Abstract
Proton-detected solid-state NMR (ssNMR) spectroscopy has dramatically improved the sensitivity and resolution of fast magic angle spinning (MAS) methods. While relatively straightforward for fibers and crystalline samples, the routine application of these techniques to membrane protein samples is still challenging. This is due to the low sensitivity of these samples, which require high lipid:protein ratios to maintain the structural and functional integrity of membrane proteins. We previously introduced a family of novel polarization optimized experiments (POE) that enable to make the best of nuclear polarization to obtain multiple-acquisitions from a single pulse sequence and one receiver. Here, we present the 1H-detected versions of POE using ultrafast MAS ssNMR. Specifically, we implemented proton detection into our three main POE strategies, H-DUMAS, H-MEIOSIS, and H-MAeSTOSO, achieving the acquisition of up to ten different experiments using a single pulse sequence. We tested these experiments on a model compound N-Acetyl-Val-Leu dipeptide and applied to a six transmembrane acetate transporter, SatP, reconstituted in lipid membranes. These new methods will speed up the spectroscopy of challenging biomacromolecules such as membrane proteins.
Keywords: Polarization Optimized Experiments, Multi-acquisition, Solid-State NMR, Ultra-fast Magic Angle Spinning, SIM-CP, H-DUMAS, H-MEIOSIS, H-MAeSTOSO, Membrane Proteins
Graphical abstract
1. INTRODUCTION
Solid-state NMR spectroscopy is a powerful tool to study the structure and dynamics of biomacromolecules that are recalcitrant to crystallization such as fibrillar and integral membrane proteins1–11. In the past decades, heteronuclear detected experiments (direct detection) have represented the main approach to determine resonance assignments and obtain internuclear distances for structure calculations10, 12–16. However, direct detection methods with 13C or 15N acquisition are inherently insensitive and require longer experimental times10, 17. On the other hand, inverse detection (i.e., 1H-detected experiments) has represented the method of choice for solution NMR spectroscopy18. However, strong 1H–1H dipolar couplings, hampered the success of inverse detection for ssNMR applications. This problem has been overcome by the introduction of fast MAS technology, which enabled dramatic sensitivity enhancement for multi-dimensional experiments particularly for microcrystalline and fibrillary protein preparations19–26. To achieve fast spinning rates, it is necessary to use rotors with smaller diameters which reduces the operative sample volumes to a range of 1–4 μl, lengthening significantly acquisition times. For membrane proteins, this problem is exacerbated by the presence of lipids and bulk water 3, 27. While low lipid-to-protein ratios and hydration levels are often used for ssNMR experiments, it is unlikely that membrane proteins maintain their functional and structural integrity under these conditions.
During the past decade, our group has been developing 13C-detected Polarization Optimized experiments (POE) aiming to speed up the spectroscopy of membrane proteins reconstituted with lipid-to-protein ratios close to physiological conditions9, 28–36. Our overall goal has been to adapt ssNMR spectroscopy samples to closely resemble physiological conditions, rather than adapting the samples to the needs of spectroscopy. POE utilize orphan (or undetected) spin operators that are discarded in classical multidimensional NMR experiments and recover them, allowing the acquisition of multiple 2D and 3D experiments in a single pulse sequence29–30, 32. A similar approach has also been utilized for solution NMR experiments (e.g., NOAH sequences) for simultaneous acquisition of 2D spectra of small organic compounds37. Unlike liquid samples, the longitudinal relaxation (T1) times of solids or solid-like biomacromolecules are significantly longer for 15N and 13C nuclei. Therefore, it is possible to store 15N and/or 13C polarization along the longitudinal axis (Nz or Cz) and subsequently utilize it for multiple acquisitions29, 34. The ease of storing the polarization in solids makes POE (e.g., DUMAS, MEIOSIS, and MAeSTOSO) unique tools for simultaneous acquisition of various types of triple-resonance experiments in ssNMR 38. Another key element for POE is the simultaneous cross-polarization (SIM-CP) scheme that matches the Hartmann-Hahn conditions for 1H, 13C, and 15N, simultaneously29–30, 39. The beauty of POE comes from its straightforward implementation on commercial spectrometers equipped with only one receiver and Low-E or E-free MAS probes40–41. As a result, POE are becoming more popular and have been successfully implemented for 1H-detected experiments, including hybrid pulse sequences with a combination of CP and INEPT polarization transfer periods28, 42–46.
In this work, we further expand our POE family and show the implementation of novel and efficient pulse sequences for multi-acquisitions using 1H-detection at ultra-fast magic angle spinning rates of up to 65 kHz. Specifically, we present the 1H-detected versions of the three main POE strategies we previously introduced29, 31, 33, i.e., proton-detected DUMAS, MEIOSIS and MAeSTOSO (H-DUMAS, H-MEIOSIS, and H-MAeSTOSO). While specific experiments are presented in this paper, the DUMAS, MEIOSIS and MAeSTOSO strategies enable one to concatenate several different pulse sequences32. We demonstrate the application of these methods using the standard dipeptide, N-Acetyl-Val-Leu (NAVL), and a six-pass transmembrane protein that is responsible for transporting succinate and acetate in bacteria (succinate-acetate permease or SatP), which was reconstituted in lipid membranes47.
2. EXPERIMENTAL
Uniformly 13C and 15N labeled succinate-acetate permease (SatP) was expressed and purified from E. coli bacteria using a SUMO construct as a fusion. The bacteria were cultured in M9 media enriched with 13C glucose and 15N ammonium chloride as reported previously47. The final sample consisting of approximately 0.8 mg of SatP was reconstituted in 1:40 protein to lipid ratio with 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) liposomes and packed into a 1.3 mm Bruker MAS rotor using our standard protocols.
All the experiments were implemented on a Bruker 600 MHz spectrometer equipped with 1.3 mm fast MAS probe. The CP and SIM-CP spectra reported in Figure 1 were acquired at 12 kHz MAS rate using 20 ms 13C and 15N acquisition times under 100 kHz SPINAL-64 decoupling and a 2 s recycle delay48; whereas all the remaining spectra were acquired using a MAS rate (ωr) of 65 kHz with 1H acquisition time set to 8.3 ms and recycle delay of 2 s. The heat induced by fast spinning on the sample was compensated by lowering the temperature. Specifically, at 65 kHz the temperature of the RF coil was set to −20 °C, which corresponds to an effective sample temperature of 25 °C as measured from the water resonance position. The RF carrier frequencies for 1H, 13C, and 15N were set to 4.7, 40, and 110 ppm, respectively. During 1H acquisition, a 10 kHz WALTZ16 decoupling was applied on 13C or 15N channels49. The 90° pulse length on 1H was set to 1.25 μs, whereas 3 μs 90° pulses were used for 13C and 15N. During CP and SIM-CP, the RF amplitudes on 13C and 15N were set to 40 kHz, and the 1H RF pulse was linearly ramped from 90 to 100% to obtain maximum signal at n=1 Hartmann-Hahn matching condition50. The optimized 1H amplitudes during CP and SIM-CP periods were 51.2 and 102 kHz at MAS rates of 12 and 65 kHz, respectively. The suppression of the 1H signals arising from water and lipids was achieved by phase-switched pulses (MISSISSIPPI)51, with 30 kHz 1H RF amplitude applied for 200 ms (tsupp). For the 2D experiments, the SIM-CP contact time was set to 750 μs. The durations of back CP transfers from 13C to 1H (TCCP) and 15N to 1H (TNCP) were set to 150 and 200 μs, respectively. The SPECIFIC-CP transfer between 15N and 13CA was obtained by using a linear ramp (85 to 100%) pulse on 13C applied at 60 ppm and a constant amplitude pulse on 15N52. The optimized RF amplitudes for SPECIFIC-CP on 13C and 15N were 18.3 and 43.3 kHz, respectively. During t1 evolution, 10 kHz WALTZ16 decoupling was applied on 1H. Simultaneous (or parallel) 13C and 15N t1 evolution periods were synchronized by using 100 13C t1 increments and two sets (n=2 in Figures 2, 4, 7 and 8) of 50 t1 increments for 15N29. The dwell-times for 13C and15 N were set to 100 and 200 μs that corresponds to a maximum t1 evolution of 10 ms for both 13C and 15N. A four-step phase cycle was applied for all the pulse sequences with ϕ1= y,-y, ϕ2= x,x,−x,−x, and receiver phase ϕrec=x,-x,-x,x. The t1 dimension of 2D spectra were acquired in States mode by switching the phase of ϕ* pulse between y and −x. For the 1D data reported in Figure 1, 13C CP and SIM-CP spectra were acquired with 32 scans, and 15N CP and SIM-CP spectra were acquired with 128 scans. 13C-detected 1D MEIOSIS spectra of Figure 3 were acquired with 256 scans using 20 ms 13C acquisition time under 10 kHz 1H WALTZ decoupling. Proton 1D spectra in Figure 5 were acquired with 16 scans. The H-DUMAS (Figure 2), H-MAeSTOSO-4 (Figure 7) spectra on SatP were acquired using 16 and 40 scans, respectively, whereas the H-MEIOSIS (Figure 4) spectrum was acquired with 40 scans and two interleaved data sets obtained with ϕ=x and −x. The total experimental times for SatP 2D H-DUMAS, H-MEIOSIS, and H-MAeSTOSO4 spectra in Figures 2, 4, and 7 were 2, 10, and 5.3 hrs, respectively. The 2D H-MAeSTOSO-10 spectra of NAVL in Figure 8 were acquired using 24 scans for a total experimental time of 6.2 hrs. During HH RFDR, 1H 180° pulse length was set to 2.5 μs. Mixing times for HH RFDR were set to 0.74 or 1.47 ms53, whereas the CC TOCSY mixing (or TCWALTZ) time in Figure8 was set to 9.6 ms. All the spectra were processed using NMRpipe scripts as described in references34, 54 for multiple acquisition data sets. Both t1 and t2 dimensions were processed with 90° shifted sine bell window functions.
