Abstract
People with Insomnia Disorder tend to underestimate their sleep compared with polysomnography or actigraphy, a phenomenon known as paradoxical insomnia or sleep‐state misperception. Previous studies suggested that night‐to‐night variability could be an important feature differentiating subtypes of misperception. This study aimed for a data‐driven definition of misperception subtypes revealed by multiple sleep features including night‐to‐night variability. We assessed features describing the mean and dispersion of misperception and objective and subjective sleep duration from 7‐night diary and actigraphy recordings of 181 people with Insomnia Disorder and 55 people without sleep complaints. A minimally collinear subset of features was submitted to latent class analysis for data‐driven subtyping. Analysis revealed three subtypes, best discriminated by three of five selected features: an individual’s shortest reported subjective sleep duration; and the mean and standard deviation of misperception. These features were on average 5.4, −0.0 and 0.5 hr in one subtype accommodating the majority of good sleepers; 4.1, −1.4 and 1.0 hr in a second subtype representing the majority of people with Insomnia Disorder; and 1.7, −2.2 and 1.5 hr in a third subtype representing a quarter of people with Insomnia Disorder and hardly any good sleepers. Subtypes did not differ on an individual’s objective sleep duration mean (6.9, 7.2 and 6.9 hr) and standard deviation (0.8, 0.8 and 0.9 hr). Data‐driven analysis of naturalistic sleep revealed three subtypes that markedly differed in misperception features. Future studies may include misperception subtype to investigate whether it contributes to the unexplained considerable individual variability in treatment response.
Keywords: clustering analysis, objective insomnia, subjective insomnia, subjective−objective sleep discrepancy
1. INTRODUCTION
Insomnia Disorder (ID) is the second most common mental disorder in Europe (Wittchen et al., 2011). The disorder is characterized by subjective reporting of difficulty initiating or maintaining sleep or early morning awakening despite adequate opportunity for sleep, accompanied by daytime impairment (American Psychiatric Association, 2013; Diagnostic Classification Steering Committee, 2014). People with ID have a tendency to overestimate their sleep‐onset latency (SOL) and underestimate their total sleep time (TST) when compared with simultaneous objective estimates of sleep recorded by polysomnography (PSG; Harvey & Tang, 2012; Means, Edinger, Glenn, & Fins, 2003; Perlis, Smith, Andrews, Orff, & Giles, 2001) or actigraphy (Tang & Harvey, 2006; Van Den Berg et al., 2008). This negative discrepancy between objectively measured and subjectively experienced sleep was referred to as sleep‐state misperception (SSM) in earlier versions of the International Classification of Sleep Disorders (ICSD), and renamed to paradoxical insomnia in the third edition of the ICSD (Diagnostic Classification Steering Committee, 2014; Harvey & Tang, 2012). Good sleepers, on the other hand, tend to accurately estimate their sleep or overestimate their sleep (Feige et al., 2008; Manconi et al., 2010; Mendelson, 1995).
No consensus has been reached on whether “sleep‐state misperception” or “paradoxical insomnia” represent a separate subtype of insomnia, or rather represent a symptom that varies along a dimensional continuum (Harvey & Tang, 2012). One study comparing sleep diary with PSG in ID and good sleepers found misperception across a continuum of which only 23% of good sleepers underestimated their TST by no more than 50 min, while 43% of ID underestimated their sleep by up to 200 min. Similarly, overestimation was no more than 100 min for good sleepers, but up to 200 min in ID (Feige et al., 2008). Several studies have found distinct subtypes using data‐driven techniques. One laboratory study found a bimodal distribution in the absolute misperception as well as the relative misperception index (MI; misperception divided by objective sleep time) derived from PSG recordings and sleep diaries (Manconi et al., 2010). The bimodal distribution was indicative of a group of ID with very high levels of misperception, distinctly different from the remaining ID in whom misperception ranged from overestimation to moderate underestimation. Another laboratory study found that misperception only occurred in ID with normal sleep duration (≥ 6 hr) but not in those with short sleep duration (< 6 hr; Fernandez‐Mendoza et al., 2011). Using hierarchical clustering on observations across two laboratory and two home PSG assessments of misperception, Means et al. (2003) found four clusters in the misperception of ID and three clusters in the misperception of good sleepers. The majority of people with ID were allocated to a cluster characterized by slight underestimates of sleep time and a second cluster with reasonably accurate sleep time estimates. The remaining ID were allocated to two clusters, one characterized by substantial underestimation of sleep and the other by an overestimation of sleep duration. The majority of normal sleepers were allocated to a cluster characterized by consistently accurate perception of their sleep duration and another cluster characterized by a consistent overestimation of sleep duration. The third cluster of normal sleepers had a “random” pattern of under‐ and overestimates during lab and home nights.
Most studies to date have compared sleep diaries with PSG across 1 or 2 nights. The variability of sleep duration and misperception observed across multiple nights may, however, contain important information not captured during a few nights or by averaging (Bei, Wiley, Trinder, & Manber, 2016; Herbert, Pratt, Emsley, & Kyle, 2017; Kay, Dzierzewski, Rowe, & McCrae, 2013; Van Someren, 2007). As much as 54% of the variance in MI has been attributed to the individual (within‐subject) variance of misperception across 7 nights (Herbert et al., 2017). Another study found larger variability in the misperception of people with sleep complaints than those without sleep complaints, indicating that the variability itself may be an important factor discriminating between good sleepers and those with ID (Kay et al., 2013).
We hypothesized that night‐to‐night variability of multiple sleep features derived from actigraphy and sleep diaries across multiple nights in a home environment may better reveal misperception subtypes. Therefore, we quantified the night‐to‐night variability of subjective and objective sleep duration, and misperception, across a large sample of ID and good sleepers. To do so, we assessed sleep features from diaries and actigraphy for 7 nights in their home environment and used them for data‐driven subtype finding.
