Abstract
Telomere length (TL) and shortening is increasingly shown to predict variation in survival and lifespan, raising the question of what causes variation in these traits. Oxidative stress is well known to accelerate telomere attrition in vitro, but its importance in vivo is largely hypothetical. We tested this hypothesis experimentally by supplementing white stork (Ciconia ciconia) chicks with antioxidants. Individuals received either a control treatment, or a supply of tocopherol (vitamin E) and selenium, which both have antioxidant properties. The antioxidant treatment increased the concentration of tocopherol for up to two weeks after treatment but did not affect growth. Using the telomere restriction fragment technique, we evaluated erythrocyte TL and its dynamics. Telomeres shortened significantly over the 21 days between the baseline and final sample, independent of sex, mass, size and hatching order. The antioxidant treatment significantly mitigated shortening rate of average TL (−31% in shorter telomeres; percentiles 10th, 20th and 30th). Thus, our results support the hypothesis that oxidative stress shortens telomeres in vivo.
Keywords: telomeres, oxidative stress, TRF, antioxidant supplementation, free-living birds
1. Introduction
Telomeres are located at the ends of linear chromosomes to protect chromosome ends from degradation and maintaining stability [1]. With each cell division, telomere length (TL) decreases; when they reach a critically short length, the cell enters a degenerative process of senescence, eventually followed by apoptosis [1,2]. Also, on the level of the whole organism, there is an association between lifespan/annual survival and TL and its attrition [3–8]. There is accumulating evidence that telomere attrition during development is of particular importance for later health and survival [9,10]. Different external factors could affect telomere attrition, but it is generally assumed that most of these exert their effect through oxidative stress [11–13].
Oxidative stress results from an imbalance between the production of reactive oxygen species (ROS) and the antioxidant capacity of an organism [14], and accelerates telomere attrition in cell cultures [15]. The nucleobase guanine is relatively sensitive to oxidation, and its high proportion in the (TTAGGG)n repeats that constitute vertebrate telomeres makes them particularly vulnerable to oxidative attack [15]. This high sensitivity, together with the deficiency in repairing single-strand breaks in telomere areas, makes telomeres particularly susceptible to oxidative damage [16]. This process, together with the oxidative stress enhancing effects of various physiological and psychological stressors, makes telomeres potential biomarkers of lifespan, and of exposure to environmental challenges and individual lifestyle [17].
However, the apparently simple relation between oxidative stress and telomere dynamics cited above is largely based on in vitro studies of cell cultures [15,18], and the extent to which oxidative stress accelerates telomere shortening in vivo has recently been questioned [19,20]. To date, few studies have attempted to evaluate this correlation using an experimental approach. Thus, the extent to which oxidative stress accelerates telomere attrition in vivo remains an open question [21].
In this study, we evaluated the effect of antioxidant supplementation on TL and their attrition in free-living white stork (Ciconia ciconia) chicks. First, we analysed whether the antioxidant supplementation affected oxidative stress biomarkers to verify its efficacy. We subsequently described telomere dynamics in relation to age, sex, hatching order, mass, size and treatment. Because we measured TL using the telomere restriction fragment (TRF) method (i.e. using a smear on a gel), we can characterize TL not only by the average TL but also by distribution. This can be useful, because telomere shortening is faster at the high end of the telomere distribution within individuals [22,23], suggesting that treatment effects may also vary depending on location in the telomere distribution.
2. Material and methods
(a). Experimental design and sampling
The study was carried out in a breeding colony located in northern Madrid, in the centre of Iberian Peninsula (40°44′ N, 3°49′ E).
In total, 55 chicks from 20 nests were used in the experiment (165 samples, of which 129 were analysed for the present study). Hatching order was established through frequent visits during the hatching period based on size differences between the nestlings within broods that are due to the high asynchrony in this species. White storks begin incubation with the first or second egg, and laying occurs at intervals of 2 days. Furthermore, egg mass also tends to decrease with laying order, and this effect, combined with hatching asynchrony, results in a marked size hierarchy among nest-mates [24]. Birds were randomly assigned to two treatment groups, while ensuring that both groups were represented in all nests. The ‘vitamins’ group was supplied with a subcutaneous dose of a commercial mixture for veterinarian use (Selevit, Syva laboratories, León, Spain). Each dose contained 5 mg of α-tocopherol acetate (aka vitamin E) and 50 µg of sodium selenite per kg body mass, calculated for each dose depending on the actual mass. Their vitamin E radical-scavenging activity causes both substances to be considered a chain-breaking antioxidant [25–27]. Selenium is a trace mineral, essential for the function of different antioxidant enzymes [25,28]. The ‘control’ group was administrated with an equivalent dose of a sterile saline solution. Sampling was carried out under a special permit of the regional park Cuenca Alta del Manzanares and of the regional government (Comunidad de Madrid).
