Abstract
Variation in the degree of sexual size dimorphism (SSD) among taxa is generally considered to arise from differences in the relative intensity of male–male competition and fecundity selection. One might predict, therefore, that SSD will vary systematically with (1) the intensity of sexual selection for increased male size, and (2) the intensity of fecundity selection for increased female size. To test these two fundamental hypotheses, we conducted a phylogenetic comparative analysis of SSD in fish. Specifically, using records of body length at first sexual maturity from FishBase, we quantified variation in the magnitude and direction of SSD in more than 600 diverse freshwater and marine fish species, from sticklebacks to sharks. Although female-biased SSD was common, and thought to be driven primarily by fecundity selection, variation in SSD was not dependent on either the allometric scaling of reproductive energy output or fecundity in female fish. Instead, systematic patterns based on habitat and life-history characteristics associated with varying degrees of male–male competition and paternal care strongly suggest that adaptive variation in SSD is driven by the intensity of sexual selection for increased male size.
Keywords: body size, life history, male–male competition, fecundity selection
1. Introduction
A difference in adult body size between males and females within a species, termed sexual size dimorphism (SSD), is widespread in the animal kingdom. Life-history theory predicts that SSD will arise from both natural and sexual selection, such that variation in reproductive success leads to differences in the optimal body size of each sex. Male-biased SSD is commonly associated with a high degree of sexual selection (e.g. male–male competition, intrasexual combat or territoriality), whereas female-biased SSD is typically attributed to the positive correlation between maternal size and fecundity (i.e. fecundity selection) [1–5]. Variation in the degree of SSD among species and taxa is generally considered to arise from differences in the relative intensity of each of these selective forces, but given such a vast array of reproductive strategies, even within closely related taxa, the extent to which SSD is driven by sexual selection in males versus fecundity selection in females continues to fascinate ecologists and evolutionary biologists. Only by quantifying variation in both the magnitude and direction of SSD, and identifying systematic patterns based on ecological and life-history characteristics, can we better understand its adaptive significance.
Here, to improve our understanding of adaptive variation in SSD and make broad inferences about its likely causation, we investigated the extent to which SSD is driven by selection for increased size in males versus in females (figure 1). Specifically, we aimed to establish whether:
-
(i)
SSD varies systematically with the intensity of sexual selection for increased male size (i.e. the magnitude and direction of SSD is associated with the degree of intrasexual competition, territoriality and/or paternal care); and
-
(ii)
SSD varies systematically with the intensity of fecundity selection for increased female size (i.e. the magnitude and direction of SSD is dependent on the allometric scaling of reproductive energy output and/or fecundity in females, and thus the extent to which larger individuals reproduce disproportionately more than smaller individuals, and theoretically have more to gain from maturing at a larger size).
Figure 1.
A summary of the key life-history characteristics that might select for increased body size in females versus in males. Female-biased sexual size dimorphism (SSD) is typically attributed to the positive correlation between body size and fecundity (i.e. fecundity selection), and variation in the degree of SSD may be dependent on the allometric scaling of reproductive energy output (REO) or fecundity in females (i.e. the extent to which larger females reproduce disproportionately more than smaller individuals). Selection for increased male size may be associated with a high degree of territoriality, sperm competition and/or paternal care. Variation in the degree of SSD among species and taxa is generally considered to arise from differences in the relative intensity of each of these selective forces.
To test these fundamental hypotheses, we conducted a comprehensive, phylogenetically controlled quantitative analysis of SSD in marine and freshwater fish species. Fish exhibit a remarkable array of reproductive strategies, from extreme female-biased SSD in many angler fish, in which dwarf males fuse to, and parasitize, the much larger females [6], to male-biased SSD in reef species characterized by intense territoriality and sperm competition (e.g. [7,8]). This makes fish excellent model organisms for investigating the adaptive significance of SSD. Whereas previous comparative studies have analysed patterns in SSD in a range of other taxa including birds [9,10], copepods [11], insects [12], mammals [13] and reptiles [14,15], to our knowledge there have been no detailed quantitative syntheses of SSD in fish, and there is a recognized need for more rigorous phylogenetically controlled comparative analyses in this group [16,17]. Some models have previously been proposed to explain the evolution of SSD in fish [5], suggesting that, whereas large female size is generally favoured because it increases fecundity, the intensity of selection for increased male size is the most important predictor of SSD. However, almost thirty years since they were proposed, these generalizations remain tentative, having only been tested qualitatively and at relatively low taxonomic resolution. Here, we provide a more robust test of these hypotheses, considering 619 marine and freshwater species from six taxonomic classes, 44 orders and 162 families. We identify systematic patterns in SSD based on habitat and key reproductive life-history characteristics, many of which can be closely associated with varying degrees of male–male competition and paternal care. In doing so, we find strong empirical support for the prediction that SSD varies according to the intensity of sexual selection for increased male size, but no support for the prediction that SSD varies with the allometric scaling of reproductive energy output or fecundity in females.
2. Material and methods
(a). Data acquisition
To quantitatively describe SSD in fish, we obtained data on length at maturity from FishBase [18]. FishBase contains almost 3000 records of body length at first sexual maturity for a wide range of marine and freshwater fish species. We screened these records to include only those studies for which mean length at maturity (Lm) was reported for both sexes separately and at the same sampling location. If reported, we also recorded mean age at maturity (tm). In each case, Lm and tm represent the point at which 50% of the population reached maturity. In addition to length data, we also used FishBase to record important ecological attributes and reproductive life-history characteristics that might explain variation in the degree of SSD between species. Specifically, we categorized species by environment (freshwater versus marine), habitat type (bathydemersal, bathypelagic, benthopelagic, demersal, pelagic and reef), reproductive mode (dioecism, protandry, protogyny), fertilization method (external versus internal), reproductive guild (oviparous brooders, oviparous guarders, oviparous non-guarders and viviparous) and level of parental care (none, biparental, maternal and paternal). Definitions for each of these terms are provided in a glossary in the electronic supplementary material.
