Abstract
Background
Williams syndrome (WS) and autism spectrum disorder (ASD) are neurodevelopmental disorders that demonstrate overlapping genetic associations, dichotomous sociobehavioral phenotypes, and dichotomous pathological differences in neuronal distribution in key social brain areas, including the prefrontal cortex and the amygdala. The serotonergic system is critical to many processes underlying neurodevelopment and is additionally an important neuromodulator associated with behavioral variation. The amygdala is heavily innervated by serotonergic projections, suggesting that the serotonergic system is a significant mediator of neuronal activity. Disruptions to the serotonergic system, and atypical structure and function of the amygdala, are implicated in both WS and ASD.
Methods
We quantified the serotonergic axon density in the four major subdivisions of the amygdala in the postmortem brains of individuals diagnosed with ASD and WS and neurotypical (NT) brains.
Results
We found opposing directions of change in serotonergic innervation in the two disorders, with ASD displaying an increase in serotonergic axons compared to NT and WS displaying a decrease. Significant differences (p < 0.05) were observed between WS and ASD data sets across multiple amygdala nuclei.
Limitations
This study is limited by the availability of human postmortem tissue. Small sample size is an unavoidable limitation of most postmortem human brain research and particularly postmortem research in rare disorders.
Conclusions
Differential alterations to serotonergic innervation of the amygdala may contribute to differences in sociobehavioral phenotype in WS and ASD. These findings will inform future work identifying targets for future therapeutics in these and other disorders characterized by atypical social behavior.
Background
Williams syndrome (WS) is a rare neurodevelopmental disorder (~ 1/10,000 [1]) caused by a hemizygous deletion on chromosome band 7q11.23 and associated with a distinct socioaffective phenotype which includes an atypically strong drive for social engagement, an uninhibited propensity to approach and socially engage with strangers, decreased social anxiety, and increased attention to faces [2, 3]. In contrast, autism spectrum disorder (ASD) is a common neurodevelopmental disorder (1/59 in the USA [4]) with a highly complex and heterogeneous genetic etiology and a behavioral phenotype characterized in part by reduced drive for social engagement and decreased attention/atypical processing of the eyes of others, an important social stimulus in humans [5, 6]. Our previous studies in the postmortem brains of individuals with ASD [7] and WS [8, 9] have demonstrated opposing patterns of difference compared to healthy controls in the number of neurons in the same key social brain areas, paralleling differences in social behavior. Together, these findings suggest that a direct comparison of these two disorders may offer a unique human model in which to examine changes in the brain that may contribute to the biological underpinnings of social behavior and, furthermore, may help elucidate critical neural targets for potential therapeutics in disorders accompanied by sociobehavioral difficulties.
The amygdala, a limbic structure located in the medial temporal lobe, is critically implicated in social behavior and emotion. Neuroimaging studies have demonstrated structural and functional abnormalities of the amygdala in many neurological disorders that are accompanied by atypical social behavior, including in the WS and ASD amygdala [10–13]. However, the relationship between structure and function of the amygdala and behavior remains elusive. The amygdala is composed of several nuclei that can be distinguished from each other based on histological criteria [14], and tracer and lesion studies in animal models suggest that the structural heterogeneity of these nuclei correspond to functional differences. Four nuclei in particular, the lateral, basal, accessory basal, and central nuclei, are significantly implicated in two distinct but overlapping processing loops. The lateral, basal, and accessory basal nuclei are thought be involved in cognitive processing given the significant bidirectional connectivity to association areas in the frontal and temporal lobes [15–17]. In contrast, the central nucleus is critical to the autonomic loop of processing in the amygdala, as it lacks connectivity to association cortex, but receives heavy intra-amygdala projections, and serves as the major output nucleus of the amygdala to brain stem and hypothalamic regulatory centers [15–17]. In our postmortem studies of the amygdala in WS [9] and ASD [7, 18], we found that the lateral nucleus was selectively vulnerable in both disorders, such that compared to NT, there was a significant increase in neuron number in the lateral nucleus in WS and a significant decrease in neuron number in the lateral nucleus in ASD. The lateral nucleus is the primary site of cortical input into the amygdala and an important region for cognitive processing of external stimuli, so these targeted alterations, in opposing directions of change, may contribute to differential atypical processing of social stimuli in WS and ASD.
While differences in neuron number likely contribute to differences in amygdala function, neuronal activity is frequently modulated by neurotransmitter systems. Serotonin is a monoamine that has been implicated in a diverse array of functions in the brain. As a neurotransmitter, serotonin plays a role in several processes of neural development and neural plasticity, including neurogenesis, neural differentiation, axon myelination, and synapse formation and remodeling [19, 20]. Serotonin is also a key neuromodulator in several processes of emotion and cognition, including anxiety and social behavior [21]. WS and ASD diagnoses share a high comorbidity with anxiety disorders, and the effective use of selective serotonin uptake inhibitors (SSRIs) in mitigating symptoms of severe anxiety in patients with WS and ASD implicates involvement of the serotonergic system in both disorders [22, 23]. Furthermore, studies in animal models have found evidence of altered serotonergic metabolism and synthesis in WS and ASD that are associated with characteristic behavioral and neuroanatomical phenotypes [24–26]. Neuronal activity in the amygdala is heavily modulated by serotonergic axons, and disruptions to amygdala serotonergic chemoarchitecture may contribute to neuropathologies underlying atypical social behavior, such as the dichotomous behavioral phenotypes of WS and ASD.
A key component of the serotonergic function within the brain is serotonin transporter (SERT), which is involved in serotonin reuptake back into the presynaptic terminal. Maternal SERT function has been demonstrated to have a profound effect on neural development in offspring in animal models [27]. Animal studies have additionally found significant associations between SERT expression and behavior [28, 29]. In humans, histological methods that label SERT expression in preserved brain tissue can give insight into the chemoarchitecture and anatomy of the serotonin system. Atypical SERT axon density in postmortem brains has been observed in the cortex in other neurological disorders with affective behavioral phenotypes, including schizophrenia [30] and victims of suicide [31, 32]. However, no study to date has quantified SERT axon density across the major subdivisions of the postmortem human amygdala in any disorder or disease, including ASD and WS. Here, we utilized immunohistochemical methods to determine the density of SERT immunoreactive (SERT-ir) axons in the lateral, basal, accessory basal, and central nuclei of the amygdala in WS and ASD, and we compared these results with our data on SERT-ir axon density in neurotypical (NT) postmortem brains, as previously reported in Lew et al. [33], in order to test the hypothesis that serotonergic chemoarchitecture of targeted amygdaloid nuclei are disrupted in ASD and WS. Specifically, given previous qualitative observations of global increases in SERT axon density in ASD [34, 35] and a pattern of opposing directions of change in WS and ASD cytoarchitecture [7, 8, 18], we predicted SERT axon density of the amygdala would be increased in ASD and decreased in WS compared to NT and that the basolateral nuclei would demonstrate the greatest differences between the two disorders.
