Abstract
An original method has been developed for the synthesis of 1,3-dyine derivatives of natural lembehyne B in high yields (50–67%) and with high selectivity (>98%). The key stage of the synthesis is new Ti-catalyzed cross-cyclomagnesiation of oxygenated and aliphatic 1,2-dienes induced by Grignard reagents. For studying the effect of the structure on the antitumor and neuritogenic activities, a series of lembehyne B analogues with different distances between the terminal hydroxy group and the 1,3-diyne moiety was prepared and tested for neuritogenic activity on mouse neuroblastoma Neuro 2A cells and for cytotoxicity, induction of apoptosis, and effects on the cell cycle using Jurkat, U937, K562, HeLa, and Hek293 tumor cell lines.
1. Introduction
Higher acetylenic alcohols and their derivatives are encountered in many plants, algae, marine invertebrates, and higher fungus species,1−8 and more than 1000 acetylenic compounds have been isolated and identified to date. For many years, increasing interest has been shown in these organic compounds, largely owing to the important biological activities they exhibit, in particular, antitumor, antimicrobial, antiparasitic, antibacterial, antifungal, and other types of activities.4−16
Among the diverse alkynol derivatives known to date, derivatives
of carbinols containing one or several triple bonds in the α-position
to the hydroxy group are the most abundant.1−5
In addition, it has been shown by Japanese researchers that higher alkynols, lembehynes A–C, containing bis-methylene-separated Z-double bonds in the molecules, isolated in nanoconcentrations from Indonesian sea sponges Haliclona sp., show a high neuritogenic activity against mouse neuroblastoma Neuro 2A cells and rat pheochromocytoma PC12 cells.17−20
Therefore, these compounds can be considered as the base for the development of modern drugs for the treatment of neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease, Huntington’s chorea and so on).
Meanwhile, studies along this line were considerably held up by the exceptionally low content of lembehynes in sea sponges and the lack of efficient methods for their total synthesis. Therefore, we developed original stereoselective approaches both for the preparation of key monomers and for the total synthesis of lembehynes A and B21−24 using the Ti-catalyzed cross-cyclomagnesiation of oxygenated and aliphatic 1,2-dienes on treatment with Grignard reagents, which we discovered.25−27 In addition, it was shown for the first time by flow cytometry that lembehyne B can induce early apoptosis in various types of leukemia cells.22
As a continuation
of the studies dealing with stereoselective synthetic
routes to lembehyne derivatives and the influence of their structure
on the antitumor and neuritogenic activities, here, we report a stereoselective
method for the synthesis of lembehyne B 1,3-diyne derivatives, close
structural analogues of natural strongylodiols,28−30 pellynols,31 and halicynones,32 which exhibit a broad range of biological activities. In addition,
we planned to pay particular attention to testing of the antitumor
and neuritogenic activities of the compounds by means of modern flow
cytometry and phase-contrast microscopy.
2. Results and Discussion
2.1. Chemistry
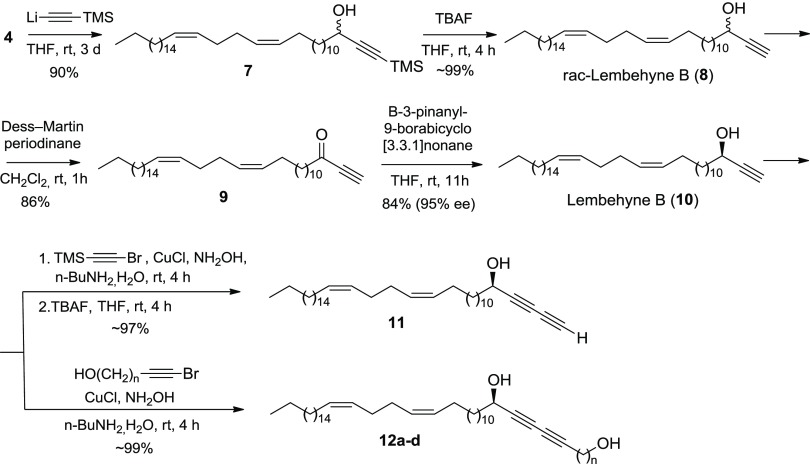
According to the previously developed scheme for the preparation of racemic lembehyne B,21 we first prepared (13Z,17Z)-tetraconta-13,17-dienal (4) in a 79% yield by cross-cyclomagnesiation of 1,2-nonadediene (1) and 2-tetradeca-12,13-dien-1-yl-1,3-dioxolane (2) on treatment with EtMgBr in the presence of Mg metal and a catalytic amount of Cp2TiCl2 (10 mol %) (1/2/EtMgBr/Mg/[Ti] = 12:10:30:20:0.1, Et2O, 20–22 °C, 7 h) (Scheme 1). This was followed by a successive reaction of aldehyde 4 with presynthesized 1-lithium-4-trimethylsilyl-1,3-butadiyne and removal of the trimethylsilyl group on treatment with trimethylbutylammonium fluoride (TBAF) in tetrahydrofuran (THF) to give the target 1,3-diyne rac-lembehyne B analogue 6 in ∼66% yield (Scheme 1).
Scheme 1. Synthesis of Racemic 1,3-Diyne’s Lembehyne B Derivative 6.
Subsequently, we developed a synthetic route to 1,3-diyne derivatives of natural lembehyne B with the R-configuration of the hydroxy group by the direct addition of 1-bromoalkynes to lembehyne B22 synthesized from aldehyde 4 according to Scheme 2:
Scheme 2. Synthesis of 1,3-Diyne’s Analogues 11 and 12a–d on the Basis of Natural Lembehyne B (10).
