Abstract
Background
Facioscapulohumeral muscular dystrophy is a progressive muscle disease which has no agreed treatment. Early suggestions that corticosteroids might be helpful were not supported by a subsequent open label study. The beta 2 adrenergic agonist albuterol, also known as salbutamol, is known to have anabolic effects which might be beneficial for facioscapulohumeral muscular dystrophy. Creatine has been used as a muscle performance enhancer by athletes and it might be helpful in muscular dystrophies including facioscapulohumeral muscular dystrophy.
Objectives
The objective of the review was to determine whether there is any drug treatment which alters the progression of facioscapulohumeral muscular dystrophy.
Search methods
We searched the Cochrane Neuromuscular Disease Group specialised register (searched August 2003), MEDLINE (January 1966 to August 2003) and EMBASE (January 1980 to August 2003) for any references to facioscapulohumeral muscular dystrophy. Abstracts from the major neurological meetings and trial bibliographies were also searched for further references to trials. Experts were contacted for information regarding unpublished trials or trials in progress.
Selection criteria
We included all randomised or quasi‐randomised trials of any drug treatment for facioscapulohumeral muscular dystrophy, in adults with a recognised diagnosis of facioscapulohumeral muscular dystrophy. Trials had to include an assessment of muscle strength at one year.
Data collection and analysis
All identified trials were independently assessed by both reviewers to ensure that they fulfilled the selection criteria and were then rated for their quality. Trial data were extracted and entered by one reviewer and checked by the other. If appropriate data existed a weighted treatment effect was to be calculated across trials using the Cochrane statistical package, Review Manager. The results were to have been expressed as relative risks and 95% confidence intervals and risk differences and 95% confidence intervals for dichotomous outcomes, and weighted mean differences and 95% confidence intervals for continuous outcomes.
Main results
Two published high quality randomised controlled trials fulfilled the selection criteria. One compared creatine supplementation with placebo and the other compared high and low‐dose albuterol with placebo. A further unpublished randomised controlled trial of albuterol in facioscapulohumeral muscular dystrophy was identified. The creatine trial showed a non‐significant difference in favour of creatine. The albuterol trial showed no significant difference in muscle strength at one year but some secondary measures such as lean body mass and handgrip strength did improve.
Authors' conclusions
There is no evidence from randomised controlled trials to support any drug treatment for facioscapulohumeral muscular dystrophy but only two randomised controlled trials have been published.
Plain language summary
The evidence from published randomised controlled trials is inadequate to establish the effectiveness of any drug for treating facioscapulohumeral muscular dystrophy. More research is needed
Facioscapulohumeral muscular dystrophy is a progressive muscle disease. Muscle weakness is often relatively mild and progression slow but around one fifth of affected people eventually become wheelchair‐bound. The muscles of the face, shoulder blades and upper arms are most severely effected, but weakness occurs in other muscles. There is no agreed treatment. Only two randomised controlled trials have been published. One small trial of albuterol (also known as salbutamol)and another small trial of creatine (a dietary supplement for building muscle) were inadequate to confirm or refute a significant effect. Further trials of albuterol, creatine and other agents are needed.
Background
Facioscapulohumeral muscular dystrophy (FSHD) is an autosomal dominant disorder with a characteristic, initially regional, pattern of involvement. It is the third most common form of muscular dystrophy with an estimated prevalence of 1:20,000 (Padberg 1982). Although muscle weakness in FSHD is often relatively mild and progression slow, about 20% of affected individuals eventually become wheelchair‐bound (Padberg 1982). The pathophysiology of FSHD remains unknown and despite genetic localization of FSHD to chromosome 4q35 and clear identification of the molecular lesion, neither the causative gene(s) nor its protein product is known (Tawil 1998).
