Abstract
Background
Ferulic acid is an antioxidant phenolic compound derived from plants, which has effects on cancer cells. This study aimed to investigate the effects of ferulic acid on HeLa and Caski human cervical carcinoma cells and the molecular mechanisms involved.
Material/Methods
HeLa and Caski human cervical carcinoma cells were grown in culture and treated with increasing doses of ferulic acid. The MTT assay was used to evaluate cell viability. Flow cytometry was performed with 4′,6-diamidino-2-phenylindole (DAPI) and Annexin V staining for cell apoptosis. The expression of myeloid leukemia cell differentiation-1 (Mcl-1) protein and MCL-1 mRNA were determined by Western blot and reverse transcription-polymerase chain reaction (RT-PCR).
Results
Ferulic acid significantly reduced HeLa and Caski cell viability in the concentration range of 4–20 μM (P<0.05). Ferulic acid treatment promoted DNA condensation and significantly increased apoptosis in Caski cells (P<0.05). Ferulic acid treatment resulted in the activation of pro-caspase-3, pro-caspase-8, pro-caspase-9, and PARP. The MTT assay showed that ferulic acid did not reduce the viability of Caski cells treated with the caspase inhibitor, z-VAD-fmk. Ferulic acid reduced the levels of Bcl-2 and Mcl-1, and increased the levels of Bax and reactive oxygen species (ROS). In Caski cells, Akt and PI3K phosphorylation were reduced by ferulic acid in a concentration-dependent manner.
Conclusions
The effects of ferulic acid were dose-dependent and resulted in cell cytotoxicity and apoptosis of HeLa and Caski cells, and the PI3K/Akt signaling pathway was down-regulated in Caski cells.
MeSH Keywords: Apoptosis, Caspase 8, Cytochrome c Group, DNA Damage
Background
Ferulic acid is a phenolic antioxidant compound derived from plants that also has anti-inflammatory properties [1]. The role of ferulic acid has been studied for its protective effects on neuronal oxidative stress and neurotoxicity induced by amyloid-β (Aβ) oligomers [2]. In mouse models, long-term treatment with ferulic acid was shown to inhibit memory deficits induced by Aβ [3]. Cell death in neuroblastoma associated with Aβ-oligomers was inhibited by ferulic acid when combined with lipid nanoparticles by the reduction of oxidative stress [4]. Ferulic acid destabilizes and inhibits the formation of the amyloid fibrils by directly binding to these structures [5,6].
Cell apoptosis is a recognized mechanism of cell elimination that involves the activation of caspases and the cleavage of associated proteins leading to biochemical and morphological changes in the cell [7–10]. Cytochrome c is released from the mitochondria in the early stages of apoptosis and initiates pro-apoptotic changes in the cell cytosol [11]. A major function of cytochrome c in the cytoplasm is the oligomerization of apoptotic protease activating factor 1 (APAF1) and the activation of pro-caspases [11]. Bcl-2 promotes the release of cytochrome c from mitochondria through the regulation of membrane permeability [12]. Pro-apoptotic factors, including Bax and Bak, and anti-apoptotic factors, including Bcl-2 and myeloid leukemia cell differentiation-1 (Mcl-1), are members of the Bcl-2 family [12–14].
The anti-apoptotic protein, Mcl-1 is different from other Bcl-2 homologs due to its ability to bind to the BH3-only subset of the proteins in the Bcl-2 family that contains a single BH3-domain [15,16]. Mcl-1 has an important role in inhibiting cell apoptosis induced by ultraviolet (UV) radiation [17]. Mcl-1 has anti-apoptotic effects by inhibiting the expression of Bak in the mitochondria [18]. Also, Mcl-1 has been shown to induce conformational changes in the structure of Bax, preventing its translocation to the mitochondria, which inhibits the release of cytochrome c [19]. Studies in medicinal chemistry have involved the investigation of heterocyclic scaffolds for the synthesis of potent bioactive compounds [20]. These compounds possess properties that inhibit the proliferation of cancer cells, have anti-microbial properties and have a role in animal models of Alzheimer’s disease [21–23]. Studies have shown that natural phenolic phytochemicals and synthetic aromatic compounds have biological activities [24]. Therefore, this study aimed to investigate the effects of ferulic acid on HeLa and Caski human cervical carcinoma cells in vitro and the molecular mechanisms involved.
Material and Methods
Cell culture
HeLa and Caski cell lines were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). The cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 μg/m). The cells were cultured in a humidified atmosphere of 5% CO2 at 37°C.
