Abstract
Streptomyces ghanaensis ATCC14672 is remarkable for its production of phosphoglycolipid compounds, moenomycins, which serve as a blueprint for the development of a novel class of antibiotics based on inhibition of peptidoglycan glycosyltransferases. Here we employed mariner transposon (Tn) mutagenesis to find new regulatory genes essential for moenomycin production. We generated a library of 3000 mutants which were screened for altered antibiotic activity. Our focus centred on a single mutant, HIM5, which accumulated lower amounts of moenomycin and was impaired in morphogenesis as compared to the parental strain. HIM5 carried the Tn insertion within gene ssfg_01967 for putative tRNA (N6-isopentenyl adenosine(37)-C2)-methylthiotransferase, or MiaB, and led to a reduced level of thiomethylation at position 37 in the anticodon of S. ghanaensis transfer ribonucleic acid (tRNA). It is likely that the mutant phenotype of HIM5 stems from the way in which ssfg_01967::Tn influences translation of the rare leucine codon UUA in several genes for moenomycin production and life cycle progression in S. ghanaensis . This is the first report showing that quantitative changes in tRNA modification status in Streptomyces have physiological consequences.
Keywords: streptomyces, tRNA modification, MiaB, moenomycin, morphogenesis
Introduction
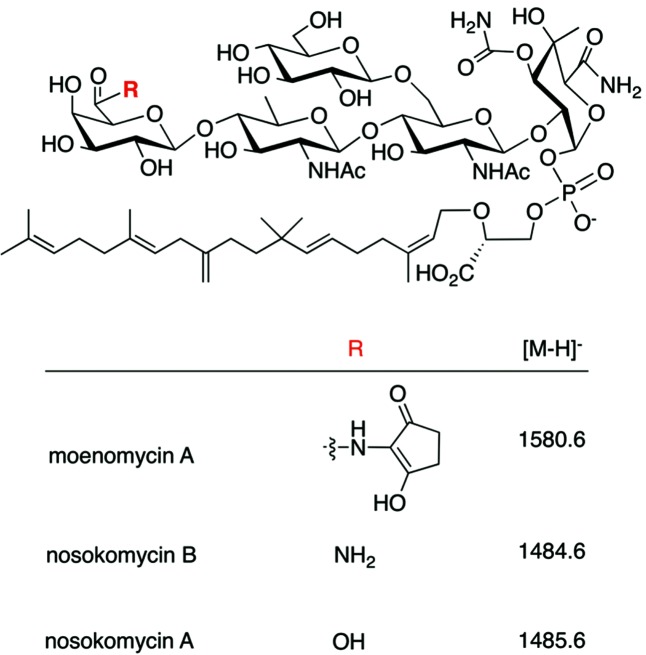
Streptomyces ghanaensis ATCC14672 produces a mixture of natural phosphoglycolipid compounds, collectively known as moenomycins. Moenomycin A (MmA; Fig. 1) is a 'founding member' of the phosphoglycolipid family of antibiotics and has attracted interest for several reasons. First, the overall chemical structure of MmA is unique as it contains unusually tailored carbohydrates and other building blocks (such as 3-phosphoglycerate) rarely found in secondary metabolites. Second, the biosynthesis of MmA comrpises many unusual features, at both the genetic and biochemical level, that are worth studying on their own [1]. For example, moenomycin biosynthesis is notable due to an absence of regulatory, resistance and building block supply genes, leading to a highly parsimonious metabolic pathway [2]. However, MmA is highly effective against Gram-positive pathogens (including vancomycin- and methicillin-resistant cocci), and its unique mode of action has fuelled interest in this class of natural products. They are considered a promising model and tool in the development of novel classes of antibiotics that act through direct inhibition of peptidoglycan glycosyltransferases, essential enzymes involved in the penultimate step of bacterial cell wall formation [3, 4].
Fig. 1.
Structures of moenomycin family antibiotics mentioned in this work: moenomycin A (MmA), nosokomycin B (NoB) and nosokomycin A (NoA). The masses of anions (in Da) monitored during LC-MS experiments are shown.
Access to reasonable amounts of various MmA analogues is key to its transformation into a drug. Due to its structural complexity, MmA remains a formidable target for total synthesis [5, 6]. It may be that the only economically viable route towards moenomycins generation would be biologically (fermentation)based. S. ghanaensis ATCC14672 produces small quantities of moenomycins, necessitating the development of improved strains. Identification of regulatory genes involved in MmA biosynthesis would help rationalize the search for moenomycin over-producers.
Over the last decade we have clarified the genetic control of MmA biosynthesis in S. ghanaensis and revealed a number of regulators of this pathway [1, 7, 8]. Notably, most of these affect not only MmA biosynthesis but also aerial mycelium formation in solid culture. This observation is in agreement with the assumption that MmA biosynthesis is not governed at the pathway-specific level, but rather relies on a circuit of pleiotropic regulators coupling secondary metabolism to morphogenesis. Given the complexity of the aforementioned circuits, as they are understood from studies of a few model strains such as S. coelicolor [9, 10], our understanding of the regulation of MmA biosynthesis is probably far from complete.
Sequence homology-driven approaches continue to reveal more players in the MmA biosynthesis regulatory network [11], yet they cannot lead to genuinely new regulators because no homologues have been described in other streptomycetes. Recently we developed the vector mariner transposon (Himar1) for Streptomyces , which efficiently provided random and uniform Tn mutagenesis of S. coelicolor and S. albus genomes [12]. We reasoned that Himar1 mutagenesis might be useful in the search for a hitherto unknown class, or classes, of genes that orchestrate antibiotic biosynthesis. The MmA producer is an excellent model for such efforts, as MmA biosynthesis is independent of cluster-situated regulators. MmA biosynthesis is also evolutionarily and biochemically distant from paradigmatic polyketides, as well as non-ribosomal peptide synthase-derived natural products. Here we report the investigation of Himar1-generated mutant S. ghanaensis HIM5 impaired in expression of the gene ssfg_01967 (miaB) for (dimethylallyl)adenosine tRNA methylthiotransferase (MiaB). HIM5 is characterized by decreased levels of moenomycin production, partially blocked aerial hyphae and spore formation as compared to the parent strain. On combining different lines of evidence, we suggest that the observed phenotype of HIM5 is largely a result of post-transcriptional under-modification of tRNALeu UAA necessary for decoding of the rare leucyl codon, UUA, within several S. ghanaensis genes. These genes are responsible for both moenomycin production and morphological development. Because miaB orthologues are omnipresent in actinobacterial genomes, it is likely that similar translational control mechanisms operate in other Streptomyces spp.
Methods
Bacterial strains and culture conditions
Bacterial strains and plasmids used in this work are listed in Table 1. Escherichia coli strains were grown at 37 °C in LB or 2×TY for routine applications. Streptomyces strains were grown at 30 °C unless otherwise stated. Solid oatmeal medium (OM) [13] or mannitol–soy medium (MS) [14] were used for sporulation of streptomycetes and plating of E. coli – Streptomyces matings. For total DNA and RNA isolation, streptomycetes were grown in tryptic soy broth (TSB) for 48–60 h. Moenomycin production by S. ghanaensis strains was assayed in TSB [8], and on solid media TSA and Bennett [13]. Total RNA was isolated from S. ghanaensis cultures grown in TSB. Where required, bacteria were grown in the presence of antibiotics and chromogenic substrates as described elsewhere [15].