There is no known effective treatment for FSHD. Early case reports suggested a role for corticosteroids in FSHD. The use of corticosteroids in FSHD was prompted by the presence of inflammatory infiltrates in a significant proportion of FSHD muscle biopsies (Arahata 1995). Munsat et al. reported improvement in strength and a reduction in serum creatine kinase levels in three of four patients with FSHD (Munsat 1972). The report, however, provides details of dosage and duration of treatment in only two patients and the reported changes in muscle strength were not quantified (Munsat 1972). Moreover, in a follow‐up report, all the treated patients eventually continued to progress (Munsat 1977). Following this report it became common practice to treat FSHD patients having inflammatory infiltrates on their muscle biopsy, at least transiently, with corticosteroids. Interest in corticosteroid treatment for dystrophies including FSHD was heightened with the demonstration of its efficacy in Duchenne dystrophy. In 1997, the results of a prospective, 12 week, open label uncontrolled trial of prednisone in FSHD were published (Tawil 1997). Eight patients with FSHD were enrolled and received 1.5 mg/kg/day of prednisone (maximum 80 mg/day). The primary outcome of efficacy was change in mean manual muscle testing scores. Secondary outcomes included change in mean standardized isometric myometry scores, change in lean body mass as assessed by dual energy x‐ray absorptiometry (DEXA) and change in muscle mass as estimated by urinary creatinine excretion. There was no significant improvement in strength measures or surrogate measures of muscle function after 12 weeks of treatment. Based on this study, there appears to be no role for the use of short‐term corticosteroids to improve strength in FSHD. The study did not have sufficient power to address the question of whether long‐term corticosteroids can slow disease progression.
Promising results of a pilot trial of albuterol in FSHD suggested that this might be a useful treatment (Kissel 1998). Albuterol (known as salbutamol in the UK) is a beta‐2 receptor agonist. This class of drugs has muscle anabolic effects in several animal models of muscle wasting as well as in normal humans in the setting of disuse atrophy (Kissel 1998).
Creatine might be another potentially useful treatment for muscular dystrophies including FSHD. It is a guanidino compound that is found in meat containing products, and is produced in the body, mainly by the liver but also by the kidneys (Guthmiller 1994). Creatine plays an important role in muscle energy metabolism (Wallimann 1992) and may play a role in the regulation of protein metabolism (Parise 2001). Patients with neuromuscular disorders (Park 1994; Park 2000; Tarnopolsky 1999) have lower skeletal muscle creatine levels thus giving the potential for creatine monohydrate supplementation to enhance muscle performance. Indeed healthy adults taking creatine supplements have shown improved muscle force (Greenhaff 1993; Kamber 1999; Maganaris 1998; Tarnopolsky 2000) and increased lean body mass (most of which is muscle) (Harris 1992; Hultman 1996; Mihic 2000).
Objectives
We reviewed the evidence from randomised trials about the efficacy of any drug treatment in facioscapulohumeral dystrophy (FSHD).
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised or quasi‐randomised trials of any drug treatment for FSHD.
Types of participants
We included all subjects (whether male or female and of any age) with a proven diagnosis of FSHD. The diagnosis was based upon a typical phenotype or else on an atypical phenotype where there was genetic confirmation of FSHD, either in the individual, or in their family.
Types of interventions
We included drug treatment with corticosteroids, beta‐2 adrenergic agonists or other drugs.
Types of outcome measures
Primary outcomes
The primary outcome measure was:
mean manual muscle strength testing based upon the Medical Research Council strength score at one year.
Secondary outcomes
Secondary outcome measures were:
mean manual muscle strength testing based upon the Medical Research Council strength score at six months;
mean standardized isometric myometry scores at one year;
change in muscle mass assessed by methods such as urinary creatine excretion, muscle imaging or dual energy x‐ray absorptiometry (DEXA) at one year;
functional assessments of muscle performance at one year;
adverse events during the intervention.
Search methods for identification of studies
See: Cochrane Neuromuscular Disease Group Search Strategy. We searched the Cochrane Neuromuscular Disease Group specialised trials register (searched August 2003) using facioscapulohumeral dystrophy as the search term. A similar search strategy was applied to MEDLINE (from January 1966 to August 2003) and EMBASE (from January 1980 to August 2003). Conference abstracts including those of the American Academy of Neurology, the International Conference on Neuromuscular Diseases, the World Muscle Society and the European Neurological Society were scanned for relevant studies. We checked the bibliographies in reports of the randomised trials and contacted their authors and other experts in the field to identify additional published or unpublished data.
Data collection and analysis
Selection of studies
All trials identified by the search strategy were read by one reviewer (MR) to confirm that they were randomised or quasi‐randomised trials and that the diagnostic criteria for FSHD were met.
Data extraction and management
Data extraction was performed by one reviewer and checked by the other and the authors of trials were contacted to provide missing data where possible. Data were checked and entered into the computer by one reviewer (MR) and checked by the other.
