Abstract
Background
Trifluoperazine is an inexpensive accessible 'high potency' antipsychotic drug, widely used to treat schizophrenia or related psychoses.
Objectives
To estimate the effects of trifluoperazine compared with placebo and other drugs.
Search methods
Searches of the Cochrane Schizophrenia Group's register of trials (March 2002), supplemented with hand searching, reference searching, personal communication and contact with industry.
Selection criteria
All clinical randomised trials involving people with schizophrenia and comparing trifluoperazine with any other treatment.
Data collection and analysis
Studies were reliably selected and quality rated and data was extracted. For dichotomous data, relative risks (RR) were estimated, with 95% confidence intervals (CI). Where possible, we undertook intention‐to‐treat analyses. For statistically significant results, the number needed to treat (NNT) was calculated. We estimated heterogeneity (I‐square technique) and publication bias.
Main results
1162 people from 13 studies were randomised to trifluoperazine or placebo. For global improvement, small short‐term studies favoured trifluoperazine (n=95, 3 RCTs, RR 0.62 CI 0.49 to 0.78 NNT 3 CI 2 to 4). Loss to follow up was about 12% in both groups (n=280, 7 RCTs, RR 0.99 CI 0.62 to 1.57) and more people allocated trifluoperazine used antiparkinson drugs to alleviate movements disorders compared with placebo (n=195, 4 RCTs, RR 5.06 CI 2.49 to 10.27, NNH 4 CI 2 to 9). 2230 people from 49 studies were randomised to trifluoperazine or another older generation antipsychotic. Trifluoperazine was not clearly different in terms of 'no substantial improvement' (n=1016, 27 RCTs, RR 1.06 CI 0.98 to 1.14) or leaving the study early (n=930, 22 RCTs, RR 1.15 CI 0.83 to 1.58). Almost identical numbers of people reported at least one adverse event (˜60%) in each group (n=585, 14 RCTs, RR 0.99 CI 0.87 to 1.13), although trifluoperazine was more likely to cause extrapyramidal adverse effects overall when compared to low potency antipsychotics such as chlorpromazine (n=130, 3 RCTs, RR 1.66 CI 1.03 to 2.67, NNH 6 CI 3 to 121). One small study (n=38) found no clear differences between trifluoperazine and the atypical drug, sulpiride.
Authors' conclusions
Although there are shortcomings and gaps in the data, there appears to be enough consistency over different outcomes and periods to confirm that trifluoperazine is an antipsychotic of similar efficacy to other commonly used neuroleptics for people with schizophrenia. Its adverse events profile is similar to that of other drugs. It has been claimed that trifluoperazine is effective at low doses for patients with schizophrenia but this does not appear to be based on good quality trial based evidence.
Plain language summary
Trifluoperazine for schizophrenia
Trifluoperazine is inexpensive and widely accessible. Thousands of people with schizophrenia have participated in studies and therefore we are reasonably sure that it is a potent antipsychotic drug and as good as similar older drugs. Most people taking it do experience adverse effects, but this also applies to other older drugs. Not enough comparisons with newer generations of drugs have been undertaken to be sure of how trifluoperazine compares to them.
Background
The most commonly prescribed antipsychotic drugs (chlorpromazine, haloperidol and trifluoperazine), are the benchmarks against which all others are compared. They have too rarely been the subjects of quantitative, systematic reviews. Now that synthesis of trials comparing placebo with chlorpromazine, and placebo with haloperidol, for people with schizophrenia are maintained in the Cochrane Library (Thornley 2001, Joy 2001), only trifluoperazine remains to be reviewed.
Trifluoperazine is a long‐established, widely used 'conventional' antipsychotic drug. It has been claimed that it is effective at low doses (Ban 1966, Bazire 2000, Reardon 1989). Trifluoperazine has been considered effective and safe since the 1960s (Reardon 1989, APA 1997), and is considered a first‐line drug for people in the acute phase of schizophrenia (APA 1997). Conventional antipsychotic medications vary, both in potency and propensity to induce adverse effects such as, the so‐called 'extrapyramidal adverse effects', or EPS. With EPS, the normally fluid movements of everyday living become stiff and tremulous, facial expression becomes limited and some repetitive movements of the mouth and face may become gross and disfiguring. These EPS are said to occur more frequently with 'high‐potency' compounds such as haloperidol and trifluoperazine. Sedation and low blood pressure, so often associated with antipsychotic drugs, is said to be less frequent with the 'high potency' trifluoperazine (APA 1997).
The search to identify antipsychotic drugs with minimal adverse effects, and a broader spectrum of efficacy, has produced a class of drug known as atypical antipsychotics. As a group, it is claimed that these cause reduced EPS and have some efficacy in the treatment of the negative symptoms of schizophrenia (apathy, inability to express emotions, poverty of speech and lack of motivation). From the whole class of 'atypicals' , only clozapine has proved to be more potent for treating refractory symptoms (Wahlbeck 2001, Duggan 2002, Hunter 2003), although even this is in question (Geddes 2000). Atypical antipsychotics are more expensive than the conventional drugs, and cost has become part of the controversy concerning their prescribing (Wood Mackenzie 1998, Meltzer 1996). Cost is a major influence on accessibility of treatments, especially for middle or low‐income countries.
Technical background Trifluoperazine is a phenothiazine derivative, 10‐[3‐(4‐methyl‐1‐piperazinyl)propyl]‐2‐trifluoromethylpheno thiazine(hydrochloride), sold as Stelazine by GlaxoSmithkline (tablets of 1mg, 2 mg, 5mg and 10 mg). The daily dose ranges from 12 to 50 mg (APA 1997). It may be administered orally once or twice a day.
Trifluoperazine is related chemically to chlorpromazine, but it has high milligram potency, and an adverse effect profile (mainly the incidence of extrapyramidal symptoms) similar to haloperidol (Luckey 1967). It has a specific action on the brain: D2 receptors blockade. The blockade of postsynaptic D2 receptors in mesolimbic and mesocortical projection is responsible for initiating the therapeutic actions of typical antipsychotic drugs. The blockade in striatum is responsible for the extrapyramidal effects and in the tuberoinfundubular system of the hypothalamus can produce hyperprolactinemia (Arana 2000). Although D2 blockade takes hours, clinical effects usually take weeks, probably because the decrease of homovallinic acid levels (principal metabolite of dopamine) is expected to take several weeks (Bazire 2000). The risk of extrapyramidal reactions (pseudo parkinsonism, acute dystonic reaction, akathisia and tardive dyskinesia) is suggested to be higher than in other phenothiazines. Trifluoperazine has a low potency of cholinergic blockade (confusion, agitation, dry mouth, blurred vision, urinary retention) and causes less sedation and orthostatic hypotension (histaminic and alpha‐adrenergic antagonism). It is usually well tolerated (Kaplan 1998).
Objectives
To examine the effects of trifluoperazine compared to placebo, and other antipsychotic drugs, for people with schizophrenia.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials were included. Where a trial was described as 'double‐blind' but it was implied that the study was randomised and the demographic details of each group were similar, it was included. Quasi‐randomised studies, such as those allocated by using alternate days of the week, were excluded.
Types of participants
People with schizophrenia and non‐affective serious/chronic mental illness irrespective of mode of diagnosis, age, sex and chronicity of illness.
Types of interventions
1. Trifluoperazine: any dose and mode or pattern of administration. If a high/low dichotomy was not provided within the trial, high dose was defined as over 30 mg/day, and low dose as any lesser dose. If different doses of trifluoperazine were randomised these studies were also of interest.
2. Placebo.
3. Other typical antipsychotics: any dose and mode or pattern of administration. Examples of such drugs are chlorpromazine and haloperidol.
4. Atypical antipsychotics: any dose and mode or pattern of administration. Examples of such drugs are clozapine, olanzapine, quetiapine, and risperidone.
Types of outcome measures
As schizophrenia is often a life‐long illness, and trifluoperazine is used as an ongoing treatment, outcomes were grouped according to time periods: immediate (seven days or less), short term (eight days to three months), medium term (> three months to one year) and long term (more than one year).
Primary outcomes
1. Service utilisation outcomes 1.1 Hospital admission.
2. Global outcomes 2.1 No clinically significant response in global state ‐ as defined by each of the studies.
3. Mental state 3.1 No clinically significant response in mental state ‐ as defined by each of the studies.
4. Extrapyramidal adverse effects 4.1 Incidence of use of antiparkinsonian drugs.
Secondary outcomes
1. Death 1.1 Suicide. 1.2 Other causes.
2. Service utilisation outcomes 2.1 Days in hospital. 3. Global outcomes 3.1 Average score/change in global state.
4. Mental state 4.1 Average score/change in mental state. 4.2 No clinically significant response on negative symptoms ‐ as defined by each of the studies. 4.3 Average score/change in negative symptoms. 4.4 Relapse as defined in the study.
5. Behaviour 5.1 Leaving the study early. 5.2 No clinically significant response in behaviour ‐ as defined by each of the studies. 5.3 Average score/change in behaviour.
6. Extrapyramidal adverse effects 6.1 No clinically significant extrapyramidal adverse effects ‐ as defined by each of the studies. 6.2 Average score/change in extrapyramidal adverse effects.
7. Other adverse effects, general and specific 7.1 Number of people dropping out due to adverse effects. 7.2 Cardiac effects. 7.3 Anticholinergic effects. 7.4 Antihistaminic effects. 7.5 Prolactin related symptoms.
8. Social functioning 8.1 No clinically significant response in social functioning ‐ as defined by each of the studies. 8.2 Average score/change in social functioning.
9. Economic outcomes
10. Quality of life/ satisfaction with care for either recipients of care or careers. 10.1 Significant change in quality of life / satisfaction ‐ as defined by each of the studies. 10.2 Average score/change in quality of life / satisfaction. 10.3 Employment status.
11. Cognitive functioning
Search methods for identification of studies
Electronic searches
Trifluoperazine is known by many names. We constructed the following search phrase to assist identification:
Trifluoperazine‐phrase = *10‐[3‐(4‐methyl‐1‐piperazinyl)propyl]‐2‐trifluoromethylpheno thiazine (hydrochloride)* or *terfluzine* or *terfluzinor discimer* or *eskazine foille* or *iremo* or *piero* or *jatroneural* or *modalina* or *oxyperazine* or *sedofren* or *sporalon* or *stelazine* or *stelazina* or *stelium* or *terflurazine* or *terfluoperazine* or *SKF 5019* or *7623 RP* or *trifluoperazine* or *Solazine*.
1. The Cochrane Schizophrenia Group's Register (March 2002) was searched using the phrase:
{[(trifluoperazine‐phrase) in title, abstract or index terms of REFERENCE] or [*trifluoperazine* in interventions of STUDY]}
This register is compiled by methodical searches of BIOSIS, CINAHL, Dissertation abstracts, EMBASE, LILACS, MEDLINE, PSYNDEX, PsycINFO, RUSSMED, Sociofile, supplemented with hand searching of relevant journals and numerous conference proceedings (see Group Module).
Searching other resources
1. Reference searching References of all identified studies were also inspected for more trials. SCISEARCH ‐ Science Citation Index (1974 to 2001) was used to trace papers that had cited included trials. These reports were inspected in order to identify further studies.
2. Personal contact The first author of each included study was contacted for information regarding unpublished trials.
3. Industry Pharmaceutical companies were contacted for additional data or reports of trials.
Data collection and analysis
1. Selection of trials All reports of identified studies were inspected by the principal reviewer (LOM). A random sample of ten percent of reports was re inspected by MSL in order to ensure reliability of selection. Where disagreement occurred this was resolved by discussion, or, when there was still doubt, reviewers acquired the full article for further inspection. Once the full articles were obtained LOM decided whether they met review criteria and this was checked by MSL on a 10% sample. Again, if disagreement occurred this was resolved by discussion and when this was not possible further information was sought. These trials were added to the list of those awaiting assessment pending acquisition of this further information. 2. Quality assessment Trials were classified into three quality categories, as described in the Cochrane Collaboration Handbook (Clarke 2001) by the lead reviewer (LOM). A random sample (10%) of trial reports was re inspected by MSL. When disputes arose regarding which category a trial should be allocated to, again, resolution was attempted by discussion. When this was not possible, and further information was necessary, data were not entered into the analyses and the study was allocated to the list of those awaiting assessment. Only trials in Category A or B were included in the review. 3. Data extraction Data from selected trials were extracted twice by LOM blind to the first extraction. A random selection were independently re inspected by MSL (10%). When disputes arose reviewers attempted resolution by discussion. If doubt remained and further information was necessary to resolve the dilemma, reviewers did not enter the data and added them to the list of those awaiting assessment, pending further information.
4. Data synthesis 4.1 Data types Outcomes are assessed using continuous (for example, average changes on a behaviour scale), categorical (for example, one of three categories on a behaviour scale, such as 'little change', 'moderate change' or 'much change') or dichotomous measures (for example, either 'no important changes' or 'important changes' in a person's behaviour). RevMan software does not currently support categorical data so they were only presented in the text of the review. 4.2 Incomplete data With the exception of the outcome of leaving the study early, trial outcomes were not included if more than 40% of people were not reported in the final analysis. Reviewers felt that such a degree of attrition would threaten the validity of any findings. 4.3 Dichotomous data Where the original authors of the studies gave outcomes such as 'clinically improved' or 'not clinically improved' based on their clinical judgment, predetermined criteria or any scale, this was recorded in RevMan. If data was from a rater not clearly stated to be independent then it was only included if it did not change the results, otherwise it was presented separately with a label 'prone to bias'.
Where possible, efforts were made to convert relevant categorical or continuous outcome measures to dichotomous data by identifying cut off points on rating scales and dividing people accordingly into groups. We used the cut off points 'moderate or severe impairment' for end of study data or 'no better or worse' for change data. For example, the Brief Psychiatric Rating Scale (BPRS ‐ Overall 1962) is frequently used as a measure of change of symptoms in studies. The reviewers defined a 50% change on this particular scale as clinically important, although it was recognised that for many people, especially those with chronic or severe illnesses, a less rigorous definition of important improvement, for example, 25% on the BPRS, would be equally valid. If individual patient data were available, the 50% cut off was used for non‐chronically ill people and 25% for those with chronic illness.
This review presents an intention to treat analysis. As long as over 60% of people completed the study, everyone allocated to the intervention was counted whether or not they completed follow up. It was assumed that those who left the study early had a negative outcome, with the exception of the outcome of death. Also with regard to 'reasons for leaving the study early' because of adverse events or relapse, only patients not accounted for were assumed to have a negative outcome. This was felt to be a more reasonable interpretation of limited data. These data were included as long as this did not change the results. This was tested by a sensitivity analysis.
Where trialists presented data as 'last result carried forward' for those who left the study early, these data were only included if they did not change the overall results, otherwise, they were presented separately with the label 'prone to bias'. Where there was genuine uncertainty as to how the trialists had handled data, this uncertainty was tested using a range of possible missing values. If this affected the results then it was presented separately with a label 'prone to bias'.
We used relative risk (RR) and 95% confidence interval (CI) based on the random effects model as the preferred statistic for summation, as this takes into account differences between studies even if heterogeneity is not statistically significant. Data were inspected to see if analysis using a Mantel‐Haenszel odds ratio and fixed effects models made a substantive difference. Where possible, reviewers estimated the number needed to treat / harm (NNT/H). 4.4 Continuous data In the case of continuous data, completer analysis is presented. 4.4.1 Rating scales A wide range of instruments is available to measure mental health outcomes. These instruments vary in quality and many are not valid, or even ad hoc. For outcome instruments some minimum standards have to be set. They are that: i. the psychometric properties of the instrument should have been described in a peer‐reviewed journal; ii. the instrument should either be a self‐report, or completed by an independent rater or relative (not the therapist); and iii. the instrument should be a global assessment of an area of functioning. 4.4.2 Normal distribution Mental health continuous data are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data the following standards were applied to all data before inclusion: i. standard deviations and means were reported in the paper or were obtainable from the authors; ii. if the data were finite measures from, for example 0‐100, when the standard deviation was multiplied by two, the result should be less than the mean, otherwise the mean was unlikely to be an appropriate measure of the centre of the distribution (Altman 1996). Continuous data, if normally distributed, were summated using a calculation of the weighted mean difference (WMD). Non‐normally distributed data were reported in the 'Other data types' tables. Endpoint scale‐derived data is finite, ranging from one score to another. Change data is more problematic and for it the rule described above does not hold. Although most change scores are likely to be skewed, it cannot be proven, so they were presented in MetaView. Where both endpoint and change were available for the same outcome the reviewers presented the former in preference. 4.5 Sensitivity analyses 4.5.1 Primary outcomes for an intention‐to‐treat analysis were compared with those from a completer‐only analysis.
4.6 Subgroup analysis 4.6.1 Trials that used low doses of trifluoperazine were compared with those where high doses (over 30 mg/day) were employed.
4.6.2 Studies with less than 40% of people leaving early were compared with those with higher rates.
5. Heterogeneity As well as inspecting the graphical presentations, the reviewers checked whether the differences between results of trials were greater than would be expected by chance alone using tests of heterogeneity (Chi squared). A significance level of less than 0.10 was interpreted as evidence of heterogeneity. When heterogeneity was present, a sensitivity analysis was undertaken. Outlying studies were removed from the pooled measure if they caused a substantive change in the overall findings and these data were presented and discussed separately.
6. Assessing the presence of publication bias Data from all included trials were entered into a funnel graph (trial effect versus trial size or 'precision') in an attempt to investigate the likelihood of overt publication bias. A formal test of funnel plot asymmetry (which suggests potential publication bias) was undertaken where appropriate, according to the methods of Egger 1997. Significance levels of p < 0.1 were set a priori to detect the presence of asymmetry. Where only 3‐4 studies reported an outcome, or there was little variety in sample size (or precision estimate) between studies, tests of asymmetry were not appropriate.
7. Tables and figures Where possible, data were entered into RevMan so the area to the left of the line of no effect indicated a favourable outcome for trifluoperazine.
Results
Description of studies
Please also see Included and Excluded studies tables.
1. Awaiting assessment Four studies have been ordered but not yet obtained (Toru 1969, Gallant 2000, Kogeorgos 1995, Ortega‐Soto 1996). A further eighteen did not fulfil inclusion criteria but we have contacted the authors and await their response before these are excluded. One Polish paper has not yet been translated (Terminska 1989).
2. Excluded studies The majority of excluded studies either did not have a control group (n=7) or were not stated to be randomised (n=32). Some randomised studies used interventions that were not the focus of this review or involved people who did not suffer from schizophrenia (n=7). Ten randomised trials had to be excluded as no data were usable, for example, one was a crossover study with no information for the first stage. Two trials did not report the number of people randomised to each group.
3. Included studies Fifty studies were included.
3.1 Methods All included studies were described as randomised. Follow‐up periods ranged from three days to 13 months. Twenty‐eight were in the short duration category (less than three months) and six were of intermediate duration (three months to one year). Only one was over one year (long duration, Donlon 1978).
3.2 Participants The number of people in the included studies ranged from 18 to 360 (mean 52 SD 50). 47% of studies had 40 or fewer participants but a total of 2583 people have participated in the 50 trials.
From the studies reporting information on the sex of participants, there were 1271 men and 971 women. Ages ranged from 14 to 74 years (mean from 18 studies was about 43 years). Only two studies specifically focused on adolescents (Bagadia 1980, Malik 1980).
Most participants had a diagnosis of schizophrenia although some studies were included if most people had schizophrenia or a schizophrenia‐like illness. For example, Coons 1962 reported that 79% of participants had schizophrenia, and Marjerrison 1964 and Denber 1972 that over 80% of included people suffered from schizophrenia. Rubin 1971 included one person with manic‐depressive disorder and Brauzer 1968 included a single person with chronic brain syndrome with psychoses. Andersen 1974 randomised 40 people with schizophrenia or "paranoid syndrome" and the participants in O'Brien 1974 were 30 people with hostility, suspiciousness, paranoia and aggressiveness.
Only nine studies described the diagnostic criteria used; the remainder appeared to have made a clinical diagnosis without the use of operational criteria. Many trials (n=30) involved only people with chronic illness, two during an acute exacerbation of this illness. Two studies involved people whose illness was designated as highly treatment resistant. The remainder included acutely ill people and first episode patients.
3.3 Setting Most studies were conducted in inpatient settings. Only six trials included only people who were not in hospital. Most trial centres were in USA (30) or Canada (10). Five were in India, two in Australia, two in Europe (Sweden, UK) and one in Peru.
3.4 Interventions The mean dose of trifluoperazine, based on the 25 studies which reported it, was about 28 mg/day (SD ˜20 mg/day, median 26 mg/day) and the range, taken from 35 studies, was 4 to 100 mg/day. Three studies used intramuscular trifluoperazine (Brauzer 1968, Gallant 1968 II, Perales 1974).
Eleven studies employed a separate placebo arm. Forty‐nine studies compared trifluoperazine with oral typical antipsychotics (for comparison compounds and their frequency of use please see Table 4). For the purposes of this review, loxapine and molindone are considered as typical drugs. Only Edwards 1980 compared trifluoperazine to an atypical antipsychotic (sulpiride). Twenty‐four studies stated they used other medications to alleviate adverse effects or problematic behaviour as required. These drugs included benztropine mesylate, biperiden, trihexphenidyl, chloral hydrate, metyprylon, phenobarbital, chlorpromazine, thioridazine, and short action sedatives. Rubin 1971 reported that some patients received psychotherapy in addition to medication.
1. Comparison compounds used in trifluoperazine studies.
| Drug | Number of studies |
| loxapine | 7 |
| pimozide | 6 |
| thiothixene | 4 |
| molindone | 4 |
| SKF 14336 ‐ acridan derivative | 4 |
| chlorpromazine | 4 |
| haloperidol | 4 |
| trifluperidol | 3 |
| fluphenazine | 1 |
| fluspirilene | 1 |
| penfluridol | 1 |
| cetbutindole | 1 |
| triperidol | 1 |
| clorprothixene | 1 |
| clothiapine | 1 |
| butaperazine | 1 |
| SKF 7261 | 1 |
| GP‐45795 | 1 |
3.5 Outcomes Most outcomes were reported as dichotomous (yes‐no/binary outcomes), and are presented as such. Scale derived data was usually categorical and therefore easily dichotomised. Four studies used the Clinical Global Impression (Guy 1976) and reported categorical data. Another 27 studies, undertaken before 1976, used comparable data to report global ratings of improvement. Thirty five studies used the Brief Psychiatric Rating Scale (Overall 1962) and 17 employed the Nurses Observational Scale of Inpatients Evaluation (NOSIE, Honingfeld 1965) but these data were either impossible to extract from graphs or so incomplete as to render them unusable. Menon 1972 reported dichotomous global state data on the Wings rating Scale. *Doongaji 1989 provided continuous data on mean daily dose of an antiparkinson agent but information collected on adverse events and side effects was not standardised.
3.5.1 Scales
3.5.1.1 Global State Clinical Global Impression ‐ CGI (Guy 1976) CGI is a rating instrument commonly used in studies on schizophrenia that enables clinicians to quantify severity of illness and overall clinical improvement during therapy. A seven‐point scoring system is usually used, with low scores indicating decreased severity and/or greater recovery. In this review, absence of clinical improvement was considered to be when patients had moderate, slight or no improvement. 3.5.1.2 Mental state Brief Psychiatric Rating Scale ‐ BPRS (Overall 1962) A brief rating scale used to assess the severity of a range of psychiatric symptoms, including psychotic symptoms. The original scale has 16 items, but a revised 18‐item scale is commonly used. Each item is defined on a seven‐point scale varying from 'not present' to 'extremely severe', scoring from 0‐6 or 1‐7. Scores can range from 0‐126, with high scores indicating more severe symptoms.
3.5.1.3 Behaviour Nurses Observational Scale of Inpatients Evaluation ‐ NOSIE (Honingfeld 1965). An 80‐item scale which ranks items from 0‐4 (0=never present). Ratings are taken from behaviour over the previous three days. The seven headings are: social competence, social interest, personal neatness, co‐operation, irritability, manifest psychosis and finally, psychotic depression. Scoring ranges from 0‐320.
Wing Behaviour Rating Scale (Wing 1961) This scale consists of two parts. The first rates four typical symptoms of schizophrenic mental state, based on a brief psychiatric interview, on a 5‐point scale. The second is a 12‐item behavioural schedule that rates on a three‐point scale. The more acute the patient's condition, the higher the score on the scale.
3.5.1.4 Adverse events Treatment Emergent Symptoms Scale ‐ TESS (Guy 1976) This side effect tool is a checklist where the degree and severity of an adverse event is recorded. The TESS records the presence or absence of a list of side effects.
3.5.2 Missing outcomes No study reported on negative symptoms, neither were there usable cognitive outcomes. Death, suicide or self‐harm were not mentioned in any of the trials. Only one included study attempted to quantify levels of satisfaction or quality of life (Malik 1980) and no trial attempted to any direct economic evaluation of trifluoperazine.
Risk of bias in included studies
1. Randomisation Only four studies described the method used to generate random allocation (Clark 1975, Edwards 1980, Perales 1974, *Pinard 1972). All used tables of random numbers. Eleven trials reported that allocation was undertaken independently (Angus 1969, Clark 1975, Coons 1962, Edwards 1980, Needham 1969, O'Brien 1974, Perales 1974, Schiele 1969, *Simpson 1971, Simpson 1976). Coons 1962 described a form of allocation concealment (sealed envelopes). Denber 1972 and Sugerman 1965 reported that the random code was unknown until the end of study. For the other studies little assurance was given that bias was minimised during the allocation procedure. Twenty‐six studies reported that the numbers allocated to each treatment group were identical, without reporting the use of block randomisation. When allocating by chance this is improbable.
2. Blinding Thirty‐six of the fifty included trials described precautions taken to make the investigation blind (identical capsules). Two studies (Gallant 1968 II, Gallant 1972) gave no indication that blinding had been attempted. Menon 1972 reported that only research workers were blind. The remaining eleven trials indicated that an attempt at blinding had been made, but they gave no description of how this had been undertaken.
3. Loss to follow‐up Thirty‐two studies gave data on loss to follow‐up and 15 reported a full breakdown of the reasons for leaving the study.
