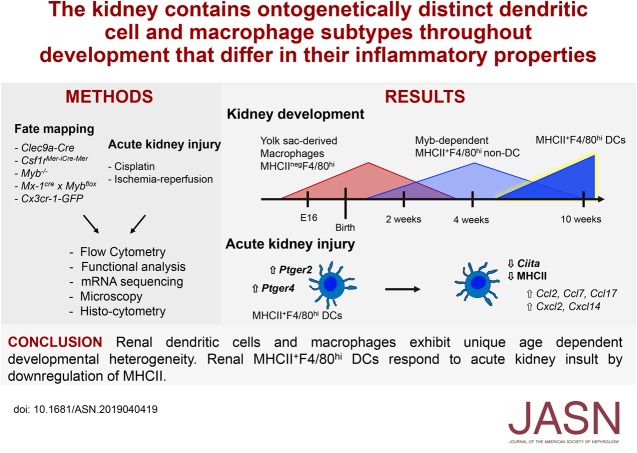
Significance Statement
The origin of kidney dendritic cells (DCs) has been highly debated because they share many phenotypic traits with macrophages in this tissue. Using fate mapping, the authors demonstrate that kidneys from adult mice contain four subsets of DCs unique age-dependent differences in DCs and macrophages. Renal embryonic-derived macrophages are replaced shortly after birth by phenotypically similar cells arising from hematopoiesis. In adults, these are generated from DC progenitors. In two models of renal injury, cells resembling embryonic-derived macrophages reappear in inflamed kidneys as a result from MHCII downregulation from renal dendritic cells. Understanding age-dependent developmental aspects in these cells of immune-modulatory and antigen-presenting function may help scientists develop therapies targeting them.
Keywords: macrophages, kidney development, immunology, dendritic cell, hematopoiesis, acute kidney injury
Visual Abstract
Abstract
Background
Mononuclear phagocytes (MPs), including macrophages, monocytes, and dendritic cells (DCs), are phagocytic cells with important roles in immunity. The developmental origin of kidney DCs has been highly debated because of the large phenotypic overlap between macrophages and DCs in this tissue.
Methods
We used fate mapping, RNA sequencing, flow cytometry, confocal microscopy, and histo-cytometry to assess the origin and phenotypic and functional properties of renal DCs in healthy kidney and of DCs after cisplatin and ischemia reperfusion–induced kidney injury.
Results
Adult kidney contains at least four subsets of MPs with prominent Clec9a-expression history indicating a DC origin. We demonstrate that these populations are phenotypically, functionally, and transcriptionally distinct from each other. We also show these kidney MPs exhibit unique age-dependent developmental heterogeneity. Kidneys from newborn mice contain a prominent population of embryonic-derived MHCIInegF4/80hiCD11blow macrophages that express T cell Ig and mucin domain containing 4 (TIM-4) and MER receptor tyrosine kinase (MERTK). These macrophages are replaced within a few weeks after birth by phenotypically similar cells that express MHCII but lack TIM-4 and MERTK. MHCII+F4/80hi cells exhibit prominent Clec9a-expression history in adulthood but not early life, indicating additional age-dependent developmental heterogeneity. In AKI, MHCIInegF4/80hi cells reappear in adult kidneys as a result of MHCII downregulation by resident MHCII+F4/80hi cells, possibly in response to prostaglandin E2 (PGE2). RNA sequencing further suggests MHCII+F4/80hi cells help coordinate the recruitment of inflammatory cells during renal injury.
Conclusions
Distinct developmental programs contribute to renal DC and macrophage populations throughout life, which could have important implications for therapies targeting these cells.
Macrophages, monocytes, and dendritic cells (DCs) are important innate immune sentinels that also play crucial roles in organ homeostasis.1–6 They are located in most tissues of the body and are collectively known as mononuclear phagocytes (MPs) because they were originally thought to share a common monocyte origin.1,4,7 Through the use of improved fate-mapping models and single-cell technologies, numerous studies have unequivocally established that macrophages, monocytes, and DCs constitute developmentally distinct cell lineages with unique functions in immunity.1 It has also become increasingly clear that even at steady state, accurate and reliable distinction of monocyte, DC, and macrophage subsets based on surface markers is complex due to the existence of distinct age-dependent developmental programs that contribute to MP networks across tissues and because MPs exhibit a degree of tissue-specific adaptation.1,6,8
Macrophage poiesis begins before birth and, in mice, primitive macrophage progenitors appear in the yolk sac (YS) as early as embryonic day (E) 7.25.8–10 From E8.25, in a second wave of macrophage poiesis, erythro-myeloid progenitors are generated in the YS that enter the embryo proper between E8.5 and E12.5, give rise to macrophages in various organs, and contribute to fetal liver hematopoiesis.11–13 Embryonic-derived macrophages, arising from primitive YS progenitors or erythro-myeloid progenitors, can persist into adulthood due to self-renewal in brain, lung, or liver in steady-state conditions.1,4,6,14 From E10.5, hematopoietic stem cells (HSCs) arise in the dorsal aorta and migrate to the liver to initiate fetal definitive hematopoiesis, which later shifts to the bone marrow.6 With increasing age, HSC-derived monocytes can also differentiate into long-lived macrophages and, in some tissues such as heart or testes, these monocyte-derived cells coexist side by side with macrophages of embryonic origin.15,16 In intestine and dermis, which are exposed to a specific microbial environment, monocyte-derived cells are thought to fully replace embryonic-derived macrophages over time,6,17 although in the gastrointestinal tract some tissue-resident macrophages appear to self-maintain independently of monocytic input.18,19 The signals that regulate the tissue-specific ontogenetic diversity of macrophages remain ill defined.
DCs and monocytes arise from definitive hematopoiesis and their differentiation is well understood in adult mice. Adult bone marrow contains a fraction of progenitor cells capable of generating monocytes, plasmacytoid DCs (pDCs), and conventional or classic DCs (cDCs) and has therefore been termed macrophage and DC progenitor.20 This bone marrow fraction further gives rise to common monocyte progenitors and so called common DC progenitors (CDPs) that exclusively generate monocytes and cDCs/pDCs, respectively.21,22 CDPs further differentiate into pre-cDCs, which terminally differentiate into the two main cDC1 and cDC2 subtypes in peripheral organs in response to environmental cues.1,2,23 Within CDPs the expression of the C-type lectin receptor DNGR-1 (encoded by the Clec9a gene) distinguishes cells with exclusive cDC potential and DNGR-1 continues to be expressed on pre-cDCs.24 Although the Clec9a promoter is active in cDC1s and pDCs, it is not active in progenitors for other lymphoid and myeloid lineages—including precursors for monocytes, granulocytes, or lymphoid cells—and therefore mice expressing CRE-recombinase (CRE) under the Clec9a promoter allow for faithful tracing of the cDC lineage in steady state as well as in inflammation.24,25
Using Clec9a-Cre–mediated fate mapping, we have demonstrated that the steady-state adult kidney, when compared with other organs, contains numerous cells with high expression of the putative monocyte/macrophage markers CD64 and F4/80 that show substantial Clec9a-expression history, indicative of a CDP origin.24 Renal CD64+ cells can further be distinguished based on differential expression of F4/80 and CD11b into F4/80hi and CD11bhi cells,24,26 thus phenotypically resembling embryonic-derived macrophages and monocyte-derived cells, respectively.12,27,28 CD11bhi cells arise from CDP in adoptive transfers,24 require the DC growth factor FMS-like tyrosine kinase 3 ligand (FLT3L) for their development and phenotypically resemble cDC2 based on high expression of the signature cDC2 transcription factor IRF4 and low levels of IRF8.26 It has therefore been suggested that renal CD11bhi cells are cDC2s that express CD64.26 However, whether renal cDC2s and CD11bhi cells share the same transcriptional requirements and resemble each other in comparative gene expression analysis has not been investigated.
Renal F4/80hi cells resemble DCs in their ability to activate naive T cells,24 but these cells also exhibit prominent phenotypic overlap with embryonic-derived macrophages.26,29–31 We and others were unable to confirm the CDP origin of renal F4/80hi cells using adoptive transfers of CDPs or pre-cDCs.24,32 However, bone marrow progenitors can contribute to renal F4/80hi cells in lethally irradiated mice and after depletion of HSCs by conditional deletion of the HSC master regulator Myb in Mx-1creMybflox/flox chimeric mice.12,24 Fate mapping of adult HSCs in Flt3-Cre mice further corroborates the HSC origin of this population in steady-state adult kidney because about 50% of renal F4/80hi cells display FLT3 expression history.12,16 In contrast to these findings, renal F4/80hi cells exchange poorly between parabiotic partners, indicating HSC-independent maintenance, although a poor exchange of blood monocytes between partner mice, as well as low chimerism in kidney compared with other organs,33–36 could have led to an underestimation of blood contribution to kidney-resident leukocyte populations. Fate mapping of YS progenitors in mice carrying a tamoxifen-inducible CRE driven by the Csfr-1 promoter (Csf1rMer-iCre-Mer) demonstrates a YS contribution to renal F4/80hi cells in early life that disappears within a few weeks after birth.11,12,16 Taken together, these data indicate an age-dependent ontogenetic diversity of renal F4/80hi cells and highlight that the precise origins of this population throughout life remain controversial.
In this study, we revisit the origin of kidney DC and macrophage populations in mice throughout development. We demonstrate that adult kidney contains at least four transcriptionally and functionally distinct cell populations with prominent Clec9a-expression history, indicative of DC origin. Of these, renal F4/80hi cells bear phenotypic and transcriptional similarities to embryonic-derived macrophages. However, using fate mapping, we demonstrate that embryonic-derived macrophages do not show Clec9a-expression history and do not persist in kidney into adulthood. Instead, we find that YS-derived macrophages, which lack MHC class 2 (MHCII) expression, dominate the kidney at birth but are lost within the first few weeks of life. Their loss coincides with the appearance of MHCII+F4/80hi cells that arise from definitive hematopoiesis but lack Clec9a-expression history in early life. At a time when MHCIIneg YS erythro-myeloid progenitor–derived macrophages and MHCII+F4/80hi cells coexist, MHCIInegF4/80hi macrophages preferentially localize to the renal medulla; whereas MHCII+F4/80hi cells spread throughout the medulla and cortex. During cisplatin and ischemia reperfusion–induced AKI, MHCIInegF4/80hi cells reappear in adult kidneys. These cells do not arise from de novo cell differentiation but are the result of MHCII downregulation by tissue-resident MHCII+F4/80hi cells. Taken together, our studies suggest that at least three distinct developmental programs contribute to the kidney MP compartment during development and reveal a previously unappreciated age-dependent ontogenetic heterogeneity of renal MPs.
Methods
Mice
Clec9a-Cre,24 Rosa26-lox-STOP-lox-EYFP,37 ROSA26-lox-STOP-lox-tdtomato,38 Cx3cr-1-green fluorescent protein (GFP),39 c-Myb−/−,40 Csf1rMer-iCre-Mer,41 Mx-1Cre,42 Mybflox/flox,43 and C57BL/6J mice were bred at the Biomedical Center or Walter Brendel Centre for Experimental Medicine (Ludwig Maximilian University of Munich, Martinsried, Germany) in specific pathogen-free conditions. For timed mating, mice of desired genotypes were mated overnight. Embryonic development was estimated considering the day of vaginal plug formation as E0.5. Mice at the age of 8–12 weeks were considered as adults. All animal procedures were performed in accordance with national and institutional guidelines for animal welfare and approved by the Regierung of Oberbayern (district government of Upper Bavaria).
Cell Isolation
Organs were isolated from mice after perfusion with cold PBS and cut into small pieces. Kidneys were digested in 2 ml of RPMI (Thermo Fisher Scientific) with 200 U/ml collagenase IV (Worthington) and 0.2 mg/ml DNAse I (Roche) for 1 hour at 37°C while shaking (120 rpm). After digestion, cells were passed through a 70-µm strainer and washed once with FACS buffer. Leukocytes were enriched using a 70%–37%–30% Percoll gradient by centrifugation (2000 rpm for 30 minutes at room temperature [RT]). Cells were collected at the 70%–37% interface. Percoll (100%) was prepared by adding nine parts of Percoll (GE Healthcare) to one part of 10× concentrated PBS. After Percoll enrichment, cells were washed once and resuspended in FACS buffer (PBS with 1% FBS, 2.5 mM EDTA [Invitrogen], 0.02% sodium azide [Sigma-Aldrich]) for analysis. For functional assays and sorting for RNA sequencing (RNA-seq), FACS buffer without sodium azide was used. Organs from mice up to 3 weeks old were collected without perfusion.