Figure 1:
(A) CP and SIM-CP pulse sequences used for quantifying relative signal intensities. For SIM-CP, 13C and 15N spectra were acquired in separate experiments with a single receiver. (B) and (C) Comparison of CP and SIM-CP on SatP membrane protein obtained from integrated intensities of 13C and 15N spectra acquired at 65 kHz and 12 kHz MAS rates, respectively.
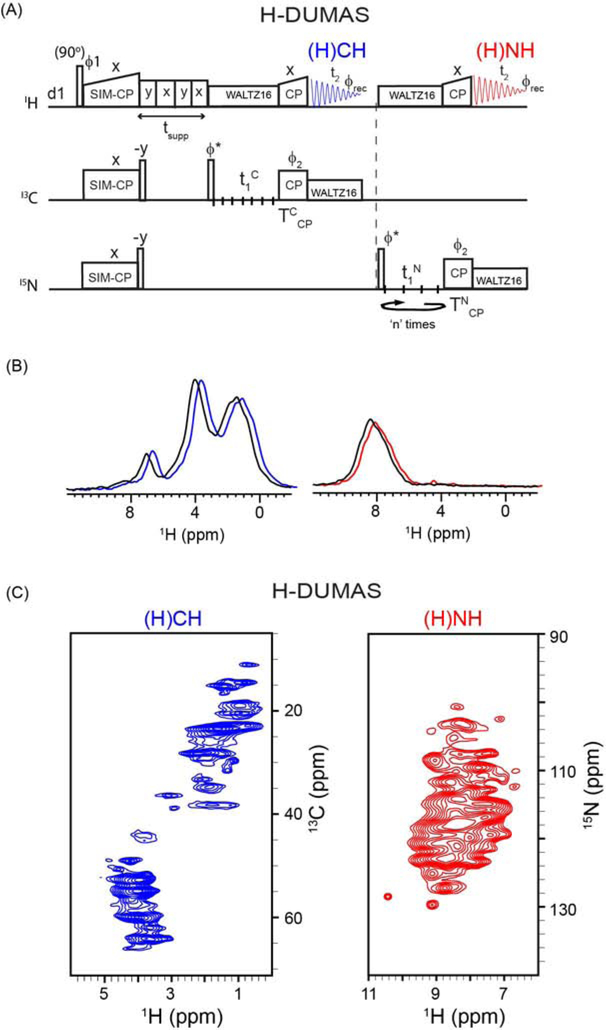
Figure 2:
(A) Pulse sequence for two-dimensional H-DUMAS (proton detected DUMAS) experiment for simultaneous acquisition of 13C- and 15N-edited experiments, (H)CH and (H)NH. (B) 1D (H)CH and (H)NH spectra of SatP membrane protein obtained from first increment (t1=0) of H-DUMAS (shown in blue and red), and the corresponding spectra (shown in black) obtained from single acquisition methods using 65 kHz MAS rate. For comparison the 1D spectra shown in blue and red are slightly shifted to the right. (C) Two-dimensional (H)CH and (H)NH spectra of SatP protein acquired using H-DUMAS pulse sequence with n=2 loops for 15N t1 evolution.
Figure 4:
(A) Two-dimensional H-MEIOSIS (proton detected MEIOSIS) pulse sequence for simultaneous acquisition of two pairs of 13C- and 15N-edited experiments, (H)CH, (H)N(CA)H, (H)NH and (H)CA(N)H. (B) 2D H-MEIOSIS spectra of SatP protein acquired at 65 kHz MAS rate using two loops (n=2) for 15N t1 evolution. A pair of 2D spectra in the 1st and 2nd acquisitions are obtained by adding and subtracting the two data sets recorded with ϕ=x and −x.
Figure 7:
(A) Pulse sequence for the H-MAeSTOSO-4 experiment for simultaneous acquisition of (H)CHH, (H)NHH (RFDR=1.47 ms), H(N)HH (RFDR=0.74ms), and (H)NH experiments. Corresponding 2D spectra on SatP membrane protein are shown in (B). Suppression of 1H signals from water and lipids is obtained by using tsupp=200ms. Prior to 3rd and 4th acquisitions 0.25*tsupp (=50 ms) period was used for eliminating waters signals. (C) 2D spectra (H)NHH and (H)NH obtained from H-MAeSTOSO-4 pulse sequence in the absence of 0.25*tsupp period, showing the residual water signals.
Figure8:
(A) Pulse sequence for the H-MAeSTOSO-10 experiment for simultaneous acquisition of ten 2D spectra. (B) Application of H-MAeSTOSO-10 on the NAVL (N-acetyl-Val-Leu) dipeptide.
Figure 3:
MEIOSIS experiment at fast MAS rates. (A) Pulse sequence for 1D MEIOSIS that records four polarization pathways (CCres, NCAtrans, NNres, and CANtrans) resulting from the first SPECIFIC-CP period τ. (B) Normalized integrated intensities of all four pathways measured for SatP protein by varying the τ mixing times. The polarization in each pathway is normalized with respect to initial value at τ=0. (C) 1D spectra of CCres, NCAtrans, NNres, and CANtrans (color coded accordingly) polarization pathways obtained at τ= 0 and 6.5 ms. (D) 1D spectra at τ=6.5 ms with corresponding intensities normalized with respect to highest intensity spectrum of CC polarization pathway.
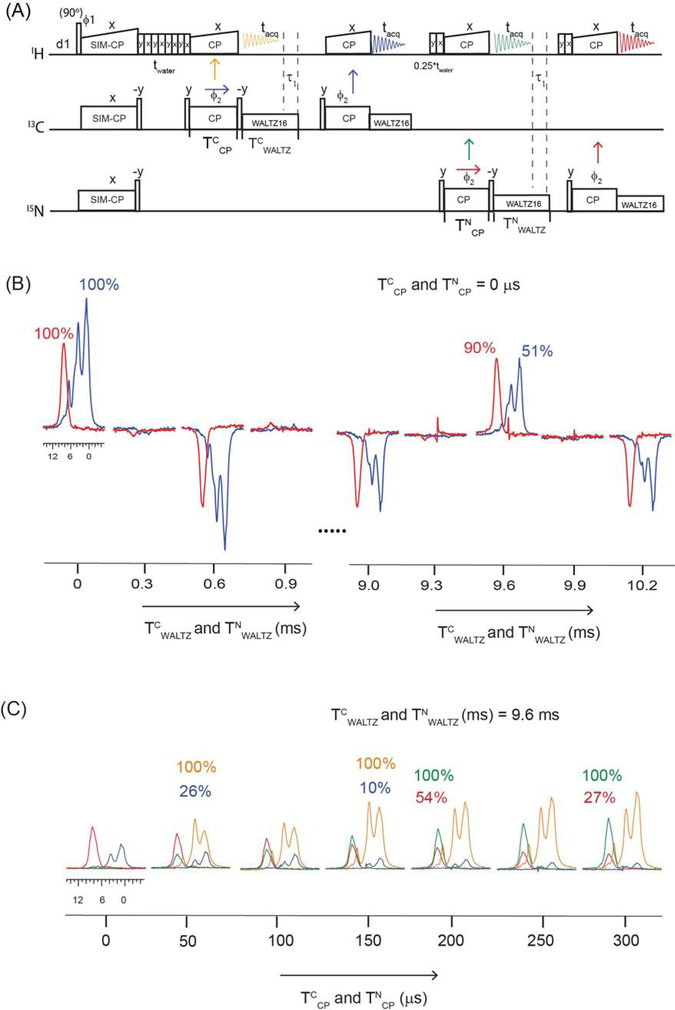
Figure 5:
(A) Pulse sequence for evaluating the residual 13C and 15N polarization that remains on 13C and 15N at the end of back CP periods (TCCP and TNCP). (B) Membrane protein SatP spectra acquired in 2nd and 4th acquisition periods by varying TCWALTZ and TNWALTZ decoupling periods. (C) Transferred and residual polarization of 13C–1H and 15N–1H back CP periods measured by varying TCCP and TNCP back CP periods.
To avoid the sharing of WALTZ16 pulse profile, we recommend using different names for each WALTZ16 period in the pulse sequences. Bruker TOPSPIN pulse programming allows up to nine CPD names (CPD1, CPD2…..CPD9) that can be recalled during the decoupling periods of a pulse program. For example, in Figures 5A and 5B the duration of TCWALTZ on 13C (or TNWALTZ on 15N) channels was varied from 0 to 10.2 ms followed by back-CP and another 13C (or 15N) WALTZ16 period. If the two 13C WALTZ16 decoupling sequences (1’st and 2nd acquisitions of Figure 5A) on 13C channel are used in a pulse sequence with the same name, then the shape or pulse profile of WALTZ16 is shared between two periods depending on the length of each period. An alternative way is to have the WALTZ16 sequence to complete an entire cycle, setting ‘n’ cycles of WALTZ16 (= 2.4, 4.8, 7.2, and 9.6 ms). In this case, the WALTZ16 profile for two periods do not overlap.
