Abstract
Understanding the neural systems that drive alcohol motivation and are disrupted in alcohol use disorders is of critical importance in developing novel treatments. The dynorphin and orexin/hypocretin neuropeptide systems are particularly relevant with respect to alcohol use and misuse. Both systems are strongly associated with alcohol-seeking behaviors, particularly in cases of high levels of alcohol use as seen in dependence. Furthermore, both systems also play a role in stress and anxiety, indicating that disruption of these systems may underlie long-term homeostatic dysregulation seen in alcohol use disorders. These systems are also closely interrelated with one another – dynorphin/kappa opioid receptors and orexin/hypocretin receptors are found in similar regions and hypocretin/orexin neurons also express dynorphin – suggesting that these two systems may work together in the regulation of alcohol seeking and may be mutually disrupted in alcohol use disorders. This chapter reviews studies demonstrating a role for each of these systems in motivated behavior, with a focus on their roles in regulating alcohol-seeking and self-administration behaviors. Consideration is also given to evidence indicating that these neuropeptide systems may be viable targets for the development of potential treatments for alcohol use disorders.
Keywords: Alcohol, Dynorphin, Ethanol, Hypocretin, Kappa opioid receptor, Orexin
1. Neuropeptides: Dynorphin and Orexin
1.1. Introduction
The dynorphin (DYN) and hypocretin/orexin (ORX) neuropeptide systems play critical roles in regulating appetitively and aversively motivated behaviors. Activation of both systems is associated with arousal, stress, and reward motivation. Both systems are also implicated in psychiatric diseases such as anxiety, depression, and addiction. In particular, DYN and ORX have been demonstrated to be major contributors to alcohol use and potentially misuse and dependence. In this chapter we first discuss the overarching roles of these systems in reward- and aversion-related behaviors followed by a consideration of their roles in alcohol use and dependence. In some cases these peptides are co-expressed, raising questions about their separate versus overlapping roles in the motivational effects of alcohol. We end with a discussion of the potential interaction between these systems and future studies that could address their unique or shared contributions to alcohol consumption and alcohol use disorders.
2. Dynorphin/Kappa Opioid System and Roles in Pharmacological and Motivational Effects of Alcohol
Dynorphins are highly potent endogenous opioids that bind preferentially to kappa opioid receptors with relatively little affinity for mu and delta opioid receptor subtypes. The precursor protein prodynorphin can be cleaved to form numerous active peptides including dynorphin A, dynorphin B, big dynorphin, α and β neoendorphins, and leumorphin (Chavkin 2013). Kappa opioid receptors are G protein-coupled receptors that are distributed widely throughout the central nervous system. When activated, KORs typically couple to inhibitory G proteins and exert their effects through multiple signal transduction pathways (Bruchas and Chavkin 2010). However, lower ligand concentrations have been reported to provoke coupling to Gs proteins, initiating stimulatory signaling cascades (Crain and Shen 2000).
2.1. Dynorphin/Kappa Opioid Receptor (DYN/KOR) System Anatomy
The anatomical distribution of DYN and KOR expression in brain regions associated with reward and stress enable this neuropeptide system to contribute to addiction and mood disorders. Dynorphin immunoreactivity has been observed throughout the cortex, nucleus accumbens (NAc), striatum, caudate-putamen, lateral division of the central nucleus of the amygdala, bed nucleus of the stria terminalis (BNST), hippocampus, multiple hypothalamic nuclei, periacqueductal gray, and numerous brainstem nuclei (Khachaturian et al. 1982; Fallon and Leslie 1986). Assessments of KOR mRNA in the human brain have revealed high expression in prefrontal cortex (PFC; particularly in deep layers), NAc, caudate-putamen, dentate gyrus of the hippocampus, thalamus, hypothalamus, amygdala, ventral tegmental area (VTA), and multiple brainstem nuclei (Peckys and Landwehrmeyer 1999; Simonin et al. 1995). Similarly, a study of KOR mRNA and KOR binding density in the rat brain reported co-expression (suggesting local receptor synthesis) in multiple brain regions including the NAc, caudate-putamen, olfactory tubercle, BNST, paraventricular nucleus of the hypothalamus (PVN), amygdala, periaqueductal gray, raphe nucleus, locus coeruleus, and nucleus of the solitary tract (Mansour et al. 1995). Within the VTA, however, few KOR binding sites are detected but KOR mRNA is highly expressed, suggesting that KORs are likely produced in this region and transported to the NAc (Mansour et al. 1995). Notably, species differences have been observed when comparing KOR expression in humans and rodents: KORs are more widely expressed in the human brain, particularly in the cortex, hippocampus, and thalamus (Peckys and Landwehrmeyer 1999).
2.2. KOR Pharmacology and Signaling
Although pharmacological evidence has suggested the existence of multiple KOR subtypes, only one KOR has been cloned (reviewed by Bruijnzeel 2009; Dietis et al. 2011). Agonist binding at these receptors can initiate the dissociation of G βγ from Gα subunits or directly interact with β arrestins (Bruchas and Chavkin 2010). Consequently, KOR activation can result in stimulation of a variety of signaling cascades (including ERK 1/2, p38 MAPK, and JNK) that depend on the nature of the ligand. The β arrestin recruitment of the p38 MAPK cascade has been implicated in dysphoric effects of KOR agonism and stress (Bruchas et al. 2007). Synthesis of ligands that favor a particular signaling pathway (biased agonism) is an emerging trend in drug development, and recent efforts have aimed to develop KOR ligands that favor G-protein coupled signaling rather than β arrestin in order to diminish aversive effects in favor of therapeutic effects (Lovell et al. 2015; Zhou et al. 2013).
Kappa opioid receptors are expressed throughout the brain, with presynaptic expression enabling modulation of neurotransmission in numerous brain regions associated with drug and alcohol reward. For example, KORs located on glutamatergic projections to the PFC, NAc, dorsal striatum, BNST, and VTA inhibit signaling when activated (Hjelmstad and Fields 2001; Margolis et al. 2005; Tejeda et al. 2013; Atwood et al. 2014; Crowley et al. 2016). Similarly, stimulation of KORs expressed on dopaminergic projections to amygdala, NAc, and PFC and on GABAergic projections to amygdala, NAc, VTA, and BNST also results in decreased transmission (Ford et al. 2007; Margolis et al. 2003, 2006, 2008; Hjelmstad and Fields 2003; Li et al. 2012). KORs located on projections to multiple brain regions can also influence serotonergic and noradrenergic signaling (Berger et al. 2006; Land et al. 2009).
2.3. DYN/KOR System and Motivational Behaviors
Whereas activation of mu opioid receptors stimulates dopamine release in the NAc, activation of KORs results in reduced dopamine release in this brain region (Di Chiara and Imperato 1988). Thus, in contrast to euphoric effects that characterize mu opioid receptor activation, effects of KOR activation are largely aversive or dysphoric. For example, studies in rodents have shown that KOR activation results in conditioned taste and place aversion, decreased reward sensitivity (i.e., increased reward thresholds in intracranial self-stimulation procedures), and increased depressive-like and anxiety-like behaviors (Mucha and Herz 1985; Todtenkopf et al. 2004; Mague et al. 2003; Bruchas et al. 2009; Valdez and Harshberger 2012). Similarly, administration of KOR agonists to humans has been reported to produce aversive effects including anxiety, racing thoughts, agitation, hallucinations, confusion, sedation, and dysphoria (Pfeiffer et al. 1986; Rimoy et al. 1994; Walsh et al. 2001).
KOR activation activates the hypothalamic-pituitary-adrenocortical (HPA) axis and stimulates glucocorticoid release (Iyengar et al. 1986; Wittmann et al. 2009). Likewise, stress exposure activates and upregulates the KOR system, and DYN signaling through KORs has been implicated in the aversive effects of stress (Land et al. 2008). On the other hand, KOR antagonists have been shown to reduce both anxiety-like and depressive-like behavior in addition to blocking the dysphoric effects of stressor exposure (Knoll et al. 2007; Carr and Lucki 2010; Land et al. 2008; Mague et al. 2003). The DYN/KOR system likely facilitates stress-related signaling through interactions with the corticotropin-releasing factor (CRF) system (Land et al. 2008; Van’t Veer and Carlezon 2013). Evidence for co-localization of DYN and CRF has been observed within neurons of the central nucleus of the amygdala (CeA), PVN, and locus coeruleus (Marchant et al. 2007; Roth et al. 1983; Kreibich et al. 2008).
2.4. Alcohol and the DYN/KOR System
Interest in DYN/KOR modulation of alcohol consumption dates back to the late 1980s, and appears to have stemmed from a body of evidence that established a role for this neuropeptide system in ingestive behaviors (Sandi et al. 1988; Morley and Levine 1983). Since that time, research efforts have expanded to assess effects of alcohol on DYN/KOR expression and function, as well as effects of the DYN/KOR system on alcohol’s rewarding and motivational effects (Anderson and Becker 2017).
2.4.1. Effects of Alcohol Exposure on DYN/KOR Expression and Function in Brain
Both acute and chronic alcohol exposures produce adaptations in the DYN/KOR system, typically reflected by an upregulation of expression and activity. For example, microdialysis and radioimmunoassay studies have revealed that, following acute systemic delivery of alcohol, dynorphin levels are increased in the NAc, CeA, VTA, and PVN (Marinelli et al. 2006; Lam et al. 2008; Jarjour et al. 2009; Chang et al. 2007). Elevated prodynorphin or dynorphin mRNA expression also has been observed in the amygdala, PFC, and PVN following acute alcohol administration (D’Addario et al. 2013; Chang et al. 2007). DYN-B expression was elevated in the NAc following repeated alcohol administration (Lindholm et al. 2000), although in another report, a similar alcohol exposure regimen resulted in decreased KOR mRNA expression in the NAc and VTA (Rosin et al. 1999). Chronic alcohol consumption has been shown to increase DYN mRNA and peptide levels in the PVN and prodynorphin levels in the NAc (Chang et al. 2007; Przewlocka et al. 1997). Adaptations in the DYN/KOR system also occur during alcohol withdrawal, with upregulated KOR signaling and DYN peptide expression observed in the CeA (Kissler et al. 2014).
2.4.2. Effects of KOR Activation and Blockade on Alcohol-Related Behaviors
A number of preclinical studies have examined the effects of KOR agonists and antagonists on alcohol-related behavior. Research examining effects of KOR ligands on home-cage alcohol consumption has yielded variable results in rats, with studies reporting increases, decreases, and no change in alcohol intake following both systemic KOR activation and blockade (Sandi et al. 1988, 1990; Nestby et al. 1999; Holter et al. 2000; Lindholm et al. 2001; Mitchell et al. 2005; Morales et al. 2014; Rorick-Kehn et al. 2014). These discrepant findings are likely due to differences in experimental parameters, including sex, strain, drug dose and timing of administration, stress experience, and history of ethanol exposure (Anderson and Becker 2017). In alcohol-preferring C57BL/6J mice, however, several reports have replicated the finding that the KOR agonist U50,488 increases alcohol intake (Sperling et al. 2010; Rose et al. 2016; Anderson et al. 2016). Likewise, KOR antagonism has been consistently reported to decrease home-cage alcohol consumption in C57BL/6J mice, though this effect is typically observed only when intake is elevated above a basal level following induction of alcohol dependence or stress exposure (Sperling et al. 2010; Rose et al. 2016; Anderson et al. 2016). Only a few studies have examined the effects of KOR activation in specific brain regions on home-cage ethanol consumption. Administration of U50,488 reduced intake in rats when infused into the lateral hypothalamus or the PVN (Chen et al. 2013; Barson et al. 2010), but had no effect in the NAc or ventral pallidum (Barson et al. 2009; Kemppainen et al. 2012).