MTT assay
Changes in the viability of HeLa and Caski cells were evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The cell lines were cultured for 24 h under a humidified atmosphere of 5% CO2 at 37°C. Fresh medium was mixed with 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, and 25 μM of ferulic acid, and the cells were cultured for a further 48 h. The cells were then incubated for 4 h with 5 mg/ml solution of MTT (100 μl). The culture medium in the plates was discarded, and 150 μl of dimethyl sulfoxide (DMSO) was added. The optical density (OD) was measured for each plate at 578 nm using a microplate reader (Molecular Devices, San Jose, CA, USA).
Analysis of DNA fragmentation
The Caski cells (1×106 cells per well) in 60 mm cultural plates were treated with 4, 8, 16, and 20 μM concentrations of ferulic acid. Following 48 h of treatment, the cells were fixed for 40 min onto glass slides with 4% paraformaldehyde at room temperature. The cells were washed three times with PBS and incubated for 15 min with 4′,6′-diamidino-2-phenylindole (DAPI), and examined using an Olympus BX53 fluorescence microscope (Olympus, Tokyo, Japan) to evaluate the DNA condensation.
Flow cytometry for apoptosis
The Caski cells were distributed at a density of 3.0×105 cells/well in six – well plates and cultured for 24 h. The cells were treated for 48 h with 4, 8, 16, and 20 μM concentrations of ferulic acid, washed three times with PBS and resuspended in 450 μl of binding buffer. The cells were treated in the dark with 5 μl of annexin V – fluorescein isothiocyanate (FITC) and propidium iodide (PI) for 20 min at room temperature. The stained cells were examined using a FACS Calibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) using an argon laser (488 nm) for fluorescence measurement. The percentage of apoptotic cells was counted using FACS Scan software version 6.0 (BD Biosciences, Franklin Lakes, NJ, USA).
Western blot
The Caski cells at a density of 1×106 cell/mL were trypsinized following 48 h of treatment with 4, 8, 16, and 20 μM concentrations of ferulic acid. The cells were lysed and resuspended in RIPA lysis buffer consisting of Tris – base (50 mM), sodium chloride (150 mM), sodium dodecyl sulfate (0.1%), EDTA (1 mM), Triton X – 100 (1%), and sodium deoxycholate (1%) for 40 min. The lysate was centrifuged at 4°C for 15 min at 12,000 x g to obtain the supernatant. The protein concentration was measured using bicinchoninic acid (BCA) protein kits.
The 5X SDS-PAGE loading buffer and 5 μg of protein samples were mixed and denatured at 100°C in water for 15 min. Protein resolution by electrophoresis was performed using 10 μl samples on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. The proteins were transferred onto polyvinylidene fluoride (PVDF) membranes that had previously been blocked for 2 h by incubation at room temperature with 50 g/l of dried skimmed milk powder. The membranes were incubated overnight at 4°C with primary antibodies. The primary rabbit or mouse antibodies were to caspase-8, caspase-9, caspase-3, cleaved PARP, Bcl-2, Bax, Mcl-1, p-AKT, and p-PI3K. After washing with PBS, the membranes were incubated for 2 h with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse IgG secondary antibodies at room temperature. Enhanced chemiluminescence (ECL) was used to visualize the proteins, and a scanning densitometer (Personal Densitometer, Molecular Dynamics, Sunnyvale, CA, USA) was used for quantification of the signals.
Detections of reactive oxygen species (ROS)
The Caski cells were treated with 4, 8, 16, and 20 μM of ferulic acid for 48 h. The cells were harvested and resuspended in 400 μl of 2,7-dichloro-dihydro-fluorescein diacetate (DCFH-DA). The production of ROS was measured by flow cytometry.
Reverse transcription-polymerase chain reaction (RT-PCR)
The total RNA from Caski cells was extracted by TRIzol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manual procedure. The 1 μg of total RNA was used for the synthesis of cDNA using a Primescript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian, China) for 20 min at 37°C. The Roche Light Cycler96 Real-time PCR system containing the SYBR Premix EX TaqII kit (Takara Biotechnology Co., Ltd., Dalian, China) was used to perform the RT-PCR assay. The reaction volume used consisted of 20 μl of SYBR Premix EX Taq II (10 μl), forward primer (0.8 μl), reverse primer (0.8 μl), cDNA (2 μl), and sterilized water (6.4 μl).