4. Data reporting This was generally poor. Overall very little of the data from the fifty included trials was usable. Continuous data were particularly problematic. The most common reason for exclusion was lack of standard deviations and/or failure to give any information about outcomes at all. Many studies also presented findings in graphs, in percentiles or by inexact p‐values. 'P'‐values are commonly used as a measure of association between intervention and outcomes instead of showing the strength of the association. We were unable to carry out an intention to treat analysis in some studies because the data on leaving the study early were not provided or were not fully or not clearly reported. These studies were preceded by a '*'.
5. Overall quality Only studies allocated to Category A or B according to the Cochrane Handbook criteria were included in this review. As only twelve studies could be allocated to Category A, the data from the remaining 38 studies must be considered to be prone to a moderate degree of bias.
Effects of interventions
1. The search The Cochrane Schizophrenia Group's Register (March 2002‐ BIOSIS, CINAHL, Dissertation abstracts, EMBASE, LILACS, MEDLINE, PSYNDEX, PsycINFO, RUSSMED, Sociofile, supplemented with hand searching of relevant journals and numerous conference proceedings) provided 124 records. Of these, after removal of duplicates, 99 were obtained as full publications. A further 25 were acquired after hand searching the references, but only two of the latter could be included. Contacting authors of included studies has not yielded further studies. To date, contacting the relevant drug company (GlaxoSmithkline) has not led to further usable data. Fifty eight publications were sufficiently close to the review's inclusion criteria but had to be excluded (see Excluded Studies table). Twenty three papers or studies still await assessment.
2. COMPARISON 1. TRIFLUOPERAZINE versus PLACEBO A total of 1162 patients from 13 studies were randomised to this comparison.
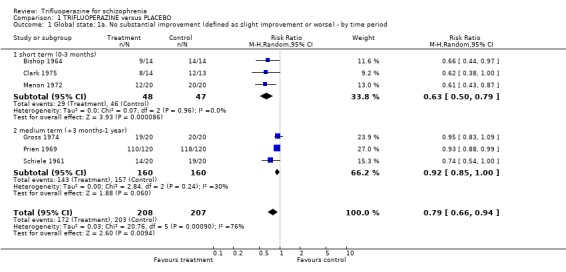
2.1 Global state Studies presented data on global impression in several ways. Three small short‐term studies (three months or less) favoured trifluoperazine (n=95, RR not substantially improved 0.62 CI 0.49 to 0.78, NNT 3 CI 2 to 4). At six months, three other studies tended to favour trifluoperazine (n=320, RR not substantially improved 0.93 CI 0.86 to 1.0). When all information was pooled, heterogeneous data (p=0.0002, I2 79%) favoured trifluoperazine (n=415, RR not substantially improved 0.79 CI 0.67 to 0.94, NNT 6.5 CI 5 to 10).
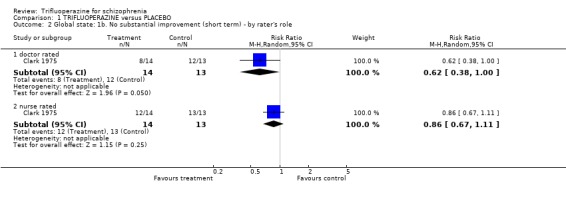
Clark 1975 compared ratings by nurses and doctors for this outcome. This small study (n=27) found no significant differences, although doctors did tend to rate the experimental group more favourably. Prien 1969 evaluated different doses of trifluoperazine compared with placebo and found no clear differences in response.
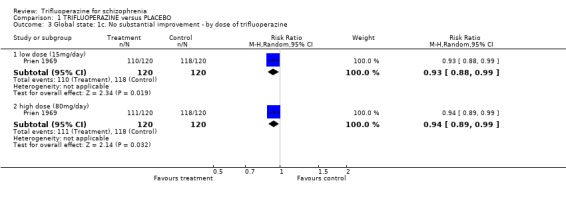
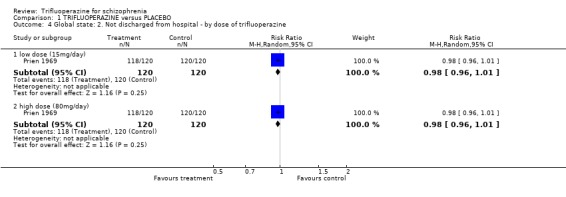
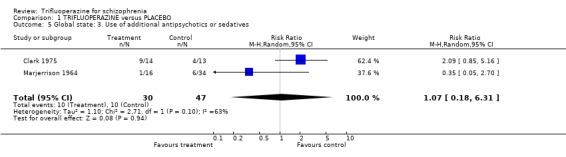
One study provided data on whether or not patients were discharged from hospital (Prien 1969). Most people were in hospital throughout this study and treatment with either high or low dose trifluoperazine made little difference (n=240, RR not discharged on low dose trifluoperazine 0.98 CI 0.96 to 1.01). Two trials reported on 'use of additional antipsychotics/sedatives'. There was no clear difference between trifluoperazine and placebo (n=77, 2 RCTs, RR 1.07 CI 0.18 to 6.31).
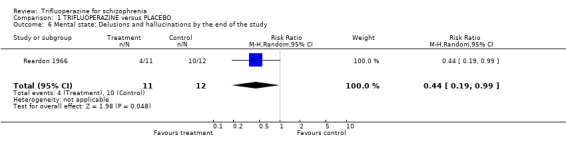
2.2 Mental state Only Reardon 1966 (n=23) directly reported mental state outcomes. Delusions and hallucinations were a little less prevalent for people given trifluoperazine (RR 0.44 CI 0.19 to 0.99, NNT 3 CI 5 to 100).
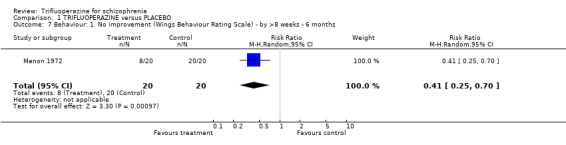
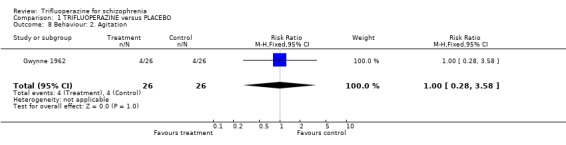
2.3 Behaviour Only one small study reported overall impression of effects on behaviour (Menon 1972). Dichotomised data from the Wings Behaviour Rating Scale favoured trifluoperazine (n=40, RR 0.4 CI 0.23 to 0.68). Another small study reported data on whether a person was agitated (Gwynne 1962, n=52). Trifluoperazine did not seem to have much advantage over placebo (RR agitation 1.0 CI 0.22 to 4.51).
2.4 Leaving the study early There was no significant difference between trifluoperazine and placebo for the outcome 'leaving for any reason' (n=280, 7 RCTs, RR 0.99 CI 0.62 to 1.57). Nearly thirteen percent of the trifluoperazine group left early compared to 11.6% of people allocated to placebo. When different reasons for leaving early the study were analysed separately, this result did not change. 2.5 Adverse events When studies reported on the non‐specific outcome of 'any adverse event', trifluoperazine was no more likely to cause this than placebo (n=77, 2 RCTs, RR 1.4 CI 0.90 to 2.18). Most adverse event data comes from one or two small studies and selective reporting could be an issue. Cardiovascular problems such as minor ECG abnormalities (˜40% of participants in the one study that recorded them, Clark 1975) and hypertension were equally common in the trifluoperazine and placebo groups.
Trifluoperazine may well cause more extrapyramidal events than placebo (n=70, 2 RCTs, RR any moment disorder 2.27 CI 1.19 to 4.33, NNH 4 CI 2 to 23) and more people in the trifluoperazine group used antiparkinson drugs to alleviate movements disorders (n=195, 4 RCTs, RR 5.06 CI 2.49 to 10.27, NNH 4 CI 2 to 9). Other studies reported on more specific movement disorders such as oculogyric crisis (n=27, 1 RCT, RR oculogyric crisis 3.71 CI 0.47 to 29.06) and akathisia but were too underpowered to highlight a real difference. Most results, however, did suggest that trifluoperazine does tend to cause a full spectrum of movement disorders.
Only one study reported haematological outcomes (Clark 1975, n=27). Such disorders were rare and trifluoperazine did not seem implicated.
Gwynne 1962 (n=52) reported neurological outcomes and trifluoperazine did not clearly cause convulsions (RR 0.50 CI 0.10 to 2.50), or faintness (RR 0.67 CI 0.12 to 3.67). Trifluoperazine may, however, be sedating (n=119, 3 RCTs, RR 2.94 CI 1.42 to 6.10, NNH 4 CI 2 to 18).
More people given trifluoperazine lost weight (7/14) than gained it (4/14) but neither effect was statistically significant (Clark 1975). Trifluoperazine may cause more blurred vision than placebo (n=119, 3 RCTs, RR 1.72 CI 0.47 to 6.26).
3. COMPARISON 2. TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTIC This comparison includes 2230 people from 49 studies.
3.1 Global state In terms of the outcome of 'no substantial improvement', trifluoperazine was not clearly different than other older generation drugs (n=1016, 27 RCTs, RR 1.06 CI 0.98 to 1.14). This applies to the short term (n=816, 21 RCTs, RR 1.04 CI 0.94 to 1.15), medium term (n=175, 5 RCTs, RR 1.07 CI 0.93 to 1.24), and to the one long term trial (n=25, RR 1.09 CI 0.92 to 1.29).
Acutely ill people responded similarly (n=351, 10 RCTs, RR 1.03 CI 0.84 to 1.27) to those with a more chronic illness (n=482, 13 RCTs, RR 1.06 CI 0.97 to 1.15). Involving people with mixed diagnoses, such as those with affective disorders, did not effect the outcome of 'no substantial improvement' (n=115, 4 RCTs RR 1.11 CI 0.8 to 1.53).
Three studies, dating from the 1970's, do not suggest that trifluoperazine has any clear advantage over other drugs for the outcome of discharge from hospital (n=121, RR 1.10 CI 0.82 to 1.47). In these trials about 40% of participants were still in hospital by one year follow up.
Trifluoperazine was no better than other drugs in avoiding the need for additional sedation (n=78, 3 RCTs, RR 0.79 CI 0.33 to 1.91).
3.2 Mental state One small study (Reardon 1966) specifically reported whether hallucinations and delusions were present in participants. Most people in both the trifluoperazine and chlorpromazine group did not complain of these key psychotic symptoms (n=22, RR delusions or hallucinations 1.33 CI 0.39 to 4.62). Several other less specific aspects of mental state were reported. Compared with other older generation drugs, trifluoperazine was not clearly better in reducing anxiety, depression or excitement.
3.3 Behaviour One study reported general effects on behaviour (Menon 1972, n=40). Compared with trifluperidol, trifluoperazine was not clearly advantageous (RR no behavioural improvement 1.00 CI 0.47 to 2.14). Similar findings were reported for specific aspects of behaviour such as lethargy and aggression.
3.4 Leaving the study early Twenty‐two short‐term studies (n=930) found no clear differences between trifluoperazine and other typical antipsychotics for the outcome 'leaving for any reason' (RR 1.15 CI 0.83 to 1.58). This held true for the nine medium term studies (n=345, RR 1.8 CI 0.99 to 3.27) and the one long term trial (Donlon 1978, n=25, RR 0.18 CI 0.03 to 1.36). For the more specific outcome of leaving due to adverse effects, trifluoperazine was no more toxic than other older generation drugs, with very few people leaving for this reason (n=320, 8 RCTs, RR leaving due to adverse effects 1.24 CI 0.49 to 3.11). Leaving due to relapse was similar (n=431, 13 RCTs, RR leaving due to relapse 1.31 CI 0.72 to 2.38) as was leaving due to treatment refusal (n=110, 3 RCTs, RR leaving due to treatment refusal 0.83 CI 0.27 to 2.50).
3.5 Adverse events A huge number of adverse events were listed in many different ways. There were no statistically differences between trifluoperazine and other drugs for all comparisons. Almost identical numbers of people reported at least one adverse event (˜60%) in each group (n=585, 14 RCTs, RR 0.99 CI 0.87 to 1.13).
We will not reiterate all adverse effects here but just highlight some of the most noteworthy. Trifluoperazine did not cause more blurred vision than other first generation drugs (n=606, 14 RCTs, RR 1.18 CI 0.91 to 1.55). It did tend to encourage considerable weight gain, but not quite to the conventional level of statistical significance (n=207, 5 RCTs, RR >10lb weight gain 1.25 CI 0.92 to 1.71). Data with a moderate degree of inconsistency (I2 46%) did not suggest that trifluoperazine causes more unspecified movement disorders than other older generation drugs (n=485, 12 RCTs, RR 1.11 CI 0.87 to 1.43). Similarly heterogeneous data (I2 56%) did not suggest that using trifluoperazine results in more need for anticholinergic drugs than typical antipsychotics (n=707, 19 RCTs, RR 1.04 CI 0.87 to 1.24). Trifluoperazine does not seem to cause any more akathisia (n=719, 18 RCTs, RR 0.91 CI 0.70 to 1.17), dystonia (n=520, 13 RCTs, RR 1.09 CI 0.72 to 1.65) or tremor (n=612, 13 RCTs, RR 0.92 CI 0.75 to 1.12) than other old antipsychotics.
We carried out subgroup analyses in some comparisons where heterogeneity was detected. Drugs used in the control group were pooled according their potency for blocking dopamine receptors. Trifluoperazine was more likely to cause extrapyramidal adverse effects overall when compared to the low potency group (n=130, 3 RCTs, RR 1.66 CI 1.03 to 2.67, NNH 6 CI 3 to 121) and there was no difference in the high potency group (n=355, 9 RCTs, RR 1.02 CI 0.78 to 1.34), but heterogeneity remained for this subgroup (p=0.047, I2 46%). The same pattern was apparent for the high versus low potency comparison sub‐groups for the outcome of rigidity and use of antiparkinsonian drugs. Finally for this sub‐group analysis, data from the high potency control group was pooled excluding loxapine because of its different extrapyramidal profile (Fenton 2002) but this made little difference (n=225, 5 RCTs, RR extrapyramidal effects 1.15 CI 0.91 to 1.46).
Trifluoperazine did not cause more nausea (n=457, 11 RCTs, RR 0.82 CI 0.51 to 1.32), or drowsiness (n=816, 20 RCTs, RR 0.89 CI 0.72 to 1.10) than the comparison drugs
3.6 Patient's preference One small study (Malik 1980, n=54) asked patients if they preferred one medication over the other. 50‐60% in each group did express a preference for the other medication.
4. COMPARISON 3. TRIFLUOPERAZINE versus ATYPICAL ANTIPSYCHOTIC Only one study compared trifluoperazine with atypical antipsychotics (sulpiride) in a six‐week trial Edwards 1980 (n=38).
4.1 Global state Raters using the Clinical Global Impression scale, found no difference between the drugs in terms of global state (RR no substantial improvement 0.89 CI 0.44 to 1.81). This also applied to the outcomes of 'severely ill or worse' (RR 1.00 CI 0.43 to 2.3) and use of additional sedatives (RR 2.00 CI 0.58 to 6.85).
4.2 Mental state The small study did not find any effects of trifluoperazine on emotional state (RR lability 0.14 CI 0.01 to 2.59, RR euphoria 0.33 CI 0.04 to 2.93).
4.3 Leaving the study early Very few people left the study. One patient left each group because of worsening symptoms and another person allocated to sulpiride left the hospital (differences not significant).
4.4 Behaviour Less people given trifluoperazine were agitated compared with those in the sulpiride group (0 vs 4) but this was not statistically significant as confidence intervals were wide (RR 0.11 CI 0.01 to 1.93).
4.5 Adverse events We have roughly grouped adverse events into movement disorders and others. Edwards 1980 involved only 38 people so any statistically significant differences would be surprising. None were found, although in keeping with the findings from the trifluoperazine versus placebo comparison it seems that the older drug is more prone to cause problems with movement.
5. Publication bias We used funnel plots to investigate the possibility of publication bias (see Methods). No asymmetry was detected but many of the outcomes had a small number of trials which limits the value of the plot. Such plots are not powerful investigative tools and are further weakened when there is little variation in study size (Egger 1997). 6. Statistical model for measuring effect The findings noted above were not changed by using odds ratios or by using a fixed effects model for relative risk.
7. Sensitivity analyses We had hoped to undertake a sensitivity analysis for intention‐to‐treat analyses versus completer‐only analyses when heterogeneity was present. This is not complete and will be presented in the updates of this review.
Discussion
1. Applicability The 50 included studies involved many people who would be recognisable in everyday medical practice. Only two studies (Bagadia 1980, Malik 1980) focused on adolescents and none on childhood or old age. These trials presented similar results to those including other scopes of participants. In most of the studies the diagnosis was clinical and only a few operational criteria were used. People with schizophrenia who also had any kind of comorbidity such as alcoholism or kidney insufficiency were, however, frequently excluded in original studies and most studies were undertaken in hospital. Applying findings to people with additional health problems and in community settings could be problematic. Overall the daily doses of trifluoperazine were probably a little higher than would be seen in everyday practice (mean 28mg/day, SD 20) but do fit with American Psychiatric Association recommendations (APA 1997).
2. Limitations and strengths The main limitation of this review is that it includes a large number of small studies from many years ago. Valuable additional information could not be obtained as it proved impossible to contact many authors. We were unable to carry out an intention to treat analysis in some studies because the data on leaving the study early were not provided or were not fully or not clearly reported. Despite the fact that most of the included trials predate the CONSORT statement (Begg 1996, Moher 2001) which encourages high standards of trial reporting, twelve trials did receive a quality rating of 'A' (good) and all remaining studies were rated 'B' (medium). This review is the most current and comprehensive synthesis of these data for a widely used antipsychotic.
3. COMPARISON 1. TRIFLUOPERAZINE versus PLACEBO With 1162 people involved in this comparison, these data must represent one of the most powerful placebo comparisons in the whole of antipsychotic research.
3.1 Global state These old placebo‐controlled studies could probably not be repeated now. Although they are dogged with inconsistency in terms of the choice of measures used for similar outcomes, their results do indicate that the number of people needed to be given trifluoperazine in order for one person to improve to an important extent in the medium term is about six. In this respect trifluoperazine is similar to other older generation antipsychotic drugs (Joy 2001, Thornley 2001).
Most of the included studies were undertaken in hospital and were carried out at a time when community care was less prevalent. However, in health systems where hospital care still dominates, it may be relevant that the use of trifluoperazine, at any dose, did not make clear differences in the rate of discharge (though it should be noted that 56% of patients in the study from which these findings are derived had been in hospital for more than ten years). Oddly, being allocated to trifluoperazine did not decrease the risk of needing additional antipsychotics/sedatives. Perhaps this was because the populations in the two studies (total n=77) were not very disturbed or that placebo is also calming.
3.2 Mental state In such a large review, with thousands of patients included, only a single small study (n=23, Reardon 1966) directly reported mental state outcomes. Even within a study of such low power, trifluoperazine did seem to be effective and delusions and hallucinations were decreased (NNT 3 CI 5 to 100). Ideally, of course, this finding should be replicated in order to increase the confidence in the result.
3.3 Behaviour Again, as for mental state outcomes, too few studies with too few participants reported on this for any firm conclusions to be taken from the data. Menon 1972 (n=40) favoured trifluoperazine but Gwynne 1962 (n=52) found no clear differences between trifluoperazine and placebo.
3.4 Leaving the study early Considerably fewer people left these studies early than is common in modern studies where attrition of 40‐50% is common (Duggan 2002, Srisurapanont 2002). This could well be due to the different research climate of three decades ago rather than some advantageous aspect of study design. Nevertheless, the latter is also possible and it could be that modern researchers have lost skills or been so constrained by regulations that design has suffered and so have those likely to benefit from the research. In seven studies (total n=280) trifluoperazine was as acceptable as placebo. It can be concluded that in the climate in which these studies were undertaken, adverse effects were not an obstacle to compliance with study protocol. 3.5 Adverse events It is difficult to interpret some of the adverse effect data. Often a single study reported on an effect that was attributed to trifluoperazine and reporting biases could well be in operation. However, the more consistently reported findings do fit with clinical impression. Trifluoperazine may well cause more extrapyramidal events than placebo (n=70, 2 RCTs, NN to cause any moment disorder 4 CI 2 to 23) and more people in the trifluoperazine group used antiparkinson drugs to alleviate movement disorders (n=195, 4 RCTs, NNH 4 CI 2 to 9). It is likely that trifluoperazine may be sedating (n=119, 3 RCTs, NNH 4 CI 2 to 18) and cause anticholinergic effects (n=119, 3 RCTs, RR blurred vision 1.72 CI 0.47 to 6.26).
4. TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTIC This comparison includes over 2000 people (49 RCTs) and so is one of the largest in the evaluation of the care of people with schizophrenia.
4.1 Global state Analyses of various measures of global state consistently failed to find clear differences between trifluoperazine and other typical antipsychotics (n=1016, 27 RCTs, RR 1.06 CI 0.98 to 1.14) and splitting the data by chronic illness versus non‐chronic, mixed diagnoses versus only people with 'pure' schizophrenia did not materially change the results. Trifluoperazine is as effective as other older generation drugs but there are no data to support its reputed 'activating effects' at low doses.
4.2 Mental state Considering the amount of data for other outcomes, it is surprising that so few have been collected on direct mental state symptoms such as delusions and hallucinations. Reardon 1966 (n=22) did record mental state outcomes but the results are too imprecise for conclusions to be drawn. Even after over 40 years of research based on randomised studies, clinicians and recipients of care have to continue to be unsure of the direct effects on delusions and hallucinations of trifluoperazine compared with other similar drugs.
4.3 Behaviour Behavioural improvement was also a rare outcome (Menon 1972, n=40) so results are imprecise and not clearly different from those of trifluperidol.
4.4 Leaving the study early Taking trifluoperazine is as acceptable as other typical antipsychotics for people with schizophrenia (14% attrition vs 11%) and, because of the relatively large numbers of people involved, we can be fairly certain of these results (short term n=930, medium term n=345). When data were reported with reasons for attrition specified, once more trifluoperazine is no different in terms of leaving the study because of adverse effects, relapse or due to due to treatment refusal. Again, because the numbers of people in these outcomes is relatively large we can be confident of the findings.
4.5 Adverse events Trifluoperazine does cause some kind of adverse effect in about 60% of people, but, according to the data from 14 trials (n=585) so do all other drugs of comparison. There was no difference between the typical antipsychotics and trifluoperazine for a whole series of adverse outcomes for which reasonable amounts of data were available (blurred vision n=606, weight gain n=207, unspecified movement disorders n=485, need for anticholinergic drugs n=707, akathisia n=719, dystonia n=520, or tremor n=612). Use of trifluoperazine carries with it a burden of adverse effects, but no more so than other similar drugs.
The sub‐group analyses must be viewed with a degree of caution as all results are heterogeneous, but the findings do coincide with clinical impression. Trifluoperazine is more likely to cause extrapyramidal adverse effects, the need for additional anticholinergic drugs, and rigidity when compared to the low potency drugs such a chlorpromazine (n˜130, 3 RCTs). This difference disappeared in comparisons with only high potency drugs such as haloperidol (n˜355, 9 RCTs). It is a pity that more studies did not ask patients if they preferred a different medication. With the above results it would be expected that people with schizophrenia would prefer the low potency drugs. Only Malik 1980 (n=54) asked patients if they preferred trifluoperazine to loxapine, an unusual low potency drug. There was no clear preference but with such small numbers the confidence in the final result is limited.
5. TRIFLUOPERAZINE versus SULPIRIDE (ATYPICAL DRUGS) Only one study compared trifluoperazine with an atypical antipsychotic (Edwards 1980, n=38, duration six weeks).
5.1 Global state, mental state, leaving the study early, and adverse effects The precision of all findings is poor as numbers are small but, in terms of measures of global state, mental state, study attrition, and adverse effects trifluoperazine seems little different to sulpiride.
This seems a little implausible and the equivocal findings are probably due to imprecision in the reporting of results. It would be interesting to compare these findings with those of other atypical studies where benchmark drugs such as clozapine have been used, particularly because there is some controversy as to whether sulpiride is really an atypical drug (Soares 2002),
6. Publication bias Even for the outcomes which many studies reported on, funnel plot analyses are limited in power, but it is heartening that no suggestion of small study‐biases were found. Perhaps, as trifluoperazine is an old drug in which there is little current commercial interest, we have managed to identify all relevant trials.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia Trifluoperazine is an antipsychotic which is as effective as other commonly used typical drugs for treating schizophrenia and, in common with the other drugs, about 60% of people do experience an adverse effect. Whether to use trifluoperazine or another typical drug may, therefore, be a matter of personal preference, and there is some indication that trifluoperazine may cause more adverse effects than drugs such as chlorpromazine.
2. For clinicians Although there are shortcomings and gaps in the data, there appears to be enough overall consistency over different outcomes and time scales to confirm that trifluoperazine is an antipsychotic of similar efficacy to other commonly used antipsychotics, such as chlorpromazine. The adverse effect profile of trifluoperazine seems similar to that of typical drugs overall, but, in common with haloperidol, it may have a higher level of extrapyramidal adverse events. The common claim that trifluoperazine is a particularly useful in the treatment of withdrawal patients is not based on trial derived evidence.
3. For managers, funders, decision makers Trifluoperazine is inexpensive and is as effective as other typical antipsychotics. It might be an alternative when newer and more expensive antipsychotics are not readily available.
Implications for research.
1. General All studies considerably preceded the CONSORT statement (Begg 1996, Moher 2001), so the quality of data reporting might be expected to be lower than at present, although some studies were very clearly presented. If data from past studies is still available, we would be most interested in hearing from the authors of trials. Future studies should rigorously apply the standards of reporting as outlined in CONSORT and also make all data freely available. Continuous data were particularly poorly reported and authors should present raw data rather than graphical format.
2. Specific Trifluoperazine does seem comparable to other typical antipsychotics in terms of efficacy but most existing randomised studies are of short‐term duration and were undertaken in hospital settings. Given the clinical dilemma as to whether to use trifluoperazine or, perhaps a drug such as chlorpromazine, there is still a need for more well designed, conducted and reported randomised studies of considerable duration undertaken in real world circumstances.
What's new
| Date | Event | Description |
|---|---|---|
| 14 April 2010 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 15 February 2010 | Amended | Contact details updated. |
| 31 October 2008 | Amended | Converted to new review format. |
Acknowledgements
LOM would like to thank Clive Adams, Nancy Owens, Mark Fenton, Gillian Rizzello, Shazia Akhtar (from the Cochrane Schizophrenia Group, UK) for their administrative help and support.