Assessment of risk of bias in included studies
Randomised trials were assessed for methodological quality by both reviewers using the following criteria which were scored using the Cochrane approach as follows: Grade A ‐ adequate, Grade B ‐ uncertain, and Grade C ‐ clearly inadequate.
explicit diagnostic criteria;
specific detail concerning randomisation;
adequacy of allocation concealment;
quality of outcome measures;
blind outcome assessment ;
blind administration of treatment.
In cases of uncertainty authors were contacted for clarification. The agreement on methodology assessment was by consensus. We adopted this method for assessing trial quality in preference to the Jadad scale proposed in our protocol because this had become the preferred method of the Cochrane Neuromuscular Disease Group in the interim.
Data synthesis
If more than one trial with the same intervention had been available we would have calculated a weighted treatment effect (using random effects) using the Cochrane statistical package, Review Manager (RevMan). The results would have been expressed as relative risks (RR) and 95% confidence intervals (CI) and risk differences (RD) with 95% CI for dichotomous outcomes and weighted mean differences (WMD) and 95% CI for continuous outcomes. It was intended that sensitivity analysis would be performed on the basis of methodological quality and to test for heterogeneity in the results.
Results
Description of studies
Only two published randomised trials were identified, one of albuterol and one of creatine (Kissel 2001; Walter 2000). We are aware of an additional randomised trial of albuterol in FSHD conducted in Holland by Padberg et al. (personal communication) (Padberg 2004). There are no randomised trials of corticosteroids for FSHD.
Albuterol
This trial (Kissel 2001) randomised 90 subjects with FSHD to either placebo, or to a low (8 mg twice daily) or high dose (16 mg twice daily) of the beta 2 adrenergic agonist, albuterol (also known as salbutamol). Treatment was titrated to full dose over two weeks and then continued for one year. Assessments were at baseline, 13, 26 and 52 weeks. Of the 90 subjects randomised (49 men, 41 women, ages 18 to 57), 84 successfully completed the trial (Kissel 2001). The six drop‐outs included one for side effects, one for unrelated chest pain, three for personal reasons and one mistakenly randomised as they were too weak. The last available data from these drop‐outs was used for statistical analysis for all subsequent time points.
Outcome measures used were:
quantitative myometry (maximal voluntary isometric contraction or MVIC) testing performed on six muscle groups bilaterally (a total of 12 measures) ; elbow extensors, elbow flexors, shoulder abductors, shoulder external rotators, knee extensors,and knee flexors. Subjects had to have at least eight testable muscles;
manual muscle testing (MMT) on 38 muscle groups; the neck flexors, neck extensors and then bilaterally for shoulder abductors, shoulder horizontal abductors, shoulder horizontal adductors, shoulder internal rotators, shoulder external rotators, elbow flexors, elbow extensors, wrist extensors, wrist flexors, hip flexors, hip abductors, hip extensors, knee extensors, knee flexors, ankle dorsiflexors, ankle evertors, ankle invertors, and ankle plantar flexors. Muscle groups were graded on a 13 point expanded version of the six point MRC scale;
functional testing; grip strength by myometer, time to walk 30 feet, time to climb four stairs and a upper and lower limb functional scale (Personius 1994);
Lean body mass by DEXA.
The primary outcome measure was change in global MVIC at 52 weeks. Change in global MVIC at other time points (13 and 26 weeks) and change in all the above outcome measures and in biochemical tests at all time points (13, 26 and 52 weeks) were secondary outcomes.
Creatine
One trial (Walter 2000) randomised 32 patients with muscular dystrophy (of whom 12 had FSHD) to creatine (as Creapure 10 g/day for adults and 5 g/day for children) or placebo for eight weeks in a double blind randomised crossover study.
Outcome measures used were:
manual muscle testing (MMT) on 30 muscle groups; neck flexors, neck extensors and then bilaterally for deltoid, biceps, brachioradialis, triceps, wrist extension and flexion, finger extension and flexion, quadrciceps, iliopsoas, hip adduction and abduction, knee extension and flexion and ankle plantar flexion and dorsiflexion. Muscle groups were graded on the standard six point MRC scale;
neuromuscular symptoms score which assessed 14 daily life activities with a maximum score of 42 (Soueidan 1993);
forced vital capacity and vital capacity as a percentage of the predicted normal;
patient rating of treatment response.
The primary outcome measure was not specifically stated.
Risk of bias in included studies
The quality of the included trials was good with details as shown in Table 3. The trial by Walter 2000 did not have specific detail on the method of randomisation nor information that would establish that there was concealment of allocation.
