Abstract
Background
Lambert‐Eaton myasthenic syndrome (LEMS) is an autoimmune disorder of neuromuscular transmission. Treatments attempt to overcome the harmful autoimmune process, or improve residual neuromuscular transmission
Objectives
The objective was to examine the efficacy of treatment in Lambert‐Eaton myasthenic syndrome.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register (12 October 2010), the Cochrane Central Register of Controlled Trials (CENTRAL) (12 October 2010, Issue 4 2010 in the Cochrane Library), MEDLINE (January 1966 to September 2010) and EMBASE (January 1980 to September 2010).
Selection criteria
All randomised or quasi‐randomised trials of adults and children with a diagnosis of Lambert‐Eaton myasthenic syndrome, with or without small‐cell lung cancer, receiving any form of pharmacological or physical treatment.
Data collection and analysis
All authors independently assessed studies for inclusion and extracted data. Study authors were contacted for missing information when possible.
Main results
Four controlled trials of 3,4‐diaminopyridine compared with placebo in a total of 54 participants with Lambert‐Eaton myasthenic syndrome were eligible: three cross‐over trials and one parallel group. Two were added at this update. One of these trials also assessed pyridostigmine in conjunction with 3,4‐diaminopyridine. A further cross‐over trial compared intravenous immunoglobulin (IVIg) to placebo in nine participants.
Four trials of 3,4‐diaminopyridine reported significant improvement in the primary outcome, muscle strength score, or myometric limb measurement for between hours and a week following treatment, and significant improvement in resting compound muscle action potential (CMAP) amplitude following 3,4‐diaminopyridine, compared with placebo.
A meta‐analysis of the primary endpoint showed Quantitative Myasthenia Gravis (QMG) muscle score assessed between three and eight days was likely to improve by a mean of 2.44 points (95% confidence interval 3.6 to 1.22). Meta‐analysis of the secondary endpoint CMAP amplitude also showed a mean improvement of 1.36 mV (95% confidence interval 0.99 to 1.72) over the same period. The risk of bias was determined to be low, and quality of evidence moderate to high.
A single cross‐over trial reported significant improvement in myometric limb strength and non‐significant improvement in mean resting CMAP amplitude with IVIg compared to placebo. Clinical improvement lasted for up to eight weeks.
Authors' conclusions
Limited but moderate to high quality evidence from randomised controlled trials showed that over days 3,4‐diaminopyridine, or for up to 8 weeks IVIg, improved muscle strength scores and CMAP amplitudes in participants with Lambert‐Eaton myasthenic syndrome. There are insufficient data at present to quantify this effect. Other possible treatments have not been tested in randomised controlled trials.
Plain language summary
Treatment for Lambert‐Eaton myasthenic syndrome
Lambert‐Eaton myasthenic syndrome (LEMS) is a rare disorder of the neuromuscular junction that causes muscle weakness (most commonly in the upper arms and legs). It is an autoimmune disease in which the body's own antibodies prevent the release of the chemical acetylcholine. This interferes with transmission of nerve impulses to the muscles. One of the main treatments is 3,4‐diaminopyridine which increases the release of acetylcholine. Four small randomised controlled trials involving 54 participants in total showed that 3,4‐diaminopyridine improves muscle strength. This was determined by measuring the compound muscle action potential (CMAP) which is a test that records the amount of electrical activity generated in a muscle when it is stimulated by its nerve. Although the number of trials is relatively small, the quality of evidence from these trials is moderate to high, which supports the findings of this review. The changes are measured over days only. A single trial involving nine participants showed that intravenous immunoglobulin also improved muscle strength up to 8 weeks from treatment. Other possible treatments such as plasma exchange, steroids and immunosuppressive agents have not been tested in randomised controlled trials. Further trials of these treatments are needed.
Summary of findings
Summary of findings for the main comparison. 3,4‐Diaminopyridine compared to placebo for LEMS.
| 3,4‐Diaminopyridine compared to placebo for Lambert‐Eaton myasthenic syndrome | ||||||
|
Patient or population: participants with Lambert‐Eaton myasthenic syndrome Settings: inpatient and outpatient setting Intervention: 3,4‐diaminopyridine Comparison: untreated | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Untreated | 3,4‐Diaminopyridine | |||||
|
Improvement in muscle strength (QMG score) Scale from: 0 to 39 Follow‐up: 3 to 8 days |
The mean untreated muscle strength score (QMG score) ranged across control groups from 11.6 to 13.2 | The mean change in muscle strength (QMG score) in the intervention groups was 2.44 lower (3.6 to 1.22 lower) | 40 (2 studies1) | +++O moderate2 | ||
|
Improvement in mean CMAP amplitude (mV) Follow‐up: 3 hours to 8 days |
The mean untreated CMAP amplitude ranged across control groups from 1.7 to 3.3 | The mean change in mean CMAP amplitude in the intervention groups was 1.36 higher (0.99 to 1.72 higher) | 94 (4 studies3) | ++++ high | ||
| Adverse events | See comment | See comment | Not estimable | 42 (4 studies) | ++++ high | 3,4‐Diaminopyridine (3,4‐DAP) was generally well tolerated, though minor side effects were noted in all 4 studies4 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; CMAP: compound muscle action potential | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There was a total of only 33 participants in these two trials. Further studies with larger patient numbers may be beneficial (see also footnote 2). 2 A previous study (Barohn et al, 1998 ‐ see references) showed that due to the variability of repeated observations when using the QMG scoring system, a treatment must produce score changes of 2.6 or greater to be considered of significance. Hence further studies are needed to truly determine any significant effect. 3 Although only 54 patients in total were recruited into all 4 trials of 3,4‐DAP, in 3 of the 4 studies a cross‐over design was used resulting in patients acting as their own controls. In one study (Sanders et al), 14 patients received placebo only. 4 In total 42 patients received 3,4‐DAP. Serious side effects were extremely rare. One patient had a generalised seizure using high dose 3,4‐DAP (McEvoy et al). Minor side effects of limb or perioral paraesthesia occurred in 19 participants, with insomnia and headache occurring in 5.
Background
Lambert‐Eaton myasthenic syndrome (LEMS) is a presynaptic disorder of neuromuscular transmission characterised by impaired quantal release of acetylcholine that causes proximal weakness, depressed tendon reflexes and post‐tetanic potentiation; additionally, autonomic changes are present (Lambert 1956; Lambert 1971). Approximately 60% of people with LEMS have a small‐cell lung cancer (SCLC) (O'Neill 1988). Evidence that LEMS is an autoimmune disease mediated by antibodies to voltage‐gated calcium channels (VGCC) at motor nerve terminals includes the clinical response to plasma exchange (Lang 1981), the passive transfer of the pathophysiological and morphological changes to mice by injection of patients' immunoglobulin (Fukunaga 1983; Lang 1981; Lang 1983; Lang 1987), and the detection by radioimmunoassay of serum antibodies in people with LEMS to P/Q‐type VGCC (Lennon 1995; Motomura 1995). The antigenic stimulus for anti‐VGCC autoantibody production in people with SCLC‐LEMS appears to be tumour VGCC (Roberts 1985).The trigger for the production of anti‐VGCC antibodies in people with LEMS with no detectable lung cancer (non‐SCLC‐LEMS) is unknown.
Symptomatic treatment for LEMS include drugs that increase neurotransmitter release at the neuromuscular junction. Guanidine was first recommended for use in LEMS by Lambert (Lambert 1966) but has not been used in large randomised controlled trials because of serious side effects of marrow suppression (Oh 1973) and renal failure (Blumhardt 1977). Low dose guanidine (less than 1000 mg/day) was used in conjunction with pyridostigmine in nine participants with LEMS in an open trial (Oh 1997). Mean treatment duration was three years, during which three participants stopped taking guanidine due to persistent gastrointestinal side effects. Combination treatment was beneficial in terms of muscle strength and electrophysiological compound muscle action potential (CMAP) amplitude measurements in all nine participants. There were no reported serious side effects.
