Abstract
Objectives:
Gamma oscillation is important for cortico-cortical coordination and the integration of information across neural networks. The 40 Hz auditory steady-state response (ASSR), which reflects neural synchrony in the gamma band (30–100 Hz), is abnormal in patients with schizophrenia (SZ). The present study used the ASSR at multiple frequencies to examine (1) gamma dysfunction in patients with SZ, schizoaffective (SA), and bipolar disorder (BD) compared with controls, (2) the relationship between ASSR measures and clinical symptom severity, and (3) the relationship between ASSR measures and real-life community functioning.
Methods:
EEG was recorded from 75 controls, 52 SZ, 55 SA, and 89 BD patients during 20–30-40-Hz binaural click trains. ANCOVA was used to compare ASSR measures between groups controlling for age, sex, and education. Associations between ASSR measures, symptom severity, and community functioning were examined using linear regression and Pearson partial correlations.
Results:
ASSR deficits at gamma frequency were observed in all patient groups. SA patients showed additional specific deficit in the 20 Hz ASSR. Severity of manic, depressive, and anxiety symptoms mediated ASSR deficits. Severity of hallucinatory symptom and community functioning, particularly independent living/meaningful activity, were significantly and independently associated with the 40 Hz ASSR.
Conclusions:
SZ, SA and BD patients are likely to share the same abnormalities in neural processes that generate gamma oscillations. 40 Hz ASSR are associated with community functioning across patients and may serve as a biomarker for predicting functional outcome.
Keywords: Schizophrenia, Affective psychosis, Cortical oscillation, Symptom severity, Community functioning
1. Introduction
Neural oscillations, especially at gamma band frequencies (30-100 Hz), are thought to play a crucial role in signal transmission and integration of information across cortical networks (Singer, 1999; Uhlhaas et al., 2008). Gamma oscillations are frequently found to be impaired in schizophrenia, and these deficits are commonly attributed to dysfunctional GABAergic neurotransmission (Gonzalez-Burgos and Lewis, 2008; Uhlhaas et al., 2008). Auditory steady-state responses (ASSRs), electrophysiological responses generated in primary auditory cortex that are entrained to the frequency and phase of a periodic auditory stimulus (Picton et al., 2003; Ross et al., 2005), have been widely used to assess neural synchrony and the integrity of auditory pathways within and between cortical regions in patients with schizophrenia (SZ) (Brenner et al., 2009; Kwon et al., 1999; Onitsuka et al., 2013; Roach et al., 2013). Patients with SZ show robustly reduced ASSR power and phase locking to gamma range stimulation, particularly at 40 Hz (Brenner et al., 2009; Hirano et al., 2015; Kirihara et al., 2012; Kwon et al., 1999; O'Donnell et al., 2013; Onitsuka et al., 2013; Roach et al., 2013; Spencer et al., 2008; Thune et al., 2016). These disturbances may reflect a loss of neural integration and effective connectivity and may mediate a host of sensory and cognitive deficits and clinical symptoms (Gonzalez-Burgos and Lewis, 2008; Uhlhaas and Singer, 2013).
Previous studies have shown that deficits in gamma ASSRs are not specific to SZ (Spencer et al., 2008). Patients with bipolar disorder (BD) also exhibit ASSR deficits (O'Donnell et al., 2004; Oda, 2014b; Rass et al., 2010; Spencer et al., 2008), suggesting that these deficits may be manifestations of neural circuit dysfunctions shared among serious psychiatric disorders. Psychotic features are present in 58–80% of BD patients and may correlate with ERP responses (Hall et al., 2009; Hall et al., 2008). Numerous large-scale studies indicate overlap in genetic susceptibility across BD, SZ, and related phenotypes (Ruderfer et al., 2014). We have found that SZ risk genes are associated with reduced ASSR at 40 Hz in patients with psychosis (Hall et al., 2015). However, to our knowledge, ASSR deficits in patients with schizoaffective (SA) disorder and comparisons between SZ, SA and BD, has not been systematically evaluated.
According to the literature, SZ patients seem to have selective gamma ASSR deficits but not at lower stimulation frequencies (Kwon et al., 1999; Light et al., 2006; Spencer et al., 2008; Thune et al., 2016). BD patients, however, show ASSR reduction across the beta and gamma range (O'Donnell et al., 2004), but deficits in lower frequency are not consistent (Isomura et al., 2016a, 2016b; Oda, 2014a; Rass et al., 2010). The specificity of 20-Hz ASSR deficits to BD, being present in SA, and whether clinical variables modulate 20-Hz ASSR calls for further investigation. In addition, multiple studies have examined the associations between gamma oscillations and clinical features, but the results have been inconsistent (Gandal et al., 2012). Some studies have reported that gamma oscillation abnormalities were associated with psychotic symptoms including hallucinations, thought disorder, and cognitive disorganization (Spencer et al., 2004), or factors such as illness duration (Gallinat et al., 2004; Hall et al., 2011). Using the ASSR paradigm, Spencer et al. found a significant relation between increased 40 Hz ASSR and more severe positive symptoms of psychosis (e.g., hallucinations, delusions) (Spencer et al., 2009; Spencer et al., 2008), but this association was not observed by a larger well-powered study (Light et al., 2006). Taken together, relationships between gamma synchrony and clinical symptoms remain inconclusive, substantiating the need for studies of specific symptom domains and ASSR dysfunction in people with psychotic conditions.
To our knowledge, no study has examined the relationship between ASSR deficits and real-life community functioning in patients with psychosis, although numerous studies have reported gamma deficits in relation to neurocognitive impairments in psychiatric patients (Cho et al., 2006; Haenschel et al., 2009; Ford et al., 2008; Johannesen et al., 2008; Light et al., 2006; Minzenberg et al., 2010; Isomura et al., 2016a, 2016b). Community functioning (such as meaningful daily activity or social interests) plays a key role in evaluating for prognosis and a person's day to day life; it is also an important outcome measure for assessing the efficacy of treatments. A link between ASSR abnormalities and poor community functioning, if exists, will provide evidence of using this phenotype as biomarker for understanding and treating psychotic disorders.
The present study assembled a large clinical cohort to investigate: i) whether differences in ASSR measures (phase-locking factor [PLF] and evoked power) at multiple frequencies distinguish among patients with SZ, SA, and BD and between patients and controls; ii) whether ASSR measures are correlated with clinical variables such as psychotic or affective symptom severity; and iii) whether ASSR measures are related to community functioning in patients.
