Abstract
The direct interactions between people and nature are critically important in many ways, with growing attention particularly on their impacts on human health and wellbeing (both positive and negative), on people's attitudes and behaviour towards nature, and on the benefits and hazards to wildlife. A growing evidence base is accelerating the understanding of different forms that these direct human–nature interactions take, novel analyses are revealing the importance of the opportunity and orientation of individual people as key drivers of these interactions, and methodological developments are increasingly making apparent their spatial, temporal and socio-economic dynamics. Here, we provide a roadmap of these advances and identify key, often interdisciplinary, research challenges that remain to be met. We identified several key challenges, including the need to characterize individual people's nature interactions through their life course, to determine in a comparable fashion how these interactions vary across much more diverse geographical, cultural and socio-economic contexts that have been explored to date, and to quantify how the relative contributions of people's opportunity and orientation vary in shaping their nature interactions. A robust research effort, guided by a focus on such unanswered questions, has the potential to yield high-impact insights into the fundamental nature of human–nature interactions and contribute to developing strategies for their appropriate management.
Keywords: extinction of experience, global change, human–nature interactions, personalized ecology, species distribution, urbanization
1. Introduction
The direct interactions between individual people and nature (hereafter human–nature interactions) have attracted growing interest. The principal reasons are fourfold. First, and foremost, there is increasing evidence that direct interactions with nature can provide people with a range of health and wellbeing benefits [1–3], and can play an important role in addressing some chronic health conditions and reducing the need for pharmaceutical interventions [4,5]. Second, there is societal fear and awareness of the negative consequences of some human–nature interactions, such as attacks on people by wild large carnivores [6–8], poisoning by venomous animals [9], wildlife–vehicle collisions [10] and the risk of vector-borne zoonoses [11]. Third, there is evidence of a progressive decline in positive human–nature interactions, the so-called ‘extinction of experience’, with potentially serious consequences for human health, childhood development and support for biodiversity conservation [12–15]. Lastly, recent technological advances in tracking and sensing devices, as well as in environmental monitoring, have greatly improved the ability to describe and quantify an individual person's interactions with nature [16–19]. This has opened the potential for a new era in this field (cf. ‘personalized ecology’ [20]), using approaches that enable studies at finer spatial and temporal resolutions and over larger areas and longer periods.
The body of research on human–nature interactions is dispersed across a wide range of research disciplines, which has made consolidation of ideas and application of findings to practise significant challenges. Further, little effort has so far been made to synthesize and generate a coherent overview of knowledge in this area. Here, we provide a roadmap of advances in understanding the ecology of human–nature interactions. We present a definition and typology of these interactions, and then a conceptual framework that aids understanding of their drivers and patterns in their spatial, temporal and socio-economic dynamics. Throughout, we highlight key knowledge gaps and recommendations for further research.
2. Definition
We consider nature to encompass individual living organisms through to ecosystems, excluding organisms that are not self-sustained. This exclusion criterion enables a focus on essentially wild organisms; including those organisms that are not self-sustaining results in a nature interaction essentially becoming one with any non-human form of life (including, for example, cultivated crops, potted houseplants, livestock and domestic pets) as it becomes hard to draw any other line. We acknowledge that this definition of nature is one of many different possibilities, and providing a precise and uniformly accepted approach is still challenging (see [1,3] for further discussion). Under this definition a given human–nature interaction occurs when a person is present in the same physical space as nature or directly perceives a stimulus from nature. This might be the individual organisms they encounter or the ecosystems that they experience, and might include visiting an urban green space or a national park, viewing trees through a window, smelling scent of wildflowers, listening to bird song or being bitten by a mosquito. In principle, the definition could include interactions with organisms that live on or in people, although we will not discuss these further as they are generally considered a rather different and distinct kind of interaction. Since our definition covers only direct sensory interactions with nature, we exclude ‘interactions’ through the media (e.g. through books, television, websites). Of course, for some groups of people, such ‘virtual’ or ‘vicarious’ interactions are important and can have positive impacts (e.g. [21,22]).
3. Typology
The many forms of human–nature interactions can be classified along five key dimensions: immediateness, consciousness, intentionality, degree of human mediation and direction of outcome (figure 1).
Figure 1.
Examples of human–nature interactions across five dimensions to their typology. Examples include (a) visiting an urban park, (b) viewing trees through a window, (c) viewing wild birds, (d) encountering vegetation while cycling, (e) participating in ecotourism activities, (f) being bitten by a mosquito, (g) feeding wild birds, (h) walking in a remote protected forest, (i) hiking in a protected area, (j) being attacked by a monkey, (k) feeding squirrels and (l) hitting a fox while driving a car. All photos are from Pixabay (https://pixabay.com/ja/). (Online version in colour.)
(a). Immediateness
Immediateness is the degree of physical proximity between a person and nature (figure 1a,b). More immediate interactions occur when a person is physically present in nature (e.g. walking in a forest) [5,19] or physically interacting with it (e.g. touching wildflowers, being stung by a wasp [23]). Less immediate interactions, on the other hand, do not require a person to be physically present, and might include such activities as having a view of roadside trees through a window or listening to outdoor bird song from inside a room (these have previously been termed ‘indirect’ interactions [24]). Although less immediate interactions probably greatly outnumber the more immediate ones [24], little is known about how this balance varies between people.
(b). Consciousness
Consciousness is the extent to which a person is aware that an interaction with nature is occurring (figure 1c,d). More conscious interactions might include actively observing wildlife or being attacked by larger animals (e.g. bears, sharks) [6–8]. Less conscious (or perhaps subconscious) interactions might include passively observing vegetation, hearing sounds of insects or smelling the scents of natural elements (e.g. flowers) while engaged in other activities (e.g. travelling, working), or being fed on by a leech. While the majority of research to date has focused on understanding the dynamics of more conscious human–nature interactions, it is likely that less conscious ones occur for much of the time that people are outdoors or have a view of the outdoors. Robust ways of quantifying the types and frequencies of interactions of different levels of consciousness are required. A key challenge is to do so in a way that avoids drawing people's attention to less conscious interactions and thus rendering these as conscious.
(c). Intentionality
Intentionality is the extent to which a person deliberately engages in a nature interaction (figure 1e,f). More intentional interactions might include visiting an urban green space or a national park [25,26], or feeding birds in a domestic garden [27,28]. At the other extreme, unintentional nature interactions occur as byproducts of other activities, such as encountering vegetation while cycling to work or accidentally hitting a bird while driving a car. The degree of intentionality can be difficult to determine. For example, it is a challenge objectively to assess a person's intention to incorporate human–nature interactions when they choose their route to a destination (which may also be influenced by knowledge of the route, its practicality, etc.).
