Abstract
We describe herein the clinical, endoscopic, computed tomography (CT), pathologic, and microbiologic features of an infection caused by an under-recognized fungal pathogen, Flavodon flavus, in a 25-y-old Australian Quarter Horse. The horse had a unilateral obstructive nasal mass, resulting in stertor and dyspnea. On endoscopy, the mass was tan, multinodular, and completely obstructed the nasal passage. CT analysis revealed a large, soft tissue–attenuating and partially mineralized mass in the right nasal passage and dorsal-conchofrontal sinus, expanding into adjacent paranasal sinuses with associated bone lysis and rhinosinusitis. Histopathology of the mass on 2 occasions revealed suppurative inflammation initially, and pyogranulomatous inflammation subsequently. The inflammatory reaction surrounded numerous spherical fungal structures (~60–80 µm diameter) that stained positively on periodic acid–Schiff and Grocott methenamine silver stains. PCR for the fungal internal transcribed spacer 1 and 2 regions followed by Sanger sequencing on a cultured isolate identified the agent as F. flavus, which has only been reported previously as pathogenic in one horse in the United States, to our knowledge. Previous reports described this fungus as a nonpathogenic, environmental commensal fungus associated with insects and plants.
Keywords: equine, fungal granuloma, fungal rhinitis, nasal granuloma, sinusitis
Mycosis of the equine respiratory tract is rare, and only a small number of fungal species have been implicated as causative agents.18 The recognized upper respiratory tract fungal infections in the horse include aspergillosis caused by Aspergillus spp. associated with guttural pouch mycosis and nasal granuloma formation4; nasal phycomycosis caused by Conidiobolus spp.,1 Basidiobolus spp.,5 and Pythium spp.17; nasal coccidioidomycosis caused by Coccidioides immitis7; sinonasal cryptococcosis caused by Cryptococcus neoformans3,18; and sinonasal maduromycosis caused by the Pseudallescheria boydii/Scedosporium apiospermum complex.2 The resultant infection often leads to the development of granulomas and/or pyogranulomas that partially or completely obstruct the upper respiratory tract.2-5 A 2018 North American report described a series of equine mycotic rhinitis cases caused by a variety of fungal agents previously not known to be pathogenic in animals, and one of these included the environmental fungus, Flavodon flavus.14 The pathogenic potential of F. flavus does not appear to have been reported prior to that study. In the present brief report, we present a case of obstructive rhinosinusitis in an Australian mare caused by F. flavus (NCBI: txid559739). We describe the clinical, radiographic, pathologic, in vitro growth, and molecular features of this fungus. With our case, we propose that F. flavus may be an under-recognized and emerging equine pathogen.
A 25-y-old Quarter Horse mare from coastal northern New South Wales, Australia, was presented to the Equine Specialist Hospital, The University of Queensland, in February 2017 (mid-summer), following a year described as generally wet and warm (Australian Bureau of Meteorology archive, http://www.bom.gov.au/climate/current/annual/nsw/archive/2016.summary.shtml). The mare had an 8-wk history of increasingly thick mucopurulent discharge from the right nostril. The owner reported discomfort in the horse during breathing, and a right facial contour deformity. A first-opinion veterinarian treated presumptively for a nasal granuloma with potassium iodide. The owner administered herbal remedies, which consisted of in-feed treatment with a mixture of garlic, turmeric, yellow sulfur, pepper, honey, colloidal silver, maritime pine, and potassium iodide, and topical treatment over the dorsal aspect of the sinuses and nose with frankincense, myrrh, and cannabis oil. The horse had no history of overseas travel.