Spleen was digested in 1 ml of RPMI for 30 minutes as above. After digestion, cells were passed through a 70-µm strainer and washed once with FACS buffer. Erythrocytes were lysed with Red Blood Cell Lysing Buffer Hybri-Max (Sigma-Aldrich) for 2 minutes at RT, washed once, and resuspended in FACS buffer for further analysis.
Liver was digested in 2 ml PBS containing magnesium and calcium ions (Sigma-Aldrich) with 1 mg/ml collagenase IV, 60 U/ml DNAse I, 2.4 mg/ml Dispase II (Roche), and 3% FBS (Sigma-Aldrich) for 30 minutes at 37°C while shaking (120 rpm). After digestion, cells were passed through a 100-µm strainer, washed once with FACS buffer, and resuspended in FACS buffer for analysis.
Flow Cytometry
For surface staining, cells were incubated with 50 μl purified anti-mouse CD16/32/FcBlock for 10 minutes at 4°C. Additional antibodies were then added in FACS buffer to a final volume of 100 μl at 4°C for 20 minutes. After staining, cells were washed twice and resuspended in FACS buffer for analysis. Dead cells were identified with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). For intracellular staining, cells were first stained with antibody against extracellular epitopes, and dead cells were identified with Fixable Viability Dye eFluor 780 (Thermo Fisher Scientific) or Zombie UV dye (Biolegend). Stained cells were fixed with 2% paraformaldehyde at RT for 15 minutes, and then washed with FACS buffer. Intranuclear staining was performed by using a Foxp3 Transcription Factor Staining Set (Thermo Fisher Scientific) according to the manufacturer’s protocol. Intracellular staining for cytokine detection was performed by using the Intracellular Fixation and Permeabilization Buffer Set (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Flow cytometry was performed on an LSR Fortessa (BD Biosciences) with subsequent data analysis using FlowJo software (Tree Star). Cell sorting was performed on an Aria III Fusion (BD Biosciences). Cells were quantified by using CountBright Absolute Counting Beads (Thermo Fisher Scientific).
The following antibodies were purchased from Biolegend: anti–CD45.2-Alexa Fluor (AF) 700 (clone 104), anti–CD45.2-R-phycoerythrin-cyanine 7 (PECy7; clone 104), anti-MHCII I-A/I-E-AF700 (clone M5/114.15.2), anti-MHCII I-A/I-E-Allophycocyanin-cyanine 7 (APC-Cy7; clone M5/114.15.2), anti-MHCII I-A/I-E-Pacific Blue (PB; clone M5/114.15.2), anti–MHCII-Brilliant Violet (BV) 510 (clone M5/114.15.2), anti–CD11b-BV421 (clone M1/70), anti–CD11b-APC-Cy7 (clone M1/70), anti–CD11c-BV786 (clone N418), anti–CD11c-APC-Cy7 (clone N418), anti–CD3ε-PECy5 (clone 145–2C11), anti–CD19-BV650 (clone 6D5), anti–Ly6G-Peridinin-chlorophyll (PerCP)-Cy5.5, anti–CD205-PECy7 (clone NLDC-145), anti–F4/80-BV605 (clone BM8), anti–F4/80-BV786 (clone BM8), anti–F4/80-AF647 (clone BM8), anti–CD24-BV605 (clone M1/69), anti–XCR1-BV650 (clone ZET), anti–CD64-PE (clone ×54–5/7.1), anti–CD64-APC (clone X54-5/7.1), anti–CD64-biotin (clone X54-5/7.1), anti–IRF4-AF647 (clone IRF42EA), anti–TNFα-PECy7 (clone MP6-XT22), anti–IL-12/IL-23 p40-PE (clone C15.6), anti–Ly6C-BV605 (clone HK1.4), and anti–Ly6C-PB (clone HK1.4). Streptavidin-BV711 and streptavidin-PE was also purchased from Biolegend.
The following antibodies were purchased from BD Biosciences: anti–CD11b-Brilliant UltraViolet (BUV) 737 (clone M1/70), purified anti-CD16/32 (clone 2.4G2), and anti–ZBTB46-PE (clone U4-1374). Anti–MERTK-PerCPeFluor710 (PerCPeFluor710; clone DS5MMER) and anti–IRF8-PE (clone V3GYWCH) were purchased from Thermo Fisher Scientific. Anti–Mer-Biotin (clone unknown, catalog number BAF591) was purchased from R&D systems.
EdU Labeling
EdU (5-ethynyl-2′-deoxyuridine; Thermo Fisher Scientific) was dissolved in sterile infusion solution (Fresenius Kabi) and injected intraperitoneally (i.p.) at a dose of 0.1 mg/g body wt. Mice were euthanized 6 hours after injection. EdU was detected by using the Click-iT EdU Alexa Fluor 647 Flow Cytometry Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Pulse Labeling of YS-Derived Macrophages
For labeling of YS-derived macrophages, Csf1rMer-iCre-Mer mice were crossed with heterozygous Rosa26-lox-STOP-lox-EYFP reporter mice. Pregnant females were injected at E8.5 with a single dose of 75 μg/g body wt of 4-hydroxytamoxifen (Sigma-Aldrich) supplemented with 37.5 μg/g body wt progesterone (Sigma-Aldrich). Kidneys and livers of F1 mice were analyzed at E18.5 and 2 weeks after birth by flow cytometry.
Conditional Deletion of Myb
Mx-1CreMybflox/flox mice received four i.p. injections of polyinosinic-polycytidylic acid (polyI:C) every other day at a dose of 10 μg/g body wt.44 Mice were then transplanted with 1×107 CD45.1+ congenic wild-type bone marrow cells. At 3 and 9 months after bone marrow transplantation, mice were euthanized, and kidney and liver were analyzed by flow cytometry.
Transwell Chemotaxis Assay
A total of 0.5×105 leukocytes from kidneys were added to the upper well of a transwell support (pore size of 5.0 μm; Corning) on a 24-well plate containing 100 ng/ml recombinant mouse CCL19 and CCL21 (Peprotech) in complete medium (RPMI supplemented with 10% FCS, penicillin/streptomycin, 1% nonessential amino acids, sodium pyruvate, l-glutamine, 0.05 mM β-mercaptoethanol). Cells were incubated at 37°C in humidified atmosphere containing 5% carbon dioxide. After 2 hours, cells were collected from the bottom well and analyzed by flow cytometry. The number of migrated cells per population was quantified by using CountBright Absolute Counting Beads (Thermo Fisher Scientific). The percentage of migrated cells per population was calculated by dividing the cell number from the bottom well to the input number of the respective population.
In Vitro Stimulation with Toll-Like Receptor Ligands
A total of 1×105 kidney leukocytes were seeded in 200 μl complete medium (RPMI supplemented with 10% FCS, penicillin/streptomycin, 1% nonessential amino acids, sodium pyruvate, l-glutamine, 0.05 mM β-mercaptoethanol) and stimulated with resiquimod (R848; 2 µg/ml; Sigma-Aldrich), LPS (lipopolysaccharide, 100 ng/ml; Enzo), or zymosan (10 µg/ml; Sigma-Aldrich). After 2 hours, brefeldin A (5 μg/ml; Biolegend) was added and cells were incubated for an additional 4 hours before analysis by intracellular cytokine staining and flow cytometry.
Cisplatin-Induced AKI
AKI was induced in 10-week-old female Clec9acre/creRosaYFP mice by i.p. injection of cisplatin (15 mg/kg body wt). Sodium chloride was injected as a control for kidney damage. Blood, kidneys, and spleen were collected 72 hours after injection. Serum was isolated from blood for creatinine and BUN measurements using a Cobas Integra 400 Plus analyzer (Roche). Creatinine was detected using a cobas c pack CREP2 (Roche) and BUN was measured with a cobas c pack UREAL (Roche). Kidneys and spleen were processed for flow cytometry.
Unilateral Ischemia-Reperfusion Injury
Unilateral ischemia-reperfusion injury was induced in 10-week-old male Clec9acre/creRosaYFP mice as previously described.45 Briefly, an anesthesia mixture containing medetomidine, midazolam, and fentanyl was applied before surgery. Online rectal temperature recording was then installed for every mouse. The left unilateral renal pedicle of each mouse was clamped for 25 minutes with a micro aneurysm clamp (Medicon) after a flank incision. Body temperature was continuously measured with a rectal probe and maintained at 36.5–38.5°C by placing the mice on a heating plate. After clamping, the kidney was placed back inside the abdomen. After clamp removal, the kidney was inspected for color change from pale (ischemia) to the original color before closing wounds with absorbable sutures for the peritoneal and cutaneous layer (Ethicon). To maintain fluid balance, all mice were supplemented with 200 µl of saline. Three days after surgery, kidneys were isolated and analyzed as described above.
RNA Isolation and Library Construction
Total RNA was isolated using the column-based PicoPure RNA Isolation Kit (Thermo Fisher Scientific). RNA quality was assessed using a 2100 Bioanalyzer (Agilent) and samples with an RNA integrity number of more than eight were used for cDNA synthesis by using the ultra-low input RNA SMART-Seq v4 Kit (Clontech) according to the manufacturer’s instructions. cDNA was transferred to AFA Fiber Pre-Slit Snap-Cap 6×16 mm microTUBEs (Covaris) and sheared by sonication using a Covaris S220 instrument. Sheared cDNA was cleaned using ethanol precipitation and sonication efficiency was determined using the Agilent 2100 Bioanalyzer. A maximum of 10 ng sheared cDNA was used to generate libraries for RNA-seq with the MicroPlex Library Preparation Kit v2 (Diagenode). The libraries were amplified until a DNA concentration >5 ng/µl was reached as determined by Qubit 2 DNA quantification (Thermo Fisher Scientific). Amplified libraries were cleaned using AMPure XP beads (Beckman Coulter) as described in the SMART-seq v4 Kit protocol, and the final concentration as well as the purity of the libraries were assessed using a 2100 Bioanalyzer. Sequencing was performed on an Illumina HiSeq1500 sequencer with 50-bp single-end reads and a sequencing depth of 20 million reads.
RNA-Seq Analysis
RNA-seq reads were mapped to the mouse genome (mm10) using STAR.46 Expression of genes in transcripts per kilobase million was calculated with RSEM.47 RNA-seq analysis was performed in R (version 3.4.3) with RStudio (version 1.1.414). DESeq2 (version 1.18.1) was used for differential gene expression analysis and principal component analysis (PCA). Genes with average gene counts of less than one were discarded and log2 fold-change shrinkage was performed using the Apeglm package.48 iCre and Gapdh DNA sequences were loaded in R using Biostrings (version 2.50.1) and manual alignment was performed with Shortread (version 1.40.0). Heatmaps were generated using pheatmap (version 1.0.10) and graphs were plotted with ggplot2 (version 2.2.1). Sequencing data have been deposited in the Gene Expression Omnibus under accession numbers GSE131751 and GSE135921.
Immunofluorescence Microscopy
Kidneys were fixed overnight at 4°C in paraformaldehyde, dehydrated in phosphate buffer containing 30% sucrose overnight at 4°C,49 transferred to Tissue-Tek O.C.T. (Sakura), and frozen on dry ice. Frozen sections (10 µm thick) were cut on a cryostat at −20°C (CM3050S; Leica), rehydrated in PBS, and permeabilized with PBS with 0.2% Triton X-100 (Sigma-Aldrich) or acetone (Sigma-Aldrich). Afterward, the sections were circled with a PAP Pen (Kisker Biotech GmbH) and blocked for 1 hour at RT in the dark with blocking buffer containing 10% goat serum in PBS. Antibodies were diluted in blocking buffer and sections were incubated for 2 hours at RT in the dark with the antibody mixture. Finally, stained sections were washed with PBS, mounted with ProLong Diamond Antifade Mountant (Thermo Fisher Scientific), cured at RT for 24 hours in the dark, and stored at 4°C until imaging. Confocal microscopy was performed at the Core Facility Bioimaging of the Biomedical Center with an upright Leica SP8X WLL microscope equipped with a 405 nm laser, WLL2 laser (470–670 nm), and acousto-optical beam splitter. Three-dimensional tile scans were acquired with a 20×0.75 objective and image voxel size was 180 nm in the x/y direction and 0.5–1.3 µm in the z direction. The following channel settings were used: DAPI/BV421 (excitation, 405 nm; emission, 415–470 nm), AF488 (500 nm; 510–542 nm), tdTomato (553 nm; 563–591 nm), AF594 (592 nm; 605–640 nm), and AF647 (646 nm; 656–718 nm). Recording was done sequentially to avoid bleed through. BV421, AF488, AF594, AF647, and tdTomato were recorded with hybrid photo detectors and DAPI with a conventional photomultiplier tube. Tile scans were merged in LAS X (version 3.4.1.17670; Leica) and deconvolved using Huygens Professional (version 17.10.0p2.64b; Scientific Volume Imaging). Deconvoluted z-stacks were imported in Fiji50 to create maximum projections, adjust brightness/contrast, and to add scale bars.