(d). Degree of human mediation
Human–nature interactions can occur in places or through processes that have been more or less modified by anthropogenic activities (figure 1g,h). Less human-mediated interactions might include observing wildlife from a distance on a remote, pristine and rarely visited island [19]. More human-mediated interactions, on the other hand, would occur where anthropogenic influences are marked, such as when observing birds at a feeding station in a city garden [27,28] or being attacked by a bear that is habituated to humans in a popular site for ecotourism [8]. The degree of human mediation of human–nature interactions can have important consequences for the form of those interactions and the ease with which they can be achieved (e.g. how artificial the environments are in which they occur, overt evidence that other people have had such interactions, the degree of habituation of animals to people) [29,30].
(e). Direction of outcomes
The direction of outcomes of human–nature interactions can be considered from the perspectives of humans and of nature (figure 1i–l). From the human perspective, positive interactions occur when a person obtains beneficial outcomes (e.g. health and wellbeing benefits), such as when viewing roadside flowers through a window or visiting a green space [1–3]. We do not generally consider eating plants and animals as positive human–nature interactions because for most people the associated beneficial outcomes are normally derived from dead organisms which lie beyond our definition of nature. Negative interactions occur when these result in physical or mental injury, such as being attacked by wildlife [7,8] or when encountering a type of organism about which one has a psychological phobia (e.g. apiphobia, arachnophobia, ophidiophobia). The direction of outcomes may be heavily moderated by personal characteristics. For example, more nature-oriented people might feel relaxed while visiting a forest (i.e. a positive interaction), whereas those with less nature orientation may feel discomfort or anxiety (i.e. a negative interaction).
From the perspective of nature, positive interactions occur when it derives beneficial effects from interacting with humans (these positive outcomes may or may not be desirable from a management or conservation perspective). This could, for example, be in the form of resources (e.g. feeding wild birds in a domestic garden; [27,28,31]) or protection from potential predators (e.g. reduced risk of nest predation due to human recreational use of urban parks [32]; the so-called ‘human shield effect’ [30]). Negative interactions might occur when wildlife suffers greater disturbance or greater mortality risk, such as when vehicles collide with deer [33] or when hikers trample rare flowering plants [34]. These benefits and costs can be evaluated at multiple levels, with, for example, those from feeding garden birds having been considered in terms of impacts on resource gain by individuals, on their disease risk, breeding success or mortality and on population sizes and species richness [31,35–38]. There is often a substantial mismatch between the direction of outcomes for humans and for nature. For example, while recreational activities (e.g. camping, hiking and mountain biking) in protected areas provide visitors with multiple health and wellbeing benefits [26,39], they often exert serious negative impacts on local biodiversity [34]. This can cause significant challenges for conservation bodies as to how best to maximize the positive outcomes for humans while minimizing the negative consequences for nature.
(f). Inter-relations
These five dimensions of human–nature interactions are interrelated in many ways. Intentional human–nature interactions, for example, are more likely to be conscious and positive. Likewise, more immediate human–nature interactions are more likely to be conscious, as these can deliver more intense, multi-sensory experiences for a person [40]. Critically, although the majority of studies to date have focused on a single type of human–nature interaction, most do not occur in isolation, but rather simultaneously with others. Visiting urban green space, for example, can lead to several differing human–nature interactions, such as viewing wildflowers, listening to bird song, being hassled by geese for food and actively feeding squirrels. Determining how such mixtures influence the outcomes of human–nature interactions is a key research frontier.
4. Dynamics
(a). Spatial dynamics
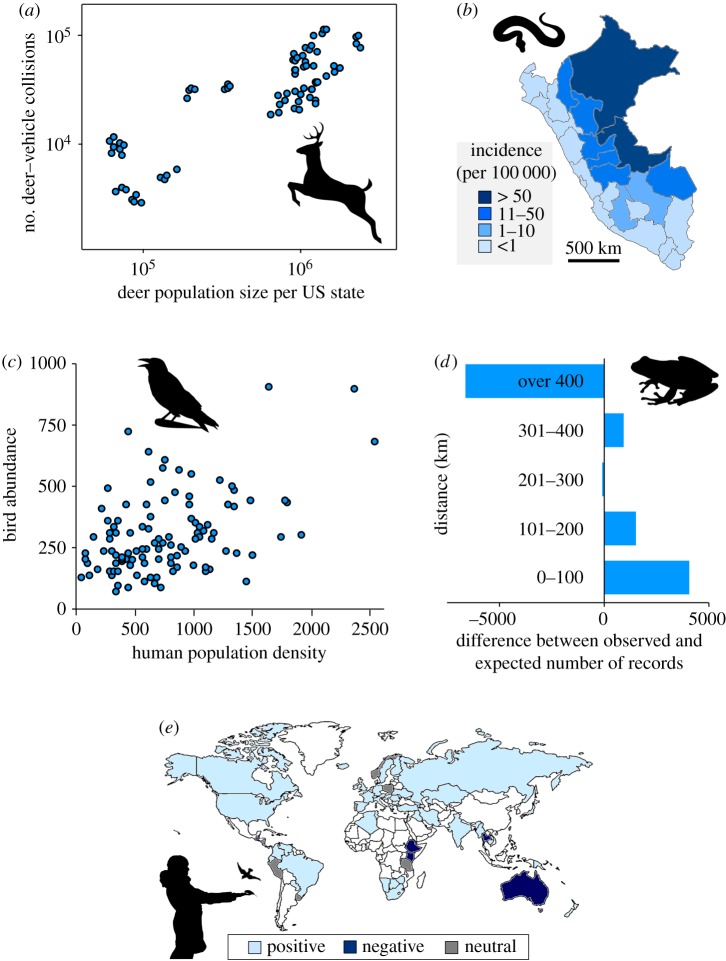
The occurrence and levels of human–nature interactions vary at multiple spatial scales ranging from local to regional, and national to international (figures 2 and 3). This is driven by three major factors, the balance of which is poorly understood (figure 2). First, spatial variation in human–nature interactions reflects differences in the opportunities that people have to experience nature. These opportunities are shaped heavily both by the availability and ease of access to more natural environments, including urban green space, countryside and protected areas, and through international ecotourism [25]. They are also shaped both by the occurrence and abundance of nature within those environments, which are dependent on such classical ecological factors as the size, shape, connectivity and abiotic conditions of habitats [17,41]. These factors influence both positive and negative human–nature interactions (figure 3a–c) [9,33,41]. In North America, for example, the annual number of deer–vehicle collisions varies approximately fourfold between states and is strongly correlated with the size of deer populations [33].