2. Methods and materials
2.1. Subjects
The sample consisted of a total of 196 patients (SZ [N = 52], schizoaffective [N = 55], BD [N = 89]) and 75 healthy controls (HC). 95% of BD patients had lifetime psychotic features. All subjects were assessed using the SCID. Patients were clinically stable and were recruited from outpatient clinics, flyers posted in a psychiatric hospital, community residential facilities, or by physician referral. All participants were between 18 and 65 years old with no known neurological disorder, no prior head injury with loss of consciousness, normal hearing confirmed by audiometry, and normal intellectual ability based on the North American Adult Reading Test (NAART) or years of education (high school completion or more). Patients were included if they had no substance abuse (excluding nicotine) in the preceding 6 months or dependence in the preceding 12 months and no history of seizures or ECT treatment in the preceding 12 months. Patients with psychiatric comorbidities (primarily panic disorders N = 7) were included as well. Clinical assessments were done by master or PhD-level clinical interviewers. In all assessments, inter-rater reliabilities were between 0.86 and 0.95. The HC sample was recruited through local advertisements. Additional inclusion criteria for HC were: no current or past history of psychotic disorder, BD, or a SZ/SA spectrum disorder, no affective disorder in the preceding 12 months, no substance abuse in the preceding 12 months or previous chronic dependence, and no first-degree relative with a history of psychosis or BD. Five controls in our sample had a lifetime (not in the past 12 months) diagnosis of single major depressive episode. One had a life time diagnosis of anorexia. None were taking psychotropic medication in the past 12 months. Demographic and clinical characteristics of the sample are presented in Table 1. This study was approved by the McLean Hospital Institutional Review Board. After a complete description of the study, written informed consent was obtained from each subject.
Table 1.
Demographic and clinical characteristics of subject groups.
| SZ patients (n = 52) |
SA patients (n = 55) |
BPD patients (n = 89) |
Controls (n = 75) | F | p-value | |
|---|---|---|---|---|---|---|
| Age, yrs | 42.7 (14.3) | 41.7 (11.7) | 38.6 (14.3) | 38.9 (11.5) | 1.61 | p = 0.19 |
| Female sex, N (%) | 41 (78.8) | 30 (54.6) | 49 (55.1) | 33 (44.0) | 5.40 | P = 0.001 |
| Education, yrs | 14.0 (1.9) | 14.4 (2.2) | 14.9 (2.3) | 16.2 (2.1) | 8.74 | p < 0.001 |
| Current smoker, N (%) | 19 (37.3) | 23 (45.0) | 29 (34.9) | 4 (5.6) | 10.58 | p < 0.001 |
| No. cigarettes/day, if smoker | 21.3 (16.9) | 16.2 (11.9) | 13.3 (10.6) | 7.3 (5.8) | 2.20 | p = 0.004 |
| Age of Onseta | 23.7 (8.3) | 23.2 (9.5) | 22.1 (8.8) | n/a | 0.52 | p = 0.59 |
| Right Handedness, N (%) | 42 (91.3) | 42 (84.0) | 62 (82.7) | 43 (93.4) | 1.36 | p = 0.25 |
| Medication | ||||||
| CPZ (mg/d) | 590.2 (560.7) | 502.1 (488.0) | 261.3 (355.5) | n/a | 4.76 | p = 0.01 |
| Bupropion | 260.9 (89.4) | 239.3 (125.7) | 300.0 (228.3) | n/a | 0.77 | p = 0.69 |
| Lithium | 1100 (346.4) | 814.3 (414.0) | 1105.2(375.2) | n/a | 2.18 | p = 0.18 |
| Divalproex sodium | 1300 (571.5) | 1857.1 (497) | 1216.3(467.9) | n/a | 1.04 | p = 0.39 |
| Lorazepam | 1.64(1.1) | 3.1 (2.0) | 2.4(2.5) | n/a | 0.58 | p = 0.59 |
| Real life functioning | ||||||
| MCAS total | 42.8 (7.5) | 45.1 (7.2) | 47.0 (5.4) | n/a | 0.74 | p = 0.48 |
| MCAS independent | 7.97 (1.6) | 8.09 (1.9) | 8.64 (1.3) | n/a | 1.23 | p = 0.30 |
| MCAS Social | 14.81 (3.4) | 15.59 (3.0) | 17.10 (2.4) | n/a | 2.77 | p = 0.07 |
| Symptom scales | ||||||
| SHPS | 1.80 (2.6) | 2.24 (2.3) | 1.52 (2.4) | 0.44(1.1) | 6.61 | p = 0.0001 |
| MASQ | 130.4 (39.5) | 141.5 (41.5) | 132.7 (36.3) | 102.5 (23.3) | 20.35 | p < 0.001 |
| MADRS | 11.2 (10.6) | 16.1 (14.1) | 11.1 (11.6) | n/a | 0.31 | p = 0.73 |
| YMRS | 8.63 (8.9) | 7.76 (5.8) | 8.11 (9.8) | n/a | 0.71 | p = 0.50 |
| PANSS-positive | 17.0 (6.7) | 14.68 (5.6) | 14.16 (6.9) | n/a | 0.17 | p = 0.85 |
| PANSS-negative | 18.0 (8.9) | 143.47 (7.5) | 12.47 (5.5) | n/a | 4.13 | p = 0.02 |
| PANSS-general | 32.36 (12.6) | 30.16 (11.0) | 28.47 (9.1) | n/a | 2.22 | p = 0.12 |
| PANSS-total | 67.36 (24.4) | 58.31 (21.5) | 55.1 (17.1) | n/a | 3.28 | p = 0.05 |
| SAPS hallucination global rating | 7.73 (6.3) | 6.0 (7.4) | 0.56 (0.9) | n/a | 4.58 | p = 0.03 |
| SAPS delusion global rating | 8.8 (8.8) | 9.78 (9.1) | 3.37 (4.8) | n/a | 2.53 | p = 0.15 |
| SAPS bizarre behaviour global rating | 2.07 (3.8) | 1.28 (3.1) | 0.67 (1.4) | n/a | 0.03 | p = 0.97 |
| SAPS thought disorder global rating b | 5.64 (6.0) | 6.0 (5.5) | 4.4 (5.8) | n/a | 0.31 | p = 0.73 |
Note: Significant differences are highlighted in bold. For medication and clinical variables, age and sex were included as covariates. Values are means (SD) unless otherwise indicated. Abbreviations: CPZ: chlorpromazine equivalents daily dose. PANSS: The Positive and Negative Syndrome Scale. SAPS: The Scale for the Assessment of Positive Symptoms. SANS: The Scale for the Assessment of Negative Symptoms. SHPS: The Snaith-Hamilton Pleasure Scale. MADRS: The Montgomery-Asberg Depression Rating Scale. MASQ: The Mood and Anxiety Symptom Questionnaire. YMRS: The Young Mania Rating Scale. Handedness were assessed using the Edinburgh Handedness Inventory.
Age at first affective/psychotic symptoms.
