Significance Statement
The human CFHR–Factor H gene cluster encodes the five FHR proteins that are emerging complement and immune modulators and the two complement regulators Factor H and FHL1. Genetic and chromosomal alterations in this cluster are associated with the human kidney diseases atypical hemolytic uremic syndrome and C3 glomerulopathy. Various genetic alterations result in the expression of mutant and altered FHR proteins, or FHR::Factor H and Factor H::FHR hybrid proteins. The modified FHR proteins together with an altered FHR and Factor H plasma repertoire, which often modify complement action in the fluid phase and cause morphologic alteration in the glomerulus, provide important views on FHR protein function in the kidney.
Keywords: Complement Factor H related, complement, glomerulonephritis, hemolytic uremic syndrome, glomerular disease
Abstract
Sequence and copy number variations in the human CFHR–Factor H gene cluster comprising the complement genes CFHR1, CFHR2, CFHR3, CFHR4, CFHR5, and Factor H are linked to the human kidney diseases atypical hemolytic uremic syndrome (aHUS) and C3 glomerulopathy. Distinct genetic and chromosomal alterations, deletions, or duplications generate hybrid or mutant CFHR genes, as well as hybrid CFHR–Factor H genes, and alter the FHR and Factor H plasma repertoire. A clear association between the genetic modifications and the pathologic outcome is emerging: CFHR1, CFHR3, and Factor H gene alterations combined with intact CFHR2, CFHR4, and CFHR5 genes are reported in atypical hemolytic uremic syndrome. But alterations in each of the five CFHR genes in the context of an intact Factor H gene are described in C3 glomerulopathy. These genetic modifications influence complement function and the interplay of the five FHR proteins with each other and with Factor H. Understanding how mutant or hybrid FHR proteins, Factor H::FHR hybrid proteins, and altered Factor H, FHR plasma profiles cause pathology is of high interest for diagnosis and therapy.
Sequence and copy number variations in the human CFHR gene cluster are linked to the kidney disorders atypical hemolytic uremic syndrome (aHUS) and C3 glomerulopathy,1–6 and are furthermore associated with IgA nephropathy (IgAN), with a retinal disease, and with infections.5–8 These copy number variations are caused by deletions, duplications, and insertions of gene or chromosomal segments and generate CFHR::CFHR, CFHR::Factor H, and Factor H::CFHR hybrid genes or CFHR genes with duplicated elements and result in FHR hybrid or mutant proteins1 and alter the FHR and Factor H plasma repertoire. One clear difference is emerging: in aHUS, ultimately the protective complement action of Factor H on the endothelial surface is altered. In C3 glomerulopathy, the FHR mutants and a modified FHR plasma repertoire apparently affect local complement regulation, inducing cell proliferation and chronic inflammation. To evaluate how alterations in the five CFHR genes and the Factor H gene cause different renal pathologies, we here link the genetic scenarios, the specific FHR variants expressed in plasma, with the glomerular changes in these kidney diseases. We summarize the role of FHR proteins as emerging complement modulators, amplifiers, and inflammatory modifiers. In aHUS FHR mutants and in autoimmune aHUS pathogenic Factor H–binding autoantibodies alter Factor H regulation on endothelial surfaces and fluid-phase regulation remains intact. In C3 glomerulopathy, FHR mutants in the context of intact Factor H and FHL1 (the Factor H–like protein) affect complement regulation in the fluid phase and on the glomerular surface, and FHR mutants with duplicated interaction segments form large oligomers, which deregulate complement and compete with Factor H for surface binding. Thus, C3 glomerulopathy develops due to unique CFHR gene variations in the context of an intact Factor H gene.
Here, we summarize how modifications in the CFHR gene cluster described for aHUS and C3 glomerulopathy cause specific FHR mutants and how they affect the FHR plasma repertoire. Thereby, this review will focus on (1) providing an overview of complement initiation, with the two central enzymatic levels and the various effector actions; (2) the characteristic morphologic features of the human kidney disorders aHUS and C3 glomerulopathy; (3) giving a brief summary on the organization of the human CFHR–Factor H gene cluster with the Factor H and the five CFHR genes and on the structure of the encoded FHR proteins; (4) explaining how, in aHUS, CFHR gene variations generate Factor H::FHR3, Factor H::FHR1, and FHR1::Factor H hybrid proteins and how they alter FHR plasma levels; (5) describing which genetic CFHR changes occur in C3 glomerulopathy; (6) describing which FHR hybrid or FHR mutant proteins are expressed in this disease; (7) describing how altered FHR plasma levels result in C3 glomerulopathy; (8) summarizing the new insights into the role of the FHR proteins in IgAN, in complement control and disease pathology; and (9) providing concluding remarks and an outlook for diagnosis, biomarker profiling, and therapy.
Complement: Initiation, Enzymatic Levels, and Effector Pathways
Complement is a central homeotic system and defective complement causes many human diseases, in particular the kidney diseases aHUS and C3 glomerulopathy.9,10 In order to link complement with CFHR gene variations a brief overview of complement, with the three activation pathways and with two central enzymatic checkpoints, is presented (Figure 1A). Activation of the alternative pathway occurs spontaneously in the fluid phase and propels on target surfaces; the lectin and the classic pathways are initiated on target surfaces by carbohydrates and antibodies bound to antigens.9–11 Upon initiation, two subsequently acting enzymatic effector systems are formed. The first enzymatic level is mediated by two C3 convertases, the AP (C3bBb) and the LP/CP (C2bC4b) convertases, which cleave the same substrate C3 and generate C3a and C3b.12 This enzymatic level includes a self-amplifying amplification loop that enhances activation. The second enzyme level generates C5 convertases. Again, two enzymes are generated; both the convertase (C3bBbC3b) of the AP and the convertase (C2bC4bC3b) of the LP/CP-pathway use C5 as substrate and generate C5a and C5b.
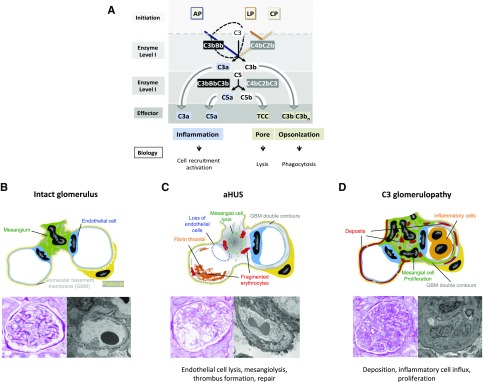
Figure 1.
Overview of complement activation and effector pathways and morphologic changes in aHUS and C3 glomerulopathy. (A) Complement activation occurs via three pathways, the alternative pathway (AP), the lectin pathway (LP), and the classic pathway (CP), which are initiated on surfaces. The type of surface influences activation and the regulator repertoire decides on cascade progression or inhibition. The LP and CP are activated on surfaces by specific carbohydrate moieties (LP), by surface-deposited components (e.g., C reactive protein, Pentraxin), and by IgGs (CP). A repertoire of regulators controls cascade progression in the fluid phase and on surfaces. The three pathways form specific surface-bound convertases; the AP results in the generation of AP-C3 convertase and the LP/CP trigger formation of the CP C3 convertase. The general role of both C3 convertases is to cleave the abundant plasma protein C3 (concentration 1000–1500 μg/ml) into the anaphylatoxin C3a and the opsonic C3b. The enzymatic response on the first enzymatic levels is frequently enhanced by the potent self-amplifying amplification loop. When activation proceeds the C3 convertases attach an additional C3b fragment, form the C3bBbC3b complex, changes substrate specificity and form a C5 convertase. C5 convertases of the AP and of the LP/CP pathways exist. The major role of the C5 convertase is to cleave C5 (plasma concentration 350 μg/ml) into the powerful anaphylatoxin C5a and to generate a surface-binding C5b. C5b subsequently initiates the TCC, which forms lytic pores. (B) Structure of an intact glomerulus. Top: Schematic view of mesangial area (green color) in the filtration unit with endothelial cells (blue), GBM (gray), and podocytes (yellow color). Bottom left: PAS staining of an intact pretransplant kidney. Right: Electron microscopic analysis of a pretransplant kidney with intact glomerular structure. (C) Glomerular changes in aHUS. Top: Schematic presentation of hypocellularity due to cell lysis and loss of endothelial and mesangial cells. Bottom left: PAS staining. Right: EM imaging reveals a pattern of thrombotic microangiopathy. (D) C3 glomerulopathy showing a proliferative pattern with hypercellularity and influx of infiltrating cells. Top: Schematic view of mesangial proliferation and thickening of GBM in MPGN (right glomerulus) and in DDD (left glomerulus). Bottom left: Glomerular changes revealed by PAS staining. Right: EM image showing thickening of the GBM and double contour formation.
Complement action occurs in the fluid phase and on surfaces and multiple regulators control activation and the transition from fluid phase to the surface.9 Both C3 convertases form the inflammatory anaphylatoxin C3a and the opsonin C3b. C3b is handled differently on self and nonself surfaces. C3b deposited on intact self surfaces is inactivated by complement proteases which act together with cofactors, and inactivated iC3b is further processed to C3dg and C3d. In addition, C3b when deposited to foreign surfaces initiates the amplification loop that amplifies C3b deposition. The second enzymatic level with the C5 convertase generates the potent anaphylatoxin C5a and deposits C5b, which initiates the pore-forming terminal complement complex (TCC) (Figure 1A).13,14 Thus, two enzymatic steps generate different effector compounds (1) in the form of anaphylatoxins C3a and C5a that induce inflammation and attract host cells; (2) by opsonization of target surfaces with C3b; and (3) by forming the TCC, which generates a pore and damages the target membrane.15,16
An important challenge is to understand complement regulation and the actions of existing regulators and new modulators. Given that FHR proteins are emerging complement modulators and amplifiers, it is of interest to define at which specific step of a pathway and at which checkpoints each FHR protein acts. The different pathologies caused by the FHR mutants and hybrids, i.e., endothelial damage in aHUS and in DEAP-HUS (homozygous CFHR1-CFHR3 Deficiency and Autoantibody to Factor H Positive), and inflammatory and proliferative action in C3 glomerulopathy already indicate different regulatory and modulatory and amplifiers roles of FHR proteins in the complement cascade.