The following antibodies were purchased from Biolegend: anti-MHCII I-A/I-E-BV421 (clone M5/114.15.2), anti–CD11b-AF647 (clone M1/70), anti–CD31-AF488 (clone MEC13.3), anti–F4/80-AF594 (clone BM8), and anti–F4/80-AF647 (clone BM8). Anti-CD64 (50086-R027, rabbit IgG) was purchased from Sino Biologic, cleaved caspase-3 (clone D3E9, rabbit IgG) was purchased from Cell Signaling Technologies, goat anti-rabbit IgG-AF555 (A21429) was purchased from Thermo Fisher, and goat anti-rabbit IgG-AF488 (111-545-144) secondary antibody was ordered from Jackson ImmunoResearch. For quantification of MHCIInegF4/80hi cells and MHCII+F4/80hi cells, 300×300 µm cutouts were randomly chosen from cortex and medulla of deconvoluted tile scans. F4/80neg cells were excluded using the Interactive Watershed plugin on the thresholded F4/80 channel. F4/80+ cells with high expression of CD11b were not counted.
Histo-Cytometry
Histo-cytometry was adapted from Li et al.51 Deconvoluted z-stacks were opened in Fiji for further processing. The z-stack was split in its channels and each channel was thresholded with the Auto Threshold plugin and default settings. The thresholded channels were recombined to a stack and transformed to a red-green-blue image. Noise was removed with the Despeckle plugin and the image was blurred with a Gaussian Blur filter and a 2-pixel radius to improve segmentation. The resulting image was transformed to an 8-bit image and segmentation was performed with the Interactive Watershed plugin. Using the same plugin, the selections for all segmented cells were imported from LabelMap to ROIManager. Finally, the selections were applied to the original deconvoluted tile scan to measure area, position, shape descriptors, and mean channel intensity of each segmented cell. Results were combined to comma-separated value files, which were then converted to a flow-cytometry standard file for gating analysis using FlowJo software.
Statistical Analysis
Statistical significance was calculated using the two-tailed t test in Prism 7 software (GraphPad). Multiple comparison was performed by using one-way ANOVA. A P value <0.05 was considered significant.
Results
The Adult Kidney Contains Four Subsets of CDP-Derived Cells
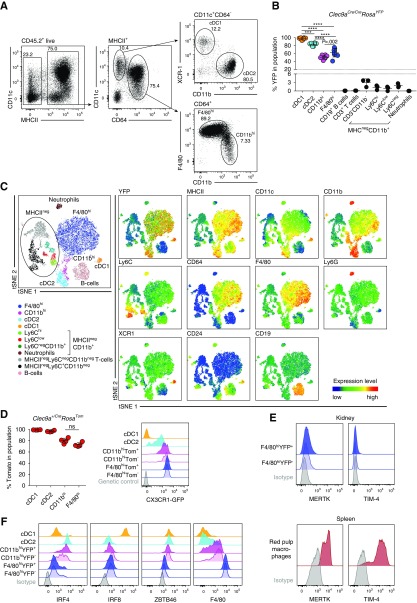
To characterize MPs of CDP origin in kidneys using flow cytometry, CD45+ kidney leukocytes from Clec9acre/creRosaYFP mice were gated and MHCII+ cells were identified independently of CD11c expression levels, because adult kidney harbors CDP-derived leukocytes that express little or no CD11c.24 Within the CD45+MHCII+ fraction, CD11c−CD64− cells constitute B cells and CD11c+CD64− cells comprise the two main XCR-1+CD11b− cDC1 and XCR-1−CD11b+ cDC2 populations (Figure 1A, Supplemental Figure 1A). CD64+ cells can further be split based on differential expression of F4/80 and CD11b into F4/80hiCD11blow and CD11bhiF4/80low cells (Figure 1A).24 For simplicity, we refer to these populations as F4/80hi and CD11bhi cells, respectively. As previously demonstrated, cDC1s exhibit near complete labeling with yellow fluorescent protein (YFP) in Clec9acre/creRosaYFP mice, whereas labeling of cDC2s is lower (83%±4%; Figure 1B). Increased labeling of cDC1s is to be expected because cDC1s express DNGR-1 in their differentiated form and thus can become labeled with YFP as fully mature cells.24 In cDC2s, which lack DNGR-1, YFP labeling is a true indicator of cell origin (Figure 1B), although labeling of cDC2s is incomplete because some Clec9a positive DC precursors escape labeling in Clec9acre mice.24 F4/80hi and CD11bhi cells also lack DNGR-1 expression24 and label with YFP at 62%±11% and 50%±5%, respectively, indicating a CDP contribution to these populations (Figure 1B). No YFP labeling was observed in other lymphoid or myeloid cell lineages in the kidney, including MHCIInegCD11b+ cells, which include monocytes and neutrophils (Figure 1B, Supplemental Figure 1A). In vivo pulse labeling of proliferating cells with the thymidine analog EdU revealed that renal cDC2s showed lower homeostatic turnover than cDC1s but were comparable to CD11bhi cells (Supplemental Figure 1B). F4/80hi cells showed little to no EdU incorporation, indicating a slow homeostatic turnover of this population in steady-state kidney (Supplemental Figure 1B).
Figure 1.
The adult kidney contains four phenotypically distinct subsets of MPs with Clec9a-expression history. (A–C) Kidney leukocytes from 10- to 12-week-old Clec9acre/creRosaYFP mice were analyzed by flow cytometry. (A) Live CD45.2+ MHCII+ cells were gated as indicated and subdivided into CD11c+CD64− and CD64+ cells. CD11c+CD64− cells were further analyzed for XCR-1 and CD11b expression to identify cDC1s and cDC2s, respectively. CD64+ cells were further divided into F4/80hi and CD11bhi cells. (B) The percentage of YFP+ cells in the indicated populations (see also Supplemental Figure 1A) is shown. (C) Representative t-distributed stochastic neighbor embedding (tSNE) of kidney leukocytes. Cells were clustered independently of their YFP labeling and manually gated populations were overlaid on the tSNE plot in the indicated colors. Blue-to-red gradient indicates increasing intensity of marker expression. (D) Left panel shows the percentage of tomato+ cells in each population; right panel, CX3CR-1-GFP expression in cDC1s, cDC2s, as well as F4/80hi and CD11bhi cells, further divided into tomato+ and tomato− cells. Gray traces represent GFP fluorescence in control mice lacking the CX3CR-1-GFP allele. (E) Renal YFP+ and YFP− F4/80hi cells were analyzed for MERTK and TIM-4 expression by flow cytometry. Expression of these markers on splenic RPMs is shown as positive control. (F) Renal cDC1s, cDC2s, YFP+ and YFP− F4/80hi cells, as well as YFP+ and YFP− CD11bhi cells from Clec9acre/creRosaYFP mice were analyzed for IRF4, IRF8, ZBTB46, and F4/80 expression. Gray traces represent staining with isotype-matched control antibodies. (B–D) Each dot represents one mouse, horizontal bars represent mean, error bars represent SD. ***P<0.001, ****P<0.0001.
Because F4/80hi and CD11bhi populations labeled with YFP to a lower extent than cDC2s, we wanted to address whether the YFP+ and YFP− F4/80hi and CD11bhi populations exhibit ontogenetic heterogeneity. Therefore, we performed phenotypic analysis of kidney leukocytes using macrophage and DC markers. We first generated triple transgenic mice by crossing Clec9a-Cre mice to Rosalox-stop-lox-tomato (RosaTom)38 and Cx3cr1-Gfp transgenic mice.39 In these mice, the expression of CX3CR1 can be assessed by GFP fluorescence and in cells of CDP origin, which are marked by tomato fluorescence. RosaTom mice are efficient fate-reporter mice due to a short distance between the loxP sites and a strong promoter driving tomato expression.52 Accordingly, the penetrance of CRE-mediated DNA rearrangement was increased in Clec9a+/creRosaTomCx3cr-1+/GFP mice compared with our observations in Clec9acreRosaYFP mice.24 Near complete labeling of renal cDC2s (97%±1%) and increased labeling of CD11bhi cells (80%±5%) and F4/80hi cells (73%±4%) was observed in Clec9a+/creRosaTom animals heterozygous for Cre (Figure 1D, left panel). Importantly, tomato signal remained restricted to CDP-derived cells in Clec9a+/creRosaTomCx3cr-1+/GFP mice (Supplemental Figure 1C). We therefore concluded that heterozygous Clec9a+/creRosaTom mice are efficient reporters of CDP origin and can be used for fate-mapping experiments interchangeably with homozygous Clec9acre/creRosaYFP mice.
CX3CR-1 is highly expressed in macrophages of embryonic origin, some tissue macrophage populations such as microglia and cardiac macrophages, and to a lower extent on monocyte-derived cells and some cDCs.53 As expected, renal cDC1s lacked CX3CR1 expression, whereas cDC2s expressed low to intermediate levels of CX3CR1 (Figure 1D, right panel, Supplemental Figure 1D). The highest CX3CR1-GFP levels were observed in F4/80hi cells (Figure 1D, right panel, Supplemental Figure 1D), consistent with previous reports,30,31 and GFP levels did not differ between tomato-labeled and unlabeled F4/80hi cells (Figure 1D, right panel, Supplemental Figure 1D). Notably, F4/80hi cells, independent of whether they were displaying Clec9a-expression history or not, lacked expression of MERTK and TIM-4 (Figure 1E, Supplemental Figure 1E), markers typically associated with embryonic-derived or self-maintaining macrophages.16,18,54,55 F4/80hi cells also expressed low levels of the cDC-specifying transcription factors IRF4, IRF8, and ZBTB46 (Figure 1F, Supplemental Figure 1F). As reported, CD11bhi cells principally resembled cDC2s based on high levels of IRF4 and ZBTB46, and concomitant low levels of IRF8 expression26 (Figure 1F, Supplemental Figure 1F), but showed higher CX3CR1 and F4/80 expression than cDC2s (Figure 1, D and F, Supplemental Figure 1, D and F). Phenotypic analysis did not show obvious differences between CD11bhi cells displaying Clec9a-expression history or not (Figure 1, D and F). Thus, F4/80hi and CD11bhi populations differ from each other and cDC1/2s in terms of marker expression, although no obvious phenotypic differences exist between the YFP+ and YFP− fractions of these populations.