2.2. Clinical assessments
The Positive and Negative Syndrome Scale [PANSS (Kay et al., 1987)], The Scales for the Assessment of Positive Symptoms [SAPS (Andreasen et al., 1995)] and Negative symtoms [SANS (Andreasen, 1989)], the Young Mania Rating Scale [YMRS (Young et al., 1978)], and the Montgomery-Asberg Depression Rating Scale [MADRS (Montgomery and Asberg, 1979)(Corruble et al., 1998)] were used to evaluate current psychotic and mood symptoms. The Mood and Anxiety Symptom Questionnaire [MASQ (Watson et al., 1995)] was used to evaluate anhedonia, anxious arousal, and general distress related to anxiety or depression. Hedonic tone was measured using the Snaith-Hamilton Pleasure Scale [SHPS (Snaith et al., 1995)]. Real-life community functioning was evaluated using an abbreviated version of the Multnomah Community Ability Scale [MCAS; (Hendryx et al., 2001)]. We administered a modified version, which included 11 items scored 1–5 (higher scores indicate better functioning) for a total of 55 points. Items were also sub-grouped into two sub-categories: 1) independence in daily living and meaningful activity (item 7 and 13), and 2) Social interest and functioning (item 9–12). Information about medications at the time of the EEG assessment was obtained from participants and chlorpromazine (CPZ) equivalents were calculated based on the recommendations of Baldessarini (Baldessarini, 2013). All participants completed the SCID, SHPS, and the MASQ on the day of their EEG. Seventy nine patients (SZ n = 22, SA n = 19, BD n = 38) had clinical scales, including the PANSS, YMRS, MCAS, and MADRS, within 100 days of the date of their EEG (80% were completed within 30 days). Forty patients (SZ n = 14, SA n = 13, BD n = 13) also had data on the SAPS and SANS.
All but 1 SA patient were receiving psychotropic medication. The most commonly prescribed non-antipsychotic medications were: lithium carbonate (N = 43), divalproex sodium (N = 32), bupropion (N = 20). Patients who were on medication including clonazepam,lamotrigine, and lorazepam were excluded in this study because these medication are likely to alter gamma oscillations given their role in altering GABAergic neurotransmission.
2.3. Electrophysiological recording and analysis
The EEG was recorded continuously online using the BioSemi Active Two system (BioSemi Inc., Amsterdam, Netherland) at a digitization rate of 512 Hz, with a bandpass of DC–104 Hz and a Common Mode Sense (CMS) as the reference (PO2 site) using a 18-channel electrode cap (for details see e-Appendix). ASSR stimuli were presented through earphones in three blocks of stimuli (150/block): 20-Hz, 30-Hz, and 40-Hz stimulation rates. Stimuli consisted of trains of 1-msec white noise clicks (500-msec duration, 1100-msec stimulus onset asynchrony, 80 dB sound pressure level [SPL]). Subjects were instructed to look at the fixation cross on the monitor and listen to the stimuli. The order of blocks was counterbalanced across subjects (Spencer et al., 2008). Signal processing was performed off-line using Brain Vision Analyzer software (Brain Products GmbH, Germany). Time-frequency analyses were performed using custom programs in the IDL environment (EXELIS Visual Information Solutions, Boulder, Colorado). Single-trial epochs were extracted (−250 to 800 ms), baseline corrected, eye-blink corrected (Gratton et al., 1983), and artifact rejected if activity exceeded >100 μV. Time-frequency decomposition was performed using the Morlet wavelet transform (frequency/duration ratio f0/σf = 6), applied in 1 Hz steps from 10 to 100 Hz at each time point. PLF and evoked power were derived from the wavelet-transformed data at the Fz electrode. At each stimulation frequency (20, 30, and 40 Hz), average PLF and evoked power were computed across the time points (20 Hz: 50–550 ms; 30 Hz: 30–530 ms; 40 Hz: 20–520 ms) and wavelet frequencies (20 Hz: 17–26 Hz; 30 Hz: 28–37 Hz; 40 Hz: 38–47 Hz) where both ASSR PLF and evoked power were maximal (Spencer et al., 2009; Spencer et al., 2008).
2.4. Statistical analyses
Analysis of variance (ANOVA) was used for comparing groups on the demographic variables. Differences between the groups (HC, SZ, SA, BD) for each ASSR outcome variable were determined by analysis of covariance (ANCOVA) using STATA (STATA version12; Stata Corp., College Station, TX), with each ASSR outcome variable as a dependent variable, group as a categorical independent variable, controlling for sex, age and education as covariates. Post hoc pairwise comparisons were done by the Tukey-Kramer method for significant group findings corrected for multiple comparisons. Partial correlations, controlling for age, gender, and time gap (days) between the EEG and clinical assessments, were conducted to examine the association of PLF or evoked power with medication, clinical symptom severity (PANSS, SAPS, SANS, YMRS, MADRS, SHPS, and MASQ), and community functioning (MCAS) across the patient groups and by diagnosis. As correlation analyses were exploratory, statistical significance for the correlation analyses was set to be p ≤ 0.05. Clinical and community functioning variables that were found to be statistically significant in the correlation analyses were then entered into stepwise regression models with forward selection to determine which candidate factors independently predicted dependent variables (PLF or evoked power) at each stimulation frequency, controlling for age, sex, and time gap (days).
3. Results
Groups differed on several demographic variables, including gender, education, and smoking status (Table 1). There were more males in the SZ group versus the other groups (Table 2); the SA, BD, and HC groups did not differ in sex distribution. Patients as a group had significantly fewer years of education than HC subjects and SZ patients had fewer years of education than BD patients. There was a significantly larger proportion of smokers in all patient groups compared with the HCs. The patient groups did not differ from each other in proportion of current smokers or mean age of illness onset (Table 2). Patient groups differed on several measures of clinical symptom severity, including PANSS Negative (SZ > SA; SZ > BD) and Total scores (SZ > BD); SAPS hallucination and delusional global scores (SZ > BD; SA > BD). Patient groups differed on MCAS total (SZ < BD) and MCAS social scores (SZ < BD; SA < BD) [Table 2].
Table 2.
Post-hoc pairwise comparisons (Tukey-Kramer) of group means for demographic, medication, and clinical variables.
