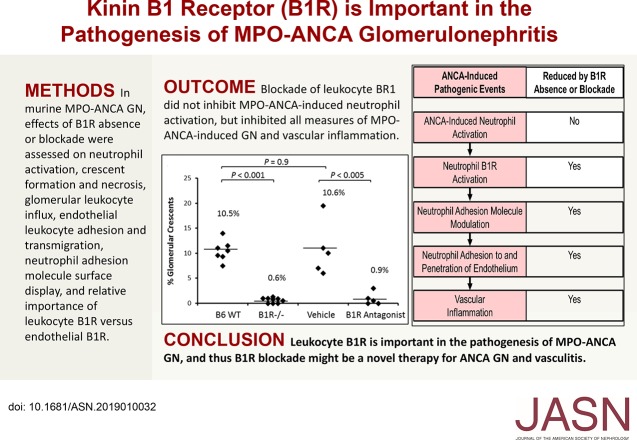
Significance Statement
ANCAs specific for myeloperoxidase (MPO) and proteinase 3 are implicated in the pathogenesis of vasculitis and GN. Kinins play a major role in mediating acute inflammation, and kinin system activation occurs in patients with ANCA vasculitis. The authors investigated the role of bradykinin receptor 1 (B1R), which modulates leukocyte adhesion and migration, in the pathogenesis of MPO-ANCA GN in a murine model. By evaluating the effects of B1R genetic ablation and pharmacologic blockade on neutrophil activation, crescent formation and necrosis, glomerular leukocyte influx and transmigration, neutrophil adhesion molecule surface display, and relative importance of leukocyte B1R versus endothelial B1R, they determined that leukocyte B1R plays a critical role in murine MPO-ANCA GN pathogenesis by modulating neutrophil-endothelial interaction. Pharmacologic blockade of B1R might be a therapeutic option for ANCA GN and vasculitis.
Keywords: ANCA, glomerulonephritis, vasculitis, beta integrins
Visual Abstract
Abstract
Background
Myeloperoxidase-specific ANCA (MPO-ANCA) are implicated in the pathogenesis of vasculitis and GN. Kinins play a major role during acute inflammation by regulating vasodilatation and vascular permeability and by modulating adhesion and migration of leukocytes. Kinin system activation occurs in patients with ANCA vasculitis. Previous studies in animal models of GN and sclerosing kidney diseases have demonstrated protective effects of bradykinin receptor 1 (B1R) blockade via interference with myeloid cell trafficking.
Methods
To investigate the role of B1R in a murine model of MPO-ANCA GN, we evaluated effects of B1R genetic ablation and pharmacologic blockade. We used bone marrow chimeric mice to determine the role of B1R in bone marrow–derived cells (leukocytes) versus nonbone marrow–derived cells. We elucidated mechanisms of B1R effects using in vitro assays for MPO-ANCA–induced neutrophil activation, endothelial adherence, endothelial transmigration, and neutrophil adhesion molecule surface display.
Results
B1R deficiency or blockade prevented or markedly reduced ANCA-induced glomerular crescents, necrosis, and leukocyte influx in mice. B1R was not required for in vitro MPO-ANCA–induced neutrophil activation. Leukocyte B1R deficiency, but not endothelial B1R deficiency, decreased glomerular neutrophil infiltration induced by MPO-ANCA in vivo. B1R enhanced ANCA-induced neutrophil endothelial adhesion and transmigration in vitro. ANCA-activated neutrophils exhibited changes in Mac-1 and LFA-1, important regulators of neutrophil endothelial adhesion and transmigration: ANCA-activated neutrophils increased surface expression of Mac-1 and increased shedding of LFA-1, whereas B1R blockade reduced these effects.
Conclusions
The leukocyte B1R plays a critical role in the pathogenesis of MPO-ANCA–induced GN in a mouse model by modulating neutrophil–endothelial interaction. B1R blockade may have potential as a therapy for ANCA GN and vasculitis.
ANCAs are associated with and may cause an aggressive form of necrotizing small-vessel vasculitis and GN.1 Advances have been made in treating ANCA disease, in part by developing more targeted therapies as the pathogenesis is better understood.2 Current therapies are not fully effective and are accompanied by adverse side effects.3 Advances in understanding molecular pathways that mediate vascular inflammation in ANCA disease could lead to more targeted and less toxic therapy.
The kinin system (kallikrein–kinin system) has been implicated in the pathogenesis of ANCA vasculitis, but the mechanism has not been elucidated.4 Multiple prior studies of animal models of GN and sclerosing kidney diseases have demonstrated protective effects of bradykinin receptor 1 (B1R) blockade via interference with myeloid cell trafficking.5–11 Classic kinin system activation comprises conversion of plasma prekallikrein to active plasma kallikrein, which cleaves high-molecular-weight kininogen, releasing bradykinin.4 Bradykinin engages the bradykinin receptor 2 (B2R), and the active metabolite of bradykinin, des-arg9-BK, engages B1R. B1R is expressed in response to pathophysiologic stimuli, including inflammation, whereas B2R is constitutively expressed in healthy tissue. Similar to complement system activation, kinin system activation amplifies inflammatory responses.
Patients with ANCA vasculitis have increased levels of circulating high-molecular-weight kininogen and bradykinin, and increased kinins in tissue at sites of vasculitis and GN.4,5 Patients with ANCA vasculitis have circulating leukocyte-derived microvesicles with surface B1R, with most microvesicles derived from neutrophils.12 Circulating microvesicles can dock with endothelial cells and transfer B1R to endothelial surfaces.12 B1R-armed endothelial microvesicles may contribute to inflammation in patients with ANCA vasculitis by neutrophil chemotaxis.13
B1R also regulates neutrophil interactions with endothelial cells by modulating neutrophil integrin function.14 Thus, B1R effects in ANCA GN might be mediated in part by influencing the function of integrins. The importance of integrins in the pathogenesis of ANCA GN is supported by in vitro studies that show that blockade of integrin function prevents adherence to human endothelial cells of neutrophils activated by human MPO-ANCA.15
Klein et al.6 observed increased B1R protein in glomeruli and renal interstitium in biopsies from patients with GN, including ANCA GN. They used a mouse model of nephrotoxic GN to demonstrate that pharmacologic B1R blockade reduced severity of GN and improved renal function.6,7
There is strong evidence that ANCA are pathogenic.16 Here, we report our observations on the role of B1R in a mouse model of GN caused by myeloperoxidase-specific ANCA (MPO-ANCA),17 which suggest that B1R blockade could be an effective therapy for ANCA vasculitis and GN.