First, racemic 8 and natural lembehyne B (10) were synthesized according to a previously developed scheme21−24 by the successive addition of lithium trimethylsilylacetylenide to aldehyde 4, deprotection of the resulting alkyne 7, oxidation of alcohol 8, and stereoselective reduction of ketone 9 at the final step. The reactions of natural lembehyne B (10) with 1-bromo-2-trimethylsilylacetylene or 1-bromo-2-(ω-hydroxyalkyl)acetylenes in the presence of CuCl afforded the target 1,3-diyne lembehyne B derivatives 11 and 12a–d in high yields.
In the second stage, we initiated a study of antitumor and neuritogenic activities of the synthesized 1,3-diynes in comparison with those of the initial lembehyne B.
2.2. Biology
The cytotoxicity of lymbehynes B and their derivatives (altogether six compounds) was evaluated using a flow cytofluorimeter and a Guava ViaCount kit (Millipore). The following cell lines were studied: Jurkat (human T-lymphoblastic leukemia), K562 (human chronic myelogenous leukemia), U937 (human histiocytic lymphoma), HeLa (human cervical carcinoma), Hek293 (human embryonic kidney cells), and Neuro 2A cells. The neuroblastoma Neuro 2A cell line of mice (albino) is a convenient model to study the mechanisms of neuronal differentiation and the action of neurotrophic factors.
We tested two 1,3-diyne derivatives 6 and 11 based on the racemic and natural lembehyne B and four ω-hydroxy-1,3-diyne lembehyne B (10) derivatives (12a–d) with different distances between the hydroxy group and the 1,3-diyne moiety. Cytotoxicity assays of these compounds against Jurkat, K562, U937, and HeLa tumor cells and Hek293 embryonic cells showed that 1,3-diyne 6 based on racemic lembehyne B has a somewhat lower cytotoxic activity against these cell lines than its analogue 11 synthesized using natural lembehyne B. The highest cytotoxicity was found for compound 12c, the cytotoxicity of which was higher for suspension cultures (Jurkat, K562, U937) than for adhesion cultures (Table 1).
Table 1. Biological Activity of Lembehyne B 1,3-Diyne Derivatives.
| cytotoxicity,
IC50, μM (24 h) |
neuritogenesisa 72 h, % | |||||
|---|---|---|---|---|---|---|
| Jurkat | K562 | U937 | HeLa | Hek293 | Neuro 2A | |
| 12a | 0.55 ± 0.041 | 0.61 ± 0.046 | 0.52 ± 0.037 | 1.17 ± 0.079 | 0.97 ± 0.069 | 54 ± 4 |
| 12b | 0.47 ± 0.037 | 0.54 ± 0.039 | 0.51 ± 0.034 | 1.01 ± 0.072 | 0.88 ± 0.071 | 61 ± 5 |
| 12c | 0.34 ± 0.031 | 0.37 ± 0.021 | 0.32 ± 0.028 | 0.79 ± 0.051 | 0.63 ± 0.044 | 72 ± 6 |
| 12d | 0.52 ± 0.038 | 0.59 ± 0.041 | 0.49 ± 0.039 | 1.35 ± 0.084 | 1.12 ± 0.075 | 68 ± 5 |
| 6 | 0.69 ± 0.047 | 0.74 ± 0.049 | 0.61 ± 0.042 | 1.42 ± 0.092 | 1.28 ± 0.081 | 44 ± 3 |
| 11 | 0.65 ± 0.044 | 0.71 ± 0.046 | 0.58 ± 0.039 | 1.38 ± 0.089 | 1.25 ± 0.079 | 49 ± 4 |
| 10 | 0.53 ± 0.039 | 0.69 ± 0.043 | 0.56 ± 0.040 | 2.84 ± 0.124 | 2.11 ± 0.097 | 56 ± 4 |
| NGS | 44 ± 3 | |||||
The maximum number of cells with neurites, whose length exceeds the body length of the neuron, observed in the concentration range 0.2 nM, incubation 72 h, in %.
The lembehyne B derivatives at a concentration of 0.60 μM/mL induced apoptosis of 87% of Jurkat tumor cells (Figure 1A), 15% being early apoptosis (Q7-4) and more than 71% being late apoptosis (Q7-2).
Figure 1.
Jurkat cells treated with different concentrations of compound 12c (1) were double-stained with annexin V/PI and analyzed by flow cytometry. (A) 0.6 μM; (B) 0.4 μM; (C) 0.2 μM; (D) 0.1 μM; (E) control. Q7-1, necrotic cells; Q7-2, late apoptotic cells; Q7-3, living cells; Q7-4, early apoptotic cells.
Thus, lembehyne B derivatives show a dose-dependent influence on the cell population and induce early and late apoptosis; meanwhile, the initial lembehyne B has been previously shown to induce, most of all, early apoptosis.22
Figure 2 shows the results of the study of the cell cycle phases for Jurkat cells carried out by flow cytometry within 24 h after the cells were treated with compound 12c at various concentrations.
Figure 2.
Cell cycle analysis in Jurkat cells after incubation with compound 12c at different concentrations for 24 h. (A) 0.6 μM; (B) 0.4 μM; (C) 0.2 μM; (D) 0.1 μM; (E) untreated cells.
