Abstract
Background
Newborn animal studies and pilot studies in humans suggest that mild hypothermia following peripartum hypoxia‐ischaemia in newborn infants may reduce neurological sequelae without adverse effects.
Objectives
To determine the effect of therapeutic hypothermia in encephalopathic asphyxiated newborn infants on mortality, long‐term neurodevelopmental disability and clinically important side effects.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group as outlined in The Cochrane Library (Issue 2, 2007). Randomised controlled trials evaluating therapeutic hypothermia in term and late preterm newborns with hypoxic ischaemic encephalopathy were identified by searching the Oxford Database of Perinatal Trials, the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, 2007, Issue 2), MEDLINE (1966 to June 2007), previous reviews including cross‐references, abstracts, conferences, symposia proceedings, expert informants and journal handsearching. We updated this search in May 2012.
Selection criteria
We included randomised controlled trials comparing the use of therapeutic hypothermia with standard care in encephalopathic term or late preterm infants with evidence of peripartum asphyxia and without recognisable major congenital anomalies. The primary outcome measure was death or long‐term major neurodevelopmental disability. Other outcomes included adverse effects of cooling and 'early' indicators of neurodevelopmental outcome.
Data collection and analysis
Four review authors independently selected, assessed the quality of and extracted data from the included studies. Study authors were contacted for further information. Meta‐analyses were performed using risk ratios (RR) and risk differences (RD) for dichotomous data, and weighted mean difference for continuous data with 95% confidence intervals (CI).
Main results
We included 11 randomised controlled trials in this updated review, comprising 1505 term and late preterm infants with moderate/severe encephalopathy and evidence of intrapartum asphyxia. Therapeutic hypothermia resulted in a statistically significant and clinically important reduction in the combined outcome of mortality or major neurodevelopmental disability to 18 months of age (typical RR 0.75 (95% CI 0.68 to 0.83); typical RD ‐0.15, 95% CI ‐0.20 to ‐0.10); number needed to treat for an additional beneficial outcome (NNTB) 7 (95% CI 5 to 10) (8 studies, 1344 infants). Cooling also resulted in statistically significant reductions in mortality (typical RR 0.75 (95% CI 0.64 to 0.88), typical RD ‐0.09 (95% CI ‐0.13 to ‐0.04); NNTB 11 (95% CI 8 to 25) (11 studies, 1468 infants) and in neurodevelopmental disability in survivors (typical RR 0.77 (95% CI 0.63 to 0.94), typical RD ‐0.13 (95% CI ‐0.19 to ‐0.07); NNTB 8 (95% CI 5 to 14) (8 studies, 917 infants). Some adverse effects of hypothermia included an increase sinus bradycardia and a significant increase in thrombocytopenia.
Authors' conclusions
There is evidence from the 11 randomised controlled trials included in this systematic review (N = 1505 infants) that therapeutic hypothermia is beneficial in term and late preterm newborns with hypoxic ischaemic encephalopathy. Cooling reduces mortality without increasing major disability in survivors. The benefits of cooling on survival and neurodevelopment outweigh the short‐term adverse effects. Hypothermia should be instituted in term and late preterm infants with moderate‐to‐severe hypoxic ischaemic encephalopathy if identified before six hours of age. Further trials to determine the appropriate techniques of cooling, including refinement of patient selection, duration of cooling and method of providing therapeutic hypothermia, will refine our understanding of this intervention.
Plain language summary
Cooling for newborns with hypoxic ischaemic encephalopathy
There is evidence that induced hypothermia (cooling) of newborn babies who may have suffered from a lack of oxygen at birth reduces death or disability, without increasing disability in survivors. This means that parents should expect that cooling will decrease their baby's chance of dying, and that if their baby survives, cooling will decrease his/her chance of major disability. A lack of oxygen before and during birth can destroy cells in a newborn baby's brain. The damage caused by the lack of oxygen continues for some time afterwards. One way to try to stop this damage is to induce hypothermia ‐ cooling the baby or just the baby's head for hours to days. This treatment may reduce the amount of damage to brain cells. This review found that there is evidence from trials to show that induced hypothermia helps to improve survival and development at 18 to 24 months for term and late preterm newborn babies at risk of brain damage. More research is needed to understand which infants need cooling and the best way of cooling, including duration of treatment and method of cooling.
Background
Description of the condition
In technically developed countries, peripartum asphyxia affects three to five newborns per 1000 live births with subsequent moderate or severe hypoxic ischaemic encephalopathy (HIE) in 0.5 to 1 per 1000 live births (Levene 1986). HIE is a major problem worldwide as 10% to 60% of affected infants die, and at least 25% of survivors have long‐term neurodevelopmental sequelae (Vannucci 1990). There are no specific treatments shown to decrease brain damage from HIE. Hypothermia is a clinically feasible manoeuvre that may improve the outcome of neonates with HIE.
Clinical and experimental studies have demonstrated that neuronal death occurs in two phases following a reversible hypoxic‐ischaemic global insult (Gluckman 1992; Lorek 1994; Penrice 1996). If the insult is severe, there may be immediate 'primary neuronal death' related to cellular hypoxia with exhaustion of the cell's high‐energy stores (primary energy failure). After a latent period of at least six hours, the secondary phase of 'delayed neuronal death' begins (Williams 1991). The mechanisms involved in delayed neuronal death include hyperaemia, cytotoxic oedema, mitochondrial failure, accumulation of excitotoxins, active cell death (analogous to developmental apoptosis), nitric oxide synthesis, free radical damage and cytotoxic actions of activated microglia (Inder 2000). The delayed phase is associated with encephalopathy and increased seizure activity, and accounts for a significant proportion of the final cell loss even after very severe insults.
In term and late preterm infants with evidence of intrapartum hypoxia and moderate‐to‐severe encephalopathy, magnetic resonance spectroscopy studies are consistent with this biphasic model of neuronal death. These studies demonstrate normal cerebral oxidative metabolism shortly after birth followed by 'secondary energy failure', the degree of which predicts outcome (mortality and neurodevelopmental outcome at both one and four years of age) (Roth 1992; Roth 1997). Therefore, a therapeutic 'window of opportunity' exists in the interval following resuscitation of the asphyxiated newborn before the secondary phase of impaired energy metabolism and injury.
Description of the intervention
Therapeutic hypothermia aims to lower the temperature of the vulnerable deep brain structures, the basal ganglia, to 32 °C to 34 °C. Two methods are being evaluated in newborn infants with HIE: whole body cooling and selective head cooling with mild systemic hypothermia. The rationale for selective head cooling is that the newborn infant's brain produces 70% of total body heat and that systemic hypothermia may be physiologically harmful to the sick neonate. Therefore, the adverse effects of systemic cooling may be minimised by selectively cooling the brain more than the body (Gunn 1998a). However, a theoretical modelling of cooling investigating temperature distribution within the neonatal head found that the only situation that resulted in a significant reduction in deep brain temperature was when the core body temperature was lowered to 34 °C, implying that it is necessary to reduce systemic temperature to achieve deep brain cooling (Van Leeuwen 2000). Whole body cooling relies on core body and deep brain temperatures being similar.
How the intervention might work
There are a number of postulated mechanisms by which hypothermia may be neuroprotective. Hypothermia may modify cells programmed for apoptosis, leading to their survival. In neonatal piglets, 12 hours of mild hypothermia after resuscitation significantly decreased the number of apoptotic cells, but not the number of necrotic cells (Edwards 1995). Hypothermia may also protect neurons by reducing cerebral metabolic rate, attenuating the release of excitatory amino acids (glutamate, dopamine), ameliorating the ischaemia‐impaired uptake of glutamate and lowering production of toxic nitric oxide (NO) and free radicals (Globus 1995).
Several term and preterm animal experimental models have demonstrated that a reduction in brain temperature of 2 °C to 3 °C immediately following a hypoxic Ischaemic insult reduces energy expenditure, improves subsequent performance testing, reduces histological neuronal loss, or a combination of these (Laptook 1994; Thoresen 1995; Laptook 1997; Gunn 2001). In the term fetal lamb, a significant reduction in histological neuronal loss was seen with extradural temperatures below 35 °C (Gunn 1997a). Temperature modelling calculations also suggest that lowering an infant's core temperature to below 35 °C is required to produce any reduction in the deep brain temperature (Van Leeuwen 2000).
For many decades, deep hypothermia to less than 28 °C has been shown to be valuable for neuroprotection during cardiac arrest for open‐heart and neurosurgical procedures. However, one Cochrane systematic review has called into question utility of hypothermia following neurological surgery. In one meta‐analysis of four trials of cooling for cerebral protection during neurological surgery, Milani 2011 did not find significant reductions in mortality or neurological outcomes, though a trend towards improvement in both outcomes was seen. The results in other studies utilising hypothermia in adults have been similarly unclear. There are five Cochrane systematic reviews of the effects of systemic cooling on outcome of adults following head injury (Sydenham 2009), acute stroke (Der Hertog 2009), coronary artery bypass surgery (Rees 2001), cardiopulmonary resuscitation (CPR) (Arrich 2012), and intracranial aneurysm surgery (Li 2012). While two of these trials found that the patients treated with hypothermia improved neurological outcomes following traumatic head injury (Sydenham 2009) and CPR (Arrich 2012), they did not demonstrate a reduction in mortality. Three reviews did not demonstrate significant improvements in death or dependency with the use of hypothermia following coronary artery bypass surgery (Rees 2001), acute stroke (Der Hertog 2009) or intracranial aneurysm surgery (Li 2012). However, there were no significant adverse events following hypothermia in any of these reviews (Der Hertog 2009; Rees 2001; Sydenham 2009), though a trend towards increased infections (Der Hertog 2009) and pneumonia (Sydenham 2009) was seen. As such, it appears that hypothermia is well tolerated, though its effectiveness is not fully established for all conditions.
Mild hypothermia appears to be well tolerated in a variety of experimental animal models, as well as in adult human studies (Thoresen 1995; Thoresen 1996; Gunn 1997b; Haaland 1997; Marion 1997). There were no reported serious adverse effects in four pilot studies of hypothermia in human newborns (Gunn 1998; Azzopardi 2000; Thoresen 2000; Shankaran 2002). Adverse effects, such as sinus bradycardia, increased blood pressure and increased oxygen requirement, were all transient and reversible with re‐warming (Thoresen 2000).
Identification of infants with hypoxic ischaemic brain injury at risk of future disability who may benefit from hypothermia is challenging. It may be particularly difficult to distinguish between encephalopathy secondary to intrapartum hypoxia and that related to antepartum factors (Badawi 1998a; Badawi 1998b) or underlying congenital abnormalities not easily recognisable at birth (Felix 2000). Newborn animal and adult human studies are consistent with the potential for rescue hypothermia being greatest following moderate, rather than severe, hypoxic ischaemic insults (Haaland 1997; Marion 1997). Aspects of cooling therapy that remain controversial include: how soon after the insult or birth does cooling need to be started, what level of hypothermia is required, what method (selective head cooling versus whole body cooling) should be used and what is the duration of cooling required?
Why it is important to do this review
Effective therapies are urgently required to prevent neurosensory impairment following peripartum asphyxia. We systematically reviewed the evidence to determine whether therapeutic hypothermia reduces adverse outcomes in encephalopathic asphyxiated newborn infants.
Objectives
To determine the effect of therapeutic hypothermia on death and long‐term neurodevelopmental disability, and to ascertain clinically important side effects in newborn infants with HIE.
Secondary objectives included assessment of the adverse effects of cooling and effects on early prognostic indicators of adverse outcome. Subgroup analyses were planned based on:
severity of HIE a) based on Sarnat score (mild, moderate, severe) (Sarnat 1976; Finer 1981); b) based on electroencephalogram (EEG) or amplitude‐integrated electroencephalogram (aEEG) at baseline;
inclusion criteria: electrophysiological plus clinical criteria versus clinical criteria alone;
method of cooling (whole body versus selective head cooling with mild systemic hypothermia);
duration of cooling (< 48 hours vs. ≥ 48 hours);
quality of outcome assessment (high quality (> 18 months with formal psychological testing and review by developmental paediatrician for diagnosis of cerebral palsy (CP)) versus lower quality).
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised and quasi‐randomised studies comparing the use of therapeutic hypothermia with standard care.
Types of participants
Newborn infants of 35 weeks' gestation or greater.
-
Evidence of peripartum asphyxia, with each enrolled infant satisfying at least one of the following criteria:
Apgar score of 5 or less at 10 minutes;
mechanical ventilation or resuscitation at 10 minutes;
cord pH < 7.1, or an arterial pH < 7.1 or base deficit of 12 or more within 60 minutes of birth.
-
Evidence of encephalopathy according to Sarnat staging (Sarnat 1976; Finer 1981):
Stage 1 (mild): hyperalertness, hyper‐reflexia, dilated pupils, tachycardia, absence of seizures;
Stage 2 (moderate): lethargy, hyper‐reflexia, miosis, bradycardia, seizures, hypotonia with weak suck and Moro;
Stage 3 (severe): stupor, flaccidity, small to mid position pupils that react poorly to light, decreased stretch reflexes, hypothermia and absent Moro.
No major congenital abnormalities recognisable at birth.
Types of interventions
Therapeutic cooling (whole body or selective head cooling) initiated prior to six hours after birth versus no cooling (standard care).
Types of outcome measures
Primary outcomes
The primary outcome measure was death or long‐term major neurodevelopmental disability (CP, developmental delay (Bayley or Griffith assessment more than two standard deviations (SD) below the mean) or intellectual impairment (intelligence quotient (IQ) more than two SD below mean), blindness (vision < 6/60 in both eyes), sensorineural deafness requiring amplification). Long‐term outcomes will be reported for all studies that have evaluated children after 18 months' chronological age. Separate analyses will be performed for children aged 18 to 24 months and > three years.
Secondary outcomes
1. Mortality.
2. Major neurodevelopmental disability (CP, developmental delay (Bayley or Griffith assessment more than two SD below the mean) or intellectual impairment (IQ more than two SD below mean), blindness (vision < 6/60 in both eyes), sensorineural deafness requiring amplification).
Each component of major neurodevelopmental disability:
a) CP;
b) developmental delay or intellectual impairment:
Bayley or Griffith assessment more than two SD below the mean or intellectual impairment (IQ more than two SD below mean);
neuromotor development (Bayley Scales of Infant Development ‐ Psychomotor Development Index (BSID PDI)) assessed in survivors;
mental development (Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI)) assessed in survivors;
c) blindness vision (< 6/60 in both eyes);
d) sensorineural deafness requiring amplification;
These components of long‐term outcome will be reported for all studies that have evaluated children after 18 months' chronological age. Separate analyses will be performed for children aged 18 to 24 months and three to five years.
3. The incidence of adverse effects of cooling:
a) heart rate:
any arrhythmia;
sinus bradycardia (heart rate < 80 beats/minute);
prolonged QT interval;
major arrhythmia (requiring medical intervention or cessation of cooling, or both);
b) blood pressure:
hypotension (mean arterial pressure (MAP) < 40 mm Hg);
need for inotrope support;
c) full blood examination:
anaemia (haemoglobin (Hb) <100 g/L with or without hematocrit (Hct) < 30);
leukopenia (white cell count (WCC) < 5 x 109/L);
thrombocytopenia (platelet count < 150 x 109/L);
d) coagulation
any coagulopathy (includes prolonged coagulation time or diagnosis of coagulopathy or disseminated intravascular coagulation (DIC));
coagulopathy resulting in major thrombosis or haemorrhage;
e) hypoglycaemia (< 2.6 mmol/L);
f) hypokalaemia (< 3.5 mmol/L);
g) elevated lactate (number > 2 mmol/L);
h) renal impairment (diagnosis of renal impairment or acute renal failure);
urea (maximum mean ± SD);
creatinine (maximum mean ± SD);
oliguria (< 1 mL/kg/hour);
i) culture‐conformed sepsis (positive blood, cerebrospinal fluid (CSF) or bladder tap urine culture);
j) hepatic dysfunction (elevated liver enzymes aspartate transaminase (AST) > 200 U/L or alanine transaminase (ALT) > 100 U/L);
k) persistent pulmonary hypertension (PPHN) (diagnosed clinically or by echocardiogram);
requiring inhaled NO.
4. Additional indicators of neurodevelopmental outcome:
a) severity of encephalopathy (Sarnat staging) (Sarnat 1976; Finer 1981);
b) severity of EEG abnormality:
severe: isoelectric or burst‐suppression pattern;
moderate: low voltage or discontinuous background;
mild: electrographic seizures, dysmaturity.
c) seizures:
seizures during initial hospitalisation;
seizures or need for anticonvulsants at follow‐up;
d) need for gavage feeds at time of discharge;
e) magnetic resonance imaging (MRI) abnormalities (moderate or severe abnormalities in the basal ganglia or thalamus, severe white matter lesions or abnormalities in the posterior limb of the internal capsule, per Rutherford 2010 ‐ see TOBY Study 2009) assessed in the neonatal period.
Search methods for identification of studies
We used the standard search strategy of the Cochrane Neonatal Review Group as outlined in The Cochrane Library (2007, Issue 2). This included searches of the Oxford Database of Perinatal Trials, the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, 2007, Issue 2), MEDLINE (Silver Platter ‐ 1966 to June, 2007: Infant, Newborn (explode) (MeSH heading) and Asphyxia (explode) (MeSH heading) or Hypoxic Ischaemic Encephalopathy and Hypothermia (explode) (MeSH heading)), previous reviews including cross‐references, abstracts, conferences, symposia proceedings, expert informants and journal handsearching. We did not apply any language restrictions.
We updated this search in May 2012.
Data collection and analysis
We used the methods of the Cochrane Neonatal Review Group for data collection and analysis.
Selection of studies
Four review authors independently identified the studies to be included, assessed the quality of the studies and extracted the data. The updated search in 2012 was reviewed by two review authors (SJ and MB).
Data extraction and management
We included all randomised and quasi‐randomised controlled trials that fulfilled the selection criteria described in the previous section.
We sought information regarding the method of randomisation, blinding and reporting of outcomes of all infants enrolled in the trial for each trial. We obtained data from the primary investigator for unpublished trials or when published data were incomplete. For the initial review, three review authors (SJ, PD and RH) independently retrieved articles, assessed and data abstracted. For the review update in 2012, four review authors (SJ, MB, PD and RH) performed data extraction. We resolved discrepancies by discussion and consensus.
For each study, final data were entered into Review Manager (RevMan 2011) by one review author and then checked for accuracy by a second review author. All review authors reviewed the draft analyses. We resolved discrepancies through discussion.
Assessment of risk of bias in included studies
Methodological quality assessment was based on 1) blinding of randomisation, 2) blinding of intervention, 3) completeness of follow‐up and 4) blinding of outcome measurement. When necessary, additional information and clarification of published data was requested from the authors of individual trials.
The review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved any disagreement by discussion.
The methodological quality of the studies was assessed using the following criteria.
1. Sequence generation (checking for possible selection bias): for each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk.
2. Allocation concealment (checking for possible selection bias): for each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; e.g. unsealed or non‐opaque envelopes; alternation; date of birth);
unclear risk.
3. Blinding (checking for possible performance bias): for each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We characterised the methods used for blinding as:
low risk, high risk or unclear risk for participants;
low risk, high risk or unclear risk for personnel;
low risk, high risk or unclear risk for outcome assessors.
4. Incomplete outcome data (checking for possible attrition bias through withdrawals, drop‐outs, protocol deviations): for each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data);
unclear risk.
5. Selective reporting bias: for each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk.
6. Other sources of bias: for each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk.
7. Overall risk of bias
We made explicit judgements regarding whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses (see 'Sensitivity analysis' below).
Measures of treatment effect
We performed meta‐analyses using the fixed‐effect model. We calculated risk ratio (RR) and risk difference (RD) for dichotomous data and weighted mean difference (WMD) for continuous data, with 95% confidence intervals (CI) for all analyses. We determined the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH) and associated 95% CI were for a statistically significant reduction in the RD.
Unit of analysis issues
We analysed the data as proportion of neonates having one or more episodes for clinical outcomes such as episodes of sepsis.
Dealing with missing data
We noted levels of attrition for included studies. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
All outcomes analyses were on an intention‐to‐treat basis i.e. we included all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We examined heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic. If noted, we planned to explore the possible causes of statistical heterogeneity using pre‐specified subgroup analysis (e.g. differences in study quality, participants, intervention regimens or outcome assessments).
Assessment of reporting biases
We assessed possible publication bias and other biases using symmetry/asymmetry of funnel plots.
For included trials, we explored possible selective reporting of study outcomes by comparing the primary and secondary outcomes in the reports with the primary and secondary outcomes proposed at trial registration, using the web sites www.clinicaltrials.gov and www.controlled‐trials.com. If such discrepancies were found, we planned to contact the primary investigators to obtain missing outcome data on outcomes pre‐specified at trial registration.
Data synthesis
Meta‐analysis was done using Review Manager software (RevMan 2011), supplied by The Cochrane Collaboration. We used the Mantel‐Haenszel method for estimates of typical RR and RD. We analysed continuous measures using the inverse variance method.
We used the fixed‐effect model for all meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses based on method of cooling, severity of encephalopathy and methodological quality.
Sensitivity analysis
Outcome data are reported and analysed in this review for all randomised participants with known outcomes. Those with missing outcome data were excluded from analysis. For the primary outcome, death or major disability, a sensitivity analysis was performed to allow for the additional uncertainty arising from missing outcome data (Gamble 2005).
Results
Description of studies
Eleven randomised controlled trials met inclusion criteria for this review, including eight previously identified (Gunn 1998; Akisu 2003; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; Lin 2006; ICE Study 2011; Shankaran 2002) and three new studies (TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010). Two studies (NICHD Study 2005; ICE Study 2011) had additional data reported, which is included in this analysis. Five were performed as pilot studies, two in single centres (Turkey (Akisu 2003) and China (Lin 2006)), two at multiple centres in North America (Shankaran 2002; Eicher 2005) and one in New Zealand (Gunn 1998). Six large multicentre randomised controlled trials have been published, one from the National Institute of Child Health and Human Development (NICHD) network in North America (NICHD Study 2005), one from multiple centres in China (Zhou 2010) and four from multiple international centres (Cool Cap Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011).
All 11 trials included term or late preterm infants with moderate or severe encephalopathy and evidence of intrapartum hypoxia ischaemia without obvious congenital abnormalities.
Infants in all studies were randomised with initiation of the intervention by six hours of age (mean age at entry range: 1.9 hours (Akisu 2003) to 4.7 hours (TOBY Study 2009)). Five studies used head cooling devices in conjunction with mild systemic hypothermia (Gunn 1998; Akisu 2003; Cool Cap Study 2005; Lin 2006; Zhou 2010), while the other six used whole body cooling alone (Shankaran 2002; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011). The duration of hypothermia was 72 hours in all but one study that cooled infants for 48 hours (Eicher 2005) and one that cooled from 48 hours to 72 hours depending on neurological recovery (Gunn 1998). Eight studies re‐warmed infants by 0.5 °C per hour with the re‐warming period of four hours (Gunn 1998; Shankaran 2002; Akisu 2003; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010), one study re‐warmed infants by 0.5 °C every second hour with a duration of eight hours for re‐warming (ICE Study 2011) and two studies allowed infants to re‐warm spontaneously at room temperature, such that re‐warming took up to 12 hours (Lin 2006; Zhou 2010).
Mortality was ascertained up to latest follow‐up in all studies, ranging from 10 days of age (Lin 2006), to hospital discharge (Shankaran 2002; Akisu 2003) or to neurodevelopmental assessment at 12 months (Eicher 2005) or 18 to 24 months (Gunn 1998; Cool Cap Study 2005; NICHD Study 2005; TOBY Study 2009; Zhou 2010; neo.nEURO Study 2010; ICE Study 2011). Decisions to withdraw care were reported to precede death in seven trials (Shankaran 2002; Eicher 2005; NICHD Study 2005; Lin 2006; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011). Short‐ and long‐term outcomes were assessed at 12 months in one study (Eicher 2005), at 18 months in three studies (Cool Cap Study 2005; TOBY Study 2009; Zhou 2010), to 18 to 22 months in three studies (Gunn 1998; NICHD Study 2005; neo.nEURO Study 2010) and to 24 months in one study (ICE Study 2011). Three studies (Shankaran 2002; Akisu 2003; Lin 2006) reported short‐term outcomes only, with last assessment at 10 days of age or discharge from hospital.
One trial reported industry sponsorship (Cool Cap Study 2005); Olympic Medical (Seattle, WA, USA) provided financial and administrative support and equipment and monitored data for accuracy but was not involved in study design, data interpretation or publication. In the other included studies, there was no disclosure of sponsorship from industry.
Results of the search
We updated the search in May 2012. Eleven studies met criteria for inclusion, including three new studies.
Included studies
Gunn 1998
Gunn 1998 reported the short‐term medical outcomes of 22 term infants with HIE (10 controls randomised to normothermia and 12 randomised to hypothermia) in a randomised controlled pilot study (Gunn 1998). The first six randomised hypothermic infants received minimal cooling (36.0 °C to 36.5 °C; N = 6) and then the next six mild cooling (35.5 °C to 35.9 °C; N = 6) as part of this 'safety' study. The study continued and the 18‐month outcome of these 22 infants, together with a further 18 infants (nine randomised (three normothermic controls, with six allocated to hypothermia to 34.5 °C to 35.4 °C) and nine non‐randomised (two controls, seven cooled to 34 °C to 35 °C)) were reported in a subsequent publication (Battin 2001). Infants were cooled for 72 hours; however, re‐warming was permitted after 48 hours if there was evidence of neurological recovery. The combined results of the 31 randomised infants (13 normothermic controls, 18 allocated to hypothermia) are presented (Gunn 1998), with individual patient data provided by the authors. The non‐randomised patients were not included in this review. There was one further report of short‐term medical outcomes arising from these studies that included the 13 randomised control infants with six infants randomised to 34.5 °C to 35.4 °C and the seven non‐randomised infants at 34 °C to 35 °C (Battin 2003). For the purpose of this systematic review, all randomised infants in the various reports (Gunn 1998, Battin 2001, Battin 2003) are included in the study referred to as Gunn 1998. This study did not report whether any infants had treatment withdrawn prior to death. Eighteen‐month neurodevelopmental outcome assessment using the BSID was performed by a psychologist blinded to treatment allocation and was complete in all patients. Neurodevelopmental outcomes were determined from Table 2 in Battin 2001, and comprised randomised infants (normothermia, numbers 1 to 13; hypothermia, numbers 16 to 33) ascertained from the author. Adverse neurodevelopmental outcome was defined by Gunn 1998 as BSID MDI or PDI < 70.
Shankaran 2002
Shankaran 2002 reported the short‐term medical outcomes to hospital discharge of 19 infants of 36 weeks' gestation or greater and less than six hours of age with evidence of peripartum asphyxia (defined by pH or base deficit within first hour of life; evidence of acute perinatal event and depressed Apgars or need for assisted ventilation at 10 minutes of life; or both) and either seizures or moderate/severe encephalopathy (assessed via level of consciousness, spontaneous activity, posture, primitive reflexes, and autonomic nervous system abnormalities). There were 10 controls and nine infants randomised to therapeutic hypothermia for 72 hours at 34.5 °C by means of a servo‐controlled cooling blanket. Adverse events reported include hypotension, PPHN, renal failure, hepatic dysfunction, DIC and data on hospital course (days on oxygen, length of stay). Withdrawal of care preceded three of five deaths (2/2 cooled and 1/3 standard care).
Akisu 2003
Akisu 2003 reported the short‐term medical outcomes to discharge from hospital of 21 term infants with peripartum asphyxia (defined by pH or base deficit shortly after birth and five‐minute Apgar score) and encephalopathy (presence of stupor, hypotonia or abnormal neonatal reflexes). Eleven infants had their temperature lowered by cooling caps with cold water at 5 °C to 10 °C placed around the scalp for 72 hours. The left external auditory canal and rectal temperatures were monitored to maintain the external auditory canal temperature at 33 °C to 33.5 °C, while the rectal temperature was maintained at 36 °C to 36.5 °C with the servo‐mechanism of the radiant warmer. Ten control infants also had their rectal temperature maintained at 36 °C to 36.5 °C with the servo‐mechanism of the radiant warmer. An additional seven non‐randomised term control infants without asphyxia were not included in this review (Akisu 2003). Adverse effects were reported, including bradycardia, arrhythmia, hypotension, renal impairment, hypoglycaemia, sepsis, thrombocytopenia and short‐term outcome to discharge from hospital (mortality, length of hospital stay, seizures, abnormal EEG, abnormal cranial ultrasound and CT scan). Decisions to withdraw care were not reported.