For each species (within single studies and sampling locations), we calculated the degree of SSD using the sexual dimorphism index (SDI) of Lovich & Gibbons [19], where
| 1 |
We followed the convention of assigning this metric a positive value when females were the larger sex, and a negative value when males were larger [20]. Similarly, to provide a measure of the relative difference in mean age at maturity between the sexes, we also calculated a sexual bimaturism index (SBM) for each species (within single studies and sampling locations), where
| 2 |
We assigned this metric a positive value when females matured later, and a negative value when males matured later. This allowed us to investigate whether the degree of SSD covaried with the relative difference in age at maturity (i.e. development time) between the sexes. Where we had multiple records for the same species, we calculated the species-specific mean SDI and SBM prior to any statistical analyses.
(b). Statistical analyses
Statistical analyses were conducted in R (v. 3.5.2) [21]. Species have shared evolutionary histories and are therefore not completely statistically independent. Thus, we began by determining the relative degree of relatedness among species in our dataset. Specifically, we used the package ‘rotl' [22], which provides an interface to the Open Tree of Life [23], to retrieve and construct a phylogenetic tree for our fish species. Branch lengths were computed following the Grafen method [24] using the package ‘ape' [25]. This phylogeny was used to create a variance-covariance matrix among species, with a Pagel's lambda correlation structure [26,27], and was incorporated in all our models to control for the phylogenetic correlation among observations. This phylogeny is provided in our electronic supplementary material (Newick file format).
We began by deriving an overall phylogenetically corrected mean SDI value for fish, calculated using an intercept-only phylogenetic generalized least-squares (PGLS) model in which SDI was the independent variable (created using package ‘nlme' [28] in combination with ‘ape'). We then determined whether the intercept (i.e. the phylogenetically corrected mean SDI) differed significantly from zero (two-sided t-test). Quantitative genetic theory predicts that the sex under historically stronger directional selection will exhibit greater interspecific variation in size, resulting in covariation across taxa between the allometric slope of log10 male versus log10 female size and the degree of SSD [29,30]. Rensch's rule also suggests a similar correlation, but one in which males are always the sex with greater interspecific size variation [31]. Thus, we also quantified the allometry of SSD by plotting a phylogenetic reduced major axis (RMA) regression of log10 male versus log10 female size. Specifically, we used the function phyl.RMA in the package ‘phytools' [32] to determine whether the RMA regression slope (β) departed from isometry (i.e. differed significantly from a slope value of 1; two-sided t-test). Where n was greater than or equal to 5, mean SDI values, RMA regressions and their significance were also examined separately by taxonomic classification (class, order, family), environment, habitat type and reproductive trait category.
Next, we compared several candidate models to best predict variation in SSD between species, based on Akaike's information criterion corrected for small samples (AICc). Using SDI as the dependent variable, we incorporated ecological attributes and reproductive life-history characteristics as independent variables in a global PGLS model. These independent variables included environment (freshwater versus marine), habitat type (bathydemersal, bathypelagic, benthopelagic, demersal, pelagic and reef), reproductive mode (dioecism, protandry, protogyny), fertilization method (external versus internal), reproductive guild (oviparous brooders, oviparous guarders, oviparous non-guarders and viviparous) and level of parental care (none, biparental, maternal and paternal care). We then compared all possible combinations of the global model terms using the ‘dredge' function in the MuMIn package [33], including an intercept-only model, which contained no independent variables and predicted that the best estimate of SDI was the intercept. The best model was identified as that with the lowest AICc value. Where the difference between a model's AICc and the lowest AICc (i.e. ΔAICc) was less than 2, a set of best-fit models, rather than a single best model, was assumed. Model averaging was then used to identify the best predictor variables across the top candidate models and determine their relative importance (computed for each variable as the sum of the Akaike weights from all models in which they appear) [34]. Specifically, using package ‘AICcmodavg’ [35], we averaged over the entire set of candidate models (i.e. global PGLS model and all possible simpler models) to calculate the ‘full' model-averaged coefficients for each of the best predictor variables and determine their significance (z-statistic, p < 0.05). Using the ‘full' average assumes that each variable is included in every candidate model, but in some models, the corresponding coefficient (and its respective variance) is set to zero. This reduces the tendency of biasing the estimated coefficients away from zero. Note that these AICc analyses were only conducted on a subset of species for which we had data for all the independent variables (n = 364), meaning species with any missing data were excluded.
In addition to AICc model selection, we also scored each species according to the categorical variables in our analyses, many of which can be associated with varying degrees of selection for increased male size (table 1). By scoring each of these traits and calculating the combined total, we generated a selection-pressure index for increased male size; a comparative measure that estimates the relative degree of selection for increased male size, predicted for each species. Total scores varied from 0 to 8, ranging from dioecious pelagic species with internal fertilization and no parental care or nest guarding (i.e. species with a relatively low likelihood of selection for increased male size), to protogynous reef species with external fertilization and paternal care (i.e. species with a relatively high likelihood of selection for increased male size). We then determined whether the SDI varied significantly as a function of the selection-pressure index for increased male size (PGLS regression).
Table 1.