2. METHODS
2.1. Participants
To evaluate the discrepancy between objective and subjective sleep duration, we retrospectively analysed actigraphy and sleep diaries collected during several studies in our sleep laboratory (Dekker et al., 2019; Wassing et al., 2019; Wei et al., 2017). All studies were approved by the ethics committee of the VU University Medical Center, Amsterdam, the Netherlands. Participants were recruited through advertisement and the Netherlands Sleep Registry (https://www.sleepregistry.org; Benjamins et al., 2016). Screening was performed using online questionnaires, a structured interview and by telephone, and included the Insomnia Severity Index (ISI; Bastien, Vallieres, & Morin, 2001). All participants provided written informed consent. Participants of multiple studies were included in the current dataset only once. Criteria for the ID group (n = 181, age range 22–69 years) were conformed to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (American Psychiatric Association, 2013), the ICSD, Third Edition (Diagnostic Classification Steering Committee, 2014) as well as to the Research Diagnostic Criteria for Insomnia Disorder (Edinger et al., 2004). We moreover added the criterion of an ISI score ≥ 10. More details can be found in the original papers (Dekker et al., 2019; Wassing et al., 2019; Wei et al., 2017). One study (Dekker et al., 2019) included only people with ID, resulting in a smaller control group. The control (CTRL) group included age‐ and sex‐matched volunteers (n = 55, age range 22–70 years) that reported to have no sleep difficulties by phone and had an ISI score ≤ 9. Additional inclusion criteria were the availability of at least 5 out of 7 consecutive nights of valid actigraphy and matching complete sleep diaries. Table 1 summarizes the demographic characteristics of the included participants and their ISI scores.
Table 1.
Participant demographics (mean ± standard deviation)
| Characteristic | Control (n = 55) | ID (n = 181) | p |
|---|---|---|---|
| Sex, female/male | 39/16 | 140/41 | .37 |
| Age, years | 46.4 ± 15.1 | 50.5 ± 12.0 | .11 |
| ISI | 2.3 ± 2.5 | 16.8 ± 3.4 | < .0001 |
Bold font highlights significant differences.
Abbreviations: ID, Insomnia Disorder; ISI, Insomnia Severity Index.
2.2. Protocol
People with ID and matched CTRL were asked to complete 7 nights of actigraphy and sleep diaries at home. Ninety‐two nights with missing or incomplete data, from 49 people with ID and 11 controls were removed from the dataset. In total 236 participants with at least 5 nights (median [range]: 7 [5–7] nights) were included: 9 participants (1 CTRL/8 ID) had 5 nights, 51 participants (10/41) had 6 nights, and 176 participants (43/133) had 7 nights. A total of 1,583 nights were included in the analyses. Actigraphy analyses were performed using custom scripts written in MATLAB 8.3 (The MathWorks, Natick, Massachusetts, USA; https://github.com/btlindert/actant-1). All other analyses were performed using R (R Core Team, 2018).
2.3. Actigraphy
Actigraphy was recorded using a microelectromechanical system accelerometer (Geneactiv, ActivInsights) at a sampling frequency of 50 Hz and uploaded to a pc using Geneactiv PC software (version 1.0, ActivInsights). Activity counts were calculated using a validated method (te Lindert & Van Someren, 2013). After conversion to counts, the counts time series were recalculated by weighing the counts in each epoch by the counts in the two epochs preceding and the two epochs following the epoch of interest (Kushida et al., 2001). Subsequently, activity counts above a predefined wake sensitivity threshold were rated as wake and below the threshold as sleep. Starting from lights off time, the first period of 5 min of consecutive sleep encountered was defined as the onset of sleep. The sum of all epochs scored as sleep after onset was summed to obtain the objective TST (oTST). We used this commonly used so‐called “sleep−wake” algorithm with a wake sensitivity threshold of 40, which was found optimal for sleep estimates in ID and in samples of unknown diagnosis (te Lindert et al., 2019).
2.4. Sleep diaries
The consensus sleep diary (Carney et al., 2012) was completed daily on paper or online (Benjamins et al., 2016). For every individual night, the subjective TST (sTST) was calculated by subtracting the subjectively reported SOL and wake after sleep onset (WASO) from the period between lights out and final wake time.
2.5. Sleep and misperception features
Misperception of TST (mTST) was calculated for each individual night as the sTST from actigraphy minus the oTST obtained from the sleep diary, resulting in negative values for the underestimation of sleep. In addition to this absolute measure, we also computed the relative MI (Manconi et al., 2010) Across the 7 days, the calculated within‐subject measures of centrality and dispersion in oTST, sTST, mTST were the mean, median, standard deviation (SD), minimum, maximum and the mean square of successive differences (MSSD; von Neumann, Kent, Bellinson, & Hart, 1941) using the ‘psych’ R package (Revelle, 2018). For sequences of random uncorrelated data, the MSSD equals twice the variance. If sequences of data points are auto‐correlated, the MSSD is smaller than twice the variance. MSSD thus provides additional information to the variance. To avoid collinearity in the features selected for cluster analyses, we focussed on TST, which correlates with SOL, WASO and other sleep features.
2.6. Feature selection
Considerable interdependence may exist between the features (Dejonckheere et al., 2019). We therefore used the Goldbricker method from the “networktools” R package (Hittner, May, & Silver, 2003; Jones, 2018) to select a subset of features optimized to have minimal collinearity for use in subsequent unsupervised latent class analysis (LCA). The Goldbricker method considers the correlations of a set of features with all other features and can be used to find the best sparse set of minimally correlated features. Because LCA assumes each pair of features to be statistically independent within each subtype, minimally correlated features are a requirement. Goldbricker may be preferred over traditional principal component analysis (PCA) where resulting components may still correlate. The Goldbricker method calculates for each pair of features whether they correlate to the same degree with other variables. If these correlations are highly similar, the pair may redundantly be measuring the same construct and one of the features in the pair should be removed to avoid collinearity. Results of the Goldbricker method depend on two parameters: the minimal percentage of significantly different correlations; and the minimal correlation evaluated. We used a threshold of 75%, i.e. a pair of features should have significantly different correlations with 75% of the other features. We accepted minimal correlations of 0.25. For every collinear pair, we choose to include the computationally simpler variable (e.g. SD instead of MSSD).
