Abstract
Adhesion molecules may contribute to the development of interstitial lung disease (ILD) and have been proposed as prognostic biomarkers in idiopathic pulmonary fibrosis. Our objective was to determine whether circulating adhesion molecules sICAM-1, sVCAM-1 and P-selectin are associated with subclinical ILD in community-dwelling adults.
The Multi-Ethnic Study of Atherosclerosis enrolled men and women aged 45–84 from six communities in the United States in 2000–2002. High attenuation areas were defined as the percentage of imaged lung volume with attenuation −600 to −250 Hounsfield Units on cardiac computed tomography. Interstitial lung abnormalities (ILA) were visually assessed on a full lung computed tomography. Spirometry was performed on a subset of individuals. ILD hospitalizations and deaths were adjudicated.
In fully adjusted analyses, higher levels of sICAM-1, sVCAM-1 and P-selectin were associated with greater high attenuation areas (2.94%, 95%CI 1.80–4.07; 1.24%, 95%CI 0.14–2.35; 1.58%, 95%CI 0.92–2.23, respectively), and greater rate of ILD hospitalizations (HR 1.36, 95%CI 1.03–1.80; 1.40, 95%CI 1.07–1.85, 2.03, 95%CI 1.16–3.5, respectively). sICAM-1 was associated with greater prevalence of interstitial lung abnormalities (OR 1.39, 95%CI 1.13–1.71). sICAM-1 and P-selectin were associated with lower forced vital capacity (44ml, 95%CI 12–76; 29ml, 95%CI 8–49, respectively). sVCAM-1 and P-selectin were associated with increased risk of ILD death (HR 2.15, 95%CI 1.26–3.64; 3.61, 95%CI 1.54–8.46, respectively).
Higher levels of circulating sICAM-1, sVCAM-1, and P-selectin are independently associated with CT and spirometric measures of subclinical ILD, and increased rate of adjudicated ILD events among community-dwelling adults.
Introduction
Interstitial lung disease (ILD) is characterized by the presence of cellular proliferation, cellular infiltration, and/or fibrosis of the lung parenchyma not due to infection or malignancy [1, 2]. Cellular-level changes that lead to ILD likely begin years prior to the onset of symptoms [3]. Fibrotic ILDs generally have a poor prognosis and few treatment options [4]. The investigation of subclinical ILD has gained momentum in recent years as a way to identify novel risk factors and early mechanisms of lung fibrosis in humans [5–9]. These studies may lead to future interventions to prevent lung fibrosis [1].
Adhesion molecules mediate leukocyte recruitment into the lung, and may play a role in the development of ILD [10, 11]. Intracellular adhesion molecule-1 (ICAM-1) is overexpressed in idiopathic pulmonary fibrosis (IPF) lungs. Serum levels of soluble ICAM-1 (sICAM-1) and soluble vascular cell adhesion molecule-1 (sVCAM-1) correlate with the extent of fibrosis and poor survival in IPF, and have been proposed as potential prognostic biomarkers [10, 12, 13]. The selectin family of adhesion molecules, including E-selectin and L-selectin, are an important part of the innate immune response [11, 14]. P-selectin has been implicated in lung injury but has not been extensively studied in ILD [15]. The role of these adhesion molecules in adults with subclinical ILD is not known.
In the current study, we sought to determine the associations of circulating sICAM-1, sVCAM-1, E-selectin, L-selectin, and P-selectin with CT measures of subclinical ILD, forced vital capacity (FVC), and clinical ILD events in a large cohort of community-dwelling adults enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA). We hypothesized that higher levels of circulating cell adhesion molecules will be associated with greater high attenuation areas (HAA), higher odds of interstitial lung abnormalities (ILA), lower FVC, and increased risk of ILD events during follow-up.
Methods
Participants
MESA is a multi-center population-based prospective cohort study that enrolled 6814 men and women aged 45–84 without evidence of clinical cardiovascular disease at study entry. MESA participants were sampled from six U.S. communities in years 2000 to 2002, regardless of lung disease, respiratory symptoms, or smoking history, as previously described [16]. Circulating adhesion molecules were measured in a random sample of MESA participants stratified by race/ethnicity. The samples sizes for each biomarker and timeline of measurements are shown in Figure S1. Written informed consent was obtained from all participants and the study was approved by Institutional Review Boards at all collaborating centers.
Circulating adhesion molecules
sICAM-1 and E-selectin were measured in blood samples collected at the MESA baseline exam in years 2000–2002. Samples were stored at −80ºC and analyzed in year 2004 after the first thaw. sICAM-1 was measured by ELISA (Parameter Human sICAM-1 Immunoassay, R&D Systems) in plasma from 2,621 participants. E-selectin was measured by ELISA (Human Soluble E-Selectin kit, R&D Systems) in serum from 999 participants. sVCAM-1, L-Selectin and P-Selectin were measured in blood samples collected at the MESA follow-up exam in years 2002–2004. Samples were stored at −80ºC, sent for processing after one thaw and analyzed in years 2010–2011 after the second thaw cycle. Serum levels of sVCAM-1 and L-Selectin were measured by ELISA (Quantikine Human Soluble VCAM Immunoassay kit, Human Soluble L-Selectin Immunoassay kit, R&D Systems) in 2,440 participants. Plasma levels of P-selectin were measured in two independent batches from 6,147 participants by ELISA (Human Soluble P-Selectin Immunoassay kit, R&D Systems). Different sets of ELISA reagents and separate human control pool were used for each batch. The inter-assay coefficients of variation were 5.0%, 3.6%, 6.7%, 7.3% and 6.7% for sICAM-1, sVCAM-1, P-selectin, E-selectin and L-selectin, respectively.
Genotyping
The sICAM-1 ELISAs can differ in their ability to recognize two of the less frequent sICAM-1 variants due to poor binding to the immunoassay [17]. Thus, the genotype of the sICAM-1 single nucleotide polymorphism (SNP) rs5491 was attained from all consenting individuals in order to ensure that the results were not affected by inclusion of individuals with the AT and TT variants. Genotyping was performed using the ITMAT-Broad-CARe (IBC) microarray (Illumina, Inc; San Diego, CA).