Methods
The data sets included in this study were obtained from the postmortem amygdala of a total of 20 subjects, composed of six age-matched adult sets (NT, ASD, WS) and one age-, sex-, and hemisphere-matched WS-NT infant pair (see Table 1 for subject background). A corresponding ASD infant could not be included in this study, as ASD is not formally diagnosed until around 2.5 years of age at the earliest [36]. The data set obtained from the six adult NT subjects was previously reported by us in an earlier publication [33]. The adult WS and ASD tissue and the WS-NT infant pair was processed and data was collected following identical methods. Only subjects free of seizures or other neurological disorders were used. Amygdala tissue from individuals diagnosed with ASD prior to death was obtained from the laboratory of Cynthia Schumann (MIND Institute, UC Davis School of Medicine). Amygdala tissue from individuals diagnosed with WS derived from the Ursula Bellugi Williams Syndrome Brain Collection, an ongoing donation-based program run by the Laboratory for Human Comparative Neuroanatomy at UC San Diego (Semendeferi, PI), in collaboration with the NIH NeuroBioBank at the University of Maryland. Fluorescence in situ hybridization (FISH) probes for elastin, a gene consistently deleted in the WS hemideletion, were used to determine genetic diagnosis in the WS cases, and all WS subjects used in this study demonstrated the typical WS genetic deletion. Mutations and deletions of the elastin gene are associated with supravalvular aortic stenosis, a heart defect that is prevalent in WS, and notably, cardiac complications was the cause of death in five of the seven WS subjects included in this study. Diagnosis for ASD subjects was assessed based on results of the Autism Diagnostic Interview-Revised and other medical records [7]. Mean age and age range for adult subjects were similar across all three groups included in the analysis (age mean, age range in years: NT = 45, 19–69; ASD = 41, 20–64; WS = 41, 17–69). One hemisphere (right or left, based on availability) was analyzed per subject. A lack of hemispheric asymmetry has been observed in the human amygdala in both histological and neuroimaging studies [37, 38] suggesting a single hemisphere is sufficient for analysis. Diagnostic groups were not matched for sex or hemisphere due to the limited availability of postmortem human brain tissue, and particularly brain tissue from individuals with neurodevelopmental disorders, which is exceptionally rare.
Table 1.
Subject background
| Subject ID | Age at death | Diagnosis | Sex | Hemisphere | Cause of death | Postmortem interval (hours) |
|---|---|---|---|---|---|---|
| 5183 | 107 days | Neurotypical | Male | Right | Sudden infant death syndrome | 13 |
| WS 7 | 114 days | Williams syndrome | Male | Right | Multiple organ failure | 30 |
| WS 10 | 17 years | Williams syndrome | Male | Right | Cardiac complications | 24 |
| 4916 | 19 years | Neurotypical | Male | Right | Drowning | 5 |
| H-10-01 | 20 years | Autism spectrum disorder | Male | Right | Motor vehicle accident | 24 |
| WS 15 | 25 years | Williams syndrome | Female | Right | Cardiac complications | 30 |
| H-11-02 | 27 years | Neurotypical | Male | Left | Cardiovascular disease | 16 |
| H-6-04 | 28 years | Autism spectrum disorder | Male | Left | Monoxide poisoning | 18 |
| WS 14 | 42 years | Williams syndrome | Female | Right | Cardiac complications | 18 |
| H-7-02 | 42 years | Autism spectrum disorder | Male | Right | Cardiac arrest | 24 |
| WS 9 | 43 years | Williams syndrome | Female | Right | Cardiac complications | 12 |
| 5758 | 43 years | Neurotypical | Female | Right | Sepsis | 22 |
| H-6-00 | 44 years | Autism spectrum disorder | Male | Left | Cardiovascular disease | 31 |
| H-19-01 | 44 years | Neurotypical | Male | Left | Unknown | 26 |
| H-1-01 | 44 years | Autism spectrum disorder | Male | Left | Pulmonary Embolism | 20 |
| WS 8 | 48 years | Williams syndrome | Male | Left | Respiratory illness | 30 |
| H-4-02 | 64 years | Autism spectrum disorder | Male | Right | Sepsis | 18.5 |
| H-11-98 | 67 years | Neurotypical | Male | Left | Unknown | ? |
| WS 13 | 69 years | Williams syndrome | Male | Right | Cardiac complications | 8 |
| 5943 | 69 years | Neurotypical | Male | Right | Acute coronary artery thrombosis | 23 |
Tissue processing
One brain hemisphere from each subject was immersed in 10% buffered formalin after autopsy (see Table 1 for postmortem interval) and remained in formalin until sectioning. Tissue blocks containing the entire rostrocaudal extent of the amygdala were extracted from the whole hemisphere of the brain. Extracted blocks were saturated in a cryoprotectant solution of sucrose and 0.1 M phosphate buffer, frozen with dry ice, and cut along the coronal plane using a Leica SM sliding microtome. Tissue was cut in either alternating 80 μm and 40 μm sections (WS tissue and NT tissue from NIH NeuroBioBank) or alternating 100 μm and 50 μm sections (ASD tissue and NT tissue from the Schumann collection). A 1-in-10 series of either 80 μm or 100 μm sections per individual was mounted and stained for Nissl substance, and a 1-in-20 series of either 40 μm or 50 μm sections per individual was stained with mouse monoclonal antibody against SERT (MAB5618, EMD Millipore, Billerica, MA) using the heat-based antigen retrieval and immunohistochemical staining protocol described in our previous publication [33].