2.7. Latent class analysis
2.7.1. Model estimation
We used LCA (implemented in the Latent GOLD software package; Vermunt & Magidson, 2002, 2016) for a data‐driven evaluation of the presence of subtypes in profiles of objective and subjective sleep. Both ID and controls (n = 236) were included in the analysis. The characteristics were entered as continuous variables and evaluated using the default settings of Latent GOLD (Vermunt & Magidson, 2002).
We determined the most probable number of subtypes by stepwise increasing the number of subtypes, and selecting the model that minimized the Bayesian information criterion (BIC; Vermunt & Magidson, 2016). Importantly, to obtain a robust solution we used fivefold cross‐validation, which splits the sample into five folds. For each possible number of classes, the model was trained on a training set consisting of four of the five folds (80% of the data) and subsequently applied on the hold‐out test set (20% of the data) to calculate the BIC. This was repeated across all five combinations of training and test sets. The number of classes with the lowest mean BIC across the five independent test sets was chosen as the most robust solution.
2.7.2. Model evaluation
Using the model with the most probable number of subtypes, we classified participants to one of the subtypes and evaluated the misclassification error by the median posterior class‐membership probabilities and the classification error. Each participant has a posterior probability to belong to each of the subtypes, which together sum to 1. Participants are assigned to the subtype for which this posterior probability is highest. The median posterior class‐membership probability indicates the certainty with which participants are assigned to a specific subtype. The overall classification error estimates the proportion of participants that are misclassified across all subtypes. It is calculated by averaging the misclassification probabilities (1—posterior probability) of each individual.
Model assumptions were evaluated using the bivariate residual. LCA assumes each pair of features to be statistically independent within a subtype. To evaluate this assumption, the residual relation among scores of two features within a class is estimated (called the bivariate residual). When the bivariate residual is substantially larger than 1, this indicates model misfit (Magidson & Vermunt, 2004). Mean values for each of the features in a subtype (i.e. class centroids), subtype size and explained variance were calculated for the final model.
2.7.3. Comparison with insomnia types
The final model was compared with recently discovered insomnia types derived from personality traits and personal history (Blanken et al., 2019) to assess if misperception subtypes were related to insomnia types.
3. RESULTS
Misperception was observed across a continuum. Figure 1 shows the distribution of individual night discrepancies between objectively measured and subjectively experienced sleep for both ID and CTRL. While good sleepers tended to accurately estimate or overestimate their sleep, the distribution was shifted leftward in ID, indicating that they were more likely to perceive the time spent asleep as shorter than suggested by actigraphy.
Figure 1.
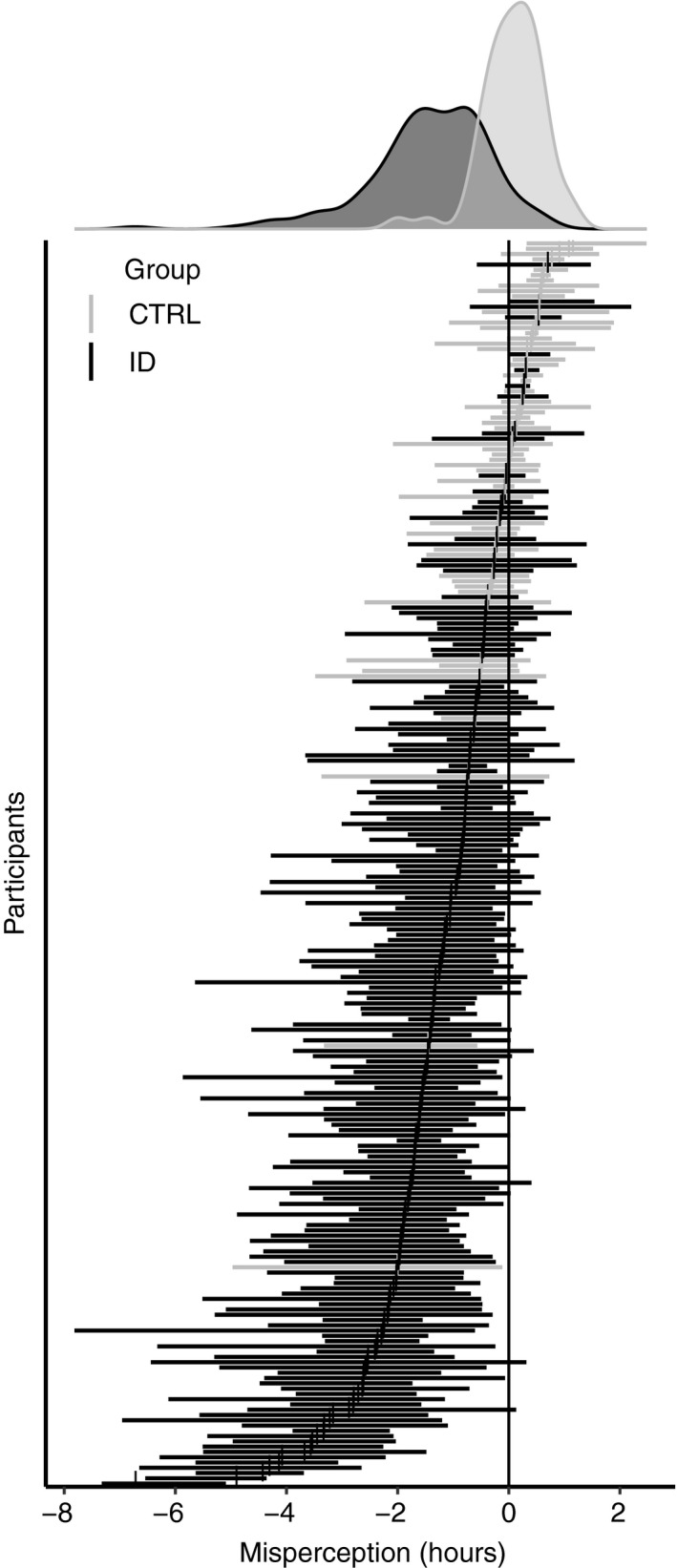
The mean and range of misperception for each individual derived from up to 7 ambulatory nights of actigraphy and sleep diaries. Both Insomnia Disorder (ID, black) and good sleepers are plotted (CTRL, grey). The density plots summarize the group distribution of subject average misperception
The Goldbricker method selected a set of five features that best covered the variance in the data while minimizing collinearity between features: the mean oTST, the SD of oTST, the shortest sTST, the mean mTST and the SD of mTST. Of note, three of the variables concern within‐subject variability across days, providing strong support for our contention that night‐to‐night variability could be an important feature differentiating subtypes.