The Jurkat, K562, U937, HeLa, and Hek293 cell cycle characteristics in the control samples showed a considerable predominance of cells in the G0–G1 phase and a balance between the synthesis (S phase) and apoptosis (sub-G0–G1) processes.
Within 24 h after exposure to compound 12c, virtually in all tumor cell lines, most of all in the suspension Jurkat, K562, and U937 cells, apoptosis processes predominated (increased sub-G0–G1 stage), with retained or even somewhat enhanced ability of cells to DNA synthesis (S phase). This was accompanied by a decreasing fraction of cells in the G0–G1 phase, together with an increasing proliferation block and a decreasing proliferation index due to a decrease in the number of cells in the G2 + M phase (Figure 2A).
All of the foregoing may be indicative of the cytotoxic activity of compound 12c against chronic myeloid, T-cell leukemia, and histiocytic lymphoma cells caused by the ability of these compounds to induce apoptosis.
A comparison of the data on the effects of natural lembehyne B and its 1,3-diyne analogue 12c on the cell cycle showed some differences. Whereas lembehyne B mainly arrests the cell cycle in the G1 phase, apart from the accumulation of hypodiploid cells (G0), the 1,3-diyne analogues directly affect the S phase by stopping it and decreasing the G2 cell population, which also finally contributes to the hypodiploid cell population.
Like other derivatives that we studied, compound 12c stimulated the growth of neurites in the Neuro 2A cell culture; however, unlike other compounds, 12c showed a very high differentiating activity toward neuroblastoma cells, which was manifested even at low concentrations (Figure 3 and Table 1). When the time of cell incubation with 1,3-diyne 12c increased, the differentiation was enhanced and neurite branching and elongation took place (Figure 3). The advantage of the neurite branching and length for the cells incubated in the medium with compound 12c can be clearly seen in Figure 3. In other words, compound 12c may prove to be not inferior in activity to neuro growth factor (NGF); however, for a better understanding of the cellular processes induced by lembehyne derivatives, further research into the molecular transformations involved in the lembehyne-induced neuritogenesis is required.
Figure 3.
Phase-contrast microscopy of neuritogenesis in the Neuro 2A cells induced by compound 12c at the concentration of 0.2 nM. (A) Cell incubation with NGF for 72 h. Cell incubation with compound 12c for (B) 24 h, (C) 48 h, (D) 72 h.
A completely different cell cycle situation is found by flow cytometry for the treatment of Neuro 2A cells with the lembehyne B derivative (Figure 4). The histograms of cell cycle phases in the Neuro 2A cells on exposure to compound 12c show, first, somewhat enhanced capability of cells for DNA synthesis (increased S phase) and then, by the end of the third day, a clearly defined decrease in the S and G2 phases, i.e., almost complete cell disappearance during mitosis.
Figure 4.
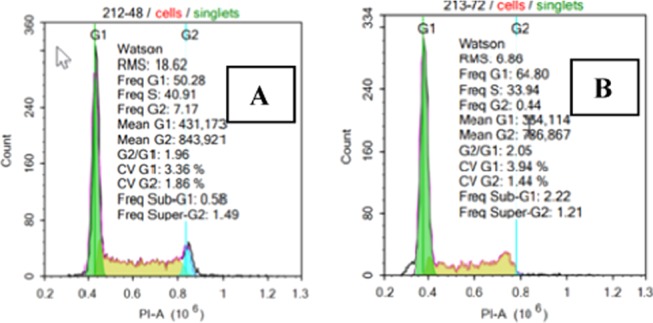
Cell cycle phases of the Neuro 2A cells treated with compound 12c (0.2 nM) and incubated with test compounds for 48 h (A) and (B) 72 h, respectively.
The obtained data suggest that under the action of lembehyne B, the mouse neuroblastoma cells are likely to undergo differentiation, which is confirmed by the activation of neuritogenesis.
3. Conclusions
Thus, as a continuation of our research on the development of original methods for the stereoselective synthesis of natural alkynols containing bis-methylene-separated Z-double bonds, we synthesized a series of 1,3-diyne analogues of natural lembehyne in high yields and with high selectivity. Biological activity assays in vitro revealed the high potential of these compounds as bases for the development of modern antitumor or neuritogenic agents for the treatment of socially significant human diseases. Currently, we are carrying out intensive research in this area dealing with the investigation of the molecular mechanisms of lembehyne-induced neuritogenesis and the antitumor activity of natural and synthetic lembehynes using advanced molecular biological approaches of flow cytometry, multiparameter analysis of signaling pathways, immunohistochemical studies, Western blotting, and fluorescence spectroscopy.
