Abstract
Purpose:
Glaucoma is the leading cause of irreversible blindness in the world. The current study aims to estimate prevalence, features, and associations of open angle glaucoma (OAG) in a rural and urban East Indian population.
Methods:
This is a population based cross sectional study with two arms, rural (28 contiguous villages from 13 Gram Panchayats in Balagarh Police Station, Hooghly district) and urban (Kolkata). Individuals residing in the study area aged 40 years and above were included using multistage random cluster sampling. All subjects underwent a detailed ophthalmic examination at our base hospitals including applanation tonometry, ultrasound pachymetry, gonioscopy, and frequency doubling technology perimetry. The primary outcome was the prevalence of POAG (95% CI). Age and gender specific prevalence estimates were calculated. Multiple logistic regressions were used to analyze the risk factors.
Results:
Data from 7128 and 6964 subjects aged 40 years or older from Kolkata city and Hooghly district, respectively were analyzed. In the urban population, 2.10% (95% CI: 1.99–2.21%) had POAG and 0.15% (95% CI: 0.13–0.17%) had secondary OAG. In the rural population, 1.45% (95% CI: 0.59–2.31%) had POAG and 0.10 ± 0.03% (95% CI: 0.07–0.13%) had secondary OAG.
Conclusion:
The study concludes that higher age, higher vertical cup disc ratio (VCDR), and lower central corneal thickness (CCT) are important independent predictors of OAG and emphasizes that increased intraocular pressure (IOP) is not POAG. Gonioscopy, disc evaluation, and screening perimetry need to be incorporated in the detection protocol for glaucoma if we intend to lighten the burden of blindness due to glaucoma.
Keywords: Central corneal thickness, frequency-doubling technology perimetry, gonioscopy, open-angle glaucoma
Glaucoma is the leading cause of irreversible blindness in the world.[1] Studies have shown that 60.5 million people worldwide had glaucoma in 2010, and this included 8.4 million people with bilateral blindness due to glaucoma.[2] According to Quigley et al., there will be 79.6 million people with glaucoma by 2020, and of these, 74% will have open-angle glaucoma (OAG).[3] The irreversible and relatively asymptomatic nature of damage caused by glaucoma makes it a greater public health challenge than cataracts, which is the leading cause of blindness globally.[4]
The Hooghly River Glaucoma Study (HRGS) was the largest population-based cross-sectional glaucoma prevalence study from a rural and urban population in Eastern India, which spanned from April 2011 to January 2014.[5] In the present paper, we discuss the prevalence, features, and associations of OAG in a rural and urban population from Eastern India.
Methods
The methodology of HRGS has been discussed in detail elsewhere.[5] This cross-sectional study was approved by the institutional ethics committee and adheres to the tenets of the Declaration of Helsinki.
Kolkata city, our urban study area, is divided into 15 boroughs and 141 wards.[6,7,8] Subjects were enumerated from 8 randomly selected divisions from each of these 15 boroughs. The rural study area consisted of 28 contiguous villages from 13 Gram Panchayats in Balagarh Police Station of Hooghly district in West Bengal. After the enumeration of subjects at field visits, residents of Kolkata were transported to our urban examination center, a tertiary eye hospital in Kolkata and those from Hooghly District were transported to our rural examination center in Kuliapara village, Balagarh Police Station for hospital-based examination. After consenting, the subjects proceeded through various ophthalmic examinations and diagnostic procedures, which have been discussed in detail elsewhere.[5]
The current paper deals with OAG. The following definitions, based on the ISGEO guidelines,[9] were used for the current work. A diagnosis of glaucoma was made in the HRGS when the subjects fulfilled two or more of the following criteria:
Intraocular pressure by Goldman Applanation Tonometry ≥22 mm Hg,
Vertical cup-disc ratio (VCDR) ≥0.6 in either eye or VCDR asymmetry of ≥0.2,
Shaffer Grading of 3 or more for at least 180° in both eyes by gonioscopy,
Frequency doubling technology perimetry (FDP) results suggestive of glaucomatous damage, as interpreted by three senior trained glaucoma specialists. Based on ISGEO guidelines, the presence of a cluster of three contiguous points at the 5% level or less on the pattern deviation plot of the N-30 threshold test was taken to be indicative of glaucomatous damage.