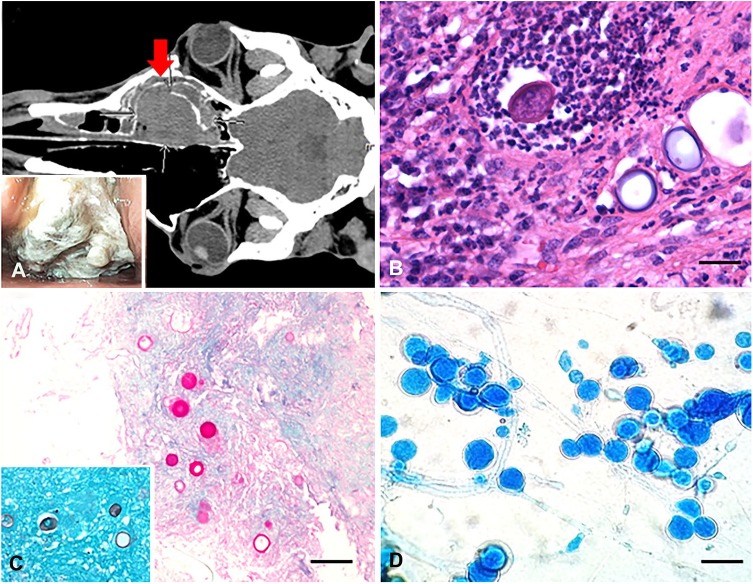
Initial physical examination identified abundant mucopurulent exudate discharging from the right nostril, with subtle facial deformity over the right nasal bone and absent airflow from the right nostril. Dyspnea and stertor were also evident. Routine blood analysis including a complete blood cell count and serum chemistry showed only a moderate increase in the acute-phase protein serum amyloid A (382 mg/L, reference interval [RI]: 0–20 mg/L). On non-contrast computed tomography of the head, the right nasal passage and dorsal-conchofrontal sinus was filled with a large (10 × 9 × 6 cm), soft tissue–attenuating (average Hounsfield units (HU), 37 HU; RI: 14–66 HU), partially mineralized mass. The mass extended into the surrounding right-sided paranasal sinuses with bone erosion, mild leftward displacement of the adjacent septum, effacement of the ethmoturbinates, and disruption of the infraorbital canal (Fig. 1A). Dependent fluid density was present in the right maxillary, frontal, and sphenopalatine sinuses, with some fluid and mucosal thickening noted in the right dorsal meatus; these findings were most consistent with rhinosinusitis. On endoscopy, a large tan multinodular mass was identified at ~10 cm from the right nostril opening, occluding the entire lumen of the right nasal passage (Fig. 1A inset).
Figure 1.
An obstructive nasal pyogranuloma caused by Flavodon flavus infection in a horse. A. Dorsal plane computed tomography image of the head (soft tissue algorithm) demonstrates the large, soft tissue–attenuating, partially mineralized mass (arrow) occupying the width of the right nasal passage and dorsal-conchofrontal sinus. Inset: nasal passage endoscopy shows a large obstructive irregular mass covered with necropurulent exudate. B. Multiple spherical fungal structures admixed with many degenerate neutrophils and other inflammatory cells. H&E. Bar = 80 µm. C. The wall of the round fungal structures is periodic acid–Schiff stain positive and Grocott methenamine silver stain positive (inset). Bar = 200 µm. D. Lactophenol cotton blue stain of the fungal colonies identified numerous conidia in clusters, associated with parallel-walled and branching hyphae. Bar = 150 µm.
Fresh samples from the right nasal mass were obtained on 2 separate occasions. An endoscopic incisional biopsy was obtained on admission. Subsequently, the mass was excised through a frontonasal sinusotomy. The mass was bluntly dissected free of its attachments and removed with sponge forceps. Hemorrhage was controlled by packing the sinus with gauze. An attempt was made to remove all visibly abnormal tissue. However, hemorrhage obscured the field and made inspection of the surgical margins difficult after the mass was removed. Therefore, as clean surgical margins could not be ascertained, potassium iodide therapy was continued for an additional 6 wk: 2 wk in hospital at a dose of 40 mg/kg/d; 4 wk following discharge at a dose of 20 mg/kg/d.
Both fresh samples were divided in half, one of which was submitted for bacterial and fungal culture, and the other for histopathology. The fresh sample from the latter collection was also submitted for fungal PCR, targeting the pan-fungal internal transcribed spacer (ITS) 1 and 2 regions, followed by conventional Sanger sequencing for fungal identification. On multiple follow-up calls until 24 mo after discharge, the owner reported complete resolution of the problem; the mare was retired, in good health, and with no indication of recurrence of the sinonasal granuloma. We consider the resolution of the infection was mainly attributable to surgical resection of the granuloma, with potassium iodide administered as an ancillary therapy for treatment of any remaining unresected infected tissue.
On histopathology, both the initial endoscopic and debulked samples contained numerous circular-to-ovoid structures of ~ 60–80 µm diameter, with a refractile, thin, basophilic wall, and morphology consistent with either spore-like structures such as chlamydospores of basidio- and ascomycetes or spherules consistent with Coccidioides spp. (Fig. 1B). Notably, hyphae were not observed in the sections examined. The wall of these spherical structures was periodic acid–Schiff and Grocott methenamine silver stains positive, supporting a fungal origin for the spherical structures (Fig. 1C and inset). Most of these round bodies contained an empty center, but occasionally they contained irregular eosinophilic contents or infiltrating degenerate neutrophils; the latter likely represented leukocytic attempts to phagocytize or destroy these structures.