Renal CD11bhi and F4/80hi Cells Are Transcriptionally Distinct from cDC1 and cDC2
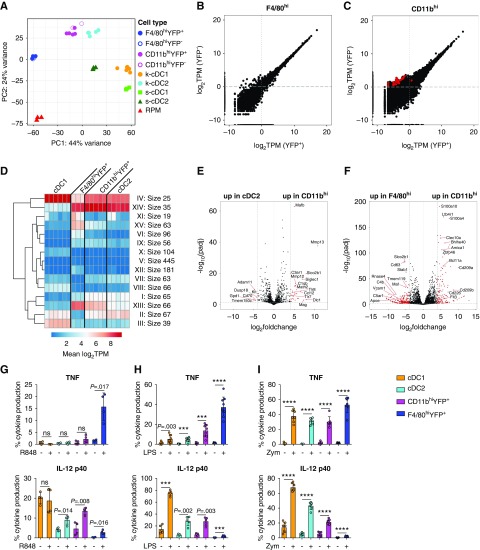
To further reveal differences between phenotypically similar cells displaying Clec9a-expression history or not, we performed transcriptome profiling by mRNA sequencing. We sort purified the YFP+ and YFP− fractions of F4/80hi and CD11bhi cells from kidneys of Clec9acre/creRosaYFP mice and compared their gene expression profiles to those of cDC1s and cDC2s sorted as YFP+ cells from the same organ (for gating strategy and sort purity see Supplemental Figure 2, A and B). As reference populations, splenic YFP+ cDC1s, YFP+ cDC2s, and red pulp macrophages (RPMs) were sorted. In PCA, reference cDC1s and cDC2s segregated from splenic RPMs on principal component 1 (PC1), revealing transcriptional differences between cDCs and macrophages (Figure 2A). PC2 identified tissue-specific differences in gene expression and uniformly separated populations sorted from spleen and kidney (Figure 2A). As expected, cDC1s and cDC2s from different organs clustered in close proximity on PC1 (Figure 2A). Notably, CD11bhi cells segregated away from cDC1s and cDC2s in PCA (Figure 2A), indicating that they are transcriptionally distinct, although they more closely resembled cDC2 than cDC1. To our surprise, YFP+ and YFP− CD11bhi cells clustered together in PCA (Figure 2A) and showed few transcriptional differences in pairwise comparison (adjusted P<0.05, log2 fold change>4; Figure 2C), demonstrating a strong transcriptional overlap. A strong resemblance was also observed between YFP+ and YFP− F4/80hi cells in PCA and pairwise comparison, and these populations unexpectedly clustered away from the other renal populations but close to RPM in PC1 (Figure 2, A and B). Because the Clec9a-expression history indicates a common origin of F4/80hi cells with cDCs,24 we would have expected a closer resemblance of F4/80hi cells to cDCs, particularly in the YFP+ fraction. Importantly, we detected no Cre expression in cDC2s, CD11bhi cells, and F4/80hi cells; whereas cDC1s showed strong expression of Cre, as expected (Supplemental Figure 2C). These data corroborate that these populations lack Clec9a promoter activity and therefore the YFP label must result from Clec9a expression at an earlier time.24
Figure 2.
Renal F4/80hi are transcriptionally distinct from cDC1s, cDC2s, and CD11bhi cells. (A–F) YFP+ and YFP− fractions of F4/80hi and CD11bhi cells as well as YFP+ cDC1s and YFP+ cDC2s were sorted from kidneys of adult Clec9acre/creRosaYFP mice. Splenic YFP+ cDC1s, YFP+ cDC2s, and RPMs were isolated as reference populations from the same mice. Sorted populations were subjected to mRNA-sequencing analysis. (A) PCA of the top 500 genes as defined by the highest variance across all samples. Each dot of the same color represents a biologic replicate. (B and C) Comparison of gene expression in (B) YFP+ and YFP− F4/80hi cells and (C) YFP+ and YFP− CD11bhi cells displayed as transcripts per kilobase million (TPM) reads. Red circles indicate genes with a log2 fold change greater than four between samples and an adjusted P (padj)<0.05. (D) k-means clustering of differentially expressed genes (log2 fold change>4; adjusted P<0.05) between renal cDC1s, cDC2s, YFP+ CD11bhi cells, and YFP+ F4/80hi cells. (E and F) Pairwise comparison of (E) cDC2s with YFP+ CD11bhi cells or (F) YFP+ F4/80hi with YFP+ CD11bhi cells was performed on RNA-seq data using the DEseq2 plugin in R. Red circles indicate genes with a log2 fold change greater than four between samples and an adjusted P value <0.05. (G–I) Renal leukocytes from adult Clec9acre/creRosaYFP mice were isolated and stimulated in vitro with (G) R848, (H) LPS, or (I) zymosan (Zym) for 6 hours. Intracellular levels of TNF and IL-12 p40 were analyzed by flow cytometry. Each dot represents one mouse, error bars represent SD. ***P<0.001, ****P<0.0001.
Because RNA-seq analysis did not reveal differences between YFP+ and YFP− populations, we focused exclusively on CD11bhi and F4/80hi samples sorted as YFP+ cells for further analyses. Using unsupervised, hierarchical k-means clustering of genes differentially expressed between renal cDC1s, cDC2s, CD11bhi cells, and F4/80hi cells, we defined 15 clusters of genes with distinct expression characteristics (Figure 2D). Of these, several clusters (II, VI, and XII) distinguished F4/80hi cells and revealed lower expression of typical DC-associated genes, such as Zbtb46, Flt3, Bcl11a, and Ccr7, in F4/80hi cells compared with the other renal populations (Figure 1F, Supplemental Table 1).26 Clusters XIII and XV contained genes with higher expression in F4/80hi and CD11bhi cells compared with cDC2s and cDC1s and included FcgR1 (CD64), Emr1 (F4/80), and Cx3cr1 (Supplemental Table 1). Pairwise comparison of cDC2s and CD11bhi cells identified 38 genes with a log2 fold change greater than four (adjusted P<0.05; Figure 2E) and 180 genes with a log2 fold change greater than two. Differentially expressed genes included several genes involved in pathogen defense such as Tlr7, Tlr8, and the complement receptor C3ar1 (Figure 2E, Supplemental Table 2). Pairwise comparison of CD11bhi cells with F4/80hi cells revealed 362 genes with a log2 fold change greater than four (adjusted P<0.05; Figure 2F, Supplemental Table 3). Higher expression of Zbtb46, Flt3, Bcl11a, and Blhe40 in CD11bhi cells confirmed a more classic cDC transcriptional signature in this population than in F4/80hi cells (Figure 2F, Supplemental Table 3). In contrast, F4/80hi cells had higher expression of several macrophage-associated genes such as Slco2b1, VCam1, and Tmem119 (Figure 2F, Supplemental Table 3). Taken together, these analyses demonstrate that CD11bhi and F4/80hi cells are transcriptionally distinct from cDC1/2s, although it is noteworthy that CD11bhi cells were more similar to cDC2s than cDC1s and F4/80hi cells.
In response to TLR ligands, cDC1s were the most potent source of interleukin (IL)-12, because we detected high amounts of preformed IL-12 in this population even without prior stimulation (Figure 2, G–I, gating strategy in Supplemental Figure 3A). cDC1s were also the strongest producers of IL-12 in response to LPS and zymosan stimulation, although they did not respond to resiquimod (R848) stimulation with production of TNF or IL-12 (Figure 2, G–I). F4/80hi cells were the most potent producers of TNF in response to R848, LPS, and zymosan (Figure 2, G–I) but they were poor producers of IL-12 under the tested conditions (Figure 2, G–I). cDC2s and CD11bhi cells did not produce TNF in response to R848 (Figure 2G). Notably, more CD11bhi cells than cDC2s produced IL-12 in response to R848 (Figure 2G), in line with higher TLR8 expression in these cells compared with cDC2s (Supplemental Figure 3B). In contrast, more cDC2s produced IL-12 in response to zymosan than CD11bhi cells (Figure 2G), despite comparable expression of TLR2 and lower expression of Dectin1 (Supplemental Figure 3B). In this analysis we found a similar response between the YFP+ and YFP− fractions of F4/80hi and CD11bhi cells, respectively, although it is noteworthy that YFP− F4/80hi cells showed slightly reduced TNF production to LPS and zymosan compared with their YFP+ counterparts (Supplemental Figure 3, C–E). CCR7 mediates migration of cDCs to lymph nodes and showed a relative paucity in expression in F4/80hi and CD11bhi cells when compared with cDC1s and cDC2s (Supplemental Table 1). Accordingly, cDC1s and cDC2s isolated from the steady-state kidney migrated toward the CCR7 ligands CCL19/21 in in vitro transwell chemotaxis assays (Supplemental Figure 3F). F4/80hi and CD11bhi cells failed to migrate toward CCL19/21 in the same assay (Supplemental Figure 3F). Taken together, these analyses show that cDC1s, cDC2s, CD11bhi cells, and F4/80hi cells are transcriptionally distinct populations of renal MPs that display the distinct ability to respond to pathogenic stimuli.
Renal Embryonic–Derived Macrophages Do Not Show Clec9a-Expression History
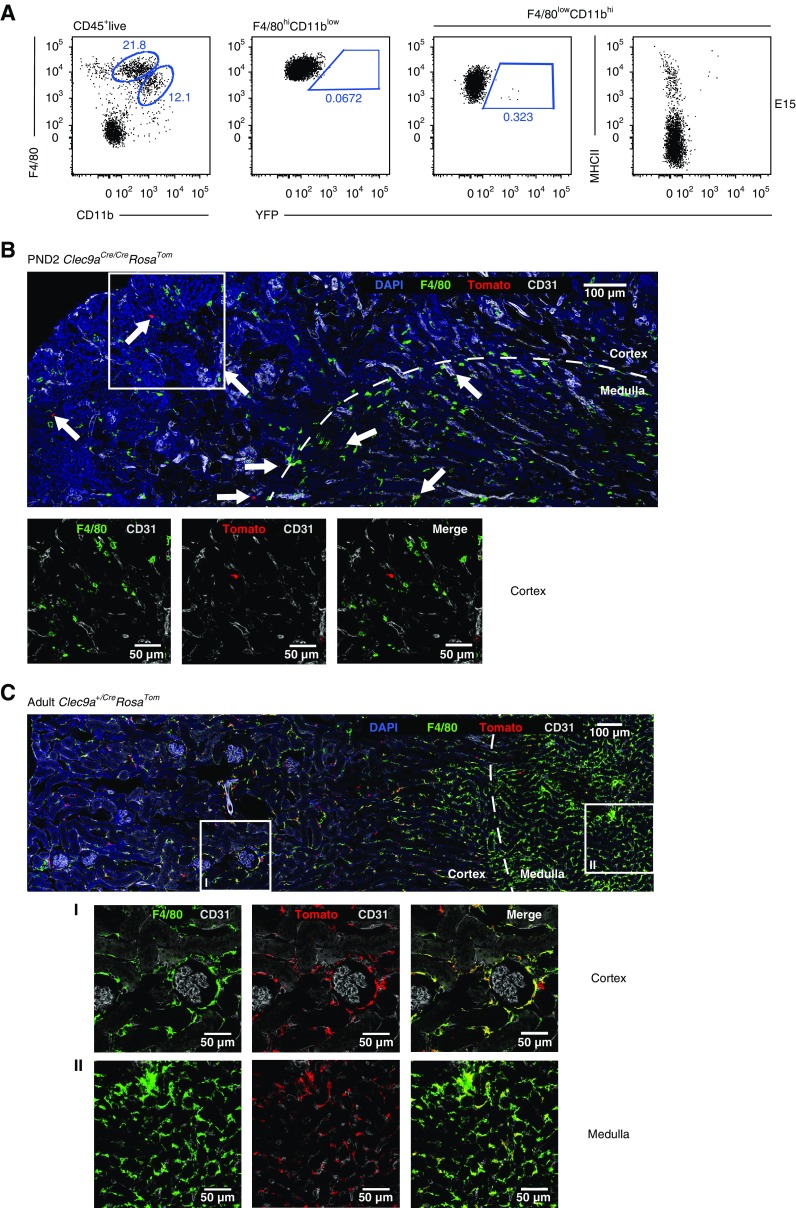
Considering that, despite exhibiting Clec9a-expression history, renal F4/80hi cells resembled macrophages in terms of phenotype (Figure 1) and gene expression (Figure 2A), we wanted to exclude the possibility that renal YS-derived macrophages aberrantly label in Clec9acre mice. We therefore isolated kidneys from Clec9acre/creRosaYFP mice on E15, when macrophages with a F4/80hi phenotype predominantly arise from YS progenitors.11–13,56,57 As expected, E15 kidneys from Clec9acre/creRosaYFP mice contained F4/80hi and CD11bhi cells, which represent YS-derived macrophages and Myb-dependent HSC-derived cells, respectively.12 Importantly, F4/80hi cells did not show any YFP labeling in Clec9acre/creRosaYFP mice (Figure 3A), whereas YFP+ cells could be found within the Myb-dependent CD11bhi fraction of E15 kidneys,12 indicating the Clec9a promoter is active at this stage of development (Figure 3A). These YFP+ cells uniformly expressed MHCII and CD11c, consistent with them being cDCs (Figure 3A and data not shown). Taken together, these data strongly support the notion that embryo-derived macrophages do not label with YFP in Clec9acre/creRosaYFP mice.
Figure 3.