The amplification procedure included: pre-degeneration for 2 min at 93°C, then 40 cycles of denaturation for 10 sec at 93°C, and annealing for 15 sec at 58°C. The 2ΔΔCT method was used for determination of the relative mRNA levels using GAPDH as the loading control. The primer sequence for MCL-1 mRNA were: forward primer, 5′-CCT TCC AAG GAT GGG TTT GT-3′; reverse primer, 5′-TCT TCA ATC AAT GGG GAG CA-3′.
Statistical analysis
Data were analyzed using SPSS version 17.0 software (IBM Corp., Armonk, NY, USA). The data were presented as the mean±standard deviation (SD) of three experiments. Comparison between groups was made using analysis of variance (ANOVA) followed by Tukey’s or Dunnett’s test. A P-value <0.05 was considered to be statistically significant.
Results
The cytotoxicity effects of ferulic acid on HeLa and Caski human cervical carcinoma cells
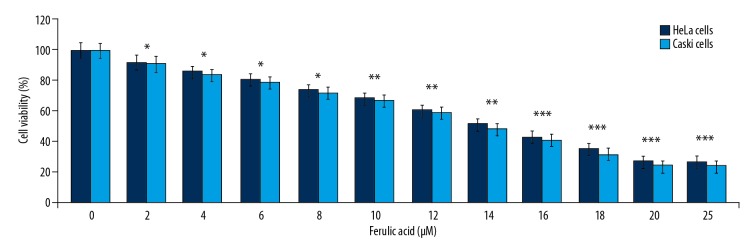
Data from the MTT assay showed that ferulic acid reduced HeLa and Caski cell viability at 48 h in a dose-dependent manner (Figure 1). Ferulic acid significantly (P<0.05) reduced HeLa and Caski cell viability in the concentration range of 2–20 μM. Treatment with 2 μM of ferulic acid significantly reduced the viability of HeLa and Caski cells (P<0.05). The viability of HeLa and Caski cells was reduced to 27% and 24%, respectively on treatment with 20 μM of ferulic acid. Treatment of HeLa and Caski cells to ferulic acid at concentrations >20 μM resulted in no further decrease in viability.
Figure 1.
The effect of ferulic acid on HeLa and Caski human cervical carcinoma cells. Ferulic acid was added to cultures of HeLa and Caski human cervical carcinoma cells at concentrations of 2–25 μM for 48 h. The viability of cells treated with ferulic acid and dimethyl sulfoxide (DMSO) was measured using the MTT assay. * P<0.05, ** P<0.02 and *** P<0.01 vs. DMSO-treated cells.
Ferulic acid resulted in DNA damage and apoptosis in Caski cells
The DAPI staining of ferulic acid-treated Caski cells showed DNA condensation in a dose-dependent manner (Figure 2A). DNA condensation was significantly increased in Caski cells following treatment with 4, 8, 16, and 20 μM of ferulic acid. The findings of 4′,6-diamidino-2-phenylindole (DAPI) staining were confirmed by Annexin-V for apoptosis (Figure 2B). The apoptotic cell population in Caski cell cultures was significantly increased following treatment with 4, 8, 16, and 20 μM of ferulic acid for 48 h (P<0.05).
Figure 2.
Induction of apoptosis by ferulic acid in Caski human cervical carcinoma cells. Ferulic acid was added to cultures of HeLa and Caski human cervical carcinoma cells at concentrations of 4, 8, 16, and 20 μM. (A) Staining with 4′,6-diamidino-2-phenylindole (DAPI) of nuclear DNA condensation of the cells is shown by flow cytometry. (B) Annexin-V staining for cell apoptosis is shown by flow cytometry. * P<0.05 and ** P<0.01 vs. DMSO-treated cells.
Ferulic acid activates caspases in Caski cells
Ferulic acid treatment at 4, 8, 16, and 20 μM for 48 h resulted in the activation of pro-caspase-8 and pro-caspase-9 in Caski cells (Figure 3A). The activation of pro-caspase-8 and pro-caspase-9 by ferulic acid in Caski cells increased in a dose-dependent manner from 4–20 μM. The level of cleaved caspase-3 increased on treatment with 4, 8, 16, and 20 μM of ferulic acid in Caski cells. Also, ferulic acid treatment of Caski cells resulted in the cleavage of PARP in a dose-dependent manner. Caski cells were treated with the caspase inhibitor, z-VAD-fmk, which confirmed the MTT findings (Figure 3B). Data from the MTT assay showed that ferulic acid did not reduce the viability of Caski cells treated with z-VAD-fmk.
