Abstract
Purpose:
Cutaneous melanoma metastatic to the vitreous is very rare. This study investigates the clinical findings, treatment and outcome of patients with metastatic cutaneous melanoma to the vitreous. The majority of patients received checkpoint inhibition for the treatment of their systemic disease and the significance of this is explored.
Participants:
14 eyes of 11 patients with metastatic cutaneous melanoma to the vitreous.
Design:
Multicenter, retrospective cohort study
Methods:
Clinical records, including fundus photography and ultrasound, were retrospectively reviewed and relevant data was recorded for each patient eye.
Main Outcome Measures:
Clinical features at presentation, ophthalmic and systemic treatments and outcome
Results:
The median age at presentation of ophthalmic disease was 66 years (range 23–88 years), and the median follow-up from diagnosis of ophthalmic disease was 23 months. Ten of eleven patients were treated with immune checkpoint inhibition, at some point in their treatment course. The median time from starting immunotherapy to ocular symptoms was 17 months (range 4.5 to 38 mos). Half the eyes had amelanotic vitreous debris. Five eyes developed elevated intraocular pressure and four eyes developed a retinal detachment. Six patients had metastatic disease in their central nervous system. Ophthalmic treatment included: external beam radiation (30–40Gy) in six eyes, intravitreous melphalan (10–20μg) in four eyes, enucleation in one eye, local observation while on systemic treatment in two eyes. Three eyes received intravitreous bevacizumab for neovascularization. The final Snellen visual acuity ranged from 20/20 to no light perception (NLP).
Conclusion:
The differential diagnosis of vitreous debris in the context of metastatic cutaneous melanoma includes intravitreal metastasis, and this appears to be particularly apparent during this era of treatment with checkpoint inhibition. External beam radiation, intravitreous melphalan and systemic checkpoint inhibition can be used in the treatment of ophthalmic disease. Neovascular glaucoma and retinal detachments may occur, and the majority of eyes have poor visual potential. Approximately one quarter of the patients had ocular disease that preceded central nervous system metastasis . Patients with visual symptoms or vitreous debris in the context of metastatic cutaneous melanoma would benefit from evaluation by an ophthalmic oncologist.
Precis:
Vitreous debris in the context of metastatic cutaneous melanoma could represent metastatic disease, and this is particularly pertinent in the era of treating cutaneous melanoma with checkpoint inhibitors.
Introduction
The most common sites of metastatic cutaneous melanoma include skin, lung, brain, liver, bone, distant lymph nodes and intestine1. Although metastases to the eye are common in some cancers (breast and lung cancer in adults and leukemias in children) the eye is an unusual site of metastasis for cutaneous melanoma, and when it occurs, it typically involves the choroid2. In contrast, the vitreous is an extremely rare site of metastasis for any cancer including cutaneous melanoma. True to its rarity, only case reports and series of intravitreous metastatic cutaneous melanoma have been reported3: with the largest series totaling 4 eyes in 3 patients4. Within the last decade, management of metastatic melanoma has shifted: immune checkpoint inhibitors (CPI) have become available, widespread and now a first-line treatment of metastatic melanoma.
CPI comprise a group of immunotherapy agents that prevent tumor cells from evading the immune system. They include cytotoxic T-lymphocyte associated antigen-4 (CTLA-4), programmed cell death (PD-1) and programmed cell death protein 1 (PD-L1) inhibitors. Checkpoint inhibitors potentiate the T-lymphocyte response generally, including reactivity against tumor cells. These drugs have had a major impact on the treatment of melanoma and a subset of other cancers, and are one of the first class of drugs proven to improve the overall survival of patients with metastatic cutaneous melanoma5.
We present a series of metastatic cutaneous melanoma patients who developed vitreous opacities, the majority of whom were receiving checkpoint inhibition. We investigate the clinical findings, treatment and outcome of these patients; and explore the potential role of immunotherapy in the occurrence and clinical features of this metastatic disease site. We also describe how known drug-induced toxicities may complicate the differential diagnosis.
Methods
This study included all eyes with metastatic cutaneous melanoma to the vitreous diagnosed between July 2010 and Feb 2019. The study centers included Memorial Sloan Kettering Cancer Center (MSKCC), New York Eye and Ear Infirmary, Massachusetts Eye and Ear Infirmary, Kellogg Eye Center and Emory Eye Center. The study was conducted under Institutional Review Board approval from Memorial Sloan Kettering Cancer Center; and was Health Insurance Portability and Accountability Act (HIPAA) compliant. All patients provided informed consent. Research adhered to the tenets of the Declaration of Helsinki.