Among the subjects diagnosed with glaucoma, patients with a history of use of the topical steroids in the last 6 months, history of trauma or ocular surgery (excluding squint or oculoplastic surgeries), history of chronic uveitis, evidence of pseudoexfoliation or pigment dispersion on slit-lamp examination and those with hyper mature or intumescent cataract were grouped under secondary glaucoma.
Statistical analysis
The data collected from both the rural and urban cohorts were analyzed using SPSS Statistics software package version 13 (SPSS Inc., Chicago, IL). P <0.05 was taken to be statistically significant and P <0.001 was taken to be statistically highly significant. The primary outcome was the prevalence of primary open-angle glaucoma (POAG) with a 95% confidence interval. Age- and gender-specific prevalence estimates of POAG were also calculated. Multiple logistic regressions were used to analyze the risk factors for POAG. The independent risk factors analyzed include age, sex, intraocular pressure (IOP), and central corneal thickness (CCT).
Results
The HRGS analyzed data from 7128 subjects from Kolkata metropolitan city in the urban phase (response rate 98%) and 6964 subjects from Hooghly district in the rural phase (response rate 94%). Data from this largest Indian glaucoma prevalence study has already been published earlier.[5] 230 subjects (3.23%; 95% confidence interval [CI]: 2.93–3.53%) were detected to have glaucoma in our urban population using the modified the International Society of Geographical and Epidemiologic Ophthalmology (ISGEO) criteria with 53.42% being male whereas 188 subjects (2.70%; 95% CI: 1.09–4.31%) were suffering from glaucoma in the rural group with 55.31% being male. The prevalence of glaucoma increased with age in both urban and rural study divisions. Findings from the subjects with POAG are discussed here in detail.
In the urban study population, 161 subjects had OAG, out of which 11 subjects had secondary OAG. In the secondary glaucoma subgroup, five subjects had pigmentary glaucoma, three had pseudoexfoliation syndrome, two had uveitic glaucoma, and a single eye had neovascular glaucoma secondary to uncontrolled diabetes mellitus. Hence, in the urban study population, 2.10% (95% CI: 1.99–2.21%) had POAG and 0.15% (95% CI: 0.13–0.17%) had secondary OAG. In the rural study population, 108 subjects had OAG, out of which 7 subjects had secondary glaucoma. In the secondary OAG subgroup, one subject had pigmentary glaucoma, three had pseudoexfoliation syndrome, two had uveitic glaucoma, and a single eye had neovascular glaucoma secondary to central retinal venous obstruction. In the rural study population, 1.45% (95% CI: 0.59-2.31%) had POAG and 0.10 ± 0.03% (95% CI: 0.07–0.13%) had secondary OAG.
Fig. 1 shows the distribution of VCDR in subjects detected with POAG in the urban and rural study divisions of the HRGS. It is evident that in the urban division, 27.3%, 31.3%, and 32% of subjects with POAG had a VCDR of 0.6, 0.7, and 0.8, respectively. Similarly, in the rural divisions, 28.7%, 26.7%, and 29.7% of subjects with POAG had a VCDR of 0.6, 0.7, and 0.8, respectively. So, in both the divisions, the majority of subjects detected with POAG had VCDR between 0.6 and 0.8 and the average VCDR in both the groups was 0.65.
Figure 1.
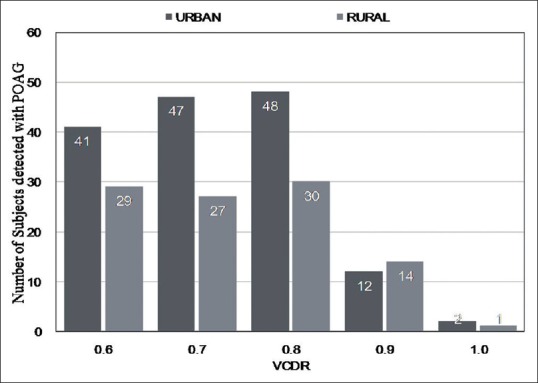
Distribution of vertical cup-disc ratio (VCDR) in subjects detected with primary open-angle glaucoma (POAG) in the urban and rural study populations of the Hooghly River Glaucoma Study (HRGS)
Table 1 shows multiple logistic regressions for age, sex, IOP, and CCT as risk factors for POAG in the urban and rural divisions of HRGS. It is evident that increasing age, IOP, and CCT are important risk factors for POAG.
