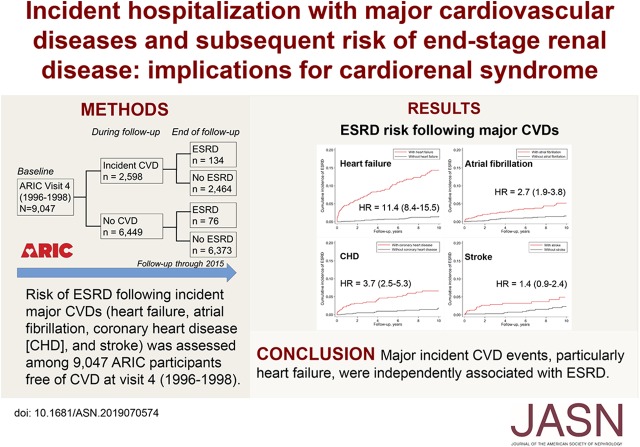
Significance Statement
Whether incident cardiovascular disease increases the long-term risk of ESKD is not well studied. The authors assessed the association of incident major cardiovascular diseases (heart failure, atrial fibrillation, coronary heart disease, and stroke) with risk of ESKD in 9047 participants of a prospective cohort study. They found that each of these major cardiovascular diseases was significantly and independently associated with the risk of ESKD, with a particularly strong association for heart failure. The association was stronger for heart failure with preserved ejection fraction compared with heart failure with reduced ejection fraction. These findings highlight the importance of managing kidney disease after cardiovascular disease. The potentially distinct contribution to ESKD of heart failure with preserved ejection fraction versus heart failure with reduced ejection fraction deserves future investigation.
Keywords: cardiovascular disease, chronic kidney disease, end-stage renal disease, end stage kidney disease
Visual Abstract
Abstract
Background
Cardiorenal syndrome is a well known concept, bolstered by extensive investigations of CKD as a risk factor of cardiovascular disease. However, data on whether cardiovascular disease increases long-term risk of ESKD are sparse.
Methods
We assessed the association of incident hospitalization with major cardiovascular diseases (heart failure, atrial fibrillation, coronary heart disease, and stroke) with subsequent risk of ESKD among individuals enrolled in the Atherosclerosis Risk in Communities study; the analysis included 9047 individuals without prevalent cardiovascular disease at their fourth study visit. Each relevant incident cardiovascular disease event was entered into multivariable Cox proportional hazard models as a time-varying exposure to estimate hazard ratios.
Results
During a median follow-up of 17.5 years, there were 2598 cases of hospitalization with cardiovascular disease (heart failure, n=1269; atrial fibrillation, n=1337; coronary heart disease, n=696; and stroke, n=559) and 210 cases of incident ESKD. The incidence of major cardiovascular disease was associated with increased risk of ESKD, with the highest risk for heart failure (hazard ratio, 11.40; 95% confidence interval, 8.38 to 15.50), followed by coronary heart disease, atrial fibrillation, and stroke. When we analyzed heart failure with preserved ejection fraction and heart failure with reduced ejection fraction separately, the risk was nominally higher for heart failure with preserved ejection fraction.
Conclusions
Major incident cardiovascular disease events were associated with ESKD, independent of kidney risk factors. In particular, heart failure showed a very strong association with ESKD. Our findings highlight the importance of monitoring and managing kidney disease in patients with cardiovascular disease. The potentially distinct contribution to ESKD of heart failure with preserved versus reduced ejection fraction deserves future investigation.
The cardio-renal syndrome, often described as a bidirectional relationship between cardiovascular disease (CVD) and CKD, is broadly recognized.1–3 However, most previous studies have investigated CKD as a risk factor of CVD,4–6 whereas data on CVD as a risk factor of incident CKD are sparse.
For example, previous studies investigating worsening kidney function after acute heart failure were mostly focused on the short-term outcome of AKI.7–11 A few population-based studies reported that persons with prevalent CVD (coronary heart disease, heart failure, and stroke) had a higher risk of kidney function decline compared with those without.12–16 However, these studies may underestimate the full effect of CVD on kidney outcomes because prevalent CVD lacks information on the time from CVD occurrence to the study enrollment. Also, none of these studies investigated the hard kidney end point of ESKD. To our knowledge, only three studies assessed the prospective association of incident CVD with risk of ESKD, but those studies investigated selected clinical populations (i.e., patients with advanced CKD with diabetes in a clinical trial,17 or patients with CKD referred to the nephrology clinic).18,19
We hypothesized that each major CVD subtype (heart failure, atrial fibrillation, coronary heart disease, and stroke) would be an independent risk factor for subsequent development of ESKD, and the association would be particularly evident for heart failure. To test our hypothesis, we investigated the association of incident hospitalization with major CVD (heart failure, atrial fibrillation, coronary heart disease, and stroke) with risk of subsequently developing ESKD, using data from the Atherosclerosis Risk in Communities (ARIC) study.
Methods
Study Population
The ARIC study is a prospective cohort study of 15,792 individuals aged 45–64 years who were enrolled in 1987–1989 (visit 1) from four United States communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland.20 Several clinic visits were conducted during follow-up. We used visit 4 as the baseline of this specific study because eGFR and urinary albumin-to-creatinine ratio (ACR), two major predictors of ESKD,5 were simultaneously assessed for the first time at this visit in ARIC. In addition, visit 4 allowed us to assure identification and exclusion of prevalent CVD at baseline by using data over approximately 9 years of follow-up between visit 1 and visit 4. Of 11,656 individuals who attended visit 4, we excluded those who were other than white or black race (n=27), had prevalent ESKD or eGFR<30 ml/min per 1.73 m2 (n=24), or had missing covariates (n=409). We also excluded those who had a previous history of CVD (n=2149), because these participants might be a selected population who were healthy enough to stay at risk and attend the clinic visits despite the previous occurrence of CVD. After the exclusion, 9047 individuals were included in analysis. Written informed consent was obtained from all ARIC participants, and the institutional review board at each study site approved the study (approval number H.34.99.07.02.A1 at Johns Hopkins University).