Studies using operant self-administration procedures in rats have reported that KOR agonists reduce alcohol self-administration, suggesting that KOR activation opposes the rewarding effects of alcohol (Holter et al. 2000; Henderson-Redmond and Czachowski 2014). KOR agonists are also consistently reported to induce reinstatement of alcohol-seeking behavior, an effect interpreted as a stress-like effect of KOR activation (Harshberger et al. 2016; Funk et al. 2014; Le et al. 2017). Conversely, KOR blockade has been shown to attenuate cue-induced reinstatement and reinstatement induced by pharmacological stressors (Berger et al. 2013; Schank et al. 2012; Funk et al. 2014). Both systemic and site-specific (NAc, BNST, CeA) administrations of the KOR antagonist nor-BNI have been shown to attenuate elevated self-administration in alcohol-dependent rats while not influencing responding in nondependent rats (Walker and Koob 2008; Walker et al. 2011; Nealey et al. 2011; Kissler et al. 2014; Erikson and Walker 2016).
Several reports have observed altered alcohol-induced conditioned place preference following KOR activation, suggesting that KOR signaling can influence the conditioned motivational effects of alcohol. However, the direction of the effect appears to be related to the timing of agonist administration. Specifically, administration of a KOR agonist shortly before alcohol treatment blocked the development of alcohol conditioned place preference whereas administration of the same agonist 90 min before alcohol conditioning sessions resulted in a potentiation of alcohol conditioned place preference (Logrip et al. 2009; Sperling et al. 2010). Although studies with cocaine have revealed time-dependent effects of KOR agonist administration on cocaine-induced dopamine release in the NAc (Ehrich et al. 2014; Chartoff et al. 2016), at present, it is unclear how dose of the KOR agonist (U50,488) and/or the timing of its administration in relation to alcohol intoxication influences the outcome of these conditioning studies. Interestingly, several reports suggest that KOR blockade has no effect on alcohol’s conditioned motivational properties. For instance, nor-BNI did not alter expression of alcohol conditioned place preference or taste aversion under standard testing conditions (Sperling et al. 2010; Roma et al. 2008; Anderson et al. 2013; Nguyen et al. 2012).
A large literature has provided evidence that stress activates the DYN/KOR system and that increased DYN/KOR activity plays an important role in mediating behavioral responses to various stress events (Crowley and Kash 2015; Knoll and Carlezon 2010; Van’t Veer and Carlezon 2013). Despite an established role for this neuropeptide system in stress-related behavior, relatively few studies have examined the influence of DYN/KOR activity in mediating the interaction between stress and alcohol reward (Becker 2017). Although these studies provide evidence to indicate such involvement, the results have not been consistent. For example, pretreatment with the KOR antagonist nor-BNI was reported to block stress-induced potentiation of alcohol conditioned place preference in mice whereas a study in rats reported that nor-BNI administration further enhanced the effects of stress on alcohol conditioned place preference (Sperling et al. 2010; Matsuzawa et al. 1999). Evidence suggests that KOR modulation of alcohol consumption is also influenced by stress conditions. Mice defeated in multiple social interactions consumed more alcohol than victorious mice, an effect that was further enhanced by administration of the KOR agonist U50,488 (Kudryavtseva et al. 2006). In another report, stress-enhanced consumption in alcohol-dependent mice was blocked by the KOR antagonist LY2444296 (Anderson et al. 2016). Similarly, a study that observed elevated alcohol consumption in adult rats exposed to isolation stress throughout adolescence found that nor-BNI administration reversed this effect (Karkhanis et al. 2016a). The same report demonstrated enhanced sensitivity to KOR agonist-induced suppression of dopamine release in the NAc of rats reared in isolation, suggesting long-lasting adaptations of the KOR system following stressful experiences (Karkhanis et al. 2016a). Taken together, a growing body of literature demonstrates that pharmacological manipulation of KORs influences the motivational effects of alcohol. This includes alcohol self-administration as well as the conditioned rewarding effects of alcohol. A host of variables, including dose, timing of drug administration, and stress experience, likely accounts for differences in outcomes. Future studies will be needed to tease apart these important variables, on both mechanistic and behavioral levels.
2.5. Brain Circuitry Analyses of DYN/KOR System Involvement in Alcohol Actions
Substantial evidence indicates that the NAc is an important site where KOR activity modulates alcohol-induced dopamine release. Indeed, systemic administration of alcohol has been shown to provoke DYN release in the NAc (Marinelli et al. 2006), and pharmacological activation of KORs has been reported to reduce alcohol-evoked dopamine release in this brain region (Lindholm et al. 2007). Although the agonist effect was independent of alcohol exposure history, KOR antagonism increased alcohol-evoked dopamine release in rats with a history of repeated alcohol treatment, but not in saline-injected controls (Lindholm et al. 2007). Additional evidence indicates that chronic alcohol exposure results in increased sensitivity of KORs in the NAc. That is, KOR agonist-induced suppression of dopamine release (measured using fast-scan cyclic voltammetry) was more pronounced in subjects exposed to chronic alcohol (Rose et al. 2016; Karkhanis et al. 2016b; Siciliano et al. 2015). These adaptations may explain why blockade of KORs in the NAc shell has been shown to selectively reduce escalated consumption in alcohol-dependent rats (Nealey et al. 2011). Interestingly, another study demonstrated that within the NAc shell, subpopulations of DYN neurons mediate reward and aversion (Al-Hasani et al. 2015). The effects of alcohol on these subpopulations have yet to be examined and are worthy of future study.
Alcohol administration also results in DYN release in the CeA (Lam et al. 2008). Induction of alcohol dependence via repeated alcohol vapor inhalation has been shown to increase both DYN peptide expression and KOR signaling within the CeA (Kissler et al. 2014). Accordingly, site-specific administration of the KOR antagonist nor-BNI into this area resulted in reduced alcohol consumption in dependent rats, but not their nondependent counterparts (Kissler et al. 2014). KOR ligands have also been reported to influence the effects of alcohol on GABAergic transmission within the CeA (Kang-Park et al. 2013; Gilpin et al. 2014).
Recent and ongoing research continues to illuminate mechanisms of DYN modulation of neural signaling in other brain regions sensitive to alcohol, although interactions with alcohol actions have not yet been well characterized. For example, KORs modulate neurotransmission within multiple projections to the BNST, a brain region implicated in alcohol seeking that shows stress-induced plasticity (Conrad et al. 2012; Pina et al. 2015). Activation of KORs on projections from the central amygdala to the BNST inhibits GABAergic transmission (Li et al. 2012), while stimulation of KORs on projections from the basolateral amygdala also inhibits glutamate transmission in the BNST (Crowley et al. 2016). Blockade of KORs in the BNST eliminates KOR agonist-induced reinstatement of operant alcohol self-administration, suggesting a role for KOR signaling in stress modulation of alcohol reward (Le et al. 2017).
The PFC may be another area where the DYN/KOR system interacts with alcohol. KORs modulate neurotransmission in the PFC, and this brain region is implicated in the role of stress in the transition to alcohol dependence (Margolis et al. 2006; Tejeda et al. 2013, 2015; Lu and Richardson 2014; Rodberg et al. 2017). Chronic alcohol exposure increases prodynorphin expression in the PFC, and a comparison of postmortem human brain tissue in alcoholic and control subjects revealed greater expression of prodynorphin and KOR mRNA in the dorsolateral PFC and orbitofrontal cortex (Bazov et al. 2013; D’Addario et al. 2013).
3. Orexin/Hypocretin Receptor System and Roles in Pharmacological and Motivational Effects of Alcohol
In contrast to the DYN/KOR system, the orexin/hypocretin (ORX) system has been less-extensively studied in the context of alcohol use/misuse. Nevertheless, in the past 10 years, interest in the role ORX plays in mediating various alcohol actions has steadily grown. There is also recognition of an intriguing overlap between ORX and DYN neuropeptides, in part due to co-localization of both peptides in the same neurons as well as the fact that both systems play fundamental roles in stress and motivation, particularly for alcohol. These and other relationships between these two neuropeptides will be considered further after a discussion of the ORX system and its role in alcohol use and dependence.
3.1. Orexin/Hypocretin Peptide/Receptor System Anatomy
The ORX system is made up of a population of neurons located exclusively in the tuberal hypothalamus, in a region typically referred to as the lateral hypothalamic area. First discovered in rodents in 1998 where it was termed orexin (Sakurai et al. 1998) and hypocretin (de Lecea et al. 1998) by different research groups, this relatively restricted population of neurons influences a wide range of behaviors. There are approximately 70,000 ORX neurons in humans and approximately 3,000 in rats (Peyron et al. 1998; Nambu et al. 1999), but these neurons project widely across the central nervous system. ORX neurons are defined by the expression of the protein precursor prepro-orexin (preprohypocretin), which is cleaved into two active peptides: the 33 amino acid orexin-A (ORX-A), also known as hypocretin-1 (HCRT-1), and the 28 amino acid orexin-B (ORX-B), also known as hypocretin-2 (HCRT-2). There are two ORX receptors (OXRs), OX1R (HCRTR1) and OX2R (HCRTR2), which exhibit differential selectivity for ORX-A vs. ORX-B, and activation of these receptors mediates numerous physiological functions.
ORX neurons residing in the hypothalamus project widely across the extent of the brain and spinal cord (Peyron et al. 1998; Date et al. 1999; Nambu et al. 1999; van den Pol 1999; Nixon and Smale 2007). Among the many projection targets, the ORX system strongly innervates a number of regions associated with motivation for natural and drug rewards, as well as those associated with emotional regulation, including stress. This includes projection sites with dense ORX terminal expression such as the noradrenergic locus coeruleus, dopaminergic midbrain areas such as the VTA and substantia nigra, the serotonergic raphe nuclei, the cholinergic laterodorsal and peducopontine nuclei, BNST, CeA, a number of thalamic nuclei, and more local projections among numerous nuclei across the extent of the hypothalamus (Peyron et al. 1998; Date et al. 1999; Nambu et al. 1999; Nixon and Smale 2007). Other brain areas, particularly those involved in regulating sleep and arousal, such as the noradrenergic locus coeruleus and the histaminergic tuberomammilary nucleus, are also heavily innervated, demonstrating an additional important role for this system in regulating arousal states (Sakurai 2007). As reviewed below, ORX activity in a number of these targets has been shown to have profound effects on reward seeking, including alcohol seeking, and emotional arousal and regulation.