Methods
Mice
C57BL/6J (B6) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Bdkrb1 knock out (B1R−/−) mice were generated as described previously and genotype confirmed.17 All mice were on a B6 background and females used at 9–12 weeks of age. Mpo knock out (MPO−/−) mice were used for immunization and as donors of anti-MPO IgG.17 Procedures were conducted in accordance with the University of North Carolina Institutional Care and Use Committee, and National Institutes of Health Guide for Care and Use of Laboratory Animals.
Preparation of Pathogenic Anti-MPO IgG
Mouse MPO purification and immunization of MPO−/− mice were performed as previously reported.17 MPO−/− mice were immunized intraperitoneally with 20 μg murine MPO in CFA and boosted twice. Development of antibodies was monitored by anti-MPO ELISA.17 Anti-MPO IgG was isolated from serum of MPO-immunized MPO−/− mice by 50% ammonium sulfate precipitation and protein G affinity chromatography. IgG fractions were concentrated and sterilized by ultrafiltration. Protein concentrations were determined by Coomassie protein assay. IgG purity was confirmed by SDS-PAGE electrophoresis.
Bone Marrow Transplantation
Bone marrow (BM) cells were harvested from femurs and tibias of wild-type (WT) B6 and B1R−/− mice.18 Recipient mice kept under sterile conditions received acidified water with 0.2% neomycin for ≥1 week before γ-irradiation. Mice were lethally irradiated with 900 rad and reconstituted 12–24 hours later with 1.5×107 BM cells. Chimeras were produced by injecting WT donor cells into B1R−/− recipients (B6→B1R−/− chimeras) or B1R−/− cells into WT recipients (B1R−/−→B6 chimeras). B6→B6 and B1R−/−→B1R−/− BM syngeneic transfers served as controls.
In Vitro Neutrophil Activation by Anti-MPO IgG
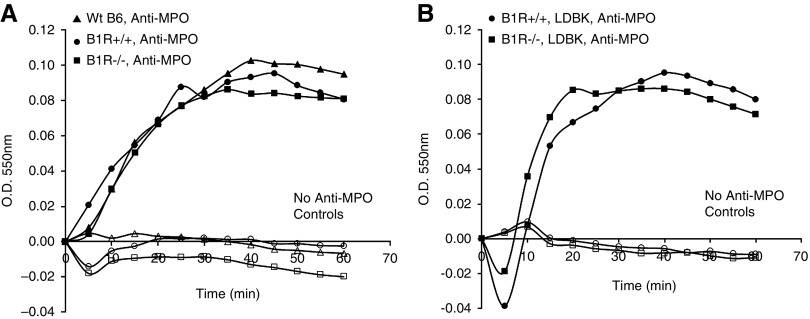
Oxidative burst induced by anti-MPO in murine neutrophils was measured by superoxide release determined by SOD-inhibitable ferricytochrome reduction. Mature neutrophils (1×107, HBSS) were isolated from BM cells by density gradient separation.19 Wright-stained cytospin preparations demonstrated 90.6% neutrophils with mature doughnut-shaped nuclei, 6.3% monocytes, 3.1% lymphocytes, and 0% immature-morphology myeloid cells. Neutrophils were pretreated with 5 μg/ml cytochalasin B for 15 minutes (at 4°C). Neutrophils (5×105) were prewarmed with 50 mmol/L ferricytochrome with/without 300 U/ml SOD for 15 minutes, then primed with 2 ng/ml TNF-α for 15 minutes (at 37°C). Primed neutrophils were incubated with 250 μg/ml anti-MPO IgG at 37°C with/without 10 nM of B1R agonist Lys-des[Arg 9]bradykinin (LDBK) (Sigma-Aldrich). Absorption was scanned at 550 nm every 5 minutes for 60 minutes (Microplate Autoreader; Molecular Devices).
Induction of MPO-ANCA GN
GN was induced by injecting anti-MPO IgG as previously reported.17 Mice were injected intravenously with 50 μg/g body wt anti-MPO IgG. After 6 days, mice were euthanized and kidneys were examined by light microscopy. In BM transplant experiments, 6 weeks after BM transfer (when ≥95% of circulating leukocytes derived from donor),18 recipient mice received anti-MPO IgG (50 μg/g) and were euthanized and evaluated 6 days later.
Functional and Histologic Evaluation of Renal Injury
Mice were placed in metabolic cages 1 day before euthanasia and urine was collected for 12 hours, and tested by dipstick (Roche Diagnostics Corp.) for hematuria, leukocyturia, and proteinuria, reported as the mean on a scale of 0 (none) to 4 (severe), on the basis of observations made without knowledge of the experimental status of the mice. Kidney tissue was collected at euthanasia, examined by light microscopy, and crescents and necrosis expressed as mean percentage of glomeruli with lesions.17
Glomerular leukocyte influx was evaluated by immunohistochemistry. Sections were stained with rat mAb directed to neutrophils (NIMP-R14; Santa Cruz Biotechnology) and monocyte/macrophages (anti-F4/80; eBioscience Inc.) using Bond Autostainer (Leica Biosystems Inc.). Slides were dewaxed and hydrated in Bond Dewax and Wash solution, respectively. NIMP-R14 antigen retrieval was performed at 100°C in Bond-Epitope retrieval solution-1 for 30 minutes. F4/80 Bond enzyme-1 digestion was done for 5 minutes (at 37°C). Slides were incubated for 1 hour with F4/80 (1:50), and 2 hours with NIMP-R14 (1:50). Detection was performed using Bond polymer refine detection system. Positive and negative controls (no primary antibody) were included. Leukocyte localization was evaluated as previously described.18
Cross-sections of the right and left kidneys were evaluated by light microscopy. All glomeruli were evaluated and the percentage with a particular finding calculated. Each cross-section had approximately 95 glomeruli for counting for a total of approximately 190 glomeruli per mouse. All histopathologic evaluations were performed without knowledge of the experimental status of the mice.
In Vivo Blockade of B1R
Using a subcutaneous osmotic minipump (DURECT), WT mice received a continuous infusion (0.7 μg/h) of B1R antagonist [Des-Arg10]-HOE140 (Phoenix Pharmaceuticals, Burlingame, CA) or PBS vehicle that was initiated the day before administration of 50 μg/g mouse anti-MPO IgG and lasted until euthanasia, 6 days after injection of anti-MPO.
In Vitro Neutrophil Adhesion and Transmigration Assays
Neutrophil adhesion and transmigration assays were performed as previously reported.20 Mouse glomerular endothelial cells (Cell Biologics) were seeded into 24-well plates (5×104/well) until confluence. Monolayers were washed with HBSS+/+ and stimulated with 10 ng/ml TNF-α for 4 hours (at 37°C). Cells were washed three times before neutrophil loading. Neutrophils were pretreated with 10 ng/ml TNF-α, 10 nM LDBK, and/or 250 μg/ml anti-MPO IgG in RPMI 1640 for 30 minutes (at 37°C). Pretreated neutrophils (0.5×106) were incubated with endothelial cells for 30 minutes (at 37°C). After washing, endothelial monolayers and adherent neutrophils were lysed in 200 μl 0.5% Triton X-100.