4. Experimental Section
4.1. Materials and Methods
All solvents were dried (1,4-dioxane, tetrahydrofuran, diethyl ether over Na) and freshly distilled before use. All reactions were carried out under a dry argon atmosphere. 1H and 13C NMR spectra were obtained using a Bruker AVANCE 400 spectrometer in CDCl3 operating at 400 MHz for 1H and 100 MHz for 13C. High-resolution mass spectra (HRMS) were measured on an instrument using a time-of-flight (TOF) mass analyzer with electrospray ionization (ESI). Elemental analyses were performed on an 1106 Carlo Erba apparatus. The individuality and purity of the synthesized compounds were controlled using thin-layer chromatography (TLC) on Sorbfil plates; anisic aldehyde in acetic acid was used as a developer. Column chromatography was carried out on an Acrus silica gel (0.060–0.200 mm). Aliphatic and oxygenated 1,2-dienes and lembehyne B have been synthesized according to previously developed procedures.21−24
4.2. Synthesis
4.2.1. Cross-Cyclomagnesiation of Nonadeca-1,2-diene (1) and 2-Tetradeca-12,13-dien-1-yl-1,3-dioxolane (6) with EtMgBr in the Presence of Mg Metal and a Cp2TiCl2 Catalyst
Diethyl ether (30 mL), nonadeca-1,2-diene (1) (1.27 g, 4.8 mmol), 2-tetradeca-12,13-dien-1-yl-1,3-dioxolane (2) (1.06 g, 4.0 mmol), EtMgBr (12.0 mmol) (as 1.5 M solution in Et2O), Mg powder (0.19 g, 8.0 mmol), and Cp2TiCl2 (0.1 g, 0.4 mmol) were placed in a glass reactor with stirring under argon (∼0 °C). The reaction mixture was warmed up to room temperature (20–22 °C) and stirred for 6 h. The reaction mixture was quenched with a 10% solution of HCl in H2O (20 mL) and extracted with diethyl ether (2 × 100 mL). The combined organic phases were then dried over MgSO4, filtered, and the solvents were removed under vacuum. Silica gel column chromatography (hexane/EtOAc, 35:1) of the residue gave dienal 4 (1.50 g, 77%) as a pale yellow oily liquid.
4.2.1.1. (13Z,17Z)-Tetraconta-13,17-dien-1-al (4)
1H NMR (400 MHz) δ 0.88 (3H, t, J = 6.6 Hz, CH3), 1.42–1.15 (44H, m, CH2), 1.56–1.66 (2H, m, CH2), 1.96–2.10 (8H, m, CH2–CH=), 2.40 (2H, t, J = 7.3 Hz,), 5.41–5.32 (4H, m, J = 5.4 Hz,), 9.74 (1H, s, O=CH). 13C NMR (100.62 MHz, CDCl3) δ 14.10, 22.08, 22.71, 27.21, 27.25, 27.42, 29.19, 29.34, 29.41, 29.47, 29.52, 29.58, 29.62, 29.65, 29.71, 29.75, 31.96, 43.89, 129.08, 130.25, 202.24. HRMS (ESI-TOF): calcd for C34H64O [M + H]+ 489.8793, found 489.8790. Anal. calcd for C34H64O: C, 83.53; H, 13.20. Found: C, 83.39; H, 13.16.
4.2.2. Procedure for the Preparation of 1,3-Diyne (5)
To a solution of 4-trimethylsilyl-1,3-butadiyne 0.73 g (6 mmol) in THF (10 mL), a solution of 4 mL n-BuLi (1.5 M in hexane) was added dropwise at −40 °C. The solution was stirred for 1 h at −40 to 0 °C and then added dropwise to THF solution of 1.5 g (3.08 mmol) dienal (4) at −10 °C. The reaction mixture was warmed up to ambient temperature and stirred for 3 days. The reaction mixture was treated with a 5% solution of NH4Cl in H2O (20 mL) and extracted with diethyl ether (2 × 100 mL). The combined organic phases were dried over MgSO4, filtered, and the solvents were removed under vacuum. Silica gel column chromatography of the residue gave compound 5 (1.59 g, 85%) as a pale yellow oily liquid.
4.2.2.1. (17Z,21Z)-1-(Trimethylsilyl)octatriaconta-17,21-dien-1,3-diyn-5-ol (5)
1H NMR (400 MHz) δ 0.22 (9H, s, CH3), 0.91 (3H, t, J = 6.6 Hz, CH3), 1.29–1.48 (46H, m, CH2), 1.67–1.90 (2H, m, CH2), 2.02–2.21 (8H, m, CH2–CH=), 4.43 (1H, t, J = 6.6, HO–CH), 5.47–5.30 (4H, m, =CH). 13C NMR (100.62 MHz, CDCl3) δ −0.47, 14.14, 22.71, 25.03, 27.28, 27.44, 29.24, 29.35, 29.39, 29.51, 29.59, 29.65, 29.68, 29.72, 29.77, 31.95, 37.49, 62.86, 69.78, 78.73, 87.22, 87.58, 129.16, 130.38. HRMS (ESI-TOF): calcd for C41H74OSi [M + H]+ 612.1191, found 612.1193. Anal. calcd for C41H74OSi: C, 80.58; H, 12.20. Found: C, 80.55; H, 12.22.
4.2.3. Procedure for the Preparation of 1,3-Diynoles (6)
To a solution of alkyne (5) 1.17 g (2 mmol) in THF (10 mL), TBAF (1 M in THF, 1.2 equiv) was added at 0 °C, the solution was stirred for 4 h at ambient temperature and then added dropwise to a THF solution of 1.5 g (3.08 mmol) dienal (6) at −10 °C. The reaction mixture was treated with saturated aq. NaCl and extracted with diethyl ether (2 × 50 mL). The combined organic phases were dried over MgSO4, filtered, and the solvents were removed under vacuum. Silica gel column chromatography of the residue gave compound 6 (1.07 g, 99%) as a colorless waxy solid.