The time between the first and last sample was limited to 20 days. Chicks were weighed, measured and bled at the start of the experiment, at which time they were on average 20 days old (s.d. = 8 days; termed ‘day 1’ below), after which the first treatment was administered. This process was repeated 14 and 21 days later, except that no treatment was provided on day 21 (electronic supplementary material, figure S1). Blood samples (±1 ml) were collected from the brachial vein with a heparinized syringe, immediately transferred to sterile tubes and kept at 4°C until centrifugation (10 min at 1800 g at 4°C). Blood cells were separated from the plasma and washed three times in ice-cold physiological (0.9%) sodium chloride solution, and both plasma and cells were aliquoted and stored at −80°C until analysis.
(b). Oxidative stress biomarkers
Levels of tocopherol in plasma and malondialdehyde (MDA, indicator of lipid peroxidation) were measured using high-performance liquid chromatography (HPLC, Agilent Technologies 1100 Series) ([29] and [30], respectively). The intra-assay coefficient variation (CV) of tocopherol and MDA were 1.52% and 3.96%, respectively.
Total antioxidant capacity (TAC) was determined spectrophotometrically using the ferric reducing ability of plasma (FRAP) method described in [31] and modified as described in [32]. The parameter was corrected for the uric acid concentration by using the residuals of the linear model between both variables [33]. Uric acid was measured with a commercial kit (Spinreact; Girona, Spain). The intra-assay and inter-assay CV were, respectively, 6.71% and 3.08%.
(c). Telomere length assays
TL was measured as described in [22]. Briefly, 7 µl of erythrocytes were suspended in an agarose solution to form an agarose plug of 0.8% (CHEF Mammalian Genomic DNA Plug kit, Bio-Rad Laboratories, USA) and digested overnight with proteinase K at 50°C. Subsequently, DNA was simultaneously digested with Hinf I (30 U), Msp I (60 U) and Hind III (60 U) restriction endonuclease overnight at 37°C in NEB2 buffer (New England Biolabs, Beverly, MA, USA). The digested DNA from each sample and the 32P-labelled size ladders (DNA Molecular Weight Marker XV, Roche Diagnostics, Basel, Switzerland; 1 kb DNA ladder, New England Biolabs, Ipswich, MA, USA) were separated through a 0.8% agarose gel by pulsed-field gel electrophoresis at 14°C for 24 h. Gels were dried using a gel dryer (model 538, Bio-Rad) and hybridized overnight using a 32P-end-labelled oligo (5′-CCCTAA-3′) that binds to the single strand overhang of telomeres. Unbound oligonucleotides were removed by washing the gel for 30 min with 0.25 x saline-sodium citrate buffer at 37°C. The radioactive signal was detected by a phosphor screen (MS, Perkin-Elmer, Waltham, MA, USA) and analysed using a phosphor imager (Cyclone TM Storage Phosphor System, Perkin-Elmer), resulting in a gel picture with a distribution of grey values in a smear, reflecting the distribution of TL in a sample. We included the three samples of each individual in the same gel next to each other in random order, and the same number of the two treatments randomized in every gel used to avoid confounding effects due to gel identity.
Individual TL size distributions were quantified through densitometry using the open-source software ImageJ v. 1.38x. This technique allows us to classify the telomere distribution of each sample into every 10th percentile from 10th to 90th, with the 10th percentile being the shortest telomeres and the 90th the longest, besides the average TL. Between-gel CV was below 5% but note that samples of the same individual were always together on a gel.
(d). Statistical analyses
We used general linear mixed models fitted with REML (restricted maximum likelihood) to analyse the data. We created the covariate hatch order, assigning 1 to the first-hatched chick, 3 to the last-hatched chick and 2 to the intermediate chick(s) (mean ± s.d., brood size on day 1 was 3.76 ± 1.03).
To assess the effect of the treatment on oxidative stress variables, we constructed three different models, with tocopherol concentration, TAC and MDA as dependent variables, respectively, treatment (control or vitamins) as factor and age of individuals (in days) as covariate. Treatment was coded ‘control’ (zero) for day 1 for all individuals because antioxidant supplementation started after sampling and coded zero or one on days thereafter for control and treated individuals respectively. Individual identity, nested in brood identity, was included as a random factor in all models to avoid pseudo-replication. Analysis of treatment effects on mass and size (tarsus length in mm) followed the same approach.
When analysing telomere dynamics, we tested effects of sex, mass, hatch order and treatment in a single model, using a stepwise backward procedure to obtain the final model. Gel identity was included as a random effect in all telomere models, in addition to individual identity nested in brood identity. In each analysis, interaction of each variable with age (included as covariate) was tested and removed from the final model if non-significant. The same analysis were carried out for each percentile (electronic supplementary material, table S1), evaluating at the same time if the loss of telomeres by percentiles differed significantly (electronic supplementary material, table S3).