1. Methodological quality of included trials.
| Criteria | Kissel 2001 | Walter 2000 |
| Diagnostic criteria | A | A |
| Detail of randomisation | A | B |
| Allocation concealment | A | B |
| Explicit outcome measures | A | A |
| Blinded outcome assessment | A | A |
| Blinded administrration of treatment | A | A |
Effects of interventions
As there were only two RCTs available which compared different interventions, no meta‐analysis or sensitivity analysis was appropriate. However when full data from an unpublished trial using albuterol become available (Padberg 2004) such analysis may be possible for the albuterol intervention.
The creatine trial published data (Walter 2000) pooled the results of 32 patients with a variety of muscular dystrophies. The authors kindly allowed us access to their original data thus enabling us to analyse the data relevant to the 12 participants with FSHD. Although this trial was only of eight weeks duration and therefore did not include any of the outcome criteria for this review we nevertheless present the results. As it was a crossover study we have analysed the results as if for a parallel study, thus counting each participant twice (once on creatine and again on placebo). The trial was negative for FSHD participants in that the difference in the MRC scores during creatine (a mean decline of ‐0.31%) and during placebo (mean decline of ‐2.64%) was not significant. Likewise the changes in the neuromuscular symptoms score during creatine treatment (mean 9.01%) compared with placebo (mean ‐0.49%) was not significant.
(1) Mean manual muscle strength testing based upon the Medical Research Council strength score at one year
Albuterol
MMT at one year did not significantly change with either 16 mg or 32 mg of daily albuterol (separately or combined) as compared with the placebo (Kissel 2001) (see Comparison 1, Outcome 1).
Creatine
The only creatine trial (Walter 2000) was of just eight weeks duration.
(2) Mean manual muscle strength testing based upon the Medical Research Council strength score at six months
Albuterol
MMT at six months did not significantly change with either 16 mg or 32 mg of daily albuterol (separately or combined) as compared with the placebo (Kissel 2001).
Creatine
The only creatine trial (Walter 2000) was of just eight weeks duration.
(3) Mean standardized isometric myometry scores at one year
Albuterol
Neither 16 mg nor 32 mg of daily albuterol significantly improved MVIC scores at one year as compared with placebo. (Kissel 2001) (see Comparison 1, Outcome 02).
Creatine
The only creatine trial (Walter 2000) was just eight weeks duration.
(4) Change in muscle mass assessed by methods such as urinary creatine excretion, muscle imaging or dual energy x‐ray absorptiometry (DEXA) at one year
Albuterol
There was a significant change lean body mass (a surrogate measure for muscle mass) for albuterol 32 mg daily with a non‐significant gain for 16 mg daily albuterol (see Comparison 1, Outcome 03) (Kissel 2001).
Creatine
There were no trials that included this outcome measure.
(5) Functional assessments of muscle performance at one year
Albuterol
There was a significant gain in grip strength for both 16 mg and 32 mg daily doses of albuterol (see Comparison 1, Outcome 04) (Kissel 2001). Other functional measures such as 30 feet walk time, time to climb four steps and an FSH specific grading scale (Personius 1994) did not improve with albuterol (Kissel 2001).
Creatine
The only creatine trial was of just eight weeks duration (Walter 2000).
(6) Adverse events during the intervention
Albuterol
This was well tolerated with no serious adverse effects reported. Subjects on high dose albuterol had a significantly higher heart rate than seen in the placebo group but without any clinical effect. Most subjects had only mild side effects that were just as frequently seen in the placebo as in the treated groups see Table 4. Moderate to severe side effects tended to occur early and to diminish with time. Incremental dosing was delayed in 4/90 subjects (two on placebo), temporarily suspended in 7/90 (three on placebo) or curtailed, but continued at lower dose in 7/90 (two on placebo). One subject in the high dose albuterol group dropped out after three months due to side effects which included tremor, headache and shakiness (Kissel 2001).
2. Adverse events of albuterol.
| Symptom | Placebo (n = 30) | Low dose (n = 30) | High dose (n = 30) |
| Tremors | 3 | 12 (p = 0.015 for low dose group alone compared to placebo) | 18 (p < 0.0001 for high dose group alone compared to placebo). |
| Cramps | 8 | 12 | 10 |
| Insomnia | 3 | 4 | 5 |
| Nervousness | 5 | 3 | 6 |
| Headache | 7 | 6 | 10 |
| Palpitations | 0 | 1 | 4 |
| Nausea/vomiting | 2 | 2 | 3 |
| Rash/pruritis | 1 | 2 | 4 |
Creatine
All subjects completed eight weeks of the creatine and placebo treatment without any particular side effects (Walter 2000).