4.2.3.1. (17Z,21Z)-Octatriaconta-17,21-dien-1,3-diyn-5-ol (6)
1H NMR (400 MHz) δ 0.90 (3H, t, J = 6.7, CH3), 1.54–1.19 (46H, m, CH2), 1.67–1.77 (2H, m, CH2), 2.03–2.10 (8H, m, CH2–CH=), 2.21 (1H, s, CH), 4.43 (1H, t, J = 6.6, HO–CH), 5.32–5.40 (4H, m, =CH). 13C NMR (100.62 MHz, CDCl3) δ 14.14, 22.72, 25.00, 27.23, 27.29, 27.42, 27.44, 29.21, 29.35, 29.39, 29.49, 29.57, 29.59, 29.64, 29.69, 29.73, 29.77, 31.95, 37.41, 62.73, 67.44, 68.40, 69.09, 129.16, 130.40. HRMS (ESI-TOF): calcd for C38H66O [M + H]+ 539.9379, found 539.9371. Anal. calcd for C38H66O: C, 84.68; H, 12.34. Found: C, 84.62; H, 12.36.
4.2.4. Procedure for the Preparation of 1,3-Diyne’s Analogues (12a–d)
To a stirred solution of n-BuNH2 (0.29 mL) and distilled H2O (0.58 mL), copper(I) chloride (5.8 mg, 0.06 mmol) was added at 0 °C under argon, which resulted in a deep blue solution. A few crystals of NH2OH·HCl were added to discharge the blue color, and a solution of enynic alcohol (lembehyne B) 10 (150 mg, 0.45 mmol) in CH2Cl2 (1.16 mL) was added via a syringe at the same temperature. Then, 3-bromoprop-2-yn-1-ol (43 mg, 0.32 mmol) was added slowly. The reaction mixture was warmed to room temperature and stirred for 30 min. A few crystals of NH2OH·HCl were added occasionally to prevent the solution from turning green or blue throughout the reaction. Upon completion, the reaction solution was extracted with CH2Cl2. The combined organic phases were dried over anhydrous MgSO4 and concentrated under reduced pressure. The residue was purified by silica gel chromatography (n-hexane/ethyl acetate, 5:1) as a colorless oil.
4.2.4.1. (18Z,22Z)-Nonatriaconta-18,22-dien-2,4-diyne-1,6-diol (12a)
Yield 99%. 1H NMR (400 MHz, CDCl3) δ 0.90 (3H, t, J = 6.7, CH3), 1.27–1.47 (46H, m, CH2), 1.79–1.70 (2H, m, CH2), 2.18–1.98 (8H, m, CH2–CH=), 4.36 (2H, s, HO–CH2), 4.45 (1H, t, J = 6.6, HO–CH), 5.44–5.38 (4H, m, =CH). 13C NMR (100.62 MHz, CDCl3) δ 14.13, 22.71, 25.06, 27.28, 27.43, 29.24, 29.35, 29.38, 29.52, 29.59, 29.68, 29.72, 31.94, 37.48, 51.38, 62.83, 68.84, 69.81, 77.53, 80.54, 129.16, 130.38, 130.40. HRMS (ESI-TOF): calcd for C39H68O2 [M + H]+ 569.9639, found 569.9631. Anal. calcd for C39H68O2: C, 82.32; H, 12.05. Found: C, 82.36; H, 12.08.
4.2.4.2. (19Z,23Z)-Tetraconta-19,23-dien-3,5-diyne-1,7-diol (12b)
Yield 98%. 1H NMR (400 MHz) δ 0.90 (3H, t, J = 6.7, CH3), 1.17–1.51 (46H, m, CH2), 1.64–1.78 (2H, m, CH2), 1.95–2.13 (8H, m, CH2–CH=), 2.58 (2H, t, J = 6.4 Hz, CH2), 3.77 (2H, t, J = 6.4 Hz, HO–CH2), 4.40 (t, J = 6.6, 1H, HO–CH), 5.37–5.43 (m, 4H, =CH). 13C NMR (100.62 MHz, CDCl3) δ 14.13, 22.71, 23.62, 25.13, 27.28, 27.43, 29.29, 29.35, 29.39, 29.57, 29.62, 29.68, 29.72, 29.78, 31.95, 37.58, 60.63, 62.73, 66.15, 69.45, 77.38, 78.00, 129.15, 130.36. HRMS (ESI-TOF): calcd for C40H70O2 [M + H]+ 583.9905, found 583.9900. Anal. calcd for C40H70O2: C, 82.40; H, 12.10. Found: C, 82.39; H, 12.15.
4.2.4.3. (20Z,24Z)-Hentetraconta-20,24-dien-4,6-diyne-1,8-diol (12c)
Yield 97%. 1H NMR (400 MHz) δ 0.90 (3H, t, J = 6.7, CH3), 1.28–1.47 (48H, m, CH2), 1.66–1.85 (2H, m, CH2), 1.95–2.10 (8H, m, CH2–CH=), 2.44 (2H, t, J = 6.4 Hz, CH2), 3.77 (2H, t, J = 6.4 Hz, HO–CH2), 4.41 (1H, t, J = 6.6, HO–CH), 5.38–5.45 (4H, m, =CH). 13C NMR (100.62 MHz, CDCl3) δ 14.14, 15.83, 22.71, 25.09, 27.28, 27.43, 29.27, 29.35, 29.39, 29.54, 29.60, 29.68, 29.72, 30.80, 31.95, 37.65, 61.30, 62.85, 64.92, 69.68, 76.87, 80.65, 129.16, 130.38. HRMS (ESI-TOF): calcd for C41H72O2 [M + H]+ 598.0171, found 598.0177. Anal. calcd for C41H72O2: C, 82.48; H, 12.16. Found: C, 82.24; H, 12.09.