Finally, the relation between telomeres and oxidative stress variables was evaluated using TL as dependent variable, and the values of the previous measurement day of tocopherol, MDA and TAC (mean centred) were used as covariates.
All models were validated by visual inspection of the residual graphs to verify the assumptions of normality of the residuals and homogeneity of the variances. In all tables, the column ‘intercept’ was added to show how it changed with the exclusion of the rejected terms. All analyses were performed in R 3.4.1 [34] using the R packages ‘lme4’ (1.1–17) and ‘lmerTest’ (3.0–1) [35,36].
3. Results
(a). Treatment effect on oxidative stress biomarkers, mass and size
Treatment elevated tocopherol concentration by approximately 10% (p = 0.017; table 1), indicating a correct absorption and assimilation of the product (figure 1a). Independent of treatment, tocopherol concentration increased with age (p < 0.001; table 1). There was a trend for treatment to increase TAC (p = 0.077; table 1 and figure 1b), further supporting the assumption that the administration of the antioxidants had a functional effect on oxidative stress. It is worth noting that these effects were measured in samples taken 2 weeks (day 14) and 1 week (day 21) after the administration of the antioxidants. It can therefore be assumed that tocopherol concentration and TAC were elevated considerably more over the whole period between administration and sampling than on the sample days. Lipid peroxidation (MDA concentration) did not differ between treatment groups (p = 0.77; table 1 and figure 1c).
Table 1.
Tocopherol, MDA and TAC in relation to age (days) and treatment (control or vitamins; note that day 1 measurements are coded as control for all nestlings). Factors in the final model have been highlighted in bold. Significant factors (p < 0.05) have an asterisk. Random terms values are in italic. TAC, total antioxidant capacity; MDA, malondialdehyde.
| oxidative stress and treatment | ||||||
|---|---|---|---|---|---|---|
| intercept | estimate (s.e.) | F (d.f.) | p-value | |||
| tocopherol | fixed terms | age | 2.65 | 1.4 (0.15) | 91.91 (79.71) | <0.001* |
| treatment (vitamins) | 2.65 | 6.32 (2.58) | 6.01 (53.47) | 0.018* | ||
| rejected terms | age : treatment | 1.25 | −0.4 (0.42) | 0.91 (91.5) | 0.342 | |
| random terms (variance) |
chick/nest nest residual |
14.71 | ||||
| 64.12 | ||||||
| 101 | ||||||
| MDA | fixed terms | age | 330 | −0.03 (0.78) | 0.01 (74.8) | 0.968 |
| treatment (vitamins) | 330 | 4.11 (14.02) | 0.09 (38.93) | 0.771 | ||
| rejected terms | age : treatment | 342 | 2.54 (2.26) | 1.27 (76.97) | 0.263 | |
| random terms (variance) |
chick/nest nest residual |
209 | ||||
| 5622 | ||||||
| 2775 | ||||||
| TAC | fixed terms | age | −0.01 | 0.01 (0.01) | 0.19 (100) | 0.665 |
| treatment (vitamins) | −0.01 | 0.04 (0.02) | 3.2 (100) | 0.077 | ||
| rejected terms | age : treatment | 0.03 | 0.01 (0.01) | 3.7 (99) | 0.057 | |
| random terms (variance) |
chick/nest
nest residual |
0 | ||||
| 0 | ||||||
| 0.01 | ||||||
Figure 1.

Effects of antioxidant supplementation on oxidative stress markers one to two weeks later. (a) Concentration of tocopherol in plasma, (b) TAC (total antioxidant capacity) of plasma, FRAP corrected by uric acid concentration and (c) MDA (malondialdehyde) concentration in erythrocytes. Box plots show the median, upper quartiles, maximum and minimum values and outliers. Data shown are the values of each variable of the days 1, 14 and 21, but coding day 1 all as control group. Data shown are the values of each variable depending of the treatment group and the day of measurement (day 1, 14 and 21, respectively). Asterisk indicates differences between groups with p < 0.05. Hash indicates differences between groups with p < 0.1. (Online version in colour.)
Treatment had no significant effect on the mass or size of the individuals in any comparison (electronic supplementary material, table S2).
(b). Telomere dynamics
Average (± s.d.) TL was 8700 ± 688 bp in 32-day-old chicks (i.e. the average age over all measurement), and telomere attrition in all individuals pooled was on average 9.8 bp day−1 (table 2, figure 2). There were no sex difference in TL (p = 0.59) or attrition (p = 0.678; table 2) while there were large and consistent differences between individuals (figure 2). Hatching order did not modulate TL (p = 0.252) or attrition (p = 0.4, table 2), and nor did weight for either TL (p = 0.814) or attrition (p = 0.395).
