Abstract
Background
Various diagnostic modalities are available to assess the problematic knee arthroplasty. Visualization of soft‐tissue structures in relation to the arthroplasty and bone remains difficult. Recent developments in MRI sequences could make MRI a viable addition to the diagnostic arsenal.
Purpose
To review the diagnostic properties of MRI, to identify certain causes of complaints that may be directly related to implant failure of total (TKA) or unicompartmental knee arthroplasty (UKA); infection, loosening and wear, instability, malalignment, arthrofibrosis, or patellofemoral problems.
Study Type
Systematic review.
Population
Twenty‐three studies were included: 16 TKA, four UKA, and three cadaveric studies. Causes of knee arthroplasty complaints analyzed were; infection (three), loosening and wear (11), malalignment (five) and instability (four).
Field Strength and Sequences
No field strength or sequence restrictions.
Assessment
PubMed, SCOPUS, and EMBASE were searched. Risk of bias was assessed using the COnsensus‐based Standards for the selection of health Measurement Instruments (COSMIN) and the QUality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2).
Statistical Tests
The results of the original research articles are stated.
Results
Fifteen studies assessed the reproducibility of analyzing infection, loosening and wear, and malalignment. Fourteen of 15 studies were deemed as adequate to good quality. Results showed a moderate to excellent agreement (ICC/K 0.55–0.97). Fourteen studies addressed the accuracy. For infection and loosening and wear the sensitivity and specificity estimates varied between 0.85–0.97 and 0.70–1.00, respectively. The accuracy for malalignment was excellent (r ≥ 0.81). For these studies QUADAS‐2 analysis suggested few risks of bias. A meta‐analysis was not possible due to the heterogeneity of the data.
Data Conclusion
This study supports that MRI can be used with overall reproducible and accurate results for diagnosing infection, loosening and wear, and malalignment after knee arthroplasty. Nonetheless, studies regarding the diagnosis of instability, arthrofibrosis or patellofemoral complaints using MRI are limited and inconclusive.
Level of Evidence: 3
Technical Efficacy: Stage 2
J. Magn. Reson. Imaging 2020;51:446–458.
Keywords: systematic review, magnetic resonance imaging, knee arthroplasty, complaints
UNICOMPARTMENTAL KNEE ARTHROPLASTY (UKA) and total knee arthroplasty (TKA) are widely accepted treatment options for endstage osteoarthritis.1 The number of UKA and TKA procedures performed is growing annually due to the aging of the population, as well as the increased incidence of osteoarthritis in younger patients, among whom there is increased demand for and acceptance of these procedures.2 Consequently, the number of revision surgeries is also increasing and likely will increase further in the coming decades.3 An important aspect that influences the success rate of revision surgery is identification of the underlying cause(s) of the failure of the problematic knee arthroplasty.3 The most common causes of a problematic knee arthroplasty for which revision surgery may offer benefits are infection, loosening and wear, instability, malalignment, and, less frequently, arthrofibrosis.4, 5 In addition to these causes, there are various problems that revision surgery cannot solve, such as periarticular causes (eg, tendinopathies or local and/or diffuse neuropathic pain) or extraarticular causes (eg, hip osteoarthritis).6
To differentiate among the potential causative factor(s), various imaging techniques are available after the basic workup, which involves extensive history, physical examination, radiographs (including long leg view), and lab tests. The imaging techniques utilized include combinations of radiographic views, stress radiographs, computed tomography (CT), magnetic resonance imaging (MRI), planar bone scintigraphy with or without single photon emission computed tomography (SPECT), and fluorodeoxyglucose (FDG)‐positron emission tomography (PET)/CT.7, 8 It would be valuable if one imaging technique could offer the same diagnostic power as two or more other imaging techniques for identifying the cause(s) of failure.
In recent decades, MRI has become the standard for the evaluation of joints and soft tissues in the native knee.9 However, MRI is considered to have limited diagnostic properties for TKA patients, due to artifacts caused by the prosthetic implant.10, 11 Interestingly, a literature study conducted by Fritz et al 5 discussed strategies for MRI around knee arthroplasty implants and demonstrated the imaging appearances of common causes of complaints. That study suggested that MRI with optimized sequences and advanced metal artifact reduction techniques could be applied to evaluate the underlying causes of failed knee arthroplasty. However, the additional diagnostic properties of MRI for diagnosing the knee after arthroplasty were not assessed.
Therefore, the aim of the current study was to critically appraise, summarize, and compare the literature on the diagnostic properties of MRI, to identify the causes of complaints that are directly related to implant failure. Hence, this systematic review focused on MRI studies that examined implant‐related issues of infection, loosening and wear, instability, malalignment, arthrofibrosis, and patellofemoral complaints after TKA or UKA.
Materials and Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic review and Meta‐Analyses (PRISMA).12
Eligibility Criteria
Studies were included that reported on: 1) the ability of MRI to diagnose (one of the) probable causes of complaints (for definitions, see Table 1) after primary TKA or UKA; 2) patients or cadaveric studies.
Table 1.
Probable Causes of Complaints After Knee Arthroplasty and Their MRI Manifestations
| Infection | Loosening and wear | Instability | Malalignment | Arthrofibrosis | Patellofemoral |
|---|---|---|---|---|---|
| Lamellated hyperintense synovitis, extracapsular soft‐tissue edema, extracapsular collections and reactive lymphadenopathy 29. |
Fibrous membrane formation between the bone and the implant or cement 5. Cytokine‐mediated inflammatory reaction due to polyethylene wear 50. |
Tendon abnormalities, tendinosis or tendon rupture of the quadriceps or patellar tendon. Deficiency of the posteromedial or lateral stabilizers 5. |
Increased internal‐external or varus‐valgus rotation of the femoral or tibial component 26, 44. | Fibrous tissue i.e. thickening along the synovial lining 5, 51. |
Patellar problems, as patellar clunk, patella baja, patella alta 5. |
Studies were excluded if they were: 1) written in a language other than English; 2) letters to the editor; 3) review articles.