Eicher 2005
In two consecutive publications, Eicher 2005 reported in‐hospital morbidity with mortality and neurodevelopmental outcomes to 12 months of 65 infants of 35 weeks' gestation or greater with evidence of fetal or postnatal hypoxia‐ischaemia (based upon pH or base deficit, 10‐minute Apgar score, need for resuscitation after five minutes, or a combination), and encephalopathy (two of: posturing; seizures; autonomic dysfunction; or abnormalities of tone, reflexes or state of consciousness) at up to six hours of life. Thirty‐two infants had their temperature lowered by the initial application of ice to head and body for up to two hours that was then maintained at 32.5 °C to 33.5 °C (rectal) on a servo‐controlled cooling blanket for 48 hours. Thirty‐three control infants had their rectal temperature maintained at 36.5 °C to 37.5 °C by servo‐controlled radiant warmer (Eicher 2005). Data presented on short‐term adverse effects of cooling included coagulopathy, cardiac arrhythmias, persistent metabolic acidosis, sepsis/pneumonia within the first seven days of life, hypokalaemia, necrotising enterocolitis (NEC), skin injury, extension of intracranial haemorrhage, PPHN of the newborn, and treatment with extracorporeal membrane oxygenation (ECMO). Eighteen of 24 deaths were preceded by withdrawal of care (9/10 cooled and 9/14 standard care). Neurodevelopmental outcome to 12 months of age was assessed in 28/41 or 68% of surviving infants (17/22 or 77% of cooled infants and 11/19 or 58% of standard care infants) using the BSID, Cognitive Adaptive Test/Clinical Linguistic and Auditory Milestone Scale (CAT/CLAMS) or Vineland examinations at 12 months of age by the developmental team blinded to study group assignment. Severe neuromotor disability was defined by Eicher 2005 as BSID PDI < 70. This study was considered to be of lower quality because the neurodevelopmental outcome assessment was at 12 months rather than 18 to 24 months, follow‐up was incomplete (81%) and only composite outcome of death or severe neuromotor impairment was reported.
CoolCap 2005
Cool Cap Study 2005, for the CoolCap Study Group, reported mortality and severe neuromotor disability to 18 months of age in 234 infants of 36 weeks' gestation or greater born with evidence of peripartum hypoxia‐ischaemia (based upon pH or base deficit, 10‐minute Apgar score and need for resuscitation after 10 minutes), moderate or severe encephalopathy (Sarnat criteria) or clinical seizures and moderate or severely abnormal background or seizures on aEEG. One hundred and sixteen infants underwent head cooling by cooling cap while receiving care on a radiant warmer servo‐controlled to the infants' abdominal skin temperature adjusted to maintain the rectal temperature at 34 °C to 35 °C for 72 hours. One hundred and eighteen infants received standard care on the radiant warmer servo‐controlled to infant's abdominal skin temperature that was adjusted to maintain rectal temperature at 36.8 °C to 37.2 °C (Cool Cap Study 2005), although several non‐cooled infants had elevation of body temperature greater than 38 °C. Adverse events were reported, including arrhythmia, hypotension, coagulopathy, abnormal renal function, hyponatraemia, hypokalaemia, bone marrow depression, abnormal liver function and metabolic acidosis. This study did not report withdrawal of care in deaths. The 18‐month neurodevelopmental assessment (neurological examination, visual and auditory assessment and BSID) was performed in 136/156 or 89.5% of surviving infants (68/76 or 89.5% of cooled infants and 68/76 or 89.5% of standard care infants) by qualified staff and developmental psychologists blinded to treatment group assignment. Severe neurodevelopmental disability was defined by Cool Cap Study 2005 as gross motor function (GMF) level 3 to 5, BSID MDI < 70, or bilateral cortical visual impairment. In this review, we subtracted "died" from "died or severe disability at 18 months" as reported in Table 3 of Cool Cap Study 2005 to obtain major neurodevelopmental disability.
NICHD Study 2005
NICHD Study 2005, for the NICHD Neonatal Research Network, reported mortality and moderate/severe disability at 18 to 22 months for 208 infants at 36 weeks' gestation or greater and less than six hours of age with either severe acidosis (or moderate acidosis with additional criteria) as well as evidence of encephalopathy (on standardised neurological examination by a qualified examiner) or clinical seizures. Acidosis was defined by pH or base deficit on cord or blood gas within one hour of birth), and additional criteria included an acute perinatal event (late or variable decelerations, cord prolapse, cord rupture, uterine rupture, maternal trauma, haemorrhage or cardiorespiratory arrest) along with an Apgar score of ≤ 5 at 10 minutes or need for assisted ventilation initiated at birth and continued for at least 10 minutes. Criteria for encephalopathy included assessment of loss of consciousness (LOC), spontaneous activity, posture, primitive reflexes and autonomic nervous system abnormalities. One hundred and two infants were placed on a pre‐cooled infant blanket servo‐controlled to oesophageal temperature of 33 °C to 34 °C for 72 hours; a second blanket was included in the cooling system to diminish oesophageal temperature variability. One hundred and six infants received standard care with skin temperature servo‐controlled to abdominal skin temperature 36.5 °C to 37 °C (NICHD Study 2005). All infants had abdominal and oesophageal temperature monitoring. Initial mean temperature of hypothermic infants on cooling was 32.7 °C. Several non‐cooled infants had elevation of body temperature greater than 38 °C. Data collected on adverse events included those during the 72‐hour intervention (cardiac arrhythmia, persistent acidosis, major thrombosis or bleeding, skin changes, death) and those prior to hospital discharge (hypotension, PPHN, renal impairment, hepatic dysfunction, sepsis, hypoglycaemia, hypokalaemia, death, length of stay, feeding status and use of anticonvulsants at discharge). Withdrawal of care preceded death in 39 of the 62 deaths (12/24 cooled and 27/38 standard care). Trained developmental examiners blinded to treatment group assignment performed the 18‐ to 22‐month neurodevelopmental assessment of growth, vision, hearing, neurological examination and development using the BSID in 205/208 or 99% of surviving infants (three control infants lost to follow‐up). Severe disability was defined by NICHD Study 2005 as any of the following: GMF level 3 to 5, BSID MDI < 70, hearing impairment requiring hearing aids or blindness. As a component of their primary outcome, NICHD Study 2005 reported "moderate or severe disability." However, as shown in Table 4 of NICHD Study 2005, among the 45 and 64 cooled and control infants who died or had moderate or severe disability, 43 and 62, respectively, either died or had BSID MDI < 70 at follow‐up at 18 to 22 months. Thus, the vast majority of survivors, if not all, who met the NICHD Study 2005 criteria for moderate or severe disability in fact had severe disability by their definition. We used the numbers reported by NICHD Study 2005 for moderate or severe disability to define major neurodevelopmental disability in this review. For this study, moderate‐to‐severe disability was defined as an IQ score 2 or more SD below the mean (scored by Wechsler Preschool and Primary Scale of Intelligence III, score 69 or lower), a GMF level of III or greater, bilateral deafness (with or without amplification), bilateral blindness or refractory epilepsy. Follow‐up at six to seven years occurred in 190/208 or 91% of patients (97/102 or 95% cooled and 93/106 or 88% controls). A number of follow‐up reports were included in this study, providing data on safety and effectiveness (Shankaran 2008), seizures (Kwon 2010), MRI findings (Shankaran 2011) and follow‐up at six to seven years (Shankaran 2012). The average age on completion of MRI was 15 ± 12 days, and data presented are from infants with moderate‐to‐severe injury as classified by the Rutherford 2010 pattern (Shankaran 2011).
Lin 2006
Lin 2006 reported short‐term outcomes to 10 days of age including mortality, moderate‐to‐severe brain injury on CT scan and neurobehavioural assessment for 62 consecutive infants of 37 weeks' gestation or greater with peripartum hypoxia‐ischaemia (Apgar < 6 at five minutes and acidosis defined by pH or base deficit) and clinical encephalopathy (decreased muscle tone, lethargy, coma or seizure). Infants were quasi‐randomised (based on odd or even day of admission) within six hours of birth. Thirty‐two infants had their temperature lowered by a cooling cap device shielded under radiant warmer with output to maintain rectal temperature at 34 °C to 35 °C for 72 hours. The 30 infants who received standard care had intermittent measurement of their rectal temperature, although the target temperature was not stated. Adverse events were not reported. Aspects of the medical treatment were standardised such that all infants received prophylactic phenobarbitone (loading and maintenance) and dopamine (5 μg/kg/minute) throughout the 72‐hour study period (Lin 2006). Decisions to withdraw life support preceded the four deaths. Mortality and short‐term neurobehavioural outcomes (using Neonatal Behavioral Neurological Assessment score) were assessed at seven to 10 days of life.
TOBY 2009
The TOBY Study Group (TOBY Study 2009) reported mortality and neurodevelopmental disability at 18 months as well as adverse outcomes for 325 infants 36 weeks' gestation or greater with perinatal asphyxial encephalopathy. Criteria for inclusion were an Apgar of ≤ 5 or continued need for resuscitation at 10 minutes, acidosis (defined by pH or base deficit within the first hour of life), and evidence of moderate‐to‐severe encephalopathy (lethargy, stupor or coma), and either hypotonia, abnormal reflexes, absent or weak suck, or clinical evidence of seizure. Additionally, infants had to have seizures or abnormal background for at least three minutes on aEEG. One‐hundred and sixty‐three infants were cooled via cooling blanket with a target rectal temperature of 33 °C to 34 °C (actual mean 33.5 °C). The thermostat of the cooling blanket was manually adjusted (not servo‐controlled) to maintain the target rectal temperature of 33 °C to 34 °C. One‐hundred and sixty‐two infants received standard care with skin temperature servo‐controlled to a target rectal temperature of 37 °C (actual mean 36.9 °C, several non‐cooled infants had elevation of body temperature greater than 38 °C). All infants had continuous skin and rectal temperature monitoring. Adverse events recorded included the presence of intracranial haemorrhage, persistent hypotension, pulmonary haemorrhage, PPHN, prolonged blood coagulation time, culture‐confirmed sepsis, NEC, thrombocytopenia, major venous thrombosis, renal failure requiring dialysis, pneumonia, pulmonary air leak and duration of hospitalisation. Withdrawal of care occurred in 63 of 86 deaths reported (34/39 or 87% in cooled and 29/39 or 74% in standard care). The 18‐month neurodevelopmental assessment (neurological examination, visual and auditory assessment and BSID) was performed in 237/239 or 99% of surviving infants (one infant lost to follow‐up in each group) by trained assessors blinded to treatment group assignment. Severe neurodevelopmental disability was defined by TOBY Study 2009 as BSID MDI < 70, GMF level 3 to 5 or bilateral cortical visual impairment. Additional data on MRI findings was presented in Rutherford 2010 and is included in this analysis, with an average age at MRI of eight days (range 2 days to 30 days). Major MRI abnormalities were defined as moderate or severe basal ganglia or thalamic lesions, severe white matter lesions or abnormalities of the posterior limb of the internal capsule.
neo.nEURO 2010
The neo.nEURO.network (neo.nEURO Study 2010) reported mortality or major sensorineural disability outcomes for 125 neonates of at least 36 weeks' gestation with evidence of birth asphyxia (Apgar < 5 at 10 minute, need for continued resuscitation after 10 minutes, presence of acidosis on cord or arterial blood gas within 60 minutes of birth) and clinical evidence of encephalopathy (hypotonia, abnormal reflexes, absent/weak suck, clinical seizures, or a combination). Sixty‐two infants were cooled via cooling mattress with a target rectal temperature of 33.5 °C (range 33.0 °C to 34.0 °C) for 72 hours. Temperature was controlled by manual adjustment of cooling mattress (not servo‐controlled). Sixty‐three infants received standard treatment with a target rectal temperature of 37 °C (range 36.5 °C to 37.5 °C). All infants received morphine (0.1 mg/kg) or an equivalent dose of fentanyl every four hours or by continuous infusion. Adverse events recorded included systemic hypotension, metabolic acidosis, seizures on EEG or clinical, intracranial haemorrhage, venous thrombosis, overt bleeding, coagulopathy, thrombocytopenia, haemoconcentration, systemic infection, arrhythmia, hypoglycaemia, hypocalcaemia, hyponatraemia, elevated liver enzymes, pathological renal function, need for ventilatory support after initiation of intervention, need for inhaled NO or death during intervention. Deaths occurred in 20/62 or 32% of infants in hypothermia group and 33/63 or 52% of controls (non‐significant). In the hypothermia group, 5% of deaths occurred while on maximal support, while 18.2% of such deaths occurred in the control group. A similar percentage of the deaths occurred in both groups following withdrawal of support (14/20 or 70.0% of cooled and 22/33 or 66.7% of infants receiving standard care). Neurological assessments were performed via neurologists blinded to treatment allocation at 18 to 21 months in 58/76 or 76% of surviving infants (33/42 or 79% of cooled and 25/30 or 83% of standard care). Major sensorineural disability was defined by neo.nEURO Study 2010 as a neurological functional score of 3 to 5, development quotient (DQ) < two SD (via Griffiths general quotient or Brunet‐Lezine quotient), severe bilateral cortical visual impairment, or a combination.
Zhou 2010
The China Study Group (Zhou 2010) reported mortality and severe neurodevelopmental disability for 194 neonates 37 weeks' gestation or greater admitted to the neonatal intensive care unit (NICU) within six hours of life with clinical evidence of exposure to perinatal hypoxia‐ischaemia (as determined by Apgar scores at one and five minutes, acidosis on cord gas, or a need for resuscitation or ventilation at five minutes) or a diagnosis of encephalopathy (Sarnat). One hundred infants underwent head cooling via a semiconductor‐controlled water circulation device for 72 hours, servo‐controlled to target nasopharyngeal temperature of 33.8 °C to 34.2 °C, with additional use of a radiant warmer to target a rectal temperature of 34.5 °C to 35 °C. Ninety‐four infants receiving standard treatment were cared for in servo‐controlled radiant warmers with rectal temperature target 36 °C to 37.5 °C. Six infants treated with hypothermia and five controls had a temperature > 38 °C. Adverse events recorded were classified as major (severe arrhythmia, major venous thrombosis, refractory hypotension, moderate or severe scleroedema, severe bleeding, scleroedema) and minor (mild arrhythmia, mild scleroedema, renal dysfunction, liver dysfunction, thrombocytopenia, serum electrolyte or biochemical abnormalities). The study initially included 235 infants (119 cooled and 116 who received standard care), but 41 of these infants (19/119 or 16% of cooled and 22/116 or 22% of standard care) were lost to follow‐up; no data were presented on short‐ or long‐term outcomes for these infants. This study did not report withdrawal of care in deaths. For infants whose data were presented in this study, the 18‐month neurodevelopmental assessment (neurological examination and developmental assessment) was performed in 138/147 surviving infants (75/80 or 93% of cooled and 63/67 or 94% of standard care) by qualified staff blinded to treatment group assignment. For infants who did not return for follow‐up, outcomes were assessed by local trained paediatricians. Severe neuromotor disability was defined by the China Study Group (Zhou 2010) as a DQ < 70 (via Gessell Child Development Age Scale) or GMF level 3 to 5.
ICE 2011
The Infant Cooling Evaluation Collaboration (ICE Study 2011) reported mortality and major sensorineural disability in 221 infants 35 weeks' gestation or greater with moderate or severe HIE (defined as lethargy, stupor, coma, abnormal tone, seizure, or a combination) and evidence of peripartum HIE (at least two of: Apgar ≤ 5 at 10 minutes, continued need for mechanical ventilation after 10 minutes and metabolic acidosis on cord or arterial gas within 60 minutes of birth). One‐hundred and ten infants were cooled by being exposed to the ambient environment (turning radiant warmer off) with refrigerated gel packs applied as required to maintain rectal temperature at 33 °C to 34 °C for 72 hours. One‐hundred and eleven infants received standard care in a radiant warmer, with rectal temperature maintained at 36.8 °C to 37.3 °C. Recruitment was halted due to loss of equipoise. Adverse events recorded included cardiac arrhythmia requiring treatment, prolonged QT interval, hypotension requiring inotropes, overt bleeding, thrombosis or coagulopathy treated with fresh frozen plasma or cryoglobulin (or both), hypoxia while receiving a fraction of inspired oxygen (FiO2) of 100% resulting in discontinuation of hypothermia, thrombocytopenia, oliguria, hepatic dysfunction, rectal bleeding or NEC, sepsis and mortality. Decisions to withdraw support preceded death in 22/27 (81.5%) of cooled and 30/42 (71.4%) of control infants. A decision not to escalate support preceded death in an additional 4/27 (14.8%) of cooled and 9/42 (21.4%) of control infants. Neurodevelopmental assessments were performed at two years of age by trained developmental paediatricians and psychologists blinded to treatment group assignment in 146/152 (96%) of surviving infants (three lost to follow‐up in each group). Major sensorineural disability was defined as neuromotor delay (moderate or severe CP, BSID PDI or MDI of less than ‐2 SD, or a disability index on the Gross Motor Function Classification System (GFMCS) of 2 to 5), developmental delay (BSID MDI, Cognitive Scale, or Language Composite Scale, score of less than ‐2 SD), blindness (vision worse than 20/200 in both eyes), deafness requiring amplification or worse, or a combination. Additional data on MRI findings were presented in Cheong 2012 and is included in this analysis, with an average age at MRI of six days (range three days to eight days). Major MRI abnormalities were defined as moderate or severe basal ganglia or thalamic lesions, severe white matter lesions or abnormalities of the posterior limb of the internal capsule according to the criteria employed in Rutherford 2010 (see TOBY Study 2009).
Excluded studies
Sixteen of the 33 excluded trials were observational case series without controls (Azzopardi 2000; Thoresen 2000; Debillon 2003; Horn 2006; Filippi 2009; Araki 2010; Filippi 2010; Kendall 2010; Massaro 2010; Meyn 2010; Filippi 2011; Thomas 2011; Wusthoff 2011; Christensen 2012; Gucuyener 2012; Tusor 2012). Seven other trials were retrospective cohort studies with historical controls (Simbruner 1999; Compagnoni 2002; Kilani 2002; Lista 2004; Róka 2007; Compagnoni 2008; Hamelin 2011). Three 'randomised' studies (Zhou 2003; Zhou 2002; Li 2009) did not describe the method of allocation, and two studies (Zhou 2002; Liu 2010) did not report any of our pre‐specified outcomes. One study did not meet pre‐specified inclusion criteria for perinatal asphyxia (Robertson 2008). Three studies (Inder 2004; Rutherford 2005; Thoresen 2010) were excluded as they comprised infants from multiple multicentre randomised controlled trials for which the data were reported elsewhere. Two additional studies (Ichiba 2003; Horan 2004) employed cooling via ECMO, but did not meet criteria because they were non‐randomised prospective cohort studies and patients did not meet pre‐specified inclusion criteria for presence of perinatal asphyxia or encephalopathy.
Risk of bias in included studies
The method of randomisation/allocation concealment in nine studies was achieved by means of computer‐generated numbers either in opaque sealed envelopes (Gunn 1998; Cool Cap Study 2005; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011) or obtained centrally from a data co‐ordinating centre (Shankaran 2002; NICHD Study 2005; TOBY Study 2009) or web‐based system (Eicher 2005; TOBY Study 2009). One study (TOBY Study 2009) used either a central data co‐ordinating centre or a web‐based system. In one study (Akisu 2003), the method of randomisation and allocation concealment may have been adequate, but details of the computer‐generated randomisation protocol were unclear. Allocation concealment was inadequate in one study (Lin 2006) because it used quasi‐randomisation by odd or even day of admission. Following randomisation of 31 infants, Gunn et al (see Gunn 1998) obtained ethics approval to cool a further seven non‐randomised infants who are not included in this review sequentially (Battin 2001). A further seven non‐randomised control term infants without asphyxia in one study were also not included in this review (Akisu 2003).
Due to the nature of the intervention, the caregivers in these trials could not be blinded. Importantly, no study reported that the assessors of short‐term outcomes were blinded to treatment allocation; this may have resulted in ascertainment bias. Short‐term follow‐up was complete in all studies. Three studies (Shankaran 2002; Akisu 2003; Lin 2006) reported only short‐term data. One trial reported neurodevelopmental outcome to 12 months (Eicher 2005), with incomplete follow‐up (81.5%). Longer‐term neurodevelopmental outcomes (18 to 22 months) were incomplete in two trials (78% in Zhou 2010 and 76% in neo.nEURO Study 2010) and complete in five others (100% in Gunn 1998, 93% in Cool Cap Study 2005, 96% in ICE Study 2011 and 99% in both NICHD Study 2005 and TOBY Study 2009), with masking of neurodevelopmental outcome assessors to study group assignment (Gunn 1998; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011). The quality of the neurodevelopmental outcome assessment was considered to be high in six studies that followed survivors to at least 18 months of age (Gunn 1998; Cool Cap Study 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011) and lower in the studies that had incomplete follow‐up to 12 months (Eicher 2005).
Effects of interventions
One thousand five hundred and five term or late preterm infants with moderate or severe encephalopathy and evidence of intrapartum asphyxia were enrolled in 11 randomised controlled trials to determine the effect of therapeutic hypothermia on mortality (Gunn 1998; Shankaran 2002; Akisu 2003; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; Lin 2006; TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011), short‐term medical (ICE Study 2011; Shankaran 2002; Akisu 2003; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010) and longer‐term neurodevelopmental outcomes (Gunn 1998; NICHD Study 2005; Cool Cap Study 2005; Eicher 2005; TOBY Study 2009; Zhou 2010; neo.nEURO Study 2010; ICE Study 2011).
Therapeutic hypothermia versus standard care (all infants): subgroup analysis by method of cooling (Comparison 1)
Death or major neurodevelopmental disability in survivors assessed (Outcome 1.1)
Data that permitted the assessment of the effect on this composite outcome were available from eight trials (Gunn 1998; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011). There were 1344 participants, of whom 721 either died or had major neurodevelopmental disability at follow‐up assessment. Five of the trials (Eicher 2005; NICHD Study 2005; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011) found a significant reduction in the incidence of death or major neurodevelopmental disability in the hypothermia groups. Meta‐analysis of all eight trials found a significant reduction in death or major neurodevelopmental disability in survivors (typical RR 0.75 (95% CI 0.68 to 0.83), typical RD ‐0.15 (95% CI ‐0.20 to ‐0.10), NNTB 7 (95% CI 5 to 10); 8 studies, 1344 infants) (Analysis 1.1). There was no evidence of heterogeneity (I2 = 0%).
1.1. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 1 Death or major disability in survivors assessed, by method of cooling.
Meta‐analysis of the three trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005; Zhou 2010) demonstrated a significant reduction in the incidence of death or major neurodevelopmental disability in the hypothermia groups (typical RR 0.77 (95% CI 0.64 to 0.92), typical RD ‐0.13 (95% CI ‐0.22 to ‐0.04), 3 studies, 443 infants)(Analysis 1.1). Meta‐analysis of the five trials (Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011) that used whole body cooling demonstrated a significant reduction in death or disability in the hypothermia groups (typical RR 0.75 (95% CI 0.66 to 0.84), typical RD ‐0.16 (95% CI ‐0.22 to ‐0.10); 5 studies, 901 infants) (Analysis 1.1). There was no evidence of heterogeneity of effect (I2 = 0%).
Data were missing for the primary outcome for this review, death or major disability, in a few participants in five trials: Eicher 2005 (five cooled, eight control), Cool Cap Study 2005 (eight cooled, eight control), NICHD Study 2005 (zero cooled, three control), neo.nEURO Study 2010 (nine cooled, five control), ICE Study 2011 (three cooled, 10 control). After allowing for uncertainty due to these missing outcome data (Gamble 2005), the reduction in the risk of death or major disability was maintained (uncertainty interval for RD ‐0.19 to ‐0.06; data not shown).
Mortality by method of cooling (Outcome 1.2)
Eleven trials reported on mortality (Gunn 1998; Shankaran 2002; Akisu 2003; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; Lin 2006; TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011). There were 1468 infants and 436 deaths in total. Only two of the trials (NICHD Study 2005; ICE Study 2011) found a statistically significant reduction in mortality in the hypothermia group. The meta‐analysis of all 11 trials demonstrated a significant reduction in mortality in the hypothermia groups (typical RR 0.75 (95% CI 0.64 to 0.88), typical RD ‐0.09 (95% CI ‐0.13 to ‐0.04), NNTB 11 (95% CI 8 to 25); 11 studies, 1468 infants) (Analysis 1.2). The effect was consistent across trials, with no important heterogeneity (I2 = 0%).
1.2. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 2 Mortality, by method of cooling.
Meta‐analysis of the five trials (Gunn 1998; Akisu 2003; Cool Cap Study 2005; Lin 2006; Zhou 2010) that used selective head cooling with mild systemic hypothermia did not show a statistically significant effect on mortality (typical RR 0.78 (95% CI 0.59 to 1.04), typical RD ‐0.06 (95% CI ‐0.14 to 0.01); 5 studies, 526 infants). However, meta‐analysis of the six trials that used whole body cooling (Shankaran 2002; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011) demonstrated a significant reduction in mortality in the hypothermia groups (typical RR 0.73 (95% CI 0.61 to 0.89), typical RD ‐0.10 (95% CI ‐0.16 to ‐0.04); NNTB 10 (95% CI 6 to 25); 6 studies, 942 infants) (Analysis 1.2).
Major neurodevelopmental disability (Outcomes 1.3 and 1.4)
Major neurodevelopmental disability by method of cooling (in all infants) (Outcome 1.3)
Eight trials reported effect on neurodevelopmental disability (Gunn 1998; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011). There were 1344 infants with known outcomes, of whom 296 had major neurodevelopmental disability. Meta‐analysis of all eight trials demonstrated a significant reduction in major neurodevelopmental disability in the hypothermia groups (typical RR 0.77 (95% CI 0.63 to 0.94), typical RD ‐0.06 (95% CI ‐0.10 to ‐0.01), NNTB 17 (95% CI 10 to 100); 8 studies, 1344 infants) (Analysis 1.3). There was no significant heterogeneity of treatment effect (I2 = 0%).
1.3. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 3 Major neurodevelopmental disability by method of cooling.
Meta‐analysis of the three trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005; Zhou 2010) demonstrated a non‐significant reduction in major neurodevelopmental disability in the hypothermia groups (typical RR 0.72 (95% CI 0.50 to 1.05), typical RD ‐0.06 (95% CI ‐0.14 to 0.01); 3 studies, 443 infants) as did the five trials that used whole body cooling (Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011) (typical RR 0.79, 95% CI 0.62 to 1.01; typical RD ‐0.05, 95% CI ‐0.11 to ‐0.00; 5 studies: 901 infants) (Analysis 1.3).
Major neurodevelopmental disability in survivors assessed by method of cooling (Outcome 1.4)
Eight trials reported effect on neurodevelopmental disability (Gunn 1998; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011). There were 917 surviving infants with known outcomes, of whom 296 had major neurodevelopmental disability. Meta‐analysis of all eight trials demonstrated a statistically significant reduction in major neurodevelopmental disability among survivors in the hypothermia groups (typical RR 0.67 (95% CI 0.55 to 0.80), typical RD ‐0.13 (95% CI ‐0.19 to ‐0.07), NNTB 8 (95% CI 5 to 14); 8 studies, 917 infants) (Analysis 1.4). There was minimal heterogeneity of treatment effect (I2 = 16%).
1.4. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 4 Major neurodevelopmental disability in survivors assessed, by method of cooling.
Meta‐analysis of the three trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005; Zhou 2010) demonstrated a statistically significant reduction in major neurodevelopmental disability among survivors in the hypothermia groups (typical RR 0.66 (95% CI 0.47 to 0.94), typical RD ‐0.12 (95% CI ‐0.22 to ‐0.02); 3 studies, 312 infants). Meta‐analysis of the five trials that used whole body cooling (Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011) also demonstrated a significant reduction in major neurodevelopmental disability among survivors in the hypothermia groups (typical RR 0.67 (95% CI 0.53 to 0.83), typical RD ‐0.14 (95% CI ‐0.21 to ‐0.06); 5 studies, 605 infants) (Analysis 1.4).