In transmigration experiments, transwell inserts (3.0 μm pores, 6.5 mm, 24-well plates; Corning) were precoated with gelatin at 4°C overnight. Mouse glomerular endothelial cells were gown in transwell inserts to confluence, then pretreated with 10 ng/ml TNF-α for 4 hours (at 37°C). Then, 4×105 TNF-α–pretreated neutrophils, LDBK, and/or anti-MPO antibodies were loaded in the upper chamber; IL-8 (10 ng/ml) was placed in the lower compartment. Transmigrated cells were collected after coincubation of neutrophils and endothelial monolayers for 2.5 hours, then washed and quantified by MPO assay. Experiments were run in triplicate; controls included nontreated neutrophils and absence of IL-8.
Estimation of adherent/transmigrated neutrophils was performed by MPO assay. After washing, cells were lysed with 0.5% Triton X-100 at 4°C for 20 minutes, acidified by 1M citrate buffer (pH 4.2, 50 μl/ml cell lysate) before the reaction. Aliquots (75 μl) from each sample were evaluated. 2.2′-Azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (0.05% in 100 mM citrate with 0.03% H2O2) was added in an equal volume as substrate for MPO. MPO was measured at OD 405 nm. The number of neutrophils in each well was calculated on the basis of standards generated from known concentrations of neutrophils.
Evaluation of Neutrophil Surface Mac-1 and LFA-1 by Flow Cytometry, and Neutrophil LFA-1 Shedding by ELISA
Purified WT B6 neutrophils (2.5×106) were pretreated with or without 100 μm B1R antagonist [Des-Arg10]-HOE140 (Phoenix Pharmaceuticals) for 30 minutes (at 37°C) and then incubated with 250 μg/ml murine anti-MPO Ig G for 40 minutes (at 37°C). Neutrophils were centrifuged and resuspended at 1×106 cells/100 μl in cold FACS buffer (HBSS, 2% FBS) (Gibco), then stained with anti-mouse Ly6G-PE clone 1A8, Mac-1 (CD11b)-BV421 clone M1/70, and LFA-1(CD11a)-Alexa Fluor 647 clone M17/4 (BioLegend, San Diego, CA) antibodies for 30 minutes on ice. Cells were washed twice with FACS buffer and fixed using a 1× 1-step Fix/Lyse solution (eBioscience). Samples were resuspended in FACS buffer and evaluated using an Attune NxT flow cytometer. Neutrophils were gated using FSC-H/FSC-A parameters, then SSC-A/FSC-A parameters, and finally, on the basis of expression of Ly6G. The median fluorescence intensity of each sample was quantified using FlowJo software.
Soluble LFA-1 shed into supernatants from anti-MPO–activated neutrophils was measured by ELISA using LFA-1–specific Mab clone M17/4 (BxCell, West Lebanon, NH) that binds to an epitope on mouse LFA-1 α-subunit extracellular domain (I-domain), which is shed from neutrophils during inflammatory responses.
Statistical Analyses
Data were expressed as mean±SD. Comparisons between groups were performed using a t test and one-way ANOVA with Bonferroni multiple comparison post-test (SAS software version 9.4; SAS Institute, Cary, NC). A P value <0.05 was considered statistically significant.
Results
B1R Deficiency or Blockade Prevents or Ameliorates Murine MPO-ANCA GN
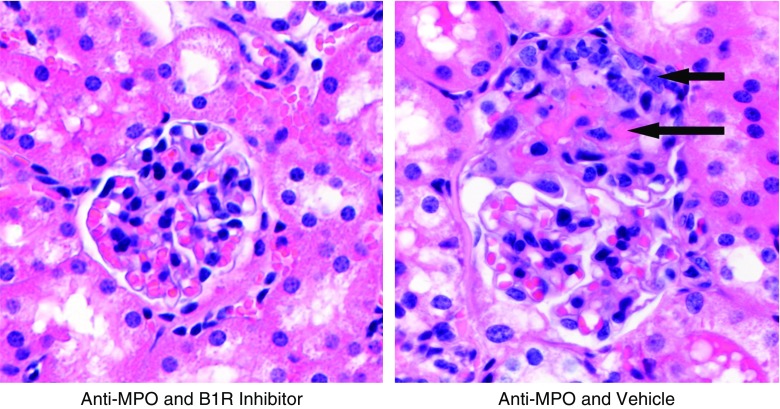
A murine model of MPO-ANCA GN was induced by intravenous injection of 50 µg/g body wt anti-mouse MPO IgG. Induction of MPO-ANCA GN was detected histopathologically by development of focal segmental fibrinoid necrosis and crescent formation (Figure 1).
Figure 1.
Segmental fibrinoid necrosis and crescent formation are the histopathologic features of murine MPO-ANCA GN and are prevented by a B1R antagonist. On the left, from a mouse pretreated with B1R antagonist before receiving anti-MPO, is a representative histologically normal glomerulus 6 days after intravenous injection with 50 µg/g body wt anti-MPO IgG. For comparison, on the right from a mouse pretreated with B1R antagonist vehicle alone before receiving anti-MPO, is a representative glomerulus with segmental fibrinoid necrosis (long arrow) and a cellular crescent (short arrow) 6 days after intravenous injection with 50 µg/g body wt anti-MPO IgG. These anti-MPO–induced crescentic and necrotizing lesions are quantified under different experimental conditions in Figures 2, 3, and 5. Original magnification ×400, hematoxylin and eosin stain.