The reviewers sent many letters to authors, asking for extra information. Dr George Simpson (University of Southern California), Dr Charles O'Brien (University of Pennsylvania, School of Medicine), Dr Maryanne O'Donnell (in memoriam of Dr Leslie Kiloh; Prince of Wales Hospital, UK) and Dr Guy Edwards (Royal South Hants Hospital, UK) were kind enough to respond, for which we are very grateful.
Data and analyses
Comparison 1. TRIFLUOPERAZINE versus PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No substantial improvement (defined as slight improvement or worse) ‐ by time period | 6 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.66, 0.94] |
| 1.1 short term (0‐3 months) | 3 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.50, 0.79] |
| 1.2 medium term (+3 months‐1 year) | 3 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.85, 1.00] |
| 2 Global state: 1b. No substantial improvement (short term) ‐ by rater's role | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 doctor rated | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.38, 1.00] |
| 2.2 nurse rated | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.67, 1.11] |
| 3 Global state: 1c. No substantial improvement ‐ by dose of trifluoperazine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 low dose (15mg/day) | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.99] |
| 3.2 high dose (80mg/day) | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.89, 0.99] |
| 4 Global state: 2. Not discharged from hospital ‐ by dose of trifluoperazine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 low dose (15mg/day) | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.96, 1.01] |
| 4.2 high dose (80mg/day) | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.96, 1.01] |
| 5 Global state: 3. Use of additional antipsychotics or sedatives | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.18, 6.31] |
| 6 Mental state: Delusions and hallucinations by the end of the study | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.19, 0.99] |
| 7 Behaviour: 1. No improvement (Wings Behaviour Rating Scale) ‐ by >8 weeks ‐ 6 months | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.25, 0.70] |
| 8 Behaviour: 2. Agitation | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.28, 3.58] |
| 9 Leaving the study early | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 any reason | 7 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.59, 1.48] |
| 9.2 due to adverse events | 3 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.24, 5.11] |
| 9.3 due to relapse or worsening | 5 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.50, 1.62] |
| 10 Adverse events: 1. At least one adverse effect | 2 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.90, 2.18] |
| 11 Adverse events: 2. Cardiovascular | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 ECG abnormalities ‐ minor | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.58, 5.94] |
| 11.2 hypertension | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.20, 20.33] |
| 12 Adverse events: 3. Extrapyramidal | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 non‐specific ‐ any movement disorder | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 2.27 [1.19, 4.33] |
| 12.2 non‐specific ‐ antiparkinson drugs needed | 4 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 5.06 [2.49, 10.27] |
| 12.3 specific ‐ akathisia (motor restlessness) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 69.52] |
| 12.4 specific ‐ dyskinesia | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [0.47, 29.06] |
| 12.5 specific ‐ oculogyric crisis | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [0.47, 29.06] |
| 12.6 specific ‐ rigidity | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [1.25, 64.59] |
| 12.7 specific ‐ tremors | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.72] |
| 13 Adverse events: 4. Haematological | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 eosinophilia >5% | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.41, 8.49] |
| 13.2 leukocytosis | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.46, 5.22] |
| 13.3 urea nitrogen increase | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.28, 7.05] |
| 14 Adverse events: 5. Neurological | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 convulsions | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.50] |
| 14.2 faintness | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.67] |
| 14.3 urinary incontinence | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.22, 4.50] |
| 15 Adverse events: 6. Somnolesence | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 drowsiness | 3 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [1.42, 6.10] |
| 15.2 insomnia | 2 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.07, 12.05] |
| 16 Adverse events: 7. Weight | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 gain >10 lb | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.34, 4.51] |
| 16.2 loss >10 lb | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [0.71, 6.66] |
| 17 Adverse events: 8. Miscellaneous | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 anorexia | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.24, 2.65] |
| 17.2 blurred vision | 3 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.47, 6.26] |
| 17.3 nausea | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.28, 7.05] |
| 17.4 oedema ‐ face | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.22, 4.50] |
| 17.5 rashes | 3 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.30, 4.01] |
| 17.6 swallowing ‐ difficulties | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.22, 4.50] |
1.1. Analysis.
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 1 Global state: 1a. No substantial improvement (defined as slight improvement or worse) ‐ by time period.
1.2. Analysis.
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 2 Global state: 1b. No substantial improvement (short term) ‐ by rater's role.
1.3. Analysis.
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 3 Global state: 1c. No substantial improvement ‐ by dose of trifluoperazine.
1.4. Analysis.
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 4 Global state: 2. Not discharged from hospital ‐ by dose of trifluoperazine.
1.5. Analysis.
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 5 Global state: 3. Use of additional antipsychotics or sedatives.
1.6. Analysis.
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 6 Mental state: Delusions and hallucinations by the end of the study.
1.7. Analysis.
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 7 Behaviour: 1. No improvement (Wings Behaviour Rating Scale) ‐ by >8 weeks ‐ 6 months.
1.8. Analysis.
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 8 Behaviour: 2. Agitation.
1.9. Analysis.
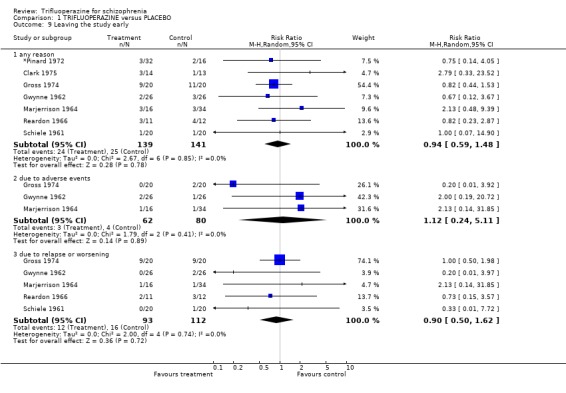
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 9 Leaving the study early.
1.10. Analysis.
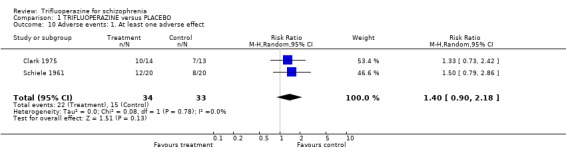
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 10 Adverse events: 1. At least one adverse effect.
1.11. Analysis.
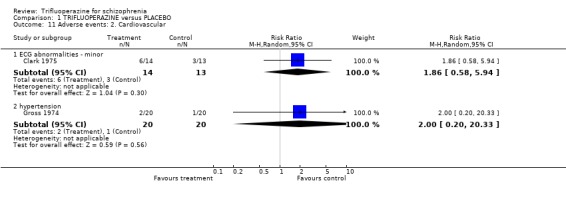
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 11 Adverse events: 2. Cardiovascular.
1.12. Analysis.
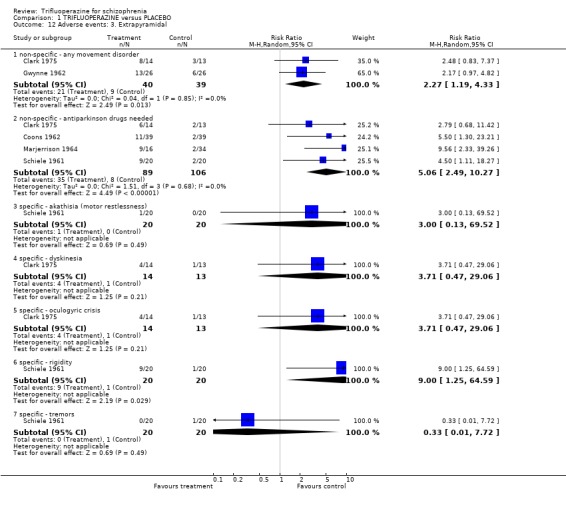
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 12 Adverse events: 3. Extrapyramidal.
1.13. Analysis.
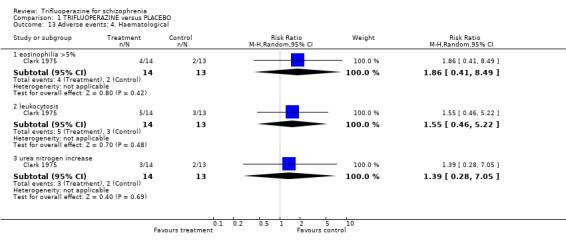
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 13 Adverse events: 4. Haematological.
1.14. Analysis.
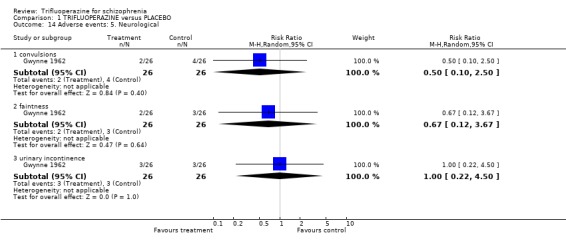
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 14 Adverse events: 5. Neurological.
1.15. Analysis.
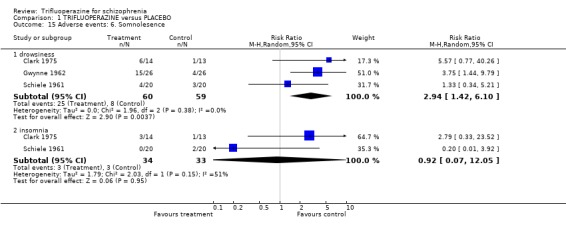
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 15 Adverse events: 6. Somnolesence.
1.16. Analysis.
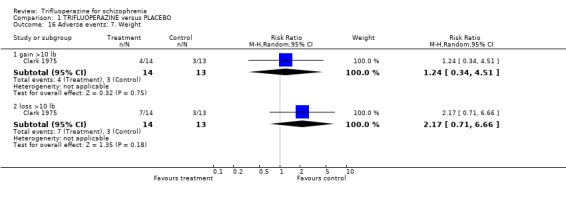
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 16 Adverse events: 7. Weight.
1.17. Analysis.
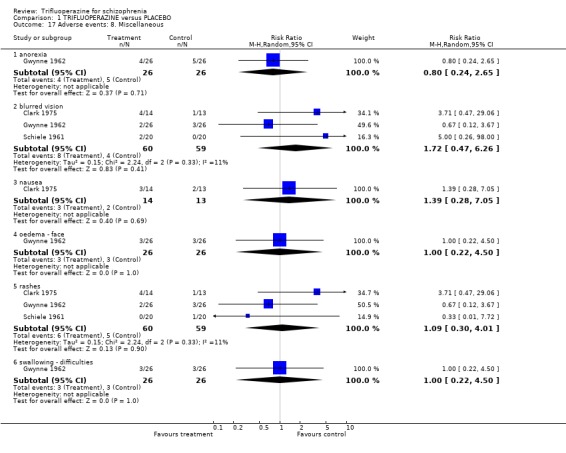
Comparison 1 TRIFLUOPERAZINE versus PLACEBO, Outcome 17 Adverse events: 8. Miscellaneous.
Comparison 2. TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No substantial improvement (defined as slight improvement or worse) ‐ by time period | 28 | 1016 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.14] |
| 1.1 by 3 months | 22 | 816 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.94, 1.15] |
| 1.2 3 months to 1 year | 5 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.93, 1.24] |
| 1.3 1 year to 2 years | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.87, 1.36] |
| 2 Global state: 1b. No substantial improvement (short term) ‐ by rater's role | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 doctor rated | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.48, 1.53] |
| 2.2 nurse rated | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.32] |
| 2.3 patent rated | 3 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.54, 1.32] |
| 3 Global state: 1d. No substantial improvement ‐ subgroup analysis ‐ acute vs chronic | 24 | 833 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.97, 1.14] |
| 3.1 acutely ill people | 10 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.84, 1.27] |
| 3.2 chronically ill people | 14 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.96, 1.15] |
| 4 Global state: 1e. No substantial improvement ‐ subgroup analysis ‐ mixed diagnoses vs schizophrenia only | 21 | 792 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.91, 1.13] |
| 4.1 mixed diagnoses ‐ short term studies only | 4 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.80, 1.53] |
| 4.2 schizophrenia only ‐ short term studies | 17 | 677 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.90, 1.12] |
| 5 Global state: 2. Not discharged from hospital ‐ by time period | 3 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.82, 1.47] |
| 5.1 by 3 months | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.79, 1.58] |
| 5.2 3 months to 1 year | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.62, 1.80] |
| 6 Global state: 3. Use of additional antipsychotics or sedatives | 3 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.33, 1.91] |
| 7 Mental state: 1. Delusions and hallucinations by the end of the study | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.39, 4.62] |
| 8 Mental state: 2. Other specific effects | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 anxious | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.02, 8.08] |
| 8.2 depressed | 5 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.33, 1.40] |
| 8.3 excitement | 5 | 273 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.17, 1.58] |
| 9 Behaviour: 1. No improvement (Wings Behaviour Rating Scale) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.47, 2.14] |
| 10 Behaviour: 2. Specific effects | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 lethargy | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.23, 2.21] |
| 10.2 restlessness | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.24, 2.66] |
| 10.3 violent and aggressive | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.19] |
| 10.4 unspecified behavioural problems | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.21, 5.49] |
| 11 Leaving the study early: 1. Any reason ‐ by time period | 32 | 1300 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.92, 1.62] |
| 11.1 by 3 months | 22 | 930 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.83, 1.58] |
| 11.2 by 3 months to 1 year | 9 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.99, 3.27] |
| 11.3 by 1 year to 2 years | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 1.36] |
| 12 Leaving the study early: 2. Due to adverse events ‐ by time period | 8 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.49, 3.11] |
| 12.1 by 3 months | 3 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.11, 4.05] |
| 12.2 by 3 months to 1 year | 5 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.53, 4.49] |
| 13 Leaving the study early: 3. Due to relapse or worsening ‐ by time period | 13 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.72, 2.38] |
| 13.1 by 3 months | 6 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.41, 2.78] |
| 13.2 by 3 months to 1 year | 6 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.78, 3.85] |
| 13.3 by 1 year to 2 years | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.52] |
| 14 Leaving the study early: 4. Due to refusal of treatment ‐ by time period | 3 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.27, 2.50] |
| 14.1 by 3 months | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.28, 3.52] |
| 14.2 by 1 year to 2 years | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.05, 4.46] |
| 15 Adverse events: 1. Reporting at least one adverse event | 14 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.88, 1.13] |
| 16 Adverse events: 2. Anticholinergic | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 blurred vision | 14 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.91, 1.55] |
| 16.2 constipation | 7 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.10] |
| 16.3 dry mouth | 11 | 466 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.77, 1.44] |
| 16.4 nasal congestion | 3 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.47, 5.48] |
| 16.5 urinary difficulties | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.20, 20.33] |
| 16.6 unspecified | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.43, 2.49] |
| 17 Adverse events: 3. Cardiovascular | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 any unspecified cardiovascular problem | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.54, 2.73] |
| 17.2 ECG abnormalities ‐ minor | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.45, 2.55] |
| 17.3 hypertension | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.21, 7.66] |
| 17.4 hypotension | 6 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.28, 1.10] |
| 17.5 palpitations | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.39, 2.58] |
| 17.6 syncope | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.06, 2.17] |
| 17.7 tachycardia | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.09, 2.10] |
| 18 Adverse events: 4. Endocrine | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 amenorrhoea | 2 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.24, 2.86] |
| 18.2 galactorrhoea | 2 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.16, 3.86] |
| 18.3 weight gain >10 lb | 5 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.92, 1.71] |
| 18.4 weight loss >10 lb | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.54, 7.45] |
| 19 Adverse events: 5a. Extrapyramidal ‐ non specific | 28 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 any unspecifed extrapramidal effect | 12 | 485 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.88, 1.42] |
| 19.2 use of antiparkinson agents | 19 | 707 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.86, 1.25] |
| 20 Adverse events: 5b. Extrapyramidal ‐ specific problems | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 akathisia | 18 | 719 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.70, 1.17] |
| 20.2 dyskinesia, tardive | 2 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [0.71, 9.02] |
| 20.3 dystonic symptoms ‐ unspecified | 14 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.72, 1.65] |
| 20.4 facial expression abnormal | 4 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.33, 1.16] |
| 20.5 gait, Parkinsonian | 5 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.67, 1.57] |
| 20.6 oculogyric crisis | 3 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.52, 2.82] |
| 20.7 Parkinsonism syndrome | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 7.24 [1.00, 52.64] |
| 20.8 rigidity | 15 | 636 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.79, 1.42] |
| 20.9 salivation, increased | 7 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.64, 1.84] |
| 20.10 tremor | 14 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.12] |
| 21 Adverse effects: 5c. Extrapyramidal ‐ average daily dose of antiparkinson agent (trihexyphenidyl) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐2.2 [‐2.78, ‐1.62] |
| 22 Adverse events: 5d‐i. Extrapyramidal ‐ subgroup analysis ‐ high vs low potency control groups ‐ any effect | 12 | 485 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.88, 1.42] |
| 22.1 vs high potency control group | 9 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.79, 1.34] |
| 22.2 vs low potency control group | 3 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.03, 2.67] |
| 23 Adverse events: 5d‐ii. Extrapyramidal ‐ subgroup analysis ‐ high vs low potency control groups ‐ rigidity | 15 | 636 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.79, 1.42] |
| 23.1 vs high potency control group | 12 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.79, 1.36] |
| 23.2 vs low potency control group | 3 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.36, 4.60] |
| 24 Adverse events: 5d‐iii. Extrapyramidal ‐ subgroup analysis ‐ high vs low potency control groups ‐ anti'p drugs | 19 | 707 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.86, 1.25] |
| 24.1 vs high potency control group | 13 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.15] |
| 24.2 vs low potency control group | 6 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.80, 3.50] |
| 25 Adverse events: 5d‐iv. Extrapyramidal ‐ subgroup analysis ‐ specific drugs ‐ any effect | 9 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.79, 1.34] |
| 25.1 vs loxapine | 5 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.91, 1.46] |
| 25.2 vs other high potency antipsichotics | 4 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.49, 1.43] |
| 26 Adverse events: 6. Gastrointestinal | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 26.1 any ‐ unspecified | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.58, 4.82] |
| 26.2 abdominal discomfort | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.22, 2.01] |
| 26.3 diarrhoea | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [0.47, 18.60] |
| 26.4 nausea or vomiting | 11 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.51, 1.32] |
| 27 Adverse events: 7. Haematological | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.1 any haematological abnormality ‐ unpsecified | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.52, 7.32] |
| 27.2 blood cell count ‐ eosinophilia >5% | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.39, 5.28] |
| 27.3 blood cell count ‐ leukocytosis | 2 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.43, 3.15] |
| 27.4 liver enzyme elevation ‐ alkaline phosphatase | 2 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.25, 1.39] |
| 27.5 liver enzyme elevation ‐ bilirubin | 2 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 0.92] |
| 27.6 liver enzyme elevation ‐ SGOT | 4 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 4.07] |
| 27.7 liver enzyme elevation ‐ SGPT | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.05, 1.62] |
| 27.8 renal function ‐ urea nitrogen elevated | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.31, 8.24] |
| 28 Adverse events: 8. Neurological | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 28.1 confusional state | 3 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.46, 2.95] |
| 28.2 convulsions | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 99.34] |
| 28.3 dizziness or giddiness | 3 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.71, 4.61] |
| 28.4 faintness or weakness | 12 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.91, 1.77] |
| 28.5 parasthesia | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.34, 2.93] |
| 28.6 urinary incontinence | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [0.38, 129.11] |
| 29 Adverse events: 9. Somnolescence | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 29.1 drowsiness | 20 | 816 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.72, 1.10] |
| 29.2 insomnia | 12 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.87, 1.73] |
| 30 Adverse events: 10. Miscellaneous | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 30.1 anorexia | 6 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.81, 2.20] |
| 30.2 breathlessness | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.69] |
| 30.3 bulimia | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.69] |
| 30.4 difficulty swallowing | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [0.38, 129.11] |
| 30.5 eye changes ‐slight pigmentation | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.09, 1.56] |
| 30.6 eye changes ‐ worsening of cataracts | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.38, 10.49] |
| 30.7 headache | 5 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.32] |
| 30.8 hiccough | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 5.38 [0.27, 106.98] |
| 30.9 joint pain | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.39, 3.99] |
| 30.10 lacrimation | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.69] |
| 30.11 local tissue reactions (IM preparations) | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.18] |
| 30.12 oedema of face | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [0.38, 129.11] |
| 30.13 rashes | 10 | 489 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.53, 2.33] |
| 30.14 sweating, facial | 2 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.47, 2.01] |
| 30.15 sweating, general | 3 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.12, 1.80] |
| 30.16 unspecified | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.52, 4.48] |
| 31 Patient's drug preference: Would prefer a different medication | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.52, 1.31] |
2.1. Analysis.
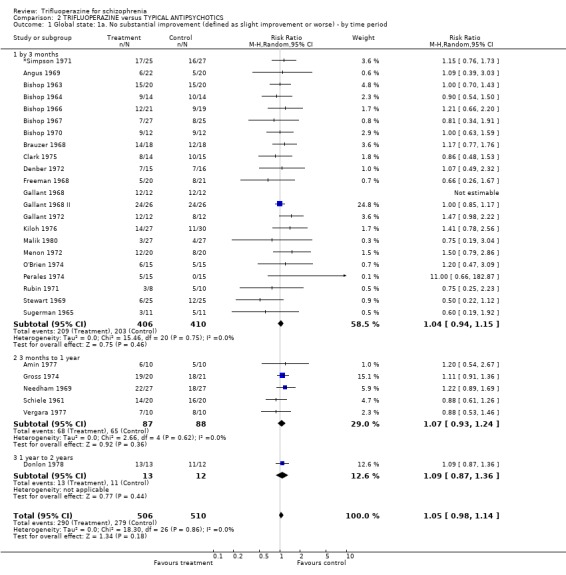
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 1 Global state: 1a. No substantial improvement (defined as slight improvement or worse) ‐ by time period.
2.2. Analysis.
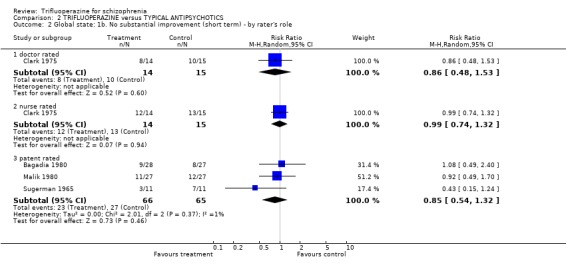
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 2 Global state: 1b. No substantial improvement (short term) ‐ by rater's role.
2.3. Analysis.
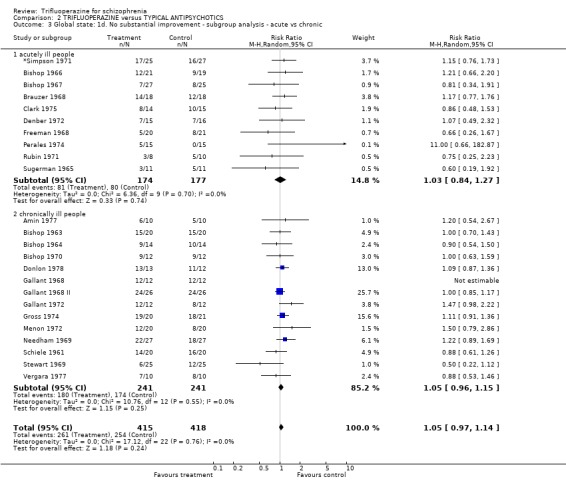
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 3 Global state: 1d. No substantial improvement ‐ subgroup analysis ‐ acute vs chronic.
2.4. Analysis.
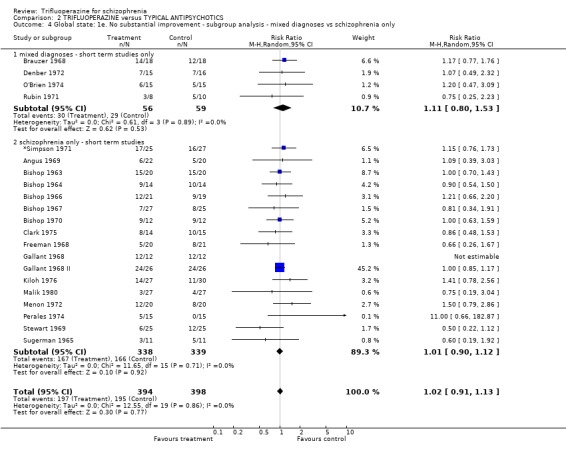
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 4 Global state: 1e. No substantial improvement ‐ subgroup analysis ‐ mixed diagnoses vs schizophrenia only.
2.5. Analysis.
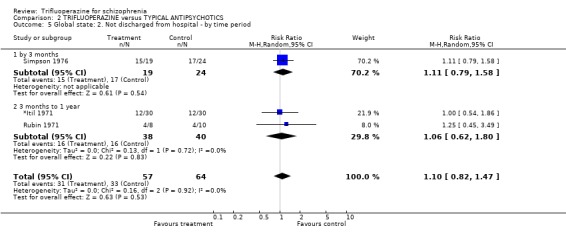
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 5 Global state: 2. Not discharged from hospital ‐ by time period.
2.6. Analysis.
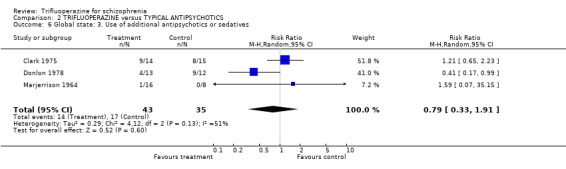
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 6 Global state: 3. Use of additional antipsychotics or sedatives.
2.7. Analysis.
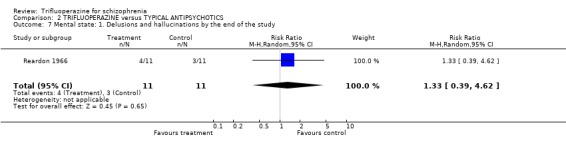
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 7 Mental state: 1. Delusions and hallucinations by the end of the study.