Neuromotor delay in survivors assessed by method of cooling (Outcome 1.5)
Six trials reported effect on neuromotor outcome (Gunn 1998; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; ICE Study 2011). There were 657 survivors, of whom 198 had neuromotor delay on the PDI more than two SD below the mean using the BSID. Meta‐analysis of the six trials demonstrated a significant reduction in neuromotor delay on PDI in the hypothermia group (typical RR 0.75 (95% CI 0.59 to 0.94), typical RD ‐0.09 (95% CI ‐0.16 to ‐0.02); NNTB 11 (95% CI 6 to 50); 6 studies, 657 infants) (Analysis 1.5). There was no significant heterogeneity of treatment effect (I2 = 0%).
1.5. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 5 Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed, by method of cooling.
Meta‐analysis of the four trials that used whole body cooling (Eicher 2005; NICHD Study 2005; TOBY Study 2009; ICE Study 2011) demonstrated a significant reduction in neuromotor delay on PDI in the hypothermia groups (typical RR 0.73 (95% CI 0.56 to 0.95), typical RD ‐0.09 (95% CI ‐0.17 to ‐0.01); 4 studies, 510 infants). The two trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005) showed no statistically significant effect (Analysis 1.5).
Developmental delay in survivors assessed by method of cooling (Outcome 1.6)
Seven trials reported developmental delay or intellectual impairment (Gunn 1998; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011); however, only six used the BSID to assess children. The neo.nEURO Study 2010 tested infants with the Griffiths Scale and reported a statistically significant reduction in developmental delay among survivors assessed. In the six studies that used the BSID, there were 667 survivors, of whom 197 had developmental delay on the MDI of more than two SD below the mean. Meta‐analysis of the six trials demonstrated a significant reduction in developmental delay on MDI in the hypothermia groups (typical RR 0.74 (95% CI 0.58 to 0.94), typical RD ‐0.09 (95% CI ‐0.16 to ‐0.02); NNTB 11 (95% CI 6 to 50); 6 studies, 667 infants) (Analysis 1.6). There was no significant heterogeneity of treatment effect (I2 = 0%).
1.6. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 6 Developmental delay (BSID MDI more than 2 SD below mean) in survivors assessed, by method of cooling.
Meta‐analysis of the four trials that used whole body cooling (Eicher 2005; NICHD Study 2005; TOBY Study 2009; ICE Study 2011) demonstrated a significant reduction in developmental delay on the MDI in the hypothermia groups (typical RR 0.70 (95% CI 0.54 to 0.93), typical RD ‐0.10 (95% CI ‐0.18 to ‐0.02); NNTB 10 (95% CI 6 to 50); 4 studies, 514 infants). The two trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005) showed no statistically significant effect (Analysis 1.6).
Neuromotor development (BSID PDI) assessed in survivors (Outcome 1.7)
Three trials comprising 271 survivors reported effect on neuromotor delay in survivors assessed on the BSID PDI (Gunn 1998; ICE Study 2011; NICHD Study 2005). Meta‐analysis of the three trials demonstrated no significant difference in mean PDI in the hypothermia group (WMD 0.77 (95% CI ‐4.39 to 5.94)) (Analysis 1.7).
1.7. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 7 Neuromotor development (BSID PDI) in survivors assessed.
Mental Development (BSID MDI) assessed in survivors (Outcome 1.8)
Three trials comprising 271 survivors reported effect on mental development in survivors assessed on the BSID MDI (Gunn 1998; NICHD Study 2005; ICE Study 2011). Meta‐analysis of the three trials demonstrated no significant difference in mean MDI in the hypothermia group (WMD 2.47 (95% CI ‐2.77 to 7.71)) (Analysis 1.8).
1.8. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 8 Mental development (BSID MDI) in survivors assessed.
Cerebral palsy in survivors assessed (Outcome 1.9)
Seven trials reported effect on CP (Gunn 1998; Cool Cap Study 2005; NICHD Study 2005; TOBY Study 2009; Zhou 2010; neo.nEURO Study 2010; ICE Study 2011). There were 881 survivors, of whom 252 had CP. Meta‐analysis of the seven trials demonstrated a significant reduction in CP in the hypothermia groups (typical RR 0.66 (95% CI 0.54 to 0.82), typical RD ‐0.12 (95% CI ‐0.18 to ‐0.06); NNTB 8 (95% CI 6 to 17); 7 studies, 881 infants) (Analysis 1.9). There was minimal heterogeneity of treatment effect (I2 = 17%).
1.9. Analysis.
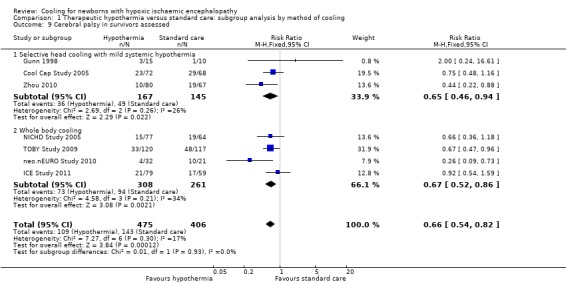
Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 9 Cerebral palsy in survivors assessed.
Meta‐analysis of the three trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005; Zhou 2010) demonstrated a significant reduction in CP in the hypothermia groups (typical RR 0.65 (95% CI 0.46 to 0.94), typical RD ‐0.12 (95% CI ‐0.21 to ‐0.02); NNTB 8 (95% CI 5 to 50); 3 studies, 312 infants) as did the four trials that used whole body cooling (NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011) (typical RR 0.67 (95% CI 0.52 to 0.86), typical RD ‐0.12 (95% CI ‐0.19 to ‐0.04); NNTB 8 (95% CI 5 to 25); 4 studies, 569 infants) (Analysis 1.9).
Blindness in survivors assessed (Outcome 1.10)
Seven trials reported effect of hypothermia on this visual outcome (Gunn 1998; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011). There were 749 survivors, of whom 58 were legally blind. Meta‐analysis of the seven trials showed a non‐significant reduction in blindness in infants who received hypothermia (typical RR 0.62 (95% CI 0.38 to 1.01), typical RD ‐0.04 (95% CI ‐0.08 to 0.00); 7 studies, 749 infants) (Analysis 1.10). There was no significant heterogeneity of treatment effect (I2 = 0%).
1.10. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 10 Blindness in survivors assessed.
Meta‐analysis of the two trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005) and the five trials that used whole body cooling (Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011) demonstrated no statistically significant effect (Analysis 1.10).
Deafness in survivors assessed (Outcome 1.11)
Seven studies reported effect on sensorineural hearing loss requiring amplification (Gunn 1998; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011). There were 720 survivors, of whom 34 had sensorineural hearing loss requiring amplification. Meta‐analysis showed no significant effect of hypothermia on aided sensorineural hearing loss (typical RR 0.66 (95% CI 0.35 to 1.26), typical RD ‐0.02 (95% CI ‐0.05 to 0.01); 7 studies, 720 infants) (Analysis 1.11). There was no significant heterogeneity of treatment effect (I2 = 0%).
1.11. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 11 Deafness in survivors assessed.
Meta‐analysis of the two trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005) and the five trials that used whole body cooling (Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011) demonstrated no statistically significant effect.
Outcome at six to seven years of age (Outcome 1.12)
One study (NICHD Study 2005) reported long‐term follow‐up data at six to seven years of age. There were 190 infants for whom data were available. No significant effect of hypothermia was seen on the primary outcome of death or moderate‐to‐severe disability or on the secondary outcomes of moderate‐to‐severe disability, CP, blindness, deafness or the presence of seizures. There was a significant decrease in death seen in infants undergoing therapeutic hypothermia (RR 0.63 (95% CI 0.43 to 0.94), typical RD ‐0.16 (‐0.30, ‐0.03); NNTB 6 (95% CI 3 to 33)) (Analysis 1.12).
1.12. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 12 Outcome at 6 to 7 years of age.
Cardiovascular adverse effects (Outcomes 1.13 to 1.16)
Eight trials reported effect on sinus bradycardia (Gunn 1998; Akisu 2003; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011). There were 1292 infants, of whom 62 had a sinus bradycardia below 80 beats/minute. Meta‐analysis of the eight trials demonstrated significantly increased sinus bradycardia in hypothermia groups (typical RR 11.59 (95% CI 4.94 to 27.17), typical RD 0.09 (95% CI 0.07 to 0.11); NNTH 11 (95% CI 9 to 14); 8 studies, 1292 infants) (Analysis 1.13). There was significant heterogeneity of treatment effect (I2 = 90%). Meta‐analysis of the four trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Akisu 2003; Cool Cap Study 2005; Zhou 2010) demonstrated a significant increase in sinus bradycardia in the hypothermia groups (typical RR 10.40 (95% CI 2.05 to 52.60), typical RD 0.06 (95% CI 0.03 to 0.10); NNTH 17 (95% CI 10 to 33); 4 studies, 476 infants) as did the four trials that used whole body cooling (Eicher 2005; NICHD Study 2005; TOBY Study 2009; ICE Study 2011) (typical RR 12.06 (95% CI 4.43 to 32.85), typical RD 0.11 (95% CI 0.08 to 0.14); NNTB 9 (95% CI 7 to 13); 4 studies, 816 infants) (Analysis 1.13).
1.13. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 13 Sinus bradycardia.
Eight trials reported the effect of hypothermia on the presence of a major cardiac arrhythmia requiring medical intervention, cessation of cooling, or both (Gunn 1998; Akisu 2003; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; Zhou 2010; ICE Study 2011). There were 1292 infants, of whom six had a major arrhythmia. Meta‐analysis of the eight trials showed no significant effect of hypothermia on the presence of a major cardiac arrhythmia (typical RR 0.55 (95% CI 0.12 to 2.56), typical RD 0.00 (95% CI ‐0.01 to 0.01); 8 studies, 1292 infants) (Analysis 1.14). There was no significant heterogeneity of treatment effect (I2 = 0%). Meta‐analysis of the four trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Akisu 2003; Cool Cap Study 2005; Zhou 2010) and the four trials that used whole body cooling (Eicher 2005; NICHD Study 2005; TOBY Study 2009; ICE Study 2011) also demonstrated no statistically significant effect (Analysis 1.14).
1.14. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 14 Major arrhythmia.
Eight trials reported the effect of hypothermia on the presence of hypotension (MAP < 40 mmHg) (Gunn 1998; Akisu 2003; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011). There were 1221 infants, of whom 742 developed hypotension. Meta‐analysis of the eight trials showed no significant effect of hypothermia on the presence of hypotension (typical RR 1.00 (95% CI 0.92 to 1.09), typical RD 0.00 (‐0.05 to 0.05); 8 studies, 1221 infants) (Analysis 1.15). There was moderate heterogeneity of treatment effect (I2 = 50%). Meta‐analysis of the three trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Akisu 2003; Cool Cap Study 2005) and the five trials that used whole body cooling (Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011) also demonstrated no statistically significant effect (Analysis 1.15).
1.15. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 15 Hypotension (mean arterial pressure < 40 mmHg).
Six trials reported the effect of hypothermia on the need for blood pressure support with inotropic agents (Gunn 1998; Shankaran 2002; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; ICE Study 2011). There were 768 infants, of whom 391 required inotrope support for hypotension. Meta‐analysis of the six trials showed no significant effect of hypothermia on the need for blood pressure support with inotropic agents (typical RR 1.09 (95% CI 0.96 to 1.24), typical RD 0.04 (‐0.02 to 0.11); 6 studies, 768 infants) (Analysis 1.16). There was mild heterogeneity of treatment effect (I2 = 22%). Meta‐analysis of the four trials that used whole body cooling (Shankaran 2002; Eicher 2005; NICHD Study 2005; ICE Study 2011) and the two trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005) demonstrated no statistically significant effect (Analysis 1.16).
1.16. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 16 Hypotension requiring inotropic support.
In this review, we did not report on the outcomes 'any arrhythmia' or 'prolonged QT interval', as insufficient data were available.
Haematological adverse effects (Outcomes 1.17 to 1.21)
Five trials reported the effect of hypothermia on anaemia requiring blood transfusion (Gunn 1998; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; ICE Study 2011). There were 749 infants, of whom 101 were transfused for anaemia. Meta‐analysis of the five trials showed no significant effect of hypothermia on anaemia requiring blood transfusion (typical RR 1.01 (95% CI 0.71 to 1.43), typical RD 0.00 (95% CI ‐0.04 to 0.05); 5 studies, 749 infants) (Analysis 1.17). There was minimal heterogeneity of treatment effect (I2 = 23%). Meta‐analysis of the two trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005) and the three trials that used whole body cooling (Eicher 2005; NICHD Study 2005; ICE Study 2011) also demonstrated no statistically significant effect (Analysis 1.17).
1.17. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 17 Anaemia requiring transfusion.
Four trials reported the effect of hypothermia on WCC (Gunn 1998; Cool Cap Study 2005; Eicher 2005; ICE Study 2011), though one (Eicher 2005) described neutropenia rather than leukopenia. There were 537 infants, of whom 15 had leukopenia (WCC below 5 x 109/L) or neutropenia. Meta‐analysis of the four trials showed no significant effect of hypothermia on the incidence of leukopenia/neutropenia (typical RR 2.40 (95% CI 0.85 to 6.79), typical RD 0.03 (95% CI ‐0.00 to 0.06); 4 studies, 537 infants) (Analysis 1.18). There was no heterogeneity of treatment effect (I2 = 0%). Meta‐analysis of the two trials employing whole body cooling (Eicher 2005; ICE Study 2011) demonstrated a statistically significant increase in leukopenia/neutropenia among infants who underwent therapeutic hypothermia (typical RR 5.70 (95% CI 1.02 to 31.82), typical RD 0.05 (95% CI 0.01 to 0.09); 2 studies, 283 infants); however, no statistically significant effect was seen in the two trials of selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005) (Analysis 1.18).
1.18. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 18 Leukopenia.
Eight trials reported the effect of hypothermia on platelet count (Gunn 1998; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011). There were 1392 infants, of whom 438 were thrombocytopenic with platelet counts below 150 x 109/L. Meta‐analysis of the eight trials showed statistically significantly increased thrombocytopenia in the hypothermic groups (typical RR 1.21 (95% CI 1.05 to 1.40), typical RD 0.06 (95% CI 0.02 to 0.10); NNTH 17 (95% CI 10 to 50); 8 studies, 1392 infants) (Analysis 1.19). There was minimal heterogeneity of treatment effect (I2 = 5%). Meta‐analysis of the three trials of selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005; Zhou 2010) demonstrated a statistically significant increase in thrombocytopenia among infants who underwent therapeutic hypothermia (typical RR 1.58 (95% CI 1.09 to 2.31), typical RD 0.08 (95% CI 0.02 to 0.15); NNTH 13 (95% CI 7 to 50); 3 studies, 455 infants) (Analysis 1.19). Meta‐analysis of the five trials employing whole body cooling (Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011) demonstrated no significant effect (Analysis 1.19).
1.19. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 19 Thrombocytopenia.
Seven trials reported the effect of hypothermia on the presence of any coagulopathy (Shankaran 2002; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011). There were 1188 infants, of whom 354 had coagulopathy. Meta‐analysis of the seven trials showed no significant effect on coagulopathy in cooled infants (typical RR 1.10 (95% CI 0.93 to 1.29), typical RD 0.03 (95% CI ‐0.02 to 0.08); 7 studies, 1188 infants). There was no significant heterogeneity of treatment effect (I2 = 0%). The one trial that used selective head cooling with mild systemic hypothermia (Cool Cap Study 2005) demonstrated no statistically significant effect. Meta‐analysis of the six trials that used whole body cooling (Shankaran 2002; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011) also demonstrated no statistically significant effect (Analysis 1.20).
1.20. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 20 Any coagulopathy.
Four trials reported the effect of hypothermia on the presence of coagulopathy resulting in major thrombosis or haemorrhage (Gunn 1998; NICHD Study 2005; Zhou 2010; ICE Study 2011). There were 689 infants, of whom 12 had coagulopathy resulting in major thrombosis or haemorrhage. Meta‐analysis of the four trials showed no significant effect on coagulopathy resulting in major thrombosis or haemorrhage in cooled infants (typical RR 1.68 (95% CI 0.58 to 4.83), typical RD 0.01 (95% CI ‐0.01 to 0.03); 4 studies, 689 infants). There was no significant heterogeneity of treatment effect (I2 = 0%). Meta‐analysis of the two trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Zhou 2010) and the two trials that used whole body cooling (NICHD Study 2005; ICE Study 2011) also demonstrated no statistically significant effect (Analysis 1.21).
1.21. Analysis.
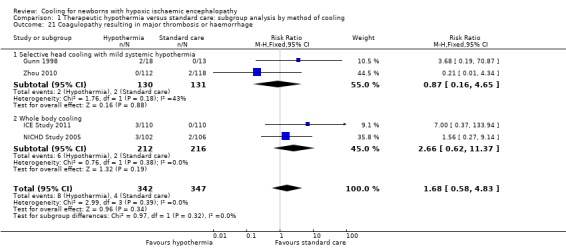
Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 21 Coagulopathy resulting in major thrombosis or haemorrhage.
Metabolic adverse effects (Outcomes 1.22 and 1.23)
Seven trials reported the effect of hypothermia on glucose homeostasis (Gunn 1998; Akisu 2003; Cool Cap Study 2005; NICHD Study 2005; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011). There were1030 infants, of whom 160 were hypoglycaemic with a blood glucose below 2.6 mmol/L. Meta‐analysis of the seven trials showed no significant hypoglycaemia in hypothermic groups (typical RR 0.80 (95% CI 0.60 to 1.06), typical RD ‐0.03 (95% CI ‐0.08 to 0.01); 7 studies, 1030 infants) (Analysis 1.22). There was no significant heterogeneity of treatment effect (I2 = 0%). Meta‐analysis of the three trials employing whole body cooling (NICHD Study 2005; neo.nEURO Study 2010; ICE Study 2011) demonstrated a statistically significant decrease in hypoglycaemia among infants who underwent therapeutic hypothermia (typical RR 0.70 (95% CI 0.49 to 0.98), typical RD ‐0.07 (95% CI ‐0.13 to ‐0.00); 3 studies, 554 infants). Meta‐analysis of the four trials employing selective head cooling with mild systemic hypothermia (Gunn 1998; Akisu 2003; Cool Cap Study 2005; Zhou 2010) demonstrated no significant effect (Analysis 1.22).
1.22. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 22 Hypoglycaemia.
Five trials reported the effect of hypothermia on serum potassium (Gunn 1998; Cool Cap Study 2005; Eicher 2005; Zhou 2010; ICE Study 2011). There were 738 infants, of whom 309 had hypokalaemia with a serum potassium below 3.5 mmol/L. Meta‐analysis of the five trials showed no statistically significant difference in the incidence of hypokalaemia in cooled infants (typical RR 0.93 (95% CI 0.79 to 1.08), typical RD ‐0.03 (95% CI ‐0.10 to 0.03); 5 studies, 738 infants) (Analysis 1.23). There was no significant heterogeneity of treatment effect (I2 = 0%). Meta‐analysis of the three trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005; Zhou 2010) and the two trials that used whole body cooling (Eicher 2005; ICE Study 2011) also demonstrated no statistically significant effect (Analysis 1.23).
1.23. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 23 Hypokalaemia.
In this review, we did not report on the outcome 'elevated lactate (number > 2 mmol/L)' as insufficient data were available.
Renal adverse effects (Outcomes 1.24 and 1.25)
Six trials reported the effect of hypothermia on a diagnosis of renal impairment or acute renal failure (Gunn 1998; Akisu 2003; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; neo.nEURO Study 2010). There were 667 infants, of whom 279 had renal impairment or a diagnosis of acute renal failure. Meta‐analysis of the six trials showed no statistically significant difference in rate of renal impairment in cooled infants (typical RR 0.87 (95% CI 0.74 to 1.02), typical RD ‐0.06 (95% CI ‐0.12 to 0.01); 6 studies, 667 infants) (Analysis 1.24). There was no significant heterogeneity of treatment effect (I2 = 0%). Meta‐analysis of the three trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Akisu 2003; Cool Cap Study 2005) and the three trials that used whole body cooling (Eicher 2005; NICHD Study 2005; neo.nEURO Study 2010) also demonstrated no statistically significant effect ((Analysis 1.24)).
1.24. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 24 Renal impairment.
Six trials reported the effect of hypothermia on urine output (Gunn 1998; Shankaran 2002; Cool Cap Study 2005; NICHD Study 2005; Zhou 2010; ICE Study 2011). There were 865 infants, of whom 201 had oliguria with urine output below 1 mL/kg/hour. Meta‐analysis of the six trials showed no statistically significant difference in rate of oliguria in cooled infants (typical RR 0.95 (95% CI 0.76 to 1.19), typical RD ‐0.01 (95% CI ‐0.06 to 0.04); 6 studies, 865 infants). There was no significant heterogeneity of treatment effect (I2 = 0%). Meta‐analysis of the three trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Cool Cap Study 2005; Zhou 2010) and the three trials that used whole body cooling (Shankaran 2002; NICHD Study 2005; ICE Study 2011) demonstrated no statistically significant effect (Analysis 1.25).
1.25. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 25 Oliguria.
In this review, we did not report on the outcomes 'urea' or 'creatinine', as insufficient data were available, and these criteria were used in the definition of renal impairment in several studies.
Sepsis (Outcome 1.26)
Eight trials reported the effect of hypothermia on sepsis (Gunn 1998; Akisu 2003; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011). There were 1222 infants, of whom 99 had culture‐confirmed sepsis. Meta‐analysis of the seven trials showed no significant effect of hypothermia on sepsis (typical RR 0.87 (95% CI 0.60 to 1.26), typical RD ‐0.01 (‐0.04 to 0.02); 8 studies, 1222 infants) (Analysis 1.26). There was no significant heterogeneity of treatment effect (I2 = 0%). Meta‐analysis of the three trials that used selective head cooling with mild systemic hypothermia (Gunn 1998; Akisu 2003; Cool Cap Study 2005) and the five trials that used whole body cooling (Eicher 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; ICE Study 2011) also demonstrated no statistically significant effect.
1.26. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 26 Sepsis.
Persistent pulmonary hypertension and the need for inhaled nitric oxide (Outcomes 1.27 and 1.28)
Four trials reported the effect of hypothermia on the presence of pulmonary hypertension of the newborn (Shankaran 2002; Eicher 2005; NICHD Study 2005; TOBY Study 2009). There were 614 infants, of whom 93 had PPHN. Meta‐analysis of the four trials showed no significant effect of hypothermia on PPHN of the newborn (typical RR 1.36 (95% CI 0.94 to 1.97), typical RD 0.05 (‐0.01 to 0.10); 4 studies, 614 infants). All studies included infants undergoing whole body cooling. There was no significant heterogeneity of treatment effect (I2 = 0%) (Analysis 1.27).
1.27. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 27 Persistent pulmonary hypertension.
Four trials reported the effect of hypothermia on the need for inhaled NO (Gunn 1998; Eicher 2005; NICHD Study 2005; neo.nEURO Study 2010). There were 426 infants, of whom 57 required inhaled NO. Meta‐analysis of the three trials showed no significant effect of hypothermia on the need for inhaled NO (typical RR 1.18 (95% CI 0.72 to 1.92), typical RD 0.02 (‐0.04 to 0.09); 4 studies, 426 infants). There was moderate heterogeneity of treatment effect (I2 = 49%). The one trial that used selective head cooling with mild systemic hypothermia (Gunn 1998) demonstrated no statistically significant effect. Meta‐analysis of the three trials that used whole body cooling (Eicher 2005; NICHD Study 2005; neo.nEURO Study 2010) also demonstrated no statistically significant effect (Analysis 1.28).
1.28. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 28 Treated with inhaled nitric oxide.
Hepatic dysfunction (Outcome 1.29)
Six trials reported the effect of hypothermia on hepatic dysfunction (elevated liver enzymes AST > 200 U/L or ALT > 100 U/L) (Shankaran 2002; Cool Cap Study 2005; NICHD Study 2005; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011). There were 975 infants, of whom 316 had evidence of hepatic dysfunction. Meta‐analysis of the six trials showed no significant effect of hypothermia on the presence of hepatic dysfunction (typical RR 0.88 (95% CI 0.74 to 1.05), typical RD ‐0.04 (‐0.10 to 0.02); 6 studies, 975 infants). There was moderate heterogeneity of treatment effect (I2 = 31%). Meta‐analysis of the two trials that used selective head cooling with mild systemic hypothermia (Cool Cap Study 2005; Zhou 2010) and the four trials that used whole body cooling (Shankaran 2002; NICHD Study 2005; neo.nEURO Study 2010, ICE Study 2011) also demonstrated no statistically significant effect.
Additional neurological outcomes (Outcome 1.30 to 1.33)
Three trials reported the effect of hypothermia on the need for gastric tube feeds at discharge (Shankaran 2002; NICHD Study 2005; ICE Study 2011). There were 330 infants, of whom 31 required gavage feeds at discharge. All included infants underwent whole body cooling. Meta‐analysis of the three trials showed no significant effect of therapeutic hypothermia on the need for gavage feeds at discharge (typical RR 1.36 (95% CI 0.70 to 2.64), typical RD 0.03 (95% CI ‐0.03 to 0.09); 3 studies, 330 infants). There was minimal heterogeneity of treatment effect (I2 = 22%) (Analysis 1.30).
1.30. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 30 Gastric tube feeds at discharge.
Eight trials reported the effect of hypothermia on seizures diagnosed during the initial hospitalisation (Gunn 1998; Shankaran 2002; Akisu 2003; Cool Cap Study 2005; Eicher 2005; NICHD Study 2005; neo.nEURO Study 2010; ICE Study 2011). There were 907 infants, of whom 565 had clinically recognised seizures. Meta‐analysis of eight trials demonstrated an effect of therapeutic hypothermia on the incidence of clinically recognised seizures that was of borderline significance (typical RR 0.91 (95% CI 0.83 to 1.00), typical RD ‐0.06 (95% CI ‐0.11 to 0.00); 8 studies, 907 infants). There was moderate heterogeneity of treatment effect (I2 = 54%). Meta‐analysis of the five trials employing whole body cooling (Shankaran 2002; Eicher 2005; NICHD Study 2005; neo.nEURO Study 2010; ICE Study 2011) demonstrated a decrease in seizures among infants who underwent therapeutic hypothermia that was of borderline significance (typical RR 0.88 (95% CI 0.78 to 1.00), typical RD ‐0.07 (95% CI ‐0.14 to ‐0.00); 5 studies, 634 infants). Meta‐analysis of the three trials employing selective head cooling with mild systemic hypothermia (Gunn 1998; Akisu 2003; Cool Cap Study 2005) demonstrated no significant effect (Analysis 1.31).
1.31. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 31 Seizures during initial hospitalisation.
Four trials reported the effect of hypothermia on the presence of seizures or anticonvulsant treatment at follow‐up (Cool Cap Study 2005; NICHD Study 2005; TOBY Study 2009; ICE Study 2011). There were 650 infants, of whom 85 had seizures or required anticonvulsants at follow‐up. Meta‐analysis of the four trials showed no significant effect of therapeutic hypothermia on the presence of seizures or need for anticonvulsant treatment at follow‐up (typical RR 0.88 (95% CI 0.59 to 1.31), typical RD ‐0.02 (95% CI ‐0.07 to 0.03); 4 studies, 650 infants). There was no significant heterogeneity of treatment effect (I2 = 0%). The one trial that used selective head cooling with mild systemic hypothermia (Cool Cap Study 2005) demonstrated no statistically significant effect. Meta‐analysis of the three trials that used whole body cooling (NICHD Study 2005; TOBY Study 2009; ICE Study 2011) also demonstrated no statistically significant effect (Analysis 1.32).
1.32. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 32 Seizures or need for anticonvulsant treatment at follow‐up.