Figure 2.
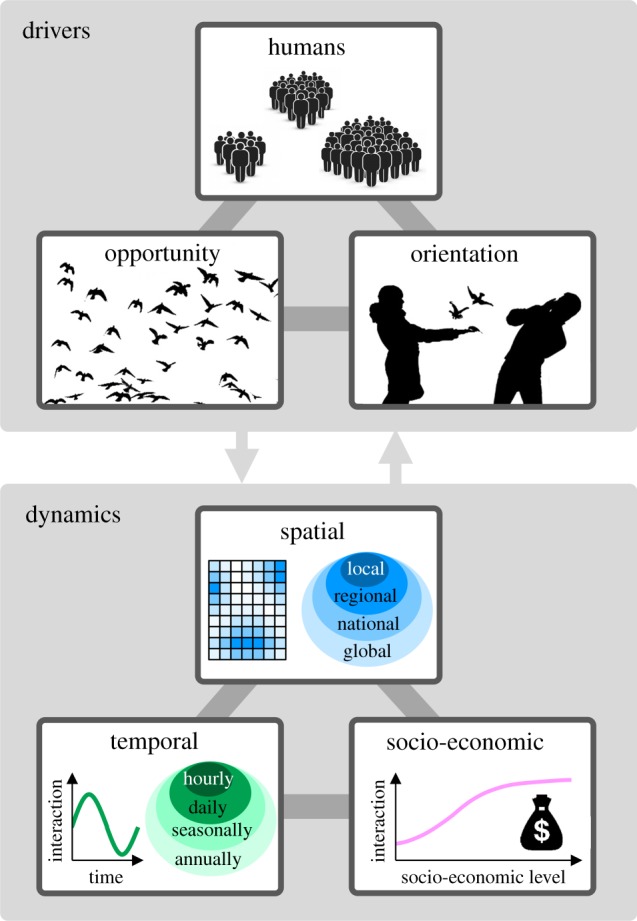
Conceptual framework to illustrate the drivers and the dynamics of human–nature interactions. The three main drivers that shape the dynamics of human–nature interactions are (i) humans (i.e. distribution and behaviour of people), (ii) opportunity to interact with nature (e.g. distribution of natural environments and wildlife, phenological patterns of plants and activity patterns of animals) and (iii) orientation towards engaging with nature. Note that the three drivers of human–nature interactions here are not likely to shape the main forms of dynamics—spatial, temporal and socio-economic—of human–nature interactions independently, but rather are closely interrelated to each other. Likewise, the three types of dynamics are also likely to be interrelated, as changes in the spatial, temporal and socio-economic patterns of these interactions often occur simultaneously or consecutively (see the main text). (Online version in colour.)
Figure 3.
Examples of spatial dynamics of human–nature interactions. (a) Annual number of deer–vehicle collisions as a function of deer population size in each US state (note that: each state has four data points) within the historical range of the eastern cougar, 2009–2012 [33]. (b) Geographical distribution of incidence of snake bites in Peru 2000–2015 [9]. (c) Relationship between human population density and abundance of sets of bird species that commonly display behaviours that are negative for human wellbeing (e.g. crow, magpie) in southern England [41]. (d) Differences between the observed and expected number of records of frog species obtained by the South African Frog Atlas Project within distance bands from cities [42]. The record data were collected through various methods and sources, such as herpetological volunteers' field survey and museum and personal databases (i.e. historical records), which covers approximately 100 years of frog sampling. (e) Distribution of countries that have policies with positive, negative or neutral views of wild bird feeding [43]. Light blue shading indicates countries where the modal policy position represents a positive view, dark blue shading represents countries where the modal policy position was negative and grey shading represents countries where the modal policy position was neutral concerning wild bird feeding activity (see [43] for more details). Countries shaded white have no information available. (Online version in colour.)
Second, spatial variation in human–nature interactions is influenced by the distribution of humans themselves (figure 2). Given the high degree of urbanization of the human population, for most people their human–nature interactions occur in cities and towns. This is despite much of the world's urban area typically having lower densities of wildlife compared with natural areas (figure 3d) [42]. For example, the distribution of species observations collected in citizen science projects (one form of intentional human–nature interaction) is generally spatially biased towards areas near major transport routes and urban areas [42], which is sometimes quite different from their actual overall distributions [44]. Along with urban areas, popular sites for ecotourism are also hotspots of human–nature interactions [19]. The high levels of human–nature interactions in intensely used areas often alter the behaviour of animals. For example, it is known that individuals of many wild animal species that have benign interactions with humans undergo habituation-like processes leading to some degree of human tolerance [30,45]. This, in turn, is likely to facilitate more frequent and intense interactions, suggesting the existence of a positive feedback loop between people and nature. Of course, species that have, or are perceived to have, less benign interactions with people (e.g. large carnivores) can also undergo such habituation, which can result in negative feedback loops when this leads to these individuals then being killed or moved elsewhere.
Third, spatial variation in human–nature interactions, particularly at larger scales, is also determined by cultural, religious and socio-economic differences in people's orientation towards engaging with nature (figure 2). These can vary from very positive to very negative attitudes towards such engagement and result in behaviour that can maximize the occurrence of human–nature interactions in environments when the opportunities are limited or minimize it in environments when the opportunities are extensive (figure 3e) [43]. For example, the intentional feeding of wild birds in domestic gardens is an extremely popular activity in some Western countries, such as the UK and the US [27,28]. However, this type of nature experience is widely discouraged in Australia, and virtually unknown in much of Africa and Asia [43].
