Abstract
Urine specific gravity (USG), which is usually measured by refractometry, is an important indicator of renal concentrating ability. Few studies have evaluated refractometers with separate scales for canine and feline urine. Variables such as protein content or storage time may influence the USG. We compared the effects of measuring USG with a refractometer with single or separate scales for canine and feline urine, investigated inter- and intra-observer variability, and measured agreement between whole urine and supernatant. We evaluated the correlation between USG and osmolality, the influence of urinary protein on USG and osmolality, and the impact of storage time up to 6 mo. We examined 252 canine and 126 feline samples. Bland–Altman analysis revealed higher USG values of the single-scale refractometer than the dual-scale refractometer, with a mean difference (bias) of < 0.001 for canine and 0.003 for feline specimens. Inter- and intra-observer variability were acceptable. Good agreement was shown between USG of whole urine and supernatant. Correlations between USG and osmolality were excellent (0.98–0.99, p < 0.001). Proteinuria up to 1 g/L had no major impact on USG or osmolality. Storage time had no significant effect on USG. The difference between the refractometers is clinically irrelevant, and the use of a refractometer with separate feline and canine scales is unnecessary. Whole urine and supernatant stored up to 6 mo can both be used for USG measurement. The influence of proteinuria <1 g/L on USG and osmolality is negligible.
Keywords: canine urine, feline urine, osmolality, proteinuria, refractometer, storage time, urine specific gravity
Introduction
Evaluation of renal concentrating ability is an important test to confirm diagnoses of chronic kidney disease or acute kidney injury.10 Osmolality is the gold standard for evaluating the kidney’s urine concentration ability, but determination of urine specific gravity (USG) is commonly used as a practical alternative and is considered a sensitive test of renal concentrating ability.24 USG is defined as the ratio of the weight of a volume of urine to the weight of the same volume of distilled water and is affected by the number, molecular mass, and chemical structure of particles.24 In contrast, osmolality is the total solute concentration and is only affected by the number of particles.7 For this reason, heavier molecules, such as proteins, should have a larger effect on USG than on osmolality. Reference methods to measure USG are pycnometry and measurement of total solids after drying. Despite inconsistencies between results obtained by reference methods and refractometry,19 the latter is used in most veterinary clinics and laboratories. Determination of USG with a refractometer is simple to perform and inexpensive.
Formulas for the relationship between total solids and USG in human, canine, and feline urine samples were developed 60 y ago.16 Urine samples of 190 humans, 21 dogs, and 22 cats were included. The refractive index for all samples together was higher than the refractive index for human samples alone. In that study, all 22 feline samples had specific gravities > 1.030; the 21 canine and 190 human urines were more dilute. Based on these calculations, refractometers with separate scales for canine and feline urine samples have been available for several years, but only a few studies have examined their usefulness for measurement of USG in feline urine samples.4,19 Previous studies have compared osmolality and USG measured by refractometry, and most had good correlation.8,9,12 Refractometry is therefore accepted as an adequate surrogate of osmolality.7 Although a 2002 study demonstrated an impact of proteinuria on USG and osmolality,23 this was not confirmed in a 2013 study.3
In human and veterinary practice, measurement of USG is usually performed by using fresh whole urine immediately after collection. However, in laboratory studies, USG or osmolality is often determined from stored urine supernatants. A difference between whole urine and supernatant USG has not been reported, nor how prolonged storage time of weeks or months influences the results, to our knowledge.
Our aims were 1) to compare 2 optical refractometers, one with a single scale and one with separate scales for canine and feline urine samples, 2) to evaluate inter- and intra-observer variability, 3) to measure the agreement between whole urine and supernatant USG, 4) to evaluate the correlation between USG and osmolality, 5) to investigate the influence of protein content on the correlation between USG and osmolality, and 6) to determine the influence of up to 6 mo of storage on USG.