Search Strategy
The search included studies published between January 1st 2003 and February 28th 2019. The reference lists were imported to Endnote 8.1 (Thompson Reuters, Eagan, MN) and duplicate articles were removed. A literature search was conducted using the following electronic databases: PubMed, SCOPUS, and EMBASE. The search terms used were "knee prosthesis" and all synonyms thereof and "MRI" and all synonyms thereof. The detailed search strategies for each database are given in Table 2.
Table 2.
Search Strategy
| Database | Search strategy | Results |
|---|---|---|
| PubMed |
|
490 |
| SCOPUS |
|
681 |
| EMBASE |
|
840 |
Study Selection and Data Collection
Two independent observers (C.P. and F.S., respectively 2 and 4 years of research experience) selected eligible studies and extracted the data. First, titles and abstracts were screened. Studies that were identified as potentially relevant by at least one reader were retrieved and the full texts were evaluated. Any disagreement between the two readers was resolved through discussion. In case of remaining disagreement, the dispute was resolved with the help of a third reviewer (R.H. with 18 years of research experience). Additionally, the references of all considered articles were hand‐searched to identify any relevant studies that may have been overlooked by the search strategy.
Study characteristics were extracted as: year of publication, study design, causes of complaints, number of subjects, number of controls, mean age, type of prosthesis (UKA or TKA), and MRI settings. It was noted when the prosthesis was made out of zirconium, because zirconium prostheses are known for their reduced metal artifacts, which may influence study results.13
The included studies were divided into three groups—TKA, UKA, and cadaveric studies—and sorted by their reported causes of complaints.
Critical Appraisal and Analysis
The included studies assessed the diagnostic properties of MRI to identify probable causes of complaints. Some studies achieved this by evaluating the reproducibility of measurements, and others assessed diagnostic accuracy. To evaluate these studies, two different critical appraisal tools were chosen.
The methodological quality of the reproducibility studies was assessed by evaluating reliability with the reliability box of the Consensus‐based Standards for the selection of health status Measurement Instruments (COSMIN).14 Reliability is a measure of the consistency between or within observers. The questions in the reliability box can be answered with "very good," "adequate," "doubtful," or "inadequate." The total score for reliability is based on the lowest rating given for any of the questions.14
Moreover, outcome measures for the reproducibility of the MRI measurements, such as the intraclass correlation coefficient (ICC) or kappa, were collected from these studies. The ICC values were defined as follows: ICC values lower than 0.5 indicate poor reliability, values between 0.5–0.75 moderate reliability, values between 0.75–0.9 good reliability, and values greater than 0.90 excellent reliability.15 Kappa values were defined as follows: 0.01–0.20 no agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 good agreement, and 0.81–1.00 almost excellent agreement.16
Diagnostic accuracy was assessed in terms of validity using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS‐2) tool.17 Validity indicates that MRI is able to accurately identify complaints compared with the reference standard (criterion validity) or compared with another standard (construct validity). The QUADAS questions can be answered with "low," "high," or "unclear." Included studies with outcome measures reporting the diagnostic accuracy for one or more of the possible causes of complaints expressed in sensitivity, specificity, P‐values, and correlations were collected. P < 0.05 was considered significant.
Studies that assessed both reproducibility and accuracy were evaluated using both critical appraisal tools.
Results
Study Selection
The search initially returned 2011 hits (Fig. 1 shows the flowchart of the study selection process). After the removal of duplicates, 1348 citations remained. After titles and abstracts were screened, a total of 56 full‐text articles remained. Of these, a total of 23 publications met the eligibility criteria. Reference checking did not yield additional relevant publications.
Figure 1.
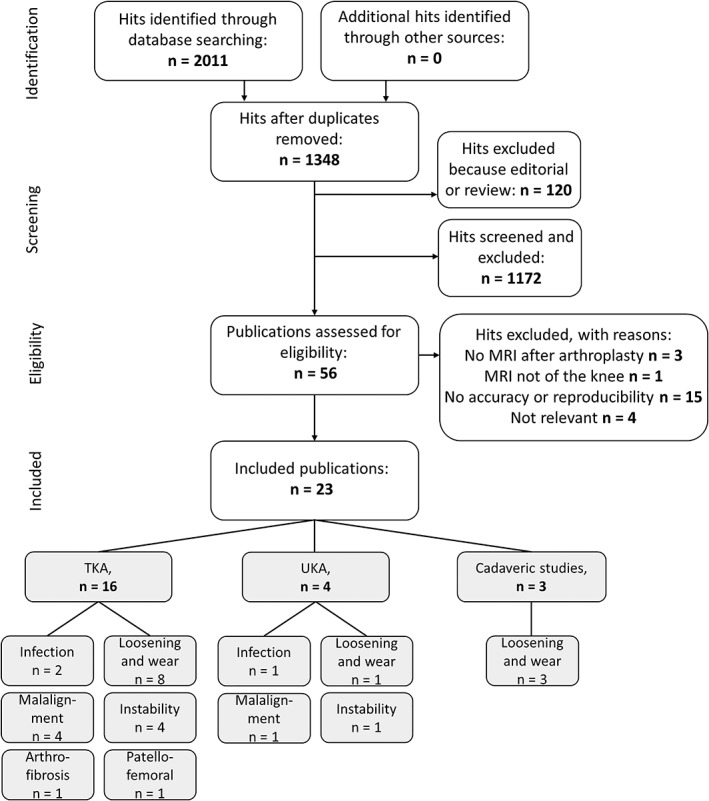
Flowchart of the study selection.
