Abstract
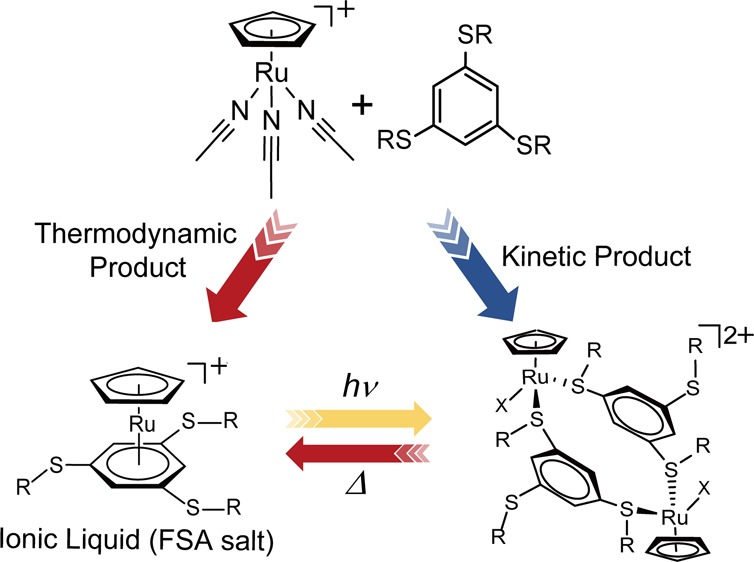
To explore the structural transformation of cyclopentadienyl ruthenium (CpRu) complexes in response to external stimuli, the reaction of [RuCp(MeCN)3][X] (X = PF6, (FSO2)2N [= FSA]) and tris(alkylthio)benzenes (1,3,5-C6H3(SR)3; L1: R = Pr, L2: R = Me) was investigated, and the crystal structures and thermal properties of the products were examined. The reaction produced the sandwich complexes [RuCpLn][X] or dinuclear complexes [Ru2Cp2(μ-Ln)2(CH3CN)m][X]2 (X = PF6, FSA) depending on the reaction conditions. The sandwich complex [RuCpL1][FSA] was an ionic liquid. The solids of dinuclear complexes transformed into the thermodynamically stable sandwich complexes upon heating accompanied by acetonitrile loss. This change resulted in a transformation from crystal to ionic liquid for complexes with the FSA anion. UV irradiation of the sandwich complex [RuCpL1][PF6] in methanol produced the dinuclear complex [Ru2Cp2(μ-L1)2L12][PF6]2. The complex transformed into the sandwich complex upon heating.
Introduction
Many cyclopentadienyl ruthenium (CpRu) complexes have been synthesized to date because of the interest in their chemical reactivities and catalytic activities.1−15 They are also used for the construction of various supramolecular assemblies.16−18 A versatile precursor for their production is the triacetonitrile complex [RuCp(MeCN)3]+,9−13 which produces cationic sandwich-type Ru complexes upon reaction with arene ligands.13−15
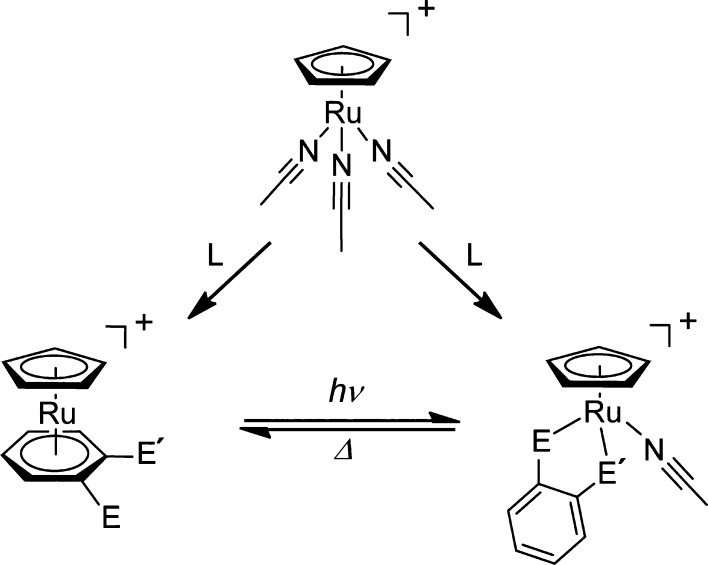
Ionic liquids are salts with melting points below 100 °C,23,24 and we have synthesized a variety of organometallic ionic liquids containing cationic sandwich complexes.19−22 In particular, ionic liquids with the formula [RuCp(arene)][X] (X = fluorinated anion) have been prepared using the reaction of [RuCp(MeCN)3]+ with arene ligands.25−28 As part of this investigation, we previously found that the reaction with ortho-substituted benzenes afforded either sandwich-type or chelate complexes depending on the reaction conditions (Figure 1), and their interconversion in solution was possible upon application of light and heat.29 Based on this mechanism, organometallic ionic liquids that transform into amorphous coordination polymers upon photo-irradiation were designed.30,31
Figure 1.
Reaction of [RuCp(MeCN)3]+ with 1,2-disubstituted benzene ligands (L). Transformation between the products occurs in acetonitrile by application of light and heat (E, E′ = SMe, NMe2).
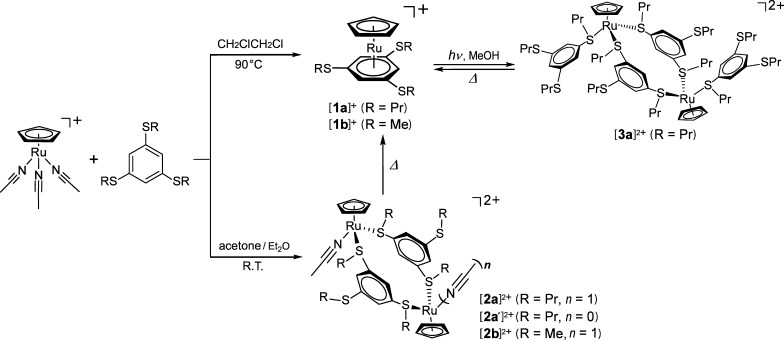
In this study, to further explore the structural transformations of CpRu complexes via external stimuli, the reaction of the triacetonitrile complex with meta-substituted ligands was performed because they cannot form chelate complexes unlike the ortho-substituted benzenes. The reaction with 1,3,5-C6H3(SR)3 ligands (L1: R = Pr; L2: R = Me) produced sandwich-type or dinuclear complexes depending on the reaction condition (Figure 2). The hexafluorophosphate (PF6–) and bis(fluorosulfonyl) amide ((FSO2)2N–; FSA) anions were used as counter anions. The FSA anion is often used for the preparation of ionic liquids.24 The thermal properties of the products were investigated using differential scanning calorimetry (DSC), along with crystal structure determination. Furthermore, the thermal reactivity of the dinuclear complexes and the photochemical reactivity of the sandwich-type complexes were investigated.
Figure 2.
Reaction scheme of [RuCp(MeCN)3]+ and tris(alkylthio) benzenes investigated in this study. The thermal reaction and photochemical reactions of the products are also shown.