2.8. Analysis.
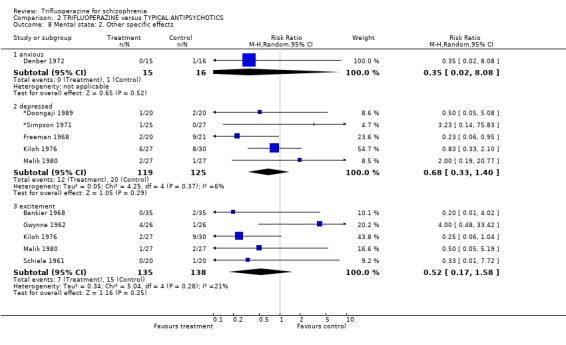
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 8 Mental state: 2. Other specific effects.
2.9. Analysis.
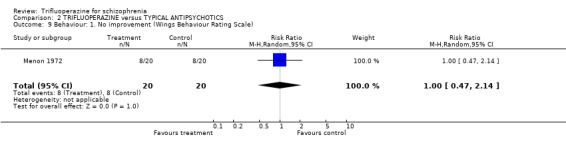
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 9 Behaviour: 1. No improvement (Wings Behaviour Rating Scale).
2.10. Analysis.
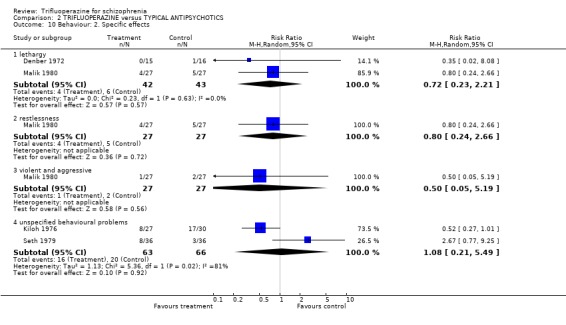
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 10 Behaviour: 2. Specific effects.
2.11. Analysis.
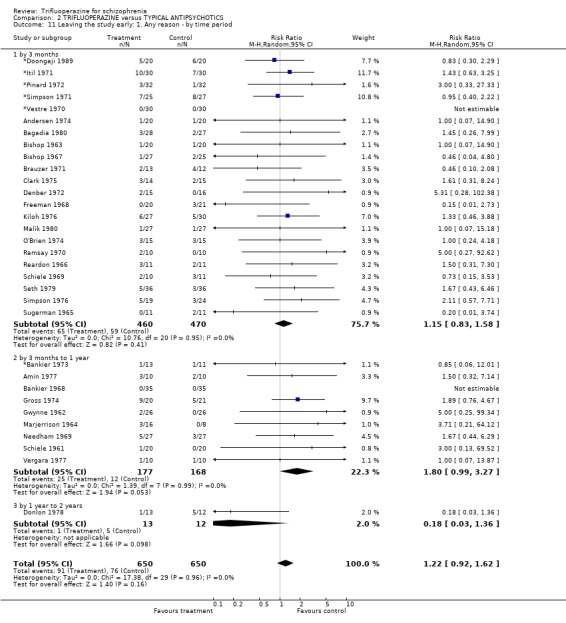
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 11 Leaving the study early: 1. Any reason ‐ by time period.
2.12. Analysis.
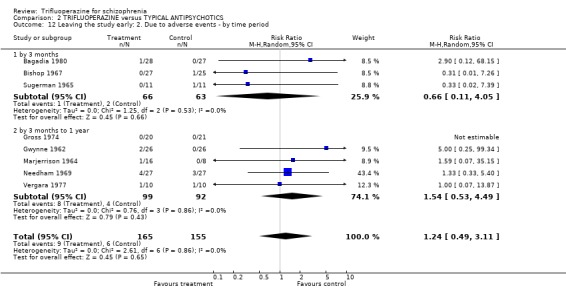
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 12 Leaving the study early: 2. Due to adverse events ‐ by time period.
2.13. Analysis.
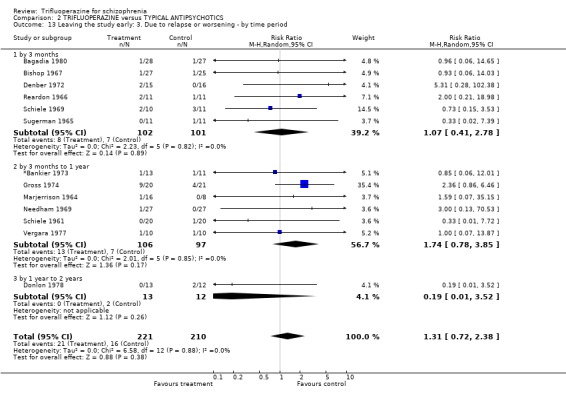
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 13 Leaving the study early: 3. Due to relapse or worsening ‐ by time period.
2.14. Analysis.
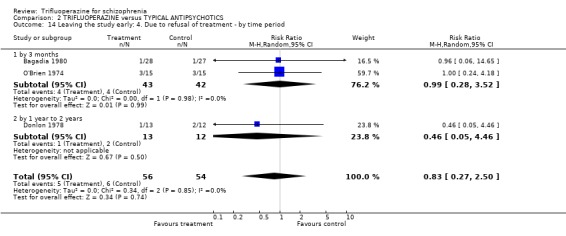
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 14 Leaving the study early: 4. Due to refusal of treatment ‐ by time period.
2.15. Analysis.
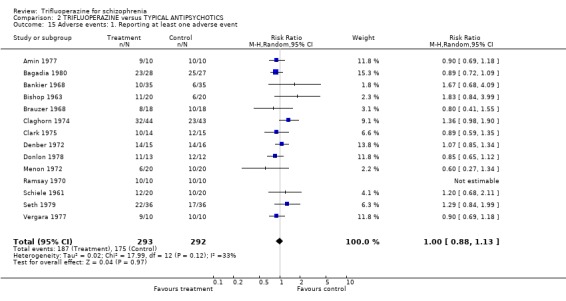
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 15 Adverse events: 1. Reporting at least one adverse event.
2.16. Analysis.
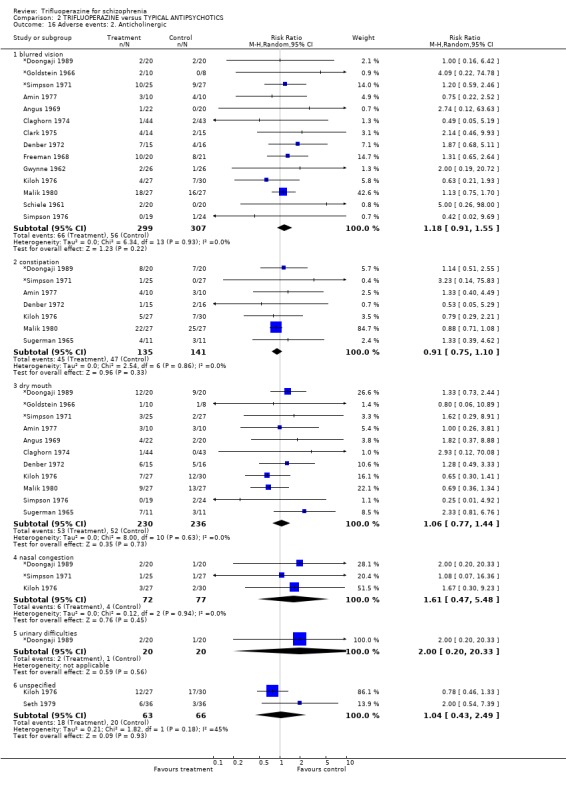
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 16 Adverse events: 2. Anticholinergic.
2.17. Analysis.
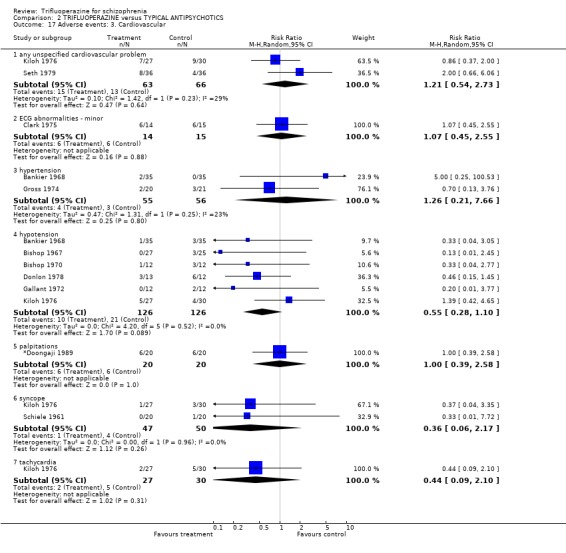
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 17 Adverse events: 3. Cardiovascular.
2.18. Analysis.
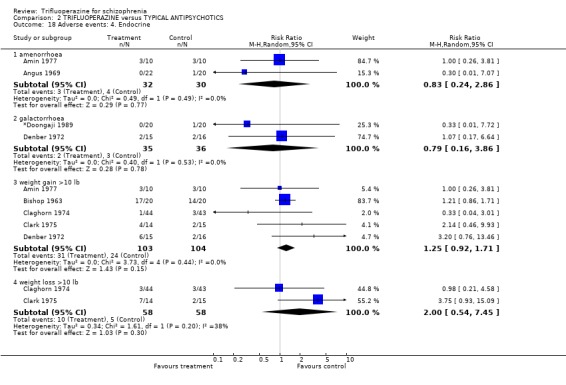
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 18 Adverse events: 4. Endocrine.
2.19. Analysis.
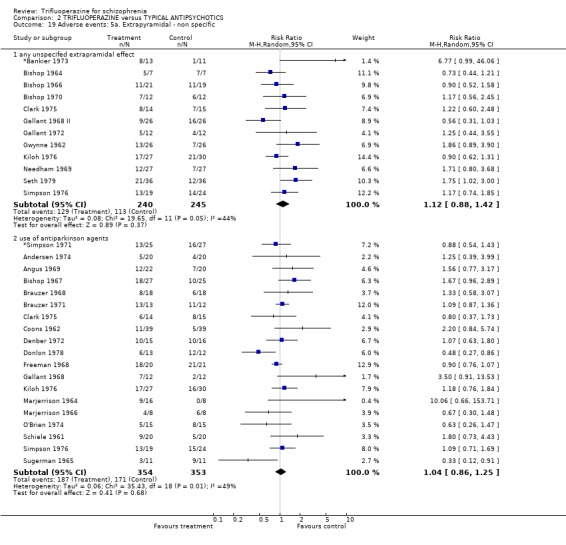
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 19 Adverse events: 5a. Extrapyramidal ‐ non specific.
2.20. Analysis.
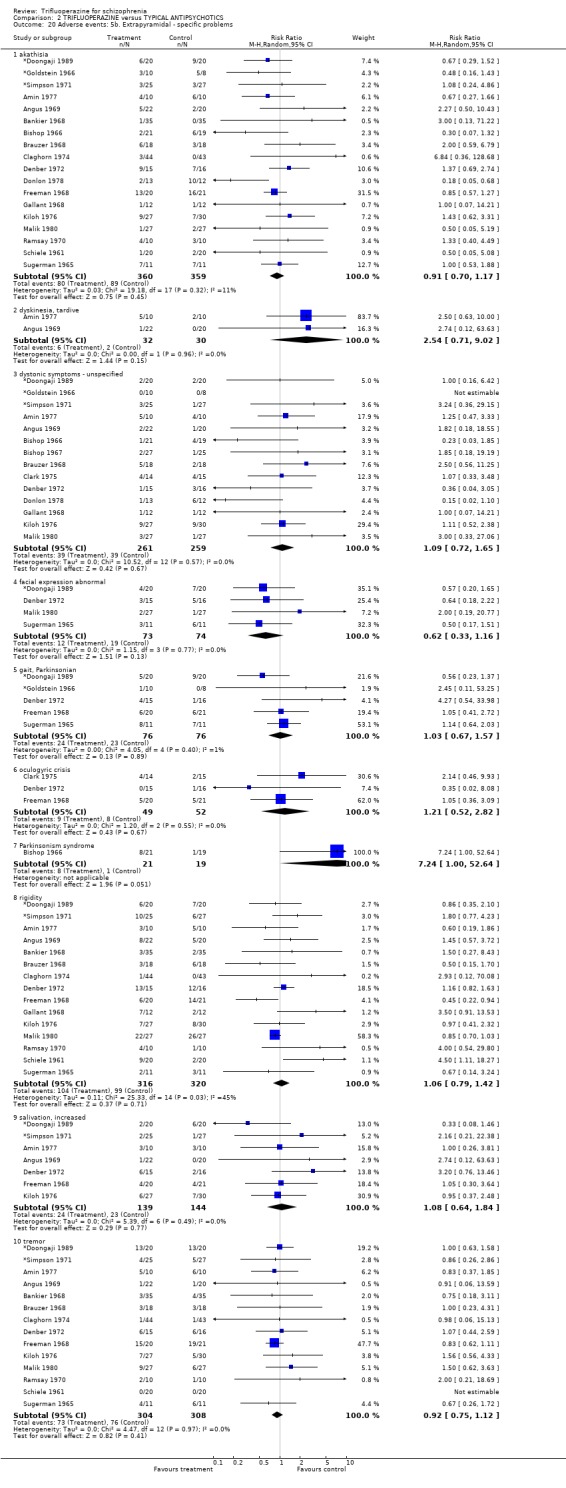
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 20 Adverse events: 5b. Extrapyramidal ‐ specific problems.
2.21. Analysis.
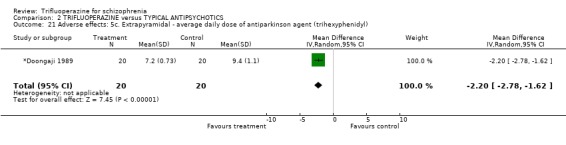
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 21 Adverse effects: 5c. Extrapyramidal ‐ average daily dose of antiparkinson agent (trihexyphenidyl).
2.22. Analysis.
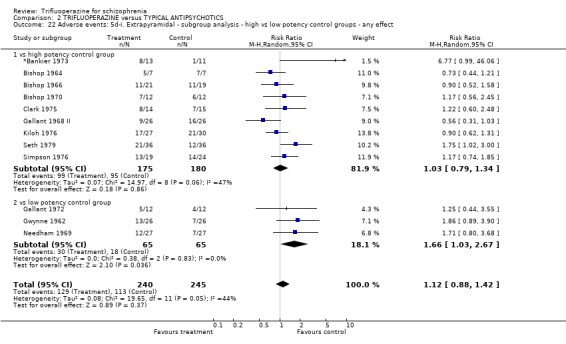
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 22 Adverse events: 5d‐i. Extrapyramidal ‐ subgroup analysis ‐ high vs low potency control groups ‐ any effect.
2.23. Analysis.
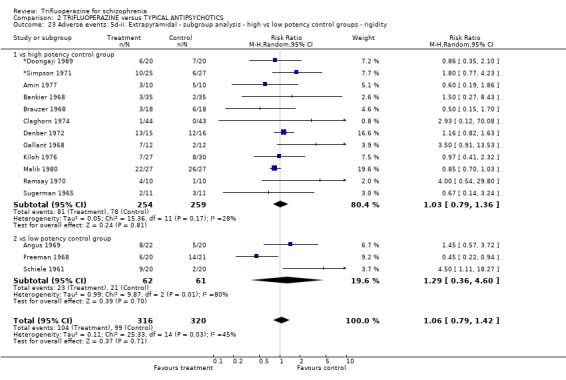
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 23 Adverse events: 5d‐ii. Extrapyramidal ‐ subgroup analysis ‐ high vs low potency control groups ‐ rigidity.
2.24. Analysis.
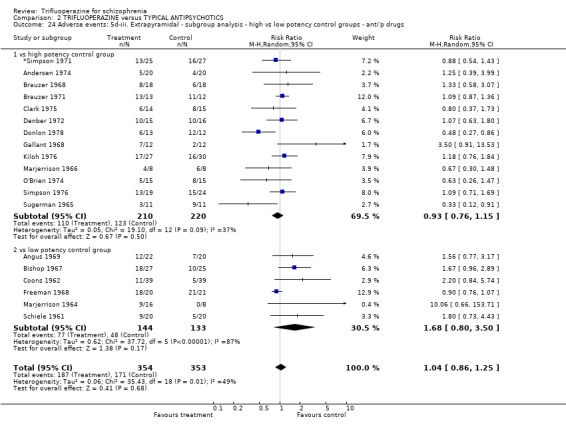
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 24 Adverse events: 5d‐iii. Extrapyramidal ‐ subgroup analysis ‐ high vs low potency control groups ‐ anti'p drugs.
2.25. Analysis.
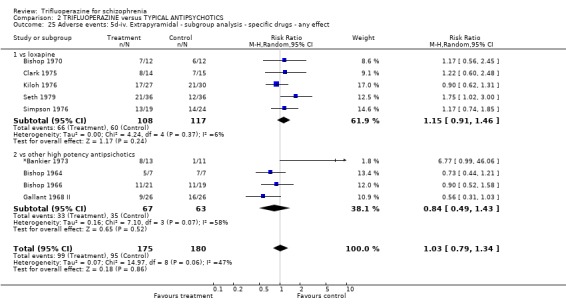
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 25 Adverse events: 5d‐iv. Extrapyramidal ‐ subgroup analysis ‐ specific drugs ‐ any effect.
2.26. Analysis.
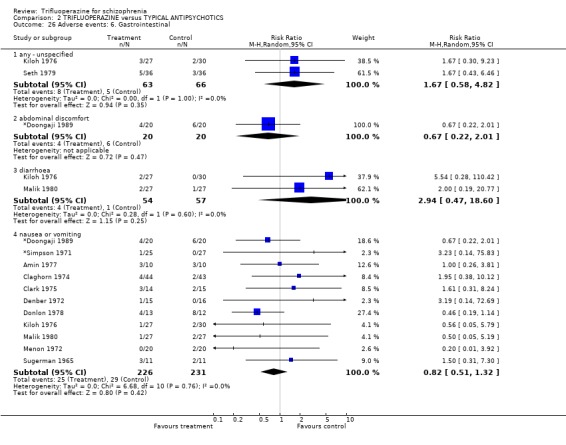
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 26 Adverse events: 6. Gastrointestinal.
2.27. Analysis.
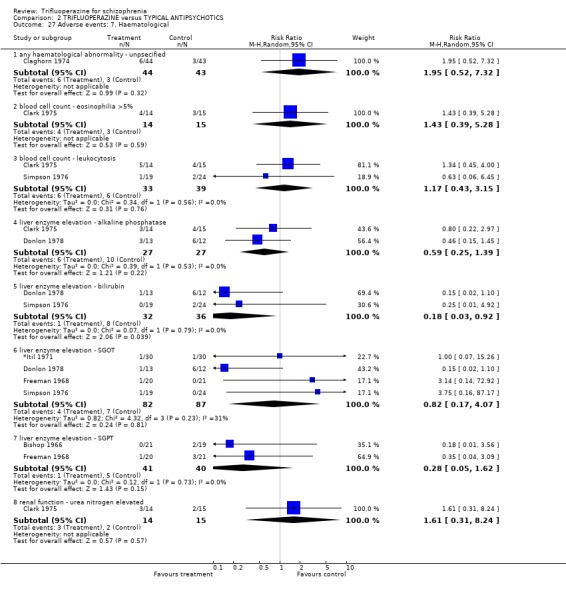
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 27 Adverse events: 7. Haematological.
2.28. Analysis.
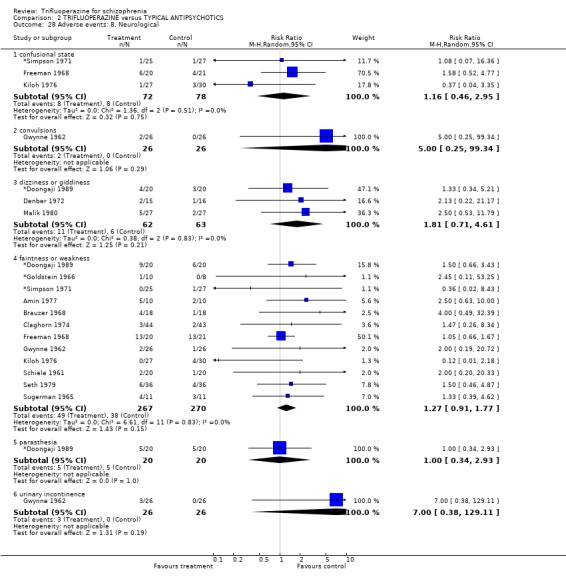
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 28 Adverse events: 8. Neurological.
2.29. Analysis.
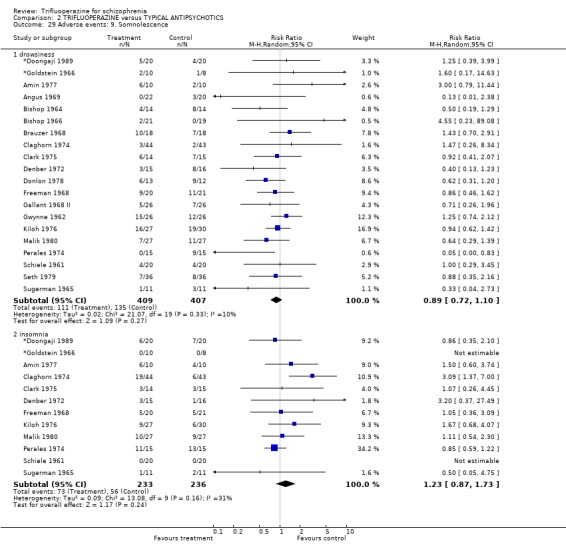
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 29 Adverse events: 9. Somnolescence.
2.30. Analysis.
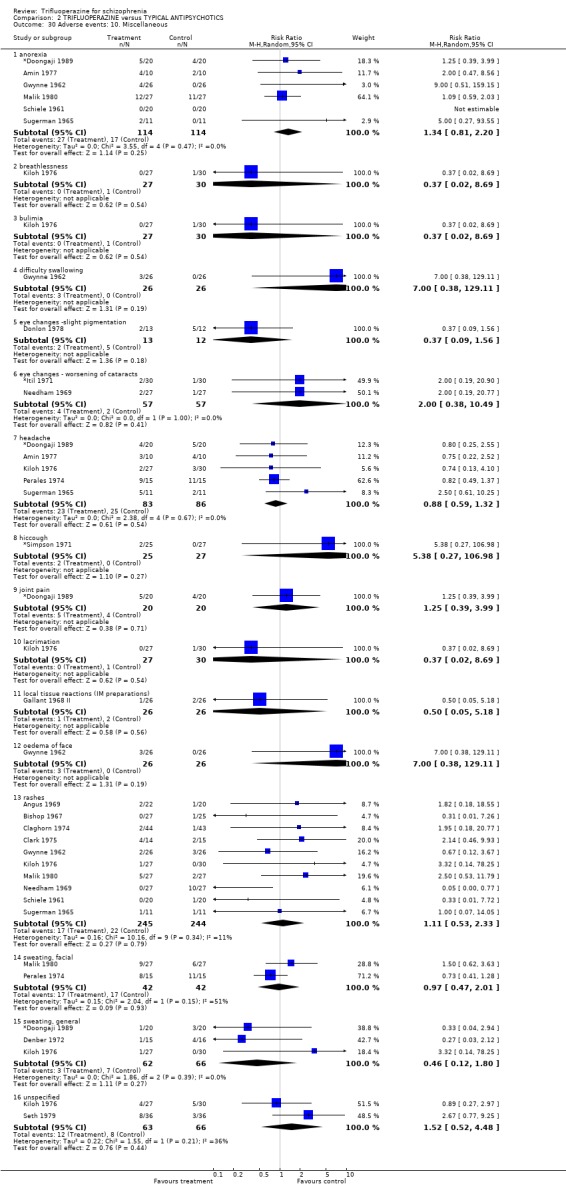
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 30 Adverse events: 10. Miscellaneous.
2.31. Analysis.
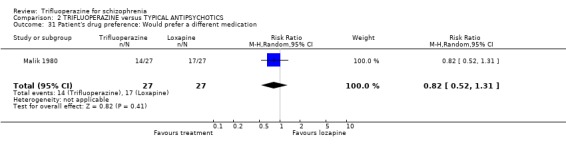
Comparison 2 TRIFLUOPERAZINE versus TYPICAL ANTIPSYCHOTICS, Outcome 31 Patient's drug preference: Would prefer a different medication.
Comparison 3. TRIFLUOPERAZINE versus ATYPICAL ANTIPSYCHOTICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No substantial improvement (defined as slight improvement or worse) | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.44, 1.81] |
| 2 Global state: 2. Severely ill or worse | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.43, 2.30] |
| 3 Global state: 3. Use of additional sedatives | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.58, 6.85] |
| 4 Mental state | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 emotional lability | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.59] |
| 4.2 euphoria | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.93] |
| 5 Leaving the study early | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 any reason | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.06] |
| 5.2 due to deterioration | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.85] |
| 6 Behaviour: Agitation | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.93] |
| 7 Adverse events: 1. Extrapyramidal | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 non‐specific ‐ use of antiparkinson drugs | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.16, 6.38] |
| 7.2 specific ‐ akathisia | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.13, 3.55] |
| 7.3 specific ‐ dyskinesia, oral | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.34, 26.33] |
| 7.4 specific ‐ dystonic reaction | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.26, 97.70] |
| 7.5 specific ‐ rigidity | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.34, 5.17] |
| 7.6 specific ‐ salivation, excessive | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.41, 9.65] |
| 7.7 specific ‐ tremor | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.29, 3.43] |
| 8 Adverse events: 2. Miscellaneous | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 dry mouth | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 69.31] |
| 8.2 headache | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.16, 6.38] |
| 8.3 nausea | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 69.31] |
| 8.4 perspiration, excessive | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.85] |
| 8.5 rash | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.70] |
| 8.6 urinary frequency | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 69.31] |
| 8.7 weakness/lethargy | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 69.31] |
3.1. Analysis.
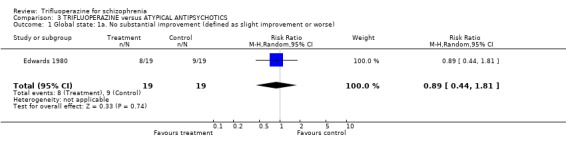
Comparison 3 TRIFLUOPERAZINE versus ATYPICAL ANTIPSYCHOTICS, Outcome 1 Global state: 1a. No substantial improvement (defined as slight improvement or worse).