Of course, the distributions of humans, opportunity and orientation do not shape the spatial dynamics of human–nature interactions independently, but rather are interrelated in multiple ways (figure 2). For example, highly populated areas generally provide fewer opportunities due to limited space for the maintenance of nature [46], suggesting the presence of a trade-off between the distributions of humans and opportunity. More importantly, however, these two factors often have a synergistic effect on the development of human–nature interactions. Two obvious examples are that areas with (i) greater human population density often attract large numbers of wild animals that are adapted to human-dominated environments (figure 3c) [41] and (ii) greater opportunities (e.g. popular ecotourism sites) can attract large numbers of people [19]. The spatial distributions of opportunity and orientation are also likely to be connected to each other in a bidirectional manner; more nature-orientated people are more likely to reside in areas with greater opportunities (e.g. greener neighbourhoods) and living in such areas may increase people's nature orientation [47]. Understanding the dynamics of the relationships among the drivers of human–nature interactions is a key challenge that has received relatively little attention.
Undoubtedly, spatial patterns of human–nature interactions are not static but change over time. Global warming is one of the major forces driving such change, as it is altering the distribution of nature [48,49], the distribution of people [50] and the orientation of people to interact with nature [51]. In Mozambique, for example, it is estimated that climate change will alter the spatial patterns of snake bite risk due to changes in the distribution of venomous snake species [52]. Such knowledge allows policy makers to develop risk management plans to minimize the consequences of negative human–nature interactions.
(b). Temporal dynamics
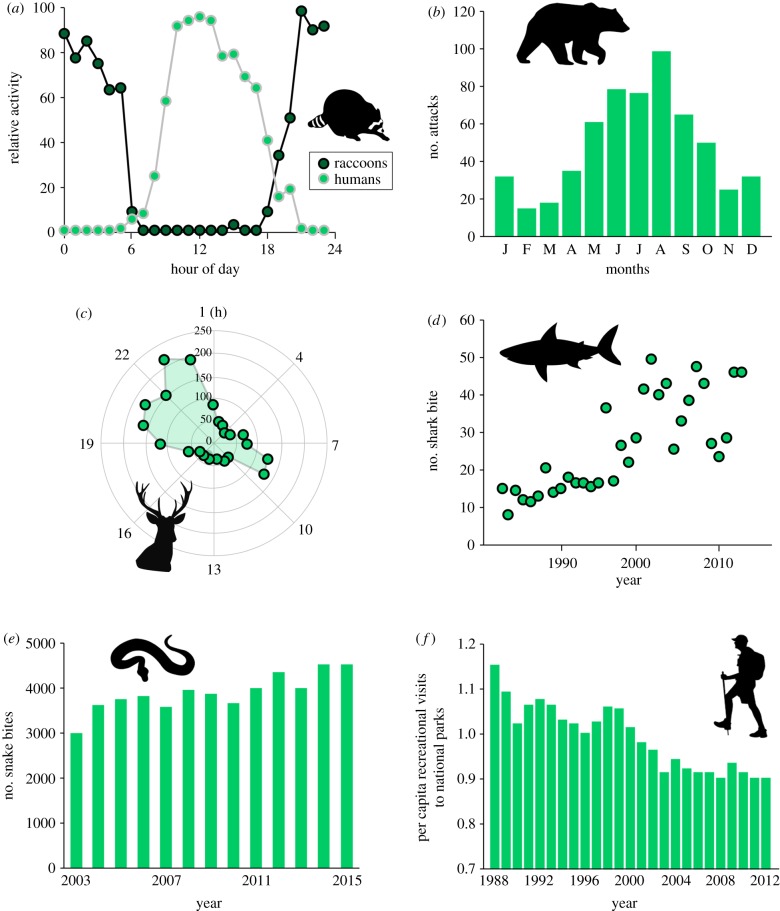
The occurrence, frequency and composition of human–nature interactions vary on multiple temporal scales, from hourly to daily, seasonally and annually (figures 2 and 4). This arises as a result of there being temporal variations in human distribution and behaviour (e.g. sleep-wake cycles, weekday/weekend variations in diurnal behaviour, seasonal differences in time spent outdoors), people's orientation towards nature (i.e. changes in individuals' motivation to engage with nature) and natural processes (e.g. occurrence and activity patterns of wildlife) (figure 2). Examples corresponding to each of these three drivers include that (i) human–nature interactions tend to be with a small subset of local birds because most are active earlier in the day than are people and with a small subset of local mammals because most of these are nocturnal (figure 4a) [53]; (ii) large carnivore attacks on people in North America and Europe occur predominantly from late spring to early autumn, because many people participate in outdoor recreational activities during this period (figure 4b) [7]; and (iii) the incidence of wildlife–vehicle collision accidents in developed countries tends to be high at dusk and during the night, as a result of increased activity of nocturnal mammals (figure 4c) [54]. Clearly, the temporal dynamics of human–nature interactions are governed by the complex interplay of these three drivers, and their relative contributions probably depend on the type of interactions.
Figure 4.
Examples of temporal dynamics of human–nature interactions. (a) Diel activity patterns of raccoons compared with humans and dogs in the Diamond Fork Area in central Utah, the US (data were collected from January to June 2015) [53]. (b) Monthly pattern in number of large carnivore attacks on humans in developed countries [7]. (c) Hourly pattern in number of wildlife–vehicle collisions in southern Belgium [54]. (d) Yearly pattern in number of shark bites in South Africa [6]. (e) Yearly pattern in number of snake bites in Mexico [9]. (f) Yearly pattern in per capita recreational visits to US national parks [13]. (Online version in colour.)
There is currently an emerging growth trend in some kinds of negative human–nature interactions, such as snake bites, shark bites and attacks by large carnivores (figure 4d,e) [6–8,55,56]. For example, the number of attacks by brown bears is on the rise in many areas around the world, increasing approximately fourfold over the last 16 years [8]. Likewise, in South Africa, the incidence rate of shark bite has increased more than fourfold over the past 30 years [6]. Although it is difficult to pinpoint exactly what has led to these changes, possible drivers include increases in human populations, increases in numbers of ecotourists, reductions in available natural undisturbed habitat due to urban and agricultural developments, increases in ecotourism to previously remote and undisturbed locations, growing familiarity of wild animals with people and inappropriate behaviour of people towards them [6–8,29,55,56]. Given the extent and severity of negative human–nature interactions worldwide, identification of the key environmental and social factors (e.g. changes in wildlife populations, loss of people's ecological knowledge) underlying their occurrence is urgently needed.