High Attenuation Areas on CT scan
Lung attenuation was measured on exam 2 or 3 cardiac CT scans in 5,703 MESA participants at the University of Iowa Imaging Lab using a modified version of Pulmonary Analysis Software Suite. HAA was defined as percentage of imaged lung with attenuation between −600 and −250 Hounsfield units (HU), as previously described [5, 18]. Percent emphysema was defined as the percentage of lung voxels below −950 HU
Interstitial Lung Abnormalities on CT scan
Full-lung CT scans were performed at follow-up exam 5, in years 2010–2012, using multi-detector CT-scanners, as previously described [7]. A total of 2,907 participants had CT scans visually assessed by one of five expert radiologists (inter-reader kappa 0.47) for the presence of ILA, which was defined as the presence of ground-glass, reticular abnormalities, diffuse centrilobular nodularities, honeycombing, traction bronchiectasis, non-emphysematous cysts or architectural distortion in at least 5% of nondependent portions of the lung, as previously described [7, 19, 20]. Of these scans, 477 were classified as indeterminate for ILA and were excluded from ILA analyses.
Spirometry
Spirometry was performed at follow-up exams 3 and 4, between years 2004 and 2007, in accordance with American Thoracic Society/European Respiratory Society guidelines, using protocol that was previously described [21, 22].
Cause of death and hospitalization
Data on hospitalizations and deaths was obtained through direct contact with MESA participants or surrogates at 9–12 month intervals, and was supplemented by review of the National Death Index. Mortality data were complete as of March 2015 and hospitalization data were complete as of December 2013. A two-member adjudication panel adjudicated all ILD deaths and hospitalizations, as previously described [9]. Respiratory death was determined based on the ICD-10 diagnosis code for underlying cause of death on the death certificate.
Statistical Analysis
We examined the distributions of each biomarker using box plots and computed pearson correlation coefficients between individual adhesion molecules. We used generalized linear models to examine the associations of circulating adhesion molecules, modeled in a continuous fashion, with HAA and FVC. We report all results per standard deviation (SD) of each adhesion molecules to facilitate interpretation. HAA was expressed as percentage of total imaged lung volume and was log-transformed. We used logistic regression to examine the association of adhesion molecules with ILA. HAA models were adjusted for age, sex, body mass index, waist circumference, height, race/ethnicity, current smoking status and pack-year smoking history, educational attainment, glomerular filtration rate (GFR), study site/scanner, radiation dose, percent emphysema, and total imaged lung volume. ILA and FVC models were adjusted for age, gender, race, smoking status, BMI, and study site, as previously described [23]. We examined models stratified by SNP rs5491, smoking-status, age and BMI, and used the likelihood ratio test to test for effect modification. To account for differences in P-selectin measurement between the two batches, we obtained residuals from a linear regression model using log-transformed raw P-selectin values as a response variable and batch number as a predictor, as previously described, and used the P-selectin residuals in our fully adjusted models [24]. We used Cox regression and proportional means models, which allow for recurrent events, to examine the associations of circulating adhesion molecules with time to ILD death and hospitalization, respectively, as previously described [9]. Due to low event rates, we controlled for confounding in these models by calculating a generalized propensity score (GPS), as previously described [9]. We used generalized additive models with loess smoothers of continuous variables to examine for non-linear associations between our predictors and outcomes, and to generate graphs. Statistical significance was defined using the Benjamini-Hochberg procedure to control the false discovery rate at 0.05. All analyses were done in Stata (version 14) and R (version 3.5.1).
Results
The baseline characteristics of MESA participants overall and by quartile of each adhesion molecule are shown in Tables 1, and S1–S4. Overall, for participants with measured sICAM-1 and HAA, the mean (SD) age was 59 (9.6) years. 56% were female, 49% were white, 18% were black, 22% were Hispanic and 12% were Chinese. 45% were current or former smokers with a median of 15 pack years smoking history (Table 1). Participants in the highest quartile of sICAM-1 were more likely to be female, white or Hispanic, ever-smokers and have higher BMI. The mean (SD) levels of sICAM-1, sVCAM-1, P-selectin, E-selectin, and L-selectin were 274 ng/mL (78.4), 740 ng/mL (232.0), 36 ng/mL (13.9), 55 ng/mL (25.3), and 891 ng/mL (200.0), respectively (Figure S2). Pearson correlation coefficients demonstrated only weakly positive relationships between the adhesion molecules (Table S5).
Table 1.
Baseline characteristics of MESA participants by quartile of sICAM-1
| Quartile of sICAM-1 | |||||
|---|---|---|---|---|---|
| Overall | Q1 | Q2 | Q3 | Q4 | |
| Number | 2233 | 559 | 558 | 558 | 558 |
| sICAM-1 | 273.8 ± 78.39 | 187.84 ± 35.21 | 248.43 ± 11.02 | 288.26 ± 12.03 | 373.29 ± 70.38 |
| Age, years | 59.1 ± 9.6 | 58.1 ± 9.5 | 59.8 ± 9.8 | 60.0± 9.9 | 60.0 ± 9.7 |
| Women, % | 56.2 | 55 | 55.2 | 55.5 | 59.2 |
| BMI, kg/m2 | 28.3 | 27.1 | 29.1 | 29.1 | 29.9 |
| Waist Circumference, cm | 97.6 | 93.8 | 96.1 | 99.8 | 102 |
| Height, cm | 166.8 | 166.9 | 167.2 | 166.4 | 165.6 |
| eGFR, mL/min/1.73 m2 | 82.9 | 80.4 | 80.4 | 81.2 | |
| Race | |||||
| White, % | 49.2 | 30 | 55.1 | 55.7 | 49.1 |
| Chinese American, % | 12.2 | 24.2 | 13.2 | 6 | 3.3 |
| Black, African American, % | 18 | 30.8 | 13 | 13.2 | 17.3 |
| Hispanic, % | 20.8 | 14.7 | 18.7 | 25.1 | 30.2 |
| Smoking | |||||
| Ever Smokers, % | 45.7 | 47.7 | 45.3 | 49.6 | 60.9 |
| Current Smokers, % | 15.1 | 7.5 | 8.3 | 12.1 | 28.8 |
| Site | |||||
| Wake Forest, % | 16.8 | 13.4 | 18.9 | 14.4 | 17.3 |
| Columbia, % | 16.7 | 17.9 | 12.6 | 16.3 | 19 |
| John’s Hopkins, % | 12.7 | 15.6 | 11.5 | 12.9 | 12.1 |
| UMN, % | 15.8 | 6.6 | 14.1 | 14.34 | 24.8 |
| Northwestern, % | 15.5 | 19.5 | 18.4 | 17.2 | 8.3 |
| UCLA, % | 22.5 | 27 | 24.5 | 22.9 | 18.4 |
Data presented as mean +/− SD, n (%) or median (interquartile range), unless otherwise stated. All parameters were collected at MESA baseline visit in years 2000–2002. HAA: high attenuation areas; sICAM: soluble intracellular adhesion molecule; BMI: body mass index; eGFR: effective glomerular filtration rate
High Attenuation Areas
In fully adjusted models, higher levels of sICAM-1, sVCAM-1 and P-selectin were associated with greater HAA. Each SD increment of sICAM-1, sVCAM-1, and P-selectin was associated with a 2.94% (95% CI 1.80–4.07, p<0.001,), 1.24% (95% CI 0.14–2.35, p=0.03), and 1.58% (CI 0.92–2.23, p<0.001) increase in HAA, respectively (Table 2, Figure 1A–C). There was no evidence of effect modification by smoking status, age or BMI on the association between these adhesion molecules and HAA (p for interaction >0.08, Table S6–8). After correction for multiple comparisons, the p-values for sICAM-1, sVCAM-1 and P-selectin remained significant. Findings were consistent in a less adjusted model (Table S9). The effect estimate for sICAM-1 was similar when the analysis was limited to individuals with the AA genotype of SNP rs5491 (Table S10). There were no significant associations of E-selectin or L-selectin with HAA.