Including these five features in the LCA with fivefold cross‐validation indicated that a model with three latent classes had the lowest BIC (Table 2). This three‐subtype model explained 81.7% of the variance and a classification accuracy of 92% (the estimated classification error was 8%) when fitted on the full dataset. Posterior class‐membership probabilities were high across all subtypes at (median [range]) 0.99 [0.59–1.00], 0.96 [0.42–1.00] and 1.00 [0.45–1.00] for subtypes 1, 2 and 3, respectively.
Table 2.
BIC, classification error and explained variance of latent class models of different cluster sizes across five independent folds
| Model | BIC | Classification error (%) | R 2 |
|---|---|---|---|
| 1 cluster | 12,519 | 0.0 | 1 |
| 2 clusters | 12,329 | 6.6 | 0.81 |
| 3 clusters | 12,321 | 9.6 | 0.79 |
| 4 clusters | 12,359 | 12.5 | 0.75 |
| 5 clusters | 12,415 | 14.8 | 0.73 |
Abbreviation: BIC, Bayesian Information Criterion.
Classification error, estimated classification error based on the posterior probabilities of individual cluster assignments.
R 2, explained variance.
The three‐cluster model resulted in the lowest BIC across five independent folds.
On average, subtype 1 can concisely be characterized as getting 6.9 hr of actigraphically estimated sleep, varying across days with an SD of 0.8 hr, and subjectively estimating their sleep duration to be no less than 5.4 hr (Figure 2). The average misperception of subtype 1 is 0.0 hr and varies across days with a SD of 0.5 hr (Figure 3). A minority of people with ID (22%) and the majority of good sleepers (87%) showed this profile.
Figure 2.
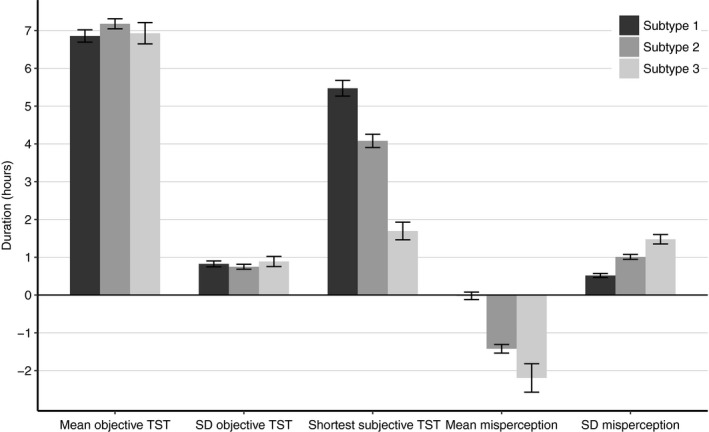
Characteristic features for individuals in each subtype. Mean ± 95% confidence interval calculated across all individuals assigned to each subtype using latent class cluster analysis (LCA). SD, standard deviation; TST, total sleep time
Figure 3.
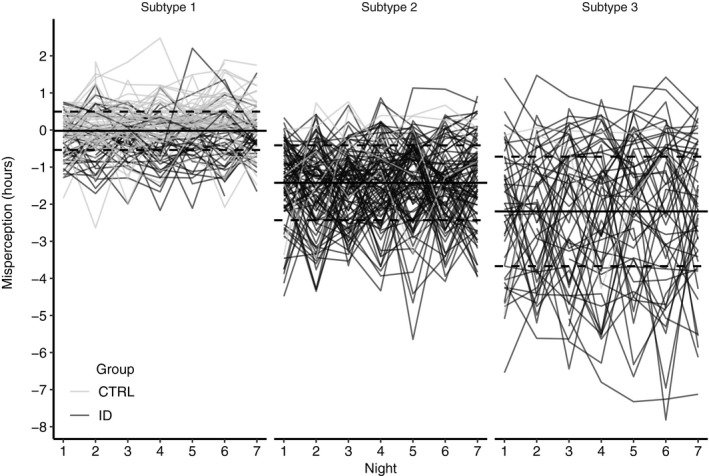
Misperception of sleep across 7 ambulatory nights for individuals assigned to each of the three classes derived from the latent class cluster analysis (LCA). Individual traces of misperception are plotted for people with Insomnia Disorder (ID, black) and good sleepers (CTRL, grey). Mean misperception (dashed lines) and ± SD (dotted lines) derived from the LCA model
On average, subtype 2 can be characterized as getting 7.2 hr of actigraphically estimated sleep, varying across days with an SD of 0.8 hr, and subjectively estimating their sleep duration to be no less than 4.1 hr. The average misperception of subtype 2 is −1.4 hr and varies across days with a SD of 1 hr. Half of the people with ID (49%) and a small number of good sleepers (9%) showed this profile. On average, subtype 3 can be characterized as getting 6.9 hr of actigraphically estimated sleep, varying across days with an SD of 0.9 hr, and subjectively estimating their sleep duration to be no less than 1.7 hr. The average misperception of subtype 3 is −2.2 hr and varies across days with a SD of 1.5 hr. A minority of people with ID (29%) and a minority of good sleepers (4%) showed this profile.