Over much longer periods one of the major trends in human–nature interactions is the progressive loss of positive interactions, the so-called ‘extinction of experience’ [12,13,57]. Regular interactions between people (especially children) and nature have been in persistent decline, particularly in developed countries (figure 4f) [13,58]. Two major factors drive this trajectory [13]. The first is the loss of opportunity to interact with nature, which is a consequence both of the rapid urbanization of human populations and of the loss of biodiversity (particularly common species) [58]. The second factor is the loss of orientation of people towards engaging with nature, which is likely to be associated with the rise in alternative leisure time activities (e.g. social media, television, internet) and increased parental concerns for safety if children play outdoors [59–61]. Although debate so far has emphasized the importance of opportunity in the extinction of experience, the influence of orientation on people's use of nature can be comparable and sometimes stronger [59,60]. Understanding the role of opportunity and orientation, and their interactions, in shaping human–nature interactions is crucial for developing policies and strategies targeted to reduce the ongoing extinction of experience and its negative consequences [25].
(c). Socio-economic dynamics
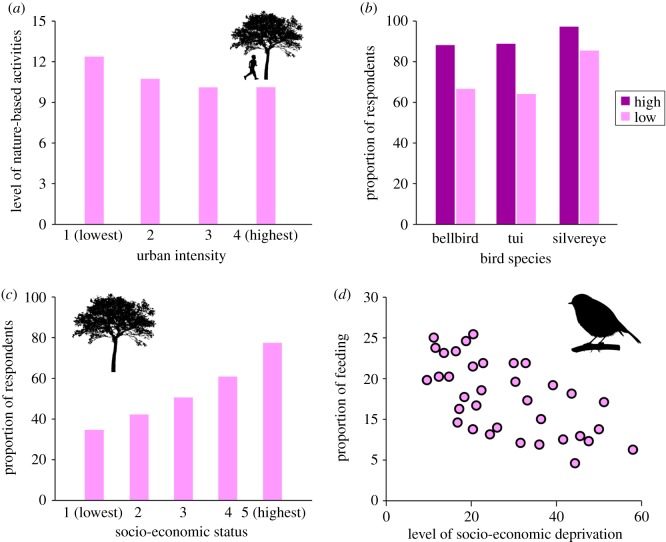
The occurrence, frequency and composition of human–nature interactions are influenced by multiple socio-economic factors, as these factors affect the three key drivers of these interactions (figures 2 and 5). Rapid economic growth coupled with urbanization and industrialization is one of the major driving forces of large-scale changes in the dynamics of human–nature interactions, as it results in (i) a decline of overall human–nature interactions, particularly positive interactions, because of a loss of opportunity (figure 5a) [58,62]; (ii) a shift towards more human-mediated nature interactions because of high levels of anthropogenic disturbance in urban ecosystems [45,65]; and (iii) a shift towards less immediate human–nature interactions because of increased levels of indoor sedentary behaviours associated with urban living [24].
Figure 5.
Examples of socio-economic dynamics of human–nature interactions. (a) Variation in level of elementary school children's immediate experiences with nature in areas of different urban intensity in China [62]. Levels of nature experiences were measured by the number of common nature-based activities (e.g. climbing trees, catching insects) in which each child participated (see [62] for more details). (b) The proportion of respondents from high and low socio-economic suburbs who fed birds and who reported seeing native birds (bellbird, tui, silvereye) in their gardens in Dunedin, New Zealand [63]. (c) Proportion of residents with trees that provide shade in the open spaces around their house across five socio-economic classes in Melbourne, Australia [64]. (d) Relationship between levels of socio-economic deprivation and the proportion of households providing food for birds in their domestic gardens in Sheffield, UK [35]. (Online version in colour.)
At least in more urbanized societies, socio-economically advantaged groups of people often have a higher frequency of positive interactions with nature, such as watching birds in a domestic garden and viewing street trees through a window (figure 5b) [63,64]. This is driven by three different, albeit interrelated, factors. First, at a larger scale, wealthier cities often have greater coverage of public green space because of their financial capacity for its retention and maintenance [46]. Second, at a more local scale, individuals from more socio-economically advantaged backgrounds tend to live in greener neighbourhoods with houses sited nearer to parks, woodland and trees (figure 5c) [64,66–68]. Third, wealthier people are more likely to use their personal resources to increase habitat for wildlife on their private land (e.g. wildlife gardening, food provision for birds; (figure 5d) [35,63,69], making their neighbourhoods richer in biodiversity (the so-called ‘luxury effect’ [69,70]).
As well as receiving fewer positive human–nature interactions in economically developed countries, in less developed countries individuals from disadvantaged backgrounds often experience more negative interactions, such as snake bites, scorpion stings and attacks by large carnivores [55,71]. At least in some developing countries, poor people tend to live in regions where there are more wildlife species that can cause them harm [55]. They are also more likely to undertake activities that place them at high risk of experiencing negative human–nature interactions. In the Americas, for example, snake bites occur predominantly in rural areas during agricultural activities, especially in developing countries where farming is an important economic activity [9]. Likewise, in Mozambique there is a high incidence of crocodile attacks in rivers across the country, which is associated with the lifestyle patterns of local communities, such as collecting water for drinking and catching fish [71].
(d). Inter-relations
The three key types of dynamics of human–nature interactions are interrelated (figure 2), as changes in the spatial, temporal and socio-economic patterns of these interactions often occur simultaneously or consecutively. Urbanization, for example, alters all three dynamics simultaneously, as it leads to changes in both the spatial (e.g. increased human-mediated interactions in populated areas [65]) and temporal patterns of human–nature interactions (e.g. long-term decline in positive interactions [58]), as well as the socio-economic inequalities in the degree to which people interact with nature (e.g. increased positive interactions among wealthier households [70]). While the majority of studies to date have focused on a single type of dynamics of human–nature interactions, more research effort should be devoted to exploring how the three types are related to, and dependent on, each other.
5. Conclusion
There is widespread recognition of the importance of direct interactions between people and nature, particularly for human health and wellbeing, but also for the future of biodiversity because of the impacts on people's attitudes and behaviour towards nature [13,20]. Somewhat ironically, this recognition has grown in a period when for much of the global human population such interactions overall are increasingly scarce [13]. Arguably, however, alongside methodological advances, this has also made it somewhat easier to characterize and study direct human–nature interactions. The major challenge for science and policy is to find ways that will maximize the positive outcomes of direct human interactions with nature while minimizing their negative consequences, both for humans and nature [20]. While some of the basic principles have been established, this requires a much improved understanding of the patterns, drivers and dynamics of human–nature interactions. This includes the need to characterize individual people's nature interactions through their life course, to determine in a comparable fashion how these nature interactions vary across much more diverse geographical, cultural and socio-economic contexts that have been explored to date, and to quantify how the relative contributions of people's opportunity and orientation vary in shaping their interactions. This constitutes an agenda that epitomizes the demand for research that crosses historically poorly connected disciplines.