Exposures of Interest
The primary exposures of interest were incident hospitalization with each major subtype of CVD, defined as heart failure, atrial fibrillation, coronary heart disease, and stroke. In ARIC, all hospitalizations are identified through annual telephone calls to participants and their proxies, and active surveillance collects hospital discharge information including International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9CM) codes. For this study, heart failure was defined as hospitalization with ICD-9CM code 428 (heart failure) in any position or a death certificate with code 428 (heart failure).21 Atrial fibrillation was defined by hospitalization with ICD-9CM code 427.31 (atrial fibrillation), but also included a small number of cases detected through electrocardiogram exams at visit 5 (2011–2013) (n=120), as done previously.22 Hospitalizations with coronary heart disease and stroke are centrally adjudicated in ARIC, and definite or probable cases were included.23
Outcome of Interest
Incident ESKD included the initiation of dialysis or kidney transplantation and was identified through linkage to the US Renal Data System. Because of a change in the coding system from the ICD-9CM to the tenth revision that occurred on October 1, 2015,24 participants who did not develop ESKD were administratively censored on September 30, 2015.
Covariates of Interest
All baseline covariates except for years of education were assessed at visit 4 (1996–1998). Years of education were on the basis of visit 1 (1987–1989). Smoking (ever versus never) and alcohol history (ever versus never) were on the basis of self-report questionnaires. Diabetes was defined by self-reported physician diagnosis, a fasting glucose ≥126 mg/dl, a random glucose ≥200 mg/dl, or taking antidiabetic drugs. GFR was estimated using the CKD Epidemiology Collaboration creatinine equation.25 ACR was calculated by dividing urine albumin by urine creatinine. Serum level of high-sensitivity C-reactive protein (CRP) was measured using the immunoturbidimetric CRP-Latex (II) assay (Denka Seiken, Tokyo, Japan). Plasma levels of total cholesterol and HDL cholesterol (HDL-C) were measured using enzymatic methods according to standard protocols.26 History of chronic obstructive pulmonary disease (COPD) and cancer were defined as hospitalization with ICD-9CM codes 490–492, 494, and 496 (COPD), and 140–165, 170–176, 179–209, and 235–239 (cancer) between visit 1 and visit 4, respectively.
We also used annually updated information on self-reported diabetes and hypertension (semi-annually since 2012) on the basis of telephone calls to participants, as well as body mass index, smoking status, drinking status, systolic BP, anti-hypertensive drugs, eGFR, ACR, total cholesterol, HDL-C, and a history of COPD and cancer obtained at visit 5 (2011–2013).
Statistical Analyses
Baseline characteristics were compared between those who over the course of follow-up did versus did not develop CVD using chi-squared tests for categorical variables and the Student t tests for continuous variables. Crude incidence rates and their 95% confidence intervals (95% CIs) were estimated using Poisson regression models. Hazard ratios (HRs) were estimated using Cox proportional hazard models. Each CVD subtype was entered into Cox proportional hazard models as a time-varying exposure. In other words, participants contributed person‐time to the “no CVD” category until the first incidence of CVD, when they began contributing person‐time to the “CVD” category for the remaining period of follow-up. The proportional hazards assumption was confirmed by plotting the log of the negative log of the estimated survival probability versus the log of follow-up time.
In multivariable Cox proportional models, model 1 was adjusted for age, sex, and race. Model 2 additionally accounted for body mass index, ever smoking, ever drinking, years of education, systolic BP, antihypertensive drugs, diabetes, eGFR, ACR, CRP, total cholesterol, HDL-C, and history of COPD and cancer, with relevant covariates updated as described above. Model 3 additionally adjusted for other CVD subtypes (e.g., atrial fibrillation, coronary heart disease, and stroke for analysis of heart failure). In this model, for individuals who had multiple CVD subtypes during follow-up (e.g., coronary heart disease and heart failure), each relevant CVD subtype was separately taken into account as a time-varying exposure at the occurrence of the corresponding event (Supplemental Figure 1). Complete cases analysis was performed because there were no evident differences between those with and without missing values and there were <5% of participants excluded because of missing variables (Supplemental Table 1). We also ran Fine and Gray competing risk regression models, treating death as a competing event.
In sensitivity analyses, we repeated the analysis excluding those who had incident heart failure within 90 days before ESKD diagnosis, because the diagnosis of heart failure in these cases might have been primarily owing to symptomatic volume overload as a result of reduced kidney function.27 We also repeated the analysis in a weighted sample using the inverse probability weighting for the propensity of developing CVD, as well as propensity score matched analysis, to account for substantial differences in the baseline characteristics between those who did and did not have CVD. Subgroup analyses were performed in categories determined a priori: visit 4 age (<65 versus ≥65 years), sex (male versus female), race (white versus black), diabetes (yes versus no), and CKD (yes versus no), defined as either eGFR<60 ml/min per 1.73 m2 or ACR≥30 mg/g.
To quantify the absolute risk of ESKD after major CVDs, we also estimated the cumulative incidence of ESKD among participants with and without major CVDs. For this analysis, we used the incidence density sampling methods to create the nested analysis cohort.28 Specifically, at each occurrence of incident CVD, we identified two participants without CVD matched on age, sex, and race as a comparator, sampled from individuals who had remained in the risk set. The cumulative incidence of developing ESKD was depicted using Kaplan–Meier method, i.e., expressing 1−S(t).