Three trials reported the effect of hypothermia on the presence of abnormal MRI findings (NICHD Study 2005; TOBY Study 2009; ICE Study 2011). There were 384 infants, of whom 195 had abnormal MRI findings (moderate or severe basal ganglia or thalamic lesions, severe white matter lesions, or abnormalities of the posterior limb of the internal capsule). All infants underwent whole body cooling. Meta‐analysis of the three trials demonstrated a significant effect of therapeutic hypothermia on the presence of abnormal MRI findings (typical RR 0.73 (95% CI 0.60 to 0.89), typical RD ‐0.16 (95% CI ‐0.26 to ‐0.06); NNTB 6 (95% CI 4 to 17); 3 studies, 384 infants) (Analysis 1.33). There was no significant heterogeneity of treatment effect (I2 = 0%).
1.33. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 33 MRI abnormalities.
Other short‐term neurological outcomes have not been reported, including severity of encephalopathy (Sarnat staging) following cooling, severity of EEG abnormality following cooling and standardised neurological assessment at seven days.
In summary, there was a significant decrease in the incidence of death or major disability, mortality, major disability (among all infants and survivors), neuromotor impairment (PDI), developmental delay (MDI), CP, and presence of abnormal MRI findings among infants treated with hypothermia. A borderline decrease in the incidence of blindness and in seizures during the initial hospitalisation was also seen. There was no significant effect of hypothermia on the presence of deafness, seizures at follow‐up or the need for gastric tube feeding at discharge. For short‐term adverse outcomes, increases in the incidence of sinus bradycardia and thrombocytopenia were seen in infants treated with hypothermia. There was no significant effect of hypothermia on other short‐term adverse outcomes, though an increase in leukopenia and a decrease in hypoglycaemia were seen in infants undergoing whole body cooling. Analyses of several secondary outcomes planned for this review were unable to be performed because they were not reported in the included trials. Planned subgroup analyses based on the degree and duration of cooling and re‐warming, as well as electrophysiological plus clinical criteria versus clinical criteria alone, were still unable to be performed because of lack of eligible data.
Therapeutic hypothermia versus standard care: subgroup analysis by baseline severity of encephalopathy (Comparison 2)
Death or major disability in survivors assessed (Outcome 2.1)
Five trials reported the effect of hypothermia on death of major disability by severity of baseline encephalopathy (Gunn 1998; Cool Cap Study 2005; NICHD Study 2005; Zhou 2010; ICE Study 2011). There were 472 infants with moderate encephalopathy, of whom 212 died or survived with major neurodevelopmental disability. Meta‐analysis of the five trials demonstrated a significant effect on death or major disability in survivors who had moderate encephalopathy (typical RR 0.68 (95% CI 0.56 to 0.84), typical RD ‐0.17 (95% CI ‐0.26 to ‐0.08), NNTB 6 (95% CI 4 to 13)). There was no significant heterogeneity of treatment effect (I2 = 0%). There were 283 infants with severe encephalopathy, of whom 220 died or survived with major neurodevelopmental disability. Meta‐analysis of the five trials found a significant reduction in death or major neurodevelopmental disability in survivors (typical RR 0.82 (95% CI 0.72 to 0.93), typical RD ‐0.16 (95% CI ‐0.25 to ‐0.06), NNTB 6 (95% CI 4 to 17)). There was no significant heterogeneity of treatment effect (I2 = 0%) (Analysis 2.1).
2.1. Analysis.
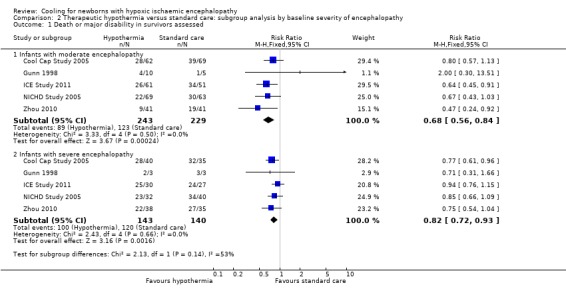
Comparison 2 Therapeutic hypothermia versus standard care: subgroup analysis by baseline severity of encephalopathy, Outcome 1 Death or major disability in survivors assessed.
Mortality (Outcome 2.2)
Five trials reported the effect of hypothermia on mortality by severity of encephalopathy (Gunn 1998; Cool Cap Study 2005; NICHD Study 2005; Zhou 2010; ICE Study 2011). There were 476 infants with moderate encephalopathy, of whom 86 died. Meta‐analysis of the five trials demonstrated a significant reduction in mortality in cooled infants with moderate encephalopathy (typical RR 0.60 (95% CI 0.41 to 0.88), typical RD ‐0.09 (95% CI ‐0.16 to ‐0.02), NNTB 11 (95% CI 6 to 50)). There was moderate heterogeneity of treatment effect (I2 = 26%). There were 285 infants with severe encephalopathy, of whom 171 died. One trial (Cool Cap Study 2005) found a significant reduction in the cooled group. Meta‐analysis of the five trials found a significant reduction in mortality in infants with severe encephalopathy (typical RR 0.77 (95% CI 0.64 to 0.93), typical RD ‐0.16 (95% CI ‐0.27 to ‐0.04), NNTB 6 (95% CI 4 to 25)). There was no significant heterogeneity of treatment effect (I2 = 0%) (Analysis 2.2).
2.2. Analysis.

Comparison 2 Therapeutic hypothermia versus standard care: subgroup analysis by baseline severity of encephalopathy, Outcome 2 Mortality.
Major disability in survivors assessed (Outcome 2.3)
Five trials reported the effect of hypothermia in infants with moderate encephalopathy on neurodevelopmental disability in survivors assessed (Gunn 1998; Cool Cap Study 2005; NICHD Study 2005; Zhou 2010; ICE Study 2011). There were 390 survivors with moderate encephalopathy, of whom 126 had major neurodevelopmental disability. Meta‐analysis of the five trials found a significant reduction in major disability in infants with moderate encephalopathy (typical RR 0.67 (95% CI 0.50 to 0.90); typical RD ‐0.13 (95% CI ‐0.22 to ‐0.03), NNTB 8 (95% CI 5 to 33)). There was no significant heterogeneity of treatment effect (I2 = 0%). There were 115 survivors with severe encephalopathy, of whom 49 had major neurodevelopmental disability. There was no significant effect of cooling on disability among survivors with severe encephalopathy (typical RR 0.75 (95% CI 0.50 to 1.12); typical RD ‐0.13 (95% CI ‐0.31 to 0.06)). There was no significant heterogeneity of treatment effect (I2 = 0%) (Analysis 2.3).
2.3. Analysis.

Comparison 2 Therapeutic hypothermia versus standard care: subgroup analysis by baseline severity of encephalopathy, Outcome 3 Major disability in survivors assessed.
Therapeutic hypothermia versus standard care: subgroup analysis by baseline aEEG findings (Comparison 3)
Death or major disability in survivors assessed (Outcome 3.1)
Two trials reported the effect of hypothermia on this composite outcome (Cool Cap Study 2005; neo.nEURO Study 2010). There were 205 infants with intermediate aEEG findings, of whom 113 died or survived with major disability. For infants with intermediate aEEG findings, meta‐analysis of the two trials demonstrated a significant reduction in death or major disability (typical RR 0.70 (95% CI 0.54 to 0.90), typical RD ‐0.19 (95% CI ‐0.33 to ‐0.06)).
There were 123 infants with severe aEEG findings, of whom 94 died or survived with major neurodevelopmental disability. Meta‐analysis of the two trials demonstrated no significant effect on death or major disability among survivors who had severe aEEG findings (typical RR 0.83 (95% CI 0.67 to 1.03), typical RD ‐0.14 (95% CI ‐0.29 to 0.01)) (Analysis 3.1).
3.1. Analysis.

Comparison 3 Therapeutic hypothermia versus standard care: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings, Outcome 1 Death or major disability in survivors assessed.
Mortality (Outcome 3.2)
Only one trial reported the effect of hypothermia on mortality (Cool Cap Study 2005). There were 171 infants with intermediate aEEG findings, of whom 58 died. For infants with intermediate aEEG findings, meta‐analysis of the two trials demonstrate no significant reduction in mortality (typical RR 0.75 (95% CI 0.49 to 1.15), typical RD ‐0.10 (95% CI ‐0.24 to 0.04)).
There were 46 infants with severe aEEG findings, of whom 20 died. Meta‐analysis of the two trials demonstrate no significant effect on mortality among infants who had severe aEEG findings (typical RR 1.38 (95% CI 0.69 to 2.72), typical RD 0.14 (95% CI ‐0.15 to 0.42)) (Analysis 3.2).
3.2. Analysis.

Comparison 3 Therapeutic hypothermia versus standard care: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings, Outcome 2 Mortality.
Major disability in survivors assessed (Outcome 3.3)
Only one trial reported the effect of hypothermia on major disability in survivors (Cool Cap Study 2005). There were 112 infants with intermediate aEEG findings, of whom 22 survived with major disability. For infants with intermediate aEEG findings, meta‐analysis of the two trials demonstrated a significant reduction major disability (typical RR 0.43 (95% CI 0.19 to 0.98), typical RD ‐0.16 (95% CI ‐0.30 to ‐0.01)).
There were 26 infants with severe aEEG findings, of whom 13 survived with major neurodevelopmental disability. Meta‐analysis of the two trials demonstrated no significant effect on major disability among survivors who had severe aEEG findings (typical RR 1.36 (95% CI 0.63 to 2.94), typical RD 0.15 (95% CI ‐0.23 to 0.54)) (Analysis 3.3).
3.3. Analysis.
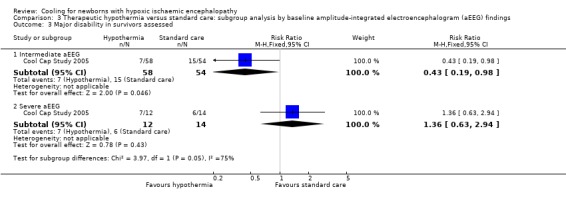
Comparison 3 Therapeutic hypothermia versus standard care: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings, Outcome 3 Major disability in survivors assessed.
Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up (Comparison 4)
Death or major neurodevelopmental disability in survivors assessed by quality of follow‐up (Outcome 4.1)
In the seven trials with high‐quality follow‐up (Gunn 1998; Cool Cap Study 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011), the reduction in death or major disability was statistically significant (typical RR 0.76 (95% CI 0.69, 0.84), typical RD ‐0.14 (95% CI ‐0.20 to ‐0.09), NNTB 7 (95% CI 5 to 11); 7 studies, 1292 infants). The effect was also significant in the one trial with lower‐quality follow‐up (Eicher 2005) (Analysis 4.1).
4.1. Analysis.

Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 1 Death or major disability in survivors assessed, by quality of follow‐up.
Major neurodevelopmental disability by quality of follow‐up (in all infants) (Outcome 4.2)
Meta‐analysis of the seven trials with high‐quality follow‐up (Gunn 1998; Cool Cap Study 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011) demonstrated a significant reduction in major neurodevelopmental disability in the hypothermia groups (typical RR 0.78 (95% CI 0.64 to 0.96), typical RD ‐0.05 (95% CI ‐0.10 to ‐0.01); NNTB 20 (95% CI 10 to 100); 7 studies, 1292 infants). In the one trial with lower quality follow‐up (Eicher 2005) there was no statistically significant reduction in major disability in the hypothermia group (Analysis 4.2).
4.2. Analysis.
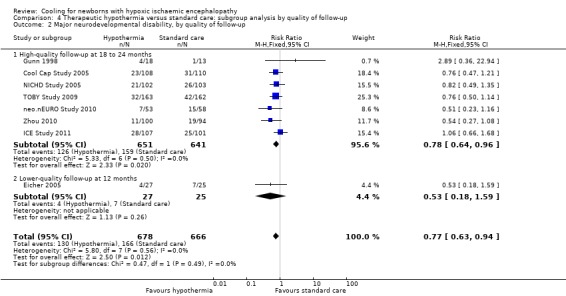
Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 2 Major neurodevelopmental disability, by quality of follow‐up.
Major neurodevelopmental disability in survivors assessed by quality of follow‐up (Outcome 4.3)
Meta‐analysis of the seven trials with high‐quality follow‐up (Gunn 1998; Cool Cap Study 2005; NICHD Study 2005; TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010; ICE Study 2011) demonstrated a significant reduction in major neurodevelopmental disability in the hypothermia groups (typical RR 0.68 (95% CI 0.56 to 0.82), typical RD ‐0.12 (95% CI ‐0.18 to ‐0.06); NNTB 8 (95% CI 6 to 17); 7 studies, 889 infants). The effect was also significant in the one trial with lower‐quality follow‐up (Eicher 2005) (Analysis 4.3).
4.3. Analysis.

Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 3 Major neurodevelopmental disability in survivors assessed, by quality of follow‐up.
Neuromotor delay in survivors assessed by method of cooling (Outcome 4.4)
Meta‐analysis of the five trials with high‐quality follow‐up (Gunn 1998; Cool Cap Study 2005; NICHD Study 2005; TOBY Study 2009; ICE Study 2011) demonstrated a borderline reduction in neuromotor delay on PDI in the hypothermia groups (typical RR 0.78 (95% CI 0.61 to 0.99), typical RD ‐0.07 (95% CI ‐0.15 to ‐0.00); 5 studies, 629 infants). The one trial with lower‐quality follow‐up (Eicher 2005) demonstrated a statistically significant effect (Analysis 4.3).
Developmental delay in survivors assessed by quality of follow‐up (Outcome 4.5)
Meta‐analysis of the five high‐quality trials (Gunn 1998; Cool Cap Study 2005; NICHD Study 2005; TOBY Study 2009; ICE Study 2011) demonstrated a significant reduction in developmental delay on MDI in the hypothermia groups (typical RR 0.75 (95% CI 0.59 to 0.95), typical RD ‐0.09 (95% CI ‐0.16 to ‐0.01); NNTB 11 (95% CI 6 to 100); 5 studies, 638 infants). The one trial with lower‐quality follow‐up (Eicher 2005) demonstrated no statistically significant effect (Analysis 4.5).
4.5. Analysis.

Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 5 Developmental delay (BSID MDI more than 2 SD below mean) in survivors assessed, by quality of follow‐up.
Discussion
Summary of main results
The previous version of this systematic review demonstrated that therapeutic hypothermia for term and late preterm newborn infants with moderate or severe HIE resulted in a clinically important reduction in the composite outcome of mortality or neurodevelopmental disability to 18 to 24 months of age (Jacobs 2007). This review update has added three new randomised controlled trials including 644 infants (TOBY Study 2009; neo.nEURO Study 2010; Zhou 2010) and has also included 204 additional infants from one trial (ICE Study 2011) to bring the total infants included in the review to 1505.
The updated review confirms these important findings and adds precision to our estimates of effect. The meta‐analysis of these 11 RCTs demonstrates a reduction in the composite outcome of mortality or neurodevelopmental disability to 18 to 24 months of age associated with hypothermia (typical relative risk reduction of 25%, absolute risk reduction of 15% and NNTB of even). To prevent one death or major disability, as many as 10 infants or as few as five infants would need to be treated. This reduction in death or major disability remains significant in the subgroup analysis for severe encephalopathy (NNTB 7, as many as 17 infants or as few as 4 infants), and for moderate encephalopathy (NNTB 6, as many as 11 infants or as few as 4 infants). In the overall analysis, the effects on each component contributing to the composite outcome (death, major neurodevelopmental disability in survivors, CP, neuromotor delay, developmental delay) were also statistically significant and clinically important. These results are consistent across the seven trials that measured the effect on death or major disability (I2 = 0%). Overall, the methodology of the included studies is strong. This is particularly true of the four largest studies (Cool Cap Study 2005; NICHD Study 2005; TOBY Study 2009; ICE Study 2011) that contributed most of the weight to the pooled analysis.
It is important to note that along with the composite measure of "death or neurodevelopmental disability to 18 to 24 months of age", each component of the measure was decreased with cooling therapy. Among all treated infants, there was a significant reduction in mortality (Outcome 1.2) with a statistically significant decrease in major disability overall and among survivors (Outcomes 1.3 and 1.4). In addition, neuromotor delay, mental developmental delay and CP were significantly reduced (Outcomes 1.5, 1.6 and 1.9) and a borderline reduction in blindness was seen (Outcome 1.10) among survivors treated with hypothermia. Therefore, cooling reduces mortality and, if an infant survives, also decreases the infant's chance of major disability.
Subgroup analyses performed in this review included method of cooling, quality of follow‐up, degree of baseline encephalopathy and severity of baseline aEEG findings. For the subgroup analysis by method of cooling, both head cooling with mild systemic hypothermia and whole body cooling resulted in a decrease in the composite outcome of death or major disability (Outcome 1.1). However, for the independent outcomes of mortality and major disability, results differed between treatment modalities. While there was no significant decrease in mortality (Outcome 1.2), neuromotor disability (Outcome 1.5) or developmental delay (Outcome 1.6) seen among infants treated with selective head cooling, a significant decrease in these outcomes was seen among infants treated with whole body cooling. For all of these non‐significant outcomes in infants treated with head cooling, there was a strong trend towards improvement as compared to control infants. For some safety outcomes as well, statistical significance was achieved for one cooling modality but not another. Thrombocytopenia was significantly increased overall and in infants treated with head cooling (Outcome 1.19), while leukopenia was significantly increased among infants treated with whole body cooling (Outcome 1.18). Infants undergoing whole body cooling were also noted to have significantly decreased hypoglycaemia (Outcome 1.22). However, it is unclear whether these findings represent a true difference between modalities or rather reflect inadequacy of sample size. Given the controversy surrounding the optimal modality of cooling, it is important to emphasise that these findings do not support any significant superiority of either modality. The better method of cooling may remain uncertain until selective head cooling and whole body cooling are directly compared in clinical trials.
Subgroup analyses by degree of baseline encephalopathy (Comparison 2) included infants with both severe and moderate encephalopathy. For both infants with severe and moderate encephalopathy, there is a significant reduction in death or major disability (Outcome 2.1) and mortality (Outcome 2.2) seen in infants treated with hypothermia. However, infants with severe encephalopathy demonstrated no reduction in major disability, while a significant reduction was seen in infants with moderate encephalopathy (Outcome 2.3). One concern is whether this supports the "death versus disability" question addressed by Barks (Barks 2008), that is, by treating with hypothermia are we preferentially saving those infants with severe injury who might otherwise have died, but instead survive with profound neurological impairment? It is important to note that these data do not support this controversy. If this were the case, a reduction in mortality among those with severe HIE would be likely to result in either a trend towards increased risk of neurodevelopmental sequelae overall in infants treated with hypothermia or, at a minimum, no difference in the risk of such sequelae. While this meta‐analysis did not demonstrate a significant reduction in major disability among surviving infants with severe HIE, there was a trend towards improvement (typical RR 0.75, 95% CI 0.50 to 1.12) and the lack of significance is likely to reflect the smaller number of infants in this category.
Subgroup analyses by severity of baseline aEEG findings demonstrated a reduction in death or major disability (Outcome 3.1) among cooled infants with intermediate but not severe aEEG findings at baseline. Given that baseline aEEG findings are used to exclude infants with severe encephalopathy at some centres, it should be noted that there was a strong trend towards reduction in death or major disability among infants with severe aEEG findings at baseline. There was a reduction in major disability (Outcome 3.3) but no significant decrease in mortality (Outcome 3.2) seen in cooled infants with either intermediate or severe aEEG findings. However, these analyses (Outcomes 3.2 and 3.3) included only a single study (Cool Cap Study 2005).
For subgroup analyses by quality of follow‐up (Comparison 4), there was a significant reduction in both the primary outcome of death or neurodevelopmental disability (Outcome 4.1), in mortality (Outcome 4.2), in major neurodevelopmental disability (Outcome 4.3), in neuromotor delay (BSID PDI ≥ two SD below mean, Outcome 4.5) for both infants with high‐quality and lower‐quality follow‐up. For developmental delay (BSID MDI ≥ two SD below mean, Outcome 4.4) significant improvement was seen only in infants with high‐quality follow‐up. While no significant reduction was seen in infants with lower‐quality follow‐up, this most likely reflects the inclusion of only a small number of infants from a single trial in this analysis.
Due to limited data, we were not able to perform subgroup analyses according to the duration of hypothermia or according to electrophysiological plus clinical criteria versus clinical criteria alone.
Additional data on neurodevelopmental outcomes reported in this analysis include MRI abnormalities, need for gavage feeds at discharge, seizures during initial hospitalisation, the need for anticonvulsants at follow‐up and developmental outcomes at school‐age of six to seven years. While the MRI findings represent a consistent pattern of injury as classified by Rutherford 2010 (TOBY Study 2009), the time at completion of MRI varied between studies. Nonetheless, there was a significant reduction in the presence of abnormal findings on MRI (Outcome 1.33). There was also borderline reduction in the presence of seizures during the initial hospitalisation (Outcome 1.31) among infants undergoing therapeutic hypothermia, but no significant decrease in the number of infants requiring tube feeds at discharge (Outcome 1.30) or with seizures at follow‐up (Outcome 1.32). Developmental outcomes at school‐age of six to seven years are reported in one study (NICHD Study 2005). While there was no significant reduction in death or moderate‐to‐severe disability at age six to seven years among those treated with hypothermia (Outcome 1.12.1), there was a clinically important trend towards improvement (RR 0.81, 95% CI 0.64 to 1.04) and a significant reduction in death at age six to seven years (Outcome 1.12.2). No significant reduction was seen in moderate‐to‐severe disability (Outcome 1.12.3), blindness (1.12.5), deafness (1.12.6) or seizures (Outcome 1.12.7) at age six to seven years. While the long‐term benefit of cooling is currently unknown, the results of this single study suggest that mortality is reduced without any increase in moderate or severe neurosensory disability with more remote follow‐up.
Reporting of many of the secondary outcomes specified in our original protocol was not adequate to assess adverse outcomes or safety. While an increase in the incidence of sinus bradycardia and thrombocytopenia were seen in infants treated with hypothermia (as well as an increase in leukopenia and a decrease in hypoglycaemia in infants undergoing whole body cooling), there was no significant effect of hypothermia on other short‐term adverse outcomes. While not statistically significant, there was a trend towards increased PPHN among all infants treated with hypothermia that may be clinically important. An additional safety outcome emerging in the literature is the occurrence of subcutaneous fat necrosis as a complication of hypothermia. This was previously reported in infants undergoing hypothermia for cardiovascular surgery (Chuang 1995), but was not noted in clinical trials of therapeutic hypothermia for HIE. Subcutaneous fat necrosis was noted in only one cooled infant in NICHD and unspecified skin changes were noted in one infant in CoolCap. Since the more widespread clinical application of therapeutic hypothermia, a number of case reports of fat necrosis in cooled infants (Wiadrowski 2001; Oza 2010; Gómez‐Fernández 2011; Akcay 2012; Sivanandan 2012). In addition, one report from the UK TOBY Cooling Register (Strohm 2011) noted 12 cases in infants treated with hypothermia, representing approximately 1% of infants registered as cooled infants. No similar cases have been reported in infants randomised to standard care, suggesting that hypothermia may be associated with an increased risk of subcutaneous fat necrosis. Interventions to reduce the incidence of this complication, such as that of Filippi 2012, may be beneficial as therapeutic hypothermia becomes more commonplace.
Quality of the evidence
In the overall analyses, the number of infants studied is substantial; the estimates of treatment effect on the primary outcome, death or major neurodevelopmental disability, and on each component of this composite outcome, were reasonably precise. However, these estimates of treatment effect were less precise in subgroup analyses based on severity of encephalopathy and aEEG at baseline, method of cooling and quality of follow‐up.
Potential biases in the review process
Several limitations of the available evidence should be noted. By clinical necessity, the caregivers could not be blinded to the intervention. Even so, in seven of the eight trials that assessed the effect of cooling on major neurodevelopmental disability, assessors of neurodevelopment were blinded to treatment group assignment. The effect of treatment group on mortality could be biased by the unblinded intervention if it resulted in fewer decisions by caregivers to withdraw intensive care in cooled babies. However, the finding that major neurodevelopmental disability was not increased in survivors who had been cooled does not support this speculation.
Authors' conclusions
Implications for practice.
There is evidence from the 11 randomised controlled trials (N = 1505) included in this systematic review that therapeutic hypothermia is beneficial to term and late preterm newborns with HIE. Death or major disability, mortality, and neurodevelopmental disability in survivors are all reduced. Importantly, mortality is reduced without increasing major disability in survivors. While there is also some evidence of harm from therapeutic hypothermia (increased thrombocytopenia), the benefits of cooling on survival and neurodevelopment outweigh the short‐term adverse effects.
Cautious application of the results of this meta‐analysis is recommended. Trials contributing participants to this meta‐analysis were conducted within strict protocols and often at centres of excellence with considerable experience in therapeutic hypothermia. However, as evidence for the therapeutic efficacy of hypothermia has emerged, a large number of centres have introduced hypothermia as standard treatment, and in 2010 the International Liaison Committee on Resuscitation (ILCOR) recommended that therapeutic hypothermia should be offered to term or near‐term infants with moderate or severe encephalopathy (Perlman 2010). While the ILCOR recommended the use of standard inclusion criteria and cooling protocols, as hypothermia has become standard treatment it appears that centres are using less rigorous inclusion criteria (Pfister 2010). These have included both tertiary centres and local hospitals with more limited experience with patient selection and cooling protocols. It remains to be seen if the use of therapeutic hypothermia in less experienced centres or using less rigorous selection criteria, or both, will blunt the efficacy of hypothermia seen here. Additionally, as hypothermia is a significant problem in the developing world, studies are underway to assess the use of hypothermia in low‐resource settings (Bhat 2006; Horn 2006; Robertson 2008; Thayyil 2010), and further analyses will need to be conducted to determine the efficacy of hypothermia in these settings.
As the use of hypothermia has become more routine, the difficulty in initiating hypothermia prior to six hours of life has provoked interest in the efficacy of late (after six hours of life) initiation of hypothermia. While animal studies have suggested that cooling is most beneficial when initiated prior to six hours of life (Gunn 1998a), studies are now underway (NICHD: Late Hypothermia) to investigate the utility of later hypothermia. In one completed study of late hypothermia (Li 2009), there was a statistically significant reduction in death or disability seen when cooling was started at up to 10 hours of life. However, the utility of such late cooling cannot be determined with the limited data now available. Cooling during transport is often considered as a means to initiate hypothermia in a more timely manner for infants born at referral hospitals where hypothermia is not available. While there is some evidence that controlled hypothermia targeted to core temperature can safely be initiated by dedicated transport teams using strict NICU protocols at referring hospitals and continued during transport (ICE Study 2011), reports of transport of infants where either active or passive cooling has been commenced by referring hospitals or on transport have demonstrated that while both active (Hobson 2011; O'Reilly 2011) and passive (Anderson 2007; Kendall 2010; Robertson 2010; O'Reilly 2011 ) cooling during transport may be effective, difficulty with temperature management (particularly with excessive hypothermia) can occur. Although cooling at the referring hospital of birth, prior to arrival of the transport team, is becoming a widely used practice, its efficacy has not yet been established by any randomised controlled trials, and the possibility of excessive hypothermia must be carefully considered when considering initiating cooling at referring hospitals prior to transport.
Implications for research.
Further well‐designed and executed studies with appropriate power are required to determine the optimal modality (head versus body), duration (≤ 48 hours versus > 48 hours or > 72 hours), and degree (≤ 33 °C versus > 33 °C) of hypothermia. While cooling for longer durations or lower temperatures, or both, is currently being studied (NICHD: Optimizing Cooling), the question of selective head cooling versus whole body cooling remains. Additionally, the application of hypothermia to additional populations of infants at risk for neurological sequelae is being pursued on multiple fronts. This includes preterm infants (32 to 35 weeks' gestation) with a diagnosis of HIE (Walsh: Preterm Infants), but also infants being treated with ECMO (Ichiba 2003; Horan 2004; NEST Study) and infants with hyperammonaemia and encephalopathy (Lichter‐Konecki).