Data collection
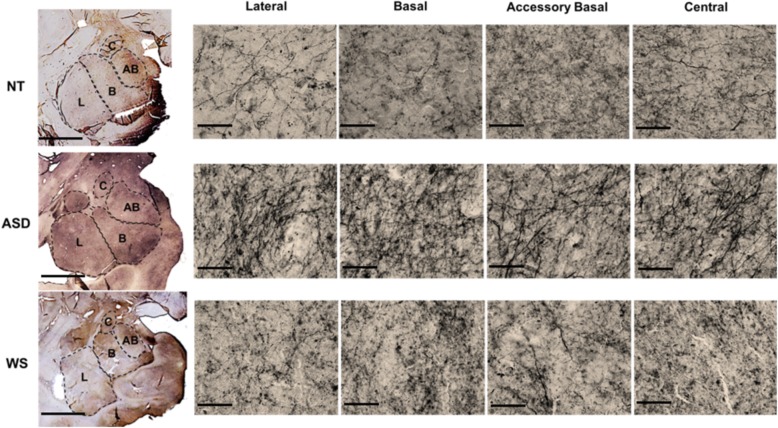
Adult data was collected by CL and infant data was collected by KG, after establishing inter-rater reliability with > 95% concordance. Data was collected using the Stereoinvestigator software suite (MBF BioScience, Williston, VT) on a Dell workstation with a 30.48 centimeter (cm) by 53.34 cm monitor, receiving live video feed from a Lumenera color video camera (Ottawa, Ontario) attached to an Eclipse 80i microscope equipped with a Ludl MAC5000 stage (Hawthorn, NY) and a Heiden z-axis encoder (Plymouth, MN). For each section examined, boundaries of the amygdaloid nuclei were first traced in Stereoinvestigator at × 1 magnification, utilizing an adjacent section from the Nissl-stained series as a visual aid during tracing to ensure precision of boundaries (described in detail in [9, 14]). After boundaries on the SERT-ir stained sections were identified (Fig. 1), the Stereoinvestigator Spaceballs probe, which utilizes systematic random sampling for accurate stereologic quantification, was employed to estimate SERT-ir axon length at × 100 magnification (1.4 numerical aperture, oil lens), using the parameters described in our previous publication [33]. Total axon length density was calculated by dividing the total axon length by the planimetric reference volume [39, 40].
Fig. 1.
Micrograph showing the four regions of interest in the amygdala in each diagnostic group. The first photo of each row shows the whole amygdala with boundaries of the lateral, basal, accessory basal, and central nuclei (scale bar = 5 mm). The remaining photos in each row show SERT-ir stained fibers in each nucleus examined at × 60 magnification (scale bar = 50 μm)
Analyses of data
All data analyses were performed using Prism statistical software (v.8, GraphPad Software, La Jolla, CA). Spearman rank-order correlation tests were used to identify any age, sex, or post-mortem interval (PMI) effects on SERT-ir axon density, and data for all subjects was run through a Grubbs’ test (P < 0.05) to detect possible outliers. Given the small sample size of the data sets, non-parametric statistical methods were used. The Kruskal-Wallis test with Dunn’s test for multiple comparisons was employed to examine differences in SERT-ir axon density in the lateral, basal, accessory basal, and central nuclei between each group. While the infant pair was included in the analyses of NT-WS comparisons, only adult subjects were included in NT-ASD and ASD-WS comparisons due to the unavailability of an ASD age-matched infant. The difference between mean density of SERT-ir axons in ASD and WS was calculated as the percentage of mean density in NT subjects (only adult NT subjects included for comparison with ASD; all NT subjects included for comparison with WS).
Results
Stereological results of mean SERT-ir axon density and standard deviation in each nucleus in NT, ASD, and WS are reported in Table 2. One subject in the WS data set, WS 14, was found to be an outlier by the Grubbs outlier test and so was excluded from the WS mean values and all statistical analyses, although individual values of this subject are included in Fig. 2. No correlations were found between age, sex, or postmortem interval and SERT-ir axon density. As observed in our previous analyses of the postmortem amygdala in WS and ASD [7, 8], no significant differences in planimetric volume of any nucleus examined were found between the three groups. SERT-ir axon densities in the WS and NT infant subjects, although lower than the adult means, were within the standard deviation of the adults in their diagnostic group (Tables 2 and 3; Figs. 2 and 3).
Table 2.
Mean SERT-ir axon density and standard deviation in micrometers (μm/μm3) in each nucleus of the amygdala in neurotypical, autism spectrum disorder, and Williams syndrome brains
| Diagnosis | Mean SERT-ir axon density and standard deviation (μm/μm3) | |||
|---|---|---|---|---|
| Lateral | Basal | Accessory basal | Central | |
| Neurotypical (adults only) | 0.00368 ± 0.00101 | 0.00458 ± 0.00182 | 0.00572 ± 0.00270 | 0.00656 ± 0.00309 |
| Neurotypical (all subjects) | 0.00347 ± 0.0011 | 0.00437 ± 0.00175 | 0.00532 ± 0.00268 | 0.00615 ± 0.00302 |
| Autism spectrum disorder | 0.00501 ± 0.00174 | 0.00528 ± 0.00154 | 0.00581 ± 0.00088 | 0.00563 ± 0.00088 |
| Williams syndrome (adults only) | 0.00384 ± 0.00240 | 0.00432 ± 0.00296 | 0.00399 ± 0.00223 | 0.00504 ± 0.00268 |
| Williams syndrome (all subjects) | 0.00289 ± 0.00067 | 0.00314 ± 0.00071 | 0.00312 ± 0.00075 | 0.00418 ± 0.00188 |
Fig. 2.