Table 2.
Telomere length (average of each sample) in relation to the covariates age (days), mass (g) and Hatch Order (1,2,3) and the factors sex (male and female) and treatment (control or vitamins; note that day 1 measurements are coded as control for all nestlings). Factors in the final model have been highlighted in bold. Significant factors (p < 0.05) have an asterisk. Random terms values are in italic.
| fixed terms |
random terms |
|||||
|---|---|---|---|---|---|---|
| terms | intercept | estimate (s.e.) | F (d.f.) | p-value | terms | variance |
| age | 9052 | −9.816 (1.779) | 30.43 (78.46) | <0.001 | chick/nest | 438.1 |
| nest | 316.7 | |||||
| treatment | 100.2 (46.76) | 4.592 (87.63) | 0.035* | gel | 496.1 | |
| residual | 126.6 | |||||
| rejected : hatch order | 9249 | −109.9 (94.34) | 1.356 (34.58) | 0.252 | ||
| rejected : sex (male) | 9289 | −113 (95.01) | 0.295 (43.94) | 0.59 | ||
| rejected : mass | 9285 | 0.013 (0.055) | 0.056 (78.4) | 0.814 | ||
| rejected : age : hatch order | 9188 | −1.657 (2.329) | 0.506 (77.8) | 0.479 | ||
| rejected : age : mass | 9028 | −0.002 (0.002) | 0.732 (75.65) | 0.395 | ||
| rejected : age : treatment | 9058 | −3206 (6122) | 0.274 (72.68) | 0.602 | ||
| rejected : age : sex (male) | 9057 | 1.378 (3.305) | 0.174 (71.79) | 0.678 | ||
Figure 2.
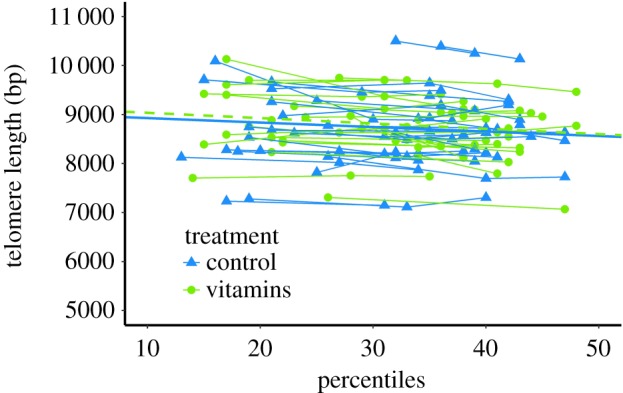
Telomere length in relation to age (days), and the best-fitting regression lines for treatment ‘vitamins’ (green dashed) and control groups (blue solid). (Online version in colour.)
Although we did not find significant differences in telomere shortening rates between percentiles (electronic supplementary material, table S3) we decided to analyse the data for every 10th percentile separately, following [23]. This allowed us not only to compare between- and within-individual differences in telomere attrition, but also to evaluate if the relation of all our variables of study with telomeres was the same regardless of the length of the telomeres. This decision is based on previous studies in which attrition was shown to differ between percentiles [22,23].
We found that attrition was faster in the longer telomeres within individuals, increasing from 6.20 bp day−1 at the 10th percentile to 10.44 at the 90th percentile (a 68% increase), but this increase did not reach statistical significance (electronic supplementary material, table S1 and S3; figure 3a).
Figure 3.
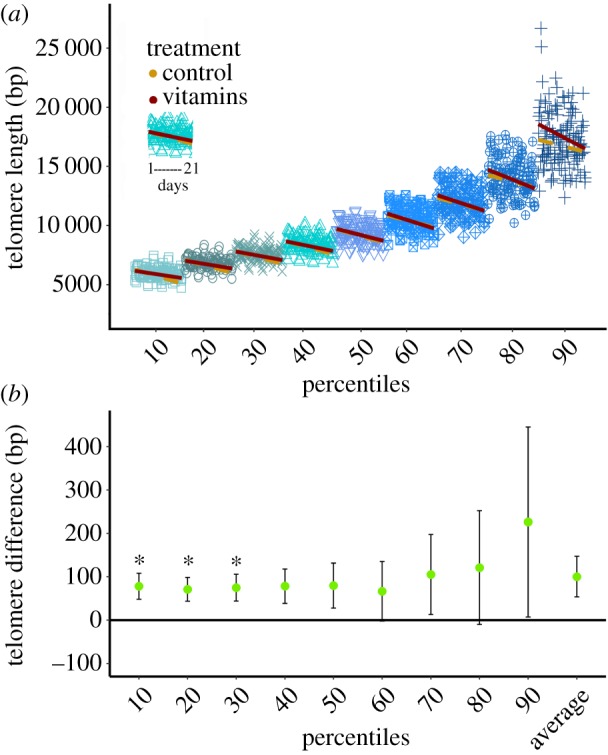
(a) Telomere shortening with age for the 10th to 90th percentile. x-axis represents the age of the individuals, repeating the values for each percentile. Regression lines of telomere length by age are yellow dashed for the control group and red solid for the vitamins group. (b) Estimate ± s.e. of telomere length in the vitamins group relative to the control group. Asterisk indicates differences between groups with p < 0.05. (Online version in colour.)