The inflammatory reaction surrounding these spherical structures consisted of an admixture of degenerate neutrophils, macrophages, and lesser numbers of lymphocytes and plasma cells in no particular arrangement in the initial biopsy. The subsequent debulked sample contained multiple pyogranulomas centered on the spherical structures. Multinucleate giant cells were frequent and often contained phagocytized fungal bodies. Within the inflammatory mass were multiple necrotic blood vessels, expanded by fibrin thrombi, consistent with mycotic vascular injury. There were also multifocal areas of necrosis, likely a result of infarction. Superficially, colonies of bacterial cocci of ~1 µm diameter were identified. Bacterial culture isolated a mixed but predominant growth of Streptococcus equi ssp. zooepidemicus, consistent with secondary superficial bacterial colonization by the most common commensal organism identified in the upper respiratory tract of horses.19
Fungal culture from the second biopsy sample performed on routine Sabouraud dextrose agar grew an initially glabrous (day 4 post-inoculation) to subsequently wrinkled (day 10 post-inoculation) white fungal colony, with light aerial mycelia, and no color change on the reverse side of the agar. Lactophenol cotton blue staining of the colonies identified numerous conidia in clusters, often associated with parallel-walled and branching hyphae (Fig. 1D). The absence of hyphal elements in the in vivo sample may represent a different growth pattern compared to that under in vitro conditions.
PCR was performed on both the fresh sample and the in vitro grown fungal colony, targeting the ITS1 and ITS2 regions. DNA sequence analysis of the ITS1, 5.8S, and ITS2 regions of the ribosomal DNA gene cluster using published primers and standard sequencing methodologies was performed on the fungal culture.20 The isolate’s sequence was compared to those in GenBank using the BLASTn search tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and showed > 99.5% identity (597 base pairs [bp]) to F. flavus (FJ478126.1). Fresh tissue was referred for pan-fungal PCR testing also targeting the ITS1 and ITS2 regions of the ribosomal DNA.12 F. flavus DNA was detected in both targets. The ITS1 (228 bp) and ITS2 (310 bp) sequences showed > 99% identity to GenBank sequences FJ478126.1 and MK075023.1. This confirmed that the intralesional mycotic agent was F. flavus.
Prior to 2018, F. flavus was only known as an environmental basidiomycete fungus with a coastal distribution associated with decaying vegetation.15 There were also reports of a symbiotic relationship with plants and insects,10 antibacterial and antifungal properties,10 and potential biotechnologic application.15 The pathogenic potential of F. flavus in a vertebrate host has been reported in a 12-y-old Fjord gelding, with a nasal granuloma similar to the one described in our report.14 The horse resided in an area of Florida with access to wetland and decaying wood (S. More and W. Castleman, pers. comm., 2019). This was comparable to the year of occurrence (2017), the coastal inhabitation, and climatic conditions at the time of presentation of our case. These similarities suggest that F. flavus is an under-recognized mycotic pathogen of the horse, and the relatively recent occurrence of these cases implies a recent emergence of F. flavus as a vertebrate-infecting fungus. Whole genome sequencing is required to identify potential newly acquired virulence determinants conducive for this host switching. Alternatively, infrequent molecular characterization of equine mycotic granulomas may have led to under-diagnosis and -reporting of F. flavus as a pathogenic fungus. Further studies into the epidemiology, genomic characterization, and in vivo pathogenesis of this fungus are required.
Despite some of the similarities mentioned above, there are a few differences between our case and the prior reported case.14 Most notable is the absence of hyphal elements associated with the spherical fungal structures in our biopsy samples. F. flavus belongs in the phylum of Basidiomycota.15 Basidiomycetes have been reported to produce chlamydospores, which are enlarged vegetative cells formed within hyphae or at hyphal tips and are hypothesized to be rest or survival structures.13 Although the prior report14 described the intralesional round structures as chlamydospores, we are cautious in identifying our structures as chlamydospores because hyphae were not observed in our samples. Alternative fungal structures such as yeast cells (e.g. Cryptococcus neoformans, a basidiomycete that can exist as yeast cells) or arthroconidia should be considered.8,11
The character of the inflammation is also slightly different in our case compared to that described in the Florida case.14 The presence of more neutrophils in our samples is consistent with chronic-active inflammation. The ongoing inflammatory stimuli could be a combination of factors within the wall of the fungal spherical structures and the presence of secondary bacterial infection with Streptococcus equi ssp. zooepidemicus. The latter was not present in the lesion of the Florida case,14 and thus may account for the difference in the inflammatory reaction. Alternatively, the variation in the inflammatory profile may reflect a difference in the chronicity of the cases or could, at least in part, have been triggered by the various topical treatments being applied by the owner.