Table 1.
Multiple logistic regressions for risk factors for primary open-angle glaucoma (POAG) in the urban and rural divisions of HRGS
| Urban | Rural | |||
|---|---|---|---|---|
| POAG | Odds ratio for POAG (95% CI) | POAG | Odds ratio for OAG (95% CI) | |
| Total | 150 | 101 | ||
| 40-49 years | 33 | 1.0 | 27 | 1.0 |
| 50-59 years | 36 | 2.6 (2.09-3.11) | 31 | 2.62 (1.18-4.06) |
| 60-69 years | 51 | 4.2 (3.84-4.56) | 25 | 4.24 (2.0-6.48) |
| >=70 Years | 30 | 5.2 (4.8-5.6) | 18 | 5.27 (2.37-8.17) |
| Male | 80 | 1.0 | 54 | 1.0 |
| Female | 70 | 0.94 (0.55-1.33) | 47 | 0.97 (0.55-1.39) |
| IOP | 150 | 2.4 (1.8-3.0) | 101 | 2.7 (1.7-3.7) |
| CCT | 150 | 2.1 (1.2-3.0) | 101 | 1.9 (0.3-3.5) |
IOP: Intraocular pressure, CCT: Central corneal thickness, POAG: Primary open-angle glaucoma, HRGS: Hooghly river glaucoma study
Fig. 2 shows the distribution of IOP in subjects diagnosed with POAG in the urban and rural divisions of the HRGS. It is evident that the majority of the subjects had IOP between 23 mm Hg and 26 mm Hg in both the divisions. Table 2 shows the age and sex distribution of subjects detected with POAG in the rural and urban divisions of the HRGS.
Figure 2.
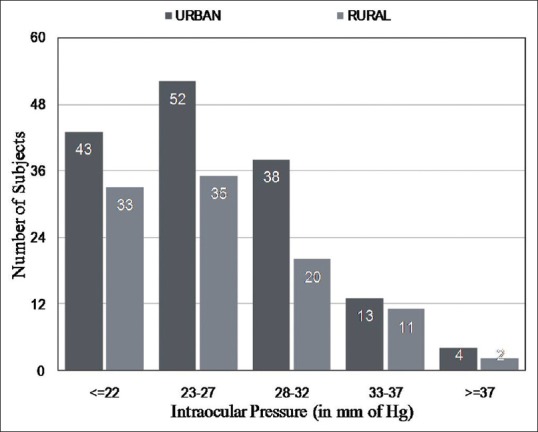
The distribution of intraocular pressure (IOP) in subjects diagnosed with POAG in the urban and rural divisions of the HRGS
Table 2.
The age and sex distribution of subjects detected with POAG in the two divisions of the HRGS
| Age groups (yrs) | Subjects detected with POAG in urban division | Subjects detected with POAG in rural division | P (urban vs rural, total n) | ||||
|---|---|---|---|---|---|---|---|
| Total | Males | Females | Total | Males | Females | ||
| Total | 150 | 80 | 70 | 101 | 54 | 47 | 0.03* |
| 40-49 | 33 | 20 | 13 | 27 | 14 | 13 | 0.19 |
| 50-59 | 36 | 18 | 18 | 31 | 17 | 14 | 0.21 |
| 60-69 | 51 | 28 | 23 | 25 | 14 | 11 | 0.04* |
| >=70 | 30 | 14 | 16 | 18 | 9 | 9 | 0.06 |
*P<0.05 taken to be statistically significant, POAG: Primary open-angle glaucoma, HRGS: Hooghly river glaucoma study
Table 3 shows the distribution of IOP in the subjects not detected to have glaucoma and comparison of the same with those detected with POAG in the rural and urban divisions of the HRGS. The IOP in the POAG group was significantly higher compared to the normal population.
Table 3.