3.2. Analysis.
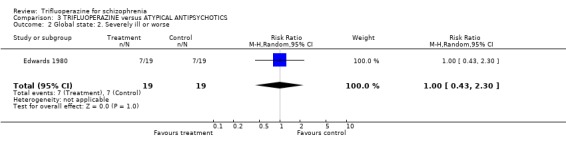
Comparison 3 TRIFLUOPERAZINE versus ATYPICAL ANTIPSYCHOTICS, Outcome 2 Global state: 2. Severely ill or worse.
3.3. Analysis.
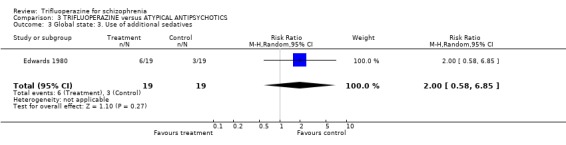
Comparison 3 TRIFLUOPERAZINE versus ATYPICAL ANTIPSYCHOTICS, Outcome 3 Global state: 3. Use of additional sedatives.
3.4. Analysis.
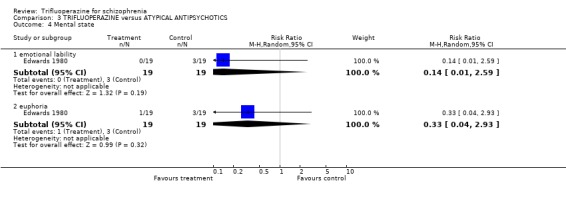
Comparison 3 TRIFLUOPERAZINE versus ATYPICAL ANTIPSYCHOTICS, Outcome 4 Mental state.
3.5. Analysis.
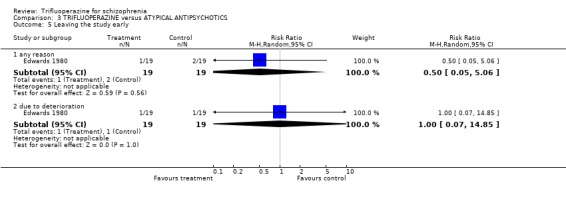
Comparison 3 TRIFLUOPERAZINE versus ATYPICAL ANTIPSYCHOTICS, Outcome 5 Leaving the study early.
3.6. Analysis.
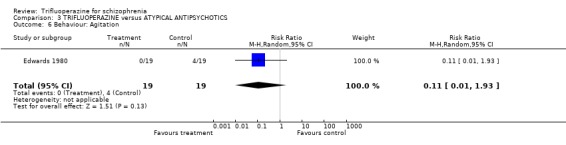
Comparison 3 TRIFLUOPERAZINE versus ATYPICAL ANTIPSYCHOTICS, Outcome 6 Behaviour: Agitation.
3.7. Analysis.
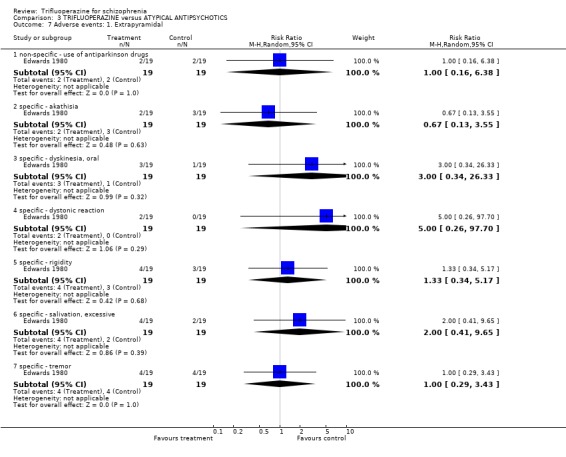
Comparison 3 TRIFLUOPERAZINE versus ATYPICAL ANTIPSYCHOTICS, Outcome 7 Adverse events: 1. Extrapyramidal.
3.8. Analysis.
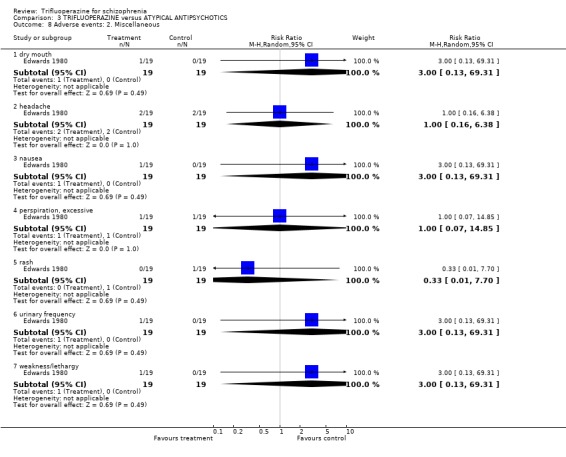
Comparison 3 TRIFLUOPERAZINE versus ATYPICAL ANTIPSYCHOTICS, Outcome 8 Adverse events: 2. Miscellaneous.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
*Bankier 1973.
| Methods | Allocation: randomly assigned, no further details. Blindness: double (identical oral preparations, but not in IM preparation ‐ saline by a senior nurse). Design: 2 parallel groups, single centre. Duration: 16 weeks. Analysis: not clearly described in the report, (ITT)?* Country: Canada. | |
| Participants | Diagnosis: schizophrenia, no further details. N=24. Age: mean 33.1 years. Sex: M 15, F 9. Setting: hospital. History: acutely disturbed (mean length of illness 98.5 months. | |
| Interventions | 1. Trifluoperazine: dose (mean 40 mg/day). N=13. 2. Fluspirilene: dose (mean 3.25 ml, 1 amp IM/week). N=11. | |
| Outcomes | Leaving the study early.
Side effects. Unable to use ‐ Mental state: (BPRS ‐ only graph; NOSIE ‐ no data). Laboratory tests ‐ no data. EKG ‐ no data. |
|
| Notes | *ITT unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
*Doongaji 1989.
| Methods | Allocation: randomly assigned, no further information. Blindness: double. Design: 2 parallel groups, single centre. Duration: 6 weeks (preceded by one placebo week). Analysis: not clearly described in the report, (ITT)?*. Country: India. | |
| Participants | Diagnosis: schizophrenia (RDC, instrument 58 New York State Psychiatric Institute, 1975). N=40. Age: range 18‐45 years, mean ˜30 years. Sex: M 22, F 7. Setting: hospital. History: acute (mean duration of illness 9.7 months). Exclusion: those who totalled 35 points on BPRS initially, or whose total score reduced by 20% at one placebo week; concurrent systemic illness; pregnant women. | |
| Interventions | 1. Trifluoperazine: range 15‐22.5 mg/day. N=20. 2. Centbutindole: range 3‐45 mg/day. N=20. | |
| Outcomes | Leaving the study early.
Side effects.
Mean dose of antiparkinson drug. Unable to use ‐ Global state: (CGI ‐ no SD). Mental state: (BPRS ‐ no SD). |
|
| Notes | *ITT unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
*Goldstein 1966.
| Methods | Allocation: randomly assigned, no further information. Blindness: double (identical capsules). Design: 2 parallel groups, single centre. Duration: no data. Analysis: no (ITT)* Country: USA. | |
| Participants | Diagnosis: schizophrenia (clinical diagnosis). N=21. Age: range 21‐55 years. Sex: no data. Setting: hospital. History: newly addmitted. Exclusion: childhood psychoses, brain syndromes, mental deficiency, alcoholism, epilepsy, drug addiction, abnormal laboratory tests. | |
| Interventions | 1.Trifluoperazine: range 7‐20 mg/day. N=10. 2. Haloperidol: range 10‐22 mg/day. N=8. | |
| Outcomes | Side effects. Unable to use ‐ Mental state: (IMPS ‐ no SD). Behaviour: (WBRS ‐ no SD). |
|
| Notes | * ITT not performed, dropouts each group not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
*Itil 1971.
| Methods | Allocation: randomly assigned, no further details. Blindness: double. Design: 2 parallel groups, single centre. Duration: 12 weeks. Analysis: not clearly described in the report, (ITT)?* Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further details. N=60. Age: range 16‐46 years, mean 28.2 years. Sex: M 44, F 14*. Setting: hospital. History: acute schizophrenics (length of illness 3 weeks to 35 months. | |
| Interventions | 1. Trifluoperazine: range 10‐160 mg/day. N=30. 2. Molindone: range 10‐160 mg/day. N=30. | |
| Outcomes | Leaving the study early.
Global state: (discharged from hospital).
Side effects: (cataracts, cardiovascular, laboratory abnormalities). Unable to use ‐ EEG: (no usable data). Mental state: (BPRS ‐ no data). Behaviour: (NOSIE ‐ no data). Global state: (ECDU ‐ no data). Side effects ‐ not known how many incidents took place in the same patient. |
|
| Notes | * ITT unclear # Demographic characteristics only reported for 58 patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
*Pinard 1972.
| Methods | Allocation: randomly assigned (random number table, blocks of 5, matched for treatment symptom severity (BPRS), sex, ward, evaluator). Blindness: double. Design: 5 parallel groups. Duration: 70 days‐preceeded by 21 days where all had chlorpromazine 100 mg/day. Analysis: not clearly described in the report, (ITT?)*. Country: Canada. | |
| Participants | Diagnosis: "schizophrenics", no further details. N=80. Age: range 20‐60 years. Sex: "equally represented". Setting: hospital. History: chronic, hospitalised for the last 2 years, no exacerbation in last year, in hospital for only social or personal reasons. | |
| Interventions | 1. Trifluoperazine: dose 5 mg/tds. N=16. 2. Trifluoperazine: dose 15 mg/day. N=16. 3. Pimozide: dose 3 mg/day. N=16. 4. Pimozide: dose 6 mg/day. N=16. 5. Placebo: N=16. Chlorpromazine, methyprylon, benztropine as required. | |
| Outcomes | Leaving the study early. Unable to use ‐ Mental state: (BPRS ‐ 'p' values only). Behavior: (NOSIE ‐ graph only). Side effects: (Extrapyramidal symptom rating BEP ‐ graph only). Insight scale: (Echelle D"Autocritique ‐ no usable data). |
|
| Notes | * ITT unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
*Simpson 1971.
| Methods | Allocation: randomly assigned acording to a code prepared in advanced and held by the pharmacist" ‐ no information how the code was prepared. Blindness: double. Duration: 4 weeks (preceeded by 3 days washout). Design: 2 parallel groups, single centre. Analysis: intention to treat performed only for global change in condition, others outcomes were not clearly described*. Country: USA. | |
| Participants | Diagnosis: described elsewhere ‐ Angus 1969. N=52. Age: range 20‐48 years, mean 37.3 years. Sex: M 16, F 36. Setting: hospital. History: acute schizophrenia or acute exacerbation of chronic schizophrenia. | |
| Interventions | 1. Trifluoperazine: range 20‐45 mg/day (mean 35mg). N=25. 2. Molindone: range 40‐120 mg/day (mean 75mg). N=27. | |
| Outcomes | Global state: (global rating of change in condition).
Side effects.
Use of antiparkinson agents.
Leaving the study early. Unable to use ‐ Mental state: (BPRS ‐ no SD). Behaviour: (NOSIE ‐ no SD). |
|
| Notes | * ITT unclear in some outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
*Vestre 1970.
| Methods | Allocation: randomised after ranking for withdrawal on Psychotic Reaction Profile, no further details. Blindness: double (raters reported as blind, identical capsules). Design: double cross‐over, single centre. Duration: 2 week placebo washout, 7 weeks on one drug, 2 weeks placebo washout, 7 weeks on second drug. Analysis: intention‐to‐treat not clearly described in the report. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further details. N=60. Age: range 23‐65 years, mean 43 years. Sex: all male. Setting: hospital. History: chronic illness (mean hospitalisation 15 years). | |
| Interventions | 1. Trifluoperazine: range 5‐40 mg/day. N=30. 2. Thioridazine: range 200‐800 mg/day. N=30. | |
| Outcomes | Leaving the study early. Unable to use ‐ Mental state: (BPRS; Psychotic Reaction Profile ‐ only significance tests). Adverse events: (only significance tests). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Amin 1977.
| Methods | Allocation: randomly assigned, no further details. Blindness: double. Design: 2 parallel groups. Duration: 16 weeks. Analysis: intention‐to‐treat not performed in the trial. Country: Canada. | |
| Participants | Diagnosis: schizophrenia, no further details. N=20. Age: range 20‐63 years, mean 38.6 years. Sex: M 6, F 14. Setting: outpatients. History: maintenance treatment of chronic schizophrenics (minimum of 2 years of illness). | |
| Interventions | 1. Trifluoperazine: range 5‐30 mg/day. N=10. 2. Pimozide: range 2‐12 mg/day. N=10. | |
| Outcomes | Global state: (CGI).
Leaving the study early.
Side effects. Unable to use ‐ Mental state: (BPRS ‐ no SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Andersen 1974.
| Methods | Allocation: randomly assigned (described on Andersen 1972 preliminary results), no further details. Blindness: double (commercial preparations but administered by people not involved in the trial). Design: 2 parallel groups. Duration: 3 months. Analysis: intention‐to‐treat not performed in the trial. Country: Sweden. | |
| Participants | Diagnosis: schizophrenia or paranoid syndrome.** N=40. Age: range 21‐65 years. Sex: M 19, F19. Setting: hospital. History: chronic patients. Inclusion: hospitalisation < 10 years, no organic disorders, no substance abuse, no depression episodes, no violent patients. | |
| Interventions | 1. Trifluoperazine: range 3‐12 mg/bid (mean 4.34 mg). N=20. 2. Pimozide: range 3‐14 mg/day (mean 3.92 mg). N=20. | |
| Outcomes | Side effects.
Use of antiparkinson agents.
Leaving the study early. Unable to use ‐ Mental state: (scale ‐ 'p' values only). Behaviour: (Wing behaviour scale ‐ 'p' values only). Side effects: (point rating scale ‐ validity unknown). |
|
| Notes | ** Mixed diagnosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Angus 1969.
| Methods | Allocation: randomly assigned (according to a code prepared in advance and held by the pharmacist). Blindness: double. Design: 2 parallel group, single centre. Duration: 6 months (results in 6 weeks). Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: Scheneider criteria. N=42. Age: range 17‐57 years, mean 38.5 years. Sex: M 14, F 28. Setting: hospital. | |
| Interventions | 1.Trifluoperazine: range 7.5‐45 mg/day. N=22. 2.SK&F 14,336: range 100‐600 mg/day. N=20. | |
| Outcomes | Global state: (global ratings of improvement).
Side‐effects.
Use of antiparkinson agents. Unable to use ‐ Mental state: (BPRS ‐ no data). Behaviour: (NOSIE ‐ no data). Leaving the study early ‐ no usable data. Global state: (degree of severity of the illness scale ‐ no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bagadia 1980.
| Methods | Allocation: randomly assigned, no further information. Blindness: double (identical capsules). Design: 2 parallel groups. Duration: 28 days. Analysis: intention‐to‐treat not performed by the authors. Country: India. | |
| Participants | Diagnosis: schizophrenia (ICD, 8th revision). N=55. Age: 14‐24 years, mean 17.4 years. Sex: M 33, F 17. Setting: outpatients. History: adolescents (onset of illness between the ages of 13 to 19). Exclusion: hypersensitivity to compounds; 3 weeks previous treatment with phenothiazines or MAOI; 8 weeks with ECT; insulin coma or subcoma therapy; acute or chronic brain syndrome; convulsive disorders; mental retardation; childhood psychoses; serious renal, hepatic, cardiovascular or metabolic diseases or history of dependences. | |
| Interventions | 1. Trifluoperazine: dose (mean 24.4 mg/day; maximum 30 mg/day). N=28. 2. Loxapine: dose (mean 96.3 mg/day; maximum 120 mg/day). N=27. | |
| Outcomes | Leaving the study early.
Side effects.
Global state: (patients' self‐evaluations). Unable to use ‐ Mental state: (BPRS ‐ no SD). Global state: (CGI ‐ no SD). Behaviour: (NOSIE ‐ no SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bankier 1968.
| Methods | Allocation: randomly assigned, no further information. Blindness: double (identical capsules and IM preparations). Design: 2 parallel groups arranged into 8 subgroups with 8 to 10 patients in each, single centre. Duration: 6 months (phase III and VI) preceded by phase II (2 weeks washout) plus phase I (2 weeks assessment period). Analysis: intention‐to‐treat. Country: Canada. | |
| Participants | Diagnosis: schizophrenia, no further details. N=70. Age: mean 47.8 years. Sex: M 48, F24 (report 2 more patients)#. Setting: hospital. History: chronic (mean 17.5 years of hospitalisation). | |
| Interventions | 1. Trifluoperazine: dose no data, only the equivalence between both drugs. N=35. 2. Fluphenazine enanthate (Prolixin): dose no data, only the equivalence between both drugs. N=35. | |
| Outcomes | Side effects.
Leaving the study early. Mental state: (psychological tests; Bender‐Gestalt Score; Hooper VOT scores, MSRPP ratings ‐ no SD). Behaviour: (daily ward reports ‐ nursing staff). Laboratory tests. |
|
| Notes | # report 48 male plus 24 female=72 patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bishop 1963.
| Methods | Allocation: randomly divided, no further details. Blindness: double (identical capsules, coded bottles). Design: 2 parallel groups, single centre. Duration: 8 weeks (preceded by one placebo week and off of study medication for at least 60 days). Analysis: intention‐to‐treat not performed. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further information. N=40. Age: range 22‐49 years. Sex: M 20, F 18. Setting: hospital. History: chronic (length of hospitalisation 3‐17 years). | |
| Interventions | 1. Trifluoperazine: dose (weeks 2 to 5, 15 mg/day, weeks 6 to 9, 24 mg/day). N=20. 2. SK&F 7261: dose (weeks 2 to 5, 3 mg/day), (weeks 6 to 9, 6 mg/day). N=20. | |
| Outcomes | Leaving the study early.
Side effects: (total number, weight gain).
Global state: (change in condition). Unable to use ‐ Psychological findings. Mental state: (Lorr psychiatric rating scale). Laboratory tests. Vital signs. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bishop 1964.
| Methods | Allocation: "randomly divided", no further details. Blindness: double (identical capsules dispensed from individual bottles). Design: 3 parallel groups, single centre. Duration: 10 weeks (preceeded by 60 days washout). Analysis: intention to treat. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further details. N=42. Age: range 21‐53 years, mean 41 years. Sex: M 21, F21. Setting: hospital. History: chronic schizophrenia (time in hospital 3‐27 years). Excluded: physical or neurologic disorders. | |
| Interventions | 1. Trifluoperazine: dose 5 mg/day (week 1) increasing to 40 mg/day by (week 5). N=14. 2. Butaperazine: dose 25 mg/day (week 1) increasing to 200 mg/day by (week 5). N=14. 3. Placebo. N=14. * Half of the patients in each group received Artane 2 mg t.i.d., and the others "placebo Artane". Use of prophylactic antiparkinson medication. | |
| Outcomes | Global state.
Side effects: (Extrapyramidal, lethargy). Unable to use ‐ Mental state: (Psychotic Reaction Profile; Beckomberga rating scale). Behaviour: (Tulane test battery; Minimal Social Behavior). Laboratory tests: (no data). Physiological measures. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bishop 1966.
| Methods | Allocation: "prearranged randomised procedure", no further details. Blindeness: double, (identical capsules supplied in individual bottles only with the subjects study number). Design: 2 parallel groups, two centres. Duration: 30 days. Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further information. N=40. Age: range 18‐54 years, mean 34.3 years. Sex: M 21, F 19. Setting: hospital. History: newly admitted patients, trifluoperazine group > paranoid diagnosis. | |
| Interventions | 1. Trifluoperazine: range 5‐30 mg/day. N=21. 2. Thiothixene: range 5‐30 mg/day. N=19. | |
| Outcomes | Global state: (global ratings of improvement).
Side effects: (EPS). Unable to use ‐ Mental state: (BPRS, only graphs). |
|
| Notes | #The groups differ on sex, age and diagnostic type. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bishop 1967.
| Methods | Allocation: "prearranged randomised procedure". Blindness: double (identical capsules in individual bottles labeled with the subjects' study number). Design: 2 parallel groups, single centre. Duration: 6 weeks (preceded for 2 weeks washout). Analysis: intention‐to‐treat described only for side effects and use of antiparkinson agents. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further information. N=52. Age: range 19‐51 years, mean 30.8 years. Sex: M 15, F 37. Setting: hospital. History: first admission, or no more than 2 hospitalisations with good remissions. | |
| Interventions | 1. Trifluoperazine: range 7.5‐30 mg/day. N=27. 2. SK&F 14336 (acridanes) range 100‐400 mg/day. N=25. | |
| Outcomes | Global state: (CGI).
Leaving the study early.
Side effects.
Use of antiparkinson agents. Unable to use ‐ Mental state: (BPRS ‐ only "p" value). Behaviour: (NOSIE ‐ only "p" value). Laboratory tests ‐ no data. Vital signs ‐ no data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bishop 1970.
| Methods | Allocation: divided on a random basis, no further details. Blindness: double, no further information. Design: 2 parallel groups, single centre. Duration: 8 weeks (preceded by 4 weeks washout + 2 weeks assessment period). Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further details. N=24. Age: range 30‐55 years, mean 43.9 years. Setting: hospital. Sex: 12 M, 12 F. History: chronic, mean length of hospitalisation ˜ 17 years, range 5‐29 years. | |
| Interventions | 1. Trifluoperazine: range 10‐60 mg/day. N=12. 2. Loxapine: range 20‐120 mg/day. N=12. | |
| Outcomes | Global state: (CGI).
Side effects: (EPS, hypotension). Unable to use ‐ Mental state: (BPRS ‐ no usable data). Behaviour: (NOSIE ‐ no usable data). Laboratory tests: (ECG ‐ no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Brauzer 1968.
| Methods | Allocation: randomly assigned, no further details. Blindness: double (identical 10cc multiple dose vials). Design: 2 parallel groups, single centre. Duration: 72 h. Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: schizophrenia (DSM). N=36. Age: at least 21 years. Sex: M 17, F 19. Setting: hospital. History: acute patients, recently hospitalised **include one chronic brain syndrome with psychosis. | |
| Interventions | 1. Trifluoperazine: dose maximum 20 mg/day IM. N=18. 2. Thiothixene: dose maximum 20 mg/day IM. N=18. | |
| Outcomes | Global state.
Side effects.
Use of antiparkinson agents. Unable to use ‐ Mental state: (IMPS; BPRS ‐ no data). Physiological measures ‐ no data available. |
|
| Notes | ** Mixed diagnosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Brauzer 1971.
| Methods | Allocation: randomly assigned, no further description. Blindness: double. Design: 2 parallel groups, single centre. Duration: 1 to 3 months. Analysis: intention‐to‐treat not performed in the trial. Country: USA. | |
| Participants | Diagnosis: schizophrenia**. N=25. Age: mean 41.8 years. Sex: M 11, F 14. Setting: outpatients. History: only 20% are paranoid schizophrenics. | |
| Interventions | 1. Trifluoperazine: dose initially 5 mg/day followed by minimum 8 mg/day and maximum of 19 mg/day, then maintenance dose 13 mg/day. N=13. 2. Molindone: dose initially 10 mg/day, followed by minimum of 18 mg/day and maximum of 38 mg/day, then maintenance dose 25 mg/day. N=12. | |
| Outcomes | Use of antiparkinson agents.
Leaving the study early. Unable to use ‐ Mental state: (BPRS ‐ no SD). Side effects ‐ no data. |
|
| Notes | **Mixed diagnosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Claghorn 1974.
| Methods | Allocation: randomly assigned, no further details. Blindness: double (identical capsules). Design: 2 parallel groups, single centre. Duration: 24 weeks. Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: schizophrenia no further details. N=87. Age: range 22‐65 years, mean 42.5 years. Sex: M 23, F 64. Setting: outpatients. History: maintenance treatment of chronic (at least 2 years duration) adults. | |
| Interventions | 1. Trifluoperazine: range: 5‐19 mg/day (mean 12.53 mg/day). N=44. 2. Pimozide: range 2‐8 mg/day (mean 5.16 mg/day). N=43. | |
| Outcomes | Side effects.
Laboratory tests. Unable to use ‐ Mental state: (BPRS). Global state: (CGI). Behaviour: (Evaluation of Social Functioning). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Clark 1975.
| Methods | Allocation: pre‐randomised list, blocks of 3, provided by drug company. Blindness: double (identical capsules). Design: 3 parallel groups, single centre. Duration: 4 weeks. Analysis: intention‐to‐treat performed only for some outcomes. Country: USA. | |
| Participants | Diagnosis: schizophrenia, confirmed by research psychiatry. N=42. Age: range: 21‐57 years. Sex: M 21, F 16. Setting: hospital. History: newly admitted chronic patients. Excluded: < 2 years of illness; < 18 years of age; mental deficiency, epilepsy, organic brain disease, metabolic disorders, liver, renal or cardiac disease. | |
| Interventions | 1. Trifluoperazine: dose 10 mg/day initially, then increasing to maximum 50 mg/day (mean 36 mg). N=14. 2. Loxapine: dose 20 mg/day initially, then increased to maximum 100 mg/day, (mean 71mg). N=15. 3. Placebo: 2‐10 capsules. N=13. | |
| Outcomes | Leaving the study early.
Side effects: (total; EPS; insomnia; weight; EKG).
Global state: (CGI; use of additional sedation).
Laboratory tests. Unable to use ‐ Mental state: (BPRS ‐ no SD). Efficacy: (analysis of covariance ‐ no usable data). Behaviour: (NOSIE ‐ no usable data). Physiological measures: (BP, pulse ‐ no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Coons 1962.
| Methods | Allocation: randomly assigned (by Dr. Coons independently, code in a sealed envelope). Blindness: double (identical capsules, coded bottles). Design: 3 parallel groups, single centre. Duration: 6 weeks (preceded by 8 days washout). Analysis: intention‐to‐treat. Country: Canada. | |
| Participants | Diagnosis: ** mixed (˜79,4% schizophrenia; ˜12% affective psychoses; ˜5% psychoneuroses; ˜3.6% outros, ˜12% leucotomy). N=117. Age: ˜47 years. Sex: female. Setting: hospital. History: long term hospital patients (mean˜141 months). | |
| Interventions | 1. Trifluoperazine: dose 15 mg/day. N=39. 2. Chlorpromazine: dose 150 mg/day. N=39. 3. Placebo. N=39. | |
| Outcomes | Use of antiparkinson agents. Unable to use ‐ Behaviour: (Hospital Adjustment Scale ‐ no data). |
|
| Notes | ** Mixed diagnosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Denber 1972.
| Methods | Allocation: randomly assigned, (random code was unknown until the end of study). Blindness: double (identical capsules dispensed by the research nurse). Design: 2 parallel groups, single centre. Duration: average of 36 days. Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: ** 26 schizophrenics; 1 psychose + mental deficiency and 3 involutional psychoses. N=31 (30 patients, one patient participated twice). Age: no data. Sex: only female. Setting: hospital. History: acute illness. | |
| Interventions | 1. Trifluoperazine: dose (mean 58.7 mg/day). N=15. 1. Thiothixene: dose (mean 49 mg/day). N=16. | |
| Outcomes | Side effects: (total number; specific).