4.2.4.4. (21Z,25Z)-Dotetraconta-21,25-dien-5,7-diyne-1,9-diol (12d)
Yield 98%. 1H NMR (400 MHz) δ 0.90 (3H,t, J = 6.7, CH3), 1.19–1.49 (50H, m, CH2), 1.61–1.78 (2H, m, CH), 2.10–1.96 (8H, m, CH2–CH=), 2.36 (2H,t, J = 6.4 Hz, CH2), 3.69 (2H,t, J = 6.4 Hz, HO–CH2), 4.40 (1H, t, J = 6.6, HO–CH), 5.31–5.44 (4H, m, =CH). 13C NMR (100.62 MHz, CDCl3) δ 14.13, 19.09, 22.70, 24.50, 25.10, 27.28, 27.43, 29.27, 29.35, 29.38, 29.54, 29.59, 29.67, 29.72, 29.77, 31.68, 31.94, 37.66, 62.22, 62.81, 64.85, 69.71, 76.86, 81.08, 129.15, 130.37. HRMS (ESI-TOF): calcd for C42H74O2 [M + H]+ 612.0437, found 612.0433. Anal. calcd for C42H74O2: C, 82.55; H, 12.20. Found: C, 82.51; H, 12.22.
4.3. Biological Assays
4.3.1. Cell Culture
Human cancer cell lines HeLa and Hek293 were obtained from the HPA Culture Collections (U.K.). Cells (U937, K562, Jurkat) were purchased from the Russian Cell Culture Collection (Institute of Cytology of the Russian Academy of Sciences) and cultured according to standard protocols and a sterile technique. The cell lines were shown to be free from viral contamination and mycoplasma. HeLa and Hek293 cell lines were cultured as monolayers and maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL) supplemented with 10% fetal bovine serum (FBS) and a 1% penicillin–streptomycin solution at 37 °C in a humidified incubator under a 5% CO2 atmosphere. Cells were maintained in RPMI 1640 (Jurkat, K562, U937) (Gibco) supplemented with 4 μM glutamine, 10% FBS (Sigma), and 100 units/mL penicillin–streptomycin (Sigma). All types of cells were grown in an atmosphere of 5% CO2 at 37 °C. The cells were subcultured at 2–3 days intervals. Adherent cells (HeLa) were suspended using trypsin/ethylenediaminetetraacetic acid (EDTA) and counted after they had reached 80% confluency. Cells were then seeded in 24-well plates at 5 × 104 cells per well and incubated overnight. Jurkat, K562, and U937 cells were subcultured at 2-day intervals with a seeding density of 1 × 105 cells per 24-well plate in RPMI with 10% FBS. Mouse neuroblastoma Neuro 2A cells were obtained from the American Type Culture Collection (ATCC). Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS, Hyclone) at 37 °C in a humidified atmosphere with 5% CO2.
4.3.2. Cytotoxicity Assay
A viability (Live/Dead) assessment was performed by staining cells with 7-aminoactinomycin D (7-AAD) (Biolegend). After treatment, cells were harvested, washed 1–2 times with phosphate-buffered saline (PBS), and centrifuged at 400g for 5 min. Cell pellets were resuspended in 200 μL of flow cytometry staining buffer (PBS without Ca2+ and Mg2+, 2.5% FBS) and stained with 5 μL of a 7-AAD staining solution for 15 min at room temperature in the dark. Samples were acquired on a NovoCyte TM 2000 Flow Cytometry System (ACEA) equipped with a 488 nm argon laser. Detection of 7-AAD emission was performed through a 675/30 nm filter in the FL4 channel.
4.3.3. Viability and Apoptosis
Apoptosis was studied using flow cytometric analysis of annexin V and 7-aminoactinomycin D staining. After treatment, cells were harvested for 24 h, washed 1–2 times with phosphate-buffered saline (PBS), and centrifuged at 400g for 5 min. Cell pellets were resuspended in 200 μL of flow cytometry staining buffer (PBS without Ca2+ and Mg2+, 2.5% FBS). Then, 200 μL of Guava Nexin reagent (Millipore, Bedford, MA) was added to 5 × 105 cells in 200 μL, and the cells were incubated with the reagent for 20 min at room temperature in the dark. At the end of incubation, the cells were analyzed on a NovoCyte TM 2000 Flow Cytometry System (ACEA).
4.3.4. Cell Cycle Analysis
The cell cycle was analyzed using the method of propidium iodide staining. After treatment, cells were harvested for 24 h, washed 1–2 times with phosphate-buffered saline (PBS), and centrifuged at 400g for 5 min. Cell pellets were resuspended in 200 μL of flow cytometry staining buffer (PBS without Ca2+ and Mg2+, 2.5% FBS). Then, cells were plated in 24-well round-bottom plates at a density of 10 × 105 cells per well, centrifuged at 450g for 5 min, and fixed with ice-cold 70% ethanol for 24 h at 0 °C. Cells were then washed with PBS and incubated for 30 min at room temperature with 250 μL of Guava Cell Cycle Reagent (Millipore) in the dark. Samples were analyzed on a NovoCyte TM 2000 Flow Cytometry System (ACEA).
4.3.5. Cell Differentiation Assay
The Neuro 2A cells were plated on 24-well plates at a density of 1 × 104 cells/cm2 with 1 mL of the culture medium. After a 24 h cultivation, the medium was exchanged for a fresh medium, and a testing sample of 10 mL of an ethanol solution was added to each well. After 12, 24, 48, and 72 h incubations, morphological changes in the cells were observed under a phase-contrast microscope. The cells that had processes longer than the diameter of the cell body were evaluated as neurite-bearing cells. The percentage of the cells with neurites in a particular culture was determined by counting at least 300 cells in the photomicrographs of the areas where the cell density was representative.