Finally, we explored heart failure with preserved ejection fraction (HFpEF) (ejection fraction ≥50%) and heart failure with reduced ejection fraction (HFrEF) (EF <50%) separately.29 For this analysis, we restricted to adjudicated acute decompensated heart failure cases in ARIC that happened after 2005 where ejection fraction data were also collected. Although details can be found elsewhere,30 in brief, heart failure has been adjudicated by a panel of physician investigators. In addition, these adjudicated heart failure cases allowed us to assess last and highest serum creatinine values during the heart failure hospitalization.31 A two-sided P<0.05 was considered statistically significant. All statistical analyses were performed using Stata version 13 (StataCorp, Collage Station, TX).
Results
Overall Incidence of CVD and ESKD
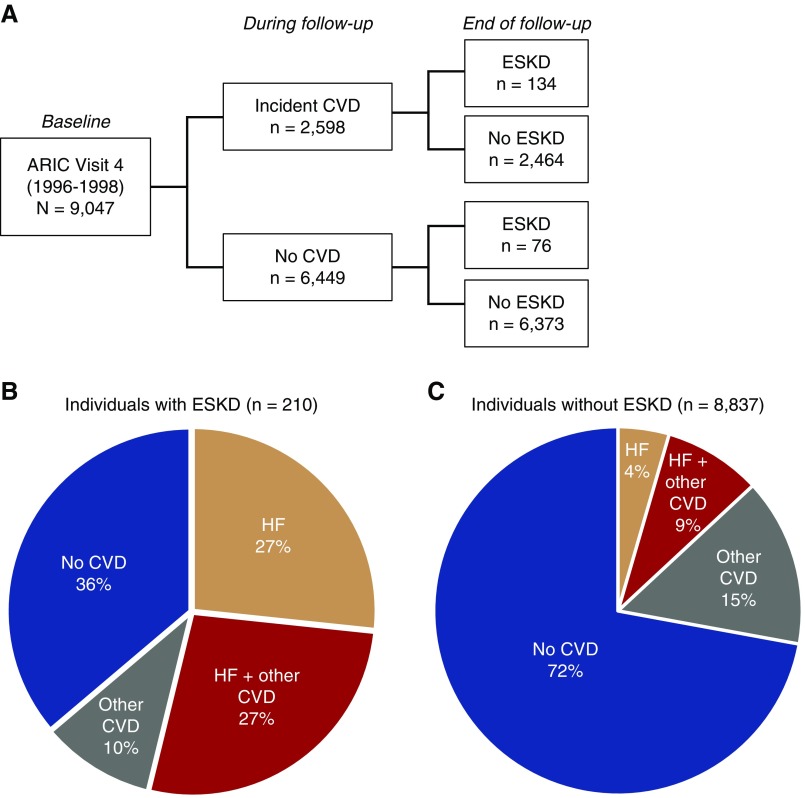
During a median follow-up of 17.5 years, 2598 (29%) had hospitalization with at least one CVD subtype, and 210 (2.3%) developed incident ESKD (n=134 after CVD, and n=76 when free of CVD) (Figure 1A). The histograms of years between CVD and ESKD were shown in Supplemental Figure 2. Among major CVDs, the most frequent CVD subtypes were heart failure (1269 events) and atrial fibrillation (1337 events), followed by coronary heart disease (696 events) and stroke (559 events). Figure 1, B and C show the pattern of CVD subtypes by the status of ESKD at the end of follow-up. Among 210 individuals with ESKD, only 36% did not develop a CVD event before ESKD, whereas 54% experienced heart failure (27% without any and 27% with one or more other CVD subtypes), and 10% had one or more CVD other than heart failure (Figure 1B). Among 8837 individuals without ESKD, 72% did not develop any CVD during follow-up, 13% experienced heart failure (4% without any and 9% with one or more other CVD subtypes), and 15% had one or more CVD other than heart failure (Figure 1C).
Figure 1.
Patterns of CVD events by the status of ESKD at end of follow-up. Among 210 individuals with incident ESKD, only 36% did not develop a CVD event before ESKD, whereas 54% experienced heart failure, and 10% had one or more CVD other than heart failure. Among 8837 individuals without ESKD, 72% did not develop any CVD during follow-up, 13% experienced heart failure, and 15% had one or more CVD other than heart failure. Other CVD indicates one or more of the following CVD subtypes: atrial fibrillation, coronary heart disease, and stroke. Considers only CVD events occurring before ESKD development. HF, heart failure.
Baseline Characteristics
Table 1 shows baseline characteristics by the presence and absence of hospitalization with major CVD during follow-up. Those with major CVD were more likely to be older, male, black race, hypertensive, and diabetic, and have lower education, eGFR, and HDL but higher body mass index, ACR, and CRP. When baseline characteristics were compared by the status of ESKD at end of follow-up, individuals with ESKD were more likely to be older, black race, hypertensive, diabetic, and have higher body mass index, systolic BP, and ACR, and lower education and eGFR (Supplemental Table 2).
Table 1.