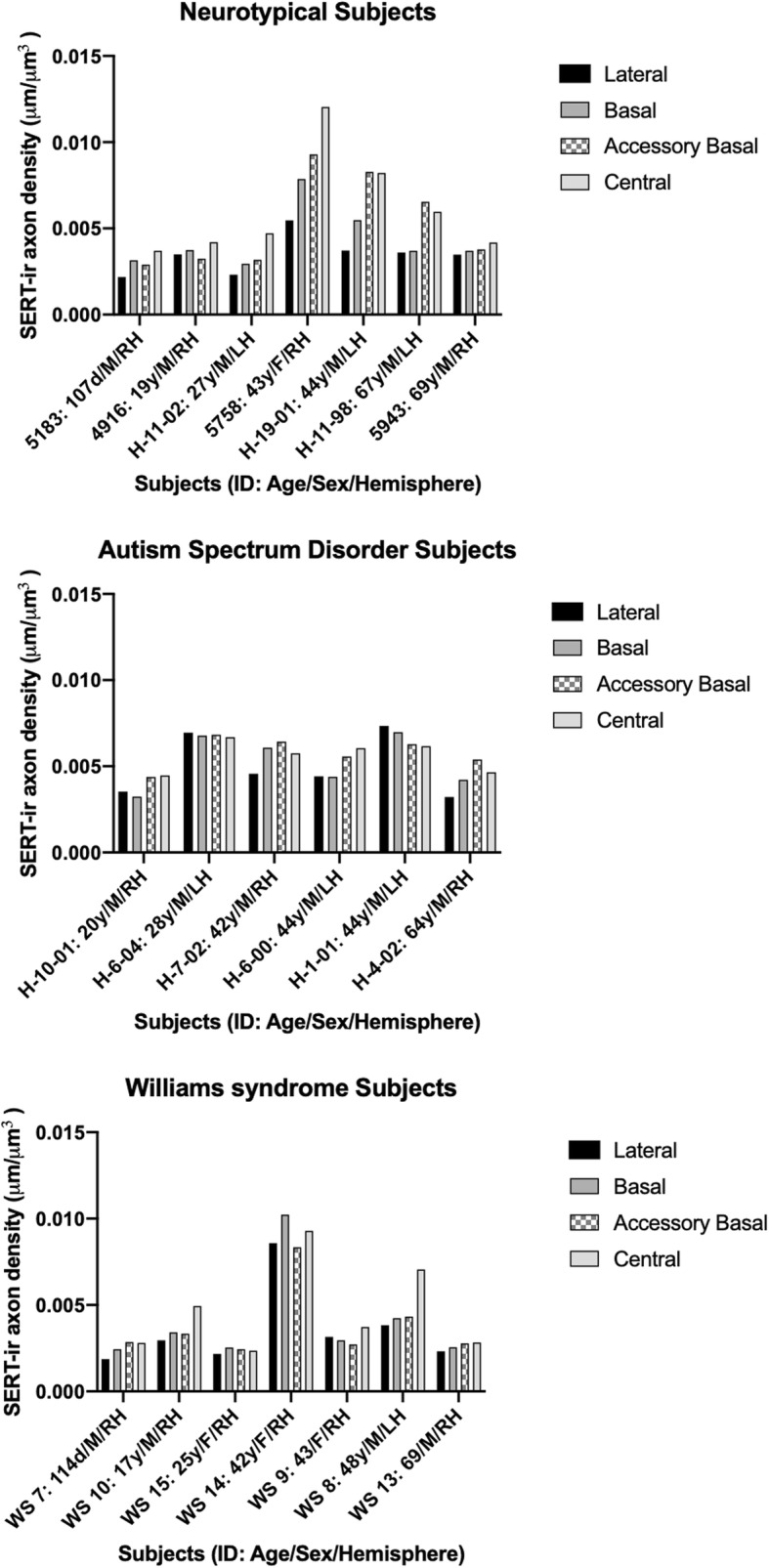
Stereological estimates of SERT-ir axon density in the lateral, basal, accessory basal, and central nuclei of the amygdala of individual subjects in each diagnostic group
Table 3.
P values of NT compared to ASD, WS, and WS. Comparisons with ASD includes only the adult subjects in NT and WS due to lack of an infant age match in the ASD data set
| Comparison | Lateral | Basal | Accessory basal | Central |
|---|---|---|---|---|
| NT vs ASD | 0.4684 | 0.8470 | > 0.9999 | > 0.9999 |
| NT vs WS | 0.4200 | 0.1950 | 0.0513 | 0.3460 |
| ASD vs WS | 0.0425* | 0.0466* | 0.0365* | 0.4693 |
* indicicates statistical significance p < 0.05
Fig. 3.
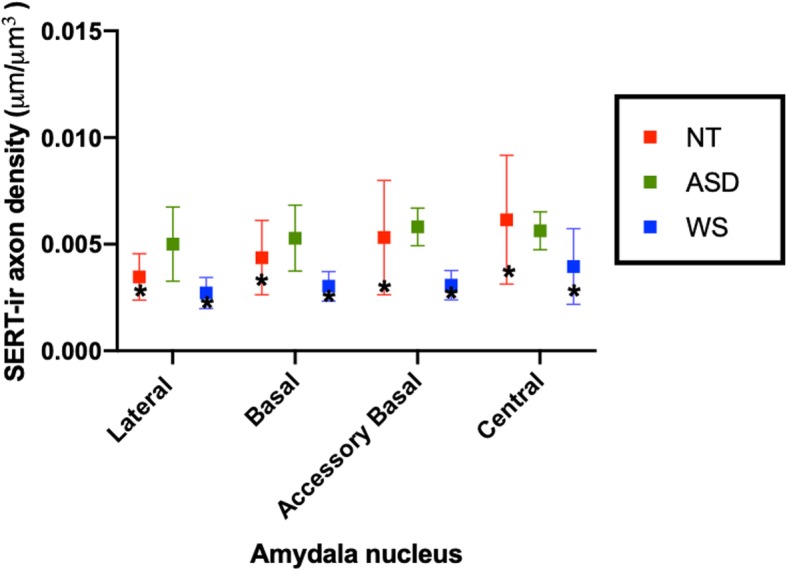
Mean SERT-ir axon density in the lateral, basal, accessory basal, and central nuclei of the amygdala in the adult subjects of each diagnostic group. The WS and NT adult means are overlaid by values of the WS and NT infant subjects (WS 7 and 5183, respectively) denoted by asterisks. Lines represent standard deviation of the mean. As observed in the figure, while WS and NT infant values are in the lower range of the adult values, they fall within the standard deviation of the adult mean
Mean SERT-ir axon density in ASD was greater than WS in all nuclei examined (Table 2; Fig. 3). The difference between ASD and WS reached significance in the lateral, basal, and accessory basal nuclei (p = 0.0425, p = 0.0466, p = 0.0365, respectively; Table 3). No significant differences were found between NT and ASD or NT and WS in any nucleus of the amygdala examined. In ASD, mean SERT-ir axon density was slightly increased in the lateral and basal nuclei, similar in the accessory basal nucleus, and slightly decreased in the central nucleus compared to NT (Fig. 3; Tables 2 and 4). Mean SERT-ir axon density was decreased in WS compared to NT in all four nuclei, and the difference between the two groups was largest and approaching statistical significance in the basal (p = 0.0513) and accessory basal nuclei (p = 0.0513; Tables 2, 3, and 4).
Table 4.