Scores allocated to each species according to the categorical variables associated with varying degrees of selection for increased male size. The total combined scores for each species, termed the selection-pressure index, were then used to predict variation in the degree of sexual size dimorphism (SSD) in fish, such that low-scoring species (i.e. species with a relatively low likelihood of selection for increased male size) generally exhibited female-biased SSD, whereas high-scoring species (i.e. species with a relatively high likelihood of selection for increased male size) generally exhibited male-biased SSD (figure 3a).
| trait | score |
|---|---|
| habitat | |
| bathypelagic | 0 (low territoriality) |
| pelagic | 0 (low territoriality) |
| bathydemersal | 1 (moderate territoriality) |
| benthopelagic | 1 (moderate territoriality) |
| demersal | 1 (moderate territoriality) |
| reef | 2 (high territoriality) |
| fertilization method | |
| internal | 0 (relatively low sperm competition) |
| external | 1 (relatively high sperm competition) |
| reproductive guild | |
| oviparous (non-guarder) | 0 (low male size advantage) |
| viviparous | 0 (low male size advantage) |
| oviparous (female-only brooder) | 0 (low male size advantage) |
| oviparous (female-only guarder) | 0 (low male size advantage) |
| oviparous (brooder) | 1 (moderate male size advantage) |
| oviparous (guarder) | 1 (moderate male size advantage) |
| oviparous (male-only brooder) | 2 (high male size advantage) |
| oviparous (male-only guarder) | 2 (high male size advantage) |
| parental care | |
| none | 0 (low male size advantage) |
| maternal | 0 (low male size advantage) |
| biparental | 1 (moderate male size advantage) |
| paternal | 2 (high male size advantage) |
| reproductive mode | |
| dioecism | 0 (low selection intensity) |
| protandry (i.e. male → female) | 0 (low selection intensity) |
| protogyny (i.e. female → male) | 1 (high selection intensity) |
To test the fecundity selection hypothesis, we investigated the extent to which SSD varied with the allometric scaling of total reproductive energy output and fecundity in females. Total reproductive energy output is a composite measure that incorporates not just estimates of fecundity, but also egg size and egg energy content, and therefore is likely to provide a more robust estimate of how reproductive investment scales with female body mass within a given species [36]. Theoretically, selection for large female size, and by extension female-biased SSD, may be more prevalent in those species with steeper allometric scaling of reproductive energy output and/or fecundity, as females may gain more from maturing at a larger size. To test these predictions, we acquired species-specific reproductive energy output and fecundity mass-scaling exponents from Barneche et al. [36], which were available for 75 and 70 of the species in our dataset, respectively. We then used a PGLS regression to predict variation in (i) female size, and (ii) SDI as a function of both the reproductive energy output and fecundity mass-scaling exponents.
Whereas we use SDI as the dependent variable in our statistical analyses, to aid in the interpretation of our findings, note that in our figures we express SDI as a percentage to indicate the degree of female- or male-biased SSD (%). For example, an SDI value of 0.5 indicates that Lm (length at maturity) in females is 50% larger than in males, whereas an SDI value of −0.5 indicates that Lm in males is 50% larger than in females.
3. Results
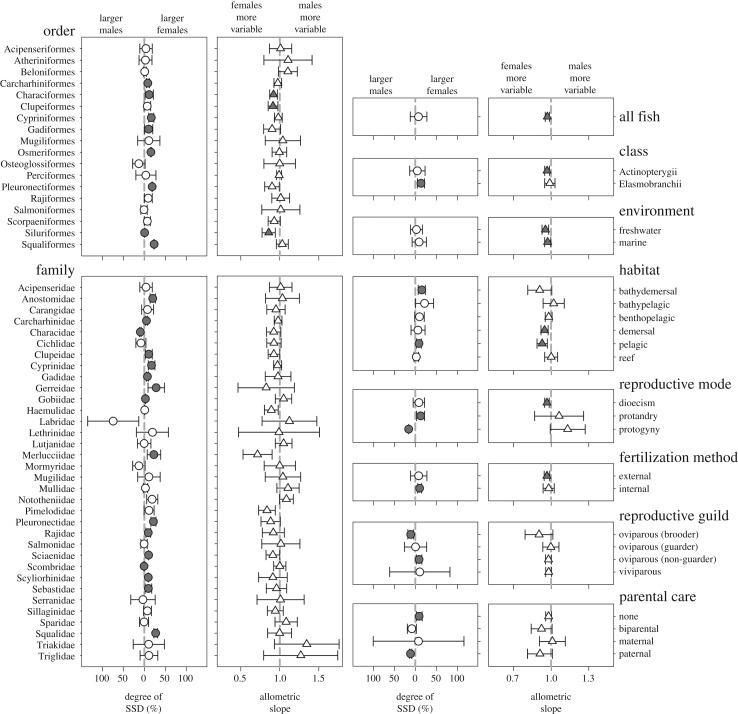
Our final screened dataset consisted of 960 SDI values, representing 619 marine and freshwater species from six taxonomic classes, 44 orders and 162 families. SSD varied considerably between species, with species-specific SDI values ranging from 1.01 (i.e. female Lm 101% larger than male Lm) to −1.34 (i.e. male Lm 134% larger than female Lm). SDI values were greater than zero in 68% of cases (female-biased), less than zero in 27% of cases (male-biased), and exactly zero in only 5% of cases (no difference in size between the sexes). However, the overall phylogenetically corrected mean SDI (0.076 ± 0.20; 95% CI) did not differ significantly from zero (t617 = −0.76, p = 0.45, λ = 0.67). Thus, Lm was not consistently larger in one sex than the other, on average. Our dataset included 34 families in which the number of species was greater than or equal to 5. Fourteen of these families exhibited an overall mean SDI significantly greater than zero, indicating female-biased SSD. Of these, the greatest difference in Lm was observed in the family Gerreidae (mojarras), in which females were approximately 28% larger than males. Male-biased SSD was much less common, with only two families having an overall mean SDI significantly less than zero (Characidae and Scombridae). The remaining 18 families exhibited no significant difference in length at maturity between the sexes, on average. A complete summary of these outcomes, where data are divided by taxonomic classification, environment, habitat and reproductive characteristics, is provided in figure 2 and electronic supplementary material, table S1.