Baseline characteristics
| Characteristics | Overall (n=9047) | Hospitalization with Major CVD | P Value | |
|---|---|---|---|---|
| No (n=6449) | Yes (n=2598) | |||
| Age, yr, mean (SD) | 63 (5.6) | 62 (5.5) | 64 (5.5) | <0.001 |
| Female, n (%) | 5295 (59) | 3894 (60) | 1401 (54) | <0.001 |
| Black race, n (%) | 1942 (22) | 1362 (21) | 580 (22) | 0.21 |
| Body mass index, kg/m2, mean (SD) | 29 (5.5) | 28 (5.3) | 29 (5.9) | <0.001 |
| Ever smoke, n (%) | 5125 (57) | 3535 (55) | 1590 (61) | <0.001 |
| Ever drink, n (%) | 7194 (80) | 5164 (80) | 2030 (78) | 0.04 |
| Received education 12 yr or more, n (%) | 7453 (82) | 5455 (85) | 1998 (77) | <0.001 |
| Systolic BP, mm Hg, mean (SD) | 127 (19) | 125 (18) | 131 (20) | <0.001 |
| Diastolic BP, mm Hg, mean (SD) | 71 (10) | 71 (9.9) | 71 (11) | 0.49 |
| Antihypertensive drugs, n (%) | 3353 (37) | 2145 (33) | 1208 (47) | <0.001 |
| Diabetes, n (%) | 1295 (14) | 739 (12) | 556 (21) | <0.001 |
| Laboratory tests | ||||
| eGFR, ml/min per 1.73 m2, n (%) | ||||
| ≥90 | 3990 (44) | 3048 (47) | 942 (36) | <0.001 |
| 60–89 | 4561 (50) | 3134 (49) | 1427 (55) | |
| 45–59 | 3,79 (4) | 208 (3) | 171 (7) | |
| 30–44 | 117 (1) | 59 (1) | 58 (2) | |
| ACR, mg/g, n (%) | ||||
| <10 | 7433 (82) | 5451 (85) | 1982 (76) | <0.001 |
| 10–29 | 1035 (11) | 707 (11) | 328 (13) | |
| 30–299 | 482 (5) | 251 (4) | 231 (9) | |
| ≥300 | 97 (1) | 40 (1) | 57 (2) | |
| CRP, mg/L, mean (SD) | 4.3 (6.2) | 4.0 (5.9) | 5.0 (7.0) | <0.001 |
| Total cholesterol, mg/dl, mean (SD) | 202 (36) | 202 (36) | 202 (37) | 0.99 |
| HDL-C, mg/dl, mean (SD) | 51 (17) | 52 (17) | 49 (16) | <0.001 |
| Past medical history, n (%) | ||||
| COPD | 223 (2.5) | 125 (1.9) | 98 (3.8) | <0.001 |
| Cancer | 514 (5.7) | 359 (5.6) | 155 (6.0) | 0.46 |
Data are presented as mean (SD) or n (%).
Incident CVD and Risk of ESKD
When comparing between person-years when free of CVD and person-years after CVD, the crude incidence rate of ESKD was consistently higher after CVD, with the risk gradient most evident for heart failure (0.8 versus 6.1 per 1000 person-years) (Table 2). When each CVD subtype was individually entered into age-, sex-, and race-adjusted cause-specific Cox model as a time-varying exposure, the HR was highest for heart failure (HR, 22.91; 95% CI, 17.13 to 30.64), followed by coronary heart disease (HR, 4.91; 95% CI, 3.42 to 7.03), atrial fibrillation (HR, 4.21; 95% CI, 2.99 to 5.93), and stroke (HR, 2.27; 95% CI, 1.35 to 3.80) (Table 2, model 1). The association was consistent and mostly remained significant after adjusting for other confounders including eGFR and ACR (HR, 11.40; 95% CI, 8.38 to 15.50 for heart failure; HR, 3.67; 95% CI, 2.53 to 5.33 for coronary heart disease; HR, 2.66; 95% CI, 1.87 to 3.79 for atrial fibrillation; and HR, 1.44; 95% CI, 0.85 to 1.85 for stroke). (Table 2, model 2). When all CVD subtypes were simultaneously entered into a Cox model as time-varying exposures (e.g., adjusting for atrial fibrillation, coronary heart disease, stroke for analysis of heart failure), the HR remained significant and highest for heart failure (HR, 9.92; 95% CI, 7.14 to 13.79), whereas the associations for atrial fibrillation and stroke were not significant (Table 2, model 3). Regarding the overall discrimination of the Cox model, the Harrell c-index for the multivariable Cox model with the covariates in model 3 was 0.88 (95% CI, 0.85 to 0.90).
Table 2.
Crude incidence rates and adjusted HRs of ESKD comparing the risk between the time-periods after CVD and free of CVD
| ESKD Risk | All CVD Subtypes Combined (2598 events) | CVD Subtypes | |||
|---|---|---|---|---|---|
| Heart Failure (1269 Events) | Atrial Fibrillation (1337 Events) | Coronary Heart Disease (696 Events) | Stroke (559 Events) | ||
| Person-yr when free of relevant CVD (×103) | 106.0 | 127.5 | 124.6 | 135.2 | 137.6 |
| ESKD cases | 76 | 97 | 163 | 172 | 194 |
| Incidence rate per 1000 person-yra | 0.7 (0.6–0.9) | 0.8 (0.6–0.9) | 1.3 (1.1–1.5) | 1.3 (1.1–1.5) | 1.4 (1.2–1.6) |
| Person-yr after relevant CVD (×103) | 40.0 | 18.5 | 21.4 | 10.8 | 8.4 |
| ESKD cases | 134 | 113 | 47 | 38 | 16 |
| Incidence rate per 1000 person-yra | 3.3 (2.8–4.0) | 6.1 (5.1–7.3) | 2.2 (1.6–2.9) | 3.5 (2.6–4.8) | 1.9 (1.2–3.1) |
| HR (95% CI) | |||||
| Model 1 | 11.61 (8.61 to 15.65) | 22.91 (17.13 to 30.64) | 4.21 (2.99 to 5.93) | 4.91 (3.42 to 7.03) | 2.27 (1.35 to 3.80) |
| Model 2 | 6.63 (4.88 to 9.00) | 11.40 (8.38 to 15.50) | 2.66 (1.87 to 3.79) | 3.67 (2.53 to 5.33) | 1.44 (0.85 to 2.44) |
| Model 3 | — | 9.92 (7.14 to 13.79) | 1.10 (0.76 to 1.60) | 1.80 (1.22 to 2.66) | 1.09 (0.65 to 1.85) |
Model 1 adjusted for age, sex, and race. Model 2 additionally adjusted for body mass index, ever smoking, ever drink, years of education, systolic BP, antihypertensive drugs, diabetes, eGFR, ACR, CRP, total cholesterol, HDL-C, and history of COPD and cancer, with covariates updated when available. Model 3 additionally adjusted for CVD subtypes (e.g., atrial fibrillation, coronary heart disease, and stroke for analysis of heart failure).
Data in parentheses displayed as 95% CI.