Among people with ID, the three subtypes were not distinguished by age or severity of insomnia (Table 3). Among CTRL, the three subtypes were not distinguished by age, while the severity of insomnia was lowest in type 1 and highest in type 3.
Table 3.
LCA cluster demographics and features for ID and good sleepers (mean ± standard deviation)
| Characteristic | Subtype 1 | Subtype 2 | Subtype 3 | Statistic | p | Effect size |
|---|---|---|---|---|---|---|
| ID | ||||||
| Subtype size | 40 (22%) | 89 (49%) | 52 (29%) | |||
| Sex, female/male | 25/15 | 73/16 | 42/10 | 6.49 | .039 | 0.019 |
| Age, years | 48.2 ± 11.5 | 50.5 ± 12.0 | 52.2 ± 12.1 | 1.27 | .28 | 0.014 |
| ISI | 15.9 ± 11.5 | 17.2 ± 3.4 | 17.0 ± 3.8 | 1.94 | .15 | 0.021 |
| Mean objective TST, min | 398.9 ± 43.2 | 427.7 ± 37.5 | 420.1 ± 53.7 | 5.98 | .003 | 0.063 |
| SD objective TST, min | 47.3 ± 22.4 | 44.5 ± 19.4 | 53.7 ± 29.9 | 2.54 | .082 | 0.028 |
| Shortest subjective TST, min | 303.6 ± 54.2 | 241.7 ± 49.7 | 101.0 ± 52.1 | 199.42 | < .0001 | 0.691 |
| Mean misperception, min | −16.6 ± 24.0 | −87.8 ± 32.2 | −134.4 ± 82.4 | 60.81 | < .0001 | 0.406 |
| SD misperception, min | 32.4 ± 13.5 | 59.8 ± 18.9 | 89.6 ± 25.2 | 93.99 | < .0001 | 0.514 |
| CTRL | ||||||
| Subtype size | 48 (87%) | 5 (9%) | 2 (4%) | |||
| Sex, female/male | 34/14 | 5/0 | 0/2 | 6.93 | .047 | 0.355 |
| Age, years | 46.2 ± 15.4 | 43.2 ± 11.6 | 58.5 ± 16.3 | 0.75 | .48 | 0.028 |
| ISI | 2.0 ± 2.3 | 3.6 ± 2.7 | 6.5 ± 0.7 | 4.65 | .014 | 0.152 |
| Mean objective TST, min | 421.8 ± 46.5 | 484.0 ± 24.8 | 302.5 ± 171.1 | 9.29 | .0004 | 0.263 |
| SD objective TST, min | 51.3 ± 21.6 | 52.4 ± 14.4 | 41.2 ± 11.1 | 0.23 | .79 | 0.009 |
| Shortest subjective TST, min | 349.0 ± 55.0 | 299.0 ± 58.8 | 121.0 ± 12.7 | 17.90 | < .0001 | 0.408 |
| Mean misperception, min | 11.6 ± 24.7 | −42.8 ± 25.8 | −61.8 ± 82.2 | 15.24 | < .0001 | 0.37 |
| SD misperception, min | 29.8 ± 16.5 | 74.1 ± 12.8 | 61.5 ± 75.1 | 14.06 | < .0001 | 0.351 |
The Chi‐squared statistic and Cramer's V were used to calculate p‐values and effect sizes for categorical variables.
The F statistic and Eta‐squared were used to calculate p‐values and effect sizes for continuous variables.
Bold font highlights significant differences.
Abbreviations: CTRL, control; ID, Insomnia Disorder; ISI, Insomnia Severity Index; SD, standard deviation; TST, total sleep time.
The subtypes of misperception were equally distributed within recently discovered insomnia subtypes (p = .84; Data S1; Blanken et al., 2019).
4. DISCUSSION
This study aimed for a data‐driven definition of SSM subtypes revealed by multiple sleep features including night‐to‐night variability. To do so, we calculated subjective, objective and misperception features of sleep obtained from 7 nights of sleep diaries and actigraphy in a large sample of people with ID and good sleepers. A data‐driven and robust solution was pursued by using the Goldbricker method for feature selection and LCA with cross‐validation.
Our findings indicate that misperception of sleep and the objective and subjective features of sleep occur across a continuum, which is in line with previous studies (Harvey & Tang, 2012). However, data‐driven analysis revealed three categorically distinguishable subtypes in the profiles (or fingerprints) of these features. Importantly, two of the three most distinguishing features concern within‐subject differences across days, providing strong support for our contention that night‐to‐night variability is an important feature differentiating subtypes.
Subtypes were best discriminated by three features; an individual’s shortest reported subjective sleep duration, and the mean and standard deviation of misperception. These features were on average 5.4, 0.0 and 0.5 hr in one subtype accommodating the majority of good sleepers; 4.1, −1.4 and 1.0 hr in a second subtype representing the majority of ID; and 1.7, −2.2 and 1.5 hr in a third subtype representing a quarter of ID and hardly any good sleepers. Subtypes did not differ on the remaining two features, which were an individual’s mean (6.9, 7.2 and 6.9 hr) and standard deviation (0.8, 0.8 and 0.9 hr) of the actigraphically estimated objective sleep duration.
