Abstract
Seabirds must often travel vast distances to exploit heterogeneously distributed oceanic resources, but how routes and destinations of foraging trips are optimized remains poorly understood. Among the seabirds, gadfly petrels (Pterodroma spp.) are supremely adapted for making efficient use of wind energy in dynamic soaring flight. We used GPS tracking data to investigate the role of wind in the flight behaviour and foraging strategy of the Desertas petrel, Pterodroma deserta. We found that rather than visiting foraging hotspots, Desertas petrels maximize prey encounter by covering some of the longest distances known in any animal in a single foraging trip (up to 12 000 km) over deep, pelagic waters. Petrels flew with consistent crosswind (relative wind angle 60°), close to that which maximizes their groundspeed. By combining state–space modelling with a series of comparisons to simulated foraging trips (reshuffled-random, rotated, time-shifted, reversed), we show that this resulted in trajectories that were close to the fastest possible, given the location and time. This wind use is thus consistent both with birds using current winds to fine-tune their routes and, impressively, with an a priori knowledge of predictable regional-scale wind regimes, facilitating efficient flight over great distances before returning to the home colony.
Keywords: flight behaviour, wind, seabird, Pterodroma, optimization, state-space model
1. Background
Optimal foraging theory predicts that animals looking for food adopt mechanisms to maximize their energy acquisition per unit time and minimize their energy loss [1–3]. The constraints faced by breeding oceanic seabirds—patchily distributed resources and having to return to their colony to alternate incubation shifts with the partner—result in sometimes spectacular foraging trips, many thousands of kilometres from the colony [4–7]. Seabirds thus have morphology and flight behaviour adapted to glean energy for these long commutes from the wind. Nevertheless, how seabirds make use of wind in combination with memory of foraging patches and adaptive search behaviour to maximally exploit oceanic resources remains poorly understood.
Wind fields intrinsically shape the energy expenditure of many seabirds' movements [8–10], affecting their flight behaviour [11,12] and ultimately, driving changes in population distributions [13] and demographic processes [14,15]. Albatrosses, and other procellariiform seabirds, adopt a flight behaviour known as dynamic soaring that exploits vertical wind speed gradients near the sea surface [16–18]. While flying with favourable winds, their energetic consumption is almost as low as when they sit on the nest or rest on the water surface [8,19]. This, in turn, might constrain the directions that dynamic soaring birds can fly efficiently. Therefore, dynamic soaring Procellariiformes must optimize their searching strategies across trade-offs between knowledge about the location and quality of resources and the wind field, which varies to different extents across space and time. Correspondingly, albatrosses and other seabirds often fly with favourable side and tail winds [8,9,20,21] that enable them to travel at high ground speeds and low energetic cost [8,9,18,22]. Their ground speed attained during flight is affected by both relative wind direction (i.e. the difference in angle between wind direction and the bird direction of movement) and relative wind speed (or tail wind component, i.e. the wind speed component in direction of the bird's flight) [8,9].
Gadfly petrels (Pterodroma spp.) are among the most threatened and least studied seabird genera in the world. As predicted by aerodynamic theory—and as suggested by their genus name (from the Greek words ‘Pteron’, wing, and ‘dromos’, run)—the high aspect ratio per wing loading (the highest of all seabirds) makes them especially anatomically adapted for efficient flight: a fast, gliding flight with low profile drag [20]. Gadfly petrels are highly mobile, capable of undertaking exceptionally long foraging trips [4–7] by spending a large proportion of time in direct flight, actively looking for prey [4,7]. For example, Murphy's petrels (Pterodroma ultima) tracked from Henderson Island did not consistently target specific areas and foraged both during directed movement and area restricted search [6], and the higher mass gains were associated with the most wide-ranging trips [6]. Despite being the largest genus of pelagic seabirds, with several threatened species, many aspects of gadfly petrels' ecology have never been investigated and, at present, the flight behaviour and the wind use of the long distance flyers par excellence remain largely unknown. Yet, due to their unparalleled motility, these birds could serve as a paradigm to understand the best performing adaptations for efficient flight.
Here we present one of the first GPS tracking datasets available for gadfly petrels, the first on Desertas petrel (Pterodroma deserta), collected across three consecutive breeding seasons. Desertas petrel, classified as Vulnerable by the IUCN [23], breeds exclusively on the Desertas Islands near Madeira, with an estimated population of ca. 200 pairs. While recent research shed some light on the species' year-round distribution [7], the processes underpinning their movement ecology during breeding are poorly understood. In this paper, we investigate gadfly petrels' foraging strategy, flight behaviour and use of wind during their long journeys at sea, using Desertas petrels' tracks as a case-study.
In the course of each trip, the birds switch between different movement behavioural modes as their internal motivation changes. For instance, when their motivation is searching for food, they might perform a slower and less direct movement compared with birds transiting between foraging patches. Depending on their motivation, birds are not equally free to optimize their use of wind. These constraints are presumably less pressing during the transit state, when the birds' motivation is to cover distance. Hence, it is when they engage in the transit mode that birds should maximize their flight efficiency and show the most refined behavioural adaptations to efficiently use the wind fields. These predictions should hold true particularly if the birds' foraging strategy does not rely on targeting stable meso- (100–1000 km2) and coarse-scale (1–100 km2) features of the seascape, i.e. if they are unconstrained in their route planning.