Study Characteristics
Of the 23 included studies described in Table 3, 16 publications concerning diagnostic MRI after TKA were retrieved, with a total number of 650 patients. Four publications (58 patients) were found concerning diagnostic MRI after UKA. Three remaining publications concerned cadaveric studies (18 human or porcine cadaveric specimens) and tried to determine the added value of MRI in diagnosing the underlying causes of loosening after arthroplasty.
Table 3.
Study Characteristics
| Study (year published) | Design | Subjects (n) | Controls (n) | Mean age (years) | Investigated cause of complaints | Prosthesis | MRI (Tesla; sequence) |
|---|---|---|---|---|---|---|---|
| Total knee arthroplasty | |||||||
| A. Li et al (2016) 19 | Retrospective, cross‐sectional | 73 | — | 65 | Infection, instability, loosening and wear | TKA | 1.5T; FSE + IR + MAVRIC |
| A. Plodkowski et al (2013) 29 | Retrospective, case control | 28 | 28 | 64 | Infection | TKA | 1.5T; FSE + IR |
| B. Raphael et al (2006) 20 | Retrospective | 21 | — | 57 | Instability | TKA, 14 Zirconium | 1.5T; FSE + IR |
| A. Jawhar (2018) 27 | Retrospective | 15 | — | 76 | Instability | TKA | 1,5T; TSE + VAT + SEMAC |
| T. Heyse et al (2011) 21 | Retrospective | 55 | — | 59 | Loosening and wear | TKA, 27 Zirconium | 1.5T; FSE + IR |
| A. Li et al (2016) 33 | Retrospective, observational | 96 | — | 64 | Loosening and wear | TKA | 1,5T; FSE + IR + MAVRIC |
| A. Li et al (2017) 25 | Retrospective | 61 | — | 66 | Loosening and wear | TKA | 1,5T; FSE + IR + MAVRIC |
| M. Meftah et al (2013) 38 | Prospective, longitudinal | 24 | — | 63 | Loosening and wear | TKA | 1,5T; FSE + MAVRIC |
| C. Sofka et al (2003) 10 | Retrospective | 41 | — | n/a | Loosening and wear, instability, arthrofibrosis | TKA | 1.5T; FSE + IR |
| R. Sutter et al (2013) 34 | Prospective | 42 | 29 | 66 | Loosening and wear | TKA | 1.5T; TSE + IR + SEMAC |
| M. Vessely et al (2006) 35 | Retrospective | 10 | — | 67 | Loosening and wear | TKA | 1.5T; FSE + IR |
| T. Heyse et al (2012) 18 | Retrospective | 55 | — | 59 | Malalignment | TKA, 27 Zirconium | 1.5T; FSE + IR |
| T. Heyse et al (2015) 24 | Retrospective | 55 | — | 65 | Malalignment | TKA | 1.5T; FSE |
| A. Murakami et al (2012) 26 | Retrospective, case‐control | 50 | 16 | 69 | Malalignment | TKA | 1.5T; FSE |
| M. Sgroi et al (2015) 30 | Prospective, cohort | 12 | 12 | 70 | Malalignment | TKA | 1.5T; TSE |
| T. Heyse et al (2012) 22 | Retrospective | 12 | — | 63 | Patellofemoral | TKA, 1 Zirconium | 1.5T; FSE + IR |
| Unicompartmental knee arthroplasty | |||||||
| C. Park et al (2015) 31 | Retrospective | 28 | — | 57 | Infection and others | UKA | 1.5T; FSE + IR |
| T. Heyse et al (2012) 11 | Retrospective | 10 | — | 65 | Instability | UKA, 10 Zirconium | 1.5T; TSE |
| D. Malcherczyk et al (2015) 28 | Retrospective | 10 | — | 65 | Loosening and wear | UKA, 10 Zirconium | 1.5T; TSE |
| T. Heyse et al (2013) 23 | Retrospective | 10 | — | 65 | Malalignment | UKA, 10 Zirconium | 1.5T; TSE |
| Cadaveric studies | |||||||
| Y. Minoda et al (2014) 32 | Proof of concept | 6 pc | — | — | Loosening and wear | FC | 1,5T |
| Y. Minoda et al (2017) 37 | Proof of concept | 6 pc | — | — | Loosening and wear | FC, Zirconium | 1,5T |
| L. Solomon et al (2012) 36 | Proof of concept | 6 hc | — | — | Loosening and wear | TKA | 1.5T; FSE + IR |
TKA = total knee arthroplasty, UKA = unicompartmental knee arthroplasty, pc = porcine cadaver, hc = human cadaver, TSE = turbo spin echo, FSE = fast spin echo, VAT = view angle tilting, IR = inversion recovery, SEMAC = Slice Encoding for Metal Artifact Correction, MAVRIC = Multi‐Acquisition Variable Resonance Image Combination.
Reproducibility
The reproducibility of MRI for diagnosing one or more of the probable causes of complaints was examined in 11 out of the 16 TKA studies, three out of the four UKA studies, and one out of the three cadaveric studies (Table 4). All studies except one18 scored adequate to very good for reliability by COSMIN. However, despite their adequate to very good methodological quality, these studies typically failed to indicate the time between repeated measurements.11, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28
Table 4.