Figure 2.
Multi-panel plot showing (i) the phylogenetically corrected mean degree of sexual size dimorphism (%), and (ii) the allometric slope values of phylogenetic reduced major axis (RMA) regressions of log10 male versus log10 female length at maturity in fish. Data are divided by taxonomic classification, environment, habitat and reproductive characteristics. Error bars denote 95% confidence intervals. Shaded data points indicate where the degree of SSD or allometric slope value differs significantly from monomorphism or isometry, respectively (dashed grey lines). These analyses were only performed when n ≥ 5. Accompanying sample sizes and p-values are presented in electronic supplementary material, table S1.
The best-supported model for explaining variation in SDI contained habitat type, reproductive mode (dioecism, protandry, protogyny) and reproductive guild (oviparous brooders, oviparous guarders, oviparous non-guarders and viviparous) as independent variables, which together accounted for 22% of the variation in SSD (AICc model selection). An alternative model that also included fertilization method and parental care, but excluded reproductive guild, had a ΔAICc less than 2, and we therefore calculated the combined parameter Akaike weights across both models to determine the relative importance of each variable (see electronic supplementary material, table S2). Habitat type and reproductive mode were the most important predictors, followed by reproductive guild, whereas fertilization and parental care were relatively less important. Model averaging revealed significant effects of both habitat type and reproductive mode on SDI, whereas none of the other independent variables had a significant effect. A summary of these outcomes, including the full model-averaged coefficients, is presented in electronic supplementary material, table S3.
A phylogenetic RMA regression of log10 male Lm versus log10 female Lm across all species had a slope value (β) significantly less than 1, suggesting greater interspecific variation in female than male size (i.e. the inverse of Rensch's rule) [31]. However, RMA regressions for individual taxa showed that most taxa exhibited isometry in SSD (figure 2; electronic supplementary material, table S1). Comparing taxonomic classes, SSD in elasmobranchs did not depart from isometry, whereas females were the more variable sex in Actinopterygii. However, male–female length isometry applied when all 34 families were examined separately, and only 3 of the 19 orders examined had β values significantly less than 1 (i.e. greater variance in female size). At no level of organization tested was β significantly greater than 1 (i.e. greater variance in male size); thus, we found no evidence to support Rensch's rule [31]. Contrary to the predictions of quantitative genetic theory [29,30], there was no significant relationship between family specific phylogenetically corrected mean SDI values and their respective β values (F1,34 = 1.51, p = 0.23; electronic supplementary material, figure S1). We also collated data on time to maturity where this was reported (n = 212). In most cases (approx. 69%), the smaller sex within a species reached maturity earlier. Those species with the greatest relative difference in age at maturity between the sexes also exhibited the strongest degree of SSD (PGLS regression; t210 = 13.60, p < 0.001, λ = −0.05; electronic supplementary material, figure S2).
(a). Importance of selection for increased male size
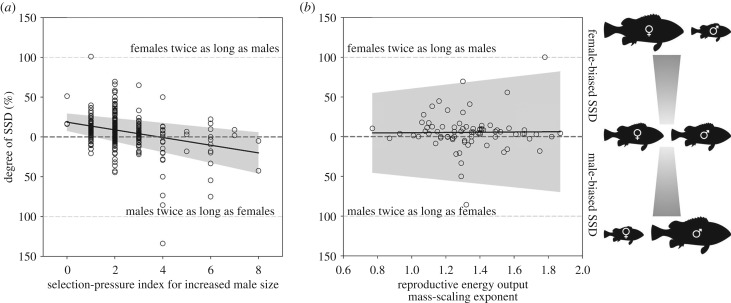
We were able to significantly predict variation in SDI when species were scored based on the selection-pressure index for increased male size (PGLS regression; t362 = 5.08, p < 0.001, λ = 0.24; figure 3a). The lowest-scoring species generally exhibited female-biased SSD (Lm in females approx. 18.5% larger than in males), whereas the highest-scoring species generally exhibited male-biased SSD (Lm in males approx. 20.0% larger than in females). The sexes were much more similar in size in those species with intermediate scores. As might be expected, SSD was female-biased in those species exhibiting protandry (i.e. switching sex from male to female), whereas those species exhibiting protogyny (i.e. switching sex from female to male) showed significant male-biased SSD. Even after removing those species in which there was evidence of sex switching (n = 35), we still found a significant relationship between the selection-pressure index for increased male size and SDI (PGLS regression; t327 = 4.16, p < 0.001, λ = 0.32). Female Lm was approximately 10.3% larger than male Lm in species with internal fertilization, whereas in those species with external fertilization, males and females were similar in body size. Significant male-biased SSD was evident in oviparous brooders (Lm in males approx. 11% larger than in females), whereas species that did not guard their eggs exhibited female-biased SSD (Lm in females approx. 8.9% larger than in males). Finally, Lm in males was approximately 11.5% larger than in females in those species with paternal care, whereas those species with no parental care exhibited female-biased SSD (Lm in females approx. 9.1% larger than in males).
Figure 3.
Variation in the degree of sexual size dimorphism (SSD) as a function of (a) the selection-pressure index for increased male size (n = 364), and (b) species-specific female reproductive energy output mass-scaling exponents reported by Barneche et al. [36]. Solid lines indicate the PGLS regression through the data; 95% CIs are contained within the shaded area. In (a), species were scored according to categorical variables associated with varying degrees of selection for increased male size (table 1). The combined total of these scores significantly predicts variation in the degree of SSD, such that low-scoring species (i.e. species with a relatively low likelihood of selection for increased male size) generally exhibit female-biased SSD, whereas high-scoring species (i.e. species with a relatively high likelihood of selection for increased male size) generally exhibit male-biased SSD. In (b), there is no significant relationship between the allometric scaling of female reproductive energy output and the degree of SSD. We postulate that this is because species with higher scaling exponents, hence a disproportionately greater reproductive energy output with increasing body size, also have a disproportionate increase in the energetic costs of reproduction, and these two components probably counteract each other. Similarly, we found no significant relationship between the intra-specific allometric scaling of fecundity and SSD in females.