Table 2.
Associations of circulating adhesion molecules with HAA, ILA and FVC.
| sICAM-1 | P value | sVCAM-1 | P value | P-selectin | P value | E-selectin | P value | L-selectin | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| HAA | ||||||||||
| N participants | 2,226 | 2,187 | 5,498 | 864 | 2187 | |||||
| % change (95% CI)* | ||||||||||
| Unadjusted | 2.87 (1.38 to 4.36) | <0.001 | 1.82 (0.34 to 3.31) | 0.02 | 1.79 (0.87 to 2.72) | <0.001 | 4.87 (2.51 to 7.23) | 0.01 | −0.96 (−2.45 to 0.53) | 0.21 |
| Adjusted | 2.94 (1.80 to 4.07) | <0.001 | 1.24 (0.14 to 2.35) | 0.03 | 1.58 (0.92 to 2.23) | <0.001 | 0.64 (−1.21 to 2.42) | 0.51 | 0.40 (−0.70 to 1.49) | 0.48 |
| ILA | ||||||||||
| N participants | 1,028 | 1,008 | 2,239 | 507 | 1,008 | |||||
| OR (95% CI)† | ||||||||||
| Unadjusted | 1.41 (1.18 to 1.67) | <0.001 | 1.33 (1.13 to 1.56) | 0.001 | 1.10 (0.97 to 1.24) | 0.14 | 0.88 (0.67 to 1.17) | 0.37 | 0.91 (0.75 to 0.09) | 0.30 |
| Adjusted | 1.39 (1.13 to 1.71) | 0.002 | 1.20 (0.99 to 1.45) | 0.07 | 1.08 (0.94 to 1.24) | 0.27 | 0.96 (0.79 to 1.30) | 0.78 | 0.93 (0.75 to 1.15) | 0.49 |
| FVC | ||||||||||
| N participants | 1,549 | 1,680 | 3,449 | 808 | 1,688 | |||||
| Change, mL (95% CI)† | ||||||||||
| Unadjusted | −47.8 (−94.5 to −0.6) | 0.05 | −77.8(−120.0 to −35.6) | 0.001 | 60.7 (30.0 to 91.5) | <0.001 | −36.81 (−102.6 to 28.9) | 0.27 | −9.47 (−51.8 to 32.9) | 0.66 |
| Adjusted | −44.1 (−75.9 to −12.4) | 0.006 | −29.1 (−57.7 to −0.4) | 0.047§ | −28.5 (−49.1 to −8.0) | 0.007 | −43.9 (−87.6 to −0.1) | 0.049§ | 10.6 (−17.7 to 38.9) | 0.46 |
sICAM-1: soluble intracellular adhesion molecule; sVCAM: soluble vascular adhesion molecule; HAA: high attenuation areas; ILA: interstitial lung abnormalities; FVC: forced vital capacity; OR: odds ratio
Effect estimates are per standard deviation of each adhesion molecule.
adjusted for age, sex, body mass index, waist circumference, race/ethnicity, eGFR, current smoking status and pack-year smoking history, educational attainment, study site/scanner, mA dose, percent emphysema, and total imaged lung volume.
adjusted for age, gender, race, smoking status, eGFR, BMI and site
p value no longer significant after Benjamini-Hochberg correction for multiple comparisons
Figure 1.
Continuous associations of adhesions molecules sICAM-1, sVCAM-1 and P-selectin with HAA (A-C, respectively), ILA (D-F, respectively), and FVC (G-I, respectively). HAA models (A-C) are adusted for age, sex, body mass index, waist circumference, height, race/ethnicity, current smoking status and pack-year smoking history, educational attainment, glomerular filtration rate (GFR), study site/scanner, radiation dose, percent emphysema, and total imaged lung volume. ILA and FVC models (D-I) are adjusted for age, gender, race, smoking status, BMI, and study site. Solid line is the overall effect estimate, and dashed lines are the 95% confidence bands. Each vertical hashmark in the rug plot along the x-axis represent one study participant.