The quaternary ammonium compound, 4‐aminopyridine (4‐AP), was also found to increase the release of acetylcholine at the neuromuscular junction (Lundh 1978), and was subsequently used for the symptomatic treatment of two patients with LEMS (Agoston 1978; Lundh 1977). A larger open study of the use of oral 4‐AP in people with LEMS resulted in clinical and electrophysiological improvement in all four participants tested, but one participant suffered a single tonic clonic seizure on a dose of 120 mg 4‐AP per day (Murray 1981). The threat of serious central nervous system side effects has thus limited the use of 4‐AP, a drug known to cross the blood‐brain barrier and result in epileptogenic effects in animals (Lemeignan 1971).
The related aminopyridine 3,4‐diaminopyridine (3,4‐DAP) has become the mainstay of symptomatic treatment of LEMS in Europe. It has been shown in animals to be more potent in improving neuromuscular transmission (Molgo 1980) and less convulsant (Lechat 1968) than 4‐AP. In addition, it has the advantage over 4‐AP of crossing the blood‐brain barrier less readily (Lemeignan 1982), resulting in fewer central nervous system side effects. The first use of 3,4‐DAP was in three people with LEMS without lung cancer who all derived significant clinical and electrophysiological benefit from intravenous and then oral preparations of 3,4‐DAP (Lundh 1983). Follow‐up data collected after a mean treatment duration of five years demonstrated prolonged beneficial clinical effects with minimal side effects at daily doses less than 60 mg of 3,4‐DAP (Lundh 1993).
There have subsequently been four randomised placebo‐controlled trials of 3,4‐DAP in people with LEMS (McEvoy 1989; Oh 2009; Sanders 2000; Wirtz 2009). A cross‐over trial of 12 participants conducted by McEvoy et al (McEvoy 1989) showed a significant improvement in isometric muscle strength and a parallel increase in resting CMAP amplitudes following 3,4‐DAP treatment in all participants compared with placebo. Sanders et al (Sanders 2000) found a significant improvement in mean Quantitative Myasthenia Gravis (QMG) score and median CMAP amplitude in people with LEMS treated with 3,4‐DAP compared with placebo. In a subsequent open‐label phase of the trial, only one of 25 participants had no symptomatic improvement on 3,4‐DAP. Wirtz et al (Wirtz 2009) in a cross‐over trial of nine participants showed that isometric muscle testing and mean CMAP amplitude improved with 3,4‐DAP treatment, and that pyridostigmine in isolation was no better than placebo, and failed to confer any additional benefit when used in conjunction with 3,4‐DAP. A further cross‐over trial of seven participants showed that CMAP amplitude, QMG score, subjective symptom score, muscle strength score and LEMS classification all improved with 3,4‐DAP when compared to baseline and placebo (Oh 2009).
The evidence indicating that LEMS was an autoimmune disorder (Lang 1981) prompted the use of immunosuppressive treatments. Prednisolone, given in conjunction with azathioprine, following a course of plasma exchanges in three LEMS participants resulted in a marked improvement in clinical and electromyographic measurements in two of them (Lang 1981). Eight of nine participants in a subsequent open study (Newsom‐Davis 1984) showed a short‐term clinical and electromyographic response to plasma exchange. However, three of these participants developed lung cancer during the course of the study. Of the remaining six people with LEMS without lung cancer, three subsequently achieved almost complete remission of symptoms within a year of beginning treatment with prednisolone and azathioprine. The remaining three participants also improved but to a lesser degree (two were intolerant of azathioprine and received prednisolone alone). Similar improvements were seen in resting CMAP amplitudes. To date, there have been no randomised controlled trials of prednisolone or other oral immunosuppressive agents in LEMS patients.
Since treatment with intravenous immunoglobulin (IVIg) has been shown to benefit patients with other autoimmune diseases, and because of a single case report of a person with LEMS improving following IVIg (Bird 1992), a randomised, double‐blind placebo‐controlled cross‐over trial was conducted in nine people with LEMS (Bain 1996). There were significant improvements in myometric strength measures associated with a significant decline in serum VGCC antibody titres.
Objectives
The objective of this review was to examine the efficacy of all forms of treatment for Lambert‐Eaton myasthenic syndrome (LEMS).
Methods
Criteria for considering studies for this review
Types of studies
We searched for all randomised or quasi‐randomised trials involving treatment of LEMS.
Types of participants
All adults and children with a diagnosis of LEMS, with or without small‐cell lung cancer. Diagnosis was based on typical electrophysiological findings of low resting CMAP amplitude in a hand muscle, and facilitation of more than 100% after 10 seconds maximal voluntary contraction (O'Neill 1988). In addition, the clinical features included proximal muscle weakness, with or without absent reflexes or autonomic disturbance.
Types of interventions
We included any form of medical (pharmacological or physical) treatment.
Types of outcome measures
Primary outcomes
The primary outcome measure was a change in:
the score on a muscle strength scale, (the QMG score) (Barohn 1998; Besinger 1983; Tindall 1987), or when not available, limb muscle strength measured by myometry.
Secondary outcomes
The secondary outcome measure was:
improvement in the amplitude of the resting CMAP(s) (mean of all muscles tested).
We included primary and secondary outcomes and adverse effects in a 'Summary of findings' table. See 'Table 1'.
Search methods for identification of studies
We searched the Cochrane Neuromuscular Disease Group Specialized Register (12 October 2010) for randomised controlled trials using 'Lambert‐Eaton (myasthenic syndrome)' or 'LEMS' or 'Eaton‐Lambert' as the search terms. We also searched the Cochrane Central Register of Controlled Trials (CENTRAL) (12 October 2010, Issue 4 2010 in the Cochrane Library), MEDLINE (January 1966 to September 2010) and EMBASE (January 1980 to September 2010). We checked the bibliographies in the randomised trial reports and contacted their authors to identify additional published or unpublished data.
Electronic searches
See Appendix 1, Appendix 2 and Appendix 3.
Data collection and analysis
Selection of studies
The three review authors checked the titles and abstracts identified from the literature search. The authors obtained the full text of all potentially relevant studies for independent assessment by all authors. The authors decided which trials fitted the inclusion criteria and graded their methodological quality. Disagreements about inclusion criteria were resolved by discussion between the authors.
Data extraction and management
All authors performed data extraction. Missing data were obtained from the trial authors whenever possible.
Assessment of risk of bias in included studies
We graded risk of bias using the Cochrane risk of bias scoring system (Higgins 2008). The risk of bias process takes into account sequence generation, allocation concealment, blinding, addressing incomplete outcome data, selective reporting or any other forms of bias. These items were graded according to the established Cochrane scale of 'Yes', 'No' or 'Unclear', with 'Yes' indicating a low risk of bias, and 'No' indicating a high risk of bias. 'Unclear' was used when there is insufficient information to make this judgement or when the item was not relevant to the study. The review authors reached agreement by consensus.
Measures of treatment effect
We performed all statistical calculations using the Cochrane statistical package 'Review Manager 5.1'. We expressed results as mean differences (MDs) and 95% confidence intervals (CIs) for continuous variable outcomes. Due to the cross‐over design of the studies, we pooled data with the generic inverse variance (GIV) method. This takes the MD between treatment and control, with standard error of the mean (SEM) for the difference. Wherever possible, we have used the published SEM; when this was not available, we used the published P value or original data obtained from the authors to estimate SEM.
For one trial when no other means of obtaining the variance were possible, we had to assume a known within‐subject correlation between the treatment effect in the two periods of the cross‐over study. We then used the subsequent calculated values in a GIV analysis.
Subgroup analysis and investigation of heterogeneity
We also identified subgroups with or without an associated small‐cell lung cancer in advance because of its prognostic importance and confounding effect on treatment.
