Abstract
Triple-negative tumors are characterized immunohistochemically by the absence of positivity to sex hormone receptors and to human epidermal growth factor receptor 2. Additionally, they are differentiated into basal-like and non-basal (or null) subtypes, based on the presence of basal cytokeratin expression (CK5/6, 14, and17). Triple-negative subtypes are yet to be characterized in male dogs, to our knowledge. We report herein the clinical and pathologic findings and molecular characterization of carcinoma in the mammary glands of 2 male dogs. Case 1 was diagnosed as a grade II tubulopapillary carcinoma; case 2 was diagnosed as a grade II carcinoma in a mixed tumor. The tumors were characterized phenotypically as triple-negative basal and triple-negative non-basal, respectively.
Keywords: cancer, immunohistochemistry, male dogs, mammary carcinoma, molecular phenotype, prognosis
Mammary gland neoplasms are rare in male dogs; males are 62 times less likely to develop mammary gland neoplasms than female dogs. However, despite the low incidence in males, neoplasm aggressiveness may be high.10,19
Triple-negative basal neoplasms that do not express estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2), but express cytokeratins (CKs; CK5/6, CK14, and CK17) or human epidermal growth factor receptor (HER1 or EGFR), have molecular profiles similar to those of myoepithelial or basal cells in the normal mammary gland.1 The epithelium throughout the ductal-lobular system is composed of a dual-cell population of luminal epithelial and basal myoepithelial cells juxtaposed to a continuous basement membrane. Luminal epithelial cells are characterized by the expression of type I acidic keratins (CK18 and CK19), and type II basic keratins (CK7 and CK8).1 Therefore, basal-like neoplasms have been suggested to arise from basal or myoepithelial cells.3
Molecular characterization of mammary neoplasms across canine breeds has shown that most triple-negative carcinomas have the basal-like phenotype.1 On the other hand, in female dogs diagnosed with mammary carcinomas within benign mixed tumors, there is a predominance of the luminal A subtype (41.4% positive for ERα and/or PR, HER2 negative, and Ki-67 < 14%) followed by triple-negative basal-like (27.6%) neoplasms.18 Other studies, however, have demonstrated a higher frequency of luminal profile B (48% positive for ERα and/or PR, HER2 negative, and Ki-67 ≥ 14%), followed by basal-like profile (28%).20 The molecular-based classification system adopted for breast cancer is a valuable tool for assessing prognosis and investigating similarities between canine and human tumor types. Our objective was to investigate the molecular profile of mammary gland carcinomas in 2 male dogs.
Case 1 was a 5-y-old male Rottweiler dog presented to the Hospital of Veterinary Medicine of the Federal University of Bahia (Brazil) with a history of mammary nodules for 2 mo. On physical examination, a > 5.0 cm diameter, non-adherent, firm, ulcerated nodule was identified in the right caudal abdominal mammary gland (M4). Enlarged right inguinal lymph nodes and nodules in the right testis were also palpated. Physical examination findings were otherwise normal, and no abnormalities were detected on complete blood count and serum biochemistry. Cytologic analysis of the mammary nodule and inguinal lymph nodes revealed aggregates of neoplastic epithelial cells with marked pleomorphism. The cells had an increased nuclear-to-cytoplasmic ratio, basophilic clear cytoplasm with fine amphophilic granules, large nuclei with a coarse chromatin pattern, and prominent nucleoli. Cytologic characteristics supported the diagnosis of carcinoma with lymph node metastasis. No evidence of distant metastasis was identified from radiographic and ultrasonographic images. Based on the size of the neoplasm (T), involvement of the regional lymph node (N), and absence of distant metastasis (M), the clinical condition was assigned TNM stage IV.16
The dog underwent unilateral radical mastectomy with removal of the inguinal lymph nodes and orchiectomy. Macroscopically, a 7.0 × 6.5 × 4.0 cm irregular, brown, firm, ulcerated nodule was observed in the right M4 mammary gland. The right inguinal lymph nodes were irregular, brown, firm, and enlarged (3.0 × 2.1 × 2.0 cm and 2.2 × 1.9 × 1.0 cm). The right testis measured 5.0 × 4.0 × 3.9 cm and had an irregular surface with raised, solid, and firm white areas.