Results and Discussion
Preparation and Thermal Properties of the Sandwich-Type Complexes
A solution of [RuCp(MeCN)3][PF6] and L1 or L2 in 1,2-dichloroethane was heated at 90 °C for 16 h to produce the sandwich-type complexes [1a][PF6] and [1b][PF6] in 83 and 76% yields, respectively (Figure 2, top), as pale yellow crystals. In addition, [1a][FSA] was synthesized using the same procedure from [RuCp(MeCN)3][FSA] in 75% yield. The complex was obtained as a pale yellow liquid, which solidified upon application of physical agitation at low temperature (−16 °C).
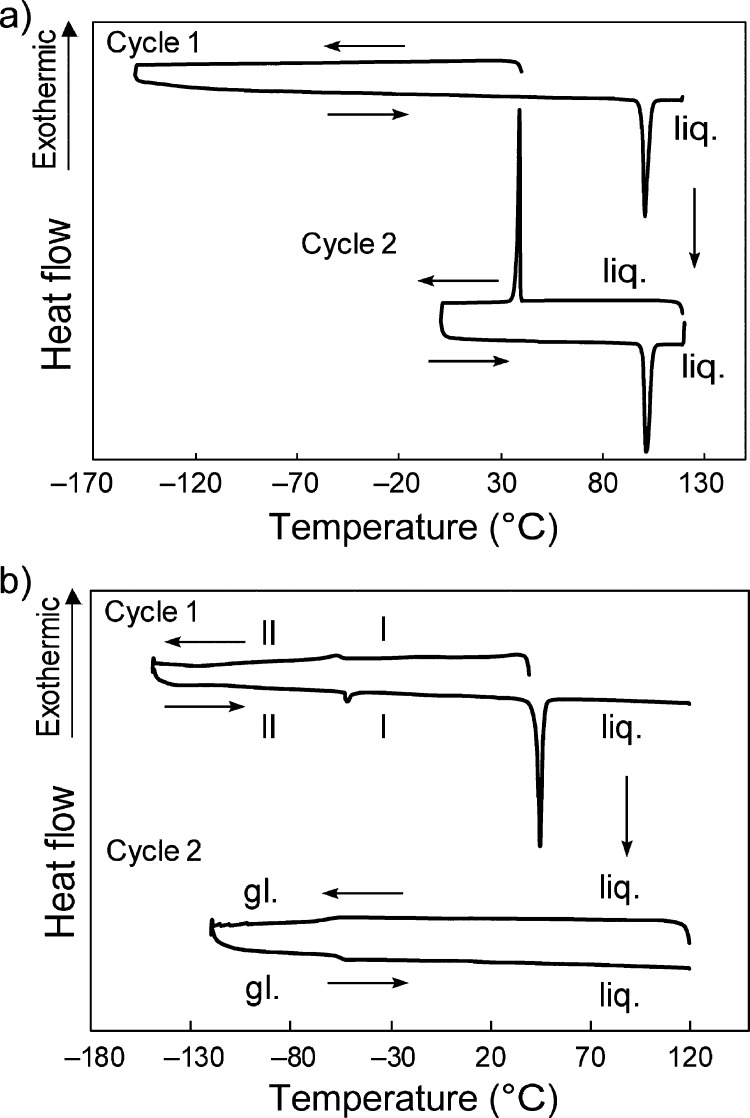
The thermal properties of the prepared complexes were investigated via DSC, and the obtained data are summarized in Table 1 and Figure 3. The melting points of [1a][PF6] and [1b][PF6] were 99.5 and 213.7 °C, respectively, and they crystallized upon cooling from the melt (Figure 3a). The melting point of [1a][FSA] was 42.7 °C, and this salt can be regarded as an ionic liquid. Once melted, this salt maintained the liquid state at room temperature, exhibiting a glass transition at −52 °C upon further cooling (Figure 3b). The ratio of the glass transition temperature to the melting point (Tg/Tm) was 0.68, in agreement with the empirical relationship (Tg/Tm ≈ 2/3).32 This complex exhibited a phase transition at −53.5 °C (ΔH = 1.3 kJ mol–1) in the solid state.
Table 1. Melting Points (Tm), Melting Enthalpies (ΔH), and Glass Transition Temperatures (Tg) of the Prepared Complexes.
| Tm (°C) | ΔH (kJ/mol) | Tg (°C) | |
|---|---|---|---|
| [1a][PF6] | 99.5 | 31.7 | |
| [1b][PF6] | 213.7 | 39.4 | |
| [1a][FSA] | 42.7 | 17.8 | –57 |
Figure 3.
DSC traces of (a) [1a][PF6] and (b) [1a][FSA] where liq. and gl. are the liquid and glassy states, respectively.
Crystal Structures of the Sandwich-Type Complexes
The crystal structures of [1a][PF6] and [1b][PF6] were determined at 90 K, crystallizing in space groups P21212 and P21/c, respectively. The packing diagrams are provided in Figure S1 (Supporting Information), and the structures of the cations are shown in Figure 4. One of the four crystallographically independent cations is shown for [1a][PF6], while the other cations are provided in Figure S2 (Supporting Information) and exhibit different substituent conformations. The Ru–Cpcentroid and Ru–Arenecentroid distances in these cations are 1.82 and 1.71 Å, respectively, which are usual values for CpRu complexes.28,31 The C–S–C angles of [1a][PF6] and [1b][PF6] were 101.5–105.1° and 102.8–103.3°, respectively. In both crystals, the cations exhibited intermolecular π–π interactions between the arene rings, forming a dimeric structure with centroid–centroid distances of 3.43 ([1a][PF6]) and 3.37 Å ([1b][PF6]), respectively.
Figure 4.
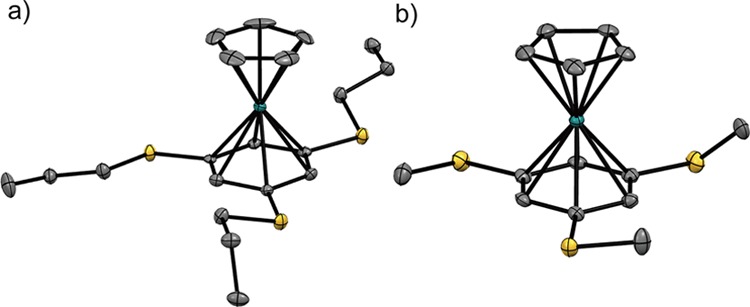
Molecular structures of the cations in (a) [1a][PF6] and (b) [1b][PF6]. The hydrogen atoms were omitted for clarity.
Preparation and Thermal Properties of the Dinuclear Complexes
The reaction of [RuCp(MeCN)3][PF6] and the ligands in acetone at room temperature produced orange crystals of the acetonitrile-coordinated dinuclear complexes [2a][PF6]2 and [2b][PF6]2 in 28 and 37% yields, respectively (Figure 2, bottom). Similarly, the reaction of [RuCp(MeCN)3][FSA] and L1 under the same conditions produced orange crystals of [2a′][FSA]2 in 22% yield.