Distribution of IOP in the subjects not detected to have glaucoma and comparison of the same with those detected with POAG in each of the two divisions of the HRGS
| Rural | Urban | |||||
|---|---|---|---|---|---|---|
| IOP in “normal subjects” | IOP in POAG group | P (IOP normal POAG) | IOP in “normal subjects” | IOP in POAG group | P (IOP normal POAG) | |
| Total | 17.20 | 24.8 | <0.001 | 17.40 | 23.8 | <0.001* |
| 40-49 | 16.20 | 23.9 | <0.001 | 16.34 | 22.9 | <0.001* |
| 50-59 | 16.71 | 24.1 | <0.001 | 16.75 | 23.2 | <0.001* |
| 60-69 | 17.10 | 24.9 | <0.001 | 17.12 | 23.7 | <0.001* |
| >=70 | 18.20 | 25.2 | <0.001 | 18.24 | 24.2 | <0.001* |
*P<0.001: Statistically highly significant, POAG: Primary open-angle glaucoma, IOP: Intraocular pressure, HRGS: Hooghly river glaucoma study
Fig. 3 shows the prevalence of POAG at various IOP Levels in the rural and urban divisions of the HRGS. Nearly all the subjects with an IOP of over 31 mm Hg in both the divisions were diagnosed with POAG.
Figure 3.
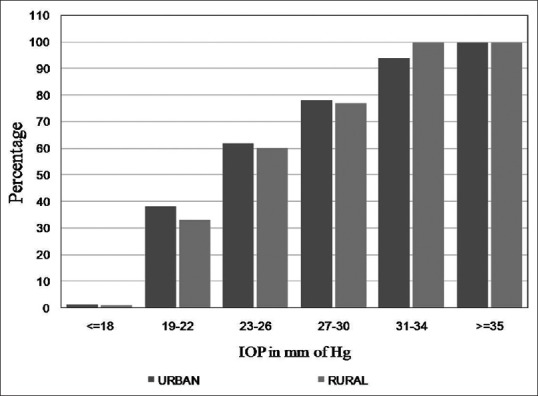
Prevalence of POAG at various IOP levels in the rural and urban divisions of the HRGS
Discussion
The prevalence of POAG was higher in the urban population as compared to the rural population and the difference was found to be statistically significant [Table 2]. Similar findings were reported in the Chennai Glaucoma Study (CGS)[10,11] and the Andhra Pradesh Eye Disease Study (APEDS).[12] Since the age and sex compositions of the rural and urban divisions in the HRGS were similar, the significant differences between the two divisions in glaucoma prevalence could be attributed to genetic differences between the two groups.
In chronic and silent disease, such as POAG, having sound knowledge of the risk factors helps in planning strategies for early detection of the disease.[11] Increasing age has been found to be an important risk factor for the incidence of POAG across the studies.[13,14,15] In both the urban and rural divisions of the HRGS, increasing age has been found to be statistically associated with the increasing prevalence of POAG. The relative risk of POAG was found to be quadrupled in those aged 60–69 years and was five times more in those aged >=70 years, very similar to the findings of previous glaucoma prevalence studies, including the CGS. This emphasizes that community glaucoma screening programs should especially screen those above 60 years. Though males were found to be at a slightly higher risk for the development of POAG in both the divisions, the association was not statistically significant, similar to the findings of the CGS and the Los Angeles Latino Eye Study.