Sensitivity analysis
We undertook a sensitivity analysis on the basis of methodological quality and tested for heterogeneity in the results, adjusting the confidence limits as appropriate.
Results
Description of studies
For this update, a search of the Cochrane Neuromuscular Disease Group Specialized Register revealed 13 papers (2 new), 138 papers (53 new) through MEDLINE, 100 (30 new) through EMBASE, and 23 through CENTRAL. After review of the new studies and removal of duplicates, we found two new studies (Oh 2009; Wirtz 2009) for this update, resulting in a total of five included randomised controlled trials. We found no other trials despite contact with the authors of previous trials. There were no exclusions. The five eligible trials included a total of 54 participants with LEMS treated with 3,4‐DAP (or placebo), and nine participants treated with IVIg (or placebo). One patient with complete stable remission was studied in the trial by Oh et al. No healthy participants were studied.
The first trial was a cross‐over study of 12 participants with LEMS that compared the effect of maximum dose oral 3,4‐DAP (100 mg/day) for six days with placebo, using a muscle strength score and electrophysiological testing at three and six days (McEvoy 1989).
A second trial with a parallel group design compared oral 3,4‐DAP (60 mg/day) with placebo (oral lactose capsules) in 26 participants (12 received 3,4‐DAP, 14 placebo). A QMG muscle strength score and electrophysiological measurements were taken on days five and six (Sanders 2000).
The third trial was a placebo‐controlled, double dummy, double blind, randomised cross‐over study of nine participants. They compared 10 mg of intravenous 3,4‐DAP against placebo infusion, IV pyridostigmine (varying doses), and a combination of 3,4‐DAP and pyridostigmine. Muscle strength and electrophysiological testing between 10 and 170 minutes post infusion were measured endpoints (Wirtz 2009).
A fourth trial was a randomised, double blind, placebo‐controlled, cross‐over study of seven participants. The treatment protocol varied for two groups of participants. The first group of three cases received an initial daily dose of 15 mg increased to 80 mg per day by the end of the eight day period. A second group received 30 mg per day increased to 75 mg/day over a three day study period due to time constraints. Endpoints assessed subjective symptoms scores, LEMS classification, Medical Research Council (MRC) muscle strength score, QMG score and CMAP (Oh 2009).
The fifth trial compared the effects of IVIg (total 2 g/kg body weight over two days) with placebo infusion (0.3% albumin) in nine participants with LEMS in a cross‐over trial (Bain 1996). An interval of eight weeks was left before administration of the second reciprocal infusion. Myometric limb muscle strength scores and anti‐VGCC antibody concentrations were measured at two‐weekly intervals over both eight‐week study periods. See 'Characteristics of included studies' table for further details.
Risk of bias in included studies
Sequence generation was considered acceptable in all five trials. Participants were assigned treatment or placebo by either random allocation table (Bain 1996; Sanders 2000; Wirtz 2009) or random number table (McEvoy 1989; Oh 2009).
Allocation concealment was on the whole infrequently described in significant detail. In three trials, insufficient information was given about the process of concealment to permit full judgement (Bain 1996; McEvoy 1989; Wirtz 2009).
Participant blinding was intended in all five trials. However, only two trials (Oh 2009; Sanders 2000) clearly stated that the active drug was identical in appearance to the placebo preparation. The details of the compound used for the placebo in one trial (McEvoy 1989) were not stated. Two trials (McEvoy 1989; Sanders 2000) recorded the effectiveness of blinding in terms of the side effects noted when taking active drug (3,4‐DAP) rather than placebo. Perioral or digital paraesthesiae were noted after active treatment (3,4‐DAP) in 4 out of 14 participants in one study (Sanders 2000) and 10 out of 12 participants in another (McEvoy 1989). The observers were described as being blinded in all five trials; however, the methods by which this was achieved was again infrequently outlined. Discussions with the clinicians involved in one of the trials (Bain 1996) revealed that the blinding of participants and observers was excellent. The only exceptions to this were one participant who had an acute meningitic reaction following active treatment (IVIg), and another who had cellulitis but was withdrawn from the study.
The five trials detailed full clinical and electrophysiological diagnostic criteria, which fulfilled accepted diagnostic guidelines for LEMS, for all participants (AAEM 2001a; AAEM 2001b; O'Neill 1988).
Explicit outcome criteria were detailed adequately in four studies (Bain 1996; Oh 2009; Sanders 2000; Wirtz 2009), but in the fifth study (McEvoy 1989), all outcome measures were simply listed, and it was not clear which of these were primary and which were secondary outcome measures. The only trial of the five that was parallel in design, and not cross‐over, detailed full baseline characteristics for the LEMS participants receiving either 3,4‐DAP or placebo. There were no significant differences between the groups in terms of participant age, sex, presence of SCLC, CMAP amplitudes or QMG scores (Sanders 2000). Incomplete outcome data were well explained and accounted for in all studies.
Follow‐up was complete for the short, intended time period for all five trials. Three of the trials reported on an extended period of follow‐up, but the long‐term effect of treatment was not a planned endpoint. Extended follow‐up of 12 to 21 months by McEvoy et al (McEvoy 1989) showed sustained benefit in favour of 3,4‐DAP. Almost all (22 of 25) participants studied by Sanders et al (Sanders 2000) gained sustained benefit from 3,4‐DAP treatment over a six‐month follow‐up period. Oh et al (Oh 2009) described patients choice for long‐term treatment at the cessation of the trial, and gave a subjective or objective account of their progress.
The baseline difference gradings were not applicable to the four cross‐over trials (Bain 1996; McEvoy 1989; Oh 2009; Wirtz 2009), because the participants acted as their own controls. For a summary of review authors' judgments about each risk of bias item for included studies see the 'Risk of bias summary' (Figure 1).
1.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
3,4‐Diaminopyridine versus placebo
Primary outcome measure: the score on a muscle strength scale (QMG score, or limb muscle strength measured by myometry)
Four trials used a muscle strength scale as an outcome measure, and this was explicitly listed as a primary outcome measure in three (Oh 2009; Sanders 2000; Wirtz 2009). All trials reported a significant improvement in either muscle strength score, or myometric limb measurement following treatment. However, a meta‐analysis of the results was not possible because of marked differences between these trials regarding primary outcome measures. Sanders et al (Sanders 2000) and Oh et al (Oh 2009) both used the QMG score as a primary outcome.The trials by McEvoy et al (McEvoy 1989) and (Bain 1996) used a different muscle strength score from the QMG score and the isometric muscle strength reported by Wirtz et al (Wirtz 2009). The scoring system and isometric limb measurements used by McEvoy (McEvoy 1989) or Wirtz (Wirtz 2009) were not detailed enough to calculate an equivalent QMG score. We failed to obtain individual participant data from the authors (McEvoy 1989).
The QMG scores in both trials (Oh 2009; Sanders 2000) were taken at the end of the treatment periods. In the trial by Sanders et al (Sanders 2000) 3,4‐DAP was given three times a day for six days, with QMG scores being recorded on days five and six of the administration phase. In the trial by Oh et al, a mixture of an eight‐day and three‐day treatment phases were used, with QMG scores assessed at the end of each of these treatment periods.
We were therefore able to compare the overall treatment effect by looking at the change in QMG score from baseline with either 3,4‐DAP treatment, or placebo treatment from the trials by Oh et al and Sanders et al (Oh 2009; Sanders 2000). A GIV analysis of these two trials showed that QMG scores decreased (improved) by 2.24 points (95% CI 1.22 to 3.65 points) after treatment with 3,4‐DAP (see Analysis 1.1, Figure 2).
1.1. Analysis.

Comparison 1 3,4‐diaminopyridine treatment versus placebo, Outcome 1 Change in QMG score with generalised inverse variance model.
2.