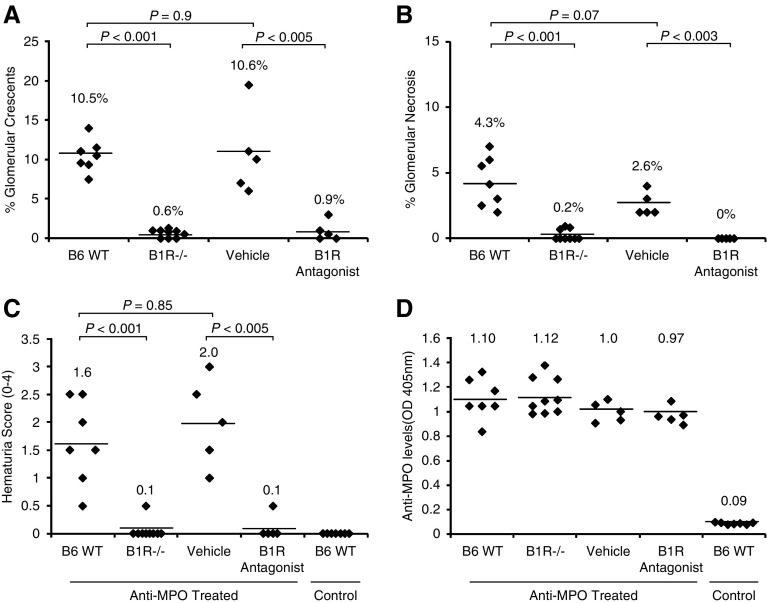
We investigated the pathogenic role of B1R in murine MPO-ANCA GN by comparing GN in mice lacking B1R to WT control mice. At 6 days after administration of anti-MPO, histologic examination of all WT animals (n=7) revealed GN with an average 10.5% crescents and 4.3% necrosis (Figure 2, A and B). In contrast, B1R−/− mice (n=9) developed minimal or no GN with an average <1% glomeruli with crescents or necrosis (P<0.001) (Figure 2, A and B). By day 6, urinalysis showed that WT mice had more hematuria than B1R−/− mice (Figure 2C). Proteinuria and leukocyturia in all groups were not significantly different from control levels, although WT mice and mice that received B1R antagonist trended higher for both. All mice had similar levels of circulating anti-MPO (Figure 2D). These results demonstrate that B1R has a critical role in the pathogenesis of murine MPO-ANCA GN.
Figure 2.
B1R deficiency or blockade ameliorates induction of murine MPO-ANCA GN. WT B6 (n=7) mice, B1R−/− mice (n=9), and mice that were pretreated with vehicle (PBS) (n=5) or specific B1R antagonist (n=5) were intravenously injected with anti-MPO IgG (50 µg/g body wt). Mice were euthanized on day 6 after injection of anti-MPO. Severity of GN was measured by calculating the percentage of glomeruli with crescents and necrosis by counting all glomeruli in cross-sections of both kidneys. (A) Glomerular crescent formation and (B) necrosis induced by anti-MPO were absent or reduced in all B1R−/− mice and B1R antagonist-treated mice compared with WT B6 and vehicle treated mice, respectively. (C) Urine hematuria (dipstick scale, 0–4+) was also reduced in B1R−/− and B1R antagonist-treated mice. (D) Measurement by ELISA of circulating anti-MPO titers at euthanasia demonstrated similar levels in all groups after anti-MPO IgG injection.
To confirm this nephritogenic role for B1R, we tested the effect of B1R pharmacologic blockade using the specific antagonist [Des-Arg10]-HOE140. Starting 1 day before injection of 50 µg/g mouse anti-MPO IgG, WT mice received a continuous infusion (0.7 μg/h) of [Des-Arg10]-HOE140 or PBS vehicle through subcutaneously implanted minipumps. At 6 days after MPO-ANCA administration, all mice in the vehicle control group (n=5) developed GN (average 10.6% crescents and 2.6% necrosis) and high-grade hematuria similar to MPO-ANCA treated WT mice (Figure 2). Pharmacologic B1R blockade (n=5) markedly ameliorated GN (average 0.9% crescents and no necrosis) and hematuria (P<0.005) (Figure 2), demonstrating that B1R blockade protects against murine MPO-ANCA GN.
B1R Deficiency in BM-Derived Cells But Not B1R Deficiency in Non-BM–Derived Cells Suppresses Nephritogenicity of MPO-ANCA
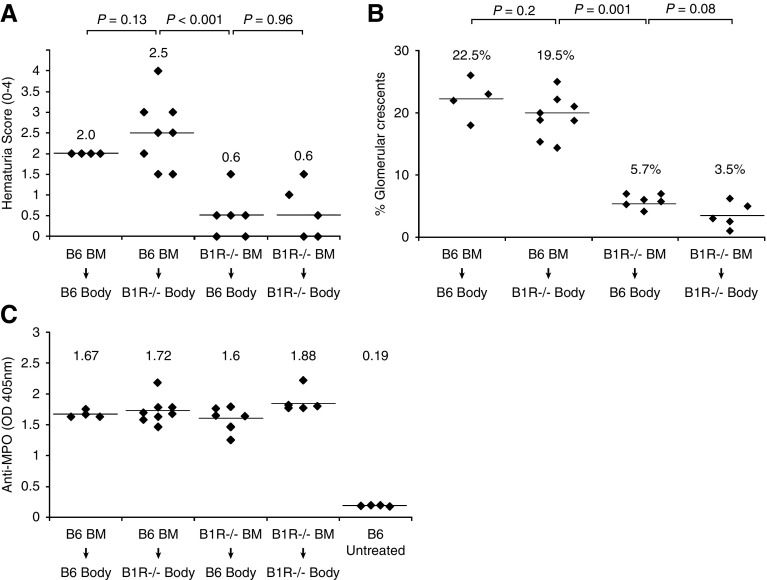
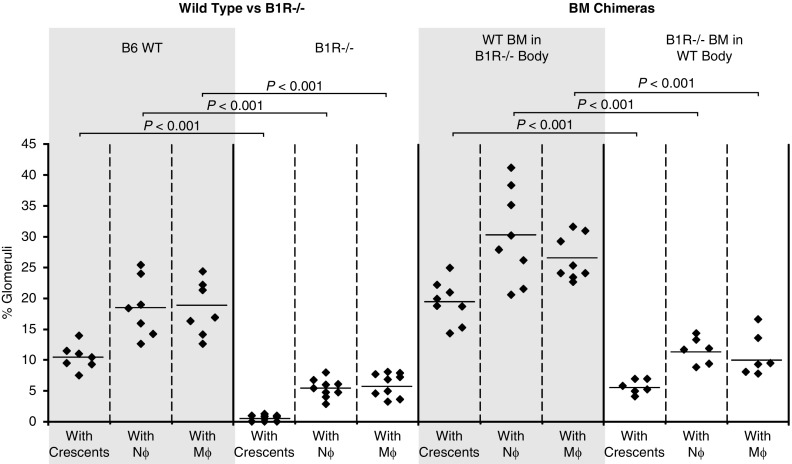
As B1R can be expressed on leukocytes and on tissue cells (e.g., endothelial cells), the pathogenicity of MPO-ANCA was compared in chimeric mice with B1R−/− leukocytes and B1R+/+ tissue to pathogenicity in chimeric mice with B1R+/+ leukocytes and B1R−/− tissue to identify B1R-bearing cell type that is most involved in pathogenesis. Anti-MPO IgG was injected 6 weeks after BM transplantation. At 6 days after anti-MPO injection, all B1R−/− mice transplanted with WT BM (B6→B1R−/− chimeras) developed hematuria and GN (average 19.5% glomerular crescents) similar to the B6→B6 control group (22.5%) (Figure 3). In contrast, B6 mice that received B1R−/− BM cells (B1R−/−→B6 chimeras) had only low-grade hematuria and mild GN (5.7% crescents) that was not significantly different from B1R−/−→B1R−/− control group. These data indicate that B1R on BM-derived cells (i.e., leukocytes) have a major role in the pathogenesis of murine MPO-ANCA GN, whereas B1R on non-BM–derived cells (e.g., endothelial cells) do not.