In our investigation, differential diagnoses for pathogenic fungal or fungal-like agents with spherical morphology within lesions included Coccidioides spp.,9 Rhinosporidium seeberi,6 and Emmonsia spp.16 The 2 former differentials are exotic to Australia, and Coccidioides spp. was especially concerning given its zoonotic potential.9 Our horse was placed in quarantine during the interim phase of the diagnostic process. Other common differentials for mycotic rhinosinusitis in the horse such as Conidiobolus spp. and Basidiobolus spp. were also considered. The possibility of nonpathogenic artefacts such as micelles originating from the herbal remedies applied by the owner, however unlikely, was also considered. Sanger sequencing results confirmed that the causative agent was F. flavus. The possibility of environmental contamination of F. flavus in our samples was excluded based on: 1) the localization of numerous fungal structures within the suppurative and pyogranulomatous lesions; 2) ITS PCR of biopsy detected the presence of a fungus that was identified as F. flavus upon DNA sequencing; and 3) fungal culture of the biopsy isolated a fungus that was also identified as F. flavus by ITS PCR and sequencing. These findings confirmed that the etiologic agent causing the sinonasal pyogranuloma in this mare was F. flavus.
Acknowledgments
We thank Sunil More and William Castleman from the University of Florida for providing details of their case for comparison with our case.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Carlos E. Medina-Torres  https://orcid.org/0000-0003-2048-8165
https://orcid.org/0000-0003-2048-8165
References
- 1. Berrocal A, van den Ingh T. S. G. A. M. Pathology of equine phycomycosis. Vet Q 1987;9:180–184. [DOI] [PubMed] [Google Scholar]
- 2. Brearly JC, et al. Nasal granuloma caused by Pseudallescheria boydii. Equine Vet J 1986;18:151–153. [DOI] [PubMed] [Google Scholar]
- 3. Cruz VC, et al. Successful treatment of a sinonasal cryptococcal granuloma in a horse. J Am Vet Med Assoc 2009;234:509–513. [DOI] [PubMed] [Google Scholar]
- 4. Greet TR. Nasal aspergillosis in three horses. Vet Rec 1981;109:487–489. [DOI] [PubMed] [Google Scholar]
- 5. Hanselka DV. Equine nasal phyco-mycosis. Vet Med Small Anim Clin 1977;72:251–253. [PubMed] [Google Scholar]
- 6. Herr RA, et al. Phylogenetic analysis of Rhinosporidium seeberi’s 18S small-subunit ribosomal DNA groups this pathogen among members of the protoctistan Mesomycetozoa clade. J Clin Microbiol 1999;37:2750–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hodgin EC, et al. Recurrence of obstructive nasal coccidioidal granuloma in a horse. J Am Vet Med Assoc 1984;184:339–340. [PubMed] [Google Scholar]
- 8. Kimura M, et al. Chlamydospores of Rhizopus microsporus var. rhizopodiformis in tissue of pulmonary mucormycosis. Mycopathologia 2012;174:441–450. [DOI] [PubMed] [Google Scholar]
- 9. Kirkland TN, Fierer J. Coccidioides immitis and posadasii; a review of their biology, genomics, pathogenesis, and host immunity. Virulence 2018;9:1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klaiklay S, et al. Flavodonfuran: a new difuranylmethane derivative from the mangrove endophytic fungus Flavodon flavus PSU-MA201. Nat Prod Res 2013;27:1722–1726. [DOI] [PubMed] [Google Scholar]
- 11. Kozubowski L, Heitman J. Profiling a killer, the development of Cryptococcus neoformans. FEMS Microbiol Rev 2012;36:78–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lau A, et al. Development and clinical application of a panfungal PCR assay to detect and identify fungal DNA in tissue specimens. J Clin Microbiol 2006;45:380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin X, Heitman J. Chlamydospore formation during hyphal growth in Cryptococcus neoformans. Eukaryot Cell 2005;4:1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. More SN, et al. Mycotic rhinitis and sinusitis in Florida horses. Vet Pathol 2019;56:586–598. [DOI] [PubMed] [Google Scholar]
- 15. Raghukumar C, et al. Lignin-modifying enzymes of Flavodon flavus, a basidiomycete isolated from a coastal marine environment. Appl Environ Microbiol 1999;65:2103–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schaffer-White A, et al. Pulmonary adiaspiromycosis in critically endangered northern hairy-nosed wombats (Lasiorhinus krefftii). Aust Vet J 2017;95:431–436. [DOI] [PubMed] [Google Scholar]
- 17. Souto EPF, et al. Pythiosis in the nasal cavity of horses. J Comp Pathol 2016;155:126–129. [DOI] [PubMed] [Google Scholar]
- 18. Stewart AJ, et al. Multimodal treatment of recurrent sinonasal cryptococcal granulomas in a horse. J Am Vet Med Assoc 2009;235:723–730. [DOI] [PubMed] [Google Scholar]
- 19. Timoney JF. The pathogenic equine streptococci. Vet Res 2004;35:397–409. [DOI] [PubMed] [Google Scholar]
- 20. White TJ, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, et al. , eds. PCR Protocols: A Guide to Methods and Applications. San Diego, CA: Academic Press, 1990:315–322. [Google Scholar]