The results confirm previously reported findings that the majority of good sleepers are able to accurately perceive their sleep (Feige et al., 2008; Manconi et al., 2010; Mendelson, 1995). The results also confirm that the majority of ID persistently underestimate their sleep (Harvey & Tang, 2012; Manconi et al., 2010; Means et al., 2003), but that overestimation of sleep occurs occasionally in a minority of ID (Figure 1; Trajanovic, Radivojevic, Kaushansky, & Shapiro, 2007). Note that we do not interpret misperception as “false perception”. On the contrary, we think that it is more likely that traditional assessment by PSG and actigraphy of sleep fall short in detecting ongoing mental activity during sleep (Siclari et al., 2017; Siclari, Larocque, Postle, & Tononi, 2013), which can be indiscriminable from the experience of being awake (Wassing et al., 2016).
Several studies have defined subtypes by manually splitting groups based on data inspection. Using the MI as a discriminating factor, a previous study (Manconi et al., 2010) defined two subtypes of misperception in ID: one with high misperception and another class with a range of misperception. Our data‐driven approach confirms the presence of a subtype with extreme misperception consisting predominantly of ID, but revealed three instead of two subtypes. Like the MI, our final feature set included oTST as well as mTST. This confirms that including oTST, either as part of MI (i.e. mTST/oTST) or as a separate variable, captures variability not accounted for by the other variables, although it remains unclear if it results in better discrimination between subtypes than mTST alone.
Objective TST was previously used in a study as a distinguishing factor to a priori split ID into groups with short sleep (< 6 hr) and normal sleep (> 6 hr) resulting in two subtypes (Fernandez‐Mendoza et al., 2011). The group with objective short sleep duration displayed no misperception or overestimation of sleep, and the group with objective normal sleep displayed clear misperception of sleep. In contrast to their study, our findings indicated that the subtype with maximal misperception had the shortest sleep duration (subtype 3), while the subtype with moderate misperception had the longest sleep duration (subtype 2). However, it should be noted that sleep duration differed by no more than 17 min, and averaged about 7 hr of sleep in all subtypes.
An unsupervised hierarchical clustering analysis on observations across two laboratory and two home assessments of misperception (Means et al., 2003) found four clusters in the misperception of ID and three clusters in the misperception of good sleepers. The majority of people with ID were allocated to a cluster characterized by slight underestimates of sleep time and a second cluster with reasonably accurate sleep time estimates. The remaining ID were allocated to two clusters, one characterized by substantial underestimation of sleep and the other by an overestimation of sleep duration. The majority of normal sleepers were allocated to a cluster characterized by consistently accurate perception of their sleep duration and another cluster characterized by a consistent overestimation of sleep duration. The third cluster of normal sleepers had a “random” pattern of under‐ and overestimates during lab versus home nights.
In line with their findings (Means et al., 2003), most of the ID in the present study could be allocated to classes with minimal or moderate misperception and a smaller group with quite severe misperception. We did not find a separate cluster of people with overestimation of misperception, although we observed its occurrence in individuals, mostly of subtype 1. The majority of the good sleepers were assigned to a class with no misperception on average.
It has been suggested that greater night‐to‐night variability in misperception is observed in adults with sleep complaints compared with adults without sleep complaints. Indeed, our findings confirm that greater night‐to‐night variability is observed in ID compared with good sleepers. However, within the ID group there appears to be no relationship with the severity of complaints as measured by the ISI across the three subtypes.
In addition, we did not observe a correlation between the distribution of recently discovered subtypes of insomnia derived from affect, personality traits and life history, which suggests that misperception subtype is not characteristic of a specific type of insomnia and captures distinct properties.
Although most individual traces of misperception appeared random across the 5−7 nights, some individuals showed increasing or decreasing trends in misperception across several nights. Studies looking at the variability of sleep over time may therefore want to include more than 7 nights to elucidate if the patterns are truly random or contain some oscillatory pattern, which may not be captured by the 7 nights included in the present study.
The effect of interventions of misperception has remained virtually unexplored and seems highly relevant. It is conceivable that effects of pharmacological and cognitive behavioural interventions differ across subtypes. Such knowledge could aid to the choice of intervention.
One recent study showed that changes in misperception were mediated by changes in self‐reported sleep quality (Pittsburgh Sleep Quality Index) as well as previous levels of misperception. Therefore, sleep quality may be a valuable target for focused interventions intent on improving the accuracy of perceptions of disturbed sleep (Dzierzewski et al., 2019).
In conclusion, the current study revealed three subtypes in the mean and dispersion of misperception and objective and subjective sleep duration of ID and good sleepers. We did so by evaluating a wide range of features derived from multiple ambulatory nights of actigraphy and sleep diaries. The results suggest that sleep diaries and ambulatory objective measurements of sleep can be used to assign good sleepers and ID to one of the classes with a high certainty. The findings may help to understand the unexplained considerable individual variability in treatment response. If the three misperception subtypes respond differentially to cognitive behavioural and pharmacological interventions, subtyping brings us one step closer to personalized medicine.
CONFLICTS OF INTEREST
All authors declare no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
BTL, TB and EVS were involved in the conception, hypothesis delineation and design of the study. WVDM, KD, RW, YVDW and JR were involved in the acquisition of the data. BTL and TB analysed the data. BTL and EVS drafted the original version of the article. All authors contributed to the final submitted version of the article.
Supporting information
ACKNOWLEDGEMENTS
This work was supported by Project NeuroSIPE 10738, of the Dutch Technology Foundation STW, which is part of the Netherlands Organization for Scientific Research (NWO) and partly funded by the Ministry of Economic Affairs, Agriculture and Innovation; by the Bial Foundation grants 190/16 and 252/12; and by the European Research Council (ERC) grants ERC‐2014‐AdG‐671084 INSOMNIA and ERC‐2016‐PoC‐737634‐INSOMNIA BEAT IT.
te Lindert BHW, Blanken TF, van der Meijden WP, et al. Actigraphic multi‐night home‐recorded sleep estimates reveal three types of sleep misperception in Insomnia Disorder and good sleepers. J Sleep Res. 2020;29:e12937 10.1111/jsr.12937
Jennifer R. Ramautar and Eus J. W. Van Someren are co‐senior authors.