Global state: (global clinical evaluation).
Weight changes.
Use of antiparkinson agents.
Leaving the study early. Unable to use ‐ Mental state: (IMPS). |
|
| Notes | ** Mixed diagnosis, one patient participated twice. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Donlon 1978.
| Methods | Allocation: randomly assigned, no further details. Blindness: double (identical drug and placebo capsules). Design: 2 parallel groups, single centre. Duration: 52 weeks. Analysis: intention‐to‐treat not performed in the trial. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further details. N=25. Age: mean 41 years. Sex: M 12, F 13. History: chronic. Inclusion: no depression, good health, no previous hospitalisation during last 6 months, symptoms stabilised with neuroleptics. | |
| Interventions | 1. Trifluoperazine: range 20‐40 mg/day. N=13. 2. Penfluridol: dose range once a week 80‐160 mg plus 6 days of placebo. N=12. | |
| Outcomes | Leaving the study early
Global state: (CGI, use of additional medication).
Adverse effects: (TESS; ophtalmologic; abnormal laboratory values). Unable to use ‐ Mental state: (BPRS ‐ only graphs, Hamilton's Psychiatric Scale for depression factors ‐ no data). Social state: (Evaluation of Social Functioning factors ‐no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Edwards 1980.
| Methods | Allocation: randomly assigned in advance and held by the hospital pharmacist (open list of random numbers). Blindness: double. Design: 2 parallel groups, single centre. Duration: 6 weeks (preceded by 1 month washout if possible). Analysis: intention‐to‐treat not performed in the trial. Country: UK. | |
| Participants | Diagnosis: schizophrenia, Schneider 1959. N=38. Age: range 25‐74 years, majority 55‐64 years. Sex: M 33, F 5. Setting: hospital. History: long‐stay inpatients, 36 ill >10 years. | |
| Interventions | 1. Trifluoperazine: range 15‐45 mg/day (mean 27.7 mg). N=19. 2. Sulpiride: range 600‐1800 mg/day (mean 1212 mg). N=19. | |
| Outcomes | Global state: (change in condition; severity of illness).
Leaving the study early.
Use of hypnotic.
Use of antiparkinson agents.
Side effects: (specific list). Unable to use ‐ Mental state: (BPRS ‐ no SD). Side effects: (Simpson and Angus Scale ‐ no SD). Physiological measures: (handwriting, weight, pulse, blood pressure ‐ no SD). Live events ‐ denominator unclear. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Freeman 1968.
| Methods | Allocation: randomly assigned, random code not broken to the end of study. Blindness: double (identical capsules). Design: 2 parallel groups, single centre. Duration: 12 weeks (preceded by 4 weeks washout). Analysis: intention‐to‐treatnot performed in the trial. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further details. N=41. Age: mean 30 years. Sex: M 14, F 27. Setting: hospital. History: majority of cases undifferentiated and paranoid. Inclusion: duration of present illness of not more than 2 years and hospital admissions not longer than 5 years. | |
| Interventions | 1. Trifluoperazine: dose 15 mg/day (first week), 30 mg/day (second week), maximum 45 mg/day (mean 26 mg). N=20. 2. SKF 14336: dose 200 mg/day (first week), 400 mg/day (second week), maximum 600 mg/day (mean 385 mg). N=21. | |
| Outcomes | Global state: (global judgement of the psychiatrists).
Use of antiparkinson agents.
Side effects.
Leaving the study early.
Side effects: (abnormal laboratory tests). Unable to use ‐ Mental state: (BPRS; IMPS ‐ only graphs). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gallant 1968.
| Methods | Allocation: randomly assigned, no further details. Blindness: double. Design: 2 parallel groups, single centre. Duration: 9 weeks (preceded by minimum of 17 days washout). Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further details. N=24. Age: average 43 years. Sex: M 12, F 12. Setting: hospital. History: chronic. | |
| Interventions | 1. Trifluoperazine: dose up to 80 mg/day. N=12. 2. Molindone: dose up to 80 mg/day. N=12. | |
| Outcomes | Global state: (global ratings of improvement).
Side effects: (EPS).
Use of antiparkinson agents. Unable to use ‐ Mental state: (BPRS ‐ no data). Behaviour: (NOSIE ‐ no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gallant 1968 II.
| Methods | Allocation: randomly assigned, no further details. Blindness: no data#. Design: 2 parallel groups, single centre. Duration: 1 week (preceded by minimum of 26 days washout). Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further details. N=52. Age: range 31‐58 years, mean 45 years. Setting: hospital. Sex: M 26, F 26. History: chronic Illness (average length of hospitalisation 16.5 years, range 4‐28 years). | |
| Interventions | 1. Trifluoperazine: dose 20 mg/day IM (maximum). N=26. 2. Thiothixene: dose 20 mg/day IM (maximum). N=26. | |
| Outcomes | Global state: (global ratings of improvment).
Side effects: (EPS, sedation, local tissue reactions). Mental state: (BPRS ‐ no data). Behaviour: (NOSIE ‐ no data). |
|
| Notes | # Blindness: no data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gallant 1972.
| Methods | Allocation: randomly assigned, no further details. Blindness: no data#. Design: 2 parallel groups, single centre. Duration: 8 weeks (preceded by 2 weeks of baseline examinations, 25 days washout). Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further details. N=24. Age: median ˜ 39.5 years. Sex: males and females, no further details. Setting: hospital. History: chronic Illness. | |
| Interventions | 1. Trifluoperazine: dose 10‐100 mg/day. N=12. 2. GP‐45795 (tricyclic dibenzothiepin): dose 50‐500 mg/day. N=12. | |
| Outcomes | Global state: (global ratings of clinical change).
Side effects: (EPS, hypotension). Unable to use ‐ Mental state: (BPRS ‐ only "p" value). Behaviour: (NOSIE ‐ only "p" value). EKG. Photosensitivity skin tests. Laboratory tests. |
|
| Notes | # Blindness: no data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gross 1974.
| Methods | Allocation: randomly assigned, no further information. Blindness: double (identical capsules). Design: 3 parallel groups, single centre. Duration: 16 weeks (preceded by 4 weeks neuroleptic treatment not exceeding chlorpromazine 500 mg/d, thioridazine 500 mg/d, trifluoperazine 30 mg/d, fluphenazine 30 mg/d) + 36 weeks (extended open evaluation)). Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further details. N=61. Age: mean ˜ 46 years. Sex: M 37, F 24. Setting: formerly hospitalised (residents of Harbor View House). History: chronic schizophrenia (ill> 2 years or past 3 months hospitalisation with continuous antipsychotic treatment ). Excluded: epilepsy, drug addiction, severe depression, mental retardation, organic brain disease, physical disease, and those receiving heavy sedation. | |
| Interventions | 1. Trifluoperazine: range 5‐30 mg/day, mean 17.5 mg. N=20. 2. Pimozide: range 2‐12 mg/day (mean 6.3 mg). N=21. 3. Placebo: N=20. Antiparkinson medication and chloral hydrate if necessary. | |
| Outcomes | Global state: (CGI).
Leaving the study early. Unable to use ‐ Mental state: (BPRS ‐ only graphs). Global state: (CGI ‐ no usable data). Social functioning: (Family Rating Form ‐ no usable data; Harbor view house Resident Rating Report ‐ unpublished scale). Adverse events: (no usable data). |
|
| Notes | ##High dropout rate(40,1%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gwynne 1962.
| Methods | Allocation: randomly assigned, no further details. Blindness: double (identical capsules). Design: 3 parallel groups, single centre. Duration: 16 weeks (preceded by at least 1 month washout). Analysis: intention‐to‐treat not performed in the trial. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further details. N=78. Age: mean 49 years. Sex: M 39, F 39. Setting: hospital. History: chronic illness (average time in hospital 20 years); withdrawal for at least 1 year; poor response to previous therapy. | |
| Interventions | 1. Trifluoperazine: range 10‐40 mg/day, mean at week‐16, 11.2 mg. N=26. 2. Chlorpromazine: range 100‐400 mg/day, mean at week‐16, 146mg. N=26. 3. Placebo: N=26. | |
| Outcomes | Leaving the study early.
Side effects. Unable to use ‐ Mental state: (Lorr Multidimensional Scale for Rating Psychiatric Patients ‐ only "p" value). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kiloh 1976.
| Methods | Allocation: randomly assigned, no further details. Blindness: double (identical capsules). Design: 2 parallel groups, single centre. Duration: 12 weeks (preceded by 2 weeks washout). Analysis: intention‐to‐treat not performed in the trial. Country: Australia. | |
| Participants | Diagnosis: schizophrenia, Clinical Psychiatry by Slater and Roth 1969. N=57. Age: range 20‐69 years. Sex: male and female, no further details. Setting: hospital at the beginning, then continued on an out‐patients basis. History: acute (ill for less than 2 years) and chronic (over than 2 years) schizophrenic patients. | |
| Interventions | 1. Trifluoperazine: mean doses, acute 23.5 mg/day (N=10), chronic 31mg/day (N=11). 2. Loxapine: mean doses, acute 37mg/day (N=17), chronic 56 mg/day (N=8). | |
| Outcomes | Use of antiparkinson agents.
Global state: (global rating changes).
Side effects.
Leaving the study early. Unable to use ‐ Mental state: (BPRS ‐ no SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Malik 1980.
| Methods | Allocation: randomly allocated. Blindness: double (identical capsules). Design: 2 parallel groups, single centre. Duration: 28 days. Analysis: intention‐to‐treat not performed in the trial. Country: India. | |
| Participants | Diagnosis: schizophrenia, no further details. N=54. Age: range 14‐19 years mean ˜17 years. Sex: M 25, F 27 (2 not reported). Setting: hospital. History: adolescent schizophrenics. Exclusion: sensivity to study drugs, ECT in the last 8 weeks, brain syndrome, convulsive disorders, mental retardation or autism. | |
| Interventions | 1. Trifluoperazine: dose mean 23.57 mg/day, maximum 35 mg. N=27. 2. Loxapine succinate: dose mean 91.5 mg/day, maximum 120 mg. N=27. Antiparkinson medication as required. | |
| Outcomes | Global state: (CGI, patient's self evaluation).
Patient's drug preference.
Leaving the study early. Unable to use ‐ Mental state: (BPRS ‐ no SD). Use of antiparkinson drugs ‐ no data. Physiological measures: (BP, pulse ‐ no data). Laboratory tests ‐ no data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marjerrison 1964.
| Methods | Allocation: randomly assigned, no further details. Blindness: double (multiplicity of forms, individual coded bottles). Design: 4 parallel groups, single centre. Duration: phase I, five‐months; phase II, two‐months. Analysis: intention‐to‐treat not performed in the trial. Country: Canada. | |
| Participants | Diagnosis: **schizophrenia but 12 with other diagnoses distributed among the groups. N=88. Age: mean 48 years. Sex: M 38, F 40. Setting: hospital. History: chronic psychotics; highly treatment resistant. | |
| Interventions | 1. Trifluoperazine: dose phase I, 29 mg/day, phase II, 27 mg/day. N=16. 2. Chlorprothixene: dose phase I, 200 mg/day, phase II, 270 mg/day. N=8. 3. Placebo. N=34. 4. Usual Phenothiazines: dose variated. N=30 (drugs were chosen clinically). | |
| Outcomes | Leaving the study early.
Global state: (numbers of subjects receiving barbiturate medication).
Use of antiparkinson agents. Unable to use ‐ Mental state: (PRP; Ward Inventory scores). |
|
| Notes | ** Mixed diagnosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marjerrison 1966.
| Methods | Allocation: randomly assigned, no further details. Blindness: double (coded bottles). Design: 3 parallel groups, single centre. Duration: 12 weeks (preceded by a 7‐month drug‐withdrawal project). Analysis: intention‐to‐treat. Country: Canada. | |
| Participants | Diagnosis: schizophrenia, no further details. N=27. Age: range 32‐63 years, median 47 years. Sex: male and female. Setting: hospital. History: highly treatment resistant, long‐term chronic patients (length of hospitalisation range 3‐33 years). | |
| Interventions | 1. Trifluoperazine: dose initial mean 27.5 mg/day, final 26 mg. N=8. 2. Triperidol: dose initial mean 10 mg/day, final 6.4 mg. N=8. 3. Haloanisone: dose initial mean 35 mg/day, final 45 mg. N=11. | |
| Outcomes | Use of antiparkinson agents. Unable to use ‐ Mental state: (PRP; IMPS ‐ no SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Menon 1972.
| Methods | Allocation: randomly assigned, no further details. Blindness: research workers blind, no further information. Design: 3 parallel groups, single centre. Duration: 10 weeks (preceded by 6 weeks washout). Analysis: intention‐to‐treat. Country: India. | |
| Participants | Diagnosis: schizophrenia (clinical criteria). N=60. Age: range 20‐60 years. Sex: M 30, F 30. Setting: hospital. History: chronic, length of hospitalisation 1.5‐10 years; continuously hospitalised > 1 year. Excluded: mental deficiency and physical complications. | |
| Interventions | 1. Trifluoperazine: dose 15 mg/day. N=20. 2. Trifluperidol: dose 1.5 mg/day. N=20. 3. Placebo: N=20. * Antiparkinson drugs if necessary. | |
| Outcomes | Global state: (QPSS scale).
Behaviour: (Wings scale).
Side effects: (EPS, nausea and vomiting). Unable to use ‐ Laboratory tests ‐ no data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Needham 1969.
| Methods | Allocation: randomly assigned by the pharmacist, no further details. Blindness: double (identical capsules). Design: 2 parallel groups, single centre. Duration: 24 weeks (preceded by 4 weeks washout). Analysis: intention‐to‐treat not performed in global state. Country: Australia. | |
| Participants | Diagnosis: schizophrenia, no further details. N=54. Age: mean 45 years. Sex: only male. History: average duration of illness 22 years; majority chronic undifferentiated schizophrenics. | |
| Interventions | 1. Trifluoperazine: range 11.25‐37.5 mg/day. N=27. 2. SKF 14336: range 150‐500 mg/day. N=27. | |
| Outcomes | Leaving the study early.
Global state: (Global evaluation of improvement).
Side effects: (EPS, skin and eyes changes). Unable to use ‐ Mental state: (BPRS ‐ no SD). Behaviour: (NOSIE ‐ no SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
O'Brien 1974.
| Methods | Allocation: randomly assigned (code held by a technician who was not responsible for allocation). Blindness: double (identical capsules, coded bottles). Design: 2 parallel groups, 2 centres. Duration: 3 weeks. Analysis: intention‐to‐treat not performed in the trial. Country: USA. | |
| Participants | Diagnosis: patients with symptoms of hostility, suspiciousness, paranoia and aggressiveness; 20 schizophrenics*. N=30. Age: mean ˜30 years. Sex: M 22, F 2. Setting: hospital. | |
| Interventions | 1. Trifluoperazine: dose 9‐48 mg/day. N=15. 2. Haloperidol: dose 3‐20 mg/day. N=15. | |
| Outcomes | Global state.
Leaving the study early.
Use of antiparkinson agents. Unable to use ‐ Mental state: (BPRS; Global Hostility; Global Paranoia ‐ no SD). Psychological Tests: (Buss‐Durkee hostility inventory; Rozenzweig picture frustration test; MMPI and Rorshach test ‐ no data). |
|
| Notes | **Mixed diagnosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Perales 1974.
| Methods | Allocation: randomly assigned by random table (independently and secretly by the head of the project). Blindness: double (treatment and evaluation made by 2 independent psychiatrists blind to drug taken). Design: 2 parallel groups, single centre. Duration: 45 days. Analysis: intention‐to‐treat. Country: Peru. | |
| Participants | Diagnosis: schizophrenia no further details. N=30. Age: range 19‐43 years, mean 31.3 years. Sex: M 26, F 4. Setting: hospital. History: acute state of paranoid schizophrenia. | |
| Interventions | 1. Trifluoperazine: dose first 5 days 2‐8 amp (2‐8 mg IM), then 10‐40 mg. N=15. 2. Clotiapine: dose first 5 days 1‐4 amp (40‐160 mg IM), then 40‐60 mg. N=15. | |
| Outcomes | Global state: (clinical evaluation).
Side effects. Unable to use ‐ Mental state: (BPRS). Side effects: (Extrapiramidal signs ‐ Bordeau scale). Use of antiparkinson agents. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Prien 1969.
| Methods | Allocation: randomly assigned, no further details. Blindness: double (identical capsules). Design: 3 parallel groups, at 6 hospitals centres. Duration: 24 weeks (no washout period). Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further details. N=360. Age: mean 41.8 years. Sex: M 180, F 180. History: chronic (55% more than ten years of hospitalisation). Exclusion: organic brain disease, mental deficiency or medical conditions contraindicated with high dose drugs. | |
| Interventions | 1.Trifluoperazine: dose 80 mg/day. N=120. 2.Trifluoperazine: dose 15 mg/day. N=120. 3. Placebo: N=120. | |
| Outcomes | Global state: (Global psychiatric change).
Side effects.
Leaving the study early.
Opthalmologic changes.
DRI (Discharge readiness Inventory). Unable to use ‐ Mental state: (BPRS; IMPS ‐ no data). Behaviour: (NOSIE ‐ no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ramsay 1970.
| Methods | Allocation: randomly assigned, no further information. Blindness: double (identical capsules). Design: 2 parallel groups. Duration: 12 weeks, single centre. Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: schizophrenics, no further details. N=20. Age: range 29‐55 years, mean 44.4 years. Sex: M 14, F 6. Setting: hospital. History: "chronic" (hospitalised for at least 2 years). | |
| Interventions | 1. Trifluoperazine: range 10‐60 mg/day. N=10. 2. Molindone: range 20‐120 mg/day. N=10. | |
| Outcomes | Leaving the study early
Side effects: (total number, EPS). Unable to use ‐ Global state: (CGI). Mental state: (BPRS). Behaviour: (NOSIE). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Reardon 1966.
| Methods | Allocation: randomly assigned, no further description. Blindness: double. Design: 3 parallel groups, single centre. Duration: minimum 4 weeks, maximum 12 weeks. Analysis: intention‐to‐treat not performed in the trial. Country: USA. | |
| Participants | Diagnosis: paranoid schizophrenia (Bleuler criteria). N=34. Age: not stated. Sex: M 22, F 12. Setting: hospital. History: "acute" illness. | |
| Interventions | 1. Trifluoperazine: dose 20 mg/day (week 1), 40 mg/day thereafter. N=11. 2. Chlorpromazine: dose 300 mg/day (week 1) 600 mg/day thereafter. N=11. 3. Placebo: 2‐4 cc/day (week 1), 5‐10 cc/day thereafter. N=12. Artane 10 mg/day prophylactically. | |
| Outcomes | Leaving study early.
Global state: (lack of previously observed delusions and hallucinations). Unable to use ‐ Mental state: (MMPI ‐ no SD). Shipley Hartford. WAIS. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rubin 1971.
| Methods | Allocation: randomly assigned, no further description. Blindness: double (identical capsules). Design: 2 parallel groups. Duration: minimum 5 days, maximum 79 days. Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: *psychotic patients: paranoid schizophrenics (13); undifferentiated schizophrenia (4); Manic‐depressive (1). N=18. Age: range 26‐49 years, mean 37.5 years. Sex: only male. Setting: hospital. History: acute illness. | |
| Interventions | 1. Trifluoperazine: range 6‐60 mg/day. N=8. 2. Haloperidol: range 2‐20 mg/day. N=10. 3 patients receiving TFP began group psychotherapy. | |
| Outcomes | Global state: (Mental Status Schedule; discharged from hospital. Unable to use ‐ Average time of onset of Therapeutic Effect: (only patients who improved). |
|
| Notes | **Mixed diagnosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Schiele 1961.
| Methods | Allocation: randomly assigned (held by the pharmacist). Blindness: double (identical capsules, coded bottles). Design: 4 parallel groups, single centre. Duration: 16 weeks (in strict double‐blind conditions) + 22 weeks, no washout period. Analysis: intention to treat. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further details. N=80. Age: mean 40.6 years. Sex: males. Setting: hospital. History: chronic (average hospitalisation 10 years). Excluded: people > 55 years and organic factors. Included: inadvertently 3 with lobotomies (2 in the thioridazine group and other in the chlorpromazine). | |
| Interventions | 1. Trifluoperazine: 10‐50 mg/day, maximum 35 mg, 12 received less than the maximum. N=20. 2. Thioridazine: 200‐1000 mg/day, maximum 958 mg, 3 received less than the maximum. N=20. 3. Chlorpromazine: 200‐1000 mg/day, maximum 894 mg/day, 3 received less than the maximum. N=20. 4. Placebo: 2‐10 capsules. Occasional additional medication, phenobarbital and benzotropine methansulfonate. | |
| Outcomes | Global state: (Clinical Evaluation).
Side effects.
Leaving the study early.
Use of antiparkinson agents. Unable to use ‐ Behaviour: (Behaviour rating scale ‐ no SD). Mental state: (MMPI ‐ only 43 patients took the tests). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Schiele 1969.
| Methods | Allocation: randomly assigned (by administrative personnel not involved with patient treatment or evaluation). Blindness: double (identical capsules). Design: 2 parallel groups. Duration: 4 weeks, but it was permitted to remove patients at the end of 2 weeks or to continue beyond the 4 weeks. Analysis: only 2 weeks ratings were analysed because of the small sample size. Country: USA. | |
| Participants | Diagnosis: schizophrenia, no further information. N=21. Age: range 16‐48 years, mean 24 years. Sex: no information. Setting: hospital. History: acute illness; patients must not have been hospitalised during the preceding 12 months, or have childhood schizophrenia, or brain syndrome, or mental deficiency, alcoholism, epilepsy or drug addiction. | |
| Interventions | 1. Trifluoperazine: range 15‐30 mg/day. N=10. 2. Trifluperidol: range 3‐6 mg/day. N=11. Without washout period; using antiparkinson drug only if dose reduction of the antipsychotic did not provide relief. | |
| Outcomes | Leaving the study early (at the end of 2 weeks). Unable to use ‐ Global state: (Global rating of Pathology; Global rating of improvement ‐ no SD). Mental state: (BPRS; MMPI; IMPS ‐ no SD). Side effects: (no data available). |
|
| Notes | # Analysis performed in 2 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Seth 1979.
| Methods | Allocation: randomly assigned, no further information. Blindness: double (identical capsules). Design: 2 parallel groups. Duration: 12 weeks. Analysis: intention‐to‐treat not performed in the trial. Country: India. | |
| Participants | Diagnosis: schizophrenics, no further details. N=72. Age: range 20‐49 years, mean 30 years. Sex: M 28, F 36. Setting: hospital. History: chronic illness. | |
| Interventions | 1. Trifluoperazine: range 5‐45 mg/day. N=36. Medication free for at least 4 weeks. 2. Loxapine: range 20‐90 mg/day. N=36. | |
| Outcomes | Leaving the study early.
Side effects. Unable to use ‐ Mental state: (BPRS). Behaviour: (NOSIE). Global state: (CGI). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Simpson 1976.
| Methods | Allocation: randomly assigned (by a person who was not responsible for recruiting patients). Blindness: double (identical capsules, coded bottles). Design: 2 parallel groups, single center. Duration: 4 weeks preceded by 3 days washout (evaluation carried out by the same physician, study lasted over 3 years). Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: acute schizophrenia, no further details. N=43. Age: range 16‐61 years, mean 31.7 years. Sex : M 27, F 16. Setting: hospital. History: newly hospitalised, mean admitted ˜2 times, 6 first admissions. Exclusion: ill health. | |
| Interventions | 1. Trifluoperazine: range 20‐50 mg/day, mean 35 mg. N=19. 2. Loxapine succinate: range 40‐80 mg/day, mean 74 mg. N=24. Antiparkinson drugs and chlorayl hydrate as required. | |
| Outcomes | Global state: (discharge).
Leaving the study early.
Side effects: (unwanted effects checklist, use of neurological rating scale).
Use of antiparkinson agents.
Laboratory tests. Unable to use ‐ Global state: (CGI ‐ no usable data). Mental state: (BPRS ‐ no usable data). Physiological measures: (BP, EEG, opthalmic tests, pulse ‐ no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Stewart 1969.
| Methods | Allocation: randomly assigned, no further information. Blindness: double (identical capsules). Design: crossover. Duration: 14 days (washout) + 28 days (1st placebo period) + 56 days (first drug period) + 28 days (2nd placebo period) + 56 days (2nd drug period). Analysis: intention‐to‐treat. Country: Canada. | |
| Participants | Diagnosis: schizophrenia, no further details. N=50. Age: range 33‐59 years, mean 48.6 years. Sex: M 17, F 8 each group. Setting: hospital. History: "chronic" (hospitalised from 3‐37 years); free of epilepsy, chronic brain syndrome and arterioesclerosis and none lobotomized. | |
| Interventions | 1. Trifluoperazine: range 4‐16 mg/day. N=25. 2. Haloperidol: range 4‐16 mg/day. N=25. | |
| Outcomes | Global state: (overall Improvement). Unable to use ‐ Mental state: (BPRS; IMPS; Rockland‐Pollin Scale). Side effects. Behaviour: (MACC ‐ modified). Laboratory tests. Vital signs. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sugerman 1965.
| Methods | Allocation: randomly assigned (according to a number code that had broken only when the study was concluded). Blindness: double (identical capsules, individual bottles). Design: 2 parallel groups, single centre. Duration: 1 month. Analysis: intention‐to‐treat. Country: USA. | |
| Participants | Diagnosis: schizophrenia (clinical criteria). N=22. Age: mean 32.5 years. Sex: M 6, F 16. Setting: hospital. History: majority was schizophrenic reaction, acute indifferentiated type. Exclusion: organic disease and pregnancy. | |
| Interventions | 1. Trifluoperazine: range 4‐30 mg/day, mean 10.4 mg. N=11. 2. Trifluperidol: range 1‐7.5 mg/day, mean 2.1 mg. N=11. | |
| Outcomes | Use of antiparkinson agents.