A vast body of literature has explored how animals optimize their movement trajectory to maximize prey encounter rate. For instance, it has been proposed that seabirds' search pattern should conform to the Lévy flight foraging hypothesis [24–26] (but see [27,28]) given the heterogeneous and patchy nature of the marine resources that they rely upon. In this paper we consider two foraging strategies that can be underpinned by covering long distances at low energetic cost. On the one hand, if the birds target predictably favourable foraging areas, a fast low cost flight allows a higher number of known productive patches to be visited, minimizing the time spent commuting between them. In this case, we expect a significant degree of foraging hotspots overlap across individuals. Further, we expect the return route between the main foraging areas and the colony to be the fastest one, irrespective of the ground covered. On the other hand, if the birds exploit a marine domain with heterogeneous resources distribution and less predictable meso- or coarse-scale features, such as the oceanic waters [29], covering large distances will increase the probability of encountering prey along the route. In this case, we expect a higher flight routes overlap across individuals—particularly if winds are predictable in the area crossed by the birds—but a low overlap in the areas used for foraging by different individuals. Further, we expect that birds choose routes that maximize ground speed, rather than minimizing commuting time between the areas of concentrated searching behaviour and the colony.
Previous studies have shown that travelling seabirds follow prevailing favourable winds [6,8], but little is known about the mechanisms with which they manage to do so. Here, we examine whether long distance fliers such as gadfly petrels are capable not only of planning their route based on a priori knowledge of the regional wind regimes, but also of refining their route based on the local wind conditions. If this holds true, we predict that the observed route should be the most favourable one at the specific time and given the local wind conditions experienced by the bird, but it may be a suboptimal solution if it had been carried out at any other time.
Specifically, in this paper we test the following hypotheses:
(Hp1) Birds exploit wind differently during the transit and searching states.
(Hp2) Birds engaging in the transit state use the most favourable relative wind direction, i.e. the one that maximizes their ground speed.
(Hp3) In the pelagic domain used by gadfly petrels, characterized by patchy and unpredictable resources distribution at the coarse- to meso-scale, their foraging strategy relies upon covering large areas at low cost in search of prey along the route, rather than targeting specific foraging grounds.
(Hp4) Petrels optimize their route to cover the largest distance in the shortest time, following predictable regional winds and adjusting their tracks to the local wind conditions experienced en route.
To test Hp1, we use hidden Markov models (HMMs) to classify movement behaviour and compare the birds' wind use during the ‘transit’ and ‘searching’ states. To test Hp2, we use generalized additive mixed effects models (hereafter GAMMs) to quantify the effect of wind on the transiting birds' ground speed and to identify the most favourable relative wind direction, which we then compare with the one used most intensively. To address Hp3, we identify and quantify the overlap across individuals in the birds' core searching (‘foraging hotspots’) and transiting (‘travelling hotspots’) areas using utilization distributions (UDs), and compare the ground speed and distance covered during the return section of the tracks with simulated ‘beeline’ homebound tracks; if our hypothesis is true, we expect the travelling hotspots to overlap more than foraging hotspots and the real homebound routes to maximize the ground speed and distance covered. To address Hp4, we adopt a novel spatial and temporal tracks' simulation framework. If petrels both follow predictable regional winds and adjust tracks to local conditions, we expect the observed routes to be more efficient than the tracks randomized spatially and temporally, respectively.
2. Methods
(a). The data
We collected GPS tracks at a 2 h temporal resolution on 20 breeding Desertas petrels during three consecutive breeding seasons (2015–2017). The tracked birds performed both long and short trips. The latter, mostly undertaken after long trips, lasted for an average duration of 28 h, covering the waters in proximity of the colony (average distance from colony = 119 km) and representing only 13.7% of the total time spent at sea by the tracked birds. As we were unsure of the significance of these tracks—possibly not primarily linked to foraging and therefore not representative of the birds' use of wind during foraging trips—we used k-means clustering to categorize trips and only retained long GPS tracks for the analysis (see electronic supplementary material for details). Gaps in the tracking datasets were linearly interpolated using the adehabitatLT package [30] in R in order to obtain tracks at 2 h time intervals. The extent of this interpolation was minimal (less than 1% of the points in the final dataset were imputed). We excluded from the analysis the fixes at the beginning or end of each trip falling within a 50 km circular buffer, centred in the colony.
A set of physiographic, oceanographic, biological, distance-related and temporal explanatory variables were extracted for each GPS relocation (electronic supplementary material). They were included in the HMM and tested as explanatory variables for the state-switching probabilities (see below). Fine scale 3 h temporal resolution wind grids were downloaded from the European Centre for Medium-Range Weather Forecasts (http://apps.ecmwf.int/datasets/data/interim-full-daily/levtype=sfc/), at a spatial resolution of 0.25°. We extracted wind intensity (expressed in km h−1), wind direction (expressed in degrees), tail wind component (hereafter ‘TWC’, calculated as in [31]), and wind direction relative to the bird bearing (hereafter ‘Δangle’, computed as in [9]). As bird bearing and wind direction were expressed using the same reference system (relative to the True North), Δangle was bounded between 0° (tail winds, blowing on the same direction of movements of the bird) and 180° (head winds, blowing in the opposite direction of movement of the bird).
(b). Spatial analysis
In order to classify the birds' relocations into ‘transiting’ or ‘searching’, the tracks were analysed using the HMM framework using the R package momentuHMM [32]. Based on the observed distance travelled (step length) and the change of movement direction (turning angle) between consecutive relocations, the model estimated the step length and turning angle distributions for the two states considered. The behavioural states of each animal along the track were then decoded using the Viterbi algorithm [33]. To enhance the model's biological realism and to avoid potential confounding effects to the final classification output, we made the following considerations. First, that the probability of transitioning between states may be affected by environmental covariates. Second, that seabirds' speed is intrinsically dependent on the TWC, with longer steps occurring with more favourable winds. We accounted for this in an HMM that modelled the effects of the environmental variables to the state-switching probabilities and simultaneously accounted (through a design matrix) for the effect of TWC on the mean parameter of the step length distributions for both states (details are provided in the electronic supplementary material). Overall, TWC had a marginal effect on the classification: only 6% of the points changed state as a result of the inclusion of wind into the HMM design matrix.