Treatment had a positive effect on TL (p = 0.035), with supplemented birds having 100 bp longer average TL than the control group (table 2 and figure 3b). When testing for treatment effects at the different percentiles separately, we found there to be a significant effect on the 10th, 20th and 30th percentile (p < 0.05), and a trend in the 40th percentile (p = 0.052; electronic supplementary material, table S1). Over the 50th to 90th percentile, the treatment effect slightly increased to approximately the same extent as at the lower percentiles, but due to the increase in variance the effects were not significant (electronic supplementary material, table S1).
There were no significant correlations between TL and any of the oxidative stress variables (electronic supplementary material, table S4) nor with mass (electronic supplementary material, table S5).
4. Discussion
Telomere attrition was 9.8 bp day−1 (table 2, figure 2), close to values obtained in other longitudinal studies of chicks using the same technique (common terns Sterna hirundo: 7.5 bp d−1 [37]; jackdaws Corvus monedula: 10.5 bp d−1 [9,22]). The rate of telomere loss appears to be higher at the higher percentiles (electronic supplementary material, table S1; figure 3a), also in accordance with findings in jackdaws [22] and common terns [38]. Longer telomeres are larger targets for damage, and a larger number of base pairs will on average be lost following a double-strand break, which may explain this pattern [39]. Previous studies found TL and their rate of loss to be affected by hatching order [40,41], sex [42] or mass gain [43], but these variables did not noticeably affect TL and/or its attrition in our study (table 2; electronic supplementary material, table S5).
Our knowledge of the accelerating effect of oxidative stress on telomere attrition is largely based on in vitro studies, raising the question whether oxidative stress also modulates telomere attrition in vivo. In our study, the treatment we administered was effective in alleviating oxidative stress, in that tocopherol (significantly) and TAC (bordering on significance) were still elevated one to two weeks after supplement administration (table 1, figure 1a,b). Our treatment also had a significant effect on TL, with this group having 100 bp longer telomeres than the control group (figure 3b). A difference of 100 bp may appear a modest effect when compared to the mean TL of 8700 bp, because it is only 1.15% of average TL on an absolute scale. However, it is a large effect when one considers that individuals experience on average 150 bp shortening during the experimental period. By percentiles, the treatment had a significant effect on shorter telomeres (from the 10th to 30th percentile, and approaching significance in the 40th (electronic supplementary material, tables S1; figure 3a). In the higher percentiles, this difference was not significant, although the ‘vitamins’ group had a longer TL at all percentiles, and this effect was larger at the longest percentiles, but so was the variance (electronic supplementary material, table S1; figure 3a).
We did not find a significant relation between any of the oxidative stress variables and TL (electronic supplementary material, table S4) although the relation between tocopherol and TL in the 10th percentile approached significance. The lack of significance for the relation between MDA and TL could be due to the fact that it is not a marker of oxidative damage specific to DNA. By contrast, 8-oxo-dG is, and its measurement could have yielded a different result. Another potential explanation of the lack of relation between oxidative stress variables and TL despite the significant effect of the treatment on the former could be that the reduction in telomere attrition is not directly related to oxidative damage prevention, but rather to changes in redox signalling. It is known that the cellular redox environment (balance between the production of reactive species of oxygen and nitrogen and their removal by antioxidants) has an active role in the control of the cell cycle, which in turn could regulate telomere dynamics [44].
Observational studies on free-living birds show both significant [45,46] and non-significant correlations between oxidative stress markers and TL or attrition [19,47]. Experimental studies in free-living animals have yielded mixed effects, with negative [20] or positive [48] results, or affecting only one sex [49], and we contribute a positive result to this small set of studies. There are different potential explanations of this variation: studies differ in methods (including the composition of the antioxidant supplements); in the technique used to measure TL, or in the species of study (which present different physiology). Ecology may be very important, because antioxidant supplementation is likely to be of benefit if there is a shortage of antioxidants, either because the diet contains few antioxidants or the need for antioxidants is very high [21,50,51]. For example, in [48], the higher shortening caused by reproduction was alleviated by the administration of tocopherol.