Leaving the study early.
Global state: (global clinical rating of change).
Side effects.
Tomkins‐Horn Picture Arrangement Test. Unable to use ‐ Mental state: (BPRS ‐ no SD). Behaviour: (NOSIE ‐ no SD). Hoffer‐Osmond Diagnostic Test. Tulane Behavioral rating Scale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Vergara 1977.
| Methods | Allocation: randomly assigned, no further details. Blindness: double. Design: 2 parallel groups. Duration: 16 weeks (pre study neuroleptic medication was discontinued between the 28th and 84th day). Analysis: intention‐to‐treat. Country: Canada. | |
| Participants | Diagnosis: schizophrenics, no further details. N=20. Age: range 20‐55 years, mean 38.4 years. Sex: M 13, F 7. Setting: outpatients. History: chronic illness. | |
| Interventions | 1. Trifluoperazine: dose 5‐30 mg/day. N=10. 2. Pimozide: dose 2‐12 mg/day. N=10. | |
| Outcomes | Global state: (CGI).
Side effects.
Leaving the study early. Unable to use ‐ Mental state: (BPRS ‐ no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
AIMS = Abnormal Involuntary Movement Scale BPRS = Brief Psychiatric Rating Scale CGI = Clinical Global Improvement DSM‐III‐R = Diagnostic and Statistical Manual, 3‐rd revised edition EKG = Electrocardiogram EPS = Extrapyraminal Rating Scale IMPS = Inpatient Multidimensional Psychiatric Scale ITT= Intention to treat MMPI = The Minnesota multiphasic personality inventory NOSIE = Nurses Observational Scale of Inpatients Evaluation TESS = Treatment Emergent Symptom Scale WAIS = Wechsler Adult Intelligence Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraham 1987 | Allocation: randomised. Participants: people with schizophrenia. Interventions: trifluoperazine and ECT versus trifluoperazine with simulated ECT. |
| Barsa 1959 | Allocation: not randomised. |
| Brannen 1969 | Allocation: randomised. Participants: people with chronic schizophrenia. Interventions: promethazine versus trifluoperazine versus placebo. Outcomes: no usable data, 57% lost to follow up. |
| Buffaloe 1961 | Allocation: unclear, double‐blind crossover study. Participants: people with schizophrenia. Interventions: first phase only trifluoperazine, second phase trifluoperazine plus parnate versus trifluoperazine. Outcomes: no data available. |
| Cabrera 1990 | Allocation: unclear, double‐blind study. Participants: people with schizophrenia. Interventions: interferon "intratecal" + oral placebo versus trifluoperazine + chlorpromazine + trihexifenidil + lumbar injection of distilled water. |
| Calwell 1964 | Allocation: "randomly divided" ‐ no further description, multiple crossover (medication was changed every 4 weeks). Participants: women with chronic schizophrenia. Interventions: oxypertine versus trifluoperazine. Outcomes: no data reported for first arm of crossover. |
| Carscallen 1968 | Allocation: "double‐blind technique" ‐ no further details. Participants: people with schizophrenia. Interventions: trifluoperazine 10 mg/day versus trifluoperazine 100 mg/day. Outcomes: no data available. |
| Casey 1961 | Allocation: randomised. Participants: people with schizophrenia. Interventions: d‐amphetamine versus isocarboxazid versus imipramine versus trifluoperazine versus placebo but chlorpromazine given to everyone. |
| Childers 1961 | Allocation: not randomised. |
| Childers 1962 | Allocation: randomised. Participants: people with extrapyramidal symptoms. Interventions: benzotropine methanesulfonate plus chlorpromazine or trifluoperazine or fluphenazine versus procyclidine plus chlorpromazine or trifluoperazine or fluphenazine, no data for trifluroperazine group alone. |
| Childres 1962 b | Allocation: not randomised, "placed in temporal sequence". |
| Crane 1970 | Allocation: randomised. Participants: people with schizophrenia. Interventions: trifluoperazine 80 mg/day versus trifluoperazine 16 mg/day versus placebo. Outcomes: no usable data. |
| D'Elia 1974 | Allocation: randomised. Participants: people with schizophrenia. Interventions: trifluoperazine versus pimozide. Outcomes: EEG study, no data available. |
| Den Boer 2000 | Allocation: double‐blind procedure. Participants: people with schizophrenia. Interventions: neuroleptic treatment (trifluoperazine, chlorpromazine, haloperidol, thioridazine) plus ritanserin versus neuroleptic treatment plus placebo. |
| Dewolfe 1971 | Allocation: randomised, crossover trial. Interventions: prolixin enanthate versus thorazine‐stelazine, not stelazine. |
| DiMascio 1965 | Allocation: randomised Participants: healthy volunteers. |
| Eitan 1992 | Allocation: "were assigned", no further details, double‐blind crossover study . Participants: people with schizophrenia. Interventions: chlorpromazine versus thioridazine versus trifluoperazine versus haloperidol versus placebo. Outcomes: no data reported for individual arms of cross‐over. |
| Fish 1966 | Allocation: not randomised. |
| Forrester 1958 | Allocation: not randomised. |
| Gardos 1968 | Allocation: "were assigned" ‐ no further details. Participants: psychotic patients ‐ no further details. Interventions: two different samples, withdrawn group (trifluoperazine 10 mg versus trifluoperazine 5 mg + chlorpromazine 50 mg) and agitated group (chlorpromazine 200 mg versus trifluoperazine 5 mg+ chlorpromazine 200 mg). Outcomes: no data available. |
| Gottfries 1971 | Allocation: not randomised. |
| Guido 1960 | Allocation: not randomised. |
| Held 1970 | Allocation: randomised. Participants: people with schizophrenia. Interventions: thorazine versus melleril versus stelazine versus prolixin versus proketazine versus trifalon versus haloperidol versus placebo. Outcomes: no usable data. |
| Janecek 1963 | Allocation: not randomised. |
| Jones 1970 | Allocation: not randomised. |
| Jones 1971 | Allocation: not randomised. |
| Joshi 1980 | Allocation: unclear ‐ double‐blind study. Participants: people with schizophrenia. Interventions: combined modality therapy (trifluoperazine + vitamins versus trifluoperazine + placebo). |
| Joshi 1982 | Allocation: unclear ‐ double‐blind. Participants: people with schizophrenia. Interventions: combined modality therapy (trifluoperazine, vitamins B1, B6, B12, chlorpromazine and placebo). |
| Kalinin 1986 | Allocation: randomised. Participants: people with schizophrenia. Interventions: insulin coma therapy versus individual pharmacotherapy (haloperidol, trifluoperazine plus sedatives ‐ amitriptyline and lithium). |
| Kline 1977 | Allocation: not randomised. |
| Knight 1979 | Allocation: double‐blind crossover trial. Participants: people with chronic schizophrenia. Interventions: thiothixene versus combination of trifluoperazine and chlorpromazine. |
| Lal 1974 | Allocation: randomised ‐ crossover study. Participants: people with oral dyskinesia. |
| Lapolla 1965 | Allocation: randomised. Participants: "preponderance of schizophrenia". Interventions: only trifluoperazine, no control group. |
| Lovett 1987 | Allocation: not randomised. |
| Luckey 1967 | Allocation: randomised. Participants: people with schizophrenia. Interventions: haloperidol versus trifluoperazine. Outcomes: 66% of people were not reported in the final analysis. |
| Macdonald 1959 | Allocation: not randomised. |
| Mandel 1962 | Allocation: randomised, crossover trial. Participants: people with schizophrenia. Interventions: trifluoperazine versus butyrylperazine. Outcomes: no usable data. |
| Mena 1964 | Allocation: double‐blind controlled study. Participants: "chronic outpatients". |
| Monroe 1965 | Allocation: randomisation not mentioned, described as controlled. Participants: mixed diagnosis. |
| Montero 1971 | Allocation: "double‐blind". Participants: people with paranoid schizophrenia. Interventions: thioridazine versus trifluoperazine plus chlorpromazine. |
| Morton 1968 | Allocation: randomised ‐ withdrawal study. |
| Moyano 1975 | Allocation: unclear, double‐blind. Participants: people with schizophrenia. Interventions: trifluoperazine versus loxapine succinate. |
| Oybir 1962 | Allocation: not randomised. |
| Pecknold 1977 | Allocation: randomised Participants: people with schizophrenia. Interventions: clomacran versus trifluoperazine. Outcomes: 50% left the study. |
| Polvan 1971 | Allocation: not randomised. |
| Rudy 1958 | Allocation: not randomised. |
| Schiele 1963 | Allocation: randomised. Participants: people with schizophrenia. Interventions: trifluoperazine plus tranilcipromine versus trifluoperazine plus placebo. Outcomes: no data reported for the individual drugs. |
| Sharpley 1964 | Allocation: "double‐blind study". Interventions: pargyline plus tranylcypromine versus pargyline plus tranylcypromine plus trifluoperazine. |
| Stanley 1961 | Allocation: not randomised, case control study. |
| Talbot 1964 | Allocation: unclear. Participants: people with schizophrenia. Interventions: trifluoperazine plus chlorpromazine versus placebo. |
| Teja 1975 | Allocation: randomised. Participants: people with schizophrenia. Interventions: trifluoperazine versus chlorpromazine versus thiothixene versus haloperidol versus placebo. Outcomes: no usable data. |
| Tetreault 1968 | Allocation: randomised. Participants: people with chronic mental disease. Interventions: trial 1 ‐ trifluperidol versus trifluoperazine, trial 2 ‐ TPS‐23 versus chlorpromazine versus placebo. Outcomes: no data available. |
| Tolan 1959 | Allocation: not randomised. |
| Uddyback 1962 | Allocation: not randomised, case series. |
| Vogt 1961 | Allocation: not randomised |
| Weckowicz 1960 | Allocation: not randomised. |
| Weston 1961 | Allocation: randomised. Participants: people with schizophrenia. Interventions: trifluoperazine versus placebo. Outcomes: no data available. |
| Wilson 1961 | Allocation: unclear, "Latin square", crossover design. Participants: people with schizophrenia. Interventions: chlorpromazine versus prochlorperazine versus trifluoperazine versus placebo. Outcomes: no data available. |
| Winnik 1962 | Allocation: not randomised. |
Contributions of authors
Luciana de Oliveira Marques ‐ wrote the protocol, carried out data extraction and analysis and wrote the review.
Bernardo Garcia de Oliveira Soares ‐ wrote the protocol and participated in the analysis and writing of the review.
Maurício Silva de Lima ‐ wrote the protocol, carried out the data extraction and helped to write the review.
Sources of support
Internal sources
Universidade Católica de Pelotas, Brazil.
External sources
Universidade Federal de Pelotas, Brazil.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
*Bankier 1973 {published data only}
- Bankier RG. A comparison of fluspirilene and trifluoperazine in the treatment of acute schizophrenic psychosis. The Journal of Clinical Pharmacology and New Drugs 1973;13(1):44‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
*Doongaji 1989 {published data only}
- Doongaji DR, Satoskar RS, Sheth AS, Apte JS, Desai AB, Shah BR. Centbutindole vs trifluoperazine: a double‐blind controlled clinical study in acute schizophrenia. Journal of Postgraduate Medicine 1989;35(1):3‐8. [PubMed] [Google Scholar]
*Goldstein 1966 {published data only}
- Goldstein BJ, Clyde DJ. Haloperidol in controlling the symptoms of acute psychoses. Part II: A double‐blind evaluation of haloperidol and trifluoperazine. Current Therapeutic Research 1966;8(5):236‐40. [MEDLINE: ] [PubMed] [Google Scholar]
*Itil 1971 {published data only}
- Itil TM, Polvan N, Ucok A, Eper E, Guven F, Hsu W. Comparison of the clinical and electroencephalographical effects of molindone and trifluoperazine in acute schizophrenic patients. Behavioral Neuropsychiatry 1971;3(5):25‐32. [PubMed] [Google Scholar]
- Itil TM, Polvan N, Ucok A, Eper E, Guven F, Hsu W. Comparison of the clinical and electroencephalographical effects of molindone and trifluoperazine in acute schizophrenic patients.. Behavioral Neuropsychiatry 1971;3(9):6‐13. [MEDLINE: ] [PubMed] [Google Scholar]
*Pinard 1972 {published data only}
- Pinard G, Prenoveau Y, Fliesen W, Elie R, Bielmann P, Lamontagne Y, Tetreault L. Pimozide: a comparative study in the treatment of chronic schizophrenic patients. International Journal of Clinical Pharmacology Therapy and Toxicology 1972;6(1):22‐27. [PubMed] [Google Scholar]
*Simpson 1971 {published data only}
- Simpson GM, Amin M, Edwards G. A Double‐blind comparison of molindone and trifluoperazine in the treatment of acute schizophrenia. The Journal of Clinical Pharmacology and New Drugs 1971;11:227‐36. [PubMed] [Google Scholar]
*Vestre 1970 {published data only}
- Vestre ND, Schiele BC. Differential drug effects of the two phenothiazines. (A controlled comparison of thioridazine and trifluoperazine in chronic schizophrenics). Diseases of the Nervous System 1970;31(12):821‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Amin 1977 {published data only}
- Amin MM, Ban TA, Lehmann HE. A standard (trifluoperazine) controlled clinical study with pimozide in the maintenance treatment of schizophrenic patients. Psychopharmacology Bulletin 1977;13(3):15‐17. [PubMed] [Google Scholar]
Andersen 1974 {published data only}
- Andersen K, D'Elia G, Hallberg B, Perris C, Rapp W, Roman G. The treatment of chronic schizophrenia. Preliminary results of a controlled comparison of pimozide (Orap) with trifluoperazine. Clinical Trials Journal 1971;8(2):72‐6. [Google Scholar]
- Andersen K, d'Elia G, Hallberg B, Perris C, Rapp W, Roman G. A controlled trial of pimozide and trifluoperazine in chronic schizophrenic syndromes. Acta Psychiatr Scand Acta Psychiatr Scand 1974;249 supplement:43‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Angus 1969 {published data only}
- Angus JW, Deutsch LJ, Edwards JG, Simpson GM. A double‐blind comparison of the acridane derivative, clomacran phosphate and trifluoperazine in schizophrenia. Behavioral Neuropsychiatry 1969;1:13‐18. [PubMed] [Google Scholar]
Bagadia 1980 {published data only}
- Bagadia VN, Shah LP, Abhyankar RR. A double‐blind controlled trial of loxapine and trifluoperazine in adolescent schizophrenia. Current Therapeutic Research Clinical & Experimental 1980;27(6):886‐896. [Google Scholar]
Bankier 1968 {published data only}
- Bankier RG, Pettit DE, Bergen B. A comparative study of fluphenazine enanthate and trifluoperazine in chronic schizophrenic patients. Diseases of The Nervous System 1968;29(1):56‐60. [PubMed] [Google Scholar]
Bishop 1963 {published data only}
- Bishop MP, Buddington RW, Robinson WG, Silva F. A comparison of SKF‐7261 and trifluoperazine (Stelazine) in the treatment of chronic schizophrenic patients. Journal of Neuropsychiatry 1963;5:23‐32. [PubMed] [Google Scholar]
Bishop 1964 {published data only}
- Bishop MP, Gallant DM, Nesselhof W, Sprehe DJ. A controlled evaluation of butaperazine in chronic schizophrenic patients. Diseases of the Nervous System 1964;25:674‐83. [PubMed] [Google Scholar]
Bishop 1966 {published data only}
- Bishop MP, Fulmer TE, Gallant DM. Thiothixene versus trifluoperazine in newly‐admitted schizophrenic patients. Current Therapeutic Research Clinical and Experimental 1966;8(11):509‐14. [PubMed] [Google Scholar]
Bishop 1967 {published data only}
- Bishop M, Gallant D. A controlled study of SKF 14336 in acute schizophrenia. Journal of Clinical Pharmacology and the Journal of New Drugs 1967;7(6):342‐7. [DOI] [PubMed] [Google Scholar]
Bishop 1970 {published data only}
- Bishop MP, Gallant DM. Loxapine: a controlled evaluation in chronic schizophrenic patients. Current Therapeutic Research Clinical and Experimental 1970;12(9):594‐7. [PubMed] [Google Scholar]
Brauzer 1968 {published data only}
- Brauzer B, Goldstein BJ. Comparative effects of intramuscular thiothixene and trifluoperazine in psychotic patients. The Journal of Clinical Pharmacology and New Drugs 1968;8(6):400‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brauzer 1971 {published data only}
- Brauzer B, Goldstein BJ. A clinical comparison of molindone hydrochloride with trifluoperazine in psychotic outpatients. Current Therapeutic Research 1971;13(3):152‐7. [PubMed] [Google Scholar]
Claghorn 1974 {published data only}
- Claghorn JL. A double‐blind comparison of pimozide vs. trifluoperazine in schizophrenic outpatients. Current Therapeutic Research Clinical and Experimental 1974;16(9):1005‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Clark 1975 {published data only}
- Clark ML, Paredes A, Costiloe JP, Wood F, Barrett A. Loxapine in newly admitted chronic schizophrenic patients. Journal of Clinical Pharmacology 1975;15(4 Pt 1):286‐94. [DOI] [PubMed] [Google Scholar]
Coons 1962 {published data only}
- Coons WH, Boyd BA, White JG. Chlorpromazine, trifluoperazine and placebo with long‐term mental hospital patients. Canadian Psychiatric Association Journal 1962;7:159‐63. [DOI] [PubMed] [Google Scholar]
Denber 1972 {published data only}
- Denber HC, Turns D. Double blind comparison of thiothixene and trifluoperazine in acute schizophrenia. Psychosomatics 1972;13(2):100‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Donlon 1978 {published data only}
- Donlon PT, Meyer JE. A twelve month comparison of penfluridol and trifluoperazine in chronic schizophrenic outpatients. Journal of Clinical Psychiatry Journal of Clinical Psychiatry 1978;39(6):582‐3, 586‐7. [PubMed] [Google Scholar]
Edwards 1980 {published data only}
- Edwards JG, Alexander JR, Alexander MS, Gordon A, Zutchi T. Controlled trial of sulpiride in chronic schizophrenic patients. British Journal of Psychiatry 1980;137:522‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Freeman 1968 {published data only}
- Freeman H, Oktem N, Oktem MR. A double‐blind study of SKF 14336 vs. trifluoperazine in schizophrenic patients. Current Therapeutic Research Clinical and Experimental 1968;10(10):537‐42. [MEDLINE: ] [PubMed] [Google Scholar]
Gallant 1968 {published data only}
- Gallant DM, Bishop MP. Molindone: a controlled evaluation in chronic schizophrenic patients. Current Therapeutic Research Clinical & Experimental 1968;10(9):441‐7. [PubMed] [Google Scholar]
Gallant 1968 II {published data only}
- Gallant DM, Bishop MP, Bishop G, Steele CA. Thiothixene: a controlled evaluation of the intramuscular antipsychotic preparation. Current Therapeutic Research Clinical and Experimental 1968;10(11):561‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Gallant 1972 {published data only}
- Gallant DM, Bishop MP, Guerrero‐Figueroa R. GP‐45795: a controlled evaluation in chronic schizophrenic patients. Current Therapeutic Research Clinical and Experimental 1972;14(4):215‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Gross 1974 {published data only}
- Gross HS. A double‐blind comparison of once‐a‐day pimozide, trifluoperazine and placebo in the maintenance care of chronic schizophrenic outpatients. Current Therapeutic Research Clinical and Experimental 1974;16(7):696‐705. [MEDLINE: ] [PubMed] [Google Scholar]
Gwynne 1962 {published data only}
- Gwynne PH, Hundziak M, Kavtschitsch J, Lefton M, Pasamanick B. Efficacy of trifluoperazine on withdrawal in chronic schizophrenia. Journal of Nervous and Mental Disease 1962;134:451‐5. [DOI] [PubMed] [Google Scholar]
Kiloh 1976 {published data only}
- Kiloh LG, Williams P, Grant DA, Whetton PS. A double‐blind comparative trial of loxapine and trifluoperazine in acute and chronic schizophrenic patients. Journal of International Medical Research 1976;4(6):441‐8. [DOI] [PubMed] [Google Scholar]
Malik 1980 {published data only}
- Malik SC, Kumar K. Loxapine in adolescent schizophrenia‐ a comparative study with trifluoperazine. Current Therapeutic Research Clinical & Experimental 1980;28(3):432‐46. [Google Scholar]
Marjerrison 1964 {published data only}
- Marjerrison G, Irvine D, Stewart CN, Williams R, Matheu H, Demay M. Withdrawal of long term phenothiazines from chronically hospitalized psychiatric patients. Canadian Psychiatric Association Journal 1964;60:290‐8. [DOI] [PubMed] [Google Scholar]
Marjerrison 1966 {published data only}
- Marjerrison G, Hrychuk W, Varsanyi EL. A comparison of two butirophenones with trifluoperazine. Canadian Psychiatric Association Journal 1966;11(1):26‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Menon 1972 {published data only}
- Menon MS, Ramachandran V. A controlled clinical trial of trifluperidol on a group of chronic schizophrenic patients. Current Therapeutic Research Clinical and Experimental 1972;14(1):17‐21. [PubMed] [Google Scholar]
Needham 1969 {published data only}
- Needham RFH, Blignault WJ. A comparison of an acridan derivative (Smith Kline and French 14336) and trifluoperazine in the treatment of chronic schizophrenia. The Medical Journal of Australia 1969;2(11):550‐3. [DOI] [PubMed] [Google Scholar]
O'Brien 1974 {published data only}
- O'Brien CP, Giacomo JN, Webb W. Management of hostile, suspicious patients: trifluoperazine versus haloperidol. Diseases of the Nervous System 1974;35(2):75‐8. [PubMed] [Google Scholar]
Perales 1974 {published data only}
- Perales JA, Garcia A, Infantes V, Valle G. Comparative study of clotiapine and trifluoperazine in acute paranoid schizophrenia [Estudio Comparativo de la Clotiapina y de la Trifluoperazina en Episodios Agudos de Esquizofrenia Paranoide]. Acta Psiquiatrica y Psicologica de America Latina 1974;20(3):207‐13. [MEDLINE: ] [PubMed] [Google Scholar]
Prien 1969 {published data only}
- Prien RF, Levine J, Cole JO. High dose trifluoperazine therapy in chronic schizophrenia. American Journal of Psychiatry 1969;126:305‐13. [DOI] [PubMed] [Google Scholar]
Ramsay 1970 {published data only}
- Ramsay RA, Ban TA, Lehmann HE, Saxena BM, Bennet J. A comparative study of molindone and trifluoperazine. Current Therapeutic Research 1970;12(7):438‐40. [PubMed] [Google Scholar]
- Ramsay RA, Lehmann HE, Ban TA, Saxena BM, Bennett J. Clinical evaluation of a new psychotropic drug‐Molindone. International Journal of Clinical Pharmacology, Therapy and Toxicology 1970;3(1):46‐8. [PubMed] [Google Scholar]
Reardon 1966 {published data only}
- Reardon JD, Abrams S. Acute paranoid schizophrenia (treatment with chlorpromazine, trifluoperazine and placebo). Diseases of the Nervous System 1966;27(4):265‐70. [MEDLINE: ] [PubMed] [Google Scholar]
Rubin 1971 {published data only}
- Rubin R. A double‐blind comparison of the onset of activity of haloperidol and trifluoperazine. Alabama Journal of Mental Science 1971;8(4):414‐18. [PubMed] [Google Scholar]
Schiele 1961 {published data only}
- Schiele BC, Vestre ND, Stein KE. A comparison of thioridazine, trifluoperazine, chlorpromazine and placebo: A double‐blind controlled study on the treatment of chronic hospitalized schizophrenic patients. Journal of Clinical and Experimental Psychopathology 1961;22:151‐62. [PubMed] [Google Scholar]
Schiele 1969 {published data only}
- Schiele BC, Janecek J, Zimmermann R. A double‐blind comparison of trifluperidol and trifluoperazine in acute schizophrenic patients. Comprehensive Psychiatry 1969;10(5):355‐60. [DOI] [PubMed] [Google Scholar]
Seth 1979 {published data only}
- Seth S, Mahal AS, Kumar KA. A double‐blind comparative trial of loxapine and trifluoperazine in chronic schizophrenic patients. Current Therapeutic Research 1979;25(2):320‐9. [Google Scholar]
Simpson 1976 {published data only}
- Simpson GM, Cuculic Z. A double‐blind comparison of loxapine succinate and trifluoperazine in newly admitted schizophrenic patients. Journal of Clinical Pharmacology 1976;16(1):60‐5. [DOI] [PubMed] [Google Scholar]
Stewart 1969 {published data only}
- Stewart A, Lafave HG, Segovia G. Haloperidol‐new addition to the drug treatment of schizophrenia. Behavioral Neuropsychiatry 1969;1(7):23‐8. [PubMed] [Google Scholar]
Sugerman 1965 {published data only}
- Sugerman AA, Williams BH. Trifluperidol and trifluoperazine in acute schizophrenic patients. Journal of New Drugs 1965;5(5):318‐26. [DOI] [PubMed] [Google Scholar]
Vergara 1977 {published data only}
- Vergara L, Amin MM, Ban TA. A standard (trifluoperazine) controlled clinical study with pimozide in the maintenance treatment of schizophrenic patients, II. Psychopharmacology Bulletin 1977;13(3):17‐19. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Abraham 1987 {published data only}
- Abraham KR, Kulhara P. The efficacy of electroconvulsive therapy in the treatment of schizophrenia. A comparative study. British Journal of Psychiatry 1987;151:152‐5. [DOI] [PubMed] [Google Scholar]
Barsa 1959 {published data only}
- Barsa JA, Saunders JC, Kline NS. Trifluoperazine in the treatment of chronic schizophrenics. American Journal of Psychiatry 1959;115:812. [DOI] [PubMed] [Google Scholar]