Similar patterns were observed when we ran the Fine and Gray competing risk models treating death as a competing event (Supplemental Table 3). When we excluded 42 individuals with incident ESKD who had incident heart failure within <90 days before ESKD, the association for heart failure was substantially attenuated but remained strong and significant (HR, 13.16; 95% CI, 9.50 to 18.22 for model 1; HR, 6.15; 95% CI, 4.36 to 8.67 for model 2; and HR, 5.06; 95% CI, 3.50 to 7.30 for model 3). The associations were mostly unchanged in a weighted sample using the inverse probability weighting (Supplemental Table 4) or propensity score matched analysis (Supplemental Table 5). The analysis excluding those whose atrial fibrillation was detected through electrocardiogram exams at visit 5 (2011–2013) (n=120) did not change the results (data not shown). In subgroup analyses, the associations were consistent in subgroups by age, sex, race, diabetes, and CKD without significant interactions except that the association of atrial fibrillation was stronger in women than men (P for interaction =0.02) (Table 3).
Table 3.
Subgroup analysis
| Subgroup | No. of Participants | ESKD Cases | CVD Subtypes, HR (95% CI) | |||
|---|---|---|---|---|---|---|
| Heart Failure | Atrial Fibrillation | Coronary Heart Disease | Stroke | |||
| Age, yr | ||||||
| <65 | 5660 | 96 | 11.12 (7.06 to 17.54) | 2.94 (1.65 to 5.23) | 2.31 (1.17 to 4.57) | 1.22 (0.53 to 2.84) |
| ≥65 | 3387 | 114 | 12.39 (8.16 to 18.82) | 2.53 (1.61 to 3.98) | 4.67 (2.96 to 7.37) | 1.45 (0.71 to 2.94) |
| P for interaction | 0.20 | 0.21 | 0.06 | 0.17 | ||
| Sex | ||||||
| Male | 3752 | 98 | 12.03 (7.64 to 18.94) | 1.82 (1.07 to 3.09) | 3.80 (2.26 to 6.40) | 1.48 (0.71 to 3.08) |
| Female | 5295 | 112 | 13.37 (8.79 to 20.33) | 4.74 (2.89 to 7.79) | 4.08 (2.35 to 7.08) | 1.30 (0.59 to 2.90) |
| P for interaction | 0.41 | 0.02 | 0.57 | 0.85 | ||
| Race | ||||||
| White | 7105 | 128 | 10.34 (6.92 to 15.46) | 3.08 (2.06 to 4.62) | 3.69 (2.36 to 5.78) | 1.57 (0.80 to 3.08) |
| Black | 1942 | 82 | 14.71 (9.08 to 23.84) | 1.62 (0.68 to 3.89) | 3.42 (1.72 to 6.80) | 1.27 (0.53 to 3.07) |
| P for interaction | 0.90 | 0.05 | 0.71 | 0.50 | ||
| Diabetes | ||||||
| No | 7752 | 119 | 11.45 (7.48 to 17.51) | 2.11 (1.32 to 3.37) | 3.55 (2.15 to 5.86) | 0.97 (0.42 to 2.26) |
| Yes | 1295 | 91 | 11.74 (7.38 to 18.68) | 3.72 (2.15 to 6.44) | 3.89 (2.19 to 6.92) | 1.87 (0.93 to 3.79) |
| P for interaction | 0.63 | 0.43 | 0.85 | 0.36 | ||
| CKD | ||||||
| No | 8067 | 111 | 9.11 (5.94 to 13.96) | 2.99 (1.87 to 4.78) | 2.68 (1.56 to 4.59) | 1.14 (0.46 to 2.82) |
| Yes | 980 | 99 | 14.67 (9.28 to 23.19) | 2.20 (1.28 to 3.76) | 5.16 (3.05 to 8.71) | 1.93 (0.97 to 3.81) |
| P for interaction | 0.98 | 0.26 | 0.35 | 0.84 | ||
The model was adjusted for age, sex, race, body mass index, ever smoking, ever drink, years of education, systolic BP, antihypertensive drugs, diabetes, eGFR, ACR, CRP, total cholesterol, HDL-C, and history of COPD and cancer. The covariates were updated when available.
Cumulative Incidence of ESKD after CVD
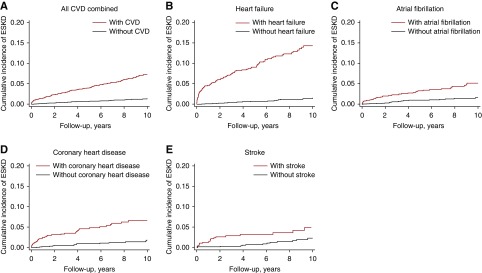
Figure 2 shows the Kaplan–Meier curves depicting the cumulative incidence of ESKD among participants with CVD and matched participants without CVD. For heart failure, the risk separation was most evident in the beginning of the follow-up, but observed consistently throughout the follow-up. The cumulative incidence of ESKD among participants with heart failure was approximately 10% at 5 years (versus approximately 1% among those without), and 15% at 10 years (versus <2% among those without).
Figure 2.
Kaplan–Meier curves for the risk of ESKD comparing between participants with CVD and matched participants without CVD. Participants with CVD had a higher risk of ESKD compared to matched participants without CVD. The cumulative incidence of ESKD among participants with heart failure was approximately 10% at 5 years (versus approximately 1% among those without), and 15% at 10 years (versus <2% among those without). Participants without CVD were sampled at the ratio of 2:1 using the incidence density sampling methods matched on age, sex, and race. The cumulative incidence was calculated as 1–S(t).