Interstitial Lung Abnormalities
In fully adjusted models, higher levels of sICAM-1 were associated with a greater prevalence of ILA on CT scan. Each SD increment of sICAM-1 was associated with 1.39 (95% CI 1.13–1.71, p=0.002) higher odds of ILA, (Table 2, Figure 1D–E). Unadjusted analyses by ILA subtype are shown in Table S11. Each SD increment of sICAM-1 was associated with 1.39 (95% CI 1.14 to 1.70, p=0.001) higher odds of fibrotic ILA subtypes, as defined by the presence of reticular abnormalities, traction bronchiectasis or honeycombing. There was no evidence of effect modification by smoking status, age or BMI on the association between sICAM-1 and ILA (p for interaction >0.05, Table S6–8). When corrected for multiple comparisons, the p-value remained significant. Results for sICAM-1 were similar in analyses restricted to individuals with AA genotype of SNP rs5491 (Table S10). There were no significant associations of sVCAM-1, P-selectin, E-selectin or L-selectin with ILA (Table 2, Figure 2F). Each SD increment of VCAM-1 was associated with 1.34 higher odds of fibrotic ILA (Table S11).
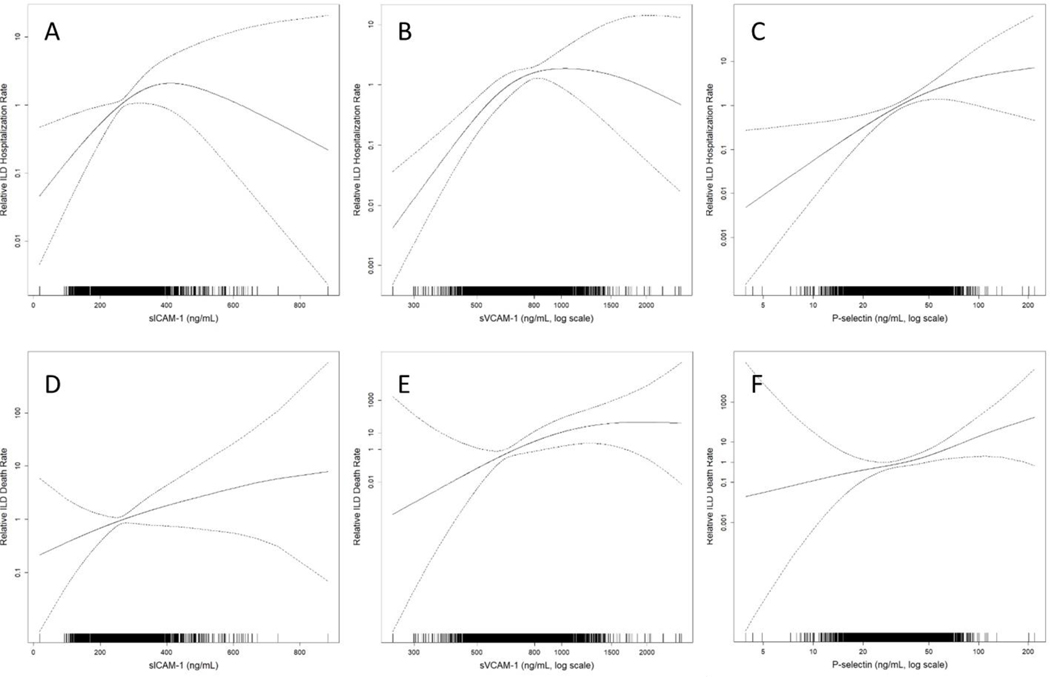
Figure 2.
Continuous associations of adhesions molecules sICAM-1, sVCAM-1 and P-selectin with ILD hospitalization (A-C, respectively), and death due to ILD (D-F, respectively). Models adjusted for generalized propensity score 2, which included age, gender, race/ethnicity, smoking status, cigarette pack-years, BMI, waist circumference, height, educational attainment, study site/scanner, glomerular filtration rate, radiation dose, alcohol use, total intentional exercise (metabolic equivalent minutes/week), coronary artery calcium, diabetes medication use, insulin use, fasting glucose, hypertension, antihypertensive medication use, systolic and diastolic blood pressures, cholesterol medication use, total and high-density lipoprotein cholesterol levels, C-reactive protein, d-dimer, and cancer history. Solid line is the overall effect estimate, and dashed lines are the 95% confidence bands. Each vertical hashmark in the rug plot along the x-axis represent one study participant.
Lung function
In fully adjusted models, higher levels of sICAM-1 and P-selectin were associated with a lower FVC. Each SD increment in sICAM-1 and P-selectin was associated with a 44.1mL (95% CI 12.4–75.9, p=0.006) and 28.5ml (95% CI 8.0–49.1, p=0.007) lower FVC, respectively (Table 2, Figures 1G–I). The association of sICAM-1 with FVC was modified by smoking status, with stronger effect estimates among ever-smokers (p for interaction 0.01, Table S6). There was no evidence of effect modification by age or BMI (p for interaction >0.05, Table S7–8). There were significant associations of sVCAM-1 and E-selectin with FVC among ever-smokers, although the p values for interaction were not significant (0.08 and 0.28, respectively, Table S6). Association between adhesion molecules with percent predicted FVC are shown in Table S12. Results were consistent when participants with an obstructive ventilator defect (FEV1/FVC ratio< 70%) were excluded from the analyses and among participants with the AA genotype of SNP rs5491 (Tables S9 and S13). There was no significant association between L-selectin and FVC.
ILD hospitalizations and death
In fully adjusted models, higher levels of sICAM-1, sVCAM-1 and P-selectin were all associated with greater rate of respiratory hospitalizations among those with ILD, as well as hospitalizations due to ILD. Each SD increment of sICAM-1, sVCAM-1 and P-selectin was associated with a HR of 1.36 (95% CI 1.03–1.80, p=0.03), 1.40 (95% CI 1.07–1.85, p=0.02), and 2.03 (95% CI 1.16–3.55, p=0.01) for ILD hospitalization, respectively (Table 3, Figure 2A–C). In fully adjusted models, both sVCAM-1 and P-selectin were associated with increased risk of death due to respiratory causes and ILD deaths. Each SD increment in sVCAM-1 and P-selectin was associated with a HR of 2.15 (95% CI 1.26–3.64, p=0.005) and 3.61(95% CI 1.54–8.46, p=0.003) for ILD death, respectively (Table 3, Figure 2E–F). There was a trend toward an increased risk for both respiratory and ILD mortality in participants with higher sICAM-1 levels, but the association did not reach statistical significance (Table 3, Figure 2D). There were no significant associations of E-selectin and L-selectin with ILD events.
Table 3.