Materials and methods
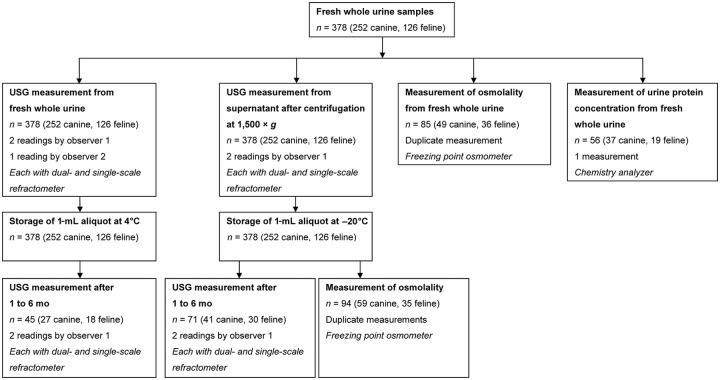
Urine specimens from dogs and cats presented to the Clinic of Small Animal Medicine at the Ludwig-Maximilians-University (LMU) in Munich, Germany because of health issues, and from healthy dogs and cats of clinic staff members, were consecutively collected from June 2015 to April 2016. Dogs and cats were included regardless of breed, age, sex, medical condition, or method of urine collection. Inclusion criteria were a minimum volume of urine of 3.0 mL and the ability to examine the samples within 1 h of collection. Analyses were performed by 2 independent blinded observers (defined as observers 1 and 2; Fig. 1). The study was approved by the ethical committee of the veterinary faculty of the LMU Munich (50-15-06-2015).
Figure 1.
Urine specimen handling scheme. Observer 1 performed 2 readings with each refractometer, the dual-scale and the single-scale refractometer. Observer 2 did one reading with each refractometer. The measurement of urine specific gravity (USG) from supernatant was only performed by observer 1 who did 2 readings with each refractometer. Urine protein concentration was measured in 56 of 85 samples in which determination of osmolality was performed. Repeated USG measurements after storage were performed by observer 1 (2 measurements with each refractometer). Osmolality after storage was measured in 94 samples. In 80 samples, osmolality of both fresh whole urine and of stored supernatant was determined. For all statistical analyses including USG, the first reading of observer 1 was used.
All statistical analyses were conducted using MedCalc statistical software v.16.4.3 (MedCalc Software, Ostend, Belgium). A p ≤ 0.05 was considered statistically significant.
Refractometer comparison
USG of fresh whole urine was determined by an optical hand-held clinical refractometer (Rhino Vet 360; Reichert Analytical Instruments, Depew, NY) with separate scales for dogs and cats, and with an optical bench-top refractometer (SPR-T2; Atago, Tokyo, Japan) with a single scale, referred to as dual-scale and single-scale refractometers, respectively. The former has a refractometer range of 1.000–1.060; the latter has a single common reading scale with a range of 1.000–1.050. Two drops (~ 0.1 mL total) of fresh whole urine were placed on the prism of each refractometer. Both devices were calibrated to 1.000 with distilled water after every 5 readings and cleaned with a soft tissue after each sample. USG was measured once with each instrument by observer 2, and twice by observer 1, using either the dog or the cat scale of the dual-scale refractometer as was species appropriate. The mean differences (bias) of USG readings between refractometers were evaluated with Bland–Altman analysis using the first readings of observer 1, against the assumption that there would be no significant difference between refractometers. Good agreement was defined relative to a refractometer’s minimum resolution of 0.005.
Evaluation of inter- and intra-observer variability
Whole urine USG was measured with each refractometer twice by observer 1 and once by observer 2. Observers 1 and 2 were blinded to each other’s readings. For evaluation of intra-observer variability, the duplicate readings of observer 1 were used. For evaluation of inter-observer variability of the refractometer results, the first reading of observer 1 and the reading of observer 2 were used. Usually, several urine samples (≤ 10 samples) were investigated at the same time by observer 1. The second measurement of observer 1 was performed on all samples directly after the first measurement within 20 min and using a different randomly selected order to ensure that observer 1 was blinded to the first reading. When only one urine sample was analyzed, observer 1 could not be blinded to the first reading. Readings were then performed by observer 2. Inter- and intra-observer variability were calculated by a coefficient of variation for repeated measurements (CV).
USG agreement between whole urine and supernatant
After USG measurement of fresh whole urine samples, urine specimens were centrifuged for 5 min at 1,500 × g. The supernatant was separated for the measurement of USG using the dual-scale and the single-scale refractometers twice by observer 1. The measurements of observer 1 were performed in the same way as described above. The agreement between results of whole urine and supernatant was evaluated with Bland–Altman analysis using the first readings of observer 1.
USG and osmolality correlation
One aliquot of the supernatant of all samples was frozen at −20°C and stored until determination of urine osmolality. Osmolality was measured in a subset of fresh whole urine samples and stored supernatant aliquots. Frozen supernatants were brought to room temperature. Urine osmolality was measured in duplicate with a freezing-point depression osmometer (Automatic semi-micro; Knauer, Berlin, Germany). Two-point calibration was done with distilled water (0 mOsm/kg) and a 400 mOsm/kg solution according to the manufacturer’s instructions. USG measurements of fresh whole urine and the frozen supernatant (first reading of observer 1 of each) were used to calculate the correlation between osmolality of fresh whole urine and USG and between osmolality of stored supernatants and USG, respectively.