The overlap between individuals' core foraging and travelling hotspots—defined as the 50% contour of the searching and transiting UDs, respectively—was calculated using Bhattacharyya's affinity index, as in Oppel et al. [34]. Moreover, following Ventura et al. [35], we bootstrapped the sample of individual UDs to obtain a robust foraging and travelling hotspots map (electronic supplementary material). The UDs were calculated using the adehabitatHR package in R, with a specified smoothing parameter equal to 120 km, i.e. the longest step length recorded in 2 h.
(c). Wind use analysis
We calculated the distribution of Δangle in the locations classified as transit and searching. GAMMs from the mgcv package [36] in R were used to investigate whether Δangle had an effect on the transiting birds' step length (i.e. their ground speed) and whether this effect is consistent across different values of wind intensity. To do that, the following gamma-based GAMM (hereafter ‘wind model’) was fitted to the transit section of the tracks:
where the functions ƒ are cubic regression splines with shrinkage, adopted in order to prevent overfitting. We structured the GAMM to include the tensor product interaction between variables. To account both for temporal autocorrelation and for the dependency between observations collected on the same animal i, the model implemented the residual auto-regressive AR1 correlation structure
applied to observations collected on each individual track at regularly spaced time-steps s and t. The generalized additive framework was adopted in order to account for nonlinearity between the response and the explanatory variables chosen.
(d). Track simulation
For this analytical step, we only retained the transit bouts of the tracks. The last point of each transit bout interrupted by searching was connected to the first point of the subsequent transit bout by a beeline. These long, connecting segments were retained to preserve the trip configuration, but their duration and length were discarded from the final calculation of trip duration and distance travelled. The values yielded by the analysis therefore only refer to ‘transit’, rather than to the overall track.
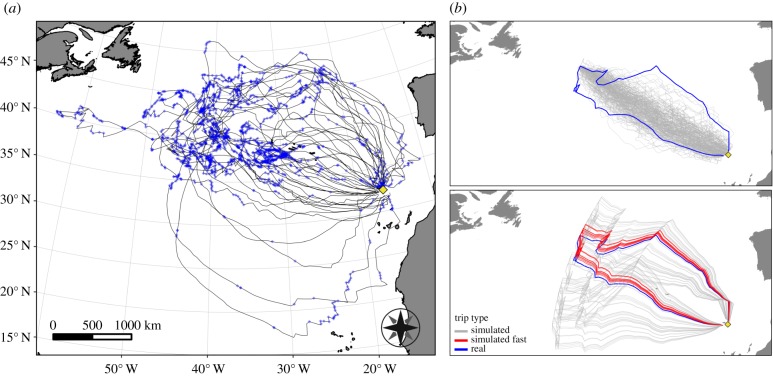
We adopted four scenarios and generated the following simulated trips for each observed track: 100 random, 100 rotated, 1 reversed and 14 time-lagged simulated trips, all characterized by the same cumulative distance travelled as their respective real trip. To generate each random trip (figure 1b, top), we split the real trip into two legs, from the colony to the furthest away foraging location and vice versa, and within each leg all segments (i.e. all steps between GPS relocations) were randomly reshuffled. The rotated trips (figure 1b, bottom) were generated using a random rotate-shift model, fixed on the colony location, using the adehabitatLT package [30]. The reversed trip had the same shape as the respective real trip, but the reverse direction, from the last GPS relocation to the first one. The time-lagged trips had the same route configuration as the observed tracks, but were shifted in time and had a timestamp delayed by 1–14 days, resulting in different wind conditions at each location. Regardless of the method used, the duration of each simulated trip was calculated as follows. The start point of the real and random trips shared the same timestamp. Beginning from the start point ‘a’, we calculated the time required to travel to the following point ‘a + 1’ based on the step length predicted by the wind model given the wind conditions (Δangle and wind intensity) experienced at ‘a’. We then calculated the time of arrival at ‘a + 1’, extracted the local wind conditions at that time, and calculated the time needed to reach point ‘a + 2’. This procedure was repeated for all points along the track until the endpoint ‘e’, and the cumulative duration of the trip was then calculated.
Figure 1.
(a) Desertas petrels' tracks. The blue dots represent the locations classified as ‘searching’. The yellow rhombus shows the breeding colony on Bugio Island. (b) The random (top) and rotated (bottom) simulated trips based on an example track. The real trip is depicted in blue, the simulated ones in light grey. Of the rotated tracks, the ones that would grant the bird a higher mean speed are depicted in red. For clarity, only a subset of the 100 rotated tracks is displayed. (Online version in colour.)
Finally, we focused on the homebound sections of each bird's track, i.e. from the furthest to the endpoint of the journey. From the furthest point ‘f’, we simulated a great-circle homeward ‘beeline’ trajectory, i.e. with the bird travelling heading directly to the colony. We calculated the hypothetical new wind conditions at ‘f’ and estimated how far along the beeline trajectory the bird would have moved in 2 h based on the wind model, defining a new point ‘f + 1’. We repeated the calculation until the bird reached the endpoint, and extracted the cumulative duration and distance travelled.
3. Results
(a). Spatial analysis
Desertas petrels undertook some of the longest foraging trips recorded for any animal species during breeding, almost reaching the continental shelf break waters off the coast of Newfoundland on a clockwise trajectory (figure 1a). On average they travelled for 14 (s.d. = 3.6) days, covering a total distance of 7891 (s.d. = 2205) km. The maximum trip duration and distance travelled were 20 days and 12 000 km, respectively. Upon departure, they left the colony travelling west-southwest (circular mean bearing and circular variance equal to 262° and 0.16, respectively). When returning to their colony, the birds approached it from a northerly direction (circular mean bearing and circular variance equal to 182° and 0.22).