The neoplastic mass, lymph node, and testis were fixed in 10% formalin and were processed routinely; 4-μm sections were stained with hematoxylin and eosin. Histologic classification was performed as recommended by the World Health Organization (WHO) scheme,13 and the histologic grade was defined by evaluating the percentage of tubular formation, nuclear pleomorphism, and mitotic count.6,11 For evaluation of mitotic activity, 10 neoplasm fields were evaluated, without necrosis or artifacts, using a BX40 Olympus microscope, 40× objective, 10× ocular field number, 22 mm field view of diameter, and 0.55 mm in the sample level fields. Each high-power field (HPF) corresponds to an area of 0.237 mm2. All typical and atypical mitoses were counted in 10 fields (i.e., 2.37 mm2 of area).
Histologically, the mammary nodule consisted of a neoplastic proliferation of epithelial cells with infiltration of the epidermis, dermis, and musculature. Neoplastic cells were arranged as irregular dilated ducts containing papillae and amorphous secretions. The cytoplasm was variably eosinophilic, with ill-defined cytoplasmic borders, and exhibited apical blebbing. Cells had medium-to-large nuclei with vesicular chromatin and single or multiple nucleoli (Fig. 1A). Binucleate cells with bizarre nuclei were present. Neoplastic emboli within dermal lymphatic vessels were also observed. The total mitotic count was 41 mitoses, with the mean count of 4 mitoses per HPF. Anisocytosis and anisokaryosis were moderate. Collagenous stroma supporting the neoplasm was abundant and richly vascularized with marked diffuse granulomatous inflammation. Based on the histologic characteristics, the mammary tumor was diagnosed as grade II tubulopapillary mammary carcinoma.
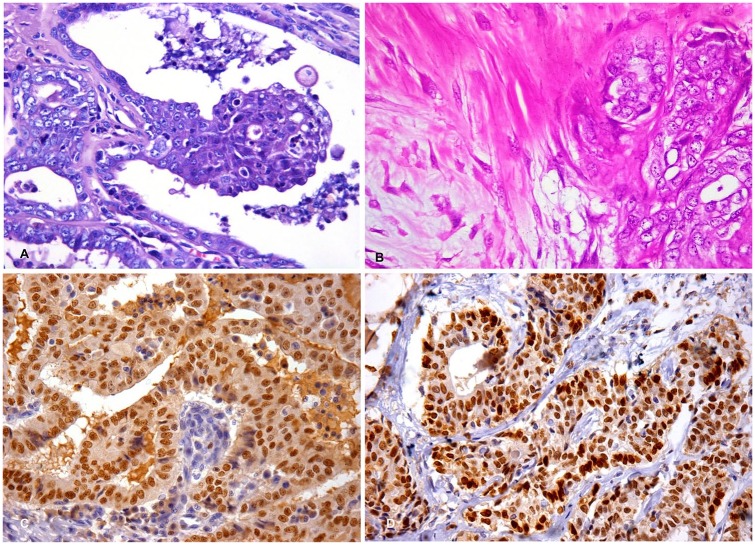
Figure 1.
Triple-negative carcinoma in the mammary gland of 2 male dogs. A, C. 5-y-old male dog (case 1). B, D. 15-y-old male dog (case 2). A. Neoplastic cells with poorly delineated eosinophilic cytoplasm, medium-to-large nuclei with vesicular chromatin, and 1 or 2 nucleoli. H&E. 40×. B. Carcinoma in mixed tumor, grade II; foci of proliferation of myoepithelial cells associated with nests of malignant epithelial cells. H&E. 20×. C. Strong and diffuse nuclear expression of GATA3. Immunohistochemistry, hematoxylin counterstain. 40×. D. Strong and diffuse nuclear expression of GATA3. Immunohistochemistry, hematoxylin counterstain. 40×.