The relationship between USG readings obtained with each refractometer and osmolality was measured by Pearson correlation coefficient. Duplicate osmolality measurements of the initially measured fresh whole urine samples were used to assess repeatability using CV.
Influence of protein on USG and osmolality
To evaluate the influence of protein on USG and osmolality, protein was determined in a subset of fresh whole urine samples in which measurement of USG and osmolality was performed as described above. Urine protein concentration was measured (Cobas INTEGRA 400 plus chemistry analyzer; Roche Diagnostics, Mannheim, Germany). Linear regression analysis was performed to examine the influence of protein on the relationship between USG and osmolality. For USG, the first readings of observer 1 were used for this analysis.
Influence of storage time on USG
To assess the influence of storage time on USG measurement, whole urine samples and supernatants were stored at 4°C and at −20°C, respectively. A subset of the samples was measured again after a storage time of 1–6 mo. Specimens were brought to room temperature prior to measuring. USG of the whole urine and the supernatant was measured twice by observer 1 using the 2 refractometers. The comparison between fresh and stored whole urine as well as between fresh and stored supernatant was performed with Bland–Altman analysis using the first readings of observer 1.
Results
Of the 252 canine and 126 feline specimens in our study, 232 were collected via cystocentesis, 6 were catheter-derived samples, and 140 samples were free-catch. All cystocentesis- and catheter-derived urine samples, as well as 115 free-catch samples, were obtained from patients in which a urinalysis was medically indicated for further investigation; 25 free-catch samples were collected from dogs and cats of staff members of the clinic. The canine sample consisted of 119 males (41 castrated, 78 intact) and 133 females (84 spayed, 49 intact). The cat sample included 75 males (51 castrated, 24 intact) and 51 females (44 spayed, 7 intact). Ages of dogs were 2 mo to 16 y (mean ± SD, 7.8 ± 4.4 y) and of cats were 7 mo to 20 y (9.0 ± 4.7 y). For patients presented because of clinical problems (Supplementary Table 1), USG of canine whole urine was 1.004–1.053, and for feline specimens was 1.002 to > 1.060. USG of clinically normal dogs and cats was 1.019 to > 1.060 and 1.020–1.042, respectively. Osmolality measurements of supernatant for clinical patients were 108–2,070 mOsm/kg for canine samples and 87–2,640 mOsm/kg for feline specimens. Osmolality of clinically normal dogs and cats was 689–2,020 mOsm/kg and 760–1,860 mOsm/kg, respectively.
Refractometer comparison
There was good agreement between the 2 refractometers for measurements of USG of fresh whole urine (p < 0.001 for dog and cat samples) and fresh supernatant (p = 0.002 for dog samples, p < 0.001 for cat samples). The mean difference (bias) in USG of fresh whole urine between the 2 refractometers was < 0.001 (95% limits of agreement (LOA): –0.001 to 0.002) in dog samples and 0.003 (95% LOA: 0.001–0.005) in cat samples (Fig. 2). The single-scale refractometer (mean 1.032 for fresh native urine) consistently had higher values than the dual-scale refractometer (mean 1.029 for fresh whole urine) for feline urine specimens. The bias in fresh supernatant was < 0.001 for canine (95% LOA: –0.001 to 0.002) and 0.003 for feline specimens (95% LOA: 0.001–0.005; Supplementary Table 2). The single-scale refractometer results (mean 1.023 for fresh whole urine) were higher on average than the dual-scale refractometer readings (mean 1.023 for fresh whole urine) for canine specimens.
Figure 2.