REFERENCES
- American Psychiatric Association . (2013). DSM‐5: Diagnostic and statistical manual of mental disorders (5th edn). Washington, DC: American Psychiatric Press. [Google Scholar]
- Bastien, C. H. , Vallieres, A. , & Morin, C. M. (2001). Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine, 2(4), 297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- Bei, B. , Wiley, J. F. , Trinder, J. , & Manber, R. (2016). Beyond the mean: A systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Medicine Reviews, 28, 108–124. 10.1016/j.smrv.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Benjamins, J. , Migliorati, F. , Dekker, K. , Wassing, R. , Moens, S. , Van Someren, E. , …, Jarkiewicz, M. (2016). The sleep registry. An international online survey and cognitive test assessment tool and database for multivariate sleep and insomnia phenotyping (vol 14S, pg e293, 2013). Sleep Medicine, 21, 178–178. 10.1016/j.sleep.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Blanken, T. F. , Benjamins, J. S. , Borsboom, D. , Vermunt, J. K. , Paquola, C. , Ramautar, J. , …, Van Someren, E. J. W. (2019). Insomnia disorder subtypes derived from life history and traits of affect and personality. Lancet Psychiatry, 6(2), 151–163. 10.1016/S2215-0366(18)30464-4 [DOI] [PubMed] [Google Scholar]
- Carney, C. E. , Buysse, D. J. , Ancoli‐Israel, S. , Edinger, J. D. , Krystal, A. D. , Lichstein, K. L. , & Morin, C. M. (2012). The consensus sleep diary: Standardizing prospective sleep self‐monitoring. Sleep, 35(2), 287–302. 10.5665/sleep.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejonckheere, E. , Mestdagh, M. , Houben, M. , Rutten, I. , Sels, L. , Kuppens, P. , & Tuerlinckx, F. (2019). Complex affect dynamics add limited information to the prediction of psychological well‐being. Nature Human Behaviour, 3(5), 478–491. 10.1038/s41562-019-0555-0 [DOI] [PubMed] [Google Scholar]
- Dekker, K. , Benjamins, J. S. , Maksimovic, T. , van Straten, A. Hofman, W. F. , & Van Someren, E. J. W. (2019). Effects of cognitive behavioral therapy, bright light, physical activity, body warming and their combinations for treatment of Insomnia Disorder. A randomized controlled trial. Psychotherapy and Psychosomatics, in press. 10.1159/000503570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnostic Classification Steering Committee . (2014). ICSD3—International classification of sleep disorders: Diagnostic and coding manual. (3rd edn). Rochester, MN: American Sleep Disorders Association. [Google Scholar]
- Dzierzewski, J. M. , Martin, J. L. , Fung, C. H. , Song, Y. , Fiorentino, L. , Jouldjian, S. , …, Alessi, C. A. (2019). CBT for late‐life insomnia and the accuracy of sleep and wake perceptions: Results from a randomized‐controlled trial. Journal of Sleep Research, 28(4), e12809 10.1111/jsr.12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger, J. D. , Bonnet, M. H. , Bootzin, R. R. , Doghramji, K. , Dorsey, C. M. , Espie, C. A. , … Stepanski, E. J. (2004). Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep, 27(8), 1567–1596. 10.1093/sleep/27.8.1567 [DOI] [PubMed] [Google Scholar]
- Feige, B. , Al‐shajlawi, A. , Nissen, C. , Voderholzer, U. , Hornyak, M. , Spiegelhalder, K. , …, Riemann, D. (2008). Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. Journal of Sleep Research, 17(2), 180–190. 10.1111/j.1365-2869.2008.00651.x [DOI] [PubMed] [Google Scholar]
- Fernandez‐Mendoza, J. , Calhoun, S. L. , Bixler, E. O. , Karataraki, M. , Liao, D. , Vela‐Bueno, A. , …, Vgontzas, A. N. (2011). Sleep misperception and chronic insomnia in the general population: Role of objective sleep duration and psychological profiles. Psychosomatic Medicine, 73(1), 88–97. 10.1097/PSY.0b013e3181fe365a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, A. G. , & Tang, N. K. (2012). (Mis)perception of sleep in insomnia: A puzzle and a resolution. Psychological Bulletin, 138(1), 77–101. 10.1037/a0025730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert, V. , Pratt, D. , Emsley, R. , & Kyle, S. D. (2017). Predictors of nightly subjective‐objective sleep discrepancy in poor sleepers over a seven‐day period. Brain Sciences, 7(3), 10.3390/brainsci7030029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hittner, J. B. , May, K. , & Silver, N. C. (2003). A Monte Carlo evaluation of tests for comparing dependent correlations. Journal of General Psychology, 130(2), 149–168. 10.1080/00221300309601282 [DOI] [PubMed] [Google Scholar]
- Jones, P. (2018). networktools: Tools for identifying important nodes in networks (Version 1.2.0). Retrieved from https://cran.r-project.org/package=networktools
- Kay, D. B. , Dzierzewski, J. M. , Rowe, M. , & McCrae, C. S. (2013). Greater night‐to‐night variability in sleep discrepancy among older adults with a sleep complaint compared to noncomplaining older adults. Behavioral Sleep Medicine, 11(2), 76–90. 10.1080/15402002.2011.602775 [DOI] [PubMed] [Google Scholar]
- Kushida, C. A. , Chang, A. , Gadkary, C. , Guilleminault, C. , Carrillo, O. , & Dement, W. C. (2001). Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep‐disordered patients. Sleep Medicine, 2(5), 389–396. 10.1016/S1389-9457(00)00098-8 [DOI] [PubMed] [Google Scholar]