Figure 3.
B1R deficiency in BM-derived cells but not B1R deficiency in non-BM–derived cells suppresses the pathogenicity of anti-MPO. BM chimeric mice were generated by transplantation of B1R−/− or WT B6 BM into lethally irradiated WT B6 or B1R−/− mice, respectively. Syngeneic BM transplant between the same genotype was used as positive (B6 BM into B6 mice) and negative (B1R−/− BM into B1R−/− mice) controls. All mice received anti-MPO antibodies 6 weeks after BM transplant and were euthanized at day 6 after anti-MPO injection. (A) Determination of hematuria by dipstick (scale, 0–4) revealed a significant reduction in B1R−/− → B6 chimeras. (B) Similarly, histopathologic evaluation of kidney samples from B1R−/− → B6 chimeras demonstrated a significant reduction in the severity of anti-MPO GN. These data indicate that B1R on BM-derived cells have a pathogenic role in the induction of murine MPO-ANCA GN. (C) Measurement of circulating anti-MPO titers by ELISA at euthanasia demonstrated similar levels in all groups after anti-MPO IgG injection.
B1R Is Not Required for In Vitro MPO-ANCA–Induced Neutrophil Activation
On the basis of current concepts of ANCA pathogenesis, B1R could be involved in ANCA GN pathogenesis by facilitating activation of neutrophils by ANCA, or by facilitating the interaction of ANCA-activated neutrophils with endothelial cells, or both. To evaluate the role of B1R in neutrophil activation by ANCA, neutrophils from WT B1R+/+ littermates, and B1R−/− mice were primed with TNF-α, incubated with anti-MPO IgG, and superoxide generation was measured to assess activation. Murine MPO-ANCA caused comparable activation of WT and B1R−/− neutrophils (Figure 4A). Further, addition of B1R agonist LDBK did not increase superoxide anion production in TNF-α–primed, MPO-ANCA–activated neutrophils (Figure 4B). These results indicate that B1R is not involved in direct MPO-ANCA–induced neutrophil activation.
Figure 4.
In vitro induction of respiratory burst by neutrophil activation by anti-MPO IgG is not dependent on B1R. (A) Superoxide anion production in TNF-α–primed neutrophils derived from WT B6 (black triangles), B1R+/+ littermates (black circles), and B1R−/− (black squares) as measured by the SOD inhibitable ferricytochrome C assay. Anti-MPO IgG induces similar superoxide anion production in these three groups. (B) TNF-α–primed B1R+/+ and B1R−/− neutrophils incubated with B1R agonist LDBK and anti-MPO IgG showed comparable levels of activation. Negative controls include neutrophils from WT B6 (white triangles), B1R+/+ littermates (white circles), and B1R−/− (white squares) treated only with LDBK (no anti-MPO IgG).
Leukocyte B1R Deficiency Decreases Glomerular Neutrophil Infiltration Induced by MPO-ANCA
To evaluate the role of B1R in mediating interaction of ANCA-activated neutrophils with glomerular capillaries in vivo, we evaluated the effect of B1R deletion on MPO-ANCA–induced glomerular leukocyte accumulation. GN was induced by injecting anti-MPO IgG. Glomerular influx of neutrophils and macrophages was measured by immunohistochemistry 6 days after anti-MPO IgG administration. Neutrophil and macrophage accumulation in glomeruli of B1R-null mice was reduced compared with WT mice (Figure 5A). Circulating white blood cell count and differential were similar within both strains (Table 1). This observation was confirmed using BM chimeras as WT mice receiving B1R−/− BM cells showed a significant lower number of intraglomerular neutrophils and macrophages compared with B1R−/− chimeric mice transplanted with B1R+/+ BM cells (Figure 5B). Circulating white blood cell count and differential were similar within all strains (data not shown). These findings indicate that B1R on BM-derived cells (i.e., leukocytes) plays an important role in MPO-ANCA–induced glomerular influx of neutrophils and monocytes.
Figure 5.
B1R deficiency in BM-derived cells reduces anti-MPO–induced glomerular crescents, and glomerular influx of neutrophils and monocytes. At 6 days after injection of anti-MPO IgG, kidney samples of experimental groups were evaluated for percent of glomeruli with crescents, and percent of glomeruli containing neutrophils and monocytes. WT B6 mice injected with anti-MPO had more glomeruli with crescents, and more with neutrophil and monocytes compared with B1R−/− mice (two panels on the left). Chimeric B1R−/− mice that received WT (B1R+/+) BM (n=8) injected with anti-MPO had more glomeruli with crescents, and more with neutrophil and monocytes compared with chimeric WT mice that received B1R−/− BM (n=6).
Table 1.
Peripheral blood cell analysis for WT and B1R−/− mice 6 days after receipt of anti-MPO IgG
| Mouse | WBCs (103/µl) | Lymphocytes (103/µl) | Neutrophils (103/µl) | Monocytes (103/µl) | Hemoglobin (g/dl) |
|---|---|---|---|---|---|
| B6 WT (n=7) | 9.8±0.3 | 8.1±0.6 | 0.6±0.3 | 0.7±0.1 | 16.6±0.4 |
| B6 B1R−/− (n=7) | 8.8±1.3 | 7.6±1.0 | 0.5±02 | 0.7±0.2 | 17.6±0.3 |
Data are means±SD. None of the comparisons showed significant differences. WBCs, white blood cells.
B1R Enhances ANCA-Induced Neutrophil Endothelial Adhesion and Transmigration
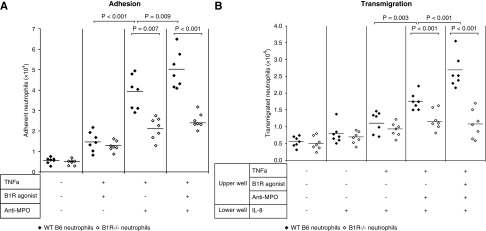
We evaluated the functional role of B1R in adhesion and transmigration of MPO-ANCA–stimulated neutrophils through glomerular endothelial monolayers. Adhesion to endothelial cells was greater for anti-MPO–treated WT neutrophils compared with B1R−/− neutrophils (Figure 6A). Neutrophils adherence increased further when B1R agonist LDBK was added. Anti-MPO antibodies caused increased transmigration of WT neutrophils compared with B1R−/− cells; this effect was enhanced by a B1R agonist (Figure 6B). These results indicate that B1R activation augments endothelial adhesion and transmigration of MPO-ANCA–activated neutrophils.
Figure 6.