Renal Histologic Changes in aHUS and in C3 Glomerulopathy
Chromosomal changes in the CFHR–Factor H gene cluster result in genetic modifications that are causative for the two kidney diseases, genetic aHUS and C3 glomerulopathy. The morphologic and cellular alterations, which are caused by deregulated complement, are summarized in recent excellent reviews.17,18 The morphologic appearance of aHUS in the glomeruli and the preglomerular arterioles can be characterized as thrombotic microangiopathy. Best understood is primary complement-mediated endothelial and mesangial cell damage due to dysregulation of the alternative complement cascade on the cell surface.19 Defective complement control on endothelial surfaces results in cell lysis, followed by thrombus formation, loss of mesangial cells, and mesangiolysis as seen in histology (Figure 1C). In the chronic or repair phase, the newly formed endothelial cells produce new extracellular matrix, leading to double contours of the glomerular basement membrane (GBM) thickening.17,18 In contrast, in C3 glomerulopathy, chronic complement activation in the fluid phase and/or on the surface leads to deposition of complement components in the mesangium and in the subendothelial space (membranoproliferative GN [MPGN] pattern), or within the GBM (intramembranous GN, dense deposit disease (DDD) pattern), and sometimes also at the outer aspects of the GBM (subepithelial deposits) (Figure 1D).20 Depot formation is followed by mesangial and endothelial cell activation as well as proliferation, leading to influx of macrophages and to endocapillary and mesangial hypercellularity. Constantly activated endothelial and mesangial cells form new matrixes leading to thickening of the GBM and double contours or multilayering, as well as mesangial matrix increase. This glomerular remodeling leads to the typical lobular appearance that is summarized under the umbrella term MPGN.5
The Factor H–CFHR Gene Cluster, FHR Proteins, and Conformation
The Human Factor H–CFHR Gene Cluster
The human Factor H–CFHR gene cluster is located on human chromosome 1q32 in the regulators of complement activation region.19,21 The five CFHR genes are positioned downstream of the Factor H gene and are arranged in the order CFHR3, CFHR1, CFHR4, CFHR2, and CFHR5.19 This cluster presents an instable, dynamic chromosomal region and a hotspot for structural rearrangements. The human CFHR gene cluster includes interspersed duplicated regions with strong nucleotide identity22–24 (H. Richter, C. Skerka, and P. Zipfel, unpublished observations) (Figure 2, left panel). The segmental duplicated regions can include coding regions, e.g., the repeat regions B and B’ include exons that encode the C-terminal SCRs of Factor H and FHR1, respectively (see below).25
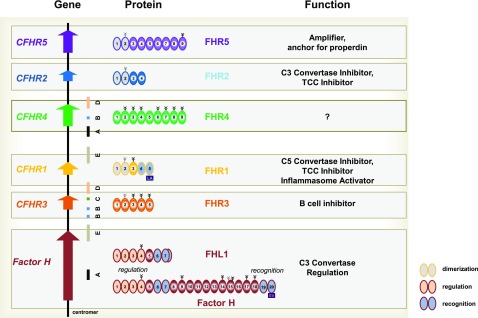
Figure 2.
The human Factor H–CFHR gene cluster and the FHR, Factor H protein family. The human Factor H–CFHR gene cluster includes the Factor H gene and the five CFHR genes, which are located on chromosome 1q32. Left side: The CFHR genes are positioned downstream of the Factor H gene (bottom) and are arranged in the order CFHR3, CFHR1, CFHR4, CFHR2, and CFHR5. The human CFHR gene cluster includes interspersed duplicated regions, or segmental duplication elements, which have a high sequence identity and are shown as colored bars below the gene structure. Middle segment: Proteins. Domain organization of the secreted FHR proteins and of Factor H gene products FHL1 and Factor H. Each SCR domain is indicated and the domains of each protein are consecutively numbered. Attached carbohydrate side chains are shown above by the tree-like structure. Constitutively attached carbohydrate side chains have black lines, and facultatively attached carbohydrates have gray lines. The N-terminal multimerization domains of FHR1, FHR2, and FHR5 represented by two SCRs are filled with dashed lines. The C-terminal surface-binding regions of FHR1 and the surface-binding regions of Factor H and FHL1 are shown with a stippled pattern. FHR1: L290A296 indicates the two FHR1-specific residues, which differ from that of Factor H. Bottom: The human Factor H gene encodes two fluid-phase C3 convertase inhibitors, FHL1 and Factor H. The complement regulatory region is located in SCRs 1–4, is shown by the orange patterns, and is shared by FHL1 and Factor H. The C-terminal recognition regions, i.e., SCRs 6–7 (FHL1/Factor H) and SCRs 19–20 (Factor H), are shown by blue patterns. The Factor H–specific two amino acids S1191V1196 in the most C-terminal recognition region are shown in the box below SCR 20. FHL1 has a unique six–amino-acid extension that follows SCR 7. Right side: Identified major functions of each FHR protein and of Factor H and FHL1.
CFHR genes are conserved in evolution indicating essential biologic functions.24,26 The four mouse genes mCFHR-A, mCFHR-B, mCFHR-C, and mCFHR-D differ in structure, domain composition, and sequence from the human genes.25–27 These substantial differences make it difficult to predict the mouse homologs of the human FHR protein.26,28 Similar to the MCP/CD46 (human) versus Crry (mouse) situation, mouse FHR homologs need to be identified by functional studies.
FHR Proteins Are Emerging Complement Modulators and Represent Immune and Inflammatory Regulators with Common and Unique Features
CFHR genes are mainly transcribed in the liver and the proteins are distributed in plasma. The five FHR proteins share structural homology with each other and with Factor H and the homology of single domains ranges from 35% to >90% (Figure 2, middle panel).25–27 Domains with high homology can share overlapping ligand-binding profiles and functions. However, domains with low sequence homology and especially the different numbers of SCR domains of the FHR proteins indicate unique functions.
FHR proteins are emerging complement modulators, activators, and immune regulators. Each FHR protein binds to C3b, iC3b, C3dg, and C3d. Some, but not all, FHR proteins interact with ligands such as glycosaminoglycans, monomeric CRP, pentraxin-3, the lipid peroxidation product MDA (malondialdehyde), and laminins of the kidney, and bind to the surfaces of apoptotic and necrotic cells.29–32 Often, two or more SCR domains form functional units or ligand-binding segments, including dimerization, multimerization, or cell binding. Extensive domain mapping for all five FHR proteins is necessary to localize such binding and interaction domains.
FHR proteins show sequence homology and share domains with the central fluid-phase regulator Factor H and also with FHL1. Factor H acts as a cofactor for the complement protease Factor I and regulates the stability of the C3 convertase.33,34,35 FHL1 is also encoded by Factor H gene and the FHL1 mRNA is generated by alternative splicing.36 FHL1 shares the regulatory N-terminal region with Factor H and has a surface-binding site in SCRs6–7 (Figure 2).
CFHR Gene Variations in aHUS
aHUS is defined by the triad hemolytic anemia, thrombocytopenia, and renal damage. The disease develops in pediatric and in adult patients, and the clinical symptoms that are associated with the various subforms and patient management are summarized in excellent reviews.3,37 aHUS is a rare disease and the frequency is one patient in a population of 500,000 (https://ghr.nlm.nih.gov/condition/atypical-hemolytic-uremic-syndrome).
HUS has many causes, and includes STEC-HUS caused by infections with Shiga toxin–producing Escherichia coli, pneumococcal HUS due to infections with Streptococcus pneumoniae, and aHUS.9,38,39 The latter is also termed genetic aHUS and accounts for about 10% of total HUS cases. About 50%–60% of patients with genetic aHUS have alterations in one or several complement genes,40–43 as well as in the DGKe (diacyl glycerine Kinase epsilon) gene, which encodes a cytoplasmic signaling protein, and in the INF2 (Inverted Formin 2) gene, an intracellular actin binding protein.44,45 Thus, the affected genes code for complement proteins that (1) form the alternative pathway C3 convertase (C3, Factor B), (2) regulate this central complement enzyme (Factor H, MCP/CD46, Factor I), (3) encode modulators (FHR1, FHR3, FHR4, thrombomodulin), and (4) code for TCC inhibitor (vitronectin) or for cytoplasmic proteins. For more detailed information on the genetic causes of aHUS see the excellent reviews that summarize these complex issues.3,37
CFHR–Factor H gene alterations are identified in aHUS.3,37,46–58 Here, we describe how the various CFHR1, CFHR3, and Factor H gene alterations affect protein structure, protein function, and the FHR and Factor H plasma repertoire.
CFHR Copy Number Variations
In aHUS, chromosomal changes affect one or several CFHR genes and may also involve the Factor H gene (Figure 3, Supplemental Table 1). FHR variants, as well as FHR1::Factor H and Factor H::FHR hybrids, are expressed and the FHR, as well as the Factor H plasma inventories, are altered, but FHL1 mRNA is transcribed from the defective Factor H allele. Four types of modifications exist: (1) deletions of chromosomal segments resulting in Factor H::CFHR3 or (2) Factor H::CFHR1 hybrid genes, (3) insertions of chromosomal segments generating extra CFHR1::Factor H hybrid genes, or (4) homozygous deletions of the CFHR3-CFHR1 or CFHR1-CFHR4 gene segments. Similar, but not identical, scenarios were identified in unrelated families.