3. RESULTS
3.1. Simultaneous cross-polarization (SIM-CP)
In POE, the initial 13C and 15N polarization is obtained from SIM-CP, where both 13C and 15N RF amplitudes (ω) are simultaneously Hartmann-Hahn matched to 1H RF (ωH)29, 32. For a given MAS rate (ωr), the 13C and 15N RF amplitudes satisfy the matching condition ωH=n·ωr ±ω, where n=1 and 2 are known as 1st and 2nd sideband Hartmann-Hahn matching conditions. Figure 1 shows the systematic comparison of CP and SIM-CP intensities for the membrane protein SatP at fast (65 kHz) and slow (12 kHz) MAS rates using separate 13C- and 15N-detected experiments (Figure 1A) with a single receiver. At 65 kHz MAS rate, heteronuclear 1H decoupling was obtained by 10 kHz WALTZ16 pulses; whereas at 12 kHz MAS rate the 1H RF was set to 100 kHz for SPINAL64 proton decoupling. During CP and SIM-CP contact time (t), 13C and 15N RF amplitudes (ω) were set to 40 kHz, and 1H RF was optimized to satisfy the first or n=1 sideband matching condition (ωr+ ω), which corresponds to 52 kHz and 105 kHz at MAS rates of 12 and 65 kHz, respectively. The 1H radio frequency (RF) was linearly ramped from 90 to 100%. The integrated intensities for 13C and 15N spectra were calculated for spectral regions spanning from 0 to 80 ppm and 95 to 140 ppm, respectively. As shown in Figure 1B and C, the 13C intensities for SIM-CP follow a trend similar to the conventional CP for both 65 and 12 kHz MAS rates. The average intensity loss with the 15N SIM-CP experiments are approximately 15 and 8% for 12 and 65 kHz spinning rates, respectively, with contact times ranging from 0.3 to 0.8 ms. Note that the data reported in Figure 1 are recorded under identical sample conditions using a 1.3 mm MAS probe. The effective sample temperatures for SatP at 12 and 65 kHz were set to 25 °C and monitored using the 1H peak position of the water signal. The relative intensities of CP and SIM-CP spectra at 12 kHz MAS are in close agreement with our previous results obtained for crystalline and membrane protein samples using a 3.2 mm probe with MAS rates of 8 and 12 kHz29–30. Interestingly the 15N SIM-CP scheme is more efficient at faster MAS rates. At contact times greater than 1 ms, the performance of SIM-CP at 65 kHz MAS gradually decreases with an average intensity loss of approximately 15% with respect to CP. Due to the complexity of 1H–1H dipolar coupling network, it is difficult to quantify the CP and SIM-CP dynamics. However, it is possible to speculate that the increased efficiency of 15N SIM-CP at lower contact times and higher MAS rates may originate from more localized polarization transfer from the directly bonded amide protons to the 15N atoms. In fact, at 65 kHz MAS rate we observe a more oscillatory buildup for the 15N polarization (Figure 1B). In contrast, at 12 kHz MAS rate the oscillations of the 15N polarization are significantly damped by the strong 1H–1H dipolar couplings (Figure 1C).
3.2. 1H-detected DUMAS (H-DUMAS): simultaneous acquisition of (H)CH and (H)NH experiments
In the original implementation of DUMAS (DUal acquisition Magic Angle Spinning) experiment29, the 13C and 15N polarization from SIM-CP was used to acquire 2D 13C- and 15N-edited experiments such as DARR and NCA with 13C detection at slow MAS rates (8–12 kHz). Analogously, the H-DUMAS experiment enables the acquisition of 2D 1H-detected 13C- and 15N- edited experiments (Figure 2A). After SIM-CP transfer from 1H to 13C and 15N, a pair of 90° pulses on 13C and 15N flips the polarization to the z-direction followed by a water suppression period. A 90° pulse on 13C brings the polarization to the transverse plane, which evolves during t1 followed by a back CP period (TCCP) from 13C to 1H. The 1H magnetization is then detected during the first t2 acquisition that records a 2D (H)CH experiment (blue). After the first acquisition, a 90° pulse on 15N flips the polarization to the transverse plane, which evolves during 15N t1 followed by an 15N–1H back CP (TNCP) and a second t2 acquisition period that records a 2D (H)NH spectrum. During the t1 and t2 evolution periods, heteronuclear decoupling is achieved by a WALTZ16 pulse sequence49.
An important feature of the DUMAS experiments is the distinct t1 evolution periods for 13C and 15N29, which enables to optimize 13C and 15N t1 evolution parameters independently. Therefore, the t1 evolution time for the 13C-edited (H)CH experiment was set with a maximum evolution time of 10 ms, with a dwell time of 100 μs and 100 increments. On the other hand, the t1 evolution time for the 15N-edited (H)NH experiment was divided into two identical t1 evolution periods with 50 increments each corresponding to a maximum t1 evolution period of 10 ms and a dwell time of 200 μs (n=2, Figure 2A). As described previously29, 34, 38, the two data sets for the (H)NH experiment are added post acquisition during the data processing. In this manner, the effective number of scans for the (H)NH is double that of the (H)CH experiment. Figure 2B displays the 1D spectra of SatP obtained from the first increment (t1 = 0) of the H-DUMAS-(H)CH-(H)NH experiment shown in blue and red, respectively. As a comparison, the single-acquisition spectra obtained from the conventional (H)CH and (H)NH pulse sequences are overlaid in black. As expected, the intensity of the H-DUMAS-(H)CH spectrum is identical to the conventional (H)CH. On the other hand, for the H-DUMAS-(H)NH spectrum shows an intensity loss of approximately 5%, which is expected from the lower performance of the SIM-CP scheme. Figure 2C shows the application of the 2D H-DUMAS-(H)CH-(H)NH experiment to SatP acquired simultaneously at 65 kHz for a total experimental time of ~2 hours. Although 1H resolution at 65 kHz MAS rate is still challenging for larger proteins such as SatP, we anticipate that it is possible to further improve sensitivity and resolution using a combination of 3D experiments, higher MAS rates (100–110 kHz) as well as sparse protein deuteration55. Note that the acquisition of 2D (H)NH and (H)CH spectra at fast MAS rates can also be obtained using single acquisition as reported in references42, 44 at slow and fast MAS rates. The H-DUMAS experiment is more flexible and enables to choose different TCCP and TNCP back CP periods and optimize sensitivity and resolution of the 1H spectra. Similar to 13C detected DUMAS experiments, H-DUMAS can easily be extended to the third dimension with homo- or hetero-nuclear (CC, HH or NC) mixing times.
3.3. 1H detected MEIOSIS (H-MEIOSIS): simultaneous acquisition of (H)CH, (H)N(CA)H, (H)CA(N)H and (H)NH experiments
We previously demonstrated that when a given 13C- or 15N-edited pulse sequence includes NC or CN polarization transfer (SPECIFIC-CP) periods, it is possible to nest into it three additional experiments using the MEIOSIS (Multiple ExperIments via Orphan SpIn operatorS) strategy31. To implement this for proton detection, we first investigated the relative intensities of the polarization pathways using 13C-detected MEIOSIS experiments at fast MAS rates (Figure 3A). In this case, the preparation period (SIM-CP) is followed by SPECIFIC-CP that creates two bidirectional polarization transfer pathways from 15N to 13C and vice versa named NCAtrans and CANtrans, respectively. The residual 13C and 15N polarization pathways (i.e., CCres and NNres) remain on 13C and 15N at the end of SPECIFIC-CP period31. The polarization from the CCres and NCAtrans pathways is recorded in the first acquisition; whereas the 15N polarization from NNres and CANtrans pathways is transferred to 13C via SPECIFIC-CP and is recorded in a second acquisition period. For each acquisition, the decoding of two pathways is obtained by altering the phase ϕ of 15N SPECIFIC-CP pulse between x and −x, and the resultant data sets are stored in two separate files. The phase ϕ inverts the transferred polarization (CNtrans and NCtrans,), whereas the CCres and NNres are not affected by flipping ϕ31. In the first acquisition, CCres and NCAtrans are decoded by adding and subtracting the two data sets recorded with ϕ= x and −x. Similarly, addition and subtraction of two data sets of the second acquisition decodes both CANtrans and NNres pathways.
Figure 3B shows the 1D normalized intensities of SatP protein spectra for all four pathways measured at 65 kHz. SPECIFIC-CP mixing times (τ) were arrayed from 0 to 8 ms. The optimal NCA and CAN transfer is obtained at a mixing time of 6.5 ms. Figure 3C shows the 1D spectra corresponding to four pathways obtained at 0 and 6.5 ms SPECIFIC-CP mixing times. The residual 13C and 15N polarization at optimal NCA or CAN mixing time (~ 6.5 ms) corresponds to approximately 52 and 40% of the normalized signal as previously shown for a 3.2 mm probe at slower MAS rates31. Note that the T1ρ relaxation times for 13C and 15N during SPECIFIC-CP are relatively longer at faster MAS rates and the transfer efficiency for NCA and CAN can vary significantly based on the MAS rates. As a result, the extent of residual polarization depends on the net effect of T1ρ and SPECIFIC-CP transfer efficiencies for a given MAS rate.
The spectra of Figure 3 were recorded using 256 scans with ϕ phase switching between x and −x, which corresponds to 512 effective scans. If the signal-to-noise achieved with ‘n’ scans is ‘So’, then k * So signal-to-noise requires ‘k2 * n’ scans. Therefore, using conventional single acquisition methods (τ=0) to obtain 52% (k=0.52) 13C CP signal it would take 128 scans (k2*512), which corresponds to approximately one fourth of the experimental time. Similarly, to obtain 40% (k=0.4) NCA signal, it would require 82 scans (k2*512), which corresponds to one sixth of the experimental time. The experimental time for 1D MEIOSIS using 512 scans and τ=6.5 ms is 18 minutes (Figure 3C). Using conventional, single acquisition methods, NCA and CAN 1D spectra require 36 minutes, whereas to obtain 52% 13C CP and 40% NCA signal it would take 4.5 and 3 minutes respectively. In other words, to achieve similar signal intensities, it would require approximately 43.5 minutes with conventional methods; whereas the MEIOSIS experiment would only take 18 minutes, with a net time saving of ~59%. Note that in Figures 3B and 3C, each of the four pathways are normalized separately to show gains and losses of transferred and residual polarization intensities with respect to the initial value at τ=0. For setting up the 2D MEIOSIS experiment, it is important to consider the relative intensities of the four 1D spectra. Figure 3D shows the relative intensities of four MEIOSIS pathways for a SPECIFIC-CP period of 6.5 ms calculated by integrating the intensity values between 45 to 75 ppm. The residual CC pathway shows the highest intensity, which was normalized to 100%, and the relative intensities of NCA, NN, and CNC spectra, corresponding to 41%, 18%, and 17% respectively.