Forest plot of comparison: 1 3,4‐diaminopyridine treatment versus placebo, outcome: 1.1 Change in QMG score with generalised inverse variance model.
Secondary outcome measure: improvement in the amplitude of the resting CMAP(s) (mean of all muscles tested)
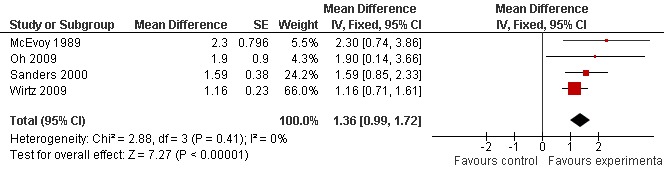
All trials recorded changes in the amplitude of resting CMAPs after active treatment or placebo. Resting CMAP values in both one arm and one leg muscle, obtained before and after treatment with 3,4‐DAP, were available for each participant in one trial (McEvoy 1989). Oh et al (Oh 2009) also gave before, during and after CMAP abductor digiti quinti values in their results section. An averaged CMAP obtained from one foot and two hand muscles was used in the trial by Sanders et al (Sanders 2000), and the averaged change in CMAP amplitude was given for the participant cohort by Wirtz et al (Wirtz 2009). The original CMAP data were subsequently kindly provided by Sanders et al (Sanders 2000) and Wirtz et al (Wirtz 2009) in order to be able to include this in the analysis. All trials recorded significant improvement in resting CMAP amplitudes following 3,4‐DAP treatment compared with placebo.
We were able to compare overall treatment effect by using an averaged overall (or hand muscle) CMAP amplitude response. As three trials were a cross‐over design (McEvoy 1989; Oh 2009; Wirtz 2009), and the other used a parallel protocol (Sanders 2000), it was necessary to employ a GIV analysis. Original data provided by the authors of three trials (Oh 2009; Sanders 2000; Wirtz 2009) were used to used in this analysis. For the paper by McEvoy et al the standard error (SE) of the mean difference was deduced by assuming that the CMAP values for individual patients, both before and after treatment had a correlation of r = 0.5. These results were then used in conjunction with the data from the three other trials (Oh 2009; Sanders 2000; Wirtz 2009) and included in the GIV analysis to assess the overall effect of treatment.
Meta‐analysis of the CMAP secondary endpoint showed a significant overall benefit in CMAP amplitude after treatment with 3,4‐DAP. The overall mean improvement on GIV analysis was 1.36 mV (95% CI 0.99 to 1.72) in favour of the treatment (see Analysis 1.2, Figure 3).
1.2. Analysis.

Comparison 1 3,4‐diaminopyridine treatment versus placebo, Outcome 2 Improvement in mean CMAP amplitude with generalised inverse variance method (assumed r = 0.5 for within‐patient treatment effects in cross‐over trials).
3.
Forest plot of comparison: 1 3,4‐diaminopyridine treatment versus placebo, outcome: 1.3 Improvement in mean CMAP amplitude with generalised inverse variance method using the assumption of r = 0.5 correlation in individual patients before and after treatment in the McEvoy trial.
All trials assessed CMAPs at one specific time point during their trial, with only the trial by McEvoy et al also providing three month follow up results. Each trial assessed CMAPs after different periods of exposure to 3,4‐DAP. Sanders et al (Sanders 2000) assessed CMAPs on day five or six of their six‐day treatment regime, though the time period post 3,4‐DAP dose administration was not recorded. McEvoy et al (McEvoy 1989) assessed CMAPs on the final day of their three‐day trial period, though again, the time the electrophysiology was performed in relation to the last dose was not stated. Oh et al (Oh 2009) also measured CMAPs on the final day of their three or eight day treatment protocol. The study by Wirtz et al performed electrophysiological testing after only one dose, and measured CMAPs in 20 minute intervals for three hours post administration of 3,4‐DAP. The values used in this analysis were the mean of all values recorded after 3,4‐DAP was given.
Testing for heterogeneity (Chi2 = 2.88, (three degrees of freedom), P value = 0.41) suggested that the significant meta‐analysis finding for the secondary endpoint may have been due to chance.
Intravenous immunoglobulin versus placebo
Primary outcome measure: the score on a muscle strength scale (QMG score, or limb muscle strength measured by myometry)
The trial of IVIg versus placebo reported a significant improvement in the primary outcome measure of limb strength as measured by myometry when participants received IVIg compared to placebo infusions (Bain 1996). No muscle strength score such as the QMG was used to measure treatment effect. Individual participant data were not available.
Secondary outcome measure: improvement in the amplitude of the resting CMAP(s) (mean of all muscles tested)
IVIg treatment resulted in an improvement in the resting CMAP amplitudes compared with placebo infusions, but this did not reach statistical significance (Bain 1996).
The number of participants with SCLC included in the trials was small (only 15 participants in three of the five trials (McEvoy 1989; Oh 2009; Sanders 2000)). In the analyses of the effects of 3,4‐DAP treatment in these three trials, the numbers of participants with an associated SCLC (15) was too small to enable statistically meaningful subgroup analysis.
Discussion
There have been only five randomised controlled trials of treatment for Lambert‐Eaton myasthenic syndrome: four of 3,4‐DAP and one of IVIg.
Effects of 3,4‐diaminopyridine
All primary endpoint measures of isometric muscle strength (Wirtz 2009), neurological disability score (McEvoy 1989) and QMG score (Oh 2009; Sanders 2000) improved significantly following the administration of oral 3,4‐DAP.
We were able to perform a meta‐analysis on QMG scores based on the data provided in the papers by Sanders et al (Sanders 2000) and Oh et al (Oh 2009). Sanders et al (Sanders 2000) was the first trial to use the QMG muscle score and showed that there was a 2.23 point improvement between the placebo and 3‐4‐DAP treated group. In the study by Oh et al (Oh 2009), QMG score improved in four of the six participants treated with 3,4‐DAP, with a mean improvement of 3.00 points. See Table 1.
The QMG scoring system ranges from a score of 0 to 39. A score of zero implies that speech, swallowing, vital capacity, facial muscle strength, external ocular muscles, and all limb muscles are normal. Barohn et al (Barohn 1998 ) tested for inter‐rater reliability of the QMG score, and found that if the QMG score is to be used as a primary efficacy measure, then a treatment must produce more than 2.6 units of change to be of clinical significance.
Our analysis shows that QMG score does indeed appear to improve with 3,4‐DAP treatment compared to baseline QMG values; however, with a mean overall improvement of 2.44 points, and 95% CI of 1.22 to 3.65 points. Therefore, this apparent improvement must be placed into the context of the reliability of the QMG score as a primary efficacy measurement in clinical trials as outlined by Barohn et al Barohn 1998, who determined that a treatment must produce more than 2.6 units of change in QMG score to be of clinical significance. It therefore remains inconclusive as to whether 3,4‐DAP treatment of LEMS definitively improves QMG muscle score. The authors believe that the QMG score should remain as the preferred measure of muscle strength testing in future trials of treatment in LEMS. The use of a uniform primary outcome measure, and data from further trials would enable a more definitive effect to be delineated. The authors also believe that in keeping with previous studies, it is appropriate to continue to assess the effect of 3,4‐DAP treatment by performing a QMG assessment 3‐4 days after the initiation of treatment.
Although we were able to demonstrate (with meta‐analysis) a significant improvement in the secondary endpoint of mean CMAP amplitude following treatment with 3,4‐DAP, some statistical assumptions had to be employed in the analysis. This was because one of the four trials (McEvoy 1989) had a cross‐over design and we did not have access to individual patient data to help determine within‐patient treatment effects in the two cross‐over periods. However, we were able to use original data from the other three trials directly in the analysis, which revealed a significant overall improvement of CMAP to be determined on GIV testing. Therefore, change in mean CMAP amplitude following treatment seems to be an ideal, objective, and reproducible secondary endpoint for trials of treatment in LEMS. The authors believe that due to the short duration of action of 3,4‐DAP CMAPs should be recorded three to six hours after a dose of 3,4‐DAP, and that future studies should record the timing of CMAP assessment in relation to doses of the drug.