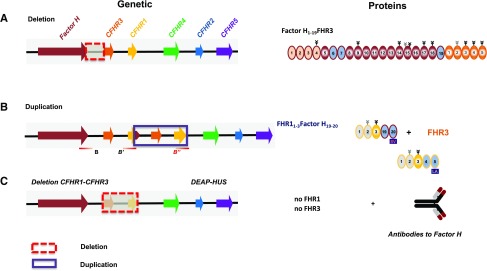
ad (i) Factor H::CFHR3 hybrids. Factor H::CFHR3 hybrid genes result from chromosomal deletions. One scenario is a Factor H::CFHR3 hybrid gene that has Factor H exons i-xxii linked to the complete CFHR3 gene46 (Figure 3A, upper line). The encoded 186-kDa, 24-domain protein has Factor H1–19 attached to FHR3. A related hybrid gene, with a different chromosomal breakpoint and generated by de novo deletion, codes for a Factor H1–17::FHR3 protein composed of 22 domains47 (Supplemental Table 1). Both Factor H::FHR3 hybrids harbor the N-terminal regulatory region, lack the C-terminal recognition region of Factor H, and have instead full-length FHR3 added at the C terminus. The hybrid proteins are expressed in plasma. The Factor H1–19FHR3 hybrid has cofactor activity, and binds with high intensity to immobilized C3b and heparin resulting in defective surface binding and regulation.46 Also, the highly related Factor H1–17::FHR3 hybrid shows impaired surface binding and defective surface regulation.47 Both hybrid genes allow transcription of FHL1 mRNA, and FHL1 is present in plasma. These genomic settings alter the FHR, Factor H plasma inventory in the same manner. FHR3 and Factor H plasma levels are reduced and intact proteins are encoded by the second, intact allele. CFHR1, CFHR2, CFHR4, and CFHR5 genes are present in two copies and the corresponding proteins are expressed at normal levels (Figure 3).
ad (ii) Factor H::FHR1 hybrids. A Factor H::CFHR1 hybrid gene that has Factor H exons i–xxi (SP, Factor H1–18) linked to CFHR1 exons v-vi (FHR14–5) was reported in a UK family with eight affected individuals48 (Supplemental Table 1). A related hybrid gene, caused by de novo deletion, has a different breakpoint and has Factor H exons i–xxii linked to CFHR1 exon vi49 (Supplemental Table 1). Again, FHL1 mRNA is transcribed from the hybrid genes. Both hybrid proteins, i.e., Factor H1–18::FHR14–5 and Factor H1–19::FHR15, have the FHR1-specific C-terminal residues and represent Factor HLA (Factor HL1191A1197).48,49 The patients have reduced Factor H, FHR3, and FHR1 plasma levels and normal FHR4, FHR2, and FHR5 levels (Figure 3, Table 1).
ad (iii) Extra FHR1::Factor H hybrids. Chromosomal duplications generating an extra CFHR1::Factor H hybrid gene and a third CFHR3 allele were reported as de novo50 and as familial cases.51 The de novo case had an extra chromosomal segment, consisting of Factor H exons xxii-xxiii, the CFHR3 gene, and CFHR1 exons i–iv50 (Figure 3B). In the familial scenario, the 49-year-old patient and the affected 20-year-old daughter had a chromosomal segment duplicated that includes Factor H exon xxiii, the CFHR3 gene, and CFHR1 exons i–v51 (Supplemental Table 1). Thus, duplicated segments are inserted within either the B or B’ segmented duplicated elements of the CFHR gene cluster. The CFHR1::Factor H hybrid genes encode an extra FHR11–3::Factor H19–20 (or FHR11–4::Factor H20) termed FHR1SV and the extra CFHR3 allele results in elevated FHR3 plasma levels (150%) (Figure 3B). Plasma levels of FHR1, FHR2, FHR4, FHR5, Factor H, and FHL1 are normal. The hybrid protein with the Factor H–specific C-terminal residues has a Janus-faced character. FHR1SV forms multimers with itself and with FHR1 and FHR2, and does compete with Factor H for surface binding.50–52
Figure 3.
CFHR gene variations in aHUS and aHUS-associated FHR protein variants. (A) Deletion of chromosomal segments: Factor H::CFHR3 hybrid gene. Genetic deletion generating a Factor H::FHR3 hybrid gene (left side) and the corresponding Factor H::FHR3 hybrid variant are shown using the color code of Figure 2. The deleted chromosomal segment is shown with the box with red, stippled lines. (B) Insertional mutagenesis (duplications) of chromosomal segments generating a CFHR1::Factor H hybrid gene. Genetic duplication (boxes with blue lines) generates an extra CFHR1::Factor H hybrid gene together with an extra CFHR3 allele (left side), and the corresponding FHR1::Factor H hybrid protein is shown. The changes affect the FHR plasma repertoire (see Table 1). (C) In patients with DEAP-HUS, the deletion of CFHR3-CFHR1 is often associated with the formation of autoantibodies that bind to the C terminus of Factor H.
Table 1.
CFHR Gene Variations in aHUS Affect FHR and Factor H but not FHL1 Plasma Levels
| Variable | Intact FHL1 | Affected | Intact | |||||
|---|---|---|---|---|---|---|---|---|
| Factor H | FHR3 | FHR1 | FHR 4 | FHR2 | FHR5 | |||
| Factor HFHR3 | 100 | 50 | Factor HFHR3 | 50 | 100 | 100 | 100 | 100 |
| Factor HFHR3 | 100 | 50 | Factor HFHR3 | 50 | 100 | 100 | 100 | 100 |
| Factor HFHR1 | 100 | 50 | Factor HFHR1 | 100 | 50 | 100 | 100 | 100 |
| Factor HFHR1 | 100 | 50 | Factor HFHR1 | 100 | 50 | 100 | 100 | 100 |
| FHR1::Factor H | 100 | 100 | FHR1::Factor H | 150 | 100 | 100 | 100 | 100 |
| FHR1::Factor H | 100 | 100 | FHR1::Factor H | 150 | 100 | 100 | 100 | 100 |
| ΔΔFHR3FHR1 | 100 | 100 | 0 | 0 | 100 | 100 | 100 | |
| ΔFHR3FHR1 | 100 | 100 | 50 | 50 | 50 | 100 | 100 | |
| ΔΔFHR1FHR4 | 100 | 100 | 100 | 0 | 0 | 100 | 100 | |
Numerical data presented as % plasma levels.
Frequency of CFHR Gene Mutations in aHUS
In the Spanish cohort of 513 patients with aHUS, six patients (1.2%) expressed FHR1SV variants.53 In an Italian aHUS cohort (154 patients), seven patients (4.5%) had heterozygous CFHR gene rearrangements.54 Five patients presented with Factor H::FHR1 hybrids (2.8%); two patients showed the extra FHR1SV hybrid and a third the CFHR3 allele (1.3%) and altered FHR1/FHR3 plasma levels.54
Functional Consequences of CFHR Gene Alterations
Familial or de novo modifications generating heterozygous CFHR gene variants cause aHUS and identify a role of FHR variant proteins in disease pathology. The CFHR1, CFHR3, and Factor H genes are affected; FHR variants are expressed; and the FHR, Factor H plasma repertoire is altered (Table 1). Three CFHR scenarios cause aHUS: (1) chromosomal deletions, (2) chromosomal duplications, and (3) homozygous FHR1-FHR3 deficiency. ad (i) chromosomal deletions generate Factor H::CFHR3 or Factor H::CFHR1 hybrid genes and the encoded hybrid proteins have the N-terminal regulatory region together with additional Factor H domains linked to either FHR3 or the recognition region of FHR1. The hybrid proteins show normal Factor H regulation in the fluid phase and lack Factor H surface binding, but use FHR3 or FHR1 domains for surface binding. Intact FHL1 mRNA is transcribed from the mutant allele. ad (ii) duplications result in extra CFHR1::Factor H hybrid genes. The encoded FHR1SV protein has dual character. This Janus-faced protein combines the N-terminal dimerization region of FHR1 with the C-terminal recognition region of Factor H. The protein lacks the discriminatory part of the FHR1, specifically the LA residues. The chromosomal duplications also generate a third CFHR3 allele, resulting in higher FHR3 plasma levels. ad (iii) homozygous deletion of an approximately 24-kb chromosomal segment that encompasses the CFHR3-CFHR1 genes is observed in about 15% of predominantly young patients with aHUS (Figure 3C).22,55–58 For these patients with DEAP-HUS, this genetic scenario is frequently associated with the presence of autoantibodies that bind to the C-terminal region of Factor H (SCRs 19–20) and block surface binding.23,56–59 The exact mechanisms whereby in HUS the homozygous deficiency of CFHR1 and CFHR3 breaks tolerance and induces antibody production are not clear. However, one link is that FHR3 binds to the C3d fragment and blocks the adjuvant effect of C3d in B cell activation.60
Glomerulopathy due to CFHR Gene Variations
C3 glomerulopathy is a very rare disorder affecting 1–2 people per million people worldwide and is equally common in men and women (https://ghr.nlm.nih.gov/condition/c3-glomerulopathy). C3 glomerulopathy is frequently associated with proteinuria, hematuria, high creatinine plasma levels, and altered complement plasma levels. Kidney function decreases with time and within 10 years about 50% of patients progress to ESKD. Two major forms are separated, DDD and C3 GN, which form related kidney problems.61,62 Precise diagnosis requires kidney biopsy specimens. This heterogeneous disease is induced by various triggering events that lead to complement activation in the fluid phase and on glomerular surfaces, thus explaining a link of defective alternative complement and proteinuria and hematuria. The genetic and autoimmune causes are carefully summarized in recent outstanding reviews63–65. Here, we focus on CFHR copy number variations in the context of an intact Factor H gene, which represent a specific genetic form of C3 glomerulopathy. All five CFHR genes can be affected and in this case the Factor H gene remains intact. Mutant FHR proteins are expressed and the FHR plasma repertoire is disturbed, whereas Factor H and FHL1 levels are normal.