Clinical records, including fundus photography and ultrasound, were retrospectively reviewed and relevant data was recorded for each patient eye. The patient data included age, gender, laterality, type and onset of symptoms, initial and final Snellen visual acuity, vital status (alive or dead) and follow-up time. Disease data included appearance of vitreous metastases, presence of other ophthalmic pathology, presence of metastasis to central nervous system. Treatment data included prior systemic treatments for metastatic melanoma, drugs at time of vitreous metastasis diagnosis, time to onset of symptoms from diagnosis of metastatic cutaneous melanoma to vitreous disease, subsequent ophthalmic and systemic treatments. All patients had confirmation of vitreous disease by vitreous biopsy.
Results
Tables 1 and 2 outline the patient, disease and treatment characteristics; and figures 1–4 demonstrate some of the clinical and histopathological findings. 14 eyes of eleven patients (6 female and 5 male) were included in this study. The median age at presentation of ophthalmic disease was 66 years (range 23–88 years), and the median follow-up was 23 mos (2–97 mos) during which time three patients died at 2, 3 and 63 months following initial ocular symptoms. Three patients had bilateral disease. Initially reported symptoms included decreased or blurry vision (5 patients), floaters (5 patients), asymptomatic (1 patient). One patient was asymptomatic and was diagnosed on routine eye exam. The Snellen visual acuity at ophthalmic presentation ranged from 20/20 to counting fingers (CF). At the time of vitreous disease diagnosis, eight patients had completed or were currently managed on checkpoint inhibition. The median time from starting immunotherapy to ocular symptoms was 17 months (range 4.5 to 38 mos).
Table 1:
Clinical characteristics of patients and their systemic disease
| eye 1 | eye 2 | eyes 3 + 4 | eye 5 | eye 6 | eyes 7 + 8 | eye 9 | eye 10 | eye 11 | eyes 12 + 13 | eyes 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| age (years), gender | 66, F | 88, F | 88, M | 64, F | 23, M | 64, F | 69, M | 66, M | 37, F | 72 F | 78 M |
| prior treatment | nivolumab, vemurafenib | none | none | none | interluekin-2, vemurafenib | none | Ipilimumab, nivolumab | None | lnterleukin-2 | Pembrolizumab | none |
| drug at time ocular symptoms | Ipilimumab | Ipilimumab, Nivolumsb | Nivolumab | Pembrolizumab | ipilimumab | none | Nivolumab | Pembrolizumab | Autologous anti-tumor lymphocyte tx | Encorafenib + Binimetinib | none |
| CNS mets* | no | Yes (pre) | no | yes (post) | yes (pre) | Yes (pre) | no | no | yes (post) | yes (post) | no |