Acknowledgments
This work was financially supported by the Russian Science Foundation (Grant No. 16-13-10172). The structural studies of the synthesized compounds were performed with the use of Collective Usage Center “Agidel” at the Institute of Petrochemistry and Catalysis of RAS. The biological studies were done in the Laboratory of Molecular Design and Drug Bioscreening at the Institute of Petrochemistry and Catalysis of RAS.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03826.
Copies of 1H, 13C NMR spectra of final products (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Nathalie L.; Fahmi E. M.; Mohamed M.; Philippe A. Marine Polyacetylenes: Distribution, biological properties, and synthesis. Stud. Nat. Prod. Chem. 2015, 45, 251–295. 10.1016/B978-0-444-63473-3.00008-3. [DOI] [Google Scholar]
- Listunov D.; Maraval V.; Chauvin R.; Génisson Y. Chiral alkynylcarbinols from marine sponges: asymmetric synthesis and biological relevance. Nat. Prod. Rep. 2015, 32, 49–75. 10.1039/C4NP00043A. [DOI] [PubMed] [Google Scholar]
- Siddiq A.; Dembitsky V. M. Acetylenic anticancer agents. Anti-Cancer Agents Med. Chem. 2008, 8, 132–170. 10.2174/187152008783497073. [DOI] [PubMed] [Google Scholar]
- Dembitsky V. M. Anticancer activity of natural and synthetic acetylenic lipids. Lipids 2006, 41, 883–924. 10.1007/s11745-006-5044-3. [DOI] [PubMed] [Google Scholar]
- Dembitsky V. M.; Levitsky D. O. Acetylenic terrestrial anticancer agents. Nat. Prod. Comm. 2006, 1, 405–429. 10.1177/1934578X0600100512. [DOI] [Google Scholar]
- Kuklev D. V.; Dembitsky V. M. Epoxy acetylenic lipids: Their analogues and derivatives. Prog. Lipid Res. 2014, 56, 67–91. 10.1016/j.plipres.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Zhou Z.-F.; Menna M.; Cai Y.-S.; Guo Y.-W. Polyacetylenes of marine origin: chemistry and bioactivity. Chem. Rev. 2015, 115, 1543–1596. 10.1021/cr4006507. [DOI] [PubMed] [Google Scholar]
- Nuzzo G.; Ciavatta M. L.; Villani G.; Manzo E.; Zanfardino A.; Varcamonti M.; Gavagnin M. Fulvynes, antimicrobial polyoxygenated acetylenes from the Mediterranean sponge Haliclona fulva. Tetrahedron 2012, 68, 754–760. 10.1016/j.tet.2011.10.068. [DOI] [Google Scholar]
- Zindo F. T.; Malan S. F.; Omoruyi S. I.; Enogieru A. B.; Ekpo O. E.; Joubert J. Design, synthesis and evaluation of pentacycloundecane and hexacycloundecane propargylamine derivatives as multifunctional neuroprotective agents. Eur. J. Med. Chem. 2019, 163, 83–94. 10.1016/j.ejmech.2018.11.051. [DOI] [PubMed] [Google Scholar]
- Zindo F. T.; Barber Q. R.; Joubert J.; Bergh J. J.; Petzer J. P.; Malan S. F. Polycyclic propargylamine and acetylene derivatives as multifunctional neuroprotective agents. Eur. J. Med. Chem. 2014, 80, 122–134. 10.1016/j.ejmech.2014.04.039. [DOI] [PubMed] [Google Scholar]
- Ramakrishna G. V.; Fernandes R. A. Total synthesis of the sensitive triyne natural product (4S,5S)-4,8-dihydroxy-3,4-dihydrovernoniyne and all of Its stereoisomers. Org. Lett. 2019, 21, 5827–5831. 10.1021/acs.orglett.9b01897. [DOI] [PubMed] [Google Scholar]
- Yang M.; Liang L.-F.; Wang T.; Wang H.-Y.; Liu H.-L.; Guo Y.-W. Further brominated polyacetylenes with pancreatic lipase inhibitory activity from Chinese marine sponge Xestospongia testudinaria. J. Asian Nat. Prod. Res. 2017, 19, 732–737. 10.1080/10286020.2016.1274308. [DOI] [PubMed] [Google Scholar]
- Negri R. Polyacetylenes from terrestrial plants and fungi: Recent phytochemical and biological advances. Fitoterapia 2015, 106, 92–109. 10.1016/j.fitote.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Schwartsmann G.; da Rocha A. B.; Berlinck R. G. S.; Jimeno J. Marine organisms as a source of new anticancer agents. Lancet Oncol. 2001, 2, 221–225. 10.1016/S1470-2045(00)00292-8. [DOI] [PubMed] [Google Scholar]
- Montaser R.; Luesch H. Marine natural products: a new wave of drugs?. Future Med. Chem. 2011, 3, 1475–1489. 10.4155/fmc.11.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar I.; Kim S.-K. Marine Antitumor Drugs: Status, Shortfalls and Strategies. Mar. Drugs 2010, 8, 2702–2720. 10.3390/md8102702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S.; Matsui K.; Tanaka K.; Satari R.; Kobayashi M. Lembehyne A, a novel neuritogenic polyacetylene, from a marine sponge of Haliclona sp. Tetrahedron 2000, 56, 9945–9948. 10.1016/S0040-4020(00)00973-X. [DOI] [Google Scholar]