The best HMM model retained depth, distance from the colony, distance from seamounts and local time of day as significant predictors of the state switching probabilities. However, the inclusion of these explanatory variables affected the final classification only marginally: overall, only 2% of the total locations changed state after the inclusion of habitat covariates (electronic supplementary material). Along the tracks, on average, 43% (s.d. = 15%) of the relocations were classified as searching. Transit and searching bouts had a median duration of 12 h (interquartile range = 6–20 h) and 9 h (interquartile range = 4–16 h), respectively. The overlap between individuals' foraging hotspots was 0.30. The overlap between individuals' travelling hotspots was equal to 0.49.
(b). Wind use analysis
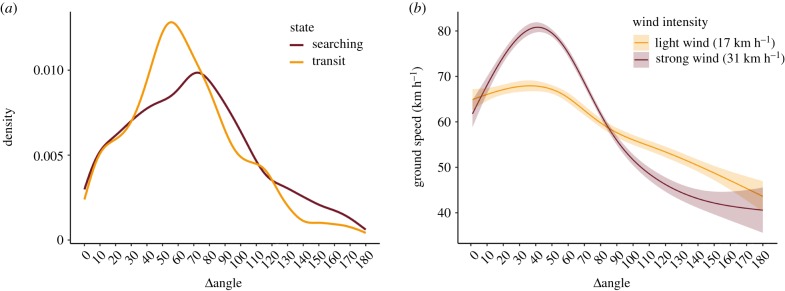
Overall, the Δangle values exploited by Desertas petrels during transit (median = 60.7°; interquartile range = 40.6°–85.7°) and searching (median 68.9°; interquartile range = 39.5°–95.8°) were significantly different (figure 2a): in the linear mixed effects model with Δangle as response variable and individual birds as random effects, the inclusion of behavioural state as explanatory variable was highly significant (p < 0.001). In particular, the transiting state was predicted to reduce Δangle by an average of 4.3°. The results of the wind model showed that the ground speed of birds was affected by Δangle in a nonlinear trend, with the predicted birds' speed peaking for values of Δangle ≈ 50°. The model also showed that this trend was particularly emphasized when the birds were travelling with strong winds (figure 2b).
Figure 2.
(a) Density curves of Δangle used by the birds along the tracks, separately for the searching and transit behavioural states. (b) Results of the generalized additive mixed effect wind model fitted to the transit portions of the tracks. The shaded areas represent the 95% confidence intervals. For visualization, the predicted effect of Δangle on ground speed was calculated for light and strong winds (the 25 and 75% quantiles of the wind speeds experienced by the birds, equal to 17 and 31 km h−1, respectively). The Δangle predicted by the wind model to maximize the ground speed (50°) is similar to that maximally exploited by Desertas petrels during the transit state (60°). (Online version in colour.)
(c). Track simulation
Real trips were significantly faster than their equivalent simulated trips. This held true for random, rotated, inverted and time-lagged trips (figure 3). Real travel duration (mean = 180.3 h, s.d. = 59 h) was 27.3 h (i.e. 15.2%) shorter than the average duration of the respective random trips (paired t-test, t = −4.37, d.f. = 24, p-value < 0.001). The observed transit bouts were 16.9 h (i.e. 9.4%) faster than the respective average rotated trips (paired t-test, t = −4.87, d.f. = 24, p-value < 0.001). The few faster rotated tracks were in close proximity to the respective observed trip (figure 1b, bottom). The inverse trips were, on average, 65.2 h (i.e. 36.2%) slower than the corresponding real trips (paired t-test, t = −7.82, d.f. = 24, p-value < 0.001). Finally, the real tracks were also significantly faster (by an average of 10.3 h, i.e. 5.7%) than the respective lagged trips (paired t-test, t = −2.80, d.f. = 24, p-value = 0.009).
Figure 3.
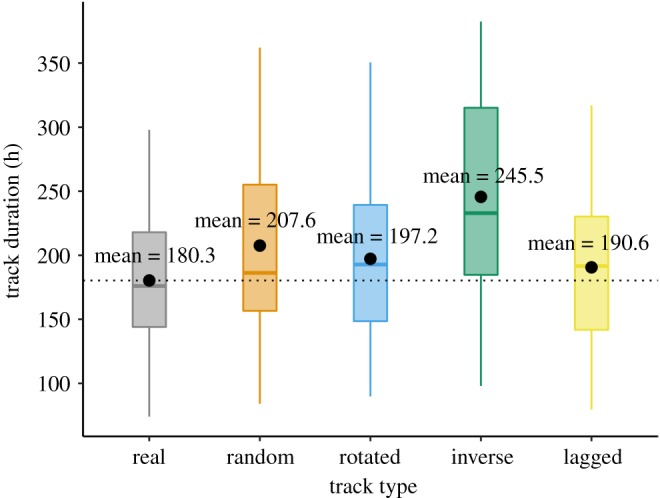
Boxplot showing the track duration (in hours) of the real, random, rotated, inverse and time-lagged trips. The black circle represents the mean and the black dotted line shows the real tracks' mean. Real trips are significantly shorter than the respective random, rotated, inverse and time-lagged ones. (Online version in colour.)
Overall, the real homecoming portions of the tracks were 27.3 h longer (s.d. = 26.2 h) than the respective predicted beeline ones. By taking the observed homecoming route, birds covered 1201 km (s.d. = 794 km) more than they would have covered by travelling directly towards the colony. The mean travelling speed in the observed homecoming tracks was significantly higher (by 3 km h−1) than the hypothetical speed attained in the beeline tracks (t = 2.31, d.f. = 24, p-value = 0.03).