B1R stimulation enhances anti-MPO–induced neutrophil–endothelial cell interaction. Neutrophils from WT B6 (n=7) or B1R−/− (n=7) mice were pretreated with TNF-α (10 ng/ml), B1R agonist LDBK (10 nM), and/or anti-MPO IgG (250 µg/ml) for 30 minutes at 37°C, and then coincubated with (A) postconfluent glomerular endothelial monolayers for 30 minutes for adhesion assay, or (B) with transwell insert coated with the endothelial cells for transmigration assay. All experiments were performed in triplicate. The bars represent the mean. Quantity of adherent or migrated neutrophils were measured by MPO activity assay after separation of neutrophils from medium.
B1R Blockade Decreased MPO-ANCA–Induced Increased Neutrophil Surface Display of Mac-1, and Increased Shedding of LFA-1 Fragments
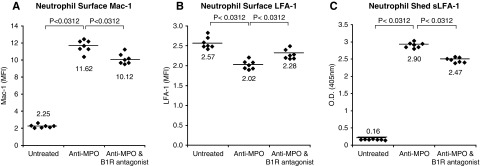
Because Mac-1 and LFA-1 are important regulators of neutrophil endothelial adhesion and transmigration,14 we evaluated the effects of B1R blockade on Mac-1 and LFA-1 surface display on ANCA-activated neutrophils. In vitro activation of mouse neutrophils resulted in increased Mac-1 surface display by FACS analysis, and this was diminished by an antagonist of B1R (Figure 7A). Conversely, in vitro activation of mouse neutrophils resulted in decreased surface display of LFA-1 as recognized by an antibody specific for the I-domain, and this also was diminished by an antagonist of B1R (Figure 7B). An I-domain fragment of LFA-1 that is recognized by the mAb used in this study (M17/4; BxCell) is shed from leukocytes during inflammatory responses.21,22 This was confirmed by demonstrating the shedding of this fragment into the supernatant after activation of neutrophils by anti-MPO IgG, which also was diminished by a B1R antagonist (Figure 7C). Thus, B1R modulates changes in neutrophil surface expression of Mac-1 and LFA-1 that are induced by MPO-ANCA activation.
Figure 7.
MPO-ANCA–induced increased neutrophil surface display of Mac-1, and increased shedding of LFA-1 are reduced by B1R blockade. WT B6 neutrophils were pretreated with or without B1R antagonist and then incubated with murine anti-MPO IgG. Surface display of (A) Mac-1 and (B) LFA-1 was measured by FACS analysis on paired untreated, anti-MPO–treated, and anti-MPO plus B1R antagonist-treated samples. Surface display was expressed as median fluorescence intensity (MFI) of each sample. (C) Soluble LFA-1 (sLFA-1) shed into supernatants from anti-MPO–activated neutrophils was measured by ELISA using an LFA-1–specific monoclonal that binds to an epitope on mouse LFA-1 α-subunit extracellular domain (I-domain) that is shed from activated neutrophils. Statistical analysis used Wilcoxon signed rank tests, with an adjusted P value reported with Bonferroni correction for multiple comparisons. The adjusted P value is 0.03 for comparisons between each group in (A–C).
Discussion
B1R plays a critical role in pathogenesis in this mouse model of MPO-ANCA GN. Our results show that (1) B1R deficiency or blockade prevents or ameliorates MPO-ANCA GN; (2) B1R is not required for in vitro MPO-ANCA–induced neutrophil activation; (3) leukocyte B1R deficiency, but not endothelial B1R deficiency, decreases glomerular neutrophil accumulation; (4) B1R activation enhances MPO-ANCA–induced neutrophil endothelial adhesion and transmigration; and (5) B1R modulates changes in surface expression of Mac-1 and LFA-1 on ANCA-activated neutrophils.
This experimental data agrees with clinical evidence suggesting that the kinin system is involved in the pathogenesis of ANCA vasculitis.4,5,8 Bradykinin is increased in serum and kidney tissue of patients with active ANCA disease.5 Renal biopsy specimens from patients with ANCA GN have increased B1R in crescentic glomeruli and in areas of severe interstitial inflammation.7 Patients with ANCA vasculitis have neutrophil-derived microvesicles bearing functional B1R in the circulation and in inflamed glomerular capillaries.12 Neutrophil-derived microvesicles can dock with endothelial cells and arm them with additional B1R, and endothelial microvesicles with B1R may contribute to vascular inflammation.13
Using BM transplants of B1R−/− or B1R+/+ hematopoietic cells into WT or B1R−/− recipients, we found that B1R expressing BM-derived cells were critical for induction of murine MPO-ANCA GN. This is consistent with our prior studies demonstrating that the severity of this model of murine MPO-ANCA GN is determined by differences in function of BM-derived cells,23 and that neutrophils are the most important effector cells.24 B1R-armed endothelial cells in B1R WT mice could play a role in pathogenesis,14 but endothelial cells in B1R−/− mice that are not armed with leukocyte-derived B1R may not have sufficient endogenous B1R to facilitate vascular inflammation.
We investigated whether B1R deficiency or agonist affect in vitro activation of neutrophils by MPO-ANCA, and found that B1R is not directly involved in direct ANCA-induced neutrophil activation. This indicates that the in vivo effect is at a later step in the pathogenesis of vascular inflammation after ANCA-induced neutrophil activation.
Acute lesions in patients with ANCA vasculitis and GN,25 and the MPO-ANCA mouse model,17,18 are characterized histologically by neutrophil infiltration and necrosis, indicating local recruitment and activation of neutrophils. We found that B1R-null mice had reduction of neutrophils and monocytes at sites of anti-MPO–induced glomerular inflammation. We further observed that absence of B1R decreased neutrophil adhesion to and transmigration through endothelial monolayers, and that activation of B1R synergized with MPO-ANCA to increase neutrophils adhesion and transmigration. These findings confirm previous studies showing that B1R has a critical role in several stages of neutrophil recruitment, and that blockade of B1R markedly attenuates the inflammatory response by reducing neutrophil migration to inflammatory stimuli.26–28 Our results are in agreement with findings in a nephrotoxic nephritis model in which B1R blockade prevents GN by reducing leukocyte recruitment.7
Activated neutrophils are induced to migrate from the vascular compartment into affected glomeruli and vessel walls by interactions with endothelium through modulation of adhesion molecules, especially Mac-1 and LFA-1.14 We demonstrated that ANCA-activated neutrophils have changes in surface display of Mac-1 and LFA-1 that have been observed in other inflammatory conditions. Of note is the demonstration that activation of neutrophils by ANCA results in the shedding of a portion of the I-domain of LFA-1, which has been observed in human inflammatory conditions.21,22 This raises the possibility that circulating soluble LFA-1 might have biologic effects in patients with ANCA GN, and should be evaluated as a possible biomarker of active disease.