CFHR Gene Variations in C3 Glomerulopathy
Chromosomal Deletions
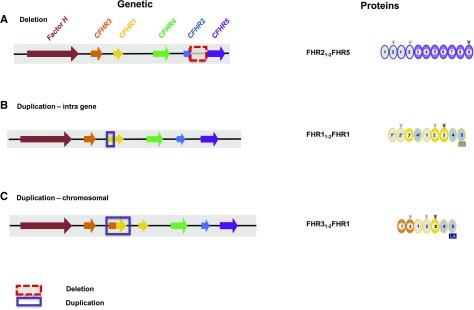
A 26-year-old Italian patient diagnosed with DDD showed variations in several CFHR genes.63 A deletion on one allele generates a CFHR3i-iv::CFHR4ix,x hybrid gene that encodes an FHR31–3::FHR48,9 hybrid protein (Supplemental Table 1). The second allele has the common CFHR3::CFHR1 deletion. In addition, the patient has two heterozygous mutations (p.Q211X and p.R254×) in the CFHR2 gene that terminate transcription. This results in a unique plasma repertoire with an FHR3::FHR4 hybrid protein; lack of FHR1, FHR2, and FHR3; reduced FHR4 levels; and normal FHR5, Factor H, and FHL1 plasma levels.
In an Indian family, the 8-year-old index patient diagnosed with glomerulopathy histologically classified as an overlap of C3 GN and DDD has a chromosomal deletion that spans from CFHR1v to the CFHR5-gene promoter.64 The CFHR1i-iv::CFHR5 hybrid gene encodes a 90-kDa FHR11–3::FHR5 protein, and is associated with low FHR1, FHR2, FHR4, and FHR5 plasma levels (Supplemental Table 1). The father and a 3-year-old sister have this deletion on one allele, combined with the common CFHR1-CFHR3 deletion on the second allele. Both patients lack FHR1, and have reduced FHR3, FHR2, FHR4, and FHR5 plasma levels. The hybrid protein, with two interacting regions, forms large oligomers, which include the hybrid, FHR1, FHR2, and FHR5.
Two related German patients diagnosed with MPGN-II had a heterozygous deletion of a 24-kbp chromosomal segment, resulting in a CFHR2i-iii::CFHR5 hybrid gene that encodes an FHR212::FHR5 hybrid protein.65 FHR2 and FHR5 plasma levels are reduced, whereas FHR1, FHR3, FHR4, FHL1, and Factor H levels are normal (Figure 4A). The kidney biopsy specimens from both patients showed C3b, C5b-9 deposition, and FHR5, as well as properdin staining.65 This FHR212FHR5 hybrid protein with two multimerization domains forms large oligomers, consisting of FHR21–2FHR5, FHR1, FHR2, and FHR5, and forms a hyperactive fluid-phase C3 convertase that consumes C3. In addition, the surface-bound hybrid anchors properdin and serves as a hyper-amplifier of complement.
Figure 4.
CFHR gene variations in C3 glomerulopathy and associated FHR protein variants. (A) Deletion of large chromosomal segments. Deletion of a large chromosomal segment, which includes exons of the CFHR2 gene and the intragenic region spanning to the CFHR5 gene, generates a CFHR2::CFHR5 hybrid gene. This gene encodes an FHR21–2::FHR5 hybrid protein which is shown on the right. (B) Insertional mutation of intragenetic segments: Duplication of CFHR1 gene segments results in an FHR1 mutant protein. Duplication of the first three exons results in a mutant CFHR1 gene. The encoded protein FHR11–2FHR1 has duplicated interaction segments (right panel). (C) Insertional mutageneses of chromosomal segments results in extra hybrid genes. A duplicated extra gene has CFHR3 exons i–iii linked to the last exons of the CFHR1 gene and generates a new CFHR3::CFHR1 hybrid gene that encodes an FHR31–2::FHR1 hybrid protein.
Insertional Mutagenesis of Intragenic Segments
Two scenarios are reported for Spanish patients with C3 glomerulopathy, which add new genetic material in the CFHR1 gene. One patient diagnosed with C3 glomerulopathy has in one CFHR1 allele a duplication, which includes the CFHR1 promoter and exons i-iii, resulting in a mutant gene with two transcription start sites (Figure 4B).66 The upstream promoter generates a longer transcript, which codes FHR11–2FHR1. The mutant proteins with two multimerization segments form large oligomeric complexes with other FHR proteins, which enhance hemolysis (Supplemental Table 1).
A 12-year-old boy with an upper respiratory tract infection by Haemophilus influenza from another Spanish family diagnosed with C3 glomerulopathy had a heterozygous intragenic duplication in one CFHR1 allele combined with the CFHR3-CFHR1 deletion on the second allele.67 Duplication of exons i-v results in an FHR1 mutant protein composed of nine SCRs (Figure 4B). The patients have normal FHR2, FHR3, FHR4, FHR5, FHL1, and Factor H levels. The affected individuals have low C3 plasma levels and the kidneys showed intense C3 deposition, mesangial hypercellularity, and osmiophilic deposits along the GBM. The FHR11–4FHR1 mutant forms large oligomers, which include the mutant, FHR1, FHR2, and FHR5, and which bound with higher intensity to surface-attached C3b and acted as effective competitors for Factor H.
FHR5::FHR5 mutants with duplicated SCRs1–2 were reported in patients of a large Cypriote cohort, who presented with microscopic hematuria, synpharyngitic macroscopic hematuria, and C3 GN, and showed slightly reduced C3 plasma levels.68–70 Kidney biopsy specimens revealed mesangial matrix expansion, capillary wall thickening, and glomerular C3, as well as C1q, IgA, and IgG staining combined with subendothelial electron dense deposits.70 In the CFHR5 gene, the patients have exons i-iii duplicated (Supplemental Table 1). The FHR51–2FHR5 mutant protein has two multimerization domains. An almost identical duplication of CFHR5 exons i-iii was identified in UK family of non-Cypriote origin. The patients expressed the same FHR51–2FHR5 mutants in plasma.71 Both the Cypriote and the UK patients have reduced FHR5 plasma levels. All other FHR proteins, FHL1, and Factor H are present at normal levels.
Duplication of Extra Gene Segments
Insertion of an extra CFHR312::CFHR1 hybrid gene between the FHR3 and FHR1 genes was reported in a UK family.72 The affected members had the biopsy-based diagnosis of MPGN. All five CFHR genes and the Factor H gene are intact; thus, the FHR31–2FHR1 hybrid is expressed together with the five FHR proteins, FHL1, and Factor H (Figure 4C). The renal biopsy specimens showed C3b staining, glomerular inflammation, and a membranoproliferative type III pattern.
A CFHR5::CFHR2 hybrid gene was identified in a US patient with familial C3 glomerulopathy.73 This extra hybrid gene has CFHR5 exons i-iii (SP, SCRs1,2) attached to the CFHR2 gene (Supplemental Table 1). The encoded FHR51–2::FHR2 protein forms large oligomers, which include the hybrid, FHR1, FHR2, and FHR5.
Glomerulopathy due to CFHR Gene Variations
The eight scenarios show heterozygous variations and are familial. Three scenarios result from deletion of large genomic segments. Two cases combine the deletion with heterozygous CFHR3-CFHR1 deletion on the second allele. Two Indian patients, but not the index patient, combine the CFHR4::CFHR2 and CFHR3-CFHR1 deletions. The Italian patient combines the mutations with CFHR2 gene mutations. Two case scenarios from Spain show duplications in the CFHR1 gene, the Cypriot patients have a CFHR5 gene duplication, and two other duplications result in extra CFHR3-CFHR1 or CFHR5-CFHR2 hybrid genes (Supplemental Table 1). CFHR gene variations cause C3 glomerulopathy and the disease develops in the context of an intact Factor H gene and presence of Factor H in plasma. One principle is emerging: CFHR gene variations in C3 glomerulopathy result in variant proteins that have two interaction segments, and for several proteins formation of large oligomeric complexes is shown. Thus, FHR mutant proteins and together with altered FHR plasma levels in the context of an intact Factor H gene cause glomerular pathology (Supplemental Table 1).
Distinct Genetic Scenarios Cause Related Glomerular Changes
C3 glomerulopathy is caused by distinct genetic scenarios; e.g., by heterozygous CFHR gene mutations in the context of intact Factor H alleles and also by homozygous (or compound-heterozygous) Factor H gene mutations and intact CFHR genes. Thus, alterations in different genes cause the same or highly related glomerular alterations and the same pathologies,20,74,75 and highlight the role of FHR proteins as complement modulators.
CFHR Gene Cluster Variations in Other (Kidney) Diseases
IgAN
CFHR1, CFHR3, and CFHR5 gene modifications and variations of FHR plasma levels cause pathology in IgAN and these scenarios develop in the context of intact Factor H and FHL1.76–79 In addition, Factor H genetic variations apparently influence complement regulation in IgAN.80,81 Furthermore, mutations in another complement gene, such as the MBL gene, are reported in IgAN.82 Genome-wide association studies identified the CFHR gene cluster as a susceptibility locus in IgAN and opposing effects are reported.83 Homozygous CFHR1/CFHR3 deletion resulting in the absence of FHR1 and FHR3 in plasma is protective.84–86 In addition, CFHR5 is an IgAN susceptibility gene and mutant FHR5 proteins show altered C3b binding.87 Current work in IgAN is focusing on FHR1 and FHR5 plasma levels.88–91 Elevated FHR1 and also FHR5 plasma levels and higher FHR1::Factor H ratios influence alternative pathway regulation, and correlate with glomerular filtration and disease severity.91
CFHR5 Gene Variations in Other Kidney and Retinal Diseases
Sequence variations in the CFHR5 gene are also reported in aHUS,91–93 in C3 glomerulopathy/DDD,94 for a case with S. pneumoniae infection,95 and for the retinal disease AMD.96 For these scenarios it will be necessary to address whether all reported changes are pathogenic or whether the exchanges represent rare polymorphic variants.