% difference of mean SERT-ir axon density in ASD and WS compared to NT in each nucleus of the amygdala
| Nucleus | ASD (%) | WS (%) |
|---|---|---|
| Lateral | + 36.1 | − 21.5 |
| Basal | + 15.3 | − 31.4 |
| Accessory basal | + 1.6 | − 45.5 |
| Central | − 14.2 | − 36.3 |
Discussion
This is the first quantitative stereological study to examine serotonergic innervation of the major amygdala subdivisions in two neurodevelopmental disorders characterized by dichotomous socio-affective behavioral phenotypes. We found significant differences in serotonergic innervation of the amygdala between WS and ASD. Furthermore, WS and ASD displayed quantitative changes in opposing directions compared to neurotypical controls. These findings contribute to a growing body of literature [7–9, 18] in WS and ASD which demonstrate that both disorders display selective vulnerability of similar targets in the social brain, but in quantitatively opposing directions of change compared to healthy controls. This pattern parallels the dichotomous sociobehavioral phenotypes of the two disorders, suggesting that the microanatomical changes in neural structure of these regions may contribute to behavioral differences.
Specifically, in the present study, we found trends of a slight increase in mean SERT-ir axon density in ASD compared to NT and a decrease in mean SERT-ir axon density in WS compared to NT (Tables 2, 3, and 4; Fig. 3). Differences between WS and NT are greater than differences between ASD and NT in most nuclei, and the decrease in mean SERT-ir axon density in WS compared to NT approaches statistical significance in the basal and accessory basal nuclei (p = 0.0513 for both nuclei). Differences between ASD and WS are more robust: mean SERT-ir axon density in ASD is greater than WS in all nuclei examined, and as we predicted, these differences are significant in the basolateral nuclei, which demonstrate significant connectivity to association cortex, including the prefrontal cortex, another region preferentially targeted in both disorders [8, 41]. Additionally, the present dichotomous findings in the basolateral nuclei of the amygdala in WS and ASD parallel the dichotomy of change in neuron number in the same regions of interest in the two disorders: neuron number in the basolateral nuclei is decreased in ASD compared to NT [7, 18] and increased in WS compared to NT [9]. Given the role of serotonin in the regulation of several neurodevelopmental processes, including neurogenesis, neuronal differentiation, neuropil formation, axon myelination, and synaptogenesis [19, 42–45], perhaps the dichotomous amygdala pathologies observed in WS and ASD in these two domains, neuron number and SERT axon density, could be related to the effect of different manifestations of serotonergic disruption to amygdala cellular development and seemingly opposing behavioral phenotypes [46].
While the similar pattern of differences in behavioral phenotype and SERT-ir axon density in WS and ASD are intriguing, the relationship between serotonergic innervation of the amygdala and behavior is unclear. A recent study found that mice with homozygous and hemizygous knockouts of the SERT gene have increased anxiety, enhanced fear acquisition, and disrupted inhibition in the amygdala [29], indicating that a possible role of SERT in social behavior may be related to the modulation of reactivity of the amygdala in response to stimuli with emotional valence. Typical activation and reactivity of the amygdala in response to emotional stimuli, such as faces, is crucial to determining the emotional valence of stimuli for appropriate behavioral response. In humans, activation of the amygdala in response to faces in general, as well as positive emotion faces (such as “happy”) and negative emotion faces (such as “angry” or “fearful”), are parts of different emotional valence cascades which contribute to appropriate sociobehavioral response. Both individuals with ASD and individuals with WS have demonstrated atypical activation of the amygdala in response to human faces. Specifically, individuals with ASD display hyperactivation of the amygdala in response to human faces and are avoidant of the eye region, in which much of emotionally relevant social cues are displayed in humans, suggesting a negatively valenced overarousal of the amygdala in response to social stimuli in ASD which may contribute to social avoidance behaviors [5, 47–49]. Individuals with WS, in contrast, display hypoactivation of the amygdala in response to negative emotion faces and hyperactivation in response to positive emotion faces, suggesting disruption of autonomic processing in response to both positive and negative emotionally valenced stimuli, which may contribute to the atypically strong prosocial drive characteristic of the disorder [10, 50, 51].
Comparative studies examining serotonergic innervation of the amygdala in closely related species may help shed light on how different patterns of serotonergic innervation might contribute to socio-affective behavior. Bonobos and chimpanzees are two closely related apes and are the closest living relatives to humans. Bonobos typically respond to conflict with prosocial strategies [52, 53], while chimpanzees more frequently respond to conflict with aggression [54]. These behavioral differences are thought to be mediated in part by differences in emotional reactivity between species [55]. SERT axon density in the postmortem amygdala is lower in chimpanzees relative to bonobos and humans and more similar between bonobos and humans than between bonobos and chimpanzees [33, 40]. While species-specific differences are not directly comparable to differences across human neuropathologies, the observation that SERT-ir axon density in the amygdala is more similar in humans and bonobos, two highly prosocial species, than in phylogenetically close chimpanzees and bonobos, implicates the role of serotonergic innervation of the amygdala in social behavior more generally.
While the association between differences in serotonergic innervation of the amygdala and behavioral phenotype in WS and ASD are speculative, genetic evidence suggests disruptions to the serotonergic system are a feature of both disorders. One possible genetic link to serotonergic disruption of the amygdala is GTF2IRD1, a general transcription factor included in the WS deletion that is linked to the characteristic WS behavioral phenotype [56] and also implicated as a common site of allelic variation in autism [57]. Genetically altered mice with a deletion of GTF2IRD1 demonstrate altered serotonergic metabolism in the amygdala and frontal cortex, as well as reduced fear and aggression compared to wild-type mice [24, 26]. Another possible mechanism could be related to genetic variation of serotonin transporter genes, which have been linked to cognitive and behavioral differences in primates [58]. In addition to GTF2IRD1, several other genetic polymorphisms linked to ASD are found in genes involved in serotonin transporter signaling and function [59–62], and high concentration of serotonin in the blood, called hyperserotonemia, occurs in about one third of autism cases [63]. Blood levels of serotonin are normal in most cases of WS [64]; however, two separate studies have reported on a total of four cases in which patients with the common WS genetic deletion display hyperserotonemia, along with social and communicative deficits diagnostic of autism rather than WS [65, 66]. The researchers of the later study [66] also genotyped the SERT polymorphism (5-HTTLPR) for the two subjects they examined and found both were homozygous for the short allele (5-HTTLPR s). Tordjman and colleagues suggest that that the deviation from typical WS phenotype displayed by the two subjects in their study could be due to an interaction of WS genetic deletion with other genetic factors, such as the 5-HTTLPR polymorphism. The 5-HTTLPR polymorphism has been linked to socio-affective behavioral variation in humans and non-human primates [67, 68], and the 5-HTTLPR s allele is associated with heightened amygdala reactivity [69, 70] and stronger amygdala-prefrontal functional connectivity [71] in healthy subjects. Furthermore, the 5-HTTLPR s allele is thought to be a genetic risk factor for neuropathologies associated with deficits in affect and social behaviors [58, 72]. This polymorphism may contribute to the present findings in the WS and ASD amygdala, as well as the characteristic behavioral phenotypes. A future project aimed at genotyping the 5-HTTLPR polymorphism in the subjects of this study would shed light on the possible effects of this polymorphism to the WS and ASD phenotypes.