Table 1. Strains and plasmids used in this work.
| Bacterial strain or plasmid | Description* | Source or reference |
|---|---|---|
| E. coli DH5α | supE44 ΔlacU169(φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | NEB |
| E. coli ET12567 (pUZ8002) | dam-13::Tn9(Cmr) dcm-6 hsdM; harbors conjugative plasmid pUZ8002; Cmr, Kmr | C.P.Smith, UMIST, UK; |
| E. coli WM6026 | lacIq rrnB3 ΔlacZ4787 hsdR514 ΔaraBAD567 ΔrhaBAD568 rph-1 attλ::pAE12 (ΔoriR6K-cat::Frt5) ΔendA::Frt uidA(ΔMluI)::pir attHK::pJK1006Δ (oriR6K-cat::Frt5 trfA::Frt) | [58] |
| E. coli BW25113 (pKD46) | (F-(φ80dΔ(lacZ)M15 recA1 endA1 gyrA96 thi1 deoR (lacZYA-argF)U169); λ bet- exo -gam; aph (Kmr) | J. Beckwith, HMS |
| S. ghanaensis ATCC14672 | Wild-type moenomycin producer | ATCC |
| S. ghanaensis B38.3 | Markerless deletion of moeH5; produces NoA | [21] |
| S. ghanaensis HIM5 | Himar1 insertion into ssfg_01967 gene of B38.3 | This work |
| Bacillus cereus ATCC19637 | Moenomycin-sensitive test culture | ATCC |
| pTES | Amr; phiC31-based Streptomyces integrative vector; expression of cloned gene from ermEp* | [59] |
| pKC1132 | Amr; conjugative suicide vector for gene disruption | [14] |
| pIJ6902 | AmrThr; phiC31-based Streptomyces integrative vector; expression of cloned gene from tipAp |
[60] |
| pHYG | pLitmus-derived vector containing hygromycin resistance cassette hyg; HyrApr | J. Salas, Universidad de Oviedo |
| patt-shyg | hygromycin cassette flanked by B-CC and P-GG sites for IMES recombination system | [61] |
| pGUS | Promoter probe vector, contains promoterless gusA; derived from pSET152 |
[20] |
| pGUSHL4aadA | pGUS derivative for translational fusions | [20] |
| padpAscript | pGUS derivative, transcriptional fusion of adpAp (0.5 kb KpnI-XbaI fragment) to gusA, AmrSpr |
[8] |
| padpAtransl | pGUSHL4aadA derivative, adpA-gusA translational fusion, AmrSpr | [8] |
| padpAcontr | pGUSHL4aadA, promoterless adpAgh-gusA fusion, AmrSpr | [8] |
| pmiaB | pKC1132 carrying 5 kb BamHI-HindIII fragment centred on ssfg_01967, Amr | This work |
| pmiaBhyg | pmiaB derivative, replacement of with hyg cassette from patt-shyg, AmrHyr | This work |
| pBluescriptIIKS | Apr; general purpose cloning vector; blue–white selection | ThermoScientific |
| pOOB120 | pBluescriptIIKS carrying 1.8 kb adpA-adpA fragment | This work |
| pOOB121 | pOOB120 with TTA codon within adpA replaced with CTC | This work |
| padpAtransl-CTC | pGUSHL4aadA carrying adpAp-adpA from pOOB121 | This work |
| pOOB106 | pTES carrying gene ssfg_01967 under ermEp* promoter | This work |
| pOOB106a | pOOB106 with aac(3)IV marker changed to hyg | This work |
| pOOB108L | pIJ6902 carrying gene ssfg_01967 under tipAp promoter | This work |
| pOOB113a | pOOB108L with aac(3)IV marker changed to hyg | This work |
*Cmr, Kmr, Amr, Spr, Thr, Hyr, Apr: chloramphenicol, kanamycin, apramycin, spectinomycin, thiostrepton, hygromycin and ampicillin resistance, respectively
DNA manipulations
Genomic and plasmid DNA from Streptomyces and plasmid DNA from E. coli were isolated using standard protocols [14, 15]. E. coli transformation and intergeneric E. coli – Streptomyces matings were performed as previously described [14]. Restriction endonucleases, Klenow fragment, bacterial alkaline phosphatase, T4 DNA ligase, Q5 DNA polymerase and AMV reverse transcriptase were purchased from standard commercial sources (Thermo Scientific, NEB, Amersham Biosciences, Invitrogen) and used according to the manufacturers’ instructions. PCR experiments were performed using thermal cyclers Mastercycler (Eppendorf) and T-100 (Biorad). DNA sequencing was carried out at the Biopolymers Facility of Harvard Medical School.
RNA isolation and semi-quantitative RT-PCR analysis
After 48 and 72 h of growth S. ghanaensis , mycelia were collected from 10 ml samples with centrifugation (3 min, 4 °C), immediately frozen in liquid nitrogen and stored at −80 °C until required for RNA isolation. The samples were thawed on ice, re-suspended in 1 ml of RNAProtect solution (Ambion) and spun down for 30 s at room temperature. Cells were re-suspended in 450 mcl of TE buffer supplemented with 4 mg of lysozyme and 10 u.a. of proteinase K, and incubated in an Eppendorf thermomixer (950 r.p.m., 37 °C) for 30 min. The resulting solution was processed according to RNAeasy (Qiagen) instructions with on-column DNA digestion. The RNA isolated was further treated with DNAse according to RNAqueous (Ambion) instructions. RNA concentration and purity were determined by measuring the ratio of OD260/OD280 and test PCR. An equal amount of RNA from each sample was used for RT reactions. cDNA was obtained using a Cloned AMV First-Strand Synthesis Kit (Invitrogen) and random hexanucleotide primers. PCR was performed using Q5 DNA polymerase (NEB) and primer pairs specific to each individual gene (Table 2). As a positive control, the hrdB primer pair specific to S. ghanaensis was used. Negative controls were carried out with hrdB primers to confirm the absence of contaminating DNA in the RNA preparations. PCR products were analysed by electrophoresis in 1.5 % agarose gel, and band intensity was established by ImageJ1.36b software (National Institutes of Health, Bethesda, MD).