| SZ vs. HC | SA vs. HC | BPD vs, HC | SZ vs. SA | SZ vs. BPD | SA vs. BPD | |
|---|---|---|---|---|---|---|
| Female sex, N (%) | χ2 = 15.3, p < 0.01 | χ2 = 1.41, n.s. | χ2 = 1.99, n.s. | χ2 = 7.07, p < 0.01 | χ2 = 8.05, p < 0.01 | χ2 = 0.01, n.s. |
| Education, yrs | t = −5.22, p < 0.01 | t = −4.67, p < 0.01 | t = −3.50, p < 0.01. | t = −0.77, n.s. | t = −2.26, p = 0.03. | t = −1.49, n.s. |
| Current smoker, N (%) | t = 40.01, p < .01 | t = 5.00, p < 0.01 | t = 4.22, p < 0.01 | t = −0.92, n.s. | t = 0.30, n.s. | t = 1.32, n.s. |
| No. cigarettes/day, if smoker | t = 2.03, p < 0.05 | t = 1.31, n.s. | t = 0.89, n.s. | t = 1.45, n.s. | t = 2.35, p < 0.05 | t = 0.89, n.s. |
| Medication | ||||||
| CPZ (mg/d) | n/a | n/a | n/a | t = 0.81, n.s. | t = 40.01, p < .01 | t = 3.24, p < 0.01 |
| Bupropiona | n/a | n/a | n/a | t = 0.45, n.s. | t = 0.02, n.s. | t = 0.61, n.s. |
| Lithiumb | n/a | n/a | n/a | t = 2.7, n.s. | t = 0.54, n.s. | t = 3.63, p < 0.05 |
| Divalproex sodiumc | n/a | n/a | n/a | t = 4.02, p < 0.05 | t = 0.72, n.s. | t = 7.03, p < 0.05 |
| Lorazepamd | n/a | n/a | n/a | t = 2.52, n.s. | t = 1.10, n.s. | t = 1.42, n.s. |
| Real life functioning | ||||||
| MCAS total | n/a | n/a | n/a | t = −1.41, n.s. | t = 3.36, p < 0.01 | t = −1.57, n.s. |
| MCAS independent | n/a | n/a | n/a | t = −0.12, n.s. | t = −0.67, n.s. | t = −0.54, n.s. |
| MCAS social | n/a | n/a | n/a | t = −1.08, n.s. | t = −4.03, p < 0.01 | t = −2.91, p < 0.01 |
| Symptom scales | ||||||
| SHPS | t = 1.95, p = 0.05 | t = 2.96, p < 0.01 | t = 2.42, p = 0.02 | t = −0.94, n.s. | t = −0.10, n.s. | t = 0.93, n.s. |
| MASQ | t = 4.17, p < 0.01 | t = 4.34, p < 0.01 | t = 3.77, p < 0.01 | t = −0.17, n.s. | t = 1.04, n.s. | t = 1.19, n.s. |
| MADRSe | n/a | n/a | n/a | t = 2.37, n.s. | t = 0.84, n.s. | t = 1.54, n.s. |
| YMRSe | n/a | n/a | n/a | t = 0.95, n.s. | t = 2.98, n.s. | t = 2.02, n.s. |
| PANSS-positivee | n/a | n/a | n/a | t = 0.97, n.s. | t = 1.75, n.s. | t = 0.79, n.s. |
| PANSS-negativee | n/a | n/a | n/a | t = 4.23, p < 0.05 | t = 6.58, p < 0.05 | t = 2.35, n.s. |
| PANSS-generale | n/a | n/a | n/a | t = 1.72, n.s. | t = 2.59, n.s. | t = 0.86, n.s. |
| PANSS-totale | n/a | n/a | n/a | t = 2.45, n.s. | t = 4.35, p < 0.05 | t = 1.90, n.s. |
| SAPS hallucination global ratingf | n/a | n/a | n/a | t = 0.68, n.s. | t = 2.92, p < 0.01 | t = 4.49 p < 0.01 |
| SAPS delusion global ratingf | n/a | n/a | n/a | t = −0.29, n.s. | t = 2.15, p < 0.05 | t = 2.45, p < 0.05 |
| SAPS bizarre behaviour global ratingf | n/a | n/a | n/a | t = 0.78, n.s. | t = 1.83, n.s. | t = 1.12, n.s. |
| SAPS thought disorder global ratingf | n/a | n/a | n/a | t = 0.42, n.s. | t = 1.40, n.s. | t = 1.02, n.s. |
Note: Significant differences are highlighted in bold.
SZ n = 5; SA n = 7; BPD n = 8.
SZ n = 3; SA n = 6; BPD n = 29.
SZ n = 4; SA n = 7; BPD n = 19.
SZ n = 11; SA n = 6; BPD n = 4.
SZ n = 22; SA n = 19; BPD n = 38.
SZ n = 15; SA n = 14; BPD n = 16.
3.1. Phase Locking Factor (PLF)
3.1.1. Between group comparison
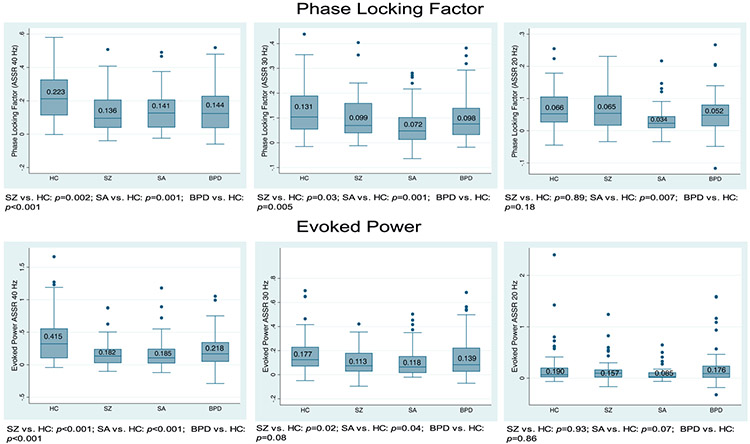
Fig. 1 shows the group comparison of the ASSR at each stimulation frequency. Fig. 2 shows the time-frequency analyses across groups. Compared with HC, patients with SZ, SA, or BD showed a significantly reduced ASSR at 40 Hz (HC vs. SZ p = 0.002, η2 = 0.019; HC vs. SA p = 0.001, η2 = 0.024; HC vs. BD p < 0.001, η2 = 0.043) and at 30 Hz (HC vs. SZ p = 0.030, η2 = 0.018; HC vs. SA p = 0.001, η2 = 0.047; HC vs. BD p = 0.005, η2 = 0.031). Patient groups did not differ from each other at either 40 Hz or 30 Hz ASSR. At 20 Hz patients with SA had reduced ASSR PLF (HC vs. SA p = 0.007, η2 = 0.039).
Fig. 1.
Group comparison of ASSR at each stimulation frequency (top: phase locking factor; bottom: evoked power).
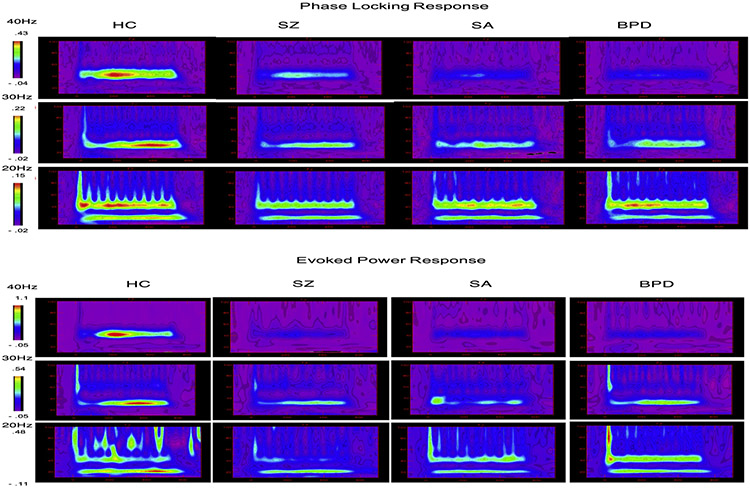
Fig. 2.
Time/frequency analysis across groups at each stimulation frequency (top: phase locking factor; bottom: evoked power).