Limitations
The sample size of this study is limited by the availability of tissue. The available material is further subjected to an elaborate immunochemical staining that often requires further exclusion of subjects to ensure that only highest quality tissue is used for data collection.
Despite this limitation, we found robust differences between the WS and ASD groups, suggesting differences between NT and the two disorders could potentially reach significance with the addition of a few more subjects.
Conclusions
The present study is the first quantitative stereological study to examine serotonergic innervation of the major amygdala nuclei in two closely linked neurodevelopmental disorders with dichotomous atypical sociobehavioral phenotypes. We found that quantitative differences in SERT-ir axon density in the amygdala in WS and ASD parallel the opposing differences between the two disorders that we previously observed in neuronal distribution of the amygdala. Additionally, these dichotomous findings of atypical microstructure of the amygdala in WS and ASD parallel the dichotomous sociobehavioral phenotype of these two disorders. The serotonergic system is crucial to both neuronal development and behavioral modulation. The present findings of opposing disruptions to the serotonergic system in ASD and WS may contribute to differential atypical development of the amygdala and subsequent differences in amygdala reactivity to social stimuli in WS and ASD. Given the frequent use of SSRIs in patients with WS and ASD [22, 23], yet the relative lack of knowledge of the mechanisms involved, more studies that examine the role of serotonin in the etiology and phenotype of WS and ASD are needed to inform treatment and identify targets of future, more effective therapeutics in these disorders.
Acknowledgements
We wish to thank the tissue donors and their families whose gift to science made this study possible, with particular gratitude to Terry Monkaba and the Williams Syndrome Association. We additionally thank Erin Carlson at the MIND Institute for her assistance with procuring ASD and NT tissue from the Schumann brain collection.
Abbreviations
- ASD
Autism spectrum disorder
- NT
Neurotypical
- SERT
Serotonin transporter
- SERT-ir
Serotonin transporter immunoreactive
- WS
Williams syndrome
Authors’ contributions
CL, KS, and CM conceived and planned the experiments. CL carried out the experiments, data collection, and analysis for all adult subjects. KG carried out the experiments, data collection, and analysis for all infant subjects. DG and DC helped with sample preparation and assisted in the experiments. KS supervised the overall project, and KH supervised the laboratory work. CL was the primary author of the manuscript, in consultation with KS and CM. All authors provided critical feedback and helped shape the research, analysis, and manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the National Institutes of Health P01 NICHD033113, 5R03MH103697, R56MH109587 and Academic Senate, UCSD.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Strømme P, Bjørnstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J Child Neurol. 2002;17(4):269–271. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- 2.Järvinen-Pasley A, Adolphs R, Yam A, Hill KJ, Grichanik M, Reilly J, et al. Affiliative behavior in Williams syndrome: social perception and real-life social behavior. Neuropsychologia. 2010;48(7):2110–2119. doi: 10.1016/j.neuropsychologia.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones W, Bellugi U, Lai Z, Chiles M, Reilly J, Lincoln A, et al. II. Hypersociability in Williams Syndrome. J Cogn Neurosci. 2000;12(Supplement):30–46. doi: 10.1162/089892900561968. [DOI] [PubMed] [Google Scholar]
- 4.Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 2018;67(SS-6):1–23. doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13(2):232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- 6.Green J, Gilchrist A, Burton D, Cox A. Social and psychiatric functioning in adolescents with Asperger syndrome compared with conduct disorder. J Autism and Dev Disorders. 2000;30:279–293. doi: 10.1023/A:1005523232106. [DOI] [PubMed] [Google Scholar]
- 7.Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26(29):7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lew CH, Brown C, Bellugi U, Semendeferi K. Neuron density is decreased in the prefrontal cortex in Williams syndrome. Autism Res. 2017;10(1):99–112. doi: 10.1002/aur.1677. [DOI] [PubMed] [Google Scholar]
- 9.Lew CH, Groeniger KM, Bellugi U, Stefanacci L, Schumann CM, Semendeferi K. A postmortem stereological study of the amygdala in Williams syndrome. Brain Struct Funct. 2018;223(4):1897–1907. doi: 10.1007/s00429-017-1592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas BW, Mills D, Yam A, Hoeft F, Bellugi U, Reiss A. Genetic influences on sociability: heightened amygdala reactivity and event-related responses to positive social stimuli in Williams syndrome. J Neurosci. 2009;29(4):1132–1139. doi: 10.1523/JNEUROSCI.5324-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, et al. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8(8):991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- 12.Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabil Res Rev. 2004;10(4):259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- 13.Reiss AL, Eliez S, Schmitt JE, Straus E, Lai Z, Jones W, et al. IV. Neuroanatomy of Williams syndrome: a high-resolution MRI study. J Cogn Neurosci. 2000;12(Suppl 1):65–73. doi: 10.1162/089892900561986. [DOI] [PubMed] [Google Scholar]
- 14.Schumann CM, Amaral DG. Stereological estimation of the number of neurons in the human amygdaloid complex. J Comp Neurol. 2005;491(4):320–329. doi: 10.1002/cne.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price JL, Russchen FT, Amaral DG. The limbic region. II. The amygdaloid complex. In: Bjorkland A, Hokfelt T, Swanson L, editors. Handbook of chemical neuroanatomy. Amsterdam: Elsevier; 1987. pp. 279–381. [Google Scholar]