A limitation of antioxidant supplementation studies with the aim to alleviate oxidative stress is that the manipulation is indirect, in that the aim is to manipulate oxidative stress through the supplementation with antioxidants rather than manipulating oxidative stress directly. This limitation could, in theory, be mitigated by manipulating or measuring the molecular cause of oxidative stress directly (e.g. the production of ROS), instead of resorting to downstream oxidative stress markers of which the interpretation is not straightforward, but this is difficult in practice, in particular in an ecologically relevant setting. Nevertheless, our results agree with the hypothesis that oxidative stress affects telomere attrition not only in cell cultures, but also in vivo, observing a beneficial effect on TL of the supplementation with antioxidants. This effect could indicate a lack of antioxidants in free-living populations of white stork, which could affect their potential lifespan.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the owners and workers of Prado Herrero farm where our experiment was carried out, Pablo Camarero for help during laboratory analysis with the HPLC, Christina Bauch for help during laboratory analyses of telomeres and suggestions to improve the study, Blanca Jimeno for help with statistical analyses, and the editor and the three referees for their constructive comments that greatly improved the quality of the paper.
Ethics
Sampling was carried out under a special permit of the regional park Cuenca Alta del Manzanares and of the regional government (Comunidad de Madrid).
Data accessibility
Supporting data are accessible in the electronic supplementary material, figure S1, tables S1–S5, R code and datasheets.
Authors' contributions
J.P.-P., A.H.-D., J.I.A., U.H. and S.V. conceived the ideas and designed methodology; J.P.-P., A.H.-D. and E.M. collected samples and data; J.P.-P. and S.V. analysed the data; J.P.-P. led the writing of the manuscript with input from the entire author team.
Competing interests
We declare we have no competing interests
Funding
Diego San Mauro for his financial support for the telomere assays (Project CGL2017-85637-P financed by the Spanish Ministry of Economy and Competitiveness). Fieldwork for this study and oxidative stress assays were partly supported by project RTA2011-00111-C03-02 financed by the Spanish National Institute for Agricultural Research INIA. J.P.-P. was funded by a grant from the Complutense University of Madrid (CT45/15-CT46/15).
References
- 1.Blackburn EH. 1991. Structure and function of telomeres. Nature 350, 569–573. ( 10.1038/350569a0) [DOI] [PubMed] [Google Scholar]
- 2.Rodier F, Kim S-H, Nijjar T, Yaswen P, Campisi J. 2005. Cancer and aging: the importance of telomeres in genome maintenance. Int. J. Biochem. Cell Biol. 37, 977–990. ( 10.1016/j.biocel.2004.10.012) [DOI] [PubMed] [Google Scholar]
- 3.Boonekamp JJ, Simons MJP, Hemerik L, Verhulst S. 2013. Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell 12, 330–332. ( 10.1111/acel.12050) [DOI] [PubMed] [Google Scholar]
- 4.Dantzer B, Fletcher QE. 2015. Telomeres shorten more slowly in slow-aging wild animals than in fast-aging ones. Exp. Gerontol. 71, 38–47. ( 10.1016/j.exger.2015.08.012) [DOI] [PubMed] [Google Scholar]
- 5.Tricola GM, et al. 2018. The rate of telomere loss is related to maximum lifespan in birds. Phil. Trans. R. Soc. B 373, 20160445 ( 10.1098/rstb.2016.0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eastwood JR, Hall ML, Teunissen N, Kingma SA, Hidalgo Aranzamendi N, Fan M, Roast M, Verhulst S, Peters A. 2019. Early-life telomere length predicts lifespan and lifetime reproductive success in a wild bird. Mol. Ecol. 28, 1127–1137. ( 10.1111/mec.15002) [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Zhan Y, Pedersen NL, Fang F, Hägg S. 2018. Telomere length and all-cause mortality: a meta-analysis. Ageing Res. Rev. 48, 11–20. ( 10.1016/j.arr.2018.09.002) [DOI] [PubMed] [Google Scholar]