Supplementary Material
Acknowledgements
We are grateful to D. T. C. Cox, S. Gaston, M. J. Evans and three anonymous reviewers for their valuable feedback on an earlier version of this paper
Data accessibility
This article has no additional data.
Authors' contributions
Both authors conceived the work, drafted the manuscript, edited and approved the final version.
Competing interests
The authors declare no competing interests.
Funding
We received no funding for this study.
References
- 1.Bratman GN, Hamilton JP, Daily GC. 2012. The impacts of nature experience on human cognitive function and mental health. Ann. NY Acad. Sci. 1249, 118–136. ( 10.1111/j.1749-6632.2011.06400.x) [DOI] [PubMed] [Google Scholar]
- 2.Keniger LE, Gaston KJ, Irvine KN, Fuller RA. 2013. What are the benefits of interacting with nature? Int. J. Environ. Res. Public Health 10, 913–935. ( 10.3390/ijerph10030913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartig T, Mitchell R, De Vries S, Frumkin H. 2014. Nature and health. Annu. Rev. Public Health 35, 207–228. ( 10.1146/annurev-publhealth-032013-182443) [DOI] [PubMed] [Google Scholar]
- 4.Kardan O, Gozdyra P, Misic B, Moola F, Palmer LJ. 2015. Neighborhood greenspace and health in a large urban center: neighborhood greenspace and health in a large urban center. Sci. Rep. 5, 11610 ( 10.1038/srep11610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanahan DF, Bush R, Gaston KJ, Lin BB, Dean J, Barber E, Fuller RA. 2016. Health benefits from nature experiences depend on dose. Sci. Rep. 6, 28551 ( 10.1038/srep28551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman BK, McPhee D. 2016. Global shark attack hotspots: identifying underlying factors behind increased unprovoked shark bite incidence. Ocean Coast. Manag. 133, 72–84. ( 10.1016/j.ocecoaman.2016.09.010) [DOI] [Google Scholar]
- 7.Penteriani V, et al. 2016. Human behaviour can trigger large carnivore attacks in developed countries. Sci. Rep. 6, 20552 ( 10.1038/srep20552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bombieri G, et al. 2019. Brown bear attacks on humans: a worldwide perspective. Sci. Rep. 9, 8573 ( 10.1038/s41598-019-44341-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chippaux JP. 2017. Incidence and mortality due to snakebite in the Americas. PLoS Negl. Trop. Dis. 11, e0005662 ( 10.1371/journal.pntd.0005662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litvaitis JA, Tash JP. 2008. An approach toward understanding wildlife–vehicle collisions. Environ. Manage. 42, 688–697. ( 10.1007/s00267-008-9108-4) [DOI] [PubMed] [Google Scholar]
- 11.Allen T, Murray KA, Zambrana-Torrelio C, Morse SS, Rondinini C, Di Marco M, Breit N, Olival KJ, Daszak P. 2017. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 8, 1124 ( 10.1038/s41467-017-00923-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JR. 2005. Biodiversity conservation and the extinction of experience. Trends Ecol. Evol. 20, 430–434. ( 10.1016/j.tree.2005.05.013) [DOI] [PubMed] [Google Scholar]
- 13.Soga M, Gaston KJ. 2016. Extinction of experience: the loss of human–nature interactions. Front. Ecol. Environ. 14, 94–101. ( 10.1002/fee.1225) [DOI] [Google Scholar]
- 14.Soga M, Gaston KJ. 2018. Shifting baseline syndrome: causes, consequences, and implications. Front. Ecol. Environ. 16, 222–230. ( 10.1002/fee.1794) [DOI] [Google Scholar]
- 15.Soga M, Gaston KJ, Koyanagi TF, Kurisu K, Hanaki K. 2016. Urban residents' perceptions of neighbourhood nature: does the extinction of experience matter? Biological Conservation 203, 143–150. [Google Scholar]
- 16.Wood SA, Guerry AD, Silver JM, Lacayo M. 2013. Using social media to quantify nature-based tourism and recreation. Sci. Rep. 3, 2976 ( 10.1038/srep02976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox DTC, Inger R, Hancock S, Anderson K, Gaston KJ. 2016. Movement of feeder-using songbirds: the influence of urban features. Sci. Rep. 6, 37669 ( 10.1038/srep37669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amati M, Parmehr EG, McCarthy C, Sita J. 2018. How eye-catching are natural features when walking through a park? Eye-tracking responses to videos of walks. Urban For. Urban Green. 31, 67–78. ( 10.1016/j.ufug.2017.12.013) [DOI] [Google Scholar]
- 19.Mancini F, Coghill GM, Lusseau D. 2018. Using social media to quantify spatial and temporal dynamics of nature-based recreational activities. PLoS ONE 13, e0200565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaston KJ, Soga M, Duffy JP, Garrett JK, Gaston S, Cox DTC. 2018. Personalised ecology. Trends Ecol. Evol. 33, 916–925. ( 10.1016/j.tree.2018.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soga M, Gaston K, Yamaura Y, Kurisu K, Hanaki K. 2016. Both direct and vicarious experiences of nature affect children's willingness to conserve biodiversity. Int. J. Environ. Res. Public Health 13, 529 ( 10.3390/ijerph13060529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadkarni NM, Hasbach PH, Thys T, Crockett EG, Schnacker L. 2017. Impacts of nature imagery on people in severely nature-deprived environments. Front. Ecol. Environ. 15, 395–403. ( 10.1002/fee.1518) [DOI] [Google Scholar]
- 23.Palliwoda J, Kowarik I, von der Lippe M. 2017. Human-biodiversity interactions in urban parks: the species level matters. Landscape and Urban Planning 157, 394–406. [Google Scholar]
- 24.Cox DTC, Hudson HL, Shanahan DF, Fuller RA, Gaston KJ. 2017. The rarity of direct experiences of nature in an urban population. Landsc. Urban Plan. 160, 79–84. ( 10.1016/j.landurbplan.2016.12.006) [DOI] [Google Scholar]
