Cardiovascular magnetic resonance imaging (CMR) with late gadolinium enhancement (LGE) has recently been examined in patients after orthotopic heart transplantation (OHT); however, the data is limited to relatively small cohorts (1-3). The objective of this study was to examine natural history and prognostic value of myocardial LGE in our prospective OHT cohort, which includes 114 consecutive adult OHT patients. After excluding 22 patients (17 non-contrast studies, 5 consent withdrawn), the final cohort comprised 92 patients (50 ± 16 years, 38% female). There were 75 patients with a baseline CMR at a median of 6.3 years post-OHT with a 1- and 2-year follow-up and 17 patients with a baseline CMR at a median of 4 months post-OHT with a 3-, 6-, and 12-month follow-up. All studies were carried out on 1.5-T magnetic resonance imaging Siemen scanners. The CMR protocol consisted of cine Steady-state free precession sequence (repetition time 3.0 ms; echo time 1.5 ms; flip angle 70°, slice thickness 6 mm, gap 2 mm, temporal resolution 35 to 40 ms) and LGE sequences (SSFP-based PSIR sequence: TR 960 ms, TE 1.2 ms, slice thickness 6 mm, and gap 2 mm; and a segmented breath-held PSIR Turbo-FLASH sequence: TR 500 to 860 ms, TE 3.3 ms, slice thickness 6 mm, and gap 4 mm). For both LGE sequences, the inversion time was selected using an inversion time scout scan to optimally null normal myocardium. Parallel imaging with generalized autocalibrating partially parallel acquisitions (GRAPPA) was used for both SSFP and Turbo-FLASH techniques. Quantification of LGE extent was performed by a level-2 physician with 1 year of experience in image post-processing who was blinded to clinical events. A 3SD greater than the mean SI of the normal remote myocardium threshold was used.
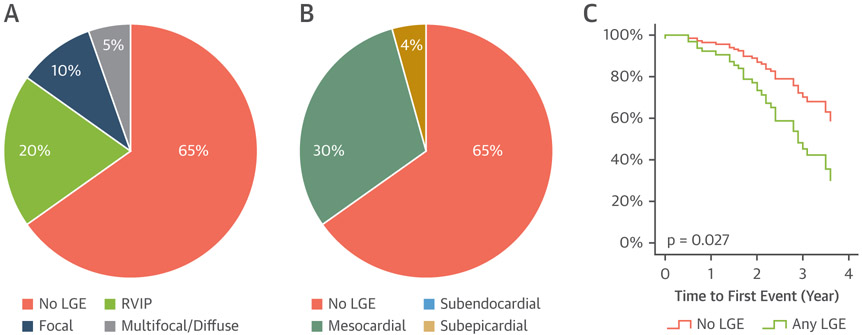
The interval between OHT and baseline magnetic resonance imaging was 5.1 years (interquartile range [IQR]: 2.1 to 8.0 years). The prevalence of LGE was 35% with LGE extent of 3.0% (IQR: 2.3% to 6.8%) of the left ventricular (LV) myocardium. All the LGE had non-coronary artery disease-related patterns predominantly in the inferior right ventricular insertion point (Figure 1A). Patients with LGE had significantly higher use of cardiovascular medications and larger LV and right ventricular (RV) sizes.
Figure 1. LGE in Heart Transplant Recipients.
Left ventricular myocardial LGE is fairly common in heart transplant patients (35%) with the most common LGE pattern located in the RVIP (A) and the most common distribution in a mesocardial (midwall) layer (B). Presence of LGE is associated with increased adverse clinical events as shown in the adjusted event-free survival curve (C). LGE = late gadolinium enhancement; RVIP = right ventricular insertion point.