Associations circulating adhesion molecules with ILD events.
| sICAM-1 | P value | sVCAM-1 | P value | P-selectin | P value | E-selectin | P value | L-selectin | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Respiratory hospitalization with concomitant ILD | ||||||||||
| Number of events | 20 | 18 | 77 | 9 | 18 | |||||
| Person-years (PY) | 30,295 | 28,014 | 70,176 | 11,457 | 28,014 | |||||
| Rate per 10,000 PY (95% CI) | 6.6 (4.3 to 10.2) | 6.4 (4.0 to 10.2) | 11.0 (8.8 to 13.7) | 7.9 (4.1 to 15.1) | 6.4 (4.0 to 10.2) | |||||
| Hazard ratio (95% CI) | ||||||||||
| Unadjusted | 1.75 (1.46 to 2.10) | <0.001 | 1.56 (1.27 to 1.90) | <0.001 | 1.73 (1.23 to 2.43) | 0.002 | 1.39 (1.02 to 1.89) | 0.04 | 1.09 (0.67 to 1.71) | 0.78 |
| Adjusted: Model 1* | 1.36 (1.13 to 1.63) | 0.001 | 1.62 (1.21 to 2.16) | 0.001 | 1.84 (1.24 to 2.72) | 0.002 | 1.29 (0.71 to 2.35) | 0.40 | 1.09 (0.65 to 1.84) | 0.75 |
| Adjusted: Model 2† | 1.34 (1.09 to 1.66) | 0.006 | 1.68 (1.25 to 2.27) | <0.001 | 2.07 (1.25 to 3.43) | 0.005 | 1.42 (0.75 to 2.70) | 0.29 | 1.09 (0.66 to 1.80) | 0.75 |
| ILD hospitalization | ||||||||||
| Number of events | 12 | 11 | 44 | 8 | 11 | |||||
| Person-years (PY) | 30,295 | 28,014 | 70,176 | 11,457 | 28,014 | |||||
| Rate per 10,000 PY (95% CI) | 4.0 (2.3 to 7.0) | 3.9 (2.2 to 7.1) | 6.3 (4.7 to 8.4) | 7.0 (3.5 to 14.0) | 3.9 (2.2 to 7.1) | |||||
| Hazard ratio (95% CI) | ||||||||||
| Unadjusted | 1.70 (1.32 to 2.21) | <0.001 | 1.57 (1.21 to 2.05) | <0.001 | 1.95 (1.32 to 2.88) | <0.001 | 1.34 (0.94 to 1.91) | 0.11 | 1.10 (0.52 to 2.33) | 0.80 |
| Adjusted: Model 1* | 1.30 (1.00 to 1.70) | 0.047§ | 1.53 (1.24 to 1.89) | <0.001 | 1.87 (1.19 to 2.92) | 0.007 | 1.25 (0.65 to 2.41) | 0.51 | 1.03 (0.58 to 1.84) | 0.91 |
| Adjusted: Model 2† | 1.36 (1.03 to 1.80) | 0.03 | 1.40 (1.07 to 1.85) | 0.02 | 2.03 (1.16 to 3.55) | 0.01 | 1.30 (0.72 to 2.36) | 0.38 | 1.05 (0.56 to 1.97) | 0.87 |
| Respiratory mortality | ||||||||||
| Number of events | 28 | 25 | 63 | 7 | 25 | |||||
| Person-years (PY) | 30,295 | 28,014 | 70,176 | 11,457 | 28,014 | |||||
| Rate per 10,000 PY (95% CI) | 9.2 (6.4 to 13.4) | 8.9 (6.0 to 13.2) | 9.0 (7.0 to 11.5) | 6.1 (2.9 to 12.8) | 8.9 (6.0 to 13.2) | |||||
| Hazard ratio (95% CI) | ||||||||||
| Unadjusted | 1.43 (1.08 to 1.91) | 0.01§ | 1.71 (1.42 to 2.06) | <0.001 | 1.48 (1.14 to 1.92) | 0.003 | 1.78 (1.13 to 2.81) | 0.01§ | 0.85 (0.56 to 1.29) | 0.43 |
| Adjusted: Model 1* | 1.25 (0.94 to 1.65) | 0.13 | 1.80 (1.38 to 2.36) | <0.001 | 1.49 (1.12 to 1.98) | 0.006 | 1.95 (0.97 to 3.94) | 0.06 | 0.85 (0.59 to 1.23) | 0.39 |
| Adjusted: Model 2† | 1.29 (0.96 to 1.72) | 0.09 | 2.06 (1.56 to 2.72) | <0.001 | 1.54 (1.15 to 2.08) | 0.004 | 1.95 (1.04 to 3.65) | 0.04§ | 0.84 (0.58 to 1.21) | 0.35 |
| ILD mortality | ||||||||||
| Number of events | 9 | 7 | 16 | 3 | 7 | |||||
| Person-years (PY) | 30,295 | 28,014 | 70,176 | 11,457 | 28,014 | |||||
| Rate per 10,000 PY (95% CI) | 3.0 (1.5 to 5.7) | 2.5 (1.2 to 5.2) | 2.3 (1.4 to 3.7) | 2.6 (0.8 to 8.1) | 2.5 (1.2 to 5.2) | |||||
| Hazard ratio (95% CI) | ||||||||||
| Unadjusted | 1.83 (1.31 to 2.55) | <0.001 | 1.71 (1.21 to 2.43) | 0.003 | 2.04 (1.25 to 3.33) | 0.004 | 1.35 (0.56 to 3.26) | 0.51 | 1.47 (0.76 to 2.87) | 0.25 |
| Adjusted: Model 1* | 1.41 (0.93 to 2.14) | 0.11 | 2.02 (1.19 to 3.41) | 0.009 | 3.30 (1.40 to 7.80) | 0.006 | 1.28 (0.45 to 3.65) | 0.64 | 1.29 (0.67 to 2.50) | 0.45 |
| Adjusted: Model 2† | 1.39 (0.94 to 2.07) | 0.10 | 2.15 (1.26 to 3.64) | 0.005 | 3.61 (1.54 to 8.46) | 0.003 | 1.36 (0.52 to 3.58) | 0.53 | 1.29 (0.66 to 2.53) | 0.45 |
ILD: interstitial lung disease; HR: hazard ratio
Effect estimates are per standard deviation of each adhesion molecule.