In the inguinal lymph nodes, neoplastic cells were arranged into irregularly dilated tubules containing amorphous secretions, replacing one-third of the parenchyma, consistent with metastatic carcinoma. In the testicular parenchyma, there was neoplastic proliferation of polygonal-to-fusiform cells without definite arrangement, with some areas arranged in bundles perpendicular to the basal membrane (palisades); the stroma was moderate and formed pseudolobules mimicking normal testicular architecture; Sertoli cell tumor was diagnosed.
The mammary neoplasm was subjected to immunohistochemical evaluation for antibodies against GATA3 and was considered positive when ≥ 10% of the cells had nuclear labeling.15 The panel for molecular phenotyping was composed of the antibodies to ERα, PR, HER2, and CK5/6 (Table 1). Samples are considered positive for ERα or PR if 1% of neoplastic cell nuclei are immunoreactive.9 Assessment of HER2 labeling was based on the Hercep test (Dako, Glostrup, Denmark), and is considered positive when 30% of the cells express complete membrane labeling.17 The anti-CK5/6 antibody was used as the basal marker, and its expression is considered positive when 5% or more of the epithelial cells express cytoplasmic or membrane labeling.1 Absence of expression of hormone receptors and HER2, and positivity for CK5/6, phenotypically characterized the neoplasm as a triple-negative basal-like carcinoma.1,7 The negative controls were represented by the reaction without the primary antibody. Adjacent normal human breast tissue was used as a positive control for GATA3, and normal canine mammary tissue was used as a control for ERα, PR, and HER2. Molecular profiles of the samples analyzed were defined as follows: luminal A (positive for ER and/or PR, and negative for HER2); luminal B (positive for ER and/or PR, and positive for HER2); HER2-like (negative for ER and PR, and positive for HER2); triple-negative basal-like carcinoma (negative for ER and/or PR, negative for HER2, and positive for CK5/6).1
Table 1.
Antibody sources, manufacturers, dilutions, clones, types, incubation times, and antigen retrieval methods.
| Antibody | Antibody source | Dilution | Clone | Type | Incubation time | Antigen retrieval |
|---|---|---|---|---|---|---|
| GATA3 | Cell Marque | 1:250 | L50-823 | Monoclonal mouse | 30 min | HIER, trilogy |
| ERα | Leica | Ready to use | 6 F11 | Monoclonal mouse | 60 min | HIER, citrate pH 6 |
| PR | Leica | Ready to use | 16 | Monoclonal mouse | 30 min | HIER, citrate pH 6 |
| HER2/neu | Ventana Medical System | 1:15 | 4B5 | Monoclonal rabbit | 30 min | HIER, TR6 |
| CK5/6 | Dako | 1:100 | D5/16 B4 | Monoclonal mouse | 45 min | HIER, EnVision FLEX target retrieval solution, high pH |
HIER = heat-induced epitope retrieval. Previously tested mammary gland samples were used as positive controls; negative controls were obtained by replacement of the primary antibody by IgG. Detection system used was NovoLink detection system (Leica Biosystems, Buffalo Grove, IL).
In case 1, 90% of the neoplastic cells were positive for GATA3 (Fig. 1C), negative for ERα, PR, and HER2, and positive for CK5/6. Given lymph node metastasis, chemotherapy was indicated; however, this procedure was not authorized by the owner. Six months after the surgical procedure, the dog died following a period of respiratory difficulty and cachexia; however, the owner did not authorize an autopsy.
Case 2 was a 15-y-old male German Shepherd dog with a history of positive serology for Leishmania chagasi, progressive weight loss, difficulty in locomotion because of degenerative joint disease, and a nodule in the left cranial abdominal mammary gland (M3). Given an unfavorable prognosis, the animal was euthanized and referred to the Pathology Sector of the School of Veterinary Medicine, Federal University of Minas Gerais (Brazil) for autopsy. At autopsy, the dog was very thin with severe osteoarthritis in the coxofemoral joints. In the left M3 mammary gland, there was a 5.1 × 4.0 × 3.8-cm nodule that was white on section, irregular, and soft, with solid areas and cystic areas containing brown fluid. The regional lymph nodes were grossly normal. The left testis was enlarged and contained a 5.0 × 3.0 × 4.0-cm white friable mass with areas of hemorrhage. The right testis contained a white, solid, firm nodule of 2.5 cm diameter.