Table 2. Primers used in this study.
| Name | Sequence (5′→3′) | Purpose |
|---|---|---|
| pMODfor | CCAACGACTACGCACTAGCCAAC | Sequencing of the rescue plasmids |
| pTn5Oksfor | ATTCAGGCTGCGCAACTG | |
| rt_hrdB_ghana_up | GAGTTCGGCGACCTCATTG | RT-PCR analysis of S. ghanaensis genes |
| rt_hrdB_ghana_rp | CGTCTTGGACTCGATCTGG (240 bp) | |
| rt_moeO5_up | GAATACCCCGCGAGGAGTG | |
| rt_moeO5_rp | GCTGTCGAGGTACTCGGTG (302 bp) | |
| rt_01967_up | TCCAGGAGGAGATCTCCTG | |
| rt_01967_rp | GCTTCTCCCAGGCGTCAC (280 bp) | |
| rt_adpA_gh_up | GGAATCGATCTGTGCCTGC | |
| rt_adpA_gh_rp | GGTGATCAGCCACTGCAGC | |
| Ssfg_01967_XbaI_up | AAATCTAGAGGAAATGATCGAGAACTCC | Cloning of miaB |
| Ssfg_01967_EcoRI_rp | AAAGAATTCGTCCGACTCCGTCACGAC | |
| ssfg_01967_HindIIIup | AAAAAGCTTGAGGGTGACGGTGACGGTAC | Cloning of 5 kb fragment for miaB knockout |
| ssfg_01967_XbaIrp | AAATCTAGAGGTCGAAGGCCACGGAGG | |
| Ssfg_01967red_up | GCGGAGGCCGTCCGCGGCACCGCCTACCCTTA | RedET-assisted replacement of miaB with hyg cassette |
| GGGCATGTTCCGGGGATCCGTCGACCC | ||
| Ssfg_01967red_rp | GCCGAGCCGTGCGTCCGCCCCCTCGTCGCGGAC | |
| CGGTCA TGTAGGCTGGAGCTGCTTCG | ||
| aqua_adpA_f | TACCTCGACCGGTCTCTCCCGGAG | TTA→CTC replacement |
| aqua_adpA_r | GCCGATCTCCTCCGGGAGAGACCG |
tRNA isolation and analysis of post-transcriptional tRNA modifications
Transfer RNA was purified as previously described [16, 17]. Analysis of nucleosides was carried out by LC-MS/MS. A UHPLC system (Vanquish Flex Quaternary, Thermo Fisher Scientific, San Jose, CA) was employed. Reversed phase chromatography was carried out using a high-strength silica column (Acquity UPLC HSS T3, 1.8 µm, 1.0×50, Waters, Milford, MA), 5.3 mM ammonium formate in water, pH 4.5, as mobile phase A, and a mixture of acetonitrile/water (40 : 60) with 5.3 mM ammonium formate as mobile phase B. The LC gradient consisted of: 0 % B (from 0 to 6.3 min), 2 % B at 13.1 min, 3 % B at 16 min, 5 % B at 21.4 min, 25 % B at 24.6 min, 50 % B at 26.9 min, 75 % B at 30.2 min (hold for 0.3 min), 99 % B at 33 min (hold for 6 min) then returning to 0 % B at 39 min, followed by a re-equilibration step at 0 % B for 16 min prior to the next injection. A flow rate of 60 µl min−1 was used. The column temperature was set at 30 °C.
An Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Fisher Scientific) interfaced with an H-ESI electrospray source (Thermo Fisher Scientific) was used. The analyses were carried out in positive polarity. Full-scan data were acquired at a resolution of 120 000, mass range 220–900 m/z, AGC 7.5×104 and IT 100 ms. Data-dependent top speed MS/MS (1 s cycle) were acquired at a resolution of 15 000, AGC 1.0×104, and IT 200 ms. CID was employed for MS/MS fragmentation. The collision energy setting for CID was 42 %, quadrupoleisolation of 1 m/z, ion funnel RF level 35 %; sheath gas, auxiliary gas and sweep gas of 30, 10 and 0 arbitrary units, respectively; ion transfer tube temperature of 289 °C; vaporizer temperature of 92 °C; and spray voltage of 3.5 kV. Two micrograms of S. ghanaensis tRNA were hydrolysed to nucleosides, suspended in mobile phase A and injected in the mass spectrometer.
Data processing was done using the Qual browser of Xcalibur 3.0. Accurate m/z values of the precursor and fragment ions were computed by ChemCalc (http://www.chemcalc.org). Relative abundance of listed modifications is shown as peak overlays in the main text.
Plasmid construction
ssfg_01967 expression
A 1780 bp fragment containing ssfg_01967 and its 202 bp upstream region was amplified from S. ghanaensis genomic DNA with primers ssfg_01967_XbaI_up and ssfg_01967_EcoRI_rp. Sequences of primers used throughout this work are given in Table 2. The resulting amplicon was digested with XbaI and EcoRI and cloned into the same sites of vectors pTES and pIJ6902 to give pOOB106 and pOOB108L, respectively. The apramycin resistance marker aac(3)IV was replaced in the pOOB106 and pOOB108L hygromycin resistance cassette hyg (plasmid pHYG) using recombineering approach and primers described in [18] to generate plasmids pOOB106a (pOOB106-derived) and pOOB113a (pOOB108L-derived).
ssfg_01967 disruption
A 5345 bp fragment centred on ssfg_01967 was amplified from S. ghanaensis genomic DNA with primers ssfg_01967_HindIIIup and ssfg_01967_XbaIrp and cloned into respective sites of pKC1132 to give pmiaB. The coding sequence of ssfg_01967 was replaced with the hyg cassette (plasmid patt-shyg) with the help of recombineering to give pmiaBhyg.
TTA-free adpA translational fusion
An XbaI-EcoRV 1.8 kb fragment containing adpAp-adpA was sub-cloned from padpAtransl into the respective sites of pBluescriptIIKS to give pOOB120a. The TTA codon within the adpA coding sequence was replaced with CTC using an AQUA cloning approach [19] and primers aqua_adpA_f and aqua_adpA_r. The resulting plasmid was labelled pOOB121. adpAp-adpA(CTC) was retrieved as a 1.8 kb XbaI-EcoRV fragment from pOOB121 and cloned into respective sites of pGUSHL4aadA, where the gene of interest is fused to gusA via helical linker HL4. The resulting plasmid was referred to as padpAtransl-CTC.
Himar1-mediated transposon mutagenesis of S. ghanaensis
E. coli ET12567 (pUZ8002) was freshly transformed with Himar1 transposon suicide vector pHAM and used for Tn mutagenesis as described in [12]. Apramycin-resistant colonies were picked onto master plates after 5 days of growth, and then analysed for antibiotic resistance markers and antibiotic activity. The Himar1 insertion site in HIM5 mutant has been rescued as re-ligated SacI and NcoI fragments of HIM5 genome in pir + strain E. coli WM6026.
Antibiotic production and resistance assays
Moenomycin production was analysed by disc assays and LC-MS as described in [8]. In LC-MS, we monitored the NoA anion [1485.6 Da (M-H)−, see Fig. 1], and the mean value of its mass peak area in strain B38.3 was taken as 100 %. The extracts were spiked with equal amounts of moenomycin A (to a final concentration of 1 µM) as an internal standard. Only the measurement series that showed equal peak areas for the moenomycin A anion (1580.6 Da) across the samples were taken into account. Amounts of NoA were referred back to equal amounts of biomass (dry weight) and were mean values from three independent experiments. Antibiotic resistance was analysed by the disc diffusion method. We used commercially available discs (for aminoglycosides, lactams, macrolides, rifampicin and cephalosporins) or prepared them from fresh antibiotic stocks and sterile ∅ 5 mm Whatman discs (for thiostrepton and kasugamycin, 50 µg per disc).