FHR Protein Deposition in Fibrillary GN and Pauci-Immune Kidneys
Proteome analyses identified FHR1, FHR2, and FHR5 deposition in glomeruli of patients with the rare kidney disease fibrillary GN along with strong deposition of DNA-JB997 and in pauci-immune necrotizing crescentic GN.98
The Challenge of Homozygous CFHR1-CFHR3 Deletion
Homozygous CFHR1-CFHR3 deficiency has many faces: it confers risk in DEAP-HUS,23 in SLE,99 and for infections with the Gram-negative bacterium Neisseria meningitidis,100 but has protective effects in IgAN78 and AMD.101,102 Importantly, homozygous CFHR1-CFHR3 deficiency is not a risk or protective marker on its own. Homozygous CFHR1-CFHR3 deficiency is present in 5%–8% of healthy whites and the frequency is even higher in the healthy Asian (approximately 18%) and African populations (approximately 28%).103,104
FHR Variants Provide Clues on FHR Protein Function and Identify Pathologic Roles of FHR Oligomers
CFHR gene alterations cause pathology, which can manifest in different tissues, and variations within the same CFHR gene can cause different pathologies in tissue compartments. Parameters affected include the following categories
The C Terminus of FHR1
FHR1 and Factor H share almost identical three C-terminal SCRs that include the recognition regions. Thus, FHR1 and Factor H bind the same or related targets and together fine-tune local complement action. However, the most C-terminal SCRs of FHR1 and Factor H differ; FHR1LA uses L290,A296 in SCR5 and Factor HSV, S1191,V1197 in SCR20 (see Figure 2). Exchange of the two residues LA and SV has pathologic consequences; FHR1SV, with the Factor HSV residues, and similarly Factor HLA, with the FHR1LA residues, cause aHUS.105–107
FHR Plasma Levels
CFHR cluster variations result in unique FHR–Factor H plasma profiles (Tables 1 and 2). FHR plasma concentration is a timely topic because plasma levels reported for healthy individuals vary by up to two orders of magnitude.108–112 For example, FHR1 plasma levels range from 0.2 μg/ml to 120 μg/ml.76,108–110,113 Complex formation of FHR proteins and association of FHR proteins in lipoprotein particles (see below) may explain such variations and also indicate assay-based detection aspects.
Table 2.
CFHR Gene Variations in C3 Glomerulopathy affect FHR Plasma Levels, but not Factor H and FHL1 plasma levels
| Variable | Intact | Affected | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FHL1 | Factor H | FHR3 | FHR1 | FHR 4 | FHR2 | FHR5 | |||||
| FHR3::FHR4 | 100 | 100 | 0 | FHR3::FHR4 | 0 | 50 | 100 Mutated | 100 | |||
| FHR1::FHR5 | 100 | 100 | 100 | 50 | FHR1::FHR5 | 50 | 50 | 50 | |||
| FHR1::FHR5 | 100 | 100 | 50 | 0 | FHR1::FHR5 | 50 | 50 | 50 | |||
| FHR2::FHR5 | 100 | 100 | 100 | 100 | 100 | 50 | FHR2::FHR5 | 50 | |||
| FHR1::FHR1 | 100 | 100 | 100 | 50 | FHR1::FHR1 | 100 | 100 | 100 | |||
| FHR1::FHR1 | 100 | 100 | 100 | 50 | FHR1::FHR1 | 100 | 100 | 100 | |||
| FHR5::FHR5 | 100 | 100 | 50 | 50 | 100 | 100 | FHR5::FHR5 | 50 | |||
| FHR3::FHR1 | 100 | 100 | 100 | FHR3::FHR1 | 100 | 100 | 100 | 100 | |||
| FHR5::FHR2 | 100 | 100 | 100 | 100 | 100 | 100 | FHR5::FHR2 | 100 | |||
Numerical data presented as %.
FHR Proteins Form Multimers that Show a Preference for Interaction Partners
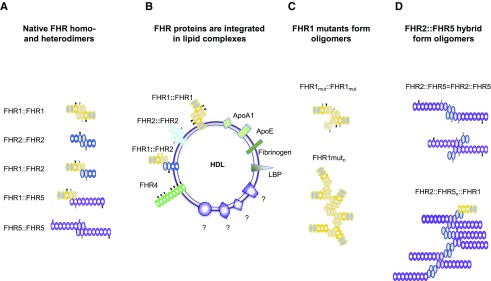
The SCR backbone provides flexibility. FHR proteins apparently interact with themselves, with other FHR proteins, and potentially also with Factor H, and bind to C3b and to plasma and cell surface constituents. In physiologic settings, FHR proteins and also Factor H adapt various conformations and can form dimers, tetramers, or multimers (Figure 5A).58,66,67,111–115 Such multimers enhance regulatory action; for example, Factor H mini-variants, which are linked via an FHR1 multimerization region, are more potent in complement control.116,117 For both FHR1 mutants with duplicated interaction segments,67,68 for the FHR1-FHR5,65 and also for the FHR2::FHR566 hybrid formation of large oligomeric complexes is reported, which enhance complement activity.
Figure 5.
FHR proteins form homodimers and heterodimers; FHR hybrid proteins with two multimerization segments form oligomers. (A) The three proteins FHR1, FHR2, and FHR5 form dimers via their N-terminal domains (gray domains). The N-terminal multimerization segments of each FHR protein interact with a second protein and form dimers and even multimers. FHR1 and FHR2 can form heterodimers. (B) FHR proteins are contained in lipid complexes. FHR1, FHR2, and FHR4-A are associated with lipids HDL and LDLD. Lipids include FHR1 and FHR2 together with ApoAI, LPS-binding protein, fibrinogen, and additional so-far-uncharacterized proteins. FHR4-A is present in triglyceride-rich lipid particles, which also include ApoE, ApoAI, ApoE, ApoAIV, ApoB48, and ApoB100. (C) The FHR1 mutants with two interaction sites form larger complexes. Upper panel: A single FHR1::FHR1 mutant with two interaction segments that bind FHR1, FHR2, and FHR5 and allow formation of larger complexes. Oligomerization or branching is disrupted when FHR1, FHR2, or FHR5 with one interaction segment is integrated. (D) The FHR2::FHR5 hybrid forms larger complexes. Upper panel: A single FHR2::FHR5 hybrid has two interaction segments that allow formation of larger complexes. The FHR2::FHR5 hybrid protein can multimerize via the FHR2 (upper constellation) or via the FHR5 multimerization domain with FHR5 (bottom scenario). Oligomerization or branching is disrupted when FHR1 or FHR2 with one interaction segment is attached to the FHR2 domain, or when FHR5 is bound to the FHR5 domain.
Furthermore, FHR1, FHR2, and FHR4A are integrated into lipid particles, which also include ApoE, ApoAI, ApoB100, LPS-binding protein, fibrinogen, and other uncharacterized proteins.118,119 Thus, unique interaction profiles and a preference for interacting partners indicate different regulatory roles of FHR oligomers (Figure 5B).
Payload Composition Influences Oligomer Function
The disease-associated FHR mutants (FHR1::FHR1, FHR1::FHR5, FHR5::FHR5) and hybrids (FHR1::FHR5-, FHR2::FHR5, FHR5::FHR2) form oligomers that contain the specific FHR variant, FHR1, FHR2, and FHR5. Apparently, payload composition and the combinations of functionally distinct FHR proteins influence oligomer function (Figures 5, C and D and 6).65–68
Figure 6.
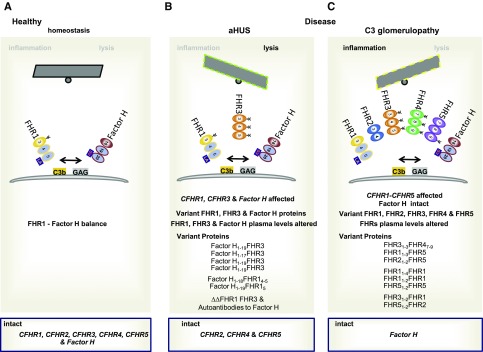
FHR mutant and hybrid proteins affect complement regulation at target sites. Gene mutations and copy number variations in the human Factor H–CFHR gene cluster in aHUS and C3 glomerulopathy affect protein structure and protein levels in plasma and influence the fate of complement control at target surfaces. (A) Normal homeotic scenario. FHR1 and Factor H with their homologous C-terminal regions bind to surface-deposited C3b at danger sites and to glycosaminoglycans (GAGs). The proteins are shown and the three C-terminal recognition region is presented, and the FHR1-specific LA and the Factor H–specific SV residues in the most C-terminal domains are shown. The balance of FHR1 and Factor H at modified target sites is influenced by the structure of the proteins; by Factor H, FHL1, and FHR plasma levels; by the binding intensities of the proteins to the ligands; and by the density of C3b and the type and composition of GAGs at sites of damage. FHR1 competes with Factor H for surface binding and, in a proper combination, the two regulators adjust and fine-tune local complement action. Thereby, FHR1 initiates inflammasome activation and Factor H dissociates the alternative pathway C3 convertase and inhibits complement progression. (B) Scenarios in aHUS and C3 glomerulopathy. aHUS scenario, left panel: Factor H::FHR3, Factor H::FHR1, and FHR1::Factor H hybrid proteins together with altered plasma levels influence the local FHR1, Factor H balance at a damage surface (left site). C3 glomerulopathy scenario, right panel: The various FHR hybrid and mutant proteins, many of which having two interacting segments and form large oligomeric complexes, influence the local FHR1, Factor H balance at damaged surfaces. Scenarios in aHUS, left panel: Various scenarios in the form of deletions; insertional mutations, including intragene; as well as chromosomal duplications generate CFHR–Factor H or Factor H–CFHR hybrid genes. In aHUS, Factor H and the CFHR1 and CFHR3 genes are affected, but CFHR4, CFHR2, and CFHR5 genes remain intact. Scenarios in C3 glomerulopathy, right panel: Various scenarios in the form of deletions; insertional mutations, including intragene; as well as chromosomal duplications generate CFHR-CFHR hybrid genes. In C3 glomerulopathy all five CFHR genes can be affected, but the Factor H gene remains intact.
FHR Proteins as Biomarkers for Diagnosis and Therapy
CFHR gene variations define specific genetic forms that integrate in the overall disease spectrum of aHUS and C3 glomerulopathy. The current diagnosis for C3 glomerulopathy is on the basis of kidney biopsy specimens. However, for both disorders minimal invasive diagnostic approaches are advantageous for biomarker profiling.