Brannen 1969 {published data only}
- Brannen JO, Jewett RE. Effects of selected phenothiazines on REM sleep in schizophrenics. Archives of General Psychiatry 1969;21(3):284‐90. [DOI] [PubMed] [Google Scholar]
Buffaloe 1961 {published data only}
- Buffaloe Wj, Sandifer Mg. A study combined therapy with stelazine and parnate(SKF 385) in chronic anergic schizophrenics. American Journal of Psychiatry 1961;117:1030‐1. [Google Scholar]
Cabrera 1990 {published data only}
- Cabrera GJ, Reyes GB, Fernandez LO, Rojas PM, Molina LM, Perez I, Lopez SP, Apezteguia R. Treatment of schizophrenia with interferon. A double‐blind study [Tratamiento de la Esquizofrenia con Interferon. Estudio a Doble‐Ciegas]. Revista del Hospital Psiquiatrico de la Habana 1990;31(2):251‐68. [Google Scholar]
Calwell 1964 {published data only}
- Calwell WPK, Jacobsen M, Skarbek A. A comparative study of oxypertine and trifluoperazine in chronic schizophrenia ‐ a new application of the Wing Rating Scale. British Journal of Psychiatry 1964;110:520‐30. [DOI] [PubMed] [Google Scholar]
Carscallen 1968 {published data only}
- Carscallen HB, Rochman H, Lovegrove TD. High dosage trifluoperazine in schizophrenia. Comparison of the efficacy of high and usual doses of trifluoperazine in the treatment of chronic schizophrenics. Canadian Psychiatric Association Journal 1968;13(5):459‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Casey 1961 {published data only}
- Casey JF, Hollister LE, Klett CJ, Lasky JJ, Caffey EM. Combined drug therapy of chronic schizophrenics. Controlled evaluation of placebo, dextroamphetamine, imipramine, isocarboxazid and trifluoperazine added to maintanence doses of chlorpromazine. American Journal of Psychiatry 1961;117:997‐1003. [DOI] [PubMed] [Google Scholar]
Childers 1961 {published data only}
- Childers RT, Therrien R. A comparison of the effectiveness of trifluoperazine and chlorpromazine in schizophrenia. American Journal of Psychiatry 1961;118:552‐4. [DOI] [PubMed] [Google Scholar]
Childers 1962 {published data only}
- Childers RT. Procyclidine and benzotropine methanesulfonate compared in drug induced extrapyramidal reactions. American Journal of Psychiatry 1962;1456:462‐3. [DOI] [PubMed] [Google Scholar]
Childres 1962 b {published data only}
- Childers RT. Selective effectiveness of chlorpromazine and trifluoperazine in schizophrenia. Disease of the Nervous System 1962;23:156‐7. [Google Scholar]
Crane 1970 {published data only}
- Crane GE, Chase C. High doses of trifluoperazine and tardive dyskinesia. Archives of Neurology 1970;22:176‐80. [DOI] [PubMed] [Google Scholar]
D'Elia 1974 {published data only}
- D'Elia G, Perris C, Rapp W. Objective evaluation of EEG amplitude in chronic schizophrenic patients during a controlled trial of pimozide and trifluoperazine. Acta Psychiatrica Scandinava Supplementum 1974;249:78‐86. [DOI] [PubMed] [Google Scholar]
Den Boer 2000 {published data only}
- Boer JA, Vahine JO, Post P, Heck AH, Daubenton F, Olbrich R. Ritanserin as add on medication to neuroleptic therapy for patients with chronic or subchronic schizophrenia. Human Psychopharmacology 2000;15(3):179‐89. [Embase 2000170004] [DOI] [PubMed] [Google Scholar]
Dewolfe 1971 {published data only}
- DeWolfe AS, Barrell RP, London L, Spaner FE. Prolixin enanthate and thorazine‐stelazine regimens in the treatment of schizophrenic patients. An experimental evaluation. Psychosomatics 1971;12:186‐90. [DOI] [PubMed] [Google Scholar]
DiMascio 1965 {published data only}
- DiMascio A, Havens LL, Klerman GL. The psychopharmacology of phenothiazine compounds: a comparative study of the effects of chlorpromazine, promethazine, trifluoperazine and perphenazine in normal males‐I. Introduction, aims and methods. Journal of Nervous and Mental Disease 1965;136:15‐28. [PubMed] [Google Scholar]
Eitan 1992 {published data only}
- Eitan N, Levin Y, Ben‐Artzi E, Levy A, Neumann M. Effects of antipsychotic drugs on memory functions of schizophrenic patients. Acta Psychiatica Scandinavica 1992;85(1):74‐6. [DOI] [PubMed] [Google Scholar]
Fish 1966 {published data only}
- Fish B, Shapiro T, Campbell M. Long‐term prognosis and the response of schizophrenic children to drug therapy: a controlled study of trifluoperazine. American Journal of Psychiatry 1966;123(1):32‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Forrester 1958 {published data only}
- Forrester ME. Disturbed chronic psychotic patients: pilot trial of stelazine. British Medical Journal 1958;2:90‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gardos 1968 {published data only}
- Gardos G, rapkin RM, DiMascio A. Trifluoperazine and chlorpromazine in combination and individually. Current Therapeutic Research Clinical and Experimental 1968;10(12):609‐12. [PubMed] [Google Scholar]
Gottfries 1971 {published data only}
- Gottfries CG. Flupenthixol and trifluoperazine: A double‐blind investigation in the treatment of schizophrenics. British Journal of Psychiatry 1971;119:547‐8. [DOI] [PubMed] [Google Scholar]
Guido 1960 {published data only}
- Guido JA, Abe GY. Trifluoperazine: a report of a clinical trial in back ward psychotic patients. American Journal of Psychiatry 1960;117:453‐5. [DOI] [PubMed] [Google Scholar]
Held 1970 {published data only}
- Held JM, Cromwell RL, Frank ET Jr, Fann WE. Effect of phenothiazines on reaction time in schizophrenics. Journal of Psychiatric Research 1970;7(3):209‐13. [DOI] [PubMed] [Google Scholar]
Janecek 1963 {published data only}
- Janecek J, Schiele BC, Bellville T, Anderson R. The effects of withdrawal of trifluoperazine on patients maintained on the combination of tranylcypromine and trifluoperazine: a double‐blind study. Current Therapeutic Research Clinical and Experimental 1963;85:608‐15. [Acc. No. 1965‐00966‐001] [PubMed] [Google Scholar]
Jones 1970 {published data only}
- Jones IH, Davies J, Buckle R, Hanna WH, Pikler N. Comparison of methylperidol and trifluoperazine in the treatment of chronic, underactive schizophrenic patients. Australian and New Zealand Journal of Psychiatry 1970;4:143‐7. [DOI] [PubMed] [Google Scholar]
Jones 1971 {published data only}
- Jones IH, Pikler N. Effects of chlorpromazine and trifluoperazine on the activity of chronic schizophrenics. British Journal of Psychiatry 1971;119:545‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Joshi 1980 {published data only}
- Joshi VG, Eswaran S. Vitamins B1, B6 and B12 in the adjunctive treatment of schizophrenia. Journal of Orthomolecular Psychiatry 1980;9(1):35‐40. [Google Scholar]
Joshi 1982 {published data only}
- Joshi VG, Nagesh R, Eswaran S. Vitamins B1, B6 and B12 in the adjunctive treatment of schizophrenia‐further studies to examine the effect of reduction of chlorpromazine dosage. Journal of Orthomolecular Psychiatry 1982;11(1):45‐9. [Google Scholar]
Kalinin 1986 {published data only}
- Kalinin VV, Rudik VA. The place of insulin coma therapy among modern methods of treatment of paroxysmal schizophrenia patients. Zhurnal Nevropatologii i Psikhiatrii imeni S.S. Korsakova 1986;86(5):745‐9. [PubMed] [Google Scholar]
Kline 1977 {published data only}
- Kline F, Burgoyne RW, Yamamoto J. Comparison of pimozide and trifluoperazine as once‐daily therapy in chronic schizophrenic outpatients. Current Therapeutic Research 1977;21(6):768‐78. [Google Scholar]
Knight 1979 {published data only}
- Knight RG, Harrison A. A double‐blind comparison of thiothixene and trifluoperazine‐chlorpromazine composite in the treatment of chronic schizophrenia. New Zealand Medical Journal 1979;89(634):302‐4. [PubMed] [Google Scholar]
Lal 1974 {published data only}
- Lal S, Ettigi P. Comparison of thiopropazate and trifluoperazine on oral dyskinesia‐ a double‐blind study. Current Therapeutic Research Clinical and Experimental 1974;16(9):990‐7. [PubMed] [Google Scholar]
Lapolla 1965 {published data only}
- Lapolla A. A study of stelazine. Western Medicine the Medical Journal of the West 1965;6(9):240‐5. [PubMed] [Google Scholar]
Lovett 1987 {published data only}
- Lovett WC, Stokes DK, Taylor LB, Young ML, Free SM, Phelan DG. Management of behaviour symptoms in disturbed elderly patients: comparison of trifluoperazine and haloperidol. Journal of Clinical Psychiatry 1987;48(6):234‐6. [PubMed] [Google Scholar]
Luckey 1967 {published data only}
- Luckey WT, Schiele BC. A comparison of haloperidol and trifluoperazine. (A double‐blind controlled study on chronic schizophrenic outpatients). Diseases of the Nervous System 1967;28(3):181‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Macdonald 1959 {published data only}
- Macdonald R, Watts TPS. Trifluoperazine dihydrochloride("Stelazine") in paranoid schizophrenia. British Medical Journal 1959;1:549‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandel 1962 {published data only}
- Mandel W, Evans P. Comparison of butyrylperazine and trifluoperazine in chronic schizophrenic subjects. American Journal of Psychiatry 1962;119:70‐1. [DOI] [PubMed] [Google Scholar]
Mena 1964 {published data only}
- Mena A, Heistad G, Schiele BC, Janecek J. A comparison of tranylcypromine alone with tranylcypromine plus trifluoperazine in the treatment of chronic outpatients: a double‐blind controlled study. Journal of Cardiovascular Nursing 1964;53:542‐50. [Acc. No. 1965‐00966‐001] [PubMed] [Google Scholar]
Monroe 1965 {published data only}
- Monroe RR, Kramer MD, Goulding R, Wise S. EEG activation of patients receiving phenothiazines and chlordiazepoxide. Journal of Nervous and Mental Disease 1965;141:100‐7. [DOI] [PubMed] [Google Scholar]
Montero 1971 {published data only}
- Montero E. Thioridazine compared with a combination of chlorpromazine and trifluoperazine hydrochloride in schizophrenic patients. 5th World Congress of Psychiatry; 1971 Nov 28‐ Dec 4, Ciudad de Mexico, Mexico. 1971:433. [MEDLINE: 72095778]
Morton 1968 {published data only}
- Morton MR. A study of the withdrawal of chlorpromazine or trifluoperazine in chronic schizophrenia. American Journal of Psychiatry 1968;124(11):1585‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moyano 1975 {published data only}
- Moyano CZ. A double‐blind comparison of loxitane‐loxapine succinate and trifluoperazine hydrocloride in chronic schizophrenic patients. Diseases of the Nervous System 1975;36:301‐4. [PubMed] [Google Scholar]
Oybir 1962 {published data only}
- Oybir F. Trifluoperazine in chronic withdrawn schizophrenics (a double‐blind study). Diseases of the Nervous System 1962;23:348‐50. [PubMed] [Google Scholar]
Pecknold 1977 {published data only}
- Pecknold JC, Ban TA, Lehmann HE. A standard (trifluoperazine) controlled clinical study with clomacran in newly admitted schizophrenic patients. Psychopharmacology Bulletin 1977;13(3):14‐15. [PubMed] [Google Scholar]
Polvan 1971 {published data only}
- Polvan N, Akpinar S, Ahmed MB, Itil TM. Different effects of trifluoperazine when administered daytime or night. British Journal of Psychiatry 1971;119(553):601‐2. [DOI] [PubMed] [Google Scholar]
Rudy 1958 {published data only}
- Rudy L, Rinaldi E, Himwich H, Tuteur W, Glotzer J. Trifluoperazine in the treatment of psychotic patients. American Journal of Psychiatry 1958;115:364‐5. [DOI] [PubMed] [Google Scholar]
Schiele 1963 {published data only}
- Schiele BC, Vestre ND. Treatment of hospitalized schizophrenics with trifluoperazine plus tranylcypromine: a double‐blind controlled study. Comprehensive Psychiatry 1963;4(2):66‐79. [DOI] [PubMed] [Google Scholar]
Sharpley 1964 {published data only}
- Sharpley P, Mena A, Schiele BC, Heistad G. A comparison of pargyline and tranylcypromine with and without the addition of trifluoperazine: a double‐blind study. Current Therapeutic Research Clinical and Experimental 1964;15:344‐52. [PubMed] [Google Scholar]
Stanley 1961 {published data only}
- Stanley WJ, Walton D. Trifluoperazine (Stelazine) a controlled clinical trial in chronic schizophrenia. Journal of Mental Science 1961;107:250‐7. [Google Scholar]
Talbot 1964 {published data only}
- Talbot DR. Are tranquilizers combinations more effective than a single tranquilizer?. American Journal of Psychiatry 1964;121:597‐600. [DOI] [PubMed] [Google Scholar]
Teja 1975 {published data only}
- Teja JS, Grey WH, Clum Jm, Warren C. Tranquilizers or anti‐depressants for chronic schizophrenics: A long term study. Australian and New Zealand Journal of Psychiatry 1975;9(4):241‐7. [DOI] [PubMed] [Google Scholar]
Tetreault 1968 {published data only}
- Tetreault L, Filotto J, Bordeleau JM. Neuroleptics and extrapyramidal effects: correlation between antipsychotic and extrapyramidal properties [Neuroleptiques et Effets Extra‐Pyramidaux: Correlation Entre Les Proprietes Antipsychotiques Et Extra‐Pyramidales]. Canadian Psychiatric Association Journal 1968;13(6):507‐12. [DOI] [PubMed] [Google Scholar]
Tolan 1959 {published data only}
- Tolan EJ, Peppel HH. Preliminary observations of trifluoperazine in schizophrenia. American Journal of Psychiatry 1959;115:935. [DOI] [PubMed] [Google Scholar]
Uddyback 1962 {published data only}
- Uddyback OT, Chen Ch. Thioridazine (Mellaril) on regressed schizophrenic patients. American Journal of Psychiatry 1962;119:740‐41. [DOI] [PubMed] [Google Scholar]
Vogt 1961 {published data only}
- Vogt AH. The use of stelazine and parnate in chronic, withdrawn patients. American journal of Psychiatry 1961;118:256‐57. [DOI] [PubMed] [Google Scholar]
Weckowicz 1960 {published data only}
- Weckowicz TE, Ward TF. Clinical trial of "stelazine"on apathetic chronic schizophrenics. Journal of Mental Science(British Journal of Psychiatry from 1963) 1960;106:1008‐15. [DOI] [PubMed] [Google Scholar]
Weston 1961 {published data only}
- Weston FK, Loftus AP. A terminal double‐blind trial of trifluoperazine ("stelazine") in chronic schizophrenia. The Medical Journal of Australia 1961;48(1):776‐80. [PubMed] [Google Scholar]
Wilson 1961 {published data only}
- Wilson IC, Mckay J, Sandifer MG. A double‐blind trial to investigate the effects of thorazine (largactil, chlorpromazine), compazine (stemetil, prochlorperazine) and stelazine (trifluoperazine) in paranoid schizophrenia. Journal of Mental Science 1961;107:90‐9. [DOI] [PubMed] [Google Scholar]
Winnik 1962 {published data only}
- Winnik HZ, Assael M, Gabbai F. Clinical trial of stelazine therapy in schizophrenia refractory to alternative treatments. Dapim Rafuiim 1962;21:161‐68. [PubMed] [Google Scholar]
References to studies awaiting assessment
Abuzzahab 1977 {published data only}
- Abuzzahab FS. The treatment of schizophrenia with long‐acting oral neuroleptics: A six‐month double‐blind investigation of penfluridol versus trifluoperazine. Psychopharmacology Bulletin 1977;13(3):26‐7. [PubMed] [Google Scholar]
Barron 1961 {published data only}
- Barron A, Beckering B, Rudy LH, Smith JA. A double‐blind study comparing RO 4‐0403, trifluoperazine and placebo in chronically ill mental patients. American Journal of Psychiatry 1961;118:347‐8. [Google Scholar]
Bora 1968 {published data only}
- Bora G. Comparison of dosage ranges of carphenazine and trifluoperazine in elderly chronic schizophrenics. Disease of the Nervous System 1968;29(10):695‐7. [PubMed] [Google Scholar]
Cahan 1960 {published data only}
- Cahan RB. Efficacy of trifluoperazine in chronic mental ilness. American Journal of Psychiatry 1960;116:838‐9. [DOI] [PubMed] [Google Scholar]
Claghorn 1969 {published data only}
- Claghorn JL. Psychopharmacologic characteristics of an indole compound‐molindone. Current Therapeutic Research Clinical and Experimental 1969;11(8):524‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Freeman 1969 {published data only}
- Freeman H, Frederick AN. Comparison of trifluoperazine and molindone in chronic schizophrenic patients. Current Therapeutic Research Clinical and Experimental 1969;11(11):670‐6. [PubMed] [Google Scholar]
Gallant 2000 {published data only}
- Gallant. GP‐45795 vs. trifluoperazine. Early Clinical Drug Evaluation Unit Reports 2000.
Gardos 1970 {published data only}
- Gardos G, Finnerty RJ, Cole JO. Thiothixene and trifluoperazine in a step system. Psychosomatics 1970;11(1):36‐40. [DOI] [PubMed] [Google Scholar]
Hamilton 1963 {published data only}
- Hamilton M, Hordern A, Waldrop FN, Lofft J. A controlled trial on the value of prochlorperazine, trifluoperazine and intensive group treatment. British Journal of Psychiatry 1963;109:510‐22. [DOI] [PubMed] [Google Scholar]
Holden 1971 {published data only}
- Holden JMC, Itil TM, Gannon PJ, Keskiner A. The clinical effects of intramuscular thiothixene and trifluoperazine in chronic schizophrenia: a comparative study. Current Therapeutic Research 1971;13(5):298‐310. [PubMed] [Google Scholar]
Hunt 1967 {published data only}
- Hunt PV. A comparison of the effects of the oxypertine and trifluoperazine in withdrawal schizophrenics. British Journal of Psychiatry 1967;113(505):1419‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khorana 1988 {published data only}
- Khorana AB, Patel Y. Comparative short term evaluation on penfluridol and trifluoperazine in chronic schizophrenia. Indian Journal of Physiology and Pharmacology 1988;32:293‐8. [PubMed] [Google Scholar]
Kogeorgos 1995 {published data only}
- Kogeorgos J, Kanellos P, Michalakeas A, Ioannidis J. Sulpiride and risperidone vs. "classical neuroleptics" in schizophrenia: a follow up study. 8th Congress of the European College of Neuropsychopharmacology; 1995 Sep 30‐ Oct 4, Venice, Italy. 1995.
Madgwick 1958 {published data only}
- Madgwick JRA, McNeill DLM, Driver M, Preston GC. Stelazine (trifluoperazine) A preliminary report on a clinical trial. Journal of Mental Science 1958;104:1195‐8. [DOI] [PubMed] [Google Scholar]
Ortega‐Soto 1996 {published data only}
- Ortega‐Soto HA, Brunner E, Apiquian R, Torre MP, Ulloa RE. Typical antipsychotics: the threshold doses strategy. 20th Congress of Collegium Internationale Neuro‐psychopharmacologicum; 1996 june 23‐27, Melbourne, Australia. 1996.
- Ortega‐Soto HA, Brunner E, Apiquian R, Torre P, Ulloa RE, Mendizabal A. Optimal minimum doses of trifluoperazine in acute schizophrenia. 149th Annual Meeting of the American Psychiatric Association; 1996 May 4‐9, New York, USA. 1996.
Platz 1967 {published data only}
- Platz AR, Klett CJ, Caffey EM. Selective drug action related to chronic schizophrenic subtype. Diseases of the Nervous System 1967;28(9):601‐5. [PubMed] [Google Scholar]
Spiegel 1967 {published data only}
- Spiegel DE, Spiegel KP. The effects of carphenazine, trifluoperazine and chlorpromazine on ward behaviour, physiological functioning and psychological test scores in chronic schizophrenic patients. The Journal of Nervous and Mental Disease 1967;144(2):111‐16. [DOI] [PubMed] [Google Scholar]
Svestka 1975 {published data only}
- Svetska J, Nahunek K, Rodova A, Ceskova E. The position of trifluoperazine in the group of neuroleptics (a controlled clinical comparative study). Activitas Nervosa Superior 1975;17(3):208. [PubMed] [Google Scholar]
Terminska 1989 {published data only}
- Terminska K, Mrowiec W. Comparison of influence of perazine, fluphenazine, trifluoperazine, chlorpromazine and haloperidol on primary and deficit schizophrenic symptoms in patients first hospitalized because of paranoid schizophrenia [Badanie porównawcze wplywu perazyny, flufenazyny, trifluoroperazyny, chloropromazyny i haloperydolu na objawy pierwotne i deficytowe pierwszego zachorowania na schizofrenie paranoidalna]. Psychiatria Polska 1989;23:24‐30. [PubMed] [Google Scholar]
Toru 1969 {published data only}
- Toru M, Kobayashi K, Shimazono Y. A double‐blind cross‐over comparison of oxypertine with trifluoperazine in chronic schizophrenia. Rinsyo Igaku 1969;11(10):787‐96. [Google Scholar]
Vinar 1966 {published data only}
- Vinar O, Taussigova D. Clinical experience with trifluoperazine. Activitas Nervosa Superior 1966;8:448‐51. [PubMed] [Google Scholar]
Vinar 1971 {published data only}
- Vinar O, Taussigova D. Thiothixene in schizophrenic psychoses. Activitas Nervosa Superior 1971;13(3):174‐7. [PubMed] [Google Scholar]
Wijsenbeek 1974 {published data only}
- Wijsenbeek H, Steiner M, Goldberg SC. Trifluoperazine: a comparison between regular and high doses. Psychopharmacologia 1974;36:147‐50. [DOI] [PubMed] [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1997
- American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia. American Journal of Psychiatry 1997;154(4):1‐63. [DOI] [PubMed] [Google Scholar]
Arana 2000
- Arana G, Rosenbaum J. Handbook of Psychiatric Drug Therapy. Fourth. Philadelphia, USA: Lippincott Williams & Wilkins, 2000. [Google Scholar]
Ban 1966
- Ban TA. Clinical pharmacology of the phenothiazines. Applied Therapeutics 1966;8(5):423‐7. [PubMed] [Google Scholar]
Bazire 2000
- Bazire S. Psychotropic Drug Directory. New York, USA: Butler and Tanner Limited, 2000. [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637‐9. [DOI] [PubMed] [Google Scholar]
Clarke 2001
- Clarke M, Oxman D. Cochrane Collaboration Handbook. Cochrane Database of Systematic Reviews. Oxford: Update software, 2001, issue 1.
Duggan 2002
- Duggan L, Fenton M, Dardennes RM, El‐Dosoky A, Indran S. Olanzapine for schizophrenia. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001359.pub2] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Zellweger‐Zähner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet 1997;350:326‐9. [DOI] [PubMed] [Google Scholar]
Fenton 2002
- Fenton M, Murphy B, Wood J, Bagnall AM, Chue P, Leitner M. Loxapine for schizophrenia. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD001943.pub2] [DOI] [PubMed] [Google Scholar]
Geddes 2000
- Geddes J, Freemantle N, Harrison P, Bebbington P. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta‐regression analysis. BMJ 2000;321:1371‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Early clinical drug evaluation (ECDEU) assessment manual for psychopharmacology. Washington DC, USA: National Institute of Mental Health, 1976:217‐22. [Google Scholar]
Honingfeld 1965
- Honingfeld G, Klett CJ. The Nurses Observation Scale for Impatient Evaluation (NOSIE): a new scale for measuring improvment in chronic schizophrenia. Journal of Clinical Psychology 1965;21:65‐71. [DOI] [PubMed] [Google Scholar]
Hunter 2003
- Hunter RH, Joy CB, Kennedy E, Gilbody SM, Song F. Risperidone versus typical antipsychotic medication for schizophrenia. Cochrane Database of Systematic Reviews 2003, Issue Issue 4. [DOI: 10.1002/14651858.CD000440] [DOI] [PubMed] [Google Scholar]
Joy 2001
- Joy CB, Adams CE, Lawrie SM. Haloperidol versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003082.pub2] [DOI] [PubMed] [Google Scholar]
Kaplan 1998
- Kaplan H, Sadock B. Pocket handbook of clinical psychiatry. 2nd Edition. New York, USA: Williams & Wilkins, 1998. [Google Scholar]
Luckey 1967
- Luckey WT, Schiele BC. A comparison of haloperidol and trifluoperazine.A double‐blind, controlled study on chronic schizophrenic outpatients. Diseases of the Nervous System 1967;28(3):181‐6. [PubMed] [Google Scholar]
Meltzer 1996
- Meltzer HY. Cost‐efectiveness of clozapine treatment. Journal of Clinical Psychiatry. Monograph Series 1996;14:16‐17. [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D, CONSORT Group. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐91. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Reardon 1989
- Reardon GT, Rifkin A, Schwartz A, Myerson A, Siris SG. Changing patterns of neuroleptic dosage over a decade. American Journal of Psychiatry 1989;146:726‐9. [DOI] [PubMed] [Google Scholar]
Soares 2002
- Soares BGO, Fenton M, Chue P. Sulpiride for schizophrenia. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD001162] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srisurapanont 2002
- Srisurapanont M, Disayavanish C, Taimkaew K. Quetiapine for schizophrenia. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD000967.pub2] [DOI] [Google Scholar]
Thornley 2001
- Thornley B, Adams CE, Awad G. Chlorpromazine versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000284.pub2] [DOI] [PubMed] [Google Scholar]
Wahlbeck 2001
- Wahlbeck K, Cheine M, Essali MA. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000059] [DOI] [PubMed] [Google Scholar]
Wing 1961
- Wing JK. A simple and reliable subclassification of chronic schizophrenia. Journal of Mental Science 1961;107:862‐75. [DOI] [PubMed] [Google Scholar]
Wood Mackenzie 1998
- Wood Mackenzie. Review of the global schizophrenia market. PharmaForum 1998;39:9. [Google Scholar]