3.2. Relationship between phase locking factor, medication, and clinical symptom severity
Table 3 shows the results of partial correlation analyses among the ASSR, clinical symptom severity, and real-life functioning, controlling for age, sex and time gap between assessments. Correlational analyses failed to identify significant effects of medication (CPZ equivalents, Lithium, Divalproex sodium, or Buproprion), age of illness onset, current smoking status, or number of cigarettes/day for either PLF or evoked power measures in patients as a group or by diagnosis. Significant correlations were found between 40 Hz PLF and depressive/anxiety symptoms, with lower PLF being associated with higher MADRS score (partial r = −0.26, p = 0.040) and the MASQ total score (partial r = −0.15, p = 0.030). Significant correlations were found between PLF at gamma frequency (both 40 and 30 Hz) and PANSS hallucinatory behavior (40 Hz r = −0.38, p = 0.01; 30 Hz r = −0.46, p = 0.003) and SAPS global hallucination scores (40 Hz r = −0.49, p = 0.02; 30 Hz r = −0.50, p = 0.030), with lower PLF being associated with more severe hallucinatory symptom. Finally, significant correlations were found between 40 Hz PLF and MCAS functioning, with higher PLF being associated with higher functioning in total score (r = 0.25, p = 0.040) and independence living and meaningful activity sub-score (r = 0.35, p = 0.006).
Table 3.
Correlations of clinical symptoms scales and real life functioning with ASSR variables.
| 40 Hz | PLF |
Evoked power |
||||
|---|---|---|---|---|---|---|
| 40 Hz | 30 Hz | 20 Hz | 40 Hz | 30 Hz | 20 Hz | |
| PANSS-positive | 0.07 n.s. | 0.02 n.s. | 0.12 n.s. | −0.08 n.s. | 0.06 n.s. | −0.14 n.s. |
| PANSS-hallucinatory Behavior | −0.38 p = 0.01 | −0.46 p = 0.003a | −0.07 n.s. | −0.41 p = 0.006a | −0.41 p = 0.01a | −0.01 n.s. |
| PANSS-Negative (*all n.s) | −0.18 n.g. | −0.07 n.s. | 0.14 n.g. | −0.15 n.g. | −0.09 n.g. | 0.06 n.g. |
| PANSS-General (*all n.s) | −0.05 n.g. | 0.01 n.g. | 0.05 n.g. | −0.08 n.g. | −0.01 n.g. | −0.04 n.g. |
| YMRS | 0.09 n.s. | −0.01 n.s. | −0.41 p = 0.01b | 0.09 n.s. | 0.01 n.s. | −0.36 p = 0.03b |
| SHPS | −0.13 n.s. | −0.03 n.s. | 0.10 n.s. | −0.17 p = 0.01b | 0.12 n.s. | 0.19 n.s. |
| MADRS | −0.26 p = 0.04 | −0.04 n.s. | 0.05 n.s. | −0.16 n.s. | 0.08 n.s. | 0.09 n.s. |
| MASQ | −0.15 p = 0.03 | −0.06 n.s. | 0.11 n.s. | −0.15 p = 0.02 | −0.15 n.s. | 0.02 n.s. |
| SAPS global hallucination rating | −0.49 p = 0.02 | −0.50 p = 0.03 | 0.24 n.s. | −0.49 p = 0.02 | −0.49 p = 0.05 | −0.05 n.s. |
| SAPS global delusion rating | 0.09 n.s. | 0.14 n.s. | 0.30 n.s. | −0.08 n.s. | 0.21 n.s. | −0.10 n.s. |
| SAPS global bizarre behavior rating | 0.01 n.s. | 0.09 n.s. | 0.02 n.s. | −0.14 n.s. | 0.02 n.s. | −0.01 n.s. |
| SAPS global thought disorder rating | 0.16 n.s. | 0.20 n.s. | −0.07 n.s. | 0.09 n.s. | 0.38 n.s. | −0.17 n.s. |
| MCAS total | 0.25 p = 0.04 | 0.05 n.s. | 0.03 n.s. | 0.29 p = 0.02 | 0.01 n.s. | 0.01 n.s. |
| MCAS independent | 0.35 p = 0.006 | 0.08 n.s. | −0.25 n.s. | 0.37 p = 0.003 | 0.02 n.s. | −0.14 n.s. |
| MCAS social | 0.16 n.s. | 0.12 n.s. | −0.23 n.s. | 0.28 p = 0.03 | 0.08 n.s. | −0.09 n.s. |
Abbreviations: CPZ: chlorpromazine equivalents daily dose. PANSS: The Positive and Negative Syndrome Scale. SAPS: The Scale for the Assessment of Positive Symptoms. SANS: The Scale for the Assessment of Negative Symptoms. SHPS: The Snaith-Hamilton Pleasure Scale. MADRS: The Montgomery-Asberg Depression Rating Scale. MASQ: The Mood and Anxiety Symptom Questionnaire. YMRS: The Young Mania Rating Scale. MCAS: Multnomah Community Ability Scale. MCAS-independent: independence in daily living and meaningful activity. MCAS-social: Social functioning. Note: Significant correlation (p < 0.05) between HC vs. all patients are highlighted in bold.
Significant between HC vs. SZ.
Significant between HC vs. BD.
Partial correlations conducted separately by diagnosis revealed that in the SZ and BD group, lower 40 Hz PLF was associated with higher MADRS scores (r = −0.36, p = 0.010); in the BD and SA groups, lower 20 Hz PLF was associated with higher YMRS (r = −0.46, p = 0.010); in the SZ group, lower 30 Hz PLF was associated with higher PANSS hallucinatory behavior (r = −0.44, p = 0.030), after accounting for the effects of age, sex, and time Gap [Table 3].
3.3. Independent predictors of phase locking factor
Regression models were used to determine whether clinical symptom severity (PANSS auditory hallucinatory behavior, MADRS, MASQ, SHPS) and community functioning (MCAS independent living & social activity) were significant predictors of PLF in the patient group after accounting for age, sex, education, and time gap. Results showed that PANSS auditory hallucinatory behavior (η2 = 0.052, p = 0.049), MASQ (η2 = 0.054, p = 0.044), and MCAS independent living/meaningful activity (η2 = 0.067, p = 0.027) were significant and independent predictors of 40 Hz. Auditory hallucinatory behavior was a significant predictor of 30 Hz (η2 = 0.19, p = 0.002). YMRS (η2 = 0.16, p = 0.007) and SHPS (η2 = 0.12, p = 0.017) were significant and independent predictors of 20 Hz ASSR.
3.4. Evoked power
3.4.1. Between group comparison
Compared with HC, patients with SZ, SA, or BD showed significantly reduced ASSR evoked power at 40 Hz (HC vs. SZ p < 0.001, η2 = 0.049; HC vs. SA p < 0.001, η2 = 0.057; HC vs. BD p = 0.001, η2 = 0.068, Fig. 1). At 30 Hz, patients with SZ and SA showed significantly reduced power (HC vs. SZ p = 0.020, η2 = 0.024; HC vs. SA p = 0.040, η2 = 0.019) whereas the reduction in BD patients was at a trend level (p = 0.080). No significant group differences were observed at 20 Hz.