(b). SSD and the allometric scaling of female reproductive energy output and fecundity
To test the prediction that SSD varies systematically with the allometric scaling of reproductive energy output and/or fecundity in females, we used species-specific mass-scaling exponents reported by Barneche et al. [36]. Reproductive energy output mass-scaling exponents were available for 75 of the species in our dataset, with scaling exponents ranging from 0.77 to 1.87. Fecundity mass-scaling exponents were available for 70 of the species in our dataset, with scaling exponents ranging from 0.67 to 1.76. We found no significant relationship between the intra-specific allometric scaling exponent for reproductive energy output and Lm in females (PGLS regression; t73 = 0.54, p = 0.59, λ = 0.60), nor with SDI (PGLS regression; t73 = −0.12, p = 0.90, λ = −0.02; figure 3b). Similarly, we found no significant relationship between the intra-specific allometric scaling exponent for fecundity and Lm in females (PGLS regression; t68 = 1.15, p = 0.25, λ = 0.70), nor with SDI (PGLS regression; t68 = 0.76, p = 0.43, λ = −0.09). Thus, variation in SSD was not dependent on the extent to which larger females reproduce disproportionately more than smaller females.
4. Discussion
Here, we tested two fundamental hypotheses to better understand the adaptive significance of SSD: that the magnitude and direction of SSD varies systematically with (i) the intensity of sexual selection for increased male size, and (ii) with the intensity of fecundity selection for increased female size. We find compelling empirical support for the former (i), but no support for the latter (ii).
Recent work has shown that, on average, both reproductive energy output and fecundity scale hyper-allometrically with body mass in female fish (i.e. larger females reproduce disproportionally more than smaller females) [36]. Yet, although increased female size is commonly favoured because it increases fecundity, selection pressures for increased male size appear to be the best predictor of variation in the degree of SSD between fish species, supporting the predictions of Parker [5]. Examples of male-biased SSD in fish are generally observed where there is a high degree of territoriality or sperm competition, or where there is evidence of paternal care (e.g. [37,38–41]). Home ranges have been documented in a wide range of coral reef fish, but this is rarely the case for pelagic species, and consequently male–male competition, in the form of territoriality, is likely to be much more intense in reef compared to pelagic habitats [42]. Differences in mating behaviour (e.g. monogamy versus polygamy, distinct pairing versus communal spawning) will lead to variation in the intensity of sperm competition, and at comparable levels of polygamy and communal spawning, internal fertilization carries a lower risk of sperm competition than does external fertilization (since the displacement of previous males' sperm and/or greater sperm mortality in internal fertilizers will tend to decrease the intensity of sperm competition) [43]. Consequently, an increase in male body size and aggression of external fertilizers may, among other factors, help to minimize multiple paternity of female eggs [44,45]. Similarly, the advantages of large male size are predicted to be greater in those species with paternal care, or where male brooding and/or nest guarding is evident [41,46]. We generated a selection-pressure index for increased male size by scoring species based on these traits. This index significantly predicted variation in SSD between fish species, not just in direction but also in magnitude. At the extremes, Lm in females was approximately 18.5% larger than in males in dioecious pelagic species with internal fertilization and no parental care, where territoriality and sperm competition are predicted to be relatively low, and where high male mortality probably selects for earlier reproduction, constraining male size, resulting in female-biased SSD. By contrast, Lm in males was approximately 20.0% larger than in females in protogynous reef species with external fertilization and paternal care, where male–male competition is predicted to be most intense. Assuming that most fish exhibit isometric growth (i.e. they increase in their length with increasing weight in cubic form) [18], these extremes would be equivalent to differences in body mass of 66.4% and 72.8%, respectively.
We recognize that the traits used in our analyses only provide a proxy for the strength of selection for increased male size, and do not capture the complexity of natural systems. For example, the intensity of sperm competition varies enormously among fish species and is by no means entirely dependent on fertilization method. Nevertheless, to make useful ecological generalizations from the available data, categorizing species based on a few key life-history characteristics provides a more comprehensive hypothesis-driven approach to explaining the observed diversity in SSD. We also acknowledge that in our analyses, scores for each of the different life-history traits were allocated the same weighting, and some traits may be much more influential in driving SSD than others. For example, in protogynous species, males will always be larger than the females they once were. However, even after removing species that switch sex, there was still a significant relationship between SSD and the selection-pressure index for increased male size. Although the underlying scoring is subjective and not truly quantitative, in combination with AICc model selection, our findings provide robust support for the prediction that adaptive variation in SSD among more than 600 diverse fish species is primarily driven by the intensity of selection for increased male size.
Having established that the intensity of selection for increased male size is an important predictor of SSD, we also examined to what extent selection for increased female size might contribute to the observed patterns (e.g. [20,47]). Specifically, one might predict that large female size, and by extension female-biased SSD, will be more prevalent in those species with particularly steep allometric scaling of female reproductive energy output and/or fecundity (i.e. where larger individuals reproduce disproportionately more than smaller individuals, and theoretically have more to gain from maturing at a larger size). Yet we found no such patterns in the species for which we had suitable data. We postulate that this is because species with higher scaling exponents, and hence disproportionately greater reproductive energy output or fecundity with increasing body size, may also have a disproportionate increase in the energetic costs of reproduction [36], and these two components may counteract each other. These findings are particularly pertinent to the recent debate on the extent to which constraints on growth versus the allometric scaling of costly reproductive output may drive mature size and SSD, especially in fish [36,48–51].