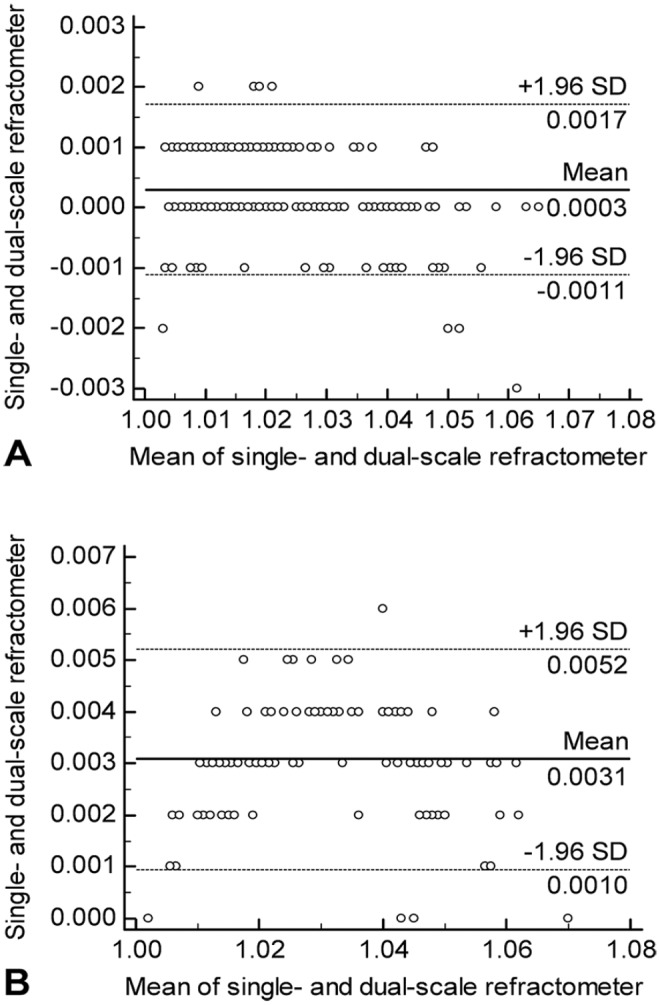
Bland–Altman plot of fresh whole canine and feline urine specific gravity (USG) measured on single-scale and dual-scale refractometers. A. Bland–Altman plot for canine urine samples. B. Bland–Altman plot for feline urine samples. The solid line represents the mean difference, and the dashed lines represent the limits of agreement (= mean difference ± 1.96 standard deviation (SD) of differences).
Inter- and intra-observer variability
The intra-observer variability of both refractometers was determined by measuring specimens of fresh whole urine and fresh supernatant in duplicate for each of the 252 canine and 126 feline specimens. The inter-observer variability for refractometer measurements showed acceptable values (CV < 0.5%) for the 2 devices for fresh whole urine. The intra-observer variability (CV) for both refractometers of both fresh whole urine and fresh supernatant was 0%.
USG agreement between whole urine and supernatant
Good agreement between USG measurements of fresh whole urine and fresh supernatant was indicated by Bland–Altman analysis on 252 dog and 126 cat samples (Table 1).
Table 1.
Agreement between canine and feline fresh whole urine and supernatant urine specific gravity (USG) measurements measured in dual-scale and single-scale refractometers.
| All samples (n = 378) | Dog samples (n = 252) | Cat samples (n = 126) | |
|---|---|---|---|
| Dual-scale | |||
| Mean difference | < –0.001 | < –0.001 | < –0.001 |
| 95% LOA | −0.002 to 0.001 | −0.002 to 0.001 | −0.001 to 0.001 |
| p value | < 0.001 | < 0.001 | < 0.001 |
| Single-scale | |||
| Mean difference | < –0.001 | < –0.001 | < –0.001 |
| 95% LOA | −0.001 to 0.001 | −0.001 to 0.001 | −0.001 to 0.001 |
| p value | < 0.001 | < 0.001 | 0.002 |
LOA = limits of agreement.
USG and osmolality correlation
There was excellent correlation between readings of both refractometers and osmolality measured in 85 fresh whole urine samples (49 canine and 36 feline samples; r = 0.98–0.99; Fig. 3) and 94 supernatants (59 canine and 35 feline samples; r = 0.98–0.99; Table 2). The instruments had excellent repeatability: CV of 0.388% for dog and cat samples, and 0.391% for dog samples and 0.378% for cat samples, respectively.
Figure 3.