Figure 3.
The effect of ferulic acid on caspases in Caski human cervical carcinoma cells. (A) Activation of caspase-8, caspase-9, caspase-3, and PARP in Caski cells treated with ferulic acid was assessed by Western blot. β-actin was used as the internal control. (B) The effect of ferulic acid on Caski cell viability following pre-treatment with the caspase inhibitor, z-VAD-fmk, was determined by the MTT assay. * P<0.05 vs. DMSO-treated cells.
Ferulic acid treatment and the expression levels of the Bcl-2 family proteins in Caski cells
The levels of Bcl-2 and Mcl-1 proteins in Caski cells were reduced following treatment with 4, 8, 16, and 20 μM of ferulic acid at 48 h (Figure 4A). Ferulic acid treatment increased the expression of Bax protein in Caski cells in a dose-dependent manner. Following reverse transcription-polymerase chain reaction (RT-PCR), the level of MCL-1 mRNA was also reduced by ferulic acid treatment in Caski cells (Figure 4B).
Figure 4.
The effect of ferulic acid on the levels of Bcl-2 family proteins in Caski human cervical carcinoma cells. (A) The levels of Bcl-2, Mcl-1, and Bax proteins were determined by Western blot following ferulic acid treatment of the Caski cells. (B) The level of MCL-1 mRNA in Caski cells following ferulic acid treatment was studied using reverse transcription-polymerase chain reaction (RT-PCR).
Ferulic acid promoted the generation of reactive oxygen species (ROS) in Caski cells
In Caski cells, the level of ROS was significantly increased following treatment with 4, 8, 16, and 20 μM of ferulic acid (Figure 5). Flow cytometry of Caski cells stained with 2,7-dichloro-dihydro-fluorescein diacetate (DCFH-DA) showed that ROS production was significantly increased on treatment with ferulic acid in a dose-dependent manner, from 4–20 μM.
Figure 5.
The effect of ferulic acid on the production of reactive oxygen species (ROS) in Caski human cervical carcinoma cells. Caski human cervical carcinoma cells following treatment with ferulic acid for 48 h were stained with 2,7-dichloro-dihydro-fluorescein diacetate (DCFH-DA) and observed by flow cytometry.
Ferulic acid inhibited the phosphorylation of Akt in Caski cells
Akt phosphorylation was reduced by ferulic acid in a dose-dependent manner in Caski cells (Figure 6). On increasing the concentration of ferulic acid from 4–20 μM, the activation of Akt was increasingly reduced. The inhibition of Akt phosphorylation by ferulic acid in Caski cells was maximum at a dose of 20 μM. Ferulic acid treatment also reduced PI3K phosphorylation in Caski cells in a dose-dependent manner.
Figure 6.

The effect of ferulic acid on the activation of the PI3K/Akt pathway in Caski human cervical carcinoma cells. Caski human cervical carcinoma cells following treatment with ferulic acid at 4, 8, 16, and 20 μM for 48 h shows Akt and PI3K phosphorylation levels determined by Western blot.
Discussion
The findings from the present study showed that treatment of HeLa and Caski human cervical carcinoma cells with ferulic acid resulted in DNA damage and cell apoptosis by activating caspases, promoting the generation of reactive oxygen species (ROS) and, in Caski cells, by down-regulation of the Akt/PI3K signaling pathway. Apoptosis a highly regulated cellular process that involves the caspase cascade [25]. The induction of cell apoptosis involves the efflux of mitochondrial membrane proteins into the cell cytoplasm [26,27]. The formation of the apoptosome and activation of pro-caspases require the release of cytochrome c from the mitochondria [26,27]. In the present study, the effect of ferulic acid was investigated on the induction of apoptosis, expression of caspase-8, caspase-9, caspase-3, and PARP in Caski cells.
This study showed that ferulic acid treatment of Caski cells resulted in DNA condensation and induction of apoptosis in a dose-dependent manner. Treatment with ferulic acid significantly reduced the levels of apoptosis initiator and executer caspases in Caski cells in a dose-dependent manner. The study findings also showed that caspase-3 was involved in the reduction of Caski cell viability and that pre-treatment of Caski cells with the caspace inhibitor, z-VAD-fmk, prevented the ferulic acid-mediated reduction in cell viability. The modulation of mitochondrial membrane integrity and negative regulation of cell apoptosis is catalyzed by several proteins of Bcl-2 family, including Bcl-2 and Mcl-1 [28–30]. Previous studies have identified several transcription factors that play an important role in controlling the expression of the MCL-1 gene, including E2F1, CREB, and ETS [31–23]. The present study showed that ferulic acid treatment of Caski cells significantly reduced the expression of Bcl-2 and Mcl-1 proteins when compared with the control. Levels of the pro-apoptotic protein, Bax, were promoted in Caski cells following treatment with ferulic acid.