| other metastatic sites | subcutaneous, pulmonary, small intestine, colon, | LN, pulmonary, small intestine, spleen | subcutaneous, LN | LN, pulmonary | subcuteanous, LN, pulmonary | none | LN, pulmonary | pulmonary | chest wall | LN, adrenal | subcutaneous |
| follow up (mos) ** | 63 | 22 | 5 | 3 | 70 | 33 | 23 | 5 | 97 | 2 | 43 |
| vital status | dead | alive | alive | dead | alive | alive | alive | alive | alive | dead | alive |
| genomics of CutMM | BRAF | NRAS,TERT | NRAS | NRAS | BRAF | BRAF | BRAF wild type | Not available | BRAF | BRAF | BRAF wild type |
= “pre” refers to CNS disease prior to ophthalmic diagnosis and “post” refers to CNS disease following ophthalmic diagnosis
= time from ocular diagnosis to death or last follow up, M = male, F = female, CNS mets = central nervous system metastasis, tx = treatment, LN = lymph node, mos = months, CutMM = cutaneous malignant melanoma, BRAF = proto-oncogene B-Raf, NRAS = Neuroblastoma RAS viral oncogene homolog, TERT = Telomerase reverse transcriptase
Table 2:
Clinical characteristics of eyes, their ophthalmic disease and their outcome
| eye 1 | eye 2 | eyes 3 + 4 | eye 5 | eye 6 | eyes 7 + 8 | eye 9 | eye 10 | eye 11 | eyes 12 + 13 | eyes 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ophthalmic symptoms | blurry vision | blurry vision | blurry vision | floaters | blurry vision | floaters | floaters | blurry vision | asymptomatic | floaters | floaters |
| time to ocular symptoms (mos)^ | 16 | 13 | 4.5 | 5 | 5 | 86 | 22 | 19 | asymptomatic | 38 | 42 |
| time to ocular diagnosis (mos)* | 17 | 13 | 6 | 6 | 6 | 92 | 23 | 21 | 12 | 38 | 46 |
| laterality of disease | left eye | left eye | both eyes | right eye | left eye | both eyes | left eye | right eye | right eye | both eyes | right |
| initial vision | OS: 20/30 | OS: CF | OD: 20/400, OS: 20/100 | OD: 20/50 | OS: 20/63 | OD: 20/20, OS: 20/20 | OS: 20/30 | OD: 20/40 | OD: 20/20 | OD: 20/25, OS: 20/30 | OD: CF |
| clinical findings | amelanotic vitreous and AC cells | amelanotic vitreous cells, yellow retrolental debris | large yellow-white vitreous snowballs, fine amelanotic cells in AC, retinal infiltration | Amelanotic vitreous clumps | pigmented choroidal lesions, vitreous seeds, pigmented hypopon | Pigmented vitreous debris | amelanotic retrolental membrane, vitreous hemorrhage; amelanotic retinal infiltrate | vitreous hemorrhage, amelanotic retinal infiltration | pigmented vitreous debris, retinal infiltration OD | Vitreous pigment | Pigment in AC and vitreous |
| retinal detachment? | yes | yes | no | yes | yes | no | no | no | no | no | no |
| neovascularization? | iris (NVG), hyphema, vit heme prior to tx | iris (NVG) | no | no | no | no | no | iris (NVG) | no | no | no |
| elevated IOP? | yes (NVG) | yes (NVG) | no | no | yes | no | no | yes (NVG) | no | no | no |