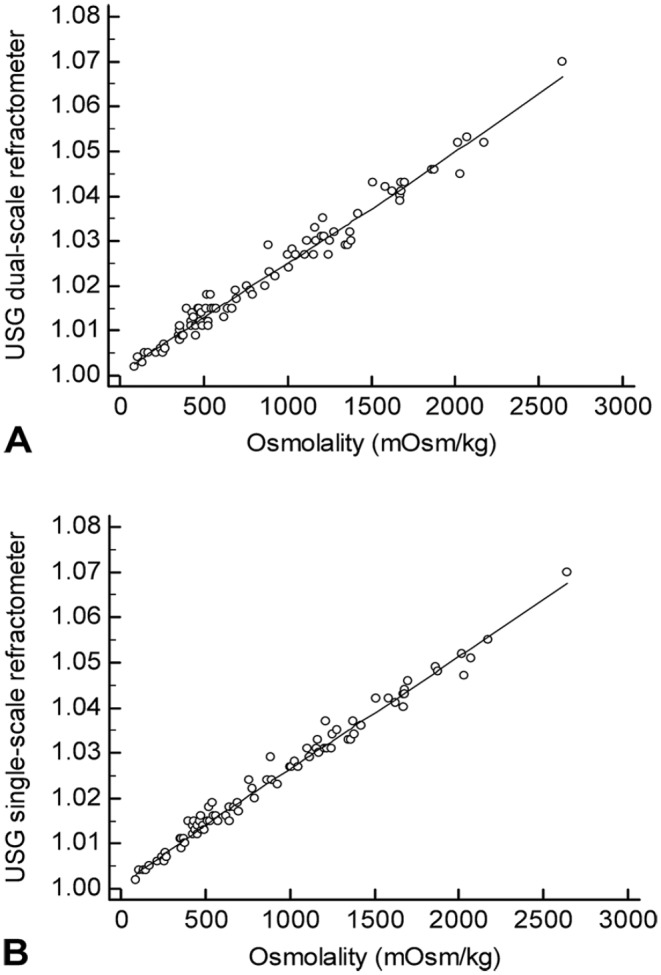
Correlation scatter plot between urine specific gravity (USG) and osmolality of fresh whole urine. A. Correlation between the dual-scale refractometer and osmolality. B. Correlation between the single-scale refractometer and osmolality.
Table 2.
Pearson correlation coefficient between canine and feline urine osmolality and urine specific gravity on fresh whole urine and urine supernatant measured on dual-scale and single-scale refractometers.
| All (n = 85) |
Dog (n = 49) |
Cat (n = 36) |
|
|---|---|---|---|
| Fresh whole urine | |||
| Dual-scale | 0.986 | 0.989 | 0.991 |
| p value | < 0.001 | < 0.001 | < 0.001 |
| Single-scale | 0.992 | 0.990 | 0.993 |
| p value | < 0.001 | < 0.001 | < 0.001 |
| All samples (n = 94) |
Dog samples (n = 59) |
Cat samples (n = 35) |
|
| Supernatant | |||
| Dual-scale | 0.984 | 0.986 | 0.990 |
| p value | < 0.001 | < 0.001 | < 0.001 |
| Single-scale | 0.990 | 0.986 | 0.992 |
| p value | < 0.001 | < 0.001 | < 0.001 |
Influence of protein USG and osmolality
The influence of urine protein on the correlation between USG and osmolality was investigated in 56 specimens (37 canine and 19 feline samples) with linear regression analysis (Fig. 4). There was no recognizable influence of urine protein up to values of 1 g/L (p = 0.087). An increase in the USG compared with osmolality for specimens containing protein concentrations > 1 g/L was significant (p = 0.007).
Figure 4.
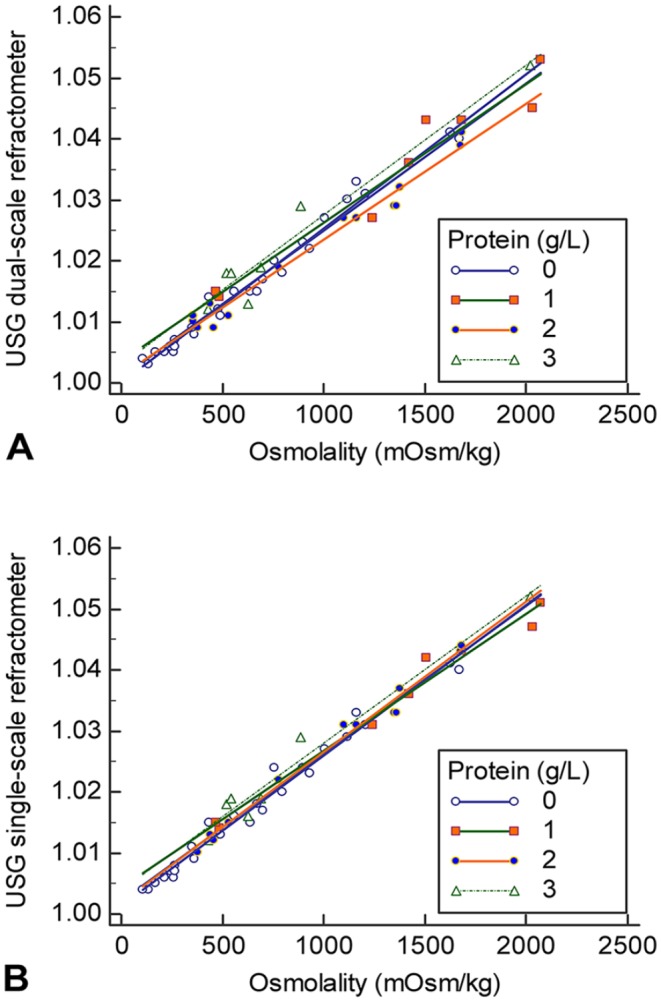
Correlation scatterplot of urine specific gravity (USG) and osmolality with various protein values. A. Scatter plot of the dual-scale refractometer and osmolality. B. Scatter plot of the single-scale refractometer and osmolality. The lines represent different protein concentrations: 0 = 0–0.14 g/L; 1 = 0.15–0.29 g/L; 2 = 0.3–1 g/L; 3 = >1 g/L.