- Magidson, J. , & Vermunt, J. K. (2004). Latent class models. In Kaplan D. (Ed.), The SAGE handbook of quantitative methodology for the social sciences (pp. 175–198). Thousand Oaks, CA: Sage Publications Inc. [Google Scholar]
- Manconi, M. , Ferri, R. , Sagrada, C. , Punjabi, N. M. , Tettamanzi, E. , Zucconi, M. , …, Ferini‐strambi, L. (2010). Measuring the error in sleep estimation in normal subjects and in patients with insomnia. Journal of Sleep Research, 19(3), 478–486. 10.1111/j.1365-2869.2009.00801.x [DOI] [PubMed] [Google Scholar]
- Means, M. K. , Edinger, J. D. , Glenn, D. M. , & Fins, A. I. (2003). Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Medicine, 4(4), 285–296. 10.1016/S1389-9457(03)00057-1 [DOI] [PubMed] [Google Scholar]
- Mendelson, W. B. (1995). Effects of flurazepam and zolpidem on the perception of sleep in normal volunteers. Sleep, 18(2), 88–91. 10.1093/sleep/18.2.88 [DOI] [PubMed] [Google Scholar]
- Perlis, M. L. , Smith, M. T. , Andrews, P. J. , Orff, H. , & Giles, D. E. (2001). Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep, 24(1), 110–117. 10.1093/sleep/24.1.110 [DOI] [PubMed] [Google Scholar]
- R Core Team . (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.r-project.org/ [Google Scholar]
- Revelle, W. (2018). psych: procedures for personality and psychological research (Version 1.8.12). Retrieved from https://cran.r-project.org/package=psych
- Siclari, F. , Baird, B. , Perogamvros, L. , Bernardi, G. , LaRocque, J. J. , Riedner, B. , …, Tononi, G. (2017). The neural correlates of dreaming. Nature Neuroscience, 20(6), 872–878. 10.1038/nn.4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siclari, F. , Larocque, J. J. , Postle, B. R. , & Tononi, G. (2013). Assessing sleep consciousness within subjects using a serial awakening paradigm. Frontiers in Psychology, 4, 542 10.3389/fpsyg.2013.00542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, N. K. , & Harvey, A. G. (2006). Altering misperception of sleep in insomnia: Behavioral experiment versus verbal feedback. Journal of Consulting and Clinical Psychology, 74(4), 767–776. 10.1037/0022-006X.74.4.767 [DOI] [PubMed] [Google Scholar]
- te Lindert, B. H. W. , van der Meijden, W. P. , Wassing, R. , Kamal, O. , Wei, Y. , Ramautar, J. R. , & Van Someren, E. J. W. (2019). (under review). Optimizing actigraphic estimates of polysomnography sleep features in Insomnia Disorder, Sleep. [DOI] [PubMed] [Google Scholar]
- te Lindert, B. H. W. , & Van Someren, E. J. W. (2013). Sleep estimates using microelectromechanical systems (MEMS). Sleep, 36(5), 781–789. 10.5665/sleep.2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajanovic, N. N. , Radivojevic, V. , Kaushansky, Y. , & Shapiro, C. M. (2007). Positive sleep state misperception—A new concept of sleep misperception. Sleep Medicine, 8(2), 111–118. 10.1016/j.sleep.2006.08.013 [DOI] [PubMed] [Google Scholar]
- Van den berg, J. F. , Van rooij, F. J. , Vos, H. , Tulen, J. H. , Hofman, A. , Miedema, H. M. , …, Tiemeier, H. (2008). Disagreement between subjective and actigraphic measures of sleep duration in a population‐based study of elderly persons. Journal of Sleep Research, 17(3), 295–302. 10.1111/j.1365-2869.2008.00638.x [DOI] [PubMed] [Google Scholar]
- Van Someren, E. J. W. (2007). Improving actigraphic sleep estimates in insomnia and dementia: How many nights? Journal of Sleep Research, 16(3), 269–275. 10.1111/j.1365-2869.2007.00592.x [DOI] [PubMed] [Google Scholar]
- Vermunt, J. K. , & Magidson, J. (2002). Latent class cluster analysis In Hagenaars J. & McCutcheon A. (Eds), Applied latent class analysis (pp. 89–106). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Vermunt, J. K. , & Magidson, J. (2016). Technical guide for Latent GOLD 5.0: basic, advanced, and syntax.
- von Neumann, J. , Kent, R. H. , Bellinson, H. R. , & Hart, B. I. (1941). The mean square successive difference. The Annals of Mathematical Statistics, 12(2), 153–162. 10.1214/aoms/1177731746. Retrieved from http://www.jstor.org/stable/2235765 [DOI] [Google Scholar]
- Wassing, R. , Benjamins, J. S. , Dekker, K. , Moens, S. , Spiegelhalder, K. , Feige, B. , …, Van Someren, E. J. W. (2016). Slow dissolving of emotional distress contributes to hyperarousal. Proceedings of the National Academy of Sciences of the United States of America, 113(9), 2538–2543. 10.1073/pnas.1522520113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassing, R. , Lakbila‐Kamal, O. , Ramautar, J. R. , Stoffers, D. , Schalkwijk, F. , & Van Someren, E. J. W. (2019). Restless REM sleep impedes overnight amygdala adaptation. Current Biology, 29(14), 2351–2358.e2354. 10.1016/j.cub.2019.06.034 [DOI] [PubMed] [Google Scholar]
- Wei, Y. , Colombo, M. A. , Ramautar, J. R. , Blanken, T. F. , van der Werf, Y. D. , Spiegelhalder, K. , …, Van Someren, E. J. W. (2017). Sleep stage transition dynamics reveal specific stage 2 vulnerability in insomnia. Sleep, 40(9), 10.1093/sleep/zsx117 [DOI] [PubMed] [Google Scholar]
- Wittchen, H. U. , Jacobi, F. , Rehm, J. , Gustavsson, A. , Svensson, M. , Jönsson, B. , …, Steinhausen, H.‐C. (2011). The size and burden of mental disorders and other disorders of the brain in Europe 2010. European Neuropsychopharmacology, 21(9), 655–679. 10.1016/j.euroneuro.2011.07.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