- 8.Wilbourn RV, Moatt JP, Froy H, Walling CA, Nussey DH, Boonekamp JJ. 2018. The relationship between telomere length and mortality risk in non-model vertebrate systems: a meta-analysis. Phil. Trans. R. Soc. B 373, 20160447 ( 10.1098/rstb.2016.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boonekamp JJ, Mulder GA, Salomons HM, Dijkstra C, Verhulst S. 2014. Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc. Biol. Sci. 281, 20133287 ( 10.1098/rspb.2013.3287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benetos A, et al. 2018. Short leukocyte telomere length precedes clinical expression of atherosclerosis: the blood-and-muscle model. Circ. Res. 122, 616–623. ( 10.1161/CIRCRESAHA.117.311751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizutani Y, Tomita N, Niizuma Y, Yoda K. 2013. Environmental perturbations influence telomere dynamics in long-lived birds in their natural habitat. Biol. Lett. 9, 20130511 ( 10.1098/rsbl.2013.0511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quirici V, Guerrero CJ, Krause JS, Wingfield JC, Vásquez RA. 2016. The relationship of telomere length to baseline corticosterone levels in nestlings of an altricial passerine bird in natural populations. Front. Zool. 13, 1 ( 10.1186/s12983-016-0133-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson H, Bolton M, Monaghan P. 2015. Variation in early-life telomere dynamics in a long-lived bird: links to environmental conditions and survival. J. Exp. Biol. 218, 668–674. ( 10.1242/jeb.104265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halliwell B. 2007. Biochemistry of oxidative stress. Biochem. Soc. Trans. 35, 1147–1150. ( 10.1042/BST0351147) [DOI] [PubMed] [Google Scholar]
- 15.von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci . 27, 339–344. ( 10.1016/S0968-0004(02)02110-2). [DOI] [PubMed] [Google Scholar]
- 16.Houben JMJ, Moonen HJJ, van Schooten FJ, Hageman GJ. 2008. Telomere length assessment: biomaker of chronic oxidative stress? Free Radic. Biol. Med. 44, 235–246. ( 10.1016/j.freeradbiomed.2007.10.001) [DOI] [PubMed] [Google Scholar]
- 17.Monaghan P, Haussmann MF. 2006. Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 21, 47–53. ( 10.1016/j.tree.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 18.Richter T, Zglinicki Tv. 2007. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp. Gerontol. 42, 1039–1042. ( 10.1016/j.exger.2007.08.005) [DOI] [PubMed] [Google Scholar]
- 19.Boonekamp JJ, Bauch C, Mulder E, Verhulst S. 2017. Does oxidative stress shorten telomeres? Biol. Lett. 13, 20170164 ( 10.1098/rsbl.2017.0164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pérez-Rodríguez L, Redondo T, Ruiz-Mata R, Camacho C, Moreno-Rueda G, Potti J. 2019. Vitamin E supplementation—but not induced oxidative stress—influences telomere dynamics during early development in wild passerines. Front. Ecol. Evol. 7, e19403 ( 10.3389/fevo.2019.00173) [DOI] [Google Scholar]
- 21.Reichert S, Stier A. 2017. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 13, 20170463 ( 10.1098/rsbl.2017.0463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MHK, Verhulst S. 2009. Telomere shortening and survival in free-living corvids. Proc. Biol. Sci. 276, 3157–3165. ( 10.1098/rspb.2009.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauch C, Becker PH, Verhulst S. 2014. Within the genome, long telomeres are more informative than short telomeres with respect to fitness components in a long-lived seabird. Mol. Ecol. 23, 300–310. ( 10.1111/mec.12602) [DOI] [PubMed] [Google Scholar]
- 24.Romero JM, Redonde T. 2016. Kind to kin: weak interference competition among white stork Ciconia Ciconia broodmates. J. Avian Biol. 47, 001–014. ( 10.1111/jav.00983) [DOI] [Google Scholar]
- 25.Cadenas E, Pacer L. 2002. Handbook of antioxidants. New York, NY: Marcel Dekker. [Google Scholar]
- 26.Traber MG, Stevens JF. 2011. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 51, 1000–1013. ( 10.1016/j.freeradbiomed.2011.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Q. 2014. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 72, 76–90. ( 10.1016/j.freeradbiomed.2014.03.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahin K, Sahin N, Yaralioglu S. 2002. Protective role of supplemental vitamin E and selenium on lipid peroxidation, vitamin E, vitamin A, and some mineral concentrations of Japanese quails reared under heat stress. BTER 85, 59–70. ( 10.1385/BTER:85:1:59) [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Estival J, Martínez-Haro M, Martín-Hernando MAP, Mateo R. 2010. Sub-chronic effects of nitrate in drinking water on red-legged partridge (Alectoris rufa). Environ. Res. 110, 469–475. ( 10.1016/j.envres.2010.03.008) [DOI] [PubMed] [Google Scholar]
- 30.Romero-Haro AA, Alonso-Alvarez C. 2014. Covariation in oxidative stress markers in the blood of nestling and adult birds. Physiol. Biochem. Zool. 87, 353–362. ( 10.1086/674432) [DOI] [PubMed] [Google Scholar]