| ophthalmic treatment | intravit bevazicumab, EBR(40Gy/ 20 fractions) | intravit. Melphalan (10μg), bevacizumab | EBROU (30Gy/10 fractions) | observation | EBR(37.5Gy/ 18 fractions), PPV, intravit melphalan (10μg × l, μg × 2), intracameral melphalan (5μg) | OD: PPVx2, Cryotherapy, endolaser, intravit melphalan 20mcg q1m x4 OS: PPV x2, endolaser | PPVxl, endolaser, EBR (40Gy/ 16 fractions), intravit melphalan 20mcg q1m x5 | EBR (40Gy/25 fractions), intravit bevacizumab x2 | observation | OS: PPV | enucleation |
| subsequent systemic treatment | dabrafenib, trametinib, encorafenib, binimetinib | nivolumab, urelumab, ipilimumab, PDL1-I, LAG3 inhibitor | nivolumab | ipilimumab, nivolumab | dabrafenib, trametinib, pembrolizumab | pembrolizumab | nivolumab | pembrolizumab | ipilimumab | ipilimumab, nivolumab | none |
| final vision^^ | NLP | LP | 20/30, 20/70 | HM | 20/70 | OD: LP; OS: 20/630 | 20/40 | 20/300 | OD HM | OD: 20/30; OS: 20/25 | enuc |
| Final ophthalmic disease status | Controlled | Responding | Controlled OU | Controlled | Controlled | Active disease OU | Controlled | Controlled | Controlled | OD: Untreated; OS: Controlled | enucfor BPE |
= time from initial diagnosis of metstatic cutaneous melanoma to first ocular symptoms
= time from initial diagnosis of metastatic cutaneous melanoma to ocular diagnosis,
+ final visual at last follow up, mos = months, CF = count fingers, OD = oculus dextrus, OS = oculus sinister, OU = oculus uterque, AC = anterior chamber, IOP = intraocular pressure, intravit = intravitreous, tx = treatment, NVG = neovascular glaucoma, PPV = pars plana vitrectomy; EL = endolaser; EBR = external beam radiotherapy; IVM = intravitreal injection of melphalan; CPC = cyclophotocoagulation; LP = light perception; enuc = enucleation; BPE = blind painful eye
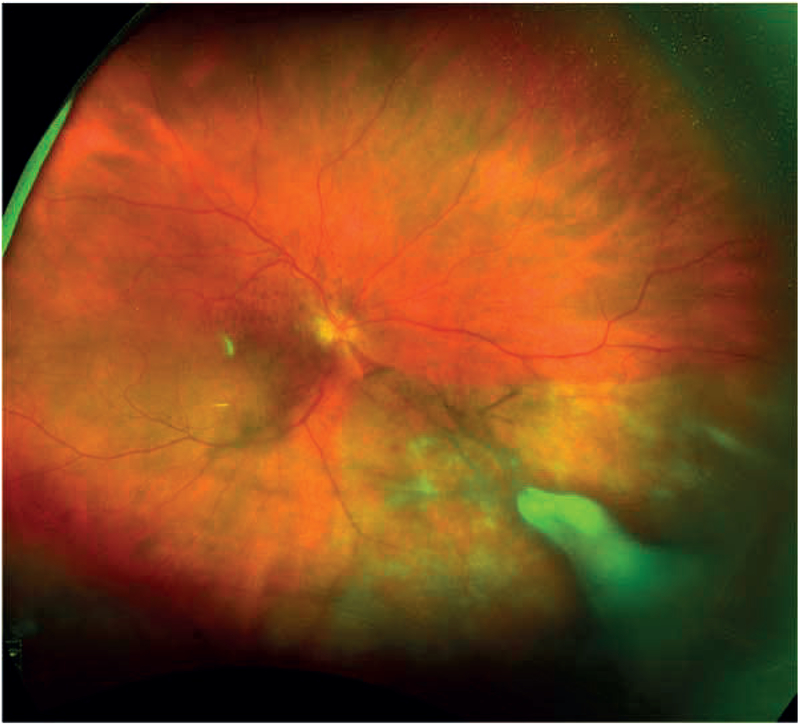
Figure 1:
Ultra wide-field fundus photograph from eye 10 demonstrating diffusely hypopigmented sectoral area inferonasally with a dense overlying white infiltrate; close examination of the macula revealed multiple clumps of preretinal pigment
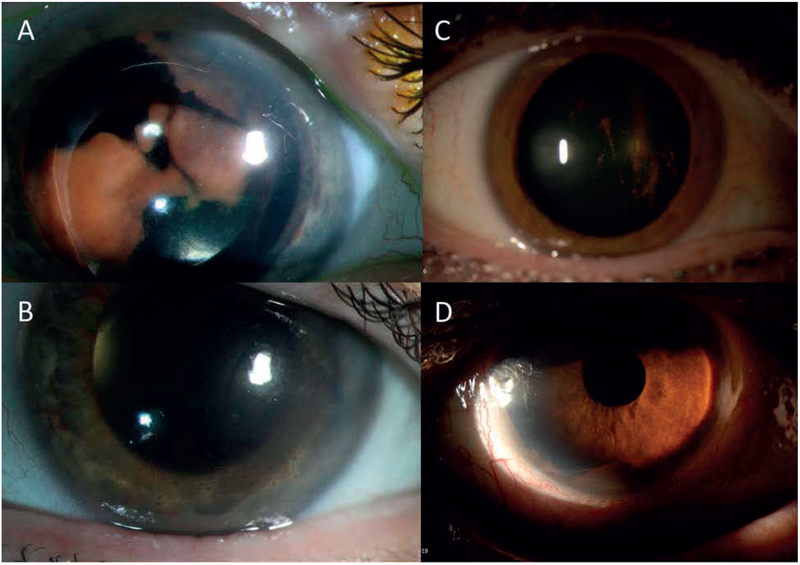
Figure 4:
Representative slit-lamp images of anterior chamber disease. A: Right eye (eye 2) demonstrating retrolental amelanotic/yellow sheet-like opacity. B: Response following 3 intravitreous melphalan injections and vitrectomy. C: Right eye (eye 7) on initial presentation demonstrating pigment deposition on the posterior surface of the crystalline lens and in the anterior vitreous D: Recurrence of disease featuring diffuse pigment clumping in the inferior angle of the anterior chamber.
Five eyes developed elevated intraocular pressure (four of five were neovascular glaucoma). Four eyes developed a retinal detachment. The final Snellen visual acuity ranged from 20/20 to NLP: eight eyes had a final vision worse than 20/200.