Reproducibility of MRI Measurements to Diagnose Probable Causes of Complaints After Knee Arthroplasty, Sorted by Pathology With Their Statistic Results and the Results of the Critical Appraisal (COSMIN) for the Reliability Box
| Author (year) | Pathology | Measurement | Interrater reliability | 95% CI | Intrarater reliability | 95% CI | COSMIN reliability box |
|---|---|---|---|---|---|---|---|
| Total knee arthroplasty | |||||||
| A. Li et al (2016) 19 | Infection, | Lamellated hyperintense synovitis | K = 0.82 | 0.72‐0.91 | K = 0.83 | 0.74‐0.93 | ++ |
| loosening and wear | Frond like hypertrophied synovitis | ||||||
| instability and other | Homogeneous effusion | ||||||
| A. Plodkowski et al (2013) 29 | Infection | Synovitis | K = 0.82 | 0.72‐0.93 | K = 0.89 | 0.78‐1.00 | ++ |
| B. Raphael et al (2006) 20 | Instability | Medial collateral ligament | ICC > 0.77 | n/a | n/a | n/a | ++ |
| Lateral collateral ligament | ICC > 0.74 | ||||||
| Joint effusion | ICC > 0.24 | ||||||
| Quadriceps tendon | ICC > 0.71 | ||||||
| Patellar tendon | ICC > 0.83 | ||||||
| Tibial component | ICC > 0.65 | ||||||
| Femoral component | ICC > 0.53 | ||||||
| Patellar component | ICC > 0.45 | ||||||
| A. Jawhar (2018) 27 | Instability | Posterior cruciate ligament | ICC > 0.90 | n/a | n/a | n/a | + |
| Medial collateral ligament | ICC > 0.34 | ||||||
| Lateral collateral ligament | ICC > 0.37 | ||||||
| Patella tendon | ICC > 0.68 | ||||||
| Popliteal vessels | ICC > 0.83 | ||||||
| Periprosthetic bone | ICC > 0.80 | ||||||
| T. Heyse et al (2011) 21 | Loosening and wear | Implant bone interface tibial | K > 0.95 | n/a | n/a | n/a | ++ |
| Implant bone interface femoral | K > 0.80 | ||||||
| Implant bone interface patellar | K > 0.94 | ||||||
| A. Li et al (2017) 25 | Loosening and wear | Synovitis | K = 0.72 | 0.65‐0.80 | n/a | n/a | + |
| T. Heyse et al (2012) 18 | Malalignment | Femoral component rotation | α = 0.82 | n/a | α = 0.95 | n/a | – |
| Tibial component rotation | α = 0.89 | α = 0.91 | |||||
| T. Heyse et al (2015) 24 | Malalignment | Tibial component rotation | ICC = 0.63‐0.97 | n/a | ICC = 0.53‐0.96 | n/a | ++ |
| A. Murakami et al (2012) 26 | Malalignment | Femoral component rotation | ICC = 0.75 | 0.63‐0.84 | n/a | n/a | ++ |
| Tibial component rotation | ICC = 0.75 | 0.62‐0.84 | |||||
| M. Sgroi et al (2015) 30 | Malalignment | Femoral component rotation | ICC = 0.55 | n/a | ICC = 0.92 | n/a | + |
| Tibial component rotation | ICC = 0.89 | ICC = 0.95 | |||||
| T. Heyse et al (2012) 22 | Patellofemoral | Patella clunk | ICC = 0.75‐0.93 | n/a | n/a | n/a | ++ |
| Unicompartmental knee arthroplasty | |||||||
| T. Heyse et al (2012) 11 | Instability | Anterior cruciate ligament | K = 1.0 | n/a | n/a | n/a | ++ |
| Posterior cruciate ligament | K = 0.76 | ||||||
| Lateral collateral ligament | K = 0.81 | ||||||
| Medial collateral ligament | K = 1.0 | ||||||
| Meniscus | K = 1.0 | ||||||
| Cartilage | K = 0.84 | ||||||
| Effusion | K = 1.0 | ||||||
| Patellar tendon | K = 1.0 | ||||||
| Quadriceps tendon | K = 1.0 | ||||||
| D. Malcherczyk et al (2015) 28 | Loosening and wear | Implant bone interface | K = 0.60‐1.00 | n/a | n/a | n/a | ++ |
| T. Heyse et al (2013) 23 | Malalignment | Femoral component rotation | ICC = 0.96 | n/a | ICC = 0.99 | n/a | ++ |
| Tibial component rotation | ICC ≥0.56 | ICC ≥0.88 | |||||
| Cadaveric studies | |||||||
| L. Solomon et al (2012) 36 | Loosening and wear | Periprosthetic osteolysis | K = 0.61 | n/a | K = 0.80‐0.86 | n/a | + |
K = Cohen's Kappa, ICC = intra class correlation coefficient, α = Cronbach's alpha, n/a = not applicable, ++ = very good, + = adequate, ‐ = doubtful, ‐‐ = inadequate.
Periprosthetic joint infections were associated with signs of lamellated hyperintense synovitis on MRI. Almost excellent reproducibility results were found regarding lamellated hyperintense synovitis, with an interrater reproducibility of (K = 0.82 and K = 0.82) and intrarater reproducibility of (K = 0.83 and K = 0.89).19, 29 Loosening was evaluated in two studies by assessing the implant–bone interface. These studies reported interrater reproducibilities that were almost excellent (K ≥ 0.80)21 and moderate (K ≥ 0.60).28 One study scored frondlike hypertrophied synovitis, associated with loosening due to wear, and concluded that interrater reproducibility was good (K = 0.72).25
In contrast, when soft‐tissue structures, which are associated with instability, were assessed, the interrater reproducibility ranged between poor and excellent (ICC between 0.24–0.85; kappa between 0.59–1.00).20, 27 However, these wide ranges could be explained by the fact that these studies assessed diverse soft‐tissue structures, which were visualized with multiple sequences around different prosthetic materials.
Regarding prosthetic malalignment, five studies18, 23, 24, 26, 30 assessed the femoral component rotation (FCR) and/or tibial component rotation (TCR). For FCR and TCR, the interrater reproducibility ranged between moderate and excellent (for FCR, an ICC between 0.55–0.96,23, 26, 30 and for TCR, an ICC between 0.56–0.9723, 24, 26, 30).