As hypothermia has become an established therapy for infants with HIE, the use of adjunct therapies has become an increasing focus of study. Trials of xenon have shown promise in animal models (Thoresen 2009; Chakkarapani 2010), and multiple randomised controlled trials employing xenon in addition to therapeutic hypothermia are underway (CoolXenon Study; TOBYXe). Other adjunctive treatments being studied include darbepoetin (DANCE) and topiramate (NeoNATI). Both erythropoietin (Iwai 2010; Fan 2011) and topiramate (Schubert 2005; Noh 2006) have previously been found to be effective in animal models of HIE. Additional therapeutic agents for future study as adjunctive therapies with hypothermia include (among others) levetiracetam, N‐acetylcysteine and melatonin, all of which have been found to be effective in animal models (Cilio 2010). Studies of the optimal management of seizures in infants with HIE and the effectiveness (or toxicity) of therapeutic agents such as phenobarbital are also needed. In keeping with various government policies (NHRMC 2005; Department of Health 2006; Washington 2011), the inclusion of parents and consumers as partners in the design and conduct of new trials and in communication of their results may enhance several aspects of future research (Tarnow‐Mordi 2012).
What's new
| Date | Event | Description |
|---|---|---|
| 25 February 2013 | Amended | Minor typographical correction Outcome 1.1 |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 19 November 2012 | New citation required and conclusions have changed | A new author (Dr. Marie Berg) was added to the authorship team. |
| 1 May 2012 | New search has been performed | This updates the review 'Cooling for newborns with hypoxic ischaemic encephalopathy' (Jacobs 2007). The 2012 update changed the following: 1. Entry criteria for trials limited to infants ≥ 35 weeks gestation who were cooled before six hours of age. 2. Added clinical outcomes PPHN, need for inhaled nitric oxide hepatic dysfunction, clarified definition of coagulopathy, seizures at follow up, outcome at six to seven years, MRI abnormalities. 3. Reordered subgroup analyses. For the updated review, we found three new randomised trials (TOBY Study 2009; Zhou 2010; neo.nEURO Study 2010) enrolling 644 infants and has also included 204 additional infants from one trial (ICE Study 2011). |
| 21 May 2008 | Amended | Converted to new review format. Updated in 2012. |
| 28 June 2007 | New citation required and conclusions have changed | Substantive amendment |
| 28 June 2007 | New search has been performed | This review updates the existing review of 'Cooling for newborns with hypoxic ischaemic encephalopathy' that was published in the Cochrane Library (Cochrane Database of Systematic Reviews), Issue 4, 2003 (Jacobs 2003). Since the first version of this review, it has become apparent that not all control infants randomised to 'normothermia' had their temperature in the normal range. Therefore, 'normothermia' has been renamed 'standard care' in this updated review. Six additional randomised controlled trials and 587 additional infants were included in the review (8 trials and 638 total infants); a further 4 studies were excluded. We are awaiting further information or publication of one completed randomised trial comprising 157 infants. Long‐term follow‐up from 3 ongoing trials is awaited, all having completed or stopped recruitment. These additional 829 infants will be incorporated into future updates of this review, and could change the results and overturn the conclusions of this review. The evidence for therapeutic hypothermia has changed significantly since the 2003 review, which concluded that there was a lack of evidence that therapeutic hypothermia was either beneficial or harmful to newborns with hypoxic ischaemic encephalopathy and that cooling should only be performed in the context of randomised controlled trials. This 2007 update concludes that there is evidence that therapeutic hypothermia is beneficial to term newborns with hypoxic ischaemic encephalopathy, and that cooling decreases death, without increasing major disability in survivors. The benefits of cooling on survival and neurodevelopment outweigh the short‐term adverse effects. |
Acknowledgements
We thank all the authors of the included studies, particularly Malcolm Battin, Dorothy Jenkins, Alistair Gunn and Seetha Shankaran for the NICHD Neonatal Research Network, who clarified existing data and provided us with additional information. We also thank Diantha Howard for her assistance with the uncertainty analysis.
Data and analyses
Comparison 1. Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or major disability in survivors assessed, by method of cooling | 8 | 1344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.83] |
| 1.1 Selective head cooling with mild systemic hypothermia | 3 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.64, 0.92] |
| 1.2 Whole body cooling | 5 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.66, 0.84] |
| 2 Mortality, by method of cooling | 11 | 1468 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.64, 0.88] |
| 2.1 Selective head cooling with mild systemic hypothermia | 5 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.04] |
| 2.2 Whole body cooling | 6 | 942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.89] |
| 3 Major neurodevelopmental disability by method of cooling | 8 | 1344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.63, 0.94] |
| 3.1 Selective head cooling with mild systemic hypothermia | 3 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.50, 1.05] |
| 3.2 Whole body cooling | 5 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.62, 1.01] |
| 4 Major neurodevelopmental disability in survivors assessed, by method of cooling | 8 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.55, 0.80] |
| 4.1 Selective head cooling with mild systemic hypothermia | 3 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.47, 0.94] |
| 4.2 Whole body cooling | 5 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.53, 0.83] |
| 5 Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed, by method of cooling | 6 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.94] |
| 5.1 Selective head cooling with mild systemic hypothermia | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.51, 1.29] |
| 5.2 Whole body cooling | 4 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.56, 0.95] |
| 6 Developmental delay (BSID MDI more than 2 SD below mean) in survivors assessed, by method of cooling | 6 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.94] |
| 6.1 Selective head cooling with mild systemic hypothermia | 2 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.54, 1.36] |
| 6.2 Whole body cooling | 4 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.93] |
| 7 Neuromotor development (BSID PDI) in survivors assessed | 3 | 271 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [‐4.39, 5.94] |
| 7.1 Selective head cooling with mild systemic hypothermia | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐6.80 [‐16.47, 2.87] |
| 7.2 Whole body cooling | 2 | 249 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [‐2.31, 9.91] |
| 8 Mental development (BSID MDI) in survivors assessed | 3 | 271 | Mean Difference (IV, Fixed, 95% CI) | 2.47 [‐2.77, 7.71] |
| 8.1 Selective head cooling with mild systemic hypothermia | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐10.30 [‐23.91, 3.31] |
| 8.2 Whole body cooling | 2 | 250 | Mean Difference (IV, Fixed, 95% CI) | 4.69 [‐0.98, 10.37] |
| 9 Cerebral palsy in survivors assessed | 7 | 881 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.54, 0.82] |
| 9.1 Selective head cooling with mild systemic hypothermia | 3 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.46, 0.94] |
| 9.2 Whole body cooling | 4 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.52, 0.86] |
| 10 Blindness in survivors assessed | 7 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.01] |
| 10.1 Selective head cooling with mild systemic hypothermia | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.23, 1.37] |
| 10.2 Whole body cooling | 5 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.35, 1.17] |
| 11 Deafness in survivors assessed | 7 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.35, 1.26] |
| 11.1 Selective head cooling with mild systemic hypothermia | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.36, 5.72] |
| 11.2 Whole body cooling | 5 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.25, 1.11] |
| 12 Outcome at 6 to 7 years of age | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Death or moderate‐to‐severe disability | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.04] |
| 12.2 Death | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.43, 0.94] |
| 12.3 Moderate‐to‐severe disability | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.57, 1.48] |
| 12.4 Cerebral palsy | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.31, 1.18] |
| 12.5 Blindness | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.03, 4.00] |
| 12.6 Deafness | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.26, 22.20] |
| 12.7 Seizures | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.25, 1.68] |
| 13 Sinus bradycardia | 8 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.59 [4.94, 27.17] |
| 13.1 Selective head cooling with mild systemic hypothermia | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.40 [2.05, 52.60] |
| 13.2 Whole body cooling | 4 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.06 [4.43, 32.85] |
| 14 Major arrhythmia | 8 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.12, 2.56] |
| 14.1 Selective head cooling with mild systemic hypothermia | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.60] |
| 14.2 Whole body cooling | 4 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.99] |
| 15 Hypotension (mean arterial pressure < 40 mmHg) | 8 | 1221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.09] |
| 15.1 Selective head cooling with mild systemic hypothermia | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.84, 1.36] |
| 15.2 Whole body cooling | 5 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.90, 1.08] |
| 16 Hypotension requiring inotropic support | 6 | 768 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.24] |
| 16.1 Selective head cooling with mild systemic hypothermia | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.97, 1.48] |
| 16.2 Whole body cooling | 4 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.87, 1.23] |
| 17 Anaemia requiring transfusion | 5 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.71, 1.43] |
| 17.1 Selective head cooling with mild systemic hypothermia | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.28, 1.80] |
| 17.2 Whole body cooling | 3 | 489 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.75, 1.58] |
| 18 Leukopenia | 4 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.85, 6.79] |
| 18.1 Selective head cooling with mild systemic hypothermia | 2 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.22, 4.33] |
| 18.2 Whole body cooling | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.70 [1.02, 31.82] |
| 19 Thrombocytopenia | 8 | 1392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.05, 1.40] |
| 19.1 Selective head cooling with mild systemic hypothermia | 3 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.09, 2.31] |
| 19.2 Whole body cooling | 5 | 937 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.98, 1.33] |
| 20 Any coagulopathy | 7 | 1188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.93, 1.29] |
| 20.1 Selective head cooling with mild systemic hypothermia | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.89, 1.56] |
| 20.2 Whole body cooling | 6 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.87, 1.30] |
| 21 Coagulopathy resulting in major thrombosis or haemorrhage | 4 | 689 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.58, 4.83] |
| 21.1 Selective head cooling with mild systemic hypothermia | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.16, 4.65] |
| 21.2 Whole body cooling | 2 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [0.62, 11.37] |
| 22 Hypoglycaemia | 7 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.60, 1.06] |
| 22.1 Selective head cooling with mild systemic hypothermia | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.63, 1.74] |
| 22.2 Whole body cooling | 3 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.49, 0.98] |
| 23 Hypokalaemia | 5 | 738 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.08] |
| 23.1 Selective head cooling with mild systemic hypothermia | 3 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.79, 1.16] |
| 23.2 Whole body cooling | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.15] |
| 24 Renal impairment | 6 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.02] |
| 24.1 Selective head cooling with mild systemic hypothermia | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| 24.2 Whole body cooling | 3 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.51, 1.06] |
| 25 Oliguria | 6 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.19] |
| 25.1 Selective head cooling with mild systemic hypothermia | 3 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.24] |
| 25.2 Whole body cooling | 3 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.70, 1.36] |
| 26 Sepsis | 8 | 1222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.60, 1.26] |
| 26.1 Selective head cooling with mild systemic hypothermia | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.25, 2.48] |
| 26.2 Whole body cooling | 5 | 940 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.30] |
| 27 Persistent pulmonary hypertension | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 Whole body cooling | 4 | 614 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.94, 1.97] |
| 28 Treated with inhaled nitric oxide | 4 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.72, 1.92] |
| 28.1 Selective head cooling with mild systemic hypothermia | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.41, 7.90] |
| 28.2 Whole body cooling | 3 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.66, 1.88] |
| 29 Hepatic dysfunction | 6 | 975 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.05] |
| 29.1 Selective head cooling with mild systemic hypothermia | 2 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.12] |
| 29.2 Whole body cooling | 4 | 551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.67, 1.14] |
| 30 Gastric tube feeds at discharge | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 Whole body cooling | 3 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.70, 2.64] |
| 31 Seizures during initial hospitalisation | 8 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.00] |
| 31.1 Selective head cooling with mild systemic hypothermia | 3 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.85, 1.10] |
| 31.2 Whole body cooling | 5 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 1.00] |
| 32 Seizures or need for anticonvulsant treatment at follow‐up | 4 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.31] |
| 32.1 Selective head cooling with mild systemic hypothermia | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.43, 2.00] |
| 32.2 Whole body cooling | 3 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.37] |
| 33 MRI abnormalities | 3 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.60, 0.89] |
1.29. Analysis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 29 Hepatic dysfunction.
Comparison 2. Therapeutic hypothermia versus standard care: subgroup analysis by baseline severity of encephalopathy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or major disability in survivors assessed | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infants with moderate encephalopathy | 5 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.84] |
| 1.2 Infants with severe encephalopathy | 5 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.93] |
| 2 Mortality | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infants with moderate encephalopathy | 5 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.41, 0.88] |
| 2.2 Infants with severe encephalopathy | 5 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.64, 0.93] |
| 3 Major disability in survivors assessed | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infants with moderate encephalopathy | 5 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.90] |
| 3.2 Infants with severe encephalopathy | 5 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.12] |
Comparison 3. Therapeutic hypothermia versus standard care: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or major disability in survivors assessed | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Intermediate aEEG | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.90] |
| 1.2 Severe aEEG | 2 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.67, 1.03] |
| 2 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Intermediate aEEG | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.15] |
| 2.2 Severe aEEG | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.69, 2.72] |
| 3 Major disability in survivors assessed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Intermediate aEEG | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.98] |
| 3.2 Severe aEEG | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.63, 2.94] |
Comparison 4. Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or major disability in survivors assessed, by quality of follow‐up | 8 | 1344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.83] |
| 1.1 High‐quality follow‐up at 18 to 24 months | 7 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.69, 0.84] |
| 1.2 Lower‐quality follow‐up at 12 months | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.41, 0.92] |
| 2 Major neurodevelopmental disability, by quality of follow‐up | 8 | 1344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.63, 0.94] |
| 2.1 High‐quality follow‐up at 18 to 24 months | 7 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.64, 0.96] |
| 2.2 Lower‐quality follow‐up at 12 months | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.18, 1.59] |
| 3 Major neurodevelopmental disability in survivors assessed, by quality of follow‐up | 8 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.55, 0.80] |
| 3.1 High‐quality follow‐up at 18 to 24 months | 7 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.82] |
| 3.2 Lower‐quality follow‐up at 12 months | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 0.97] |
| 4 Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed, by quality of follow‐up | 6 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.94] |
| 4.1 High‐quality follow‐up at 18 to 24 months | 5 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.61, 0.99] |
| 4.2 Lower‐quality follow‐up at 12 months | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 0.97] |
| 5 Developmental delay (BSID MDI more than 2 SD below mean) in survivors assessed, by quality of follow‐up | 6 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.94] |
| 5.1 High‐quality follow‐up at 18 to 24 months | 5 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.95] |
| 5.2 Lower‐quality follow‐up at 12 months | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.68] |
4.4. Analysis.

Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 4 Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed, by quality of follow‐up.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akisu 2003.
| Methods | Single‐centre randomised controlled trial in Turkey | |
| Participants | Included 21 term infants with peripartum asphyxia (5‐minute Apgar score < 6, with acidosis on cord or arterial blood shortly after delivery (pH < 7.1 or base deficit > 10 mmol/L) and encephalopathy) without congenital abnormality (metabolic, malformations, chromosomal, congenital infection) or transitory drug depression | |
| Interventions | Hypothermia: temperature lowered in 11 infants by cooling cap for 72 hours (left external auditory canal temperature lowered to 33 to 33.5 °C and rectal temperature maintained at 36 to 36.5 °C by servo‐mechanism of radiant warmer). Infants re‐warmed at 0.5 °C/hour Standard care: 10 infants had rectal temperature maintained at 36 to 36.5 °C by servo‐mechanism of radiant warmer |
|
| Outcomes | Primary outcome: platelet‐activating factor in cerebrospinal fluid Secondary outcomes: adverse effects of hypothermia (bradycardia, arrhythmia, hypotension, renal impairment, hypoglycaemia, sepsis, thrombocytopenia) and short‐term outcome to discharge from hospital (mortality, length of hospital stay, seizures, abnormal electroencephalogram (EEG), abnormal cranial ultrasound and computerised tomography (CT) scan) | |
| Notes | Age at initiation of cooling included (1.9 hours), but not age at randomisation for infants allocated to standard care 7 non‐randomised control term infants without asphyxia not included in review Not stated if death followed decision to withdraw care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated protocol number |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) long‐term outcomes | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete to discharge |
| Selective reporting (reporting bias) | Low risk | |
Cool Cap Study 2005.
| Methods | Multicentre international randomised controlled trial | |
| Participants | Included 234 infants born at ≥ 36 weeks' gestation with clinical evidence of peripartum hypoxia‐ischaemia (Apgar score ≤ 5 at 10 minutes, continued need for resuscitation at 10 minutes, or severe acidosis (pH < 7 or base deficit ≥ 16 in cord blood or arterial/venous blood within 60 minutes of birth)) AND moderate or severe encephalopathy (Sarnat criteria) or clinical seizures AND moderate or severely abnormal background or seizures on amplitude integrated electroencephalography Excluded infants were older than 5.5 hours at randomisation, or had received prophylactic anticonvulsants, or had major congenital abnormalities, or had head trauma, or had severe growth restriction (< 1800 g birthweight), or were considered too critically unwell to benefit from intensive care, or equipment was unavailable, or were planned to participate in other trials |
|
| Interventions | Hypothermia (N = 116): head cooling by cooling cap (Olympic Medical Cool Care System) on a radiant warmer servo‐controlled to infant's abdominal skin temperature adjusted to maintain rectal temperature at 34 to 35 °C for 72 hours. Infants re‐warmed at no more than 0.5 °C per hour Standard care (N = 118): radiant warmer servo‐controlled to infant's abdominal skin temperature adjusted to maintain rectal temperature at 36.8 to 37.2 °C |
|
| Outcomes | Primary: combined frequency of mortality and severe neurodevelopmental disability in survivors at 18 months of age (gross motor function 3 to 5; Mental Development Index < 70 or bilateral cortical visual impairment) Secondary: adverse events in first 7 days of life including mortality, arrhythmia, hypotension, coagulopathy, abnormal renal function, hyponatraemia, hypokalaemia, bone marrow depression, abnormal liver function, metabolic acidosis | |
| Notes | Randomised at 4.6 hours Several non‐cooled infants had elevation of body temperature greater than 38 °C Sponsored by Olympic Medical who funded study, supplied all equipment including amplitude integrated electroencephalogram (EEG) monitors and provided administrative support. Scientific advisory committee responsible for other aspects of design, data analysis and publication Not stated if any deaths followed withdrawal of care Additional information provided by authors on short‐term morbidity (prolonged QT interval, hypotension treated with inotropes, anaemia, leukopenia, thrombocytopenia, oliguria), short‐term neurological outcomes (severity of hypoxic ischaemic encephalopathy (HIE), seizures, anticonvulsant therapy) and long‐term neurodevelopmental outcome (according to severity of HIE, cerebral palsy, Bayley Scales of Infant Development ‐ Psychomotor Development Index (BSID PDI) and Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI), confirmation of major neurodevelopmental disability in 23 cooled and 31 control infants) Later reports: follow‐up at 7 to 8 years (Guillet 2011) as well as secondary analyses of primary outcome by severity of encephalopathy at randomisation (Wyatt 2007) and of the prognostic value of clinical assessment of encephalopathy (Gunn 2008) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate, with block randomisation by computer‐generated numbers in opaque sealed envelopes stratified by participating centre |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) long‐term outcomes | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18‐month follow‐up in 218/234 (93%) |
| Selective reporting (reporting bias) | Low risk | |
Eicher 2005.
| Methods | Multicentre randomised controlled trial in US | |
| Participants | Included 65 infants ≥ 35 weeks' gestation, > 2000 g birthweight, who were ≤ 6 hours of age with ≥ 1 clinical sign of a hypoxic‐Ischaemic insult (cord gas ≤ 7.0 or base deficit ≥ 13, initial infant gas pH < 7.1, Apgar score ≤ 5 at 10 minutes, continued resuscitation after 5 minutes, fetal bradycardia lasting ≥ 15 minutes, or postnatal hypoxic Ischaemic event with oxygen desaturation < 70% or arterial oxygen tension < 35 mmHg for 20 minutes with evidence of Ischaemic (chest compressions, hypotension, haemorrhage)) and 2 features of neonatal encephalopathy (posturing, seizures, autonomic dysfunction, or abnormalities of tone, reflexes or state of consciousness) Infants excluded with sepsis at birth (2 infants allocated to standard care), maternal chorioamnionitis, birthweight or head circumference < 10th centile for gestational age, or congenital abnormalities |
|
| Interventions | Hypothermia: temperature lowered in 32 infants by application of ice to head and body for up to 2 hours and then maintained at 32.5 °C to 33.5 °C (rectal) on a servo‐controlled cooling blanket for 48 hours (Blanketrol II, Cincinnati Sub‐Zero). Re‐warmed by 0.5 °C per hour after 48 hours Standard care: 33 had rectal temperature maintained at 36.5 °C to 37.5 °C by servo‐controlled radiant warmer |
|
| Outcomes | Efficacy and safety outcomes published in 2 consecutive reports. Primary outcomes in efficacy report included death or 12‐month neurodevelopmental outcome (Bayley Scales of Infant Development ‐ Psychomotor Development Index (PDI) and Mental Development Index (MDI), Cognitive Adaptive Test/Clinical Linguistic and Auditory Milestone Scale (CAT/CLAMS) or Vineland assessments). Primary outcomes in safety report included bradycardia, disseminated intravascular coagulopathy and sepsis. Additional data collected on short‐term adverse effects of cooling included coagulopathy, cardiac arrhythmias, persistent metabolic acidosis, sepsis/pneumonia within the first 7 days of life, hypokalaemia, necrotising enterocolitis, skin injury, extension of intracranial haemorrhage, persistent pulmonary hypertension of the newborn, and treatment with extracorporeal membrane oxygenation (ECMO) | |
| Notes | Randomised at 3.1 (standard care)/3.4 (hypothermia) hours Considered to be lower‐quality study as only 12‐month neuromotor outcome reported. In addition, follow‐up was incomplete (81%) and only composite outcome of death or severe neuromotor impairment was reported 3 infants were excluded after randomisation, with no data available. Denominators are 31 for both groups as reported by authors Of 24 deaths, 18 followed withdrawal of support (9 cooled, 9 standard care) Additional information provided by authors for age at randomisation, all short‐term adverse effects, and neurodevelopmental outcome at 12 months (but not according to a priori definition as per review) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate, with web‐based centralised online blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) long‐term outcomes | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Assessment of short‐term outcomes nearly complete (62/65), but incomplete 12‐month assessment (53/65 = 81%) |
| Selective reporting (reporting bias) | Low risk | |
Gunn 1998.
| Methods | Single‐centre randomised controlled trial in New Zealand | |
| Participants | Included infants in the report of Gunn 1998 and additional infants randomised in the reports of Battin 2001 and Battin 2003. In total, the trials enrolled 31 infants of 37 weeks' gestation or greater with perinatal asphyxia (pH ≤ 7.09 or Apgar ≤ 6 at 5 minutes) plus evidence of encephalopathy (lethargy/stupor, hypotonia, abnormal reflexes including absent or weak suck. Infants with major congenital abnormalities were excluded | |
| Interventions | Hypothermia: 18 infants underwent cooling via cooling cap (Silclear tubing cap in first 17 infants, Olympic Medical Cool Care System for remainder), with target temperature determined by sequential randomisation with 6 infants in a minimal cooling group (36 °C to 36.5 °C), followed by 6 infants in a mild hypothermia group (35.5 °C to 35.9 °C), 6 infants at 34.5 °C to 35.4 °C Standard care: 15 infants had rectal temperature maintained at 36.8 °C to 37.2 °C with servo‐controlled radiant warmer (10 in initial study and 3 in follow‐up) |
|
| Outcomes | Acute adverse events such as seizures or evidence of multi‐system involvement (hypotension (mean arterial pressure < 40 mmHg), bradycardia (< 80 beats/minute), cardiac arrhythmia, persistent pulmonary hypertension (requiring nitric oxide), meconium aspiration syndrome requiring respiratory support, infection, thrombocytopenia, hypoglycaemia, maximum acidosis during cooling, electrolyte imbalance (hyponatraemia, hypokalaemia), and acute renal failure. Short‐term outcomes (death, cranial ultrasound, electroencephalogram, head computerised tomography) and 18‐month neurodevelopmental follow‐up (Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI) and Psychomotor Development Index (BSID PDI)) also assessed | |
| Notes | 40 infants reported, with 31 randomised. Non‐randomised infants (7 cooled to 34 °C to 35.5 °C, 2 controls) not included in review Randomised at a mean of 4.4 hours (hypothermia) and 3.8 hours (control) of age Not stated if deaths followed withdrawal of care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Low risk, by sequential, computer‐generated numbers |
| Allocation concealment (selection bias) | Unclear risk | Adequate |
| Blinding of outcome assessment (detection bias) short‐term outcomes | Unclear risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) long‐term outcomes | Unclear risk | Caregivers not blinded to treatment assignment for short‐term outcomes, but assessors of neurodevelopment at 12 months were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Assessment of short‐term and 12‐month outcomes complete. Follow‐up at 18 months complete for 14/15 surviving cooled infants and 9/10 surviving control infants |
ICE Study 2011.
| Methods | Multicentre international study | |
| Participants | Included 221 infants 35 weeks' gestation or greater with moderate or severe hypoxic ischaemic encephalopathy (HIE) (defined as lethargy, stupor, coma, abnormal tone, seizure, or a combination) and evidence of peripartum HIE (at least 2 of: Apgar ≤ 5 at 10 minutes, continued need for mechanical ventilation after 10 minutes, with or without metabolic acidosis with cord or arterial pH of ≤ 7 with or without base deficit of ≥ 12 within 60 minutes of birth) Infants for whom hypothermia could not be initiated within 6 hours of life, who weighed < 2000 g, who had major congenital abnormalities suspected, who had overt bleeding, who required > 80% fraction of inspired oxygen (FiO2), who started hypothermia before randomisation, or for whom death was imminent were excluded |
|
| Interventions | Hypothermia: 110 infants cooled by being exposed to the ambient environment (turning radiant warmer off) with refrigerated gel packs applied as required to maintain rectal temperature at 33 °C to 34 °C for 72 hours Infants re‐warmed by 0.5 °C every 2 hours Standard care: 111 standard care infants rectal temperature was maintained at 36.8 °C to 37.3 °C |
|
| Outcomes | Primary outcomes: composite of mortality or major sensorineural disability at 2 years. Major sensorineural disability was defined as neuromotor delay (moderate or severe cerebral palsy, Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI) and Psychomotor Development Index (BSID PDI) of less than ‐2 standard deviations (SD), or a disability index on the Gross Motor Function Classification System (GFMCS) of 2 to 5), developmental delay (BSID MDI, Cognitive Scale, or Language Composite Scale, score of less than ‐2 SD), blindness (vision worse than 20/200 in both eyes), deafness requiring amplification or worse, or a combination Secondary outcomes: mortality, major sensorineural disability and individual components (neuromotor delay, developmental delay, blindness, deafness, or a combination), and survival free of any sensorineural disability Adverse events recorded included cardiac arrhythmia requiring treatment, prolonged QT interval, hypotension requiring inotropic agents, overt bleeding, thrombosis or coagulopathy treated with fresh frozen plasma, with or without cryoglobulin, hypoxia in 100% O2 resulting in discontinuation of hypothermia, thrombocytopenia, oliguria, hepatic dysfunction, gastrointestinal bleeding or necrotising enterocolitis, sepsis and mortality |
|
| Notes | Recruitment halted due to loss of equipoise Enrolled and randomised at 3.9 (cooled) and 4 (control) hours Decisions to withdraw support preceded death in 22/27 (81.5%) of cooled and 30/42 of control infants. A decision not to escalate support preceded death in an additional 4/27 (14.8%) of cooled and 9/42 (21.4%) of control infants Additional data reported on magnetic resonance imaging findings (Cheong 2012) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequentially numbered, sealed envelopes with computer‐generated random numbers stratified by study centre |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) long‐term outcomes | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Assessment of outcomes nearly complete (107/110 cooled and 101/111 control infants) |
| Selective reporting (reporting bias) | Low risk | |
Lin 2006.
| Methods | Single‐centre study in China | |
| Participants | Included 62 consecutive infants of 37 weeks' gestation or greater with peripartum hypoxia‐ischaemic (Apgar < 6 at 5 minutes with first postnatal arterial pH < 7.1 or base deficit > 15) and clinical encephalopathy quasi‐randomised within 6 hours of birth. Infants with major congenital abnormalities and severe hypoxaemia due to severe persistent fetal circulation were excluded | |
| Interventions | Hypothermia: 32 infants cooled by cooling cap device (SDL‐V, Tianyuan Scientific Development) shielded under radiant warmer with output to maintain rectal temperature at 34 °C to 35 °C for 72 hours. Infants re‐warmed spontaneously, radiant warmer used if temperature remained less than 36° C after 12 hours Standard care: 30 standard care infants had intermittent measurement of rectal temperature ‐ target temperature not stated All infants received prophylactic phenobarbital (loading and maintenance) and dopamine (5 μg/kg/minute) throughout 72‐hour study period |
|
| Outcomes | Mortality, neuroimaging (computerised tomography scan) and neurobehavioural assessment at 7 to 10 days of life using Neonatal Behavioral Neurological Assessment score | |
| Notes | Enrolled at 3.6 (hypothermia)/3.8 (standard care) hours 4 deaths all followed withdrawal of care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Inadequate, quasi‐randomised (alternate day allocation according to odd or even day of admission) |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) long‐term outcomes | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up incomplete: analysis not intention to treat, and followed to 10 days of age |
| Selective reporting (reporting bias) | Low risk | |
| Overall risk of bias | High risk | |
neo.nEURO Study 2010.