4. Discussion
The gadfly petrels' impressive motility challenges our intuitive understanding of the central tenet of the optimal foraging theory, which predicts that animals should minimize foraging costs (in time and energy) and maximize energy intake. This is particularly true for Desertas petrels, which do not target the highly productive waters in the Canary Upwelling system [37] in proximity to their breeding site (potentially as a result of displacement due to competition or kleptoparasitism), but rather embark on their trips—among the longest in the animal kingdom—that take them thousands of kilometres away from their colony, into high-sea waters.
Owing to the petrels' high manoeuvrability, state-changes may occur over smaller scales than the temporal resolution of our data. However, on the one hand, during transiting, birds travelled at a ground speed of 33 km h−1 (s.d. = 8 km h−1, see electronic supplementary material for details), suggesting a fast, direct movement, which we expect the HMM to detect with a low rate of false positives. Importantly, as the ground speed calculation does not account for the sinuosity of the dynamic soaring flight within the 2 h movement steps, the speed values are likely to be underestimated. On the other hand, while searching, the birds' ground speed was 14 km h−1 (s.d. = 8 km h−1), excluding—at least for most of the movement step—the occurrence of transit behaviour. Thus, while acknowledging the limitation of inferring behavioural modes at 2 h resolution, we argue that the results of our analysis are robust and only minimally affected by the data coarse temporal resolution.
(a). Hp1: birds exploit wind differently during the transit and searching states
During the transit phases of their trips, Desertas petrels most intensively travelled with a wind Δangle of approximately 60°, similar to the value (approx. 50°) predicted by the wind model to maximize their ground speed. With this angle, wandering [8] and black-browed [19] albatrosses were also found to travel at low energy expenditure per time unit. During the searching sections of their trips, birds showed a wind direction preference that peaked at higher Δangle values (approx. 68°), i.e. for less favourable winds blowing more from the side. Interestingly, although the Δangle selectivity during searching was lower (broader interquartile range), searching birds still showed a degree of wind selectivity. This supports the conclusion (presented below) that, when engaging in the searching activity, birds do not confine their search to specific areas, which would result in a higher variance of wind Δangle used. Rather, they continue following their routes that allow a low cost movement, exploiting foraging opportunities encountered along the way. The differences in wind use between transiting and searching could be driven by a series of factors. Firstly, searching birds are predicted to make shorter, less directional (but see above) movements characteristic of the search behaviour, hence experiencing a wider range of Δangle. Secondly, birds flying with less favourable winds can decrease their ground speed while still attaining adequate airspeeds to sustain flight, which could facilitate visual prey detection [20,38,39]. Thirdly, while looking for food, birds rely—to various extents—on odour plume detection, which in seabirds [40,41] and in a wide range of other animals [42,43] was found to benefit from orthogonal or oblique movements with respect to the direction of flow.
(b). Hp2: during transit, birds fly with the most favourable relative wind direction
The wind model showed that the ground speed of transiting Desertas petrels was affected by wind relative direction, wind speed and their interaction, with greatest speeds attained when the birds travelled with strong, quartering tail winds (with Δangle around 50°). Hence, our results revealed that transiting gadfly petrels efficiently exploit the wind fields, preferentially using quartering tail winds and thus maximizing ground speed. Seabirds flying with these favourable wind conditions were found to travel at minimum energetic cost [8,19]. Dynamic soaring and gliding, which characterize Pterodroma's flight and are the least energetically expensive flight behaviour [19,20,44], are indeed strongly associated with intense cross- and tailwinds [12,45].
(c). Hp3: the foraging strategy is based on covering large distances to increase the probability of finding prey
The tracked birds search state occurred in waters that were on average deeper than 3600 m. The petrels performed ‘looping’ trips [29], departing from the colony travelling southwest and returning from a northerly direction, a movement type—documented in pelagic species—suggesting a continuous search along the trip, which was explained in light of the food resources unpredictability at the coarse to meso-scale [29]. Desertas petrels' foraging hotspots were all located in deep, high-sea waters (electronic supplementary material) and there was a limited among-individual foraging hotspots overlap compared with the travelling hotspots. Foraging site consistency in seabirds is related to predictability in marine resources, which are likely to be patchy and, at the coarse to meso-scale, unpredictable in the oceanic domain exploited by these birds [29]. Hence, to capitalize net energy gain, Desertas petrels seemed to rely on a foraging strategy based on covering large distances to increase the probability of encountering foraging opportunities along the route rather than consistently targeting known foraging areas [8,46]. Our results corroborate and build mechanistically upon the findings of previous studies, in which breeding gadfly petrels were found to have a similar foraging strategy, with long foraging trips [4–7] characterized by low foraging site consistency [6], and long periods of direct flight [4,7], and in which birds travelling for longer distances had higher mass gains [6]. It is important to note that the birds preferentially headed west when leaving the colony and used more intensely the waters in the region to the west-northwest of the Azores. This region is part of the North Atlantic Current and mid-Atlantic Subpolar frontal system (NAC-mASPF), characterized by the presence of thermal fronts and eddies and enhanced productivity at the large scale [47,48]. This suggests that, along their long and efficiently-designed routes, Desertas petrels did target a broad area of enhanced productivity, but at a large oceanic scale (greater than 1000 km2), continuously searching for food and maximizing the probability of prey encounter by covering more ground within this region and also while travelling to and from this region.