- 25.Soga M, Akasaka M. 2019. Multiple landscape-management and social-policy approaches are essential to mitigate the extinction of experience. Landsc. Urban Plan. 191, 103634 ( 10.1016/j.landurbplan.2019.103634) [DOI] [Google Scholar]
- 26.Romagosa F, Eagles PF, Lemieux CJ. 2015. From the inside out to the outside in: exploring the role of parks and protected areas as providers of human health and well-being. J. Outdoor Recreat. Tour. 10, 70–77. ( 10.1016/j.jort.2015.06.009) [DOI] [Google Scholar]
- 27.Jones DN, James Reynolds S. 2008. Feeding birds in our towns and cities: a global research opportunity. J. Avian Biol. 39, 265–271. ( 10.1111/j.0908-8857.2008.04271.x) [DOI] [Google Scholar]
- 28.Davies ZG, Fuller RA, Dallimer M, Loram A, Gaston KJ. 2012. Household factors influencing participation in bird feeding activity: a national scale analysis. PLoS ONE 7, e39692 ( 10.1371/journal.pone.0039692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kojola I, Heikkinen S. 2012. Problem brown bears Ursusarctos in Finland in relation to bear feeding for tourism purposes and the density of bears and humans. Wildl. Biol. 18, 258–264. ( 10.2981/11-052) [DOI] [Google Scholar]
- 30.Geffroy B, Samia DS, Bessa E, Blumstein DT. 2015. How nature-based tourism might increase prey vulnerability to predators. Trends Ecol. Evol. 30, 755–765. ( 10.1016/j.tree.2015.09.010) [DOI] [PubMed] [Google Scholar]
- 31.Galbraith JA, Beggs JR, Jones DN, Stanley MC. 2015. Supplementary feeding restructures urban bird communities. Proc. Natl Acad. Sci. USA 112, E2648–E2657. ( 10.1073/pnas.1501489112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller JR, Hobbs NT. 2000. Recreational trails, human activity, and nest predation in lowland riparian areas. Landsc. Urban Plan. 50, 227–236. ( 10.1016/S0169-2046(00)00091-8) [DOI] [Google Scholar]
- 33.Gilbert SL, Sivy KJ, Pozzanghera CB, DuBour A, Overduijn K, Smith MM, Zhou J, Little JM, Prugh LR. 2017. Socioeconomic benefits of large carnivore recolonization through reduced wildlife–vehicle collisions. Conserv. Lett. 10, 431–439. ( 10.1111/conl.12280) [DOI] [Google Scholar]
- 34.Pickering CM, Hill W. 2007. Impacts of recreation and tourism on plant biodiversity and vegetation in protected areas in Australia. J. Environ. Manage. 85, 791–800. ( 10.1016/j.jenvman.2006.11.021) [DOI] [PubMed] [Google Scholar]
- 35.Fuller RA, Warren PH, Armsworth PR, Barbosa O, Gaston KJ. 2008. Garden bird feeding predicts the structure of urban avian assemblages. Divers. Distrib. 14, 131–137. ( 10.1111/j.1472-4642.2007.00439.x) [DOI] [Google Scholar]
- 36.Robb GN, McDonald RA, Chamberlain DE, Reynolds SJ, Harrison TJ, Bearhop S. 2008. Winter feeding of birds increases productivity in the subsequent breeding season. Biol. Lett. 4, 220–223. ( 10.1098/rsbl.2007.0622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanmer HJ, Thomas RL, Fellowes MD. 2017. Provision of supplementary food for wild birds may increase the risk of local nest predation. Ibis 159, 158–167. ( 10.1111/ibi.12432) [DOI] [Google Scholar]
- 38.Lawson B, Robinson RA, Toms MP, Risely K, MacDonald S, Cunningham AA. 2018. Health hazards to wild birds and risk factors associated with anthropogenic food provisioning. Phil. Trans. R. Soc. B 373, 20170091 ( 10.1098/rstb.2017.0091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terraube J, Fernández-Llamazares Á, Cabeza M. 2017. The role of protected areas in supporting human health: a call to broaden the assessment of conservation outcomes. Curr. Opin. Environ. Sustain. 25, 50–58. ( 10.1016/j.cosust.2017.08.005) [DOI] [Google Scholar]
- 40.Franco LS, Shanahan DF, Fuller RA. 2017. A review of the benefits of nature experiences: more than meets the eye. Int. J. Environ. Res. Public Health 14, 864 ( 10.3390/ijerph14080864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox DTC, Hudson HL, Plummer KE, Siriwardena GM, Anderson K, Hancock S, Devine-Wright P, Gaston KJ. 2018. Covariation in urban birds providing cultural services or disservices and people. J. Appl. Ecol 55, 2308–2319. ( 10.1111/1365-2664.13146) [DOI] [Google Scholar]
- 42.Botts EA, Erasmus BF, Alexander GJ. 2011. Geographic sampling bias in the South African Frog Atlas Project: implications for conservation planning. Biodivers. Conserv. 20, 119–139. ( 10.1007/s10531-010-9950-6) [DOI] [Google Scholar]
- 43.Baverstock S, Weston MA, Miller KK. 2018. A global paucity of wild bird feeding policy. Sci. Total Environ. 25, 105–111. ( 10.1016/j.scitotenv.2018.10.338) [DOI] [PubMed] [Google Scholar]
- 44.Higa M, Yamaura Y, Koizumi I, Yabuhara Y, Senzaki M, Ono S. 2015. Mapping large-scale bird distributions using occupancy models and citizen data with spatially biased sampling effort. Divers. Distrib. 21, 46–54. ( 10.1111/ddi.12255) [DOI] [Google Scholar]
- 45.Møller AP, Tryjanowski P, Díaz M, Kwieciński Z, Indykiewicz P, Mitrus C, Goławski A, Polakowski M. 2015. Urban habitats and feeders both contribute to flight initiation distance reduction in birds. Behav. Ecol. 26, 861–865. ( 10.1093/beheco/arv024) [DOI] [Google Scholar]
- 46.Richards DR, Passy P, Oh RR. 2017. Impacts of population density and wealth on the quantity and structure of urban green space in tropical Southeast Asia. Landsc. Urban Plan. 157, 553–560. ( 10.1016/j.landurbplan.2016.09.005) [DOI] [Google Scholar]
- 47.Shanahan DF, Cox DT, Fuller RA, Hancock S, Lin BB, Anderson K, Bush R, Gaston KJ. 2017. Variation in experiences of nature across gradients of tree cover in compact and sprawling cities. Landsc. Urban Plan. 157, 231–238. ( 10.1016/j.landurbplan.2016.07.004) [DOI] [Google Scholar]