Ophthalmic treatment included: external beam radiation (30–40Gy) in six eyes intravitreous melphalan (10–20μg) (figure 4) in four eyes, enucleation in one eye, local observation while on systemic treatment in two eyes. The single enucleation was in a blind painful eye, which developed high intraocular pressure following the diagnostic vitrectomy. Three eyes received intravitreous bevacizumab for neovascularization, and the final vision in these eyes was less than 20/200.
Six patients had metastasis in their central nervous system (CNS), three of which had CNS disease diagnosed subsequent to the ocular diagnosis. Nine patients received checkpoint inhibition as their subsequent systemic treatment following the diagnosis of intraocular metastasis.
Discussion
This series of fourteen eyes with metastatic cutaneous melanoma to the vitreous is the largest series to our knowledge on this topic. The majority of these cases occurred in the context of systemic treatment with checkpoint blockade immunotherapy (8 of 11 cases occurred following or during CPI), and this may suggest three things: 1) the vitreous opacities of metastatic disease may masquerade as drug-associated inflammation; 2) the systemic treatment may explain the unmasking of vitreous disease (treatment-related immune activation allows previously subclinical disease to become manifest, or prolonged survival allows for more distant sites of disease); or 3) surveillance for potential drug-associated ophthalmic toxicities have prompted referrals to ophthalmic oncologists and increased diagnosis.
Checkpoint inhibitors are intended to potentiate the T-lymphocyte response against tumor cells but these T-lymphocytes can also target normal tissue. This latter phenomenon can result in a range of inflammatory adverse events. With regard to ophthalmic toxicities, it is reported that approximately 1% of patients on checkpoint inhibition have ophthalmic inflammation including keratitis, uveitis, vitritis, autoimmune choroidopathies, optic neuropathies and orbitopathies6. 7 eyes (50%) of eyes in our series presented with amelanotic vitreous opacities ranging from single cells (1 eye), cellular clumps (2 eyes), cellular sheets (2 eyes) and a retrolental membrane (2 eyes). Drug-associated inflammation was a clinical suspicion in these eyes, but all patients were found to have biopsy-confirmed metastatic melanoma to the vitreous. This series highlights that intraocular amelanotic cellular opacities (uveitis/vitritis), in the context of checkpoint inhibition, may not always represent drug-associated inflammation. Instead, metastasis is part of the differential diagnosis, warranting consideration of confirmatory diagnostics to facilitate appropriate management.
A vitreous biopsy is particularly useful in establishing a definitive diagnosis. For instance, pigmented vitreous cells would point towards metastatic melanoma. However, cases of amelanotic vitreous cells in this context can be difficult to distinguish based on clinical findings: both metastatic disease and drug-induced inflammation can be non-painful, with fine or sheet-like or conglomerations/gobules of white cells. On histopathology, the metastatic cutaneous melanoma can be found entering to the retina/vitreous enters via the retinal circulation (figure 3). The nuclei of the melanoma cells contain prominent nucleoli. Immunohistochemical stains for HMB45 (Human Melanoma Black), Melan A, or Sox 10 (SRY-related HMG-box 10) may be used for confirmation (figures 2 and 3). A red chromagen can be used because it can be difficult to see a brown chromagen due to the melanin pigment.