HFpEF versus HFrEF and Risk of ESKD
Between 2005 and 2015, there were 529 adjudicated incident cases of acute decompensated heart failure, including 247 events of HFpEF and 220 events of HFrEF (62 events were missing ejection fraction). Among those with available serum creatinine during admission for heart failure (n=365), both eGFR on the basis of last and highest serum creatinine were similar between participants with HFpEF and HFrEF (52.1 versus 53.7 ml/min per 1.73 m2; P=0.58, and 43.2 versus 43.2 ml/min per 1.73 m2; P=0.99, respectively). Overall, the association for adjudicated heart failure was largely consistent with the primary results shown above (Table 4), although the 95% CIs in this analysis were wider than the primary analysis because we did not take into account potential heart failure cases before 2005. Comparing HFpEF and HFrEF, the association was particularly evident for HFpEF (HR, 22.57; 95% CI, 14.66 to 34.77 for HFpEF versus HR, 15.94; 95% CI, 9.19 to 27.65 for HFrEF in model 1; HR, 9.85; 95% CI, 6.17 to 15.72 versus HR, 9.81; 95% CI, 5.55 to 17.32 in model 2; and HR, 7.58; 95% CI, 4.69 to 12.23 versus HR, 5.56; 95% CI, 2.99 to 10.36 in model 3) (Table 4).
Table 4.
HRs of incident ESKD associated with validated events of hospitalization with acute decompensated heart failure
| Model | Overall (529 Events) | Subtype of Heart Failure, HR (95% CI) | |
|---|---|---|---|
| HFpEF (247 Events)a | HFrEF (220 Events)a | ||
| Model 1 | 19.17 (13.56 to 27.11) | 22.57 (14.66 to 34.77) | 15.94 (9.19 to 27.65) |
| Model 2 | 9.12 (6.34 to 13.12) | 9.85 (6.17 to 15.72) | 9.81 (5.55 to 17.32) |
| Model 3 | 6.81 (4.60 to 10.08) | 7.58 (4.69 to 12.23) | 5.56 (2.99 to 10.36) |
Model 1 adjusted for age, sex, and race. Model 2 additionally adjusted for body mass index, ever smoking, ever drink, years of education, systolic BP, antihypertensive drugs, diabetes, eGFR, ACR, CRP, total cholesterol, HDL-C, and history of COPD and cancer, with covariates updated when available. Model 3 additionally adjusted for CVD subtypes (e.g., atrial fibrillation, coronary heart disease, and stroke).
Sixty two individuals were excluded from analysis because of a lack of information on ejection fraction.
Discussion
In this prospective analysis of 9047 middle to older age black and white adults, incident CVD was significantly associated with subsequent risk of ESKD across subtypes of heart failure, coronary heart disease, atrial fibrillation, and stroke. The risk of ESKD was particularly high for heart failure: the HR was >20 in demographically adjusted models and approximately 10 even after accounting for other potential confounders. When the absolute risk was estimated, the cumulative incidence of ESKD among participants with heart failure was approximately 10% at 5 years, and 15% at 10 years. Finally, when analyzing separately by HFpEF versus HFrEF, the HR was nominally higher for HFpEF than HFrEF.
Our results are largely consistent with a limited number of studies reporting on the prospective association of CVD with kidney outcomes. In a secondary analysis of the Trial to Reduce Cardiovascular Events with Aranesp Therapy study, a clinical trial among patients with CKD with diabetes and anemia, the risk of ESKD was higher for heart failure than for other CVD subtypes including myocardial infarction and stroke.17 A strong association of heart failure (i.e., HRs of >5) was also observed in two other studies of CKD populations.18,19 We extended the previous literature in various ways. Using time varying exposure analysis and adequately excluding those with prevalent CVD, we showed prospective associations of CVD with risk of ESKD in a general population consisting of racially diverse middle to older age adults. Additionally, collective analyses across CVD subtypes revealed a particularly high risk of ESKD associated with heart failure, although other CVD subtypes were also significant.
Although our observational study cannot conclude causality, there are several plausible mechanisms behind the strong association between heart failure and ESKD. For example, heart failure and kidney disease often share common pathways involving inflammation, neuro-hormonal activation, metabolic changes (e.g., bone mineral disorders), and anemia.32,33 Indeed, increased renin-angiotensin-aldosterone system activity during heart failure may cause inflammation, oxidative stress, and endothelial dysfunction, which have deleterious effects on the kidney.34 Also, low cardiac output may elevate central venous pressure and reduce renal blood flow, leading to a lower kidney function.35,36 Furthermore, several interventions such as use of loop diuretics can be nephrotoxic.37,38 Also, diagnosis of heart failure may be made because of volume overload resulting from ESKD.27 Nonetheless, it is of note that our sensitivity analysis showed consistent and significant associations when excluding individuals who had heart failure within <90 days before ESKD.
The association of heart failure with the risk of ESKD was particularly evident for HFpEF. Underlying mechanisms of the stronger association of HFpEF over HFrEF with ESKD are unclear. Interestingly, kidney function during the episode of heart failure was comparable between HFpEF and HFrEF in our study. Previous studies showed that patients with HFpEF tended to be older, and have comorbidities such as hypertension and diabetes, as compared with patients with HFrEF.39–41 Thus, it is possible that HFpEF reflects systemic comorbid conditions, which share risk factors for CKD progression. Additionally, endothelial dysfunction,42 oxidative stress,43 and inflammation44 are believed to play a major role in HFpEF relative to that in HFrEF,45 which may also be relevant in the susceptibility to the development of ESKD.