There were 55 patients who had first follow-up CMR (interval 12 months; IQR: 6 to 22 months). At the first follow-up CMR, 19 patients had LGE. All of them also had LGE at baseline CMR with similar LGE pattern and extent (3%; IQR: 2% to 6%; p = 0.79). At the second follow-up CMR, there were 23 patients (interval 25 months; IQR: 10 to 29 months). Five patients had LGE and all of them also had LGE at baseline and first follow-up CMR with similar pattern and extent (3%; IQR: 2% to 5%; p = 0.21). There was no new LGE observed at both follow-up CMR.
The median follow-up duration for clinical events was 2.4 years (IQR: 1.5 to 3.5 years). Clinical events occurred in 33 patients, including 3 deaths (1 cardiogenic shock, 1 septic shock, and 1 massive pulmonary embolism), 6 heart failure hospitalizations, 3 percutaneous coronary interventions, and 21 non-cardiac hospitalization. Clinical events occurred 2.0 years (IQR: 1.4 to 2.8 years) after the baseline CMR. Patients who had clinical events had significantly higher prevalence of baseline LGE (52% vs. 25%; p = 0.012) with higher pulmonary (16 ± 8 mm Hg vs. 12 ± mm Hg; p = 0.025), RV (35 ± 10 mm Hg vs. 30 ± mm Hg; p = 0.029), and RA pressures (11 ± 7 mm Hg vs. 8 ± 5 mm Hg; p = 0.015). Univariate Cox analysis showed significant association between clinical events and presence of LGE, larger LV end-systolic volume index, and higher RA, RV, and pulmonary pressures. Multivariate Cox analysis demonstrates significant association of adverse clinical events with higher pulmonary capillary wedge pressure (hazard ratio: 1.06; confidence interval 1.02 to 1.11; p = 0.008) and with presence of LGE (hazard ratio: 2.25; confidence interval 1.10 to 4.60; p = 0.027) (Figure 1B).
Our study demonstrates that LGE is fairly common (35%) after OHT. LGE is present at initial post-OHT CMR and it is stable over time. Additionally, LGE is associated with clinical events, which are primarily composed of non-cardiac hospitalization. The predominant distribution and pattern of LGE are similar to what was observed in patients with pulmonary hypertension (4). Although the etiology of myocardial LGE is unknown, it is likely multifactorial. Myocardial LGE in OHT patients possibly represents cumulative myocardial insults prior to the baseline CMR.
Acknowledgments
Please note: The authors have received a National Institutes of Health grant: NHLBI grant R01 HL117888. Dr. Carr has received research grants from Guerbet, Bayer, and Siemens; has been a consultant for GE, Siemens, Circle Cardiovascular Imaging, and Bayer, and has been a speaker for Guerbet. Dr. Carr has received research funds, travel, and honorarium from Siemens, GE, Bayer, Guerbet, and Circle. Dr. Khan has received a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute (KL2TR001424). Dr. Markl has received a consulting and research grant from Circle Cardiovascular Imaging; a research grant from Cryolife Inc.; and research support from Siemens Healthineers. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Butler CR, Kim DH, Chow K, et al. Cardiovascular MRI predicts 5-year adverse clinical outcome in heart transplant recipients. Am J Transplan 2014;14:2055–61. [DOI] [PubMed] [Google Scholar]
- 2.Butler CR, Kumar A, Toma M, et al. Late gadolinium enhancement in cardiac transplant patients is associated with adverse ventricular functional parameters and clinical outcomes. Can J Cardiol 2013;29:1076–83. [DOI] [PubMed] [Google Scholar]
- 3.Pedrotti P, Vittori C, Facchetti R, et al. Prognostic impact of late gadolinium enhancement in the risk stratification of heart transplant patients. Eur Heart J Cardiovasc Imaging 2017;18:130–7. [DOI] [PubMed] [Google Scholar]
- 4.Blyth KG, Groenning BA, Martin TN, et al. Contrast enhanced-cardiovascular magnetic resonance imaging in patients with pulmonary hypertension. Eur Heart J 2005;26:1993–9. [DOI] [PubMed] [Google Scholar]