The Akt/PI3K pathway is upregulated in several types of cancer cells, and its roles include the inhibition of cell apoptosis [34]. Increased expression of p-PI3K/p-Akt in carcinoma cells has previously been shown to be directly associated with resistance to chemotherapeutic agents [35]. Studies have shown that apoptosis in dexamethasone-resistant myeloma cells can be promoted by the novel small molecule drug, PS-341 (bortezomib), by down-regulation of the PI3K/Akt/nuclear factor-κB pathway [36]. PI3K/Akt pathway down-regulation has also been shown to reduce the viability of drug-resistant leukemia cells [37]. In the present study, the levels of p-PI3K and p-Akt in Caski cells were significantly increased following ferulic acid treatment, which also reduced the expression of p-PI3K and p-Akt in a dose-dependent manner in Caski cells. These findings showed that ferulic acid treatment induced apoptosis in Caski cells by targeting p-PI3K/p-Akt activation.
Conclusions
This study aimed to investigate the effects of ferulic acid on HeLa and Caski human cervical carcinoma cells and the molecular mechanisms involved. The effects of ferulic acid were dose-dependent and resulted in cell cytotoxicity and apoptosis of HeLa and Caski cells, and the PI3K/Akt signaling pathway was down-regulated in Caski cells. Following these preliminary in vitro findings, functional studies in cell lines and tumor tissues are recommended to further investigate the molecular mechanisms for the effects of ferulic acid in cervical carcinoma.
Acknowledgments
The authors are highly thankful to the Head Department of Obstetrics and Gynecology, Jiangxi Provincial People’s Hospital Affiliated to Nanchang University, Nanchang, Jiangxi, China for his support.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Di Carlo M, Giacomazza D, San Biagio PL. Alzheimer’s disease: Biological aspects, therapeutic perspectives and diagnostic tools. J Phys Condens Matter. 2012;24:244102. doi: 10.1088/0953-8984/24/24/244102. [DOI] [PubMed] [Google Scholar]
- 2.Kim HS, Cho JY, Kim DH, et al. Inhibitory effects of long term administration of ferulic acid on microgilal activation induced by intercerebro-ventricular injection of beta amyloid peptide (1–42) in mice. Biol Pharm Bull. 2004;27:120–21. doi: 10.1248/bpb.27.120. [DOI] [PubMed] [Google Scholar]
- 3.Sultana R, Ravagna A, Mohmmad-Abdul H, et al. Ferulic acid ethyl ester protects neurons against amyloid beta peptide (1–42)-induced oxidative stress and neurotoxicity: Relationship to antioxidant activity. J Neurochem. 2005;92:749–58. doi: 10.1111/j.1471-4159.2004.02899.x. [DOI] [PubMed] [Google Scholar]
- 4.Picone P, Bondi ML, Montana G, et al. Ferulic acid inhibits oxidative stress and cell death induced by Aβ oligomers: Improved delivery by solid lipid nanoparticles. Free Radic Res. 2009;43:1133–45. doi: 10.1080/10715760903214454. [DOI] [PubMed] [Google Scholar]
- 5.Ono K, Hirohata M, Yamada M. Ferulic acid destabilizes preformed beta-amyloid fibrils in vitro. Biochem Biophys Res Commun. 2005;336:444–49. doi: 10.1016/j.bbrc.2005.08.148. [DOI] [PubMed] [Google Scholar]
- 6.Ono K, Li L, Takamura Y, et al. Phenolic compounds prevent amyloid beta-protein oligomerization and synaptic dysfunction by site-specific binding. J Biol Chem. 2012;287:14631–43. doi: 10.1074/jbc.M111.325456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen JJ. Apoptosis. Immunol Today. 1993;14:126–30. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- 8.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Williams GT, Smith CA. Molecular regulation of apoptosis: Genetic controls on cell death. Cell. 1993;74:777–79. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- 10.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 11.Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 12.Desagher S, Osen-Sand A, Nichols A, et al. Bid induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zong WX, Lindsten T, Ross AJ, et al. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–86. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Willis SN, Wei A, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 16.Kuwana T, Bouchier-Hayes L, Chipuk JE, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–35. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Nijhawan D, Fang M, Traer E, et al. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–86. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willis SN, Chen L, Dewson G, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Germain M, Milburn J, Duronio V. MCL-1 inhibits BAX in the absence of MCL-1/BAX Interaction. J Biol Chem. 2008;283:6384–92. doi: 10.1074/jbc.M707762200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang HZ, Zhao ZL, Zhou CH. Recent advance in oxazole-based medicinal chemistry. Eur J Med Chem. 2018;144:444–92. doi: 10.1016/j.ejmech.2017.12.044. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, Shen YF, Wu XH, et al. Synthesis and biological evaluation of coumarin derivatives containing imidazole skeleton as potential antibacterial agents. Eur J Med Chem. 2018;143:958–69. doi: 10.1016/j.ejmech.2017.11.100. [DOI] [PubMed] [Google Scholar]
- 22.Bistrović A, Krstulović L, Harej A, et al. Design, synthesis and biological evaluation of novel benzimidazole amidines as potent multi-target inhibitors for the treatment of non-small cell lung cancer. Eur J Med Chem. 2018;143:1616–34. doi: 10.1016/j.ejmech.2017.10.061. [DOI] [PubMed] [Google Scholar]
- 23.Xu YX, Wang H, Li XK, et al. Discovery of novel propargylamine-modified 4-aminoalkyl imidazole substituted pyrimidinyl thiourea derivatives as multifunctional agents for the treatment of Alzheimer’s disease. Eur J Med Chem. 2018;143:33–47. doi: 10.1016/j.ejmech.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Akhtar J, Khan AA, Ali Z, et al. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur J Med Chem. 2017;125:143–89. doi: 10.1016/j.ejmech.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Cohen GM. Caspases: The executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fulda S, Debatin KM. Apoptosis signaling in tumor therapy. Ann NY Acad Sci. 2004;1028:150–56. doi: 10.1196/annals.1322.016. [DOI] [PubMed] [Google Scholar]
- 27.Fulda S, Debatin KM. Targeting apoptosis pathways in cancer therapy. Curr Cancer Drug Targets. 2004;4:569–76. doi: 10.2174/1568009043332763. [DOI] [PubMed] [Google Scholar]
- 28.Adams JM, Cory S. The Bcl-2 protein family: Arbiters of cell survival. Science. 1998;281:1322–26. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 29.Zhang B, Gojo I, Fenton RG. Myeloid cell factor-1 is a critical survival factor for multiple myeloma. Blood. 2002;99:1885–93. doi: 10.1182/blood.v99.6.1885. [DOI] [PubMed] [Google Scholar]
- 30.Festjens N, van Gurp M, van Loo G, et al. Bcl-2 family members as sentinels of cellular integrity and role of mitochondrial intermembrane space proteins in apoptotic cell death. Acta Haematologica. 2004;111:7–27. doi: 10.1159/000074483. [DOI] [PubMed] [Google Scholar]
- 31.Croxton R, Ma Y, Song L, et al. Direct repression of the Mcl-1 promoter by E2F1. Oncogene. 2002;21:1359–69. doi: 10.1038/sj.onc.1205157. [DOI] [PubMed] [Google Scholar]
- 32.Wang JM, Lai MZ, Yang-Yen HF. Interleukin-3 stimulation of mcl-1 gene transcription involves activation of the PU.1 transcription factor through a p38 mitogen-activated protein kinase-dependent pathway. Mol Cell Biol. 2003;23:1896–909. doi: 10.1128/MCB.23.6.1896-1909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JM, Chao JR, Chen W, et al. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19:6195–206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–79. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Yang M, Huang J, Pan HZ, Jin J. Triptolide overcomes dexamethasone resistance and enhanced PS-341-induced apoptosis via PI3k/Akt/NF-kappaB pathways in human multiple myeloma cells. Int J Mol Med. 2008;22:489–96. [PubMed] [Google Scholar]
- 37.Shi X, Jin Y, Cheng C, et al. Triptolide inhibits Bcr-Abl transcription and induces apoptosis in STI571-resistant chronic myelogenous leukemia cells harboring T315I mutation. Clin Cancer Res. 2009;15:1686–97. doi: 10.1158/1078-0432.CCR-08-2141. [DOI] [PubMed] [Google Scholar]