Influence of storage time on USG
Whole urine samples (27 canine samples, 18 feline samples) and supernatants (41 canine samples, 30 feline samples) were stored for a median of 3 mo (range: 1–6 mo) and 4 mo (range: 1–6 mo), respectively. Significant differences in USG before and after storage were not observed for the readings of either refractometer (p = 0.08–1.00; Table 3).
Table 3.
Agreement between fresh and stored canine and feline urine specific gravity whole urine and urine supernatant measured on dual-scale and single-scale refractometers.
| Fresh vs. stored whole urine |
|||
|---|---|---|---|
| All samples (n = 45) |
Dog samples (n = 27) |
Cat samples (n = 18) |
|
| Dual-scale | |||
| Mean difference | < –0.001 | < –0.001 | < –0.001 |
| 95% LOA | −0.001 to 0.001 | −0.002 to 0.001 | −0.001 to 0.001 |
| p value | 0.090 | 0.294 | 0.083 |
| Single-scale | |||
| Mean difference | < –0.001 | < –0.001 | < –0.001 |
| 95% LOA | −0.001 to 0.001 | −0.001 to 0.001 | −0.001 to 0.001 |
| p value | 0.090 | 0.327 | 0.163 |
| Fresh vs. stored supernatant |
|||
| All samples (n = 71) |
Dog samples (n = 41) |
Cat samples (n = 30) |
|
| Dual-scale | |||
| Mean difference | < 0.001 | < 0.001 | < 0.001 |
| 95% LOA | −0.001 to 0.001 | −0.001 to 0.001 | −0.001 to 0.001 |
| p value | 0.621 | 1.000 | 0.424 |
| Single-scale | |||
| Mean difference | < –0.001 | < –0.001 | < –0.001 |
| 95% LOA | −0.001 to 0.001 | −0.001 to 0.001 | −0.001 to 0.001 |
| p value | 0.203 | 0.291 | 0.488 |
LOA = limits of agreement.
Discussion
The mean difference (bias) for the measurements of fresh whole urine was < 0.001 for canine specimens. Given that refractometers have a resolution of 0.005, this difference is not clinically relevant because it falls outside the limit of detection. The mean difference (bias) of 0.003 for cat urine was 10-fold larger than dogs, but there was still good agreement between the 2 refractometers. For a specific patient, interpretation of the USG using a defined cutoff for concentrating ability (USG > 1.030 for dogs, USG > 1.035 for cats)14 could lead to different clinical conclusions with the use of different refractometers. However, USG in healthy animals can vary widely. This was also shown in our study; USG of clinically normal dogs and cats ranged from 1.019 to > 1.060 and 1.020–1.042, respectively. Furthermore, USG can vary widely during a 24-h period.20 Therefore, the assessment of kidney concentrating ability should never be based on a single measurement of USG and strict cutoff values, and the mean difference of 0.003 in USG between refractometers in feline samples is unlikely to change diagnostic and therapeutic plans in the individual patient. There are only a few studies that have investigated the usefulness of a separate scale for feline urine. In one study, USG measurements of 5 refractometers, including 1 refractometer with separate scales for cats and dogs and another refractometer for measuring only feline urine, were compared to the results with 2 reference methods for measuring USG (pycnometry and measurement of total solids after drying).19 Interestingly, the 2 refractometers designed for cat urine gave consistently lower values than the reference methods and consistently revealed the lowest USG of all refractometers, leading to the conclusion that the feline refractometers gave falsely low values of USG. Two previous studies compared USG measurements of an optical and a digital refractometer for canine urine samples15 and for feline urine specimens,4 resulting in a mean difference of 0.001 and 0.003, respectively. These results are very similar to the results obtained in our study.