3.5. Relationship between evoked power, medication, and clinical symptom severity
Significant correlations were found between 40 Hz evoked power and mood/anxiety symptoms, with lower evoked power being associated with higher SHPS score (r = −0.17, p = 0.010) or MASQ total score (r = −0.15, p = 0.020), and between 40 Hz evoked power and MCAS functioning, with higher evoked power being associated with higher real-life functioning (total score: r = 0.25, p = 0.020, independence living/meaningful activity: r = 0.37, p = 0.003, and social functioning: r = 0.28, p = 0.030). Significant correlations were also found between evoked power at gamma frequency (40 and 30 Hz) and PANSS hallucinatory behavior (40 Hz r = −0.41, p = 0.006; 30 Hz r = −0.41, p = 0.010) and SAPS global hallucination scores (40 Hz r = −0.49, p = 0.020; 30 Hz r = −0.49, p = 0.050), with lower evoked power being associated with more severe hallucinatory symptom [Table 3].
Partial correlations separately by diagnosis showed that in the BD group, lower 40 Hz evoked power was associated with higher SHPS scores (r = −0.46, p = 0.030); in both SZ and BD groups, lower 40 Hz was associated with higher MADRS scores (r = −0.36, p = 0.010); in both BD and SA groups, lower 20 Hz was associated with higher YMRS (r = −0.45, p = 0.010); in the SZ group, lower gamma frequency (40 & 30 Hz) were associated with higher PANSS hallucinatory behavior (40 Hz r = −0.41, p = 0.006; 30 Hz r = −0.41, p = 0.010), after accounting for the effects of age, sex, and time Gap [Table 3].
3.6. Independent predictors of evoked power
Results of regression models showed that MCAS independent living/meaningful activity (η2 = 0.10, p = 0.009) was a significant predictor of 40 Hz and auditory hallucinatory behavior was at a trend level (η2 = 0.04, p = 0.080). Auditory hallucinatory behavior was a significant predictor of 30 Hz (η2 = 0.18, p = 0.004). YMRS (η2 = 0.07, p = 0.070) was at a trend level as a predictor of 20 Hz ASSR.
4. Discussion
The results of the present study, based on a large sample of patients with diagnoses of SZ, SA, and BD, extend previous findings showing that SZ and BD patients have selective deficits in generating gamma frequency oscillations in response to steady-state auditory stimulation (Kwon et al., 1999; Light et al., 2006; O'Donnell et al., 2004; Spencer et al., 2008), whereas SA patients appear to have deficits across beta and gamma frequency. In addition to the deficits in gamma frequency oscillations, SA patients also exhibited specific deficit at the 20 Hz beta frequency. To our knowledge, this study is the first to examine the 20-Hz ASSR deficits in SA. The additional and selective deficit at 20 Hz in SA patients were present in both PLF and evoked-power measures, although the latter was at the trend level significance (Fig. 1). These results support the interpretation that patients with SZ, SA and BD are likely to share some of the same neural circuit abnormalities probed by the ASSR paradigm at gamma frequency and are in accord with postmortem reports showing considerable overlap in inhibitory interneuron-related neural circuit abnormalities in SZ and BD (Benes and Berretta, 2001; Torrey et al., 2005; Woo et al., 2004).
Prior studies of ASSR deficits in BD found inconsistent results. O'Donnell et al. reported that BD subjects had reduced ASSR across the beta and gamma frequency bands (O'Donnell et al., 2004), whereas Rass et al. did not (Rass et al., 2010). In patients with first episode psychosis, Spencer et al. observed no group differences at 20 Hz ASSR among SZ, affective psychosis (mainly BD) and HCs (Spencer et al., 2008). It is possible that the discrepancy among studies in BD may be due to differences in clinical state. Although previous studies have included BD patients at different stage of illness (acute vs. euthymic; psychotic vs. non-psychotic), the relationships between mood symptom severity and ASSR deficits were not examined. In the present study, results showed that, in both PLF and evoked power, severity of YMRS was significantly associated with 20 Hz ASSR. Analysis by diagnosis revealed that this association was significant in SA and BD patients but not in SZ patients, suggesting that the reduction of the 20 Hz ASSR may mediate the severity of manic symptoms in affective disorders. Beta oscillations are important in long-range cortico-cortical synchronization (Schnitzler and Gross, 2005) and in mediating feed-back (top-down) prediction of upcoming sensory events (Arnal and Giraud, 2012). Our result raises the possibility that severity of manic symptoms might be linked with long-range communication between cortical areas.
40 Hz ASSR may play an important role in mediating the severity of depression and anxiety symptoms. Our results showed that lower 40 Hz ASSR was associated with higher MADRS and MASQ scores. In relation to MADRS score, this association was driven primarily by SZ and BD patients whereas in MASQ this association was not driven by a specific diagnostic group. MADRS and MASQ measures are correlated (r = 0.41, p = 0.002) but each measure also tabs into a specific mood component. These results suggest that 40 Hz ASSR deficit reflects, in part, the severity of depression and anxiety symptom in patients with psychosis. A recent magnetoencephalography MEG-ASSR study found significant negative correlations between the Hamilton depression rating scale score and 80 Hz MEG-ASSR in both MDD and BD patients (Isomura et al., 2016a, 2016b). Although this MEG-ASSR study did not find such an association at 40 Hz, the clinical symptom scale used and the small sample size might account for the non-significant p value. The observed negative correlation is in line with a recent report showing that resting EEG gamma power correlates with inattention in patients with major depressive disorder (Roh et al., 2016). Many previous studies have shown a robust relationship between gamma oscillation deficit and neurocognitive impairment in psychiatric patients, including attention (Shuai and Elhilali, 2014), working memory (Corinna Haenschel et al., 2009; Light et al., 2006), and perceptual processing (Ford et al., 2008; J.K. Johannesen et al., 2008). Impaired ASSR is believed to reflect a loss of neural integration and effective connectivity which may mediate a host of sensory and cognitive deficits and clinical symptoms in psychosis disorders (Gonzalez-Burgos and Lewis, 2008; Uhlhaas and Singer, 2013). The link between 40 Hz ASSR deficit, neurocognitive impairment, and severity of depression and anxiety symptom warrant further investigation in the future.