| Methods | International multicentre study | |
| Participants | Included 129 neonates of at least 36 weeks' gestation with evidence of birth asphyxia (Apgar < 5 at 10 minute, need for continued resuscitation after 10 minutes, cord or arterial pH of ≤ 7 or base deficit of ≥ 16 within 60 minutes of birth or both) and clinical evidence of encephalopathy (hypotonia, abnormal reflexes, absent/weak suck, clinical seizures, or a combination) Excluded infants > 5.5 hours of age, administration of > 20 mg/kg phenobarbital, weight < 1800 g, head circumference < 3rd percentile (if other growth parameters > 3rd percentile), presence of major congenital anomalies, imperforate anus, presence of gross haemorrhage, or infants who were "in extremis" |
|
| Interventions | Hypothermia: 64 infants cooled via a cooling mattress (Tecotherm TS Med 200, TecCom) with a target rectal temperature of 33.5 °C (range 33.0 °C to 34.0 °C) for 72 hours. Temperature was controlled by manual adjustment of cooling mattress (not servo‐controlled). Infants re‐warmed by less than 0.5 °C per hour Standard care: 65 infants received standard treatment with a target rectal temperature of 37 °C (range 36.5 °C to 37.5 °C) All infants received morphine (0.1 mg/kg) every 4 hours or an equivalent dose of fentanyl |
|
| Outcomes | Primary outcome: death or severe disability (neurological functional score 3 to 5, development quotient (DQ) < 2 standard deviations (SD), with or without severe bilateral cortical visual impairment) at 18 to 21 months Secondary outcomes: death or severe disability within infants with moderate or severe hypoxic ischaemic encephalopathy, death, DQ < 2 SD, disabling cerebral palsy, bilateral cortical visual impairment or severe hearing loss Additional adverse events recorded included systemic hypotension, metabolic acidosis, seizures on electroencephalogram or clinical, intracranial haemorrhage, venous thrombosis, overt bleeding, coagulopathy, thrombocytopenia, haemoconcentration, systemic infection, arrhythmia, hypoglycaemia, hypocalcaemia, hyponatraemia, elevated liver enzymes, pathological renal function, need for ventilator support after initiation of intervention, need for inhaled nitric oxide or death during intervention |
|
| Notes | Randomised at 4.6 (hypothermia) and 4.1 (control) hours Follow‐up data were incomplete (79% hypothermia and 83% control) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation using numbered, sealed envelopes, stratified by centre and severity |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) long‐term outcomes | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Assessment of short‐term outcomes nearly complete (62/64 cooled and 63/65 control infants), but incomplete follow‐up data (53/64 (83%) of cooled and 58/65 (89%) of control infants) |
| Selective reporting (reporting bias) | Low risk | |
NICHD Study 2005.
| Methods | Multicentre randomised controlled trial within National Institute of Child Health and Human Development (NICHD) network in the US | |
| Participants | Included 208 infants ≥ 36 weeks' gestation < 6 hours of age with evidence of seizures or encephalopathy and either (a) pH ≤ 7.0 or base deficit ≥ 16 mmol/L on cord blood or blood gas within 1 hour of birth OR (b) if no blood gas or if pH 7.01 to 7.15 or base deficit 10 to 15.9 mmol/L then additional criteria required: acute perinatal event (late or variable decelerations, cord prolapse, cord rupture, uterine rupture, maternal trauma, haemorrhage or cardiorespiratory arrest) AND either a 10‐minute Apgar score ≤ 5 or assisted ventilation initiated at birth and continued for at least 10 minutes. Criteria for encephalopathy included assessment of loss of consciousness, spontaneous activity, posture, primitive reflexes and autonomic nervous system abnormalities Excluded infants that were unable to be enrolled by 6 hours of age, had chromosomal or major congenital abnormalities, had growth restriction (birthweight ≤ 1800 g) or had consent refused by parent or neonatologist or who were moribund |
|
| Interventions | Hypothermia: 102 infants were placed on a pre‐cooled infant blanket (Blanketrol II Hyper‐Hypothermia System, Cincinnati Sub‐Zero) servo‐controlled to a target oesophageal temperature of 33.5 °C for 72 hours (25th and 75th percentiles at 33.2 °C and 33.5 °C). Re‐warming occurred by 0.5 °C per hour Standard care: 106 infants received standard care with skin temperature servo‐controlled to abdominal skin temperature 36.5 °C to 37 °C (25th and 75th percentiles at 36.9 °C and 37.5 °C) All infants had abdominal and oesophageal temperature monitoring |
|
| Outcomes | Primary outcome: composite of death or moderate/severe disability at 18 to 22 months according to Gross Motor Function Classification System (GMFCS), Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI) and Psychomotor Development Index (BSID PDI), aided hearing loss or presence of persistent seizures (Moderate disability ‐ BSID MDI 70 to 84 and at least 1 of: GMFCS 2, hearing impaired without amplification or persistent seizure disorder. Severe disability ‐ BSID MDI < 70, GMFCS 3 to 5, aided hearing loss or blindness)
Secondary outcomes: death, a composite of death or disability (among infants with moderate OR severe encephalopathy), survival, BSID MDI score, BSID PDI score, disabling cerebral palsy, blindness or severe hearing impairment Data collected on adverse events included those during the 72‐hour intervention (cardiac arrhythmia, persistent acidosis, major thrombosis or bleeding, skin changes, death) and those prior to hospital discharge (hypotension, persistent pulmonary hypertension, renal impairment, hepatic dysfunction, sepsis, hypoglycaemia, hypokalaemia, death, length of stay, feeding status and use of anticonvulsants at discharge) |
|
| Notes | Initial mean temperature of hypothermic infants on cooling was 32.7 °C. Several non‐cooled infants had elevation of body temperature > 38 °C Randomisation occurred at a mean 4.3 hours of age for all infants Of 62 deaths, 39 followed withdrawal of care (12/24 cooled, 27/38 standard care) Follow‐up reports included additional data on safety and effectiveness (Shankaran 2008) and magnetic resonance imaging (MRI) findings (Shankaran 2012). Secondary analysis reports included the evolution of encephalopathy during cooling (Shankaran 2012), predictors of death and disability (Ambalavanan 2006) including the 10‐minute Apgar (Laptook 2009), clinical seizures (Kwon 2010), and the effects of elevated temperature (Laptook 2008) and hypocarbia (Pappas 2010) on outcomes. Additionally, single‐centre studies using infants enrolled in the NICHD trial included data on volumetric MRI findings (Parikh 2008), prognostic utility of urinary lactate/creatinine ratio (Oh 2008) and follow‐up auditory assessments (Mietzsch 2008) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation by telephone by data‐coordinating centre, stratified by centre and generated by random, permuted block algorithm |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) long‐term outcomes | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete 18 to 22 months' follow‐up in 205/208 (98.6%) |
| Selective reporting (reporting bias) | Low risk | |
Shankaran 2002.
| Methods | Multicentre randomised controlled trial in the US | |
| Participants | Included 19 infants ≥ 36 weeks' gestation and < 6 hours of age with evidence of seizures or encephalopathy and either (a) pH ≤ 7.0 or base deficit ≥ 16 mmol/L on cord blood or blood gas within 1 hour of birth OR (b) if no blood gas or if pH 7.01 to 7.15 or base deficit 10 to 15.9 mmol/L then additional criteria required: acute perinatal event (late or variable decelerations, cord prolapse, cord rupture, uterine rupture, maternal trauma, haemorrhage or cardiorespiratory arrest) AND either a 10‐minute Apgar score ≤ 5 or assisted ventilation initiated at birth and continued for at least 10 minutes. Criteria for encephalopathy included assessment of loss of consciousness, spontaneous activity, posture, primitive reflexes and autonomic nervous system abnormalities Excluded infants unable to be enrolled by 6 hours of age, had chromosomal or major congenital abnormalities, had growth restriction (birthweight ≤ 1800 g) or had consent refused by parent or neonatologist |
|
| Interventions | Hypothermia: 9 infants were placed on a pre‐cooled infant blanket (Blanketrol II Hyper‐Hypothermia System, Cincinnati Sub‐Zero) servo‐controlled to a target oesophageal temperature of 34.5 °C for 72 hours. Re‐warming occurred by 0.5 °C per hour Standard care: 10 infants received standard care with skin temperature servo‐controlled to abdominal skin temperature 36.5 °C (oesophageal temperature 37.0 °C to 37.5 °C) All infants had abdominal and oesophageal temperature monitoring |
|
| Outcomes | Primary outcomes: temperature, heart rate and diastolic blood pressure during cooling as well as adverse events such as cardiac arrhythmia, persistent acidosis, major thrombosis or bleeding, skin changes and death Secondary outcomes: adverse events (hypotension, persistent pulmonary hypertension, renal failure, hepatic dysfunction, disseminated intravascular coagulation), data on hospital course (days on oxygen, length of stay) and discharge status (need for gavage feeds, abnormal neurological examination, seizures requiring anticonvulsants or abnormal magnetic resonance imaging) |
|
| Notes | Initial mean temperature of hypothermic infants on cooling was 32.9 °C Randomisation occurred at a mean 4.4 hours of age for cooled and 3.9 hours of age for control infants 2 deaths occurred in the hypothermia group, both following withdrawal of care. 3 deaths occurred in the control group, including 1 following withdrawal of care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation by telephone by a data co‐ordinating centre, stratified by centre and generated by random, permuted block algorithm |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) long‐term outcomes | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data complete |
| Selective reporting (reporting bias) | Low risk | |
TOBY Study 2009.
| Methods | Multicentre international randomised controlled trial | |
| Participants | Included 325 infants ≥ 36 weeks' gestation with an Apgar of < 5 or continued need for resuscitation at 10 minutes, a pH < 7 or base deficit ≥ 16 mmol/L within the 1st hour of life, and evidence of moderate‐to‐severe encephalopathy (lethargy, stupor or coma), and either hypotonia, abnormal reflexes, absent or weak suck, or clinical evidence of seizure. Additionally, infants had to have seizures or abnormal background for at least 3 minutes on amplitude‐integrated electroencephalogram (aEEG) | |
| Interventions | Hypothermia: 163 infants were cooled via cooling blanket (Tecotherm TS Med 200, TecCom) with a manually adjusted (non‐servo controlled) target rectal temperature of 33 °C to 34 °C (actual mean 33.5 °C). Re‐warming occurred by 0.5 °C per hour Standard care: 162 infants received standard care with skin temperature servo‐controlled to a target rectal temperature of 37 °C (actual mean 36.9 °C) All infants had continuous skin and rectal temperature monitoring. Uniform guidance provided on respiratory and circulatory care, fluid requirements, management of seizures and sedation |
|
| Outcomes | Primary outcome: composite of death or severe disability at 18 months (as determined by Gross Motor Function Classification System (GMFCS) level III to V, Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI) < 70, bilateral cortical visual impairment Secondary outcomes (at 18 months): death, severe disability at 18 months, survival without neurological abnormality, multiple neurodevelopmental disabilities, BSID MDI score, Bayley Scales of Infant Development ‐ Psychomotor Development Index (BSID PDI) score, GMFCS score, cerebral palsy, hearing loss, no useful vision, seizures requiring anticonvulsants or microcephaly Adverse events recorded included the presence of intracranial haemorrhage, persistent hypotension, pulmonary haemorrhage, persistent pulmonary hypertension, prolonged blood coagulation time, culture‐confirmed sepsis, necrotising enterocolitis, thrombocytopenia, major venous thrombosis, renal failure requiring dialysis, pneumonia, pulmonary air leak and duration of hospitalisation |
|
| Notes | Several non‐cooled infants had elevation of body temperature > 38 °C Randomisation occurred at a mean 4.7 hours of age for all infants Of 86 deaths, 63 followed withdrawal of care (34/39 (87%) in cooled, 29/39 (74%) in standard care) A follow‐up secondary analysis of the relationship of hypothermia to cerebral lesions on magnetic resonance imaging (Rutherford 2010) was submitted. Additionally, single‐centre studies using infants enrolled in the TOBY trial included data on laboratory indicators of organ dysfunction (Roka 2010) and oxidative stress (Perrone 2010) during hypothermia as well as elevated serum morphine concentrations during hypothermia (Roka 2008) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By telephone from data co‐ordinating centre or via web‐based system. Minimisation used to ensure balance by centre and degree of aEEG abnormality |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: not possible. Uniform guidelines for care provided to minimise potential confounding |
| Blinding of outcome assessment (detection bias) long‐term outcomes | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Both short‐term and follow‐up outcome data complete |
| Selective reporting (reporting bias) | Low risk | |
Zhou 2010.
| Methods | Multicentre randomised controlled trial in China | |
| Participants | Included 194 infants ≥ 37 weeks' gestation and bodyweight ≥ 2.5 kg, admitted to neonatal intensive care unit (NICU) within 6 hours with clinical evidence of exposure to perinatal hypoxic ischaemia (Apgar score of ≤ 3 at 1 minute and or < 5 at 5 minute, cord pH < 7 or base deficit ≥ 16 mmol/L, or a need for resuscitation or ventilation at 5 minutes) or a diagnosis of encephalopathy (mild, moderate or severe) Excluded infants with major congenital anomalies, infection, other encephalopathy (neonatal stroke, central nervous system abnormality, intracranial haemorrhage), or severe anaemia |
|
| Interventions | Hypothermia: 100 infants underwent head cooling (YJW608‐04B, Henyang Radio Manufactory) for 72 hours, with target a nasopharyngeal temperature of 34.0 °C, with additional use of a radiant warmer to target a rectal temperature of 34.5 °C to 35 °C. Following cooling, infants underwent spontaneous re‐warming Standard care: 94 infants cared for in servo‐controlled radiant warmers with rectal temperature target 36 °C to 37.5 °C. Laboratory and other monitoring parameters identical for both groups |
|
| Outcomes | Primary outcome: composite of death or severe disability (Gessell Child Development Age Scale, developmental quotient (DQ) < 70, Gross Motor Function Classification System (GMFCS) score 3 to 5), death or severe disability at 18 months Secondary outcomes: death or survival with severe disability in infants with moderate‐to‐severe, moderate, or severe hypoxic ischaemic encephalopathy, DQ Adverse events recorded included major (severe arrhythmia, major venous thrombosis, refractory hypotension, moderate or severe scleroedema, severe bleeding, scleroedema) and minor (mild arrhythmia, mild scleroedema, renal dysfunction, liver dysfunction, thrombocytopenia, serum electrolyte or biochemical abnormalities) |
|
| Notes | 6 infants treated with hypothermia and 5 controls had a temperature > 38 °C Onset of treatment occurred at a mean 4.1 hours of age for cooled infants and 4.0 hours of age for controls 20/100 infants in the hypothermia group and 27/94 of controls died. It is unclear whether these deaths were following withdrawal of care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By numbered, sealed envelopes containing random computer‐generated numbers Stratified by centre in blocks of 6 |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: not possible. Uniform guidelines for laboratory and other monitoring provided |
| Blinding of outcome assessment (detection bias) long‐term outcomes | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcome data (both short and long term) incomplete on 16% of those cooled and 22% of those who received standard care. Of those infants for whom short‐term data were reported, follow‐up data were complete (93% of cooled and 94% of control infants followed up, including some infants whose follow‐up was by telephone or at local paediatrician) |
| Selective reporting (reporting bias) | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Araki 2010 | Case series (without controls) |
| Azzopardi 2000 | Case series (without controls) |
| Christensen 2012 | Case series (without controls) |
| Compagnoni 2002 | Retrospective cohort study with historical controls |
| Compagnoni 2008 | Retrospective cohort study with historical controls. Included deep hypothermia (33 °C) |
| Debillon 2003 | Case series (without controls) |
| Filippi 2009 | Case series (without controls) |
| Filippi 2010 | Case series (without controls) |
| Filippi 2011 | Case series (without controls) |
| Gucuyener 2012 | Case series (without controls) |
| Hamelin 2011 | Retrospective cohort study with historical controls |
| Horan 2004 | Non‐randomised prospective study employing cooling via extracorporeal membrane oxygenation (ECMO), cohorts treated at sequentially decreasing temperatures. Did not meet pre‐specified inclusion criteria for presence of perinatal asphyxia or encephalopathy |
| Horn 2006 | Feasibility study using solid ice cap, halted after 4 patients cooled, technical data only |
| Ichiba 2003 | Non‐randomised prospective pilot study employing cooling via extracorporeal membrane oxygenation (ECMO), cohorts treated at sequentially decreasing temperatures. Did not meet pre‐specified inclusion criteria for presence of perinatal asphyxia or encephalopathy |
| Inder 2004 | Randomised controlled trial, outcomes included magnetic resonance imaging findings but not mortality, neurodevelopmental disability or adverse events |
| Kendall 2010 | Case series (without controls) |
| Kilani 2002 | Retrospective cohort study with historical controls |
| Li 2009 | Method of allocation not able to be determined, although described as 'random assignment'. Excluded as did not meet modified inclusion criteria (hypothermia initiated at up to 10 hours of life) |
| Lista 2004 | Retrospective cohort study with historical controls |
| Liu 2010 | Randomised controlled trial, but outcomes did not include data on pre‐specified outcomes |
| Massaro 2010 | Case series (without controls) |
| Meyn 2010 | Case series (without controls) |
| Robertson 2008 | Randomised controlled trial of whole body cooling using water bottles. Outcomes included rectal temperature during cooling period, neurological assessment up to day 17, seizures and death before discharge. Not included in analysis because study inclusion criteria do not meet the pre‐defined definition of peripartum asphyxia |
| Rutherford 2005 | Infants enrolled from composite of TOBY and CoolCap and several pilot studies. Outcomes included magnetic resonance imaging findings but not mortality or neurodevelopmental disability. Excluded from review as it included infants from multiple randomised controlled trials (with previously reported data) and multiple modalities of cooling |
| Róka 2007 | Open cohort series with historical controls |
| Simbruner 1999 | Retrospective cohort study with historical controls |
| Thomas 2011 | Case series (without controls) |
| Thoresen 2000 | Case series (without controls) |
| Thoresen 2010 | Single‐centre randomised controlled trial (RCT), included infants from multiple international RCTs (CoolCap, TOBY) as well as additional infants. Outcomes included use of baseline amplitude‐integrated electroencephalogram to predict mortality and neurodevelopmental outcomes in normothermic and hypothermic infants. Mortality and the composite outcome of death or severe disability were reported. Excluded from review as it included infants from multiple RCTs (with previously reported data) and multiple modalities of cooling |
| Tusor 2012 | Case series (without controls) |
| Wusthoff 2011 | Case series (without controls) |
| Zhou 2002 | Safety and efficacy study of selective head cooling. Method of allocation not able to be determined, although described as 'random assignment'. No pre‐defined outcomes for this review were reported |
| Zhou 2003 | Study of the effects of selective head cooling on cardiac function. Method of allocation not able to be determined, although described as 'randomly divided' |
Characteristics of studies awaiting assessment [ordered by study ID]
Bharadwaj 2012.
| Methods | Single‐centre randomised controlled trial in India Blinding of randomisation: not specified Blinding of intervention: not possible Blinding of outcome measurement: not specified Follow‐up: reported to 6 months of age. Loss to follow‐up not reported in abstract |
| Participants | Included infants with hypoxic ischaemic encephalopathy (criteria not specified in abstract). Other criteria (gestational age, age at enrolment, criteria for peripartum asphyxia) not stated and exclusion criteria not specified in abstract |
| Interventions | Hypothermia: infants underwent whole body cooling via cooling gel packs for target rectal temperature of rectal temperature 33 °C to 34 °C for 72 hours. Re‐warming protocol not specified Control treatment: standard care, not specified. Number of infants included not stated |
| Outcomes | Primary outcome was death or developmental delay at 6 months. Other outcomes reported not specified in abstract |
| Notes |
Bhat 2006.
| Methods | Single‐centre randomised controlled trial in India Blinding of randomisation: not specified Blinding of intervention: not possible Blinding of outcome measurement: not specified Follow‐up: complete to discharge |
| Participants | Included 35 infants with severe perinatal asphyxia (criteria not specified in letter). Other criteria (gestational age, age at enrolment, criteria for peripartum asphyxia) not stated and exclusion criteria not specified in letter |
| Interventions | Hypothermia: 20 Infants underwent whole body cooling (method not stated) with a target rectal temperature of rectal temperature 33.5 °C for 72 hours. Re‐warming protocol not specified Control treatment: 15 infants received standard care, treatment not specified |
| Outcomes | Primary outcome was death or abnormal neurological examination at time of discharge. Other outcomes reported not specified in abstract |
| Notes |
Sun 2012.
| Methods | Single‐centre randomised controlled trial in China Most likely a subset of trial of Zhou 2010 |
| Participants | Included 51 infants ≥ 37 weeks with a birthweight of ≥ 2.5 kg, admitted to the neonatal intensive care unit within 6 hours with clinical evidence of exposure to perinatal hypoxic ischaemia (Apgar score ≤ 3 at 1 minute and ≤ 5 at < 5 minutes, cord pH < 7 or base deficit ≥ 16 mmol/L, need for resuscitation or ventilation at 5 minutes, or a combination) or a diagnosis of encephalopathy (mild, moderate or severe). Excluded infants with major congenital abnormalities, intracranial haemorrhage and severe anaemia |
| Interventions | Hypothermia: 23 infants were treated via selective head cooling (YJW608‐04B, Henyang Radio Manufactory) for 72 hours, with target nasopharyngeal temperature of 34.0 °C, with additional use of a radiant warmer to target a rectal temperature of 34.5 °C to 35 °C. Re‐warming occurred over 8 hours Control treatment: 28 infants cared for in servo‐controlled radiant warmers with rectal temperature target 36 °C to 37.5 °C |
| Outcomes | Outcomes included cerebrospinal fluid levels of neuron‐specific enolase, S‐100 and amino acid neurotransmitters as well as Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI) < 70 and Bayley Scales of Infant Development ‐ Psychomotor Development Index (BSID PDI) < 70 at 12 months |
| Notes | Most likely a subset of trial of Zhou 2010. This needs to be confirmed 1 control infant died, unclear whether death was following withdrawal of support Age at treatment onset 4.1 hours of life (hypothermia) and 4.0 hours of life (controls) Blinding of assessment at follow‐up unclear |
Thayyil 2010.
| Methods | Single‐centre randomised controlled trial in India Blinding of randomisation: not specified Blinding of intervention: not possible Blinding of outcome measurement: not specified Follow‐up: complete to discharge |
| Participants | Included 21 newborn infants with neonatal encephalopathy (Thompson score < 5). Other criteria (gestational age, age at enrolment, criteria for peripartum asphyxia) not stated. Exclusion criteria not specified |
| Interventions | Hypothermia: 11 Infants underwent whole body cooling via a mattress containing phase‐changing material for target rectal temperature of rectal temperature 33 °C to 34 ° C for 72 hours. Re‐warming protocol not specified Control treatment: 10 infants received standard care, treatment not specified |
| Outcomes | Primary and secondary outcomes not specified. Outcomes reported included: temperature, time to cooling and re‐warming, number of blanket changes required, heart rate, respiratory rate, blood pressure, platelet counts, C‐reactive protein, liver enzymes or coagulation profile and mortality |
| Notes | Excluded as detail on methods and results insufficient to warrant inclusion |
Characteristics of ongoing studies [ordered by study ID]
CoolXenon Study.
| Trial name or title | Xenon and Cooling Therapy in Babies at High Risk of Brain Injury Following Poor Condition at Birth: Randomised Pilot Study (The CoolXenon2 Study) |
| Methods | Randomised controlled single‐centre pilot study in UK |
| Participants | Includes: infants born at ≥ 36 weeks' gestation WITH clinical evidence of peripartum hypoxia‐ischaemia (Apgar score ≤ 5 at 10 minutes, continued need for resuscitation at 10 minutes, or severe acidosis (pH < 7 or base deficit ≥ 16 mmol/L in cord blood or arterial/venous blood within 60 minutes of birth)) AND abnormal amplitude‐integrated electroencephalogram background AND moderate or severe encephalopathy (Sarnat criteria) with 1 of hypotonia, abnormal reflexes, absent or weak suck, clinical seizures, or a combination. For xenon therapy, infants must be intubated with normal partial pressure of CO2 (pCO2), a positive end‐expiratory pressure of < 6 cmH2O and fraction of inspired oxygen (FiO2) < 40%, seizures under control, be > 2.3 kg in weight and < 5 hours old, have a birthweight greater than the 2nd percentile for age, have no major congenital anomalies and be haemodynamically stable with no evidence of infection Excludes: infants considered futile, infants not meeting above criteria |
| Interventions | All infants cooled using whole body hypothermia for 72 hours at 33.5 °C. Enrolled infants randomised to 50% Xenon inhalation for 18 hours (using a closed loop xenon‐delivery system (cooling protocol not specified) or to standard cooling therapy |
| Outcomes | Primary outcome: physiological changes during and within 24 hours after end treatment Secondary outcomes: Bayley III, measured at 18 or 24 months, magnetic resonance imaging within 14 days after treatment |
| Starting date | March 2010 |
| Contact information | Marianne Thoresen, M.D. |
| Notes | Currently recruiting, estimated completion April 2014 with 24 patients anticipated. Follows non‐randomised single‐centre CoolXenon study. All infants cooled |
DANCE.
| Trial name or title | Darbe Administration in Newborns Undergoing Cooling for Encephalopathy (DANCE trial) |
| Methods | Randomised controlled trial |
| Participants | Includes: infants > 36 weeks' gestation and < 12 hours old with evidence of moderate‐to‐severe perinatal hypoxic ischaemic encephalopathy and evidence of an acute perinatal event and either (a) pH ≤ 7.0 or base deficit ≥ 16 mmol/L on cord blood or blood gas within 1 hour of birth OR (b) if no blood gas or if pH 7.01 to 7.15 or base deficit 10 to 15.9 mmol/L then additional criteria required: acute perinatal event AND either a 10‐minute Apgar score ≤ 5 or assisted ventilation initiated at birth and continued for at least 10 minutes Excludes: infants with chromosomal or major congenital abnormalities, had growth restriction (birthweight ≤ 1800 g), had a central venous haematocrit > 65%, platelet count > 600,000/dL, neutropenia (absolute neutrophil count < 500 µL), had a maternal history of major vascular thrombosis or multiple fetal losses, or were receiving extracorporeal membrane oxygenation (ECMO), had a core temperature < 33.5 °C for > 1 hour prior to screening, or were determined to be critically ill and unlikely to benefit from intensive care by the attending neonatologist |
| Interventions | All infants undergoing cooling, hypothermia protocol not specified. Enrolled infants will be randomised to receive either: a) high‐dose darbepoetin (10 µg/kg/dose), b) low‐dose darbepoetin (2 µg/kg/dose) or c) placebo. All infants were given 2 doses of Darbe or placebo, with the first dose within 12 hours of delivery and the second dose at 7 days |
| Outcomes | Primary outcomes: presence of adverse events such as alterations in blood pressure, secondary infections, neutropenia, thrombotic/vascular events, haematological events (platelets, haematocrit level, polycythaemia), and hepatic/renal dysfunction Secondary outcomes: pharmacokinetic profile of darbepoetin |
| Starting date | June 2012 |
| Contact information | Mariana Baserga, M.D. |
| Notes | Not yet recruiting patients. Estimated completion March 2014 with 45 patients anticipated. Phase I/II dose safety and pharmacokinetic trial. All infants to be cooled |
Lichter‐Konecki.