The results of the four trials of 3,4‐DAP treatment for LEMS showing significant benefit concur with earlier reports of 3,4‐DAP being beneficial for LEMS, and mirror current practice of using this drug for symptomatic first‐line treatment for people with LEMS.
Adverse events reported from 3,4‐DAP treatment during the trials (McEvoy 1989; Oh 2009; Sanders 2000; Wirtz 2009) included brief perioral tingling and digital paraesthesiae, insomnia, and epigastric discomfort, and Wirtz et al (Wirtz 2009) described a case of cellulitis following 3,4‐DAP infusion. In one study, a participant suffered from a seizure at a daily dose of 100 mg 3,4‐DAP (McEvoy 1989). No other major side effects have been reported.
Effects of IVIg
A single randomised placebo‐controlled cross‐over study showed a significant improvement in limb strength measured by myometry following IVIg treatment, compared with placebo (Bain 1996). Aside from the single randomised controlled trial detailed in this review (Bain 1996), there are very little data regarding the use of IVIg treatment for LEMS. Evidence from case reports (Bird 1992; Muchnik 1997; Takano 1994) and expert opinion would suggest that this is a potentially useful short‐term and long‐term treatment. However, it is likely that people with LEMS who have not responded favourably to IVIg have not been reported widely in the literature.
Adverse events reported from IVIg treatment during the single randomised trial (Bain 1996) include acute meningism in one participant and self‐limiting headache in four other participants. Other side effects have previously been reported in the literature (Dalakas 1999) and include neutropenia, leukopenia, cerebral or cardiac infarction due to hypercoaguable state, renal tubular damage, eczema, erythema multiforme and skin vasculitis.
Cost benefit considerations
Until recently, oral 3,4‐DAP was available as an unlicensed formulation as 3,4‐DAP base. However, a phosphate salt formulation of oral 3,4‐DAP (amifampridine) has recently been licensed in the UK for the treatment of LEMS in adults (BioMarin 2010).The original 3,4‐DAP base preparation cost approximately UK 1£ for a 20 mg tablet, and with the average dose used in one trial with long‐term follow‐up being 40 mg per day (Sanders 2000), the yearly expenditure for each person with LEMS equated to UK £730. The new licensed medication, amifampridine, is however far more expensive. Currently, the cost of one hundred 10 mg tablets in the UK is £2,017, and therefore using a 40 mg per day average dose would result in a yearly expenditure of £29,448 per patient (UKMi Pharmacists 2010). The increased cost of amifampridine may provide significant cost pressures for organisations.
The average cost of a two‐day course of IVIg using a dose of 1 g/kg/day (Bain 1996) is currently approximately UK £2,800. The muscle strength measurements recorded in the single randomised trial of IVIg in LEMS (Bain 1996) showed that the beneficial effect had dissipated by approximately eight weeks. If ongoing IVIg treatment were to be used for people who responded favourably, they would possibly require six similar treatment courses per year, at a total cost of UK £16,800. There are no direct comparison data regarding IVIg and 3,4‐DAP, but our standard deviation analysis (see above) would suggest no significant difference between the two treatments, and with the introduction of amifampridine and its increased cost, it may now potentially be a more viable treatment economically than amifampridine. However, it must be noted, that in the IVIg trial, participants were still weak despite treatment with 3,4‐DAP and immunosuppression, so the use of IVIg in these people was as a second (or third) line option for resistant muscle weakness. By contrast, 3,4‐DAP was the first line option in most of the participants in the two trials of that agent.
Authors' conclusions
Implications for practice.
Limited but moderate to high quality evidence from randomised controlled trials showed that either 3,4‐DAP or IVIg improved muscle strength scores and compound muscle action potential amplitudes in people with LEMS. There are insufficient data at present to quantify this treatment effect.
Implications for research.
Further trials of treatment for LEMS should use the QMG score as the primary outcome, and change in CMAP amplitude as the secondary outcome. The possible beneficial effect of IVIg should be validated in a further trial. Other possible treatments, such as plasma exchange, steroids and immunosuppressive agents should be tested in RCTs.
What's new
| Date | Event | Description |
|---|---|---|
| 12 October 2010 | New search has been performed | Review updated. We updated the search of the Cochrane Neuromuscular Disease Trials in October 2010, MEDLINE (January 1966 to September 2010) and EMBASE (January 1980 to September 2010). Two new trials were identified in addition to the three previous trials. |
| 14 September 2010 | New citation required but conclusions have not changed | Michael Keogh and Sam Sedehizadeh replace John Newsom‐Davis as authors. |
| 12 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 2 April 2007 | New search has been performed | We updated the search of the Cochrane Neuromuscular Disease Trials Register in April 2007, MEDLINE (January 1966 to February 2007) and EMBASE (January 1980 to February 2007). Two new trials were identified which are currently only available in abstract form and the data are not available for inclusion. |
| 6 January 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are indebted to the considerable statistical support and advice given by Dr A Swan, Statistician, Cochrane Neuromuscular Disease Group. We are also grateful for the help and support previously given by Professor John Newsom‐Davis (deceased) in the first version of this review. John Newsom Davis was a co‐author of the trial of IVIg in people with LEMS.
Appendices
Appendix 1. MEDLINE (OvidSP) search strategy
1 randomized controlled trial.pt. 2 controlled clinical trial.pt. 3 randomized.ab. 4 placebo.ab. 5 drug therapy.fs. 6 randomly.ab. 7 trial.ab. 8 groups.ab. 9 or/1‐8 10 (animals not (animals and humans)).sh. 11 9 not 10 12 Lambert‐Eaton Myasthenic Syndrome/ 13 LEMS.mp. 14 ((Lambert and Eaton and myasthen$) or (lambert and eaton and syndrom$)).mp. 15 or/12‐14 16 exp PYRIDINES/ 17 GUANIDINE/ 18 exp IMMUNOSUPPRESSIVE AGENTS/ or exp Immunosuppression/ 19 exp STEROIDS/ 20 AZATHIOPRINE/ 21 exp Immunoglobulins/ 22 or/16‐21 23 (guanidin$ or pyridostigmin$ or aminopyridin$ or AP or DAP or diaminopyridin$ or immunosuppres$ or steroid$ or prednisolone or azathioprine or (intravenous and immunoglobulin$) or IVIg).mp. 24 22 or 23 25 11 and 15 and 24
Appendix 2. EMBASE (OvidSP) search strategy
1 crossover‐procedure/ 2 double‐blind procedure/ 3 randomized controlled trial/ 4 single‐blind procedure/ 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. 6 clinical trial/ 7 or/1‐6 8 human/ or nonhuman/ 9 7 not 8 10 7 and human/ 11 9 or 10 12 Eaton Lambert Syndrome/ 13 LEMS.mp. (353) 14 ((Lambert and Eaton and myastheni$) or (lambert and eaton and syndrome$)).mp. 15 or/12‐14 16 exp Pyridine Derivative/ 17 Pyridostigmine/ 18 3,4 DIAMINOPYRIDINE/ 19 GUANIDINE/ 20 exp IMMUNOSUPPRESSIVE AGENT/ or exp IMMUNOSUPPRESSIVE TREATMENT/ 21 exp Steroid/ (886472) 22 azathioprine/ or azathioprine derivative/ 23 exp Immunoglobulin/ 24 or/16‐23 25 (guanidin$ or pyridostigmin$ or aminopyridin$ or AP or DAP or diaminopyridin$ or immunosuppres$ or steroid$ or prednisolone or azathioprine or (intravenous and immunoglobulin$) or IVIg).mp. 26 or/16‐25 27 11 and 15 and 26
Appendix 3. Cochrane Central Register of Controlled Trials search strategy
#1MeSH descriptor Lambert‐Eaton Myasthenic Syndrome, this term only #2LEMS #3Lambert and Eaton and (myasthenic or syndrome) #4(#1 OR #2 OR #3) #5MeSH descriptor Pyridines explode all trees #6MeSH descriptor Guanidine, this term only #7MeSH descriptor Immunosuppressive Agents explode all trees #8MeSH descriptor Immunosuppression explode all trees #9MeSH descriptor Steroids explode all trees #10MeSH descriptor Azathioprine, this term only #11MeSH descriptor Immunoglobulins explode all trees #12guanidin* or pyridostigmin* or aminopyridin* or AP or DAP or diaminopyridin* or immunosuppres* or steroid* or prednisolone or azathioprine or (intravenous and immunoglobulin) OR IVIg #13(#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #14(#4 AND #13)
Data and analyses
Comparison 1. 3,4‐diaminopyridine treatment versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in QMG score with generalised inverse variance model | 2 | Mean Difference (Fixed, 95% CI) | ‐2.44 [‐3.65, ‐1.22] | |
| 2 Improvement in mean CMAP amplitude with generalised inverse variance method (assumed r = 0.5 for within‐patient treatment effects in cross‐over trials) | 4 | Mean Difference (Fixed, 95% CI) | 1.36 [0.99, 1.72] | |
| 3 Improvement in mean CMAP amplitude (assumed r = 0.5 for within‐patient treatment effects in cross‐over trials) | 4 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.75 [0.93, 2.57] |