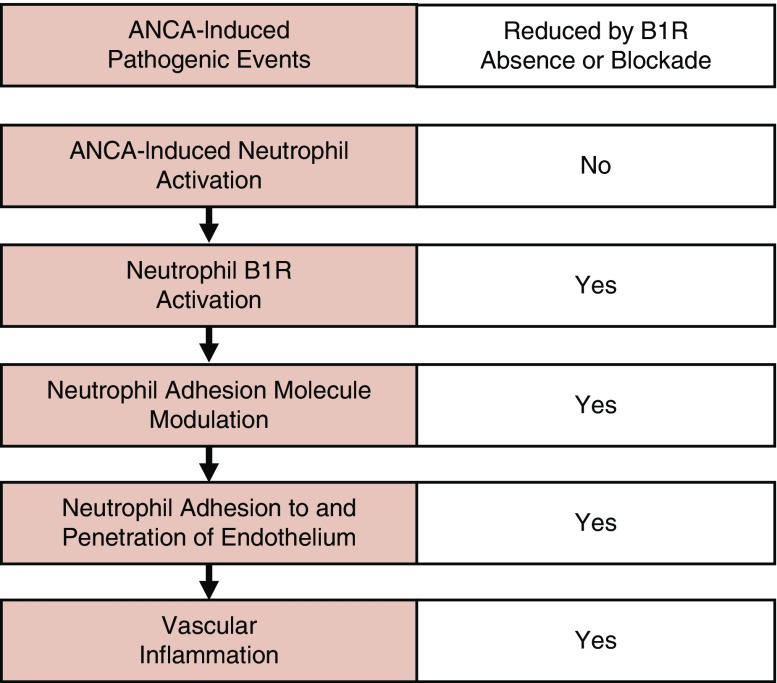
On the basis of these observations, we hypothesize that B1R activation has an important role in the pathogenesis of ANCA vasculitis and GN via a role that is downstream of ANCA-induced neutrophil activation, and that primarily modulates neutrophil adherence to and penetration of vessel walls (Figure 8). Kinin activation may have a similar role to alternative complement pathway activation29 in amplifying inflammatory injury that is initiated by ANCA-induced neutrophil activation. Blockade of the kinin system has been shown to reduce complement activation on endothelial surfaces during vascular inflammation.30
Figure 8.
B1R deficiency or blockade does not prevent ANCA-induced neutrophil activation, but does reduce ANCA-induced neutrophil adhesion molecule modulation, diminishes ANCA-induced neutrophil adhesion to and penetration of endothelial cells, and prevents or markedly suppresses murine MPO-ANCA–induced GN.
The source of kinin activation is not addressed by our study. We speculate that a major source of kinin activation occurs on the surface of ANCA-activated neutrophils and the surface of nearby endothelial cells. Activation of neutrophils causes release of proteases, e.g., PR3, elastase, azurocidin, and cathepsin, that can cleave kininogen on the surface of neutrophils and endothelial cells to produce active kinins.31–35 Neutrophil-derived PR3 can proteolyze kininogen and liberate a PR3-kinin that activates B1R.34 Neutrophil extracellular traps are filamentous extrusions of DNA, histones, and granular enzymes that are deposited at sites of ANCA-induced inflammation and seem to be involved in tissue injury36; they provide a negatively charged surface that initiates activation of the kallikrein–kinin system and the complement systems, both of which can amplify ANCA-induced inflammation.37
Shedding of B1R-bearing, neutrophil-derived microvesicles can transfer B1R onto glomerular and vessel wall cells (e.g., endothelial and smooth muscle cells), and could contribute to kinin-mediated inflammation.12 B1R activation on neutrophils and macrophages also stimulates proinflammatory cytokine release that can further amplified and sustain ANCA-induced inflammation.38–41
In conclusion, our data support an important role for the kinin system in the pathogenesis of ANCA-induced vasculitis and GN. Blockade of B1R may prove to be a novel adjunct to therapy for ANCA disease, as well as other inflammatory diseases.42
Disclosures
Dr. Jennette reports grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease, during the conduct of the study; personal fees from Genentech, personal fees from ChemoCentryx, grants from MedImmune, grants from AbbVie, outside the submitted work.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Disease grant DOI DK058335.
Acknowledgments
Hu, Su, Xiao, and Jennette designed the study. Hu, Su, and Xiao designed and carried out experiments. Hu, Su, Xiao, Herrera, Alba, Kakoki, Falk, and Jennette analyzed the data. Hu, Su, Alba, and Jennette wrote the paper. All authors approved the final version of the manuscript.
We thank Susan Hogan and Yichun Hu for assistance with statistical analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al.: 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum 65: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Jayne D, Rasmussen N: Twenty-five years of European Union collaboration in ANCA-associated vasculitis research. Nephrol Dial Transplant 30[Suppl 1]: i1–i7, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Robson J, Doll H, Suppiah R, Flossmann O, Harper L, Höglund P, et al.: Damage in the anca-associated vasculitides: Long-term data from the European vasculitis study group (EUVAS) therapeutic trials. Ann Rheum Dis 74: 177–184, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Karpman D, Kahn R: The contact/kinin and complement systems in vasculitis. APMIS Suppl 127: 48–54, 2009 [DOI] [PubMed]
- 5.Kahn R, Herwald H, Müller-Esterl W, Schmitt R, Sjögren AC, Truedsson L, et al.: Contact-system activation in children with vasculitis. Lancet 360: 535–541, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Klein J, Gonzalez J, Decramer S, Bandin F, Neau E, Salant DJ, et al.: Blockade of the kinin B1 receptor ameloriates glomerulonephritis. J Am Soc Nephrol 21: 1157–1164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein J, Gonzalez J, Duchene J, Esposito L, Pradère JP, Neau E, et al.: Delayed blockade of the kinin B1 receptor reduces renal inflammation and fibrosis in obstructive nephropathy. FASEB J 23: 134–142, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Pereira RL, Buscariollo BN, Corrêa-Costa M, Semedo P, Oliveira CD, Reis VO, et al.: Bradykinin receptor 1 activation exacerbates experimental focal and segmental glomerulosclerosis. Kidney Int 79: 1217–1227, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Wang PH, Campanholle G, Cenedeze MA, Feitoza CQ, Gonçalves GM, Landgraf RG, et al.: Bradykinin [corrected] B1 receptor antagonism is beneficial in renal ischemia-reperfusion injury. PLoS One 3: e3050, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakoki M, McGarrah RW, Kim HS, Smithies O: Bradykinin B1 and B2 receptors both have protective roles in renal ischemia/reperfusion injury. Proc Natl Acad Sci U S A 104: 7576–7581, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estrela GR, Wasinski F, Almeida DC, Amano MT, Castoldi A, Dias CC, et al.: Kinin B1 receptor deficiency attenuates cisplatin-induced acute kidney injury by modulating immune cell migration. J Mol Med (Berl) 92: 399–409, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Kahn R, Mossberg M, Ståhl AL, Johansson K, Lopatko Lindman I, Heijl C, et al.: Microvesicle transfer of kinin B1-receptors is a novel inflammatory mechanism in vasculitis. Kidney Int 91: 96–105, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Mossberg M, Ståhl AL, Kahn R, Kristoffersson AC, Tati R, Heijl C, et al.: C1-inhibitor decreases the release of vasculitis-like chemotactic endothelial microvesicles. J Am Soc Nephrol 28: 2472–2481, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figueroa CD, Matus CE, Pavicic F, Sarmiento J, Hidalgo MA, Burgos RA, et al.: Kinin B1 receptor regulates interactions between neutrophils and endothelial cells by modulating the levels of Mac-1, LFA-1 