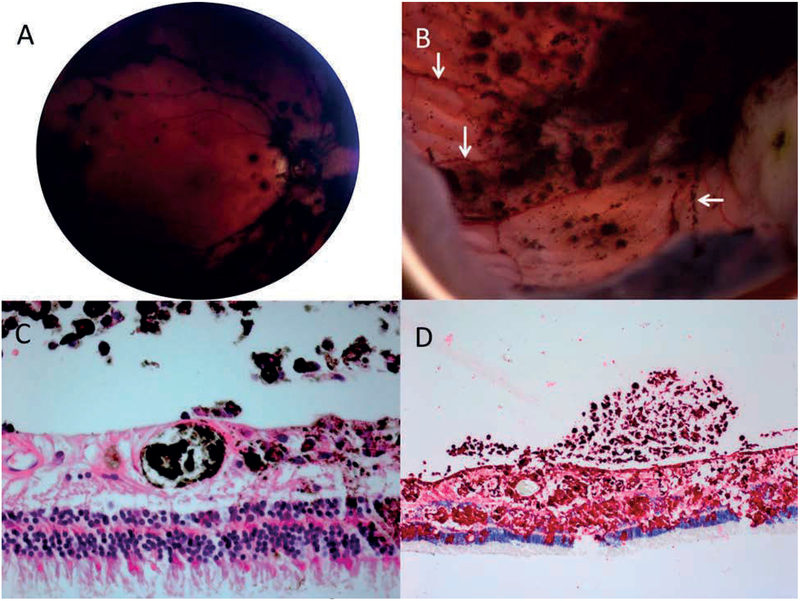
Figure 3:
Representative eye 14. A: The post-vitrectomy fundus image shows diffuse pigment on the surface of the retina, particularly in the distribution of the blood vessels. B: Gross pathologic examination of enucleated specimen revealed the retina to be carpeted with budding threads of fine, pigmented clumps, including in linear arrangements along the distribution of retinal blood vessels (white arrows). C: Melanoma cells and pigmented macrophages are present in a retinal vessels, the retina and vitreous (haematoxylin eosin stain, 100X) D: Immunohistochemical stains show melanoma cells in the retina and vitreous (peroxidase anti-peroxidase, HMB45 red chromagen, 100X)
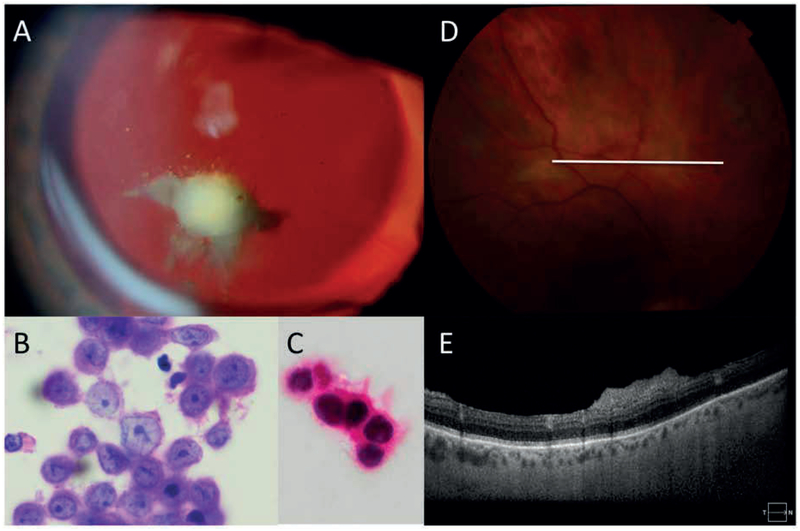
Figure 2:
Representative eyes 3 and 4: A: Slit lamp photography of the left eye demonstrating amelanotic vitreous opacities which were present in both eyes. B: Cytopathology of vitreous biopsy samples reveals atypical melanocytes harboring large irregularly bordered nuclei with prominent eosinophilic nucleoli. The attached pink cytoplasm also displays foci of melanin pigment deposition (haematoxylin eosin stain, 100X). C: Lesional cells reveal SOX-10 nuclear immunoreactivity (oil immersion, 100X) D: fundus photograph of right eye demonstrating amelanotic retinal infiltrates E: Optical coherence tomography of corresponding area demonstrating pre-retinal opacities.
The occurrence of fourteen eyes with metastatic cutaneous melanoma to the vitreous over an 8-year period is notable because of the paucity of reports of this in the pre-immune checkpoint era. This is by no means an accurate assessment of disease occurrence, but it is suggestive of an increased incidence of vitreous metastases in the recent years. It is intriguing to explore whether this is related to immunotherapy directly or simply due to longer survival leading to increased detection of previously rare sites of metastases. In terms of direct relationship to immunotherapy, we believe there are two conceivable explanations for this. First, it is possibly related to both the central nervous system and the eye being so-called immune privileged sites. In the context of CNS metastasis, it has been suggested that the initial response to checkpoint inhibition elicits an immune activation and modulation of the blood brain barrier, and by extension, the blood retinal barrier7. In turn, this “unmasks” previously clinically undetected and asymptomatic disease, such as in the vitreous. A correlate to this is the well-recognized “pseudoprogression” in response to CPI8: specifically, the apparent initial enlargement or “flare” of metastatic sites in response to the immune activation, followed by the regression of disease.