During the inbound phase of the journeys birds are more limited both in their choice of flight directions and in their remaining travelling time, as not only must they return to the colony, but also they must make sure to do so within the temporal constraints dictated by their parental duties [49]. However, we found that during the return section of the tracks, Desertas petrels do not fly towards the colony following a beeline, which would take them to the nest in a shorter time. Rather, they choose to undertake a longer route, characterized by better winds that enable them to fly at higher speed. This result, together with the HMM classification showing that the percentage of searching locations was similar in the outbound (46%) and inbound (41%) sections of the trips, strongly supports the idea that they continue looking for food also during the return phase of their journeys. To further increase the chances of encountering foraging opportunities, they keep travelling with favourable winds, selecting a longer path that allows them to cover more ground at high speed, also on their way back to the colony.
(d). Hp4: travelling birds optimize their route to cover the largest distance in the shortest time by adjusting to regional and local wind conditions
The results on the random, inverse, rotated and time-lagged tracks offer further insight into Desertas petrels' flight behaviour. First, the clockwise observed tracks were faster than all their equivalent simulated trips. In particular, they were on average 65.2 h faster (i.e. more than one-third of the average duration of the observed tracks) than the respective inverse trips. Second, while the birds' observed route was significantly shorter than the corresponding rotated tracks, some rotated tracks near the observed trip would have granted the bird a slightly higher speed. Moreover, there was a high overlap between the individuals' travelling hotspots, indicating a significant extent of travelling route consistency among individuals. The selection of fast clockwise trajectories, the concentration of favourable simulated tracks around the observed one and the spatial consistency in the individuals' travelling routes all suggest that the predictable clockwise North Atlantic winds generate, at the large scale, predictable favourable flyways preferentially used by the birds. Furthermore, the real trips were significantly faster than the respective lagged tracks. In other words, if the observed trip were performed at another time—i.e. with different wind conditions—more time would have been required to complete it. This suggests that, while birds undertake trips that take advantage of a priori knowledge of the clockwise regional wind regimes prevailing in the North Atlantic, they are also capable of refining their route, adjusting it to the local wind conditions, in order to maximize their speed and distance travelled, ultimately maximizing the probability of finding prey.
5. Conclusion
Our research revealed new insights into the foraging strategy and flight behaviour of gadfly petrels, which are arguably the best performing flyers in the animal kingdom. These oceanic birds show a strategic use of wind, which enables them to travel at high speed and low cost. In turn, efficiently using winds allows them to exhibit a foraging strategy based on covering large distances while searching for food along the route rather than selecting specific foraging hotspots. Our findings suggest that the mechanisms at the core of this strategy, particularly advantageous in the oceanic marine domain, characterized by unpredictable resources distribution at the coarse to meso-scale, seem to be a prior knowledge of the—predictable—prevailing winds and the capability of efficiently refining their route to the local wind conditions. We argue that our quantitative analysis and our novel simulation framework can be successfully extended to reveal aspects of the ecology and movement behaviour of other wide-ranging animals moving through dynamic fluids such as air or water.
Supplementary Material
Acknowledgements
Thanks to Tegan Newman, Patrícia Pedro, Isamberto Silva, João Morgado, Filipe Moniz, Teresa Catry and the wardens of Desertas Islands Nature Reserve for excellent fieldwork support. Instituto das Florestas e da Conservação da Natureza (and particularly Dília Menezes) gave permissions and support for the work at Desertas Islands. We are grateful to Mónica Silva for helping making resources available for data analysis and write-up and to Maria Dias and Ewan Wakefield for useful and inspiring discussions. We also thank two anonymous referees for helpful comments on an earlier version of this manuscript.
Data accessibility
The GPS tracking dataset is available in the BirdLife Seabird Tracking Database (http://seabirdtracking.org/mapper/?dataset_id=1463). The supporting data and R scripts to reproduce the analysis are available as part of the electronic supplementary material.
Authors' contributions
F.V. performed the research, developed the analytical approach, analysed the data and drafted the manuscript; O.P. discussed data analysis and helped draft the manuscript; J.P.G. conceived the research, collected field data, developed the analytical approach and critically revised the manuscript; P.C. conceived and performed the research, collected field data, developed the analytical approach and helped draft the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the Fundação para a Ciência e a Tecnologia (FCT, Portugal) through the projects: Oceanwebs (grant no. PTDC/MAR-PRO/0929/2014) and Oceantree (grant no. PTDC/BIA-EVL/28565/2017); strategic project MARE (grant no. UID/MAR/04292/2019), granted to MARE; Bolsa de Gestão de Ciência e Tecnologia (grant no. UID/AMB/50017/2019), granted to CESAM.