Accuracy
The accuracy of MRI in diagnosing one or more of the probable causes of complaints was examined in 10 out of the 16 TKA publications, one out of the four UKA publications, and three out of the three cadaveric studies (Table 5). The methodological quality of the accuracy studies assessed with QUADAS‐2 varied from a high risk of bias10, 31, 32, 33 to a low risk of bias.19, 25, 29, 30, 34 Criterion validity was assessed by comparing MRI findings with perioperative findings.10, 19, 25, 29, 31, 32, 33, 35, 36, 37 Construct validity was determined by using different standards as comparators, such as CT,30, 34 knee pain,38 and healthy controls.26 Due to the retrospective designs of the included studies, which is thought to increase susceptibility to selection bias, none of the retrospective studies scored "low risk" for the patient selection bias by QUADAS‐2. In addition, concerns were raised regarding the applicability of patient selection, because some studies did not describe patient selection clearly.10, 31, 33, 35, 38
Table 5.
Accuracy of MRI to Diagnose Probable Causes of Complaints After Knee Arthroplasty, Sorted by Pathology for Different Comparators (Measurements on MRI Compared With the Reference Standard or Other) With Their Statistic Results and the Results of the Critical Appraisal (QUADAS‐2) for the Risk of Bias and the Applicability of Concerns.
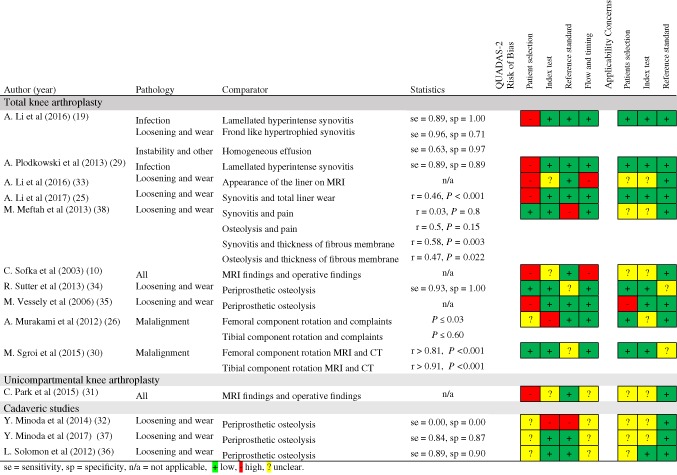
|
The sensitivity and specificity for diagnosing infection by the signs of lamellated hyperintense synovitis on MRI when taking culture results of perioperativy obtained tissue as the reference standard for periprosthetic joint infections varied between 0.89 (0.750–0.970, 95% confidence interval [CI])29 and 0.85–0.92 (0.537–0.996, 95% CI)19 for sensitivity and between 0.89 (0.559–1.00, 95% CI)29 and 1.00 (0.93–1.00)19 for specificity.
A relation was found between the presence of frondlike hypertrophied synovitis on MRI and perioperative findings of loosening due to wear.25 When these MRI findings were compared with the reference standard perioperative findings, the sensitivity and specificity of the diagnoses varied between 0.94–0.97 and 0.70–0.73.19
Diagnosing aseptic loosening by signs of periprosthetic osteolysis on MRI compared with perioperative findings was evaluated in one good‐quality study, with sensitivity and specificity of 0.93 and 1.00.34
Malalignment measurement on MRI and CT showed an excellent correlation for FCR (r = 0.81) and TCR (r = 0.91).30 Strikingly, one of the malalignment studies also included healthy controls and found significant differences between patients after TKA and healthy controls for FCR, P < 0.03.26
Discussion
The aim of this study was to critically appraise, summarize, and compare the literature on the diagnostic properties of MRI for identifying the causes of complaints in patients or cadaveric studies in terms of infection, loosening and wear, instability, malalignment, arthrofibrosis, and patellofemoral complaints after TKA or UKA. The available good‐quality studies showed good to excellent reproducibility for MRI for diagnosing infection, loosening and wear, or malalignment after TKA. Studies in which accuracy was assessed were highly varied in terms of methodological quality.
The MRI properties to assess various arthroplasty failure causations were evaluated in this systematic review. First, MRI to identify periprosthetic joint infection based on MRI findings of hypertrophied synovitis compared with the reference standard was evaluated by two studies of adequate quality. Diagnostic properties were found in terms of sensitivity and specificity (0.89 and 0.89; 0.96 and 0.71) with "almost excellent" reliability.19, 29 Nonetheless, it should be noted that both TKA studies were conducted by the same research group. Currently, the reference standard to diagnose infection is the diagnosis of a pathogen via multiple intraoperative cultures.39 In the literature, numerous preoperative and intraoperative tests for diagnosing periprosthetic joint infection were evaluated, as were several imaging modalities. Unfortunately, no test or modality has perfect sensitivity and specificity.40 Overall, MRI may be considered a possible preoperative imaging technique that can contribute to diagnosing infection.
Second, regarding loosening due to liner wear, the results showed that osteolysis can be recognized on MRI,34 and wear can be diagnosed based on synovitis patterns.19 Moreover, there is a significant relation between synovitis on MRI and liner wear.25 These findings are analogous to the literature regarding the diagnostic properties of MRI for diagnosing liner wear in total hip arthroplasty.41 In clinical practice, early loosening is very difficult to diagnose on X‐ray, and diagnosis usually becomes clearer only upon follow‐up X‐rays.42 When X‐ray is inconclusive, other imaging modalities may be used,42 and based on these results, MRI may be considered as a possible modality.