| Trial name or title | Hypothermia Treatment in Hyperammonemia and Encephalopathy |
| Methods | Non‐randomised safety study, cohort study with historic controls |
| Participants | Includes: infants > 36 weeks' gestation and ≥ 2200 g birthweight who are up to 2 months of age with clinical signs and symptoms of a urea cycle disorder or propionic, methylmalonic, or isovaleric acidaemia and hyperammonaemia and encephalopathy requiring renal replacement therapy Excludes: hyperammonaemia due to other disorders (lysinuric protein intolerance, mitochondrial disorders, congenital lactic acidosis, and fatty acid oxidation disorders), unrelated serious co‐morbidities, genetic disease, intraventricular haemorrhage, traumatic brain injury, low birthweight (< 2200 g at > 36 weeks' gestation) and infants in extremis |
| Interventions | All enrolled infants will be cooled to 33.5 °C (± 1 °C ) for 72 hours and then re‐warmed by 0.5 °C every 3 hours over 18 hours. Patients will also receive standard of care therapy (renal replacement). Historic controls will also receive renal replacement therapy |
| Outcomes | Primary outcomes: presence of unexpected serious adverse events, feasibility Secondary outcome: time to normalisation of ammonia level |
| Starting date | August 2007 |
| Contact information | Uta Lichter‐Konecki, M.D., Ph.D. |
| Notes | Currently recruiting, estimated enrolment 24 patients, estimated completion July 2015 |
NeoNATI.
| Trial name or title | Safety and Efficacy of Oral Topiramate in Neonates With Hypoxic Ischemic Encephalopathy Treated With Hypothermia: a Pilot Study of the Neonatal Neuroprotection of Asphyxiated Tuscan Infants (NeoNATI) Network |
| Methods | Randomised controlled trial |
| Participants | Includes: infants > 36 weeks' gestation and birthweight > 1800 g with evidence of asphyxia (at least 1 of: Apgar score < 5 at 10 minutes, need for resuscitation at 10 minutes after birth, acidosis (pH < 7.0, base deficit > 16 mmol/L within 60 minutes from birth), moderate‐to‐severe encephalopathy (altered state of consciousness (irritability, lethargy, stupor or coma) and > 1 of: hypotonia; abnormal reflexes, including oculomotor or pupil abnormalities; absent or weak suck, clinical seizures), and an abnormal amplitude‐integrated electroencephalogram. Excludes: infants with congenital abnormalities, congenital viral infections or evidence encephalopathy other than hypoxic ischaemic encephalopathy |
| Interventions | All infants will undergo hypothermia. Enrolled infants randomised either to a treatment arm, receiving topiramate (10 mg/kg) once daily from the time of initiation of cooling and for 3 doses, or to standard care. Hypothermia protocol not specified |
| Outcomes | Primary outcome: neurological outcome at 6, 12 and 18 months of life Secondary outcomes: neuroradiological outcome at 3 and 12 months of life |
| Starting date | February 2010 |
| Contact information | Luca Filippi, M.D. |
| Notes | Currently recruiting, estimated enrolment 60 patients, estimated completion August 2013 |
NEST Study.
| Trial name or title | Assessing the Neuro‐protective Effect of Mild Cooling in Neonates Receiving Extracorporeal Membrane Oxygenation (ECMO): a Randomised Controlled Trial (NEST Study) |
| Methods | Randomised controlled trial |
| Participants | Includes: infants of at least 35 weeks' gestation and 2000 g birthweight who are < 29 days of age at recruitment and who meet existing standard criteria for ECMO eligibility (evidence of severe cardiorespiratory failure, suffering from a condition that is potentially reversible, no more than 7 consecutive days of high‐pressure ventilation prior to referral for ECMO) Excludes: infants cooled prior to ECMO, those requiring ECMO for postoperative cardiac support, and those with an uncontrolled bleeding disorder, a congenital or acquired central nervous system disorder, or a congenital diaphragmatic hernia |
| Interventions | Infants receiving ECMO randomised to standard ECMO or ECMO with mild cooling |
| Outcomes | Primary outcome: cognitive score from the Bayley scales of Infant and toddler Development, 3rd edition (Bayley‐III) at age of 2 years (24 to 27 months) Secondary outcomes: death and multiple metrics of neurological dysfunction |
| Starting date | January 2005 |
| Contact information | David Field, M.D. |
| Notes | Excludes infants with hypoxic ischaemic encephalopathy (cooling is for prevention of ECMO‐associated morbidity). Completed, in follow‐up phase |
NICHD: Late Hypothermia.
| Trial name or title | Evaluation of Systemic Hypothermia Initiated After 6 Hours of Age in Infants ≥36 Weeks Gestation With Hypoxic‐Ischemic Encephalopathy: A Bayesian Evaluation. A Protocol for the NICHD Neonatal Research Network |
| Methods | Multicentre randomised controlled trial |
| Participants | Incudes: infants meeting National Institute of Child Health and Human Development (NICHD) 2005 criteria for encephalopathy and perinatal asphyxia enrolled between 6 and 24 hours of age. Excludes: chromosomal or major congenital abnormalities, birthweight ≤ 1800 g, moribund infants, core temp < 34 °C for > 1 hour prior to screening or consent refused by parent or neonatologist |
| Interventions | Enrolled infants are randomised to either a hypothermia (with a target oesophageal temperature of 33.5 °C using pre‐cooled infant blanket) or control (37.0 °C) for 96 hours. Temperature monitoring and re‐warming as per NICHD 2005 protocol |
| Outcomes | Primary outcome: death or moderate or severe disability at 18 to 24 months Secondary outcomes: death, mild, moderate and severe disability, non‐central nervous system organ system dysfunction, presence of a do not resuscitate (DNR) order with or without withdrawal of support, presence of neonatal seizures, with and without EEG abnormalities |
| Starting date | April 2008 |
| Contact information | Abbot R. Laptook, M.D. and Rosemary D. Higgins, M.D. |
| Notes | Currently recruiting, enrolment of 168 subjects anticipated, anticipated completion March 2014 |
NICHD: Optimizing Cooling.
| Trial name or title | Optimizing Cooling Strategies at < 6 hours of Age for Neonatal Hypoxic‐Ischemic Encephalopathy |
| Methods | Multicentre randomised controlled trial |
| Participants | Incudes: infants ≥ 36 weeks' gestation < 6 hours of age with evidence of encephalopathy and either (a) pH ≤ 7.0 or base deficit ≥ 16 mmol/L on cord blood or blood gas within 1 hour of birth OR (b) if no blood gas or if pH 7.01 to 7.15 or base deficit 10 to 15.9 mmol/L then additional criteria required: acute perinatal event AND either a 10‐minute Apgar score ≤ 5 or assisted ventilation initiated at birth and continued for at least 10 minutes. Criteria for encephalopathy included assessment of loss of consciousness, spontaneous activity, posture, tone, primitive reflexes, and autonomic nervous system abnormalities. Excludes: unable to be enrolled by 6 hours of age, presence of chromosomal or major congenital abnormalities, presence of growth restriction (birthweight ≤ 1800 g), moribund infants, those with a core temp < 33.5 °C for > 1 hour prior to screening, or consent refused by parent or neonatologist |
| Interventions | Enrolled infants placed in 1 of 4 cooling groups using a cooling blanket: (1) cooling for 72 hours to 33.5 °C; (2) cooling for 120 hours to 33.5 °C; (3) cooling for 72 hours to 32.0 °C or (4) cooling for 120 hours to 32.0 °C, all followed by slow re‐warming. Temperatures monitored by both oesophageal and skin probes. Infants will be examined at 18 to 22 months corrected age to assess their neurodevelopmental outcomes |
| Outcomes | Primary outcome: death or moderate‐to‐severe disability at 18 to 22 months Secondary outcomes: death; mild, moderate and severe disability; withdrawal of care; acute adverse events; clinical neonatal seizures; severe neonatal brain abnormalities; magnetic resonance imaging between 7 and 14 days; cognitive outcome; cerebral palsy; visual impairment; hearing impairment; multi‐organ dysfunction |
| Starting date | September 2010 |
| Contact information | Seetha Shankaran, M.D. and Rosemary D. Higgins, M.D. |
| Notes | Currently recruiting, estimated enrolment 726 subjects, anticipated completion 2017. All infants cooled |
TOBYXe.
| Trial name or title | Neuroprotective Effects of Hypothermia Combined With Inhaled Xenon Following Perinatal Asphyxia |
| Methods | Randomised controlled trial |
| Participants | Includes: infants 36 to 43 weeks' gestation with at least 1 of: Apgar score of < 5 at 10 minutes, continued need for resuscitation at 10 minutes, a pH < 7 or base deficit ≥ 16 within the first hour of life, and evidence of moderate‐to‐severe encephalopathy (lethargy, stupor or coma), and hypotonia or abnormal primitive reflexes. Additionally, infants had to have abnormal or suppressed background or seizures for at least 3 minutes on amplitude‐integrated electroencephalogram Excludes: initiation of hypothermia after 6 hours, randomisation after 12 hours of age, oxygen requirement > 70%, presence of other serious congenital abnormalities or the infant's condition appears terminal |
| Interventions | Enrolled infants randomised to either a treatment arm with 30% inhaled xenon for 24 hours or a control arm receiving standard care for hypoxic ischaemic encephalopathy. All infants receive hypothermia as well as standard intensive care |
| Outcomes | Primary outcome: reduction in lactate/N‐acetylaspartate (Lac/NAA) ratio on magnetic resonance spectroscopy or preserved fractional anisotropy measured on diffusion weighted magnetic resonance imaging Secondary outcome: clinical outcomes at hospital discharge |
| Starting date | February 2012 |
| Contact information | Denis Azzopardi, M.D. |
| Notes | Currently recruiting, estimated enrolment 130 subjects, anticipated completion 2013. All infants cooled |
Walsh: Thermal Imaging.
| Trial name or title | MRI Thermal Imaging of Infants Undergoing Cooling for HIE |
| Methods | Non‐randomised observational study |
| Participants | Includes: infants in the neonatal intensive care unit who are treated with hypothermia for hypoxic ischaemic encephalopathy and who are scheduled to have magnetic resonance imaging (MRI) for evaluation of the extent of their injury Excludes: infants too unstable to have MRI scan (on cardiac pressor medications or more than 40% oxygen) or too active to obtain MRI without sedation |
| Interventions | Enrolled infants will undergo MRI evaluation of the N‐acetylaspartate (NAA)‐H20 frequency shift (for measuring relative temperature changes) from at least 5 regions of the brain during cooling and again after re‐warming |
| Outcomes | Primary outcome: brain temperature during cooling Secondary outcome: brain temperature on re‐warming Uniformity and patterns of temperature will be analysed and variations by modality of cooling will also be explored. MRI findings (temperature distribution) will also be compared to the MRI injury patterns and infant outcomes in order to determine if distribution of cooling is related to outcome |
| Starting date | May 2010 |
| Contact information | William F Walsh, M.D. |
| Notes | Currently recruiting, estimated enrolment 10 subjects, anticipated completion 2013. All infants cooled |
Walsh: Preterm Infants.
| Trial name or title | Pilot Study of Head Cooling in Preterm Infants With Hypoxic Ischemic Encephalopathy |
| Methods | Non‐randomised feasibility study |
| Participants | Includes: intubated infants 32 0/7‐35 6/7 weeks who are < 6 hours old gestation meeting criteria for hypoxic ischaemic encephalopathy (HIE) (Apgar 0 to 3 at 1, 5 and 10 minutes, pH < 7.0, base deficit > 15, with or without need for continued resuscitation due to hypoxia at 10 minutes, AND a physical examination with evidence of hypotonia or lethargy or seizures indicative of evolving HIE) Excludes: infants in extremis on clinical examination or survival not expected, evidence of head trauma or skull fracture causing major intracranial haemorrhage, intraventricular haemorrhage, weight less than the 5th percentile for gestational age, imperforate anus, refusal of consent |
| Interventions | Enrolled infants to undergo hypothermia via Olympic Cool Cap for up to 72 hours, with body temperature maintained in the normal range (36.1 °C to 37 °C rectally). Infants tracked until discharge with follow‐up at 6, 12 and 24 months of age |
| Outcomes | Primary outcome: measurement of rectal temperature in relation to cap temperature |
| Starting date | February 2008 |
| Contact information | William F Walsh, M.D. |
| Notes | Currently recruiting, estimated enrolment 5 subjects, anticipated completion 2013. Feasibility study, all infants cooled |
Differences between protocol and review
Modifications were made to the protocol in both the Objectives and Methods. For the Objectives, the inclusion criteria were modified to exclude infants < 35 weeks' gestation and cooling initiated after six hours. These modifications were made in order to streamline the review as data emerge on cooling in infants < 35 weeks' gestation and who undergo later cooling and to improve generalisability of results. As such, the Objectives were modified to remove the category "Inclusion criteria: term or late preterm (≥ 35 weeks' gestation) infants versus more preterm (< 35 weeks' gestation)" and the Methods section "Types of participants" was modified to include infants "35 weeks' gestation or greater." It is well‐described in animal models that the effectiveness of hypothermia is dramatically reduced when initiated after six hours of life (Gunn 1998), although studies are emerging (Li 2009) exploring the use of late hypothermia. As data emerge in this field, it is felt that this will warrant a separate review. Therefore, the Objectives were modified to remove the category "Timing of commencement of intervention (< three hours versus three to six hours versus more than six hours)". The Methods section "Types of interventions" was modified to include cooling initiated prior to six hours after birth. Given that infants undergoing head cooling with mild systemic hypothermia may have relatively higher core temperatures but lower intranasal and scalp temperatures than infants treated with whole body cooling, it is difficult to compare temperatures in these treatment modalities in a meaningful way. Therefore, the Objectives were modified to remove "Degree of cooling (core temperature ≤ 34.5 °C versus > 34.5 °C)." In addition, Objective 1 (Severity of HIE) was modified to include both clinical staging of encephalopathy and staging based on baseline aEEG findings.
Additional changes to the Methods included clarification of definitions for secondary outcomes, additions to secondary outcomes and changes in the pre‐specified outcomes reported. Definitions were clarified for "Any coagulopathy" and "Renal impairment." The outcome "Seizure" was modified to include both seizures during the initial hospitalisation and at follow‐up. Three additional outcomes were added, as they were felt to be clinically important. These were 'outcome at six to seven years,' 'hepatic dysfunction,' and 'persistent pulmonary hypertension,' which also included the outcome 'requiring inhaled NO. For 'outcome at six to seven years,' both mortality and multiple neurodevelopmental findings are reported. Additionally, the outcome 'days to sucking feeds' was modified to the more binary 'need for gavage feeds at time of discharge. The outcome 'diffusion weighted imaging (DWI) on early MRI (< day 4)' and 'basal ganglia, posterior limb of internal capsule (PLIC) and/or white matter (WM) injury, parasagittal neuronal necrosis on late MRI (> day 4) were replaced with 'MRI abnormalities (moderate or severe abnormalities in the basal ganglia or thalamus, severe white matter lesions or abnormalities in the posterior limb of the internal capsule).'
Contributions of authors
Sue Jacobs: responsible for all aspects of review ‐ search, data abstraction, entry and analysis; manuscript and editing of review.
Rod Hunt: data abstraction and reviewed manuscript.
William Tarnow‐Mordi: reviewed manuscript.
Terrie Inder: reviewed manuscript.
Peter Davis: data abstraction and entry, manuscript preparation.
2012 update:
New contributor: Marie Berg: updated methods and objectives, identified new studies for inclusion, abstracted and entered data from these new studies, drafted new results and discussion based on added information.
Sue Jacobs: identified new studies for inclusion, abstracted and provided additional data, provided commentary and critical review of manuscript.
Rod Hunt: data abstraction and critical review of manuscript.
Peter Davis: data abstraction and critical review of manuscript.
William Tarnow‐Mordi: commentary and critical review of manuscript and data.
Terrie Inder: reviewed manuscript and provided commentary.
Sources of support
Internal sources
Neonatal Services, Royal Women's Hospital, Melbourne, Australia.
Departments of Pediatrics, Neurology and Radiology, St Louis Childrens Hospital, University of Washington, USA.
Westmead Hospital, Sydney, Australia.
Department of Obstetrics and Gynaecology, University of Melbourne, Australia.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
Declarations of interest
Dr Sue Jacobs is the principal investigator for one of the included randomised controlled trials, the Infant Cooling Evaluation (ICE) trial.
Edited (no change to conclusions)
References
References to studies included in this review
Akisu 2003 {published data only}
- Akisu M, Huseyinov A, Yalaz M, Cetin H, Kultursay N. Selective head cooling with hypothermia suppresses the generation of platelet‐activating factor in cerebrospinal fluid of newborn infants with perinatal asphyxia. Prostaglandins, Leukotrienes and Essential Fatty Acids 2003;69(1):45‐50. [DOI] [PubMed] [Google Scholar]
Cool Cap Study 2005 {published and unpublished data}
- Battin MR, Thoresen M, Robinson E, Polin RA, Edwards AD, Gunn AJ on behalf of the CoolCap Study Group. Does head cooling with mild systemic hypothermia affect requirement for blood pressure support?. Pediatrics 2009;123(3):1031‐6. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365(9460):663‐70. [DOI] [PubMed] [Google Scholar]
- Guillet R, Edwards AD, Thoresen M, Ferriero DM, Gluckman PD, Whitelaw A, et al on behalf of the CoolCap Trial Group. Seven‐to‐eight‐year follow‐up of the CoolCap trial of head cooling for neonatal encephalopathy. Pediatric Research 2012;71(2):205‐9. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Wyatt JS, Whitelaw A, Barks J, Azzopardi D, Ballard R, et al on behalf of the CoolCap Study Group. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. Journal of Pediatrics 2008;152(1):55‐8. [DOI] [PubMed] [Google Scholar]
- Wyatt JS, Gluckman PD, Liu PY, Azzopardi D, Ballard R, Edwards AD, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 2007;119(5):912‐21. [DOI] [PubMed] [Google Scholar]
Eicher 2005 {published and unpublished data}
- Eicher D, Wagner C, Katikaneni L, Hulsey T, Bass T, Kaufman D, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatric Neurology 2005;32(1):11‐7. [DOI] [PubMed] [Google Scholar]
- Eicher D, Wagner C, Katikaneni L, Hulsey T, Bass T, Kaufman D, et al. Moderate hypothermia in neonatal encephalopathy: safety outcomes. Pediatric Neurology 2005;32(1):18‐24. [DOI] [PubMed] [Google Scholar]
Gunn 1998 {published data only}
- Battin MR, Dezoete JA, Gunn TR, Gluckman PD, Gunn AJ. Neurodevelopmental outcome of infants treated with head cooling and mild hypothermia after perinatal asphyxia. Pediatrics 2001;107(3):480‐4. [DOI] [PubMed] [Google Scholar]
- Battin MR, Penrice J, Gunn TR, Gunn AJ. Treatment of term infants with head cooling and mild systemic hypothermia (35.0 degrees C and 34.5 degrees C) after perinatal asphyxia. Pediatrics 2003;111(2):244‐51. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Gluckman PD, Gunn TR. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics 1998;102(4 Pt 1):885‐92. [DOI] [PubMed] [Google Scholar]
ICE Study 2011 {published and unpublished data}
- Cheong JL, Coleman L, Hunt RW, Lee KJ, Doyle LW, Inder TE, et al. Prognostic utility of magnetic resonance imaging in neonatal hypoxic‐ischemic encephalopathy: substudy of a randomized trial. Archives of Pediatric and Adolescent Medicine 2012;166(7):634‐40. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Stewart M, Inder T, Doyle L, Morley C. Feasibility of a pragmatic randomised controlled trial of whole body cooling for term newborns with hypoxic‐ischaemic encephalopathy. Hot Topics in Neonatology. 2002.
- Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, et al. Whole‐body hypothermia for term and near‐term newborns with hypoxic‐ischemic encephalopathy: a randomized controlled trial. Archives of Pediatrics and Adolescent Medicine 2011;165(8):692‐700. [DOI] [PubMed] [Google Scholar]
Lin 2006 {published data only}
- Lin Z, Yu H, Lin J, Chen S, Liang Z, Zhang Z. Mild hypothermia via selective head cooling as neuroprotective therapy in term neonates with perinatal asphyxia: an experience from a single neonatal intensive care unit. Journal of Perinatology 2006;26(3):180‐4. [DOI] [PubMed] [Google Scholar]
neo.nEURO Study 2010 {published data only}
- Simbruner G, Mittal RA, Rohlmann F, Muche R, neo.nEURO.network Trial Participants. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics 2010;126(4):e771‐8. [DOI] [PubMed] [Google Scholar]
NICHD Study 2005 {published and unpublished data}
- Kwon JM, Guillet R, Shankaran S, Laptook AR, McDonald SA, Ehrenkranz RA, et al. Clinical seizures in neonatal hypoxic‐ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analysis of data from the Neonatal Research Network Hypothermia Trial. Journal of Child Neurology 2010;26(3):322‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laptook A, Tyson J, Shankaran S, McDonald S, Ehrenkranz R, Fanaroff A, et al. Elevated temperature after hypoxic‐ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics 2008;122(3):491‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laptook AR, Shankaran S, Ambalavanan N, Carlo WA, McDonald SA, Higgins RD, et al. Outcome of term infants using Apgar scores at 10 minutes following hypoxic‐ischemic encephalopathy. Pediatrics 2009;124(5):1619‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzsch U, Parikh NA, Williams AL, Shankaran S, Lasky RE. Effects of hypoxic‐ischemic encephalopathy and whole‐body hypothermia on neonatal auditory function: a pilot study. American Journal of Perinatology 2008;25(7):435‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh W, Perritt R, Shankaran S, Merritts M, Donovan EF, Ehrenkranz RA, et al. Association between urinary lactate to creatinine ratio and neurodevelopmental outcome in term infants with hypoxic‐ischemic encephalopathy. Journal of Pediatrics 2008;153(3):375‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas A, Shankaran S, Laptook AR, Langer JC, Bara R, Ehrenkranz RA, et al. Hypocarbia and adverse outcome in neonatal hypoxic‐ischemic encephalopathy. The Journal of Pediatrics 2011;158(5):752‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh NA, Lasky RE, Garza CN, Bonfante‐Mejia E, Shankaran S, Tyson JE. Volumetric and anatomical MRI for hypoxic‐ischemic encephalopathy: relationship to hypothermia therapy and neurosensory impairments. Journal of Perinatology 2009;29(2):143‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Barnes PD, Hintz SR, Laptook AR, Zaterka‐Baxter KM, McDonald SA, et al. Brain injury following trial of hypothermia for neonatal hypoxic‐ischaemic encephalopathy. Archives of Diseases in Childhood 2012;97(6):F398‐404. [DOI: 10.1136/archdischild-2011-301524] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole‐body hypothermia for neonates with hypoxic‐ischemic encephalopathy. New England Journal of Medicine 2005;353(15):1574‐84. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, McDonald SA, Higgins RD, Tyson JE, Ehrenkranz RA, et al. Temperature profile and outcomes of neonates undergoing whole body hypothermia for neonatal hypoxic‐ischemic encephalopathy. Pediatric Critical Care Medicine 2012;13(1):53‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Tyson JE, Ehrenkranz RA, Bann CA, Das A, et al. Evolution of encephalopathy during whole‐body hypothermia for neonatal hypoxic‐ischemic encephalopathy. Journal of Pediatrics 2012;160(4):567‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Pappas A, Laptook AR, McDonald SA, Ehrenkranz RA, Tyson JE, et al. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole‐body hypothermia for neonatal hypoxic‐ischemic encephalopathy. Pediatrics 2008;122(4):e791‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. New England Journal of Medicine 2012;366(22):2085‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shankaran 2002 {published data only}
- Shankaran S, Laptook A, Wright LL, Ehrenkranz RA, Donovan EF, Fanaroff AA, et al. Whole‐body hypothermia for neonatal encephalopathy: animal observations as a basis for a randomized, controlled pilot study in term infants. Pediatrics 2002;110(2 Pt 1):377‐85. [DOI] [PubMed] [Google Scholar]
TOBY Study 2009 {published data only}
- Azzopardi D, Brocklehurst P, Edwards D, Halliday H, Levene M, Thoresen M, et al. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatrics 2008;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. New England Journal of Medicine 2009;361(14):1349‐58. [DOI] [PubMed] [Google Scholar]
- Perrone S, Szabó M, Bellieni CV, Longini M, Bangó M, Kelen D, et al. Whole body hypothermia and oxidative stress in babies with hypoxic‐ischemic brain injury. Pediatric Neurology 2010;43(4):236‐40. [DOI] [PubMed] [Google Scholar]
- Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue after moderate hypothermia in neonates with hypoxic‐ischemic encephalopathy: a nested study of a randomized controlled trial. Lancet Neurology 2010;9(1):39‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Róka A, Melinda KT, Vásárhelyi B, Machay T, Azzopardi D, Szabó M. Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics 2008;121(4):e844‐9. [DOI] [PubMed] [Google Scholar]
- Róka A, Vásárhelyi B, Bodrogi E, Machay T, Szabó M. Changes in laboratory parameters indicating cell necrosis and organ dysfunction in asphyxiated neonates on moderate systemic hypothermia. Acta Paediatrica 2007;96(8):1118‐21. [DOI] [PubMed] [Google Scholar]
Zhou 2010 {published data only}
- Zhou WH, Cheng GQ, Shao XM, Liu XZ, Shan RB, Zhuang DY, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic‐ischemic encephalopathy: a multicenter randomized controlled trial in China. Journal of Pediatrics 2010;157(3):367‐72. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Araki 2010 {published data only}
- Araki S, Takahashi D, Matsui M, Saito R, Morita H, Ishii M, et al. Brain hypothermia therapy for newborns with severe birth asphyxia: an experience from a single neonatal intensive care unit [in Japanese]. Journal of UOEH 2010;32(2):205‐11. [DOI] [PubMed] [Google Scholar]
Azzopardi 2000 {published data only}
- Azzopardi D, Robertson NJ, Cowan FM, Rutherford MA, Rampling M, Edwards AD. Pilot study of treatment with whole body hypothermia for neonatal encephalopathy. Pediatrics 2000;106(4):684‐94. [DOI] [PubMed] [Google Scholar]
Christensen 2012 {published data only}
- Christensen RD, Sheffield MJ, Lambert DK, Baer VL. Effect of therapeutic hypothermia in neonates with hypoxic‐ischemic encephalopathy on platelet function. Neonatology 2012;101(2):91‐4. [DOI] [PubMed] [Google Scholar]