Embryonic macrophages do not show Clec9a-Cre expression history. (A) Kidneys from Clec9acre/creRosaYFP mice were isolated at E15 and analyzed for F4/80 and CD11b expression by flow cytometry to identify F4/80hi YS-derived macrophages and CD11bhiF4/80low cells. Populations were further analyzed for YFP and MHCII expression. (B and C) Immunofluorescence staining of kidneys from Clec9acreRosaTom mice (B) at PND2 and (C) at 10 weeks of age. Kidney sections were stained for the following markers: tomato (red), F4/80 (green), CD31 (gray), and DAPI (blue). Deconvoluted confocal tile scans were generated. (B) Arrows indicate tomato+ cells. Scale bar, 100 µm. Square marks the inset that is magnified below the tile scan to indicate absence of F4/80 signal on tomato+ cells. (C) Squares mark magnified areas of (I) the cortex or (II) the medulla that are shown below the tile scan image (scale bar, 50 µm) to indicate colocalization of F4/80 and tomato signal.
To confirm this, we performed microscopic analysis of kidney sections from Clec9acre/creRosaTom mice 2 days after birth (postnatal day 2 [PND2]; Figure 3B). As expected, we found F4/80-expressing cells in newborn kidney that were spread throughout the medulla and cortex (Figure 3B), whereas tomato-positive cells were fewer in number and mostly lacked F4/80 expression (Figure 3B, white arrows). This was in striking contrast to kidney sections from adult Clec9a+/creRosaTom mice, where tomato-positive cells costained with F4/80 showed dendritic shape and spread throughout the entire kidney interstitium, as expected (Figure 3C).24 Notably, in adult kidneys, a fraction of Tomato+ F4/80hi cells formed a discrete population around glomeruli (Figure 3C), which may indicate a role for these cells in monitoring the transendothelial transport of proteins and particles.33 Taken together, these data demonstrate that the failure to detect Clec9a-expression history in renal F4/80hi YS-derived macrophages at E15 by flow cytometry was not due to an inability to isolate these cells from tissues. These data unequivocally establish that embryonic-derived macrophages are not aberrantly labeled in Clec9a-Cre mice, although we cannot formally exclude at this point that a persistent population of embryonic-derived macrophages acquires Clec9a expression and consequently YFP/tomato labeling in later life.
Dynamic Age-Dependent Changes in the Renal MP Network
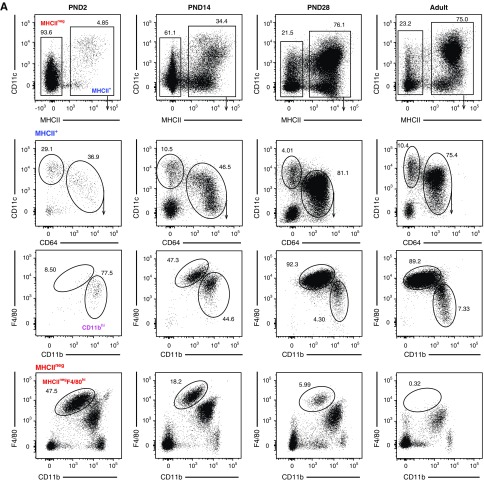
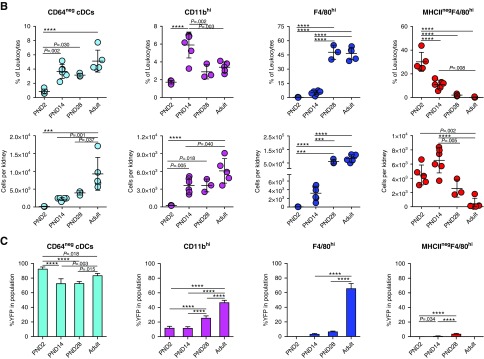
We next asked when YFP+F4/80hi cells arise in kidneys of Clec9acre/creRosaYFP mice (Figure 4, A and B). CD45+MHCII+ cells could be identified as early as PND2 in the kidney, but their relative frequency was low compared with adult kidney (Figure 4, A and B). CD45+MHCII+ cells divided into the expected CD64+ and CD64− fractions on PND2 (Figure 4, A and B). MHCII+CD64− cells encompassed XCR-1+ cDC1s and CD11b+ cDC2s at all ages examined, as expected (not shown). Surprisingly, MHCII+CD64+ kidney leukocytes uniformly displayed a CD11bhi surface phenotype on PND2 (Figure 4A). The MHCII+CD64+ F4/80hi subset was observed 2 weeks after birth and, by 4 weeks of age, had become the dominant population of MHCII+CD64+ cells (Figure 4A). Notably, F4/80hi cells were present in kidneys of Clec9acre/creRosaYFP mice on PND2 but they lacked MHCII expression (Figure 4, A and B). In newborn kidney, these MHCIInegF4/80hi cells were more abundant in frequency and number than the other MP subtypes analyzed, but this population showed an age-dependent decrease and became a minority population of kidney leukocytes by 4 weeks of age and was not found in adult kidneys (Figure 4, A and B).
Figure 4.
Renal MP populations exhibit dynamic age-dependent changes. (A–C) Kidneys isolated from Clec9acre/creRosaYFP mice on PND2, PND14, PND28, and 10–12 weeks after birth were analyzed by flow cytometry. (A) Cells were first gated on live CD45.2+ cells and further subdivided into MHCIIneg and MHCII+ cells (top row). MHCII+ cells were further analyzed for CD11c and CD64 expression (second row). MHCII+CD64+ cells (third row) and MHCIIneg cells (bottom row) were further analyzed for CD11b and F4/80 expression. (B) Frequency and total number of kidney CD64− cDCs, CD11bhi, F4/80hi and MHCIInegF4/80hi cells at the indicated ages are shown. (C) Percentage of YFP+ cells in each population from Clec9acre/creRosaYFP mice at the indicated ages. Each dot represents one mouse, horizontal lines indicate mean, error bars represent SD. ***P<0.001, ****P<0.0001, only statistically significant differences are marked.
We next analyzed YFP expression in each of these populations in Clec9acre/creRosaYFP mice over time. MHCII+CD64− cDCs, which encompass both cDC1 and cDC2, served as our reference population and, expectedly, showed near complete YFP labeling (93%±3%) in Clec9acre/creRosaYFP mice as early as PND2 (Figure 4C). MHCIInegF4/80hi cells did not label with YFP in Clec9acre/creRosaYFP mice at all ages examined (Figure 4C). To our surprise and in contrast to our findings in adult mice, MHCII+ F4/80hi and CD11bhi cells showed a paucity in YFP labeling in early life when compared with their adult counterparts (Figure 4C). The lack of YFP labeling was especially pronounced in F4/80hi cells, which acquired most of their YFP label after 4 weeks of age (Figure 4C). These data demonstrate that F4/80hi cells do not arise from Clec9a-expressing cDC progenitors in early life. Importantly, the temporal differences in population expansion between MHCIInegF4/80hi cells and MHCII+F4/80hi cells in early life suggest a different ontogeny of these cells.
Renal MHCIInegF4/80hi Cells but Not F4/80hi Cells Expressing MHCII Are Derived From Erythro-Myeloid Progenitors
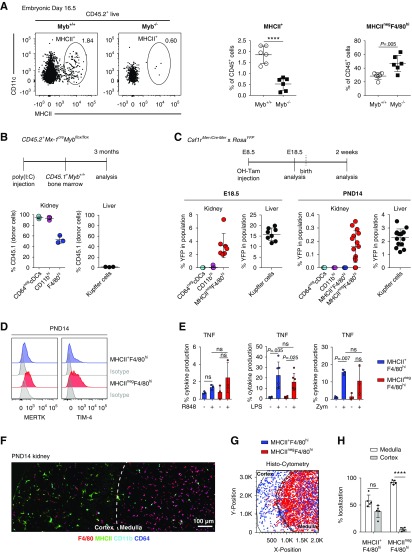
Because the age-dependent loss of MHCIInegF4/80hi cells correlated with an increase in F4/80hi cells (Figure 4B), we hypothesized that MHCIInegF4/80hi cells may represent YS-derived macrophages, which are described to lack MHCII in some tissues.15,16,55 The transcription factor MYB is dispensable for YS myelopoiesis but it is required for the development of definitive HSCs and late erythro-myeloid progenitors arising from hemogenic endothelium, which generate macrophages via fetal liver monocytic intermediates.11,40 MHCIInegF4/80hi cells developed normally in kidneys from Myb-deficient embryos at E16.5, confirming these cells arise independently of definitive hematopoiesis or Myb-dependent late erythro-myeloid progenitors (Figure 5A). In contrast, MHCII+ kidney leukocytes were strongly decreased in the absence of MYB (Figure 5A). Because F4/80hi cells expressing MHCII are first established after birth (Figure 4, A and B), their MYB dependence cannot be analyzed in full knock-out mice, which are embryonically lethal. We therefore induced conditional deletion of the Myb gene in adult mice. To this end, CD45.2+Mx-1CreMybflox/flox mice were treated with polyI:C, which leads to a rapid loss of HSCs and their progeny that can be rescued without further preconditioning by transfer of congenic CD45.1+ bone marrow.12,44 Three months after bone marrow rescue, chimeras were analyzed for bone marrow contribution to kidney MP subsets (Figure 5B). As expected, because of their HSC origin, cDCs were completely donor derived (Figure 5B). Similarly, CD11bhi cells showed full donor origin, demonstrating they are HSC derived (Figure 5B). MHCII+F4/80hi cells were also strongly donor derived (53%±7%), although a considerable proportion of these cells remained of host origin 3 months after bone marrow rescue (Figure 5B). Notably, the replacement of F4/80hi cells increased with time and, 9 months after bone marrow rescue, 74%±7% of cells had turned over (Supplemental Figure 4A). These data indicate that the incomplete donor chimerism is likely related to the relative longevity and low homeostatic turnover of this population in adult kidneys (Supplemental Figure 1B). Thus, although F4/80hi cells are not fully replaced by HSCs in the time frame analyzed, HSCs contribute to the maintenance of MHCII+F4/80hi cells in steady-state adult kidney.
Figure 5.
MHCII+ cells in the kidney are Myb dependent and do not arise from embryonic progenitors. (A) Kidneys from Myb−/− and wild-type littermate control mice were analyzed by flow cytometry on E16.5. Left: CD11c and MHCII expression of live CD45.2+ cells in mouse embryos of the indicated genotype is shown. Right: The frequency of MHCII+ and MHCIInegF4/80hi cells among live CD45.2+ cells is shown. (B) CD45.2+Mx-1creMybflox/flox were treated with serial polyI:C injections to induce deletion of HSCs and subsequently transplanted with bone marrow from congenic CD45.1+ wild-type mice. The percentage of donor-derived CD45.1+ cells in the indicated populations in the liver and kidney is shown. (C) Csf1rMer-iCre-Mer females were mated with male Rosa+/YFP mice and injected with 4-hydroxytamoxifen (OH-TAM) on E8.5. On E18.5 and 2 weeks after birth, kidneys and liver from offspring mice were analyzed for YFP expression by flow cytometry. Renal populations were identified as in Figure 4A. Liver Kupffer cells were identified as live CD45.2+F4/80hi cells. The percentage of YFP-positive cells in the indicated populations in kidneys and liver is shown. (D) Renal MHCII+F4/80hi and MHCIInegF4/80hi cells from 2-week-old mice were analyzed for MERTK and TIM-4 expression by flow cytometry. Gray traces represent staining with isotype-matched control antibodies. (E) Renal leukocytes from 2-week-old mice were stimulated in vitro with R848, LPS, or zymosan (Zym) for 6 hours and analyzed for intracellular TNF production by flow cytometry. The frequency of TNF-positive MHCII+F4/80hi and MHCIInegF4/80hi cells was calculated and plotted. (F–H) Kidney sections from two-week-old Clec9acreRosaTom mice were analyzed by histo-cytometry. (F) Immunofluorescence image of the following markers: F4/80 (red), MHCII (green), CD11b (magenta), and CD64 (blue). Scale bar, 100 µm. (G) Histo-cytometry was used to identify the x and y position of MHCIInegF4/80hi and MHCII+F4/80hi cells in the kidney sections. Lines separating renal medulla and cortex were drawn by hand based on tissue structure, autofluorescence properties, and presence of glomeruli. (H) The frequency of MHCIIneg F4/80hi and MHCII+F4/80hi cells located in the renal cortex and medulla was quantified using gates on the renal cortex or medulla in histo-cytometry analysis and plotted. Each dot represents one mouse. Horizontal bars represent mean, error bars represent SD. ****P<0.0001.