Third, femoral and tibial component malalignment measurements can reliably be performed based on MRI after TKA or UKA.23, 24, 26, 30 At present, a combination of the imaging modalities of long leg view and CT is preferred for evaluating malalignment.43 However, CT scanning results in a radiation load for the patient. Fortunately, MRI and CT show an excellent correlation regarding malalignment measurements in TKA.30 Moreover, a significant relation between complaints and internal rotation of the femur component on MRI was found.26 This was confirmed by the recent research of Panni et al,44 which concluded that excessive internal rotation of the tibial TKA component represents an important risk factor for pain and inferior functional outcomes.
Fourth, regarding the other probable causes of complaints, the number of studies or their methodological quality was limited. Results regarding instability were inconsistent,11, 20, 27 probably due to the material of the scanned prostheses. Some of the instability studies that were included used a femoral component made from zirconium.11, 20 Soft‐tissue structures surrounding a zirconium prosthesis are more visible on MRI, because zirconium is nonferromagnetic and therefore less hampered by metal artifacts.13 This may be the reason for the inconclusive results of the instability studies. Moreover, all the instability studies that were included only evaluated reproducibility and not accuracy.
Fifth, arthrofibrosis was only assessed in the more explorative studies, together with all other probable causes of complaints. In clinical practice, arthrofibrosis is diagnosed when patients experience stiffness and a restricted range of motion following knee arthroplasty.45 If other possible causes are not suspected, there is no need for additional diagnostic images such as MRI. However, the two studies included in this review that also evaluated MRI‐based diagnoses of arthrofibrosis suggest that MRI performs well in this domain.10, 31
Sixth, patellofemoral problems can be evaluated by several patellofemoral parameters, and MRI can be used in the native knee to assess the patellofemoral joint.46 However, studies that used MRI to evaluate patellofemoral complaints after TKA were not available. Only one of the included studies assessed the reproducibility of diagnosing patellar clunk and reported good results.22 However, patella clunk is a rare finding in modern‐day TKA designs.
This review included studies published after 2002. It is notable that MRI after TKA is a young field of research: 19 of the 23 studies were published in 2012 or thereafter. This can be explained by the fact that traditional MRI is not capable of adequately imaging the structures, bone, and soft tissue that surround metal implants.47 In recent decades, MRI sequences have greatly improved, partly due to the introduction of metal artifact reducing sequences (MARS) such as Slice Encoding for Metal Artifact Correction (SEMAC) and Multi‐Acquisition Variable Resonance Image Combination (MAVRIC).48, 49 The literature shows that when SEMAC is used, distortions caused by metal artifacts are significantly reduced, resulting in more reliable evaluation of soft‐tissue structures.27, 34 Similarly, increased sensitivity and specificity values are found for diagnosing loosening based on periprosthetic osteolysis.34 Therefore, it is conceivable that the use of MARS sequences may further improve the diagnostic properties of MRI after arthroplasty and resolve the inconclusiveness regarding MRI diagnoses of soft tissue and patellofemoral problems.
Many issues in the design and conduct of diagnostic studies can lead to bias or variation. The results of the critical appraisal revealed some interesting methodological challenges related to examining the diagnostic properties of MRI for identifying the causes of complaints after TKA or UKA. When evaluating criterion validity, it is noticeable that the studies' retrospective design10, 19, 25, 29, 31, 33, 35 made them susceptible to selection bias. Due to the retrospective design, the study inclusion criteria occasionally only allowed revision surgery patients who had had a preoperative MRI to be included, with a lack of healthy controls. This made evaluation with the reference standard possible. However, it induces selection bias, and leads to the possibility that sensitivity and specificity values were overestimated.19, 25 Moreover, if image observers had known that there was always some pathology to find on the MRI, this certainly may have led them to overestimate the inter‐ and intrareproducibility values.
Therefore, the optimal study design should be prospective, and the spectrum of patients should include individuals who are likely to undergo imaging to diagnose complaints after knee arthroplasty. However, it is not ethical to evaluate MRI findings with the reference standard perioperative findings when surgery is not indicated. The tension between using a study design that reduces patient selection bias and the possibility of assessing criterion validity justifies the selection of a retrospective design to assess criterion validity. Other general methodological limitations of the studies that were reviewed included insufficient descriptions of sample size determination.
We performed a systematic review to focus on the diagnostic properties of MRI after knee arthroplasty to identify probable causes of complaints (including infection, loosening and wear, instability, malalignment, arthrofibrosis, and patellofemoral complaints). However, the study has some inherent limitations. First, the heterogeneity of the studies included made it impossible to conduct a meta‐analysis. Moreover, this heterogeneity made it difficult to compare the study results and to categorize them according to probable causes of complaints. Second, this study included and compared various types of studies: patient studies, cadaveric studies, TKA studies, and UKA studies. Hence, this review is among the first to systematically present this heterogeneity by categorizing the availability of MRI knowledge per pathology associated with complaints after knee arthroplasty. We believe this study presents a systematic and practical indication of the properties of MRI for diagnosing various causes of complaints after knee replacement.
In conclusion, this study supports that MRI can be used with overall reproducible and accurate results for diagnosing infection, loosening and wear, and malalignment after knee arthroplasty. Nonetheless, definitive conclusions cannot be drawn regarding the diagnostic properties of MRI for diagnosing all probable causes of complaints after knee arthroplasty. Studies regarding the diagnosis of instability, arthrofibrosis or patellofemoral complaints using MRI are limited and inconclusive. When comparing MRI to other diagnostic modalities that asses a problematic TKA, MRI is noninvasive and does not expose the patient to harmful radiation. This makes MRI a promising alternative for assessing a problematic TKA in clinical practice and for further research. Future research should focus on the diagnostic accuracy of MRI for diagnosing complaints after knee arthroplasty in a prospective cohort study using state‐of‐the‐art MRI sequences.