Adjusted for generalized propensity score 1, which includes age, gender, race/ethnicity, smoking status, cigarette pack-years, BMI, waist circumference, height, educational attainment, study site, and glomerular filtration rate.
Adjusted for generalized propensity score 2, which includes age, gender, race/ethnicity, smoking status, cigarette pack-years, BMI, waist circumference, height, educational attainment, study site, glomerular filtration rate, percent emphysema on CT, alcohol use, total intentional exercise (metabolic equivalent minutes/week), coronary artery calcium, diabetes medication use, insulin use, fasting glucose, hypertension, antihypertensive medication use, systolic and diastolic blood pressures, cholesterol medication use, total and high-density lipoprotein cholesterol levels, C-reactive protein, d-dimer, and cancer history.
p value no longer significant after correcting for multiple comparisons
Discussion
We found that higher levels of circulating sICAM-1, sVCAM-1, and P-selectin were each independently associated with CT measures of subclinical ILD, lower FVC, increased rate of adjudicated ILD hospitalizations, and ILD death among community-dwelling adults. E-selectin was associated with lower FVC and respiratory mortality, but not our other outcome measures. These findings strongly support roles for circulating adhesion molecules sICAM-1, sVCAM-1 and P-selectin in the development of ILD and offer important insights into the biologic processes behind previous studies showing association of HAA and ILA with disease progression and mortality[9, 25].
ICAM-1 is essential for leukocyte diapedesis, which may be important in the earliest stages of pulmonary fibrosis [10, 12]. In the healthy lung, ICAM-1 is expressed in alveolar macrophages and, at low levels, in type II alveolar epithelial cells (AEC’s) and capillary endothelial cells [26, 27]. In mouse models, administration of bleomycin leads to upregulation of ICAM-1 expression on macrophages and type II AEC’s [10]. In humans with IPF, plasma concentrations of sICAM-1 are associated with poor progression-free and overall survival [12]. Ours is the first study to demonstrate associations between circulating levels of sICAM-1 and subclinical ILD. These findings may point to a role for sICAM-1 as a serum marker of type II AEC injury, or perhaps of activated macrophages, both of which have been implicated in the pathobiology of early ILD. Aaron et al previously showed that higher sICAM-1 levels are associated with greater progression of emphysema, but not with longitudinal change in lung function, as measured by FEV1 or FEV1/FVC [28]. We demonstrate an association of sICAM-1 with both CT measures of early ILD and lower FVC, even after adjusting for emphysema. Further studies are needed to determine whether sICAM-1 is directly involved in the pathobiology of early ILD, or is a biomarker of the disease process.
VCAM-1 is expressed within the vascular endothelium and functions similarly to ICAM-1 with regard to leukocyte recruitment [27]. Exposure to radiation increases endothelial expression of endothelial VCAM-1 in mouse models of pulmonary fibrosis [29]. In humans with IPF, both lung tissue expression of VCAM-1 and plasma levels of sVCAM-1 are significantly increased compared to controls [30]. Plasma levels of sVCAM-1 have also been shown to be predictive of mortality [12]. VCAM-1 is densely expressed in fibrotic foci of IPF lungs and its protein and mRNA levels significantly increase when human lung fibroblasts are treated with TGF-β [30]. Therefore, VCAM-1 may play a role in fibroblast proliferation in addition to leukocyte recruitment.
Ours is the first study to implicate P-selectin in the development and progression of ILD in humans. P-selectin is expressed on the surface of both activated endothelial cells and platelets [31]. When platelets adhere to endothelium at a site of injury, P-selectin on the surface of platelets binds neutrophils, monocytes and lymphocytes and slows their flow [31]. Activated platelets, bound to the capillary endothelium, also induce fibrin binding and upregulate endothelial expression of ICAM-1 and VCAM-1 [32]. Little is known about the role of P-selectin in the development of fibrosis, but there is substantial data supporting the role of platelets and P-selectin in acute lung injury, which is particularly relevant given that repetitive injury to alveolar membrane has been implicated in the development of ILD, and subclinical ILD has been linked to increased risk of acute respiratory distress syndrome [33]. In animal models of acute lung injury, plasma levels of P-selectin correlated with the extent of lung inflammation after infusion of lipopolysaccharide, and an increase in platelet-derived P-selectin led to downstream ICAM-1 expression and neutrophil activation [34, 35]. In patients with acute respiratory distress syndrome, plasma P-selectin levels are associated with both higher lung injury scores and increased mortality [2, 15]. Our findings of an association of plasma P-selectin levels with subclinical ILD may therefore reflect increased platelet activity, fibroblast activation and upregulation of other adhesion molecules in early ILD.
We found that E-selectin was associated with lower FVC and respiratory mortality, but not with other measures of subclinical ILD, and that L-selectin was not associated with any of our outcomes. E-selectin is expressed on endothelial cells and has been shown to be upregulated in bleomycin-induced endothelial injury [36]. Given the relatively low sample size of the E-selectin analyses, it is possible that our study was not powered to detect an association between E-selectin and subclinical ILD. Alternatively, it is possible that E-selectin plays a role in more advanced stage of ILD, especially given prior data, which showed that in IPF lungs, E-selectin expression is restricted to areas of honeycombing [37]. L-selectin is constitutively expressed on the surface of leukocytes and its expression is not affected by endothelial perturbation, which may be one explanation for our null findings [38].
The association between sICAM-1and lung function was modified by smoking status. Smoking is a well-known risk factor for development of IPF and tobacco use is associated with both decreased FVC as well as increased HAA in the MESA cohort [5]. Underlying mechanisms may include oxidative stress to alveolar epithelium, and the release of profibrotic factors like TGF-β from pulmonary fibroblasts [39]. Schaberg et al demonstrated that smoking upregulates endothelial expression of ICAM-1 within the peripheral pulmonary vasculature, suggesting that smoking may lead to recruitment of leukocytes [40]. Thus, while higher sICAM-1 levels are associated with a decrease in FVC when adjusted for smoking status, smoking and sICAM-1 may interact to jointly cause additional oxidative stress to the epithelium and recruitment of leukocytes.