We acknowledge that there are several caveats associated with our test of the fecundity selection hypothesis. First, our analyses examining selection for increased size in males versus in females differ markedly in their sample size, and this may increase the probability of a type 2 error when examining the latter. Yet, were we to include only species for which we have both a selection-pressure index score and a reproductive energy output mass-scaling exponent, this would have reduced the amount of available data by more than 88% (to n = 42). We also note that similar tests for other ectotherms, including broad-scale analyses across lizards and snakes, find mixed support for the fecundity selection hypothesis, but do find an association between SSD and proxies for the degree of sexual selection in males [14,15]. Whether relationships between SSD and the allometric scaling of reproductive energy output are evident in other taxa remains to be investigated. Second, despite potentially variable selection for increased female size among species from different habitats and with different reproductive traits, the high prevalence of female-biased SSD and evidence for the inverse of Rensch's rule [31] (i.e. allometric slope values less than 1, indicating greater variation in female size; figure 2) suggests that directional selection has generally favoured large female size. These outcomes are consistent with findings for other taxa exhibiting female-biased SSD, such as birds [52] and insects [12]. Directional selection for large female size is predicted to result from the positive correlation between body size and fecundity [3,53,54]. Consequently, although we cannot explain variation in the degree of SSD based on the allometric scaling of reproductive energy output or fecundity in females, fecundity selection is still likely to play an important role in maintaining large female size and driving SSD. Indeed, only when the selection pressures for increased male size intensify do we begin to observe deviations away from female-biased SSD towards monomorphism and male-biased SSD (figure 3a). Ultimately, although selection for increased male size predicts variation in SSD among fish species, we cannot dismiss the effect of fecundity selection, and these drivers are by no means mutually exclusive.
SSD can arise due to differences in development time and/or growth rate between the sexes, and the relative importance of each of these proximate mechanisms in generating SSD has been debated, particularly in insects [17,20,55]. We found that the sex with the longer development time also tended to mature at a larger size, and those species with the greatest relative difference in age at maturity between the sexes also exhibited the strongest degree of SSD. Therefore, while we cannot rule out that males and females may also differ in their growth rates, variation in development time appears to be an important proximate correlate of SSD in fish (electronic supplementary material, figure S2). We note that many fish exhibit indeterminate growth and, consequently, mean adult body size can be much larger than size at maturation. As a result, sex-specific growth patterns beyond maturation could lead to differences in SSD at maturity versus SSD at asymptotic size, potentially confounding our overall conclusions. Of the 619 species in our dataset, we found paired male and female estimates of asymptotic length for 240 species on FishBase [18], and therefore tested whether SSD derived from asymptotic length (SSD LInf) differed significantly from SSD derived from length at maturity (SSD Lm). On average, we found no significant difference between SSD LInf and SSD Lm, both when using a phylogenetic paired t-test (t237 = 0.015, p = 0.988), and also when plotting a phylogenetically corrected RMA regression of SSD LInf versus SSD Lm, such that the slope of the regression did not differ significantly from 1 (t232 = 1.00, p = 0.32). Furthermore, in agreement with our overall conclusions, there was a significant positive relationship between SSD LInf and the selection-pressure index from increased male size (PGLS regression; t167 = 2.79, p = 0.006), but not with the allometric scaling of reproductive energy output (PGLS regression; t,33 = 0.14, p = 0.89) or fecundity in females (PGLS regression; t32 = −0.24, p = 0.81, respectively). Thus, whereas many fish continue to grow beyond maturation, we are confident in our use of length at maturity to identify systematic patterns in SSD.
5. Conclusion
Previous phylogenetic comparative analyses have investigated the extent to which SSD is driven by selection for increased size in males versus in females, including birds [9,10], insects [12], mammals [13] and reptiles [14,15]. In the majority of cases, there were significant correlations between the degree of SSD and various measures of sexual selection for large male size, whereas those that incorporated estimates of fecundity selection (e.g. by quantifying the slope of the relationship between clutch size and maternal size) found inconsistent support for this hypothesis. Yet, studies of SSD in fish are under-represented in the literature [17], and to our knowledge, this is the first rigorous, comparative analyses of SSD in this group. Whereas Parker [5] used a categorical measure of SSD to test his predictions, our quantitative approach provides much greater statistical power, while also accounting for the phylogenetic correlation among taxa. Adopting a trait-based approach, combined with an increase in the availability of novel physiological data, such as intra-specific reproductive energy output mass-scaling exponents, allows for a more robust test of the fundamental hypotheses proposed to explain adaptive variation in SSD. In this regard, we believe our dataset and analyses provide a methodological template for future studies examining diversity in SSD in other taxa.
Supplementary Material
Supplementary Material
Acknowledgements
We wish to thank the editor and three anonymous reviewers for their detailed and constructive comments, which helped to improve this work. We are most grateful to Geoff Parker FRS for his valuable feedback on an earlier version of the manuscript.
Data accessibility
Raw data used in this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.9zw3r229m [56].
Author' contributions
C.R.H., A.G.H. and D.A. designed the study and wrote the paper. C.R.H. collated the data and performed the statistical analyses.
Competing interests
We declare we have no competing interests.
Funding
The Centre for Ocean Life is a VKR Centre of Excellence funded by the Villum Foundation.