To address whether YS-derived progenitors contribute to F4/80hi cells, we took advantage of an established genetic model to fate map these cells in Csf1rMer-iCre-Mer mice crossed to RosaYFP mice.11,12,16 We treated pregnant dams at E8.5 with a single injection of 4-hydroxytamoxifen12 and then analyzed the kidneys from offspring mice at E18.5 and 2 weeks after birth by flow cytometry (Figure 5C). Liver Kupffer cells, which derive in large proportions from YS erythro-myeloid progenitors11,12 readily labeled with YFP at both ages examined (Figure 5C). On E18.5, renal MHCIInegF4/80hi cells also labeled with YFP, albeit at lower frequency than liver Kupffer cells and consistent with previous publications.11–13 In contrast, CD64− cDCs and CD11bhi cells did not show YFP expression, as expected because of their HSC origin (Figure 5C). Importantly, 2 weeks after birth, when both MHCII-expressing and -nonexpressing F4/80hi cells are present in kidneys, YFP signal was found exclusively on MHCIInegF4/80hi but not on MHCII+F4/80hicells (Figure 5C), supporting that MHCIInegF4/80hi cells but not MHCII+F4/80hi cells arise from YS erythro-myeloid progenitors. Consistently, MHCIInegF4/80hi cells expressed higher levels of MERTK and TIM-4 than MHCII+F4/80hi cells, although expression levels of CX3CR1, F4/80, CD11c, and CD64 were similar (Figure 5D, Supplemental Figure 4B).
Because F4/80hi cells were the most potent producers of TNF in response to several TLR ligands in adults (Figure 2, G–I), we analyzed TNF production of F4/80hi cells and MHCIInegF4/80hi cells from 2- to 3-week-old mice in response to LPS, R848, and zymosan. Interestingly, neither MHCII+F4/80hi nor MHCIInegF4/80hi cells from 2-week-old mice produced TNF in response to R848 stimulation (Figure 5E), which is in contrast to F4/80hi cells from adults (Figure 2G), although these cells express similar levels of TLR7/8 message (data not shown). MHCII+F4/80hi and MHCIInegF4/80hi cells from 2-week-old mice responded similarly to LPS and zymosan in terms of TNF production (Figure 5E), but again fewer cells produced TNF in response to these stimuli when compared with F4/80hi cells from adult mice (Figures 2, H and I and 5E). We stained kidney sections from 2-week-old Clec9aCreRosaTom mice with F4/80, CD11b, MHCII, and CD64 for microscopy (Figure 5F) and used histo-cytometry51,58 to identify MHCII+F4/80hi and MHCIInegF4/80hi cells and distinguish them from CD11bhi cells and cDC2s (Supplemental Figure 4, C and D). Notably, we found a strong bias for MHCIInegF4/80hi cells to localize in the renal medulla, whereas MHCII+F4/80hi cells spread more evenly across the kidney cortex and medulla (Figure 5G). Thus, early life MHCIInegF4/80hi cells represent YS erythro-myeloid progenitor–derived macrophages that are replaced over time with MHCII+F4/80hi cells arising in a Myb-dependent manner.
Renal F4/80hi Cells from Adult Mice Downregulate MHCII Expression in Response to Renal Inflammation
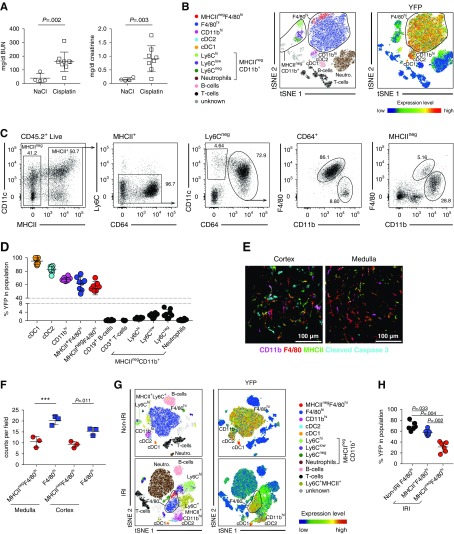
To investigate DC dynamics after kidney injury, we injected Clec9acre/creRosaYFP mice with cisplatin to induce AKI59 and analyzed renal leukocyte populations for YFP expression 3 days later (Figure 6, Supplemental Figure 5). As in steady state, MHCII+ renal leukocytes contained cDC1, cDC2, CD11bhi, and F4/80hi DC populations, with prominent Clec9a-expression history, as well as B cells (Figure 6, B–D). Interestingly, upon cisplatin treatment, a small population of MHCIInegF4/80hi cells was found in kidneys from Clec9acre/creRosaYFP mice (Figure 6B) and these cells labeled with YFP to the same extent as their MHCII+ counterparts (55%±10% versus 62%±10%, respectively; Figure 6, C and D). MHCIIneg and MHCII+F4/80hi cells were found in the cortex and medulla, although the renal cortex showed increased signs of apoptosis as measured by cleaved caspase-3 staining and indicative of higher tissue damage in this area (Figure 6, E and F). YFP labeling in other leukocytes, including MHCIInegCD11b+ cells that include Ly6G+ neutrophils, Ly6C+ monocytes, and some Ly6Cint/low cells, remained <5% (Figure 6, B–D, Supplemental Figure 5A). When identifying renal F4/80hi cells independent of MHCII expression, we found lower levels of MHCII expression on F4/80hi cells from cisplatin compared with control-treated mice (Supplemental Figure 5B). Thus, MHCIInegF4/80hi cells that arise after cisplatin injury appear related to the DC lineage and we reasoned that they may be the result of MHCII downregulation from renal F4/80hi cells, as has been observed in a model of ischemia-reperfusion injury.35 Three days after subjecting Clec9acre/creRosaYFP mice to unilateral ischemia-reperfusion injury, YFP expression remained restricted to DCs (Figure 6G, Supplemental Figure 5C). Notably, MHCIInegF4/80hi cells were found in ischemic kidneys and labeled with YFP at 33%±10% (Figure 6H, Supplemental Figure 5, C and D), although labeling of MHCII+F4/80hi cells from the same kidney (57%±10%) or nonischemic kidneys from the same mouse (69%±8%) was higher (Figure 6H). Labeling of monocytes remained low (2%±2%; Figure 6H, Supplemental Figure 5D). These data confirm a DC contribution to MHCIInegF4/80hi cells after ischemia reperfusion, although in this model an additional cell type without Clec9a-expression history also appears to contribute.
Figure 6.
Renal MHCIInegF4/80hi cells appear in adult kidneys after cisplatin and ischemia-induced AKI due to a phenotypic switch of MHCII+F4/80hi cells. (A–D) Clec9acre/creRosaYFP mice, 10 weeks of age, were injected i.p. with 15 mg/kg cisplatin and analyzed 72 hours later. (A) Serum creatinine and BUN levels are shown. (B–D) Kidney leukocytes from cisplatin-treated Clec9acre/creRosaYFP mice were analyzed by flow cytometry. (B) Representative tSNE analysis of kidney leukocytes 3 days after cisplatin treatment. Left panel: Cells were clustered independently of YFP labeling and manually gated populations were overlaid on the tSNE plot in the indicated colors. Right panel: The intensity of YFP expression in the indicated populations. Blue-to-red gradient indicates level of marker expression. (C and D) CD45.2+ cells were subdivided into MHCIIneg and MHCII+ cells. MHCII+ cells were further analyzed for CD11c and CD64 expression. After exclusion of Ly6Cneg cells, MHCII+CD64+ cells (first row) and MHCIIneg cells (second row) were further analyzed for CD11b and F4/80 expression. (D) The percentage of YFP+ labeling in the indicated renal leukocyte populations from cisplatin-treated mice is shown. (E) Kidney sections from cisplatin-treated mice were analyzed by immunofluorescence microscopy for the following markers: CD11b (magenta), F4/80 (red), MHCII (green), and cleaved caspase-3 (cyan). Representative cutouts of renal cortex and medulla are shown. Scale bar, 100 µm. (F) Quantification of MHCII+F4/80hi and MHCIInegF4/80hi cells in the renal cortex and medulla in cisplatin-treated mice. Each dot represents the average amount of cells per field from one biologic replicate. (G and H) FACS analysis of renal leukocytes from Clec9acre/creRosaYFP mouse 72 hours after unilateral ischemia-reperfusion injury. (G) Representative tSNE analyses of kidney leukocytes from ischemic and nonischemic control kidneys. Left panel: Cells were clustered independently of YFP labeling and manually gated populations were overlaid on the tSNE plot in the indicated colors. Right panel: YFP expression in the indicated populations. Blue-to-red gradient indicates increasing intensity of marker expression. (H) The percentage of YFP+ cells in renal MHCII+F4/80hi cells from nonischemic control kidneys and MHCIIneg and MHCII+F4/80hi cells from ischemic kidneys is shown. Each dot represents one mouse. Horizontal bars represent mean, error bars represent SD. ***P<0.001. IRI, ischemic-reperfusion injury; NaCl, sodium chloride.
Renal F4/80hi Leukocytes Transcriptionally Downregulate MHCII Expression after Cisplatin Treatment and Show Altered Expression of Inflammatory Chemokines
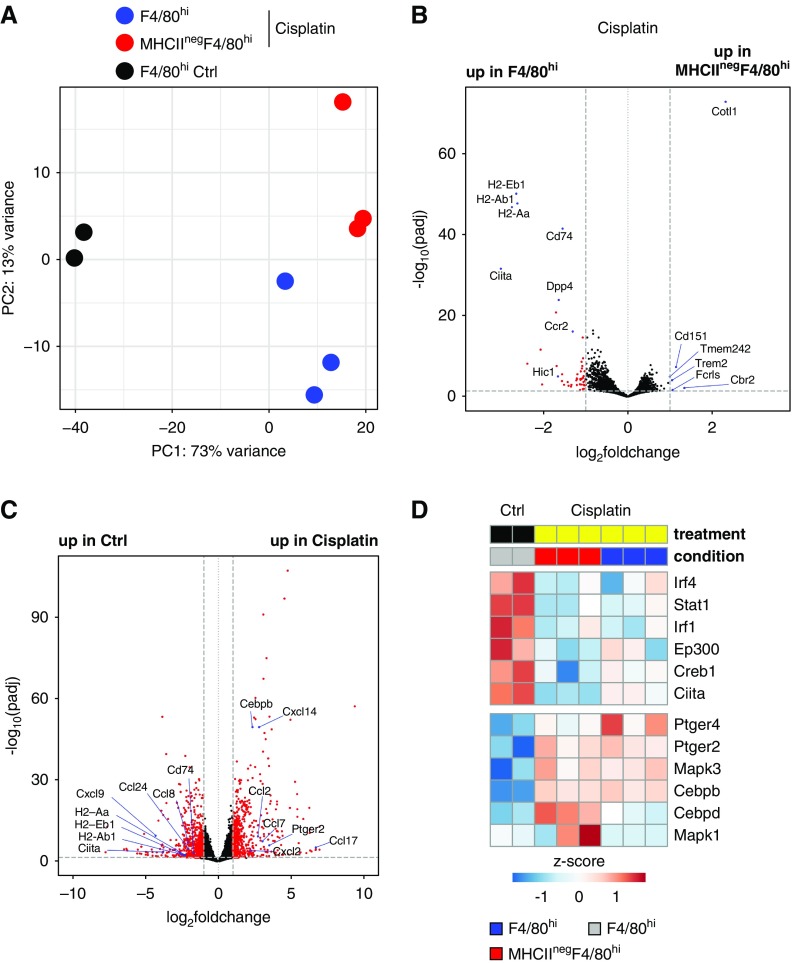
To gain a better understanding of the mechanism of MHCII downregulation from F4/80hi cells after cisplatin-induced AKI, we performed transcriptional analysis of MHCII+F4/80hi and MHCIInegF4/80hi cells from cisplatin-treated mice and MHCII+F4/80hi cells from control-treated mice (Figure 7, Supplemental Figure 6, Supplemental Tables 4–6). PCA revealed the strongest variance between populations from control and cisplatin-treated mice (Figure 7A). Genes involved in antigen presentation—including MHCII, CD74 (invariant chain), and the master regulator of MHCII expression Ciita—showed higher expression in MHCII+ than MHCIInegF4/80hi cells from cisplatin-treated mice (Figure 7, B–D), indicating that the antigen-presentation machinery is transcriptionally repressed after cisplatin treatment. Notably, when compared with F4/80hi cells from control-injected mice, MHCII+F4/80hi cells from cisplatin-treated mice showed reduced levels of positive regulators of MCHII expression, including Ciita, Irf4, Stat1, Irf1, Ep300, and Creb1,60 as well as increased expression of Cepdb and Cebpd, which inhibit Ciita promoter activity.61 Cepdb and Cebpd regulate Ciita transcription downstream of PGE2 signaling.62 Notably, renal F4/80hi cells upregulated the PGE2 receptors Ptger2 (EP2) and Ptger4 (EP4) in response to cisplatin treatment (Figure 7D), suggesting PG signaling may be involved in mediating MHCII downregulation. Interestingly, renal F4/80hi cells from control and cisplatin-treated mice showed differential expression of several inflammatory chemokines, such as Ccl2, Ccl7, Ccl17, Cxcl2, and Cxcl14 (Figure 7C), indicating these cells may play a role in coordinating recruitment of inflammatory cells. Importantly, renal F4/80hi cells show no evidence of Clec9a expression after cisplatin treatment (Supplemental Table 4), further supporting that YFP expression does not result from de novo expression of this marker, for instance on infiltrating monocytes (Figure 6, B and D).