One group has suggested that the immune privileged status of the eye protects the vitreous from the host immune system, but also from the active effects of immunotherapy9. This theory has been proposed to explained a single case, in which immunotherapy failed to control metastatic cutaneous melanoma to the eye in the context of a good systemic response9. However, the published data clearly shows that immunotherapy is active in the CNS, despite its immune privilege. Patients with metastatic brain melanoma treated with checkpoint blockade immunotherapy have 4-year survival estimates of 51.5% compared to the comparative historical rate of 16.9%10. In fact, in some subgroups of patients, the beneficial effects of immunotherapy on CNS metastasis are striking with similar intracranial and extracranial response rates to nivolumab + ipilimumab11,12. This, of course, begs the question as to whether the eye responds similarly13, and therefore whether local treatments (radiation, intravitreous melphalan etc) are necessary for these eyes. On the other hand, perhaps radiation to the eye potentiates the effects of the checkpoint inhibition, as has been anecdotally demonstrated in other metastatic melanoma sites14, and allows for enhanced local tumor control or altered blood retinal barrier.
Secondly, the increase in intravitreous metastatic melanoma may be related to the effects of immunotherapy on overall survival, which has increased significantly15. For instance, in patients treated with combination checkpoint inhibition, the four-year survival rate is 53%15; which is dramatically different from the 4-year survival rate in the pre-immunotherapy era. Perhaps longer survival gives more time for the disease to progress to the central nervous system and to less common sites such as the vitreous.
Historically, eyes with intravitreous metastasis from cutaneous melanoma were treated with enucleation or external beam radiotherapy, followed by a high rate of secondary enucleation for neovascular glaucoma. This approach was likely driven by historically poor prognosis for patients with ocular metastasis. However, now that CPIs are allowing patients to evade life-threatening metastasis, questions remain on how to best manage vision-threatening eye disease. In this series, five eyes received external beam radiation. Interestingly, only one eye developed secondary neovascular glaucoma following radiation. Eyes with neovascularization occurred either prior to radiation or in the absence of radiation, and are suggestive of disease rather than a toxic effect of treatment. 90% (9 of 10) of patients continued on systemic treatment, predominantly consisting molecularly targeted therapy or checkpoint inhibition (as shown in Table 2).
A novel treatment used in this series was intravitreous melphalan. This drug and delivery have been used extensively in the treatment of retinoblastoma (typically a dose of 20–30μg)16 and sparingly in vitreoretinal lymphoma (10μg/mL)17. One report describes its use in a single case (12 injections of 10μg/0.05mL) for the treatment of vitreous seeding from uveal melanoma with a satisfactory outcome18. The premise behind this technique is to deliver a high dose of local drug, while sparing the body the toxicity of systemic delivery. It borrows from isolated limb perfusion of melphalan, which was first described as a single case report by Creech et al. in 1957, and is now a well-established technique for inoperable recurrent cutaneous melanoma affecting an extremity19. In our series, intravitreous melphalan 10μg/0.05mL yielded mixed results: two of three eyes responded dramatically to intravitreous melphalan as a single local treatment. However, a third eye demonstrated progression of disease following four 20μg injections given monthly. The treatment dose for retinoblastoma in pediatric eyes is 20–30μg, suggesting possible room for increasing the dose for intravitreous cutaneous melanoma; and thereby perhaps yielding more favorable and sustained responses.
How the metastatic cutaneous melanoma entered into the vitreous remains in question. Access to the choroid is presumably obtained through hematogenous spread. However, in the absence of choroidal involvement, as was true in these eyes (figure 2), the point of access is less clear. Many of these patients had concomitant brain metastasis, suggesting a possible association and transfer of metastatic disease from the cerebral spinal fluid into the vitreous by way of the optic nerve. However, the optic nerve clinically appeared normal in all of these patients. Permeation of tumor from retinal blood vessels is another possible source of intraocular access and our histopatholgical observations of an enucleated specimen are suggestive of this. We observed melanoma in retinal vessels and migrating into the vitreous, with the appearance of “co-opting” the retinal blood vessels for nutrition/oxygen (figure 3). Entrance through the vasculature may explain compromised or leaky blood vessels and possibly explain the association with neovascular glaucoma. Finally, the retinal infiltrates observed in some eyes appeared to be retinal implantations from vitreous disease, suggesting the route to the retina was accessed via the vitreous (figure 2).