Discussion
Albuterol
The authors of the albuterol trial (Kissel 2001) point out that although there was no increase in strength nor improvement in function as a result of taking albuterol there were significant increases in lean body mass and handgrip strength suggesting that albuterol was having some anabolic effect. The sustained improvement in handgrip strength but not in the other muscles tested might suggest that benefit was more likely to be seen in distal rather than proximal muscles, either because proximal, and especially axial muscles, may have been too weak to benefit, or because albuterol preferentially affects distal versus proximal muscles, if there was differential expression of beta‐2 adrenergic receptors. The increased lean body mass seen with albuterol may not have translated to increased muscle strength because the average increase of 1.5 kg may not have been sufficient to produce a detectable change in strength, particularly as some of the increase may have been in the non‐muscle tissues that contribute to as much as 30% of lean body mass. The authors suggested that a greater benefit from albuterol might be achieved either by combining albuterol with other anabolic agents or instituting a regime of exercise together with the albuterol. A further suggestion for increasing the possible benefit from albuterol was to "pulse" the treatment because intermittent treatment might prevent the de‐sensitisation or down‐regulation of beta 2 adrenergic receptors seen in animal studies (Rothwell 1987). Such receptor de‐sensitisation may explain why in this trial there was a (non‐significant) trend to improvement in MVIC and MMT at three months which then declined.
We will combine the data from the unpublished trial when they become available.
Side effects of the beta 2 agonists such as albuterol include fine tremor (particularly in the hands), nervous tension, headache, peripheral dilatation and palpitations (BNF 2003). Other side effect include tachycardia and arrhythmias and disturbances of sleep and behaviour in children. Muscle cramps and hypersensitivity reactions including paradoxical bronchospasm, urticaria, and angioedema have also been reported. Beta 2 agonists are associated with hypokalaemia after high doses.
Creatine
The creatine trial (Walter 2000) did show a small but significant improvement of 3% in muscle strength (MRC) and 10% in daily life activities when all 32 subjects with various muscular dystrophies were analysed together. The sub‐group with FSHD would have been too small a sample to show anything other than a large treatment effect. The authors called for further studies of creatine for muscular dystrophies generally. A larger or longer trial of creatine might show more conclusively whether or not creatine is a worthwhile treatment for FSHD.
Creatine may cause gastrointestinal side effects such as abdominal cramps, nausea and vomiting. In rare cases it may cause renal failure, mostly in those with impaired renal function. Muscle cramps have been reported but this may relate to its use when taken by dehydrated athletes (Natural Med 2003).
Future trial methodology
The two RCTs that have been published were high quality trials with methodology (in terms of inclusion/exclusion criteria and outcome measures) that would be a good model for the design of future trials. Additional outcome measures that should be considered in future trials would include quality of life.
Therapies for future trials
There are a number of treatment options that could be studied in future trials including, but not limited to, pulsed albuterol, other anabolic agents, combinations of anabolic agents and anabolic agents in combination with exercise. The effects of exercise and muscle strength training in muscle disease including FSHD are the subject of an ongoing Cochrane review (van der Kooi 2003). The negative results of the open label uncontrolled trial of corticosteroids for FSHD (Tawil 1997), together with the appreciation that the inflammatory pathology seen in some muscle biopsies of FSHD patients is unlikely to be a primary pathology, do not encourage an RCT of corticosteroids for FSHD.
Authors' conclusions
Implications for practice.
One small trial of albuterol and another small trial of creatine were inadequate to confirm or refute a significant effect of FSHD. Other agents have not been subjected to randomised trials.
Implications for research.
There is a need for further trials of albuterol, creatine and other agents in FSHD.
What's new
| Date | Event | Description |
|---|---|---|
| 31 May 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 10 January 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Dr Maggie Walter for allowing access to original trial data.