3.2. ORX Receptor Pharmacology and Signaling
As noted above, ORX peptides exert their effects through two receptor subtypes: the orexin-1 and orexin-2 receptors (OX1R and OX2R, also referred to as HcrtR1 and HcrtR2). OX1R binds ORX-A with high affinity and ORX-B with low affinity, whereas OX2R binds equally to both peptide subtypes. OX1R couples with Gq proteins, resulting in excitation via nonselective cation channels, voltage-gated Ca2+ channels, Na+/Ca2+ exchange, and inhibition of K+ channels, whereas OX2R is Gq- and/or Gi/Go-protein coupled, resulting in more complex signaling outcomes depending on G protein profile (Tang et al. 2008; Kukkonen and Leonard 2014; Sakurai 2014; Kukkonen 2017; Schone and Burdakov 2017). OXRs can also have indirect effects via regulation of NMDA receptors, or via altering presynaptic glutamate or GABA release (Li et al. 2002; Liu et al. 2002; Borgland et al. 2006, 2008; Baimel and Borgland 2012). OX1Rs and OX2Rs are widely distributed across the brain. Within hypothalamic nuclei there is some overlap between receptor subtypes, but in many other regions the two receptor subtypes appear to exhibit complementary expression, with either exclusive or biased expression of one subtype over another (Trivedi et al. 1998; Hervieu et al. 2001; Marcus et al. 2001; Cluderay et al. 2002). For example, OX1R expression is more predominant in areas such as PFC, amygdala nuclei, CA1 andCA2 (but not CA3) hippocampal regions, laterodorsal tegmental area, and locus coeruleus. In other areas, such as the VTA, as well as a number of thalamic nuclei, receptor distribution is approximately equivalent, and OX2Rs predominate in other regions, notably in a number of hypothalamic nuclei, brainstem nuclei, lateral habenula, and other regions. This semi-differential distribution has led some investigators to propose that signaling through the OX1R is more related to emotional and motivational control whereas OX2R signaling conveys the influence of the ORX system on arousal (Sakurai 2014). However, this dichotomy is far from exclusive. For example, the locus coeruleus, which regulates sleep and arousal, exhibits extremely dense expression of OX1Rs. In contrast, the shell of the NAc, an area closely associated with appetitively motivated behaviors, preferentially expresses OX2Rs (Trivedi et al. 1998; Hervieu et al. 2001; Marcus et al. 2001; Cluderay et al. 2002). Thus, attributing specific behavioral effects to actions at OX1Rs vs. OX2Rs remains a challenge, and this includes ORX-mediated effects on alcohol-related behaviors.
The effects of ORX on postsynaptic neurons are largely excitatory, via mechanisms noted above. The extensive projections of the ORX system, particularly to areas such as the VTA, locus coeruleus, BNST, and multiple thalamic, hypothalamic, and amygdalar nuclei, indicate that these neurons produce a potent excitatory drive on a number of regions influential in arousal, emotion, and motivation. For example, ORX release in the VTA produces excitatory plasticity and increases firing of dopamine neurons in vitro and in vivo (Borgland et al. 2006; Korotkova et al. 2006; Muschamp et al. 2007; Moorman and Aston-Jones 2010), as well as elevating dopamine release in VTA targets such as the PFC and NAc (Vittoz and Berridge 2006; Calipari and Espana 2012; Prince et al. 2015). ORX neurons co-release glutamate, in addition to other peptides such as DYN (Chou et al. 2001; Rosin et al. 2003; Schone et al. 2012; Muschamp et al. 2014). Consequently, the effects of ORX neuron activation on downstream targets are complex and may be multimodal depending on the nature of neurotransmitter/peptide cocktail released (Schone et al. 2014; Schone and Burdakov 2017). Exactly what might control this complex signaling is poorly understood, and may derive from activation of differential inputs. In addition, at least two subtypes of ORX neurons have been described based on physiological responses to glucose (Williams et al. 2008; Schone et al. 2011), which may contribute to heterogeneous output. The overall influence of ORX neuron activation and, in particular, ORX signaling through OX1R and OX2R is excitatory. However, multiple factors including co-transmission of glutamate and inhibitory neuropeptides such as DYN result in complex signaling profiles, which likely contribute to dynamic effects on behavior. In addition, OX2R signaling can work through Gi/o pathways which can have an inhibitory effect on target neurons (Muroya et al. 2004). However, exactly how Gi/o interacts with Gq is unclear, and the Gi/o effects on alcohol use and other motivated behaviors are largely unknown.
3.3. ORX System and Motivational Behaviors
The ORX system has been implicated in a wide range of different behavioral functions (Willie et al. 2001; Sutcliffe and de Lecea 2002; Kuwaki and Zhang 2012; Giardino and de Lecea 2014; Mahler et al. 2014; Sakurai 2014; Flores et al. 2015; James et al. 2017a; Schone and Burdakov 2017). Early work focused on the role of ORX activity in the regulation of feeding and control of sleep and arousal. The association of the ORX system with feeding was initially based on the observation that intracerebroventricular ORX administration increased food intake (Sakurai et al. 1998). The relationship with sleep and arousal was initially based on the findings that the absence of ORX neurons and ORX in the CSF is a major, if not primary, factor in the disrupted sleep-wake balance seen in narcolepsy (Lin et al. 1999; Nishino et al. 2000; Thannickal et al. 2000). Many independent lines of research have validated both of these associations. Of particular interest with respect to the role of ORX in regulating motivation, ORX signaling is especially engaged when behavior is directed at highly palatable (rewarding) food such as chocolate, as opposed to rodent chow (Clegg et al. 2002; Cason et al. 2010). Thus, palatable foods that are sweet and high in fat drive activation of ORX neurons, and seeking of these rewarding substances are blocked with treatment with the OX1R antagonist SB334867 (Nair et al. 2008; Borgland et al. 2009; Choi et al. 2010). This finding that the ORX system is closely associated with highly reinforcing food rewards is relevant in the context of drugs of abuse and particularly alcohol. For both alcohol and other drugs of abuse, a number of studies have demonstrated parallel findings – that the ORX system plays an important role when motivation for the drug outcome is high (Borgland et al. 2009; Moorman and Aston-Jones 2009; Espana et al. 2010; Hollander et al. 2012; Mahler et al. 2014; Bentzley and Aston-Jones 2015; Lopez et al. 2016).
The ORX system also plays a critical role in regulating emotional state. In particular, this system has been strongly connected with regulation of stress, anxiety, and fear (Johnson et al. 2012; Kuwaki and Zhang 2012; Giardino and de Lecea 2014; Flores et al. 2015; James et al. 2017a), in part due to its influence on some of the systems that also control arousal (e.g., locus coeruleus) and motivation (e.g., BNST and amygdala). ORX neurons are activated following acute stress, and pharmacological or genetic decreases in ORX signaling result in blunted responses to stress challenges. ORX neurons also regulate fundamental physiological processes such as respiration, cardiovascular function, and temperature, via control of autonomic nuclei in the hypothalamus and brainstem (Madden et al. 2012; Kuwaki 2015; Carrive and Kuwaki 2017). Many of these functions are linked in order to regulate an overall adaptive active coping response to internal or external challenges.
3.4. Alcohol and the ORX System
A large body of evidence implicates a role for ORX signaling in alcohol- and drug-seeking/taking behaviors (Mahler et al. 2012; Baimel et al. 2015; Baimel and Borgland 2017; James et al. 2017b). In general, studies have shown that the ORX system is particularly involved in alcohol/drug-seeking behavior when motivation, demand, or effort requirements are high. This has led to the proposal that a major function of the ORX system is motivational activation, or to energize an individual to respond to needs, challenges, or potential rewards (Mahler et al. 2014). That this fundamental process gets coopted by alcohol and other drugs of abuse is important for understanding fundamental mechanisms of the addiction process. The relationship between ORX system function and motivational effects of alcohol was first investigated by Lawrence and colleagues in 2006 (Lawrence et al. 2006). Since then, there has been a growing interest in understanding contributions of the ORX system to alcohol use/misuse (Lawrence 2010; Mahler et al. 2012; Brown and Lawrence 2013; Barson and Leibowitz 2016; Walker and Lawrence 2017).
3.4.1. Effects of Alcohol Exposure on ORX Expression and Function in Brain
As noted above, the earliest demonstrations of a contribution of ORX signaling to alcohol seeking came from Lawrence and colleagues who reported increased prepro-ORX mRNA after chronic alcohol consumption (Lawrence et al. 2006). This was observed exclusively in alcohol-preferring iP rats originally derived from the Indiana University selectively bred line (Lumeng et al. 1977), but not in genetically selected non-preferring rats, supporting the notion that one link between ORX and alcohol is intensity of motivation or preference. More recent work by this group revealed no effect of alcohol self-administration on number of ORX positive neurons (Kastman et al. 2016), but a separate group demonstrated increased ORX mRNA following chronic alcohol, with expression levels correlated with preference (Barson et al. 2015). Other studies have reported mixed results as well. For example, both decreases and increases in ORX mRNA and peptide levels have been reported after acute or chronic alcohol administration in outbred rats (Morganstern et al. 2010). In studies involving binge-like and chronic alcohol drinking in mice, no changes in mRNA expression were noted, but decreases in ORX peptide levels were observed (Olney et al. 2015, 2017). Finally, zebrafish given chronic alcohol exposure exhibited signs of alcohol preference and increased ORX mRNA expression (Sterling et al. 2015), suggesting conservation of coarse aspects of encoding for this neuropeptide across species. Thus, while a number of studies have demonstrated that alcohol exposure influences ORX mRNA and peptide expression, differences in outcome likely reflect a number of differences in experimental parameters (e.g., species, alcohol dose, exposure duration).
A number of studies have demonstrated an association between alcohol exposure and activation of ORX neurons, primarily using the immediate early gene c-Fos as a measure of activation. For example, rats exhibiting relapse-like responding for alcoholic beer exhibited increased activation of ORX neurons, particularly in the lateral hypothalamus, which correlated significantly with intensity of responding (Hamlin et al. 2007; Millan et al. 2010). Evidence for ORX neuron activation was also demonstrated in studies involving cue and context-related alcohol-seeking behavior in rats. In these studies, Fos activation of ORX neurons in the lateral hypothalamus was correlated with home-cage alcohol-seeking responses whereas context-driven reinstatement responding was correlated with ORX neuron activation in the dorsomedial and perifornical hypothalamic nuclei (Moorman et al. 2016). Discriminative-stimulus driven reinstatement in Wistar rats produced significant increases in c-Fos activation of ORX neurons across the lateral hypothalamus (Dayas et al. 2008), as did stress-induced reinstatement of alcohol responding in iP rats (Kastman et al. 2016) and sensitization following repeated alcohol injections in mice (Macedo et al. 2013). As with ORX mRNA expression, immunohistochemical measurements of ORX neuron activity present somewhat variable results across studies, particularly with respect to the relationship between specific behaviors and hypothalamic subregions, but they reliably show an impact of alcohol responding and consumption.
A small number of studies have investigated the relationship between ORX expression and alcohol use/dependence. Alcohol-dependent patients have been shown to exhibit higher levels of ORX expression during early (1–7 days) vs. late (multiple weeks) abstinence (Bayerlein et al. 2011). Similarly, plasma ORX levels were correlated with depression-like symptoms in early withdrawal in alcohol-dependent patients, but these correlations diminished after several weeks of continued abstinence (von der Goltz et al. 2011). Thus, although limited in scope, there is some clinical evidence to indicate a correlative relationship between ORX system activity and chronic alcohol use and withdrawal. The exact factors underlying these correlations (e.g., craving, stress of withdrawal, etc.) remain to be elucidated, but these studies point to the ORX system as a potential target for dependence treatment.
3.4.2. Effects of ORX Receptor Activation and Blockade on Alcohol-Related Behaviors
The majority of studies investigating the influence of the ORX system on alcohol-related behaviors have involved pharmacologically manipulating OXRs in mice and rats. In general, these studies have shown that antagonism of OX1Rs and, in some cases, OX2Rs results in reduced alcohol self-administration and relapse-like behavior. In many cases, the effect of OXR antagonism was found to be most robust in animals exhibiting high motivation for alcohol, suggesting a role for the ORX system in heightened levels of seeking and drinking as typically seen in alcohol dependence.