We also demonstrated that the risk of ESKD was nearly 2.7-, 3.7-, and 1.4-fold higher after atrial fibrillation, coronary heart disease, and stroke, respectively. These findings suggest that health care providers managing these CVD subtypes (e.g., cardiologists) should carefully monitor kidney function in patients with these conditions. From an etiological point of view, the attenuation of the associations after accounting for heart failure indicate somewhat limited contributions of these subtypes to the development of ESKD. However, we should keep in mind that these CVD subtypes (especially atrial fibrillation and coronary heart disease) are leading causes of heart failure.46–48
This study may provide several important clinical and research implications. First, individuals with CVD should be recognized as a high-risk population for ESKD. Particularly, heart failure was a strong predictor of ESKD. In this context, physicians should be aware of CVD as an important risk condition, and thereby minimize the nephrotoxic exposures (e.g., nonsteroidal anti-inflammatory drugs, nephrotoxic antibiotics) for such individuals. Additionally, our findings may have an implication on monitoring kidney function, although current CVD guidelines do not necessarily specify the frequency of evaluating kidney function after incidence of CVD.49–51 Finally, the potentially distinct pattern in the association of HFpEF versus HFrEF with ESKD risk would warrant future studies to explore the mechanistic pathways of CKD progression after heart failure.
This study also has several limitations. First, this was observational study and therefore causal inference is limited because of the possibility of residual confounding and time-dependent confounding.52 Second, it is possible that reverse causality occurred, although we prospectively analyzed the data. Future validation studies, such as within-individual changes in the eGFR slope after incidence of CVD, may help better understand the impact of incident CVD on the kidney. Third, we did not consider recurrent events of the same CVD subtype such as additional episodes of heart failure after the incident heart failure.18 Fourth, our definition of incident CVD predominantly relied on CVD requiring hospitalization and did not capture mild CVD case. However, patients with myocardial infarction and stroke, once diagnosed at acute phase, should be hospitalized, and our approach of comparing hospitalized CVD events may have some advantage for fair comparison across the CVD subtypes. Fifth, our study could not fully assess interim eGFR decline. Finally, although our study population was racially diverse consisting of middle to older age adults and thus is considered to have broad implications, ARIC participants were not representative of the full United States population, potentially limiting the generalizability of our findings.
In conclusion, in this community-based cohort, incidence of heart failure, atrial fibrillation, coronary heart disease, and stroke were associated with increased risk of ESKD independent of conventional kidney risk factors, with the strongest association for heart failure. These findings provide important clinical implications for the management of patients after an incident CVD. Underlying mechanisms of the potentially distinct pattern in the association of HFpEF versus HFrEF with ESKD risk deserve future investigation.
Disclosures
Dr. Carrero reports support from the Swedish Research Council (2019-01059), grants from Astellas, AstraZeneca, Merck Sharp & Dohme, Swedish Research Council, and ViforPharma, outside the submitted work. Dr. Coresh reports grants from National Institutes of Health, grants from National Kidney Foundation, during the conduct of the study. Dr. Grams reports nonfinancial support from Dialysis Clinics Incorporated, nonfinancial support from Kidney Disease: Improving Global Outcomes, grants from National Institute of Diabetes and Digestive and Kidney Diseases, grants from National Kidney Foundation, outside the submitted work. Dr. Lutsey reports grants from National Institutes of Health, during the conduct of the study. Dr. Matsushita reports personal fees from Akebia, grants and personal fees from Kyowa Kirin, outside the submitted work.
Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services (contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I).
Supplementary Material
Acknowledgments
Research idea and study design: Dr. Ishigami and Dr. Matsushita. Data acquisition: Dr. Ishigami, Dr. Matsushita, and Dr. Coresh. Data analysis/interpretation: Dr. Ishigami, Dr. Cowan, Dr. Demmer, Dr. Lutsey, Dr. Grams, Dr. Carrero, Dr. Coresh, and Dr. Matsushita. Statistical analysis: Dr. Ishigami and Dr. Matsushita. Supervision or mentorship: Dr. Coresh and Dr. Matsushita. All authors approved the final version of the manuscript.
The authors thank the staff and participants of the Atherosclerosis Risk in Communities study for their important contributions.
The sponsors had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019060574/-/DCSupplemental.
Supplemental Table 1. Baseline characteristics comparing between participants with and without missing values.
Supplemental Table 2. Baseline characteristics by ESKD status at end of follow-up.
Supplemental Table 3. HRs of ESKD in Fine and Gray model treating death as a competing event.
Supplemental Table 4. Adjusted HRs of ESKD comparing the risk between the time-periods after CVD and free of CVD using the inverse probability weighting.
Supplemental Table 5. Adjusted HRs of ESKD comparing the risk between the time-periods after CVD and free of CVD using the propensity score–matched analysis.
Supplemental Figure 1. Hypothetical examples of how participants contributed person-time to CVD status.
Supplemental Figure 2. Histograms for years from CVD to ESKD.