Similar to 13C-detected MEIOSIS experiments at slow MAS rates, the four polarization pathways can be used to design various combinations of H-MEIOSIS experiments at fast MAS rates38. Figure 4A shows one example of two-dimensional H-MEIOSIS, where 13C and 15N t1 evolution is followed by SPECIFIC-CP to create CCres, NCAtrans, CANtrans, and NNres pathways to record (H)CH and (H)N(CA)H spectra in the first acquisition, and (H)CA(N)H and (H)NH spectra in the second acquisition. The 13C and 15N t1 evolutions of H-MEIOSIS are synchronized by using two loops (n=2) for 15N t1 evolution. By addition and subtraction of two data sets acquired with phase ϕ set to x and −x, it is possible to decode the two pathways for the first and second acquisitions. Figure 4B shows four 2D spectra [(H)CH, (H)N(CA)H, (H)CA(N)H, and (H)NH] of SatP acquired simultaneously using H-MEIOSIS. The total experimental time was approximately 10 hours. The 2D spectra (H)CA(N)H and (H)N(CA)H are obtained from transferred polarization pathways (CNtrans and NCtrans) under optimal SPECIFIC-CP conditions; while the 2D (H)CH and (H)NH spectra are obtained from 40–50% orphan polarization. Thus the relative intensities of (H)CH and (H)NH spectra are 40–50% lower than the corresponding H-DUMAS or single acquisition spectra. Note that the peaks in the 2D (H)N(CA)H and (H)CA(N)H experiments have lower intensity due to NCA (or CAN) transfer period, and thus require higher number of scans. The latter produces sufficient residual polarization that enables the acquisition of the additional (H)CH and (H)NH spectra. In this way, using the H-MEIOSIS strategy we combined a pair of low sensitive experiments [(H)N(CA)H and (H)CA(N)H] with another pair of high sensitive experiments [(H)NH and (H)CH].
3.4. How much residual 13C and 15N polarization remains after back CP transfer periods? Can it be used for additional experiments?
To quantify the residual 13C and 15N polarization at the end of back CP periods (TCCP and TNCP), we modified the H-DUMAS experiment. The 1D pulse sequence reported in Figure 5A consists of four acquisition periods, where the first and third FIDs (orange and green) correspond to H-DUMAS polarization pathways obtained from SIM-CP. After 13C–1H and 15N–1H back CP periods, a 90° pulse on 13C and 15N stores the residual polarization along the z-direction followed by 13C and 15N WALTZ16 decoupling periods, TCWALTZ and TNWALTZ, respectively. After a WALTZ16 decoupling period, the longitudinal 13C and 15N residual polarization is transferred to 1H by applying a 90° pulse followed by another 13C–1H and 15N–1H back CP period that records aliphatic (HC) and amide (HN) proton spectra. In this way, it is possible to quantify the net residual polarization by varying TCP and TWALTZ decoupling periods. Typically, for fully protonated samples under 40–100 kHz MAS rates the back-CP are set to 100 to 400 μs and the 13C and 15N WALTZ16 decoupling periods are set between 7 and 10 ms. The H-DUMAS and H-MEIOSIS spectra reported in Figures 2 and 4 were obtained using 13C–1H and 15N–1H back CP periods set to 150 and 200 μs respectively; whereas t2 WALTZ16 decoupling on 13C and 15N was set to 8.3 ms.
We first evaluated the effect of WALTZ16 decoupling pulses on 13C and 15N residual z-polarization during TWALTZ (TCWALTZ and TWALTZ). Figure 5B shows HC (blue) and HN (red) 1D spectra of SatP protein acquired at various TWALTZ periods with TCP (TCCP and TNCP) set to zero. Note that the second back-CP periods prior to 3rd and 4th acquisitions were set to a fixed value of 200 μs. The RF amplitudes of both 13C and 15N WALTZ16 pulses were set to 10 kHz, corresponding to a 25 μs 90° pulse. The WALTZ16 decoupling is based on composite rotation and consists of ninety-six 90° pulses with four phase switched Q blocks, namely, 49. The total time of WALTZ16 cycle corresponds to 2.4 ms (= 96 × 25 μs). Whereas each Q unit is a spin inversion sequence with twenty-four 90° pulses that corresponds to 600 μs (=24×25μs). The patterns of the 1D spectra in Figure 5B show a remarkable agreement with the WALTZ16 cycle. The latter demonstrates that the residual 13C and 15N z-polarization can be preserved at the end each Q unit (i.e., at multiple values of 600 μs) as well as 2.4, 4.8, 7.2, and 9.6 ms WALTZ16 periods, which correspond to 1, 2, 3, and 4 WALTZ16 cycles. Therefore, the residual 13C and 15N polarization can be recovered even in the presence of 13C and 15N decoupling pulses by synchronizing the total length of TWALTZ to multiples of the (n/4) of cycle time, where ‘n’ is an integer. As shown in Figure 5B, the loss of 15N signal intensity is only 10% even after a WALTZ16 period of 9.6 ms. On the other hand, the WALTZ16 period on 13C acts as homonuclear TOCSY mixing, which causes ~ 50% of signal loss for TCWALTZ of 9.6 ms.
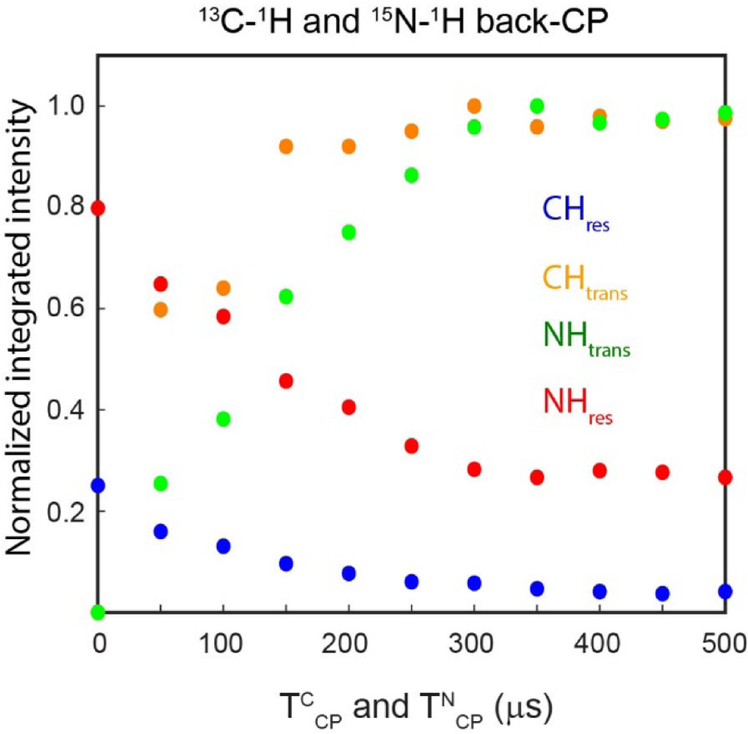
We then evaluated the effect of back-CP or TCP (TCCP and TNCP) periods on the four pathways, namely, 13C–1H and 15N–1H transferred polarization pathways (orange and green, Figure 5A) detected in the 1st and 3rd acquisitions, and 13C and 15N residual pathways (blue and red) detected in 2nd and 4th acquisitions. The four acquisition periods were set to 8.3 ms. To preserve the residual polarization after 1st and 3rd acquisitions, the WALTZ16 decoupling was set on for another 1.3 ms (τ1 in Figure 5A) to complete four WALTZ16 cycles for a total duration of TCwaltz and TNWALTZ periods of 9.6 ms. When TCP is set to zero, no signal was observed for the 1st and 3rd acquisitions, due to the absence of 13C–1H and 15N–1H back CP transfer (Figure 5C). On the other hand, when TCP is zero, full 13C and 15N polarization is used for residual pathways, showing maximum signal intensities. At higher TCP values, efficient 13C–1H and 15N–1H transfers lead to maximum intensities for 1st and 3rd acquisitions, whereas residual polarization (2nd and 4th acquisitions) gradually decreases. Typically, for U-13C,15N labeled proteins selective transfer from 13C to 1H is is obtained by using 100 to 200 μs back CP period. Whereas 15N to 1H back CP transfer is obtained by using the TCP values between 200 to 500 μs21. As shown in Figure 4C, at TCCP value of 150 μs, we observed approximately 10% 13C residual polarization. On the other hand, we observed 54 and 27% 15N residual polarization for TNCP values of 200 and 300 μs, respectively. Figure 6 shows the normalized intensities for the HC and HN spectra illustrated in Figure 5C. The transferred and residual polarization from the CH back-CP are CHtrans and CHres, respectively. Whereas NH back-CP transferred and residual polarization are represented by NHtrans and NHres, respectively. Note that the efficiency of transferred and residual polarization pathways of back-CP periods depends on sample conditions as well as the MAS rate. The NH back-CP periods of 400 to 600 μs were reported for crystalline proteins with fast MAS rates and perdeuteration sample conditions. On the other hand, for SatP protein, the back-CP transfer efficiency reached a plateau at 300 μs.
Figure 6:
Normalized integrated intensities of transferred and residual polarization pathways of 13C–1H and 15N–1H back CP obtained from the spectra reported in Figure 5C using the pulse sequences of Figure 5A.