Antagonism of OX1Rs, particularly through systemic administration of drugs such as SB334867 (SB), has been shown to decrease motivational aspects of alcohol self-administration. For example, SB administration significantly decreased both operant responding for 10% alcohol and cue-induced reinstatement of alcohol seeking (Lawrence et al. 2006), findings that have been replicated in various rat strains at varying alcohol concentrations (Richards et al. 2008; Jupp et al. 2011b; Martin-Fardon and Weiss 2014; Moorman et al. 2017). Systemic SB treatment also decreased stress (yohimbine)-induced alcohol relapse-like behavior (Richards et al. 2008) and discriminative stimulus-induced reinstatement responding (Jupp et al. 2011a). Neuropeptide S infused into the lateral hypothalamus also induced reinstatement of alcohol seeking, and this behavior was significantly reduced by pretreatment with SB (Cannella et al. 2009). Neuropeptide S may have direct effects on ORX neurons via a Gq/s protein-coupled receptor (NPSR), as over 40% of ORX neurons exhibit NPSR expression and neuropeptide S axons are found in apposition to ORX neurons (Ubaldi et al. 2016). OX1R antagonism decreased progressive ratio breakpoint for alcohol, but not sucrose, suggesting a potentially unique role in alcohol vs. natural reward motivation (Jupp et al. 2011b). SB treatment also decreased alcohol consumption when it was offered in the home-cage, as did the dual OX1R/OX2R antagonist almorexant. In contrast, selective antagonism of OX2Rs (with LSN2424100) had no effect on alcohol drinking (Anderson et al. 2014; Moorman and Aston-Jones 2009). Another study corroborated these findings, demonstrating that systemic almorexant treatment decreased operant alcohol self-administration, although sucrose self-administration was also influenced (Srinivasan et al. 2012). Decreased alcohol consumption following OX1R antagonism has also been shown in mouse models of heavy drinking, such as binge-like consumption and escalated alcohol intake resulting from repeated cycles of chronic intermittent ethanol (CIE) vapor exposure (Carvajal et al. 2015; Olney et al. 2015; Lopez et al. 2016). In a model of compulsive-like alcohol drinking (C57BL/6J mice exhibiting aversion resistance to quinine-adulterated alcohol), OX1R antagonism (SB), but not OX2R antagonism (TSC-OX2-29) reduced intake of alcohol presented alone or in combination with quinine (Lei et al. 2016a). The OX1R antagonist SB pretreatment also blocked alcohol conditioned place preference and alcohol sensitized hyperlocomotion in mice (Voorhees and Cunningham 2011; Macedo et al. 2013). Taken together, a substantial body of evidence has emerged indicating that OXR antagonism, in particular OX1R antagonism, decreases motivational aspects of alcohol self-administration behavior.
One observation that appears consistent across a number of studies is the finding that OXR antagonism is more potent and/or efficacious when motivation for alcohol seeking and consumption is at a high level, either due to natural variation in alcohol preference, or through measures employed to produce dependence-like states. Support for this contention comes from studies demonstrating that the OX1R antagonist SB produces more robust decreases in alcohol self-administration and relapse-like behavior in rats genetically selected for high alcohol preference (Lawrence et al. 2006; Dhaher et al. 2010; Anderson et al. 2014), as well as outbred rats with a high propensity for alcohol taking behavior (Moorman and Aston-Jones 2009; Moorman et al. 2017). Further, OX1R antagonism, using either SB or another selective antagonist (GSK1059865), selectively decreased escalated drinking in dependent (CIE-exposed) mice without altering more moderate levels of alcohol intake in nondependent mice (Lopez et al. 2016). Finally, OX1R antagonism was found to be more effective in reducing compulsive-like alcohol drinking in quinine-resistant (but not quinine-sensitive) mice (Lei et al. 2016a). Collectively, these findings may have important clinical implications, as the ORX system may be a particularly attractive target for treatment of individuals that have transitioned to heavy, compulsive-like alcohol drinking.
Despite this growing and compelling evidence, there are some instances in which either OX1R antagonism has had limited effects or OX2R antagonism has been shown to more prominently influence alcohol self-administration behavior. For example, in one study SB treatment had no effect on Pavlovian spontaneous recovery of alcohol seeking following a period of extinction training, but the drug did decrease renewal of rewarded alcohol self-administration in female alcohol-preferring rats (Dhaher et al. 2010). This raises questions about potential sex differences in a line of research primarily dominated by studies of male rodents. Other studies have found no effect of OX1R antagonism (using the SB-408124 compound) on alcohol self-administration or conditioned place preference but, instead, have observed an influence of OX2R antagonism, using the JNJ-10397049 compound (Shoblock et al. 2011). In contrast to some studies described above, progressive ratio breakpoints in alcohol preferring P rats were not affected by SB treatment (nor by OX2R antagonism), but were decreased by the dual OX1R/OX2R antagonist almorexant (Anderson et al. 2014). Mice in this study exhibited decreased alcohol and sucrose drinking with all OXR antagonists, suggesting a potent effect of OXR manipulation on reward consumption in general. In a separate study in mice, alcohol conditioned place preference was only modestly influenced by SB treatment, although alcohol-induced hyperlocomotion was decreased (Voorhees and Cunningham 2011). Knockdown of ORX expression using morpholinos had limited effect on responding for alcoholic beer, raising questions about the exact nature of ORX control over alcohol-seeking behavior (Prasad and McNally 2014). Thus, although the majority of pharmacological findings relating ORX to motivational effects of alcohol implicate a role for OX1Rs, there are certainly exceptions to this rule. These divergent findings suggest a complex mechanism by which the ORX system regulates alcohol seeking and consumption, potentially by signaling at different receptors in different brain areas.
3.5. Brain Circuitry Analyses of ORX System Involvement in Alcohol
As noted above, the ORX system projects widely across the brain. Systemic treatment with OXR antagonists has been shown to exert direct and indirect effects on behaviors related to the rewarding effects of alcohol. To further investigate these effects, recent studies have begun probing brain region-specific OXR signaling in the context of alcohol-related behaviors. Results from these studies demonstrate a complex framework in which OX1R signaling in some brain areas regulates alcohol self-administration behavior whereas OX2R signaling influences it in other brain regions.
Perhaps the most salient target region for ORX signaling is the VTA, given its prominent role in reward and alcohol/drug-motivated behaviors (Aston-Jones et al. 2010; Brown and Lawrence 2013; Baimel and Borgland 2017). The OX1R antagonist SB applied directly into the VTA decreased cue-induced reinstatement of alcohol responding in iP preferring rats (Brown et al. 2016). The dual OX1R/OX2R antagonist almorexant injected into the VTA decreased self-administration of both alcohol (20%) and sucrose (5%) in Long-Evans rats (Srinivasan et al. 2012; Prasad and McNally 2014). In contrast to these results, SB application to the VTA did not reduce Neuropeptide S-enhanced reinstatement of alcohol seeking in rats, nor did administration of the drug into the noradrenergic locus coeruleus (Ubaldi et al. 2016). Further, SB (and not the OX2R antagonist TCS-OX2-29) injected into the VTA decreased alcohol consumption during the first hour of consumption in a mouse model of binge-like drinking (Olney et al. 2017). Taken together, there is some evidence indicating that ORX signaling in the VTA may contribute to regulation of alcohol self-administration and relapse-like behavior.
Other brain regions where ORX signaling could potentially influence alcohol-seeking/drinking behaviors include the PFC and NAc (Kalivas et al. 2005; Kalivas 2008; Barker et al. 2015; Marchant et al. 2015). Systemic SB treatment that decreased reinstatement of alcohol seeking also decreased c-Fos expression in the NAc core, medial prefrontal cortex (mPFC), orbitofrontal cortex, and piriform cortex (Jupp et al. 2011a). Targeted SB application into the mPFC in iP rats significantly decreased cue-induced reinstatement of alcohol (but not sucrose) responding (Brown et al. 2016). SB injected into the shell subdivision of the NAc or the mPFC decreased alcohol intake in mice (Lei et al. 2016b), and direct administration of SB into the NAc shell region decreased alcohol self-administration in mice (Lei et al. 2016b) and rats (Mayannavar et al. 2016). In contrast, no effect of SB treatment to the insula was observed (Lei et al. 2016b), which is interesting given the important role for the insula in regulating alcohol-seeking behavior (Seif et al. 2013) and the noted influence of ORX in the insula on nicotine seeking (Hollander et al. 2008). In total, however, these findings demonstrate a role for ORX signaling in both cortical (mPFC) and striatal (NAc) regions in regulation of alcohol seeking and consumption.
A number of other brain regions have been implicated in mediating effects of ORX system activity on alcohol-related behaviors. Injections of ORX-A in both the paraventricular nucleus of the hypothalamus (PVN) and the lateral hypothalamus increased alcohol consumption (Schneider et al. 2007), potentially through increasing the frequency of drinking bouts (Chen et al. 2014). SB infusion into the PVN blocked the effects of intra-lateral hypothalamus injection of Neuropeptide S on reinstatement of alcohol responding, as did SB infusion into the BNST (Ubaldi et al. 2016). Antagonism of both OX1Rs and OX2Rs in the CeA reduced alcohol intake in a mouse binge-drinking model (Olney et al. 2017). ORX also interacts with relaxin-3/RXFP3, another peptide system implicated in alcohol-seeking behavior (Ryan et al. 2014). ORX-A, signaling through OX2Rs, excites relaxin-3 neurons in the nucleus incertus, and OX2R antagonists (but not OX1R antagonists) infused into the nucleus incertus decrease stress-induced reinstatement of alcohol responding in alcohol preferring iP rats (Kastman et al. 2016). Another nucleus that may regulate alcohol seeking through OXR2 signaling is the paraventricular nucleus of the thalamus (PVT). This area receives strong ORX projections and is gaining attention as a potential major regulator of motivated behavior and drug seeking (Martin-Fardon and Boutrel 2012; James and Dayas 2013; Matzeu et al. 2014, 2016), including alcohol-seeking behavior (Hamlin et al. 2009). Recent work has shown that alcohol drinking increases ORX peptide and OX2R expression in the anterior PVT, that ORX-A and ORX-B infusions into the anterior PVT increase alcohol intake, and that OXR antagonists, particularly those targeting OX2Rs, in the anterior PVT decrease alcohol consumption (Barson et al. 2015), potentially through interaction with the substance P system (Barson et al. 2017). Thus, ORX system activity within a number of brain regions appears to play a role in modulating alcohol-related behaviors. Future work in this area will be critical for understanding the anatomical and network basis for these effects.