There were several limitations to our study. First, all adhesion molecules were measured in serum or plasma rather than lung tissue or single lung cells and thus may not fully reflect pathobiological changes in the lung. Moreover, measurements were done in stored samples and were not run in duplicate. Therefore, there is some concern about protein degradation or even estimate inflation. However, we believe that the relative differences are important even if the absolute values are not completely accurate. Second, adhesion molecules were measured at a single time point, limiting our ability to study the associations of longitudinal trends in these adhesion molecules with both subclinical and clinical measures of ILD. Third, analyses of clinical ILD events are limited by low event rates. Fourth, multiple comparisons increase the chances of type I error while varying sample sizes limits our ability to compare results between biomarkers. Finally, while we adjusted for a number of demographic and clinical variables in our models, there remains the potential that residual confounding may affect some or all of our results.
In summary, we found that circulating adhesion molecules sICAM-1, sVCAM-1 and P-selectin are linked to CT measures of early lung injury, inflammation and fibrosis, lower FVC, and clinical ILD events in community-dwelling adults, supporting their role as a potential peripheral blood biomarkers of subclinical ILD. These findings suggest that sICAM-1, sVCAM-1 and P-Selectin may be important to the pathogenesis to ILD, and warrant future studies of the role these adhesion molecules play in the development and progression of pulmonary fibrosis.
Supplementary Material
Footnotes
Take-Home Message
In community-dwelling adults, adhesion molecules sICAM-1, sVCAM-1 and P-selectin are associated with CT measures of early lung injury, lower FVC, ILD hospitalizations and deaths, suggesting they may contribute to the development of pulmonary fibrosis.
Publisher's Disclaimer: “This is an author-submitted, peer-reviewed version of a manuscript that has been accepted for publication in the European Respiratory Journal, prior to copy-editing, formatting and typesetting. This version of the manuscript may not be duplicated or reproduced without prior permission from the copyright owner, the European Respiratory Society. The publisher is not responsible or liable for any errors or omissions in this version of the manuscript or in any version derived from it by any other parties. The final, copy-edited, published article, which is the version of record, is available without a subscription 18 months after the date of issue publication.”
REFERENCES
- 1.Rosas IO, Dellaripa PF, Lederer DJ, Khanna D, Young LR, Martinez FJ. Interstitial lung disease: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc 2014: 11 Suppl 3: S169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bone RC, Francis PB, Pierce AK. Intravascular coagulation associated with the adult respiratory distress syndrome. Am J Med 1976: 61(5): 585–589. [DOI] [PubMed] [Google Scholar]
- 3.Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP 3rd. Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs 2004: 64(4): 405–430. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ, Fibrosis AEJACoIP. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011: 183(6): 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, Austin JH, Jiang R, Lovasi GS, Barr RG. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med 2009: 180(5): 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Podolanczuk AJ, Oelsner EC, Barr RG, Hoffman EA, Armstrong HF, Austin JH, Basner RC, Bartels MN, Christie JD, Enright PL, Gochuico BR, Hinckley Stukovsky K, Kaufman JD, Hrudaya Nath P, Newell JD Jr, Palmer SM, Rabinowitz D, Raghu G, Sell JL, Sieren J, Sonavane SK, Tracy RP, Watts JR, Williams K, Kawut SM, Lederer DJ. High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study. Eur Respir J 2016: 48(5): 1442–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estepar RS, Lynch DA, Brehm JM, Andriole KP, Diaz AA, Khorasani R, D’Aco K, Sciurba FC, Silverman EK, Hatabu H, Rosas IO, Investigators CO. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med 2011: 364(10): 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, Okajima Y, Dupuis J, Latourelle JC, Cho MH, El-Chemaly S, Coxson HO, Celli BR, Fernandez IE, Zazueta OE, Ross JC, Harmouche R, Estepar RS, Diaz AA, Sigurdsson S, Gudmundsson EF, Eiriksdottir G, Aspelund T, Budoff MJ, Kinney GL, Hokanson JE, Williams MC, Murchison JT, MacNee W, Hoffmann U, O’Donnell CJ, Launer LJ, Harrris TB, Gudnason V, Silverman EK, O’Connor GT, Washko GR, Rosas IO, Hunninghake GM, Evaluation of CLtIPSEI, Investigators CO. Association Between Interstitial Lung Abnormalities and All-Cause Mortality. JAMA 2016: 315(7): 672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podolanczuk AJ, Oelsner EC, Barr RG, Bernstein EJ, Hoffman EA, Easthausen IJ, Stukovsky KH, RoyChoudhury A, Michos ED, Raghu G, Kawut SM, Lederer DJ. High-Attenuation Areas on Chest Computed Tomography and Clinical Respiratory Outcomes in Community-Dwelling Adults. Am J Respir Crit Care Med 2017: 196(11): 1434–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez IE, Amarie OV, Mutze K, Konigshoff M, Yildirim AO, Eickelberg O. Systematic phenotyping and correlation of biomarkers with lung function and histology in lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2016: 310(10): L919–927. [DOI] [PubMed] [Google Scholar]
- 11.Bless NM, Tojo SJ, Kawarai H, Natsume Y, Lentsch AB, Padgaonkar VA, Czermak BJ, Schmal H, Friedl HP, Ward PA. Differing patterns of P-selectin expression in lung injury. Am J Pathol 1998: 153(4): 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, Li K, Choi J, Vuga LJ, Lindell KO, Klesen M, Zhang Y, Gibson KF. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2012: 185(1): 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakao A, Hasegawa Y, Tsuchiya Y, Shimokata K. Expression of cell adhesion molecules in the lungs of patients with idiopathic pulmonary fibrosis. Chest 1995: 108(1): 233–239. [DOI] [PubMed] [Google Scholar]