- 31.Benzie IF, Strain JJ. 1996. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’. Anal. Biochem. 239, 70–76. ( 10.1006/abio.1996.0292) [DOI] [PubMed] [Google Scholar]
- 32.Herrera-Dueñas A, Pineda-Pampliega J, Antonio-García MT, Aguirre JI. 2017. The influence of urban environments on oxidative stress balance. Front. Ecol. Evol. 5, 363 ( 10.3389/fevo.2017.00106) [DOI] [Google Scholar]
- 33.Costantini D. 2011. On the measurement of circulating antioxidant capacity and the nightmare of uric acid. Methods Ecol. Evol. 2, 321–325. ( 10.1111/j.2041-210X.2010.00080.x) [DOI] [Google Scholar]
- 34.Core Team R. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 35.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1– 48 ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 36.Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. J. Stat. Soft. 82, 1–26. ( 10.18637/jss.v082.i13) [DOI] [Google Scholar]
- 37.Vedder O, Verhulst S, Bauch C, Bouwhuis S. 2017. Telomere attrition and growth: a life-history framework and case study in common terns. J. Evol. Biol. 30, 1409–1419. ( 10.1111/jeb.13119) [DOI] [PubMed] [Google Scholar]
- 38.Bauch C, Becker PH, Verhulst S. 2013. Telomere length reflects phenotypic quality and costs of reproduction in a long-lived seabird. Proc. R. Soc. B 280, 20122540 ( 10.1098/rspb.2012.2540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grasman J, Salomons HM, Verhulst S. 2011. Stochastic modeling of length-dependent telomere shortening in Corvus monedula. J. Theor. Biol. 282, 1–6. ( 10.1016/j.jtbi.2011.04.026) [DOI] [PubMed] [Google Scholar]
- 40.Noguera JC, Metcalfe NB, Reichert S, Monaghan P. 2016. Embryonic and postnatal telomere length decrease with ovulation order within clutches. Sci. Rep. 6, 25915 ( 10.1038/srep25915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stier A, Massemin S, Zahn S, Tissier ML, Criscuolo F. 2015. Starting with a handicap. Effects of asynchronous hatching on growth rate, oxidative stress and telomere dynamics in free-living great tits. Oecologia 179, 999–1010. ( 10.1007/s00442-015-3429-9) [DOI] [PubMed] [Google Scholar]
- 42.Barrett ELB, Richardson DS. 2011. Sex differences in telomeres and lifespan. Aging Cell 10, 913–921. ( 10.1111/j.1474-9726.2011.00741.x) [DOI] [PubMed] [Google Scholar]
- 43.Monaghan P, Ozanne SE. 2018. Somatic growth and telomere dynamics in vertebrates: realtionships, mechanisms and consequences. Phil. Trans. R. Soc. B 373, 20160446 ( 10.1098/rstb.2016.0446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarsour EH, Kumar MG, Chaudhuri LC, Kalen AL, Goswami PC. 2009. Redox control of the cell cycle in health and disease. Antioxid. Redox Signal 11, 2985–3011. ( 10.1089/ars.2009.2513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geiger S, Le Vaillant M, Lebard T, Reichert S, Stier A, Le Maho Y, Criscuolo F. 2012. Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol. Ecol. 21, 1500–1510. ( 10.1111/j.1365-294X.2011.05331.x) [DOI] [PubMed] [Google Scholar]
- 46.Taff CC, Freeman-Gallant CR. 2017. Sexual signals reflect telomere dynamics in a wild bird. Ecol. Evol. 7, 3436–3442. ( 10.1002/ece3.2948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stier A, Reichert S, Criscuolo F, Bize P. 2015. Red blood cells open promising avenues for longitudinal studies of ageing in laboratory, non-model and wild animals. Exp. Gerontol. 71, 118–134. ( 10.1016/j.exger.2015.09.001) [DOI] [PubMed] [Google Scholar]
- 48.Badás EP, Martínez J, Rivero de Aguilar Cachafeiro J, Miranda F, Figuerola J, Merino S. 2015. Ageing and reproduction: antioxidant supplementation alleviates telomere loss in wild birds. J. Evol. Biol. 28, 896–905 . ( 10.1111/jeb.12615) [DOI] [PubMed] [Google Scholar]
- 49.Noguera JC, Metcalfe NB, Boner W, Monaghan P. 2015. Sex-dependent effects of nutrition on telomere dynamics in zebra finches (Taeniopygia guttata). Biol. Lett. 11, 20140938 ( 10.1098/rsbl.2014.0938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beaulieu M, Schaefer HM. 2013. Rethinking the role of dietary antioxidants through the lens of self-medication. Anim. Behav. 86, 17–24. ( 10.1016/j.anbehav.2013.05.022) [DOI] [Google Scholar]
- 51.Simons MJP, Briga M, Leenknegt B, Verhulst S. 2014. Context-dependent effects of carotenoid supplementation on reproduction in zebra finches. Behav. Ecol. 25, 945–950. ( 10.1093/beheco/aru062) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting data are accessible in the electronic supplementary material, figure S1, tables S1–S5, R code and datasheets.