References
- 1. Burn E, Liddle AD, Hamilton TW, et al. Choosing between unicompartmental and total knee replacement: What can economic evaluations tell us? A systematic review. Pharmacoecon Open 2017;1:241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am 2005;87:1487–1497. [DOI] [PubMed] [Google Scholar]
- 3. Lum ZC, Shieh AK, Dorr LD. Why total knees fail‐A modern perspective review. World J Orthop 2018;9:60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirschmann MT, Henckel J, Rasch H. SPECT/CT in patients with painful knee arthroplasty—What is the evidence? Skeletal Radiol 2013;42:1201–1207. [DOI] [PubMed] [Google Scholar]
- 5. Fritz J, Lurie B, Potter HG. MR imaging of knee arthroplasty implants. RadioGraphics 2015;35:1483–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seil R, Pape D. Causes of failure and etiology of painful primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2011;19:1418–1432. [DOI] [PubMed] [Google Scholar]
- 7. Van den Wyngaert T, Palli SR, Imhoff RJ, Hirschmann MT. Cost‐effectiveness of bone SPECT/CT in painful total knee arthroplasty. J Nucl Med 2018;59:1742–1750. [DOI] [PubMed] [Google Scholar]
- 8. Signore A, Sconfienza LM, Borens O, et al. Consensus document for the diagnosis of prosthetic joint infections: A joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement). Eur J Nucl Med Mol Imaging 2019;46:971–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dean Deyle G. The role of MRI in musculoskeletal practice: A clinical perspective. J Man Manip Ther 2011;19:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sofka CM, Potter HG, Figgie M, Laskin R. Magnetic resonance imaging of total knee arthroplasty. Clin Orthop Relat Res 2003:129–135. [DOI] [PubMed] [Google Scholar]
- 11. Heyse TJ, Figiel J, Hähnlein U, et al. MRI after unicondylar knee arthroplasty: The preserved compartments. Knee 2012;19:923–926. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta–analyses: The PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee KY, Slavinsky JP, Ries MD, Blumenkrantz G, Majumdar S. Magnetic resonance imaging of in vivo kinematics after total knee arthroplasty. J Magn Reson Imaging 2005;21:172–178. [DOI] [PubMed] [Google Scholar]
- 14. Prinsen CAC, Mokkink LB, Bouter LM, et al. COSMIN guideline for systematic reviews of patient‐reported outcome measures. Qual Life Res 2018;27:1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McHugh ML. Interrater reliability: The kappa statistic. Biochem Med 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 17. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS‐2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–536. [DOI] [PubMed] [Google Scholar]
- 18. Heyse TJ, Chong LR, Davis J, Boettner F, Haas SB, Potter HG. MRI analysis for rotation of total knee components. Knee 2012;19:571–575. [DOI] [PubMed] [Google Scholar]
- 19. Li AE, Sneag DB, Greditzer HG, Johnson CC, Miller TT, Potter HG. Total knee arthroplasty: Diagnostic accuracy of patterns of synovitis at MR imaging. Radiology 2016;281:499–506. [DOI] [PubMed] [Google Scholar]
- 20. Raphael B, Haims AH, Wu JS, Katz LD, White LM, Lynch K. MRI comparison of periprosthetic structures around zirconium knee prostheses and cobalt chrome prostheses. Am J Roentgenol 2006;186:1771–1777. [DOI] [PubMed] [Google Scholar]
- 21. Heyse TJ, Chong LR, Davis J, Boettner F, Haas SB, Potter HG. MRI analysis of the component‐bone interface after TKA. Knee 2012;19:290–294. [DOI] [PubMed] [Google Scholar]
- 22. Heyse TJ, Chong LR, Davis J, Haas SB, Figgie MP, Potter HG. MRI diagnosis of patellar clunk syndrome following total knee arthroplasty. HSS J 2012;8:92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heyse TJ, Figiel J, Hähnlein U, et al. MRI after unicondylar knee arthroplasty: Rotational alignment of components. Arch Orthop Trauma Surg 2013;133:1579–1586. [DOI] [PubMed] [Google Scholar]
- 24. Heyse TJ, Stiehl JB, Tibesku CO. Measuring tibial component rotation of TKA in MRI: What is reproducible? Knee 2015;22:604–608. [DOI] [PubMed] [Google Scholar]
- 25. Li AE, Johnson CC, Sneag DB, et al. Frondlike synovitis on MRI and correlation with polyethylene surface damage of total knee arthroplasty. Am J Roentgenol 2017;209:W231–W237. [DOI] [PubMed] [Google Scholar]
- 26. Murakami AM, Hash TW, Hepinstall MS, Lyman S, Nestor BJ, Potter HG. MRI evaluation of rotational alignment and synovitis in patients with pain after total knee replacement. J Bone Joint Surg B 2012;94 B:1209–1215. [DOI] [PubMed] [Google Scholar]
- 27. Jawhar A, Reichert M, Kostrzewa M, et al. Usefulness of slice encoding for metal artifact correction (SEMAC) technique for reducing metal artifacts after total knee arthroplasty. European J Orthop Surg Traumatol 2019;29:659–666. [DOI] [PubMed] [Google Scholar]
- 28. Malcherczyk D, Figiel J, Hähnlein U, Susanne FW, Efe T, Heyse TJ. MRI following UKA: The component‐bone interface. Acta Orthop Belg 2015;81:84–89. [PubMed] [Google Scholar]