The excellent intra-observer variability in our study was similar to those reported in a previous study.15 However, in our study, we measured all samples in duplicate, whereas in that study, the USG of only 7 urine specimens was determined 8 times with the refractometers.
Contrary to the textbook recommendation to avoid the use of urine supernatant for the measurement of USG,11 which is followed in many clinical studies that use whole urine,18,25 supernatant is used for the USG measurement and dipstick analysis in other more recent clinical studies.13,22 Based on our results, either whole urine or supernatant can be used for measuring USG.
The excellent correlation between USG and osmolality in our study is in agreement with earlier studies that examined this correlation.3,4,8,9,15 A 2015 study revealed poor correlation between USG and osmolality in urine samples obtained from healthy human volunteers and patients with kidney diseases, with a correlation coefficient of r = 0.462 when all urine samples were considered together.17 The correlation remained poor when urine samples without proteinuria or glucosuria were analyzed (r = 0.572). Correlation was even poor in the healthy control group (r = 0.609). The reason that the relationship between USG and osmolality is less consistent than in other studies is unclear to us. The calculation of the correlation coefficient has often been used in human medical studies and is still often used in veterinary medical studies as an indicator of agreement between 2 methods of measurement,5 but it only reflects the association between 2 methods of measurements.2 The best statistical approach for calculating the agreement between 2 methods is Bland–Altman analysis. The comparison between USG and osmolality using Bland–Altman difference plots, however, is not possible because of different units of measurement.
When examining the relationship between osmolality and USG, most studies excluded specimens with protein content because of the widespread opinion that good correlation between osmolality and USG only exists in samples without proteinuria.8,12,26 Our results are in agreement with a 1982 study that examined the impact of proteinuria on USG measured by refractometry.6 In that study, the USG also did not exceed the osmolality until the protein content reached 1 g/L. However, that study examined the effect of protein by adding albumin to pooled human urine that contained no protein whereas our study investigated the correlation between USG and osmolality in naturally proteinuric dogs and cats. In our study, 14 of 56 samples (25%) had an elevated urine protein concentration of 0.3–1 g/L. A protein content of 1 g/L already represents a high protein content, which is rarely seen in patients. This is confirmed by our study in which only 7 of 56 samples (12%) had a protein content > 1 g/L. Therefore, in our study population, mild-to-moderate proteinuria was more common than severe proteinuria. The results of our study are clinically helpful in patients with a urine protein content of 0.3–1.0 g/L given that we demonstrated that USG is not affected by this small amount of protein.
The influence of shorter storage times (up to 24 h) has been investigated previously, and no significant changes were found in USG.1,21 However, the impact of long-term storage has not been validated. In our study, the good agreement between measurements of fresh and stored urine and supernatant demonstrates that urine can be also stored for further evaluation for longer periods of time. A limitation of our study is that the period of storage time was not exactly defined but varied from 1 to 6 mo; it can, however, be presumed that a more precisely defined storage time would not have changed the results.
Supplemental Material
Supplemental material, Supplemental_material for Influence of preanalytic and analytic variables in canine and feline urine specific gravity measurement by refractometer by Martina Mösch, Sven Reese, Karin Weber, Katrin Hartmann and Roswitha Dorsch in Journal of Veterinary Diagnostic Investigation
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Martina Mösch  https://orcid.org/0000-0002-8558-6252
https://orcid.org/0000-0002-8558-6252
Roswitha Dorsch  https://orcid.org/0000-0002-7508-5411
https://orcid.org/0000-0002-7508-5411
Supplementary material: Supplementary material for this article is available online.