Glucuronidase activity assay
This was carried out as described in [8, 20]. All experiments were done in triplicate. Values were back-referred to equal amounts of dry biomass (10 mg) and are presented as mean±2 standard errors of the mean (SEM).
Scanning electron microscopy
This was carried out as described in [13].
Results
Himar1-based transposon mutagenesis of S. ghanaensis
We selected nosokomycin A (NoA, see Fig. 1) producer S. ghanaensis B38.3 [21] as a host for Himar1 mutagenesis, for two reasons. First, this strain produces a simplified spectrum of moenomycins (reduced virtually to a single compound, in contrast to a mixture of five to seven moenomycins in ATCC14672), which greatly facilitates downstream quantification of antibiotic production. Second, NoA is produced in trace amounts necessitating the strain improvement. We therefore reasoned that transposon mutagenesis might directly lead to improved strains. Because the initial screening of the mutants is to be based on high-throughput but low-resolution agar plug antibiotic activity assay, we expected to identify S. ghanaensis mutants displaying large (at least threefold) changes in antibiotic activity.
The Himar1 transposon was transferred into the B38.3 strain from E. coli , and apramycin-resistant (Amr) transconjugants were initially selected. In total we generated a library of 3000 Amr colonies, which occurred at a mean frequency of 10−5. These colonies were screened for hygromycin susceptibility (Hys), an indicator of transposon vector loss. All presumable Himar1+ colonies turned out to be Hys, attesting to the high efficiency of transposition and vector loss that we observed previously for model streptomycetes [12]. To assess the diversity of the mutant library generated, it was replica-plated (in a 96-well format) onto minimal agar. This revealed 18 auxotrophs, of which 13 were deficient in amino acid synthesis, 2 were deficient in vitamin synthesis and 3 were deficient in the synthesis of nucleotide-related metabolites. These results led us to believe that the transposant library carries a reasonable fraction of random Himar1 insertions and is suitable for locating mutants with altereded moenomycin titre.
The Himar1+ strains were grown individually in 96-well plates, and agar plugs resulting from 120 h of growth were tested against moenomycin-sensitive Bacillus cereus . We found no strains with increased total antibiotic production, but discovered five mutants with no or significantly decreased antibiotic activity. Further work showed that four of these mutants carry Himar1 insertions in either moenomycin biosynthesis (moeGT1) or known pleiotropic regulators of secondary metabolism (adpA, ssfg_05897 for RNA polymerase omega subunit), or genes for uncharacterized protein Ssfg_05414, and thus their further study was not pursued (data not shown). We finally focused our work on a single mutant, referred to here as HIM5.
HIM5 mutant: initial characterization
Besides apramycin resistance (transposon marker gene), HIM5 possesses an antibiotic resistance profile identical to that of the parental B38.3 strain (data not shown). In liquid cultures (rich TSB and minimal defined SMMS media), HIM5 and B38.3 exhibited similar growth dynamics (Fig. S1, ESM, available in the online version of this article), while on a number of solid media the mutant displayed impaired formation of spores, as evident from visual inspection of plates and scanning microscopy (Figs 2 and S2, ESM). The latter revealed sparse formation of spore chains; those that occured rarely resembled wild-type spores in their morphology. LC-MS analysis confirmed that the decrease in total antibiotic activity of HIM5 is due to reduced levels of NoA production (Fig. 3). Sequencing of rescue plasmids revealed that HIM5 carries the Himar1 insertion within the gene ssfg_01967, encoding putative tRNA (N6-isopentenyl adenosine(37)-C2)-methylthiotransferase. We noted that the transposon insertion site is located proximally to the start codon of ssfg_01967 (Fig. 4). The Ssfg_01967 homologues, known as MiaB, are well studied in model organisms such as Salmonella and E. coli [15–17], where they control the second step (thiomethylation) of the three-enzyme cascade of hypermodification of adenosine in position 37 (A37) of tRNAs carrying anticodons XXA (Cys; Ser; Tyr; Trp; Leu; Phe) [18]. The gene miaB is omnipresent in proteobacteria; our cursory BLASTP-assisted analysis showed that miaB (ssfg_01967) orthologues are also universally present in Streptomyces genomes. Introduction of an intact copy of ssfg_01967 (plasmids pOOB108L, pOOB106a) into HIM5 restored morphogenesis and moenomycin production (Figs 2 and 3), implying that the mutant phenotype of HIM5 indeed arose from Himar1 insertion into ssfg_01967.
Fig. 2.
Sporulation is impaired in the HIM5 mutant. Lawns of S. ghanaensis strains (168 h of incubation) on OM (a) and SFM (b) agars. The lawns from OM plates were used to obtain scanning electron microscopic views of colonial surfaces of B38.3 (c, e) and HIM5 (d, f) strains at different magnification. Note that HIM5 produced sparse spore chains which have not undergone complete differentiation [spores are oblongate as if not all septa were formed; denoted by arrows in (f)]. Scale bars are 10 µm (c, d) and 5 µm (e, f).
Fig. 3.
Titres of NoA accumulated in the biomass of S. ghanaensis parental (B38.3), HIM5 mutant (HIM5) and its pOOB108L-complemented derivative after 72 h of growth in TSB at 37 °C. The data represent mean values of four independent experiments, referred back to the same amount of dry biomass. Under the above-mentioned conditons, 100 % NoA production by B38.3 corresponds approximately to 0.7 µg of NoA extracted from 1 ml of fermentation medium. Error bars indicate two standard deviations.
Fig. 4.
Genetic organization of Himar1 insertion site in chromosome of S. ghanaensis HIM5. The transposon structure is shown in the upper diagram, flanked by inverted terminal repeats (labelled R). The numbers below ssfg_01967 (miaB) refer to gene size, in codons. Himar1 is located between the 11th and 12th codons of the annotated ssfg_01967 sequence. The black quadrangle below the ssfg_01967 gene denotes the fragment amplified in RT-PCR experiments. Genetic symbols are not drawn to scale. Grey arrows indicate genes ssfg_01963 and ssfg_02154 encoding putative orthologues of miaA and miaE, respectively (see main text for further details).
Further insights into ssfg_01967 function
We performed semi-quantitative RT-PCR analysis of expression on ssfg_01967 and several other S. ghanaensis genes implicated in morphogenesis (adpA) and moenomycin biosynthesis (adpA, moeO5) [8]. There was no significant difference in adpA and moeO5 transcription when strains HIM5 and B38.3 were cultivated in TSB for 48 h. We failed to detect ssfg_01967 transcription in either S. ghanaensis strain under the afove-mentioned conditions (Fig. 5a). A faint band of ssfg_01967 transcript was detected when the amount of cDNA in B38.3 in the reaction mixture was increased threefold; unexpectedly, the same band was observed in the case of HIM5 (Fig. 5b). We sequenced this band and confirmed its identity as belonging to a segment of the ssfg_01967 gene expected to be amplified with the RT-PCR primers (data not shown). In summary, our data point to the possibility that protein Ssfg_01967 may still be expressed in HIM5, most likely in a truncated form. The coding sequence of ssfg_01967 contains ATG, GTG and TTG codons of 39, 45 and 63 bp, respectively, downstream of the Himar insertion, and these may serve as alternative translation start points.