Samples of the mammary nodule, regional lymph nodes, and testes were fixed in 10% formalin and examined histologically. The mammary neoplasm was also evaluated immunohistochemically, as in case 1 (Table 1).
Histologically, the mammary nodule consisted of neoplastic proliferation of epithelial cells arranged in nests and papillae, with infiltrative growth, and multiple foci of myoepithelial cell proliferation with myxoid matrix production (Fig. 1B). The cells had a heterogeneous appearance, characterized by moderate amounts of clear eosinophilic cytoplasm, occasional apical blebbing, and nuclei with dispersed chromatin and several nucleoli (Fig. 1B). The mitotic count in case 2 was obtained following the same methodology used in case 1, with a total count of 39 mitoses, with ~4 mitoses per HPF. The diagnosis was grade II carcinoma in a mixed tumor, which is a tumor type with a significant proliferation of malignant epithelial cells and benign myoepithelial cells that produce myxoid matrix associated with small areas of immature chondroid areas.18 No microscopic evidence of lymph node metastasis was identified. TNM clinical stage III was assigned.
The left testis had neoplastic proliferation of interstitial cells with expansile and compressive growth; Leydig cell neoplasm was diagnosed. The right testis contained an expansile and compressive growth of neoplastic germinative cells arranged diffusely and within tubules; diffuse intratubular seminoma was diagnosed.
Immunohistochemical evaluation of the mammary neoplasm was similar to that for case 1 (Table 1), with the mammary neoplastic cells staining positively for GATA3 (80% of neoplastic cells labeled; Fig. 1D) and negatively for ERα, PR, and HER2. Absence of CK5/6 expression characterized the neoplasm phenotypically as a triple-negative non-basal type.
GATA3 is an important transcription factor in the differentiation of breast epithelium, and the antibody is considered sensitive for the identification of primary and metastatic neoplasms of mammary glands in rats and humans.15 However, GATA3 has not been reported as an immunohistochemical marker in mammary neoplasms of dogs. Our study has demonstrated the antibody reactivity in dogs. In both cases, histologic features of the neoplasms and immunoreactivity for GATA3 suggested mammary origin of the neoplasms.
Absence of expression of immunohistochemical markers ER, PR, and HER2 characterized the 2 mammary tumors as triple-negative. The neoplasms were further subclassified into triple-negative basal-like phenotype in case 1 and triple-negative non-basal phenotype in case 2, based on the presence or absence of staining for CK5/6,1 respectively. Triple-negative mammary neoplasms of men often show aggressive behavior, exhibit lymph node metastasis with histologic grades II or III, and have limited therapeutic options.2 We used the histologic grade defined for human carcinomas,6 given that it has good reproducibility and similar models are already developed for animal studies.13,18
Tumors classified as luminal A subtype have positive ER and/or PR, and are negative for HER2 amplification and/or overexpression. This subtype should still have a Ki-67 index, evaluated by immunohistochemistry, of < 14%.8 Tumors of the luminal B subtype, for the most part, exhibit positive hormone receptors.1,7 This divergence in results may be related to the heterogeneity of carcinoma in mixed tumors, which may have different patterns of epithelial growth and, consequently, different molecular profiles. In our study, both carcinoma in a mixed tumor and tubulopapillary mammary carcinoma were characterized as triple-negative, although differing in basal and non-basal phenotypes.
Factors related to hormonal changes, especially in testicular neoplasms, are suggested as predisposing factors in the development of mammary neoplasms in males.4 Our 2 cases had testicular neoplasms including seminoma, Leydig cell tumor, and Sertoli cell tumor, the latter of which can be hormonally active resulting in hyperestrogenism.12 Unfortunately, measurement of serum hormone levels and the evaluation of hormone receptor expression in the testicular tumors were not performed in our study. In addition, given the scarcity of cases of mammary tumors in male dogs,19 the correlation between hormone receptor expression and prognosis is not as well established as in bitches.14,17
The dog in case 1 died 6 mo after surgery, and the dog in case 2 was euthanized at the time of diagnosis. The prognosis in cases of triple-negative neoplasms is guarded in men, with a global survival of 6 mo to 3 y, lower than that observed in women with these neoplasms, possibly because of late diagnosis in men, who may delay seeking medical assistance.5
Acknowledgments
We thank the Postgraduate Program in Animal Science in the Tropics (PPGCAT).