and intercellular adhesion molecule-1. Innate Immun 21: 289–304, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Ewert BH, Becker ME, Jennette JC, Falk RJ: Antimyeloperoxidase antibodies induce neutrophil adherence to cultured human endothelial cells. Ren Fail 17: 125–133, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Jennette JC, Falk RJ: Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol 10: 463–473, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, et al.: Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110: 955–963, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiber A, Xiao H, Falk RJ, Jennette JC: Bone marrow-derived cells are sufficient and necessary targets to mediate glomerulonephritis and vasculitis induced by anti-myeloperoxidase antibodies. J Am Soc Nephrol 17: 3355–3364, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Boxio R, Bossenmeyer-Pourié C, Steinckwich N, Dournon C, Nüsse O: Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol 75: 604–611, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Hu N, Westra J, Rutgers A, Doornbos-Van der Meer B, Huitema MG, Stegeman CA, et al.: Decreased CXCR1 and CXCR2 expression on neutrophils in anti-neutrophil cytoplasmic autoantibody-associated vasculitides potentially increases neutrophil adhesion and impairs migration. Arthritis Res Ther 13: R201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans BJ, McDowall A, Taylor PC, Hogg N, Haskard DO, Landis RC: Shedding of lymphocyte function-associated antigen-1 (LFA-1) in a human inflammatory response. Blood 107: 3593–3599, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Gjelstrup LC, Boesen T, Kragstrup TW, Jørgensen A, Klein NJ, Thiel S, et al.: Shedding of large functionally active CD11/CD18 Integrin complexes from leukocyte membranes during synovial inflammation distinguishes three types of arthritis through differential epitope exposure. J Immunol 185: 4154–4168, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Xiao H, Ciavatta D, Aylor DL, Hu P, de Villena FP, Falk RJ, et al.: Genetically determined severity of anti-myeloperoxidase glomerulonephritis. Am J Pathol 182: 1219–1226, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao H, Heeringa P, Liu Z, Huugen D, Hu P, Maeda N, et al.: The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol 167: 39–45, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jennette JC, Falk RJ: The role of pathology in the classification and diagnosis of ANCA associated vasculitis. Nephron 129[suppl 2]: 26–29, 2015 [Google Scholar]
- 26.Duchene J, Lecomte F, Ahmed S, Cayla C, Pesquero J, Bader M, et al.: A novel inflammatory pathway involved in leukocyte recruitment: Role for the kinin B1 receptor and the chemokine CXCL5. J Immunol 179: 4849–4856, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLean PG, Ahluwalia A, Perretti M: Association between kinin B(1) receptor expression and leukocyte trafficking across mouse mesenteric postcapillary venules. J Exp Med 192: 367–380, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araújo RC, Kettritz R, Fichtner I, Paiva AC, Pesquero JB, Bader M: Altered neutrophil homeostasis in kinin B1 receptor-deficient mice. Biol Chem 382: 91–95, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Charles Jennette J, Xiao H, Hu P: Complement in ANCA-associated vasculitis. Semin Nephrol 33: 557–564, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopatko Fagerström I, Ståhl AL, Mossberg M, Tati R, Kristoffersson AC, Kahn R, et al.: Blockade of the kallikrein-kinin system reduces endothelial complement activation in vascular inflammation. EBioMedicine 47: 319–328, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imamura T, Tanase S, Hayashi I, Potempa J, Kozik A, Travis J: Release of a new vascular permeability enhancing peptide from kininogens by human neutrophil elastase. Biochem Biophys Res Commun 294: 423–428, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Imamura T, Potempa J, Travis J: Activation of the kallikrein-kinin system and release of new kinins through alternative cleavage of kininogens by microbial and human cell proteinases. Biol Chem 385: 989–996, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Puzer L, Vercesi J, Alves MF, Barros NM, Araujo MS, Aparecida Juliano M, et al.: A possible alternative mechanism of kinin generation in vivo by cathepsin L. Biol Chem 386: 699–704, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Kahn R, Hellmark T, Leeb-Lundberg LM, Akbari N, Todiras M, Olofsson T, et al.: Neutrophil-derived proteinase 3 induces kallikrein-independent release of a novel vasoactive kinin. J Immunol 182: 7906–7915, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Kenne E, Rasmuson J, Renne T, Vieira ML, Muller-Esterl W, Herwald H, et al.: Neutrophils engage the kallikrein-kinin system to open up the endothelial barrier in acute inflammation. FASEB J 33: 2599–2609, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Heeringa P, Rutgers A, Kallenberg CGM: The net effect of ANCA on neutrophil extracellular trap formation. Kidney Int 94: 14–16, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Oehmcke S, Mörgelin M, Herwald H: Activation of the human contact system on neutrophil extracellular traps. J Innate Immun 1: 225–230, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehrenfeld P, Millan C, Matus CE, Figueroa JE, Burgos RA, Nualart F, et al.: Activation of kinin B1 receptors induces chemotaxis of human neutrophils. J Leukoc Biol 80: 117–124, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Stuardo M, Gonzalez CB, Nualart F, Boric M, Corthorn J, Bhoola KD, et al.: Stimulated human neutrophils form biologically active kinin peptides from high and low molecular weight kininogens. J Leukoc Biol 75: 631–640, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Tiffany CW, Burch RM: Bradykinin stimulates tumor necrosis factor and interleukin-1 release from macrophages. FEBS Lett 247: 189–192, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Bhoola KD, Figueroa CD, Worthy K: Bioregulation of kinins: Kallikreins, kininogens, and kininases. Pharmacol Rev 44: 1–80, 1992 [PubMed] [Google Scholar]
- 42.Whalley ET, Figueroa CD, Gera L, Bhoola KD: Discovery and therapeutic potential of kinin receptor antagonists. Expert Opin Drug Discov 7: 1129–1148, 2012 [DOI] [PubMed] [Google Scholar]