3.5. 1H detected MAeSTOSO-4 (H-MAeSTOSO-4): simultaneous acquisition of four experiments
The 13C and 15N residual polarization of the back-CP periods can be used to implement the experiments based on the MAeSTOSO (Multiple Acquisitions via Sequential Transfer of Orphan Spin polarizatiOn) strategy33. The 2D pulse sequence for H-MAESTOSO-4 is obtained by extending the 2D H-DUMAS pulse sequence for four acquisition periods as shown in Figure 7A. As in H-DUMAS, the 13C and 15N polarization from SIM-CP is used for recording 13C- and 15N-edited experiments in the 1st and 2nd acquisitions, respectively, where a 1H–1H RFDR period was incorporated for homonuclear transfer between 1H nuclei prior to the second acquisition period. The resulting signal in the 1st and 2nd acquisitions corresponds to the (H)CHH and (H)NHH 2D spectra, respectively. The residual 15N polarization after the first 15N–1H back-CP (TNCP) is stored along the z-direction by applying a 15N 90° pulse followed by WALTZ16 decoupling. As shown in Figure 5, the total length of the TNWALTZ period can be adjusted to 9.6 ms to retrieve the residual 15N polarization. Similarly, in the H-MAeSTOSO-4 pulse sequence the TNWALTZ period is set to 9.6 ms. After the TNWALTZ period, the 15N residual polarization is transferred to 1H followed by a 1H–1H RFDR transfer 53 that records another (H)NHH experiment in the third t2 acquisition. After the 3rd acquisition, the 15N residual polarization resulting from second 15N–1H back CP is transferred to 1H followed by a 4th acquisition that records a (H)NH 2D experiment. At TNCP of 200us, The residual 15N polarization after the first 15N–1H CP is approximately 54%, which is utilized in the 3rd acquisition of the H-MAESTOSO-4 sequence. On the other hand, the residual polarization after the second 15N–1H CP becomes approximately 29% (54% of 54% of the polarization), which is recorded in the 4th acquisition period. Note that in Figure 7, for TNCP value of 300 μs, the signal intensities of the 3rd and 4th acquisitions become 27 and 7 %, respectively, due to lower residual polarization (Figure 5c). To increase the signal intensities of 3rd and 4th acquisitions, a lower TNCP duration (equal to 200 μs) was used, which reduces the polarization transfer for the 2nd acquisition.
Figure 7B shows the SatP 2D spectra (H)CHH (RFDR=1.47 ms), (H)NHH (RFDR=1.47 ms), (H)NHH (RFDR=0.74 ms), and (H)NH acquired simultaneously using the H-MAeSTOSO-4 pulse sequence (Figure 7A) with a total experimental time of 5.3 hours. At 1.47 ms RFDR mixing, intense cross peaks were observed between HN and HC groups, whereas 0.74 ms mixing resulted in more resolved peaks, particularly for the aliphatic proton region (0 to 5 ppm). The HH cross peaks in the 15N-edited spectra recorded at two mixing times can be compared with the reference (H)NH spectrum recorded in the 4th acquisition. During the execution of these pulse sequences, some of the water signal can interfere with the 2D spectra acquisition. Therefore, the spectra in Figure 7B were acquired with two additional water suppression periods (0.25*tsupp in Figure 7A) set to 50 ms, prior to second and third 15N–1H back CP periods. As shown in Figure 7C, in the absence of second and third water suppression periods (0.25*tsupp in Figure 7A) we observed a weak water peak signal in (H)NHH (RFDR=0.74 ms) and (H)NH 2D spectra acquired in 3rd and 4th acquisitions respectively. Note that the solvent suppression element (tsupp) can also be incorporated prior to TNCP periods, which may improve water suppression as well as lipid signal intensities57. Similarly, a constant time version can be used keeping the total number of 1H pulses constant for all t1 increments, which will improve water suppression19, 21.
3.6. 1H detected MAeSTOSO-10 (H-MAeSTOSO-10): simultaneous acquisition of ten experiments
By exploiting the 13C–1H and 15N–1H back CP residual polarization pathways, we also designed the H-MAeSTOSO-10 sequence for simultaneous acquisition of ten 2D spectra using five acquisition periods. The H-MAeSTOSO-10 (Figure8) experiment records one pair of 13C- and 2D 15N-edited spectra in each of the five acquisition periods by addition and subtraction of data sets recorded with phase ϕ = x and −x. The 1st and 3rd acquisitions are respectively obtained from H-MEIOSIS pathways (CCres, NCAtrans, CANtrans, and NNres) followed by HH RFDR mixing periods to record (H)CHH and (H)N(CA)HH spectra in the 1st acquisition, and (H)CA(N)HH and (H)NHH spectra in the 3rd acquisition. During the 1st acquisition, two residual 13C polarization pathways of first 13C–1H back CP (TCCP) period are stored along the z-direction by synchronizing the TCWALTZ period to 9.6 ms. Unlike 15N, at fast MAS rates 13C z-polarization undergoes TOCSY 13C–13C homonuclear transfer under 13C WALTZ pulses (TCWALTZ period). After a TCWALTZ period the 13C polarization is flipped to the transverse plane by a 90° pulse and then transferred to 1H by another 13C–1H back CP followed by the 2nd acquisition. The corresponding 13C- and 15N-edited 2D spectra of 2nd acquisition are (H)CCH and (H)N(CAC)H, where CC TOCSY mixing is achieved during 13C TCWALTZ period57. The two 15N residual polarization pathways of the first 15N–1H back-CP period (TNCP) are recovered after the 3rd acquisition by setting the TNWALTZ period to 9.6 ms. A 90° pulse is then applied on 15N followed by a second 15N–1H back-CP and HH RFDR mixing that records (H)CA(N)HH and (H)NHH 2D spectra in the 4th acquisition. Similarly, two residual 15N polarization pathways resulting from a second 15N–1H back-CP (TNCP) are recovered for acquiring a fifth pair of 2D 13C- and 15N-edited spectra (H)CA(N)H and (H)NH in the 5th acquisition period.
Figure8B shows ten 2D spectra of the NAVL dipeptide acquired simultaneously using H-MAeSTOSO-10 pulse sequence in Figure8A. Depending on the signal intensities of the 1st increment (t1 = 0), one can choose appropriate durations for HH RFDR or CC TOCSY mixing periods. In general, experiments with longer mixing times are less sensitive and require relatively higher spectral intensities compared to lower mixing times experiments. The signal intensities of H-MEIOSIS pathways recorded in the 1st and 3rd acquisition periods are relatively higher, hence a longer RFDR mixing time (1.47 ms) was used prior to 1st and 3rd acquisitions spectra as reported in Figure8B. The 4th acquisition uses 15N residual signal from first 15N–1H back CP, hence only 0.74 ms RFDR mixing time was used prior to the 4th acquisition. On the other hand, the 5th acquisition uses weak residual polarization after two 15N–1H back CP periods, hence the spectra in the 5th acquisition were recorded without RFDR mixing that gives (H)NH and (H)CA(N)H 2D data sets. For the spectra acquired in the 2nd acquisition, the TOCSY mixing or WALTZ16 period (TCWALTZ) was set to 9.6 ms. Note that the TOCSY mixing can also be increased by setting the TCWALTZ period to multiples of 600 μs, as shown in Figure 5. The 2D spectra, (H)NH and (H)CA(N)H, recorded in 5th acquisition are used as a reference to identify the cross peaks in the spectra acquired in the first four acquisition periods with RFDR and TOCSY mixing periods. The CC TOCSY transfer is mediated by the J-coupling Hamiltonian that achieves intra-residue CC transfer in the 2D spectra [(H)C(C)H and (H)N(CAC)H] as shown in Figure8B. At a RFDR mixing time of 1.47 ms, both 13C- and 15N-edited 2D spectra showed both intra- and inter-residue HH cross peaks between Val and Leu protons. On the other hand, more selective intra-reside HH transfer was observed with a RFDR mixing period of 0.74 ms. As expected, the TOCSY transfer only showed intra-residue CC correlations. The 1H peak at 12.5 corresponds to carboxyl-terminal group of leucine58. Note that the HH transfer efficiency can vary depending on the sample conditions and MAS rates. Although we used RFDR mixing periods for HH transfer, H-MAeSTOSO sequences can easily be modified with other mixing schemes59. For example, DQ-SQ pulse sequences using BABA-XY16 recoupling is shown to be useful for assigning the proton spectra at 100 kHz MAS rate60–61. The H-MAeSTOSO-10 spectra (Figure8) on NAVL were acquired using 24 scans per t1 increment. Although four scans were sufficient to see most of the cross peaks, we used 24 scans to detect the lowest intensity cross-peaks (Vγ1 and Vγ2) in the (H)CHH and (H)C(C)H spectra. Of course, the application of H-MAeSTOSO-10 to proteins requires highly sensitive samples and careful optimization of the mixing periods. Note that in this case, long-range correlations might not be feasible due to limited signal-to-noise ratio.
4. DISCUSSION
Solid-state NMR experiments utilize 5–10% of the total experimental time for pulse sequence execution and acquisition of the signal (FID), while for 90–95% of the time the spectrometer is idle (2–4 seconds) waiting for the spin system to return to the equilibrium through longitudinal relaxation, T1. Classical ssNMR methods are designed for single acquisition period, where each 2D or 3D experiment is acquired using a single coherence transfer pathway. The introduction of POE with the DUMAS, MEIOSIS, and MAeSTOSO strategies made it possible to recover orphan spin operators and utilize the full potential of nuclear spin polarization generated by CP29–34, 38, 46–47. Using these strategies, we have successfully acquired two to eight 2D and 3D spectra using 13C detected multiple acquisition experiments. The expansion of the POE family to 1H-detection experiments and ultrafast magic angle spinning is a stepping-stone to advance the potential of ssNMR to study challenging systems such as membrane proteins.