Figure 7.
Renal F4/80hi cells downregulate MHCII expression and induce inflammatory chemokine expression in response to cisplatin treatment. At 72 hours after cisplatin treatment, MHCII+F4/80hi and MHCIInegF4/80hi cells were sorted from 10-week-old C57BL/6J mice and subjected to mRNA-sequencing analysis. As control (Ctrl), MHCII+F4/80hi cells were sorted from sodium chloride–injected mice. (A) PCA of the top 5000 genes with highest variance across all samples. Each dot of the same color represents a biologic replicate. (B) Pairwise comparison of MHCII+F4/80hi and MHCIInegF4/80hi populations from cisplatin-treated mice. (C) Pairwise comparison of F4/80hi populations from control and cisplatin-treated mice independent of MHCII expression. Red circles indicate genes with a log2 fold change greater than one between samples and an adjusted P (padj) value of <0.05. (D) Heatmap of genes implicated in inducing (upper block) or repressing (lower block) MHCII expression.
Discussion
The origin of kidney MPs has been highly debated because of the large phenotypic overlap of macrophages and DCs in this tissue.63,64 Adult kidney contains at least four subsets of MPs with prominent Clec9a-expression history, indicative of DC origin.24 These include the main cDC1 and cDC2 subsets, as well as CD64-expressing CD11bhi and F4/80hi cells. Here we show that these populations are phenotypically, functionally, and transcriptionally distinct from each other, suggesting they may constitute distinct DC subsets. Of these, renal F4/80hi cells have been suggested to constitute macrophages24,32,30,33 as well as DCs.24,31 We further demonstrate that renal F4/80hi cells exhibit a unique age-dependent developmental heterogeneity. Kidneys from newborn mice contain a prominent population of macrophages derived from YS erythro-myeloid progenitor that exhibit an F4/80hiCD11blow surface phenotype, express TIM-4 and MERTK, but lack MHCII expression. We show that renal YS erythro-myeloid progenitor–derived macrophages persist only for a few weeks after birth, when they are replaced by phenotypically similar cells that can be distinguished by MHCII expression and a relative lack of TIM-4 and MERTK. Csf1rMer-iCre-Mer–mediated fate mapping of YS progenitors demonstrates that MHCIInegF4/80hi and MHCII+F4/80hi cells are ontogenetically distinct cell lineages because YFP labeling in this model is restricted to MHCIInegF4/80hi cells, indicating that YS-derived MHCIInegF4/80hi cells do not acquire MHCII expression with time.
Mx-1CreMybflox/flox bone marrow rescue chimeras were used to demonstrate replenishment of MHCII+F4/80hi cells from HSCs in adults.12,44 It has recently been suggested that Mx-1CreMybflox/flox bone marrow rescue chimeras may not mimic steady-state cell differentiation in the kidney as polyI:C treatment induces inflammatory changes in renal leukocyte populations.35 Why systemic inflammation would induce a loss of resident leukocyte populations in specific tissues is unclear, but it is possible that polyI:C mediates this effect by acting directly on renal epithelial cells.65 We find a substantial increase in donor chimerism of F4/80hi cells between 3 and 9 months after bone marrow reconstitution. It is likely that, at 9 months after insult, niche-specific inflammatory effects of polyI:C treatment have subsided, substantiating the conclusion that F4/80hi cells are maintained by a low-level, steady-state input from HSCs.
Clec9a-Cre–mediated fate mapping further suggests that MHCII+F4/80hi cells exhibit age-dependent developmental heterogeneity. These cells show Clec9a-expression history in adult but not young mice, when compared with CD64neg cDCs, which labeled with YFP at both ages; indicating that DC progenitors contribute to F4/80hi cells in adulthood but not early life. Because Clec9a-cre–mediated fate mapping has some limitations, this interpretation needs to be approached with caution. Some DC precursors escape labeling in Clec9a-Cre mice and therefore YFP expression needs to be considered at the population level and no definitive conclusion can be made about the origin of YFP-negative cells.24 Incomplete penetrance of CRE-mediated DNA rearrangement could explain the lack of obvious transcriptional differences between YFP− and YFP+ F4/80hi cells in adults. However, it is important to note that a failure to detect transcriptional differences in ontogenetically distinct cell types could also be due to environmental imprinting.66,67 The transcriptional resemblance of MHCII+F4/80hi cells to macrophages in adult mice may additionally raise some doubt about the lineage-restricted expression of Clec9a. However, in steady state as well as in inflammation, Clec9a-expression history does not exceed a 5% background level in other lymphoid or myeloid cells, including monocytes, which are putative progenitors of MHCII+F4/80hi cells in other tissues (Figures 1B and 6D, Supplemental Figure 5D). These data strongly support that Clec9a expression is DC-lineage restricted.24,68 Additionally, we have not found evidence of Clec9a promoter activity in renal F4/80hi cells in steady state and after renal inflammation (Supplemental Figure 2C, Supplemental Table 4),24 indicating that renal MHCII+F4/80hi cells do not acquire Clec9a expression in their differentiated state. Thus, despite possible caveats of Clec9a-Cre–mediated fate mapping, the age-dependent differences in Clec9a-expression history of MCHII+F4/80hi cells strongly suggest a distinct origin of MCHII+F4/80hi cells in early and adult life. Notably, YFP labeling also suggests an age-dependent heterogeneity of CD11bhi cells. Because monocytes and lymphoid progenitors can contribute to macrophage and DC-like cells,1,11,17,69–71 these cells could also be putative progenitors of CD11bhi and F4/80hi cells in early life, which needs to be investigated.
Layered development of immune cells has been demonstrated across tissues, although we are just beginning to decipher the functional consequences of this ontogenetic diversity.5,6,72 Because kidney development in mice is not complete at birth, it is possible that renal macrophage and DC development is coordinated in distinct developmental waves to accommodate unique phases in organ growth and physiology, as well as an increasing need for immune defense with age. Our observation that MHCII+F4/80hi and MHCIInegF4/80hi cells from 2-week-old mice respond to TLR ligands with lower TNF production than their adult counterparts implies that F4/80hi cells in early life may have lower inflammatory capacity than in adulthood. Whether such age-dependent functional differences are rooted in distinct cell ontogeny or are secondary to environmental differences between young and adult mice73,74 needs to be investigated. The differential expression of MHCII on YS and HSC-derived F4/80hi cells further implies differences in antigen presentation and T cell activation, and the preferential localization of MHCIInegF4/80hi YS-derived macrophages in medulla from 2-week-old mice could place these cells in position to defend against ascending pathogens, as has been described for adult medullary DCs.31 Macrophages favorably influence kidney growth through apoptotic cell uptake and by promoting vascularization.75,76 Intriguingly however, unlike in other organs where self-maintaining MHCIIneg macrophages promote vascular integrity and tissue repair in adulthood,19,77 MHCIInegF4/80hi cells are not found in steady-state adult kidney, although the kidney is highly vascularized. It is possible that MHCII+F4/80hi cells from adult kidney have a unique role in antigen presentation, because circulating antigens are constitutively filtered and concentrated in the kidney and these cells can induce T cell activation and effector differentiation in vitro.24
Particularly intriguing are our findings that, in response to AKI, MHCIInegF4/80hi cells appear in kidneys that show prominent YFP labeling in Clec9aCreRosaYFP mice. We demonstrate that YFP expression remains restricted to the DC lineage after renal inflammation and no labeling is observed in other lymphoid and myeloid lineages, including monocytes. These data strongly support a DC origin of MHCIInegF4/80hi cells. Although it is possible that MHCIInegF4/80hi cells result from de novo differentiation of Clec9a-expressing progenitors that enter the tissue, we do not believe this is the case because transcriptional profiling indicates that F4/80hi cells shut off MHCII transcription in response to cisplatin. These data are consistent with a recent study reporting downregulation of MHCII on renal F4/80hi cells 6 days after bilateral ischemia-reperfusion injury.35 Our data further suggest that MHCII expression may be regulated by PGE2, which is produced by renal tubular cells after cisplatin treatment.78 Because MHCIIneg macrophages have been implicated in wound healing,35,55,77 it is possible that kidney-resident F4/80hi cells respond to inflammation by contributing to tissue repair, possibly coordinating the trafficking of other immune cells in the kidney through the production of chemokines.
Future studies are necessary to decipher the tissue-specific signals that regulate age-dependent aspects of DC and macrophage development in the kidney. Because the kidney is not a barrier organ, the microbiota is unlikely to play a role. However, the distinct salt environment of the kidney could influence hematopoietic programs, for instance by functional imprinting or modulating the recruitment of progenitor cells.79,80 Understanding age-dependent developmental aspects of long-lived, tissue-resident MPs with putative immune-modulatory and antigen-presenting function has important implications for targeting these cells for therapeutic intervention.
Disclosures
Dr. Schraml reports grants from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) and the European Research Council (ERC) during the conduct of the study. Dr. Schulz reports grants from the German Centre for Cardiovascular Research (Deutsches Zentrum für Herz-Kreislaufforschung, DZHK) and grants from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG), during the conduct of the study. Dr. Stremmel reports grants from the DFG, during the conduct of the study.
Funding
This work was supported by the DFG Emmy Noether grant: Schr 1444/1-1 (to Dr. Schraml), SFB914 projects A11 (to Dr. Schraml), A02 (to Dr. Walzog), A10 (to Dr. Schulz), and project number 360372040 – SFB 1335 (project 8, to Dr. Schraml). Work in the Schraml laboratory is also funded by a European Research Council Starting Grant awarded to Dr. Schraml (ERC-2016-STG-715182). Dr. Schulz received funding from the DZHK and German Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF, 81Z0600204). Dr. Anders was supported by the DFG (AN372/23-1, 24-1). Ms. Li received support from the Chinese Scholarship Council (CSC NO.201906380147).
Supplementary Material
Acknowledgments
We thank members of the Schraml laboratory for helpful discussions and critical reading of the manuscript. We thank Ronald N. Germain for his guidance in establishing histo-cytometry.
We acknowledge the Core Facility Flow Cytometry and the Core Facility Bioimaging at the Biomedical Center, Ludwig Maximilian University of Munich for providing equipment and expertise. High throughput sequencing was performed by the Laboratory for Functional Genome Analysis of the LMU Munich.
Dr. Salei, Mr. Rambichler, and Dr. Schraml planned experiments and wrote the manuscript. Dr. Salei, Mr. Rambichler, Ms. J. Salvermoser, Mr. Papaioannou, Dr. Schuchert, Dr. Cernilogar, Ms. Li, Mr. Marschner, and Dr. Schraml performed experiments. Mr. Rambichler, Dr. Straub, and Dr. Schotta performed sequencing analysis. Dr. Pakalniškytė, Mr. Rambichler, and Dr. Salei optimized the protocol for AKI. Dr. M. Salvermoser and Dr. Walzog helped with migration assays. Dr. Stremmel, Dr. Anders, Dr. Lichtnekert, and Dr. Schotta provided valuable intellectual input. Dr. Schraml designed and supervised the study.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Unraveling the Complexity of the Renal Mononuclear Phagocyte System by Genetic Cell Lineage Tracing,” on pages 233–235.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019040419/-/DCSupplemental.
Supplemental Figure 1. Clec9a-Cre mice faithfully trace renal dendritic cells.
Supplemental Figure 2. Gating strategy and sort purity of YFP+ and YFP− F4/80hi and CD11bhi cells from kidney.
Supplemental Figure 3. Cytokine production in renal YFP+ and YFP− F4/80hi and YFP+ and YFP− CD11bhi cells after TLR ligand stimulation.
Supplemental Figure 4. MHCII+ cells in the kidney are Myb-dependent and do not arise from embryonic progenitors.