Thermogravimetric (TG) analysis showed that the acetonitrile ligands in the complexes are released upon heating. The TG curves of [2a][PF6]2 and [2b][PF6]2 measured at 10 °C min–1 are shown in Figure 5. Weight losses of approximately 6 wt% at 140–160 °C (Figure 5a) and 10 wt% at 158–180 °C (Figure 5b) corresponded to the loss of acetonitrile (calculated values: 6.3 and 7.2 wt%, respectively). At higher temperatures (approximately 200–350 °C), both complexes showed a gradual weight loss of approximately 60 wt %, corresponding to the arene ligand loss (calculated values: 50.9 and 61.5 wt%, respectively).
Figure 5.
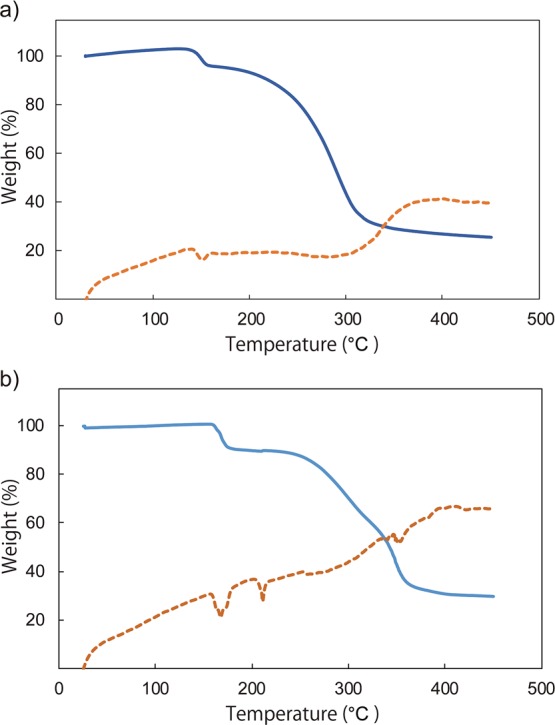
TG–DTA traces of (a) [2a][PF6]2 and (b) [2b][PF6]2 at 10 °C min–1 under a nitrogen atmosphere. The TG and DTA curves are shown in solid and dashed lines, respectively.
[2a][PF6]2 exhibited melting and acetonitrile loss simultaneously, which was observed as an endothermic peak at approximately 152 °C (onset: 141 °C) in the differential thermal analysis (DTA) curve (Figure 5a), whereas [2b][PF6] melted at a higher temperature than the acetonitrile loss, exhibiting two peaks at approximately 168 and 202 °C (onset) in the DTA curve (Figure 5b). Their thermal properties were also investigated using DSC (see below), which revealed that the loss of acetonitrile was accompanied by a structural transformation to the sandwich complexes.
Crystal Structures of the Dinuclear Complexes
The crystal structures of [2a][PF6] and [2b][PF6] were determined at 90 K, and the dinuclear cationic complex structures are shown in Figure 6. The two Ru ions in each cation are bridged by two arene ligands, where each Ru ion is coordinated with an acetonitrile and the two sulfur atoms of the arene ligands. Therefore, one of the three sulfur atoms in each arene ligand remained uncoordinated. In [2a][PF6]2, three of the six propyl substituents exhibited twofold disorder with occupancies of 0.57:0.43–0.76:0.24. One Cp ring exhibited rotational disorder over two sites with an occupancy of 0.58:0.42. The coordination bond lengths in [2a][PF6]2 (Ru–S = 2.35–2.39 Å, Ru–N = 2.06 Å) and [2b][PF6]2 (Ru–S = 2.37 Å, Ru–N = 2.06 Å) were almost identical. However, the coordination angles of S–Ru–N (84.1–93.4° in [2a][PF6]2; 92.9° in [2b][PF6]2) and S–Ru–S (93.4 and 94.9° in [2a][PF6]2; 98.7° in [2b][PF6]2) differed slightly probably because of the packing effect. The two arene rings in the cation of [2a][PF6] exhibited a 11.6° dihedral angle, whereas those in [2b][PF6]2 were oriented in a parallel manner because the cation is located on the inversion center. The centroid–centroid distances between the two arene rings in these complexes were 3.65 and 3.53 Å, respectively, and were likely stabilized by intramolecular π–π interactions. The dinuclear structure resembles the structural motif of the previously reported AgI coordination polymer [Ag2(bsb)2(ClO4)2]n [bsb = 1,3,5-tris(benzylsulfanyl)benzene].33
Figure 6.
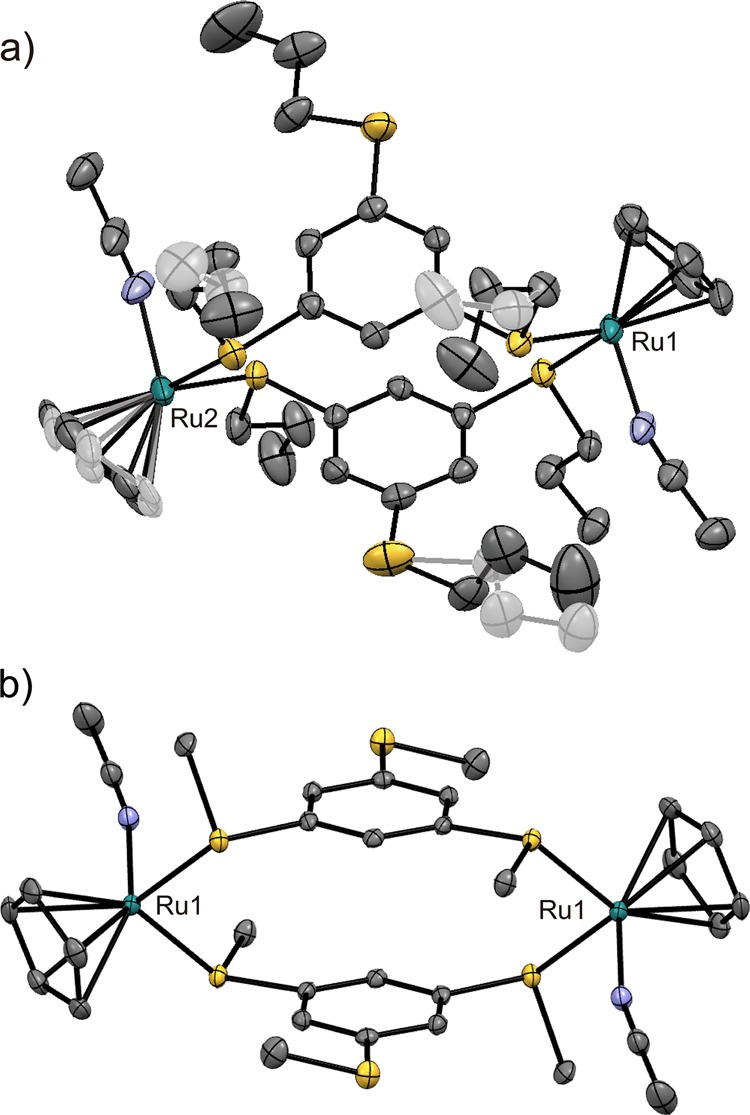
Molecular structures of the cations in (a) [2a][PF6]2 and (b) [2b][PF6]2.