1.3. Analysis.

Comparison 1 3,4‐diaminopyridine treatment versus placebo, Outcome 3 Improvement in mean CMAP amplitude (assumed r = 0.5 for within‐patient treatment effects in cross‐over trials).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bain 1996.
| Methods | Double‐blind randomised cross‐over controlled trial | |
| Participants | 10 adults with LEMS1 fulfilling diagnostic criteria of AAEM2 (2001). One withdrawal after the placebo phase | |
| Interventions | Intravenous immunoglobulin 1 g/kg body weight/day for 2 days or 0.3% albumin placebo infusions. 8 weeks later, participants who received IVIg infusions were given placebo infusions, and vice versa | |
| Outcomes | Myometric limb strength, respiratory and bulbar strength measures, and calcium channel antibody titres. Measurements made at 2‐weekly intervals for the 8 week period following treatment or placebo infusions | |
| Notes | Significant improvement in myometric limb strength after IVIg3 compared with placebo infusion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A random allocation table was used |
| Allocation concealment? | Unclear risk | No information is given in the text as to the method of concealment used, and therefore insufficient to permit judgement |
| Blinding? All outcomes | Low risk | Though not outlined in the study text, discussions with the clinicians in this trial revealed an excellent standard of blinding both observers and participants |
| Incomplete outcome data addressed? All outcomes | Low risk | 1 participant was withdrawn from the study due to side effects after the first infusion. A good explanation of this was given, and the omission is unlikely to have clinically relevant impact on observed effect size |
| Free of selective reporting? | Low risk | The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
McEvoy 1989.
| Methods | Double‐blind randomised cross‐over controlled trial | |
| Participants | 12 adults with LEMS fulfilling diagnostic criteria of AAEM (2001) | |
| Interventions | Oral 3,4‐DAP up to 20 mg four times a day for 3 days or placebo tablets for 3 days. Participants received 3,4‐DAP first then received placebo for 3 days, and vice versa | |
| Outcomes | Improvement in a neurological disability score comprising muscle strength and reflexes; isometric myometry limb strength measures; compound muscle action potential amplitude change; autonomic function testing change. Measurements made at days 1, 3, 5, 9, 12 and 15 | |
| Notes | Significant improvement in neurological disability score and resting compound muscle action potential amplitude following 3,4‐DAP treatment compared with placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A random number table was used |
| Allocation concealment? | Unclear risk | Not enough information included to permit judgement |
| Blinding? All outcomes | Low risk | Blinding of participants and study personnel was adequate, however it was not clear whether placebo capsules were aesthetically similar to 3,4‐DAP4 capsules |
| Incomplete outcome data addressed? All outcomes | Low risk | 2 participants failed to tolerate a full dose of 3,4‐DAP due to side effects. It is unlikely that these missing outcomes have a clinically relevant impact on observed effect size |
| Free of selective reporting? | Low risk | The study protocol is available and all of the pre‐specified outcomes were included |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Oh 2009.
| Methods | Prospective randomised double‐blind cross‐over trial | |
| Participants | 8 participants with LEMS fulfilling diagnostic criteria of AAEM (2001). Three participants had small cell lung cancer. 1 participant was in complete stable remission and was used as control subject | |
| Interventions | Oral 3,4‐DAP was given to 7 participants. Trial length and doses varied. Three participants received 15 mg on day one increasing to 80 mg by day 8. Four participants received 30 mg increasing to 75mg over 3 days | |
| Outcomes | Several outcomes including subjective symptom score, LEMS classification, muscle strength score, QMG5 Score and CMAP6 amplitude were assessed over the study period | |
| Notes | Significant improvement in subjective symptom score, LEMS classification, muscle strength score, QMG score, and CMAP amplitude versus placebo and baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A random number table was used |
| Allocation concealment? | Low risk | Pharmacy controlled central allocation of assignment into groups, resulting in effective blinding to both investigators and participants |
| Blinding? All outcomes | Low risk | Blinding of participants and key study personnel was ensured |
| Incomplete outcome data addressed? All outcomes | Low risk | 1 participant withdrew from study, with appropriate reasons explained |
| Free of selective reporting? | Low risk | The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes have been reported in the pre‐specified way |
| Free of other bias? | Low risk | The study appears free from other sources of bias |
Sanders 2000.
| Methods | Double‐blind randomised parallel group controlled trial | |
| Participants | 26 adults with LEMS fulfilling diagnostic criteria of AAEM (2001) | |
| Interventions | Oral 3,4‐DAP 20 mg three times daily for 6 days. Control participants received identical appearing lactose placebo tablets for 6 days | |
| Outcomes | Primary: change from baseline QMG score. Secondary: changes in the amplitudes of compound muscle action potentials in abductor digiti minimi, abductor pollicis brevis, and extensor digitorum brevis | |
| Notes | Significant improvement in QMG score and resting CMAP amplitude following 3,4‐DAP treatment compared with placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A random allocation table was used |
| Allocation concealment? | Unclear risk | The random allocation table was maintained by the pharmacy involved in the study, but little further information is given to directly determine the degree of allocation concealment |
| Blinding? All outcomes | Low risk | Attempts to ensure participant blinding were good (ensuring that the placebo was aesthetically similar to the active drug). The only exception was that 4 of 14 participants on 3,4‐DAP experienced limb tingling giving a possible means of identification, though this would not have affected objective neurophysiology |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | All of the study's pre‐specified (primary and secondary) outcomes were reported in the pre‐specified way in keeping with their methods |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Wirtz 2009.