- Aoki S.; Matsui K.; Takata T.; Hong W.; Kobayashi M. Lembehyne A, a spongean polyacetylene, induces neuronal differentiation in neuroblastoma cell. Biochem. Biophys. Res. Commun. 2001, 289, 558–563. 10.1006/bbrc.2001.6012. [DOI] [PubMed] [Google Scholar]
- Aoki S.; Matsui K.; Wei H.; Murakami N.; Kobayashi M. Structure–activity relationship of neuritogenic spongean acetylene alcohols, lembehynes. Tetrahedron 2002, 58, 5417–5422. 10.1016/S0040-4020(02)00519-7. [DOI] [Google Scholar]
- Murakami N.; Nakajima T.; Kobayashi M. Total synthesis of lembehyne A, a neuritogenic spongean polyacetylene. Tetrahedron Lett. 2001, 42, 1941–1943. 10.1016/S0040-4039(01)00038-7. [DOI] [Google Scholar]
- D’yakonov V. A.; Dzhemileva L. U.; Makarov A. A.; Andreev E. N.; Dzhemilev U. M. Short and efficient synthetic route to lembehyne B possessing neuritogenic activity. Russ. J. Org. Chem. 2016, 52, 1844–1846. 10.1134/S107042801612023X. [DOI] [Google Scholar]
- Dzhemileva L. U.; D’yakonov V. A.; Makarov A. A.; Andreev E. N.; Yunusbaeva M. M.; Dzhemilev U. M. The first total synthesis of the marine acetylenic alcohol, lembehyne B – a selective inducer of early apoptosis in leukemia cancer cells. Org. Biomol. Chem. 2017, 15, 470–476. 10.1039/C6OB02346K. [DOI] [PubMed] [Google Scholar]
- D’yakonov V. A.; Makarov A. A.; Dzhemileva L. U.; Andreev E. N.; Dzhemilev U. M. The first total synthesis of Lembehyne B. Mendeleev Commun. 2017, 27, 122–124. 10.1016/j.mencom.2017.03.004. [DOI] [Google Scholar]
- D’yakonov V. A.; Makarov A. A.; Dzhemileva L. U.; Andreev E. N.; Dzhemilev U. M. Total Synthesis of Neuritogenic Alkynes: Lembehyne B and Key Intermediate of Lembehyne A. ChemistrySelect 2017, 2, 1211–1213. 10.1002/slct.201601988. [DOI] [Google Scholar]
- D’yakonov V. A.; Makarov A. A.; Ibragimov A. G.; Khalilov L. M.; Dzhemilev U. M. Novel Mg-organic reagents in organic synthesis. Cp2TiCl2 catalyzed intermolecular cyclomagnesiation of cyclic and acyclic 1,2-dienes using Grignard reagents. Tetrahedron 2008, 64, 10188–10194. 10.1016/j.tet.2008.08.041. [DOI] [Google Scholar]
- D’yakonov V. A.; Makarov A. A.; Dzhemileva L. U.; Makarova E. K.; Khusnutdinova E. K.; Dzhemilev U. M. The facile synthesis of the 5Z,9Z-dienoic acids and their topoisomerase I inhibitory activity. Chem. Commun. 2013, 49, 8401–8403. 10.1039/c3cc44926b. [DOI] [PubMed] [Google Scholar]
- D’yakonov V. A.; Makarov A. A.; Makarova E. K.; Dzhemilev U. M. Novel organomagnesium reagents in synthesis. Catalytic cyclomagnesiation of allenes in the synthesis of N-, O-, and Si-substituted 1Z,5Z-dienes. Tetrahedron 2013, 69, 8516–8526. 10.1016/j.tet.2013.06.106. [DOI] [Google Scholar]
- Gangadhar P.; Ramakrishna S.; Venkateswarlu P.; Srihari P. Stereoselective total synthesis and structural revision of the diacetylenic diol natural products strongylodiols H and I. Beilstein J. Org. Chem. 2018, 14, 2313–2320. 10.3762/bjoc.14.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K.; Tsuda Y.; Yamane Y.; Takahashi H.; Iguchi K.; Naoki H.; Fujita T.; Van Soest R. W. M. Strongylodiols A, B and C, new cytotoxic acetylenic alcohols isolated from the Okinawan marine sponge of the genus Strongylophora as each enantiomeric mixture with a different ratio. Tetrahedron Lett. 2000, 41, 9271–9276. 10.1016/S0040-4039(00)01692-0. [DOI] [Google Scholar]
- Yadav J. S.; Mishra R. K. First total synthesis of strongylodiol A. Tetrahedron Lett. 2002, 43, 1739–1741. 10.1016/S0040-4039(02)00066-7. [DOI] [Google Scholar]
- Rashid M. A.; Gustafson K. R.; Boyd M. R. Pellynol I, a new cytotoxic polyacetylene from the sponge Pellina sp. Nat. Prod. Lett. 2000, 14, 387–392. 10.1080/10575630008043772. [DOI] [Google Scholar]
- Zhou G.-X.; Molinski T. F. Long-chain acetylenic ketones from the micronesian sponge Haliclona sp. importance of the 1-yn-3-ol group for antitumor activity. Mar. Drugs 2003, 1, 46–53. 10.3390/md101046. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.