The crystal structure of [2a′][FSA]2 could not be determined, but it likely has a similar dinuclear structure. This complex contains only one acetonitrile ligand in the cation, in contrast to [2a][PF6] and [2b][PF6]; hence, a sulfide moiety of an adjacent unit in the crystal probably coordinates to the metal center instead of acetonitrile. This structure seems plausible considering the structure of [3a][PF6]2 (see below).
Thermal Conversion from a Dinuclear to Sandwich-Type Complex
The formation of the sandwich-type and dinuclear complexes by the reactions at 90 °C and room temperature, respectively, indicates that they are the thermodynamic and kinetic products, respectively. DFT calculations also indicated that the sandwich-type complex is thermodynamically more stable (Figure S3, Supporting Information). Based on this feature, we could observe a thermal transformation from the dinuclear complex to the sandwich-type complex.
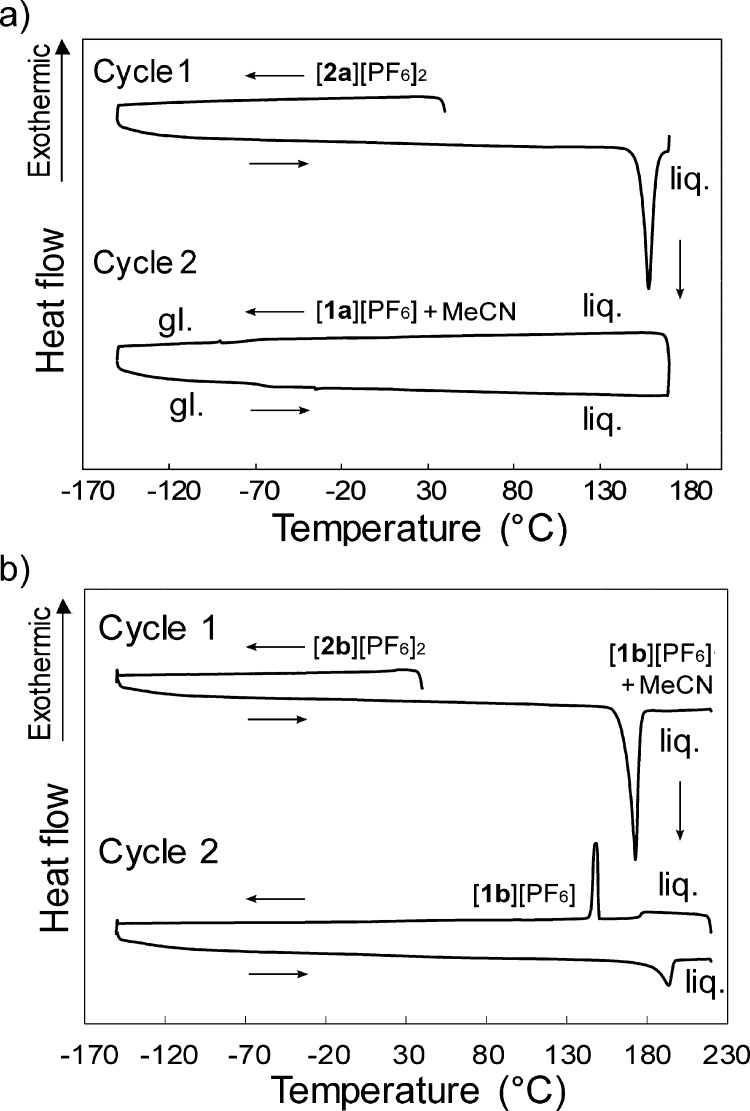
The photographs taken upon heating the crystals of [2a][PF6]2 are shown in Figure 7a. Upon heating, the orange crystals melted at approximately 150 °C, affording an orange liquid [Figure 7a(ii)]. The liquid turned to a pale yellow liquid of [1a][PF6] upon further heating [154–159 °C; Figure 7a(iii–iv)], and this color change is consistent with the coordination transformation to the sandwich complex accompanied by acetonitrile loss. Complete transformation to [1a][PF6] was confirmed by 1H NMR spectroscopy. The DSC trace of this complex measured in a sealed pan is shown in Figure 8a. A large endothermic peak was observed at 153.5 °C (ΔH = 128 kJ mol–1), which corresponds to melting and concomitant structural transformation. Upon cooling, the resultant mixture of [1a][PF6] and acetonitrile did not solidify and exhibited a glass transition at −66 °C, whereas removal of acetonitrile by vacuum drying left a solid of [1a][PF6].
Figure 7.
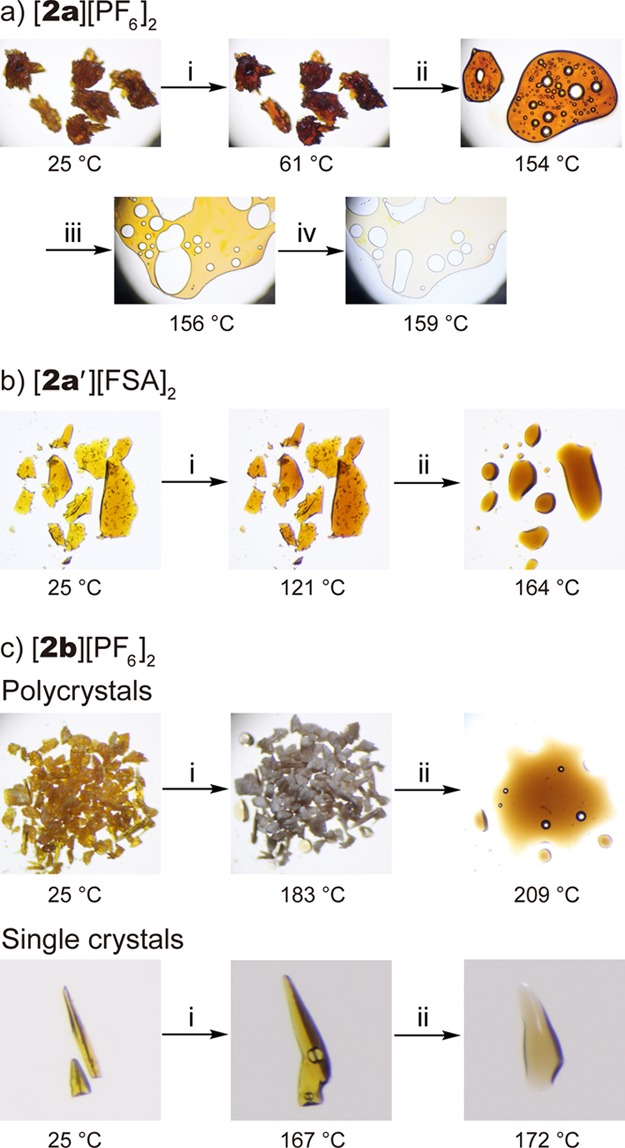
Photographs of (a) [2a][PF6]2, (b) [2a′][FSA]2, and (c) [2b][PF6]2 (polycrystals and single crystals) taken upon heating.