Data and analyses
Comparison 1. Albuterol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global manual muscle strength at 52 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 8mg BD | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.06, 0.08] |
| 1.2 16mg BD | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.04, 0.12] |
| 2 Average Maximal Voluntary Isometric Contraction (MVIC) strength at 52 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 8 mg BD | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.68, 0.20] |
| 2.2 16 mg BD | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.60, 0.36] |
| 3 Lean body mass by DEXA at 52 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 8 mg BD | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [‐0.22, 1.70] |
| 3.2 16 mg BD | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.32 [0.31, 2.33] |
| 4 Grip strength at 52 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 8 mg BD | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.43 [0.53, 4.33] |
| 4.2 16 mg BD | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.23 [0.14, 4.32] |
1.1. Analysis.
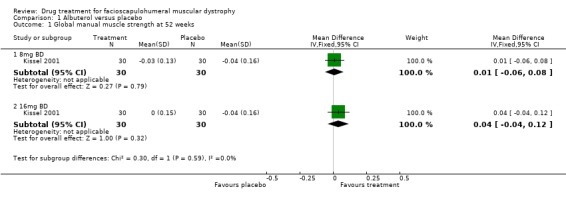
Comparison 1 Albuterol versus placebo, Outcome 1 Global manual muscle strength at 52 weeks.
1.2. Analysis.
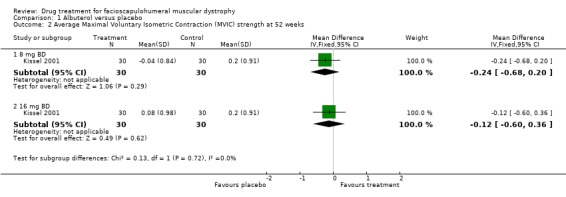
Comparison 1 Albuterol versus placebo, Outcome 2 Average Maximal Voluntary Isometric Contraction (MVIC) strength at 52 weeks.
1.3. Analysis.
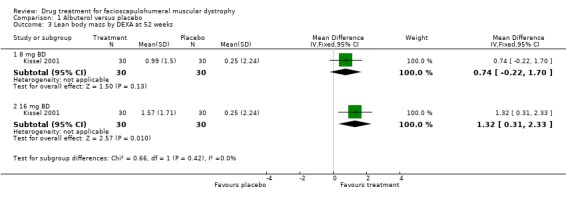
Comparison 1 Albuterol versus placebo, Outcome 3 Lean body mass by DEXA at 52 weeks.
1.4. Analysis.
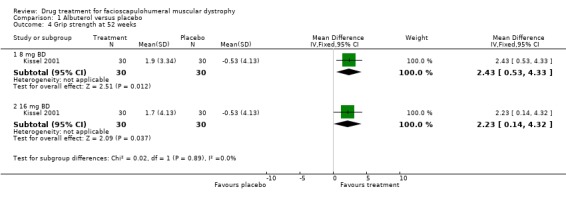
Comparison 1 Albuterol versus placebo, Outcome 4 Grip strength at 52 weeks.
Comparison 2. Creatine v Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Average manual muscle strength at 8 weeks | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 2.92 [‐1.46, 7.30] |
| 2 Neuromuscular symptoms score at 8 weeks | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.75 [‐1.58, 5.08] |
2.1. Analysis.
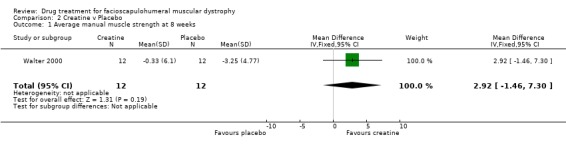
Comparison 2 Creatine v Placebo, Outcome 1 Average manual muscle strength at 8 weeks.
2.2. Analysis.
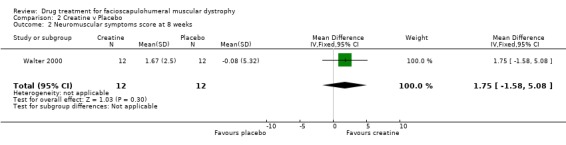
Comparison 2 Creatine v Placebo, Outcome 2 Neuromuscular symptoms score at 8 weeks.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kissel 2001.
| Methods | Double blind randomised controlled trial | |
| Participants | 90 subjects with FSHD | |
| Interventions | Placebo Albuterol 8 mg BD Albuterol 16 mg BD | |
| Outcomes | Maximal Voluntary Isometric Contraction. Manual Muscle Testing. Lean body mass by Dual Energy X‐ray. Absorptometry. Grip strength. Functional tests. | |
| Notes | Primary outcome MMT change at 6 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Walter 2000.
| Methods | Double blind randomised controlled trial | |
| Participants | 32 subjects of whom 12 had FSHD | |
| Interventions | Placebo Creatine (as Creapure 10 g/day for adults and 5 g/day for children) | |
| Outcomes | Manual Muscle Testing. Neuromuscular Symptoms Score. Forced Vital Capacity and Vital Capacity. Patient rating of treatment effect. | |
| Notes | No primary outcome measure stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Contributions of authors
MR agreed the protocol, extracted the data, wrote the final text of the review and revised the review following the peer review process. RT wrote the protocol, checked the data extraction in the full review and agreed the final text of the review.