CFHR gene variations are valuable genetic markers and, when combined with altered FHR protein mobility, variations of FHR plasma levels provide important diagnostic information. FHR plasma levels, the evaluation of complement split products, TCC levels, complement activity, and C3 levels allow monitoring of complement in a direct and timely manner. Such parameters can also be used evaluate the efficacy of approved complement therapeutics and new inhibitors that are developed and tested in clinical trials.120,121 For renal biopsy specimens, a correlation of FHR protein deposition with morphologic alterations and immunohistologic staining patterns will provide important diagnostic information. In this regard, FHR-specific mABs are advantageous and should be developed.
Conclusions, Perspective, and Future Directions
CFHR–Factor H gene cluster variations are associated with several kidney disorders, including aHUS, C3 glomerulopathy, and also IgAN. These associations show important roles of the FHR proteins in maintaining glomerular integrity. FHR proteins are complement modulators and complement activators, and FHR1 regulates inflammasome activity. The proteins act alone, and also cooperate with each other and with Factor H. For example, FHR1 balances the local action of the complement inhibitor Factor H, and when attached to the necrotic surfaces activates the inflammasome. The unique roles of the individual FHR proteins in the complete cascade suggest that patients with CFHR gene defects may benefit from treatment with one of the new complement inhibitors that are currently in development.122
The major challenges ahead are (1) to link the genetic changes in the CFHR cluster with precise morphologic and immunohistochemical staining of renal biopsy specimens, with cellular and morphologic alterations, and with plasma biomarkers; (2) to understand the exact physiologic role of each FHR protein; and (3) to define the interplay of the FHR proteins as complement modulators or amplifiers with each other and with Factor H. A strong interdisciplinary approach including geneticists, complementologists, nephropathologists, and clinicians is required to establish precise diagnostic and prognostic parameters for the FHR-associated disorders. Advanced understanding of the disease mechanisms will guide physicians and patients through this complex disease spectrum.
DISCLOSURES
Dr. Zipfel provided advice on complement to Vifor Fresenius Medical Care Renal Pharma, Generic Assays and received speaker honorarium from Alexion. All of the remaining authors have nothing to disclose.
Funding
The study was supported by the German Research Foundation Deutsche Forschungsgemeinschaft, SFB 1192 “Immune mediated glomerular diseases”, projects B06 and SK46/2. Dr. Zipfel and Dr. Skerka acknowledge support by Kidneeds. The research leading to these results has received funding from the European Community’s Seventh Framework Programme under grant agreement no. 2012-305608, “European Consortium for High-Throughput Research in Rare Kidney Diseases (EURenOmics)”. Dr. Stea was supported by a scholarship from the Società Italiana di Nefrologia.
Supplementary Material
Acknowledgments
We apologize to all colleagues and groups whose work and important data could not be cited or discussed in greater detail due to space limitations.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019050515-/DCSupplemental.
Supplemental Table 1. Morphologic differences in atypical hemolytic uremic syndrome (aHUS) and C3 glomerulopathy.
Supplemental Table 2. Alterations in the Factor H–CFHR gene cluster in aHUS/dEAP-HUS and C3 glomerulopathy.
References
- 1.Zipfel PF, Skerka C, Chen Q, Wiech T, Goodship T, Johnson S, et al.: The role of complement in C3 glomerulopathy. Mol Immunol 67: 21–30, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Nester CM, Barbour T, de Cordoba SR, Dragon-Durey MA, Fremeaux-Bacchi V, Goodship TH, et al.: Atypical aHUS: State of the art. Mol Immunol 67: 31–42, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Noris M, Mescia F, Remuzzi G: STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol 8: 622–633, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Frémeaux-Bacchi V, Kavanagh D, et al.Conference Participants ;: Atypical hemolytic uremic syndrome and C3 glomerulopathy: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) controversies conference. Kidney Int 91: 539–551, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, et al.: C3 glomerulopathy: Consensus report. Kidney Int 84: 1079–1089, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, et al.: Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet 43: 321–327, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zipfel PF, Skerka C: Complement factor H and related proteins: An expanding family of complement-regulatory proteins? Immunol Today 15: 121–126, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Medjeral-Thomas N, Pickering MC: The complement factor H-related proteins. Immunol Rev 274: 191–201, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Zipfel PF, Skerka C: Complement regulators and inhibitory proteins. Nat Rev Immunol 9: 729–740, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Hajishengallis G, Reis ES, Mastellos DC, Ricklin D, Lambris JD: Novel mechanisms and functions of complement. Nat Immunol 18: 1288–1298, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough JW, Renner B, Thurman JM: The role of the complement system in acute kidney injury. Semin Nephrol 33: 543–556, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulthard LG, Woodruff TM: Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J Immunol 194: 3542–3548, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Liszewski MK, Java A, Schramm EC, Atkinson JP: Complement dysregulation and disease: Insights from contemporary genetics. Annu Rev Pathol 12: 25–52, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holers VM, Banda NK: Complement in the initiation and evolution of rheumatoid arthritis. Front Immunol 9: 1057, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpman D, Loos S, Tati R, Arvidsson I: Haemolytic uraemic syndrome. J Intern Med 281: 123–148, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Sethi S, Fervenza FC: Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med 366: 1119–1131, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Sethi S, Fervenza FC: Pathology of renal diseases associated with dysfunction of the alternative pathway of complement: C3 glomerulopathy and atypical hemolytic uremic syndrome (aHUS). Semin Thromb Hemost 40: 416–421, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Cook HT, Pickering MC: Histopathology of MPGN and C3 glomerulopathies. Nat Rev Nephrol 11: 14–22, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Zipfel PF, Skerka C, Hellwage J, Jokiranta ST, Meri S, Brade V, et al.: Factor H family proteins: On complement, microbes and human diseases. Biochem Soc Trans 30: 971–978, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Bomback AS, Santoriello D, Avasare RS, Regunathan-Shenk R, Canetta PA, Ahn W, et al.: C3 glomerulonephritis and dense deposit disease share a similar disease course in a large United States cohort of patients with C3 glomerulopathy. Kidney Int 93: 977–985, 2018. [DOI] [PubMed] [Google Scholar]
- 21.Díaz-Guillén MA, Rodríguez de Córdoba S, Heine-Suñer D: A radiation hybrid map of complement factor H and factor H-related genes. Immunogenetics 49: 549–552, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Male DA, Ormsby RJ, Ranganathan S, Giannakis E, Gordon DL: Complement factor H: Sequence analysis of 221 kb of human genomic DNA containing the entire fH, fHR-1 and fHR-3 genes. Mol Immunol 37: 41–52, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Zipfel PF, Edey M, Heinen S, Józsi M, Richter H, Misselwitz J, et al.: Deletion of complement factor H-related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PLoS Genet 3: e41, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantsilieris S, Nelson BJ, Huddleston J, Baker C, Harshman L, Penewit K, et al.: Recurrent structural variation, clustered sites of selection, and disease risk for the complement factor H (CFH) gene family. Proc Natl Acad Sci U S A 115: E4433–E4442, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skerka C, Chen Q, Fremeaux-Bacchi V, Roumenina LT: Complement factor H related proteins (CFHRs). Mol Immunol 56: 170–180, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Vik DP, Muñoz-Cánoves P, Kozono H, Martin LG, Tack BF, Chaplin DD: Identification and sequence analysis of four complement factor H-related transcripts in mouse liver. J Biol Chem 265: 3193–3201, 1990. [PubMed] [Google Scholar]
- 27.Józsi M, Zipfel PF: Factor H family proteins and human diseases. Trends Immunol 29: 380–387, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Antonioli AH, White J, Crawford F, Renner B, Marchbank KJ, Hannan JP, et al.: Modulation of the alternative pathway of complement by murine Factor H-related proteins. J Immunol 200: 316–326, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zipfel PF, Hallström T, Hammerschmidt S, Skerka C: The complement fitness factor H: Role in human diseases and for immune escape of pathogens, like pneumococci. Vaccine 26[Suppl 8]: I67–I74, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Boon CJ, van de Kar NC, Klevering BJ, Keunen JE, Cremers FP, Klaver CC, et al.: The spectrum of phenotypes caused by variants in the CFH gene. Mol Immunol 46: 1573–1594, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Zipfel PF, Skerka C: FHL-1/reconectin: A human complement and immune regulator with cell-adhesive function. Immunol Today 20: 135–141, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Csincsi Á, Kopp A, Zöldi M, Bánlaki Z, Uzonyi B, Hebecker M, et al.: Factor H-related protein 5 interacts with pentraxin 3 and the extracellular matrix and modulates complement activation. J Immunol 194: 4963–4973, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Q, Manzke M, Hartmann A, Büttner M, Amann K, Pauly D, et al.: Complement Factor H-Related 5-Hybrid proteins anchor properdin and activate complement at self-surfaces. J Am Soc Nephrol 27: 1413–1425, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudnick RB, Chen Q, Stea ED, Hartmann A, Papac-Milicevic N, Person F, et al.: FHR5 binds to laminins, uses separate C3b and surface-binding sites and activates complement on malondialdehyde-acetaldehyde surfaces. J Immunol 200: 2280–2290, 2018. [DOI] [PubMed] [Google Scholar]