Checkpoint blockade immunotherapy has had a major impact on the treatment of a subset of cancers and has led to a highly favorable effect on survival in patients with metastatic cutaneous melanoma. It is important for ophthalmologists to be aware that prolonged survival can allow more time for distant metastasis, and this may be associated with an increased likelihood of intravitreous metastatic melanoma. In many cases, metastatic disease will present with amelanotic vitreous debris, which can be confused with immunotherapy-related intraocular inflammation. Therefore, vitritis in the context of metastatic cutaneous melanoma treated with immunotherapy is best viewed with a high suspicion for possible metastasis, and referred to a specialist for appropriate diagnostics and treatment including evaluation of the central nervous system.
Acknowledgments
The Fund for Ophthalmic Knowledge and the Cancer Center Support Grant (P30 CA008748), Cycle for Survival, Ludwig Collaborative and Swim Across America Laboratory, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA. Parker Institute for Cancer Immunotherapy, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crowley NJ, Seigler HF. Late recurrence of malignant melanoma. Analysis of 168 patients. Ann Surg 1990;212:173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shields CL, Shields JA, Gross NE, et al. Survey of 520 eyes with uveal metastases. Ophthalmology 1997;104:1265–1276. [DOI] [PubMed] [Google Scholar]
- 3.Zografos L, Mirimanoff R-O, Angeletti CA, et al. Systemic melanoma metastatic to the retina and vitreous. Ophthalmologica 2004;218:424–433. [DOI] [PubMed] [Google Scholar]
- 4.Gündüz K, Shields JA, Shields CL, Eagle RC. Cutaneous melanoma metastatic to the vitreous cavity. Ophthalmology 1998;105:600–605. [DOI] [PubMed] [Google Scholar]
- 5.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoun J, Titah C, Cochereau I. Ocular and orbital side-effects of checkpoint inhibitors: a review article. Curr Opin Oncol 2016;28:288–294. [DOI] [PubMed] [Google Scholar]
- 7.McDonald MA, Sanghvi P, Bykowski J, Daniels GA. Unmasking of intracranial metastatic melanoma during ipilimumab/nivolumab therapy: case report and literature review. BMC Cancer 2018;18:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borcoman E, Nandikolla A, Long G, et al. Patterns of Response and Progression to Immunotherapy. Am Soc Clin Oncol Educ Book 2018;38:169–178. [DOI] [PubMed] [Google Scholar]
- 9.Sia DIT, Thaung C, O’Hanlon-Brown C, et al. Immune privilege: failure of immunotherapy in controlling metastatic cutaneous melanoma to the eye. Melanoma Res 2018;28:359–362. [DOI] [PubMed] [Google Scholar]
- 10.Iorgulescu JB, Harary M, Zogg CK, et al. Improved Risk-Adjusted Survival for Melanoma Brain Metastases in the Era of Checkpoint Blockade Immunotherapies: Results from a National Cohort. Cancer Immunol Res 2018;6:1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tawbi HA, Forsyth PA, Algazi A, et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med 2018;379:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 2018;19:672–681. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Diaz AB, García-Medina A, Ferrer-Guillen B, Berrocal A. Eye immune privilege? Nivolumab plus ipilimumab: successful treatment in a patient with cutaneous melanoma and ocular metastases. Melanoma Res 2019;29:345–347. [DOI] [PubMed] [Google Scholar]
- 14.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:1480–1492. [DOI] [PubMed] [Google Scholar]
- 16.Francis JH, Brodie SE, Marr B, et al. Efficacy and Toxicity of Intravitreous Chemotherapy for Retinoblastoma: Four-Year Experience. Ophthalmology 2017;124:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shields CL, Sioufi K, Mashayekhi A, Shields JA. Intravitreal Melphalan for Treatment of Primary Vitreoretinal Lymphoma: A New Indication for an Old Drug. JAMA Ophthalmol 2017;135:815–818. [DOI] [PubMed] [Google Scholar]
- 18.Masoomian B, Mashayekhi A, Malik K, Shields CL. INTRAVITREAL MELPHALAN FOR TREATMENT OF VITREOUS SEEDING FROM CHOROIDAL MELANOMA. Retin Cases Brief Rep 2018:1. [DOI] [PubMed] [Google Scholar]
- 19.Nieweg OE, Kroon BBR. Isolated limb perfusion with melphalan for melanoma Thompson JF, ed. J Surg Oncol 2014;109:332–337. [DOI] [PubMed] [Google Scholar]