4. Potential Overlap Between ORX and DYN/KOR Systems in Mediating Alcohol-Related Behaviors
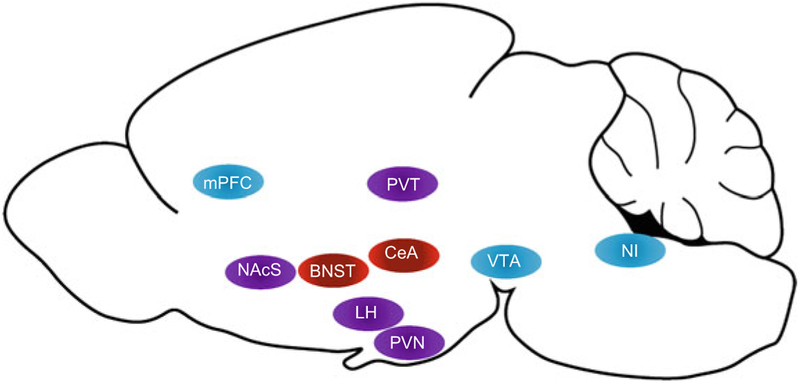
At first glance it may seem counterintuitive to group the ORX and DYN systems when considering peptidergic regulation of alcohol seeking, particularly when considering the diversity of neuropeptides that influence alcohol actions (Barson and Leibowitz 2016). However, there are a number of interesting intersection points to consider in this regard. Most prominently, almost all ORX neurons co-express DYN, and both peptides are packaged in the same vesicles and are co-released (Chou et al. 2001; Crocker et al. 2005; Li and van den Pol 2006; Muschamp et al. 2014; Baimel and Borgland 2017), although ORX is not found in DYN neuron populations outside the lateral hypothalamus. These findings indicate a close degree of coupling between ORX and at least one population of DYN neurons. That is, when “ORX” neurons in the lateral hypothalamus are activated, so are “DYN” neurons. Furthermore, OXRs and KORs are located in many of the same regions, including those in which both peptides are known to regulate motivational effects of alcohol (e.g., VTA, NAc, BNST, CeA, and PFC) (Fig. 1). Although DYN projections to each of these regions may originate from multiple sources, the shared receptor profiles across the two systems suggest a possible interaction in signaling.
Fig. 1.
Brain regions in which pharmacological manipulation of kappa opioid receptors (red), orexin receptors (blue), or both (purple) influences ethanol consumption are shown. Blockade of orexin receptors in the medial prefrontal cortex (mPFC), ventral tegmental area (VTA), nucleus incertus (NI), and paraventricular nucleus of the thalamus (PVT) results in decreased drinking. Conversely, activation of orexin receptors in the PVT increases drinking. Orexin and kappa opioid receptor agonists exert opposing effects on ethanol intake in the lateral hypothalamus (LH) and paraventricular nucleus of the hypothalamus (PVN), with orexin agonists increasing drinking and kappa opioid receptor agonists decreasing drinking. Antagonism of both orexin and kappa opioid receptors in the nucleus accumbens shell (NAcS) reduce ethanol consumption. Within the central nucleus of the amygdala (CeA), blockade of kappa opioid receptors reduces ethanol intake. Mouse brain outline by Jonas Töle
ORX and DYN have largely opposing physiological effects, with OXR signaling primarily producing excitatory effects and KOR signaling producing inhibitory responses in postsynaptic neurons. This raises an interesting question about what purpose is served by co-release of these peptides. Individual dopamine neurons of the VTA are most commonly responsive to both peptides, though some neurons are selectively responsive to ORX vs. DYN (Muschamp et al. 2014). Similar effects have been seen in the basal forebrain (Ferrari et al. 2016), and will presumably be discovered in other co-target regions. The interplay between these systems may also serve as feedback or gating mechanisms within the lateral hypothalamus, as ORX activates ORX/DYN neurons via glutamatergic interneurons (Li et al. 2002), and these neurons are directly inhibited by DYN (Li and van den Pol 2006). These initial studies suggest that either a balance of ORX/DYN release or a balance of OXR/KOR expression and function regulates excitatory/inhibitory profiles. Alternately, it is possible that co-release is precisely balanced to produce a hybrid response that is neither purely excitatory nor inhibitory, but perhaps involves more fine-tuned responses mediated by specific intracellular signaling pathways (Robinson and McDonald 2015). The interaction may also involve differential regulation of inputs or outputs depending on which other pathways are engaged during behavior (Baimel et al. 2017). It is also of note that expression of these peptides is under control of different promoters, which may be activated at different times during behavior. So, synthesis and release may be differentially regulated by the ORX/DYN neurons themselves.
The ORX and DYN systems also mediate different behavioral profiles – ORX more appetitive, DYN more aversive. ORX may facilitate reward motivation by occluding DYN anti-reward signaling, as recently demonstrated in the first behavioral study to directly address this question (Muschamp et al. 2014). Systemic or intra-VTA SB increased brain stimulation threshold levels and decreased impulsivity, and either SB treatment in rats or OXR1 knockdown in mice reduced cocaine self-administration. Interestingly, pretreatment with the KOR antagonist nor-BNI ameliorated all of these changes in behavior. This provides support for the fact that, at least in the VTA and probably elsewhere, the ORX and DYN systems serve opposing or regulatory roles over one another. On the other hand, binary distinctions between the two systems may be unrealistic, given that ORX signaling is also associated with stress and arousal. Despite knowing for over 15 years that these systems overlap, we are still at the beginning of understanding the meaning of ORX/DYN co-expression and co-release. Exactly how these interactions contribute to motivational effects of alcohol, and the therapeutic potential of these interactions, remains to be investigated.
Despite some physiological and behavioral differences, there are significant commonalities between ORX and DYN systems that are particularly relevant with regard to their influence on the motivational effects of alcohol. Orexins are known to play a role in the regulation of food/water intake (e.g., Sakurai et al. 1998; Kunii et al. 1999) and dynorphins have been shown to influence consumption of food and palatable solutions (e.g., Morley and Levine 1983; Beczkowska et al. 1992). These peptides may exert their effects to a greater degree under conditions of high motivation (hunger or thirst resulting from deprivation). Likewise, both systems have been shown to be especially effective in altering alcohol drinking when motivation for the drug is high; the effects of OXR and KOR manipulation on alcohol consumption are greater in subjects that exhibit higher levels of drinking and in models of binge-drinking and dependence-related escalated drinking (Lindholm et al. 2001; Lawrence et al. 2006; Walker and Koob 2008; Moorman and Aston-Jones 2009; Anderson et al. 2014, 2016; Olney et al. 2015; Lopez et al. 2016). Interestingly, under these conditions of apparent high motivation for alcohol, pharmacological manipulation of ORX and KOR receptor systems is selective in influencing alcohol intake relative to motivation for natural rewards (e.g., sucrose). Further, ORX and DYN may play an enhanced role in signaling stress and arousal in these circumstances, as these behavioral components are integral features of motivated action that help focus attention on the target goal. Thus, motivation for high levels of alcohol consumption may result, at least in part, from activation of these peptide systems in a manner that redirects their role from regulating motivation towards natural rewards to driving elevated motivational states that engender high alcohol intake.
Although the exact mechanisms by which ORX and DYN systems influence alcohol seeking and consumption are not fully understood, it is reasonable to assume that chronic alcohol-induced adaptations in these systems contribute to the more selective effects on self-administration associated with dependence. Synaptic plasticity of DYN and ORX neurons induced by chronic alcohol, as observed in studies of other drugs of abuse, is a likely mechanism (Li and van den Pol 2008; Sirohi et al. 2012; Yeoh et al. 2012; Rao et al. 2013). However, the precise adaptations that may occur upstream to alter excitability in DYN and ORX neurons remain unknown. One possibility may involve selective alteration of different pathways for these peptides following chronic alcohol exposure. That is, chronic alcohol may weaken the synaptic strength of certain inputs associated with pathways that subserve motivational behavior directed at natural rewards while simultaneously enhancing synapses from regions that are especially activated by alcohol-associated cues or stress. Alternately, chronic alcohol may produce synaptic changes in downstream targets to promote enhanced motivation for alcohol, as has been seen in studies of psychostimulants and opioids (Baimel et al. 2015), either through direct changes in peptidergic signaling at targets or indirectly through enhancement or suppression of glutamatergic or GABAergic signaling, as observed in ORX and DYN signaling in the VTA (Margolis et al. 2005; Borgland et al. 2006, 2008). Yet another possibility is that chronic alcohol exposure may produce changes at a genomic level within these neural populations. That is, promoters may be up- or down-regulated, biasing regulation and activity of specific DYN and ORX pathways that underlie motivational behavior. Supporting this idea, alcohol exposure in the dorsal striatum was shown to activate brain-derived neurotrophic factor (BDNF) signaling cascades that result in elevated preprodynorphin mRNA and increased DYN translation; in turn, DYN signaling was shown to mediate the decreased alcohol consumption associated with increased BDNF (Logrip et al. 2008).
These considerations are relatively speculative, largely because there is a general paucity of information regarding mechanisms by which chronic alcohol exposure functionally alters DYN and ORX systems. Future work utilizing contemporary experimental approaches will no doubt further advance our understanding of how chronic alcohol influences these peptide systems at the molecular, neuronal, and circuitry levels of analyses. This work, in turn, will shed valuable insight regarding the viability of targets within the DYN and ORX systems as potential therapeutics for tempering excessive alcohol consumption.
5. Summary
A large body of evidence indicates that both DYN and ORX are associated with stress and reward motivation, with implications that these neuropeptide systems play a significant role in contributing to psychiatric disorders including anxiety, depression, and addiction. The neuroanatomical distribution of both neuropeptide systems overlaps in brain regions implicated in the motivational effects of alcohol, including the PFC, NAc, BNST, CeA, and VTA. Accordingly, numerous reports have indicated that both DYN and ORX modulate alcohol intake, particularly when motivation to consume alcohol is high, suggesting that both neuropeptide systems may be promising therapeutic targets for the treatment of alcohol dependence. Interestingly, despite evidence that ORX is typically colocalized with DYN, and that co-release of these neuropeptides can produce opposing effects on dopamine neurons in the VTA, the implications of these interactions have not been studied within the context of alcohol reward. Future work disentangling selective vs. interactive contributions of these neuropeptide systems holds great promise for development of new and novel treatment approaches for alcohol use disorders.
Acknowledgments
This work was supported by NIH grants F32 AA023700 (RIA), P50 AA010761 (HCB), U01 AA014095 (HCB), U01 AA020929 (HCB), AA024571 (DEM), AA025481 (DEM), DA041674 (DEM), VA Medical Research BLR&D BX000813 (HCB), and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (DEM).