References
- 1.Tucker VA. 1970. Energetic cost of locomotion in animals. Comp. Biochem. Physiol. 34, 841–846. ( 10.1016/0010-406X(70)91006-6) [DOI] [PubMed] [Google Scholar]
- 2.Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, Lehman S. 2000. How animals move: an integrative view. Science 288, 100–106. ( 10.1126/science.288.5463.100) [DOI] [PubMed] [Google Scholar]
- 3.Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. 2008. A movement ecology paradigm for unifying organismal movement research. Proc. Natl Acad. Sci. USA 105, 19 052–19 059. ( 10.1073/pnas.0800375105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rayner MJ, Taylor GA, Gummer HD, Phillips RA, Sagar PM, Shaffer SA, Thompson DR. 2012. The breeding cycle, year-round distribution and activity patterns of the endangered Chatham petrel (Pterodroma axillaris). Emu 112, 107–116. ( 10.1071/MU11066) [DOI] [Google Scholar]
- 5.Ramos R, et al. 2017. It is the time for oceanic seabirds: tracking year-round distribution of gadfly petrels across the Atlantic Ocean. Divers. Distrib. 23, 794–805. ( 10.1111/ddi.12569) [DOI] [Google Scholar]
- 6.Clay TA, Oppel S, Lavers JL, Phillips RA, de Brooke ML. 2019. Divergent foraging strategies during incubation of an unusually wide-ranging seabird, the Murphy's petrel. Mar. Biol. 166, 8 ( 10.1007/s00227-018-3451-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramírez I, Paiva VH, Menezes D, Silva I, Phillips RA, Ramos JA, Garthe S. 2013. Year-round distribution and habitat preferences of the Bugio petrel. Mar. Ecol. Prog. Ser. 476, 269–284. ( 10.3354/meps10083) [DOI] [Google Scholar]
- 8.Weimerskirch H, Guionnet T, Martin J, Shaffer SA, Costa DP. 2000. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proc. R. Soc. Lond. B 267, 1869–1874. ( 10.1098/rspb.2000.1223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakefield ED, Phillips RA, Matthiopoulos J, Fukuda A, Higuchi H, Marshall GJ, Trathan PN. 2009. Wind field and sex constrain the flight speeds of central-place foraging albatrosses. Ecol. Monogr. 79, 663–679. ( 10.1890/07-2111.1) [DOI] [Google Scholar]
- 10.Amelineau F, Peron C, Lescroel A, Authier M, Provost P, Gremillet D. 2014. Windscape and tortuosity shape the flight costs of northern gannets. J. Exp. Biol. 217, 876–885. ( 10.1242/jeb.097915) [DOI] [PubMed] [Google Scholar]
- 11.Adams J, Flora S. 2010. Correlating seabird movements with ocean winds: linking satellite telemetry with ocean scatterometry. Mar. Biol. 157, 915–929. ( 10.1007/s00227-009-1367-y) [DOI] [Google Scholar]
- 12.Gibb R, Shoji A, Fayet AL, Perrins CM, Guilford T, Freeman R. 2017. Remotely sensed wind speed predicts soaring behaviour in a wide-ranging pelagic seabird. J. R. Soc. Interface 14, 20170262 ( 10.1098/rsif.2017.0262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suryan RM, et al. 2008. Wind, waves, and wing loading: morphological specialization may limit range expansion of endangered albatrosses. PLoS ONE 3, e4016 ( 10.1371/journal.pone.0004016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaffer SA, Hazen EL, Thorne LH, Antolos M, Bograd SJ, Costa DP, Conners MG. 2016. Effects of El Niño-driven changes in wind patterns on North Pacific albatrosses. J. R. Soc. Interface 13, 20160196 ( 10.1098/rsif.2016.0196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weimerskirch H, Louzao M, de Grissac S, Delord K. 2012. Changes in wind pattern alter life-history traits. Science 335, 211–214. ( 10.1126/science.1210270) [DOI] [PubMed] [Google Scholar]
- 16.Pennycuick CJ. 2002. Gust soaring as a basis for the flight of petrels and albatrosses (Procellariiformes). Avian Sci. 2, 1–12. [Google Scholar]
- 17.Sachs G, Traugott J, Nesterova AP, Dell'Omo G, Kümmeth F, Heidrich W, Vyssotski AL, Bonadonna F. 2012. Flying at no mechanical energy cost: disclosing the secret of wandering albatrosses. PLoS ONE 7, e41449 ( 10.1371/journal.pone.0041449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson PL. 2011. How do albatrosses fly around the world without flapping their wings? Prog. Oceanogr. 88, 46–58. ( 10.1016/j.pocean.2010.08.001) [DOI] [Google Scholar]
- 19.Sakamoto KQ, Takahashi A, Iwata T, Yamamoto T, Yamamoto M, Trathan PN. 2013. Heart rate and estimated energy expenditure of flapping and gliding in black-browed albatrosses. J. Exp. Biol. 216, 3175–3182. ( 10.1242/jeb.079905) [DOI] [PubMed] [Google Scholar]
- 20.Spear LB, Ainley DG. 1997. Flight behaviour of seabirds in relation to wind direction and wing morphology. Ibis 139, 221–233. ( 10.1111/j.1474-919X.1997.tb04620.x) [DOI] [Google Scholar]
- 21.Paiva VH, Guilford T, Meade J, Geraldes P, Ramos JA, Garthe S. 2010. Flight dynamics of Cory's shearwater foraging in a coastal environment. Zoology 113, 47–56. ( 10.1016/j.zool.2009.05.003) [DOI] [PubMed] [Google Scholar]
- 22.Richardson PL, Wakefield ED, Phillips RA. 2018. Flight speed and performance of the wandering albatross with respect to wind. Mov. Ecol. 6, 3 ( 10.1186/s40462-018-0121-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.BirdLife International. 2017. Pterodroma deserta. The IUCN Red List of Threatened Species 2017 ( 10.2305/IUCN.UK.2017-3.RLTS.T22736135A119180790.en) [DOI]
- 24.Viswanathan GM, Raposo EP, da Luz MGE. 2008. Lévy flights and superdiffusion in the context of biological encounters and random searches. Phys. Life Rev. 5, 133–150. ( 10.1016/j.plrev.2008.03.002) [DOI] [Google Scholar]
- 25.Viswanathan GM, Afanasyev V, Buldyrev SV, Murphy EJ, Prince PA, Stanley HE. 1996. Lévy flight search patterns of wandering albatrosses. Nature 381, 413 ( 10.1038/381413a0) [DOI] [PubMed] [Google Scholar]
- 26.Humphries NE, Weimerskirch H, Queiroz N, Southall EJ, Sims DW. 2012. Foraging success of biological Lévy flights recorded in situ. Proc. Natl Acad. Sci. USA 109, 7169–7174. ( 10.1073/pnas.1121201109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards AM, et al. 2007. Revisiting Lévy flight search patterns of wandering albatrosses, bumblebees and deer. Nature 449, 1044–1048. ( 10.1038/nature06199) [DOI] [PubMed] [Google Scholar]
- 28.Palyulin VV, Chechkin AV, Metzler R. 2014. Lévy flights do not always optimize random blind search for sparse targets. Proc. Natl Acad. Sci. USA 111, 2931–2936. ( 10.1073/pnas.1320424111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weimerskirch H. 2007. Are seabirds foraging for unpredictable resources? Deep Sea Res. Part II Top. Stud. Oceanogr. 54, 211–223. ( 10.1016/j.dsr2.2006.11.013) [DOI] [Google Scholar]
- 30.Calenge C. 2016. Analysis of animal movements in R: the adehabitatLT package. See https://cran.r-project.org/web/packages/adehabitatLT/vignettes/adehabitatLT.pdf.