- 16.Stefanacci L, Amaral DG. Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: a retrograde tracing study. J Comp Neurol. 2000;421(January):52–79. doi: 10.1002/(SICI)1096-9861(20000522)421:1<52::AID-CNE4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 17.Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol. 2002;451(May):301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- 18.Avino Thomas A., Barger Nicole, Vargas Martha V., Carlson Erin L., Amaral David G., Bauman Melissa D., Schumann Cynthia M. Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proceedings of the National Academy of Sciences. 2018;115(14):3710–3715. doi: 10.1073/pnas.1801912115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauder JM, Krebs H. Serotonin as a difference signal in early neurogenesis. Dev Neurosci. 1978;1:15–30. doi: 10.1159/000112549. [DOI] [PubMed] [Google Scholar]
- 20.Sodhi MSK, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- 21.Asan E, Steinke M, Lesch K-P. Serotonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histochem Cell Biol. 2013;139(6):785–813. doi: 10.1007/s00418-013-1081-1. [DOI] [PubMed] [Google Scholar]
- 22.Martens MA, Seyfer DL, Andridge RR, Foster JEA, Chowdhury M, McClure KE, et al. Parent report of antidepressant, anxiolytic, and antipsychotic medication use in individuals with Williams syndrome: effectiveness and adverse effects. Res Dev Disabil. 2012;33(6):2106–2121. doi: 10.1016/j.ridd.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Williams MR, Marsh R, Macdonald CD, Jain J, Pearce RKB, Hirsch SR, et al. Neuropathological changes in the nucleus basalis in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2013;263(6):485–495. doi: 10.1007/s00406-012-0387-7. [DOI] [PubMed] [Google Scholar]
- 24.Young EJ, Lipina T, Tam E, Mandel A, Clapcote SJ, Bechard AR, et al. Reduced fear and aggression and altered serotonin metabolism in Gtf2ird1-targeted mice. Genes Brain Behav. 2008;7(2):224–234. doi: 10.1111/j.1601-183X.2007.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zafeiriou D, Ververi A, Vargiami E. The serotonergic system: its role in pathogenesis and early developmental treatment of autism. Curr Neuropharmacol. 2009;7(2):150–157. doi: 10.2174/157015909788848848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proulx E, Young EJ, Osborne LR, Lambe EK. Enhanced prefrontal serotonin 5-HT1A currents in a mouse model of Williams-Beuren syndrome with low innate anxiety. J Neurodev Disord. 2010;2(2):99–108. doi: 10.1007/s11689-010-9044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller CL, Anacker AM, Rogers TD, Goeden N, Keller EH, Forsberg CG, et al. Impact of maternal serotonin transporter genotype on placental serotonin, fetal forebrain serotonin, and neurodevelopment. Neuropsychopharmacology. 2017;42(2):427–436. doi: 10.1038/npp.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellegood J, Yee Y, Kerr TM, Muller CL, Blakely RD, Henkelman RM, et al. Analysis of neuroanatomical differences in mice with genetically modified serotonin transporters assessed by structural magnetic resonance imaging. Mol Autism. 2018;9(1):1–12. doi: 10.1186/s13229-018-0210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson PL, Molosh AI, Federici LM, Bernabe C, Haggerty D, Fitz SD, et al. Assessment of fear and anxiety associated behaviors, physiology and neural circuits in rats with reduced serotonin transporter (SERT) levels. Transl Psychiatry. 2019;9(1):33. doi: 10.1038/s41398-019-0368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muguruza C, Moreno JL, Umali A, Callado LF, Meana JJ, González-Maeso J. Dysregulated 5-HT2A receptor binding in postmortem frontal cortex of schizophrenic subjects. Eur Neuropsychopharmacol. 2013;23(8):852–864. doi: 10.1016/j.euroneuro.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin MC, Whitehead RE, Edgar CL, Janosky JE, Lewis DA. Localized decrease in serotonin transporter-immunoreactive axons in the prefrontal cortex of depressed subjects committing suicide. Neuroscience. 2002;114(3):807–815. doi: 10.1016/S0306-4522(02)00289-0. [DOI] [PubMed] [Google Scholar]
- 32.Rajkowska G, Mahajan G, Legutko B, Challagundla L, Griswold M, Albert PR, et al. Length of axons expressing the serotonin transporter in orbitofrontal cortex is lower with age in depression. Neuroscience. 2017;359:30–39. doi: 10.1016/j.neuroscience.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lew CH, Hanson KL, Groeniger KM, Greiner D, Cuevas D, Hrvoj-Mihic B, et al. Serotonergic innervation of the human amygdala and evolutionary implications. Am J Phys Anthropol. 2019;170:351–360. doi: 10.1002/ajpa.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azmitia EC, Singh JS, Hou XP, Wegiel J. Dystrophic serotonin axons in postmortem brains from young autism patients. Anat Rec. 2011;294(10):1653–1662. doi: 10.1002/ar.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azmitia EC, Singh JS, Whitaker-Azmitia PM. Increased serotonin axons (immunoreactive to 5-HT transporter) in postmortem brains from young autism donors. Neuropharmacology. 2011;60(7–8):1347–1354. doi: 10.1016/j.neuropharm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Charman T, Baird G. Practitioner review: diagnosis of autism spectrum disorder in 2- and 3-year-old children. J Child Psychology Psychiatry. 2002;43:289–305. doi: 10.1111/1469-7610.00022. [DOI] [PubMed] [Google Scholar]
- 37.Barger N, Stefanacci L, Semendeferi K. A comparative volumetric analysis of the amygdaloid complex and basolateral division in the human and ape brain. Am J Phys Anthropol. 2007;134:393–403. doi: 10.1002/ajpa.20684. [DOI] [PubMed] [Google Scholar]
- 38.Brierley B, Shaw P, David AS. The human amygdala: a systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain Res Brain Res Rev. 2002;39(1):84–105. doi: 10.1016/S0165-0173(02)00160-1. [DOI] [PubMed] [Google Scholar]
- 39.Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Differences in cortical serotonergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Cereb Cortex. 2008;18(3):584–597. doi: 10.1093/cercor/bhm089. [DOI] [PubMed] [Google Scholar]
- 40.Stimpson Cheryl D., Barger Nicole, Taglialatela Jared P., Gendron-Fitzpatrick Annette, Hof Patrick R., Hopkins William D., Sherwood Chet C. Differential serotonergic innervation of the amygdala in bonobos and chimpanzees. Social Cognitive and Affective Neuroscience. 2015;11(3):413–422. doi: 10.1093/scan/nsv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306(18):2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 42.Chubakov AR, Gromova EA, Konovalov GV, Chumasov EI, Sarkisova EF. Effect of serotonin on the development of a rat cerebral cortex tissue culture. Neurosci Behav Physiol. 1986;16(6):490–497. doi: 10.1007/BF01191453. [DOI] [PubMed] [Google Scholar]