Contributor Information
Rachel I. Anderson, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA; Science and Technology Policy Fellowships, American Association for the Advancement of Science, Washington, DC, USA
David E. Moorman, Department of Psychological and Brain Sciences, Neuroscience and Behavior Graduate Program, University of Massachusetts Amherst, Amherst, MA, USA
Howard C. Becker, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA; Charleston Alcohol Research Center, Medical University of South Carolina, Charleston, SC, USA; Department of Neuroscience, Medical University of South Carolina, Charleston, SC, USA; Department of Veterans Affairs, Ralph H. Johnson VA Medical Center, Charleston, SC, USA
References
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park SI, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL, Rogers JA, Bruchas MR (2015) Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87:1063–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Becker HC (2017) Role of the dynorphin/kappa opioid receptor system in the motivational effects of ethanol. Alcohol Clin Exp Res 41:1402–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Agoglia AE, Morales M, Varlinskaya EI, Spear LP (2013) Stress, kappa manipulations, and aversive effects of ethanol in adolescent and adult male rats. Neuroscience 249:214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Becker HC, Adams BL, Jesudason CD, Rorick-Kehn LM (2014) Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models. Front Neurosci 8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, Becker HC (2016) Stress-induced enhancement of ethanol intake in C57BL/6J mice with a history of chronic ethanol exposure: involvement of kappa opioid receptors. Front Cell Neurosci 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA (2010) Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res 1314:74–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Kupferschmidt DA, Lovinger DM (2014) Opioids induce dissociable forms of long-term depression of excitatory inputs to the dorsal striatum. Nat Neurosci 17:540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Borgland SL (2012) Hypocretin modulation of drug-induced synaptic plasticity. Prog Brain Res 198:123–131 [DOI] [PubMed] [Google Scholar]
- Baimel C, Borgland SL (2017) Hypocretin/orexin and plastic adaptations associated with drug abuse. Curr Top Behav Neurosci 33:283–304 [DOI] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, Tung LW, Borgland SL (2015) Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol 172:334–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Lau BK, Qiao M, Borgland SL (2017) Projection-target-defined effects of orexin and dynorphin on VTA dopamine neurons. Cell Rep 18:1346–1355 [DOI] [PubMed] [Google Scholar]
- Barker JM, Corbit LH, Robinson DL, Gremel CM, Gonzales RA, Chandler LJ (2015) Corticostriatal circuitry and habitual ethanol seeking. Alcohol 49:817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Leibowitz SF (2016) Hypothalamic neuropeptide signaling in alcohol addiction. Prog Neuro-Psychopharmacol Biol Psychiatry 65:321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Leibowitz SF, Hoebel BG (2009) Opioids in the nucleus accumbens stimulate ethanol intake. Physiol Behav 98:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Rada P, Leibowitz SF, Hoebel BG (2010) Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. Alcohol Clin Exp Res 34:214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Ho HT, Leibowitz SF (2015) Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol 20:469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Poon K, Ho HT, Alam MI, Sanzalone L, Leibowitz SF (2017) Substance P in the anterior thalamic paraventricular nucleus: promotion of ethanol drinking in response to orexin from the hypothalamus. Addict Biol 22:58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayerlein K, Kraus T, Leinonen I, Pilniok D, Rotter A, Hofner B, Schwitulla J, Sperling W, Kornhuber J, Biermann T (2011) Orexin A expression and promoter methylation in patients with alcohol dependence comparing acute and protracted withdrawal. Alcohol 45:541–547 [DOI] [PubMed] [Google Scholar]
- Bazov I, Kononenko O, Watanabe H, Kuntic V, Sarkisyan D, Taqi MM, Hussain MZ, Nyberg F, Yakovleva T, Bakalkin G (2013) The endogenous opioid system in human alcoholics: molecular adaptations in brain areas involved in cognitive control of addiction. Addict Biol 18:161–169 [DOI] [PubMed] [Google Scholar]
- Becker HC (2017) Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology 122:115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beczkowska IW, Bowen WD, Bodnar RJ (1992) Central opioid receptor subtype antagonists differentially alter sucrose and deprivation-induced water intake in rats. Brain Res 589:291–301 [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G (2015) Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci 41:1149–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B, Rothmaier AK, Wedekind F, Zentner J, Feuerstein TJ, Jackisch R (2006) Presynaptic opioid receptors on noradrenergic and serotonergic neurons in the human as compared to the rat neocortex. Br J Pharmacol 148:795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AL, Williams AM, McGinnis MM, Walker BM (2013) Affective cue-induced escalation of alcohol self-administration and increased 22-kHz ultrasonic vocalizations during alcohol withdrawal: role of kappa-opioid receptors. Neuropsychopharmacology 38:647–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A (2006) Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49:589–601 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Storm E, Bonci A (2008) Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci 28:1545–1556 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A (2009) Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci 29:11215–11225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Lawrence AJ (2013) Ascending orexinergic pathways and alcohol-seeking. Curr Opin Neurobiol 23:467–472 [DOI] [PubMed] [Google Scholar]
- Brown RM, Kim AK, Khoo SY, Kim JH, Jupp B, Lawrence AJ (2016) Orexin-1 receptor signalling in the prelimbic cortex and ventral tegmental area regulates cue-induced reinstatement of ethanol-seeking in iP rats. Addict Biol 21:603–612 [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C (2010) Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology 210:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C (2007) Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci 27:11614–11623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Lemos JC, Chavkin C (2009) CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One 4:e8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW (2009) Kappa-opioid receptor signaling and brain reward function. Brain Res Rev 62:127–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Espana RA (2012) Hypocretin/orexin regulation of dopamine signaling: implications for reward and reinforcement mechanisms. Front Behav Neurosci 6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N, Economidou D, Kallupi M, Stopponi S, Heilig M, Massi M, Ciccocioppo R (2009) Persistent increase of alcohol-seeking evoked by neuropeptide S: an effect mediated by the hypothalamic hypocretin system. Neuropsychopharmacology 34:2125–2134 [DOI] [PubMed] [Google Scholar]
- Carr GV, Lucki I (2010) Comparison of the kappa-opioid receptor antagonist DIPPA in tests of anxiety-like behavior between Wistar Kyoto and Sprague Dawley rats. Psychopharmacology 210:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrive P, Kuwaki T (2017) Orexin and central modulation of cardiovascular and respiratory function. Curr Top Behav Neurosci 33:157–196 [DOI] [PubMed] [Google Scholar]
- Carvajal F, Alcaraz-Iborra M, Lerma-Cabrera JM, Valor LM, de la Fuente L, Sanchez-Amate Mdel C, Cubero I (2015) Orexin receptor 1 signaling contributes to ethanol binge-like drinking: pharmacological and molecular evidence. Behav Brain Res 287:230–237 [DOI] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G (2010) Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav 100:419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, Hoebel BG, Leibowitz SF (2007) Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcohol Clin Exp Res 31:249–259 [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Ebner SR, Sparrow A, Potter D, Baker PM, Ragozzino ME, Roitman MF (2016) Relative timing between kappa opioid receptor activation and cocaine determines the impact on reward and dopamine release. Neuropsychopharmacology 41:989–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C (2013) Dynorphin – still an extraordinarily potent opioid peptide. Mol Pharmacol 83:729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Barson JR, Chen A, Hoebel BG, Leibowitz SF (2013) Opioids in the perifornical lateral hypothalamus suppress ethanol drinking. Alcohol 47:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Barson JR, Chen A, Hoebel BG, Leibowitz SF (2014) Hypothalamic peptides controlling alcohol intake: differential effects on microstructure of drinking bouts. Alcohol 48:657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC (2010) The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience 167:11–20 [DOI] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE (2001) Orexin (hypocretin) neurons contain dynorphin. J Neurosci 21:RC168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Woods SC, Seeley RJ (2002) Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology 143:2995–3000 [DOI] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ (2002) Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept 104:131–144 [DOI] [PubMed] [Google Scholar]
- Conrad KL, Davis AR, Silberman Y, Sheffler DJ, Shields AD, Saleh SA, Sen N, Matthies HJ, Javitch JA, Lindsley CW, Winder DG (2012) Yohimbine depresses excitatory transmission in BNST and impairs extinction of cocaine place preference through orexin-dependent, norepinephrine-independent processes. Neuropsychopharmacology 37:2253–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain SM, Shen KF (2000) Antagonists of excitatory opioid receptor functions enhance morphine’s analgesic potency and attenuate opioid tolerance/dependence liability. Pain 84:121–131 [DOI] [PubMed] [Google Scholar]
- Crocker A, Espana RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, Honda M, Mignot E, Scammell TE (2005) Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology 65:1184–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley NA, Kash TL (2015) Kappa opioid receptor signaling in the brain: circuitry and implications for treatment. Prog Neuro-Psychopharmacol Biol Psychiatry 62:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley NA, Bloodgood DW, Hardaway JA, Kendra AM, McCall JG, Al-Hasani R, McCall NM, Yu W, Schools ZL, Krashes MJ, Lowell BB, Whistler JL, Bruchas MR, Kash TL (2016) Dynorphin controls the gain of an amygdalar anxiety circuit. Cell Rep 14:2774–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Addario C, Caputi FF, Rimondini R, Gandolfi O, Del Borrello E, Candeletti S, Romualdi P (2013) Different alcohol exposures induce selective alterations on the expression of dynorphin and nociceptin systems related genes in rat brain. Addict Biol 18:425–433 [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M (1999) Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A 96:748–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F (2008) Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry 63:152–157 [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95:322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, Rodd ZA (2010) The orexin-1 receptor antagonist SB-334867 reduces alcohol relapse drinking, but not alcohol-seeking, in alcohol-preferring (P) rats. J Addict Med 4:153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988) Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther 244:1067–1080 [PubMed] [Google Scholar]
- Dietis N, Rowbotham DJ, Lambert DG (2011) Opioid receptor subtypes: fact or artifact? Br J Anaesth 107:8–18 [DOI] [PubMed] [Google Scholar]
- Ehrich JM, Phillips PE, Chavkin C (2014) Kappa opioid receptor activation potentiates the cocaine-induced increase in evoked dopamine release recorded in vivo in the mouse nucleus accumbens. Neuropsychopharmacology 39:3036–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson C, Walker BM (2016) Maladaptive behavioral regulation in alcohol dependence: role of dynorphin/kappa-opioid receptor modifications in the bed nucleus of the stria terminalis. In: 2016 neuroscience meeting planner, Society for Neuroscience, San Diego, Program No. 826.13 (Online) [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR (2010) The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci 31:336–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Leslie FM (1986) Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol 249:293–336 [DOI] [PubMed] [Google Scholar]
- Ferrari LL, Agostinelli LJ, Krashes MJ, Lowell BB, Scammell TE, Arrigoni E (2016) Dynorphin inhibits basal forebrain cholinergic neurons by pre- and postsynaptic mechanisms. J Physiol 594:1069–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Saravia R, Maldonado R, Berrendero F (2015) Orexins and fear: implications for the treatment of anxiety disorders. Trends Neurosci 38:550–559 [DOI] [PubMed] [Google Scholar]
- Ford CP, Beckstead MJ, Williams JT (2007) Kappa opioid inhibition of somatodendritic dopamine inhibitory postsynaptic currents. J Neurophysiol 97:883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Le AD (2014) The role of kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats. Brain Behav 4:356–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, de Lecea L (2014) Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr Opin Neurobiol 29:103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Roberto M, Koob GF, Schweitzer P (2014) Kappa opioid receptor activation decreases inhibitory transmission and antagonizes alcohol effects in rat central amygdala. Neuropharmacology 77:294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP (2007) The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience 146:525–536 [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP (2009) Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci 29:802–812 [DOI] [PubMed] [Google Scholar]