Fig. 5.
Transposon insertion in the HIM5 mutant abolished transcription of neither miaB nor adpA and moeO5 genes essential for moenomycin production. (a). The expression of miaB, adpA and moeO5 was analysed in 48 h cultures; 200 ng of RNA sample were used per reaction. Lower band in each lane represents positive control (hrdB). C-, negative control (RNA without RT step). (b). 600 ng of RNA were used to detect miaB expression in 48 h cultures. C+, positive control (genomic DNA of ATCC14672 strain). The images represent the typical result of four independent RT-PCR experiments.
Introduction of an additional copy of ssfg_01967 (plasmids pOOB106a, pOOB113a) into genomes B38.3 and ATCC14672 had no influence on moenomycin production levels, yet led to increased formation of spores on OM agar (Fig. S3, ESM). We generated an ssfg_01967 disruption plasmid, pmiaBhyg, and attempted to knock out this gene. Hygromycin- and apramycin-resistant (HyrAmr) colonies, an indicator of a single crossover event, were readily obtained for strains B38.3 and ATCC14672. We failed to isolate hygromycin-resistant and apramycin-sensitive colonies (replacement of ssfg_01967 with hyg cassette), either directly after conjugal transfer of pmiaBhyg or after multiple passages of initial HyrAmr clones under non-selective conditions. In the latter case we isolated either colonies susceptible to both antibiotics (loss of pmiaBhyg) or small, feeble colonies that did not grow following re-streaking onto fresh hygromycin-supplemented agar plates.
Deficiency in ssfg_01967 affected the translation of adpA
RT-PCR analysis suggested that impaired transcription of the adpA regulatory gene is not the reason for the mutant phenotype of HIM5. We took advantage of previously published [8] β-glucuronidase reporter gene fusions of the S. ghanaensis adpA gene (padpAscript, padpAtransl) to discriminate between the potential effects of miaB mutation on transcriptional and translational steps of adpA expression. In agreement with RT-PCR data, there was no significant difference in adpA transcription between parental (B38.3) and HIM5 strains when grown in tryptic soy broth. However, we found that β-glucuronidase reporter activity from the adpA translational fusion construct was decreased in the HIM5 strain as compared to the B38.3 strain (Fig. 6). We also compared the reporter activity in B38.3 and HIM5 strains carrying translational fusion of the TTA-free version of adpA to gusA (plasmid padpAtransl-CTC). According to available data [15–18], the CTC codon is not subject to a Mia-assisted tRNA modification pathway. Removal of the TTA codon boosted the reporter activity in HIM5 significantly above the level observed for HIM5 carrying padpAtransl, yet it did not match the levels observed for B38.3 carrying either TTA- or CTC-based translational adpA-gusA reporters (Fig. 6).
Fig. 6.
Expression of adpA gene in HIM5 mutant is affected at the level of translation. Glucuronidase (GUS) activity was measured in biomass samples collected after 48 h of growth in TSB at 37 °C. Error bars, ±2 SD. C_B38.3, C_HIM5: strains carrying control plasmids, pGUS and padpAcontr in the case of transcriptional and translational fusions, respectively. The adpA(TTA-) is a gusA fusion to adpA carrying the TTA→CTC replacement.
Comparative analysis of post-transcriptional modifications in strains HIM5 and B38.3
LC-MS/MS-based analysis revealed that S. ghanaensis B38.3 tRNAs contain known modified ribonucleosides (Table S1, ESM). We observed all possible combinations of Mia-dependent post-transcriptional modifications of adenosine, e.g. N6-isopentenyladenosine (i6A), its oxidized derivative io6A, as well as the thiomethylated derivatives 2-methylthio-N6-isopentenyladenosine (ms2i6A) and its oxidized version 2-methylthio-N6-(cis-hydroxyisopentenyl) adenosine (ms2io6A); (Fig. S4, ESM). Our results suggest that S. ghanaensis expresses the MiaE oxidase homologue along with MiaA and MiaB, whose genes were found to be located in the moenomycin producer genome via a BLASTP search [19]. We therefore undertook a more careful in silico analysis of the S. ghanaensis genome, which revealed the gene ssfg_02514 that encodes an enzyme closely resembling several enterobacterial MiaE proteins. There were no qualitative differences in modified nucleosides found in strains B38.3 and HIM5. Nevertheless, relative quantification of the ms2i6A peak revealed that it was present at levels almost twofold less in HIM5 tRNA extracts as compared to B38.3 (Fig. 7a). Introduction of an intact ssfg_01967 copy into HIM5 (plasmids pOOB108L, pOOB106a) led to levels of ms2i6A production equal to or greater than those observed in B38.3. The relative abundance of i6A and io6A in HIM5 was also higher than for B38.3. The increases in i6A and io6A and decreases in ms2i6A and ms2io6A are consistent with miaB impairment. Fig. 7b summarizes the pathway of Mia-dependent modifications of tRNAXXA as suggested by our in vivo and in silico data.
Fig. 7.
Hydrolysates of total tRNA isolated from HIM5 strain are depleted in thiomethylated adenosine residues. (a). Overlaid trace chromatograms showing the abundance of i6A, io6A, ms2i6A and ms2io6A residues in B38.3 (WT), HIM5 and HIM5 complemented with plasmids pOOB106a (106a) and pOOB108L (108L). As a control, the abundance of adenosine (a) was measured in these samples and found to be equal for all extracts (see Fig. S5, ESM). (b). Proposed pathway of Mia-assisted modification of A37 of tRNAXXA.
Discussion
In this work we describe the first application of transposon mutagenesis to S. ghanaensis for the purpose of revealing novel genes involved in the regulation of moenomycin production. Our attention was directed to a single mutant, referred to as HIM5, due to its deficiency in sporulation and antibiotic production, and to a Himar1 insertion in gene ssfg_01967 previously not associated with the aforementioned biological pathways. The ssfg_01967 encodes an orthologue of the MiaB enzyme, which catalyses thiomethylation of the adenosine residue in position 37 of a number of tRNAs with XXA anticodons [22, 23]. To the best of our knowledge, this is the first report showing that a proper level of expression of a tRNA modification gene is important for the morphogenetic and secondary metabolic pathways of actinobacteria. Although being novel for actinobacteria [24], our finding is consistent with investigations carried out on proteobacteria [25–27], yeast [28, 28] and human cultured cells [29–31], where perturbations of tRNA modification led to highly specific phenotypic changes.