- 31.Dell'Ariccia G, Benhamou S, Dias MP, Granadeiro JP, Sudre J, Catry P, Bonadonna F. 2018. Flexible migratory choices of Cory's shearwaters are not driven by shifts in prevailing air currents. Scient. Rep. 8, 3376 ( 10.1038/s41598-018-21608-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClintock BT, Michelot T. 2018. momentuHMM: R package for generalized hidden Markov models of animal movement. Methods Ecol. Evol. 9, 1518–1530. ( 10.1111/2041-210X.12995) [DOI] [Google Scholar]
- 33.Zucchini W, MacDonald IL, Langrock R. 2016. Hidden Markov models for time series: an introduction using R. Boca Raton, FL: Chapman & Hall/CRC. [Google Scholar]
- 34.Oppel S, et al. 2018. Spatial scales of marine conservation management for breeding seabirds. Mar. Policy 98, 37–46. ( 10.1016/j.marpol.2018.08.024) [DOI] [Google Scholar]
- 35.Ventura F, Matthiopoulos J, Jeglinski JWE. 2019. Minimal overlap between areas of high conservation priority for endangered Galapagos pinnipeds and the conservation zone of the Galapagos Marine Reserve. Aquat. Conserv. Mar. Freshw. Ecosyst. 29, 115–126. ( 10.1002/aqc.2943) [DOI] [Google Scholar]
- 36.Wood SN. 2006. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- 37.Grecian WJ, et al. 2016. Seabird diversity hotspot linked to ocean productivity in the Canary Current Large Marine Ecosystem. Biol. Lett. 12, 201600024 ( 10.1098/rsbl.2016.0024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alerstam T, Larsson B. 1993. Flight tracks and speeds of Antarctic and Atlantic seabirds : radar and optical measurements. Phil. Trans. R. Soc. Lond. B 340, 55–67. ( 10.1098/rstb.1993.0048) [DOI] [Google Scholar]
- 39.Machovsky-Capuska GE, Howland HC, Raubenheimer D, Vaughn-Hirshorn R, Wursig B, Hauber ME, Katzir G. 2012. Visual accommodation and active pursuit of prey underwater in a plunge-diving bird: the Australasian gannet. Proc. R. Soc. B 279, 4118–4125. ( 10.1098/rspb.2012.1519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nevitt GA, Losekoot M, Weimerskirch H. 2008. Evidence for olfactory search in wandering albatross, Diomedea exulans. Proc. Natl Acad. Sci. USA 105, 4576–4581. ( 10.1073/pnas.0709047105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds AM, Cecere JG, Paiva VH, Ramos JA, Focardi S. 2015. Pelagic seabird flight patterns are consistent with a reliance on olfactory maps for oceanic navigation. Proc. R. Soc. B 282, 20150468 ( 10.1098/rspb.2015.0468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardé RT. 2007. Odour plumes and odour-mediated flight in insects. In Ciba Foundation Symp. 200 – Olfaction in Mosquito–Host Interactions (eds Bock GR, Cardew G), pp. 54–70. ( 10.1002/9780470514948.ch6) [DOI] [PubMed] [Google Scholar]
- 43.Vickers NJ. 2000. Mechanisms of animal navigation in odor plumes. Biol. Bull. 198, 203–212. ( 10.2307/1542524) [DOI] [PubMed] [Google Scholar]
- 44.Pennycuick CJ. 1982. The flight of petrels and albatrosses (Procellariiformes), observed in South Georgia and its vicinity. Phil. Trans. R. Soc. Lond. B 300, 75–106. ( 10.1098/rstb.1982.0158) [DOI] [Google Scholar]
- 45.Ainley DG, Porzig E, Zajanc D, Spear LB. 2015. Seabird flight behavior and height in response to altered wind strength and direction. Mar. Ornithol. 43, 25–36. [Google Scholar]
- 46.Weimerskirch H, Gault A, Cherel Y. 2005. Prey distribution and patchiness: factors in foraging success and efficiency of wandering albatrosses. Ecology 86, 2611–2622. ( 10.1890/04-1866) [DOI] [Google Scholar]
- 47.Rossby T. 1996. The North Atlantic Current and surrounding waters: at the crossroads. Rev. Geophys. 34, 463–481. ( 10.1029/96RG02214) [DOI] [Google Scholar]
- 48.Belkin IM, Levitus S. 1996. Temporal variability of the subarctic front near the Charlie-Gibbs fracture zone. J. Geophys. Res. Oceans 101, 28 317–28 324. ( 10.1029/96JC02794) [DOI] [Google Scholar]
- 49.Stephens DW, Krebs JR. 1986. Foraging theory. Princeton, NJ: Princeton University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The GPS tracking dataset is available in the BirdLife Seabird Tracking Database (http://seabirdtracking.org/mapper/?dataset_id=1463). The supporting data and R scripts to reproduce the analysis are available as part of the electronic supplementary material.