References
- 1. Albasan H, et al. Effects of storage time and temperature on pH, specific gravity, and crystal formation in urine samples from dogs and cats. J Am Vet Med Assoc 2003;222:176–179. [DOI] [PubMed] [Google Scholar]
- 2. Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. Statistician 1983;32:307–317. [Google Scholar]
- 3. Ayoub JA, et al. Association between urine osmolality and specific gravity in dogs and the effect of commonly measured urine solutes on that association. Am J Vet Res 2013;74:1542–1545. [DOI] [PubMed] [Google Scholar]
- 4. Bennett AD, et al. Comparison of digital and optical hand-held refractometers for the measurement of feline urine specific gravity. J Feline Med Surg 2011;13:152–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 6. Burkhardt AE, et al. A reagent strip for measuring the specific gravity of urine. Clin Chem 1982;28:2068–2072. [PubMed] [Google Scholar]
- 7. Chadha VU, et al. Measurement of urinary concentration: a critical appraisal of methodologies. Pediatr Nephrol 2001;16:374–382. [DOI] [PubMed] [Google Scholar]
- 8. Dorizzi R, et al. Refractometry, test strip, and osmometry compared as measures of relative density of urine. Clin Chem 1987;33:190. [PubMed] [Google Scholar]
- 9. Dossin O, et al. Comparison of the techniques of evaluation of urine dilution/concentration in the dog. J Vet Med A Physiol Pathol Clin Med 2003;50:322–325. [DOI] [PubMed] [Google Scholar]
- 10. Gounden D, Newall RG. Urine specific gravity measurements: comparison of a new reagent strip method with existing methodologies, as applied to the water concentration/dilution tests. Curr Med Res Opin 1983;8:375–381. [DOI] [PubMed] [Google Scholar]
- 11. Kindrachuck RW, Stamey TA. Urinalysis. In: Campbell’s Urology. 5th ed. Philadelphia, PA: Saunders, 1986:285. [Google Scholar]
- 12. Leech S, Penney MD. Correlation of specific gravity and osmolality of urine in neonates and adults. Arch Dis Child 1987;62:671–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meindl AG, et al. Relationships among urinary protein-to-creatinine ratio, urine specific gravity, and bacteriuria in canine urine samples. J Vet Intern Med 2019;33:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osborne CA, et al. A clinician’s analysis of urinalysis. In: Osborne CA, Finco DF, eds. Canine and Feline Nephrology and Urology. 1st ed Baltimore, MD: Williams and Wilkins, 1995:136–205. [Google Scholar]
- 15. Paris JK, et al. Comparison of a digital and an optical analogue hand-held refractometer for the measurement of canine urine specific gravity. Vet Rec 2012;170:463. [DOI] [PubMed] [Google Scholar]
- 16. Rubini ME, Wolf AV. Refractometric determination of total solids and water of serum and urine. J Biol Chem 1957;225:869–876. [PubMed] [Google Scholar]
- 17. Souza AC, et al. Is urinary density an adequate predictor of urinary osmolality? BMC Nephrol 2015;16:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Treutlein G, et al. Follow-up protein profiles in urine samples during the course of obstructive feline idiopathic cystitis. Vet J 2013;198:625–630. [DOI] [PubMed] [Google Scholar]
- 19. Tvedten HW, et al. Comparison of specific gravity analysis of feline and canine urine, using five refractometers, to pycnometric analysis and total solids by drying. N Z Vet 2015;63:254–259. [DOI] [PubMed] [Google Scholar]
- 20. van Vonderen IK, et al. Intra- and interindividual variation in urine osmolality and urine specific gravity in healthy pet dogs of various ages. J Vet Intern Med 1997;11:30–35. [DOI] [PubMed] [Google Scholar]
- 21. Veljkovic K, et al. Assessment of a four hour delay for urine samples stored without preservatives at room temperature for urinalysis. Clin Biochem 2012;45:856–858. [DOI] [PubMed] [Google Scholar]
- 22. Vientos-Plotts AI, et al. Effect of blood contamination on results of dipstick evaluation and urine protein-to-urine creatinine ratio for urine samples from dogs and cats. Am J Vet Res 2018;79:525–531. [DOI] [PubMed] [Google Scholar]
- 23. Voinescu GC, et al. The relationship between urine osmolality and specific gravity. Am J Med Sci 2002;323:39–42. [DOI] [PubMed] [Google Scholar]
- 24. Watson AD. Urine specific gravity in practice. Aust Vet J 1998;76:392–398. [DOI] [PubMed] [Google Scholar]
- 25. White JD, et al. Subclinical bacteriuria in older cats and its association with survival. J Vet Intern Med 2016;30:1824–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolf AV, Pillay VK. Renal concentration tests; osmotic pressure, specific gravity, refraction and electrical conductivity compared. Am J Med 1969;46:837–843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_material for Influence of preanalytic and analytic variables in canine and feline urine specific gravity measurement by refractometer by Martina Mösch, Sven Reese, Karin Weber, Katrin Hartmann and Roswitha Dorsch in Journal of Veterinary Diagnostic Investigation