| Methods | Randomised double‐blind placebo controlled cross‐over trial | |
| Participants | 9 adults with LEMS fulfilling the diagnostic criteria of AAEM (2001) | |
| Interventions | 10 mg IV 3,4‐DAP infused over 60 mins for one treatment session.Then varying doses of IV pyridostigmine were infused over 1 minute, 40 minutes apart during the previously described 3,4‐DAP infusion. A further session of IV pyridostigmine only was conducted, together with double dummy placebos for the infusions and boluses | |
| Outcomes | Primary: (1) Isometric muscle strength (hip flexion) (2) Changes in the CMAP of the hypothenar muscles of the nondominant hand Secondary: (1) Decrement of CMAP amplitude during 3 Hz repetitive nerve stimulation and its increment after 10 s of maximum voluntary contraction |
|
| Notes | Significant improvement in isometric muscle testing and resting CMAP amplitude following 3,4‐DAP treatment. No additional benefit with the addition of pyridostigmine, and pyridostigmine in isolation showed no difference to the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A random assignment table was used |
| Allocation concealment? | Unclear risk | No information given about the randomisation process in either the full article or in the supplementary information online, and it was unclear who 'held' the random assignment table |
| Blinding? All outcomes | Low risk | Blinding of participants and key study personnel was ensured. However, it was not stated whether placebo infusions appeared identical to 3,4‐DAP infusions, though this would not affect objective neurophysiological testing |
| Incomplete outcome data addressed? All outcomes | Low risk | 2 participants were unable to undertake the final session for reasons that were well explained. It is unlikely that these missing outcomes have a clinically relevant impact on observed effect size |
| Free of selective reporting? | Low risk | All of the study's pre‐specified (primary and secondary) outcomes were reported in the pre‐specified way in keeping with their methods |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
1. LEMS: Lambert‐Eaton myasthenic syndrome 2. AAEM: American Association of Electrodiagnostic Medicine 3. IVIg: intravenous immunoglobulin 4. 3,4‐DAP: 3,4‐diaminopyridine 5. CMAP: compound muscle action potential
Differences between protocol and review
For this update we used revised risk of bias methodology (Higgins 2008) and added a 'Summary of findings' table. We also used a GIV analysis to analyse the effect of treatment due to the cross‐over study design of the two added studies in this review and of one existing study.
Contributions of authors
Michael Keogh performed the literature search, amended the original text, identified and assessed new trials and collated the data.
Saam Sedehizadeh reviewed the text, helped identify and assess all relevant trials, and critically reviewed the data.
Paul Maddison wrote the text original version, identified and assessed all relevant trials, and collated all the data.
Declarations of interest
Michael Keogh ‐ none known.
Saam Sedehizadeh ‐ none known.
Paul Maddison has received an honorarium from BioMarin, manufacturers of Firdapse, for an advisory meeting..
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bain 1996 {published data only}
- Bain PG, Motomura M, Newsom‐Davis J, Misbah SA, Chapel HM, Lee ML, et al. Effects of intravenous immunoglobulin on muscle weakness and calcium‐channel autoantibodies in the Lambert‐Eaton myasthenic syndrome. Neurology 1996;47(3):678‐83. [PUBMED: 8797464 ] [DOI] [PubMed] [Google Scholar]
McEvoy 1989 {published data only}
- McEvoy KE, Windebank AJ, Daube JR. 3,4‐diaminopyridine in Lambert‐Eaton myasthenic syndrome. Annals of Neurology 1988;24(1):122. [Google Scholar]
- McEvoy KM, Windebank AJ, Daube JR, Low PA. 3,4‐Diaminopyridine in the treatment of Lambert‐Eaton myasthenic syndrome. New England Journal of Medicine 1989;321(23):1567‐71. [PUBMED: 2555713] [DOI] [PubMed] [Google Scholar]
Oh 2009 {published and unpublished data}
- Oh SJ, Claussen GG, Hatanaka Y, Morgan MB. 3,4 ‐ Diaminopyridine is more effective than placebo in a randomized, double‐blind, cross‐over drug study in LEMS. Muscle and Nerve 2009;40(5):795‐800. [PUBMED: 19722254 ] [DOI] [PubMed] [Google Scholar]
Sanders 2000 {published data only}
- Sanders DB, Massey JM, Sanders LL, Edwards LJ. A randomized trial of 3,4‐diaminopyridine in Lambert‐Eaton myasthenic syndrome. Neurology 2000;54(3):603‐7. [PUBMED: 10680790] [DOI] [PubMed] [Google Scholar]
Wirtz 2009 {published and unpublished data}
- Wirtz PW, Verschuuren JJ, Dijk JG, Kam ML, Schoemaker RC, Hasselt JGC, Titulaer MJ, Tjaden UR, Hartigh J, Gerven JM. Efficacy of 3,4‐diaminopyridine and pyridostigmine in the treatment of Lambert‐Eaton myasthenic syndrome: a randomized, double‐blind, placebo‐controlled, crossover study.. Clinical Pharmacology and Therpautics July 2009;86(1):44‐8. [PUBMED: 19357643] [DOI] [PubMed] [Google Scholar]
Additional references
AAEM 2001a
- AAEM Quality Assurance Committee. American Association of Electrodiagnostic Medicine. Practice parameter for repetitive nerve stimulation and single fiber EMG evaluation of adults with suspected myasthenia gravis or Lambert‐Eaton myasthenic syndrome: summary statement. Muscle and Nerve 2001;24(9):1236‐8. [PUBMED: 11494280] [DOI] [PubMed] [Google Scholar]
AAEM 2001b
- AAEM Quality Assurance Committee. American Association of Electrodiagnostic Medicine. Literature review of the usefulness of repetitive nerve stimulation and single fiber EMG in the electrodiagnostic evaluation of patients with suspected myasthenia gravis or Lambert‐Eaton myasthenic syndrome. Muscle and Nerve 2001;24(9):1239‐47. [PUBMED: 11494281] [DOI] [PubMed] [Google Scholar]
Agoston 1978
- Agoston S, Weerden T, Westra P, Broekert A. Effects of 4‐aminopyridine in Eaton Lambert Syndrome. British Journal of Anaesthesia 1978;50(4):383‐5. [PUBMED: 656256] [DOI] [PubMed] [Google Scholar]
Barohn 1998
- Barohn RJ, McIntire D, Herbelin L, Wolfe GI, Nations S, Bryan WW. Reliability testing of the quantitative myasthenia gravis score. Annals of the New York Academy of Sciences 1998;841:769‐72. [PUBMED: 9668327] [DOI] [PubMed] [Google Scholar]
Besinger 1983
- Besinger UA, Toyka KV, Hömberg M, Heininger K, Hohlfeld R, Fateh‐Moghadam A. Myasthenia gravis: long‐term correlation of binding and bungarotoxin blocking antibodies against acetylcholine receptors with changes in disease severity. Neurology 1983;33(10):1316‐21. [PUBMED: 6684226] [DOI] [PubMed] [Google Scholar]
BioMarin 2010
- BioMarin Europe Ltd. Summary of Product Characteristics. Firdapse® (amifampridine). Date of revision of the text February 2010.