The overall presence of psychotic symptoms as measured by the PANSS-positive total scores or by the SAPS global ratings (including delusions, bizarre behavior, and thought disorder) was not significantly related to ASSR. However, more severe hallucinations were significantly associated with reduced ASSR using both PANSS subscale (Hallucinatory behavior) and the SAPS global hallucination rating measures and that this association was specific at the gamma frequency (Table 3). No evidence was found of a correlation between ASSR 20 Hz and hallucination severity. These results thus indicate a specific relationship between hallucinatory symptom and ASSR gamma oscillations. In the present study, negative correlations between ASSR gamma oscillation and hallucinations were found. In contrast, Spencer et al. found positive correlations between ASSR gamma oscillation and hallucinations (Spencer et al., 2009). We are not sure what factors account for this inconsistency. In this sample, both patients at an early stage of illness and those at a more chronic stage showed negative correlations between ASSR gamma and hallucinations. Patients in each diagnostic group also showed inverse relations between ASSR gamma oscillations and hallucinations. Source analysis was performed in Spencer et al. report. Unfortunately, the number of EEG channels (18) used in this study prohibits us from applying source analysis.
Little is known about the functional correlates and consequences of ASSR deficits in patients with psychotic disorders. To our knowledge, the present study is the first to examine the effect of ASSR on real-life community function in patients with SZ, SA, or BD diagnoses. We found that 40 Hz ASSR is specifically correlated with real-life functioning across diagnoses. Patients with better overall community functioning, as well as with better independence and social functioning, generate larger ASSR (Table 3).
Results of model prediction revealed that, among all the candidate predictors, including clinical symptom severity (PANSS auditory hallucinatory behavior, MADRS, MASQ, SHPS) and community functioning (MCAS independent living & social activity), auditory hallucination and independent living/meaningful activity were significant and independent predictors of 40 Hz; auditory hallucinatory behavior alone was a significant predictor of 30 Hz; and that YMRS and SHPS symptoms were significant predictors of 20 Hz ASSR. These results indicate that the severity of hallucinatory symptoms as well as a person's ability to take care of him/herself independently may be influenced by the generation of cortical gamma oscillations, particularly at the 40 Hz.
Recently, there is a paradigm shift toward studying and treating psychiatric disorders (Insel, 2012; Swerdlow, 2011), focusing more on using neuroscience based translational quantitative biomarkers to guide and predict treatment outcome. Our results suggest that 40 Hz ASSR, a simple low-cost and translatable EEG measure, may be informative as a biomarker for predicting functional outcome and informing the pathophysiology of the disorder. Studies exploring the clinical utility of ASSR and demonstrating predictive power of functional status by means of simple ASSR recordings are rare but critically needed.
This study has several limitations. First, a small number of patients had a more than one-month temporal lag between the symptom ratings and the EEG assessment. Symptoms can fluctuate over time and may not reflect the clinical symptom profile at the time of the EEG recording. However, the temporal lag variable was included as a covariate in all of the analyses and we observed no significant effects on any results. Furthermore, when we restricted the analyses to individuals who had both clinical and EEG data collected within a one month period, the results remained unchanged. Second, a majority of our participants with BP had psychotic symptoms. As a result, our findings may not be generalizable to non-psychotic BP populations. Third, community functioning was evaluated using MCAS only. As functional outcome represents a multifaceted construct, it is desirable in future study to use additional measures such as the UCSD Performance-Based Skills Assessment (UPSA) to probe different domains of functioning. Fourth, a few controls had a life-time diagnosis of single major depressive episode or anorexia. However, results remained unchanged when excluding these controls from the main analyses, suggesting that findings are robust.
In conclusion, the present results support the hypothesis that patients with SZ, SA and BD share some of the same abnormalities in neural circuits and processes that generate gamma ASSR. There is also a diagnosis specific impairment in the 20 Hz beta frequency in SA. Severity of hallucinations, depressive and anxiety symptoms mediated ASSR deficits in patients with psychosis. 40 Hz ASSR are associated with community functioning across patients and may serve as a biomarker for predicting functional outcome.
Acknowledgments
This work was supported by the National Institute of Mental Health [1R01MH109687] to M-HH and [K24MH104449] to DO, the Ellison Foundation, Anonymous Foundation, the Carmela and Menachem Abraham Fund, and Team Daniel to DLL.
Role of the funding source
National Institute of Mental Health [1R01MH109687]: Mei-Hua Hall, PI; [K24MH104449]: Dost Öngür, PI. The Ellison Foundation, Anonymous Foundation, the Carmela and Menachem Abraham Fund, and Team Daniel: Deborah L. Levy, PI.
Footnotes
Conflict of interests
The authors reported no biomedical financial conflict of interests. Dr. Dost Öngür received research support from Roche Genentech in 2014.
Financial disclosures
The authors reported no biomedical financial interests or potential conflicts of interest. Dr. Dost Öngür received research support from Roche Genentech in 2014.
References
- Andreasen NC, 1989. The scale for the assessment of negative symptoms (SANS): conceptual and theoretical foundations. Br. J. Psychiatry (7), 49–58 Supplement. [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Miller D, Flaum M, Nopoulos P, 1995. Correlational studies of the scale for the assessment of negative symptoms and the scale for the assessment of positive symptoms: an overview and update. Psychopathology 28 (1), 7–17. [DOI] [PubMed] [Google Scholar]
- Arnal LH, Giraud AL, 2012. Cortical oscillations and sensory predictions. Trends Cogn. Sci 16 (7), 390–398. [DOI] [PubMed] [Google Scholar]
- Baldessarini R, 2013. Chemotherapy in Psychiatry: Pharmacologic Basis of Treatments of Major Mental Illness. Springer Verlag; (3rd), New York. [Google Scholar]
- Benes FM, Berretta S, 2001. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25 (1), 1–27. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, O'Donnell BF, 2009. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr. Bull 35 (6), 1065–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS, 2006. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc. Natl. Acad. Sci. U. S. A 103 (52), 19878–19883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corruble E, Purper D, Payan C, Guelfi J, 1998. Inter-rater reliability of two depression rating scales, MADRS and DRRS, based on videotape records of structured interviews. Eur. Psychiatry 13 (5), 264–266. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Faustman WO, Mathalon DH, 2008. Out-of-synch and out-of-sorts: dysfunction of motor-sensory communication in schizophrenia. Biol. Psychiatry 63 (8), 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D, 2004. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin. Neurophysiol 115 (8), 1863–1874. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Klook K, Siegel SJ, 2012. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology 62 (3), 1504–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA, 2008. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr. Bull 34 (5), 944–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E, 1983. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol 55 (4), 468–484. [DOI] [PubMed] [Google Scholar]