- 14.Hamaguchi Y, Nishizawa Y, Yasui M, Hasegawa M, Kaburagi Y, Komura K, Nagaoka T, Saito E, Shimada Y, Takehara K, Kadono T, Steeber DA, Tedder TF, Sato S. Intercellular adhesion molecule-1 and L-selectin regulate bleomycin-induced lung fibrosis. Am J Pathol 2002: 161(5): 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakamaki F, Ishizaka A, Handa M, Fujishima S, Urano T, Sayama K, Nakamura H, Kanazawa M, Kawashiro T, Katayama M, et al. Soluble form of P-selectin in plasma is elevated in acute lung injury. Am J Respir Crit Care Med 1995: 151(6): 1821–1826. [DOI] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002: 156(9): 871–881. [DOI] [PubMed] [Google Scholar]
- 17.Register TC, Burdon KP, Lenchik L, Bowden DW, Hawkins GA, Nicklas BJ, Lohman K, Hsu FC, Langefeld CD, Carr JJ. Variability of serum soluble intercellular adhesion molecule-1 measurements attributable to a common polymorphism. Clin Chem 2004: 50(11): 2185–2187. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, Reinhardt J, Rodriguez J, Stukovsky K, Wong ND, Barr RG. Reproducibility and validity of lung density measures from cardiac CT Scans--The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol 2009: 16(6): 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, Sciurba FC, Hunninghake GM, San Jose Estepar R, Silverman EK, Rosas IO, Hatabu H. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol 2010: 17(1): 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, Nishino M, Araki T, Zazueta OE, Kurugol S, Ross JC, San Jose Estepar R, Murphy E, Steele MP, Loyd JE, Schwarz MI, Fingerlin TE, Rosas IO, Washko GR, O’Connor GT, Schwartz DA. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med 2013: 368(23): 2192–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, Force AET. Standardisation of spirometry. Eur Respir J 2005: 26(2): 319–338. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest 2010: 137(1): 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong HF, Podolanczuk AJ, Barr RG, Oelsner EC, Kawut SM, Hoffman EA, Tracy R, Kaminski N, McClelland RL, Lederer DJ. Serum Matrix Metalloproteinase-7, Respiratory Symptoms, and Mortality in Community-Dwelling Adults. MESA (Multi-Ethnic Study of Atherosclerosis). Am J Respir Crit Care Med 2017: 196(10): 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bielinski SJ, Berardi C, Decker PA, Kirsch PS, Larson NB, Pankow JS, Sale M, de Andrade M, Sicotte H, Tang W, Hanson NQ, Wassel CL, Polak JF, Tsai MY. P-selectin and subclinical and clinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2015: 240(1): 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller ER, Putman RK, Vivero M, Hung Y, Araki T, Nishino M, Washko GR, Rosas IO, Hatabu H, Sholl LM, Hunninghake GM. Histopathology of Interstitial Lung Abnormalities in the Context of Lung Nodule Resections. Am J Respir Crit Care Med 2018: 197(7): 955–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paine R 3rd, Christensen P, Toews GB, Simon RH. Regulation of alveolar epithelial cell ICAM-1 expression by cell shape and cell-cell interactions. Am J Physiol 1994: 266(4 Pt 1): L476–484. [DOI] [PubMed] [Google Scholar]
- 27.Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell 1990: 62(1): 3–6. [DOI] [PubMed] [Google Scholar]
- 28.Aaron CP, Schwartz JE, Bielinski SJ, Hoffman EA, Austin JH, Oelsner EC, Donohue KM, Kalhan R, Berardi C, Kaufman JD, Jacobs DR Jr, Tracy RP, Barr RG. Intercellular adhesion molecule 1 and progression of percent emphysema: the MESA Lung Study. Respir Med 2015: 109(2): 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epperly MW, Sikora CA, DeFilippi SJ, Gretton JE, Bar-Sagi D, Archer H, Carlos T, Guo H, Greenberger JS. Pulmonary irradiation-induced expression of VCAM-I and ICAM-I is decreased by manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) gene therapy. Biol Blood Marrow Transplant 2002: 8(4): 175–187. [DOI] [PubMed] [Google Scholar]
- 30.Agassandian M, Tedrow JR, Sembrat J, Kass DJ, Zhang Y, Goncharova EA, Kaminski N, Mallampalli RK, Vuga LJ. VCAM-1 is a TGF-beta1 inducible gene upregulated in idiopathic pulmonary fibrosis. Cell Signal 2015: 27(12): 2467–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz JN, Kolappa KP, Becker RC. Beyond thrombosis: the versatile platelet in critical illness. Chest 2011: 139(3): 658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammwohner M, Ittenson A, Dierkes J, Bukowska A, Klein HU, Lendeckel U, Goette A. Platelet expression of CD40/CD40 ligand and its relation to inflammatory markers and adhesion molecules in patients with atrial fibrillation. Exp Biol Med (Maywood) 2007: 232(4): 581–589. [PubMed] [Google Scholar]
- 33.Selman M, King TE, Pardo A, American Thoracic S, European Respiratory S, American College of Chest P. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001: 134(2): 136–151. [DOI] [PubMed] [Google Scholar]
- 34.Hirose M, Murai T, Kawashima H. Elevation of rat plasma P-selectin in acute lung injury. Biochim Biophys Acta 2007: 1772(3): 382–389. [DOI] [PubMed] [Google Scholar]
- 35.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest 2006: 116(12): 3211–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao JK. Linking endothelial dysfunction with endothelial cell activation. J Clin Invest 2013: 123(2): 540–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi S, Abe K, Matsuoka H, Goya S, Morishita H, Mori M, Arai T, Kida H, Nishino K, Takeda Y, Osaki T, Tachibana I, Kimura K, Yokota S, Inoue Y, Sakatani M. Increased level of soluble E-selectin in the serum from patients with idiopathic pulmonary fibrosis. Inflammation 2004: 28(1): 1–5. [DOI] [PubMed] [Google Scholar]
- 38.Kelly M, Hwang JM, Kubes P. Modulating leukocyte recruitment in inflammation. J Allergy Clin Immunol 2007: 120(1): 3–10. [DOI] [PubMed] [Google Scholar]
- 39.Nagy J, Demaster EG, Wittmann I, Shultz P, Raij L. Induction of endothelial cell injury by cigarette smoke. Endothelium 1997: 5(4): 251–263. [DOI] [PubMed] [Google Scholar]
- 40.Schaberg T, Rau M, Oerter R, Liebers U, Rahn W, Kaiser D, Witt C, Lode H. Expression of adhesion molecules in peripheral pulmonary vessels from smokers and nonsmokers. Lung 1996: 174(2): 71–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.