Several reports show that mutant phenotypes of tRNA modification-deficient strains are directly linked to inefficient translation of codons whose decoding normally relies on properly modified tRNA. Particularly relevant to this study are recent works on E. coli , where the absence of Cm/Um34 and s2U34 tRNA modifications (introduced by methylase TrmL and thiouridine synthetase TusA, respectively) interfered with translation of UUX leucyl codons within mRNA for sigma factor RpoS [32]. Likewise, knockout of the isopentenyl transferase gene miaA was shown to affect the translation of UUX codons within mRNA for IraP, an anti-adaptor protein that increases RpoS stability [33]. An important conclusion from these works is that the dependence of certain transcripts on appropriately modified tRNA becomes significant only if that transcript is enriched with codons recognized by that tRNA. This explains why, despite the ubiquitous presence of UUX codons in all E. coli genes, only some are affected at the level of translation in tRNA modification mutants [34]. We suggest that a similar mechanism operates in our case, and GusA reporter studies provide circumstantial evidence for that. In its metabolic and morphological defects, strain HIM5 resembles S. ghanaensis with deleted gene bldA [13]. The latter encodes tRNALeu UAA, the only tRNA capable of decoding leucyl codon UUA in streptomycetes. Codon TTA is extremely rare (60–170 per entire 9 Mbp genome of Streptomyces ) [35, 36] and is found exclusively in dispensable genes, such as those for antibiotic production, colony development and polymer decomposition. Importantly, TTA codons are present in S. ghanaensis adpA and moenomycin biosynthesis genes (moeO5, moeE5, moeR5). Results of translational fusion experiments suggest that, at least partially, the miaB mutation exerts its effects on S. ghanaensis via less efficient decoding of UUA codons in adpA and several moe genes. Nevertheless, we recognize that decreased thiomethylation of A37 may trigger other processes directly responsible for the HIM5 phenotype. For example, it is possible that undermodified A37 interferes with U34 ribose methylation by TrmL, which in turn decreases codon–anticodon interaction, as described for E. coli [32, 33]. We also cannot rule out that undermodified A37 affects the signalling pathway independent of codon translation defects, as was proposed for MiaE-deficient Salmonella [27] and MSUM (MnmA)-null yeast [37].
It is not known why a seemingly moderate quantitative change in the tRNA modification pattern led to such a strong response in S. ghanaensis . We note here that it was possible to observe differences in the morphology of HIM5 and B38.3 only on certain solid media (see Fig. S2, ESM). This probably reflects the fact that the modification profile of a cell may change, qualitatively and quantitatively, under different conditions, as has recently been reported for E. coli [38, 39], mycobacteria [40] and yeast [41].
A possibility that the HIM5 mutant phenotype stems from inefficient decoding of UUA codons reignites questions surrounding the bldA gene for tRNALeu UAA. Although bldA mutants were first described as early as the 1960s [42], their phenotypic complexity and conditionality led to an intense debate about the exact chain of events that links the bldATTA duo to phenotypic change [43, 44]. Much of the uncertainty in this regard comes from the absence of a structure of mature Streptomyces tRNALeu UAA [45]. Our work suggests that tRNALeu UAA is subject to Mia-controlled modifications and that these are important for UUA decoding. However, the actual set of anticodons carrying Mia-dependent modifications could actually be more restricted than anticipated [46]. We revealed several nucleoside modifications expected to be present in wobble U34 position (nm5U, Um; see Table S1, ESM), although their actual distribution across tRNAXXA and genetic control in Streptomyces is not known. The temporal pattern of nascent BldA modification is also unknown [47, 48]. The development of tools and approaches towards purification and monitoring of tRNALeu UAA from Streptomyces will help address the above issues. Overall, this will expand not only structural and mechanistic understanding of tRNA modifications [49–54], but also their downstream effects on bacterial physiology [55–57].
Supplementary Data
Funding information
The work was supported by grant Bg-41Nr from the Ministry of Education and Science of Ukraine (to B.O.), NIH grant R03TW009424 (to S.W. and V.F.) and NIH grant R01GM088843 (to P.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Publication in part is based on the research provided by the grant support of the State Fund For Fundamental Research of Ukraine (project F80/2-2018, to B.O.).
Acknowledgements
We thank A. Pavlenko for generating the strains presented in Fig. S3.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: MmA, moenomycin; NoA, nosokomycin A; PTTM, post-transcriptional tRNA modification.
Five supplementary figures and one supplementary table are available with the online version of this article.
Edited by: H. Gramajo and S. V. Gordon
References
- 1.Makitrynskyy R, Rebets Y, Ostash B, Zaburannyi N, Rabyk M, et al. Genetic factors that influence moenomycin production in streptomycetes. J Ind Microbiol Biotechnol. 2010;37:559–566. doi: 10.1007/s10295-010-0701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostash B, Saghatelian A, Walker S. A streamlined metabolic pathway for the biosynthesis of moenomycin A. Chem Biol. 2007;14:257–267. doi: 10.1016/j.chembiol.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gampe CM, Tsukamoto H, Doud EH, Walker S, Kahne D. Tuning the moenomycin pharmacophore to enable discovery of bacterial cell wall synthesis inhibitors. J Am Chem Soc. 2013;135:3776–3779. doi: 10.1021/ja4000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesleh MF, Rajaratnam P, Conrad M, Chandrasekaran V, Liu CM, et al. Targeting bacterial cell wall peptidoglycan synthesis by inhibition of glycosyltransferase activity. Chem Biol Drug Des. 2016;87:190–199. doi: 10.1111/cbdd.12662. [DOI] [PubMed] [Google Scholar]
- 5.Taylor JG, Li X, Oberthür M, Zhu W, Kahne DE. The total synthesis of moenomycin A. J Am Chem Soc. 2006;128:15084–15085. doi: 10.1021/ja065907x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuse S, Tsukamoto H, Yuan Y, Wang TS, Zhang Y, et al. Functional and structural analysis of a key region of the cell wall inhibitor moenomycin. ACS Chem Biol. 2010;5:701–711. doi: 10.1021/cb100048q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabyk M, Ostash B, Rebets Y, Walker S, Fedorenko V. Streptomyces ghanaensis pleiotropic regulatory gene wblAgh influences morphogenesis and moenomycin production. Biotechnol Lett. 2011;33:2481–2486. doi: 10.1007/s10529-011-0728-z. [DOI] [PubMed] [Google Scholar]