Supplemental Figure 5. Leukocyte gating strategy and fate mapping of DCs in cisplatin- and ischemia- reperfusion-induced AKI.
Supplemental Figure 6. Gating strategy and sort purity of F4/80hi and MHCIInegF4/80hi cells after cisplatin-induced AKI.
Supplemental Table 1. Differentially expressed genes between F4/80hi YFP+, CD11bhi YFP+, cDC2 and cDC1 analyzed with DESeq2 and used for k-means clustering.
Supplemental Table 2. Differentially expressed genes between cDC2 YFP+ and CD11bhi YFP+ analyzed with DESeq2.
Supplemental Table 3. Differentially expressed genes between F4/80hi YFP+ and CD11bhi YFP+ analyzed with DESeq2.
Supplemental Table 4. TPM values of RNAseq from MHCII+/neg F4/80hi cells after cisplatin-induced AKI.
Supplemental Table 5. Differentially expressed genes between MHCIInegF4/80hi and MHCII+F4/80hi after cisplatin-induced AKI analyzed with DESeq2.
Supplemental Table 6. Differentially expressed genes between F4/80hi cells and F4/80hi cells after cisplatin-induced AKI analyzed with DESeq2.
References
- 1.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al.: Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat Rev Immunol 14: 571–578, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merad M, Sathe P, Helft J, Miller J, Mortha A: The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 31: 563–604, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy TL, Grajales-Reyes GE, Wu X, Tussiwand R, Briseño CG, Iwata A, et al.: Transcriptional control of dendritic cell development. Annu Rev Immunol 34: 93–119, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mildner A, Yona S, Jung S: A close encounter of the third kind: Monocyte-derived cells. Adv Immunol 120: 69–103, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Ginhoux F, Jung S: Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat Rev Immunol 14: 392–404, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Ginhoux F, Guilliams M: Tissue-resident macrophage ontogeny and homeostasis. Immunity 44: 439–449, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Jenkins SJ, Hume DA: Homeostasis in the mononuclear phagocyte system. Trends Immunol 35: 358–367, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al.: Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palis J, Robertson S, Kennedy M, Wall C, Keller G: Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126: 5073–5084, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A, Godin I: Three pathways to mature macrophages in the early mouse yolk sac. Blood 106: 3004–3011, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, et al.: C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42: 665–678, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al.: A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336: 86–90, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Perdiguero EG, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. : Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature Publishing Group 518: 1–17, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentek R, Molawi K, Sieweke MH: Tissue macrophage identity and self-renewal. Immunol Rev 262: 56–73, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Mossadegh-Keller N, Gentek R, Gimenez G, Bigot S, Mailfert S, Sieweke MH: Developmental origin and maintenance of distinct testicular macrophage populations. J Exp Med 214: 2829–2841, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, et al.: Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40: 91–104, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, et al.: Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 15: 929–937, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw TN, Houston SA, Wemyss K, Bridgeman HM, Barbera TA, Zangerle-Murray T, et al. : Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J Exp Med 215: 1507–1518, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, et al.: Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 175: 400–415.e13, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, et al.: A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311: 83–87, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Naik SH, Sathe P, Park H-Y, Metcalf D, Proietto AI, Dakic A, et al.: Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol 8: 1217–1226, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG: Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol 8: 1207–1216, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, et al.: The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med 206: 3115–3130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schraml BU, van Blijswijk J, Zelenay S, Whitney PG, Filby A, Acton SE, et al.: Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell 154: 843–858, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Cabeza-Cabrerizo M, van Blijswijk J, Wienert S, Heim D, Jenkins RP, Chakravarty P, et al. : Tissue clonality of dendritic cell subsets and emergency DCpoiesis revealed by multicolor fate mapping of DC progenitors. Sci Immunol 4: eaaw1941, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guilliams M, Dutertre C-A, Scott CL, McGovern N, Sichien D, Chakarov S, et al.: Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 45: 669–684, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langlet C, Tamoutounour S, Henri S, Luche H, Ardouin L, Grégoire C, et al.: CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J Immunol 188: 1751–1760, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, et al.: CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol 42: 3150–3166, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Brähler S, Zinselmeyer BH, Raju S, Nitschke M, Suleiman H, Saunders BT, et al.: Opposing roles of dendritic cell subsets in experimental GN. J Am Soc Nephrol 29: 138–154, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, et al.: CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J Clin Invest 123: 5035–5051, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hochheiser K, Heuser C, Krause TA, Teteris S, Ilias A, Weisheit C, et al.: Exclusive CX3CR1 dependence of kidney DCs impacts glomerulonephritis progression. J Clin Invest 123: 4242–4254, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Q, Wang Y, Wang XM, Lu J, Lee VWS, Ye Q, et al.: Renal F4/80+ CD11c+ mononuclear phagocytes display phenotypic and functional characteristics of macrophages in health and in adriamycin nephropathy. J Am Soc Nephrol 26: 349–363, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamatiades EG, Tremblay M-E, Bohm M, Crozet L, Bisht K, Kao D, et al.: Immune monitoring of trans-endothelial transport by kidney-resident macrophages. Cell 166: 991–1003, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lever JM, Yang Z, Boddu R, Adedoyin OO, Guo L, Joseph R, et al.: Parabiosis reveals leukocyte dynamics in the kidney. Lab Invest 98: 391–402, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lever JM, Hull TD, Boddu R, Pepin ME, Black LM, Adedoyin OO, et al.: Resident macrophages reprogram toward a developmental state after acute kidney injury. JCI Insight 4: 833, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puranik AS, Leaf IA, Jensen MA, Hedayat AF, Saad A, Kim K-W, et al.: Kidney-resident macrophages promote a proangiogenic environment in the normal and chronically ischemic mouse kidney. Sci Rep 8: 13948, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, et al.: Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1: 4, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al.: A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, et al.: Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20: 4106–4114, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mucenski ML, McLain K, Kier AB, Swerdlow SH, Schreiner CM, Miller TA, et al.: A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell 65: 677–689, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Qian B-Z, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al.: CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475: 222–225, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kühn R, Schwenk F, Aguet M, Rajewsky K: Inducible gene targeting in mice. Science 269: 1427–1429, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Lieu YK, Kumar A, Pajerowski AG, Rogers TJ, Reddy EP: Requirement of c-myb in T cell development and in mature T cell function. Proc Natl Acad Sci U S A 101: 14853–14858, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stremmel C, Schuchert R, Schneider V, Weinberger T, Thaler R, Messerer D, et al.: Inducible disruption of the c-myb gene allows allogeneic bone marrow transplantation without irradiation. J Immunol Methods 457: 66–72, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marschner JA, Schäfer H, Holderied A, Anders H-J: Optimizing mouse surgery with online rectal temperature monitoring and preoperative heat supply. Effects on post-ischemic acute kidney injury. PLoS One 11: e0149489, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al.: STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li B, Dewey CN: RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12: 323, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu A, Ibrahim JG, Love MI: Heavy-tailed prior distributions for sequence count data: Removing the noise and preserving large differences. Bioinformatics 35: 2084–2092, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bajénoff M, Glaichenhaus N, Germain RN: Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J Immunol 181: 3947–3954, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al.: Fiji: An open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W, Germain RN, Gerner MY: Multiplex, quantitative cellular analysis in large tissue volumes with clearing-enhanced 3D microscopy (Ce3D). Proc Natl Acad Sci U S A 114: E7321–E7330, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Willet SG, Bankaitis ED, Xu Y, Wright CVE, Gu G: Non-parallel recombination limits Cre-LoxP-based reporters as precise indicators of conditional genetic manipulation. Genesis 51: 436–442, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yona S, Kim K-W, Wolf Y, Mildner A, Varol D, Breker M, et al.: Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38: 79–91, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, et al.: Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun 7: 10321, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, et al.: Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol 20: 29–39, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheng J, Ruedl C, Karjalainen K: Most tissue-resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity 43: 382–393, 2015 [DOI] [PubMed] [Google Scholar]
- 57.Busch K, Klapproth K, Barile M, Flossdorf M, Holland-Letz T, Schlenner SM, et al.: Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 518: 542–546, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN: Histo-cytometry: A method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37: 364–376, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramesh G, Ranganathan P: Mouse models and methods for studying human disease, acute kidney injury (AKI). Methods Mol Biol 1194: 421–436, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Boss JM, Jensen PE: Transcriptional regulation of the MHC class II antigen presentation pathway. Curr Opin Immunol 15: 105–111, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Pennini ME, Liu Y, Yang J, Croniger CM, Boom WH, Harding CV: CCAAT/enhancer-binding protein beta and delta binding to CIITA promoters is associated with the inhibition of CIITA expression in response to Mycobacterium tuberculosis 19-kDa lipoprotein. J Immunol 179: 6910–6918, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li G, Harton JA, Zhu X, Ting JPY: Downregulation of CIITA function by protein kinase a (PKA)-mediated phosphorylation: Mechanism of prostaglandin E, cyclic AMP, and PKA inhibition of class II major histocompatibility complex expression in monocytic lines. Mol Cell Biol 21: 4626–4635, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nelson PJ, Rees AJ, Griffin MD, Hughes J, Kurts C, Duffield J: The renal mononuclear phagocytic system. J Am Soc Nephrol 23: 194–203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gottschalk C, Kurts C: The debate about dendritic cells and macrophages in the kidney. Front Immunol 6: 435, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hägele H, Allam R, Pawar RD, Anders HJ: Double-stranded RNA activates type I interferon secretion in glomerular endothelial cells via retinoic acid-inducible gene (RIG)-1. Nephrol Dial Transplant 24: 3312–3318, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, et al.: Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159: 1327–1340, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al.: Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159: 1312–1326, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joffre OP, Sancho D, Zelenay S, Keller AM, Reis e Sousa C: Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A. Eur J Immunol 40: 1255–1265, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salvermoser J, van Blijswijk J, Papaioannou NE, Rambichler S, Pasztoi M, Pakalniškytė D, et al. : Clec9a-mediated ablation of conventional dendritic cells suggests a lymphoid path to generating dendritic cells in vivo. Front Immunol 9: 563–615, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodrigues PF, Alberti-Servera L, Eremin A, Grajales-Reyes GE, Ivanek R, Tussiwand R: Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells. Nat Immunol 392: 1–18, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Audzevich T, Bashford-Rogers R, Mabbott NA, Frampton D, Freeman TC, Potocnik A, et al. : Pre/pro-B cells generate macrophage populations during homeostasis and inflammation. Proceedings of the National Academy of Sciences 114: E3954–E3963, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoeffel G, Ginhoux F: Ontogeny of tissue-resident macrophages. Front Immunol 6: 486, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Kleer IM, Kool M, de Bruijn MJW, Willart M, van Moorleghem J, Schuijs MJ, et al.: Perinatal activation of the interleukin-33 pathway promotes type 2 immunity in the developing lung. Immunity 45: 1285–1298, 2016 [DOI] [PubMed] [Google Scholar]
- 74.Bachus H, Kaur K, Papillion AM, Marquez-Lago TT, Yu Z, Ballesteros-Tato A, et al.: Impaired tumor-necrosis-factor-α-driven dendritic cell activation limits lipopolysaccharide-induced protection from allergic inflammation in infants. Immunity 50:225–240.e4, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rae F, Woods K, Sasmono T, Campanale N, Taylor D, Ovchinnikov DA, et al.: Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol 308: 232–246, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Munro DAD, Wineberg Y, Tarnick J, Vink CS, Li Z, Pridans C, et al. : Macrophages restrict the nephrogenic field and promote endothelial connections during kidney development. Elife 8: e43271, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J, et al. : Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363: eaau0964, 2019 [DOI] [PubMed] [Google Scholar]
- 78.Jia Z, Wang N, Aoyagi T, Wang H, Liu H, Yang T: Amelioration of cisplatin nephrotoxicity by genetic or pharmacologic blockade of prostaglandin synthesis. Kidney Int 79: 77–88, 2011 [DOI] [PubMed] [Google Scholar]
- 79.Berry MR, Mathews RJ, Ferdinand JR, Jing C, Loudon KW, Wlodek E, et al.: Renal sodium gradient orchestrates a dynamic antibacterial defense zone. Cell 170: 860–874.e19, 2017 [DOI] [PubMed] [Google Scholar]
- 80.Chessa F, Mathow D, Wang S, Hielscher T, Atzberger A, Porubsky S, et al.: The renal microenvironment modifies dendritic cell phenotype. Kidney Int 89: 82–94, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.