Figure 8.
DSC traces of (a) [2a][PF6]2 and (b) [2b][PF6]2 measured in a sealed pan where liq. and gl. are the liquid and glassy states, respectively. The discontinuity observed at 180 °C on the cooling runs is an artifact.
The photographs of [2a′][FSA]2 taken upon heating are shown in Figure 7b. The orange crystals melted at around 145 °C and concomitantly transformed to the sandwich complex [1a][FSA] [Figure 7b(ii)], which was a pale brown liquid. Therefore, the conversion from the solid to ionic liquid was achieved.
In contrast, the crystals of [2b][PF6]2 did not melt because the cation contained no alkyl chains. The photographs of this complex are shown in Figure 7c. The orange crystals exhibited the acetonitrile loss and coordination transformation at approximately 160 °C in the solid state, affording [1b][PF6] as a white solid [Figure 7c, top row, (i)]. The solid melted upon further heating to approximately 210 °C. On the other hand, upon heating single crystals, melting was observed at around 165 °C [Figure 7c, bottom row, (i)]. This was due to the presence of acetonitrile in the product. Consistently, when DSC measurements of [2b][PF6]2 was performed in a sealed pan (Figure 8b), melting occurred at 166.4 °C with structural transformation, yielding a large enthalpy change (ΔH = 93.7 kJ mol–1). In the second cycle, melting was observed at around 200 °C because most of the acetonitrile has escaped during subsequent heating.
Photochemical Reaction of the Sandwich-type Complex
The sandwich complex [1a][PF6] underwent a photochemical reaction in solution (365 nm), yielding a dinuclear complex (Figure 2, upper right). [1b][PF6] was insoluble in methanol and unsuitable for photoreaction investigation.
UV irradiation of [1a][PF6] in methanol for 3 h produced orange crystals of [Ru2Cp2(μ-L1)2L12][PF6]2 ([3a][PF6]2) in 28% yield. The structure of the cation was determined by X-ray crystallography at 90 K and is shown in Figure 9 (space group P-1). The cation exhibited a dinuclear structure similar to that of [2a][PF6]2, but with four arene ligands, two of which were coordinated to the Ru ions instead of acetonitrile. The cation adopted a centrosymmetric structure, and the two arene rings bridging the Ru ions were arranged in parallel. The centroid–centroid and interplane distances between the two were 3.80 and 3.21 Å, respectively. The average Ru–S bond lengths and S–Ru–S angles were 2.38 Å and 84.6–93.3°, respectively. Four of the six crystallographically independent propylthio substituents exhibited twofold disorder, with occupancy ratios of 0.50:0.50–0.81:0.19.
Figure 9.
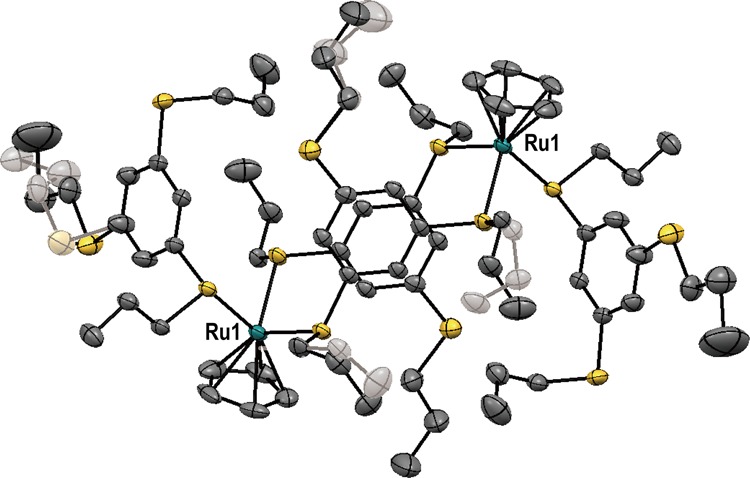
Molecular structure of the cation in [3a][PF6]2.
Heating the [3a][PF6]2 solid at 170 °C for 5 min quantitatively afforded a mixture of [1a][PF6] and L1. Therefore, this dinuclear complex was thermally transformed to a sandwich complex, similar to the other dinuclear complexes.
Conclusions
The reaction of the triacetonitrile CpRu complex [RuCp(MeCN)3]+ with 1,3,5-tris(alkylthio)benzenes produced either sandwich-type complexes or dinuclear complexes depending on reaction conditions. Dinuclear complex formation from the meta-substituted ligands is a striking contrast to the formation of mononuclear chelate complexes from the ortho-substituted ligands. The dinuclear complexes exhibited a novel structural type and are interesting from a supramolecular perspective. Furthermore, the dinuclear complexes transformed to thermodynamically stable sandwich complexes upon heating concomitant with the loss of acetonitrile. The sandwich complex photochemically produced a dinuclear complex with four ligands in solution.
We explored the phase change phenomenon coupled with structural changes of metal complexes upon application of external stimuli based on the unique reactivities of CpRu complexes. The transformation between the ionic liquid and amorphous solid upon application of light and heat was previously reported. In this study, a thermal conversion from the crystal to ionic liquid was achieved, adding versatility to the material transformation phenomenon of CpRu complexes.
Experimental Section
General
1H NMR spectra were recorded using a Bruker AVANCE 400 spectrometer, and IR spectra were recorded using a Thermo Nicolet Avatar 360 FT-IR spectrometer (ATR method). Elemental analyses were performed using a PerkinElmer 2400II elemental analyzer. DSC measurements were performed using a TA instruments Q100 differential scanning calorimeter at a 10 °C min–1 scan rate. TG analyses were performed under a nitrogen atmosphere at a 10 °C min–1 heating rate using a Rigaku TG8120 TG analyzer. DFT calculations were performed at the ωB97-D/LanL2DZ level using Spartan′18 software. Single-crystal X-ray diffraction data were collected using a Bruker APEX II Ultra diffractometer with Mo Kα radiation at 90 K. The structures were solved using SHELXS,34 and the crystallographic parameters are provided in Tables S1 and S2 (Supporting Information).
Synthesis of the Ligands
1,3,5-Tris(propylthio)benzene (L1)
K2CO3 (79 mg, 0.57 mmol) and 1-bromopropane (71 mg, 0.57 mmol) were added to a DMSO (1.5 mL) solution of 1,3,5-benzenetrithiol (20 mg, 0.12 mmol) and stirred at room temperature for 10 min under a nitrogen atmosphere. The reaction mixture was subsequently heated to 50 °C and stirred for 1 d. Water (7.5 mL) was added to the mixture, and the solution was extracted with diethyl ether. The organic layer was washed with water and a saturated saline solution. The solution was dried over anhydrous MgSO4, and the solvent was then evaporated under reduced pressure. The resultant pale yellow liquid was dried under vacuum for 5 h at room temperature (28 mg, 81%). 1H NMR (400 MHz, CDCl3): δ 1.03 (t, 9H, CH3, J = 14.7 Hz), 1.67 (sext, 6H, CH2, J = 7.59 Hz), 2.88 (t, 6H, CH2, J = 14.7 Hz), 7.02 (s, 3H, C6H3). ESI-MS (m/z): [M + H]+ calcd for C15H25S3, 301.1118; found, 301.1109.