References
- 1.Hedrick AV, Temeles EJ. 1989. The evolution of sexual dimorphism in animals: hypotheses and tests. Trends Ecol. Evol. 4, 136–138. ( 10.1016/0169-5347(89)90212-7) [DOI] [PubMed] [Google Scholar]
- 2.Slatkin M. 1984. Ecological causes of sexual dimorphism. Evolution 38, 622–630. ( 10.1111/j.1558-5646.1984.tb00327.x) [DOI] [PubMed] [Google Scholar]
- 3.Darwin C. 1874. The descent of man and selection in relation to sex. New York, NY: AL Burt Co. [Google Scholar]
- 4.Lande R. 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305. ( 10.1111/j.1558-5646.1980.tb04817.x) [DOI] [PubMed] [Google Scholar]
- 5.Parker GA. 1992. The evolution of sexual size dimorphism in fish. J. Fish Biol. 41, 1–20. ( 10.1111/j.1095-8649.1992.tb03864.x) [DOI] [Google Scholar]
- 6.Pietsch TW. 1976. Dimorphism, parasitism and sex: reproductive strategies among deepsea Ceratioid anglerfishes. Copeia 1976, 781–793. ( 10.2307/1443462) [DOI] [Google Scholar]
- 7.Reese ES. 1973. Duration of residence by coral reef fishes on ‘home’ reefs. Copeia 1973, 145–149. ( 10.2307/1442375) [DOI] [Google Scholar]
- 8.Keenleyside MHA. 2012. Diversity and adaptation in fish behaviour. Berlin, Germany: Springer. [Google Scholar]
- 9.Lislevand T, Figuerola J, Székely T. 2009. Evolution of sexual size dimorphism in grouse and allies (Aves: Phasianidae) in relation to mating competition, fecundity demands and resource division. J. Evol. Biol. 22, 1895–1905. ( 10.1111/j.1420-9101.2009.01802.x) [DOI] [PubMed] [Google Scholar]
- 10.Serrano-Meneses MA, Székely T. 2006. Sexual size dimorphism in seabirds: sexual selection, fecundity selection and differential niche-utilisation. Oikos 113, 385–394. ( 10.1111/j.0030-1299.2006.14246.x) [DOI] [Google Scholar]
- 11.Hirst AG, Kiørboe T. 2014. Macroevolutionary patterns of sexual size dimorphism in copepods. Proc. R. Soc. B 281, 20140739 ( 10.1098/rspb.2014.0739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanckenhorn WU, Meier R, Teder T. 2007. Rensch's rule in insects: patterns among and within species. In Sex, size and gender roles: evolutionary studies of sexual size dimorphism (eds Fairbairn DJ, Blanckenhorn WU, Székely T). Oxford, UK: Oxford University Press. [Google Scholar]
- 13.Lindenfors P, Gittleman JL, Jones KE. 2007. Sexual size dimorphism in mammals. In Sex, size and gender roles: evolutionary studies of sexual size dimorphism (eds Fairbairn DJ, Blanckenhorn WU, Székely T), pp. 16–26. Oxford, UK: Oxford University Press. [Google Scholar]
- 14.Cox RM, Butler MA, John-Alder HB. 2007. The evolution of sexual size dimorphism in reptiles. In Sex, size and gender roles: evolutionary studies of sexual size dimorphism (eds Fairbairn DJ, Blanckenhorn WU, Székely T), pp. 38–49. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Shine R. 1994. Sexual size dimorphism in snakes revisited. Copeia 1994, 326–346. ( 10.2307/1446982) [DOI] [Google Scholar]
- 16.Blanckenhorn WU. 2005. Behavioral causes and consequences of sexual size dimorphism. Ethology 111, 977–1016. ( 10.1111/j.1439-0310.2005.01147.x) [DOI] [Google Scholar]
- 17.Fairbairn DJ, Blanckenhorn WU, Székely T. 2007. Sex, size and gender roles: evolutionary studies of sexual size dimorphism. Oxford, UK: Oxford University Press. [Google Scholar]
- 18.Froese R, Pauly D. 2019. FishBase. See www.fishbase.org.
- 19.Lovich JE, Gibbons JW. 1992. A review of techniques for quantifying sexual size dimorphism. Growth Dev. Aging 56, 269–281. [PubMed] [Google Scholar]
- 20.Blanckenhorn WU, et al. 2007. Proximate causes of Rensch's rule: does sexual size dimorphism in arthropods result from sex differences in development time? Am. Nat. 169, 245–257. ( 10.1086/510597) [DOI] [PubMed] [Google Scholar]
- 21.Core Team R. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 22.Michonneau F, Brown JW, Winter DJ. 2016. rotl: an R package to interact with the Open Tree of Life data. Methods Ecol. Evol. 7, 1476–1481. ( 10.1111/2041-210X.12593) [DOI] [Google Scholar]
- 23.Hinchliff CE, et al. 2015. Synthesis of phylogeny and taxonomy into a comprehensive tree of life. Proc. Natl Acad. Sci. USA 112, 12 764–12 769. ( 10.1073/pnas.1423041112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grafen A. 1989. The phylogenetic regression. Phil. Trans. R. Soc. B 326, 119–157. ( 10.1098/rstb.1989.0106) [DOI] [PubMed] [Google Scholar]
- 25.Paradis E, Schliep K. 2018. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528. ( 10.1093/bioinformatics/bty633) [DOI] [PubMed] [Google Scholar]
- 26.Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726. ( 10.1086/343873) [DOI] [PubMed] [Google Scholar]
- 27.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877 ( 10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 28.Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. 2019. nlme: linear and nonlinear mixed effects models. R package version 3.1-140.