A key element in the POE pulse sequences is the preparation of both 13C and 15N polarization pathways using SIM-CP that satisfies the Hartmann-Hahn condition simultaneously for 13C and 15N with respect to the 1H spin bath. We have found that the performance of SIM-CP at a 65kHz MAS rate is more efficient compared to slow MAS rates. Other elements of POE involve the recovery of both transferred and residual polarization pathways during NC SPECIFIC-CP (τ), and 13C–1H and 15N–1H back-CP periods (TCCH and TNNH). MEIOSIS and H-MAeSTOSO 2D pulse sequences were implemented using optimal parameters for SPECIFIC-CP transfer periods as described in Figure 3. Thus, the sensitivity of transferred polarization pathways is similar to conventional single acquisition experiments. On the other hand, the sensitivity of residual polarization pathways depends on the number of CP or SPECIFIC-CP periods, as well the efficiency of back CP periods. Figures 3 and 5 quantifies the signal intensities of transferred and residual polarization pathways. Both transferred and residual polarization pathways of NC SPECIFIC-CP, NH back-CP, and CH back-CP were successfully used for the simultaneous acquisition of four to ten two-dimensional experiments using H-MAeSTOSO-4 and HMAeSTOSO-10 experiments. Although, in this work the 2D 13C-edited spectra were acquired with TCCP set to 150 μs, one could use lower TCCP values to increase the residual polarization. For instance, as shown by Rienstra and co-workers, spectral overlap in (H)CH spectra can be reduced by using as low as a 50 μs back CP (TCCP) transfer period21. Note that the shorter back-CP periods achieve selective transfer at the cost of lower 1H signal intensities for transfer pathways 13C to 1H and 15N to 1H. As shown in Figure 5C, at a 50 μs TCCP period one can retain up to 26% 13C residual polarization even in the presence of WALTZ16 pulses. WALTZ16 decoupling is routinely used for both protonated as well as perdeuterated protein samples at a range of MAS rates of 40 to 110 kHz24, 55, 57. Note that in the absence of 13C and 15N WALTZ decoupling pulses it is possible to retain higher residual polarization, but such an approach leads to lower sensitivity and resolution.
One of the objectives of this work was to investigate the extent of the orphan spin-polarization pathways using optimal experimental conditions. We found that approximately 30 to 50% 15N residual polarization was obtained from 15N–1H back CP periods; while 13C residual polarization drops to 10% due to CC TOCSY mixing during WALTZ16 pulses (Figure8). Note that the efficiency of CC TOCSY mixing can be improved under fast MAS rates. In fact, intra-residue CC correlations were obtained by WALTZ pulse sequence on fully protonated membrane protein at 100 kHz MAS rate57. Also, the residual polarization from back-CP periods can also be preserved by applying other decoupling schemes in place of WALTZ that use back to back 180° pulses. In fact, we have recently developed a hybrid 2D and 3D TEDOR-NCX experiments in which the 15N z-polarization is stored under XY-4 15N decoupling with 180° pulses47. Similarly, continuous wave (CW) decoupling can also be used to preserve the residual polarization for simultaneous acquisition of multiple experiments45, 62–63. In this work, we used fully protonated samples with 1H acquisition times set to 8.3 ms. As reported by Pintacuda and co-workers57, 64, even at 100 kHz MAS rate, 1H acquisition times of 10 ms are sufficient for fully protonated protein samples. However, proton resolution can be further improved by using fast MAS rates together with protein perdeuteration, which increases the 1H T2 relaxation times. For samples with longer T2 relaxation, 1H acquisition times are typically set to 20 to 30 ms. Note that for longer acquisition times the residual polarization may be lower, decreasing the sensitivity for the subsequent acquisitions. The pulse sequences shown here lay the groundwork for future investigation of transferred and residual polarization pathways under various sample conditions, including 1H dilution or perdeuteration as well as different decoupling conditions.
In principle, there are multiple combinations of pulse sequences that are possible for each of the H-DUMAS, H-MEIOSIS, and H-MAeSTOSO strategies by incorporating various homonuclear pulse sequence blocks with different mixing times38. However, to best optimize the experimental time it is recommended to record the first increment (t1 = 0) and then choose the appropriate homonuclear recoupling scheme and mixing times. Of course, as the number of back-CP periods increases, the signal decreases due to the lower amount of residual polarization. A key for the successful application of MAeSTOSO experiments is the use of larger number of scans to accumulate sufficient residual polarization to drive the 4th and 5th acquisitions33. To this extent, it is important to code the low-sensitivity experiments, which require a larger number of scans and enable the accumulation of residual polarization, during the first two acquisitions of the pulse scheme and the more sensitive experiments for the following acquisitions. In this way, it is possible to accumulate sufficient residual polarization to drive the acquisition of the other experiments. The power of these experimental strategies were tested on the NAVL model peptide, as well as the SatP membrane protein. As shown in Figure8, for small peptides with resolved peaks, sequential assignment can be obtained from a single H-MAeSTOSO-10 (or H-MAeSTOSO-4) experiment with multiple mixing periods. For larger proteins such as SatP, the 1H resolution in the 2D spectra is still challenging for resonance assignment. Nevertheless, these 2D fingerprints are useful in setting up the corresponding 3D experiments. This new class of POE are not alternatives to the existing approaches to speed up data acquisition; rather it provides a clever strategy to concatenate multiple experiments into a single pulse sequence to optimize the timing of the spectrometers and speed up data acquisition through the acquisition of multiple 2D and 3D experiments. We also anticipate further applications of POE at fast MAS rates (e.g., 100 kHz) using other sensitivity enhancement approaches such as DNP, PRE, and non-uniform sampling65–67. In fact, recent work by Pintacuda, Reif, and Meier groups demonstrates the feasibility of resonance assignment of large proteins using 100–110 kHz MAS rates55, 68–70.
Pines and Waugh were the first to implement multiple acquisition solid-state NMR, also known as proton enhanced experiments, using a rich 1H spin bath of unlabeled organic molecules63. POE pulse sequences are built on a similar philosophy with two basic observations in ssNMR, (1) the rich 1H spin bath enables the simultaneous generation of 13C and 15N polarization pathways, and (2) storage of 15N z-polarization which is unique for solid samples where the T1 relaxation times are significantly longer 29. In the previous POE pulse sequences, we utilized 13C and 15N residual polarization pathways resulting from NC cross polarization periods via MEIOSIS and MAeSTOSO experiments at slow spinning speeds. In this work, we systematically used similar experimental protocols with respect to slow MAS experiments to understand the relative efficiency of SIM-CP and SPECIFIC-CP residual polarization pathways at fast MAS rates. Furthermore, we also designed new strategies for recovering residual polarization from 13C–1H or 15N–1H back CP transfer periods. The ‘afterglow’ pulse sequence developed by Traaseth lab also exploits residual 15N polarization of SPECIFIC-CP and simultaneously records two 2D spectra NCA and NCO using CP as a preparation period71. Similarly, the residual 15N polarization resulting from hetero-nuclear NC mixing sequences has also been utilized at fast MAS rates43. Proton detected H-DUMAS and H-MEIOSIS experiments are indeed extensions of its 13C-detected counterparts namely, DUMAS and MEIOSIS, and other versions have also been proposed by other research groups using proton detection experiments at slow and fast MAS rates42–44.
Finally, we would like to point out that POE can also be modified for dual receiver experiments using 13C and 1H detection. In fact simultaneous acquisition of 1H and 13C detected dual receiver experiments have already been reported by other research groups72. Multi-nuclear acquisition experiments can also be implemented using single receiver systems as demonstrated in UTOPIA experiment73. From a technical point of view, at slow MAS rates POE may cause RF heating due to high power decoupling (~100 kHz), hence, it is strongly recommended to use low-E or E-free probes MAS probes34. On the other hand, the low power decoupling (~10 kHz) at fast MAS rates make the POE more promising for multi-acquisition pulse sequences.
5. CONCLUSIONS
In this work, we have developed a new set of 1H detected POE (Polarization Optimized experiments) that simultaneously acquire two to ten two-dimensional experiments. POE represent a general strategy for multiple acquisitions of almost all types of triple-resonance ssNMR experiments at slow and fast MAS rates. Proton detected H-DUMAS and H-MEIOSIS experiments are simple extensions of its 13C-detected counterparts namely, DUMAS and MEIOSIS. On the other hand, MAeSTOSO experiments require synchronized WALTZ decoupling sequences to preserve the residual polarization pathways. The proposed methods can be extended to theeand four-dimensional experiments together with multi-receiver technology. These new class of experiments are not alternatives to the existing approaches to speed up data acquisition; rather it provides a clever strategy to concatenate multiple experiments into a single pulse sequence to optimize the timing of the spectrometers and speed up data acquisition through the acquisition of multiple 2D and 3D experiments. When applied in concert with other fast acquisition and sensitivity enhancement techniques (e.g., Dynamic Nuclear Polarization, Paramagnetic Relaxation Enhancements, etc.) this approach can further push the boundaries of ssNMR applications to structural biology.
Highlights.