Bird 1992
- Bird SJ. Clinical and electrophysiologic improvement in Lambert‐Eaton syndrome with intravenous immunoglobulin therapy. Neurology 1992;42(7):1422‐3. [PUBMED: 1620360] [DOI] [PubMed] [Google Scholar]
Blumhardt 1977
- Blumhardt LD, Joekes AM, Marshall J, Philalithis PE. Guanidine treatment and impaired renal function in the Eaton‐Lambert syndrome. British Medical Journal 1977;1(6066):946‐7. [PUBMED: 851795] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalakas 1999
- Dalakas MC. Intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: present status and practical therapeutic guidelines. Muscle and Nerve 1999;22(11):1479‐97. [PUBMED: 10514226] [DOI] [PubMed] [Google Scholar]
Fukunaga 1983
- Fukunaga H, Engel AG, Lang B, Newsom‐Davis J, Vincent A. Passive transfer of Lambert‐Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proceedings of the National Academy of Sciences of the United States of America 1983;80(24):7636‐40. [PUBMED: 6584877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Lambert 1956
- Lambert EH, Eaton LM, Rooke ED. Defect of neuromuscular conduction associated with malignant neoplasms. American Journal of Physiology 1956;187(3):612‐3. [Google Scholar]
Lambert 1966
- Lambert EH. Defects of neuromuscular transmission in syndromes other than myasthenia gravis. Annals of the New York Academy of Sciences 1966;135(1):367‐84. [PUBMED: 5221351] [DOI] [PubMed] [Google Scholar]
Lambert 1971
- Lambert EH, Elmqvist D. Quantal components of end‐plate potentials in the myasthenic syndrome. Annals of the New York Academy of Sciences 1971;183:183‐99. [PUBMED: 4330759] [DOI] [PubMed] [Google Scholar]
Lang 1981
- Lang B, Newsom‐Davis J, Wray D, Vincent A, Murray N. Autoimmune aetiology for myasthenic (Eaton‐Lambert) syndrome. Lancet 1981;2(8240):224‐6. [PUBMED: 6114283] [DOI] [PubMed] [Google Scholar]
Lang 1983
- Lang B, Newsom‐Davis J, Prior C, Wray D. Antibodies to motor nerve terminals: an electrophysiological study of a human myasthenic syndrome transferred to mouse. Journal of Physiology 1983;344:335‐45. [PUBMED: 6655585] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lang 1987
- Lang B, Newsom‐Davis J, Peers C, Prior C, Wray DW. The effect of myasthenic syndrome antibody on presynaptic calcium channels in the mouse. Journal of Physiology 1987;390:257‐70. [PUBMED: 2450991] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lechat 1968
- Lechat P, Deysson G, Lemeignan M, Adolphe M. Comparison of acute toxicity of some aminopyridines in vivo (mice) and in vitro (tissue culture) [Toxicité aigue compareé de quelques aminopyridines in vivo (souris) et in vitro (cultures cellulaires)]. Annales Pharmaceutiques Francaises 1968;26(5):345‐9. [PUBMED: 5716404] [PubMed] [Google Scholar]
Lemeignan 1971
- Lemeignan M. Pharmacological approach to the study of convulsive action mechanism of amino‐4 pyridine [Abord pharmacologique de l'étude du mécanisme de l'action convulsivante de l'amino‐4 pyridine]. Therapie 1971;26(5):927‐40. [PUBMED: 4400759] [PubMed] [Google Scholar]
Lemeignan 1982
- Lemeignan M, Millart H, Letteron N, Liamable D, Josso J, Choisy H, et al. The ability of 4‐aminopyridine and 3,4‐diaminopyridine to cross the blood‐brain barrier can account for their difference in toxicity. In: Lechat P, Thesleff S, Bowman WC editor(s). Aminopyridines and similarly acting drugs: Effects on nerves, muscles and synapses. Advances in the Biosciences. Vol. 35, Oxford: Pergamon Press, 1982:222‐9. [Google Scholar]
Lennon 1995
- Lennon VA, Kryzer TJ, Griesmann GE, O'Suilleabhain PE, Windebank AJ, Woppmann A, et al. Calcium‐channel antibodies in the Lambert‐Eaton syndrome and other paraneoplastic syndromes. New England Journal of Medicine 1995;332(22):1467‐74. [PUBMED: 7739683] [DOI] [PubMed] [Google Scholar]
Lundh 1977
- Lundh H, Nilsson O, Rosén I. 4‐Aminopyridine ‐ a new drug tested in the treatment of Eaton‐Lambert syndrome. Journal of Neurology, Neurosurgery and Psychiatry 1977;40(11):1109‐12. [PUBMED: 202680] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 1978
- Lundh H. Effects of 4‐aminopyridine on neuromuscular transmission. Brain Research 1978;153(2):307‐18. [PUBMED: 210882] [DOI] [PubMed] [Google Scholar]
Lundh 1983
- Lundh H, Nilsson O, Rosén I. Novel drug of choice in Eaton‐Lambert syndrome. Journal of Neurology, Neurosurgery and Psychiatry 1983;46(7):684‐5. [PUBMED: 6310051] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 1993
- Lundh H, Nilsson O, Rosén I, Johansson S. Practical aspects of 3,4‐diaminopyridine treatment of the Lambert‐Eaton myasthenic syndrome. Acta Neurologica Scandinavica 1993;88(2):136‐40. [PUBMED: 8213058] [DOI] [PubMed] [Google Scholar]
Molgo 1980
- Molgó J, Lundh H, Thesleff S. Potency of 3,4‐diaminopyridine and 4‐aminopyridine on mammalian neuromuscular transmission and the effect of pH changes. European Journal of Pharmacology 1980;61(1):25‐34. [PUBMED: 6101553] [DOI] [PubMed] [Google Scholar]
Motomura 1995
- Motomura M, Johnston I, Lang B, Vincent A, Newsom‐Davis J. An improved diagnostic assay for Lambert‐Eaton myasthenic syndrome. Journal of Neurology, Neurosurgery and Psychiatry 1995;58(1):85‐7. [PUBMED: 7823075] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muchnik 1997
- Muchnik S, Losavio AS, Vidal A, Cura L, Mazia C. Long‐term follow‐up of Lambert‐Eaton syndrome treated with intravenous immunoglobulin. Muscle and Nerve 1997;20(6):674‐8. [PUBMED: 9149073] [DOI] [PubMed] [Google Scholar]
Murray 1981
- Murray NM, Newsom‐Davis J. Treatment with oral 4‐aminopyridine in disorders of neuromuscular transmission. Neurology 1981;31(3):265‐71. [PUBMED: 6259555] [DOI] [PubMed] [Google Scholar]
Newsom‐Davis 1984
- Newsom‐Davis J, Murray NM. Plasma exchange and immunosuppressive drug treatment in the Lambert‐Eaton myasthenic syndrome. Neurology 1984;34(4):480‐5. [PUBMED: 6322050] [DOI] [PubMed] [Google Scholar]
O'Neill 1988
- O'Neill JH, Murray NM, Newsom‐Davis J. The Lambert‐Eaton myasthenic syndrome: a review of 50 cases. Brain 1988;111(Pt 3):577‐96. [PUBMED: 2838124] [DOI] [PubMed] [Google Scholar]
Oh 1973
- Oh SJ, Kim KW. Guanidine hydrochloride in the Eaton‐Lambert syndrome. Electrophysiologic improvement. Neurology 1973;23(10):1084‐90. [PUBMED: 4355063] [DOI] [PubMed] [Google Scholar]
Oh 1997
- Oh SJ, Kim DS, Head TC, Claussen GC. Low‐dose guanidine and pyridostigmine: relatively safe and effective long‐term symptomatic therapy in Lambert‐Eaton myasthenic syndrome. Muscle and Nerve 1997;20(9):1146‐52. [PUBMED: 9270671] [DOI] [PubMed] [Google Scholar]
Roberts 1985
- Roberts A, Perera S, Lang B, Vincent A, Newsom‐Davis J. Paraneoplastic myasthenic syndrome IgG inhibits 45Ca2+ flux in a human small cell carcinoma line. Nature 1985;317(6039):737‐9. [PUBMED: 2414666] [DOI] [PubMed] [Google Scholar]
Takano 1994
- Takano H, Tanaka M, Koike R, Nagai H, Arakawa M, Tsuji S. Effect of intravenous immunoglobulin in Lambert‐Eaton myasthenic syndrome with small‐cell cancer: correlation with the titer of anti‐voltage‐gated calcium channel antibody. Muscle and Nerve 1994;17(9):1073‐5. [PUBMED: 8065398] [DOI] [PubMed] [Google Scholar]
Tindall 1987
- Tindall RS, Rollins JA, Phillips JT, Greenlee RG, Wells L, Belendiuk G. Preliminary results of a double‐blind, randomized, placebo‐controlled trial of cyclosporine in myasthenia gravis. New England Journal of Medicine 1987;316(12):719‐24. [PUBMED: 3547126] [DOI] [PubMed] [Google Scholar]
UKMi Pharmacists 2010
- UK Medicines information (UKMi) Pharmacists for NHE professionals. What is the difference between amifampridine and 3,4‐diaminopyridine base for Lambert‐Eaton myasthenic syndrome in adults?. Accessed via www.nelm.nhs.uk May 24th 2010.