Compagnoni 2002 {published data only}
- Compagnoni G, Pogliani L, Lista G, Castoldi F, Fontana P, Mosca F. Hypothermia reduces neurological damage in asphyxiated newborn infants. Biology of the Neonate 2002;82(4):222‐7. [DOI] [PubMed] [Google Scholar]
Compagnoni 2008 {published data only}
- Compagnoni G, Bottura C, Cavallaro G, Cristofori G, Lista G, Mosca F. Safety of deep hypothermia in treating neonatal asphyxia. Neonatology 2008;93(4):230‐5. [DOI] [PubMed] [Google Scholar]
Debillon 2003 {published data only}
- Debillon T, Daoud P, Durand P, Cantagrel S, Jouvet P, Saizou C, et al. Whole‐body cooling after perinatal asphyxia: a pilot study in term neonates. Developmental Medicine and Child Neurology 2003;45(1):17‐23. [PubMed] [Google Scholar]
Filippi 2009 {published data only}
- Filippi L, Marca G, Fiorini P, Poggi C, Cavallaro G, Malvagia S, et al. Topiramate concentrations in neonates treated with prolonged whole body hypothermia for hypoxic ischemic encephalopathy. Epilepsia 2009;50(11):2355‐61. [DOI] [PubMed] [Google Scholar]
Filippi 2010 {published data only}
- Filippi L, Poggi C, Marca G, Furlanetto S, Fiorini P, Cavallaro G, et al. Oral topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia: a safety study. Journal of Pediatrics 2010;157(3):361‐6. [DOI] [PubMed] [Google Scholar]
Filippi 2011 {published data only}
- Filippi L, Marca G, Cavallaro G, Fiorini P, Favelli F, Malvagia S, et al. Phenobarbital for neonatal seizures in hypoxic ischemic encephalopathy: a pharmacokinetic study during whole body hypothermia. Epilepsia 2011;52(4):794‐801. [DOI] [PubMed] [Google Scholar]
Gucuyener 2012 {published data only}
- Gucuyener K, Beken S, Ergenekon E, Soysal S, Hirfanoglu I, Turan O, et al. Use of amplitude‐integrated electroencephalography (aEEG) and near infrared spectroscopy findings in neonates with asphyxia during selective head cooling. Brain Development 2012;34(4):280‐6. [DOI] [PubMed] [Google Scholar]
Hamelin 2011 {published data only}
- Hamelin S, Delnard N, Cneude F, Debillon T, Vercueil L. Influence of hypothermia on the prognostic value of early EEG in full‐term neonates with hypoxic ischemic encephalopathy. Neurophysiologie Clinique 2011;41(1):19‐27. [DOI] [PubMed] [Google Scholar]
Horan 2004 {published data only}
- Horan M, Ichiba S, Firmin RK, Killer HM, Edwards D, Azzopardi D, et al. A pilot investigation of mild hypothermia in neonates receiving extracorporeal membrane oxygenation (ECMO). The Journal of Pediatrics 2004;144(3):301‐8. [DOI] [PubMed] [Google Scholar]
Horn 2006 {published data only}
- Horn AR, Woods DL, Thompson C, Eis I, Kroon M. Selective cerebral hypothermia for post‐hypoxic neuroprotection in neonates using a solid ice cap. South African Medical Journal 2006;96(9 Pt 2):976‐81. [PubMed] [Google Scholar]
Ichiba 2003 {published data only}
- Ichiba S, Killer HM, Firmin RK, Kotecha S, Edwards AD, Field D. Pilot investigation of hypothermia in neonates receiving extracorporeal membrane oxygenation. Archives of Diseases in Childhood. Fetal and Neonatal Edition 2003;88(2):F128‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inder 2004 {published data only}
- Inder T, Hunt R, Morley C, Coleman L, Stewart M, Doyle L, et al. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic‐ischemic encephalopathy. Journal of Pediatrics 2004;145(6):835‐7. [DOI] [PubMed] [Google Scholar]
Kendall 2010 {published data only}
- Kendall GS, Kapetanakis A, Ratnavel N, Azzopardi D, Robertson NJ, Cooling on Retrieval Study Group. Passive cooling for initiation of therapeutic hypothermia in neonatal encephalopathy. Archives of Diseases in Childhood. Fetal and Neonatal Edition 2010;95(6):F408‐12. [DOI] [PubMed] [Google Scholar]
Kilani 2002 {published data only}
- Kilani RA. The safety and practicality of selective head cooling in asphyxiated human newborn infants, a retrospective study. Lebanese Medical Journal 2002;50(1‐2):17‐22. [PubMed] [Google Scholar]
Li 2009 {published data only}
- Li T, Xu F, Cheng X, Guo X, Ji L, Zhang Z, et al. Systemic hypothermia within 10 hours after birth improved neurological outcome in newborns with hypoxic‐ischemic encephalopathy. Hospital Practice 2009;37(1):147‐52. [DOI] [PubMed] [Google Scholar]
Lista 2004 {published data only}
- Lista G, Pogliani P, Fontana P, Castoldi F, Compagnoni G. Cardiovascular and respiratory status in mechanically ventilated asphyxiated term infants: comparison between hypothermic and control group. Acta Bio‐Medica 2004;75(2):107‐13. [PubMed] [Google Scholar]
Liu 2010 {published data only}
- Liu CQ, Xia YF, Yuan YX, Li L, Qiu XL. Effects of selective head cooling with mild hypothermia on serum levels of caspase‐3 and IL‐18 in neonates with hypoxic‐ischemic encephalopathy. Zhongguo Dang Dai Er Ke Za Zhi 2010;12(9):690‐2. [PubMed] [Google Scholar]
Massaro 2010 {published data only}
- Massaro A, Rais‐Bahrami K, Chang T, Glass P, Short BL, Baumgart S. Therapeutic hypothermia for neonatal encephalopathy and extracorporeal membrane oxygenation. Journal of Pediatrics 2010;157(3):499‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meyn 2010 {published data only}
- Meyn DF Jr, Ness J, Ambalavanan N, Carlo WA. Prophylactic phenobarbital and whole‐body cooling for neonatal hypoxic‐ischemic encephalopathy. Journal of Pediatrics 2010;157(2):334‐6. [DOI] [PubMed] [Google Scholar]
Robertson 2008 {published data only}
- Robertson NJ, Nakakeeto M, Hagmann C, Cowan FM, Acolet D, Iwata O, et al. Therapeutic hypothermia for birth asphyxia in low‐resource settings: a pilot randomised controlled trial. Lancet 2008;372(9641):801‐3. [DOI] [PubMed] [Google Scholar]
Róka 2007 {published data only}
- Róka A, Bodrogi E, Szabó M, Machay T. Whole body hypothermia for the treatment of hypoxic‐ischaemic encephalopathy in term infants ‐ a safety study in Hungary. Orvosi Hetilap 2007;148(21):993‐8. [DOI] [PubMed] [Google Scholar]
Rutherford 2005 {published data only}
- Rutherford MA, Azzopardi D, Whitelaw A, Cowan F, Renowden S, Edwards AD, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic‐ischemic encephalopathy. Pediatrics 2005;116(6):1001‐6. [DOI] [PubMed] [Google Scholar]
Simbruner 1999 {published data only}
- Simbruner G, Haberl C, Harrison V, Linley L, Willeitner AE. Induced brain hypothermia in asphyxiated human newborn infants: a retrospective chart analysis of physiological and adverse effects. Intensive Care Medicine 1999;25(10):1111‐7. [DOI] [PubMed] [Google Scholar]
Thomas 2011 {published data only}
- Thomas N, George KC, Sridhar S, Kumar M, Kuruvilla KA, Jana AK. Whole body cooling in newborn infants with perinatal asphyxial encephalopathy in a low resource setting: a feasibility trial. Indian Pediatrics 2011;48(6):445‐51. [DOI] [PubMed] [Google Scholar]
Thoresen 2000 {published data only}
- Thoresen M, Whitelaw A. Cardiovascular changes during mild therapeutic hypothermia and rewarming in infants with hypoxic‐ischemic encephalopathy. Pediatrics 2000;106(1 Pt 1):92‐9. [DOI] [PubMed] [Google Scholar]
Thoresen 2010 {published data only}
- Thoresen M, Hellström‐Westas L, Liu X, Vries LS. Effect of hypothermia on amplitude‐integrated electroencephalogram in infants with asphyxia. Pediatrics 2010;126(1):e131‐9. [DOI] [PubMed] [Google Scholar]
Tusor 2012 {published data only}
- Tusor N, Wusthoff C, Smee N, Merchant N, Arichi T, Allsop JM, et al. Prediction of neurodevelopmental outcome after hypoxic‐ischemic encephalopathy treated with hypothermia by diffusion tensor imaging analyzed using tract‐based spatial statistics. Pediatric Research 2012;72(1):63‐9. [DOI] [PubMed] [Google Scholar]
Wusthoff 2011 {published data only}
- Wusthoff CJ, Dlugos DJ, Gutierrez‐Colina A, Wang A, Cook N, Donnelly M, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic‐ischemic encephalopathy. Journal of Child Neurology 2011;26(6):724‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2002 {published data only}
- Zhou WH, Shao XM, Cao Y, Chen C, Zhang XD. Safety study of hypothermia for treatment of hypoxic‐ischemic brain damage in term neonates. Acta Pharmacologica Sinica 2002;23(Supplement):64‐8. [Google Scholar]
Zhou 2003 {published data only}
- Zhou WH, Shao XM, Zhang XD, Chen C, Huang GY. Effects of hypothermia on cardiac function in neonates with asphyxia [in Chinese]. Zhonghua Er Ke Za Zhi 2003;41(6):460‐2. [PubMed] [Google Scholar]
References to studies awaiting assessment
Bharadwaj 2012 {published data only}
- Bharadwaj SK, Vishnu Bhat B. Therapeutic hypothermia using gel packs for term neonates with hypoxic ischaemic encephalopathy in resource‐limited settings: a randomized controlled trial. Journal of Tropical Pediatrics 2012;58(5):382‐8. [DOI] [PubMed] [Google Scholar]
Bhat 2006 {published data only}
- Bhat MA. Re: therapeutic hypothermia following perinatal asphyxia. Archives of Diseases in Childhood. Fetal and Neonatal Edition 2006;91(6):F464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2012 {published data only}
- Sun J, Li J, Cheng G, Sha B, Zhou W. Effects of hypothermia on NSE and S‐100 protein levels in CSF in neonates following hypoxic‐ischaemic brain damage. Acta Paediatrica 2012;101(8):e316‐20. [DOI: 10.1111/j.1651-2227.2012.02679.x] [DOI] [PubMed] [Google Scholar]
Thayyil 2010 {published data only}
- Thayyil S, Ayer M, Guhan B, Marlow N, Jacobs I, Costello A, et al. Whole body cooling using phase changing material in neonatal encephalopathy: a pilot randomised control trial. Proceedings of the Neonatal Society Autumn Conference; 2009 Nov 29; London. London: Neonatal Society, 2009.
References to ongoing studies
CoolXenon Study {published data only}
- Thoresen M. Xenon and cooling therapy in babies at high risk of brain injury following poor condition at birth: randomised pilot study (the CoolXenon2 Study). clinicaltrials.gov/show/NCT01545271 (accessed 7 December 2012).
DANCE {published data only}
- Baserga M. Darbe Administration in Newborns undergoing Cooling for Encephalopathy (DANCE trial). clinicaltrials.gov/ct2/show/NCT01471015 (accessed 7 December 2012).
Lichter‐Konecki {published data only}
- Lichter‐Konecki U. Pilot study for hypothermia treatment in hyperammonemia and encephalopathy in neonates and very young infants. clinicaltrials.gov/ct2/show/NCT01624311 (accessed 7 December 2012).
NeoNATI {published data only}
- Filippi L. Safety and efficacy of oral topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia: a pilot study of the Neonatal Neuroprotection of Asphyxiated Tuscan Infants (NeoNATI) Network. clinicaltrials.gov/show/NCT01241019 (accessed 7 December 2012). [Google Scholar]
NEST Study {published data only}
- Field DJ, Firmin R, Azzopardi DV, Cowan F, Juszczak E, Brocklehurst P, NEST Study Group. Neonatal ECMO Study of Temperature (NEST) ‐ a randomised controlled trial. BMC Pediatrics 2010;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICHD: Late Hypothermia {published data only}
- Laptook AR, Higgins RD. Evaluation of systemic hypothermia initiated after 6 hours of age in infants ≥36 weeks gestation with hypoxic‐ischemic encephalopathy: a Bayesian evaluation. A protocol for the NICHD Neonatal Research Network. www.healthetreatment.com/clinical‐trial/NCT00614744/ (accessed 7 December 2012).
NICHD: Optimizing Cooling {published data only}
- Shankaran S, Higgins RD. Optimizing cooling strategies at < 6 hours of age for neonatal hypoxic‐ischemic encephalopathy. clinicaltrials.gov/ct2/show/NCT01192776 (accessed 7 December 2012).
TOBYXe {published data only}
- Azzopardi D. Neuroprotective effects of hypothermia combined with inhaled xenon following perinatal asphyxia. clinicaltrials.gov/ct2/show/NCT00934700 (accessed 7 December 2012).
Walsh: Preterm Infants {published data only}
- Walsh WF. Pilot Study of Head Cooling in Preterm Infants With Hypoxic Ischemic Encephalopathy. clinicaltrials.gov/show/NCT00620711 (accessed 7 December 2012).
Walsh: Thermal Imaging {published data only}
- Walsh WF. MRI thermal imaging of infants undergoing cooling for hypoxic ischemic encephalopathy (HIE). www.clinicaltrials.gov/ct2/show/NCT01128673 (accessed 7 December 2012).
Additional references
Akcay 2012
- Akcay A, Akar M, Oncel MY, Kızılelma A, Erdeve O, Oguz SS, et al. Hypercalcemia due to subcutaneous fat necrosis in a newborn after total body cooling. Pediatric Dermatology 2012;Feb 22:Epub ahead of print. [DOI: 10.1111/j.1525-1470.2011.01716.x] [DOI] [PubMed] [Google Scholar]
Anderson 2007
- Anderson ME, Longhofer TA, Phillips W, McRay DE. Passive cooling to initiate hypothermia for transported encephalopathic newborns. Journal of Perinatology 2007;27(9):592‐3. [DOI] [PubMed] [Google Scholar]
Arrich 2012
- Arrich J, Holzer M, Herkner, H Müllner. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD004128.pub3] [DOI] [PubMed] [Google Scholar]
Azzopardi 2009
- Azzopardi D, Strohm B, Edwards AD, Halliday H, Juszczak E, Levene M, et al. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(4):F260‐4. [DOI] [PubMed] [Google Scholar]
Badawi 1998a
- Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O'Sullivan F, Burton PR, et al. Antenatal risk factors for newborn encephalopathy: the Western Australian case‐control study. British Medical Journal 1998;317(7172):1549‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Badawi 1998b
- Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O'Sullivan F, Burton PR, et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case‐control study. British Medical Journal 1998;317(7172):1554‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barks 2008
- Barks JD. Current controversies in hypothermic neuroprotection. Seminars in Fetal and Neonatal Medicine 2008;13(1):30‐4. [DOI] [PubMed] [Google Scholar]
Chakkarapani 2010
- Chakkarapani E, Dingley J, Liu X, Hoque N, Aquilina K, Porter H, et al. Xenon enhances hypothermic neuroprotection in asphyxiated newborn pigs. Annals of Neurology 2010;68(3):330‐41. [DOI] [PubMed] [Google Scholar]
Chuang 1995
- Chuang SD, Chiu HC, Chang CC. Subcutaneous fat necrosis of the newborn complicating hypothermic cardiac surgery. British Journal of Dermatology 1995;132(5):805‐10. [DOI] [PubMed] [Google Scholar]
Cilio 2010
- Cilio MR, Ferriero DM. Synergistic neuroprotective therapies with hypothermia. Seminars in Fetal and Neonatal Medicine 2010;15(5):293‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Department of Health 2006
- Department of Health (UK). Best research for best health: a new national health research strategy, 2006. www.dh.gov.uk/assetRoot/04/12/71/52/04127152.pdf (accessed 7 December 2012).
Der Hertog 2009
- Hertog HM, Worp HB, Tseng M‐C, Dippel DWJ. Cooling therapy for acute stroke. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001247.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dubowitz 1998
- Dubowitz L, Mercuri E, Dubowitz V. An optimality score for the neurologic examination of the term newborn. Journal of Pediatrics 1998;133(3):406‐16. [DOI] [PubMed] [Google Scholar]
Edwards 1995
- Edwards AD, Yue X, Squier MV, Thoresen M, Cady EB, Penrice J, et al. Specific inhibition of apoptosis after cerebral hypoxic‐ischemia by moderate post‐insult hypothermia. Biochemical and Biophysical Research Communications 1995;217(3):1193‐9. [DOI] [PubMed] [Google Scholar]
Edwards 2010
- Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta‐analysis of trial data. BMJ 2010;340:c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fan 2011
- Fan X, Heijnen CJ, KOOIJ MA, Groenendaal F, Bel F. Beneficial effect of erythropoietin on sensorimotor function and white matter after hypoxia‐ischemia in neonatal mice. Pediatric Research 2011;69(1):56‐61. [DOI] [PubMed] [Google Scholar]
Felix 2000
- Felix JF, Badawi N, Kurinczuk JJ, Bower C, Keogh JM, Pemberton PJ. Birth defects in children with newborn encephalopathy. Developmental Medicine and Child Neurology 2000;42(12):803‐8. [DOI] [PubMed] [Google Scholar]
Filippi 2012
- Filippi L, Catarzi S, Padrini L, Fiorini P, Marca G, Guerrini R, et al. Strategies for reducing the incidence of skin complications in newborns treated with whole‐body hypothermia. Journal of Maternal, Fetal and Neonatal Medicine 2012;25(10):2115‐21. [DOI] [PubMed] [Google Scholar]
Finer 1981
- Finer NN, Robertson CM, Richards RT, Pinnell LE, Peters KL. Hypoxic ischemic encephalopathy in term neonates: perinatal factors and outcome. Journal of Pediatrics 1981;98(1):112‐7. [DOI] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. [DOI] [PubMed] [Google Scholar]
Globus 1995
- Globus M, Alonso O, Dietrich W, Busto R, Ginsberg M. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. Journal of Neurochemistry 1995;65(4):1704‐11. [DOI] [PubMed] [Google Scholar]
Gluckman 1992
- Gluckman PD, Williams CE. When and why do brain cells die?. Developmental Medicine and Child Neurology 1992;34(11):1010‐4. [DOI] [PubMed] [Google Scholar]
Gunn 1997a
- Gunn TR, Gunn AJ, Gluckman PD. Substantial neuronal loss with prolonged selective head cooling begun 5.5h after cerebral ischemia in the fetal sheep. Pediatric Research 1997;41:152A. [Google Scholar]
Gunn 1997b
- Gunn AJ, Gunn TR, Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia. Journal of Clinical Investigation 1997;99(2):248‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunn 1998a
- Gunn AJ, Gunn TR. The 'pharmacology' of neuronal rescue with cerebral hypothermia. Early Human Development 1998;53(1):19‐35. [DOI] [PubMed] [Google Scholar]
Gunn 2001
- Gunn AJ, Gunn TR, Roelfema V, Guan J, George S, Gluckman P, et al. Is cerebral hypothermia a possible neuroprotective strategy after asphyxia in the premature fetus?. Pediatric Research 2001;49:435A. [Google Scholar]
Gómez‐Fernández 2011
- Gómez‐Fernández C, Feito Rodríguez M, Collantes Bellido E, Ybarra Zabala M, Lucas Laguna R. Indurated plaque on the back of a newborn after undergoing whole‐body cooling [in Spanish]. Anales de Pediatría 2011;74(1):64‐6. [DOI] [PubMed] [Google Scholar]
Haaland 1997
- Haaland K, Loberg EM, Steen PA, Thoresen M. Posthypoxic hypothermia in newborn piglets. Pediatric Research 1997;41(4 Pt 1):505‐12. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins RD, Raju T, Edwards AD, Azzopardi DV, Bose CL, Clark RH, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. Journal of Pediatrics 2011;159(5):851‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hobson 2011
- Hobson A, Sussman C, Knight J, Perkins J, Irwin L, Larsen V, et al. Active cooling during transport of neonates with hypoxic‐ischemic encephalopathy. Air Medical Journal 2011;30(4):197‐200. [DOI] [PubMed] [Google Scholar]
Hoehn 2008
- Hoehn T, Hansmann G, Bührer C, Simbruner G, Gunn AJ, Yager J. Therapeutic hypothermia in neonates. Review of current clinical data, ILCOR recommendations and suggestions for implementation in neonatal intensive care units. Resuscitation 2008;78(1):7‐12. [DOI] [PubMed] [Google Scholar]
Inder 2000
- Inder TE, Volpe JJ. Mechanisms of perinatal brain injury. Seminars in Neonatology 2000;5(1):3‐16. [DOI] [PubMed] [Google Scholar]
Iwai 2010
- Iwai M, Stetler RA, Xing J, Hu X, Gao Y, Zhang W, et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke 2010;41(5):1032‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laptook 1994
- Laptook AR, Corbett RJT, Sterett R, Burns DF, Tollefsbol G, Garcia D. Modest hypothermia provides partial neuroprotection for ischemic neonatal brain. Pediatric Research 1994;35(4 Pt 1):436‐42. [PubMed] [Google Scholar]
Laptook 1997
- Laptook AR, Corbett R, Sterett R, Burns D, Garcia D, Tollefsbol G. Modest hypothermia provides partial neuroprotection when used for immediate resuscitation after brain ischemia. Pediatric Research 1997;42(1):17‐23. [DOI] [PubMed] [Google Scholar]
Levene 1986
- Levene MI, Sands C, Grindulis H, Moore JR. Comparison of two methods of predicting outcome in perinatal asphyxia. Lancet 1986;1(8472):67‐9. [DOI] [PubMed] [Google Scholar]
Li 2012
- Li LR, You C, Chaudhary B. Intraoperative mild hypothermia for postoperative neurological deficits in intracranial aneurysm patients. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008445.pub2] [DOI] [PubMed] [Google Scholar]
Lorek 1994
- Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards D, et al. Delayed ("secondary") cerebral energy failure after acute hypoxia‐ischemia in the newborn piglet: continuous 48‐hour studies by phosphorus magnetic resonance spectroscopy. Pediatric Research 1994;36(6):699‐706. [DOI] [PubMed] [Google Scholar]
Marion 1997
- Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, et al. Treatment of traumatic brain injury with moderate hypothermia. New England Journal of Medicine 1997;336(8):540‐6. [DOI] [PubMed] [Google Scholar]
Milani 2011
- Milani WR, Antibas PL, Prado GF. Cooling for cerebral protection during brain surgery. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD006638.pub2] [DOI] [PubMed] [Google Scholar]
NHRMC 2005
- National Health and Medical Research Council, Consumer's Health Forum of Australia. A model framework for consumer and community participation in health and medical research, 2005. www.nhmrc.gov.au/_files_nhmrc/publications/attachments/r33.pdf. (accessed 7 December 2012).
Noh 2006
- Noh MR, Kim SK, Sun W, Park SK, Choi HC, Lim JH, et al. Neuroprotective effect of topiramate on hypoxic ischemic brain injury in neonatal rats. Experimental Neurology 2006;201(2):470‐8. [DOI] [PubMed] [Google Scholar]
O'Reilly 2011
- O'Reilly KM, Tooley J, Winterbottom S. Therapeutic hypothermia during neonatal transport. Acta Paediatrica 2011;100(8):1084‐6. [DOI] [PubMed] [Google Scholar]
Oza 2010
- Oza V, Treat J, Cook N, Tetzlaff MT, Yan A. Subcutaneous fat necrosis as a complication of whole‐body cooling for birth asphyxia. Archives of Dermatology 2010;146(8):882‐5. [DOI] [PubMed] [Google Scholar]
Penrice 1996
- Penrice J, Cady EB, Lorek A, Wylezinska M, Amess PN, Aldridge RF, et al. Proton magnetic resonance spectroscopy of the brain in normal preterm and term infants, and early changes after perinatal hypoxia‐ischemia. Pediatric Research 1996;40(1):6‐14. [DOI] [PubMed] [Google Scholar]
Perlman 2010
- Perlman J, Davis P, Wyllie J, Kattwinkel J. Therapeutic hypothermia following intrapartum hypoxia‐ischemia. An advisory statement from the Neonatal Task Force of the International Liaison Committee on Resuscitation. Resuscitation 2010;81(11):1459‐61. [Google Scholar]
Pfister 2010
- Pfister R, Bingham P, Carpenter J, Horbar J, Kenny M, Inder T. Hypothermia in practice, initial observations from the Vermont Oxford Network. Proceeding of the Pediatric Academic Society Conference. 2010 May 2; Vancouver. E‐PAS2010:26.32.5. 2010.
Rees 2001
- Rees K, Beranek‐Stanley M, Burke M, Ebrahim S. Hypothermia to reduce neurological damage following coronary artery bypass surgery. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002138] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Robertson 2010
- Robertson NJ, Kendall GS, Thayyil S. Techniques for therapeutic hypothermia during transport and in hospital for perinatal asphyxial encephalopathy. Seminars in Fetal and Neonatal Medicine 2010;15(5):276‐86. [DOI] [PubMed] [Google Scholar]
Roth 1992
- Roth SC, Edwards AD, Cady EB, Delpy DT, Wyatt JS, Azzopardi D, et al. Relation between cerebral oxidative metabolism following birth asphyxia, and neurodevelopmental outcome and brain growth at one year. Developmental Medicine and Child Neurology 1992;34(4):285‐95. [DOI] [PubMed] [Google Scholar]
Roth 1997
- Roth SC, Baudin J, Cady E, Johal K, Townsend JP, Wyatt JS, et al. Relation of deranged cerebral oxidative metabolism with neurodevelopmental outcome and head circumference at 4 years. Developmental Medicine and Child Neurology 1997;39(11):718‐25. [DOI] [PubMed] [Google Scholar]
Sarnat 1976
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of Neurology 1976;33(10):696‐705. [DOI] [PubMed] [Google Scholar]
Schubert 2005
- Schubert S, Brandl U, Brodhun M, Ulrich C, Spaltmann J, Fiedler N, et al. Neuroprotective effects of topiramate after hypoxia‐ischemia in newborn piglets. Brain Research 2005;1058(1‐2):129‐36. [DOI] [PubMed] [Google Scholar]
Schulzke 2007
- Schulzke SM, Rao S, Patole SK. A systematic review of cooling for neuroprotection in neonates with hypoxic ischemic encephalopathy – are we there yet?. BMC Pediatrics 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sivanandan 2012
- Sivanandan S, Rabi Y, Kamaluddeen M, Akierman A, Lodha A. Subcutaneous fat necrosis as a complication of therapeutic hypothermia in a term neonate. Indian Journal of Pediatrics 2012;79(5):664‐6. [DOI] [PubMed] [Google Scholar]
Strohm 2011
- Strohm B, Hobson A, Brocklehurst P, Edwards AD, Azzopardi D, UK TOBY Cooling Register. Subcutaneous fat necrosis after moderate therapeutic hypothermia in neonates.. Pediatrics 2011;128(2):e450‐2. [DOI] [PubMed] [Google Scholar]
Sydenham 2009
- Sydenham E, Riberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001048.pub2] [DOI] [PubMed] [Google Scholar]
Tagin 2012
- Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta‐analysis. Archives of Pediatrics and Adolescent Medicine 2012;166(6):558‐66. [DOI] [PubMed] [Google Scholar]
Tarnow‐Mordi 2012
- Tarnow‐Mordi WO, Cruz M, Wilkinson D. Evaluating therapeutic hypothermia: parental perspectives should be explicitly represented in future research. Archives of Pediatric and Adolescent Medicine 2012;166(6):578‐9. [DOI] [PubMed] [Google Scholar]
Thoresen 1995
- Thoresen M, Penrice J, Lorek A, Cady EB, Wylezinska M, Kirkbride V, et al. Mild hypothermia after severe transient hypoxia‐ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatric Research 1995;37(5):667‐70. [DOI] [PubMed] [Google Scholar]
Thoresen 1996
- Thoresen M, Bagenholm R, Loberg EM, Apricena F, Kjellmer I. Posthypoxic cooling of neonatal rats provides protection against brain injury. Archives of Disease in Childhood 1996;74(1):F3‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thoresen 2009
- Thoresen M, Hobbs CE, Wood T, Chakkarapani E, Dingley J. Cooling combined with immediate or delayed xenon inhalation provides equivalent long‐term neuroprotection after neonatal hypoxia‐ischemia. Journal of Cerebral Blood Flow and Metabolism 2009;29(4):707‐14. [DOI] [PubMed] [Google Scholar]
Van Leeuwen 2000
- Leeuwen GM, Hand JW, Lagendijk JW, Azzopardi DV, Edwards AD. Numerical modeling of temperature distributions within the neonatal head. Pediatric Research 2000;48(3):351‐6. [DOI] [PubMed] [Google Scholar]
Vannucci 1990
- Vannucci RC. Current and potentially new management strategies for perinatal hypoxic‐ischemic encephalopathy. Pediatrics 1990;85(6):961‐8. [PubMed] [Google Scholar]
Washington 2011
- Washington AE, Lipstein SH. The Patient‐Centered Outcomes Research Institute ‐ promoting better information, decisions, and health. New England Journal of Medicine 2011;365(15):e31. [DOI] [PubMed] [Google Scholar]
Wiadrowski 2001
- Wiadrowski TP, Marshman G. Subcutaneous fat necrosis of the newborn following hypothermia and complicated by pain and hypercalcaemia. Australasian Journal of Dermatology 2001;42(3):207‐10. [DOI] [PubMed] [Google Scholar]
Williams 1991
- Williams CE, Gunn A, Gluckman PD. Time course of intracellular edema and epileptiform activity following prenatal cerebral ischemia in sheep. Stroke 1991;22(4):516‐21. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jacobs 2003
- Jacobs S, Hunt R, Tarnow‐Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003311] [DOI] [PubMed] [Google Scholar]
Jacobs 2007
- Jacobs SE, Hunt R, Tarnow‐Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003311.pub2] [DOI] [PubMed] [Google Scholar]