- 35.Hannan JP, Laskowski J, Thurman JM, Hageman GS, Holers VM: Mapping the complement Factor H-related protein 1 (CFHR1):C3b/C3d interactions. PLoS One 11: e0166200, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halder LD, Abdelfatah MA, Jo EA, Jacobsen ID, Westermann M, Beyersdorf N, et al. : Factor H binds to extracellular DNA traps released from human blood monocytes in response to Candida albicans. Front Immunol 7: 671, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loirat C, Frémeaux-Bacchi V: Atypical hemolytic uremic syndrome. Orphanet J Rare Dis 6: 60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szilágyi A, Kiss N, Bereczki C, Tálosi G, Rácz K, Túri S, et al.: The role of complement in Streptococcus pneumoniae-associated haemolytic uraemic syndrome. Nephrol Dial Transplant 28: 2237–2245, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Meinel C, Spartà G, Dahse HM, Hörhold F, König R, Westermann M, et al.: Streptococcus pneumoniae from patients with hemolytic uremic syndrome binds human plasminogen via the surface protein PspC and uses plasmin to damage human endothelial cells. J Infect Dis 217: 358–370, 2018. [DOI] [PubMed] [Google Scholar]
- 40.Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, et al.: Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 5: 1844–1859, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osborne AJ, Breno M, Borsa NG, Bu F, Frémeaux-Bacchi V, Gale DP, et al.: Statistical validation of rare complement variants provides insights into the molecular basis of atypical hemolytic uremic syndrome and C3 glomerulopathy. J Immunol 200: 2464–2478, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maga TK, Nishimura CJ, Weaver AE, Frees KL, Smith RJ: Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat 31: E1445–E1460, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Challis RC, Ring T, Xu Y, Wong EK, Flossmann O, Roberts ISD, et al.: Thrombotic microangiopathy in inverted Formin 2-mediated renal disease. J Am Soc Nephrol 28: 1084–1091, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemaire M, Frémeaux-Bacchi V, Schaefer F, Choi M, Tang WH, Le Quintrec M, et al.: Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet 45: 531–536, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bu F, Zhang Y, Wang K, Borsa NG, Jones MB, Taylor AO, et al.: Genetic analysis of 400 patients refines understanding and implicates a new gene in atypical hemolytic uremic syndrome. J Am Soc Nephrol 29: 2809–2819, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Francis NJ, McNicholas B, Awan A, Waldron M, Reddan D, Sadlier D, et al.: A novel hybrid CFH/CFHR3 gene generated by a microhomology-mediated deletion in familial atypical hemolytic uremic syndrome. Blood 119: 591–601, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Challis RC, Araujo GS, Wong EK, Anderson HE, Awan A, Dorman AM, et al.: A de novo deletion in the regulators of complement activation cluster producing a hybrid complement Factor H/complement Factor H-related 3 gene in atypical hemolytic uremic syndrome. J Am Soc Nephrol 27: 1617–1624, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venables JP, Strain L, Routledge D, Bourn D, Powell HM, Warwicker P, et al.: Atypical haemolytic uraemic syndrome associated with a hybrid complement gene. PLoS Med 3: e431, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maga TK, Meyer NC, Belsha C, Nishimura CJ, Zhang Y, Smith RJ: A novel deletion in the RCA gene cluster causes atypical hemolytic uremic syndrome. Nephrol Dial Transplant 26: 739–741, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eyler SJ, Meyer NC, Zhang Y, Xiao X, Nester CM, Smith RJ: A novel hybrid CFHR1/CFH gene causes atypical hemolytic uremic syndrome. Pediatr Nephrol 28: 2221–2225, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valoti E, Alberti M, Tortajada A, Garcia-Fernandez J, Gastoldi S, Besso L, et al.: A novel atypical hemolytic uremic syndrome-associated hybrid CFHR1/CFH gene encoding a fusion protein that antagonizes factor H-dependent complement regulation. J Am Soc Nephrol 26: 209–219, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goicoechea de Jorge E, Tortajada A, García SP, Gastoldi S, Merinero HM, García-Fernández J, et al.: Factor H competitor generated by gene conversion events associates with atypical hemolytic uremic syndrome. J Am Soc Nephrol 29: 240–249, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abarrategui-Garrido C, Martínez-Barricarte R, López-Trascasa M, de Córdoba SR, Sánchez-Corral P: Characterization of complement factor H-related (CFHR) proteins in plasma reveals novel genetic variations of CFHR1 associated with atypical hemolytic uremic syndrome. Blood 114: 4261–4271, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Bernabéu-Herrero ME, Jiménez-Alcázar M, Anter J, Pinto S, Sánchez Chinchilla D, Garrido S, et al.: Complement factor H, FHR-3 and FHR-1 variants associate in an extended haplotype conferring increased risk of atypical hemolytic uremic syndrome. Mol Immunol 67: 276–286, 2015. [DOI] [PubMed] [Google Scholar]
- 55.Jokiranta TS: HUS and atypical HUS. Blood 129: 2847–2856, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skerka C, Józsi M, Zipfel PF, Dragon-Durey MA, Fremeaux-Bacchi V: Autoantibodies in haemolytic uraemic syndrome (HUS). Thromb Haemost 101: 227–232, 2009. [PubMed] [Google Scholar]
- 57.Józsi M, Licht C, Strobel S, Zipfel SL, Richter H, Heinen S, et al.: Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 111: 1512–1514, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Moore I, Strain L, Pappworth I, Kavanagh D, Barlow PN, Herbert AP, et al.: Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4, and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood 115: 379–387, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Durey MA, Sinha A, Togarsimalemath SK, Bagga A: Anti-complement-factor H-associated glomerulopathies. Nat Rev Nephrol 12: 563–578, 2016. [DOI] [PubMed] [Google Scholar]
- 60.Buhlmann D, Eberhardt HU, Medyukhina A, Prodinger WM, Figge MT, Zipfel PF, et al.: FHR3 Blocks C3d-Mediated Coactivation of Human B Cells. J Immunol 197: 620–629, 2016. [DOI] [PubMed] [Google Scholar]
- 61.Ravindran A, Fervenza FC, Smith RJH, De Vriese AS, Sethi S: C3 glomerulopathy: Ten years’ experience at mayo clinic. Mayo Clin Proc 93: 991–1008, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith RJH, Appel GB, Blom AM, Cook HT, D’Agati VD, Fakhouri F, et al.: C3 glomerulopathy—understanding a rare complement-driven renal disease. Nat Rev Nephrol 15: 129–143, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piras R, Martin B, Valoti B, Borsa N, Alberti M, et al. : Multiple abnormalities in the CFHR-gene cluster in a patient with DDD. Mol Immunol 67: 171, 2015. 25900877 [Google Scholar]
- 64.Togarsimalemath SK, Sethi SK, Duggal R, Le Quintrec M, Jha P, Daniel R, et al.: A novel CFHR1-CFHR5 hybrid leads to a familial dominant C3 glomerulopathy. Kidney Int 92: 876–887, 2017. [DOI] [PubMed] [Google Scholar]
- 65.Chen Q, Wiesener M, Eberhardt HU, Hartmann A, Uzonyi B, Kirschfink M, et al.: Complement factor H-related hybrid protein deregulates complement in dense deposit disease. J Clin Invest 124: 145–155, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tortajada A, Guitierrez-Tenoria J, Gonzalez AS, Letosa RM, Bouthelier A, Sanchez-Coral P, et al. : Novel duplication of the FHRs dimerization domain associated with C3G. Mol Immunol 89: 181, 2017 [Google Scholar]
- 67.Tortajada A, Yébenes H, Abarrategui-Garrido C, Anter J, García-Fernández JM, Martínez-Barricarte R, et al.: C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J Clin Invest 123: 2434–2446, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gale DP, de Jorge EG, Cook HT, Martinez-Barricarte R, Hadjisavvas A, McLean AG, et al.: Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet 376: 794–801, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Athanasiou Y, Voskarides K, Gale DP, Damianou L, Patsias C, Zavros M, et al.: Familial C3 glomerulopathy associated with CFHR5 mutations: Clinical characteristics of 91 patients in 16 pedigrees. Clin J Am Soc Nephrol 6: 1436–1446, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gale DP, Maxwell PH: C3 glomerulonephritis and CFHR5 nephropathy. Nephrol Dial Transplant 28: 282–288, 2013. [DOI] [PubMed] [Google Scholar]
- 71.Malik TH, Lavin PJ, Goicoechea de Jorge E, Vernon KA, Rose KL, Patel MP, et al.: A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol 23: 1155–1160, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Medjeral-Thomas N, Malik TH, Patel MP, Toth T, Cook HT, Tomson C, et al.: A novel CFHR5 fusion protein causes C3 glomerulopathy in a family without Cypriot ancestry. Kidney Int 85: 933–937, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao X, Ghossein C, Tortajada A, Zhang Y, Meyer N, Jones M, et al.: Familial C3 glomerulonephritis caused by a novel CFHR5-CFHR2 fusion gene. Mol Immunol 77: 89–96, 2016. [DOI] [PubMed] [Google Scholar]
- 74.Wong EKS, Kavanagh D: Diseases of complement dysregulation-an overview. Semin Immunopathol 40: 49–64, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Medjeral-Thomas NR, O’Shaughnessy MM, O’Regan JA, Traynor C, Flanagan M, Wong L, et al.: C3 glomerulopathy: Clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol 9: 46–53, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, et al.STOP-IgAN Investigators ;: Intensive Supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 373: 2225–2236, 2015. [DOI] [PubMed] [Google Scholar]
- 77.Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, et al.: Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet 46: 1187–1196, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kiryluk K, Novak J: The genetics and immunobiology of IgA nephropathy. J Clin Invest 124: 2325–2332, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Groopman EE, Rasouly HM, Gharavi AG: Genomic medicine for kidney disease. Nat Rev Nephrol 14: 83–104, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu L, Zhai YL, Wang FM, Hou P, Lv JC, Xu DM, et al.: Variants in complement factor H and complement factor H-related protein genes, CFHR3 and CFHR1, affect complement activation in IgA nephropathy. J Am Soc Nephrol 26: 1195–1204, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu L, Guo WY, Shi SF, Liu LJ, Lv JC, Medjeral-Thomas NR, et al.: Circulating complement factor H-related protein 5 levels contribute to development and progression of IgA nephropathy. Kidney Int 94: 150–158, 2018. [DOI] [PubMed] [Google Scholar]