References
- 1.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, et al. Acute Dialysis Quality Initiative (ADQI) consensus group : Cardio-renal syndromes: Report from the consensus conference of the acute dialysis quality initiative. Eur Heart J 31: 703–711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Damman K, Testani JM: The kidney in heart failure: An update. Eur Heart J 36: 1437–1444, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner-La Rocca HP, Chioncel O, et al.: Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 19: 821–836, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishigami J, Grams ME, Naik RP, Caughey MC, Loehr LR, Uchida S, et al.: Hemoglobin, albuminuria, and kidney function in cardiovascular risk: The ARIC (Atherosclerosis Risk in Communities) study. J Am Heart Assoc 7: e007209, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, et al.: Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 43: 61–67, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Akhter MW, Aronson D, Bitar F, Khan S, Singh H, Singh RP, et al.: Effect of elevated admission serum creatinine and its worsening on outcome in hospitalized patients with decompensated heart failure. Am J Cardiol 94: 957–960, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, et al.: Worsening renal function and prognosis in heart failure: Systematic review and meta-analysis. J Card Fail 13: 599–608, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Logeart D, Tabet JY, Hittinger L, Thabut G, Jourdain P, Maison P, et al.: Transient worsening of renal function during hospitalization for acute heart failure alters outcome. Int J Cardiol 127: 228–232, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL: Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur Heart J 35: 455–469, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem D, et al.: Cardiovascular disease and subsequent kidney disease. Arch Intern Med 167: 1130–1136, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Shlipak MG, Katz R, Kestenbaum B, Fried LF, Siscovick D, Sarnak MJ: Clinical and subclinical cardiovascular disease and kidney function decline in the elderly. Atherosclerosis 204: 298–303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George LK, Koshy SKG, Molnar MZ, Thomas F, Lu JL, Kalantar-Zadeh K, et al.: Heart failure increases the risk of adverse renal outcomes in patients with normal kidney function. Circ Heart Fail 10: e003825, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dini FL, Demmer RT, Simioniuc A, Morrone D, Donati F, Guarini G, et al.: Right ventricular dysfunction is associated with chronic kidney disease and predicts survival in patients with chronic systolic heart failure. Eur J Heart Fail 14: 287–294, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillege HL, van Gilst WH, van Veldhuisen DJ, Navis G, Grobbee DE, de Graeff PA, et al. CATS Randomized Trial : Accelerated decline and prognostic impact of renal function after myocardial infarction and the benefits of ACE inhibition: The CATS randomized trial. Eur Heart J 24: 412–420, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Charytan DM, Solomon SD, Ivanovich P, Remuzzi G, Cooper ME, McGill JB, et al.: ESRD after heart failure, myocardial infarction, or stroke in Type 2 diabetic patients with CKD. Am J Kidney Dis 70: 522–531, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Sud M, Tangri N, Pintilie M, Levey AS, Naimark DM: ESRD and death after heart failure in CKD. J Am Soc Nephrol 26: 715–722, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sud M, Tangri N, Pintilie M, Levey AS, Naimark D: Risk of end-stage renal disease and death after cardiovascular events in chronic kidney disease. Circulation 130: 458–465, 2014 [DOI] [PubMed] [Google Scholar]
- 20.The Atherosclerosis Risk in Communities (ARIC) study: Design and objectives. The ARIC investigators. Am J Epidemiol 129: 687–702, 1989 [PubMed] [Google Scholar]
- 21.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE: Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 101: 1016–1022, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al.: Incidence of atrial fibrillation in whites and African-Americans: The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 158: 111–117, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; ARIC Study Investigators : Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 57: 1690–1696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Medicare & Medicaid Services: CMS Releases ICD-10 Assessment and Maintenance Toolkit. Available at: https://www.cms.gov/Medicare/Coding/ICD10/Downloads/ICD-10NextStepsToolkit20170324.pdf. Accessed December 7, 2019
- 25.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siedel J, Hägele EO, Ziegenhorn J, Wahlefeld AW: Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem 29: 1075–1080, 1983 [PubMed] [Google Scholar]
- 27.Crews DC, Scialla JJ, Liu J, Guo H, Bandeen-Roche K, Ephraim PL, et al. Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) Patient Outcomes in End Stage Renal Disease Study Investigators : Predialysis health, dialysis timing, and outcomes among older United States adults. J Am Soc Nephrol 25: 370–379, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langholz B, Thomas DC: Nested case-control and case-cohort methods of sampling from a cohort: A critical comparison. Am J Epidemiol 131: 169–176, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Chang PP, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD, et al.: Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005-2014): ARIC study community surveillance. Circulation 138: 12–24, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, et al.: Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: A comparison of diagnostic criteria. Circ Heart Fail 5: 152–159, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushita K, Kwak L, Hyun N, Bessel M, Agarwal SK, Loehr LR, et al.: Community burden and prognostic impact of reduced kidney function among patients hospitalized with acute decompensated heart failure: The Atherosclerosis Risk in Communities (ARIC) Study Community Surveillance. PLoS One 12: e0181373, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S: Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat Rev Nephrol 12: 610–623, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Lam CS, Lund LH: Microvascular endothelial dysfunction in heart failure with preserved ejection fraction. Heart 102: 257–259, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Szymanski MK, de Boer RA, Navis GJ, van Gilst WH, Hillege HL: Animal models of cardiorenal syndrome: A review. Heart Fail Rev 17: 411–420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al.: Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 53: 589–596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Afsar B, Ortiz A, Covic A, Solak Y, Goldsmith D, Kanbay M: Focus on renal congestion in heart failure. Clin Kidney J 9: 39–47, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP: Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 122: 265–272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. NHLBI Heart Failure Clinical Research Network : Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 364: 797–805, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silverman MG, Patel B, Blankstein R, Lima JA, Blumenthal RS, Nasir K, et al.: Impact of race, ethnicity, and multimodality biomarkers on the incidence of new-onset heart failure with preserved ejection fraction (from the multi-ethnic study of atherosclerosis). Am J Cardiol 117: 1474–1481, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM: Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251–259, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, et al.: Predictors of new-onset heart failure: Differences in preserved versus reduced ejection fraction. Circ Heart Fail 6: 279–286, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, et al.: Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol 60: 1778–1786, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Paulus WJ, Tschöpe C: A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, et al.: Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail 4: 312–324, 2016 [DOI] [PubMed] [Google Scholar]
- 45.Borlaug BA, Redfield MM: Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 123: 2006–2013; discussion 2014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massie BM, Shah NB: Evolving trends in the epidemiologic factors of heart failure: Rationale for preventive strategies and comprehensive disease management. Am Heart J 133: 703–712, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW: Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: A retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol 32: 695–703, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Cuadrado-Godia E, Ois A, Roquer J: Heart failure in acute ischemic stroke. Curr Cardiol Rev 6: 202–213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. ESC Scientific Document Group : 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39: 119–177, 2018 [DOI] [PubMed] [Google Scholar]
- 50.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. American Heart Association Stroke Council : 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke 49: e46–e110, 2018 [DOI] [PubMed] [Google Scholar]
- 51.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al.: 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American college of cardiology/American heart association task force on practice guidelines, and the American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. Circulation 130: 1749–1767, 2014 [DOI] [PubMed] [Google Scholar]
- 52.Mansournia MA, Etminan M, Danaei G, Kaufman JS, Collins G: Handling time varying confounding in observational research. BMJ 359: j4587, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.