- 43.Chubakov AR, Tsyganova VG, Sarkisova EF. The stimulating influence of the raphe nuclei on the morphofunctional development of the hippocampus during their combined cultivation. Neurosci Behav Physiol. 1993;23(3):271–276. doi: 10.1007/BF01182928. [DOI] [PubMed] [Google Scholar]
- 44.Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. [DOI] [PubMed] [Google Scholar]
- 45.Whitaker-azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479–485. doi: 10.1016/S0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 46.Haas BW, Reiss AL. Social brain development in Williams syndrome: the current status and directions for future research. Front Psychol. 2012;3(June):186. doi: 10.3389/fpsyg.2012.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Green SA, Rudie JD, Colich NL, Wood JJ, Shirinyan D, Hernandez L, et al. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2014;52(11):1158–1172. doi: 10.1016/j.jaac.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kliemann D, Dziobek I, Hatri A, Baudewig J, Heekeren HR. The role of the amygdala in atypical gaze on emotional faces in autism spectrum disorders. J Neurosci. 2012;32(28):9469–9476. doi: 10.1523/JNEUROSCI.5294-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tottenham N, Hertzig ME, Gillespie-Lynch K, Gilhooly T, Millner AJ, Casey BJ. Elevated amygdala response to faces and gaze aversion in autism spectrum disorder. Soc Cogn Affect Neurosci. 2014;9(1):106–117. doi: 10.1093/scan/nst050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haas BW, Hoeft F, Searcy YM, Mills D, Bellugi U, Reiss A. Individual differences in social behavior predict amygdala response to fearful facial expressions in Williams syndrome. Neuropsychologia. 2010;48(5):1283–1288. doi: 10.1016/j.neuropsychologia.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paul BM, Snyder AZ, Haist F, Raichle ME, Bellugi U, Stiles J. Amygdala response to faces parallels social behavior in Williams syndrome. Soc Cogn Affect Neurosci. 2009;4(3):278–285. doi: 10.1093/scan/nsp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Waal FB. Sex as an alternative to aggression in the bonobo. In: Abramson P, Pinkerton S, editors. Sexual nature, sexual culture. Chicago: University of Chicago Press; 1995. pp. 37–56. [Google Scholar]
- 53.Palagi E. Social play in bonobos (Pan paniscus) and chimpanzees (Pan troglodytes): implications for natural social systems and interindividual relationships. Am J Phys Anthro. 2006;129:418–426. doi: 10.1002/ajpa.20289. [DOI] [PubMed] [Google Scholar]
- 54.Parish AR, de Waal FB. The other “closest living relative”. How bonobos (Pan paniscus) challenge traditional assumptions about females, dominance, intra- and intersexual interactions, and hominid evolution. Ann NY Academ Sci. 2000;907:97–113. doi: 10.1111/j.1749-6632.2000.tb06618.x. [DOI] [PubMed] [Google Scholar]
- 55.Hare B, Melis A, Woods V, Hastings S, Wrangham RW. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr Biol. 2007;17:619–623. doi: 10.1016/j.cub.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 56.van Hagen JM, van der Geest JN, van der Giessen RS, Lagers-van Haselen GC, Eussen HJFMM, Gille JJP, et al. Contribution of CYLN2 and GTF2IRD1 to neurological and cognitive symptoms in Williams syndrome. Neurobiol Dis. 2007;26(1):112–124. doi: 10.1016/j.nbd.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Weiss Lauren A., Arking Dan E. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461(7265):802–808. doi: 10.1038/nature08490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, et al. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol Psychiatry. 2010;15(5):512–522. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cook EH, Jr, Courchesne R, Lord C, Cox NJ, Yan S, et al. Evidence of linkage between the serotonin transporter and autistic disorder. Mol Psychiatry. 1997;2:247–250. doi: 10.1038/sj.mp.4000266. [DOI] [PubMed] [Google Scholar]
- 60.Huang CH, Santangelo SL. Autism and serotonin transporter gene polymorphisms: a systematic review and meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:903–913. doi: 10.1002/ajmg.b.30720. [DOI] [PubMed] [Google Scholar]
- 61.Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55(6):932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiss LA, Kosova G, Delahanty RJ, Jiang L, Cook EH, Ober C, et al. Variation in ITGB3 is associated with whole-blood serotonin level and autism susceptibility. Eur J Hum Genet. 2006;14:923–931. doi: 10.1038/sj.ejhg.5201644. [DOI] [PubMed] [Google Scholar]
- 63.Campbell M, Friedman E, DeVito E, Greenspan L, Collins PJ. Blood serotonin in psychotic and brain damaged children. J Autism Child Schizophr. 1974;4(1):33–41. doi: 10.1007/BF02104998. [DOI] [PubMed] [Google Scholar]
- 64.August GJ, Realmuto GM. Williams syndrome: serotonin’s association with developmental disabilities. J Autism Dev Disord. 1989;19(1):137–141. doi: 10.1007/BF02212725. [DOI] [PubMed] [Google Scholar]
- 65.Reiss AL, Feinstein C, Rosenbaum KN, Borengasser-caruso MA, Ph D, Goldsmith BM. Autism associated with Williams syndrome. Clin Lab Obs. 1985;106(2):247–249. doi: 10.1016/s0022-3476(85)80296-1. [DOI] [PubMed] [Google Scholar]
- 66.Tordjman S, Anderson GM, Cohen D, Kermarrec S, Carlier M, Touitou Y, et al. Presence of autism, hyperserotonemia, and severe expressive language impairment in Williams-Beuren syndrome. Mol Autism. 2013;4:29. doi: 10.1186/2040-2392-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lesch KP, Meyer J, Glatz K, Flügge G, Hinney A, Hebebrand J, et al. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. J Neural Transm. 1997;104(11–12):1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- 68.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 69.Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, et al. Variation of human amygdala response during threatening stimuli as a function of 5′HTTLPR genotype and personality style. Biol Psychiatry. 2005;57(12):1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 70.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 71.Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8(1):20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- 72.Murphy SE, Norbury R, Godlewska BR, Cowen PJ, Mannie ZM, Harmer CJ, et al. The effect of the serotonin transporter polymorphism (5-HTTLPR) on amygdala function: a meta-analysis. Mol Psychiatry. 2013;18(4):512–520. doi: 10.1038/mp.2012.19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.