- 29. Plodkowski AJ, Hayter CL, Miller TT, Nguyen JT, Potter HG. Lamellated hyperintense synovitis: Potential MR imaging sign of an infected knee arthroplasty. Radiology 2013;266:256–260. [DOI] [PubMed] [Google Scholar]
- 30. Sgroi M, Faschingbauer M, Javaheripour‐Otto K, Reichel H, Kappe T. Can rotational alignment of total knee arthroplasty be measured on MRI? Arch Orthop Trauma Surg 2015;135:1589–1594. [DOI] [PubMed] [Google Scholar]
- 31. Park CN, Zuiderbaan HA, Chang A, Khamaisy S, Pearle AD, Ranawat AS. Role of magnetic resonance imaging in the diagnosis of the painful unicompartmental knee arthroplasty. Knee 2015;22:341–346. [DOI] [PubMed] [Google Scholar]
- 32. Minoda Y, Yoshida T, Sugimoto K, Baba S, Ikebuchi M, Nakamura H. Detection of small periprosthetic bone defects after total knee arthroplasty. J Arthroplasty 2014;29:2280–2284. [DOI] [PubMed] [Google Scholar]
- 33. Li AE, Sneag DB, Miller TT, Lipman JD, Padgett DE, Potter HG. MRI of polyethylene tibial inserts in total knee arthroplasty: Normal and abnormal appearances. AJR Am J Roentgenol 2016;206:1264–1271. [DOI] [PubMed] [Google Scholar]
- 34. Sutter R, Hodek R, Fucentese SF, Nittka M, Pfirrmann CW. Total knee arthroplasty MRI featuring slice‐encoding for metal artifact correction: Reduction of artifacts for STIR and proton density‐weighted sequences. AJR Am J Roentgenol 2013;201:1315–1324. [DOI] [PubMed] [Google Scholar]
- 35. Vessely MB, Frick MA, Oakes D, Wenger DE, Berry DJ. Magnetic resonance imaging with metal suppression for evaluation of periprosthetic osteolysis after total knee arthroplasty. J Arthroplasty 2006;21:826–831. [DOI] [PubMed] [Google Scholar]
- 36. Solomon LB, Stamenkov RB, MacDonald AJ, et al. Imaging periprosthetic osteolysis around total knee arthroplasties using a human cadaver model. J Arthroplasty 2012;27:1069–1074. [DOI] [PubMed] [Google Scholar]
- 37. Minoda Y, Yamamura K, Sugimoto K, Mizokawa S, Baba S, Nakamura H. Detection of bone defects around zirconium component after total knee arthroplasty. Knee 2017;24:844–850. [DOI] [PubMed] [Google Scholar]
- 38. Meftah M, Potter HG, Gold S, Ranawat AS, Ranawat AS, Ranawat CS. Assessment of reactive synovitis in rotating‐platform posterior‐stabilized design: A 10‐year prospective matched‐pair mri study. J Arthroplasty 2013;28:1551–1555. [DOI] [PubMed] [Google Scholar]
- 39. Atkins BL, Athanasou N, Deeks JJ, et al. Prospective evaluation of criteria for microbiological diagnosis of prosthetic‐joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol 1998;36:2932–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pakos EE, Trikalinos TA, Fotopoulos AD, Ioannidis JP. Prosthesis infection: Diagnosis after total joint arthroplasty with antigranulocyte scintigraphy with 99mTc‐labeled monoclonal antibodies—A meta‐analysis. Radiology 2007;242:101–108. [DOI] [PubMed] [Google Scholar]
- 41. Koff MF, Esposito C, Shah P, et al. MRI of THA correlates with implant wear and tissue reactions: A cross‐sectional study. Clin Orthop Relat Res 2019;477:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Higuera C, Parvizi J. 18 Causes and Diagnosis of Aseptic Loosening AfterTotal Knee Replacement. In: Hirschmann MT, Becker R, editors. The Unhappy Total KneeReplacement: A Comprehensive Review and Management Guide. Cham: SpringerInternational Publishing; 2015. p 225–237.
- 43. Victor J. Rotational alignment of the distal femur: A literature review. Orthop Traumatol Surg Res 2009;95:365–372. [DOI] [PubMed] [Google Scholar]
- 44. Panni AS, Ascione F, Rossini M, et al. Tibial internal rotation negatively affects clinical outcomes in total knee arthroplasty: A systematic review. Knee Surg Sports Traumatol Arthrosc 2018;26:1636–1644. [DOI] [PubMed] [Google Scholar]
- 45. Thompson R, Novikov D, Cizmic Z, et al. Arthrofibrosis after total knee arthroplasty: Pathophysiology, diagnosis, and management. Orthop Clin N Am 2019;50:269–279. [DOI] [PubMed] [Google Scholar]
- 46. Mariani S, La Marra A, Arrigoni F, et al. Dynamic measurement of patello‐femoral joint alignment using weight‐bearing magnetic resonance imaging (WB‐MRI). Eur J Radiol 2015;84:2571–2578. [DOI] [PubMed] [Google Scholar]
- 47. Wendt RE 3rd, Wilcott MR 3rd, Nitz W, Murphy PH, Bryan RN. MR imaging of susceptibility‐induced magnetic field inhomogeneities. Radiology 1988;168:837–841. [DOI] [PubMed] [Google Scholar]
- 48. Koch KM, Lorbiecki JE, Hinks RS, King KF. A multispectral three‐dimensional acquisition technique for imaging near metal implants. Magn Reson Med 2009;61:381–390. [DOI] [PubMed] [Google Scholar]
- 49. Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: Slice encoding for metal artifact correction in MRI. Magn Reson Med 2009;62:66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Potter HG, Foo LF. Magnetic resonance imaging of joint arthroplasty. Orthop Clin 2006;37:361–373. [DOI] [PubMed] [Google Scholar]
- 51. Kurosaka M, Yoshiya S, Mizuno K, Yamamoto T. Maximizing flexion after total knee arthroplasty: The need and the pitfalls. J Arthroplasty 2002;17(4 Suppl 1):59–62. [DOI] [PubMed] [Google Scholar]