- 82.Xie J, Kiryluk K, Li Y, Mladkova N, Zhu L, Hou P, et al.: Fine mapping implicates a deletion of CFHR1 and CFHR3 in protection from IgA nephropathy in Han Chinese. J Am Soc Nephrol 27: 3187–3194, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jia M, Zhu L, Zhai YL, Chen P, Xu BY, Guo WY, et al. : Variation in complement factor H affects complement activation in immunoglobulin A vasculitis with nephritis [published online ahead of print March 6, 2019]. Nephrology (Carlton) doi:10.1111/nep.13580 [DOI] [PubMed] [Google Scholar]
- 84.Guo WY, Liu QZ, Zhu L, Li ZY, Meng SJ, Shi SF, et al.: Coding and noncoding variants in CFH act synergistically for complement activation in immunoglobulin A nephropathy. Am J Med Sci 356: 114–120, 2018. [DOI] [PubMed] [Google Scholar]
- 85.Shi B, Wang L, Mou S, Zhang M, Wang Q, Qi C, et al.: Identification of mannose-binding lectin as a mechanism in progressive immunoglobulin A nephropathy. Int J Clin Exp Pathol 8: 1889–1899, 2015. [PMC free article] [PubMed] [Google Scholar]
- 86.Merinero HM, García SP, García-Fernández J, Arjona E, Tortajada A, Rodríguez de Córdoba S: Complete functional characterization of disease-associated genetic variants in the complement factor H gene. Kidney Int 93: 470–481, 2018. [DOI] [PubMed] [Google Scholar]
- 87.Medjeral-Thomas NR, Lomax-Browne HJ, Beckwith H, Willicombe M, McLean AG, Brookes P, et al.: Circulating complement factor H-related proteins 1 and 5 correlate with disease activity in IgA nephropathy. Kidney Int 92: 942–952, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thurman JM, Laskowski J: Complement factor H-related proteins in IgA nephropathy-sometimes a gentle nudge does the trick. Kidney Int 92: 790–793, 2017. [DOI] [PubMed] [Google Scholar]
- 89.Jullien P, Laurent B, Claisse G, Masson I, Dinic M, Thibaudin D, et al.: Deletion variants of CFHR1 and CFHR3 associate with mesangial immune deposits but not with progression of IgA nephropathy. J Am Soc Nephrol 29: 661–669, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhai YL, Meng SJ, Zhu L, Shi SF, Wang SX, Liu LJ, et al.: Rare variants in the complement Factor H-related protein 5 gene contribute to genetic susceptibility to IgA nephropathy. J Am Soc Nephrol 27: 2894–2905, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tortajada A, Gutiérrez E, Goicoechea de Jorge E, Anter J, Segarra A, Espinosa M, et al.: Elevated factor H-related protein 1 and factor H pathogenic variants decrease complement regulation in IgA nephropathy. Kidney Int 92: 953–963, 2017. [DOI] [PubMed] [Google Scholar]
- 92.Monteferrante G, Brioschi S, Caprioli J, Pianetti G, Bettinaglio P, Bresin E, et al.: Genetic analysis of the complement factor H related 5 gene in haemolytic uraemic syndrome. Mol Immunol 44: 1704–1708, 2007. [DOI] [PubMed] [Google Scholar]
- 93.Westra D, Vernon KA, Volokhina EB, Pickering MC, van de Kar NC, van den Heuvel LP: Atypical hemolytic uremic syndrome and genetic aberrations in the complement factor H-related 5 gene. J Hum Genet 57: 459–464, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abrera-Abeleda MA, Nishimura C, Smith JL, Sethi S, McRae JL, Murphy BF, et al.: Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease). J Med Genet 43: 582–589, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vernon KA, Goicoechea de Jorge E, Hall AE, Fremeaux-Bacchi V, Aitman TJ, Cook HT, et al.: Acute presentation and persistent glomerulonephritis following streptococcal infection in a patient with heterozygous complement factor H-related protein 5 deficiency. Am J Kidney Dis 60: 121–125, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Narendra U, Pauer GJ, Hagstrom SA: Genetic analysis of complement factor H related 5, CFHR5, in patients with age-related macular degeneration. Mol Vis 15: 731–736, 2009. [PMC free article] [PubMed] [Google Scholar]
- 97.Dasari S, Alexander MP, Vrana JA, Theis JD, Mills JR, Negron V, et al.: DnaJ heat shock protein family B member 9 is a novel biomarker for fibrillary GN. J Am Soc Nephrol 29: 51–56, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sethi S, Zand L, De Vriese AS, Specks U, Vrana JA, Kanwar S, et al. : Complement activation in pauci-immune necrotizing and crescentic glomerulonephritis: Results of a proteomic analysis. Nephrol Dial Transplant 32[Suppl 1]: i139–i145, 2017 [DOI] [PubMed] [Google Scholar]
- 99.Zhao J, Wu H, Khosravi M, Cui H, Qian X, Kelly JA, et al.BIOLUPUS Network; GENLES Network ;: Association of genetic variants in complement factor H and factor H-related genes with systemic lupus erythematosus susceptibility. PLoS Genet 7: e1002079, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davila S, Wright VJ, Khor CC, Sim KS, Binder A, Breunis WB, et al.International Meningococcal Genetics Consortium ;: Genome-wide association study identifies variants in the CFH region associated with host susceptibility to meningococcal disease. Nat Genet 42: 772–776, 2010. [DOI] [PubMed] [Google Scholar]
- 101.Hughes AE, Orr N, Esfandiary H, Diaz-Torres M, Goodship T, Chakravarthy U: A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat Genet 38: 1173–1177, 2006. [DOI] [PubMed] [Google Scholar]
- 102.Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA: Complement factor H polymorphism and age-related macular degeneration. Science 308: 421–424, 2005. [DOI] [PubMed] [Google Scholar]
- 103.Holmes LV, Strain L, Staniforth SJ, Moore I, Marchbank K, Kavanagh D, et al.: Determining the population frequency of the CFHR3/CFHR1 deletion at 1q32. PLoS One 8: e60352, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zipfel PF, Mache C, Müller D, Licht C, Wigger M, Skerka C; European DEAP-HUS Study Group : DEAP-HUS: Deficiency of CFHR plasma proteins and autoantibody-positive form of hemolytic uremic syndrome. Pediatr Nephrol 25: 2009–2019, 2010. [DOI] [PubMed] [Google Scholar]
- 105.Heinen S, Sanchez-Corral P, Jackson MS, Strain L, Goodship JA, Kemp EJ, et al.: De novo gene conversion in the RCA gene cluster (1q32) causes mutations in complement factor H associated with atypical hemolytic uremic syndrome. Hum Mutat 27: 292–293, 2006. [DOI] [PubMed] [Google Scholar]
- 106.Pangburn MK, Rawal N, Cortes C, Alam MN, Ferreira VP, Atkinson MA: Polyanion-induced self-association of complement factor H. J Immunol 182: 1061–1068, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Manuelian T, Hellwage J, Meri S, Caprioli J, Noris M, Heinen S, et al.: Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest 111: 1181–1190, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang P, Zhu M, Geng-Spyropoulos M, Shardell M, Gonzalez-Freire M, Gudnason V, et al.: A novel, multiplexed targeted mass spectrometry assay for quantification of complement factor H (CFH) variants and CFH-related proteins 1-5 in human plasma. Proteomics 17: 6, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Beek AE, Kamp A, Kruithof S, Nieuwenhuys EJ, Wouters D, Jongerius I, et al.: Reference intervals of factor H and factor H-related proteins in healthy Children. Front Immunol 9: 1727, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schäfer N, Grosche A, Reinders J, Hauck SM, Pouw RB, Kuijpers TW, et al.: Complement regulator FHR-3 is elevated either locally or systemically in a selection of autoimmune diseases. Front Immunol 7: 542, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prodinger WM, Hellwage J, Spruth M, Dierich MP, Zipfel PF: The C-terminus of factor H: Monoclonal antibodies inhibit heparin binding and identify epitopes common to factor H and factor H-related proteins. Biochem J 331: 41–47, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.DiScipio RG: Ultrastructures and interactions of complement factors H and I. J Immunol 149: 2592–2599, 1992. [PubMed] [Google Scholar]
- 113.Pouw RB, Gómez Delgado I, López Lera A, Rodríguez de Córdoba S, Wouters D, Kuijpers TW, et al.: High complement Factor H-related (fhr)-3 levels are associated with the atypical hemolytic-uremic syndrome-risk allele cfhr3*b. Front Immunol 9: 848, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blaum BS, Hannan JP, Herbert AP, Kavanagh D, Uhrín D, Stehle T: Structural basis for sialic acid-mediated self-recognition by complement factor H. Nat Chem Biol 11: 77–82, 2015. [DOI] [PubMed] [Google Scholar]
- 115.Goicoechea de Jorge E, Caesar JJ, Malik TH, Patel M, Colledge M, Johnson S, et al.: Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc Natl Acad Sci U S A 110: 4685–4690, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Michelfelder S, Fischer F, Wäldin A, Hörle KV, Pohl M, Parsons J, et al.: The MFHR1 fusion protein is a novel synthetic multitarget complement inhibitor with therapeutic potential. J Am Soc Nephrol 29: 1141–1153, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang Y, Denton H, Davies OR, Smith-Jackson K, Kerr H, Herbert AP, et al.: An engineered complement Factor H construct for treatment of C3 Glomerulopathy. J Am Soc Nephrol 29: 1649–1661, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park CT, Wright SD: Fibrinogen is a component of a novel lipoprotein particle: Factor H-related protein (FHRP)-associated lipoprotein particle (FALP). Blood 95: 198–204, 2000. [PubMed] [Google Scholar]
- 119.Park CT, Wright SD: Plasma lipopolysaccharide-binding protein is found associated with a particle containing apolipoprotein A-I, phospholipid, and factor H-related proteins. J Biol Chem 271: 18054–18060, 1996 [DOI] [PubMed] [Google Scholar]
- 120. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/home. Accessed August 15, 2019.
- 121.Nester CM, Smith RJ: Complement inhibition in C3 glomerulopathy. Semin Immunol 28: 241–249, 2016. [DOI] [PubMed] [Google Scholar]
- 122.Zipfel PF, Wiech T, Rudnick R, Afonso S, Person F, Skerka C: Complement inhibitors in clinical trials for glomerular diseases. Front Immunol 10: 2166, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.