- Harshberger E, Gilson EA, Gillett K, Stone JH, El Amrani L, Valdez GR (2016) Nor-BNI antagonism of kappa opioid agonist-induced reinstatement of ethanol-seeking behavior. J Addict 2016:1084235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson-Redmond A, Czachowski C (2014) Effects of systemic opioid receptor ligands on ethanol- and sucrose seeking and drinking in alcohol-preferring (P) and Long Evans rats. Psychopharmacology 231:4309–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA (2001) Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience 103:777–797 [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO, Fields HL (2001) Kappa opioid receptor inhibition of glutamatergic transmission in the nucleus accumbens shell. J Neurophysiol 85:1153–1158 [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO, Fields HL (2003) Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol 89:2389–2395 [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ (2008) Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A 105:19480–19485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Pham D, Fowler CD, Kenny PJ (2012) Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci 6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter SM, Henniger MS, Lipkowski AW, Spanagel R (2000) Kappa-opioid receptors and relapse-like drinking in long-term ethanol-experienced rats. Psychopharmacology 153:93–102 [DOI] [PubMed] [Google Scholar]
- Iyengar S, Kim HS, Wood PL (1986) Kappa opiate agonists modulate the hypothalamic-pituitary-adrenocortical axis in the rat. J Pharmacol Exp Ther 238:429–436 [PubMed] [Google Scholar]
- James MH, Dayas CV (2013) What about me...? The PVT: a role for the paraventricular thalamus (PVT) in drug-seeking behavior. Front Behav Neurosci 7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Campbell EJ, Dayas CV (2017a) Role of the orexin/hypocretin system in stress-related psychiatric disorders. Curr Top Behav Neurosci 33:197–219 [DOI] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G (2017b) A decade of orexin/hypocretin and addiction: where are we now? Curr Top Behav Neurosci 33:247–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjour S, Bai L, Gianoulakis C (2009) Effect of acute ethanol administration on the release of opioid peptides from the midbrain including the ventral tegmental area. Alcohol Clin Exp Res 33:1033–1043 [DOI] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A (2012) Orexin, stress, and anxiety/panic states. Prog Brain Res 198:133–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, Lawrence AJ (2011a) Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin(1) receptors. Br J Pharmacol 162:880–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Krivdic B, Krstew E, Lawrence AJ (2011b) The orexin(1) receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res 1391:54–59 [DOI] [PubMed] [Google Scholar]
- Kalivas PW (2008) Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res 14:185–189 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J (2005) Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45:647–650 [DOI] [PubMed] [Google Scholar]
- Kang-Park M, Kieffer BL, Roberts AJ, Siggins GR, Moore SD (2013) Kappa-opioid receptors in the central amygdala regulate ethanol actions at presynaptic GABAergic sites. J Pharmacol Exp Ther 346:130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Huggins KN, Rose JH, Jones SR (2016a) Switch from excitatory to inhibitory actions of ethanol on dopamine levels after chronic exposure: role of kappa opioid receptors. Neuropharmacology 110:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Weiner JL, Jones SR (2016b) Early-life social isolation stress increases kappa opioid receptor responsiveness and downregulates the dopamine system. Neuropsychopharmacology 41:2263–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastman HE, Blasiak A, Walker L, Siwiec M, Krstew EV, Gundlach AL, Lawrence AJ (2016) Nucleus incertus orexin2 receptors mediate alcohol seeking in rats. Neuropharmacology 110:82–91 [DOI] [PubMed] [Google Scholar]
- Kemppainen H, Raivio N, Suo-Yrjo V, Kiianmaa K (2012) Opioidergic modulation of ethanol self-administration in the ventral pallidum. Alcohol Clin Exp Res 36:286–293 [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Watson SJ, Lewis ME, Coy D, Goldstein A, Akil H (1982) Dynorphin immunocytochemistry in the rat central nervous system. Peptides 3:941–954 [DOI] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, Walker BM (2014) The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiatry 75:774–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA Jr (2010) Dynorphin, stress, and depression. Brain Res 1314:56–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA Jr (2007) Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther 323:838–845 [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL (2006) Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci 23:2677–2685 [DOI] [PubMed] [Google Scholar]
- Kreibich A, Reyes BA, Curtis AL, Ecke L, Chavkin C, Van Bockstaele EJ, Valentino RJ (2008) Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci 28:6516–6525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryavtseva N, Gerrits MA, Avgustinovich DF, Tenditnik MV, Van Ree JM (2006) Anxiety and ethanol consumption in victorious and defeated mice; effect of kappa-opioid receptor activation. Eur Neuropsychopharmacol 16:504–511 [DOI] [PubMed] [Google Scholar]
- Kukkonen JP (2017) Orexin/hypocretin signaling. Curr Top Behav Neurosci 33:17–50 [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, Leonard CS (2014) Orexin/hypocretin receptor signalling cascades. Br J Pharmacol 171:314–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunii K, Yamanaka A, Nambu T, Matsuzaki I, Goto K, Sakurai T (1999) Orexins/hypocretins regulate drinking behaviour. Brain Res 842:256–261 [DOI] [PubMed] [Google Scholar]
- Kuwaki T (2015) Thermoregulation under pressure: a role for orexin neurons. Temperature 2:379–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaki T, Zhang W (2012) Orexin neurons and emotional stress. Vitam Horm 89:135–158 [DOI] [PubMed] [Google Scholar]
- Lam MP, Marinelli PW, Bai L, Gianoulakis C (2008) Effects of acute ethanol on opioid peptide release in the central amygdala: an in vivo microdialysis study. Psychopharmacology 201:261–271 [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C (2008) The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci 28:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C (2009) Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A 106:19168–19173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ (2010) Regulation of alcohol-seeking by orexin (hypocretin) neurons. Brain Res 1314:124–129 [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B (2006) The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 148:752–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Coen K, Tamadon S, Shaham Y (2017) Role of kappa-opioid receptors in the bed nucleus of stria terminalis in reinstatement of alcohol seeking. Neuropsychopharmacology 10.1038/npp.2017.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Wegner SA, Yu JH, Hopf FW (2016a) Orexin-1 receptor blockade suppresses compulsive-like alcohol drinking in mice. Neuropharmacology 110:431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Wegner SA, Yu JH, Mototake A, Hu B, Hopf FW (2016b) Nucleus accumbens shell and mPFC but not insula orexin-1 receptors promote excessive alcohol drinking. Front Neurosci 10:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, van den Pol AN (2006) Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci 26:13037–13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, van den Pol AN (2008) Mu-opioid receptor-mediated depression of the hypothalamic hypocretin/orexin arousal system. J Neurosci 28:2814–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN (2002) Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron – a potential mechanism for orchestrating the hypothalamic arousal system. Neuron 36:1169–1181 [DOI] [PubMed] [Google Scholar]
- Li C, Pleil KE, Stamatakis AM, Busan S, Vong L, Lowell BB, Stuber GD, Kash TL (2012) Presynaptic inhibition of gamma-aminobutyric acid release in the bed nucleus of the stria terminalis by kappa opioid receptor signaling. Biol Psychiatry 71:725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E (1999) The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98:365–376 [DOI] [PubMed] [Google Scholar]
- Lindholm S, Ploj K, Franck J, Nylander I (2000) Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol 22:165–171 [DOI] [PubMed] [Google Scholar]
- Lindholm S, Werme M, Brene S, Franck J (2001) The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav Brain Res 120:137–146 [DOI] [PubMed] [Google Scholar]
- Lindholm S, Rosin A, Dahlin I, Georgieva J, Franck J (2007) Ethanol alters the effect of kappa receptor ligands on dopamine release in the nucleus accumbens. Physiol Behav 92:167–171 [DOI] [PubMed] [Google Scholar]
- Liu RJ, van den Pol AN, Aghajanian GK (2002) Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci 22:9453–9464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D (2008) Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J 22:2393–2404 [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D (2009) Blockade of ethanol reward by the kappa opioid receptor agonist U50,488H. Alcohol 43:359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Moorman DE, Aston-Jones G, Becker HC (2016) The highly selective orexin/hypocretin 1 receptor antagonist GSK1059865 potently reduces ethanol drinking in ethanol dependent mice. Brain Res 1636:74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell KM, Frankowski KJ, Stahl EL, Slauson SR, Yoo E, Prisinzano TE, Aube J, Bohn LM (2015) Structure-activity relationship studies of functionally selective kappa opioid receptor agonists that modulate ERK 1/2 phosphorylation while preserving G protein over betaarrestin2 signaling bias. ACS Chem Neurosci 6:1411–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YL, Richardson HN (2014) Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience 277:139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng L, Hawkins TD, Li T-K (1977) New strains of rats with alcohol preference and nonpreference In: Thurman RG, Williamson JR, Drott H, Chance B (eds) Alcohol and aldehyde metabolizing systems. Academic, New York, pp 537–544 [Google Scholar]
- Macedo GC, Kawakami SE, Vignoli T, Sinigaglia-Coimbra R, Suchecki D (2013) The influence of orexins on ethanol-induced behavioral sensitization in male mice. Neurosci Lett 551:84–88 [DOI] [PubMed] [Google Scholar]
- Madden CJ, Tupone D, Morrison SF (2012) Orexin modulates brown adipose tissue thermogenesis. Biomol Concepts 3:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC Jr, Jones RM, Portoghese PS, Carlezon WA Jr (2003) Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther 305:323–330 [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G (2012) Multiple roles for orexin/hypocretin in addiction. Prog Brain Res 198:79–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G (2014) Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci 17:1298–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ (1995) Opioid-receptor mRNA expression in the rat CNS:anatomical and functional implications. Trends Neurosci 18:22–29 [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Densmore VS, Osborne PB (2007) Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J Comp Neurol 504:702–715 [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM (2015) Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res 1628:219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435:6–25 [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL (2003) Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci 23:9981–9986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL (2005) Both kappa and mu opioid agonists inhibit glutamatergic input to ventral tegmental area neurons. J Neurophysiol 93:3086–3093 [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL (2006) Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A 103:2938–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL (2008) Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci 28:8908–8913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C (2006) A microdialysis profile of dynorphin A(1-8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res 30:982–990 [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Boutrel B (2012) Orexin/hypocretin (Orx/Hcrt) transmission and drug-seeking behavior: is the paraventricular nucleus of the thalamus (PVT) part of the drug seeking circuitry? Front Behav Neurosci 6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F (2014) N-(2-methyl-6-benzoxazolyl)-N′-1,5-naphthyridin-4-yl urea (SB334867), a hypocretin receptor-1 antagonist, preferentially prevents ethanol seeking: comparison with natural reward seeking. Addict Biol 19:233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H (1999) Different roles of mu-, delta- and kappa-opioid receptors in ethanol-associated place preference in rats exposed to conditioned fear stress. Eur J Pharmacol 368:9–16 [DOI] [PubMed] [Google Scholar]
- Matzeu A, Zamora-Martinez ER, Martin-Fardon R (2014) The paraventricular nucleus of the thalamus is recruited by both natural rewards and drugs of abuse: recent evidence of a pivotal role for orexin/hypocretin signaling in this thalamic nucleus in drug-seeking behavior. Front Behav Neurosci 8:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Kerr TM, Weiss F, Martin-Fardon R (2016) Orexin-A/hypocretin-1 mediates cocaine-seeking behavior in the posterior paraventricular nucleus of the thalamus via orexin/hypocretin receptor-2. J Pharmacol Exp Ther 359:273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayannavar S, Rashmi KS, Rao YD, Yadav S, Ganaraja B (2016) Effect of orexin A antagonist (SB-334867) infusion into the nucleus accumbens on consummately behavior and alcohol preference in Wistar rats. Indian J Pharmacol 48:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Furlong TM, McNally GP (2010) Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci 30:4626–4635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL (2005) A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology 182:384–392 [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G (2009) Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol 43:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G (2010) Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. J Neurosci 30:15585–15599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, Kilroy EA, Aston-Jones G (2016) Orexin/hypocretin neuron activation is correlated with alcohol seeking and preference in a topographically specific manner. Eur J Neurosci 43:710–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, Kilroy EA, Aston-Jones G (2017) Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats. Brain Res 1654:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Anderson RI, Spear LP, Varlinskaya EI (2014) Effects of the kappa opioid receptor antagonist, nor-binaltorphimine, on ethanol intake: impact of age and sex. Dev Psychobiol 56:700–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF (2010) Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcohol Clin Exp Res 34:886–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Levine AS (1983) Involvement of dynorphin and the kappa opioid receptor in feeding. Peptides 4:797–800 [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A (1985) Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology 86:274–280 [DOI] [PubMed] [Google Scholar]
- Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, Shibahara M, Kuramochi M, Takigawa M, Yanagisawa M, Sakurai T, Shioda S, Yada T (2004) Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci 19:1524–1534 [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM (2007) A role for hypocretin (orexin) in male sexual behavior. J Neurosci 27:2837–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA Jr (2014) Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A 111:E1648–E1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y (2008) Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol 154:406–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K (1999) Distribution of orexin neurons in the adult rat brain. Brain Res 827:243–260 [DOI] [PubMed] [Google Scholar]
- Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM (2011) Kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology 61:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestby P, Schoffelmeer AN, Homberg JR, Wardeh G, De Vries TJ, Mulder AH, Vanderschuren LJ (1999) Bremazocine reduces unrestricted free-choice ethanol self-administration in rats without affecting sucrose preference. Psychopharmacology 142:309–317 [DOI] [PubMed] [Google Scholar]
- Nguyen K, Tseng A, Marquez P, Hamid A, Lutfy K (2012) The role of endogenous dynorphin in ethanol-induced state-dependent CPP. Behav Brain Res 227:58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E (2000) Hypocretin (orexin) deficiency in human narcolepsy. Lancet 355:39–40 [DOI] [PubMed] [Google Scholar]
- Nixon JP, Smale L (2007) A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav Brain Funct 3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE (2015) Binge-like consumption of ethanol and other salient reinforcers is blocked by orexin-1 receptor inhibition and leads to a reduction of hypothalamic orexin immunoreactivity. Alcohol Clin Exp Res 39:21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE (2017) The role of orexin signaling in the ventral tegmental area and central amygdala in modulating binge-like ethanol drinking behavior. Alcohol Clin Exp Res 41:551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB (1999) Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience 88:1093–1135 [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18:9996–10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233:774–776 [DOI] [PubMed] [Google Scholar]
- Pina MM, Young EA, Ryabinin AE, Cunningham CL (2015) The bed nucleus of the stria terminalis regulates ethanol-seeking behavior in mice. Neuropharmacology 99:627–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AA, McNally GP (2014) Effects of vivo morpholino knockdown of lateral hypothalamus orexin/hypocretin on renewal of alcohol seeking. PLoS One 9:e110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince CD, Rau AR, Yorgason JT, Espana RA (2015) Hypocretin/orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci 6:138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewlocka B, Turchan J, Lason W, Przewlocki R (1997) Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neurosci Lett 238:13–16 [DOI] [PubMed] [Google Scholar]
- Rao Y, Mineur YS, Gan G, Wang AH, Liu ZW, Wu X, Suyama S, de Lecea L, Horvath TL, Picciotto MR, Gao XB (2013) Repeated in vivo exposure of cocaine induces long-lasting synaptic plasticity in hypocretin/orexin-producing neurons in the lateral hypothalamus in mice. J Physiol 591:1951–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE (2008) Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology 199:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoy GH, Wright DM, Bhaskar NK, Rubin PC (1994) The cardiovascular and central nervous system effects in the human of U-62066E. A selective opioid receptor agonist. Eur J Clin Pharmacol 46:203–207 [DOI] [PubMed] [Google Scholar]
- Robinson JD, McDonald PH (2015) The orexin 1 receptor modulates kappa opioid receptor function via a JNK-dependent mechanism. Cell Signal 27:1449–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodberg EM, den Hartog CR, Anderson RI, Becker HC, Moorman DE, Vazey EM (2017) Stress facilitates the development of cognitive dysfunction after chronic ethanol exposure. Alcohol Clin Exp Res 41:1574–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roma PG, Rinker JA, Serafine KM, Chen SA, Barr CS, Cheng K, Rice KC, Riley AL (2008) Genetic and early environmental contributions to alcohol’s aversive and physiological effects. Pharmacol Biochem Behav 91:134–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM et al. (2014) LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology 77:131–144 [DOI] [PubMed] [Google Scholar]
- Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, McCool BA, Jones SR (2016) Supersensitive kappa opioid receptors promotes ethanol withdrawal-related behaviors and reduce dopamine signaling in the nucleus accumbens. Int J Neuropsychopharmacol 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin A, Lindholm S, Franck J, Georgieva J (1999) Downregulation of kappa opioid receptor mRNA levels by chronic ethanol and repetitive cocaine in rat ventral tegmentum and nucleus accumbens. Neurosci Lett 275:1–4 [DOI] [PubMed] [Google Scholar]
- Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG (2003) Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol 465:593–603 [DOI] [PubMed] [Google Scholar]
- Roth KA, Weber E, Barchas JD, Chang D, Chang JK (1983) Immunoreactive dynorphin-(1-8) and corticotropin-releasing factor in subpopulation of hypothalamic neurons. Science 219:189–191 [DOI] [PubMed] [Google Scholar]
- Ryan PJ, Krstew EV, Sarwar M, Gundlach AL, Lawrence AJ (2014) Relaxin-3 mRNA levels in nucleus incertus correlate with alcohol and sucrose intake in rats. Drug Alcohol Depend 140:8–16 [DOI] [PubMed] [Google Scholar]
- Sakurai T (2007) The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 8:171–181 [DOI] [PubMed] [Google Scholar]
- Sakurai T (2014) The role of orexin in motivated behaviours. Nat Rev Neurosci 15:719–731 [DOI] [PubMed] [Google Scholar]
- Sakurai T et al. (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585. (see comments) [DOI] [PubMed] [Google Scholar]
- Sandi C, Borrell J, Guaza C (1988) Involvement of kappa type opioids on ethanol drinking. Life Sci 42:1067–1075 [DOI] [PubMed] [Google Scholar]
- Sandi C, Borrell J, Guaza C (1990) Effects of the kappa opioid receptor antagonist MR-2266-BS on the acquisition of ethanol preference. Life Sci 46:1119–1129 [DOI] [PubMed] [Google Scholar]
- Schank JR, Goldstein AL, Rowe KE, King CE, Marusich JA, Wiley JL, Carroll FI, Thorsell A, Heilig M (2012) The kappa opioid receptor antagonist JDTic attenuates alcohol seeking and withdrawal anxiety. Addict Biol 17:634–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG (2007) Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res 31:1858–1865 [DOI] [PubMed] [Google Scholar]
- Schone C, Burdakov D (2017) Orexin/hypocretin and organizing principles for a diversity of wake-promoting neurons in the brain. Curr Top Behav Neurosci 33:51–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schone C, Venner A, Knowles D, Karnani MM, Burdakov D (2011) Dichotomous cellular properties of mouse orexin/hypocretin neurons. J Physiol 589:2767–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schone C, Cao ZF, Apergis-Schoute J, Adamantidis A, Sakurai T, Burdakov D (2012) Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. J Neurosci 32:12437–12443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schone C, Apergis-Schoute J, Sakurai T, Adamantidis A, Burdakov D (2014) Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep 7:697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW (2013) Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci 16:1094–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, Palmer J, Bonaventure P, Carruthers NI, Lovenberg TW, Boggs J, Galici R (2011) Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology 215:191–203 [DOI] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Cuzon Carlson VC, Helms CM, Lovinger DM, Grant KA, Jones SR (2015) Voluntary ethanol intake predicts kappa-opioid receptor supersensitivity and regionally distinct dopaminergic adaptations in macaques. J Neurosci 35:5959–5968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Gaveriaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattei MG, Charron G, Bloch B, Kieffer B (1995) Kappa-opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc Natl Acad Sci U S A 92:7006–7010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Bakalkin G, Walker BM (2012) Alcohol-induced plasticity in the dynorphin/kappa-opioid receptor system. Front Mol Neurosci 5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP (2010) Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology 210:199–209 [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, Hopf FW, Bonci A, Bartlett SE (2012) The dual orexin/hypocretin receptor antagonist, almorexant, in the ventral tegmental area attenuates ethanol self-administration. PLoS One 7:e44726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling ME, Karatayev O, Chang GQ, Algava DB, Leibowitz SF (2015) Model of voluntary ethanol intake in zebrafish: effect on behavior and hypothalamic orexigenic peptides. Behav Brain Res 278:29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L (2002) The hypocretins: setting the arousal threshold. Nat Rev Neurosci 3:339–349 [DOI] [PubMed] [Google Scholar]
- Tang J, Chen J, Ramanjaneya M, Punn A, Conner AC, Randeva HS (2008) The signalling profile of recombinant human orexin-2 receptor. Cell Signal 20:1651–1661 [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Counotte DS, Oh E, Ramamoorthy S, Schultz-Kuszak KN, Backman CM, Chefer V, O’Donnell P, Shippenberg TS (2013) Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology 38:1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Hanks AN, Scott L, Mejias-Aponte C, Hughes ZA, O’Donnell P (2015) Prefrontal cortical kappa opioid receptors attenuate responses to amygdala inputs. Neuropsychopharmacology 40:2856–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM (2000) Reduced number of hypocretin neurons in human narcolepsy. Neuron 27:469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA Jr (2004) Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology 172:463–470 [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM (1998) Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438:71–75 [DOI] [PubMed] [Google Scholar]
- Ubaldi M, Giordano A, Severi I, Li H, Kallupi M, de Guglielmo G, Ruggeri B, Stopponi S, Ciccocioppo R, Cannella N (2016) Activation of hypocretin-1/orexin-A neurons projecting to the bed nucleus of the stria terminalis and paraventricular nucleus is critical for reinstatement of alcohol seeking by neuropeptide S. Biol Psychiatry 79:452–462 [DOI] [PubMed] [Google Scholar]
- Valdez GR, Harshberger E (2012) Kappa opioid regulation of anxiety-like behavior during acute ethanol withdrawal. Pharmacol Biochem Behav 102:44–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN (1999) Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci 19:3171–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WA Jr (2013) Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology 229:435–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW (2006) Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology 31:384–395 [DOI] [PubMed] [Google Scholar]
- von der Goltz C, Koopmann A, Dinter C, Richter A, Grosshans M, Fink T, Wiedemann K, Kiefer F (2011) Involvement of orexin in the regulation of stress, depression and reward in alcohol dependence. Horm Behav 60:644–650 [DOI] [PubMed] [Google Scholar]
- Voorhees CM, Cunningham CL (2011) Involvement of the orexin/hypocretin system in ethanol conditioned place preference. Psychopharmacology 214:805–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF (2008) Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology 33:643–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Lawrence AJ (2017) The role of orexins/hypocretins in alcohol use and abuse. Curr Top Behav Neurosci 33:221–246 [DOI] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF (2011) Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol 16:116–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Abreu ME, Bigelow GE (2001) Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology 157:151–162 [DOI] [PubMed] [Google Scholar]
- Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D (2008) Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci U S A 105:11975–11980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M (2001) To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci 24:429–458 [DOI] [PubMed] [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C (2009) Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology 34:775–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh JW, James MH, Jobling P, Bains JS, Graham BA, Dayas CV (2012) Cocaine potentiates excitatory drive in the perifornical/lateral hypothalamus. J Physiol 590:3677–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lovell KM, Frankowski KJ, Slauson SR, Phillips AM, Streicher JM, Stahl E, Schmid CL, Hodder P, Madoux F, Cameron MD, Prisinzano TE, Aube J, Bohn LM (2013) Development of functionally selective, small molecule agonists at kappa opioid receptors. J Biol Chem 288:36703–36716 [DOI] [PMC free article] [PubMed] [Google Scholar]