles in Aqueous Environments. J. Disper. Sci. Technol. 2012, 33, 728. 10.1080/01932691.2011.567886. [DOI] [Google Scholar]
- Fu W.; Hua L.; Zhang W. Experimental and Modeling Assessment of the Roles of Hydrophobicity and Zeta Potential in Chemically Modified Poly(ether sulfone) Membrane Fouling Kinetics. Ind. Eng. Chem. Res. 2017, 56, 8580. 10.1021/acs.iecr.7b02203. [DOI] [Google Scholar]
- Mahlalela L. C.; Ngila J. C.; Dlamini L. N. Characterization and Stability of TiO2 Nanoparticles in Industrial Dye Stuff Effluent. J. Disper. Sci. Technol. 2017, 38, 584. 10.1080/01932691.2016.1183501. [DOI] [PubMed] [Google Scholar]
- Tsai W.-B.; Kao J.-Y.; Wu T.-M.; Cheng W.-T. Dispersion of Titanium Oxide Nanoparticles in Aqueous Solution with Anionic Stabilizer via Ultrasonic Wave. J. Nanopart. 2016, 2016, 6539581. 10.1155/2016/6539581. [DOI] [Google Scholar]
- Chen F.; Ma M.; Wang J.; Wang F.; Chern S.-X.; Zhao E. R.; Jhunjhunwala A.; Darmadi S.; Chen H.; Jokerst J. V. Exosome-like Silica Nanoparticles: a Novel Ultrasound Contrast Agent for Stem Cell Imaging. Nanoscale 2017, 9, 402. 10.1039/c6nr08177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban M. O.; Ferrentino G.. Dense Phase Carbon Dioxide: Food and Pharmaceutical Applications; Wiley: New York, 2012. [Google Scholar]
- Kreibig U.; Genzel L. Optical absorption of small metallic particles. Surf. Sci. 1985, 156, 678. 10.1016/0039-6028(85)90239-0. [DOI] [Google Scholar]