- 8.Makitrynskyy R, Ostash B, Tsypik O, Rebets Y, Doud E, et al. Pleiotropic regulatory genes bldA, adpA and absB are implicated in production of phosphoglycolipid antibiotic moenomycin. Open Biol. 2013;3:130121. doi: 10.1098/rsob.130121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu G, Chater KF, Chandra G, Niu G, Tan H. Molecular regulation of antibiotic biosynthesis in Streptomyces . Microbiol Mol Biol Rev. 2013;77:112–143. doi: 10.1128/MMBR.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoraz S, Rico S, Rodríguez H, Sevillano L, Alzate JF, et al. The orphan response regulator Aor1 is a new relevant piece in the complex puzzle of Streptomyces coelicolor antibiotic regulatory network. Front Microbiol. 2017;8:2444. doi: 10.3389/fmicb.2017.02444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutenko H, Makitrinskyy R, Tsypik O, Walker S, Ostash B, et al. Genes for biosynthesis of butenolide-like signalling molecules in Streptomyces ghanaensis, their role in moenomycin production. Russ J Genet. 2014;50:563–568. doi: 10.1134/S1022795414060076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilyk B, Weber S, Myronovskyi M, Bilyk O, Petzke L, et al. In vivo random mutagenesis of streptomycetes using mariner-based transposon Himar1. Appl Microbiol Biotechnol. 2013;97:351–359. doi: 10.1007/s00253-012-4550-x. [DOI] [PubMed] [Google Scholar]
- 13.Koshla O, Lopatniuk M, Rokytskyy I, Yushchuk O, Dacyuk Y, et al. Properties of Streptomyces albus J1074 mutant deficient in tRNALeu UAA gene bldA . Arch Microbiol. 2017;199:1175–1183. doi: 10.1007/s00203-017-1389-7. [DOI] [PubMed] [Google Scholar]
- 14.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA, et al. Practical streptomyces Genetics. Norwich, United Kingdom: John Innes Foundation; 2000. [Google Scholar]
- 15.Sambrook J, Russell DW. Molecular Cloning: a Laboratory Manual. 3rd ed. Cold Spring Harbor: NY: Cold Spring Harbor Laboratory; 2001. [Google Scholar]
- 16.Cayama E, Yépez A, Rotondo F, Bandeira E, Ferreras AC, et al. New chromatographic and biochemical strategies for quick preparative isolation of tRNA. Nucleic Acids Res. 2000;28:E64. doi: 10.1093/nar/28.12.e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross R, Cao X, Yu N, Limbach PA. Sequence mapping of transfer RNA chemical modifications by liquid chromatography tandem mass spectrometry. Methods. 2016;107:73–78. doi: 10.1016/j.ymeth.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostash B, Ostash I, Zhu L, Kharel MK, Luzhetskiĭ A, et al. Properties of lanK-based regulatory circuit involved in landomycin biosynthesis in Streptomyces cyanogenus S136. Genetika. 2010;46:604–609. [PMC free article] [PubMed] [Google Scholar]
- 19.Beyer HM, Gonschorek P, Samodelov SL, Meier M, Weber W, et al. AQUA cloning: a versatile and simple enzyme-free cloning approach. PLoS One. 2015;10:e0137652. doi: 10.1371/journal.pone.0137652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myronovskyi M, Welle E, Fedorenko V, Luzhetskyy A. Beta-glucuronidase as a sensitive and versatile reporter in actinomycetes. Appl Environ Microbiol. 2011;77:5370–5383. doi: 10.1128/AEM.00434-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopatniuk M, Ostash B, Makitrynskyy R, Walker S, Luzhetskyy A, et al. Testing the utility of site-specific recombinases for manipulations of genome of moenomycin producer Streptomyces ghanaensis ATCC14672. J Appl Genet. 2015;56:547–550. doi: 10.1007/s13353-015-0283-8. [DOI] [PubMed] [Google Scholar]
- 22.Esberg B, Leung HC, Tsui HC, Björk GR, Winkler ME. Identification of the miaB gene, involved in methylthiolation of isopentenylated A37 derivatives in the tRNA of Salmonella typhimurium and Escherichia coli . J Bacteriol. 1999;181:7256–7265. doi: 10.1128/jb.181.23.7256-7265.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng C, Black KA, dos Santos PC. Diverse mechanisms of sulfur decoration in bacterial trna and their cellular functions. Biomolecules. 2017;7:E33. doi: 10.3390/biom7010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rokytskyy I, Koshla O, Fedorenko V, Ostash B. Decoding options and accuracy of translation of developmentally regulated UUA codon in Streptomyces: bioinformatic analysis. Springerplus. 2016;5:982. doi: 10.1186/s40064-016-2683-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agris PF, Narendran A, Sarachan K, Väre VYP, Eruysal E. The importance of being modified: The role of rna modifications in translational fidelity. Enzymes. 2017;41:1–50. doi: 10.1016/bs.enz.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierrel F, Douki T, Fontecave M, Atta M. MiaB protein is a bifunctional radical-S-adenosylmethionine enzyme involved in thiolation and methylation of tRNA. J Biol Chem. 2004;279:47555–47563. doi: 10.1074/jbc.M408562200. [DOI] [PubMed] [Google Scholar]
- 27.Persson BC, Olafsson O, Lundgren HK, Hederstedt L, Björk GR. The ms2io6A37 modification of tRNA in Salmonella typhimurium regulates growth on citric acid cycle intermediates. J Bacteriol. 1998;180:3144–3151. doi: 10.1128/jb.180.12.3144-3151.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamichhane TN, Blewett NH, Crawford AK, Cherkasova VA, Iben JR, et al. Lack of tRNA modification isopentenyl-A37 alters mRNA decoding and causes metabolic deficiencies in fission yeast. Mol Cell Biol. 2013;33:2918–2929. doi: 10.1128/MCB.00278-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeharia A, Shaag A, Pappo O, Mager-Heckel AM, Saada A, et al. Acute infantile liver failure due to mutations in the TRMU gene. Am J Hum Genet. 2009;85:401–407. doi: 10.1016/j.ajhg.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlsborn T, Tükenmez H, Chen C, Byström AS. Familial dysautonomia (FD) patients have reduced levels of the modified wobble nucleoside mcm(5)s(2)U in tRNA. Biochem Biophys Res Commun. 2014;454:441–445. doi: 10.1016/j.bbrc.2014.10.116. [DOI] [PubMed] [Google Scholar]
- 31.Morscher RJ, Ducker GS, Li SH, Mayer JA, Gitai Z, et al. Mitochondrial translation requires folate-dependent tRNA methylation. Nature. 2018;554:128–132. doi: 10.1038/nature25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aubee JI, Olu M, Thompson KM. TrmL and TusA are necessary for rpoS and MiaA Is required for hfq expression in Escherichia coli . Biomolecules. 2017;7:39. doi: 10.3390/biom7020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson KM, Gottesman S. The MiaA tRNA modification enzyme is necessary for robust RpoS expression in Escherichia coli . J Bacteriol. 2014;196:754–761. doi: 10.1128/JB.01013-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aubee JI, Olu M, Thompson KM. The i6A37 tRNA modification is essential for proper decoding of UUX-Leucine codons during rpoS and iraP translation. RNA. 2016;22:729–742. doi: 10.1261/rna.053165.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chater KF, Chandra G. The use of the rare UUA codon to define "expression space" for genes involved in secondary metabolism, development and environmental adaptation in Streptomyces . J Microbiol. 2008;46:1–11. doi: 10.1007/s12275-007-0233-1. [DOI] [PubMed] [Google Scholar]