- Haenschel Corinna, Bittner Robert A., Waltz James, Haertling Fabian, Wibral Michael, Singer Wolf, Linden D'Avid, E. J., Rodriguez E, 2009. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J. Neurosci 29 (30), 9481–9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Sham P, Kalidindi S, McDonald C, Bramon E, Levy DL, Murray RM, Rijsdijk F, 2008. Further evidence for shared genetic effects between psychotic bipolar disorder and P50 suppression: a combined twin and family study. Am. J. Med. Genet. B. Neuropsychiatr. Genet 147B (5), 619–627. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Kalidindi S, McDonald C, Bramon E, Murray RM, Sham P, 2009. Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley bipolar twin and family study. Psychol. Med 39 (8), 1277–1287. [DOI] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Salisbury DF, Levy DL, 2011. Sensory gating event-related potentials and oscillations in schizophrenia patients and their unaffected relatives. Schizophr. Bull 37 (6), 1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Chen CY, Cohen BM, Spencer KM, Levy DL, Ongur D, Smoller JW, 2015. Genomewide association analyses of electrophysiological endophenotypes for schizophrenia and psychotic bipolar disorders: a preliminary report. Am. J. Med. Genet. B. Neuropsychiatr. Genet 168B (3), 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendryx M, Dyck DG, McBride D, Whitbeck J, 2001. A test of the reliability and validity of the Multnomah community ability scale. Community Ment. Health J 37 (2), 157–168. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Oribe N, Kanba S, Onitsuka T, Nestor PG, Spencer KM, 2015. Spontaneous gamma activity in schizophrenia. JAMA Psychiat. 72 (8), 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, 2012. Next-generation treatments for mental disorders. Sci. Transl. Med 4 (155) 155ps119. [DOI] [PubMed] [Google Scholar]
- Isomura S, Onitsuka T, Tsuchimoto R, Nakamura I, Hirano S, Oda Y, Oribe N, Hirano Y, Ueno T, Kanba S, 2016a. Differentiation between major depressive disorder and bipolar disorder by auditory steady-state responses. J. Affect. Disord 190, 800–806. [DOI] [PubMed] [Google Scholar]
- Isomura Shuichi, Onitsuka Toshiaki, Tsuchimoto Rikako, Nakamura Itta, Hirano Shogo, Oda Yuko, Oribe Naoya, Hirano Yoji, Ueno Takefumi, Kanba S, 2016b. Differentiation between major depressive disorder and bipolar disorder by auditory steady-state responses. J. Affect. Disord 190,800–806. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Bodkins M, O'Donnell BF, Shekhar A, Hetruck WP, 2008. Perceptual anomalies in schizophrenia co-occur with selective impairments in the gamma frequency component of midlatency auditory ERPs. J. Abnorm. Psychol 117 (1), 106–118. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA, 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull 13 (2), 261–276. [DOI] [PubMed] [Google Scholar]
- Kirihara K, Rissling AJ, Swerdlow NR, Braff DL, Light GA, 2012. Hierarchical organization of gamma and theta oscillatory dynamics in schizophrenia. Biol. Psychiatry 71 (10), 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW, 1999. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch. Gen. Psychiatry 56 (11), 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL, 2006. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol. Psychiatry 60 (11), 1231–1240. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS, 2010. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology 35 (13), 2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M, 1979. A new depression scale designed to be sensitive to change. Br. J. Psychiatry J. Ment. Sci 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Oda Y, 2014a. Gamma band neural synchronization deficits for auditory steady state responses in bipolar disorder patients. Seishin Shinkeigaku Zasshi 116 (3), 245–253. [PubMed] [Google Scholar]
- Oda Y, 2014b. Gamma band neural synchronization deficits for auditory steady state responses in bipolar disorder patients. Psychiatr. Neurol. Jpn. (Seishin Shinkeigaky Zasshi) 116 (3), 245–253. [PubMed] [Google Scholar]
- O'Donnell BF, Hetrick WP, Vohs JL, Krishnan GP, Carroll CA, Shekhar A, 2004. Neural synchronization deficits to auditory stimulation in bipolar disorder. Neuroreport 15 (8), 1369–1372. [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, Vohs JL, Krishnan GP, Rass O, Hetrick WP, Morzorati SL, 2013. The auditory steady-state response (ASSR): a translational biomarker for schizophrenia. Suppl. Clin. Neurophysiol 62,101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onitsuka T, Oribe N, Kanba S, 2013. Neurophysiological findings in patients with bipolar disorder. Suppl. Clin. Neurophysiol 62, 197–206. [DOI] [PubMed] [Google Scholar]
- Picton TW, John MS, Dimitrijevic A, Purcell D, 2003. Human auditory steady-state responses. Int. J. Audiol 42 (4), 177–219. [DOI] [PubMed] [Google Scholar]
- Rass O, Krishnan G, Brenner CA, Hetrick WP, Merrill CC, Shekhar A, O'Donnell BF, 2010. Auditory steady state response in bipolar disorder: relation to clinical state, cognitive performance, medication status, and substance disorders. Bipolar Disord. 12 (8), 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach BJ, Ford JM, Hoffman RE, Mathalon DH, 2013. Converging evidence for gamma synchrony deficits in schizophrenia. Suppl. Clin. Neurophysiol 62, 163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh SC, Park EJ, Shim M, Lee SH, 2016. EEG beta and low gamma power correlates with inattention in patients with major depressive disorder. J. Affect. Disord 204, 124–130. [DOI] [PubMed] [Google Scholar]
- Ross B, Herdman AT, Pantev C, 2005. Stimulus induced desynchronization of human auditory 40-Hz steady-state responses. J. Neurophysiol 94 (6), 4082–4093. [DOI] [PubMed] [Google Scholar]
- Ruderfer DM, Fanous AH, Ripke S, Mcquillin A, Amdur RL, Gejman PV, O'Donovan MC, Andreassen OA, Djurovic S, Hultman CM, Kelsoe JR, Jamain S, Landen M, Leboyer M, Nimgaonkar V, Nurnberger J, Smoller JW, Craddock N, Corvin A, Sullivan PF, Holmans P, Sklar P, Kendler KS, 2014. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol. Psychiatry 19 (9), 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A, Gross J, 2005. Functional connectivity analysis in magnetoencephalography. Int. Rev. Neurobiol 68,173–195. [DOI] [PubMed] [Google Scholar]
- Shuai L, Elhilali M, 2014. Task-dependent neural representations of salient events in dynamic auditory scenes. Front. Neurosci 8, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, 1999. Neuronal synchrony: a versatile code for the definition of relations? Neuron 24 (1), 49–65 (111-125). [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P, 1995. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br. J. Psychiatry J. Ment. Sci 167 (1), 99–103. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW, 2004. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc. Natl. Acad. Sci. U. S. A 101 (49), 17288–17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW, 2008. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol. Psychiatry 64 (5), 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, Mccarley RW, 2009. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 10, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow N, 2011. Are we studying and treating schizophrenia correctly? Schizophr. Res 130 (1–3), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thune H, Recasens M, Uhlhaas PJ, 2016. The 40-Hz auditory steady-state response in patients with schizophrenia: a meta-analysis. JAMA Psychiat. 73 (11), 1145–1153. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB, 2005. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol. Psychiatry 57 (3), 252–260. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W, 2013. High-frequency oscillations and the neurobiology of schizophrenia. Dialogues Clin. Neurosci 15 (3), 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W, 2008. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull 34 (5), 927–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA, 1995. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J. Abnorm. Psychol 104(1), 3–14. [DOI] [PubMed] [Google Scholar]
- Woo TU, Walsh JP, Benes FM, 2004. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch. Gen. Psychiatry 61 (7), 649–657. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry 133, 429–435. [DOI] [PubMed] [Google Scholar]