1,3,5-Tris(methylthio)benzene (L2)
This ligand was prepared by a modification of the published procedure.35 1,3,5-Trifluorobenzene (40 mg, 0.3 mmol) was added dropwise to a NaSCH3 (135 mg, 1.9 mmol) suspension in dehydrated 1,3-dimethyl-2-imidazolidinone (4 mL) with stirring for 16 h under a nitrogen atmosphere. Then, the reaction mixture was added to water and a white precipitate was collected by filtration, washed successively with water and cold methanol, and vacuum-dried (55 mg, 86%). 1H NMR (400 MHz, CDCl3): δ 2.47 (s, 9H, CH3), 6.88 (s, 3H, C6H3).
Synthesis of the Sandwich-Type Complexes
[RuCpL1][PF6] ([1a][PF6])
L1 (77 mg, 0.26 mmol) was added to a dehydrated 1,2-dichloroethane solution (2 mL) of [RuCp(MeCN)3][PF6] (56 mg, 0.11 mmol), and the resulting solution was refluxed for 20 h under a nitrogen atmosphere. The resulting solution was evaporated under reduced pressure, and the residue was dissolved in a small amount of dichloromethane before being subjected to column chromatography (alumina, eluent: diethyl ether and then acetonitrile). The fraction containing the desired salt was collected and evaporated. The residue was dissolved in a small amount of acetone, to which excess of diethyl ether was added to precipitate the product as a white powder (65 mg, 83%). Pale yellow crystals were obtained upon recrystallization from acetone–diethyl ether at −40 °C (36 mg, 46%). 1H NMR (400 MHz, CD3CN): δ 1.07 (t, 9H, CH3, J = 7.4 Hz), 1.73 (sext, 6H, CH2), 3.03 (t, 6H, CH2, J = 7.4 Hz), 5.30 (s, 5H, Cp-H5), 6.33 (s, 3H, C6H3). FT-IR (ATR, cm–1): 641, 902, 1143, 1242, 1292, 1346, 1379, 2873, 2934, 2965, 3117. Anal. Calcd for C20H29F6PRuS3: C, 39.27; H, 4.78; N, 0.00. Found: C, 39.26; H, 4.84; N, 0.09.
[RuCpL2][PF6] ([1b][PF6])
[1b][PF6] was synthesized using the same procedure as [1a][PF6] using [RuCp(MeCN)3][PF6] (51.2 mg, 0.12 mmol) and L2 (50.0 mg, 0.23 mmol). Recrystallization of the product (55 mg, 76%) from acetone–diethyl ether at −40 °C afforded pale yellow crystals (15 mg, 27%). 1H NMR (400 MHz, CD3CN): δ 2.57 (s, 9H, CH3), 5.34 (s, 5H, Cp-H5), 6.33 (s, 3H, Ar-H3). FT-IR (ATR, cm–1): 1092, 1350, 1413, 1484. Anal. Calcd for C14H17F6PRuS3: C, 31.88; H, 3.25; N, 0.00. Found: C, 31.86; H, 2.98; N, 0.03.
[RuCpL1][FSA] ([1a][FSA])
An aqueous solution (0.5 mL) of K[FSA] (53 mg, 0.245 mmol) was added to an acetone (2.5 mL) solution of [RuCpL1][PF6] (38 mg, 0.062 mmol) and stirred at room temperature for 6 h. The solution was extracted with dichloromethane (20 mL), and the organic layer was dried over MgSO4 before being evaporated. The procedure was repeated seven times until the complete disappearance of PF6– was confirmed by 19F NMR spectra (CD3CN). The resulting yellow oil was purified via column chromatography (activated alumina, dichloromethane (Rf = 0.1)/acetonitrile (Rf = 0.9), gradient from 1:0 to 0:1). The solution was evaporated and dried in vacuo for 6 h to afford a yellow liquid (32 mg, 28%). 1H NMR (400 MHz, CD3CN): δ 1.06 (t, 9H, CH3, J = 7.4 Hz), 1.72 (sext, 6H, CH2), 3.02 (t, 6H, CH2, J = 7.4 Hz), 5.29 (s, 5H, Cp-H5), 6.32 (s, 3H, C6H3). Anal. Calcd for C20H29F2O4S5NRu: C, 37.14; H, 4.52; N, 2.17. Found: C, 37.59; H, 4.54; N, 2.26. FT-IR (ATR, cm–1):787, 823, 846, 1094, 1214, 1480, 2962. Alternatively, this salt was also synthesized using the same procedure as [1a][PF6], with [RuCp(MeCN)3][FSA] (82 mg, 0.18 mmol) and L1 (111 mg, 0.37 mmol) in 75% yield (84.9 mg).
Synthesis of the Dinuclear Complexes
[Ru2Cp2(μ-L1)2(MeCN)2][PF6]2 ([2a][PF6]2)
[RuCp(MeCN)3][PF6] (18.5 mg, 0.042 mmol) and L1 (25.1 mg, 0.083 mmol) were dissolved in acetone (0.5 mL) in a test tube. Diethyl ether (10 mL) was then carefully layered onto the solution. Orange crystals of [2a][PF6]2 were formed by storing the solution in the dark at room temperature for 2 days (16.1 mg, 29%). The product dissociated in CD3CN to yield a mixture of [RuCp(MeCN)(CD3CN)2]+ and L1 in a 1:1 ratio, as confirmed by the 1H NMR spectrum. 1H NMR (400 MHz, CD3CN): δ 1.03 (t, 9H, CH3, J = 7.4 Hz), 1.66 (sext, 6H, CH2), 1.99 (s, 3H), 2.96 (t, 6H, CH2, J = 7.4 Hz), 4.30 (s, 5H, Cp-H5), 7.03 (s, 3H, C6H3). FT-IR (ATR, cm–1): 555, 824, 1092, 1350, 1413, 1484.
[Ru2Cp2(μ-L2)2(MeCN)2][PF6]2 ([2b][PF6]2)
[2b][PF6]2 was synthesized using the same procedure as [2a][PF6]2 with [RuCp(MeCN)3][PF6] (12.4 mg, 0.030 mmol) and L2 (12.1 mg, 0.056 mmol). The product was obtained as orange crystals (12 mg, 37%). Anal. Calcd for C44H64F12N2P2Ru2S6: C, 40.48; H, 4.94; N, 2.15. Found: C, 40.77; H, 5.07; N, 2.09. The product dissociated in CD3CN to afford a mixture of [RuCp(MeCN)(CD3CN)2]+ and L2 in a 1:1 ratio, as confirmed by the 1H NMR spectrum. 1H NMR (400 MHz, CD3CN): δ 1.98 (s, 3H), 2.50 (s, 9H, CH3), 4.30 (s, 5H, Cp-H5), 6.91 (s, 3H, Ar-H3). FT-IR (ATR, cm–1): 555, 676 (Ar), 827, 982, 1415, 1549. Anal. Calcd for C32H40F12S6N2Ru2P2: C, 33.80; H, 3.55; N, 2.46. Found: C, 34.77; H, 3.53; N, 2.28.