- 48.Hickling R, Roy DB, Hill JK, Fox R, Thomas CD. 2006. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Change Biol. 12, 450–455. ( 10.1111/j.1365-2486.2006.01116.x) [DOI] [Google Scholar]
- 49.Kelly AE, Goulden ML. 2008. Rapid shifts in plant distribution with recent climate change. Proc. Natl Acad. Sci. USA 105, 11823–11826. ( 10.1073/pnas.0802891105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breckheimer IK, Theobald EJ, Cristea NC, Wilson AK, Lundquist JD, Rochefort RM, HilleRisLambers J. In press Crowd-sourced data reveal social-ecological mismatches in phenology driven by climate. Front. Ecol. Environ. [Google Scholar]
- 51.Fisichelli NA, Schuurman GW, Monahan WB, Ziesler PS. 2015. Protected area tourism in a changing climate: will visitation at US national parks warm up or overheat? PLoS ONE 10, e0128226 ( 10.1371/journal.pone.0128226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zacarias D, Loyola R. 2019. Climate change impacts on the distribution of venomous snakes and snakebite risk in Mozambique. Clim. Change 152, 195–207. ( 10.1007/s10584-018-2338-4) [DOI] [Google Scholar]
- 53.Nix JH, Howell RG, Hall LK, McMillan BR. 2018. The influence of periodic increases of human activity on crepuscular and nocturnal mammals: testing the weekend effect. Behav. Process. 146, 16–21. ( 10.1016/j.beproc.2017.11.002) [DOI] [PubMed] [Google Scholar]
- 54.Morelle K, Lehaire F, Lejeune P. 2013. Spatio-temporal patterns of wildlife–vehicle collisions in a region with a high-density road network. Nat. Conserv. 5, 53–73. ( 10.3897/natureconservation.5.4634) [DOI] [Google Scholar]
- 55.Chaves LF, Chuang TW, Sasa M, Gutiérrez JM. 2015. Snakebites are associated with poverty, weather fluctuations, and El Niño. Sci. Adv. 1, e1500249 ( 10.1126/sciadv.1500249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodrich JM. 2010. Human–tiger conflict: a review and call for comprehensive plans. Integr. Zool. 5, 300–312. ( 10.1111/j.1749-4877.2010.00218.x) [DOI] [PubMed] [Google Scholar]
- 57.Pyle RM. 1993. The thunder tree: lessons from an urban wildland. Boston, MA: Houghton Mifflin. [Google Scholar]
- 58.Soga M, Gaston KJ, Kubo T. 2018. Cross-generational decline in childhood experiences of neighborhood flowering plants in Japan. Landsc. Urban Plan. 174, 55–62. ( 10.1016/j.landurbplan.2018.02.009) [DOI] [Google Scholar]
- 59.Soga M, Yamanoi T, Tsuchiya K, Koyanagi TF, Kanai T. 2018. What are the drivers of and barriers to children's direct experiences of nature? Landsc. Urban Plan. 180, 114–120. ( 10.1016/j.landurbplan.2018.08.015) [DOI] [Google Scholar]
- 60.Lin BB, Fuller RA, Bush R, Gaston KJ, Shanahan DF. 2014. Opportunity or orientation? Who uses urban parks and why. PLoS ONE 9, e87422 ( 10.1371/journal.pone.0087422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pergams OR, Zaradic PA. 2006. Is love of nature in the US becoming love of electronic media? 16-year downtrend in national park visits explained by watching movies, playing video games, internet use, and oil prices. J. Environ. Manage. 80, 387–393. ( 10.1016/j.jenvman.2006.02.001) [DOI] [PubMed] [Google Scholar]
- 62.Zhang W, Goodale E, Chen J. 2014. How contact with nature affects children's biophilia, biophobia and conservation attitude in China. Biol. Conserv. 177, 109–116. ( 10.1016/j.biocon.2014.06.011) [DOI] [Google Scholar]
- 63.van Heezik Y, Hight SR. 2017. Socio-economic-driven differences in bird-feeding practices exacerbate existing inequities in opportunities to see native birds in cities. J. Urban Ecol. 3, jux011 ( 10.1093/jue/jux011) [DOI] [Google Scholar]
- 64.Crawford D, Timperio A, Giles-Corti B, Ball K, Hume C, Roberts R, Andrianopoulos N, Salmon J. 2008. Do features of public open spaces vary according to neighbourhood socio-economic status? Health Place 14, 889–893. ( 10.1016/j.healthplace.2007.11.002) [DOI] [PubMed] [Google Scholar]
- 65.Kumar N, Jhala YV, Qureshi Q, Gosler AG, Sergio F. 2019. Human-attacks by an urban raptor are tied to human subsidies and religious practices. Sci. Rep. 9, 2545 ( 10.1038/s41598-019-38662-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shanahan DF, Lin BB, Gaston KJ, Bush R, Fuller RA. 2014. Socio-economic inequalities in access to nature on public and private lands: a case study from Brisbane, Australia. Landsc. Urban Plan. 130, 14–23. ( 10.1016/j.landurbplan.2014.06.005) [DOI] [Google Scholar]
- 67.Gerrish E, Watkins SL. 2018. The relationship between urban forests and income: a meta-analysis. Landsc. Urban Plan. 170, 293–308. ( 10.1016/j.landurbplan.2017.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belaire JA, Westphal LM, Minor ES. 2016. Different social drivers, including perceptions of urban wildlife, explain the ecological resources in residential landscapes. Landsc. Ecol. 31, 401–413. ( 10.1007/s10980-015-0256-7) [DOI] [Google Scholar]
- 69.Hope D, Gries C, Zhu W, Fagan WF, Redman CL, Grimm NB, Nelson AL, Martin C, Kinzig A. 2003. Socioeconomics drive urban plant diversity. Proc. Natl Acad. Sci. USA 100, 8788–8792. ( 10.1073/pnas.1537557100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leong M, Dunn RR, Trautwein MD. 2018. Biodiversity and socioeconomics in the city: a review of the luxury effect. Biol. Lett. 14, 20180082 ( 10.1098/rsbl.2018.0082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dunham KM, Ghiurghi A, Cumbi R, Urbano F. 2010. Human–wildlife conflict in Mozambique: a national perspective, with emphasis on wildlife attacks on humans. Oryx 44, 185–193. ( 10.1017/S003060530999086X) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.