- 29.De Lisle SP, Rowe L. 2013. Correlated evolution of allometry and sexual dimorphism across higher taxa. Am. Nat. 182, 630–639. ( 10.1086/673282) [DOI] [PubMed] [Google Scholar]
- 30.Zeng ZB. 1988. Long-term correlated response, interpopulation covariation, and interspecific allometry. Evolution 42, 363–374. ( 10.1111/j.1558-5646.1988.tb04139.x) [DOI] [PubMed] [Google Scholar]
- 31.Rensch B. 1960. Evolution above the species level. New York, NY: Columbia University Press. [Google Scholar]
- 32.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–233. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 33.Barton K.2017. MuMIn; multi-model inference. R package version 1.40.0.
- 34.Burnham KP, Anderson DM. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn New York, NY: Springer. [Google Scholar]
- 35.Mazerolle M.J. 2014. Model Selection and Multimodel Inference Based on (Q)AIC(c). R package version 2.0-1.
- 36.Barneche DR, Robertson DR, White CR, Marshall DJ. 2018. Fish reproductive-energy output increases disproportionately with body size. Science 360, 642–645. ( 10.1126/science.aao6868) [DOI] [PubMed] [Google Scholar]
- 37.Erlandsson A, Ribbink A. 1997. Patterns of sexual size dimorphism in African cichlid fishes. S. Afr. J. Sci. 93, 498–508. [Google Scholar]
- 38.Schütz D, Taborsky M. 2000. Giant males or dwarf females: what determines the extreme sexual size dimorphism in Lamprologus callipterus? J. Fish Biol. 57, 1254–1265. ( 10.1111/j.1095-8649.2000.tb00485.x) [DOI] [Google Scholar]
- 39.Kodric-Brown A. 1990. Mechanisms of sexual selection: insights from fishes. Ann. Zool. Fenn. 27, 87–100. [Google Scholar]
- 40.Kolm N. 2002. Male size determines reproductive output in a paternal mouthbrooding fish. Anim. Behav. 63, 727–733. ( 10.1006/anbe.2001.1959) [DOI] [Google Scholar]
- 41.Gagliardi-Seeley JL, Itzkowitz M. 2006. Male size predicts the ability to defend offspring in the biparental convict cichlid Archocentrus nigrofasciatus. J. Fish Biol. 69, 1239–1244. ( 10.1111/j.1095-8649.2006.01174.x) [DOI] [Google Scholar]
- 42.Hixon MA. 2015. Territory area as a determinant of mating systems. Integr. Comp. Biol. 27, 229–249. ( 10.1093/icb/27.2.229) [DOI] [Google Scholar]
- 43.Stockley P, Gage M, Parker G, Møller A. 1997. Sperm competition in fishes: the evolution of testis size and ejaculate characteristics. Am. Nat. 149, 933–954. ( 10.1086/286031) [DOI] [PubMed] [Google Scholar]
- 44.Smith RL. 2012. Sperm competition and the evolution of animal mating systems. London, UK: Elsevier. [Google Scholar]
- 45.Constantz GD. 1984. Sperm competition in poeciliid fishes. In Sperm competition and the evolution of animal mating systems (ed. Smith RL.), pp. 465–485. London, UK: Elsevier. [Google Scholar]
- 46.Gross MR, Sargent RC. 1985. The evolution of male and female parental care in fishes. Am. Zool. 25, 807–822. ( 10.1093/icb/25.3.807) [DOI] [Google Scholar]
- 47.Clarke T. 1983. Sex ratios and sexual differences in size among mesopelagic fishes from the central Pacific Ocean. Mar. Biol. 73, 203–209. ( 10.1007/BF00406889) [DOI] [Google Scholar]
- 48.Marshall DJ, White CR. 2018. Have we outgrown the existing models of growth? Trends Ecol. Evol. 34, 102–111. ( 10.1016/j.tree.2018.10.005) [DOI] [PubMed] [Google Scholar]
- 49.Marshall DJ, White CR. 2019. Aquatic life history trajectories are shaped by selection, not oxygen limitation. Trends Ecol. Evol. 34, 182–184. ( 10.1016/j.tree.2018.12.015) [DOI] [PubMed] [Google Scholar]
- 50.Pauly D. 2019. Female fish grow bigger–let's deal with it. Trends Ecol. Evol. 34, 181–182. ( 10.1016/j.tree.2018.12.007) [DOI] [PubMed] [Google Scholar]
- 51.Kearney M. 2019. Reproductive hyperallometry does not challenge mechanistic growth models. Trends Ecol. Evol. 34, 275–276. ( 10.1016/j.tree.2018.12.006) [DOI] [PubMed] [Google Scholar]
- 52.Webb TJ, Freckleton RP. 2007. Only half right: species with female-biased sexual size dimorphism consistently break Rensch's rule. PLoS ONE 2, e897 ( 10.1371/journal.pone.0000897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fairbairn DJ. 1997. Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in males and females. Annu. Rev. Ecol. Syst. 28, 659–687. ( 10.1146/annurev.ecolsys.28.1.659) [DOI] [Google Scholar]
- 54.Abouheif E, Fairbairn DJ. 1997. A comparative analysis of allometry for sexual size dimorphism: assessing Rensch's rule. Am. Nat. 149, 540–562. ( 10.1086/286004) [DOI] [Google Scholar]
- 55.Teder T. 2014. Sexual size dimorphism requires a corresponding sex difference in development time: a meta-analysis in insects. Funct. Ecol. 28, 479–486. ( 10.1111/1365-2435.12172) [DOI] [Google Scholar]
- 56.Horne CR, Hirst AG, Atkinson D. 2020. Data from: Selection for increased male size predicts variation in sexual size dimorphism among fish species. Dryad Digital Repository ( 10.5061/dryad.9zw3r229m) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Horne CR, Hirst AG, Atkinson D. 2020. Data from: Selection for increased male size predicts variation in sexual size dimorphism among fish species. Dryad Digital Repository ( 10.5061/dryad.9zw3r229m) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw data used in this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.9zw3r229m [56].