- 36.Zaburannyy N, Ostash B, Fedorenko V. TTA Lynx: a web-based service for analysis of actinomycete genes containing rare TTA codon. Bioinformatics. 2009;25:2432–2433. doi: 10.1093/bioinformatics/btp402. [DOI] [PubMed] [Google Scholar]
- 37.Zinshteyn B, Gilbert WV. Loss of a conserved tRNA anticodon modification perturbs cellular signaling. PLoS Genet. 2013;9:e1003675. doi: 10.1371/journal.pgen.1003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armengod ME, Meseguer S, Villarroya M, Prado S, Moukadiri I, et al. Modification of the wobble uridine in bacterial and mitochondrial tRNAs reading NNA/NNG triplets of 2-codon boxes. RNA Biol. 2014;11:1495–1507. doi: 10.4161/15476286.2014.992269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moukadiri I, Garzón MJ, Björk GR, Armengod ME. The output of the tRNA modification pathways controlled by the Escherichia coli MnmEG and MnmC enzymes depends on the growth conditions and the tRNA species. Nucleic Acids Res. 2014;42:2602–2623. doi: 10.1093/nar/gkt1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chionh YH, McBee M, Babu IR, Hia F, Lin W, et al. tRNA-mediated codon-biased translation in mycobacterial hypoxic persistence. Nat Commun. 2016;7:13302. doi: 10.1038/ncomms13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan CT, Deng W, Li F, Demott MS, Babu IR, et al. Highly predictive reprogramming of tRNA modifications is linked to selective expression of codon-biased genes. Chem Res Toxicol. 2015;28:978–988. doi: 10.1021/acs.chemrestox.5b00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hopwood DA. Genetic analysis and genome structure in Streptomyces coelicolor . Bacteriol Rev. 1967;31:373–403. doi: 10.1128/br.31.4.373-403.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gramajo HC, Takano E, Bibb MJ. Stationary-phase production of the antibiotic actinorhodin in Streptomyces coelicolor A3(2) is transcriptionally regulated. Mol Microbiol. 1993;7:837–845. doi: 10.1111/j.1365-2958.1993.tb01174.x. [DOI] [PubMed] [Google Scholar]
- 44.den Hengst CD, Tran NT, Bibb MJ, Chandra G, Leskiw BK, et al. Genes essential for morphological development and antibiotic production in Streptomyces coelicolor are targets of BldD during vegetative growth. Mol Microbiol. 2010;78:361–379. doi: 10.1111/j.1365-2958.2010.07338.x. [DOI] [PubMed] [Google Scholar]
- 45.Pettersson BM, Kirsebom LA. tRNA accumulation and suppression of the bldA phenotype during development in Streptomyces coelicolor . Mol Microbiol. 2011;79:1602–1614. doi: 10.1111/j.1365-2958.2011.07543.x. [DOI] [PubMed] [Google Scholar]
- 46.Lamichhane TN, Mattijssen S, Maraia RJ. Human cells have a limited set of tRNA anticodon loop substrates of the tRNA isopentenyltransferase TRIT1 tumor suppressor. Mol Cell Biol. 2013;33:4900–4908. doi: 10.1128/MCB.01041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawlor EJ, Baylis HA, Chater KF. Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a tRNA-like product in Streptomyces coelicolor A3(2) Genes Dev. 1987;1:1305–1310. doi: 10.1101/gad.1.10.1305. [DOI] [PubMed] [Google Scholar]
- 48.Leskiw BK, Mah R, Lawlor EJ, Chater KF. Accumulation of bldA-specified tRNA is temporally regulated in Streptomyces coelicolor A3(2) J Bacteriol. 1993;175:1995–2005. doi: 10.1128/jb.175.7.1995-2005.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaminska KH, Baraniak U, Boniecki M, Nowaczyk K, Czerwoniec A, et al. Structural bioinformatics analysis of enzymes involved in the biosynthesis pathway of the hypermodified nucleoside ms(2)io(6)A37 in tRNA. Proteins. 2008;70:1–18. doi: 10.1002/prot.21640. [DOI] [PubMed] [Google Scholar]
- 50.Boccaletto P, Machnicka MA, Purta E, Piatkowski P, Baginski B, et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46:D303–D307. doi: 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hori H. Transfer RNA methyltransferases with a SpoU-TrmD (SPOUT) fold and their modified nucleosides in tRNA. Biomolecules. 2017;7:1. [Google Scholar]
- 52.Shepherd J, Ibba M. Bacterial transfer RNAs. FEMS Microbiol Rev. 2015;39:280–300. doi: 10.1093/femsre/fuv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Väre VY, Eruysal ER, Narendran A, Sarachan KL, Agris PF. Chemical and conformational diversity of modified nucleosides affects tRNA structure and function. Biomolecules. 2017;7:29–32. doi: 10.3390/biom7010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schweizer U, Bohleber S, Fradejas-Villar N. The modified base isopentenyladenosine and its derivatives in tRNA. RNA Biol. 2017;14:1197–1208. doi: 10.1080/15476286.2017.1294309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ranjan N, Rodnina MV. Thio-modification of tRNA at the wobble position as regulator of the kinetics of decoding and translocation on the ribosome. J Am Chem Soc. 2017;139:5857–5864. doi: 10.1021/jacs.7b00727. [DOI] [PubMed] [Google Scholar]
- 56.Persson BC. Modification of tRNA as a regulatory device. Mol Microbiol. 1993;8:1011–1016. doi: 10.1111/j.1365-2958.1993.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 57.El Yacoubi B, Bailly M, de Crécy-Lagard V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu Rev Genet. 2012;46:69–95. doi: 10.1146/annurev-genet-110711-155641. [DOI] [PubMed] [Google Scholar]
- 58.Luo Y, Huang H, Liang J, Wang M, Lu L, et al. Activation and characterization of a cryptic polycyclic tetramate macrolactam biosynthetic gene cluster. Nat Commun. 2013;4:2894–2909. doi: 10.1038/ncomms3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herrmann S, Siegl T, Luzhetska M, Petzke L, Jilg C, et al. Site-specific recombination strategies for engineering actinomycete genomes. Appl Environ Microbiol. 2012;78:1804–1812. doi: 10.1128/AEM.06054-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J, Shi J, Molle V, Sohlberg B, Weaver D, et al. Cross-regulation among disparate antibiotic biosynthetic pathways of Streptomyces coelicolor . Mol Microbiol. 2005;58:1276–1287. doi: 10.1111/j.1365-2958.2005.04879.x. [DOI] [PubMed] [Google Scholar]
- 61.Myronovskyi M, Rosenkränzer B, Luzhetskyy A. Iterative marker excision system. Appl Microbiol Biotechnol. 2014;98:4557–4570. doi: 10.1007/s00253-014-5523-z. [DOI] [PubMed] [Google Scholar]
- 62.Karlsborn T, Tükenmez H, Mahmud AK, Xu F, Xu H, et al. Elongator, a conserved complex required for wobble uridine modifications in eukaryotes. RNA Biol. 2014;11:1519–1528. doi: 10.4161/15476286.2014.992276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.