Footnotes
Authors’ note: The data that support the findings of this study are available from the corresponding author upon request.
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: A. Estrela-Lima is grateful for the National Council for Scientific and Technological Development (CNPq) research fellowship (PQ). C. H. Cruz was granted a PNPD scholarship from the Coordination of Superior Level Staff Improvement (CAPES).
ORCID iD: Alessandra Estrela-Lima  https://orcid.org/0000-0002-9879-7999
https://orcid.org/0000-0002-9879-7999
References
- 1. Abadie J, et al. Canine invasive mammary carcinomas as models of human breast cancer. Part 2: immunophenotypes and prognostic significance. Breast Cancer Res Treat 2018;167:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdelrahman AE, et al. Prognostic impact of EGFR and cytokeratin 5/6 immunohistochemical expression in triple-negative breast cancer. Ann Diagn Pathol 2017;28:43–53. [DOI] [PubMed] [Google Scholar]
- 3. Badve S, et al. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol 2011;24:157–167. [DOI] [PubMed] [Google Scholar]
- 4. Bearss JJ, et al. Histologic, immunohistochemical, and clinical features of 27 mammary tumors in 18 male dogs. Vet Pathol 2012;49:602–607. [DOI] [PubMed] [Google Scholar]
- 5. Chavez-Macgregor M, et al. Male breast cancer according to tumor subtype and race: a population-based study. Cancer 2013;119:1611–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403–410. [DOI] [PubMed] [Google Scholar]
- 7. Gama A, et al. Identification of molecular phenotypes in canine mammary carcinomas with clinical implications: application of the human classification. Virchows Arch 2008;453:123–132. [DOI] [PubMed] [Google Scholar]
- 8. Goldhirsch A, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast Cancer 2013. Ann Oncol 2013;24:2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hammond ME, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract 2010;6:195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim HW, et al. Breed- and age-related differences in canine mammary tumors. Can J Vet Res 2016;80:146–155. [PMC free article] [PubMed] [Google Scholar]
- 11. Meuten DJ, et al. Mitotic count and the field of view area: time to standardize. Vet Pathol 2016;53:7–9. [DOI] [PubMed] [Google Scholar]
- 12. Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, IA: Blackwell, 2016:689–765. [Google Scholar]
- 13. Misdorp W, et al. Histological classification of mammary tumors of the dog and cat. 2nd ed. Washington, DC: Armed Forces Institute of Pathology, 1999:1–59. [Google Scholar]
- 14. Nguyen F, et al. Canine invasive mammary carcinomas as models of human breast cancer. Part 1: natural history and prognostic factors. Breast Cancer Res Treat 2018;167:635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ni Y-B, et al. GATA-3 is superior to GCDFP-15 and mammaglobin to identify primary and metastatic breast cancer. Breast Cancer Res Treat 2018;169:25–32. [DOI] [PubMed] [Google Scholar]
- 16. Owen LN. TNM Classification of Tumours in Domestic Animal. Geneva: Veterinary Public Health Unit & WHO Collaborating Center for Comparative Oncology, 1980:1–53. [Google Scholar]
- 17. Peña L, et al. Canine mammary tumors: a review and consensus of standard guidelines on epithelial and myoepithelial phenotype markers, HER2, and hormone receptor assessment using immunohistochemistry. Vet Pathol 2014;51:127–145. [DOI] [PubMed] [Google Scholar]
- 18. Ribeiro GM, et al. Morphological aspects and immunophenotypic profiles of mammary carcinomas in benign-mixed tumors of female dogs. Vet Med Int 2012;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saba CF, et al. Mammary gland tumors in male dogs. J Vet Intern Med 2007;21:1056–1059. [DOI] [PubMed] [Google Scholar]
- 20. Sassi F, et al. Molecular-based tumour subtypes of canine mammary carcinomas assessed by immunohistochemistry. BMC Vet Res 2010;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]