New polarization optimized experiments for proton detection of biomacromolecules
Proton detection enables up to 10 experiments acquired with one pulse sequence
Application to six transmembrane protein transporter SaTP
ACKNOWLEDGEMENTS
The National Institute of Health (GM 64742, HL 144130, 1S10OD021536 to G.V) supported this work. All the experiments were carried out at the Minnesota NMR Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.Hu F; Luo W; Hong M, Mechanisms of proton conduction and gating in influenza M2 proton channels from solid-state NMR. Science 2010, 330 (6003), 505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinn CM; Wang M; Fritz MP; Runge B; Ahn J; Xu C; Perilla JR; Gronenborn AM; Polenova T, Dynamic regulation of HIV-1 capsid interaction with the restriction factor TRIM5alpha identified by magic-angle spinning NMR and molecular dynamics simulations. Proc Natl Acad Sci U S A 2018, 115 (45), 11519–11524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou HX; Cross TA, Influences of membrane mimetic environments on membrane protein structures. Annu Rev Biophys 2013, 42, 361–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldus M, GPCR: Lock and key become flexible. Nat Chem Biol 2018, 14 (3), 201–202. [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick AW; Debelouchina GT; Bayro MJ; Clare DK; Caporini MA; Bajaj VS; Jaroniec CP; Wang L; Ladizhansky V; Muller SA; MacPhee CE; Waudby CA; Mott HR; De Simone A; Knowles TP; Saibil HR; Vendruscolo M; Orlova EV; Griffin RG; Dobson CM, Atomic structure and hierarchical assembly of a cross-beta amyloid fibril. Proc Natl Acad Sci U S A 2013, 110 (14), 5468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watt ED; Rienstra CM, Recent advances in solid-state nuclear magnetic resonance techniques to quantify biomolecular dynamics. Anal Chem 2014, 86 (1), 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiang W; Yau WM; Lu JX; Collinge J; Tycko R, Structural variation in amyloid-beta fibrils from Alzheimer’s disease clinical subtypes. Nature 2017, 541 (7636), 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SH; Das BB; Casagrande F; Tian Y; Nothnagel HJ; Chu M; Kiefer H; Maier K; De Angelis AA; Marassi FM; Opella SJ, Structure of the chemokine receptor CXCR1 in phospholipid bilayers. Nature 2012, 491 (7426), 779–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopinath T; Nelson SED; Soller KJ; Veglia G, Probing the Conformationally Excited States of Membrane Proteins via 1H-Detected MAS Solid-State NMR Spectroscopy. J Phys Chem B 2017, 121 (17), 4456–4465. [DOI] [PubMed] [Google Scholar]
- 10.Gustavsson M; Verardi R; Mullen DG; Mote KR; Traaseth NJ; Gopinath T; Veglia G, Allosteric regulation of SERCA by phosphorylation-mediated conformational shift of phospholamban. Proc Natl Acad Sci U S A 2013, 110 (43), 17338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ha KN; Gustavsson M; Veglia G, Tuning the structural coupling between the transmembrane and cytoplasmic domains of phospholamban to control sarcoplasmic reticulum Ca(2+)-ATPase (SERCA) function. J Muscle Res Cell Motil 2012, 33 (6), 485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fusco G; De Simone A; Gopinath T; Vostrikov V; Vendruscolo M; Dobson CM; Veglia G, Direct observation of the three regions in alpha-synuclein that determine its membrane-bound behaviour. Nat Commun 2014, 5, 3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellani F; van Rossum B; Diehl A; Schubert M; Rehbein K; Oschkinat H, Structure of a protein determined by solid-state magic-angle-spinning NMR spectroscopy. Nature 2002, 420 (6911), 98–102. [DOI] [PubMed] [Google Scholar]
- 14.Wang S; Ing C; Emami S; Jiang Y; Liang H; Pomes R; Brown LS; Ladizhansky V, Structure and Dynamics of Extracellular Loops in Human Aquaporin-1 from Solid-State NMR and Molecular Dynamics. J Phys Chem B 2016, 120 (37), 9887–902. [DOI] [PubMed] [Google Scholar]
- 15.Wasmer C; Lange A; Van Melckebeke H; Siemer AB; Riek R; Meier BH, Amyloid fibrils of the HET-s(218–289) prion form a beta solenoid with a triangular hydrophobic core. Science 2008, 319 (5869), 1523–6. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y; Doherty T; Li J; Lu W; Barinka C; Lubkowski J; Hong M, Resonance assignment and three-dimensional structure determination of a human alpha-defensin, HNP-1, by solid-state NMR. J Mol Biol 2010, 397 (2), 408–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franks WT; Kloepper KD; Wylie BJ; Rienstra CM, Four-dimensional heteronuclear correlation experiments for chemical shift assignment of solid proteins. J Biomol NMR 2007, 39 (2), 107–31. [DOI] [PubMed] [Google Scholar]
- 18.Cavanagh J; Fairbrother WJ; Palmer AG; Rance M; Skelton NJ, Protein NMR Spectroscopy: Principles and Practice, 2nd Edition Protein Nmr Spectroscopy: Principles and Practice, 2nd Edition 2007, 1–888. [Google Scholar]
- 19.Paulson EK; Morcombe CR; Gaponenko V; Dancheck B; Byrd RA; Zilm KW, Sensitive high resolution inverse detection NMR spectroscopy of proteins in the solid state. J Am Chem Soc 2003, 125 (51), 15831–6. [DOI] [PubMed] [Google Scholar]
- 20.Chevelkov V; Xiang S; Giller K; Becker S; Lange A; Reif B, Perspectives for sensitivity enhancement in proton-detected solid-state NMR of highly deuterated proteins by preserving water magnetization. J Biomol NMR 2015, 61 (2), 151–60. [DOI] [PubMed] [Google Scholar]
- 21.Zhou DH; Shah G; Cormos M; Mullen C; Sandoz D; Rienstra CM, Proton-detected solid-state NMR spectroscopy of fully protonated proteins at 40 kHz magic-angle spinning. J Am Chem Soc 2007, 129 (38), 11791–801. [DOI] [PubMed] [Google Scholar]
- 22.Zhou DH; Shea JJ; Nieuwkoop AJ; Franks WT; Wylie BJ; Mullen C; Sandoz D; Rienstra CM, Solid-state protein-structure determination with proton-detected triple-resonance 3D magic-angle-spinning NMR spectroscopy. Angew Chem Int Ed Engl 2007, 46 (44), 8380–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loquet A; El Mammeri N; Stanek J; Berbon M; Bardiaux B; Pintacuda G; Habenstein B, 3D structure determination of amyloid fibrils using solid-state NMR spectroscopy. Methods 2018, 138–139, 26–38. [DOI] [PubMed] [Google Scholar]
- 24.Fricke P; Chevelkov V; Zinke M; Giller K; Becker S; Lange A, Backbone assignment of perdeuterated proteins by solid-state NMR using proton detection and ultrafast magic-angle spinning. Nat Protoc 2017, 12 (4), 764–782. [DOI] [PubMed] [Google Scholar]
- 25.Zhang R; Mroue KH; Ramamoorthy A, Proton-Based Ultrafast Magic Angle Spinning Solid-State NMR Spectroscopy. Acc Chem Res 2017, 50 (4), 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang S; Parthasarathy S; Nishiyama Y; Endo Y; Nemoto T; Yamauchi K; Asakura T; Takeda M; Terauchi T; Kainosho M; Ishii Y, Nano-mole scale side-chain signal assignment by 1H-detected protein solid-state NMR by ultra-fast magic-angle spinning and stereo-array isotope labeling. PLoS One 2015, 10 (4), e0122714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chipot C; Dehez F; Schnell JR; Zitzmann N; Pebay-Peyroula E; Catoire LJ; Miroux B; Kunji ERS; Veglia G; Cross TA; Schanda P, Perturbations of Native Membrane Protein Structure in Alkyl Phosphocholine Detergents: A Critical Assessment of NMR and Biophysical Studies. Chem Rev 2018, 118 (7), 3559–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gopinath T; Nelson SED; Veglia G, (1)H-detected MAS solid-state NMR experiments enable the simultaneous mapping of rigid and dynamic domains of membrane proteins. J Magn Reson 2017, 285, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gopinath T; Veglia G, Dual acquisition magic-angle spinning solid-state NMR-spectroscopy: simultaneous acquisition of multidimensional spectra of biomacromolecules. Angew Chem Int Ed Engl 2012, 51 (11), 2731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gopinath T; Veglia G, 3D DUMAS: simultaneous acquisition of three-dimensional magic angle spinning solid-state NMR experiments of proteins. J Magn Reson 2012, 220, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gopinath T; Veglia G, Orphan spin operators enable the acquisition of multiple 2D and 3D magic angle spinning solid-state NMR spectra. J Chem Phys 2013, 138 (18), 184201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gopinath T; Veglia G, Orphan Spin Polarization: A Catalyst for High-Throughput Solid-State NMR Spectroscopy of Proteins. Annual Reports on NMR Spectroscopy 2016, 89, 103–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gopinath T; Veglia G, Multiple acquisitions via sequential transfer of orphan spin polarization (MAeSTOSO): How far can we push residual spin polarization in solid-state NMR? J Magn Reson 2016, 267, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gopinath T; Veglia G, Experimental Aspects of Polarization Optimized Experiments (POE) for Magic Angle Spinning Solid-State NMR of Microcrystalline and Membrane-Bound Proteins. Methods Mol Biol 2018, 1688, 37–53. [DOI] [PubMed] [Google Scholar]