Declarations of interest
RT was an investigator in the Kissel 2001trial. Both RT and MR are investigators in a forthcoming RCT of pulsed albuterol and oxandrolone for FSH.
Edited (no change to conclusions)
References
References to studies included in this review
Kissel 2001 {published data only}
- Kissel JT, McDermott MP, Mendell JR, King WM, Pandya S, Griggs RC, et al. Randomized, double‐blind, placebo‐controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology 2001;57:1434‐40. [DOI] [PubMed] [Google Scholar]
Walter 2000 {published and unpublished data}
- Walter MC, Lochmuller H, Reilich P, Klopstock T, Huber R, Hartard M, et al. Creatine monohydrate in muscular dystrophies: a double‐blind, placebo‐controlled clinical study. Neurology 2000;54(9):1848‐50. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Padberg 2004 {published data only (unpublished sought but not used)}
- Padberg G. Personal communication 2004.
- Kooi EL, Vogels OJ, Asseldonk RJ, Lindeman E, Hendriks JC, Wohlgemuth M, et al. Strength training and albuterol in facioscapulohumeral muscular dystrophy. Neurology 2004;63(4):702‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Arahata 1995
- Arahata K, Ishihara T, Fukunaga H, Orimo S, Lee JH, Goto K, et al. Inflammatory response in facioscapulohumeral muscular dystrophy (FSHD): immunocytochemical and genetic analyses. Muscle & Nerve 1995;2 Suppl:56‐66. [MEDLINE: ] [PubMed] [Google Scholar]
BNF 2003
- British Medical Association and the Royal Pharmaceutical Society of Great Britain. British National Formulary 46. London: British Medical Association, 2003. [Google Scholar]
Greenhaff 1993
- Greenhaff PL, Casey A, Short AH, Harris R, Soderlund K, Hultman E. Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in man. Clinical Science (London, England) 1993;84(5):565‐71. [DOI] [PubMed] [Google Scholar]
Guthmiller 1994
- Guthmiller P, Pilsum JF, Boen JR, McGuire DM. Cloning and sequencing of rat kidney L‐arginine: glycine amidinotransferase. Studies on the mechanism of regulation by growth hormone and creatine. The Journal of Biological Chemistry 1994;269(26):17556‐60. [PubMed] [Google Scholar]
Harris 1992
- Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clinical Science 1992;83(3):367‐74. [DOI] [PubMed] [Google Scholar]
Hultman 1996
- Hultman E, Soderlund K, Timmons JA, Cederlad G, Greenhaff PL. Muscle creatine loading in men. Journal of Applied Physiology 1996;81(1):232‐7. [DOI] [PubMed] [Google Scholar]
Kamber 1999
- Kamber M, Koster M, Kreis R, Walker G, Boesch C, Hoppeler H. Creatine supplementation ‐ part I: performance, clinical chemistry, and muscle volume. Medicine & Science in Sports & Exercise 1999;31(12):1763‐9. [DOI] [PubMed] [Google Scholar]
Kissel 1997
- Kissel JT, Mendell JR, Griggs RC, McDermott M, Tawil R. Open label trial of albuterol in facioscapulohumeral muscular dystrophy. Neurology 1997;48:A194. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kissel 1998
- Kissel JT, McDermott MP, Natarajan R, Mendell JR, Pandya S, King WM, Griggs RC, Tawil R. Pilot trial of albuterol in facioscapulohumeral muscular dystrophy. FSH‐ DY Group. Neurology 1998;50(5):1402‐06. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maganaris 1998
- Maganaris CN, Maughan RJ. Creatine supplementation enhances maximum voluntary isometric force and endurance capacity in resistance trained men. Acta Physiologica Scandinavica 1998;163(3):279‐87. [DOI] [PubMed] [Google Scholar]
Mihic 2000
- Mihic S, MacDonald JR, McKenzie S, Tarnopolsky MA. Acute creatine loading increases fat‐free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Medicine & Science in Sports & Exercise 2000;32(2):291‐6. [DOI] [PubMed] [Google Scholar]
Munsat 1972
- Munsat TL, Piper D, Cancilla P, Mednick J. Inflammatory myopathy with facioscapulohumeral distribution. Neurology 1972;22(4):335‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Munsat 1977