[Ru2Cp2(μ-L1)2(MeCN)][FSA]2 ([2a′][FSA]2)
[2a′][FSA]2 was synthesized using the same procedure as [2a][PF6]2 with [RuCp(MeCN)3][FSA] (15 mg, 0.032 mmol) and L1 (19 mg, 0.064 mmol). Acetone and diethyl ether were bubbled with nitrogen gas for 15 min before use. The product was obtained as an orange solid (10 mg, 22%) and dissociated in CD3CN to give a mixture of [RuCp(MeCN)(CD3CN)2]+ and L1 in a 1:1 ratio, as confirmed by the 1H NMR spectrum. The ratio of the Cp ligand to acetonitrile in the spectrum was 2:1. 1H NMR (400 MHz, CD3CN): δ 1.03 (t, 9H, CH3, J = 7.4 Hz), 1.66 (sext, 6H, CH2), 1.99 (s, 3H), 2.96 (t, 6H, CH2, J = 7.4 Hz), 4.30 (s, 5H, Cp-H5), 7.03 (s, 3H, C6H3). Anal. Calcd for C42H61N5Ru2S10O8F4: C, 37.79; H, 4.61; N, 3.15. Found: C, 38.41; H, 4.57; N, 2.88. FT-IR (ATR, cm–1): 586, 675 (Ar), 826, 965, 1215, 1417, 1556, 2009, 2927.
Photochemical Reaction of [1a][PF6]
A solution of [1a][PF6] (10.1 mg, 0.019 mmol) in MeOH (0.6 mL) was irradiated with UV light (365 nm LED light, 100 mW cm–2) for 3 h, gradually forming orange needle crystals of [Ru2Cp2(μ-L1)2L12][PF6]2 ([3a][PF6]2). The crystals were washed with cold methanol and dried under air (5.1 mg, 28%). Anal. Calcd for C70H106F12P2Ru2S12: C, 46.08; H, 5.86; N, 0.00. Found: C, 45.87; H, 5.94; N, 0.11. 1H NMR (400 MHz, CD3CN): δ 1.03 (t, 9H, CH3, J = 7.4 Hz), 1.66 (sext, 6H, CH2), 2.96 (t, 6H, CH2, J = 7.4 Hz), 4.30 (s, 5H, Cp-H5), 7.03 (s, 3H, C6H3). FT-IR (ATR, cm–1): 535, 543, 580, 676 (Ar), 1129, 1239, 1380, 1456, 1554, 2873, 2931, 2962. Heating [3a][PF6]2 at 170 °C for 5 min produced a liquid mixture of [1a][PF6] and L1 as confirmed by 1H NMR spectroscopy.
Acknowledgments
This work was supported financially by the CANON foundation and KAKENHI (grant number: 18H04516) from the Japan Society for the Promotion of Science (JSPS).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04272.
Accession Codes
CCDC 1911657−1911660, 1920912 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.
The authors declare no competing financial interest.
Supplementary Material
References
- Gimeno J.; Cadierno V.; Crochet P.. Comprehensive Organometallic Chemistry III; Mingos D. M. P., Crabtree R. H., Bruce M., Eds.; Elsevier: Amsterdam, 2007; Vol. 6, pp 465–550. [Google Scholar]
- Bennett M. A.; Khan K.; Wenger E.. Comprehensive Organometallic Chemistry II; Shriver D. F., Bruce M. I., Eds.; Elsevier: Oxford, U.K., 1995; Vol. 5, pp 473–548. [Google Scholar]
- Consiglio G.; Morandini F. Half-Sandwich Chiral Ruthenium Complexes. Chem. Rev. 1987, 87, 761–778. 10.1021/cr00080a005. [DOI] [Google Scholar]
- Hintermann L.; Xiao L.; Labonne A.; Englert U. [CpRu(η6-naphthalene)]PF6 as Precursor in Complex Synthesis and Catalysis with the Cyclopentadienyl-Ruthenium(II) Cation. Organometallics 2009, 28, 5739–5748. 10.1021/om900333t. [DOI] [Google Scholar]
- Jung K.; Ahmed T. S.; Lee J.; Sung J.-C.; Keum H.; Grubbs R. H.; Choi T.-L. Living β-Selective Cyclopolymerization using Ru Dithiolate Catalysts. Chem. Sci. 2019, 10, 8955–8963. 10.1039/c9sc01326a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dérien S.C–C Bond Formation on Activation of Alkynes and Alkenes with (C5R5)Ru Catalysts. In Ruthenium in Catalysis; Dixneuf P., Bruneau C., Eds.; Topics in Organometallic Chemistry; Springer: Cham, 2014; Vol. 48, pp 289–318. [Google Scholar]
- Perekalin D. S.; Kudinov A. R. Cyclopentadienyl ruthenium complexes with naphthalene and other polycyclic aromatic ligands. Coord. Chem. Rev. 2014, 276, 153–173. 10.1016/j.ccr.2014.06.017. [DOI] [Google Scholar]
- Gutierrez A. C.; Jamison T. F. Continuous Photochemical Generation of Catalytically Active [CpRu]+ Complexes from CpRu(η6-C6H6)PF6. Org. Lett. 2011, 13, 6414–6417. 10.1021/ol2027015. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Older C. M. A Convenient Synthetic Route to [CpRu(CH3CN)3]PF6. Organometallics 2002, 21, 2544–2546. 10.1021/om020143p. [DOI] [Google Scholar]
- Kündig E. P.Synthesis of Transition Metal η6-Arene Complexes. Transition Metal Arene π-Complexes in Organic Synthesis and Catalysis; Topics in Organometallic Chemistry; Springer, 2004; Vol. 7, pp 3–20. [Google Scholar]
- Gill T. P.; Mann K. R. Photochemical properties of the cyclopentadienyl(η6-benzene)ruthenium(II) cation. The synthesis and reactions of a synthetically useful intermediate: the cyclopentadienyltris(acetonitrile)ruthenium(II) cation. Organometallics 1982, 1, 485–488. 10.1021/om00063a014. [DOI] [Google Scholar]
- Kündig E. P.; Monnier F. R. Efficient Synthesis of Tris(acetonitrile)-(η5-cyclopentadienyl)-ruthenium(II) Hexafluorophosphate via Ruthenocene. Adv. Synth. Catal. 2004, 346, 901–904. 10.1002/adsc.200404124. [DOI] [Google Scholar]
- Rüba E.; Schmid R.; Kirchner K.; Calhorda M. J. Ruthenium-Mediated Cyclotrimerization of Alkynes Utilizing the Cationic Complex [RuCp(CH3CN)3]PF6. J. Organomet. Chem. 2003, 682, 204–211. 10.1016/s0022-328x(03)00783-6. [DOI] [Google Scholar]
- Pigge F.; Coniglio J. Stoichiometric Applications of η6-Arene Ruthenium(II) Complexes in Organic Chemistry. Curr. Org. Chem. 2001, 5, 757–784. 10.2174/1385272013375201. [DOI] [Google Scholar]