- 35.Mote KR; Gopinath T; Veglia G, Determination of structural topology of a membrane protein in lipid bilayers using polarization optimized experiments (POE) for static and MAS solid state NMR spectroscopy. J Biomol NMR 2013, 57 (2), 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson SED; Ha KN; Gopinath T; Exline MH; Mascioni A; Thomas DD; Veglia G, Effects of the Arg9Cys and Arg25Cys mutations on phospholamban’s conformational equilibrium in membrane bilayers. Biochim Biophys Acta 2018, 1860 (6), 1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kupce E; Claridge TDW, NOAH: NMR Supersequences for Small Molecule Analysis and Structure Elucidation. Angew Chem Int Ed Engl 2017, 56 (39), 11779–11783. [DOI] [PubMed] [Google Scholar]
- 38.Gopinath T; Veglia G, Multiple acquisition of magic angle spinning solid-state NMR experiments using one receiver: application to microcrystalline and membrane protein preparations. J Magn Reson 2015, 253, 143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen AB; Szekely K; Gath J; Ernst M; Nielsen NC; Meier BH, Simultaneous acquisition of PAR and PAIN spectra. J Biomol NMR 2012, 52 (4), 283–8. [DOI] [PubMed] [Google Scholar]
- 40.Stringer JA; Bronnimann CE; Mullen CG; Zhou DH; Stellfox SA; Li Y; Williams EH; Rienstra CM, Reduction of RF-induced sample heating with a scroll coil resonator structure for solid-state NMR probes. J Magn Reson 2005, 173 (1), 40–8. [DOI] [PubMed] [Google Scholar]
- 41.Gor’kov PL; Chekmenev EY; Li C; Cotten M; Buffy JJ; Traaseth NJ; Veglia G; Brey WW, Using low-E resonators to reduce RF heating in biological samples for static solid-state NMR up to 900 MHz. J Magn Reson 2007, 185 (1), 77–93. [DOI] [PubMed] [Google Scholar]
- 42.Das BB; Opella SJ, Simultaneous cross polarization to (13)C and (15)N with (1)H detection at 60kHz MAS solid-state NMR. J Magn Reson 2016, 262, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bellstedt P; Herbst C; Hafner S; Leppert J; Gorlach M; Ramachandran R, Solid state NMR of proteins at high MAS frequencies: symmetry-based mixing and simultaneous acquisition of chemical shift correlation spectra. J Biomol NMR 2012, 54 (4), 325–35. [DOI] [PubMed] [Google Scholar]
- 44.Sharma K; Madhu PK; Mote KR, A suite of pulse sequences based on multiple sequential acquisitions at one and two radiofrequency channels for solid-state magic-angle spinning NMR studies of proteins. J Biomol NMR 2016, 65 (3–4), 127–141. [DOI] [PubMed] [Google Scholar]
- 45.Zhang R; Mroue KH; Ramamoorthy A, Hybridizing cross-polarization with NOE or refocused-INEPT enhances the sensitivity of MAS NMR spectroscopy. J Magn Reson 2016, 266, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gopinath T; Veglia G, Probing membrane protein ground and conformationally excited states using dipolar- and J-coupling mediated MAS solid state NMR experiments. Methods 2018, 148, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gopinath T; Wang S; Lee J; Aihara H; Veglia G, Hybridization of TEDOR and NCX MAS solid-state NMR experiments for simultaneous acquisition of heteronuclear correlation spectra and distance measurements. J Biomol NMR 2019, 73 (3–4), 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fung BM; Khitrin AK; Ermolaev K, An improved broadband decoupling sequence for liquid crystals and solids. J Magn Reson 2000, 142 (1), 97–101. [DOI] [PubMed] [Google Scholar]
- 49.Shaka AJ; Keeler J; Frenkiel T; Freeman R, An improved sequence for broadband decoupling: WALTZ-16. J Magn Reson 1983, 52, 335–38. [Google Scholar]
- 50.Hartmann SR; Hahn EL, Nuclear Double Resonance in the Rotating Frame. Physical Review 1962, 128 (5), 2042–2053. [Google Scholar]
- 51.Zhou DH; Rienstra CM, High-performance solvent suppression for proton detected solid-state NMR. J Magn Reson 2008, 192 (1), 167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baldus M; Petkova AT; Herzfeld J; Griffin RG, Cross polarization in the tilted frame: assignment and spectral simplification in heteronuclear spin systems. Molecular Physics 1998, 95 (6), 1197–1207. [Google Scholar]
- 53.Bennett AER, C. M; Auger M; Lakshmi KV;; Griffin RG, Heteronuclear decoupling in rotating solids. J. Chem. Phys 1995, 103, 6951–6958. [Google Scholar]
- 54.Delaglio FGS; Vuister GW; Zhu G; Pfeifer J; and Bax A, NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 1995, 6, 277–293. [DOI] [PubMed] [Google Scholar]
- 55.Xue K; Sarkar R; Motz C; Asami S; Camargo DCR; Decker V; Wegner S; Tosner Z; Reif B, Limits of Resolution and Sensitivity of Proton Detected MAS Solid-State NMR Experiments at 111 kHz in Deuterated and Protonated Proteins. Sci Rep 2017, 7 (1), 7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S; Parthasarathy S; Xiao Y; Nishiyama Y; Long F; Matsuda I; Endo Y; Nemoto T; Yamauchi K; Asakura T; Takeda M; Terauchi T; Kainosho M; Ishii Y, Nano-mole scale sequential signal assignment by (1)H-detected protein solid-state NMR. Chem Commun (Camb) 2015, 51 (81), 15055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lalli D; Idso MN; Andreas LB; Hussain S; Baxter N; Han S; Chmelka BF; Pintacuda G, Proton-Based Structural Analysis of a Heptahelical Transmembrane Protein in Lipid Bilayers. J Am Chem Soc 2017, 139 (37), 13006–13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang R; Pandey MK; Nishiyama Y; Ramamoorthy A, A Novel High-Resolution and Sensitivity-Enhanced Three-Dimensional Solid-State NMR Experiment Under Ultrafast Magic Angle Spinning Conditions. Sci Rep 2015, 5, 11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wittmann JJ; Agarwal V; Hellwagner J; Lends A; Cadalbert R; Meier BH; Ernst M, Accelerating proton spin diffusion in perdeuterated proteins at 100 kHz MAS. J Biomol NMR 2016, 66 (4), 233–242. [DOI] [PubMed] [Google Scholar]
- 60.Zhang R; Duong NT; Nishiyama Y; Ramamoorthy A, 3D Double-Quantum/Double-Quantum Exchange Spectroscopy of Protons under 100 kHz Magic Angle Spinning. J Phys Chem B 2017, 121 (24), 5944–5952. [DOI] [PubMed] [Google Scholar]
- 61.Saalwachter K; Lange F; Matyjaszewski K; Huang CF; Graf R, BaBa-xy16: robust and broadband homonuclear DQ recoupling for applications in rigid and soft solids up to the highest MAS frequencies. J Magn Reson 2011, 212 (1), 204–15. [DOI] [PubMed] [Google Scholar]
- 62.Demers JP; Vijayan V; Lange A, Recovery of bulk proton magnetization and sensitivity enhancement in ultrafast magic-angle spinning solid-state NMR. J Phys Chem B 2015, 119 (7), 2908–20. [DOI] [PubMed] [Google Scholar]
- 63.Pines A; Gibby GM; Waugh JS, Proton-enhanced NMR of dilute spins in solids. J. Chem. Phys 1973, 59, 569–590. [Google Scholar]
- 64.Andreas LB; Jaudzems K; Stanek J; Lalli D; Bertarello A; Le Marchand T; Cala-De Paepe D; Kotelovica S; Akopjana I; Knott B; Wegner S; Engelke F; Lesage A; Emsley L; Tars K; Herrmann T; Pintacuda G, Structure of fully protonated proteins by proton-detected magic-angle spinning NMR. Proc Natl Acad Sci U S A 2016, 113 (33), 9187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maly T; Debelouchina GT; Bajaj VS; Hu KN; Joo CG; Mak-Jurkauskas ML; Sirigiri JR; van der Wel PC; Herzfeld J; Temkin RJ; Griffin RG, Dynamic nuclear polarization at high magnetic fields. J Chem Phys 2008, 128 (5), 052211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wickramasinghe NP; Parthasarathy S; Jones CR; Bhardwaj C; Long F; Kotecha M; Mehboob S; Fung LW; Past J; Samoson A; Ishii Y, Nanomole-scale protein solid-state NMR by breaking intrinsic 1HT1 boundaries. Nat Methods 2009, 6 (3), 215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suiter CL; Paramasivam S; Hou G; Sun S; Rice D; Hoch JC; Rovnyak D; Polenova T, Sensitivity gains, linearity, and spectral reproducibility in nonuniformly sampled multidimensional MAS NMR spectra of high dynamic range. J Biomol NMR 2014, 59 (2), 57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lecoq L; Schledorn M; Wang S; Smith-Penzel S; Malar AA; Callon M; Nassal M; Meier BH; Bockmann A, 100 kHz MAS Proton-Detected NMR Spectroscopy of Hepatitis B Virus Capsids. Front Mol Biosci 2019, 6, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Penzel S; Oss A; Org ML; Samoson A; Bockmann A; Ernst M; Meier BH, Spinning faster: protein NMR at MAS frequencies up to 126 kHz. J Biomol NMR 2019, 73 (1–2), 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Agarwal V; Penzel S; Szekely K; Cadalbert R; Testori E; Oss A; Past J; Samoson A; Ernst M; Bockmann A; Meier BH, De novo 3D structure determination from sub-milligram protein samples by solid-state 100 kHz MAS NMR spectroscopy. Angew Chem Int Ed Engl 2014, 53 (45), 12253–6. [DOI] [PubMed] [Google Scholar]
- 71.Banigan JR; Traaseth NJ, Utilizing afterglow magnetization from cross-polarization magic-angle-spinning solid-state NMR spectroscopy to obtain simultaneous heteronuclear multidimensional spectra. J Phys Chem B 2012, 116 (24), 7138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gallo A; Franks WT; Lewandowski JR, A suite of solid-state NMR experiments to utilize orphaned magnetization for assignment of proteins using parallel high and low gamma detection. J Magn Reson 2019, 305, 219–231. [DOI] [PubMed] [Google Scholar]
- 73.Viegas A; Viennet T; Yu TY; Schumann F; Bermel W; Wagner G; Etzkorn M, UTOPIA NMR: activating unexploited magnetization using interleaved low-gamma detection. J Biomol NMR 2016, 64 (1), 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]