The mean VCDR in both divisions was 0.4 with 0.6 being the 97.5th percentile. However, in the POAG group, the average VCDR was 0.65 for both the rural and urban divisions and this was significantly higher than the overall population examined by us (P < 0.001). The 97.5th VCDR percentile was 0.8. In the ocular hypertension study, thinner CCT was associated with the development of POAG[16] and the EMGT also found thinner CCT to be a risk factor for progression in eyes with higher baseline IOP.[17] Our study also found a significant relationship between lower CCT and POAG in both the divisions (P < 0.05). It is postulated that eyes with a thinner CCT may have increased elasticity of the lamina cribrosa and susceptibility for optic nerve damage.[18]
IOP is universally recognized as one of the most important modifiable risk factors for the development of POAG, our study also concludes similarly. The average IOP across various age groups was statistically significantly higher (P < 0.001) in those with POAG as compared to normal subjects. More than 50% of all included subjects with an IOP of more than 23 mm Hg were detected with POAG. However, it is also worth mentioning that about 1% of the subjects detected with POAG in both the urban and rural groups had an IOP of <=18 mm Hg. Hence, IOP assessment alone as a primary screening tool is likely to have poor yield, similar to the findings reported in the Chennai Eye Disease Incidence Study.[11]
Conclusion
Based on the results of our study, we strongly recommend that increased IOP is not to be considered as POAG. Gonioscopy, disc evaluation, and screening perimetry need to be incorporated in the detection protocol for glaucoma if we intend to lighten the burden of blindness due to glaucoma. Clinical assessment of the optic disc and further substantiation with a portable perimeter like the FDP is likely to substantially increase the yield of glaucoma screening programs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Waisbourd M, Pruzan NL, Johnson D, Ugorets A, Crews JE, Saaddine JB, et al. The Philadelphia glaucoma detection and treatment project: Detection rates and initial management. Ophthalmology. 2016;123:1667–74. doi: 10.1016/j.ophtha.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–93. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul C, Sengupta S, Choudhury S, Banerjee S, Sleath BL. Prevalence of glaucoma in Eastern India: The Hooghly river glaucoma study. Indian J Ophthalmol. 2016;64:578–83. doi: 10.4103/0301-4738.191497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. [Last accessed on 2015 Nov 10]. Available from: http://www.westbengal.gov.in/BanglarMukh/Download?FilePath=/alfresco/d/d/workspace/SpacesStore/329ba5f1-5753-4c45-af30-a191c289fb92/Chap-p_03_08_15.pdf .
- 7. [Last accessed on 2013 Dec 06]. Available from: http://www.censusindia.gov.in/PopulationFinder/District_Master.aspx?state_code=19 .
- 8.Hugli (Hooghly) District Population Census 2011, West Bengal Literacy, Sex Ratio and Density. [Last accessed on 2014 Nov 20]. Available from: http://www.census2011.co.in/census/district/12-Hooghly.html .
- 9.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijaya L, George R, Baskaran M, Arvind H, Raju P, Ramesh SV, et al. Prevalence of primary open-angle glaucoma in an urban south Indian population and comparison with a rural population. The Chennai glaucoma study. Ophthalmology. 2008;115:648–54. doi: 10.1016/j.ophtha.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 11.Vijaya L, Rashima A, Panday M, Choudhari NS, Ramesh SV, Lokapavani V, et al. Predictors for incidence of primary open-angle glaucoma in a South Indian population. The Chennai eye disease incidence study. Ophthalmology. 2014;121:1370–6. doi: 10.1016/j.ophtha.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Dandona L, Dandona R, Srinivas M, Mandal P, John RK, McCarty CA, et al. Open-angle glaucoma in an urban population in southern India: The Andhra Pradesh eye disease study. Ophthalmology. 2000;107:1702–9. doi: 10.1016/s0161-6420(00)00275-x. [DOI] [PubMed] [Google Scholar]
- 13.Jiang X, Varma R, Wu S, Torres M, Azen SP, Francis BA, et al. Los Angeles Latino Eye Study Group. Baseline risk factors that predict the development of open-angle glaucoma in a population: The Los eye study. Ophthalmology. 2012;119:2245–53. doi: 10.1016/j.ophtha.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le A, Mukesh BN, McCarty CA, Taylor HR. Risk factors associated with the incidence of open-angle glaucoma: The visual impairment project. Invest Ophthalmol Vis Sci. 2003;44:3783–9. doi: 10.1167/iovs.03-0077. [DOI] [PubMed] [Google Scholar]
- 15.Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B BESs Study Group. Risk factors for incident open-angle glaucoma: The Barbados eye studies. Ophthalmology. 2008;115:85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. Ocular Hypertension Treatment Study Group. The ocular hypertension treatment study: Baseline factors that predict the onset of primary open angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 17.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z EMGT group. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Brown KE, Congdon NG. Corneal structure and biomechanics: Impact on the diagnosis and management of glaucoma. Curr Opin Ophthalmol. 2006;17:338–43. doi: 10.1097/01.icu.0000233951.01971.5b. [DOI] [PubMed] [Google Scholar]