- Moriarty R. M.; Ku Y. Y.; Gill U. S. Synthesis and Characterization of Cyclopentadienylruthenium(II) Complexes of Aromatic Amino acid Derivatives. J. Organomet. Chem. 1989, 362, 187–191. 10.1016/0022-328x(89)85292-1. [DOI] [Google Scholar]
- Li P.; Xu Y.-M.; Deng W.; Yao Z.-J. Self-Assembly of Supramolecular Coordination Complexes Based on Half-Sandwich Metal Corner with Tunable Host Cavities. J. Organomet. Chem. 2019, 884, 36–43. 10.1016/j.jorganchem.2019.01.022. [DOI] [Google Scholar]
- Cheng B.; Tehrani A. A.; Hu M.-L.; Morsali A. Supramolecular Assemblies of Ru(II) Organometallic Half-Sandwich Complexes. CrystEngComm 2014, 16, 9125–9134. 10.1039/c4ce01214c. [DOI] [Google Scholar]
- Han Y.-F.; Jia W.-G.; Yu W.-B.; Jin G.-X. Stepwise formation of organometallic macrocycles, prisms and boxes from Ir, Rh and Ru-based half-sandwich units. Chem. Soc. Rev. 2009, 38, 3419–3434. 10.1039/b901649j. [DOI] [PubMed] [Google Scholar]
- Funasako Y.; Inagaki T.; Mochida T.; Sakurai T.; Ohta H.; Furukawa K.; Nakamura T. Organometallic ionic liquids from alkyloctamethylferrocenium cations: thermal properties, crystal structures, and magnetic properties. Dalton Trans. 2013, 42, 8317–8327. 10.1039/c3dt00084b. [DOI] [PubMed] [Google Scholar]
- Inagaki T.; Mochida T.; Takahashi M.; Kanadani C.; Saito T.; Kuwahara D. Ionic Liquids of Cationic Sandwich Complexes. Chem.—Eur. J. 2012, 18, 6795–6804. 10.1002/chem.201200151. [DOI] [PubMed] [Google Scholar]
- Inagaki T.; Mochida T. Metallocenium Ionic Liquids. Chem. Lett. 2010, 39, 572–573. 10.1246/cl.2010.572. [DOI] [Google Scholar]
- Inagaki T.; Mochida T. Reactive Half-Metallocenium Ionic Liquids That Undergo Solventless Ligand Exchange. Chem.—Eur. J. 2012, 18, 8070–8075. 10.1002/chem.201200157. [DOI] [PubMed] [Google Scholar]
- Armand M.; Endres F.; MacFarlane D. R.; Ohno H.; Scrosati B. Ionic-Liquid Materials for the Electrochemical Challenges of the Future. Nat. Mater. 2009, 8, 621–629. 10.1038/nmat2448. [DOI] [PubMed] [Google Scholar]
- Kar M.; Matuszek K.; MacFarlane D. R.. Ionic Liquids. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc., 2019. [Google Scholar]
- Komurasaki A.; Funasako Y.; Mochida T. Colorless Organometallic Ionic Liquids from Cationic Ruthenium Sandwich Complexes: Thermal Properties, Liquid Properties, and Crystal Structures of [Ru(η5-C5H5)(η6-C6H5R)][X] (X = N(SO2CF3)2, N(SO2F)2, PF6). Dalton Trans. 2015, 44, 7595–7605. 10.1039/c5dt00723b. [DOI] [PubMed] [Google Scholar]
- Higashi T.; Ueda T.; Mochida T. Effects of Substituent Branching and Chirality on the Physical Properties of Ionic Liquids Based on Cationic Ruthenium Sandwich Complexes. Phys. Chem. Chem. Phys. 2016, 18, 10041–10048. 10.1039/c6cp00643d. [DOI] [PubMed] [Google Scholar]
- Tominaga T.; Ueda T.; Mochida T. Effect of Substituents and Anions on the Phase Behavior of Ru(II) Sandwich Complexes: Exploring the Boundaries between Ionic Liquids and Ionic Plastic Crystals. Phys. Chem. Chem. Phys. 2017, 19, 4352–4359. 10.1039/c6cp08308k. [DOI] [PubMed] [Google Scholar]
- Ueda T.; Mochida T. Thermal Properties and Crystal Structures of Ionic Liquids from Ruthenium Sandwich Complexes with Trialkoxybenzene Ligands: Effects of Substituent Positions and Alkyl Chain Lengths. Organometallics 2015, 34, 1279–1286. 10.1021/acs.organomet.5b00021. [DOI] [Google Scholar]
- Mori S.; Mochida T. Preparation and Properties of Cyclopentadienyl Ruthenocenium Complexes with 1,2-Disubstituted Benzene Ligands: Competition between Chelate Coordination and Sandwich Coordination. Organometallics 2013, 32, 283–288. 10.1021/om301073z. [DOI] [Google Scholar]
- Funasako Y.; Mori S.; Mochida T. Reversible Transformation between Ionic Liquids and Coordination Polymers by Application of Light and Heat. Chem. Commun. 2016, 52, 6277–6279. 10.1039/c6cc02807a. [DOI] [PubMed] [Google Scholar]
- Ueda T.; Tominaga T.; Mochida T.; Takahashi K.; Kimura S. Photogeneration of Microporous Amorphous Coordination Polymers from Organometallic Ionic Liquids. Chem.—Eur. J. 2018, 24, 9490–9493. 10.1002/chem.201801365. [DOI] [PubMed] [Google Scholar]
- Yamamuro O.; Minamimoto Y.; Inamura Y.; Hayashi S.; Hamaguchi H.-o. Heat Capacity and Glass Transition of an Ionic Liquid 1-Butyl-3-methylimidazolium Chloride. Chem. Phys. Lett. 2006, 423, 371–375. 10.1016/j.cplett.2006.03.074. [DOI] [Google Scholar]
- Suenaga Y.; Konaka H.; Sugimoto T.; Kuroda-Sowa T.; Maekawa M.; Munakata M. Crystal Structure and Photo-induced Property of Two-dimensional Silver(I) Complex with 1,3,5-Tris(benzylsulfanyl)benzene. Inorg. Chem. Commun. 2003, 6, 389–393. 10.1016/s1387-7003(02)00780-3. [DOI] [Google Scholar]
- Sheldrick G. M. A Short History of SHELX. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64, 112–122. 10.1107/s0108767307043930. [DOI] [PubMed] [Google Scholar]
- Maiolo F.; Testaferri L.; Tiecco M.; Tingoli M. Fragmentation of aryl alkyl sulfides. A simple, one-pot synthesis of polymercaptobenzenes from polychlorobenzenes. J. Org. Chem. 1981, 46, 3070–3073. 10.1021/jo00328a016. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.