- Munsat TL, Bradley WG. Serum creatine phosphokinase levels and prednisone treated muscle weakness. Neurology 1977;27(1):96‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Natural Med 2003
- Compiled by the Editors of Pharmacist's Letter, Prescriber's Letter. Natural Medicines Comprehensive Database. Stockton, California: Therapeutic Research Faculty, 2003. [Google Scholar]
Padberg 1982
- Padberg GW. Facioscapulohumeral disease: Thesis. University of Leiden, 1982. [MEDLINE: 2246] [Google Scholar]
Parise 2001
- Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed‐muscle protein synthesis. Journal of Applied Physiology 2001;91(3):1041‐7. [DOI] [PubMed] [Google Scholar]
Park 1994
- Park JH, Vital TL, Ryder NM, Hernanz‐Schulman M, Partain CL, Price RR, et al. Magnetic resonance imaging and P‐31 magnetic resonance spectroscopy provide unique quantitative data useful in the longitudinal management of patients with dermatomyositis. Arthritis & Rheumatism 1994;37(5):736‐46. [DOI] [PubMed] [Google Scholar]
Park 2000
- Park JH, Niermann KJ, Ryder NM, Nelson AE, Das A, Lawton AR, et al. Muscle abnormalities in juvenile dermatomyositis patients: P‐31 magnetic resonance spectroscopy studies. Arthritis & Rheumatism 2000;43(10):2359‐67. [DOI] [PubMed] [Google Scholar]
Personius 1994
- Personius KE, Pandya S, King WM, Tawil R, McDermott MP. Facioscapulohumeral dystrophy natural history study: standardization of testing procedures and reliability of measurements. The FSH DY Group. Physical Therapy 1994;74(3):253‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rothwell 1987
- Rothwell NJ, Stock MJ, Sudera DK. Changes in tissue blood flow and beta‐receptor density of skeletal muscle in rats treated with the beta2‐adrenoceptor agonist clenbuterol. British Journal of Pharmacology 1987;90(3):601‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soueidan 1993
- Soueidan SA, Dalakas MC. Treatment of inclusion body myositis with high dose intravenous immunoglobulin. Neurology 1993;43(5):876‐9. [DOI] [PubMed] [Google Scholar]
Tarnopolsky 1999
- Tarnopolsky MA, Parise G. Direct measurement of high‐energy phosphate compounds in patients with neuromuscular disease. Muscle & Nerve 1999;22(9):1228‐33. [DOI] [PubMed] [Google Scholar]
Tarnopolsky 2000
- Tarnopolsky MA, MacLennan DP. Creatine monohydrate supplementation enhances high‐intensity exercise performance in males and females. International Journal of Sport Nutrition & Exercise Metabolism 2000;10(4):452‐63. [DOI] [PubMed] [Google Scholar]
Tawil 1995
- Tawil R, McDermott M, Griggs RC, FSHDY Group. Open label trial of prednisone in facioscapulohumeral dystrophy. Neurology 1995;45:A408. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tawil 1997
- Tawil R, McDermott MP, Pandya S, King W, Kissel J, Mendell JR, et al. A pilot trial of prednisone in facioscapulohumeral muscular dystrophy. FSH‐DY Group. Neurology 1997;48(1):46‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tawil 1998
- Tawil R, Figlewicz DA, Griggs RC, Weiffenbach B. Facioscapulohumeral dystrophy: a distinct regional myopathy with a novel molecular pathogenesis. FSH Consortium. Annals of Neurology 1998;43(3):279‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van der Kooi 2003
- Kooi EL, Lindeman E, Riphagen I van der Kooi EL, Lindeman E, Riphagen I. Strength training and aerobic exercise training for muscle disease (Protocol for a Cochrane Review). Cochrane Library 2004, Issue 1. [DOI] [PubMed] [Google Scholar]
Wallimann 1992
- Wallimann T, Wyss M, Brdiczka D, Nikolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ´phosphocreatine circuit´ for cellular energy homeostasis. Biochemical Journal 1992;281(Pt 1):21‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]


